Abstract
Alcohol withdrawal syndrome (AWS) is a medical emergency situation which appears after abrupt cessation of ethanol intake. Decreased GABA-A function and increased glutamate function are known to exist in the AWS. However, the involvement of glutamate transporters in the context of AWS requires further investigation. In this study, we used a model of ethanol withdrawal involving abrupt cessation of binge ethanol administration (4 g/kg/gavage three times a day for three days) using male alcohol-preferring (P) rats. After 48 hours of withdrawal, P rats were re-exposed to voluntary ethanol intake. The amount of ethanol consumed was measured during post-withdrawal phase. In addition, the expression of GLT-1, GLAST and xCT were determined in both medial prefrontal cortex (mPFC) and nucleus accumbens (NAc). We also measured glutamine synthetase (GS) activity, and the tissue content of glutamate, glutamine, dopamine and serotonin in both mPFC and NAc. We found that binge ethanol withdrawal escalated post-withdrawal ethanol intake, which was associated with downregulation of GLT-1 expression in both mPFC and NAc. The expression of GLAST and xCT were unchanged in the ethanol-withdrawal (EW) group compared to control group. Tissue content of glutamate was significantly lower in both mPFC and NAc, whereas tissue content of glutamine was higher in mPFC but unchanged in NAc in the EW group compared to control group. The GS activity was unchanged in both mPFC and NAc. The tissue content of DA was significantly lower in both mPFC and NAc, whereas tissue content of serotonin was unchanged in both mPFC and NAc. These findings provide important information of the critical role of GLT-1 in context of AWS.
Keywords: Binge ethanol, GLT-1, glutamate, dopamine, glutamine synthetase
1. INTRODUCTION
Alcohol withdrawal syndrome (AWS) comprises of signs and symptoms (autonomic/neuropsychiatric) that appear for up to 48 hours after abrupt cessation of binge ethanol drinking (Murdoch and Marsden, 2014). The underlying neuropathology of AWS involves decreased GABA-A inhibitory function and increased glutamatergic excitatory activity leading to rebound hyper-neuroexcitability, irritability, and in some cases seizures (Finn and Crabbe, 1997, Longo et al., 2011, Abulseoud et al., 2014). The first line treatment for AWS is benzodiazepines, which target GABAergic pathways (Mayo-Smith, 1997, Amato et al., 2011, Abulseoud et al., 2014). Other approaches to deal with AWS include blockade of the NMDA receptor (e.g. acamprosate) (Brust, 2014, Liang and Olsen, 2014) and the use of conjunctive agents (cardiovascular agents such as clonidine, propranolol and magnesium sulfate; and vitamins) for symptomatic relief.
The ultimate target of most neurochemical agents when treating the AWS is to restore balance between excitatory and inhibitory neurotransmission. The homeostasis of glutamate, the major excitatory neurotransmitter, is maintained through equilibrium between glutamate uptake and glutamate release in or out of neighboring astrocytes, respectively (Kalivas, 2009). Glutamate uptake into astrogial cells and neurons is mediated through Na+-dependent transmembrane proteins belonging to the solute carrier 1 (SLC1) family. To date, five subtypes of excitatory amino acid transporters (EAAT) have been identified: EAAT 1–5. EAAT-1/glutamate aspartate transporter (GLAST) expressed in astroglial cells (Lehre et al., 1995), EAAT 2 (GLT-1) is expressed primarily in astroglial cells (also expressed to a lower extent in neurons) (Chen et al., 2004, Fontana, 2015), EAAT 3 is expressed in neuronal cell bodies and dendrites (Rothstein et al., 1994), EAAT 4 is primarily expressed in cerebellar Purkinje cells (Gincel et al., 2007) and EAAT 5 is expressed in vertebrate retina (Arriza et al., 1997). Thus, EAAT 1/GLAST and EAAT 2/GLT-1 play a pivotal role for tight regulation of extra-synaptic glutamate in medial prefrontal cortex (mPFC) and nucleus accumbens (NAc); and are a focus of this study. It is important to note that GLT-1 removes the majority of extracellular glutamate (Danbolt, 2001, Mitani and Tanaka, 2003). Furthermore, there is the existence of another glial transporter protein, cystine/glutamate exchanger (xCT), which regulates the release of glutamate from astrocytes in exchange for cystine (Warr et al., 1999, Melendez et al., 2005).
In clinics, less is known about the involvement of glutamate transporters in hyper-neuroexcitation in patients undergoing AWS, which may be a promising target for drug treatment. The effects of imported glutamate within astrocytes include: formation of glutamine by glutamine synthetase (GS) enzyme, exchanging for cystine to the extracellular space through xCT, and subsequent formation of glutathione. The formed glutamine within astrocytes is further used up by neurons, and this recycle process is termed the glutamate-glutamine cycle (Thoma et al., 2011). Thus, GLT-1, xCT and GLAST play key roles in regulating extracellular glutamate concentration in the brain.
