Abstract
The present study examined whether the developmental transition from facilitation-based orienting mechanisms available very early in life to selective attention orienting (e.g., inhibition of return, IOR) promotes better learning and memory in infancy. We tested a single age group (4-month-olds) undergoing rapid development of attention orienting mechanisms. Infants completed a spatial cueing task designed to elicit IOR, in which cat or dog category exemplars consistently appeared in either the cued or noncued locations. Infants were subsequently tested on a visual paired comparison of exemplars from these cued and noncued animal categories. As expected, infants showed either facilitation-based orienting or the more mature IOR-based orienting during spatial cueing/encoding. Infants who demonstrated IOR-based orienting showed memory for both specific exemplars and broader category learning, whereas those who showed facilitation-based orienting showed weaker evidence of learning. Attention orienting also interacted with previous pet experience, such that the number of pets at home influenced learning only when infants engaged facilitation-based orienting during encoding. Learning in the context of IOR-based orienting was stable regardless of pet experience, suggesting that selective attention serves as an online learning mechanism during visual exploration that is less sensitive to prior experience.
Keywords: selective attention, learning and memory, inhibition of return, pet experience
Attention and memory are inherently linked but is the development of selective attention orienting a catalyst for change in learning and memory efficacy? Attention orienting influences what information is selected for learning and memory (Ross-Sheehy, Oakes, & Luck, 2011; Wu, Gopnik, Richardson, & Kirkham, 2011; Wu & Kirkham, 2010) and orienting to a target while simultaneously suppressing distraction affects how well information is learned and remembered (Markant & Amso, 2013, 2014). The present study examined the latter mechanism and the hypothesis that the development emergence of selective attention orienting is an agent of change in the efficacy of learning and memory during infancy. We addressed this question by focusing on a single age group (4-month-olds) undergoing marked development of attention orienting mechanisms (Amso & Johnson, 2008; Butcher, Kalverboer, & Geuze, 1999; Hood, 1993; Johnson & Tucker, 1996; Richards, 2000). Thus, we were able to hold age constant while examining differences in learning and memory that are associated with the emergence of attention selection via suppression.
Attention orienting has been studied in infancy and across development using the spatial cueing task (Posner & Cohen, 1984; Posner, 1980). In this task, participants covertly orient to brief peripheral cues resulting in engagement of attention at the cued location. The cue is followed by targets appearing in either the same cued location or in the opposite, noncued location. When the cue-to-target delay is short (< 250 ms), attention orienting benefits from the already existing attention engagement at the cued location, an effect known as facilitation (Posner & Cohen, 1984). Facilitation-based orienting is evident among very young infants (Butcher et al., 1999; Johnson & Tucker, 1996). When the cue-to-target delay is extended (> 250 ms), the cued location becomes suppressed and attention is biased to the noncued location, an effect known as inhibition of return (IOR) (Posner, Rafal, & Choate, 1985). Unlike orienting powered by facilitation at the cued location, IOR-based orienting involves an attention bias to a target location that is paired with suppression at the previously cued location, which has classically been viewed as a mechanism that promotes orienting to novel locations in visual search (Klein & MacInnes, 1999; Klein, 1988, 2000).
Visual exploration is a powerful information-gathering tool early in postnatal life. Selective attention results in improved quality of early vision, including enhanced contrast sensitivity and acuity for attended information (Carrasco, 2011, 2013). Extensive previous research has established that selective attention involves enhanced processing at the attended location and simultaneous distractor suppression, which together resolve visual noise and shift the balance such that there is a perceptual enhancement at the attended location and the attended object is seen better (Carrasco, 2011; Desimone & Duncan, 1995; Kastner & Ungerleider, 2000). However, as we execute successive eye movements during visual search and exploration, a lingering attentional trace remains at the previously attended (cued) location (Golomb, Nguyen-Phuc, Mazer, McCarthy, & Chun, 2010; Golomb, Pulido, Albrecht, Chun, & Mazer, 2010), which could potentially interfere with encoding of information at the currently attended location. Previous work examined whether target encoding in the context of suppression at the previously attended location (i.e., IOR) would result in a more robust signal for learning and memory processing relative to encoding contexts that did not involve this interference suppression (i.e., facilitation) (Markant & Amso, 2013, 2014). Consistent with these ideas, results showed that target objects encoded in the context of IOR-based attention orienting were remembered better at test than target objects encoded in the context of facilitation-based attention orienting, which showed no memory benefit beyond that observed at baseline without attention orienting (Markant & Amso, 2013, 2014). This was true at multiple ages and despite identical task demands except for the delay length between covert orienting to the cue and the overt eye movement to the target.
The selective attention mechanisms that allow for suppression of competing information during orienting (i.e., IOR) develop between 4 and 6 months of age (Amso & Johnson, 2008; Butcher et al., 1999; Hood, 1993; Johnson & Tucker, 1996; Richards, 2000). Prior to this age, attention orienting is primarily driven by simpler facilitation-based orienting mechanisms that drive infants’ attention to arousing or perceptually salient information (Butcher et al., 1999; Johnson & Tucker, 1996; Posner, 2001). The natural question is whether this transition from relying on facilitation-based orienting mechanisms to orienting mechanisms that engage suppression (i.e., IOR) improves the efficacy of infants’ encoding and subsequent recognition memory. Although previous work has documented enhanced learning in the context of IOR-based orienting relative to facilitation-based orienting among 9-month-old infants and children (Markant & Amso, 2013, 2014), these older groups were all capable of robust IOR-based orienting, making it difficult to address whether the emergence of IOR-based orienting mechanisms promotes more effective learning and memory during early development.
We examined this question through the lens of infants’ learning and memory for cat and dog categories. Previous work has shown that 3- to 4-month-old infants can form unique categories for cats and dogs (Oakes & Ribar, 2005; Quinn, Eimas, & Rosenkrantz, 1993). Mareschal and colleagues (French, Mareschal, Mermillod, & Quinn, 2004; Mareschal, French, & Quinn, 2000; Mareschal & Quinn, 2001) have shown that the primary differences in feature distributions that distinguish cats and dogs are found in the face and head region (e.g., spacing of the ears), making the head region diagnostic during learning of cat and dog categories. By 4 months of age infants preferentially look at the head region relative to non-head/body regions when viewing images of cats and dog, suggesting that they have formed an attentional bias favoring the diagnostic head region by this age (Hurley & Oakes, 2015; Quinn, Doran, Reiss, & Hoffman, 2009). Furthermore, information from the head region, but not the body, was critical for successful cat/dog category learning at this age (Quinn & Eimas, 1996), particularly when the familiarization period was short (Spencer & Quinn, 1997) and even when the internal facial features were absent and only the silhouette of the head was available (Quinn, Eimas, & Tarr, 2001).
