Abstract
Objective:
To determine the effects of application of anti-adhesive films (OrthoWrap™) in traumatic decompressive craniectomy on prevention of adhesion formation and facilitation of subsequent cranioplasty.
Methods:
This was a retrospective cohort study being performed in ShahidRajaei hospital (Shiraz Level I trauma center) during a 12-month period (from March 2012 to April 2013) including 93 patients undergoing traumatic decompressivecraniectomy.Patients who received OrthoWrap™ during the initial craniectomy (n=44) were compared to those who did not (n=49). Two study groups were matched regarding the baseline characteristics. The perioperative indices including the surgical time, amount of bleeding, transfusion and 6-month Glasgow Outcome Scale (GOS) were compared between two study groups.
Results:
There was no significant difference between two study groups regarding the baseline characteristics. We found that the cranioplasty duration (113.3±33.2 vs. 146.9±34.9 minutes; p<0.001) and amount of intraoperative bleeding (182.1±98.3 vs. 270.6±77.6 mL; p=0.043) was significantly lower in those who had OrthoWrap™ compared to control group. The final GCS (p=0.052) as well as GOSE (p=0.653) was comparable between groups. The infection rate was comparable between two study groups (p=0.263).
Conclusion:
Application of OrthoWrap™ during decompressive craniectomy in those with severe traumatic brain injury is associated with shorter duration of operation and less intraoperative bleeding in subsequent cranioplasty. Infection rate and neurologic outcome was comparable between study groups.
Key Words: Decompressive craniectomy, Anti-adhesive Films (OrthoWrap™), Craniplasty
Introduction
Decompressive craniectomy (DC) has been established as an effective treatment modality in the management intracranial hypertension secondary to traumatic brain injury (TBI), stroke, subarachnoid hemorrhage (SAH) and intraparanchymal hemorrhage (IPH) [1, 2] . Most technical notes on decompressive craniectomy have focused on the effects of the operation on decreasing the intracranial pressure (ICP) [3, 4] . Data regarding the techniques for reducing the complication and morbidities of DC is scarce. This should be kept in mind that DC is the first stage of a 2-step procedure; the bone flap replacement is performed for survivors about 6-8 weeks after the first operation[5]. Thus the complication of the DC should be extended to the second operation which h would be the cranioplasty[6-8] . The complications of cranioplasty include cerebral injury, postoperative infections, subdural and epidural hematoma formations, IPH and CSF leakage [7, 9] . Most of these complications are result of adhesion formation between the dura, dural patch and the subcuraneous tissue which harden the soft tissue dissection that is required for exposing the cranial edges. The outcomes are worse when we have scarring between the brain surface and overlyinggaleaaponeurotica and temporalis muscle[10-12] . Releasing these adhesion increases the duration of the operation as well as the amount of intraoperative bleeding and the complication. The adhesion rate increases as the interval between the two operations increases[6-8] . During the past decade, several studies have investigated the use of materials for adhesion prevention and have reported promising results in terms of reducing the duration of the operation and in the amount of blood loss [10, 12-18] .OrthoWrap™ (bio-absorbable sheet; MAST Biosurgery, USA) is a sheet made of an amorphous bioabsorbable copolymer 70:30 poly (L-lactide-co-D, L-lactide) commonly referred to as polylactic acid (PLA) (Figure 1). PLA has no side effects and is associated with minimal risk of inflammatory reaction. It is known that the non-porous hydrophobic nature of the material resists attachments[19]. OrthoWrap™ is utilized in orthopedic surgery to protect or repair tendons and minimize soft tissue attachments[20]. In the current study we tried to investigate the effects of application of anti-adhesive films (OrthoWrap™) in traumatic DC on prevention of adhesion formation and facilitation of subsequent cranioplasty.
