Abstract
Chemotherapy-induced peripheral neuropathy (CIPN) is a major dose limiting side effect that can lead to long-term morbidity. Approximately one-third of patients receiving chemotherapy with taxanes, vinca alkaloids, platinum compounds or proteasome inhibitors develop this toxic side effect. It is not possible to predict who will get CIPN, however, genetic susceptibility may play a role. We explored this hypothesis using an established in vitro dorsal root ganglia neurite outgrowth (DRG-NOG) assay to assess possible genetic influences for cisplatin- and bortezomib-induced neurotoxicity. Almost all previous in vitro studies have used rats or mice. We compared DRG-NOG between four genetically defined, inbred mouse strains (C57BL/6J, DBA/2J, BALB/cJ, and C3H/HeJ) and one rat strain (Sprague Dawley). Our studies found differences in cisplatin and bortezomib-induced neurotoxicity between mouse and rat strains and between the different mouse strains. C57BL/6J and Balb/cJ DRG-NOG was more sensitive to cisplatin than DBA/2J and C3H/HeJ DRG-NOG, and all mouse strains were more sensitive to cisplatin than rat. Bortezomib induced a biphasic dose response in DBA/2J and C3H/H3J mice. C57BL/6J DRG-NOG was most sensitive and Balb/cJ DRG-NOG was least sensitive to bortezomib. Our animal data supports the hypothesis that genetic background may play a role in CIPN and care must be taken when rodent models are used to better understand the contribution of genetics in patient susceptibility to CIPN.
Keywords: Cisplatin, bortezomib, mouse strain, neurite outgrowth, neurotoxicity, genetic variations
1. Introduction
The genealogy of the laboratory mouse has been well documented with most strains of inbred mice originating from the colony of Abbie Lathrop [1]. These mice were distributed and independent inbred colonies were generated around the world. A mouse strain is considered inbred when it has been bred brother to sister for 20 generation and can be traced back to one breeder pair at the 20th or subsequent generation (Goios et al., 2007). Outbred mice, on the other hand are a closed population of mice maintained for high heterozygosity for at least four generations [2]. Despite a common inbred mouse ancestry, significant genome sequences and their genetic variations have been found and cataloged in 17 different mouse strains from various inbred colonies. Next-gen sequencing found a total of 56.7M single nucleotide polymorphisms (SNPs), approximately 9.45M small insertions and deletions and 711,920 structural variations within the different mouse strains [3]. MtDNA sequencing confirmed a high sequence similarity consistent with a common female ancestor, with 15 base substitutions between 11 mouse strains sequenced [4]. Genetic variability between mouse strains in combination with genetic homogeneity within mouse strains makes inbred mice good models to study genetic influences on drug response.
Genetic variations between mouse strains can influence response to drug treatment and is associated with different behavioral phenotypes. In streptocozin (STZ) treated mice, genetic strain variations showed a significant difference in renal injury. DBA/2J and KK/H1J mice showed significantly greater renal injury then C57BL/6J, AJ and MRL/MpJ mice [5]. In models of chemotherapy-induced neurotoxicity, differences in behavior responses were observed between paclitaxel treated mouse strains. Ten different mouse strains were exposed to paclitaxel for 7 days and tested for mechanical allodynia using the Von Frey assay. DBA/2J mice with high response and C57BL/6 mice with low response to paclitaxel treatment were further studied for thermal hyperalgesia and cold allodynia. DBA/2J had a significantly longer response time than C57BL/6J when tested for response to heat, however, there was no difference in response to cold [6].
Chemotherapy induced peripheral neuropathy (CIPN) is a serious side effect of cancer treatment for many patients and can affect long-term quality of life. Cisplatin and bortezomib are effective agents for the treatment of germ line cancers and multiple myeloma that induce peripheral neuropathy in 20–40% of patients. Peripheral neuropathy is linked to the cumulative dose of cisplatin administered to patients and for bortezomib development of neuropathy appears to be dose-related [7]. It has been reported that patients with mild or subclinical inherited neuropathies have exaggerated neurotoxic responses to chemotherapy drugs [8]. However, it is not well understood why some patients get neuropathy while others do not.
