Abstract
Microglia are active players in inflammation, but also have important supporting roles in CNS maintenance and function, including modulation of neuronal activity. We previously observed an increase in the frequency of excitatory postsynaptic current in organotypic brain slices after depletion of microglia using clodronate. Here, we describe that local hippocampal depletion of microglia by clodronate alters performance in tests of spatial memory and sociability. Global depletion of microglia by high-dose oral administration of a Csf1R inhibitor transiently altered spatial memory but produced no change in sociability behavior. Microglia depletion and behavior effects were both reversible, consistent with a dynamic role for microglia in the regulation of such behaviors.
Keywords: microglia depletion, hippocampus, memory, clodronate, Csf1 inhibition
1. INTRODUCTION
Microglia, the immunocompetent cells of the CNS, originate from yolk-sac precursors that populate the brain prior to the formation of the blood-brain barrier (Kierdorf et al., 2013). Once they fully colonize the brain parenchyma Csf-1, among other factors, contributes to their maintenance and self-renewal (Wegiel et al., 1998). Csf-1 and its receptor Csf1R are essential for microglial proliferation, differentiation, survival, and migration. Mice that lack Csf1R display severe microglia deficiency as well as other developmental defects (Erblich et al., 2011; Michaelson et al., 1996; Nandi et al., 2012). Conversely, overexpression of Csf-1 increases microglia numbers by increasing microglial proliferation (De et al., 2014).
Besides their known roles in mediating inflammation (Colton and Wilcock, 2010; Kraft and Harry, 2011), microglia play an important supporting role in the postnatal CNS (Nayak et al., 2014; Paolicelli et al., 2011; Schwartz et al., 2013). During early neurogenesis microglia phagocytose apoptotic cells and eliminate unwanted neuronal projections (Paolicelli et al., 2011; Schafer et al., 2012). They also control production of cortical neurons through phagocytosis of neural precursor cells (Cunningham, 2013).
Microglia provide trophic support for the formation of neuronal circuits and are indispensible for neuronal survival (Ueno et al., 2013). They also directly modulate synaptic activity by contacting synapses in a CR3/C3-dependent manner (Paolicelli et al., 2011; Schafer et al., 2012; Wake et al., 2009). Neuronal stimulation results in extension of microglial processes towards highly active neurons, which in turn reduces neuronal activity (Li et al., 2012). Previous work from our lab showed an increase in the frequency of excitatory postsynaptic currents in hippocampal slices (Ji et al., 2013) and the complementary effect in neuronal/microglial co-cultures. Together, these data support a role for microglia in the regulation of neuronal activity in the healthy brain by affecting the number of active synapses.
Due to their extensive functions as supporting cells of the CNS, microglia are now considered active regulators of neurological function. Specific depletion of brain microglia upon diphtheria toxin administration in CX3CR1CreER mice significantly reduces motor-learning-dependent synapse formation as well as performance in learning and memory tasks including auditory-cued fear conditioning and novel object recognition (Parkhurst et al., 2013). Long term depletion of microglia via systemic inhibition of Csf1R improves spatial memory as assessed with the Barnes maze test (Elmore et al., 2014). Mice that lack the chemokine receptor CX3CR1 have reduced numbers of microglia along with deficits in social behavior and spatial learning (Rogers et al., 2011; Zhan et al., 2014). Finally, mice deficient in Fractalkine (CX3CL1), a chemokine that directs microglia towards developing synapses (Hoshiko et al., 2012), have reduced performance in contextual fear conditioning and cued fear conditioning (Rogers et al., 2011)
In this study, we investigate the functional significance of microglia depletion in vivo using two different methods to deplete microglia: bilateral microinjections of clodronate in the dorsal hippocampus, and systemic inhibition of Csf1R. The first method provides anatomical specificity, allowing us to examine the functional consequences of loss of microglia in hippocampal-related behaviors. The second allowed us to compare the results to a method that produced more widespread effects. We show that absence of microglia leads to alterations in spatial memory task performance and social behavior. These effects are reversed when microglia are allowed to repopulate the brain, supporting an active role for microglia in such physiological processes.
2. MATERIALS AND METHODS
2.1. Animals
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) and the Department of Laboratory Animal Research at Stony Brook University. Two to three month-old C57BL/6 (wild-type) male mice were used in this study.
2.2. Drug Administration
2.2.1. Intrahippocampal clodronate injection
Injections were performed bilaterally in the hippocampus. Mice were anesthetized with 1.25% Avertin and injected with phosphate buffered saline (PBS) or clodronate disodium salt (10 mg/ml, Calbiochem) at stereotactic coordinates −2.5 mm from bregma and −1.7 mm lateral using a Hamilton syringe (0.485 mm I.D., Hamilton, Reno, NV) connected to a motorized stereotaxic injector (Stoelting, Wood Dale, IL). Following surgery, animals were injected i.p. with 0.03 mg/kg of buprenorphine (Bedford labs) and left on a heating pad until they were fully recovered from anesthesia.
2.2.2. Administration of the Csf1R inhibitor
PLX3397 was obtained from Plexxikon Inc. and mixed into AIN-76A standard chow by Research Diets Inc. at the dose of 290 mg of drug per kg of chow (Elmore et al., 2014). Mice were fed with chow containing either control- or PLX3397-chow for either 7 or 21 days.
2.3 (Immuno)histological analysis
2.3.1 Diaminobenzidine (DAB) staining
Brain sections were treated with 3% H2O2 followed by three washes with 1x PBS-T. Sections were blocked in 1% BSA and incubated with the primary antibody overnight (Iba-1 (Wako, 1:1000), GFAP (AbCam, 1:1000). The sections were washed and incubated with the appropriate secondary antibodies (biotinylated anti-rabbit IgG, Vector Laboratories) and the ABC reagent was added (Vector Laboratories) for one hour. The sections were washed 3 times with PBS-T. The signal was visualized using 3,3′-Diaminobenzidine (DAB) (Sigma Chemical Co) solution (DAB/0.1M PB/H2O2) until the desired strength was reached. The slices were mounted on slides and dehydrated in graded ethanol, defatted in xylenes, and washed with 0.1M Phosphate Buffer before mounting.
2.3.2 Cresyl violet staining
Brain sections were dipped in 100% ethanol and washed in xylenes for two minutes. Serial hydration was performed in ethanol solutions ranging from 100% to 20%. The sections were dipped in cresyl violet dye for 5 minutes, dipped twice in distilled water, and washed in 70% ethanol and 10% acetic acid. The sections were then dipped in 100% ethanol and 10% acetic acid, dehydrated with 100% ethanol, defatted in xylenes, and mounted.
2.3.3 Immunofluorescence
40μm-thick brain sections were blocked for one hour (3% BSA and 0.02% triton-X100 in PBS) and incubated with primary antibodies overnight at 4°C. The primary antibodies used were: Iba-1 (Wako, 1:500), GFAP (AbCam, 1:1000), and NeuN (Millipore, 1:100). Sections were washed and incubated with conjugated secondary antibodies for one hour. Slides were washed and mounted with DAPI fluoromount (Southern Biotech).
2.4. Imaging
The stained sections were photographed under bright-field optics using a digital camera (Nikon CoolPix 990; Nikon, Tokyo, Japan) connected to a Nikon Eclipse E600 microscope. Immunofluorescently stained sections were photographed at a digital resolution of 1024 × 1024 with a Zeiss confocal microscope using LSM 510 Meta software.
