Abstract
The global HIV/AIDS pandemic has claimed the lives of an estimated 35 million people. A significant barrier for combating this global pandemic is substance use since it is associated with HIV transmission, delayed diagnosis/initiation of therapy, and poor adherence to therapy. Clinical studies also suggest a link between substance use and HIV-disease progression/AIDS-associated mortality. Methamphetamine (METH) use is one of the fastest-growing substance use problems in the world. METH use enhances high-risk sexual behaviors, therefore increases the likelihood of HIV-1 acquisition. METH use is also associated with higher viral loads, immune dysfunction, and antiretroviral resistance. Moreover, METH use has also been correlated with rapid progression to AIDS. However, direct effects of METH on HIV-1 disease progression remains poorly understood because use of METH and other illicit drugs is often associated with reduced/non adherence to ART. Nevertheless, in vitro studies demonstrate that METH increases HIV-1 replication in cell cultures and animal models. Thus, it has been proposed that METH’s potentiating effects on HIV-1 replication may in part contribute to the worsening of HIV-1 pathogenesis. However, our recent data demonstrate that METH inhibits HIV-1 replication in CD4+ T cells and challenges this paradigm. Thus, the goal of this review is to systematically examine the published literature to better understand the complex interaction between METH abuse and HIV-1 disease progression.
Introduction
The HIV/AIDS pandemic has caused approximately 35 million deaths worldwide1. In addition, an estimated 34 million people are living with the virus worldwide [1]. In the US there are ~1.2 million people who are infected with HIV [2]. Even though HIV research has witnessed tremendous progress in last three decades a preventative vaccine has eluded the scientific community. Therefore, antiretroviral therapy (ART) continues to be the only treatment option available to HIV infected patients [3]. The effectiveness of ART has dramatically reduced HIV/AIDS related mortality [3]. Unfortunately, only ~25% of the HIV-1 infected patients in the US have their virus under control [2]. Thus majority of HIV-1 infected people in the US are either unaware of their infection status or not connected/retained to care or are non-adherent to ART [2]. The problem of this ongoing worldwide pandemic is further accentuated by substance use that serves as a powerful cofactor at every stage of HIV/AIDS disease including transmission, diagnosis, pathogenesis, and treatment [4,5]. Clinical studies suggest that substance use may increase viral load, accelerate disease progression and worsen AIDS-related mortality even among ART adherent patients [4,5]. However, establishing a direct link between substance use and HIV/AIDS in human patients remains highly challenging. This is in part because drug use is often associated with reduced/non adherence to ART that severely complicates a direct correlation between substance use and worsening of HIV-1 disease [6–8]. In addition, there are also confounding factors associated with studying drug abusing patients that can influence outcomes of HIV disease. These include history and route of drug use, amount and formulation of drug used, single or concurrent use of other drugs, and poor nutrition [6–8]. These multifaceted problems have posed serious obstacles to researchers for delineating the mechanisms by which substance abuse exacerbates HIV disease. Nevertheless, it is becoming clear that illicit drugs such as METH, cocaine, opiates, marijuana, alcohol and others regulate HIV-1 infection/replication in vitro and in animal models [9]. Moreover, published data is shedding light on the negative effects of substance use on immune system that controls viral infections [9]. These studies are paving the way for better understanding the comorbid condition of drug use and HIV/AIDS.
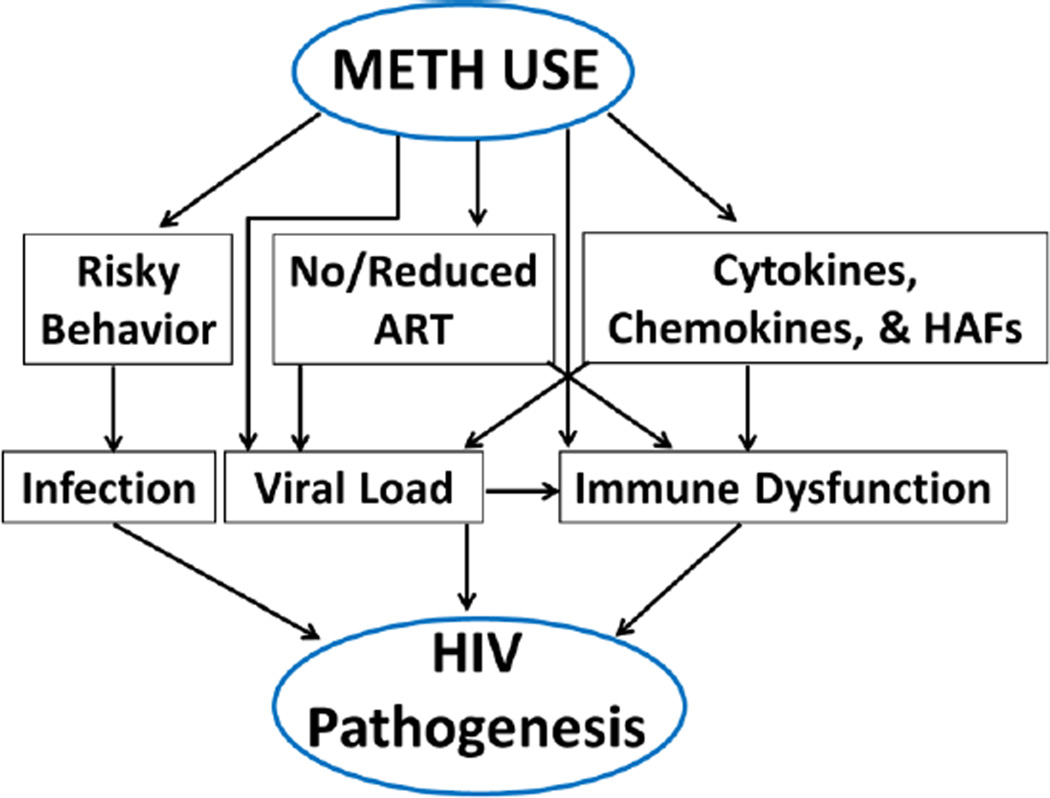
METH is one of the fastest-growing substance use problems in the world and continues to rise to epidemic conditions [10]. METH use has been linked to worsening of HIV-1 disease and AIDS-related outcomes [7, 30, 31, 32, 33]. Moreover, in vitro and animal studies demonstrate that METH enhances HIV-1 infection/replication [9]. We have recently reported that METH at physiological concentrations has limited impact on HIV-1 replication in CD4+ T cells [11] that serves as the primary targets of HIV-1 in vivo [12]. Therefore, in this review we will highlight the interplay between METH abuse and HIV/AIDS and discuss how this interaction may contribute to HIV disease (Fig. 1). Specifically, we will describe the prevalence and impact of METH abuse on; HIV-1 transmission, replication, viral load, alteration in immune function, adherence to ART, and AIDS related outcomes. We will also summarize the potential mechanisms by which METH regulates HIV-1 replication. Finally, we will discuss the unanswered questions on the synergy between METH and HIV/AIDS as part of the future prospective. The impact of METH abuse on neurological manifestations of HIV-1 infected and un-infected patients has been extensively reviewed in recent years [13–15], therefore will not be covered in this review.