In experimental models, the mechanisms of neurotoxicity appear to be different between cisplatin and bortezomib. Cisplatin kills dorsal root ganglion (DRG) neurons, in vitro, by binding both nuclear and mitochondrial DNA, inducing DNA damage and apoptosis [9–15]. Bortezomib inhibits proteasome function, in cultured DRG neurons, inducing accumulation of polymerized tubulin and inhibition of mitochondrial axonal transport [15]. Both cisplatin and bortezomib have also been shown to have significant effects on the mitochondria. Cisplatin binds to mtDNA inhibiting mtDNA replication and transcription leading to mitochondrial vacuolization and degradation, in vitro and in vivo [14]. Bortezomib has been shown to induce deficits in mitochondrial complex I and II function and significantly decreased ATP production in vivo [16]. None of these studies have looked at genetic variation in relationship to developing CIPN.
Measurement of neurite outgrowth (NOG) from embryonic DRG neurons in vitro is an established model to study the neurotoxic effects of various agents including chemotherapy drugs and can be used for genetic screens. [11–14, 17–19]. It is a rapid and reproducible way to look at general neurotoxicity of compounds. Our studies were designed to look at the effects of cisplatin and bortezomib on NOG and determine whether genetic variations between mouse strains would alter the sensitivity of the DRG neurons.
2. Materials and Methods
2.1 Animals
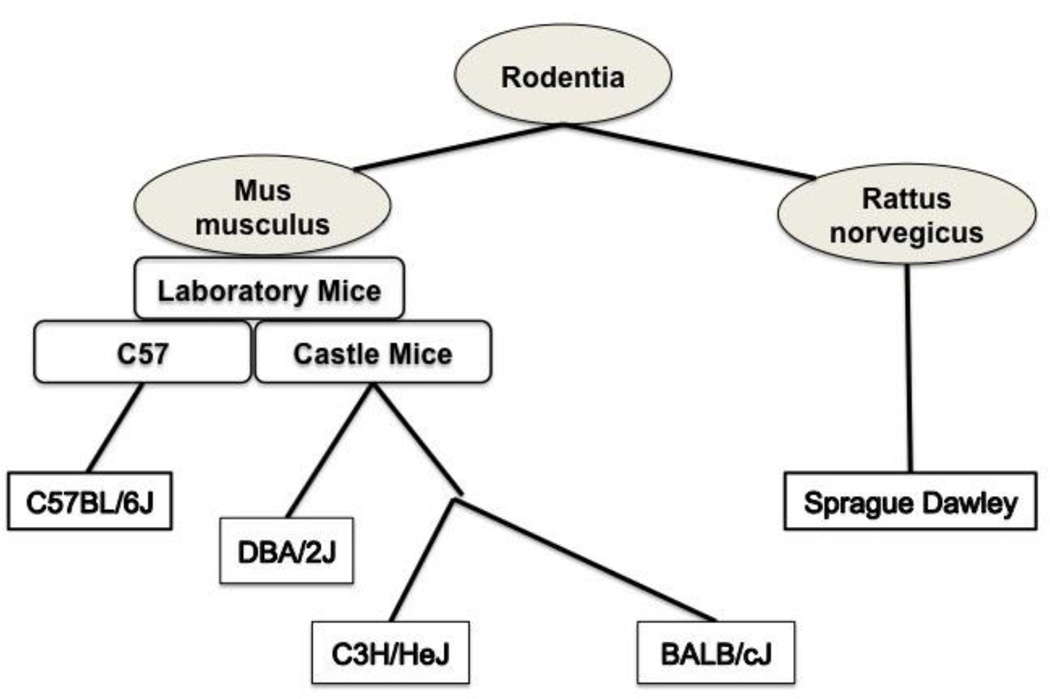
A total of 25 timed-pregnant mice from 4 strains, C57BL/6J, DBA/2J, BALB/cJ, and C3H/HeJ (Jackson Laboratory, Bar Harbor, ME) and 3 timed pregnant Sprague Dawley rats (Harlan, Indianapolis, IN) were used for the experiments. All animal studies were in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care International. Mouse strains were chosen from different hereditary lineages and NOG compared to each other as well as compared to the rat model we routinely use in our laboratory (Fig. 1).
Figure 1.
Diagram of the genetic relationship between different mouse strains and rat. Abby Lathrop from 1903–1915 bred mice from which the C57 related and Castle mouse lines were generated. DBA/2J, C3H/HeJ and Balb/cJ are different strains derived from the Castle mouse line and C57BL/6J are from the C57 related line [1]. Sprague Dawley Rats are a common outbred laboratory rat developed by the Sprague Dawley Animal Company (Madison, WI).
