Abstract
Investigations on the therapeutic effects of intravenous immunoglobulin (IVIG) have focused on the suppression of autoantibody- and immune complex-mediated inflammatory pathogenesis. Inflammatory diseases such as rheumatoid arthritis are often accompanied by excessive bone erosion but the effect of IVIG on osteoclasts, bone-resorbing cells, has not been studied. Here, we investigate whether IVIG directly regulates osteoclast differentiation and has therapeutic potential for suppressing osteoclast-mediated pathologic bone resorption. IVIG or cross-linking of Fcγ receptors with plate-bound IgG suppressed receptor activator of nuclear factor-kappa B ligand (RANKL)-induced osteoclastogenesis and expression of osteoclast-related genes such as integrin β3 and cathepsin K in a dose-dependent manner. Mechanistically, IVIG or plate-bound IgG suppressed osteoclastogenesis by downregulating RANKL-induced expression of NFATC1, the master regulator of osteoclastogenesis. IVIG suppressed NFATC1 expression by attenuating RANKL-induced NF-κB signaling, explained in part by induction of the inflammatory signaling inhibitor A20. IVIG administration attenuated in vivo osteoclastogenesis and suppressed bone resorption in the tumor necrosis factor (TNF)-induced calvarial osteolysis model. Our findings show that, in addition to suppressing inflammation, IVIG directly inhibits osteoclastogenesis through a mechanism involving suppression of RANK signaling. Direct suppression of osteoclast differentiation may provide beneficial effects on preserving bone mass when IVIG is used to treat rheumatic disorders.
Keywords: Osteoclasts, IVIG, A20
Introduction
Intravenous immunoglobulin (IVIG) contains pooled immunoglobulins from plasma of thousands of healthy donors, and IVIG therapy has been effective in a variety of autoimmune and chronic inflammatory diseases (Kazatchkine and Kaveri, 2001; Schwab and Nimmerjahn, 2013). IVIG delivers its signals via various receptors including Fc gamma receptors (FcγR), which bind to the Fc portion of immunoglobulin G, and CD209 (also known as DC-SIGN, Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin) (Anthony et al., 2011). The mechanisms of anti-inflammatory function of IVIG are complex, and a number of possible mechanisms have been described (Kazatchkine and Kaveri, 2001; Schwab and Nimmerjahn, 2013). However, the exact mechanisms for the immunomodulatory and anti-inflammatory effects of IVIG therapy have not been fully elucidated.
IVIG therapy ameliorates symptoms in rheumatoid arthritis patients (Muscat et al., 1995) and protects mice from developing inflammatory arthritis (Campbell et al., 2014; Lee et al., 2014). IVIG consists predominantly of monomeric IgG, but contains a small fraction of polymeric IgG (immune complexes), both of which are important for anti-inflammatory and immunomodulatory effects in various diseases (Nimmerjahn and Ravetch, 2008; Park-Min et al., 2007; Siragam et al., 2006). However, immune complexes can activate innate immune cells and drive inflammation and thus also have pathogenic properties. Immune complexes in which IgG forms a complex with other proteins or nucleic acids have been implicated in autoimmune and other diseases and are formed during infections, tissue injury and various inflammatory conditions (Hoiby et al., 1986; Waldman and Madaio, 2005; Zvaifler, 1973). Chronic immune complex-mediated inflammatory conditions are, in many cases, associated with bone loss and induce uncoupling of osteoclast and osteoblast function, leading to excessive, pathologic bone resorption (Harre et al., 2012; McInnes and Schett, 2011; Novack and Teitelbaum, 2008; Schett and Gravallese, 2012). However, the effect of immune complexes on osteoclastogenesis in inflammatory bone diseases remains controversial. While immune complexes are one of the key inducers in promoting bone resorption during inflammatory responses, immune complexes are capable of suppressing osteoclast differentiation in mouse bone marrow cells (Grevers et al., 2013; Seeling et al., 2013). Fcγ receptors play an important role in immune-complex mediated diseases, especially murine activating receptor FcγRIV, which has a prominent role in the pathogenesis of autoantibody-induced arthritis (Ji et al., 2002; Mancardi et al., 2011). In murine models of inflammatory arthritis, deficiency of activating Fcγ receptors decreases inflammation (Mancardi et al., 2011), but the role of activating Fcγ receptors for bone destruction remains controversial, illustrating the difficulty of developing therapy for targeting Fcγ receptors.