2.5. Quantification of microglia and astrocytes
Hippocampal microglia and astrocytes were counted using bright field images of brain sections stained with Iba-1 or GFAP, respectively. Images captured from three comparable sections per animal were quantified manually using ImageJ’s automated cell counter. The average number of cells per animal were used for analysis.
2.6. Behavior Tests
We used the Barnes maze, open field (Bukhari et al., 2011), Crawley’s sociability and preference for social novelty test (Kaidanovich-Beilin et al., 2011), and Rotarod test (Bukhari et al., 2011) to evaluate the functional consequences of microglia depletion.
2.6.1. Barnes maze
Barnes maze was performed to assess spatial memory after microglia depletion. Mice were placed on a circular maze containing eight equally spaced holes. An escape box was placed underneath a randomly selected hole. The amount of time taken by the mouse to find the escape box (latency to find) was recorded. The session ended when the mouse entered the escape box or after five minutes. Two trials were performed daily for five consecutive days. The average latencies to find and enter the escape box were used for analysis.
2.6.2. Open Field Activity
Motor activity was measured using the Opto-Varimex-Minor animal activity meter (Columbus Instruments) as described in (Bukhari et al., 2011). Mice were placed in an empty rat cage (44 cm × 21 cm) located inside the activity meter. Infrared beams (15 × 15) ran in the x-y coordinates. The total number of beams broken by the mouse as it moved around the cage as well as the animal’s supported and unsupported motor movements (rearings) were recorded during the five- minute observation period.
2.6.3. Sociability and preference for social novelty
Sociability was measured using the Crawley’s sociability and preference for social novelty test described in (Kaidanovich-Beilin et al., 2011). A rectangular three-chamber box with an open middle section was used. Two identical wired cups were placed in each side of the chamber. The test was divided into two- ten minute sessions. In session I, a mouse (stranger mouse1) was placed under one of the wired cups, while the second wired cup located in the opposite chamber was left empty. The number of active contacts as well as the duration of the active contacts between the test mouse and both the empty cup and the cup containing stranger mouse 1 were recorded. An active contact was defined as any instance in which the mouse touched the wired cup with its snout or paws. In session II, a second (novel) mouse was placed under the cup that had been empty during session I. The number of active contacts as well as the duration of active contacts between the test mouse and both the familiar mouse and the novel mouse was recorded. The time spent by the subject mice in each chamber during each session was recorded in order to determine whether the subject mice explored both chambers. Both Sessions were videotaped. The stranger mice and the subject mice were the same sex, weight, and age, but were not littermates of each other. This test was conducted every day for five days starting approximately 24 hours after clodronate treatment, and once on day 7 after ongoing administration of PLX3397.
2.6.4. Rotarod test
Motor performance was assessed in wild-type mice after administration of PLX3397 for 21 days using a Rotarod (Med Associates, Inc.) as described elsewhere (Bukhari et al., 2011). Briefly, mice were placed on a moving rod that accelerated from 4 to 40 RPM over the course of five minutes. Once the mouse fell off, both the speed of the rod and the latency to fall were automatically recorded by the apparatus. Mice were subjected to three consecutive trials and the average of the trials was used for subsequent analysis. Rotarod performance was recorded on the last day of Barnes maze testing during ongoing treatment with PLX3397.
2.7. Statistics
All statistics were performed using Statview (v4.0) or GraphPad Prism 6 for Windows. Data are presented as mean ± SEM. Initial descriptive analysis of the Barnes maze data revealed heterogeneity in the shape of the functions for the individual animals. Therefore, the nonparametric Mann-Whitney U test was used to determine significance between experimental and control groups at each time point in the Barnes maze test. Open field, and microglial quantification were analyzed by Student’s t-tests with Welch’s correction for samples having possibly unequal variances. The social behavior test data were analyzed by two-way ANOVA followed by specific two group comparisons where ANOVAs were significant. Data were considered statistically significant when p < 0.05.
3. RESULTS
3.1. Hippocampal depletion of Iba-1+ microglia in vivo resulted in alterations in spatial learning and sociability
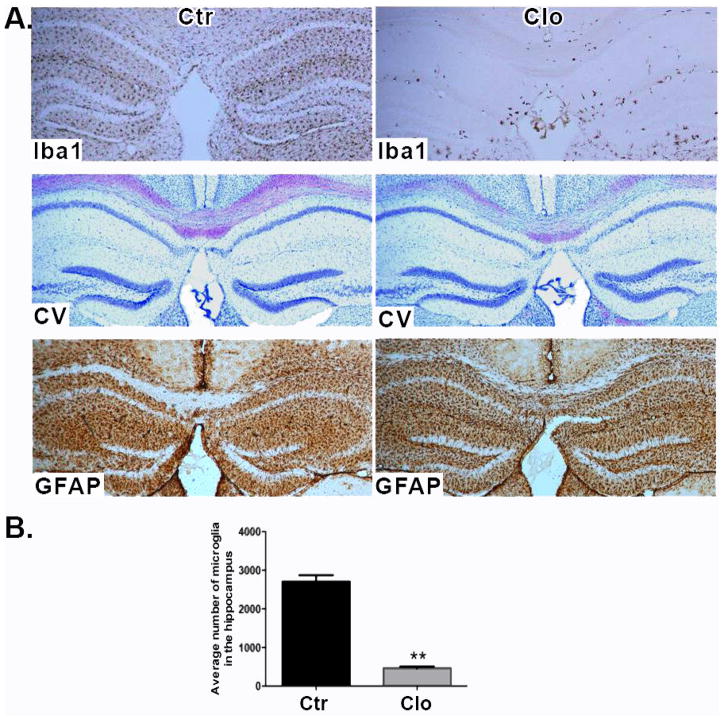
Our previous work showed that the density of active synapses is increased after microglia depletion ex vivo (in hippocampal brain slices). We attributed these effects to microglia specifically since the number of synapses and neuronal firing frequency returned to control levels when microglia-depleted brain slices were overlaid with cultured microglia cells (Ji et al., 2013). We wished to determine whether there were measurable behavioral effects with in vivo microglia depletion. Initially we investigated whether clodronate depletes microglia in vivo. Clodronate is pinocytosed by microglia and metabolized by the cell into adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, an ATP analog (Frith et al., 1997). Mice were given unilateral clodronate injections into the CA1 hippocampus, and at days 1–7 post injection the tissue microglia were analyzed using the marker Iba-1. Microglia depletion was apparent at day 1 and continued up to day 5, but by day 7 microglia started to reappear (Supplemental Information Figure 1). In mice given a bilateral clodronate injection into the CA1 hippocampus and euthanized 5 days later, the numbers of Iba-1+ microglia were significantly reduced in the hippocampus (p= 0.003. Figure 1A, top panel and Figure 1B), while astrocytes stained by GFAP immunofluorescence were clearly visible throughout the brain (Figure 1A, bottom panel). Neurons appeared normal and viable by cresyl violet staining (Figure 1A, middle panel). Whole brain images show microglial depletion in the hippocampus only (Supplemental Information Figure 2)
Figure 1. Hippocampal depletion of microglia by clodronate.