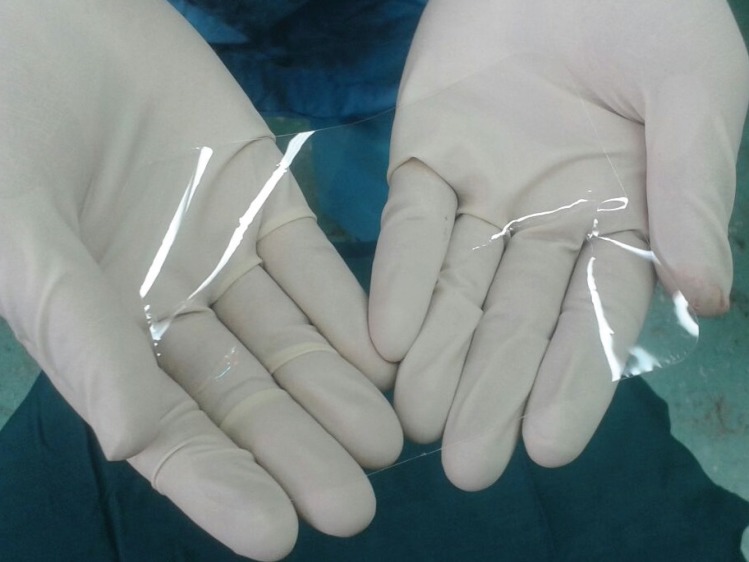
Fig. 1.
One sheet of bioresorbable adhesion barrier film (OrthoWrapTM) measuring 130mm Í 200mm Í 0.05mm
Materials and Methods
Study population
This was a retrospective cohort study being performed during a during a 12-month period from March 2012 to April 2013 in ShahidRajaei hospital, Level I trauma center affiliated with Shiraz University of Medical Sciences. We included those patients who underwent DC and subsequent cranioplastyduring the study period due to traumatic brain injury. DC was performed in cases of acute intractable brain swelling due to mass effect, in patients with a large intracranial hematoma and in those with intractable elevated ICP (>20 mmHg, despite medical therapy) during the course of intensive care treatment[1]. The decision to performed unilateral or bilateral hemicraniectomy was made by the attending physician based on the pattern of intracranial hemorrhage, compression of basal cisterns, and the presence of midline shift.Patients were categorized as OrthoWrap™ group and control group based on the use of material during the primary DC. The choice to use OrthoWrap™ was based on the surgeon’s and the patient’s guardians choice. In order to minimize the selection bias we included those patients in two study groups who had similar demographic and baseline clinical characteristics. In other words patients were matched regarding baseline characteristics before inclusion in the study. The study protocol was approved by institutional review board (IRB) and medical ethics committee of Shiraz University of Medical Sciences. As the study was retrospective, no informed written consents were required to be filled by the patients or their guardians.
Decompressive Craniectomy technique
All the patients underwent a frontotemporoparietalhemicraniectomyusing a standard, large, question mark–shaped musculocutaneous flap based on the root of the zygoma. Subtemporal decompression for maximizing temporal lobe space and middle fossa decompression was also performed in all patients by resecting the lateral sphenoid wing and the squamous portion of the temporal bone [21]. The skin, galea, fascia, and temporal muscle were elevated as a single flap. The bone flap was excised and removed using 3 bur holes, one at the base of the temporal bone; one behind the lambdoid suture; and the last 1.0–1.5 cm lateral to the bregma. Durawas opened by a C-shape incision with the base toward the superior sagittal sinus. Duraplastywas also performed with pericranial fascia in a watertight fashion. In first group, a sheet of OrthoWrap™ was placed between dura and the temporalis muscle to prevent adhesion formation in patients of the case group. Then, the subcutaneous tissue and skin were sutured in separated layers (Figure 2). In second group temporalis muscle was continuously repaired laying on the dural flap and the subcutaneous and sin were continuously repaired. The bone flaps were stored in standard refrigerator to be used for subsequent cranioplasty.