Activating Fcγ receptors had no direct effect on bone destruction in antigen-induced arthritis models (MacLellan et al., 2011; van Lent et al., 2006), but FcγRIV, one of the activating Fcγ receptors, plays a positive role in immune complex-mediated bone destruction in the K/BXN serum-induced arthritis model (Ochi et al., 2007; Seeling et al., 2013). FcγR-deficient mice have normal bone phenotype (Seeling et al., 2013), demonstrating that Fcγ receptors minimally modulate bone homeostasis in physiological conditions. Importantly, humans and mice have a different repertoire of Fcγ receptors and FcγRIV is not expressed in human cells. Therefore, the receptors responsible for IVIG’s action in human cells remain elusive.
Despite extensive studies of the beneficial effects of IVIG on inflammatory diseases, the effects of IVIG on osteoclastogenesis in physiological and pathological conditions are not known. Osteoclasts are multinucleated cells that differentiate from myeloid lineage cells and are responsible for resorbing bone and maintaining bone homeostasis (Lorenzo et al., 2008; Takayanagi, 2007). RANKL (receptor activator of nuclear factor-kappa B ligand), a key inducer of osteoclast differentiation and function, binds to its receptor RANK and activates downstream signaling pathways including NF-κB (Wada et al., 2006). NF-κB is a well-known transcription factor that regulates expression of genes important for cancer, autoimmune diseases, and immune responses (May and Ghosh, 1998; Sen and Baltimore, 1986; Tak and Firestein, 2001). NF-κB pathways play an important role in osteoclast differentiation, function, and survival (Soysa and Alles, 2009), and suppressing NF-κB signaling pathway blocks osteoclastogenesis (Boyce et al., 2010; Novack, 2011; Wada et al., 2006). The activity of NF-κB is tightly regulated by interaction with inhibitory I-κB proteins, multiple negative feedback mechanisms, and regulators including A20 (also known as tumor necrosis factor alpha-induced protein 3, TNFAIP3). In this study, we wished to test whether IVIG directly affects osteoclasts and thus can affect bone resorption independently of its effects on inflammation. We found that IVIG suppressed human osteoclast differentiation in a dose -dependent manner and suppressed pathologic inflammation-induced bone resorption in vivo. In addition, IVIG prevented osteoclast precursor cells (OCPs) from responding to RANKL and expressing NFATc1, a master transcription factor of osteoclastogenesis. Crosslinking of Fcγ receptors with plate-bound IgG also inhibited osteoclastogenesis, suggesting that the suppressive effect of IVIG on human osteoclastogenesis is mediated, in part, by polymeric IgG (immune complexes) contained within IVIG preparations. Mechanistically, IVIG suppressed RANKL-mediated NF-κB activation by inducing A20 expression, resulting in the suppression of RANKL-induced NFATc1 expression. These results identify a new mechanism of action of IVIG with therapeutic implications for suppressing bone resorption in rheumatic diseases.
MATERIALS AND METHODS
Mice and analysis of bone phenotype
Six week-old female C57BL/6 were purchased from The Jackson Laboratory (Bar Harbor, ME). All animal experiments were approved by the Hospital for Special Surgery IACUC. All animals were randomly assigned into experimental groups. For inflammatory osteolysis experiments, we used an established mouse model, the TNF-induced supracalvarial osteolysis model (Kitaura et al., 2005) with minor modifications. In brief, TNF was administered daily at the doses indicated in the figure legends to the calvarial periosteum of mice for four to five consecutive days. The mice were then sacrificed for the collection of calvarial bones for sectioning. For histopathological assessment, mice were euthanized, and calvarial bones were harvested and fixed in 4% paraformaldehyde for 2 days. These samples were then decalcified with 10% neutral buffered EDTA (Sigma-Aldrich) and embedded in paraffin. To assess osteoclastogenesis and bone resorption, sections were stained with TRAP (tartrate-resistant acid phosphatase) and hematoxylin for osteoclast visualization, and histomorphometric analysis was performed with the BioQuant image analysis system (Nashville, TN, USA) using standard procedures (Parfitt et al., 1987). Osteoclasts were identified as TRAP+ cells that were multinucleated and adjacent to bone.