(A) Brain sections stained for Iba-1 (top panel), cresyl violet (middle panel), and GFAP (bottom panel) 5 days after clodronate injection. n=9 per group. Scale bar = 100μm. (B) Numbers of microglia were quantified in three sections per biological replicate. n= 3–4 per group. Data are shown as mean ± SEM. ** p<0.005
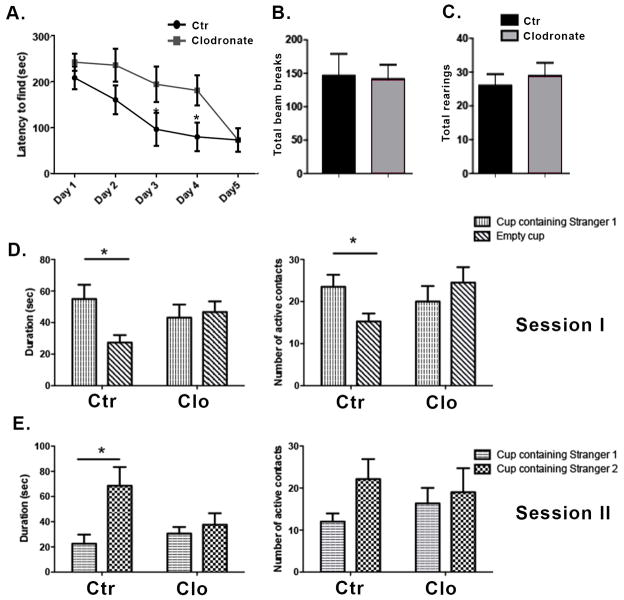
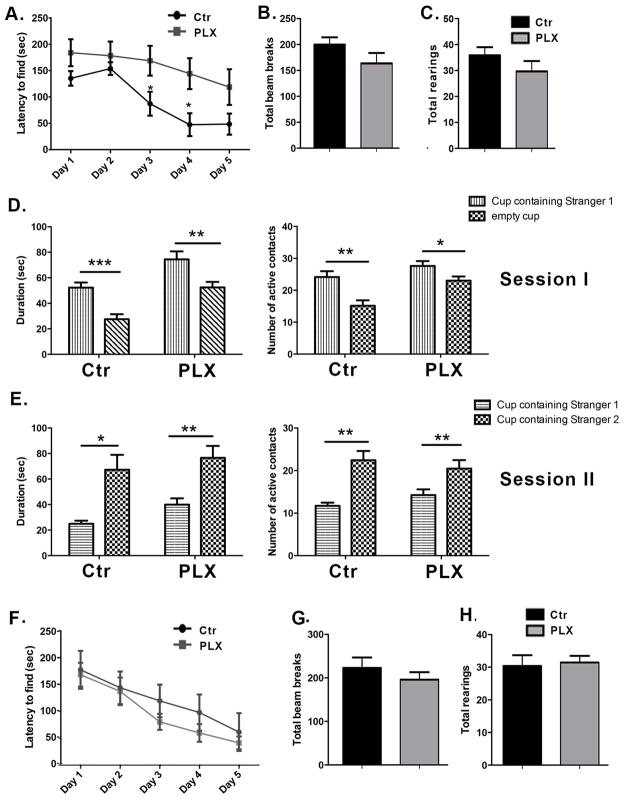
We tested the effect of microglia depletion on spatial learning and exploration using the Barnes maze, a task whose measures are known to be affected by hippocampal manipulations (Harrison et al., 2006). Two to three month old wild-type male mice were given a bilateral dorsal hippocampal injection of clodronate and tested on the Barnes maze twice per day for five consecutive days. Testing sessions were separated by fifteen minutes and data from both sessions were averaged for analysis. Mice treated with clodronate found the location of the escape box more slowly compared to the saline controls (Figure 2A), and this difference was significant at days 3 and 4 (p = 0.04 and p= 0.03, respectively). There was no significant difference at day 5 suggesting a transient deficiency in the absence of microglia. Consistent with a slower rate of learning, clodronate treated animals made significantly more visits to holes prior to entering the escape box compared to saline-treated mice at day 4 of testing (not shown, p=0.01). To assess whether hyperactivity or motor impairments in the injected animals were mediating the observed result, we assessed activity in an open field setup. Both clodronate- and saline-treated mice displayed similar numbers of photobeam breaks per session (Figure 2B, p=0.90) and a similar number of total rearings per session (Figure 2C, p=0.57).
Figure 2. Hippocampal depletion of microglia by clodronate results in alterations in spatial learning and deficits in social behavior.
(A) Mice were tested in the Barnes maze for five consecutive days starting at day 1 post injection. The time taken by the mice to find the escape box is shown. n=9 per group. (B–C) The locomotor activity of the mice was assessed on day 5 after clodronate injection using the open field activity test. n=9–13 per group. (D–E) Mice were tested for five consecutive days on the Crawley’s sociability test starting at day 1 post injection. Day 2 of testing is shown. n= 8–6 per group. Data are shown as mean ± SEM. *P<0.05.
The hippocampus has recently been identified as a critical area for social memory processing (Hitti and Siegelbaum, 2014; Stevenson and Caldwell, 2014; Uekita and Okanoya, 2011). To determine whether hippocampal loss of microglia extended to social behavior, we used Crawley’s sociability and preference for social novelty test (Kaidanovich-Beilin et al., 2011). Two-way ANOVAs revealed significant active contacts for session 1 (target x treatment interaction, p<.04) though not session 2 and only for session 1 for the duration of contacts measure (target x treatment interaction, p<.04; p=.07 for session 2). Following up with specific two-group comparisons revealed that saline-treated controls made significantly more active contacts (p=0.03) and spent more time with the cup containing a conspecific (stranger mouse 1) (Figure 2D, p= 0.02) in session 1. In contrast, clodronate treated animals did not display a preference for either cup (Figure 2D–E). Subject mice spent a similar amount of time in both chambers, indicating equal exploration of the maze (not shown). Together these results suggest a role for hippocampal microglia in spatial learning and social behavior.