2.2 Surgical procedure
On embryonic day 13, timed pregnant female mice were anesthetized with sodium pentobarbital. E-13 mouse embryos were removed from the uterus and placenta then placed into L-15 medium (Life Technologies, Grand Island, NY). The pups were euthanized by decapitation and the spinal column was removed using a dissecting microscope (Carl Zeiss, Jena, Germany). Dorsal root ganglia (DRG) attached to the spinal cord cervical, thoracic, lumbar and sacral segments were removed and placed into a separate dish. 30–40 DRG were isolated from each pup. All surgical procedures were performed using aseptic precautions and sterile technique under a laminar flow clean hood. Embryonic day 15 rat pups were processed in the same manner.
2.3 Cell culture
Whole DRG explants were cultured on 35-mm collagen coated, plastic dishes with AN2 medium (MEM plus 10% calf bovine serum, 200 mM L-glutamine, 20% glucose) in the presence of 10 ng/ml NGF and incubated at 37°C. Initial plating was done in a small volume of medium for 1–2 hours to allow for attachment followed by additional medium up to 1 ml.
2.4 Neurite outgrowth assay
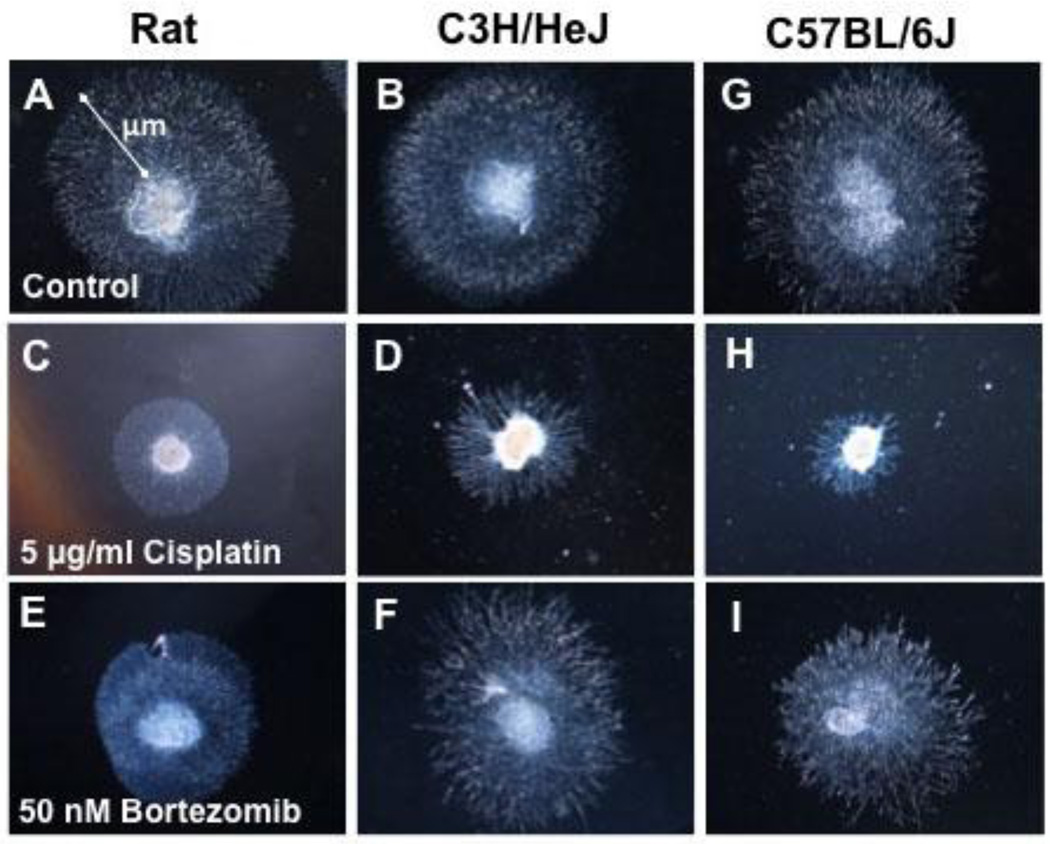
In each dish 3–4 DRG were plated into AN2 medium media with or without 1, 5, 10, and 50 µg/ml cisplatin or 25, 50, 100 and 200 nM bortezomib. Each rodent strain had 3–4 replicate dishes with a total of 17–35 DRG explants per condition. Cultures were incubated for 48 hours at 37°C. NOG was evaluated by acquiring images using a Nikon digital camera (Nikon, Melville, NY) and measuring the length of the longest neurite of each DRG using ImageJ software (NIH, Bethesda, MD, USA) at 48 hours [18, 19]. Shown are images of neurite outgrowth of rat, C3H/HeJ and C57BL/6J DRG neurons under control conditions, (Fig. 2A, B, G) treated with 5 µg/ml cisplatin (Fig. 2C, D, E) or 50 nM bortezomib (Fig. 2E, F).