In this study, we used a model of ethanol withdrawal to simulate the context of AWS involving abrupt cessation of binge ethanol intake (4 g/kg/gavage three times a day for three days) in alcohol preferring (P) rats. We tested the effects of binge ethanol withdrawal on post-withdrawal ethanol intake as well as expression of GLT-1, xCT and GLAST in mPFC and NAc. It is noteworthy that glutamatergic projections from the PFC to the NAc has been shown to initiate adaptive behaviors, and stimulation of either region increases drug seeking (Moussawi and Kalivas, 2010). Thus, mPFC and NAc were areas of focus in the current study because of their crucial role in drug dependence. Beside glutamate transporter expression, we determined the tissue content of glutamate and glutamine as well as GS activity to test the effects of binge ethanol withdrawal on the glutamate-glutamine cycle. Since ethanol acts as a positive reinforcer, we hypothesized that binge ethanol withdrawal would affect dopamine (DA) and serotonin (5-HT) concentrations. Therefore, we also determined the tissue content of DA and 5-HT in both mPFC and NAc.
2. METHODS AND MATERIALS
2.1. Animals
Male P rats were acquired from Indiana University School of Medicine, Indianapolis; IN. Rats were singly housed in a room with 21°C temperature and 50% humidity in a 12h light/dark cycle. Rats had unrestricted food and water throughout the whole experimental procedure. Institutional Animal Care and Use Committee (IACUC) of The University of Toledo approved all the experimental procedures in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission of Life sciences, 1996).
2.2. Voluntary Ethanol Drinking and Ethanol Gavage Procedures
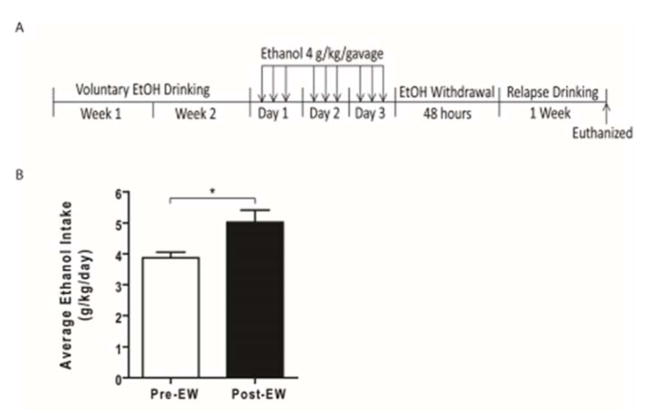
Three-month-old male P rats were exposed to voluntary drinking of ethanol (15% and 30%, v/v, concurrently), and/or water for two weeks (Fig. 1A). P rats then received ethanol through oral gavage needle at a dose of 4 g/kg/gavage (infusion volume: 4–5.7 ml) three times a day (12 g ethanol/kg/day, made from 40% v/v ethanol) for three days. After completion of the ethanol gavage treatment, rats went through 48 hours of ethanol withdrawal. After 48 hours of withdrawal, ethanol-gavaged P rats were re-exposed to voluntary ethanol drinking (15% and 30%, concurrently) for one week. Control (Ctrl) rats received water through oral gavage at same time-points as the ethanol-gavaged group.
Figure 1.
Effect of EW on post-withdrawal ethanol drinking. A) Timeline of ethanol drinking and withdrawal period. B) EW significantly increased the post-withdrawal ethanol drinking compared to pre-withdrawal drinking (*, p<0.05). All data are expressed as mean ± SEM. n =7. EW, ethanol withdrawal.
2.3. Western Blot
After one-week of post-withdrawal voluntary ethanol drinking, P rats were euthanized with carbon dioxide inhalation and brains were removed, and flash frozen in dry ice. mPFC and NAc were dissected in a cryostat apparatus according to Paxinos and Watson (1998). Expression of GLT-1, xCT, GLAST and GAPDH were determined through western blot procedure as previously described (Alhaddad et al., 2014, Das et al., 2015). Briefly, extracted proteins were separated through SDS-PAGE and transferred onto PVDF membrane electrophoretically. Then PVDF membranes were incubated overnight at 4°C with one of the following primary antibodies: guinea pig anti-GLT-1 (Millipore), rabbit anti-xCT (Abcam), rabbit anti-GLAST (Abcam), and mouse anti-GAPDH (Millipore). Protein detection was performed using a chemiluminescent kit and developed onto HyBlot CL film (Denville Scientific Inc). Blots were digitized and quantified with MCID system. Data were calculated as ratios of GLT-1/, xCT/and GLAST/GAPDH.
2.4. HPLC Quantification of Glutamate and Glutamine
Glutamate and glutamine concentrations in mPFC and NAc were simultaneously analyzed using an HPLC system with electrochemical detection as previously described (Reader et al., 1998, Das et al., 2015). Briefly, dissected brain samples were homogenized in distilled water with pestle followed by heating at 98°C for 5 min. The samples were then centrifuged at 10,000 rpm at 4°C for 5 min. After centrifugation, supernatants were collected and filtered through 0.22 μm filters, and pellets were tested for protein quantification. O-phthalaldehyde and sodium sulfite were used for pre-column derivatization of filtered supernatants with an ESA Model 540 autosampler before injecting onto a C18 column (3.0 × 50 mm, 2.5 μm particle size, Waters, Inc.). The mobile phase consisted of 0.1M Na2HPO4, 0.1mM EDTA, and 7.5% Methanol (pH 3.0). The CoulArray coulometric detector (model 5600A, ESA, Inc.) was used for detection of glutamate and glutamine, and the chromatograms were collected through CoulArray software. The concentrations of glutamate and glutamine were analyzed by peak area and compared with external standards. Tissue pellets were resuspended in 1N NaOH and protein concentration was determined using the Lowry method (Lowry et al., 1951). The concentrations of glutamate and glutamine were normalized relative to protein content.