Figure 1.
Accumulating evidence suggest that METH use can contribute to accelerated HIV-1 disease progression via multiple mechanisms. First, METH use induces release of neurotransmitters that promoter at-risk sexual behavior and predisposes METH users to acquire HIV-1 at higher rate than the general population. Second, HIV-1 positive METH users have been shown to have higher viral loads compared to non-users either due to no/reduced adherence to ART or due to direct effect of METH on viral load even among ART adherent patients. Finally, METH due to its immune-modulatory properties has been shown to regulate cytokines, chemokines and other HIV-1 associated cellular factors (HAFs). These molecules can increase viral transmission, replication, and modulate immune-dysfunction. Collectively, these complex viral, cellular, molecular and clinical alterations have been proposed to worsen disease progression among METH abusing HIV-1 infected individuals. Therefore, understanding the molecular synergy between METH and HIV-1 is critical to manage the health outcomes of HIV-1 infected patients.
Prevalence of METH use and increased risk of HIV transmission
METH is the second most popular illicit drug used in the world with an annual prevalence estimated at 35 million or 0.4% of the global population [10]. This unprecedented number of METH users exceeds that for cocaine and heroin [10]. Moreover, use of this drug is especially common amongst young individuals in US and Canada [16]. For example, in the US METH use has reached epidemic levels with an estimated 1.5 million regular users and ~11 million reported to use it at least once in their lifetime [17,18]. This high prevalence has been attributed to wide availability, low cost, long duration of psychoactive effects, and the ease of use of METH by smoking, snorting, chewing, swallowing or injecting [10,16–18]. Since METH use has serious social, economic, biological, and health effects, this ever increasing number of METH users underscore the serious drug abuse challenge in the US and the world.
METH is known to induce the release of neurotransmitters that produce sensations of pleasure and enhance at-risk sexual behavior [19]. Therefore, use of METH has been linked to increased likelihood of acquiring sexually transmitted infections including that of HIV [18–23]. For example, METH is commonly used among men who have sex with men (MSM) compared to the general population. In a study conducted by Thied et al. in 7 US urban areas, 20% of young MSM (aged 15–22 years) reported METH use during the past 6 months [24]. In addition, Mansergh et al reported an estimated 15% of MSM in San Francisco had used METH within the past 3 months [25]. Additionally, in a multicenter prospective cohort study of MSM, Plankey et al. found that METH users were more likely than non-users to acquire HIV [26]. Therefore, METH use has been associated with doubling the risk of HIV-1 acquisition among MSM [26–29]. Furthermore, METH is also used widely as a recreational drug among heterosexual men [22] and female sex workers [21]. The association between METH use and HIV-1 transmission is demonstrated in a reasonably wide demographic of people by Patterson et al [21]. These authors reported that smoking, snorting, or inhalation of METH was independently associated with HIV infection [21]. A 2002 case-control study by Wohl et al. enrolling African-American heterosexual men showed that METH use was positively associated with HIV infection [22]. These studies strongly suggest a link between METH use and HIV-1 infection. Even though this interaction is heightened in MSM it extends across multiple populations, including female sex workers, and heterosexual men. These studies also propose that the increased transmission is driven by METH use associated high-risk sexual practices, such as sex with anonymous and multiple partners, marathon sex, and unprotected sex. Most importantly, the high prevalence of METH use among HIV-1 infected and at-risk population underscore the critical need to examine the impact of METH use on every aspect of HIV-1 disease.
METH use is associated with worsening of HIV disease progression
Epidemiological studies suggest that substance use including that of METH is associated with accelerated disease progression [7, 30, 31, 32, 33, 36]. This could be attributed to several mechanisms including increased viral load, accelerated CD4+ T cell decline, immune modulation, and reduced/non adherence to ART (Fig. 1). Published studies described below and summarized in Table 1 provide evidence that METH use may affect many of these factors individually or in combination to worsen HIV-1 disease progression.
Table 1.
Relationship between METH use and HIV outcomes
| Indicator | Study, Year, Country |
Population | Sample Size |
Study Design | Study Purpose | Findings |
|---|---|---|---|---|---|---|
| ART adherence | Parsons et al. (2013) USA | HIV-positive gay and bisexual men ART regimen | 210 | RCT – baseline data | To examine aggregate, threshold, and day-level associations of METH use and non-adherence. | There was a significant association between days of meth use and 90% dose adherence, such that for every additional day of meth use, the odds of being less than 90% adherent increased by 20%. |
| Carrico et al. (2010) USA | MSM or transgendered people on ART | 122 | Cross-sectional | To examine affective correlates of stimulant use and ART adherence among HIV-positive methamphetamine users. | Elevated negative effect was independently associated with a greater likelihood of reporting METH use at least weekly. | |
| Hinkin et al. (2007) USA | HIV-positive individuals receiving highly active ART | 150 | Prospective cohort study | To examine the impact of drug use and abuse on medication adherence | Results of logistic regression revealed that drug use was associated with a 4.1 times greater risk of being a poor adherance. 70% of drug users in this study used METH | |
| Viral Load | Fairbairn et al. (2011) Canada | HIV-positive, injection drug users initiating ART | 384 | Prospective RCT | To demonstrate the association (if any) between crystal METH use and HIV RNA suppression. | Crystal methamphetamine injection was negatively associated with viral load suppression (RH, 0.63; 95% CI, 0.40–0.98). |
| King et al. (2009) USA | MSM and/or drug users | 835 | Cross-sectional | To examine the predisposing, enabling, and need factors associated with detectable viral load (VL). | Participants reporting drug use in the past 30 days, were significantly more likely to have detectable viral loads. The association of recent reported METH use with viral load is about 45% stronger than recent use of other drugs. | |
| Ellis et al. (2003) USA | HIV-positive METH users and nonusers | 230 | Cross-sectional – lab measures | To determine whether meth increases HIV replication in humans | METH-use was significantly related to plasma virus load among the subgroup receiving ART. | |
| Carrico et al. (2007) USA | HIV-positive individuals | 936 | Randomized behavioral prevention trial | To examine the association among affect regulation, substance use, non-adherence to ART, and immune status. | Among individuals currently on ART, regular stimulant users had a five-fold higher HIV viral load than those who denied regular stimulant use. | |
| CD4+ Count | Shoptaw et al. (2012) USA | Gay and bisexual men | 2789 | Prospective Cohort – Multicenter AIDS Cohort Study | To examine associations between stimulant use and other substances with immune function biomarkers among HIV+ | Significant negative associations were noted for HIV+ ART-using men between CD4+/CD8+ ratios and METH use. |
Given that METH use is prevalent among HIV-1 infected people; several studies have evaluated the effects of METH use on HIV-1 viral load in these patients. In a 2003 study, Ellis et al. reported that among a subgroup of patients receiving ART, METH use was significantly correlated to increased plasma virus load[30]. This was further supported by Carrico et al. describing that among ART adherent patients, regular stimulant users including that of METH had a five-fold higher viral load than the nonusers [31]. Similarly, Fairbairn et al. illustrated a negative association between METH use and viral load suppression [32]. Notably, a study by King et al. reported that regardless of ART adherence participants reporting drug use in the past 30 days were significantly more likely to have detectable viral loads[33]. The association between METH use and viral load in this study was about 45% stronger than use of other drugs [33]. This study highlights the unique nature of METH abuse compared to other illicit drugs such as cocaine/crack, heroine, and other drugs. Collectively, these studies indicate an association between METH use and elevated viral load that could contribute to accelerated disease progression.