The attentional bias in favor of the diagnostic head region is further modulated by infants’ prior experiences with pets. Although all infants show a bias to look at the head region by 4 months of age, this bias was stronger for infants who had experience with pets at home (Hurley & Oakes, 2015; Kovack-Lesh, McMurray, & Oakes, 2014). Moreover, this effect of pet experience is specific to cat/dog images, as there was no difference in looking to diagnostic regions of human faces or vehicles across infants with and without pets (Hurley & Oakes, 2015). Pet experience was similarly related to 6-month-old infants’ looking patterns, as infants with pets at home showed longer look durations and more frequent glances between two paired cat/dog images (Hurley, Kovack-Lesh, & Oakes, 2010). Additional work has shown that infants’ previous experience with pets interacts with their online attentional biases and looking patterns to influence learning of cat and dog categories. Specifically, Kovack-Lesh and colleagues found that 4-month-old infants’ learning about images of cats was influenced by both their previous experience with pets and their looking patterns during familiarization, including the frequency with which they looked back and forth between two paired images (Kovack-Lesh, Horst, & Oakes, 2008; Kovack-Lesh et al., 2014; Kovack-Lesh, Oakes, & McMurray, 2012). Specifically, infants who had pets at home and also showed high levels of shifting gaze during familiarization were the only group who showed successful learning; those who did not have experience with pets or showed low shift rates did not learn about the cat images presented during familiarization (Kovack-Lesh et al., 2008, 2012). Thus, pet experience may serve to enhance the attentional bias favoring the diagnostic head region and potentially induce changes in information-gathering/scanning strategies in support of category learning (Hurley & Oakes, 2015).
In the present study we asked 1) whether the emergence of attention orienting mechanisms involving suppression (i.e., IOR) promoted more effective cat/dog category learning among 4-month-old infants, and 2) whether the nature of the underlying orienting mechanism interacted with infants’ prior pet experience to influence successful category learning. In the spatial cueing/encoding phase, 4-month-old infants saw cat and dog exemplars presented either in the cued or noncued locations with long cue-target delays intended to elicit IOR (Markant & Amso, 2013). After the initial spatial cueing phase, infants saw four visual paired comparison test trials, each with exemplars from the cued and noncued animal categories on either side of the screen. We paired familiar exemplars from the cued (e.g., dog) and noncued categories (e.g., cat) in one test trial to examine item memory, and novel exemplars from the same categories in a separate test trial to examine broader category learning. Our predictions were as follows: First, we predicted that 4-month-old infants would show either facilitation-based orienting or the more mature selective attention IOR-based orienting during spatial cueing/encoding. The cue-target delay was very long (1 sec) and as such should elicit IOR in infants (Markant & Amso, 2013; Richards, 2000), if infants have developed the more mature attention mechanisms supporting IOR. Faster orienting to the cued relative to noncued locations would indicate that infants relied on the less mature facilitation-based orienting mechanism that benefits from attention engagement at the cued location (Johnson & Tucker, 1996; Richards, 2000). Thus, for these infants attention was biased to the cued location, which should support learning of exemplars presented in that location. In contrast, slower orienting to the cued location relative to the noncued location (i.e., IOR) reflects suppression at the cued location during the long delay and a subsequent attention bias to the noncued location, which should support learning of exemplars presented in the noncued location. Thus we expected that infants would learn best from the location of their attention bias. Our primary prediction was that learning from this attention bias, and subsequent memory, would be enhanced in the context of IOR-based orienting relative to facilitation-based orienting. Although we differentiate infants who showed facilitation versus IOR to consider the underlying mechanisms driving their orienting behavior, the majority of our analyses treated the spatial cueing score as a continuous variable.
We secondarily examined whether pet ownership interacted with the development of attention orienting mechanisms in contributing to infants’ learning of cat and dog categories. Consistent with previous research showing that pet experience interacted with online looking patterns during familiarization to influence learning (Kovack-Lesh et al., 2008, 2014, 2012) we predicted that the influence of pet experience on cat/dog category learning may depend on the nature of the attention orienting mechanism (i.e., facilitation vs. IOR) that was engaged during encoding. We presented infants with paired novel animal category exemplars at test from both the cued and noncued categories. In this case, the test items consisted of only the head region or only the body region, as previous research indicated that infants with pet experience show a stronger bias to attend to the diagnostic head region (Hurley & Oakes, 2015; Kovack-Lesh et al., 2014). We addressed the possibility that experiential variables buffer learning and memory when attention orienting mechanisms are immature, or facilitation-based, but that selective attention allows for learning from the diagnostic head information for subsequent memory even in the absence of pet experience.
Method
Participants
The final sample included thirty-nine 4-month-old infants (15 M, 24 F; MAge = 4 months, 12 days, SD = 10.26 days, range = 3 months, 25 days – 5 months, 11 days). Thirteen additional infants were tested but excluded due to fussiness (10) or lack of attention to the stimulus display (3). Parents reported that 71.8% of participants were Caucasian, 10.3% were African-American, 10.3% were Asian, and 7.7% were Hispanic.
Pet Experience
We assessed infants’ previous pet experience by asking parents whether they had any cats or dogs at home. Twelve families (31% of the sample) reported that they did not own any pets. The remaining 27 parents who did own pets were further asked to indicate how many and whether they owned cats, dogs, or both cats and dogs. Fifteen families reported that they owned 1 pet, 7 families owned 2 pets, 4 families owned 3 pets, and 1 family owned 5 pets. Of the families that owned pets, ten owned at least one dog, 13 families owned at least one cat, and four families owned at least one cat and one dog.
Eye tracking apparatus
We recorded eye movements using a remote eye tracker (SensoMotoric Instruments 60 Hz RED system). Infants sat on their parent’s lap 70 cm from a 22” monitor. A digital video camera (Canon ZR960) recorded infants’ head movements and allowed for online coding during the test phase. The video output was also recorded as a digital file.
Stimuli were presented using the SMI Experiment Center software. We used a 2-point calibration and 4-point calibration accuracy check protocol. Average deviation was 3.5° (SD = 2.1°). The digital eye recording was used for offline coding of left/right eye movements if an accurate calibration/stable point of gaze (POG) was not obtained. To confirm the accuracy of these data, reliability between coded and POG data was calculated for a subset of videos for infants who had successful eye movement recordings, R > .95, p < .01.