Figure 2.
Neurite outgrowth of rat, mouse C3H/HeJ and mouse C57BL/6J DRG under control conditions (Fig. 2A–C), treated with 5 µg/ml cisplatin (Fig. 2 C–H) and 50 nM bortezomib (Fig. 2 E–I). NOG is measured from the outside edge of the DRG to the longest neurite length and expressed in µm (white arrow).
2.5 Data Analysis and Statistics
Data was analyzed and graphed using Prism Software (Graph Pad, La Jolla, CA). Statistical analysis was done using One Way ANOVA and Bonferroni’s multiple comparisons test. To calculate the half maximal inhibitory concentration (IC50), the dose response curve of cisplatin was transformed and expressed as a percent inhibition of neurite outgrowth and the log of the concentration. Quantitation of IC50 was done using non-linear regression-log agonist vs. normalized response of neurite outgrowth inhibition.
3. Results
3.1 Cisplatin on NOG
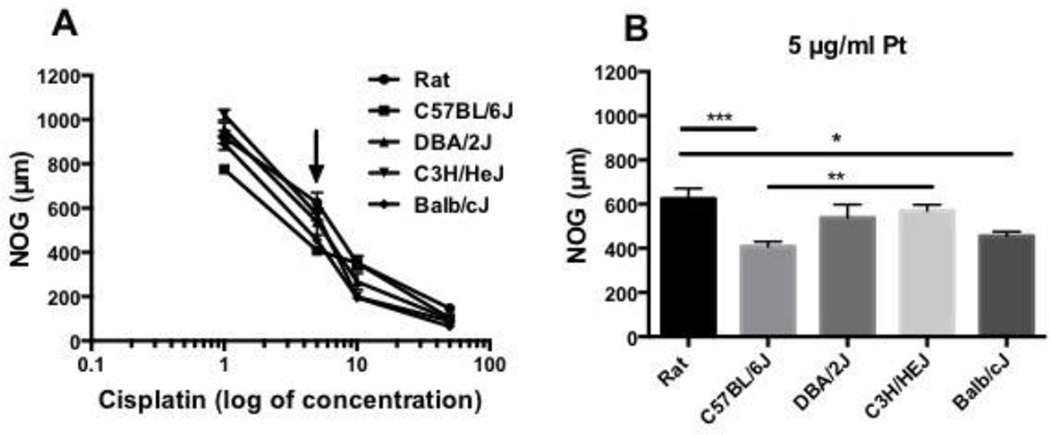
Cisplatin inhibited NOG in a dose dependent manner. DRG neurons were treated with 0, 1, 5, 10 and 50 µg/ml cisplatin for 48 hours and measured for the longest neurite length. Dose response curves for cisplatin treated DRG-NOG were plotted as a log of concentration (Fig 3A). Significant differences in NOG were observed at 1 (p<0.05-p<0.0001), 5 (black arrow, p<0.05-p<0.001) and 10 µg/ml (p<0.01-0.001) cisplatin. No significant difference was observed with 50 µg/ml cisplatin. At 5 µg/ml cisplatin, closer observation showed that rat DRG-NOG was significantly longer than C57BL/6J (p<0.001) and Balb/cJ (p<0.05) DRG-NOG (Fig.3B). DRG-NOG in rat was 625 µm (SEM, 47), C57BL/6J was 408.9 µm (SEM, 21) and Balb/cJ was 455.6 µm (SEM, 20). Significant differences were also found between C57BL/6J and C3H/HeJ DRG-NOG (p<0.01) with DRG-NOG in C3H/H3J, 571 µm (SEM, 27). There was no significant difference in DRG-NOG between mouse strains or rat under control non-drug treated conditions.
Figure 3.