2.5. HPLC Quantification of Monoamines
DA and 5-HT concentrations in mPFC and NAc were analyzed with an HPLC-EC system as previously described (Breier et al., 2006, Halpin and Yamamoto, 2012). Briefly, dissected brain regions were sonicated in 0.25N perchloric acid followed by centrifugation at 14000 × g for 20 min at 4°C. The supernatants were filtered (0.22 μm filter) and injected onto a C18 column (3.2 × 150 mm, 3μm particle size, Thermo Scientific). The mobile phase consisted of 32 mM citric acid, 54.3 mM sodium phosphate, 0.215 mM octyl sodium sulphate, and 11% methanol (pH 4.4). The CoulArray coulometric detector (model 5600A, ESA, Inc.) was used for detection of DA and 5-HT. The concentrations of DA and 5-HT were determined by peak area and compared with external standards. Pellets obtained through centrifugation were resuspended in 1N NaOH and protein content was determined using the Lowry method. The concentrations of DA and 5-HT were normalized relative to respective protein content.
2.6. Glutamine Synthetase (GS) Activity Assay
GS activity was measured as previously described (Miller et al., 1978, Das et al., 2015). Briefly, tissue homogenates were mixed with assay mixtures (50 mM imidazole chloride (pH 7.4), 50 mM L-glutamine, 25 mM hydroxylamine, 25 mM Na-arsenate, 2 mM MnCl2 and 0.16 mM ADP) followed by incubation at 37°C for 30 min. The reaction was then stopped by addition of GS stop solution (2.42% FeCl3, 1.45% trichloroacetic acid and 1.82 N HCl). Parallel incubations of tissue homogenates with assay mixtures lacking Na-arsenate and ADP served as control.
2.7. Statistical Analysis
All statistical analyses were performed using GraphPad Prism software. Pre- and post-withdrawal ethanol intakes were compared with paired t-test. The comparison between control and ethanol-withdrawal (EW) groups were conducted with unpaired t-test. The statistical significance was set at p< 0.05.
3. RESULTS
3.1. Effect of binge ethanol withdrawal on post-withdrawal ethanol intake
We measured post-withdrawal ethanol intake during one-week period of relapse to ethanol drinking. Paired t-test revealed that post-withdrawal ethanol drinking was significantly higher compared to pre-withdrawal drinking (t(12) = 2.960, p=0.025) (Fig. 1B).
3.2. Effect of binge ethanol withdrawal on GLT-1, xCT and GLAST expression as well as GS activity in mPFC and NAc
Expression of GLT-1, xCT and GLAST were determined using Western blot assay. Unpaired t-test revealed that GLT-1 expression was significantly lower in mPFC of the EW group compared to control group (t(13) = 3.316, p= 0.005) (Fig. 2A). However, unpaired t-test showed no significant difference in expression of GLAST (t(13) = 0.079, p=0.938) (Fig. 2B) and xCT (t(13) = 0.225, p= 0.825) (Fig. 3A) in mPFC of the EW group compared to control group.
Figure 2.
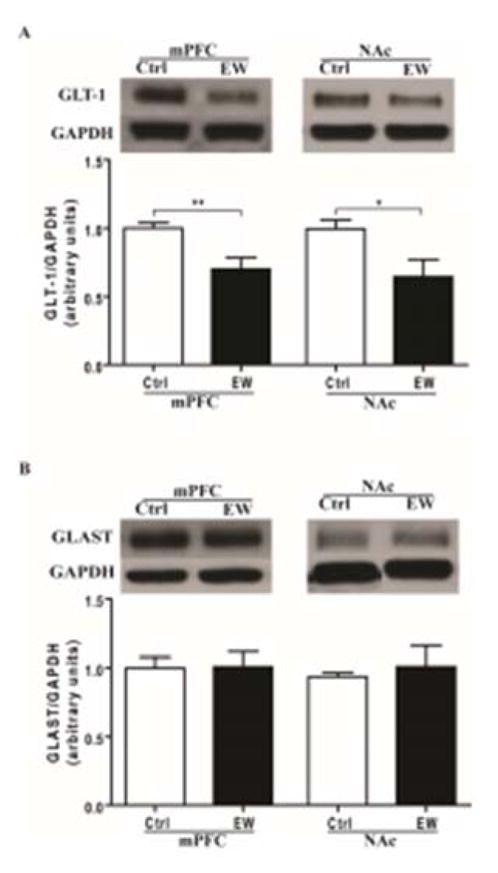
Effects of EW on expression of GLT-1 and GLAST in mPFC and NAc. A) GLT-1 expression was significantly downregulated in the EW group compared to control group in both mPFC (**, p<0.01) and NAc (*, p<0.05). B) GLAST expression was unchanged in the EW group compared to control group in both mPFC and NAc. All data are expressed as mean ± SEM. n= 7–8 rats/group. Ctrl, control; EW, ethanol-withdrawal.