Stimuli
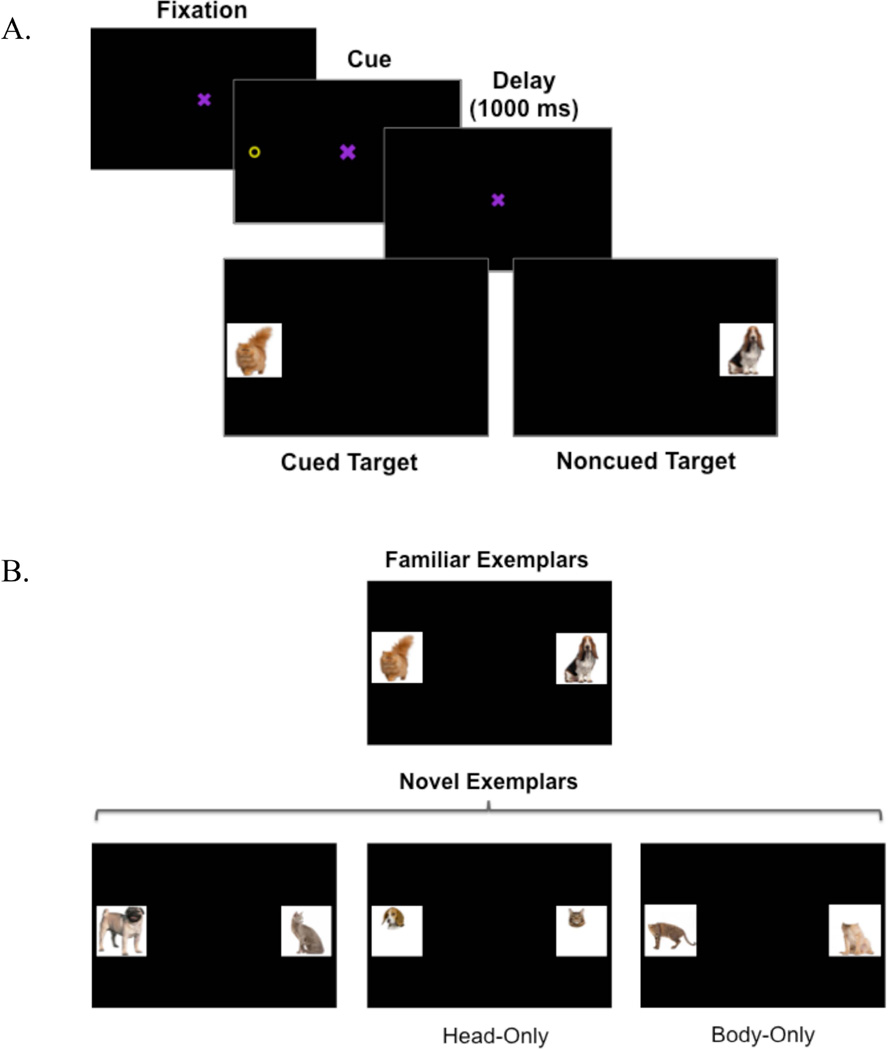
The task consisted of a spatial cueing/encoding phase followed by a subsequent memory test phase (see Figure 2). For the spatial cueing/encoding phase, stimuli included a central fixation, a cue, and a set of target objects (abstracts objects or faces). The fixation shape was a purple × (3.5 cm2) that rotated in the center of the display to engage infants’ attention. The cue was a yellow ring (2.5 cm diameter). The cue and targets appeared 10° (12.1 cm) to the left or right of the fixation. Targets were 7.1 cm2 color images of cats and dogs drawn from a set of 14 unique cat images and 14 unique dog images (see Figure 1A and B). These cat and dog images were selected to include a range of breeds, colors, and body positions (i.e., face view or profile view).
Figure 2.
(A) An example of one of the 56 spatial cueing/encoding trials. (B) Each of the four trials presented at test.
Figure 1.
Complete set of cat (A) and dog (B) category exemplars used as stimuli during the spatial cueing/encoding phase. (C) Cat and dog stimuli used in each of the four test trials.
Test stimuli included four cats and four dogs (Figure 1C). One cat and one dog were drawn from the set of stimuli that infants viewed during the spatial cueing/encoding phase (Familiar Exemplars). The remaining stimuli were novel cat and dog images that had not previously been seen by the infants. All infants saw the same cat and dog stimuli at test.
Procedure
Spatial Cueing/Encoding
The task included a spatial cueing/encoding phase followed by a subsequent memory test. The initial spatial cueing phase included 56 trials (see Figure 2A for an example trial). Each trial began with presentation of the central fixation for 1100 ms, followed by presentation of the peripheral cue for 100 ms and a subsequent 1000 ms delay period in which only the fixation stimulus was visible. A 1000 ms delay between cue offset and target onset would be sufficient to elicit IOR among older infants and adults (Johnson & Tucker, 1996; Klein, 2000). Indeed, previous versions of the spatial cueing task with 9-month-olds elicited IOR with a 600 ms delay interval (Markant & Amso, 2013). After this delay period the target cat/dog stimulus appeared in either the cued or noncued location and remained visible for 1500 ms.
An equal number of cued and noncued targets were presented in random order. Infants were randomly assigned to one of two conditions: in the ‘Noncued Dog’ condition, dogs were presented in the noncued location and cats were presented in the cued location. Conversely, in the ‘Noncued Cat’ condition, cats were presented in the noncued location and dogs were presented in the cued location. For each infant, the fourteen unique dogs and fourteen unique cats were used as category exemplars (each 7.1 cm2 on the screen). Each category exemplar was randomly presented once on the left and once on the right during encoding. Thus, every infant received 2 trials for each cat exemplar, 2 trials for each dog exemplar and a total of 28 cued trials and 28 noncued trials. For example, an infant in the Noncued Dog condition saw each of the 14 dogs in Figure 1B appear twice in the noncued location and each of the 14 cats in Figure 1A appear twice in the cued location. Conversely, an infant in the Noncued Cat condition saw each of the cats appear in the noncued location twice and each of the dogs appear in the cued location twice.
Test
After the spatial cueing phase, infants saw four paired comparison test trials. On each test trial, an exemplar from the previously cued category was paired with an exemplar from the previously noncued category. There were four trial types at test (Figure 2B). During Familiar test trials, we examined infants’ learning of the specific exemplars seen during encoding by presenting a familiar exemplar from the cued and noncued categories. During Novel test trials, we examined infants’ learning of the broader cat/dog categories by presenting a new exemplar from the cued and noncued categories. Finally, we also examined whether infants primarily relied on information from the head region during learning of the cat/dog categories. To do so, we included two additional test trials: During Head-Only test trials, infants saw a new exemplar from the cued and noncued categories that included the animals’ heads from the neck up and removed all body information. Conversely, during Body-Only test trials, infants saw a new exemplar from the cued and noncued categories that included only the animals’ bodies from the neck down. Unique novel cat/dog stimuli were used for the Head-Only and Body-Only test trials. The order of trial type presentation was counterbalanced across participants.
Each paired comparison test display was presented for 10 s. Look durations were validated offline.
Preliminary data processing
Eye tracking measures
Our primary variables of interest for the spatial cueing phase were saccade latencies and duration of looking to the targets. Initial processing of the eye movement data utilized the SMI BeGaze analysis software. Three areas of interest (AOIs) were identified based on the central, left, and right stimulus locations. These AOIs were equivalent 9.7 × 14.2 regions over each of these locations. Usable looks were defined as segments of the data in which the POG remained within 7.1 cm2 (the size of the target) for at least 100 ms. This dispersion criterion was less than a third of the distance between the opposing target locations (24 cm/20°), allowing us to maximize usable data while clearly identifying left/right looks. Saccade latencies were based on the time when a look first entered the AOI. Duration of looking was computed by summing the duration of all looks that occurred within the AOI following target onset.
Saccade latency calculation
We sought the cleanest possible measure of spatial cueing from each infant, given the importance of the Spatial Cueing score (and whether it indicates facilitation- or IOR-based orienting) to our investigation. Thus, for each infant individual trials were excluded for the saccade latency calculation if the infant looked at the cue prior to target onset (M = 9.7 trials, SD = 6.5 trials), looked away from the screen before looking at a target (M = 16.3 trials, SD = 7.6 trials), or if POG data was unavailable (M = 6.5 trials, SD = 6.8 trials). We anticipated these high rates of data exclusion (M = 32 trials overall, SD = 8 trials) because of the young age of the sample. However, given the large number of encoding trials (a total of 56 trials) spatial cueing effects were computed based on an average of 24 trials (SD = 8) per infant even after these exclusions.
