Abstract
Objective
Proposals for pediatric biobanks have prompted questions of whether parental permission is sufficient to continue to use biological samples and data after the children become adults. The objective of this study was to examine adults’ attitudes about continued research with their pediatric samples/data, particularly when they could not be located to provide consent.
Study design
Telephone interviews were conducted with 1186 patients from 5 academic medical centers by using a hypothetical scenario.
Results
Most respondents, 799 (67%), would not be concerned about the use of their sample/data after they reached adulthood. Those respondents who were concerned were more likely to be more private about their medical records, less trusting of medical researchers, or African-American. A total of 543 respondents (46%) believed their consent should be obtained to continue using their sample/data for research. Of these, 407 respondents (75%) would be at least moderately willing to give consent, when asked. Of the 1186 respondents, 310 (26%) would not want researchers to use their sample/data when they could not be located to ask for consent.
Conclusion
The data are consistent with the normative view that when feasible, adults should be asked for consent for continued research on their data collected during childhood, but it is generally acceptable to continue to conduct research when adults cannot be located. Further public engagement may help determine how best to balance the potential social value of continued research using pediatric samples/data and the expectations for explicit consent expressed by a minority of respondents that may reflect concerns about privacy and trust.
There is emerging interest in prospectively collecting DNA and phenotypic information from children who receive care at academic medical centers,1,2 have specific diseases,3-5 and/or are enrolled in population-based research such as the National Children's Study.6 The creation of large scale repositories (commonly referred to as “biobanks”) to facilitate use of children's samples and data to study genetic and environmental links to common and rare diseases raises interesting ethical questions.7-11 Of particular interest—both ethically and logistically—is whether parental permission is sufficient for ongoing research once the child becomes an adult.
Parental permission is generally accepted as both necessary and sufficient for the participation of young children in most research because they lack both the capacity and legal standing to consent on their own behalf.12-15 When children enrolled in research turn 18 years old, their ongoing direct participation requires their consent. However, in many studies, from oncology clinical trials to cross-sectional environmental exposure studies, data analysis continues long after the direct involvement of the child has ended. Some researchers have recommended that as part of any pediatric biobanking project, researchers should consider contacting children once they turn 18 years old and allow them to withdraw.16 When parents have already given permission to use their children's samples/data, how important is obtaining consent from these children when they become adults, but are not actively engaging with researchers? If such consent is desirable, should samples/data be discarded when obtaining consent is not feasible?
An assessment of public attitudes can help address these questions. Public attitudes are reflective of the potential, or at least perceived, impact of continued research on the rights and welfare of participants and this information may help to develop more nuanced and responsive policies. Although a number of recent studies have explored public attitudes toward the storage and use of biological samples,17-23 few have focused on children. Additionally, those studies that looked at children have focused on parents’ attitudes about the banking of their children's samples/data rather than their own samples banked by their parents.24,25 We report results from a survey of the attitudes of adults responding to a hypothetical scenario related to the continued research on their own samples collected during childhood.
Methods
In 2002 to 2003, 1193 patients from 5 academic health centers (Duke University, Johns Hopkins University, and the Universities of Arizona, North Carolina at Chapel Hill [UNC], and Utah) were interviewed via telephone about their attitudes toward genetic research. A more detailed description of these methods has been published.26 Respondents were enrolled from a convenience sample of 1395 patients seen at primary care, thoracic surgery, or medical oncology clinics, or patients who previously had been asked to store blood for research (UNC), for an average participation rate of 86%. Respondents received a $25 honorarium for their participation.
The questionnaire, which was developed through expert review, in-person cognitive interviews, pretest telephone interviews, and a focus group, took approximately 30 minutes to complete. The questionnaire can be found in the Appendix (available at www.jpeds.com). The questionnaire defined genetic diseases as “diseases that run in families tend to be passed from generation to generation through genes,” and genetic research as research that “connects a person's genes with the diseases he or she might get.” The questionnaire assessed patients’ attitudes about participation in genetic research and included a series of hypothetical scenarios.
One of the hypothetical scenarios focused on the collection of samples and data during childhood that were stored for future research, followed by 4 questions on attitudes about future use (Figure 1). There were 1186 respondents who answered the questions related to the pediatric scenario.
Figure 1.
Pediatric scenario and questions on adult attitudes towards the use of their childhood samples for research.
The study was approved by the institutional review boards of the National Human Genome Research Institute, University of Massachusetts at Boston, University of Utah, University of Arizona, Johns Hopkins University, Duke University, and UNC. A written description of the research was given to all individuals during recruitment, and consent was obtained verbally before each interview.
Data Analysis
Descriptive statistics for each of the 4 questions were examined. A multivariate logistic regression analysis was performed to identify predictors of the initial question about concern for research being done on their sample. Independent variables used for this regression included age, sex, race, income, education, respondent's chronic disease status, and whether they have had major surgery. Along with these demographic characteristics, the independent variables also included survey responses to trust in medical researchers, privacy about medical information, and general attitude toward genetic research. With this set of independent variables, a backward regression procedure was performed by using SPSS software version 16.0 (SPSS Inc., Chicago, Illinois). Variables with a pvalue <.02 were considered to be independent predictors of the level of concern.
Results
Of the 1186 patients interviewed, most were female (70%), >50 years old (59%), and had at least some college education (72%). Seventy-six percent of respondents identified themselves as Caucasian, and 16% identified themselves as African American. Eighty percent of the respondents had had surgery as an adult, and 61% described themselves as having a chronic medical condition (Table I; available at www.jpeds.com).
Table I.