Figure 3.
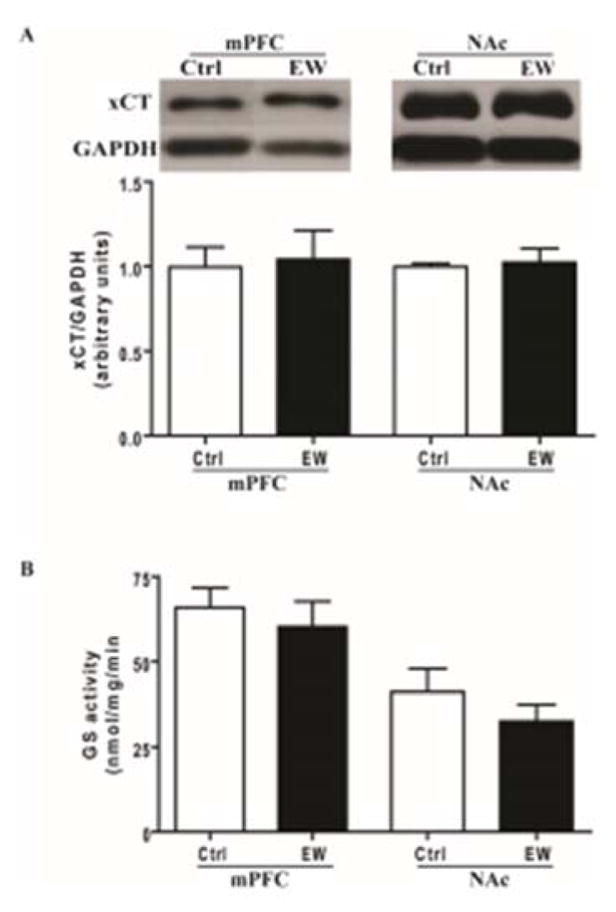
Effects of EW on expression of xCT and GS activity in both mPFC and NAc. A) The expression of xCT was not significantly different in the EW group compared to control group in both mPFC and NAc. B) The GS activity was not significantly different in EW group compared to control group in both mPFC and NAc. All data are expressed as mean ± SEM. n= 7–8 rats/group. Ctrl, control; EW, ethanol-withdrawal.
Unpaired t-test showed no significant difference in expression of GLAST (t(13) = 0.507, p=0.620) (Fig. 2A) and xCT (t(13) = 0.336, p=0.741) (Fig. 3A) in NAc of the EW group compared to control group. In addition, unpaired t-test showed no significant difference in GS activity in mPFC (t(13) = 0.611, p=0.551) and NAc (t(13) = 0.982, p=0.344) of the EW group compared to control group (Fig. 3B).
3.3. Effects of binge ethanol withdrawal on tissue content of glutamate and glutamine in mPFC and NAc
Tissue content of glutamate and glutamine was determined in mPFC and NAc using HPLC-EC system. Unpaired t-test revealed that tissue content of glutamate was significantly lower in mPFC of the EW group compared to control group (t(12) = 2.795, p= 0.016) (Fig. 4A). However, unpaired t-test showed significantly higher tissue content of glutamine in mPFC of the EW group compared to control group (t(13) = 2.504, p=0.026) (Fig. 4B).
Figure 4.
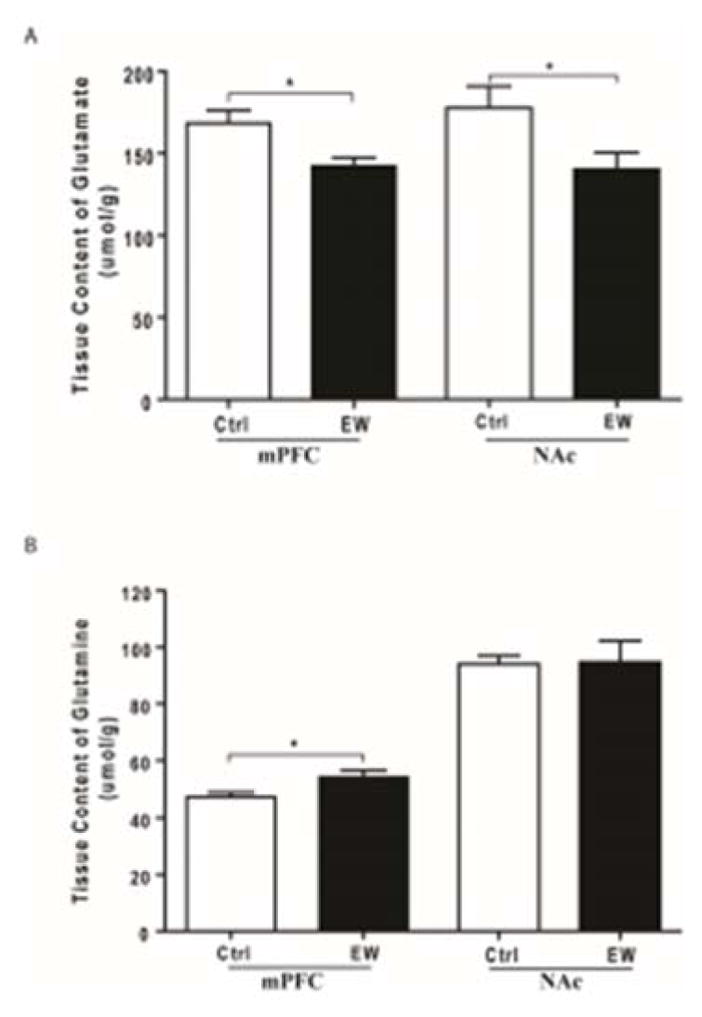
Effects of EW on tissue content of glutamate (A) and glutamine (B) in both mPFC and NAc. A) Tissue content of glutamate was significantly lower in both mPFC and NAc of the EW group compared to control group (*, p<0.05). B) Tissue content of glutamine was significantly higher in mPFC of the EW group compared to control group (*, p<0.05). Tissue content of glutamine was unchanged in NAc of the EW group compared to control group. All data are expressed as mean ± SEM. All data are expressed as mean ± SEM. n=6–8 rats/group. Ctrl, control; EW, ethanol-withdrawal.