Spatial cueing scores
We selected this age group because we anticipated that there would be wide variation in orienting responses and predicted that infants who showed evidence of emerging IOR would demonstrate enhanced learning relative to those who showed facilitation. To determine whether infants showed facilitation versus IOR, we generated a Spatial Cueing score for each infant by subtracting their mean response latency to targets appearing in the noncued location from their mean latency to targets in the cued location. Negative Spatial Cueing scores reflect faster response times to the cued location (i.e., facilitation), whereas positive Spatial Cueing scores reflect faster response times to the noncued location (i.e., IOR).
Test
For each test trial, we computed the proportion of total looking time to each of the category exemplars by dividing the sum of all looks to the exemplar by the total duration of the trial. Proportion of looking time was computed separately for the cued and noncued exemplars for each of the four test trials. All test data were included in the analyses.
Results
Spatial Cueing/Encoding Phase
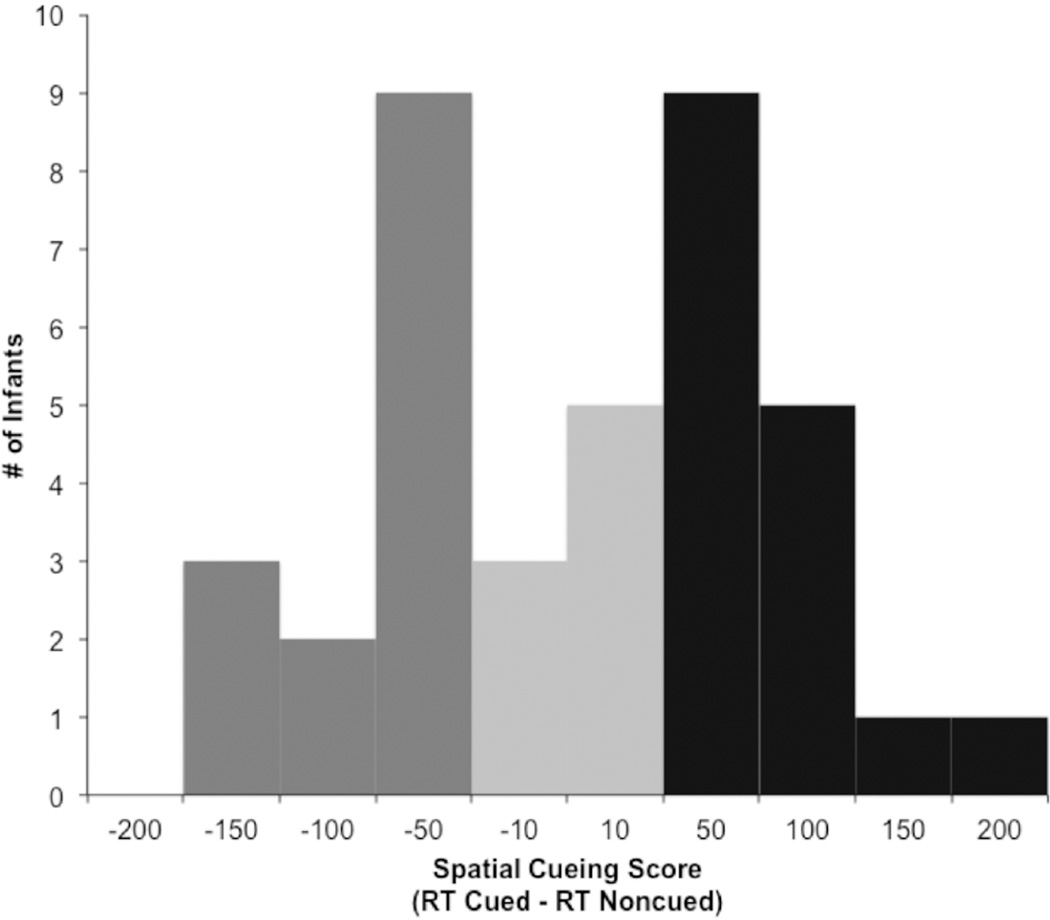
As anticipated, there was a great deal of variability in infants’ Spatial Cueing scores, as individual Spatial Cueing scores ranged from −135.58 ms to 192.20 ms (see Figure 3). Infants were considered to show facilitation, with an attention bias to the cued location, if their Spatial Cueing score was > 1 SEM (10.15 ms) below zero (N = 14 infants; 36%). Similarly, infants were considered to show IOR, with suppression at the previously cued location and an attention bias to the noncued location, if their Spatial Cueing core was > 1 SEM above zero (N = 17 infants; 44%). The remaining infants (N = 8; 20%) had Spatial Cueing scores around zero and were considered to show neither facilitation nor IOR. Despite this variability, Spatial Cueing scores were not correlated with Age (r(39) = .01, p = .972), proportion of trials excluded during encoding (r(39) = .15, p = .366), or the duration of looking to the targets during encoding (r(39) = −.14, p = .383). Analysis of infants’ eye movement latencies indicated that, due to the expected individual differences in facilitation and IOR in this age group, there was no overall spatial cueing effect at the group level (MCued = 433.48, SD = 117.94 ms; MNoncued = 428.20, SD = 109.52 ms; F(1,38) = 0.27, p = .606). Paired t-tests indicated that the group of infants showing facilitation were reliably faster to the cued location relative to the noncued location (Mcued = 373.35, SD = 79.16 ms, Mnoncued = 429.31, SD = 104.51 ms, t(13) = −5.20, p < .000). Conversely, the group of infants who showed IOR were reliably faster to the noncued location relative to the cued location (Mcued = 486.16, SD = 127.93 ms, Mnoncued = 428.70, SD = 96.51 ms, t(16) = 5.25, p < .000).
Figure 3.
Distribution of infants showing facilitation (dark grey), IOR (black), or no spatial cueing effect (light grey).
Paired Comparison Memory Test
Memory test trials were designed to examine proportion of looking times to animal category exemplars as a function of the location in which they appeared during the spatial cueing/encoding phase. Recall that 4-month-old infants saw both cat and dog categories, but they were presented either in the cued or in the noncued locations during spatial cueing/encoding. The omnibus ANCOVA examined Target Location at spatial cueing/encoding (cued, noncued) and Test Trial Type (familiar exemplar, novel exemplar, Head-Only novel exemplar, Body-Only novel exemplar) as within-subject factors and Animal Condition (i.e., whether infants had been in the Noncued-Dog vs. Noncued-Cat condition during encoding) as a between-subjects factor. We also included three continuous variables in the model, including 1) Age, 2) Duration of Looking (i.e., the mean duration of looking at the targets during encoding), and 3) Spatial Cueing Score. The continuous variables were centered, as in regression, by subtracting the mean value of the variable from each participant’s raw score. The omnibus ANCOVA resulted in only a significant three-way Target Location × Test Trial Type × Spatial Cueing Score interaction (F(3,102) = 2.90, p = .039, ηp2 = .08). There were no other reliable main effects or interactions (all ps > .09). In order to further probe the significant three-way interaction we conducted follow-up analyses that were designed to test our original predictions and were thus conducted separately for the whole animal test trials and Head/Body-Only test trials.