Characteristics of respondents (n = 1186)
| n (% of total sample) | |
|---|---|
| Sex | |
| Male | 353 (30%) |
| Female | 833 (70%) |
| Age (years) | |
| 18-29 | 106 (9%) |
| 30-39 | 154 (13%) |
| 40-49 | 232 (20%) |
| 50-64 | 426 (36%) |
| 65+ | 268 (23%) |
| Education | |
| Less than high school | 97 (8%) |
| High school | 234 (19%) |
| Some college | 330 (28%) |
| College graduate or beyond | 522 (44%) |
| No answer | 3 (<1%) |
| Race | |
| White/Caucasian | 898 (75%) |
| Black/African American | 190 (16%) |
| Asian American | 20 (2%) |
| American Indian | 20 (2%) |
| Native Hawaiian/Pacific Islander | 4 (<1%) |
| Other | 49 (4%) |
| No answer | 5 (<1%) |
| Household income previous year | |
| <$20 K | 218 (18%) |
| $20 K-39.9 K | 260 (22%) |
| $40 K-59.9 K | 215 (18%) |
| $60 K-79.9 K | 119 (10%) |
| $80K+ | 271 (23%) |
| No answer | 103 (9%) |
| Have serious or chronic medical condition | |
| Yes | 725 (61%) |
| No | 458 (38%) |
| No answer | 3 (<1%) |
| Ever had surgery as adult | |
| Yes | 951 (80%) |
| No | 235 (20%) |
Table II shows attitudes related to privacy, trust, and genetic research. Thirty percent of respondents considered themselves to be “private” or “very private” about their medical records, and 54% described themselves as “open” or “very open” about their medical information.. Most respondents (93%) answered that they were either “completely” or “somewhat” trusting of medical researchers, and only 7% trusted researchers “a little” or “not at all.” Most respondents (90%) had heard at least a little about genetic research, and 10% had never heard about such research. Of those 1063 respondents who had heard at least a little about genetic research, 90% had either “somewhat” or “very positive” feelings toward this research.
Table II.
General attitudes about medical information and research
| n (%) | |
|---|---|
| Private about medical information | 1186 |
| Very open | 236 (20%) |
| Open | 403 (40%) |
| Neither private nor open | 184 (15%) |
| Private | 182 (24%) |
| Very private | 75 (6%) |
| No answer | 6 (<1%) |
| Level of trust in medical researchers | 1186 |
| Completely | 349 (32%) |
| Somewhat | 670 (61%) |
| A little | 62 (6%) |
| Not at all | 8 (<1%) |
| Depends | 9 (<1%) |
| Don't know | 7 (<1%) |
| No answer | 2 (<1%) |
| Ever heard about genetic research | 1186 |
| Heard a lot | 318 (27%) |
| Heard a little | 745 (63%) |
| Heard nothing about it | 123 (10%) |
| Attitude toward genetic research | 1063 |
| Very positive | 504 (47%) |
| Positive | 449 (43%) |
| Neither positive nor negative | 67 (6%) |
| Negative | 29 (3%) |
| Very negative | 10 (<1%) |
| No answer | 3 (<1%) |
*Only asked of those respondents who had heard about genetic research.
Concern About Research on Their Pediatric Sample/data
When asked about their level of concern about hypothetical research with their childhood sample/data with their parents’ permission, 33% of the 1186 respondents (n = 387) were either “moderately concerned” or “very concerned,” whereas most respondents (67%, n = 799) were either “not very concerned” (26%, n = 308) or “not at all concerned” (41%, n = 491) about the use of their sample.
In multivariate regression analysis (Table III), patients with higher levels of concern about the use of pediatric samples/data (n = 387), compared with patients with lower concern, were less trusting of medical researchers, more private about their medical information, and more likely to be African American. For this regression, trust was dichotomized to “not at all/a little” and “somewhat/completely.” Privacy was dichotomized to “not at all/a little” and “somewhat/completely.”
Table III.
Predictors of concern about the use of pediatric samples*
| Independent variable (group predicting higher levels of concern) | Odds ratio | 95% CI |
|---|---|---|
| Trust in researchers (lower trust of medical researchers) | 3.22 | 1.46-7.09 |
| Private about medical information (more private about medical information) | 2.07 | 1.40-3.05 |
| Race—black versus white (African-American respondents) | 2.71 | 1.69-4.32 |
Independent variables included in the regression that were not statistically significant included age, income, sex, education, ever had major surgery, feelings about genetic research, and having a chronic medical condition.
Adults’ Attitudes Toward Consent for Use of Their Pediatric Sample/data
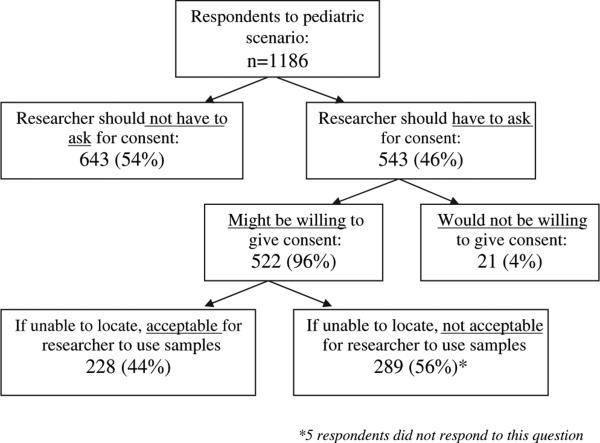
Responses to the 3 questions about attitudes on consent for continued use of sample/data are presented in Figure 2.
Figure 2.
Attitudes about consent and use of their pediatric samples and data for research.
When asked whether researchers should have to ask for consent to continue to use samples, 543 of the 1186 respondents (46%) answered yes.
The 543 respondents who believed researchers should have to get consent were then asked about their willingness to give consent if they were contacted by researchers. Of these respondents, most (75%, n = 407) were either “very willing” (43%, n = 233) or “moderately willing” (32%, n = 174,) to give consent. Twenty-four percent (n = 131) were either “somewhat or very hesitant” (20%, n = 111) or “completely unwilling” to give consent (4% n = 21). Four individuals did not respond to the questions about willingness.
All the respondents who might be willing to consent for continued use of their samples (n = 522) were then asked whether it would be acceptable for researchers to use their blood sample if they could not be located as adults. Of these respondents, 56% (n = 289) found it unacceptable for researchers to use their sample when they could not be located. By combining these 289 respondents with the 21 respondents from the earlier question who would be completely unwilling to give consent if asked, a total of 26% of respondents (n = 310) would find it unacceptable to continue to use samples and data collected during childhood if they could not be located by researchers.
Discussion
This study found generally favorable attitudes in adults toward the continued use of pediatric samples/data for research, with most respondents (67%) not very concerned about this continued use. While nearly half of the respondents (46%) believe that researchers should ask for their consent as adults. Seventy-five percent of these respondents were at least moderately willing to provide consent for continued research.