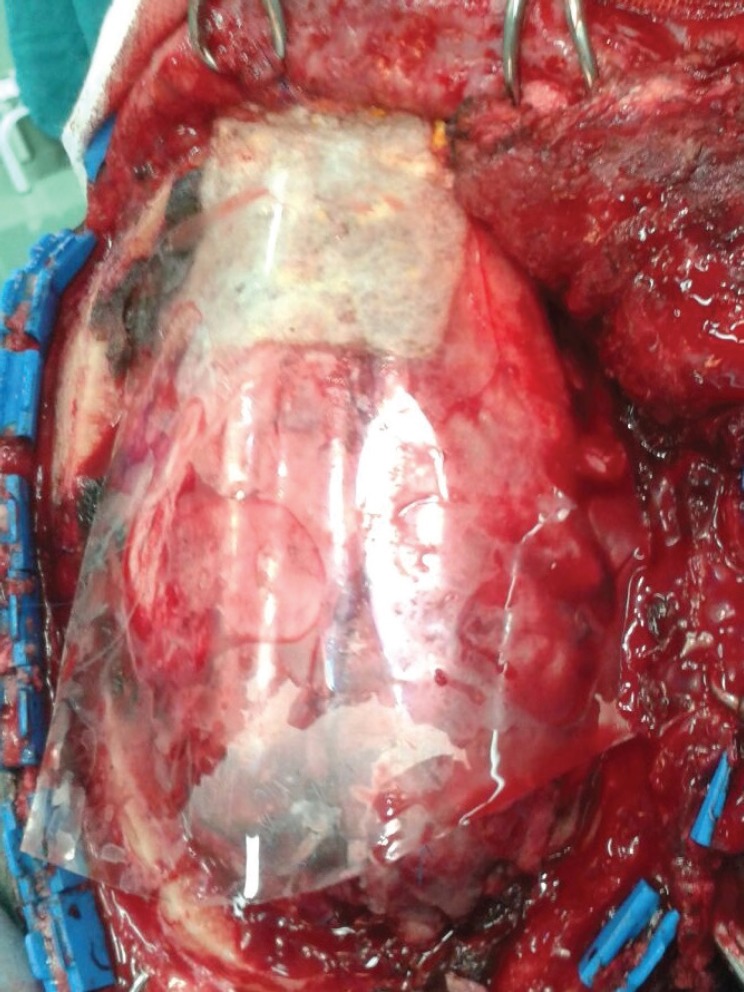
Fig. 2.
Intraoperative application of bioresorbable adhesion barrier film (OrthoWrapTM) in a patietns with subdural hematoma undergoing decompressive craniectomy. Note the placement of the film between the dura and the temporalis muscle
Cranioplasty technique
Cranioplasty was done for all the patients in a timely fashion with the mean interval of 52.9 ± 29.9 (ranging from 12 to 202)days following the craniectomy.During the cranioplasty, the layer for the replacement ofthe bone fragment was dissected between the myocutaneous flap and the OrthoWrap™ layer in case group and dura-like layer (neo-dura) covering thebrain in control group. The bone margins encasing the craniectomydefect were exposed, which was facilitated bythe cross-midline skin incision. The temporalis muscle was dissected as a separate layer and subsequently fixed at the bone flap after the bone flap was fixed with titanium plates and screws. All the surgical procedures were performed by residents in training, supervised by an attending neurosurgeon.
Outcome measures
The medical charts of the patients were reviewed and the demographic and baseline characteristics were recorded in the data gathering form. The operation and anesthesiology notes were used to extract the data regarding the intraoperative bleeding, operation duration and hemodynamics. Patients were followed 1, 3 and 6 months after the operation and were checked for Glasgow Coma Scale (GCS), Glasgow Outcome Scale - Extended (GOSE) and the infection of the flap. Preoperative and postoperative hemoglobin levels as well as number of transfused packed cells were also recorded. The outcome measured were compared between two study groups.
Statistical analysis
All the statistical analyses were performed using statistical package for social sciences (SPSS Inc., Chicago, USA) version 16.0. Data are presented as mean ± SD and proportions as appropriate. Non-parametric data were compared between two study groups using chi-square test while parametric data were compared using independent t-test. A two-sided p-value of less than 0.05 was considered statistically significant.