Stimulant use including that of METH is associated with impaired adherence to ART among HIV-positive individuals. A 2013 study by Parsons et al., demonstrated that METH use resulted in a significant reduction in ART adherence[7]. Hinkin et al. reported similar results showing that drug users including that of METH were four times less likely to adhere to ART [34]. In another study, Carrico et al. also reported a negative association between METH use and ART adherence among HIV-positive METH users [35]. These studies demonstrate that, reduced adherence to ART among METH users may play a critical role in increasing viral load and worsening of HIV-1 disease.
Decline in CD4+ T cell counts and an increase in CD8+ T cell counts serve as an important diagnostic for HIV-1 disease progression. A study by Shoptaw et al. examined the association between stimulant use including that of METH with immune function biomarkers among both HIV-positive and HIV-negative men [36]. Data from this study noted significant negative associations between METH use and CD4+/CD8+ ratios for HIV-positive men [36]. Notably, these authors also described that METH use decreased CD4+/CD8+ cell count ratio for those HIV-infected men on an ART regimen [36]. This study indicated that METH use can contribute to HIV-1 disease progression even among ART adherent patients.
In addition to affecting viral load, ART adherence and CD4+ T cell numbers, METH use has been suggested to exacerbate HIV-1 disease by other mechanisms. For example, Cachay et al. reported that in newly diagnosed, ART naïve individuals, those who reported using METH within the 30 days were more likely to have transmission of drug resistant HIV-1 [37]. In another case study, Markowitz et al. reported an association between METH use with infection of a multidrug resistant, dual-tropic HIV-1 that resulted in rapid progression to AIDS[38]. Furthermore, Colfax et al. demonstrated that in a multivariate model controlling for multiple sex partners, race/ethnicity, and other illicit drug use, frequent METH use associated with HIV-1 drug resistance [39].
Collectively, these studies describe an association between METH use and increased viral load/decreased CD4+ T cell counts and other mechanisms that have the potential to worsen HIV-1 disease progression. However, a direct link between METH use and HIV disease in human patients remains contentious. This is because use of METH and other illicit drugs is often associated with reduced/non adherence to ART [6–8,40,41]. In addition, history and route of drug use, amount and formulation of drug used, single or concurrent use of other drugs, and poor nutrition confounds establishing a functional correlation between drug use and HIV-1 disease [6–8,40,41]. Therefore, further research in well-defined cohorts is warranted to examine the direct impact of METH use on HIV-1 disease progression.
METH treatment enhances viral load in animal models
To address some of the caveats associated with clinical studies described in the previous section, Marcondes et al. examined effects of chronic METH treatment on pathogenesis of Simian Immunodeficiency Virus (SIV) in a rhesus macaque model [42]. The infection of rhesus macaques with SIV results in disease conditions that mimic that of HIV-1 disease in humans including progressive loss or dysfunction of the immune system resulting in immune deficiency [42]. Therefore, this study investigated effects of METH on viral load as well as differences in immune cells in chronically infected rhesus macaques. These authors reported that METH administration did not alter levels of virus in the plasma. Intriguingly, data in this report demonstrated that viral load in the brain was significantly increased in METH-treated animals compared with control animals. Moreover, these authors also provided evidence that viral infection resulted in a reduction in CD4+ T cells in METH treated animals. Given that this study mimicked the amounts of drug used by chronic METH abusers, the authors emphasized that METH abuse may directly impact AIDS progression by reducing CD4+ T cells even though METH’s effect on peripheral viral load was negligible.
In another study, Toussi et al. evaluated the direct effects of METH on HIV-1 replication using the JR-CSF/hu-CycT1 HIV transgenic mouse model [43]. This transgenic mouse model has an integrated copy of R5 tropic HIV-1 provirus that expresses HIV-1 proteins and develops plasma viremia. Specifically these mice express human cyclin T1 gene enabling support of Tat-mediated HIV-1 production in the mouse CD4+ T cells and myeloid-lineage cells [43]. Using this model, Toussi et al. reported that in comparison to splenocytes from control mice, the HIV-1 p24 antigen content in the splenocytes of METH-treated mice was increased significantly [43]. They also measured the impact of METH exposure on plasma HIV-1 RNA levels in these mice. Data in this report demonstrated that in contrast to control mice, administration of METH significantly increased HIV-1 viremia by almost 6-fold. However, these authors highlighted that these transgenic mice are populated with mouse CD4-expressing T cells and macrophages that cannot be infected with HIV-1 [43]. Therefore, the increased HIV-1 production and viremia was hypothesized to be dependent on METH’s effect on the post-integration stages of HIV-1 replication6. Nevertheless, these data provided some insights into the mechanisms by which METH can accelerate HIV-1 disease.