Repopulation of the CNS by Iba-1+ cells reversed microglia depletion effects on spatial memory and social behavior
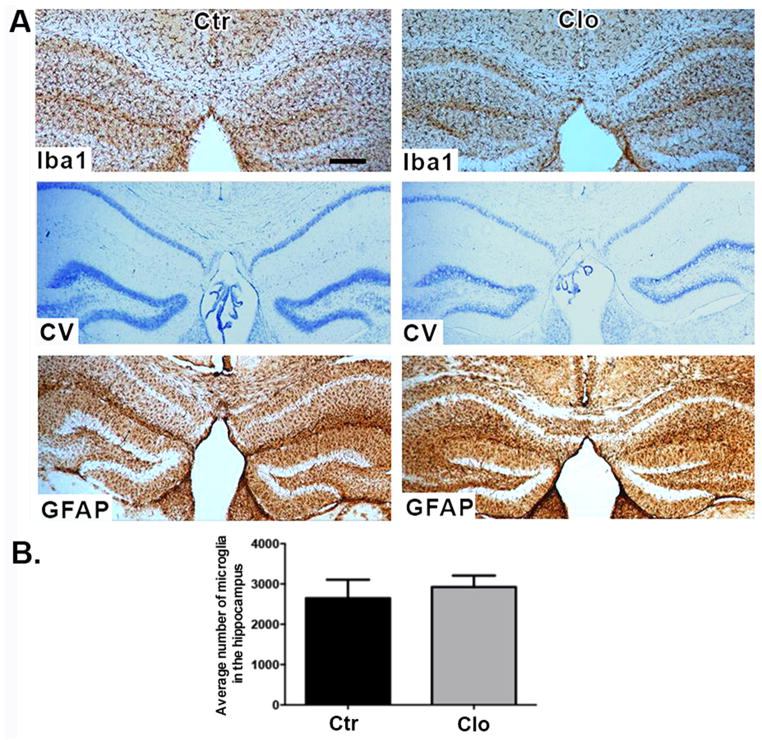
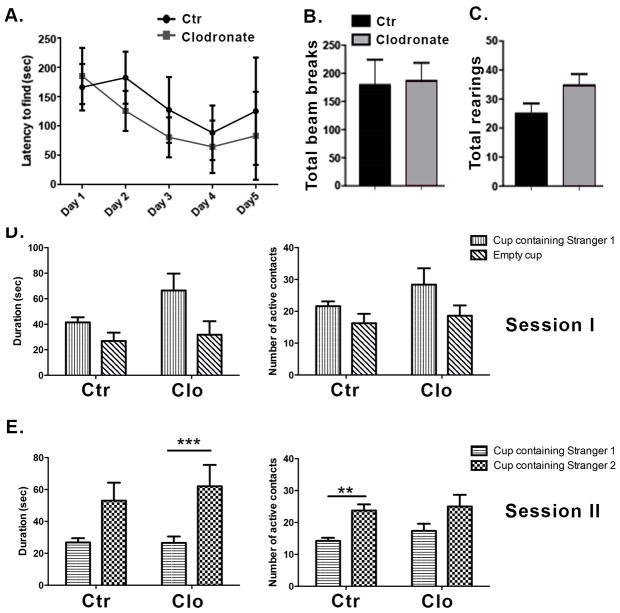
To determine whether microglia are able to repopulate the hippocampus after discontinuation of the treatment with clodronate we performed a single bilateral clodronate injection into the CA1 hippocampus of wild-type mice and euthanized the animals at day 20 post-treatment. This time point was chosen based on a previous report showing that microglia repopulate the brain of HSVTK mice within two weeks after ganciclovir treatment (Varvel et al., 2012). Figure 3 shows that microglia fully repopulated the mouse hippocampus by day 20 after clodronate treatment. The newly repopulated Iba1+ cells do not differ in number from the microglia in control brains (Figure 3B, p= 0.6405). Astrocytes and neurons remained viable. To examine whether normal behavior would also return after microglial repopulation, mice were tested on the Barnes maze as described above starting on day 20 after clodronate injection. On the last day of Barnes maze testing, open field activity and social behavior were quantified. Clodronate treated mice displayed similar latencies to find the escape box (Figure 4A, p=0.50 and p= 0.72 at days 3 and 4, respectively) and made a similar number of visits compared to controls (data not shown, p=0.33). This is in contrast to days 3 and 4 where microglia-depleted mice displayed a significant delay in spatial learning (Figure 2A). Activity levels between treated and control animals remained the same even after microglial repopulation (Figure 4B–C, p=0.8933 for total beam breaks and p=0.08 for total rearings). Two-way ANOVAs revealed no significant main effects or target x treatment interaction for number of active contacts or duration of contacts measure for session 1 or session 2. Clodronate treated animals showed effectively normal social behavior after repopulation by microglia (Figure 4D–E.). Subjects from both groups spent a similar amount of time in the chambers during Session I, indicating equal exploration of the maze (not shown).
Figure 3. Repopulation of the hippocampus by microglia.
(A) Brain sections stained for Iba-1 (top panel), cresyl violet (middle panel), and GFAP (bottom panel) 20 days after clodronate injection. n=4 per group. Scale bar = 100μm. (B) Numbers of microglia were quantified in three sections per biological replicate. n= 3 per group. Data are shown as mean ± SEM.
Figure 4. Repopulation of the hippocampus by microglia reverses the behavioral effects observed with microglia depletion.
(A) Mice were tested in the Barnes maze for five consecutive days starting on day 20 post injection. The time to find the escape box is shown. n=4 per group. (B–C) Locomotor activity was assessed on the last day of Barnes maze testing using the open field activity test. n=7 per group. (D–E) Social behavior was measured using the Crawley’s sociability test. n=5–8 per group. Data are shown as mean ± SEM. ** p<0.005, ***P≤0.0001.
Systemic Csf1R inhibition depleted Iba-1+ cells in the brain within 7 days and resulted in alterations in spatial memory
To extend the findings from the local intrahippocampal injection studies, we employed a second, systemic method of microglial depletion that takes advantage of their dependency on Csf1R signaling for their development and survival (Elmore et al., 2014; Erblich et al., 2011; Michaelson et al., 1996; Nandi et al., 2012; Wegiel et al., 1998). PLX3397 is an oral, selective tyrosine kinase inhibitor of CSF-1 that successfully crosses the blood-brain barrier (Elmore et al., 2014). Its ability to specifically target microglia and macrophages has allowed for its use in different disease models in which microglia and/or macrophages play a key role in disease progression. These include melanoma, glioma, astrocytoma, and pancreatic cancer (Coniglio et al., 2012; Nicolaides et al., 2011; Sluijter et al., 2014; Zhu et al., 2014).
PLX3397 was added to food pellets at a concentration of 290 mg/kg of chow as previously described (De et al., 2014; Elmore et al., 2014). This concentration exceeds the relevant clinical dose, as we aimed to achieve maximal reduction of microglia. Wild-type mice were fed PLX3397 or control chow for 7 days. Their brains were collected, sectioned, and immunofluorescently labeled for Iba-1, GFAP and NeuN, and DAB-stained for Iba-1. Microglia present in the hippocampus were counted using bright field images from three comparable sections per biological replicate. Consistent with previous findings (Elmore et al., 2014), Supplemental Information Figure 3A and 3B shows depletion of microglia 7 days after Csf1R inhibition (p=0.0019). While neurons were preserved, astrocytes appeared to have thicker processes as well as higher intensity of GFAP immunostaining (Supplemental Information Figure 4). This is consistent with previous reports showing increased protein levels of astrocytic proteins GFAP and S100β in mice treated with PLX3397 (Elmore et al., 2014). These results are also consistent with the higher expression of GFAP and the higher astrocytic density observed in mice lacking Csf1R (Erblich et al., 2011).
To compare the behavioral effects observed with clodronate treatment with those in animals that systemically lacked microglia, another group of wild-type mice were fed with PLX3397- or control- chow for 7 days. Testing began on day 7 and the animals remained on their respective chow till the end of the experiment. They were tested for 5 consecutive days using Barnes maze starting on day 7 and the sociability and open field tests on the last day of Barnes maze testing. Consistent with our previous results, PLX3397 treated animals took longer times to find the escape box at days 3 and 4 of testing (Figure 5A, p= 0.02 and p=0.01, respectively). PLX3397 treated animals also made significantly more visits to holes prior to entering the escape box at day 4 (data not shown, p= 0.01). Both groups had similar numbers of photobeam breaks and total rearings (Figures 5B and 5C, p=0.1540 and p=0.2352, respectively), indicating that systemic loss of microglia did not produce profound widespread effects on motor abilities, general activity level, temperament, or other aspects of well-being in the mice that would be reflected in diminished activity and exploration of a novel environment. However, in contrast to our observations in mice given clodronate treatment, PLX3397 treated mice displayed normal social behavior in the Crawley’s sociability and preference for social novelty test (Figure 5D–E). Both groups made significantly more active contacts and showed longer durations of contact with stranger mouse 1 during session I (Main effect p<.002 by two–way ANOVA; p=0.003 and p=0.03 for control and PLX3397 treated mice, respectively; Main effect p<.0001 by two–way ANOVA; p=0.0008 and p=0.008 for duration of active contacts during session I in control and PLX3397 treated mice, respectively) and with stranger mouse 2 during session II. (Main effect p<.02 by two–way ANOVA; p=0.002 and p=0.01 for control and PLX3397 treated mice, respectively). Neither number (p=.23) nor duration (p=.15) of active contacts during session II in control and PLX3397 treated mice reached significance for session II by two-way ANOVA. Both control and PLX3397 treated mice spent a similar amount of time in the chambers during Session I, indicating equal exploration of the maze (not shown).