Results
Overall we included 93 patients who underwent cranioplasty using OrthoWrap™ (n=44) and conventional method (n=49). The mean age of the patients was 35.1±14.3 (ranging from 16 to 70) years including 80 (86.0%) men and 13 (14.0%) women.The initial diagnosis was acute subdural hematoma in 60 (64.5%) patients and intraparanchymal hematoma in 5 (5.4%) patients. Decompression was performed in 28 (30.1%) patients following intracranial pressure monitoring and intractable intracranial hypertension. The procedures and techniques that the surgeons used did not differ between the groups. The baseline characteristics were comparable between two study groups. The baseline characteristics of patients in two study groups is summarized in Table 1.There was no significant difference between two study groups regarding the baseline characteristics.
Table 1.
Baseline characteristics of 93 patients undergoing decompressive craniectomy using OrthoWrap™or conventional method
| OrthoWrap™group (n=44) | Control group (n=49) | p-value | |
|---|---|---|---|
| Age (years) | 34.5 ± 14.3 | 35.4 ± 14.5 | 0.785 |
| Sex | |||
| Men (%) | 41 (83.7%) | 39 (88.6%) | 0.560 |
| Women (%) | 8 (16.3%) | 5 (11.4%) | |
| Interval (days) | 53.6 ± 28.1 | 52.4 ± 31.7 | 0.850 |
| GCS a | 8.00 ± 3.29 | 9.33 ± 3.64 | 0.070 |
GCS: Glasgow Coma Scale
We found that the cranioplasty duration was significantly lower in those who hadOrthoWrap™compared to control group (113.3±33.2 vs. 146.9±34.9 minutes; p<0.001). We also found that the amount of intraoperative bleeding was significantly lower in OrthoWrap™ group compared to control group (182.1±98.3 vs. 270.6±77.6 mL; p=0.043). The final GCS (p=0.052) as well as GOSE (p=0.653) was comparable between groups. We also found that the infection rate was comparable between two study groups (p=0.263). The study results are summarized in Table 2.
Table 2.
Cranioplasty results after using OrthoWrap™or conventional decompressive craniectomy.
| OrthoWrap™group (n=44) | Control group (n=49) | p -value | |
|---|---|---|---|
| Duration (minutes) | 113.3 ± 33.2 | 146.9 ± 34.9 | <0.001 |
| Bleeding (mL) | 182.1 ± 98.3 | 270.6 ± 77.6 | 0.043 |
| Hb a drop (mg/dL) | 1.73 ± 1.06 | 2.03 ± 1.4 | 0.272 |
| Transfusion (%) | 12 (27.2%) | 16 (32.6%) | 0.232 |
| Final GCS b | 10.9 ± 3.3 | 13.1 ± 3.4 | 0.052 |
| GOSE | 4.57 ± 2.44 | 4.82 ± 2.82 | 0.653 |
| Dead | 12 (24.5%) | 8 (18.2%) | |
| Vegetative state | 4 (8.2%) | 3 (6.8%) | |
| Lower Severe Disability | 3 (6.1%) | 6 (13.6%) | 0.304 |
| Upper Severe Disability | 3 (6.1%) | 5 (11.4%) | |
| Lower Moderate Disability | 0 (0.0%) | 1 (2.3%) | |
| Upper Moderate Disability | 2 (4.1%) | 6 (13.6%) | |
| Low Good Recovery | 17 (34.7%) | 12 (27.3%) | |
| Upper Good Recovery | 8 (16.3%) | 3 (6.8%) | |
| Infection rate (%) | 2 (4.54%) | 3 (6.12%) | 0.263 |
Hb: Hemoglobin;
GCS: Glasgow Coma Scale
Discussion
Cranioplasty after DC for patients with TBI is commonly associated with complications such as dural or brain injury due to peridural fibrosis and more soft tissue remnants under the bone flap[6, 12] . Moreover, more adhesion formation causes more difficult and time consuming dissection of the dura, thereby causing more amount of blood loss, longer duration of the operation and surgeon's frustration[17] . Using materials which prevent dural adhesion is a method that prevents formation of multiple adhesions between dura, temporalis muscle and galea. In this study we determined the effects of OrthoWrap™ application during DC on subsequent cranioplasty. We showed that application of OrthoWrap™ during DC is associated with shorter duration and less bleeding during the subsequent cranioplasty. The infection rate as well as the outcome was comparable between two study groups.