These two animal studies support the potentiating effects of METH on HIV-1 infection and provide some mechanistic insights into the effects of METH abuse on HIV-1 pathogenesis. However, there are no follow up studies that support the effects of METH on HIV-1 infection, replication/viral load and disease progression. Therefore, additional studies are needed to clearly demonstrate the potentiating effects of METH on HIV-1 pathogenesis.
METH enhances HIV-1 infection/replication in the cultures of permissive cells
CD4+ T cells serve as the primary targets of HIV-1 infection and replication in vivo even though other permissive cells such as monocytes, macrophages, dendritic cells and to some extent astrocytes support HIV-1 infection/replication. In vitro data provide strong evidence that METH potentiates HIV-1 infection/replication in these permissive cells [44], [45]. Nair et al have reported that METH at concentrations 10–100 µM increased infection of X4 tropic HIV-1 virions in monocyte derived dendritic cells [44]. These authors proposed that METH enhanced HIV-1 infection in these cells by upregulating the HIV-1 co-receptors, CXCR4 and CCR5 [44]. In addition, data in this report suggested that METH mediated increase in HIV-1 infection is dependent on the downregulation of extracellular-regulated kinase (ERK2) and the upregulation of p38 mitogen-activated protein kinase (MAPK) [44]. Notably, dendritic cells are susceptible to infection by both R5 and X4 tropic HIV-1 virions since they express the HIV receptors and co-receptors such as CD4, CCR5, CXCR4, and others [44]. Moreover, R5 HIV-1 virions are known to infect dendritic cells more efficiently than X4 tropic virions [44]. Therefore, elucidating the effects of METH on R5 tropic virions in dendritic cells is warranted to gain better insights into the potentiating effects of METH on HIV-1 infection and replication.
Liang et al. reported that METH at concentrations 1–250 µM enhanced HIV infection of monocyte derived macrophages (MDMs) [45]. These authors demonstrated that METH treatment resulted in a significant and dose-dependent increase of reverse transcriptase activity in MDMs infected with R5 tropic HIV-1 virions. They also illustrated that METH’s potentiating effects on HIV-1 was dependent on dopamine D1 receptor and the entry co-receptor CCR5 [45]. Additionally, data in this report demonstrated that METH inhibited interferon-α (INF- α) and signal transducer and activator of transcription-1 (STAT1) [45]. Similarly, another study by Gavrilin et al. demonstrated that METH increases infection/replication of feline immunodeficiency virus (FIV) in feline astrocytes [46]. These authors proposed that the effect of METH is dependent on the viral entry or integration step, but not at the translational level [46]. Given that astrocytes to some extent support HIV-1 in the central nervous system (CNS), this data corroborated the findings of Marcondes et al. [42] that METH enhances viral replication in the brain.
Even though CD4+ T cells are the primary targets of HIV-1 in vivo, studies examining the effects of METH on HIV-1 replication in human CD4+ T cells are limited. Recently, we reported the impact of METH treatment on HIV-1 replication in primary activated CD4+ T cells isolated from the PBMCs of healthy donors [11]. Our data revealed that METH at concentrations 1–50 µM had no effect on HIV-1 replication in CD4+ T cells [11]. This observation corroborated the findings of Marcondes et al. that METH administration did not alter levels of virus in the plasma of SIV infected rhesus macaques [42]. Surprisingly, our studies illustrated that at concentrations 100–1000 µM, METH treatment inhibited viral replication in CD4+ T cells in a dose dependent manner. We used these concentrations to mimic that of METH abusers that varies between 10–50 µM in blood and 240–1000 µM in spleen and brain [11]. The inhibitory effects of METH at 100–1000 µM is contrary to the study by Toussi et al that described METH at concentrations 10–150 µM enhances HIV-1 replication in the CD4+ T cells of HIV-1 transgenic mouse [43]. It is important to point out that this double transgenic mice has an integrated HIV-1 provirus in every cell type [47]. Therefore, the systemic effects of METH on viral gene expression from every mouse cells cannot be differentiated from the direct effects of METH on HIV-1 in CD4+ T cells. Given that this mouse model is not a humanized mouse model, the mouse cells in this model cannot be infected with HIV-1. Therefore, data reported by Toussi et al. only examines effects of METH on HIV-1 LTR-driven transcription but not infection or replication of the virus. Furthermore, the integrated provirus in this animal model is a R5 tropic virion in contrast to the X4 tropic virions used in our studies. We must point out that our data was derived by infecting of purified CD4+ T cells but not in the mixed culture of PBMCs. Whether similar effects of METH on HIV could be obtained with PBMCs are currently underway.
Toussi et al also reported that METH at 10–150 µM also increased HIV-1 replication in primary activated CD4+ T cells [43]. However, these authors used R5 tropic HIV-1 virions to infect human CD4+ T cells in contrast to X4 tropic HIV-1 virions used in our study. It is well established that X4 virions utilize the CXCR4 co-receptor whereas R5 tropic virions utilize CCR5 co-receptor for cellular entry. Since primary CD4+ T cells express both CXCR4 and CCR5 co-receptors these cells can be infected by both X4 and R5 virions. Surprisingly, our studies also showed inhibitory effects of METH on replication of R5 HIV-1 virions in primary CD4+ T cells [11]. One major difference in our infection studies with R5 virions is that we measured intracellular p24 in contrast to extracellular p24 measurements by Toussi et al. We speculate that the methods used cannot explain the contrasting data since extracellular p24 levels is dependent on intracellular p24 production. Furthermore, when the co-receptor requirement for viral entry was abrogated by pseudotyping [11], METH also inhibited replication of HIV-1 virions in CD4+ T cells. These data strongly suggest that METH’s effect on HIV-1 replication in CD4+ T cells may not depend on co-receptor requirement. A major difference is that Toussi et al added METH to the cells every day, whereas in our study cells were treated with METH only once. Whether, these distinct pattern of METH exposure is responsible for the contrasting effects of METH on HIV-1 replication is currently under investigation. Given that these studies used primary cells from human donors, the contribution of genetic variability among donors cannot be ruled out. The genetic variability among donors may affect the expression of cellular factors that modulate HIV-1 infection and replication in the CD4+ T cells. Nevertheless, these discrepancies in METH’s effect on HIV-1 replication highlight the critical need for comprehensive research to better understand the complex interaction between METH and HIV-1 in CD4+ T cells and other permissive cells.