These results may reflect a desire for participation in decisions about research, rather than an expression of negative attitudes about the research itself. The interest in decision-making is corroborated by the finding that 56% of respondents who were at least willing to consider giving consent were unwilling to allow further use if they could not be located. These data support making efforts, when feasible, to contact the adults to ask for their consent. Such a request demonstrates respect for their interest in decision-making and is likely to be a welcome communication for most people.
In some research studies, it will not be feasible to contact these adults. Some collections of pediatric samples/data may have personal information removed, making contact impossible. Other collections simply will not have current contact information for all participants. In this study, 26% would find it unacceptable for researchers to use their sample/data if they could not be located to ask for consent.
The potential scientific and social value of many ongoing analyses of samples and data collected from children might, in at least some cases, justify doing such research without consent when they cannot be located or do not respond to a request for consent to continued use. There are many large sample and datasets, from clinical trials for human immunodeficiency virus, cancer, and cystic fibrosis and from cross-sectional epidemiology studies that have been developed with the contributions of many children and with the investment of significant time and resources thatmay continue tobear fruitful knowledge for years to come.27-30 It is likely that many of these children cannot be located now, and a requirement to get affirmative consent from them as adults could significantly limit the scientific usefulness of these samples and datasets.
Informed consent is not an absolute requirement for all research with stored samples and data. One common solution for ongoing research is to eliminate all individual identifiers. When the samples/data are de-identified, then further research poses no appreciable risk to the sample/data sources and the use of de-identified samples/data may not constitute human subjects research under federal regulations.13 This approach eliminates the regulatory need for, and feasibility of, informed consent for all subsequent uses of the tissues/data.
However, de-identification may not be an important distinction to participants, considering that, in a separate report from this study, only 16% considered the distinction to be ethically relevant to their attitudes about wanting to know about ongoing research with their data.26 Most respondents wanted to know about ongoing research in both cases, and <10% of respondents did not want to know in either case. De-identification may also be scientifically undesirable because it becomes impossible to update clinical information at a later time. It may also be insufficient to address residual concerns about risk to population groups posed by the research when group identities, like race or Native American tribe, are retained with the samples/data.31
Even when identifiers are maintained, the regulatory requirement for informed consent can be waived in minimal risk human subjects research when the waiver will not “adversely affect the rights and welfare of the subjects” and when it is not practicable to get informed consent.32 Other criteria, such as evaluating the social value and scientific validity of the research, minimizing risks (eg, to confidentiality), and obtaining independent review (eg, by an institutional review board), are essential in the conduct of ethical research.33 When these criteria have been satisfied, and investigators cannot locate adult individuals to obtain their consent for ongoing use of these samples/data, waiving the requirements to obtain informed consent from those individuals is ethically reasonable We believe that in many contexts, this continuing research can be considered “minimal risk.” We appreciate that although such determinations of risk levels in pediatrics have both normative and empirical dimensions and remain contested,34 the likelihood of even moderate harms appears to be sufficiently remote to consider many projects with stored tissues to be minimal risk.
However, even when an institutional review board decides affirmative consent is not required (ie, can be waived), it may still be ethically preferable to attempt to obtain consent from subjects who can be located. Attempting to reach participants and honoring the dissent of those who do not want further research done with their data/samples acknowledges their interest in decision-making and is respectful of their wishes. This may be not feasible for those studies that lack the funding and administrative support to keep track of children years after the initial participation.
Public engagement through focus groups, community meetings, or other forms of dialogue provides an alternative mechanism to discuss the processes and policies associated with using pediatric samples/data, including the potential risks and benefits of such research. These efforts might increase the public's comfort with the use of pediatric samples for ongoing research and engage those who have concerns. Such dialogue might also help researchers identify any particular populations, conditions, or other circumstances for which explicit consent from adults would be an absolute requirement for ongoing use of their pediatric samples/data.
To achieve a meaningful level of engagement, it is important to know the characteristics of those within the general public who might be concerned with the use of pediatric samples/data and what kinds of concerns are most pertinent. For example, we found that patients who were less trusting of medical researchers, were more private, or were African-American were more concerned. Our study does not reveal the deeper reasons about why some participants would not give consent if asked, and why many participants would not want samples to be used if they could not be located. However, by having more in-depth discussions with the public through this kind of engagement, researchers may be able to better address their concerns.
It is important to acknowledge the financial, organizational, and methodological challenges raised by this recommendation for public engagement. Funds specifically appropriated to public engagement activities would be necessary. It may be useful to partner with community-based organizations with experience developing and implementing these types of engagement activities. There will also be challenges to better define and identify who within communities should be engaged, and how to assess that engagement, once it is carried out.
This study has a number of limitations. Our sample was not representative of the US adult population, but was derived from people who seek medical care at academic medical centers. As a group, the respondents were disproportionately female, better educated, more affluent, and better informed about genetic issues than the general population. However, because many of the currently proposed pediatric biobanks are also based at academic medical centers, the views of this sample are likely to be relevant. A second limitation is that the scenario was hypothetical and may not represent individuals’ responses to actual experiences. The scenario did not contain detailed information about the kinds of studies their sample would be used for, the potential for residual identifiability of samples, or the potential risks and benefits of the research, all which could affect their attitudes. The questions were posed in the context of a larger set of questions that focused on genetic research, and these may also have influenced some respondent's attitudes about use of their samples. Finally, these were responses to closed-ended questions, and it is possible that the concerns expressed would be attenuated in the process of an engaged discussion about this topic.
When adults can not be located to provide consent, it is generally ethically acceptable to continue to use identified samples after children have grown up. These questions addressed in this paper are relevant in similar contexts, such as the use of residual newborn screening bloodspots for research.35 Although there is an important distinction between samples that are initially used for research with parental permission and those newborn screening samples that are typically collected in most states without parental permission, such screening programs will face similar issues with the continued use of those samples that may shed light on the underlying issues for pediatric biobanks. Further research would be useful to understand how children (and eventually adults) who are actually enrolled in pediatric biobanks view these issues, and how willing they are to provide consent for ongoing use of samples/data when contacted as adults.