Figure 5. Systemic microglia depletion recapitulates the effects on spatial memory observed with localized hippocampal depletion, and microglial repopulation of the brain reverses the performance in the Barnes maze.
(A) Barnes maze test was conducted for 5 consecutive days one week after administration of PLX3397. The time taken by the mice to find the escape box is shown. n=13 per group. (B–C) The locomotor activity of the mice was assessed using the open field activity test on the last day of Barnes maze testing. n=13 per group. (D–E) Social behavior was tested using the Crawleys’s sociability test on the last day of Barnes maze testing. n=7–13 per group. Data are shown as mean ± SEM. *p<0.05, ** p<0.01, *** p<0.001. (F) C57BL/6 mice were fed control or PLX3397-chow for 7 days. They were then returned to their regular diet for 14 days and tested on the Barnes maze. The time taken to find the escape box is shown. n=8–13 per group. (G–H) Locomotor activity was assessed using the open field activity test on the last day of Barnes maze testing. n=8–13 per group. Data are shown as mean ± SEM.
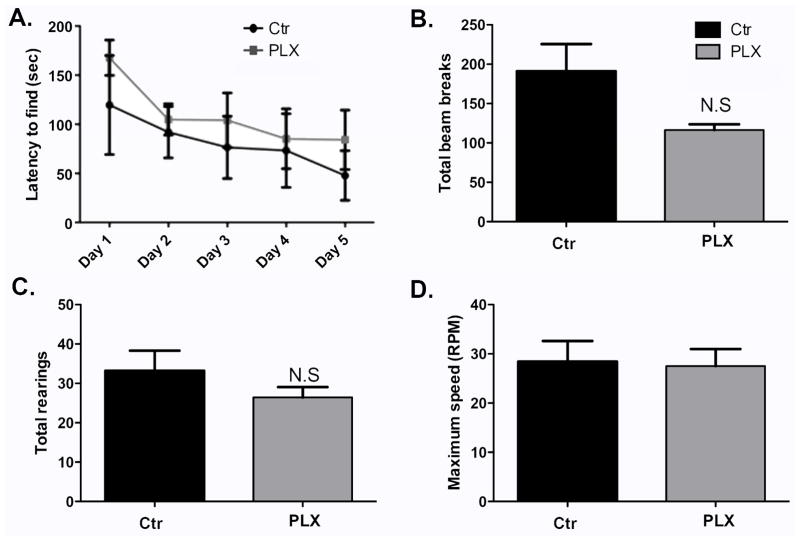
The effects of systemic microglia depletion via administration of PLX3397 may be transient
In a previous report Elmore et al (2014) showed that administration of PLX3397 for 21 days did not affect spatial memory as measured by Barnes maze (Elmore et al., 2014). In this work, we showed that treatment with PLX3397 for 7 days causes alterations in Barnes maze performance (Figure 5A). We hypothesized that the effect of microglia depletion on spatial memory is a transient one and that therefore Elmore et al missed it by testing at a later time point. To test this, another group of wild-type mice were fed with PLX3397- or control- chow for 21 days. Barnes maze testing began on day 21 and the animals remained on their respective chow till the end of the experiment. Brains from experimental and control groups were sectioned and DAB-stained for Iba-1. Microglia present in the hippocampus were counted using bright field images from three comparable sections per biological replicate. Supplemental Figure 5A–B shows depletion of microglia in the brain with PLX3397 treatment (p=0.0114). As shown in Figure 6A, PLX3397-treated mice performed similarly compared to control animal in the Barnes maze, indicating that the alterations in spatial memory observed previously with microglia depletion for 7 days may be transient, and that long term microglia depletion does not necessarily worsen spatial memory task performance. Mice treated with PLX3397 for 21 days had similar numbers of photobeam breaks and total rearings compared to control animals (Figure 6B–C, p= 0.0941 and p=0.2828, repectively), indicating good overall health in both groups. They also performed similarly to control animals in the Rotorod test (Figure 6D, p=0.8683), further supporting the lack of motor deficits in these mice. These last findings are in agreement with those of Elmore et al who found no motor defects in animals fed with PLX3397 for 21 days (Elmore et al., 2014).
Figure 6. Systemic microglia depletion via administration of PLX3397 may lead to temporary alterations in spatial memory.
(A) Barnes maze test was conducted for 5 consecutive days 21 days after administration of PLX3397. The time taken by the mice to find the escape box is shown. n=5–7 per group. Data are shown as mean ± SEM. ** p<0.01 (B–C) The locomotor activity of the mice was assessed using the open field activity test on the last day of Barnes maze testing. n=5–7 per group. (D). Lower and upper limb strength was measured in the Rotorod on the last day of Barnes maze testing. n=5–7 per group. Data are shown as mean ± SEM.
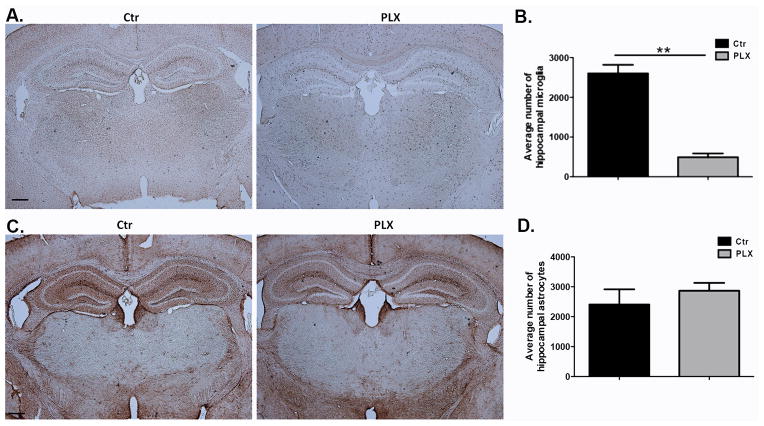
Numbers of astrocytes were not altered by microglia depletion via Csf1R inhibition
We have shown alterations in Barnes maze performance in mice after microglia depletion both after clodronate treatment and after Csf1R inhibition. In both models of depletion astrocytes display an activated morphology, supporting a possible inflammatory reaction caused by dying microglia. To address whether the alterations in Barnes maze performance are due to an inflammatory response elicited by dying cells rather than to loss of microglia per se, another group of mice were treated with PLX3397 and numbers of hippocampal astrocytes were quantified at a time point at which behavior differences were observed in Barnes maze (day 10 after ongoing treatment with PLX3397) Figure 7A–D show similar numbers of astrocytes between control and PLX3397 treated animals at day 10 after ongoing treatment with PLX3397 indicating that the behavioral changes observed are not likely to be the product of inflammation caused by microglia cell death.