Similarly, unpaired t-test revealed that tissue content of glutamate was significantly lower in NAc of the EW group compared to control group (t(10) = 2.230, p= 0.049) (Fig. 4A). Surprisingly, unpaired t-test showed no significant difference in tissue content of glutamine in NAc of the EW group compared to control group (t(14) = 0.092, p= 0.927) (Fig. 4B).
3.4. Effects of binge ethanol withdrawal on tissue content of dopamine in mPFC and NAc
Tissue content of DA and 5-HT was measured in mPFC and NAc using HPLC-EC system. Unpaired t-test revealed that tissue content of DA was significantly lower in mPFC (t(14) = 3.185, p=0.006) and NAc (t(11) = 2.462, p=0.031) of the EW group compared to control group (Fig. 5A). However, unpaired t-test revealed no significant difference in tissue content of 5-HT in mPFC (t(13) = 0.155, p=0.878) and NAc (t(12) = 1.151, p=0.272) of the EW group compared to control group (Fig. 5B).
Figure 5.
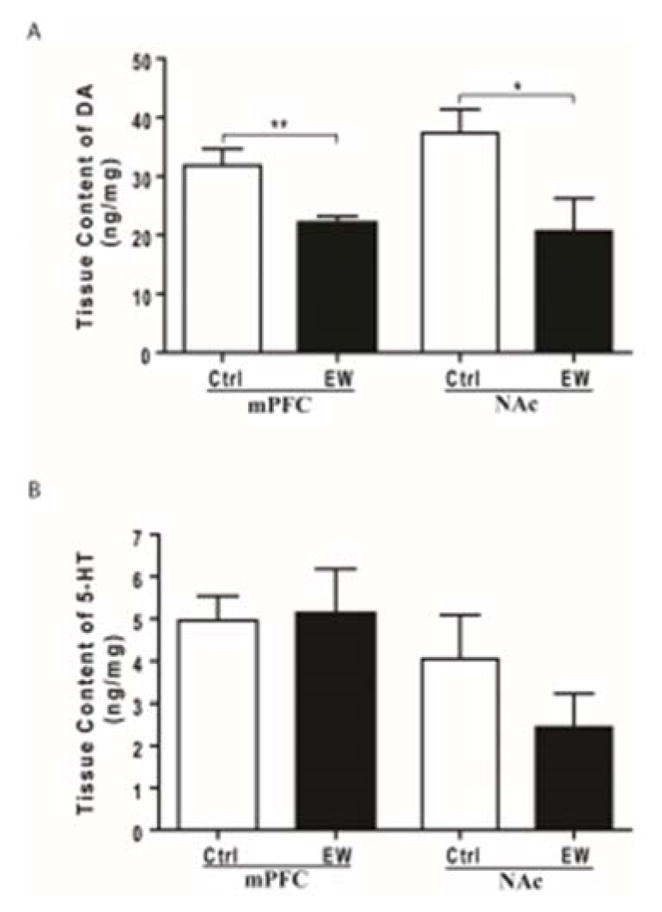
Effects of EW on tissue content of DA (A) and 5-HT (B) in both mPFC and NAc. A) Tissue content of dopamine (DA) was significantly lower in both PFC (**, p<0.01) and NAc (*, p<0.05) of the EW group compared to control group. B) Tissue content of serotonin (5-HT) was unaltered in both mPFC and NAc of the EW group compared to control group. All data are represented as mean ± SEM. n= 6–8 rats/group. Ctrl, control; EW, ethanol-withdrawal.