Acknowledgments
We thank Richard R. Sharp, Mark Brown, Mark Hughes, Debra Schwinn, Pamela Sankar, Jeremy Sugarman, Dragana Bolcic-Jankovic, and Brian R. Clarridge for their participation in the development and recruitment for the Patient Attitudes about Genetics Research Survey, (supported by intramural research funds from the NHGRI). We thank Lainie Ross for comments on a previous version of this manuscript.
The study was supported by National Human Genome Research Institute (S.H and B.W.). J.B. received support for the recruitment of subjects. A. G. was supported by the National Institutes of Health (IH P50HG003390, The Center for Genetic Research Ethics and Law). No statement in this article should be construed as an official position of the National Human Genome Research Institute, National Institutes of Health, or Department of Health and Human Services.
Appendix
CONFIDENTIALITY STATEMENT: Before we begin, there are a couple of important things I need to go over with you. Your participation in this interview is, of course, voluntary. If you decide not to participate, it will not affect you in any way.
Your answers will be kept confidential. The information from this study will not be presented or published in any way that would allow the identification of any respondent. Your answers will be combined with the answers of other people for statistical analysis.
It is important that your answers be accurate. Take your time and be sure to ask me if you are not sure what a question means or what kind of answer is wanted. It is very important that you answer as honestly and as accurately as you can. If there is any question you would rather not answer, just tell me and I will skip it.
Do you have any questions about who is doing the study or anything else pertaining to the study? May we proceed with the interview?
A1. I would like to start by asking about your family's health.
Are you aware of any diseases that run in your family?
1. YES
5. NO [skip to A2]
7. DK [skip to A2]
9. NA [skip to A2]
A1b. Do you have any of these diseases yourself?
1. YES
5. NO [skip to A2]
7. DK [skip to A2]
9. NA [skip to A2]
A1c. Which do you have?
A2. In recent years, researchers have learned that diseases that run in families tend to be passed from generation to generation through genes. For the rest of this interview we will be asking for your views on research that connects a person's genes with the diseases he or she might get.
How much have you heard about health related genetic research: would you say you have heard a lot about it, heard a little about it, or heard nothing about it?
1. HEARD A LOT ABOUT IT
2. HEARD A LITTLE ABOUT IT
3. HEARD NOTHING ABOUT IT [skip to A4]
9. NA [skip to A4]
A3. From what you have heard and read, how positive or negative do you feel about recent efforts made by genetic researchers to learn how diseases are connected to genes, would you say you feel very positive, somewhat positive, neither positive nor negative, somewhat negative, or very negative?
1. VERY POSITIVE
2. SOMEWHAT POSITIVE
3. NEITHER POSITIVE NOR NEGATIVE
4. SOMEWHAT NEGATIVE
5. VERY NEGATIVE
9. NA
A4. If you were asked today to give a blood sample and information from your medical records to a genetic researcher studying diseases, how willing do you think you would be to do so: would you say very willing, moderately willing, somewhat hesitant, very hesitant or completely unwilling?
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
5. COMPLETELY UNWILLING [skip to B1]
6 DEPENDS
7. DK
9. NA
A5. Can you tell me the number one thing you would want to know before giving a blood sample for genetic research?
1. WHAT DISEASE IS BEING STUDIED?
2. WHO IS DOING THE RESEARCH?
3. WILL I GET THE RESULTS?
4. WHAT ARE THE RISKS?
5. CONFIDENTIALITY ISSUE
6. OTHER
9. NA
Anything else?
1. WHAT DISEASE IS BEING STUDIED?
2. WHO IS DOING THE RESEARCH?
3. WILL I GET THE RESULTS?
4. WHAT ARE THE RISKS?
5. CONFIDENTIALITY ISSUE
6. OTHER
9. NA
A6a. Suppose for a minute you have made some of your blood available for research. I am going to read a short list of research topics. For each topic I mention, please tell me how willing you would be to have your blood used in genetic research for that topic.
For example, how willing would you be to have your blood used to study cancer? Would you say very willing, moderately willing, somewhat hesitant, or very hesitant?
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
9. NA
A6b. What about heart disease?
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
9. NA
A6c. What about depression?
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
9. NA
A6d. What about alcoholism?
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
9. NA
A6e. What about acne? (IF DEFINITION NEEDED: NON-LIFE THREATENING SKIN DISEASE)
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
9. NA
A6f. What about schizophrenia? (IF DEFINITION NEEDED: MENTAL ILLNESS)
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
9. NA
A6g. What about genetic research on why only some people have side effects from certain medicines?
(IF NEEDED: SIDE EFFECTS FROM MEDICINES)
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
9. NA
A6h. What about genetic research on why only some people become sick from things in the air, water, or their work environment?
(IF NEEDED: ENVIRONMENTALLY CAUSED ILLNESSES)
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
9. NA
A7. Can you say anything about why you are somewhat less willing to have your blood used for research on than you are for the other(s)?
B1. Now I would like to shift topics and ask about your personal experience giving blood. Have you ever had blood drawn from your arm with a needle?
1. YES
5. NO [skip to B4]
9. NA [skip to B4]
B2. On a zero to 10 scale, with zero being not bothersome at all and 10 being extremely bothersome, how bothersome was it to have blood drawn with a needle from your arm?
0-10.
99. NA
B3. Have you ever given blood for any kind of medical research study?
1. YES
5. NO
7. DK
9. NA
B4. [if A4 is not equal to 5 and B1 is equal to 1 skip to introC1a] Is there any way you can think of that you could be persuaded to allow your blood to be used in genetic research?
1. YES
5. NO [skip to D1]
9. NA [skip to D1]
introC1a. For the next questions we want you to suppose that a medical researcher wants to do genetic research with some blood of yours leftover from a doctor visit, together with some information from your medical records.
We will be asking about two different situations.
C1a. In the first situation, suppose that your name is removed from both the blood sample and from the information from your medical records so you cannot be identified by any of the researchers or anyone else. In this situation, how important would it be for you to know that genetic research is being done with your leftover blood? For you, would it be very important to know that research is being done, moderately important, not very important, or not important at all?
1. VERY IMPORTANT
2. MODERATELY IMPORTANT
3. NOT VERY IMPORTANT [skip to C1d]
4. NOT IMPORTANT AT ALL [skip to C1d]
9. NA [skip to C1d]
C1b. Since the research we are talking about is being done without anyone's knowledge that you were the one who gave the blood, can you say more about why it is important that you be told about the research?