Figure 7. Astrocyte numbers between control and PLX-3397-treated animals are similar at a time point at which differences in Barnes maze performance were observed.
(A–B) Mice were fed with PLX3397 or control chow for 10 days. Brain sections were stained for Iba-1 and hippocampal microglia were quantified in three sections per biological replicate. n=3 per group. (C–D) Mice were fed with PLX3397 or control chow for 10 days. Brain sections were stained for GFAP and hippocampal astrocytes were quantified in three sections per biological replicate. n=3 per group. Data are shown as mean ± SEM. ** p<0.01. Scale = 100μm.
Iba-1+ cells replenished the brain, after discontinuation of Csf1R inhibition, and reversed the effect of microglia depletion on spatial memory
Another group of mice were fed PLX3397 or control chow for 7 days to deplete microglia. The mice were then returned to their normal diet for 14 days as previously described (Elmore et al., 2014). Brains from experimental and control groups were sectioned and fluorescently stained for Iba-1, GFAP, and NeuN and DAB-stained for Iba-1. Microglia present in the hippocampus were counted using bright field images from three comparable sections per biological replicate. Supplemental Information Figure 6A and 6B shows complete replenishment of Iba-1+ microglia in the brain. This new microglia population displays an activated morphology although there was no significant difference in cell numbers (p=0.8381). Similarly, GFAP+ displayed thickened processes. NeuN+ neurons remained comparable to the control treatments (Supplemental Information Figure 4).
Another group of mice were treated as above and tested in the Barnes maze and in the open field at day 14 after discontinuation of Csf1R inhibition. Control and PLX3397 treated mice had similar latencies to find the escape box (Figure 5F, p=0.27 and p= 0.33 for days 3 and 4, respectively). Control mice made significantly more visits to holes prior to entering the escape box on day 2 (data not shown, p=0.007), consistent with a trend towards slightly higher activity levels in the open field test (Figure 5G–H, p=0.37 and p=0.78 for total photobeam breaks and rearings, respectively).
4. DISCUSSION
In this study we used two methods of microglia depletion, one ablating microglia locally at its site of administration (clodronate in the hippocampus), and one systemic (PLX3397). Both methods were effective in depleting microglia as shown by the quantification analysis. We further show that microglia are dynamic modulators of performance in the Barnes maze, a task known to be sensitive to hippocampal disturbance. Since the animals showed normal levels of activity when tested in the open field anxiety-like behavior, hyper or hypo-activity, motor defects, and changes in temperament are unlikely explanations for the altered spatial task performance.
Our results are consistent with other studies indicating a role for immune cells in learning and memory. Mice lacking the chemokine receptor CX3CR1 display a temporary reduction of microglia concomitant with deficient synaptic pruning and deficits in social behavior and spatial learning (Rogers et al., 2011; Zhan et al., 2014). CX3CR1ERT3 Cre mice show reduced spine remodeling along with memory impairments in auditory-fear conditioning and object recognition (Parkhurst et al., 2013). Mice deficient in Fractalkine (CX3CL1), a chemokine that directs microglia towards developing synapses (Hoshiko et al., 2012), reduces performance in contextual fear conditioning and cued fear conditioning (Rogers et al., 2011). Lack of microglial receptor CCR2 accelerates the appearance of memory deficits in models of Alzheimer’s disease (El Khoury et al., 2007; Naert and Rivest, 2011). Lastly, mice deficient in CNS-specific T cells exhibit deficits in spatial learning (Kipnis et al., 2004; Ziv et al., 2006), and immune deficiency in mice causes a spatial memory defect that can be rescued by immune reconstitution (Ron-Harel et al., 2008).
We found alterations in spatial learning in mice after ongoing administration of PLX3397 for 7 days but not in mice treated for 21 days. This is in agreement with a previous study that reported no changes in spatial memory in animals treated with PLX3397 for 21days (Elmore et al., 2014). It is conceivable that the alterations in spatial memory we observed after 7 days of Csf1R inhibition are simply transient and not present when Csf1R inhibition is maintained for a longer period.
We also show social behavior alterations with ablation of microglia via clodronate. This is in agreement with other studies that have linked reduced microglial density with reduced sociability (Prinz and Priller, 2014; Zhan et al., 2014). However, systemic microglia depletion did not affect sociability presently. One explanation for the differences in behavioral responses between the two models is that systemic Csf1R inhibition affects different cell populations such as perivascular and meningeal macrophages. In addition, systemic microglia depletion compromises additional areas of the brain that have a role in the regulation of social behavior, other than the hippocampus, such as the prefrontal cortex (Szczepanski and Knight, 2014) and the amygdala (Felix-Ortiz and Tye, 2014).
Another reason for this difference is the fact that behavior was assessed at different time points (7 days after systemic depletion onset versus evaluation during the first days after localized clodronate administration). The differences in social behavior were observed on day 2 post clodronate injection, a time point at which microglia are in the process of dying. The same social behavior test was conducted in mice with systemic microglia depletion on day 7 when most microglia were depleted. Therefore, we cannot discard the possibility that dying microglia in the clodronate model cause an inflammatory response which in turn causes changes in behavior. In both models of depletion astrocytes display an activated morphology, further supporting a possible inflammatory reaction caused by dying microglia. We addressed this last point by quantifying numbers of astrocytes in brain sections from mice fed PLX3397 for 10 days. This time point corresponds to day 4 of Barnes maze testing. We observed similar numbers of astrocytes in control and PLX3397 treated animals which might indicate that the alterations in Barnes maze performance we observed are due to loss of microglia and not to an astrocyte-driven inflammatory reaction caused by dying cells. Consistent with our findings, Elmore et al did not find significant changes in the numbers of GFAP+ or S100+ astrocytes after PLX3397 treatment, although they reported a significant increase in GFAP and S100 protein levels with Csf1R inhibition (Elmore et al., 2014). In contrast, Bruttger et al observed significant astrogliosis as well as a marked increase in chemokine production after microglia ablation (Bruttger et al., 2015). These studies used different models of microglia depletion, which could account for the difference in outcome.
We found that Iba1+ cells can repopulate the brain, consistent with previous studies (Elmore et al., 2015; Elmore et al., 2014; Varvel et al., 2012). In the case of clodronate treatment, microglia numbers return to control levels and the alterations in Barnes maze exploration and social behavior are reversed, consistent with an active role for microglia in regulating these behaviors. Withdrawal of the Csf1R inhibitor reverses the effects on spatial memory task performance and causes microglia to return to control numbers. It was recently shown that microglial repopulation in the Csf1R inhibition model does not affect motor behavior or cognition in mice, indicating that the nature of the newly repopulated microglia is similar to that of the resident cells (Elmore et al., 2015). In the case of Csf1R inhibition, microglial repopulation occurs from the proliferation and differentiation of Nestin+ progenitors, rather than infiltration of circulating cells (Elmore et al., 2014). This is in agreement with studies that have shown the capacity of microglia to self-renew (Ajami et al., 2007; Hashimoto et al., 2013).