Various methods and anti-adhesive materials have been used over time to reduce the amount of DC adhesions after the operation. Polytetrafluoroethylenedural substitute [11], silicone elastomer sheet[10, 22] , Seprafilm[14, 17] , and expanded polytetrafluoroethylene membrane [15] were all used as barriers for prevention of adhesions which have shown promising results in terms of decreasing total cranioplasty time and amount of blood loss. However, Huang et al.,[13] used of Neuropatch®, a non-absorbable synthetic dural substitute, in patients with TBI and reported that it was not associated with any significant difference in the amount of blood loss and the duration of the operation . In our study, DC with the use of OrthoWrap™bioabsorbable sheet was associated with decreased length of operation (reduced by 23%) and decreased amount of intraoperative bleeding.
The discordance between the results of the aforementioned studies can be attributed to several factors. First, there is heterogeneity among the pathologies that have eventually led to DC. Vakiset al.,[12] reported that the patients were mostly suffered from traumatic brain contusion and subdural hematoma[23], while in a study conducted by Kawaguchi et al.,[15] the patients were exclusively diagnosed with subarachnoid hemorrhage. Second, the time interval between craniectomy and cranioplasty was different among these studies. Vakiset al.,[12] reported that the mean interval was 4.8 months in their study, but in our study cranioplasties were performed within less than 4 months (mean: 53 days) following the craniectomy. Last but not least, our study is limited due to the small number of patients. Although our sample size was small, a statistically non-significant decrease in the duration of the operation and amount of blood loss was detected that we think that it may become significant in larger series.
In conclusion, application of OrthoWrap™ during decompressive craniectomy in those with severe traumatic brain injury is associated withshorter duration of operation and less intraoperative bleeding in subsequent cranioplasty. Infection rate and neurologic outcome was comparable between study groups. Further research is required for confirming the results of the current research.
Acknowledgment
We would like to acknowledge all the patients and their families who patiently participated in the study. The authors would like to thank Ms. Hosseini and Ms. Gholami of the Shiraz Neurosciences Research Center for their assistant.
Conflict of Interest: There isn’t any conflict of interest to be declared regarding the manuscript.
References
- 1.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D'Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493–502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 2.Alvis-Miranda H, Castellar-Leones SM, Moscote-Salazar LR. Decompressive Craniectomy and Traumatic Brain Injury: A Review. Bull Emerg Trauma. 2013;1(2):60–68. [PMC free article] [PubMed] [Google Scholar]
- 3.Ragel BT, Klimo P Jr, Martin JE, Teff RJ, Bakken HE, Armonda RA. Wartime decompressive craniectomy: technique and lessons learned. Neurosurg Focus. 2010;28(5):E2. doi: 10.3171/2010.3.FOCUS1028. [DOI] [PubMed] [Google Scholar]
- 4.Skoglund TS, Eriksson-Ritzen C, Jensen C, Rydenhag B. Aspects on decompressive craniectomy in patients with traumatic head injuries. J Neurotrauma. 2006;23(10):1502–9. doi: 10.1089/neu.2006.23.1502. [DOI] [PubMed] [Google Scholar]
- 5.Piedra MP, Nemecek AN, Ragel BT. Timing of cranioplasty after decompressive craniectomy for trauma. Surg Neurol Int. 2014;5:25. doi: 10.4103/2152-7806.127762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bobinski L, Koskinen LO, Lindvall P. Complications following cranioplasty using autologous bone or polymethylmethacrylate--retrospective experience from a single center. Clin Neurol Neurosurg. 2013;115(9):1788–91. doi: 10.1016/j.clineuro.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 7.Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010;112(5):1120–4. doi: 10.3171/2009.6.JNS09133. [DOI] [PubMed] [Google Scholar]