METH regulates both entry and post entry steps of HIV-1 replication
Published data provide strong evidence that METH potentiates HIV-1 replication by modulating the entry of virions into target cells. For example, Nair et al. proposed that METH enhanced HIV-1 entry into dendritic cells by upregulating the co-receptors, CXCR4 and CCR5 [44]. In another study, Nair et al. suggested that METH modulates HIV-1 entry into dendritic cells by regulating the receptor DC-SIGN [48]. In addition, Liang et al has illustrated that METH can increase HIV-1 entry into in MDMs by regulating expression of CXCR4 and CCR5 co-receptors45. METH also has been shown to reduce secretion of the chemokines MIP-1α, MIP-1β, and RANTES which can bind to the CCR5 co-receptor and prevent HIV-1 entry into the target cells [49]. Moreover, our data on VSV-G pseudotyped HIV-1 virions suggested that METH may also target viral post entry steps to regulate HIV-1 replication [11]. HIV-1 post entry steps include reverse transcription, integration, transcription, translation and assembly. There is evidence that METH regulates HIV-1 LTR driven transcription in microglial cells [50]. We have demonstrated that METH regulates HIV-1 protein translation in CD4 + T cells [11]. Interestingly, our data also revealed that METH achieves this by targeting the cellular anti-HIV-1 miRNAs that are known to negatively regulate HIV-1 protein translation [11]. Given that HIV-1 post entry steps are dependent on a complex interaction between the virus and cellular host factors, METH can influence HIV-1 post entry steps by targeting HIV-1 associated cellular factors [44,45]. In sum, these published data suggest that METH can modulate HIV-1 infection/replication via direct and indirect mechanisms. However, the molecular details and the underlying mechanisms by which METH regulates HIV-1 entry and post-entry steps are yet to be clearly understood.
Immunomodulatory functions of METH may modulate HIV-1 infection and replication
In addition to directly modulating various entry and post entry steps of HIV-1 infection, the immunomodulatory effects of METH may also contribute towards its effects on HIV-1 life cycle. METH is known to stimulate release of dopamine, serotonin, and norepinephrine [19,51]. The receptors and transporters that mediate effects of these neurotransmitters are also expressed on various immune cells. Therefore, METH use has been shown to alter or suppress functions of immune cells that are implicated in HIV-1 pathogenesis. For example, METH is known to regulate T cell function including proliferation, cytokine production and T cell-mediated immune response [52,53]. In addition, METH is demonstrated to downregulate the expression of anti-viral cytokine IFN-α in macrophages and dendritic cells [45,54]. Moreover, Gavrilin et al have shown that METH suppresses interlukin-2 (IL-2) and IFN-γ expression [46]. There is also evidence that METH stimulates secretion of Tumor Necrosis Factor-alpha (TNF-α) in the splenocytes of retrovirus-infected mice [55]. METH’s immunomodulatory effect is further supported by Yu et al demonstrating that METH exposure inhibited macrophage-mediated antiviral activities by reducing production of nitric oxide (NO)/TNF- α [56]. In addition, METH has been shown to reduce the expression of the dendritic cell marker CD83 that plays an important role in antigen presentation and T cell activation [48]. Microarray analysis of brain tissue from HIV-infected METH users by Everall et al. showed significant up-regulation of inflammatory genes [57]. In another microarray study on dendritic cells, Mahajan et al have illustrated that METH alters expression of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-8 [54]. Talloczy et al. have also demonstrated that METH alters the function of the murine immune system including that of dendritic cells and macrophages, and T-cell antigen presentation [58]. In addition, these authors reported that use of METH increases cytokines, chemokines, and cellular adhesion molecules in murine models and human participants [59]. Furthermore, data from chronically SIV-infected rhesus macaques showed that METH treatment induces activation of natural killer cells [42]. Taken together, these studies provide strong evidence that the immunomodulatory functions of METH could contribute to HIV infection and exacerbate disease progression.
Recent studies indicate that METH’s immune suppressive function may also depend on its ability to alkalize acidic organelles within immune cells. METH’s ability to alkalize acidic organelles may inhibit antigen presentation, impairs phagocytosis, and likely compromises the immune response to pathogen inactivation [60–62]. For example, it has been showed that METH inhibits endosomal acidification in macrophages [60–62]. A neutral pH of endosomes has been shown to have beneficial effects for pathogens during infection of macrophages [63]. In addition, METH has also been reported to destabilize the capacity of infected macrophages to regulate their intracellular milieu [60–62]. In macrophages endosomal trafficking plays critical roles in HIV-1 replication [64]. Therefore, effects of METH on endosomal pathways may directly and indirectly contribute to HIV-1 infection. In addition, acidification of organelles can potentially have devastating effects in HIV-infected METH abusers with reference to opportunistic infections. Whether the effects of METH on endosomal pathway may contribute to the discrepant data reported in the literature is yet to be determined.
Concluding Remarks and Future Prospective
METH is a highly addictive psychostimulant that modulates release of neurotransmitters in the brain and causes euphoria. Therefore, METH use and HIV-1 infection frequently coexist because of the association of this drug with engagement of high-risk behaviors. Moreover, clinical studies suggest that METH users may display higher levels of HIV loads than nonusers. Even though the biological effects of METH in the CNS have been extensively studied, the interaction between METH use and HIV/AIDS remains poorly understood. Published studies from cell culture models and animals implicate increased viral replication for the potential effects of METH on HIV-1 pathogenesis. However, the direct effects of METH use on HIV infection and HIV disease progression are still poorly understood and remain contentious. In particular, the deleterious effect of METH on the host’s immune response and its role in the immunopathogenesis of HIV remain to be elucidated. Even though a plethora of data are available on the biochemical and molecular effects of METH on an array of cell types, there are few studies that examine molecular effects of METH on immune cells specifically on T cells. Most importantly, the mechanisms by which METH regulates HIV-1 infection and replication in various permissive cells including CD4+ T cells in the periphery remain largely unclear. The contrasting data on the potentiating and inhibitory effects of METH on HIV-1 replication in vitro underscore the need to investigate the interaction between METH and HIV-1 at molecular level. The published data that METH has no/limited impact on peripheral viral load in SIV infected animals add to the biological and molecular intricacy of this drug. Given that there are only two animal studies that examine METH’s effect on HIV-1 infection and replication, whether the reported differential effects of METH on HIV-1 is dependent on the type of cells infected and/or the tropism of virions should be further investigated. Most importantly, whether the contrasting effect of METH on HIV-1 is dependent on local concentrations of METH in the tissues is yet to be determined.