(A) Cisplatin dose response curve plotted as a log of concentration. At 1, 5 (black arrow) and 10 µg/ml cisplatin, differences in DRG-NOG can be seen between strains. Comparison of rat and mouse DRG-NOG at (B) 5 µg/ml cisplatin showed significant differences between rat and C67BL6/J mouse DRG NOG (p<0.001), rat and Balb/cJ DRG-NOG (p<0.05) and C57BL6/J and C3HHE/J mouse DRG-NOG (p<0.01).
3.2 Bortezomb on NOG
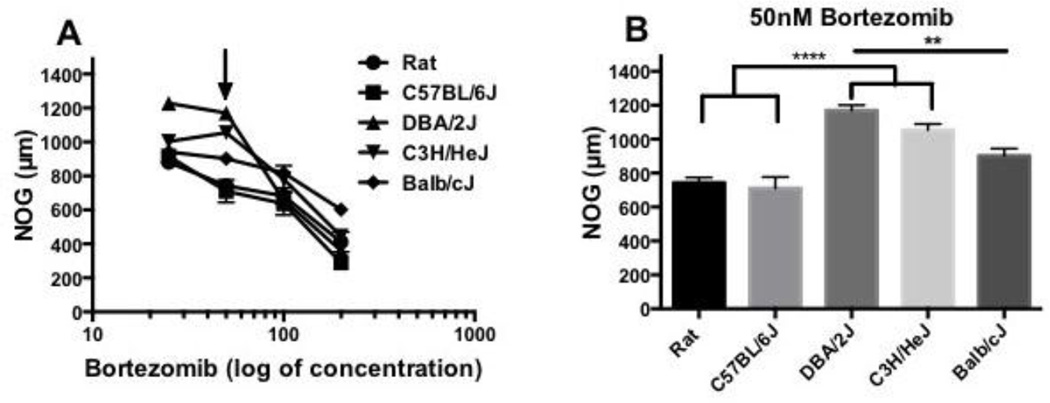
Bortezomib had different effects on DRG-NOG between rodent strains. Dose response curves of bortezomib treated DRG-NOG were plotted as log of concentration (Fig. 4A). DRG were treated with 25, 50 (black arrow), 100 and 200 nM bortezomib. Bortezomib induced inhibition of DRG-NOG in a dose dependent manner for all strains except DBA/2J and C3H/HeJ mouse DRG. In DBA/2J and C3H/H3J DRG, bortezomib had a biphasic effect with an increase in DRG-NOG at 25 and 50 nM followed by a decrease in DRG-NOG at higher concentrations. DBA/2J. DRG-NOG increased from 0 nM, 855.9 µm (SEM, 34.7) to 25 nM, 1228 µm (SEM, 33) bortezomib and decreased when the concentration of bortezomib was increased to 50 nM, 1170 µm (SEM, 31.1). DRG-NOG in C3H/H3J mouse, increased between 25 nM, 1003 µm (SEM, 27.8) and 50 nM, 1054 µm (SEM, 35) followed by decrease in DRG-NOG at 100 nM, 781.9 µm (SEM, 78.6). Closer observation of DRG-NOG at 50 nM bortezomib between rat and mouse DRG-NOG (Fig. 4B) showed DBA/2J and C3H/HeJ had significantly longer DRG-NOG (p<0.0001) than rat DRG-NOG, 744 µm (SEM, 30.9). There was no significant difference between rat and C57BL/6J, 710 µm (SEM, 67.4), or Balb/cJ, 902.2 µm (SEM, 42.6), DRG-NOG. DBA/2J and C3H/HeJ DRG also had longer NOG than C57BL/6J mouse (p<0.0001) and DBA/2J had longer DRG-NOG than Balb/cJ (p<0.01).
Figure 4.
(A) Bortezomib dose response curve plotted as log of concentration. Different rodent strains had different responses to bortezomib. DBA/2J and C3H/HeJ DRG-NOG had biphasic dose response curves while rat, C57BL/6J and Balb/cJ dose response consistently decreased. Comparison of rat and mouse DRG-NOG at (B) 50 nM bortezomib treatment showed DBA/2J and C3HHE/J mouse DRG are less sensitive to bortezomib than rat, C57BL6/J and Balb/cJ DRG-NOG. (p<0.01-p<0.0001).