Learning Whole Animal Exemplars
Our first prediction was that infants would best learn the exemplars presented in the location of their attention bias (i.e., the cued location for infants showing facilitation-based orienting and the noncued location for infants showing IOR-based orienting). Our next prediction was that 4-month-old infants who showed evidence of IOR-based orienting would show more effective learning and memory for exemplars presented in the location of their attention bias (indicated by proportionally less looking at the exemplars presented in the noncued location) relative to those who showed evidence of facilitation-based orienting. We examined infants’ proportion of looking to the familiar and novel whole-body exemplars from the cued and noncued location categories using repeated-measures ANCOVAs with category Target Location (cued, noncued) and Novelty (familiar, novel) as within-subject factors and Animal Condition as a between-subjects factor. We again included Age, Duration of Looking, and Spatial Cueing Score as continuous variables in the model. Results indicated a significant Novelty × Age interaction (F(1,34) = 4.41, p = .043, ηp2 = .24), reflecting longer looking to the Familiar exemplars relative to the Novel exemplars among older infants (r(33) = .35, p = .04). Results also indicated a significant Target Location × Spatial Cueing Score interaction (F(1,34) = 10.90, p = .002, ηp2 = .24). There were no other reliable main effects or interactions (all ps > .06). Importantly, the Target Location × Novelty × Spatial Cueing Score interaction was not significant (F(1,34) = 1.37, p = .251), indicating that this relationship was similar across the Familiar and Novel test trials that indexed memory for familiar exemplars seen during encoding and learning of the broader categories, respectively.
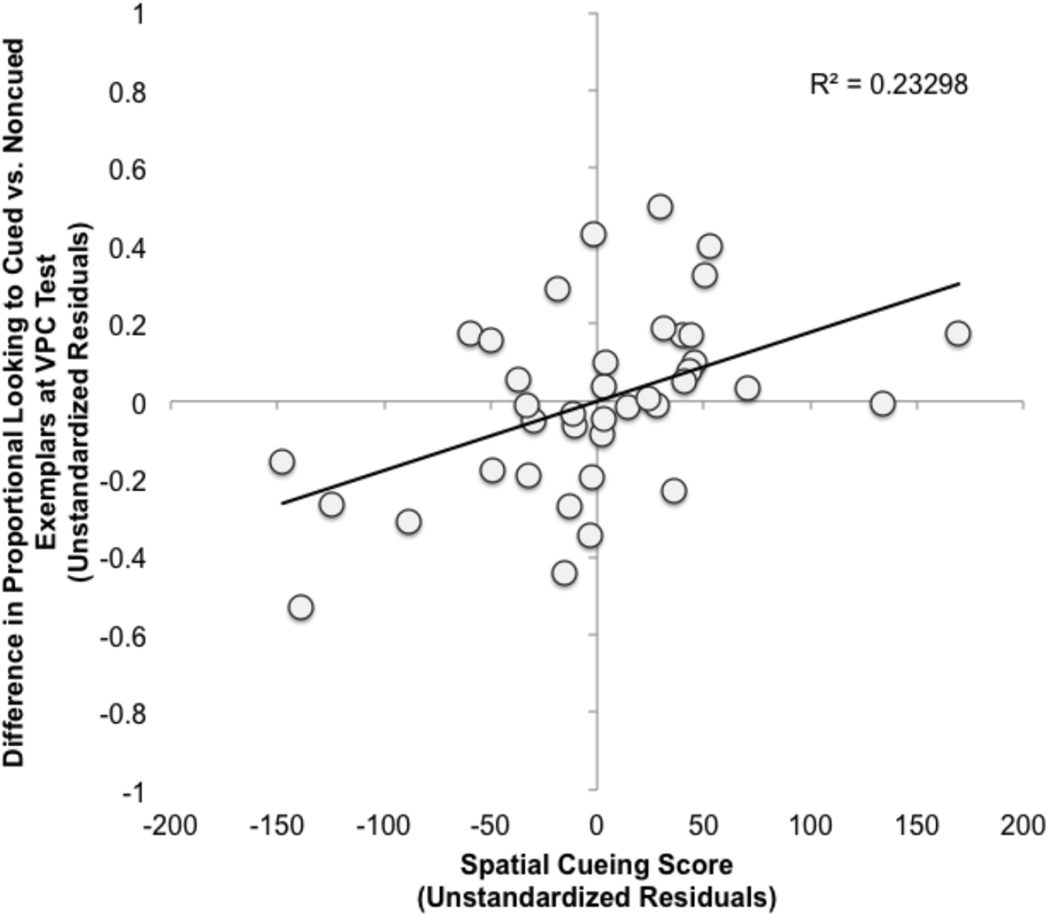
Recall that negative Spatial Cueing scores reflect faster response times to the cued location (i.e., facilitation), whereas positive Spatial Cueing scores reflect faster response times to the noncued location (i.e., IOR). We computed a difference score for each infant by subtracting their proportional looking to the noncued category exemplars from their proportional looking to the cued category exemplars. Positive scores indicated preferential looking to the noncued exemplars whereas negative scores indicated preferential looking to the cued exemplars. Partial correlations accounting for Animal Condition, Age, and Duration of Looking indicated that Spatial Cueing score was reliably positively related to this difference score (r(34) = .49, p = .002, Figure 4). Figure 4 shows that infants clustered into two groups. Four-month-olds who engaged the more mature IOR-based attention orienting mechanism had shorter looking times to category exemplars (familiar and novel) encoded in the noncued location. Four-month-olds who engaged the more immature facilitation-based orienting mechanism at encoding had shorter looking times to the cued items, relative to the paired items presented in the noncued location at encoding. These data demonstrate that both the facilitation and IOR orienting mechanisms supported learning at the individual level and thus confirmed our first prediction that infants would best learn the category information presented in the location of their attention bias.
Figure 4.
Partial plot reflecting the relationship between Spatial Cueing score and infants’ looking to category exemplars at test, expressed as a subtraction of proportion of looking to the exemplars encoded in the cued – noncued locations during the spatial cueing/encoding phase.
We next dichotomized infants into two groups, those who showed facilitation (i.e., Spatial Cueing scores > 1 SEM below zero, N = 14) and those who showed IOR (i.e., Spatial Cueing scores > 1 SEM above zero, N = 17). Paired sample t-tests examining looking to the cued versus noncued locations during the memory test trials indicated that only the group of infants who engaged IOR-based orienting showed reliably different looking to the exemplars from the cued versus noncued categories. Specifically, these infants showed reduced looking to the exemplars from the noncued category (M = .23, SD = .10) and greater looking to the exemplars from the cued category (M = .34, SD = .17; t(16) = 2.47, p = .025; Cohen’s dz = .60), indicating greater learning of the noncued category during encoding. Infants whose Spatial Cueing score indicated facilitation-based orienting did not show a reliable difference at test (MCued = .27, SD = .16, MNoncued = .33, SD = .17; t(13) = −0.92, p = .374, Cohen’s dz = .25). As predicted, IOR-based orienting was shown here to be a more powerful learning mechanism at the group level for subsequent memory and categorization than facilitation-based orienting.