In summary, clinical studies suggest an association between METH abuse and HIV-1 pathogenesis. One animal study using transgenic mice indicate that METH potentiates HIV-1 replication, whereas the SIV study suggest that METH has minimal or no effects on the viral load in the periphery but enhances viral load in the brain. There is evidence that METH enhances HIV-1 infection in the cultures of astrocytes, dendritic cells and macrophages. Our data illustrate that METH’s effect on HIV-1 in CD4+ T cells may depend on concentrations used and to some extent tropism of the HIV-1 virions. Collectively, these clinical, animal-based and in vitro studies highlight that METH’s effect on HIV-1 pathogenesis is complex and comprehensive studies are warranted for unequivocal data. Given that humanized mouse models are extensively used to study HIV-1 pathogenesis, greater insights into METH’s effect on HIV-1 can be derived using such a model. Furthermore, our understanding on the molecular interplay between METH and HIV-1 will be strengthened by investigating METH’s effect on dopaminergic system of immune cells. Therefore, additional research using in vitro and in vivo models are needed to address these outstanding questions on the complex interaction between METH use and HIV-1 pathogenesis.
Acknowledgments
Grant numbers and sources of support: This work is partly supported by grants DA024558, DA30896, DA033892 and the DIDARP grant R24DA021471 from NIDA/NIH to CD. We also acknowledge the RCMI Grant G12MD007586, the Vanderbilt CTSA grant UL1RR024975, the Meharry Translational Research Center (MeTRC) CTSA grant (U54 RR026140 from NCRR/NIH, the U54 grant MD007593 from NIMHD/NIH and the DA029506 grant that established the patient samples.
ABBREVIATIONS
- HIV
human immunodeficiency virus
- AIDS
acquired immunodeficiency syndrome
- ART
antiretroviral therapy
- METH
methamphetamine
- MSM
men who have sex with men
- STIs
sexually transmitted infections
- SIV
simian immunodeficiency virus
- RNA
ribonucleic acid
- CXCR4
chemokine (C-X-C motif) receptor 4
- CCR5
C-C chemokine receptor type 5
- ERK2
extracellular-regulated kinase
- MAPK
mitogen-activated protein kinase
- MDM
monocyte derived macrophage
- INF- α
interferon-alpha
- STAT1
signal transducer and activator of transcription-1
- FIV
feline immunodeficiency virus
- CNS
central nervous system
- LTR
Long terminal repeats
- DC-SIGN
dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin
- MIP-1
macrophage inflammatory protein 1
- RANTES
regulated on activation T cell expressed and secreted
- VSV-G
vesicular stomatitis virus g protein
- miRNA
microRNA
- IL-2
interlukin 2
- IFN-γ
interferon-gamma
- TNF-α
tumor necrosis factor alpha
- NO
nitric oxide
- IL-1β
interlukin beta
- IL-8
interlukin 8
Footnotes
ETHICAL STATEMENT: The authors declare that the manuscript has not been submitted to more than one journal for simultaneous consideration. No data have been fabricated or manipulated (including images) to support your conclusions.
Author contributions: RC and HQ carried out search of clinical literatures. JP and CD carried literature survey of cellular and molecular studies. CD designed and directed the entire study. All authors contributed to write and review the manuscript.
Competing Interests: The authors declare no competing financial and non-financial interests.
References
- 1. http://www.who.int/mediacentre/factsheets/fs360/en/index.html.
- 2.CDC. HIV Surveillance Report. 2012:22. http://wwwcdcgov/hiv/topics/surveillance/resources/
- 3.Walensky RP, Paltiel AD, Losina E, Mercincavage LM, Schackman BR, et al. The survival benefits of AIDS treatment in the United States. J Infect Dis. 2006;194:11–19. doi: 10.1086/505147. [DOI] [PubMed] [Google Scholar]
- 4.Kipp AM, Desruisseau AJ, Qian HZ. Non-injection drug use and HIV disease progression in the era of combination antiretroviral therapy. J Subst Abuse Treat. 2011;40:386–396. doi: 10.1016/j.jsat.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol. 2006;47:330–342. doi: 10.1111/j.1574-695X.2006.00097.x. [DOI] [PubMed] [Google Scholar]
- 6.Qian HZ, McGowan CC, Mitchell VJ, Cassell H, Perez G, et al. Rome, Italy: 2011. Jul 17–20, Non-injection substance abuse, depression, and adherence to antiretroviral therapy among HIV-infected patients. [Google Scholar]
- 7.Parsons JT, Kowalczyk WJ, Botsko M, Tomassilli J, Golub SA. Aggregate Versus Day Level Association Between Methamphetamine Use and HIV Medication Non-adherence Among Gay and Bisexual Men. AIDS Behav. 2013;17:1478–1487. doi: 10.1007/s10461-013-0463-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marquez C, Mitchell SJ, Hare CB, John M, Klausner JD. Methamphetamine use, sexual activity, patient-provider communication, and medication adherence among HIV-infected patients in care, San Francisco 2004–2006. AIDS Care. 2009;21:575–582. doi: 10.1080/09540120802385579. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Ho WZ. Drugs of abuse and HIV infection/replication: implications for mother-fetus transmission. Life Sci. 2011;88:972–979. doi: 10.1016/j.lfs.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Misawa F, Shimizu K, Fujii Y, Miyata R, Koshiishi F, et al. Is antipsychotic polypharmacy associated with metabolic syndrome even after adjustment for lifestyle effects?: a cross-sectional study. BMC Psychiatry. 2011;11:118. doi: 10.1186/1471-244X-11-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mantri CK, Mantri JV, Pandhare J, Dash C. Methamphetamine inhibits HIV-1 replication in CD4+ T cells by modulating anti-HIV-1 miRNA expression. Am J Pathol. 2014;184:92–100. doi: 10.1016/j.ajpath.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lane HC. Pathogenesis of HIV infection: total CD4+ T-cell pool, immune activation, and inflammation. Top HIV Med. 2010;18:2–6. [PubMed] [Google Scholar]
- 13.Gaskill PJ, Calderon TM, Coley JS, Berman JW. Drug induced increases in CNS dopamine alter monocyte, macrophage and T cell functions: implications for HAND. J Neuroimmune Pharmacol. 2013;8:621–642. doi: 10.1007/s11481-013-9443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cisneros IE, Ghorpade A. HIV-1, methamphetamine and astrocyte glutamate regulation: combined excitotoxic implications for neuro-AIDS. Curr HIV Res. 2012;10:392–406. doi: 10.2174/157016212802138832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nair MP, Samikkannu T. Differential regulation of neurotoxin in HIV clades: role of cocaine and methamphetamine. Curr HIV Res. 2012;10:429–434. doi: 10.2174/157016212802138742. [DOI] [PubMed] [Google Scholar]