C1c. There is a difference between being told about the genetic research that is being done using your blood, and being asked permission for its use by the researchers ahead of time. Still thinking about genetic research with names removed, should researchers be required to get your permission before they use your leftover blood, or would it be enough for you that they notify you by phone or mail that they are going to use it with your name removed?
1. GET PERMISSION
5. NOTIFICATION ONLY
9. NA
C1d. If genetic researchers actually asked for your permission today, do you think you would or do you think you would not allow them to use, with your name removed, a sample of your blood together with some information from your medical records?
1. WOULD [skip to introC2a]
5. WOULD NOT
6. DEPENDS
7. DK
9. NA
C1e. What is the main reason you might be hesitant to have your leftover blood, with your name removed, used in genetic research?
introC2a. Now I want you to consider the second situation. Suppose the researchers need to be able to identify the leftover blood sample as your blood and they also need some more detailed information from your medical records in order to do the research. To protect your confidentiality, your name will be replaced with a unique identification number that could be traced back to you and your medical records, if the researcher needs to do so.
C2a. In this situation, how important to you would it be for someone to tell you that genetic research is being done with your leftover blood and medical record information?
Would you say very important, moderately important, not very important or not important at all?
1. VERY IMPORTANT
2. MODERATELY IMPORTANT
3. NOT VERY IMPORTANT [skip to C2c]
4. NOT IMPORTANT AT ALL [skip to C2c]
9. NA [skip to C2c]
C2b. [if C1a is less than or equal to 2 skip to C2c] Can you say more about why you feel it is important that you be told about the research in this situation?
C2c. Suppose that the researchers have already pledged in a legally binding way to both the hospital and federal authorities that they will carefully protect participants’ names and personal information. In this situation, would you want researchers to be required to get your permission before they use your leftover blood and your medical records, or would simple notification by mail or phone that they are using your blood be enough for you?
1. GET PERMISSION
5. NOTIFICATION ONLY [skip to introC3a]
9. NA [skip to introC3a]
C2d. In this situation, where researchers would be using your leftover blood and information from your medical records without your name but with an identification number attached, do you think you would or do you think you would not participate if you were asked today?
1. WOULD [skip to introC3a]
5. WOULD NOT
6. DEPENDS
7. DK
9. NA [skip to introC3a]
C2e. What would be the main reason you might be hesitant to have your leftover blood sample with an ID number together with your medical records used in genetic research?
C2f. Is there any way you can think of that you could be persuaded to allow both your medical records and leftover blood sample with an ID number to be used in genetic research?
1. YES
5. NO [skip to D1]
9. NA [skip to D1]
C2g. What would need to be done to persuade you?
introC3a. I would like to shift the discussion a bit and have you now assume that any reservations you may have had about providing a sample have been successfully dealt with. That is, you have been convinced the research is worthy and whatever safeguards you need are now in place.
C3a. People who willingly provide a blood sample for genetic research, do so for a variety of reasons. Some of the reasons have to do with learning something about their own health, some have to do with getting information about diseases that may help their families at some point in the future, and some have to do with helping people in general. As you think about these different reasons how much of your willingness to provide a blood sample for genetics research would you say comes from your interest in finding out something about your own health?
Would you say a big part, a moderate part, a small part or no part at all?
1. A BIG PART
2. A MODERATE PART
3. A SMALL PART
4. NO PART AT ALL
9. NA
C3b. How much of your willingness to provide a blood sample for genetics research would you say comes from your interest in helping researchers learn about diseases that might affect your family? Would you say a big part, a moderate part, a small part or no part at all?
1. A BIG PART
2. A MODERATE PART
3. A SMALL PART
4. NO PART AT ALL
9. NA
C3c. How much of your willingness to provide a blood sample for genetics research would you say comes from your interest in helping people in general? Would you say a big part, a moderate part, a small part or no part at all?
1. A BIG PART
2. A MODERATE PART
3. A SMALL PART
4. NO PART AT ALL
9. NA
C3d. If the researcher told you that, for his study, he cannot give you back the specific results for yourself, do you think you would still be willing to give a blood sample for the sole purpose of helping people in general?
1. YES
5. NO
9. NA
C4. Typically, researchers doing genetic research work very hard to make sure that what they learn about an individual is not revealed to anyone without first getting permission. However, people are sometimes reluctant to give a blood sample to researchers for fear that the research results could be given to someone who shouldn't have them.
In general, how concerned are you that researchers might allow the genetic research results for you to be given to someone who shouldn't have them, would you say very concerned, moderately concerned, not very concerned or not at all concerned?
1. VERY CONCERNED
2. MODERATELY CONCERNED
3. NOT VERY CONCERNED
4. NOT AT ALL CONCERNED
9. NA
C5. Often a person's perception of this problem depends on “to whom the genetic research results are given.” If the results from genetic research on your leftover blood were given to your doctor without your permission would it be a big problem, a moderate problem, small problem, or no problem at all?
1. A BIG PROBLEM
2. MODERATE PROBLEM
3. SMALL PROBLEM
4. NO PROBLEM AT ALL
6. DEPENDS
9. NA
C6. If your results were given to a close family member, without your permission would it be a big problem, a moderate problem, small problem, or no problem at all?
1. A BIG PROBLEM
2. MODERATE PROBLEM
3. SMALL PROBLEM
4. NO PROBLEM AT ALL
6. DEPENDS
9. NA
C7. If your results were given to your employer without your permission would it be a big problem, a moderate problem, small problem, or no problem at all?
1. A BIG PROBLEM
2. MODERATE PROBLEM
3. SMALL PROBLEM
4. NO PROBLEM AT ALL
6. DEPENDS
9. NA
C8. If your results for you were given to your health insurer or HMO without your permission would it be a big problem, a moderate problem, small problem, or no problem at all?
1. A BIG PROBLEM
2. MODERATE PROBLEM
3. SMALL PROBLEM
4. NO PROBLEM AT ALL
6. DEPENDS
9. NA
C9. Do you think the possiblity of your results being given to someone without your permission would ever cause you to withhold your blood sample from a researcher?