Reagents
Human M-CSF and sRANKL were purchased from Peprotech (Rocky Hill, NJ). The clinically used IVIG preparation Carimune NF® was purchased from Cardinal Health (Dublin, OH). The antibodies used for immunoblotting are as follows: NFATc1 (BD Pharmagen); p38 (Santa Cruz Biotechnology); Lamin B (Abcam); p65, p100/p52, p100/p50, and I-κBα (Cell Signaling).
Cell culture
Peripheral blood mononuclear cells were obtained from blood leukocyte preparations purchased from the New York Blood Center by density gradient centrifugation with Ficoll (Invitrogen, Carlsbad, CA) using a protocol approved by the Hospital for Special Surgery Institutional Review Board. Monocytes were obtained from peripheral blood, using anti-CD14 magnetic beads, as recommended by the manufacturer (Miltenyi Biotec Auburn, CA). Monocyte-derived osteoclast precursors (OCPs) that express RANK were obtained by culture for one day with 20 ng/ml of M-CSF (Peprotech) in α-MEM medium (Invitrogen) supplemented with 10 % fetal bovine serum (FBS, Hyclone), and purity of monocytes was >97%, as verified by flow cytometric analysis. For ligation of Fcγ receptors, plates were coated with IgG by incubating with IVIG at the indicated concentrations for 1 hr at room temperature and washed with phosphate-buffered saline (PBS) before cells were plated. OCPs were harvested and added to IgG-coated plates for 1 hr. Polymyxin B (14 µg/ml; Sigma Aldrich), which was verified to not directly stimulate macrophages but essentially completely blocked exogenous LPS up to concentrations of 10 ng/ml in our system, was used to ensure that contaminating endotoxin did not contribute to the observed effects, as previously described (Wang et al., 2010).
Osteoclast differentiation
Human CD14+ cells were incubated with 20 ng/ml of M-CSF for one day to generate OCPs. For human osteoclastogenesis assays, cells were added to 96 well plates in triplicate at a seeding density of 5 ×104 cells per well. Osteoclast precursors were incubated with 20 ng/ml of M-CSF and 40 ng/ml of human soluble RANKL for an additional 5 days in α-MEM supplemented with 10 % FBS. Cytokines were replenished every 3 days. On day 6, cells were fixed and stained for TRAP using the Acid Phosphatase Leukocyte diagnostic kit (Sigma) as recommended by the manufacturer. Multinucleated (more than 3 nuclei) TRAP-positive osteoclasts were counted in triplicate wells.
Gene expression analysis
For real time PCR, DNA-free RNA was obtained using the RNeasy Mini Kit from QIAGEN with DNase treatment, and 1 µg of total RNA was reverse transcribed using a First Strand cDNA Synthesis kit (Fermentas, Hanover, MD). Real time PCR was performed in triplicate using the iCycler iQ thermal cycler and detection system (Applied Biosystems, Carlsbad, CA) following the manufacturer’s protocols. Expression of the tested gene was normalized relative to levels of GAPDH. Primers used in this study are as follows: hA20 5’-CCGGCTGCGTGTATTTTGGGACTC-3’, 5’-GGAACCTGGACGCTGTGGGACTGA-3; hNFATc1 5’-’ CTTCTTCCAGTATTCCACCTAT-3’, 5’-TTGCCCTAATTACCTGTTGAAG-3’; hITGB3 5’-GGAAGAACGCGCCAGAGCAAAATG-3’ 5’-CCCCAAATCCCTCCCCACAAATAC-3’; hCTSK 5’-CTCTTCCATTTCTTCCACGAT-3’, 5’-ACA CCA ACT CCC TTC CAA AG-3’; hBCL6 5’-CCTCGCCAGCCACAAGACCG-3’, 5’-CTGGCTCCGCAGGTTTCGCA-3’; hIRF8 5’-TGCGCTCCAAACTCATTCTCGTG-3’, 5’-GTCTGGCGGCGGCTCCTC-3’; hMAFB 5’-CTCAGCACTCCGTGTAGCTC-3’, 5’-GTAGTTGCTCGCCATCCAGT-3’; hFcγRIIa 5’-CATCACTGTCCAAGTGCCCA-3’, 5’-CCACAATGATCCCCATTGGT-3’; hGADPH 5’-ATCAAGAAGGTGGTGAAGCA-3’, 5’-GTCGCTGTTGAAGTCAGAGG-3’
RNA interference
0.2 nmol of three short interfering RNAs (siRNAs), specifically targeting human FcγRIIa (L-014152-00-0005, Dharmacon), human A20 (TNFAIP3: M-009919-99-0005, Dharmacon), or non-targeting control siRNA (Dharmacon) were transfected into primary human CD14+ monocytes with the Amaxa Nucleofector device set to program Y-001 using the Human Monocyte Nucleofector kit (Amaxa), as previously described (Park-Min et al., 2007).