Attention to Head and Body Features
Our final analysis was based on prior research showing that previous pet experience interacted with infants’ attention to the diagnostic head region during cat/dog category learning. We predicted that selective attention would support learning from the diagnostic head information for subsequent memory even in the absence of pet experience. We examined infants’ responses during the Head-Only and Body-Only test trials, in which infants saw novel exemplars from the cued and noncued categories that limited the features available for processing. To do so, we entered proportion of looking data into an ANCOVA with Target Location (cued, noncued) and Feature (Head-Only, Body-Only) as within-subject factors, Animal Condition (cat, dog) as a between-subjects factor, and the same continuous covariates used previously, including Age, Duration of Looking, and Spatial Cueing score. We additionally included the number of pets in infants’ homes (‘Number of Pets’), and the Spatial Cueing Score × Number of Pets interaction into these analyses.
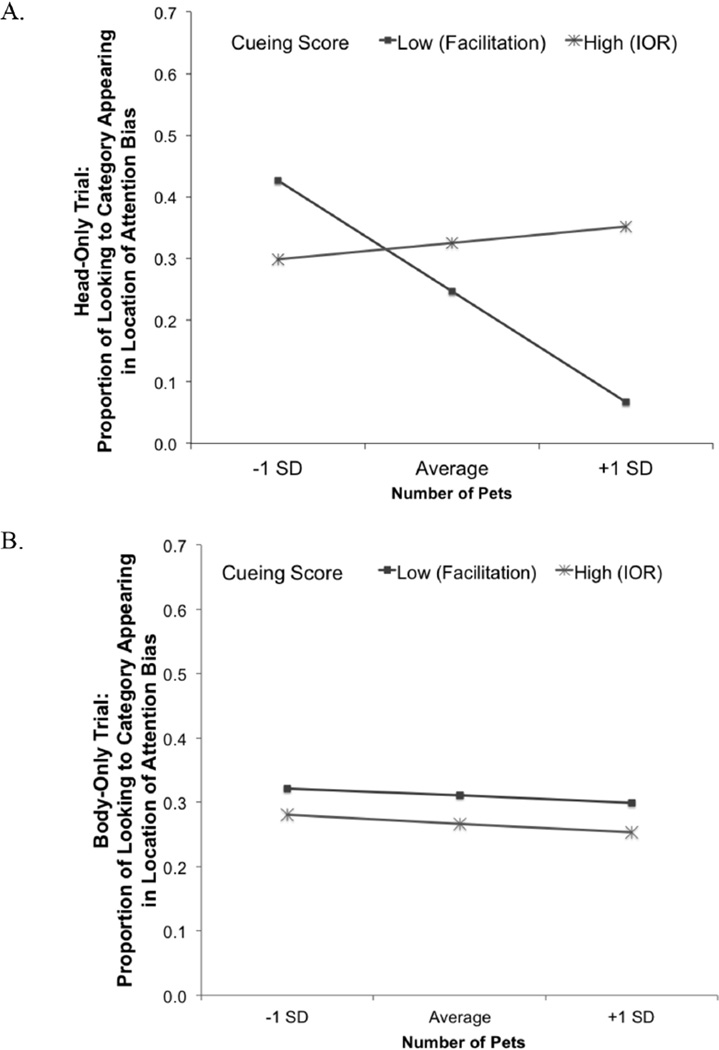
Results indicated a significant Target Location × Feature × Duration of Looking interaction (F(1,32) = 6.85, p = .013, ηp2 = .18), which reflected a relationship between longer overall looking times during encoding and greater interest in the Head-Only item from the cued category (r(32) = .45, p = .008) and the Body-Only item from the noncued category at test (r(32) = .35, p = .04). However this effect did not interact with Spatial Cueing score, Animal Condition, or infant’s prior pet experience. Results from the omnibus ANCOVA also indicated a significant Target Location × Feature × Spatial Cueing Score × Number of Pets interaction (F(1,32) = 4.96, p = .033, ηp2 = .13). Follow-up analyses revealed a significant Target Location × Spatial Cueing Score × Number of Pets interaction effect on responses to the Head-Only exemplars (F(1,32) = 5.11, p = .031, ηp2 = .14, Figure 5A) but not Body-only exemplars (F(1,32) < .001, p = .986, Figure 5B). Partial correlations revealed that the Spatial Cueing Score × Number of Pets interaction term was positively related to looking to the exemplar from the cued (r(32) = .48, p = .005) but not noncued category (r(32) = −.19, p = .275) during the Head-Only trial. We used simple slopes analyses to further examine the relationship between Number of Pets and looking to the Head-Only exemplar from the category presented in the location of the predicted attention-bias during spatial cueing/encoding (i.e., the cued location for infants showing facilitation-based orienting, the noncued location for infants showing IOR-based orienting). Results revealed that the relationship between Number of Pets and looking to cued Head-Only exemplar was significantly steeper when infants showed facilitation (B = −0.14, SEM = 0.06; t(35) = −2.37, p = .024) relative to the relationship between Number of Pets and looking to the noncued Head-Only exemplar when infants showed IOR (B = 0.02, SEM = 0.05; t(35) = 0.49, p = .629). Figure 5A shows that the 4-month-olds who engaged more immature facilitation-based orienting relied on pet experience to learn items presented in the location of their attention bias, as indicated by shorter look durations to category exemplars encoded in the cued location among infants with a higher number of pets at home. Looking time to the Head-Only exemplars presented in the location of the IOR attention bias was stable among infants who showed IOR, regardless of pet ownership.
Figure 5.
Simple slopes plots illustrating the interaction between Spatial Cueing score and Number of Pets on infants’ looking to (A) the Head-Only and (B) Body-Only exemplars for infants whose Spatial Cueing scores indicated facilitation-based orienting at encoding (spatial cueing scores 1 SD below the mean), and IOR-based orienting at encoding (spatial cueing scores 1 SD above the mean).
Discussion
We asked whether the development of selective attention orienting is an agent of developmental change in learning and memory. We focused on a single age group (4-month-olds) undergoing rapid development of attention orienting mechanisms (Amso & Johnson, 2008; Butcher et al., 1999; Hood, 1993; Johnson & Tucker, 1996; Richards, 2000). Our results showed that the transition from simple facilitation-based orienting mechanisms to more mature selective attention IOR-based orienting had implications for learning of information appearing in the attended locations. Positive spatial cueing scores, indicative of IOR, were related to shorter look durations at test to animal category exemplars presented in the attended, noncued location at encoding, indicating learning of the noncued category. This was true whether the test exemplar was a familiar or novel item from the familiar category, indicating that IOR-based orienting, supported both simple memory and broader category learning. In contrast, infants who had negative spatial cueing scores, indicative of facilitation-based orienting, showed weaker evidence of memory for familiar exemplars and category learning from information presented in the cued location. Although individual infants who showed the strongest facilitation-based orienting learned about the category appearing in the cued location, additional analyses indicated that the more basic facilitation-based orienting was not strongly related to improvements in learning and memory at the group level. This finding extends previous work showing that IOR-based orienting supported enhanced encoding and recognition memory among older infants and children who were capable of robust IOR (Markant & Amso, 2013, 2014). The present data further show that the emergence of selective attention orienting mechanisms during early development promoted more effective learning and memory.