3.3 IC50 of Cisplatin and Bortezomib
The IC50 indicates that Balb/cJ DRG-NOG is most sensitive to cisplatin and DBA2J DRG-NOG is the most resistant to cisplatin (Table 1). All mouse strains were more sensitive to cisplatin than rat DRG-NOG. With respects to Bortezomib C57BL/6J DRG-NOG is most sensitive and Balb/cJ DRG-NOG is most resistant to bortezomib.
Table 1.
The IC50 for rat and each mouse strain was calculated using the non-linear regression-log agonist vs. normalized response of neurite outgrowth inhibition.
| Species Strain | IC50 Cisplatin (µg/ml) | IC50 Bortezomib (nM) |
|---|---|---|
| Rat Sprague Dawley | 7.4 | 176.7 |
| Mouse C57BL/6J | 5.8 | 134.1 |
| Mouse DBA/2J | 6.5 | 177.0 |
| Mouse C3H/HeJ | 5.5 | 178.1 |
| Mouse Balb/cJ | 5.0 | 255.9 |
4. Discussion
Rodents are important experimental models for understanding the mechanism of CIPN. Rodents of different species or strains have been used extensively to study CIPN in vivo and in vitro, with little attention to strain differences. Transgenic mice are important tools for determining the role of a gene in CIPN mechanism [20]. Rats are good models for the study of nerve conduction velocities and behavior in relation to CIPN [21]. Both rat and mouse DRG can be used in vitro, however, we have found significant differences in how sensitive they are to cisplatin and bortezomib. We also found significant differences in response to drug between the different mouse strains. When designing experiments our study shows it is important to do independent drug dose response curves for each rodent strain and use matching genetic backgrounds. It is also important to determine if outbred or inbred mice should be used for a study. Outbred mice are better for studies that are designed to study the effect of a drug on a more heterogeneous genome. The effects of individual genetic variations are better studied in inbred mice where the background is controlled.
Our studies looked at four different mouse strains, one the C57 related line and three from the Castle Mice line. This allowed us to look at DRG-NOG response between less related and closer related mouse strains. Castle mice used in our experiments, DBA/2J, C3H/HeJ and Balb/cJ mice, were derived from C. C. Little’s laboratory. DBA/2J mice are a colony derived from Sub-line 212 and C3H/HeJ and Balb/cJ mice are different sub-stocks derived from Stock D. We made the assumption that the more closely related mice would be more similar in their genetic makeup and in their response to drugs. C3H/HeJ and DBA2J were not significantly different in their response to either cisplatin or bortezomib. However, there were significant differences in drug response between Balb/cJ and the other two Castle strains. Balb/cJ mice were bred through two more stock transitions (Stock A and then Stock B) and may be more genetically different from C3H/HeJ and DBA/2J mice. C57BL/6J mice derived from a different mouse colony had a significantly different response to cisplatin and bortezomib than C3H/HeJ and DBA/2J but not Balb/cJ.
Goios et al, showed a high mtDNA sequence similarity between 11 different inbred mouse strains [4]. However, small sequence differences in mtDNA between C57BL/6 and C3H/H3 mice contributed to acute cardiac volume-overload sensitivity [22]. Both cisplatin and bortezomib affect the mitochondria. Pt-mtDNA adducts prevents mtDNA replication and transcription leading to mitochondrial degradation and vacuolization [14]. Bortezomib interferes with mitochondrial axonal trafficking and induces vacuolization of mitochondria in a small number of rat DRG neurons, in vivo [23]. Cisplatin and botezomib both lead to mitochondrial degradation, however, cisplatin results in apoptosis in DRG neurons while bortezomib does not. It is possible the difference we see in sensitivity to cisplatin and bortezomib is associated with the level of mitochondrial stress induced by the drugs.
Genetic variability in patient backgrounds may be involved in determining susceptibility to CIPN. The most common inherited neuropathy that may be present in subclinical form is Charcot-Marie-Tooth (CMT) disease which is due to duplications, deletions and mutations in at least 80 disease-causing genes [24]. Next Gen sequencing of CMT genes in non-CMT patients observed for taxol-induced CIPN showed two genes shared between the two diseases [25]. Beutler et al, identified heterozygous single nucleotide variants (SNV) in PRX, involved in stabilization of myelin in the peripheral nervous system and ARHGEF10, a gene involved in cytoskeleton and microtubule dynamics. In a separate study Johnson and colleagues found over-representation of polymorphisms in the glutathione peroxidase 7 (GPX7) and ATP-binding cassette sub-family C member 4 (ABCC4) genes in patients developing CIPN when treated with the combination of cisplatin and paclitaxel [26].