1. YES
5. NO
9. NA
C10. How much do you trust the doctor you currently see for your health care to keep personally sensitive medical information confidential, would you say completely, somewhat, a little, or not at all?
(IF THEY HAVE MORE THAN ONE DOCTOR ASK FOR THE MAIN DOCTOR)
1. COMPLETELY
2. SOMEWHAT
3. A LITTLE
4. NOT AT ALL
6. DEPENDS
7. DK
9. NA
C11. How much do you trust the doctor you currently see for your health care to put your well being above all other considerations when treating your medical problems? Would you say completely, somewhat, a little, or not at all?
1. COMPLETELY
2. SOMEWHAT
3. A LITTLE
4. NOT AT ALL
6. DEPENDS
7. DK
9. NA
C12. Do you have one main doctor you go to for most of your health care?
1. YES
5. NO [skip to C14]
9. NA [skip to C14]
C13. Overall, how much would you say you trust that person? Would you say completely, somewhat, a little, or not at all?
1. COMPLETELY
2. SOMEWHAT
3. A LITTLE
4. NOT AT ALL
6. DEPENDS
7. DK
9. NA
C14. How much do you trust medical researchers to keep confidential personally sensitive medical information about the people in their studies?
1. COMPLETELY
2. SOMEWHAT
3. A LITTLE
4. NOT AT ALL
6. DEPENDS
7. DK
9. NA
C15. How much do you trust medical researchers to put their subjects well being above all other considerations as they do their research?
1. COMPLETELY
2. SOMEWHAT
3. A LITTLE
4. NOT AT ALL
6. DEPENDS
7. DK
9. NA
C16. So, overall, how much would you say you trust medical researchers?
1. COMPLETELY
2. SOMEWHAT
3. A LITTLE
4. NOT AT ALL
6. DEPENDS
7. DK
9. NA
C17a. Sometimes who is paying for the research makes a difference in a person's willingness to provide a sample.
If you were given the choice of having your blood sample used for a study paid for by a government agency, such as the National Institutes of Health, a study paid for by a private sector drug company such as Merck or Pfizer, or a study paid for by a consumer advocacy group, such as the American Heart Association, which would you pick?
1. THE GOVERNMENT
2. PRIVATE INDUSTRY
3. A CONSUMER ADVOCACY GROUP
6. DEPENDS
9. NA
C17b. To which of these groups would you least want to give your blood sample?
1. COMPLETELY
2. SOMEWHAT
3. A LITTLE
4. NOT AT ALL
6. DEPENDS
7. DK
9. NA
C17c. How much of your willingness to give a blood sample would you say depends on who is paying for the research—a big part, a moderate part, a small part, or no part at all?
1. A BIG PART
2. A MODERATE PART
3. A SMALL PART
4. NO PART AT ALL [skip to C18a]
9. NA [skip to C18a]
C17d. Can you say more about why it matters to you who is paying for the research?
C18a. Sometimes, no matter where a researcher is working, he might make a lot of extra money from selling a patent based on research findings.
Would it make a difference in your willingness to give a blood sample if the researcher using it were going to make a lot of money from patents related to the research using your sample?
1. YES
5. NO [skip to C18c]
9. NA [skip to C18c]
C18b. Can you say more about how the thought of researchers making money from patents affects your willingness to give a blood sample?
C18c. Do you think researchers are likely to change their interpretation of the research findings based on whether they can make a lot of money from the research?
1. YES
5. NO
9. NA
C18d. Do you think that researchers are more likely to be more careless about confidentiality based on whether or not they can make a lot of money from the research?
1. YES
5. NO
9. NA
C19a. So far in this interview, we have assumed that researchers collect blood samples at the time they do their research. However, there are some researchers collecting blood samples now to be used in research later. The idea is to collect samples that may be stored a long time and may be used in research about different diseases.
Once you have given a blood sample, are you willing to have the researcher study a variety of different diseases with it over time, or would you prefer it to be used only for the original disease that your blood was collected to study?
1. DIFFERENT DISEASES
5. ONLY ORIGINAL DISEASE [skip to C19c]
9. NA [skip to C19c]
C19b. Do you think you would be willing to sign a one-time release to allow a variety of research to be done with your blood in the future, or would you require researchers to get a new permission each time a different disease was going to be studied?
1. ONE TIME RELEASE
5. NEW PERMISSION EACH TIME
9. NA
C19c. Would it be okay for different researchers to use your blood to research the original disease that your blood was collected to study, or would you prefer that only the original researchers use it?
1. DIFFERENT RESEARCHERS [skip to C20a]
5. ONLY ORIGINAL RESEARCHER
6. DEPENDS
9. NA [skip to C20a]
C19d. What concerns, if any, would you have about your blood being used by more than one researcher?
C20a. Genetic research may help us learn that certain diseases vary by ethnic or racial background. Thus, it is possible that research could one day help groups of people who are defined by ethnic or racial characteristics.
How much of your willingness to provide a blood sample for genetic research would you say comes from your interest in helping researchers learn about diseases that might affect people of the same race or ethnicity as you? Would you say a big part, a moderate part, a small part, or no part at all?
1. A BIG PART
2. A MODERATE PART
3. A SMALL PART
4. NO PART AT ALL
9. NA
C20b. Some people worry that the research results could be used to discriminate against selected groups. Is this something you have thought about?
1. YES
5. NO
9. NA
C20c. How seriously do you take the concerns expressed by others about research information being used to discriminate against people by race or ethnicity? Would you say very seriously, moderately seriously, not very seriously or not seriously at all?
1. VERY SERIOUSLY
2. MODERATELY SERIOUSLY
3. NOT VERY SERIOUSLY
4. NOT SERIOUSLY AT ALL [skip to D1]
9. NA [skip to D1]
C20d. By how much, if any, is your willingness to provide blood samples for research reduced by these concerns, a big part, a moderate part, small part or no part at all?
1. A BIG PART
2. A MODERATE PART
3. A SMALL PART
4. NO PART AT ALL
9. NA
D1. Now I'd like you to suppose that when you were an infant, your parents gave their permission for a blood sample of yours to be used in research on children's health. Your doctor collected samples from hundreds of infants this way. Since then, your blood sample has been stored in a freezer along with a unique identification number and some background medical information about you. Several decades have passed and all of the infants whose blood samples were collected are adults. The researcher now wishes to continue to use your sample for research.