4. DISCUSSION
In this study, we found that binge ethanol withdrawal significantly increased the post-withdrawal ethanol drinking compared to pre-withdrawal drinking. This withdrawal model used in this experiment has been successfully used in previous studies investigating the induction of physical dependence and withdrawal signs through abrupt cessation of binge ethanol intake (9–15 g/kg/day) for 3–4 days (Majchrowicz, 1975, Abulseoud et al., 2014). Withdrawal-induced escalation of ethanol drinking, observed in this study, is consistent with previous studies showing elevated ethanol drinking following intermittent ethanol exposure and/or withdrawal period (Carrara-Nascimento et al., 2013, Abulseoud et al., 2014).
Interestingly, we observed in this study that binge ethanol withdrawal was associated with downregulation of GLT-1 expression in both mPFC and NAc. In contrast to this present finding, we previously reported that five weeks of voluntary ethanol drinking downregulated GLT-1 expression in NAc, but not in mPFC (Sari and Sreemantula, 2012, Goodwani et al., 2015). This discrepancy might be attributed to the use of binge ethanol exposure and severe withdrawal vs five weeks of voluntary ethanol drinking. Some other previous reports showed no change in expression of GLT-1 in PFC (Alele and Devaud, 2005) or NAc (Melendez et al., 2005, Ding et al., 2013) following systemic administration or voluntary drinking of ethanol. However, our present finding of downregulated GLT-1 expression is consistent with a previous report showing downregulation of GLT-1 expression in mPFC and NAc with similar ethanol withdrawal paradigm (Abulseoud et al., 2014). GLT-1 downregulation or knockdown was frequently reported to be associated with increased extracellular glutamate concentration (Rothstein et al., 1996, Das et al., 2015). Since AWS is well known to be associated with increased excitatory and decreased inhibitory neurotransmission in the brain, our findings provide evidence that GLT-1 could be a potential therapeutic target for decreasing excitatory neurotransmission and alleviating AWS.
However, in this current study, we didn’t find any changes in expression of GLAST in mPFC and NAc. GLAST plays a predominant role for glutamate uptake in cerebellum, whereas GLT-1 plays predominant role for glutamate uptake in mPFC and NAc (Abulseoud et al., 2014). Thus, GLAST might play a different role in mPFC and NAc following voluntary ethanol drinking. Our current finding with GLAST expression is consistent with previous reports showing no change of GLAST expression following voluntary ethanol drinking (chronic or relapse-like) (Alhaddad et al., 2014) or systemic ethanol administration (Melendez et al., 2005).
Similar to GLAST expression, the current study revealed that the expression of xCT was unaltered in both mPFC and NAc. xCT, catalytic subunit of system , pump out astrocytic glutamate into extracellular space in exchange for cystine. Thus, xCT-activity contributes about 60% to the extracellular glutamate concentration (Kalivas, 2009). There is compelling evidence that basal glutamate concentration increased in various brain regions following ethanol dependence (Melendez et al., 2005, Ding et al., 2013, Das et al., 2015). Our present finding suggests that the expression of xCT doesn’t contribute to increased basal glutamate concentration, at least not in rats that have undergone severe ethanol withdrawal. Our present finding with xCT expression is consistent with previous literature showing unchanged xCT expression or activity following ethanol dependence (Ding et al., 2013, Alhaddad et al., 2014, Griffin et al., 2015). Taken together, only GLT-1 expression was downregulated among GLT-1, GLAST and xCT expression following severe withdrawal in both the mPFC and NAc of P rats.
GLT-1 clears the majority of extracellular glutamate into astrocytes and imported glutamate is converted into glutamine by GS. Thus, we hypothesized that GS activity would have been affected following severe ethanol withdrawal. Surprisingly, GS activity was not altered in either mPFC or NAc. Our finding differs from a previous report showing increased packing density of GS-immunoreactive astrocytes in cortex of P rats withdrawn from two- and six-months ethanol exposure (Miguel-Hidalgo, 2006). This difference can be attributed to severe withdrawal (48 hours) followed by one week relapse vs withdrawal (three days) from voluntary ethanol drinking with no relapse drinking. However, our current finding is consistent with our previous report showing no change in GS activity following five weeks of voluntary ethanol drinking (Das et al., 2015).
In this current study, we also determined the tissue content of glutamate and glutamine in both the mPFC and NAc. Interestingly, tissue content of glutamate was significantly lower in both mPFC and NAc. Tissue content of glutamine was significantly higher in mPFC, but unchanged in NAc. It is well established that ethanol withdrawal is associated with increased extracellular glutamate concentration in striatum (Rossetti and Carboni, 1995, Rossetti et al., 1999), NAc (Hinton et al., 2012) and hippocampus (Dahchour and De Witte, 2003), but not in mPFC (Hinton et al., 2012). However, tissue content of glutamate doesn’t differentiate between the extracellular and intracellular pool, and thus the results become difficult to interpret. The reason behind the present finding of decreased tissue content of glutamate remains unknown, but the findings confirm altered glutamate homeostasis following severe withdrawal. The increased tissue content of glutamine in PFC might be due to the decreased conversion of glutamine into glutamate since GS activity was unchanged. The unchanged tissue concentration of glutamine in NAc suggests differential adaptations in mPFC and NAc following severe withdrawal. However, our current finding of decreased glutamate and increased glutamine content is consistent with a previous study conducted on human volunteers, through proton magnetic resonance spectroscopy, with alcohol dependence (Thoma et al., 2011).