Additionally, individual differences in the orienting mechanism engaged during encoding interacted with infants’ prior experience with pets to impact category learning. Specifically, previous experience with pets influenced learning about the diagnostic head region, but only when basic facilitation-based orienting was engaged during encoding. It is important to note here that the spatial cueing/encoding phase included only whole animals. As such, test trials with Head- and Body-Only familiar category but novel-item exemplars were quite challenging and our data do not show strong group-level learning effects based on these features alone. Nonetheless, we did find differences in the relationship between pet experience and the attention orienting mechanism engaged during encoding. Infants without pets at home who engaged in facilitation-based orienting showed poorer learning of the head region of exemplars presented in the cued location during encoding, as indicated by longer looking times at test. However, looking times to novel Head-Only exemplars from the familiar category decreased as a function of pet experience, indicating that experience bootstraps learning in the absence of a robust selection via suppression attention orienting mechanism. In contrast, the number of pets at home had little impact among infants who engaged suppression/IOR during encoding, as these infants showed the same level of learning about the head region regardless of their previous experience with pets.
The interaction between attention orienting mechanisms and prior experience with pets was specific to infants’ learning about the head region. This is consistent with prior research indicating that features of the head region are diagnostic in distinguishing cat/dog categories (French et al., 2004; Mareschal et al., 2000; Mareschal & Quinn, 2001) and that attention to the head region is critical for successful learning about these categories at 4 months of age (Quinn et al., 2001; Quinn & Eimas, 1996; Spencer & Quinn, 1997). Previous research has also shown that infants at this age were biased towards looking at the head region (Hurley & Oakes, 2015; Quinn et al., 2009) and that infants who had previous pet experience were more likely to allocate attention to the head region (Hurley & Oakes, 2015; Kovack-Lesh et al., 2014). In the present study, previous pet experience similarly modulated learning about the head region among infants who engaged in facilitation-based orienting during encoding. Moreover, the lack of any effects for the Body-Only test exemplars suggests that infants learned less about the animals’ bodies during encoding, further underscoring the important role of the head region during category learning at this age.
The present results are also consistent with previous work showing that individual differences in online attention processing interact with pet experience to influence 4-month-old infants’ learning of cat/dog categories (Kovack-Lesh et al., 2008, 2014, 2012). In these studies infants’ prior experience with pets interacted with their specific looking patterns during familiarization, including the frequency of gaze shifts between two paired images (Kovack-Lesh et al., 2008, 2012) and the proportion of time spent looking at the head region (Kovack-Lesh et al., 2014). The present study extends this work by showing that infants’ prior experience with pets interacts not just with overt patterns of eye movements but also with the underlying, covert orienting mechanisms that support attention processing during encoding.
More broadly, these results are consistent with data showing that attention orienting mechanisms interact with both intrinsic and extrinsic factors to impact learning at multiple developmental time points. Previous research demonstrated that attention orienting mechanisms interacted with IQ to predict recognition memory among children and adolescents (Markant & Amso, 2014) and with parental socioeconomic status (SES) to predict recognition memory among 9-month-old infants (Markant, Ackerman, Nussenbaum, & Amso, under review). Specifically, IQ was the only predictor of recognition memory among children who engaged facilitation-based orienting during encoding. In contrast, IQ did not predict recognition memory among children who engaged IOR-based orienting involving distractor suppression during encoding; instead, the extent of suppression was the only predictor of recognition memory. Similarly, SES predicted infants’ recognition memory when they engaged in facilitation-based orienting during encoding, with infants from lower SES environments showing poorer recognition memory relative to those from high SES environments. However, this effect of SES was mitigated when infants engaged in IOR-based orienting during encoding, as infants from low SES environments performed at the same level as those from high SES environments (Markant et al., under review). The present data indicating that infants’ prior pet experience influences learning in the context of facilitation-based orienting but not IOR-based orienting replicates this pattern and suggests that selective attention functions as a robust online learning mechanism during visual exploration. Together, this work suggests that prior to the emergence of selective attention mechanisms, infants’ learning is more dependent on frequent exposure to environmental features (e.g., experience with pets). This reliance on frequent or salient environmental features may be adaptive as it allows very young infants to clearly prioritize objects of value in their world. However, the development of more mature selective attention mechanisms allows for online learning that is less sensitive to frequency/salience but supports flexible learning across multiple contexts.
IOR has been classically studied as a mechanism that promotes novelty during visual search, as suppression at previously attended locations biases attention to novel, previously unexplored locations (Klein & MacInnes, 1999; Klein, 1988, 2000; Posner et al., 1985). Eye movement sequences during visual search and exploration are executed on a millisecond basis. During these sequences, each successive eye movement leaves behind a lingering attentional trace at the previously attended (cued) location (Golomb, Nguyen-Phuc, et al., 2010; Golomb, Pulido, et al., 2010), which can interfere with encoding of information at the currently attended location. Previous work has shown that engaging an IOR-based orienting mechanism during encoding, which effectively suppresses the attention trace at the previously attended/cued location, supported enhanced encoding and subsequent recognition memory at 9 months of age, during childhood and adolescence, and in adulthood (Markant & Amso, 2013, 2014; Markant, Worden, & Amso, 2015). The present results add to this work by demonstrating that 4-month-old infants who engaged the more mature IOR-based orienting during encoding showed more robust category learning than those who failed to engage IOR-based orienting during encoding. Thus IOR benefits both visual search and learning and memory, suggesting that the emergence of selective attention mechanisms at 4- to 6-months of age may be critical not just for the developing attention system but also for the learning and memory system. Given that eye movements are one of premotor infants’ primary means of exploration in early postnatal life, the emergence of IOR in early infancy may improve the efficiency of infants’ learning as they execute series of successive eye movements during visual exploration.
Acknowledgements
The authors gratefully acknowledge funding from the National Institutes of Health (NIGMS-NIH P20 GM103645 to DA) and the James S. McDonnell Foundation (Scholar Award in Understanding Human Cognition to DA). We would also like to thank Lisa Oakes for discussions and comments regarding the role of previous pet experience in infants’ learning of cat and dog categories.