How concerned would you be that research had been done with your parent's permission on your childhood blood sample? Would you say very concerned, moderately concerned, not very concerned, or not concerned at all?
1. VERY CONCERNED
2. MODERATELY CONCERNED
3. NOT VERY CONCERNED
4. NOT CONCERNED AT ALL
9. NA
D1b. Should the researcher have to get your permission now to continue to use your sample?
1. YES
5. NO [skip to E1]
7. DK [skip to E1]
9. NA [skip to E1]
D1c. How willing would you be to give your permission for a researcher to continue to use your sample? Would you say very willing, moderately willing, somewhat hesitant, very hesitant or completely unwilling?
1. VERY WILLING
2. MODERATELY WILLING
3. SOMEWHAT HESITANT
4. VERY HESITANT
5. COMPLETELY UNWILLING [skip to E1]
6. DEPENDS
7. DK
9. NA
D1d. Suppose that the researcher could not locate you. Would it be acceptable to you for the researcher to use your sample anyway?
1. YES
5. NO
9. NA
E1. Now I would like to ask a few questions for background.
In general, would you say that your current health is:
1. EXCELLENT
2. VERY GOOD
3. GOOD
4. FAIR
5. POOR
9. NA
E2. Do you have any serious or chronic medical conditions?
1. YES
5. NO [skip to E4]
9. NA [skip to E4]
E3. What condition(s)?
E4. Have you ever had surgery, as an adult? 1. YES 5. NO
9. NA
E5. [if B3 is equal to 1 skip to E6] Have you ever participated in any kind of medical research study?
1. YES
5. NO
7. DK
9. NA
E6. How private a person would you say you are in general? Would you say you are a very private person, a private person, neither private nor open, an open person or a very open person?
1. A VERY PRIVATE PERSON
2. A PRIVATE PERSON
3. NEITHER PRIVATE NOR OPEN
4. AN OPEN PERSON
5. A VERY OPEN PERSON
9. NA
E7. How private would you say you are about your medical information? Would you say you are a very private person, a private person, neither private nor open, an open person or a very open person?
1. A VERY PRIVATE PERSON
2. A PRIVATE PERSON
3. NEITHER PRIVATE NOR OPEN
4. AN OPEN PERSON
5. A VERY OPEN PERSON
9. NA
E8. Is respondent male or female?
1. MALE
5. FEMALE
9. NA
E9. What is your current age in years? 1-100. YEARS OLD
E10. What is the highest level of schooling you have completed-?
1. LESS THAN HIGH SCHOOL
2. HIGH SCHOOL GRADUATE/GED
3. SOME COLLEGE
4. COLLEGE GRADUATE
5. SOME GRADUATE SCHOOL
6. GRADUATE DEGREE
9. NA
E11. Are you Hispanic or Latino?
E12. [if E11 is equal to 1](In addition to being Hispanic or Latino) Which of the following racial categories fits you best?
1. BLACK OR AFRICAN AMERICAN
2. ASIAN
3. WHITE OR CAUCASIAN
4. AMERICAN INDIAN OR ALASKA NATIVE
5. NATIVE HAWAIIAN OR OTHER PACIFIC ISLANDER
6. OTHER
E13. What is your religious affiliation, if any?
1. JEWISH
2. CATHOLIC
3. PROTESTANT
4. MUSLIM
5. MORMON
6. OTHER
9. NA
E14. Would you classify yourself as:
1. NOT RELIGIOUS
2. SOMEWHAT RELIGIOUS
3. VERY RELIGIOUS
4. OTHER
9. NA
E15. Approximately what was your household's total income last year before taxes?
1. LESS THAN $5K
2. $5K-$19 999
3. $20K-$39 999
4. $40K-$59 999
5. $60K-$79 999
6. $80K OR MORE
9. NA
E16. Were there any questions in this interview that touched on topics you hadn't thought about before?
1. YES
5. NO [skip to E18]
9. NA [skip to E18]
E17. Among the topics you hadn't thought about before, which comes to mind first? (IF CLARIFICATION NEEDED: WHAT IS THE MOST IMPORTANT)
E18. Do you think your willingness to provide blood samples for genetic research has changed as a result of participating in this interview?
1. YES
5. NO [skip to pay]
9. NA [skip to pay]
E19. Would you say your willingness to provide a blood sample has increased or decreased?
1. INCREASED [skip to pay]
5. DECREASED
9. NA [skip to pay]
E20. Would you say your willingness to provide a blood sample has decreased a little or a lot?
1. A LITTLE
5. A LOT
9. NA
Thank you for taking the time to answer my questions.
To what address would you like us to send your honorarium of $25?
Footnotes
The authors declare no conflicts of interest.