- 16.Gruenewald PJ, Johnson FW, Ponicki WR, Remer LG, Lascala EA. Assessing correlates of the growth and extent of methamphetamine abuse and dependence in California. Subst Use Misuse. 2010;45:1948–1970. doi: 10.3109/10826081003682867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colfax G, Shoptaw S. The methamphetamine epidemic: implications for HIV prevention and treatment. Curr HIV/AIDS Rep. 2005;2:194–199. doi: 10.1007/s11904-005-0016-4. [DOI] [PubMed] [Google Scholar]
- 18.Rawson RA, Anglin MD, Ling W. Will the methamphetamine problem go away? J Addict Dis. 2002;21:5–19. doi: 10.1300/j069v21n01_02. [DOI] [PubMed] [Google Scholar]
- 19.Semple SJ, Patterson TL, Grant I. Motivations associated with methamphetamine use among HIV+ men who have sex with men. J Subst Abuse Treat. 2002;22:149–156. doi: 10.1016/s0740-5472(02)00223-4. [DOI] [PubMed] [Google Scholar]
- 20.Hurt CB, Torrone E, Green K, Foust E, Leone P, et al. Methamphetamine use among newly diagnosed HIV-positive young men in North Carolina, United States, from 2000 to 2005. PLoS One. 2010;5:e11314. doi: 10.1371/journal.pone.0011314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson TL, Semple SJ, Staines H, Lozada R, Orozovich P, et al. Prevalence and correlates of HIV infection among female sex workers in 2 Mexico-US border cities. J Infect Dis. 2008;197:728–732. doi: 10.1086/527379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wohl AR, Johnson DF, Lu S, Jordan W, Beall G, et al. HIV risk behaviors among African American men in Los Angeles County who self-identify as heterosexual. J Acquir Immune Defic Syndr. 2002;31:354–360. doi: 10.1097/00126334-200211010-00013. [DOI] [PubMed] [Google Scholar]
- 23.Peck JA, Reback CJ, Yang X, Rotheram-Fuller E, Shoptaw S. Sustained reductions in drug use and depression symptoms from treatment for drug abuse in methamphetamine-dependent gay and bisexual men. J Urban Health. 2005;82:i100–i108. doi: 10.1093/jurban/jti029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thiede H, Valleroy LA, MacKellar DA, Celentano DD, Ford WL, et al. Regional patterns and correlates of substance use among young men who have sex with men in 7 US urban areas. Am J Public Health. 2003;93:1915–1921. doi: 10.2105/ajph.93.11.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansergh G, Shouse RL, Marks G, Guzman R, Rader M, et al. Methamphetamine and sildenafil (Viagra) use are linked to unprotected receptive and insertive anal sex, respectively, in a sample of men who have sex with men. Sex Transm Infect. 2006;82:131–134. doi: 10.1136/sti.2005.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plankey MW, Ostrow DG, Stall R, Cox C, Li X, et al. The relationship between methamphetamine and popper use and risk of HIV seroconversion in the multicenter AIDS cohort study. J Acquir Immune Defic Syndr. 2007;45:85–92. doi: 10.1097/QAI.0b013e3180417c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex. 2001;41:17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- 28.Drumright LN, Little SJ, Strathdee SA, Slymen DJ, Araneta MR, et al. Unprotected anal intercourse and substance use among men who have sex with men with recent HIV infection. J Acquir Immune Defic Syndr. 2006;43:344–350. doi: 10.1097/01.qai.0000230530.02212.86. [DOI] [PubMed] [Google Scholar]
- 29.Shoptaw S, Reback CJ. Associations between methamphetamine use and HIV among men who have sex with men: a model for guiding public policy. J Urban Health. 2006;83:1151–1157. doi: 10.1007/s11524-006-9119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ellis RJ, Childers ME, Cherner M, Lazzaretto D, Letendre S, et al. Increased human immunodeficiency virus loads in active methamphetamine users are explained by reduced effectiveness of antiretroviral therapy. J Infect Dis. 2003;188:1820–1826. doi: 10.1086/379894. [DOI] [PubMed] [Google Scholar]
- 31.Carrico AW, Johnson MO, Moskowitz JT, Neilands TB, Morin SF, et al. Affect regulation, stimulant use, and viral load among HIV-positive persons on anti-retroviral therapy. Psychosom Med. 2007;69:785–792. doi: 10.1097/PSY.0b013e318157b142. [DOI] [PubMed] [Google Scholar]
- 32.Fairbairn N, Kerr T, Milloy MJ, Zhang R, Montaner J, et al. Crystal methamphetamine injection predicts slower HIV RNA suppression among injection drug users. Addict Behav. 2011;36:762–763. doi: 10.1016/j.addbeh.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.King WD, Larkins S, Hucks-Ortiz C, Wang PC, Gorbach PM, et al. Factors associated with HIV viral load in a respondent driven sample in Los Angeles. AIDS Behav. 2009;13:145–153. doi: 10.1007/s10461-007-9337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, et al. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11:185–194. doi: 10.1007/s10461-006-9152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrico AW, Johnson MO, Colfax GN, Moskowitz JT. Affective correlates of stimulant use and adherence to anti-retroviral therapy among HIV-positive methamphetamine users. AIDS Behav. 2010;14:769–777. doi: 10.1007/s10461-008-9513-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shoptaw S, Stall R, Bordon J, Kao U, Cox C, et al. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. Int J STD AIDS. 2012;23:576–580. doi: 10.1258/ijsa.2012.011322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cachay ER, Moini N, Kosakovsky Pond SL, Pesano R, Lie YS, et al. Active methamphetamine use is associated with transmitted drug resistance to non-nucleoside reverse transcriptase inhibitors in individuals with HIV infection of unknown duration. Open AIDS J. 2007;1:5–10. doi: 10.2174/1874613600701010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markowitz M, Mohri H, Mehandru S, Shet A, Berry L, et al. Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: a case report. Lancet. 2005;365:1031–1038. doi: 10.1016/S0140-6736(05)71139-9. [DOI] [PubMed] [Google Scholar]