References
- Amso D, Johnson SP. Development of visual selection in 3- to 9-month-olds: Evidence from saccades to previously ignored locations. Infancy. 2008;13(6):675–686. doi: 10.1080/15250000802459060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher PR, Kalverboer AF, Geuze RH. Inhibition of return in very young infants: A longitudinal study. Infant Behavior and Development. 1999;22:303–319. [Google Scholar]
- Carrasco M. Visual attention: The past 25 years. Vision Research. 2011;51:1484–1525. doi: 10.1016/j.visres.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco M. Spatial covert attention: Perceptual modulation. In: Nobre AC, Kastner S, editors. The Oxford Handbook of Attention. Oxford University Press; 2013. p. 183. [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- French RM, Mareschal D, Mermillod M, Quinn PC. The role of bottom-up processing in perceptual categorization by 3- to 4-month-old infants: simulations and data. Journal of Experimental Psychology. General. 2004;133:382–397. doi: 10.1037/0096-3445.133.3.382. [DOI] [PubMed] [Google Scholar]
- Golomb JD, Nguyen-Phuc AY, Mazer JA, McCarthy G, Chun MM. Attentional facilitation throughout human visual cortex lingers in retinotopic coordinates after eye movements. The Journal of Neuroscience. 2010;30:10493–10506. doi: 10.1523/JNEUROSCI.1546-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb JD, Pulido VZ, Albrecht AR, Chun MM, Mazer JA. Robustness of the retinotopic attentional trace after eye movements. Journal of Vision. 2010;10:1–12. doi: 10.1167/10.3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood BM. Inhibition of return produced by covert shifts of visual attention in 6-month-old infants. Infant Behavior & Development. 1993;16:245–254. [Google Scholar]
- Hurley KB, Kovack-Lesh KA, Oakes LM. The influence of pets on infants’ processing of cat and dog images. Infant Behavior & Development. 2010;33:619–628. doi: 10.1016/j.infbeh.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KB, Oakes LM. Experience and distribution of attention: Pet exposure and infants’ scanning of animal images. Journal of Cognition and Development. 2015;16:11–30. doi: 10.1080/15248372.2013.833922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MH, Tucker LA. The development and temporal dynamics of spatial orienting in infants. Journal of Experimental Child Psychology. 1996;63:171–188. doi: 10.1006/jecp.1996.0046. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibitory tagging system facilitates visual search. Nature. 1988;334:430–431. doi: 10.1038/334430a0. [DOI] [PubMed] [Google Scholar]
- Klein RM. Inhibition of return. Trends in Cognitive Sciences. 2000;4:138–147. doi: 10.1016/s1364-6613(00)01452-2. [DOI] [PubMed] [Google Scholar]
- Klein RM, MacInnes WJ. Inhibition of return is a foraging facilitator in visual search. Psychological Science. 1999;10:346–352. [Google Scholar]
- Kovack-Lesh KA, Horst J, Oakes LM. The cat is out of the bag: The joint influence of previous experience and looking behavior on infant categorization. Infancy. 2008;3:285–307. [Google Scholar]
- Kovack-Lesh KA, McMurray B, Oakes LM. Four-month-old infants’ visual investigation of cats and dogs: relations with pet experience and attentional strategy. Developmental Psychology. 2014;50:402–413. doi: 10.1037/a0033195. [DOI] [PubMed] [Google Scholar]
- Kovack-Lesh KA, Oakes LM, McMurray B. Contributions of attentional style and previous experience to 4-month-old infants’ categorization. Infancy. 2012;17:324–338. doi: 10.1111/j.1532-7078.2011.00073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mareschal D, French RM, Quinn PC. A connectionist account of asymmetric category learning in early infancy. Developmental Psychology. 2000;36:635–645. doi: 10.1037/0012-1649.36.5.635. [DOI] [PubMed] [Google Scholar]
- Mareschal D, Quinn PC. Categorization in infancy. Trends in Cognitive Sciences. 2001;5:443–450. doi: 10.1016/s1364-6613(00)01752-6. [DOI] [PubMed] [Google Scholar]
- Markant J, Ackerman L, Nussenbaum K, Amso D. Selective attention as a mechanism of resilience: Attention neutralizes the adverse effects of socioeconomic status on memory in 9-month-old infants. doi: 10.1016/j.dcn.2015.10.009. (n.d.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Amso D. Selective memories: Infants’ encoding is enhanced in selection via suppression. Developmental Science. 2013;16:926–940. doi: 10.1111/desc.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Amso D. Leveling the playing field: Attention mitigates the effects of intelligence on memory. Cognition. 2014;131:195–204. doi: 10.1016/j.cognition.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant J, Worden MS, Amso D. Not all attention orienting is created equal: Recognition memory is enhanced when attention involves distractor suppression. Neurobiology of Learning and Memory. 2015 doi: 10.1016/j.nlm.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakes LM, Ribar RJ. A comparison of infants’ categorization in paired and successive presentation familiarization tasks. Infancy. 2005;7:85–98. doi: 10.1207/s15327078in0701_7. [DOI] [PubMed] [Google Scholar]
- Posner MI. Orienting of attention. Quarterly Journal of Experimental Psychology. 1980;32:3–25. doi: 10.1080/00335558008248231. [DOI] [PubMed] [Google Scholar]
- Posner MI. Perception and attention. Developmental Science. 2001;4:270–292. [Google Scholar]
- Posner MI, Cohen Y. Components of visual orienting. In: Bouma H, Bouwhuis D, editors. Attention and Performance. X. Hillsdale, NJ: Erlbaum Lawrence Associates; 1984. pp. 531–556. [Google Scholar]
- Posner MI, Rafal RD, Choate LS. Inhibition of return: Neural basis and function. Cognitive Neuropsychology. 1985;2:211–228. [Google Scholar]
- Quinn PC, Doran MM, Reiss JE, Hoffman JE. Time course of visual attention in infant categorization of cats versus dogs: Evidence for a head bias as revealed through eye tracking. Child Development. 2009;80:151–161. doi: 10.1111/j.1467-8624.2008.01251.x. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Eimas PD. Perceptual cues that permit categorical differentiation of animal species by infants. Journal of Experimental Child Psychology. 1996;211:189–211. doi: 10.1006/jecp.1996.0047. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Eimas PD, Rosenkrantz SL. Evidence for representations of perceptually similar natural categories by 3-month-old and 4-month-old infants. Perception. 1993;22:463–475. doi: 10.1068/p220463. [DOI] [PubMed] [Google Scholar]
- Quinn PC, Eimas PD, Tarr MJ. Perceptual categorization of cat and dog silhouettes by 3- to 4-month-old infants. Journal of Experimental Child Psychology. 2001;79:78–94. doi: 10.1006/jecp.2000.2609. [DOI] [PubMed] [Google Scholar]
- Richards JE. Localizing the development of covert attention in infants with scalp event-related potentials. Developmental Psychology. 2000;36:91–108. [PubMed] [Google Scholar]
- Ross-Sheehy S, Oakes LM, Luck SJ. Exogenous attention influences visual short-term memory in infants. Developmental Science. 2011;14:490–501. doi: 10.1111/j.1467-7687.2010.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer J, Quinn PC. Heads you win, tails you lose: Evidence for young infants categorizing mammals by head and facial attributes. Early Development and Parenting. 1997;6:113–126. [Google Scholar]
- Wu R, Gopnik A, Richardson DC, Kirkham NZ. Infants learn about objects from statistics and people. Developmental Psychology. 2011;47:1220–1229. doi: 10.1037/a0024023. [DOI] [PubMed] [Google Scholar]
- Wu R, Kirkham NZ. No two cues are alike: Depth of learning during infancy is dependent on what orients attention. Journal of Experimental Child Psychology. 2010;107:118–136. doi: 10.1016/j.jecp.2010.04.014. [DOI] [PubMed] [Google Scholar]