- 8.Guresir E, Vatter H, Schuss P, Oszvald A, Raabe A, Seifert V, et al. Rapid closure technique in decompressive craniectomy. J Neurosurg. 2011;114(4):954–60. doi: 10.3171/2009.12.JNS091065. [DOI] [PubMed] [Google Scholar]
- 9.Wachter D, Reineke K, Behm T, Rohde V. Cranioplasty after decompressive hemicraniectomy: underestimated surgery-associated complications? Clin Neurol Neurosurg. 2013;115(8):1293–7. doi: 10.1016/j.clineuro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Bulters D, Belli A. Placement of silicone sheeting at decompressive craniectomy to prevent adhesions at cranioplasty. Br J Neurosurg. 2010;24(1):75–6. doi: 10.3109/02688690903506135. [DOI] [PubMed] [Google Scholar]
- 11.Missori P, Polli FM, Peschillo S, D'Avella E, Paolini S, Miscusi M. Double dural patch in decompressive craniectomy to preserve the temporal muscle: technical note. Surg Neurol. 2008;70(4):437–9. doi: 10.1016/j.surneu.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 12.Vakis A, Koutentakis D, Karabetsos D, Kalostos G. Use of polytetrafluoroethylene dural substitute as adhesion preventive material during craniectomies. Clin Neurol Neurosurg. 2006;108(8):798–802. doi: 10.1016/j.clineuro.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Huang YH, Lee TC, Chen WF, Wang YM. Safety of the nonabsorbable dural substitute in decompressive craniectomy for severe traumatic brain injury. J Trauma. 2011;71(3):533–7. doi: 10.1097/TA.0b013e318203208a. [DOI] [PubMed] [Google Scholar]
- 14.Ichinose T, Uda T, Kusakabe T, Murata K, Sakaguchi M. Effectiveness of antiadhesion barriers in preventing adhesion for external decompression and subsequent cranioplasty. No Shinkei Geka. 2007;35(2):151–4. [PubMed] [Google Scholar]
- 15.Kawaguchi T, Hosoda K, Shibata Y, Koyama J. Expanded polytetrafluoroethylene membrane for prevention of adhesions in patients undergoing external decompression and subsequent cranioplasty. Neurol Med Chir (Tokyo) 2003;43(6):320–3. doi: 10.2176/nmc.43.320. [DOI] [PubMed] [Google Scholar]
- 16.Meier U, Zeilinger FS, Henzka O. The use of decompressive craniectomy for the management of severe head injuries. Acta Neurochir Suppl. 2000;76:475–8. doi: 10.1007/978-3-7091-6346-7_99. [DOI] [PubMed] [Google Scholar]
- 17.Mumert ML, Altay T, Couldwell WT. Technique for decompressive craniectomy using Seprafilm as a dural substitute and anti-adhesion barrier. J Clin Neurosci. 2012;19(3):455–7. doi: 10.1016/j.jocn.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Oladunjoye AO, Schrot RJ, Zwienenberg-Lee M, Muizelaar JP, Shahlaie K. Decompressive craniectomy using gelatin film and future bone flap replacement. J Neurosurg. 2013;118(4):776–82. doi: 10.3171/2013.1.JNS121475. [DOI] [PubMed] [Google Scholar]
- 19.Harris J, Ace C, Serafica G. Minimal tissue attachment implantable materials. Google Patents. 2012 [Google Scholar]
- 20.Ace C, Harris J, Serafica G. Minimal tissue attachment implantable materials. Google Patents. 2009 [Google Scholar]
- 21.Quinn TM, Taylor JJ, Magarik JA, Vought E, Kindy MS, Ellegala DB. Decompressive craniectomy: technical note. Acta Neurol Scand. 2011;123(4):239–44. doi: 10.1111/j.1600-0404.2010.01397.x. [DOI] [PubMed] [Google Scholar]
- 22.Lee CH, Cho DS, Jin SC, Kim SH, Park DB. Usefulness of silicone elastomer sheet as another option of adhesion preventive material during craniectomies. Clin Neurol Neurosurg. 2007;109(8):667–71. doi: 10.1016/j.clineuro.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Vakis A, Koutentakis D, Karabetsos D, Kalostos G. Use of polytetrafluoroethylene dural substitute as adhesion preventive material during craniectomies. Clinical neurology and neurosurgery. 2006;108(8):798–802. doi: 10.1016/j.clineuro.2005.11.026. [DOI] [PubMed] [Google Scholar]