- 39.Colfax G, Coates TJ, Husnik MJ, Huang Y, Buchbinder S, et al. Longitudinal patterns of methamphetamine, popper (amyl nitrite), and cocaine use and high-risk sexual behavior among a cohort of san francisco men who have sex with men. J Urban Health. 2005;82:i62–i70. doi: 10.1093/jurban/jti025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reback CJ, Larkins S, Shoptaw S. Methamphetamine abuse as a barrier to HIV medication adherence among gay and bisexual men. AIDS Care. 2003;15:775–785. doi: 10.1080/09540120310001618621. [DOI] [PubMed] [Google Scholar]
- 42.Marcondes MC, Flynn C, Watry DD, Zandonatti M, Fox HS. Methamphetamine increases brain viral load and activates natural killer cells in simian immunodeficiency virus-infected monkeys. Am J Pathol. 2010;177:355–361. doi: 10.2353/ajpath.2010.090953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toussi SS, Joseph A, Zheng JH, Dutta M, Santambrogio L, et al. Short communication: Methamphetamine treatment increases in vitro and in vivo HIV replication. AIDS Res Hum Retroviruses. 2009;25:1117–1121. doi: 10.1089/aid.2008.0282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nair MP, Saiyed ZM, Nair N, Gandhi NH, Rodriguez JW, et al. Methamphetamine enhances HIV-1 infectivity in monocyte derived dendritic cells. J Neuroimmune Pharmacol. 2009;4:129–139. doi: 10.1007/s11481-008-9128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liang H, Wang X, Chen H, Song L, Ye L, et al. Methamphetamine enhances HIV infection of macrophages. Am J Pathol. 2008;172:1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gavrilin MA, Mathes LE, Podell M. Methamphetamine enhances cell-associated feline immunodeficiency virus replication in astrocytes. J Neurovirol. 2002;8:240–249. doi: 10.1080/13550280290049660. [DOI] [PubMed] [Google Scholar]
- 47.Sun J, Soos T, Kewalramani VN, Osiecki K, Zheng JH, et al. CD4-specific transgenic expression of human cyclin T1 markedly increases human immunodeficiency virus type 1 (HIV-1) production by CD4+ T lymphocytes and myeloid cells in mice transgenic for a provirus encoding a monocyte-tropic HIV-1 isolate. J Virol. 2006;80:1850–1862. doi: 10.1128/JVI.80.4.1850-1862.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nair MP, Mahajan S, Sykes D, Bapardekar MV, Reynolds JL. Methamphetamine modulates DC-SIGN expression by mature dendritic cells. J Neuroimmune Pharmacol. 2006;1:296–304. doi: 10.1007/s11481-006-9027-1. [DOI] [PubMed] [Google Scholar]
- 49.Nair MP, Saiyed ZM. Effect of methamphetamine on expression of HIV coreceptors and CC-chemokines by dendritic cells. Life Sci. 2011;88:987–994. doi: 10.1016/j.lfs.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wires ES, Alvarez D, Dobrowolski C, Wang Y, Morales M, et al. Methamphetamine activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB) and induces human immunodeficiency virus (HIV) transcription in human microglial cells. J Neurovirol. 2012;18:400–410. doi: 10.1007/s13365-012-0103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiner BC, Keblesh JP, Xiong H. Methamphetamine abuse, HIV infection, and neurotoxicity. Int J Physiol Pathophysiol Pharmacol. 2009;1:162–179. [PMC free article] [PubMed] [Google Scholar]
- 52.Potula R, Persidsky Y. Adding fuel to the fire: methamphetamine enhances HIV infection. Am J Pathol. 2008;172:1467–1470. doi: 10.2353/ajpath.2008.080130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harms R, Morsey B, Boyer CW, Fox HS, Sarvetnick N. Methamphetamine administration targets multiple immune subsets and induces phenotypic alterations suggestive of immunosuppression. PLoS One. 2012;7:e49897. doi: 10.1371/journal.pone.0049897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mahajan SD, Hu Z, Reynolds JL, Aalinkeel R, Schwartz SA, et al. Methamphetamine modulates gene expression patterns in monocyte derived mature dendritic cells: implications for HIV-1 pathogenesis. Mol Diagn Ther. 2006;10:257–269. doi: 10.1007/BF03256465. [DOI] [PubMed] [Google Scholar]
- 55.Yu Q, Zhang D, Walston M, Zhang J, Liu Y, et al. Chronic methamphetamine exposure alters immune function in normal and retrovirus-infected mice. Int Immunopharmacol. 2002;2:951–962. doi: 10.1016/s1567-5769(02)00047-4. [DOI] [PubMed] [Google Scholar]
- 56.In SW, Son EW, Rhee DK, Pyo S. Modulation of murine macrophage function by methamphetamine. J Toxicol Environ Health A. 2004;67:1923–1937. doi: 10.1080/15287390490514589. [DOI] [PubMed] [Google Scholar]
- 57.Everall I, Salaria S, Roberts E, Corbeil J, Sasik R, et al. Methamphetamine stimulates interferon inducible genes in HIV infected brain. J Neuroimmunol. 2005;170:158–171. doi: 10.1016/j.jneuroim.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Talloczy Z, Martinez J, Joset D, Ray Y, Gacser A, et al. Methamphetamine inhibits antigen processing, presentation, and phagocytosis. PLoS Pathog. 2008;4:e28. doi: 10.1371/journal.ppat.0040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Loftis JM, Choi D, Hoffman W, Huckans MS. Methamphetamine causes persistent immune dysregulation: a cross-species, translational report. Neurotox Res. 2011;20:59–68. doi: 10.1007/s12640-010-9223-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martinez LR, Mihu MR, Gacser A, Santambrogio L, Nosanchuk JD. Methamphetamine enhances histoplasmosis by immunosuppression of the host. J Infect Dis. 2009;200:131–141. doi: 10.1086/599328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Newman SL. Macrophages in host defense against Histoplasma capsulatum. Trends Microbiol. 1999;7:67–71. doi: 10.1016/s0966-842x(98)01431-0. [DOI] [PubMed] [Google Scholar]
- 62.Chaturvedi S, Frame P, Newman SL. Macrophages from human immunodeficiency virus-positive persons are defective in host defense against Histoplasma capsulatum. J Infect Dis. 1995;171:320–327. doi: 10.1093/infdis/171.2.320. [DOI] [PubMed] [Google Scholar]
- 63.Eissenberg LG, Goldman WE, Schlesinger PH. Histoplasma capsulatum modulates the acidification of phagolysosomes. J Exp Med. 1993;177:1605–1611. doi: 10.1084/jem.177.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pelchen-Matthews A, Kramer B, Marsh M. Infectious HIV-1 assembles in late endosomes in primary macrophages. J Cell Biol. 2003;162:443–455. doi: 10.1083/jcb.200304008. [DOI] [PMC free article] [PubMed] [Google Scholar]