Finally, we tested the hypothesis that relapse to ethanol following severe withdrawal would affect the tissue content of dopamine (DA) and serotonin (5-HT) in mPFC and/or NAc. Tissue content of DA was significantly lower in both the mPFC and NAc. Ethanol (as a positive reinforcer) is known to increase dopamine in NAc whereas ethanol withdrawal is associated with marked decreases in DA concentration in both mPFC and NAc (Weiss and Porrino, 2002, Carlson and Drew Stevens, 2006). Our present finding with DA suggests that severe withdrawal disrupts dopamine concentration, which might be a driving force to relapse. It is also evident that depleted DA was not reversed by one-week relapse to ethanol drinking. However, tissue content of 5-HT was unchanged in both mPFC and NAc. Withdrawal from chronic ethanol intake had been shown to markedly deplete 5-HT in NAc of ethanol-dependent rats and depleted 5-HT was reversed through ethanol re-exposure (Weiss et al., 1996). Thus, unchanged tissue content of 5-HT in both mPFC and NAc in the current study might be attributed to one-week relapse to ethanol drinking.
We conclude that binge ethanol withdrawal escalated post-withdrawal ethanol drinking in association with downregulation of GLT-1 in both mPFC and NAc. Besides, differential alteration in tissue contents of glutamate and glutamine in mPFC and NAc suggested the disruption of glutamate-glutamine cycle with unchanged GS activity. Binge ethanol withdrawal was also associated with marked depletion of DA in both mPFC and NAc. Taken together, the present study implicates GLT-1 as a potential target of drugs for treating AWS.
HIGHLIGHTS.
Binge ethanol withdrawal escalated post-withdrawal ethanol intake
Binge ethanol withdrawal down-regulated GLT-1 in both mPFC and NAc
Binge ethanol withdrawal altered tissue content of glutamate and glutamine in mPFC and NAc
Binge ethanol withdrawal depleted DA content in both mPFC and NAc
Acknowledgments
This research study was conducted with the support of Award Number R01AA019458 (Y.S.) from National Institutes on Alcohol Abuse and Alcoholism (NIAAA). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism or the National Institutes of Health (NIH). The authors would like to thank Dr. Osama A Abulseoud for his valuable suggestions for ethanol gavage procedure.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abulseoud OA, Camsari UM, Ruby CL, Kasasbeh A, Choi S, Choi DS. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:1674–1684. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alele PE, Devaud LL. Differential adaptations in GABAergic and glutamatergic systems during ethanol withdrawal in male and female rats. Alcoholism, clinical and experimental research. 2005;29:1027–1034. doi: 10.1097/01.alc.0000167743.96121.40. [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology. 2014;231:4049–4057. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. The Cochrane database of systematic reviews. 2011:CD008537. doi: 10.1002/14651858.CD008537.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breier JM, Bankson MG, Yamamoto BK. L-tyrosine contributes to (+)-3,4-methylenedioxymethamphetamine-induced serotonin depletions. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:290–299. doi: 10.1523/JNEUROSCI.3353-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brust JC. Acute withdrawal: diagnosis and treatment. Handbook of clinical neurology. 2014;125:123–131. doi: 10.1016/B978-0-444-62619-6.00008-2. [DOI] [PubMed] [Google Scholar]
- Carlson JN, Drew Stevens K. Individual differences in ethanol self-administration following withdrawal are associated with asymmetric changes in dopamine and serotonin in the medial prefrontal cortex and amygdala. Alcoholism, clinical and experimental research. 2006;30:1678–1692. doi: 10.1111/j.1530-0277.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Carrara-Nascimento PF, Lopez MF, Becker HC, Olive MF, Camarini R. Similar ethanol drinking in adolescent and adult C57BL/6J mice after chronic ethanol exposure and withdrawal. Alcoholism, clinical and experimental research. 2013;37:961–968. doi: 10.1111/acer.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Mahadomrongkul V, Berger UV, Bassan M, DeSilva T, Tanaka K, Irwin N, Aoki C, Rosenberg PA. The glutamate transporter GLT1a is expressed in excitatory axon terminals of mature hippocampal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:1136–1148. doi: 10.1523/JNEUROSCI.1586-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahchour A, De Witte P. Excitatory and inhibitory amino acid changes during repeated episodes of ethanol withdrawal: an in vivo microdialysis study. European journal of pharmacology. 2003;459:171–178. doi: 10.1016/s0014-2999(02)02851-0. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progress in neurobiology. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Das SC, Yamamoto BK, Hristov AM, Sari Y. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, Rodd ZA, Engleman EA, Bailey JA, Lahiri DK, McBride WJ. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addiction biology. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Crabbe JC. Exploring alcohol withdrawal syndrome. Alcohol health and research world. 1997;21:149–156. [PMC free article] [PubMed] [Google Scholar]
- Fontana AC. Current approaches to enhance glutamate transporter function and expression. Journal of neurochemistry. 2015;134:982–1007. doi: 10.1111/jnc.13200. [DOI] [PubMed] [Google Scholar]