References
- 1.Kaiser J. Genetics. US hospital launches large biobank of children's DNA. Science. 2006;312:1584–5. doi: 10.1126/science.312.5780.1584a. [DOI] [PubMed] [Google Scholar]
- 2.Winickoff DE, Winickoff RN. The charitable trust as a model for genomic biobanks. N Engl J Med. 2003;349:1180–4. doi: 10.1056/NEJMsb030036. [DOI] [PubMed] [Google Scholar]
- 3.Geschwind DH, Sowinski J, Lord C, Iversen P, Shestack J, Jones P, et al. The autism genetic resource exchange: a resource for the study of autism and related neuropsychiatric conditions. Am J Hum Genet. 2001;69:463–6. doi: 10.1086/321292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terry SF, Terry PF, Rauen KA, Uitto J, Bercovitch LG. Advocacy groups as research organizations: the PXE International example. Nat Rev Genet. 2007;8:157–64. doi: 10.1038/nrg1991. [DOI] [PubMed] [Google Scholar]
- 5.Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, et al. Genetic modifiers of lung disease in cystic fibrosis. N Engl J Med. 2005;353:1443–53. doi: 10.1056/NEJMoa051469. [DOI] [PubMed] [Google Scholar]
- 6.National Children's Study (homepage on the Internet) The National Children's Study; Bethesda: [Oct 9, 2008]. pp. c2007–2008. (updated Oct 3, 2008). Available at: http://www.nationalchildrensstudy.gov/. [Google Scholar]
- 7.Cambon-Thomsen A. The social and ethical issues of post-genomic human biobanks. Nat Rev Genet. 2004;5:866–73. doi: 10.1038/nrg1473. [DOI] [PubMed] [Google Scholar]
- 8.Caulfield T. Tissue banking, patient rights, and confidentiality: tensions in law and policy. Med Law. 2004;23:39–49. [PubMed] [Google Scholar]
- 9.Clayton EW. Informed consent and biobanks. J Law Med Ethics. 2005;33:15–21. doi: 10.1111/j.1748-720x.2005.tb00206.x. [DOI] [PubMed] [Google Scholar]
- 10.Clayton EW, Steinberg KK, Khoury MJ, Thomson E, Andrews L, Kahn MJ, et al. Informed consent for genetic research on stored tissue samples. JAMA. 1995;274:1786–92. [PubMed] [Google Scholar]
- 11.Meslin EM, Quaid KA. Ethical issues in the collection, storage, and research use of human biological materials. J Lab Clin Med. 2004;144:229–34. doi: 10.1016/j.lab.2004.08.003. discussion 6. [DOI] [PubMed] [Google Scholar]
- 12.Diekema DS. Conducting ethical research in pediatrics: a brief historical overview and review of pediatric regulations. J Pediatr. 2006;149:S3–11. doi: 10.1016/j.jpeds.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 13.US Department of Health and Human Services Code of Federal Regulations. 45 CFR 46, subpart A. [Google Scholar]
- 14.Informed consent, parental permission, and assent in pediatric practice. Committee on Bioethics, American Academy of Pediatrics. Pediatrics. 1995;95:314–317. [PubMed] [Google Scholar]
- 15.Wendler DS. Assent in paediatric research: theoretical and practical considerations. J Med Ethics. 2006;32:229–234. doi: 10.1136/jme.2004.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merlo DF, Knudsen LE, Matusiewicz K, Niebroj L, Vahakangas KH. Ethics in studies on children and environmental health. J Med Ethics. 2007;33:408–13. doi: 10.1136/jme.2006.016212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kettis-Lindblad A, Ring L, Viberth E, Hansson MG. Genetic research and donation of tissue samples to biobanks. What do potential sample donors in the Swedish general public think? Eur J Public Health. 2006;16:433–40. doi: 10.1093/eurpub/cki198. [DOI] [PubMed] [Google Scholar]
- 18.Wang SS, Fridinger F, Sheedy KM, Khoury MJ. Public attitudes regarding the donation and storage of blood specimens for genetic research. Community Genet. 2001;4:18–26. doi: 10.1159/000051152. [DOI] [PubMed] [Google Scholar]
- 19.Wendler D, Emanuel E. The debate over research on stored biological samples: what do sources think? Arch Intern Med. 2002;162:1457–62. doi: 10.1001/archinte.162.13.1457. [DOI] [PubMed] [Google Scholar]
- 20.Hoeyer K, Olofsson BO, Mjorndal T, Lynoe N. Informed consent and biobanks: a population-based study of attitudes towards tissue donation for genetic research. Scand J Public Health. 2004;32:224–9. doi: 10.1080/14034940310019506. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz MD, Rothenberg K, Joseph L, Benkendorf J, Lerman C. Consent to the use of stored DNA for genetics research: a survey of attitudes in the Jewish population. Am J Med Genet. 2001;98:336–42. doi: 10.1002/1096-8628(20010201)98:4<336::aid-ajmg1100>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.McQuillan GM, Pan Q, Porter KS. Consent for genetic research in a general population: an update on the National Health and Nutrition Examination Survey experience. Genet Med. 2006;8:354–60. doi: 10.1097/01.gim.0000223552.70393.08. [DOI] [PubMed] [Google Scholar]
- 23.Genetic Testing and DNA Biobanks—For Whom, and When? CS Mott Children's Hospital National Poll on Children's Health. 2007;1:1–2. [Google Scholar]
- 24.Neidich AB, Joseph JW, Ober C, Ross LF. Empirical data about women's attitudes towards a hypothetical pediatric biobank. Am J Med Genet A. 2008;146:297–304. doi: 10.1002/ajmg.a.32145. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman D, Geller G, Leroy L, Murphy J, Scott J, Hudson K. Ethical implications of including children in a large biobank for genetic-epidemiologic research: a qualitative study of public opinion. Am J Med Genet. 2008;148C:31–9. doi: 10.1002/ajmg.c.30159. [DOI] [PubMed] [Google Scholar]
- 26.Hull SC, Sharp RR, Botkin JR, Brown M, Hughes M, Sugarman J, et al. Patients’ views on identifiability of samples and informed consent for genetic research. Am J Bioeth. 2008;8:62–70. doi: 10.1080/15265160802478404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38:229–39. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 28.Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, et al. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21:619–25. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stiller CA. Epidemiology and genetics of childhood cancer. Oncogene. 2004;23:6429–44. doi: 10.1038/sj.onc.1207717. [DOI] [PubMed] [Google Scholar]
- 30.Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson Children's Respiratory Study: 1980 to present. J Allergy Clin Immunol. 2003;111:661–75. doi: 10.1067/mai.2003.162. [DOI] [PubMed] [Google Scholar]
- 31.Hausman DM. Group risks, risks to groups, and group engagement in genetics research. Kennedy Institute of Ethics journal. 2007;17:351–69. doi: 10.1353/ken.2008.0009. [DOI] [PubMed] [Google Scholar]
- 32.US Department of Health and Human Services Code of Federal Regulations. 45 CFR 46, subpart D. [Google Scholar]
- 33.Emanuel EJ, Wendler D, Grady C. What makes clinical research ethical? JAMA. 2000;283:2701–11. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 34.Wendler D, Varma S. Minimal risk in pediatric research. J Pediatr. 2006;149:855–61. doi: 10.1016/j.jpeds.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 35.Olney RS, Moore CA, Ojodu JA, Lindegren ML, Hannon WH. Storage and use of residual dried blood spots from state newborn screening programs. J Pediatr. 2006;148:618–22. doi: 10.1016/j.jpeds.2005.12.053. [DOI] [PubMed] [Google Scholar]