In our study local microglia ablation led to behavior phenotypes that evoke comparison to several neuropsychiatric disorders. Postmortem studies have described reduced glia numbers, including astrocytes and oligodendrocytes, in cortical regions in depressed individuals (Banasr and Duman, 2008; Raison et al., 2006; Rajkowska and Miguel-Hidalgo, 2007). Cancer or hepatitis C patients who receive therapy with immune stimulators such as IFN-α or IL-2, develop major depression (Raison et al., 2006). Exposure to LPS leads to symptoms of depression in both healthy and experimental animals (Yirmiya et al., 2001). The anti-depressant effect of microglia stimulation was shown in a model of stress-induced depression in which numbers of microglia drastically decrease as the depression develops (Kreisel et al., 2014). Microglia numbers went back to normal and the depression-like symptoms disappeared after treatment with GM-CSF.
We provide evidence that microglia can dynamically modulate behavior and that they thus play an important physiological role in the CNS. However, the mechanism through which microglia affect learning and social behavior is not well defined. It has been suggested that microglia-derived BDNF has a role in memory consolidation and that glial cell loss correlates with depression-like behavior (Bambah-Mukku et al., 2014; Parkhurst et al., 2013; Rajkowska and Miguel-Hidalgo, 2007). In light of what is known about modulation of synaptic activity by microglia, our findings suggest that disruption of communication between neurons and microglia contributes to these behavioral outcomes and represents a disturbance of normal functioning.
Supplementary Material
Wild-type mice were injected with clodronate in the right hemisphere of the CA1 hippocampus. Red arrows point to the injection site. Mice were euthanized at days 1–7 post injection and brain sections were stained for Cresyl violet (left panel), Iba-1 (middle panel), and GFAP (right panel). n=3 per group. Scale bar = 100μm
Wild-type mice were injected with clodronate in both hemispheres of the CA1 hippocampus. Mice were euthanized at day 5 post injection and brain sections were stained for Iba-1. n=3 per group. Scale bar = 100μm
(A) Wild-type mice were fed with control (Ctr) or PLX3397-chow for 7 days. The tissue was removed and microglia were visualized on brain sections by immunofluorescence for Iba-1. n=3 per group. (B) Numbers of microglia were quantified in three sections per biological replicate. n= 3 per group. Data are shown as mean ± SEM. ** p<0.005. Scale = 50μm.
Top: Wild-type mice were fed with control (Ctr) or PLX3397-chow for 7 days. The tissue was removed and microglia were visualized on brain sections by immunofluorescence for Iba-1 (left panel). The presence of astrocytes (GFAP, middle panel), and neurons (NeuN, right panel) was assessed. n=3 per group. Scale = 50μm. Bottom: another group of wild-type mice were fed with control (Ctr) or PLX3397-chow for 7 days. Mice were then returned to their regular diet and euthanized two weeks later. Brain sections were stained for Iba-1 (left panel), GFAP (middle panel), and NeuN (right panel) n=3 per group. Scale = 50μm.
(A) Wild-type mice were fed with control (Ctr) or PLX3397-chow for 21 days. The tissue was removed and microglia were visualized on brain sections by immunofluorescence for Iba-1. n=3 per group. Scale = 100μm. (B) Numbers of microglia were quantified in three sections per biological replicate. n= 3 per group. Data are shown as mean ± SEM. *p<0.05
Top: Wild-type mice were fed with control (Ctr) or PLX3397-chow for 7 days. Mice were then returned to their regular diet and euthanized two weeks later. Brain sections were stained for Iba-1. n=3 per group. Scale = 100μm. (B) Numbers of microglia were quantified in three sections per biological replicate. n= 3 per group. Data are shown as mean ± SEM.
Highlights.
Local hippocampal microglial depletion alters learning and social behavior
Systemic microglia depletion alters learning but not social behavior
These behavioral changes are reversed upon microglial repopulation
Acknowledgments
We are grateful to members of the Tsirka and Robinson labs for advice and suggestions. This work was supported by NIH R01NS42168 to SET and Turner Dissertation funds to LT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- Bambah-Mukku D, Travaglia A, Chen DY, Pollonini G, Alberini CM. A Positive Autoregulatory BDNF Feedback Loop via C/EBPbeta Mediates Hippocampal Memory Consolidation. J Neurosci. 2014;34:12547–12559. doi: 10.1523/JNEUROSCI.0324-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Keeping ‘trk’ of antidepressant actions. Neuron. 2008;59:349–351. doi: 10.1016/j.neuron.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Bruttger J, Karram K, Wortge S, Regen T, Marini F, Hoppmann N, Klein M, Blank T, Yona S, Wolf Y, Mack M, Pinteaux E, Muller W, Zipp F, Binder H, Bopp T, Prinz M, Jung S, Waisman A. Genetic Cell Ablation Reveals Clusters of Local Self-Renewing Microglia in the Mammalian Central Nervous System. Immunity. 2015;43:92–106. doi: 10.1016/j.immuni.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Bukhari N, Torres L, Robinson JK, Tsirka SE. Axonal Regrowth after Spinal Cord Injury via Chondroitinase and the Tissue Plasminogen Activator (tPA)/Plasmin System. J Neurosci. 2011;31:14931–14943. doi: 10.1523/JNEUROSCI.3339-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- Coniglio SJ, Eugenin E, Dobrenis K, Stanley ER, West BL, Symons MH, Segall JE. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Molecular medicine. 2012;18:519–527. doi: 10.2119/molmed.2011.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- De I, Nikodemova M, Steffen MD, Sokn E, Maklakova VI, Watters JJ, Collier LS. CSF1 overexpression has pleiotropic effects on microglia in vivo. Glia. 2014 doi: 10.1002/glia.22717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Elmore MR, Lee RJ, West BL, Green KN. Characterizing newly repopulated microglia in the adult mouse: impacts on animal behavior, cell morphology, and neuroinflammation. PLoS One. 2015;10:e0122912. doi: 10.1371/journal.pone.0122912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, Kitazawa M, Matusow B, Nguyen H, West BL, Green KN. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron. 2014;82:380–397. doi: 10.1016/j.neuron.2014.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erblich B, Zhu L, Etgen AM, Dobrenis K, Pollard JW. Absence of colony stimulation factor-1 receptor results in loss of microglia, disrupted brain development and olfactory deficits. PLoS One. 2011;6:e26317. doi: 10.1371/journal.pone.0026317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci. 2014;34:586–595. doi: 10.1523/JNEUROSCI.4257-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith JC, Monkkonen J, Blackburn GM, Russell RG, Rogers MJ. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J Bone Miner Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- Harrison FE, Reiserer RS, Tomarken AJ, McDonald MP. Spatial and nonspatial escape strategies in the Barnes maze. Learn Mem. 2006;13:809–819. doi: 10.1101/lm.334306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, Becker CD, See P, Price J, Lucas D, Greter M, Mortha A, Boyer SW, Forsberg EC, Tanaka M, van Rooijen N, Garcia-Sastre A, Stanley ER, Ginhoux F, Frenette PS, Merad M. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38:792–804. doi: 10.1016/j.immuni.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitti FL, Siegelbaum SA. The hippocampal CA2 region is essential for social memory. Nature. 2014;508:88–92. doi: 10.1038/nature13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshiko M, Arnoux I, Avignone E, Yamamoto N, Audinat E. Deficiency of the microglial receptor CX3CR1 impairs postnatal functional development of thalamocortical synapses in the barrel cortex. J Neurosci. 2012;32:15106–15111. doi: 10.1523/JNEUROSCI.1167-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji K, Akgul G, Wollmuth LP, Tsirka SE. Microglia actively regulate the number of functional synapses. PLoS One. 2013;8:e56293. doi: 10.1371/journal.pone.0056293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Lipina T, Vukobradovic I, Roder J, Woodgett JR. Assessment of social interaction behaviors. J Vis Exp. 2011 doi: 10.3791/2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kierdorf K, Erny D, Goldmann T, Sander V, Schulz C, Perdiguero EG, Wieghofer P, Heinrich A, Riemke P, Holscher C, Muller DN, Luckow B, Brocker T, Debowski K, Fritz G, Opdenakker G, Diefenbach A, Biber K, Heikenwalder M, Geissmann F, Rosenbauer F, Prinz M. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–280. doi: 10.1038/nn.3318. [DOI] [PubMed] [Google Scholar]
- Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc Natl Acad Sci U S A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AD, Harry GJ. Features of microglia and neuroinflammation relevant to environmental exposure and neurotoxicity. Int J Environ Res Public Health. 2011;8:2980–3018. doi: 10.3390/ijerph8072980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, Maier SF, Yirmiya R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- Michaelson MD, Bieri PL, Mehler MF, Xu H, Arezzo JC, Pollard JW, Kessler JA. CSF-1 deficiency in mice results in abnormal brain development. Development. 1996;122:2661–2672. doi: 10.1242/dev.122.9.2661. [DOI] [PubMed] [Google Scholar]
- Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. J Neurosci. 2011;31:6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi S, Gokhan S, Dai XM, Wei S, Enikolopov G, Lin H, Mehler MF, Stanley ER. The CSF-1 receptor ligands IL-34 and CSF-1 exhibit distinct developmental brain expression patterns and regulate neural progenitor cell maintenance and maturation. Dev Biol. 2012;367:100–113. doi: 10.1016/j.ydbio.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides TP, Li H, Solomon DA, Hariono S, Hashizume R, Barkovich K, Baker SJ, Paugh BS, Jones C, Forshew T, Hindley GF, Hodgson JG, Kim JS, Rowitch DH, Weiss WA, Waldman TA, James CD. Targeted therapy for BRAFV600E malignant astrocytoma. Clin Cancer Res. 2011;17:7595–7604. doi: 10.1158/1078-0432.CCR-11-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, Hempstead BL, Littman DR, Gan WB. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinz M, Priller J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat Rev Neurosci. 2014;15:300–312. doi: 10.1038/nrn3722. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JT, Morganti JM, Bachstetter AD, Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC, Gemma C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J Neurosci. 2011;31:16241–16250. doi: 10.1523/JNEUROSCI.3667-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron-Harel N, Segev Y, Lewitus GM, Cardon M, Ziv Y, Netanely D, Jacob-Hirsch J, Amariglio N, Rechavi G, Domany E, Schwartz M. Age-dependent spatial memory loss can be partially restored by immune activation. Rejuvenation Res. 2008;11:903–913. doi: 10.1089/rej.2008.0755. [DOI] [PubMed] [Google Scholar]
- Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci. 2013;33:17587–17596. doi: 10.1523/JNEUROSCI.3241-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluijter M, van der Sluis TC, van der Velden PA, Versluis M, West BL, van der Burg SH, van Hall T. Inhibition of CSF-1R supports T-cell mediated melanoma therapy. PLoS One. 2014;9:e104230. doi: 10.1371/journal.pone.0104230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson EL, Caldwell HK. Lesions to the CA2 region of the hippocampus impair social memory in mice. Eur J Neurosci. 2014;40:3294–3301. doi: 10.1111/ejn.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczepanski SM, Knight RT. Insights into Human Behavior from Lesions to the Prefrontal Cortex. Neuron. 2014;83:1002–1018. doi: 10.1016/j.neuron.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekita T, Okanoya K. Hippocampus lesions induced deficits in social and spatial recognition in Octodon degus. Behav Brain Res. 2011;219:302–309. doi: 10.1016/j.bbr.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Ueno M, Fujita Y, Tanaka T, Nakamura Y, Kikuta J, Ishii M, Yamashita T. Layer V cortical neurons require microglial support for survival during postnatal development. Nat Neurosci. 2013;16:543–551. doi: 10.1038/nn.3358. [DOI] [PubMed] [Google Scholar]
- Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, Thal DR, Charo IF, Heppner FL, Aguzzi A, Garaschuk O, Ransohoff RM, Jucker M. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A. 2012;109:18150–18155. doi: 10.1073/pnas.1210150109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegiel J, Wisniewski HM, Dziewiatkowski J, Tarnawski M, Kozielski R, Trenkner E, Wiktor-Jedrzejczak W. Reduced number and altered morphology of microglial cells in colony stimulating factor-1-deficient osteopetrotic op/op mice. Brain Res. 1998;804:135–139. doi: 10.1016/s0006-8993(98)00618-0. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Paolicelli RC, Sforazzini F, Weinhard L, Bolasco G, Pagani F, Vyssotski AL, Bifone A, Gozzi A, Ragozzino D, Gross CT. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014;17:400–406. doi: 10.1038/nn.3641. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, Wang-Gillam A, Goedegebuure SP, Linehan DC, DeNardo DG. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 2014;74:5057–5069. doi: 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wild-type mice were injected with clodronate in the right hemisphere of the CA1 hippocampus. Red arrows point to the injection site. Mice were euthanized at days 1–7 post injection and brain sections were stained for Cresyl violet (left panel), Iba-1 (middle panel), and GFAP (right panel). n=3 per group. Scale bar = 100μm
Wild-type mice were injected with clodronate in both hemispheres of the CA1 hippocampus. Mice were euthanized at day 5 post injection and brain sections were stained for Iba-1. n=3 per group. Scale bar = 100μm
(A) Wild-type mice were fed with control (Ctr) or PLX3397-chow for 7 days. The tissue was removed and microglia were visualized on brain sections by immunofluorescence for Iba-1. n=3 per group. (B) Numbers of microglia were quantified in three sections per biological replicate. n= 3 per group. Data are shown as mean ± SEM. ** p<0.005. Scale = 50μm.
Top: Wild-type mice were fed with control (Ctr) or PLX3397-chow for 7 days. The tissue was removed and microglia were visualized on brain sections by immunofluorescence for Iba-1 (left panel). The presence of astrocytes (GFAP, middle panel), and neurons (NeuN, right panel) was assessed. n=3 per group. Scale = 50μm. Bottom: another group of wild-type mice were fed with control (Ctr) or PLX3397-chow for 7 days. Mice were then returned to their regular diet and euthanized two weeks later. Brain sections were stained for Iba-1 (left panel), GFAP (middle panel), and NeuN (right panel) n=3 per group. Scale = 50μm.
(A) Wild-type mice were fed with control (Ctr) or PLX3397-chow for 21 days. The tissue was removed and microglia were visualized on brain sections by immunofluorescence for Iba-1. n=3 per group. Scale = 100μm. (B) Numbers of microglia were quantified in three sections per biological replicate. n= 3 per group. Data are shown as mean ± SEM. *p<0.05
Top: Wild-type mice were fed with control (Ctr) or PLX3397-chow for 7 days. Mice were then returned to their regular diet and euthanized two weeks later. Brain sections were stained for Iba-1. n=3 per group. Scale = 100μm. (B) Numbers of microglia were quantified in three sections per biological replicate. n= 3 per group. Data are shown as mean ± SEM.