- Gincel D, Regan MR, Jin L, Watkins AM, Bergles DE, Rothstein JD. Analysis of cerebellar Purkinje cells using EAAT4 glutamate transporter promoter reporter in mice generated via bacterial artificial chromosome-mediated transgenesis. Experimental neurology. 2007;203:205–212. doi: 10.1016/j.expneurol.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Goodwani S, Rao PS, Bell RL, Sari Y. Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain research. 2015;1622:397–408. doi: 10.1016/j.brainres.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin WC, Ramachandra VS, Knackstedt LA, Becker HC. Repeated cycles of chronic intermittent ethanol exposure increases basal glutamate in the nucleus accumbens of mice without affecting glutamate transport. Frontiers in pharmacology. 2015;6:27. doi: 10.3389/fphar.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin LE, Yamamoto BK. Peripheral ammonia as a mediator of methamphetamine neurotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:13155–13163. doi: 10.1523/JNEUROSCI.2530-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DJ, Lee MR, Jacobson TL, Mishra PK, Frye MA, Mrazek DA, Macura SI, Choi DS. Ethanol withdrawal-induced brain metabolites and the pharmacological effects of acamprosate in mice lacking ENT1. Neuropharmacology. 2012;62:2480–2488. doi: 10.1016/j.neuropharm.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nature reviews Neuroscience. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Olsen RW. Alcohol use disorders and current pharmacological therapies: the role of GABA(A) receptors. Acta pharmacologica Sinica. 2014;35:981–993. doi: 10.1038/aps.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo D, Fauci A, Kasper D, Hauser S. Harrison’s Principles of Internal Medicine. 18. McGraw-Hill Professional; 2011. [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. The Journal of biological chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacologia. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. Jama. 1997;278:144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- Melendez RI, Hicks MP, Cagle SS, Kalivas PW. Ethanol exposure decreases glutamate uptake in the nucleus accumbens. Alcoholism, clinical and experimental research. 2005;29:326–333. doi: 10.1097/01.alc.0000156086.65665.4d. [DOI] [PubMed] [Google Scholar]
- Miguel-Hidalgo JJ. Withdrawal from free-choice ethanol consumption results in increased packing density of glutamine synthetase-immunoreactive astrocytes in the prelimbic cortex of alcohol-preferring rats. Alcohol and alcoholism. 2006;41:379–385. doi: 10.1093/alcalc/agl006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RE, Hackenberg R, Gershman H. Regulation of glutamine synthetase in cultured 3T3-L1 cells by insulin, hydrocortisone, and dibutyryl cyclic AMP. Proceedings of the National Academy of Sciences of the United States of America. 1978;75:1418–1422. doi: 10.1073/pnas.75.3.1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani A, Tanaka K. Functional changes of glial glutamate transporter GLT-1 during ischemia: an in vivo study in the hippocampal CA1 of normal mice and mutant mice lacking GLT-1. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7176–7182. doi: 10.1523/JNEUROSCI.23-18-07176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Kalivas PW. Group II metabotropic glutamate receptors (mGlu2/3) in drug addiction. European journal of pharmacology. 2010;639:115–122. doi: 10.1016/j.ejphar.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch J, Marsden J. A ‘symptom-triggered’ approach to alcohol withdrawal management. British journal of nursing. 2014;23:198–202. doi: 10.12968/bjon.2014.23.4.198. [DOI] [PubMed] [Google Scholar]
- Reader TA, Strazielle C, Botez MI, Lalonde R. Brain dopamine and amino acid concentrations in Lurcher mutant mice. Brain research bulletin. 1998;45:489–493. doi: 10.1016/s0361-9230(97)00430-9. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S. Ethanol withdrawal is associated with increased extracellular glutamate in the rat striatum. European journal of pharmacology. 1995;283:177–183. doi: 10.1016/0014-2999(95)00344-k. [DOI] [PubMed] [Google Scholar]
- Rossetti ZL, Carboni S, Fadda F. Glutamate-induced increase of extracellular glutamate through N-methyl-D-aspartate receptors in ethanol withdrawal. Neuroscience. 1999;93:1135–1140. doi: 10.1016/s0306-4522(99)00250-x. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Sari Y, Sreemantula SN. Neuroimmunophilin GPI-1046 reduces ethanol consumption in part through activation of GLT1 in alcohol-preferring rats. Neuroscience. 2012;227:327–335. doi: 10.1016/j.neuroscience.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2011;36:1359–1365. doi: 10.1038/npp.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warr O, Takahashi M, Attwell D. Modulation of extracellular glutamate concentration in rat brain slices by cystine-glutamate exchange. The Journal of physiology. 1999;514(Pt 3):783–793. doi: 10.1111/j.1469-7793.1999.783ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Parsons LH, Schulteis G, Hyytia P, Lorang MT, Bloom FE, Koob GF. Ethanol self-administration restores withdrawal-associated deficiencies in accumbal dopamine and 5-hydroxytryptamine release in dependent rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996;16:3474–3485. doi: 10.1523/JNEUROSCI.16-10-03474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Porrino LJ. Behavioral neurobiology of alcohol addiction: recent advances and challenges. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:3332–3337. doi: 10.1523/JNEUROSCI.22-09-03332.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]