5.0 Conclusions
We found measureable differences in DRG-NOG response to cisplatin and bortezomib. In cisplatin treated DRG the IC50 of mouse DRG-NOG was lower than the IC50 of rat DRG-NOG indicating that mice overall are more sensitive to cisplatin than rat. DRG treated with 50 µg/ml cisplatin had significantly shorter neurites in C57BL/6J and Balb/cJ mice than in rat and between mouse strains C57BL/6J mice had significantly shorter neurites then C3H/HeJ mice. The biphasic effects of bortezomib on DRG-NOG made it more difficult to interpret the differences in IC50, however, Balb/cJ mice had a very different curve from rat and the other mouse strains and a much higher IC50 indicating they may be more resistant to bortezomib. At 50 nM bortezomib, C3H/HeJ and DBA2J DRG had significantly longer neurites than rat, C57BL/6J and Balb/cJ DRG indicating they are more resistant to bortezomib at specific drug concentrations. Our data shows there are differences in drug response between mouse and rat and between the various mouse strains. Genetic background should be considered when setting up experimental models. It also provides additional evidence that genetic influences may be critical in determining whether individual cancer patients develop CIPN when treated with a drug.
Highlights.
Measureable differences in NOG were found between rat and mouse DRG as well as between the different mouse strains.
C57BL/6J mice DRG were most sensitive to cisplatin at concentrations below IC50.
Balb/cJ mice DRG were overall the most sensitive to cisplatin.
C57BL/6J mice DRG were most sensitive to bortezomib and Balb/cJ least sensitive to bortezomib.
The largest difference between mouse strains was observed at cisplatin and bortezomib concentrations below IC50.
Acknowledgments
This study was funded by NPS: NIH (K08 CA169443), Mayo Clinic Center for Regenerative Medicine and Richard M. Schulze Family Foundation. We thank Jane Meyer for her administrative role in manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, et al. Genealogies of mouse inbred strains. Nat Genet. 2000;24(1):23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 2.Chia R, Achilli F, Festing MF, Fisher EM. The origins and uses of mouse outbred stocks. Nat Genet. 2005;37(11):1181–1186. doi: 10.1038/ng1665. [DOI] [PubMed] [Google Scholar]
- 3.Yalcin B, Adams DJ, Flint J, Keane TM. Next-generation sequencing of experimental mouse strains. Mamm Genome. 2012;23(9–10):490–498. doi: 10.1007/s00335-012-9402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goios A, Pereira L, Bogue M, Macaulay V, Amorim A. mtDNA phylogeny and evolution of laboratory mouse strains. Genome Res. 2007;17(3):293–298. doi: 10.1101/gr.5941007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi Z, Fujita H, Jin J, Davis LS, Wang Y, Fogo AB, et al. Characterization of susceptibility of inbred mouse strains to diabetic nephropathy. Diabetes. 2005;54(9):2628–2637. doi: 10.2337/diabetes.54.9.2628. [DOI] [PubMed] [Google Scholar]
- 6.Smith SB, Crager SE, Mogil JS. Paclitaxel-induced neuropathic hypersensitivity in mice: responses in 10 inbred mouse strains. Life Sci. 2004;74(21):2593–2604. doi: 10.1016/j.lfs.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Grisold W, Cavaletti G, Windebank AJ. Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. 2012;14(Suppl 4):iv45–iv54. doi: 10.1093/neuonc/nos203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verstappen CC, Heimans JJ, Hoekman K, Postma TJ. Neurotoxic complications of chemotherapy in patients with cancer: clinical signs and optimal management. Drugs. 2003;63(15):1549–1563. doi: 10.2165/00003495-200363150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Gill JS, Windebank AJ. Cisplatin-induced apoptosis in rat dorsal root ganglion neurons is associated with attempted entry into the cell cycle. J Clin Invest. 1998;101:2842–2850. doi: 10.1172/JCI1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fischer SJ, Podratz JL, Windebank AJ. Nerve growth factor rescue of cisplatin neurotoxicity is mediated through the high affinity receptor: studies in PC12 cells and p75 null mouse dorsal root ganglia. Neurosci Lett. 2001;308:1–4. doi: 10.1016/s0304-3940(01)01956-5. [DOI] [PubMed] [Google Scholar]
- 11.Ta LE, Espeset L, Podratz J, Windebank AJ. Neurotoxicity of oxaliplatin and cisplatin for dorsal root ganglion neurons correlates with platinum-DNA binding. Neurotoxicology. 2006;27(6):992–1002. doi: 10.1016/j.neuro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 12.McDonald ES, Randon KR, Knight A, Windebank AJ. Cisplatin preferentially binds to DNA in dorsal root ganglion neurons in vitro and in vivo: a potential mechanism for neurotoxicity. Neurobiol Dis. 2005;18(2):305–313. doi: 10.1016/j.nbd.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 13.McDonald ES, Windebank AJ. Cisplatin-induced apoptosis of DRG neurons involves Bax redistribution and cytochrome c release but not fas receptor signaling. Neurobiol Dis. 2002;9:220–233. doi: 10.1006/nbdi.2001.0468. [DOI] [PubMed] [Google Scholar]
- 14.Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, et al. Cisplatin induced Mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41(3):661–668. doi: 10.1016/j.nbd.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staff NP, Podratz JL, Grassner L, Bader M, Paz J, Knight AM, et al. Bortezomib alters microtubule polymerization and axonal transport in rat dorsal root ganglion neurons. Neurotoxicology. 2013;39:124–131. doi: 10.1016/j.neuro.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H, Xiao WH, Bennett GJ. Mitotoxicity and bortezomib-induced chronic painful peripheral neuropathy. Exp Neurol. 2012;238(2):225–234. doi: 10.1016/j.expneurol.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Dadsetan M, Knight AM, Lu L, Windebank AJ, Yaszemski MJ. Stimulation of neurite outgrowth using positively charged hydrogels. Biomaterials. 2009;30(23–24):3874–3881. doi: 10.1016/j.biomaterials.2009.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conti AM, Brimijoin S, Miller LJ, Windebank AJ. Suppression of neurite outgrowth by high-dose nerve growth factor is independent of functional p75NTR receptors. Neurobiol Dis. 2004;15(1):106–114. doi: 10.1016/j.nbd.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Conti AM, Fischer SJ, Windebank AJ. Inhibition of axonal growth from sensory neurons by excess nerve growth factor. Ann Neurol. 1997;42:838–846. doi: 10.1002/ana.410420604. [DOI] [PubMed] [Google Scholar]
- 20.Deng L, Cornett BL, Mackie K, Hohmann AG. CB1 Knockout Mice Unveil Sustained CB2-Mediated Antiallodynic Effects of the Mixed CB1/CB2 Agonist CP55,940 in a Mouse Model of Paclitaxel-Induced Neuropathic Pain. Mol Pharmacol. 2015;88(1):64–74. doi: 10.1124/mol.115.098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meregalli C, Canta A, Carozzi VA, Chiorazzi A, Oggioni N, Gilardini A, et al. Bortezomib-induced painful neuropathy in rats: a behavioral, neurophysiological and pathological study in rats. Eur J Pain. 2010;14(4):343–350. doi: 10.1016/j.ejpain.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Fetterman JL, Zelickson BR, Johnson LW, Moellering DR, Westbrook DG, Pompilius M, et al. Mitochondrial genetic background modulates bioenergetics and susceptibility to acute cardiac volume overload. Biochem J. 2013;455(2):157–167. doi: 10.1042/BJ20130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavaletti G, Gilardini A, Canta A, Rigamonti L, Rodriguez-Menendez V, Ceresa C, et al. Bortezomib-induced peripheral neurotoxicity: a neurophysiological and pathological study in the rat. Exp Neurol. 2007;204(1):317–325. doi: 10.1016/j.expneurol.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Timmerman V, Strickland AV, Zuchner S. Genetics of Charcot-Marie-Tooth (CMT) Disease within the Frame of the Human Genome Project Success. Genes (Basel) 2014;5(1):13–32. doi: 10.3390/genes5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beutler AS, Kulkarni AA, Kanwar R, Klein CJ, Therneau TM, Qin R, et al. Sequencing of Charcot-Marie-Tooth disease genes in a toxic polyneuropathy. Ann Neurol. 2014 doi: 10.1002/ana.24265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson C, Pankratz VS, Velazquez AI, Aakre JA, Loprinzi CL, Staff NP, et al. Candidate pathway-based genetic association study of platinum and platinum-taxane related toxicity in a cohort of primary lung cancer patients. J Neurol Sci. 2015;349(1–2):124–128. doi: 10.1016/j.jns.2014.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]