Flow Cytometry
Staining for cell surface expression of various proteins was performed using antibodies against human CD64, CD32, and CD16 (BD Parmingen). A FACSCalibur flow cytometer with CELLQuest software (Becton Dickinson) was used.
Statistical analysis
All statistical analyses were performed with Graphpad Prism 5.0 software using the 2-tailed, paired t-test (two conditions) or one-way ANOVA with Tukey’s post t-test for multiple comparisons (more than two conditions). p<0.05 was taken as statistically significant.
Results
Inhibition of RANKL-induced human osteoclastogenesis by IVIG
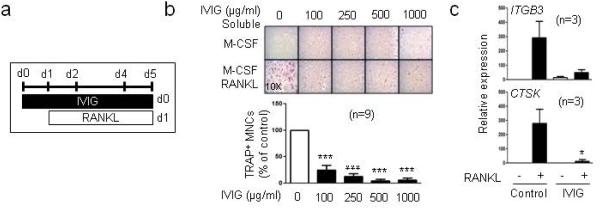
IVIG has been applied as a treatment for a broad range of diseases (Kazatchkine and Kaveri, 2001; Schwab and Nimmerjahn, 2013). Here, we wished to test the effect of IVIG treatment on human osteoclast differentiation. Human CD14-positive (CD14+) monocytes were cultured with M-CSF and IVIG for 24 hours, and cells were subsequently treated with RANKL for five additional days. IVIG treatment before RANKL stimulation significantly inhibited osteoclastogenesis in a dose-dependent manner (Fig. 1a and b). Accordingly, IVIG inhibited osteoclast-specific gene expressions such as CTSK (encodes cathepsin K) and ITGB3 (encodes integrin β3) when it was added before RANKL stimulation (Fig. 1c). The highest dose of IVIG we used (1 mg/ml) is relevant to the therapeutic dose for patients (20 mg/kg of body weight) and completely inhibited in vitro osteoclastogenesis. IVIG is endotoxin-free, and we further confirmed that this suppressive effect did not result from LPS contamination (Supplementary Fig. 1). Our results indicate that IVIG directly suppresses osteoclast differentiation of osteoclast precursor cells.
Fig.1. IVIG inhibits RANKL-induced human osteoclastogenesis.
(a -c) Human monocytes were cultured with the indicated doses of IVIG (100-1000 µg/ml) in the presence of human M-CSF (20 ng/ml) for one day and then 40 ng/ml of human RANKL was added to the culture for 4 days, and TRAP+ multinucleated (more than three nuclei) cells were counted in triplicate on day 5. (a) Experimental scheme, IVIG was added prior to RANKL. (b) Upper panel, Representative results obtained from one experiment. Lower panel, The number of osteoclasts generated by RANKL alone is set as 100%. Data are shown as mean ± SEM from 9 independent donors. (c) mRNA was measured using reverse transcription quantitative polymerase chain reaction (RT-qPCR) and normalized relative to the expression of GAPDH. *p < 0.05, *** p <0.001 by one-way ANOVA.
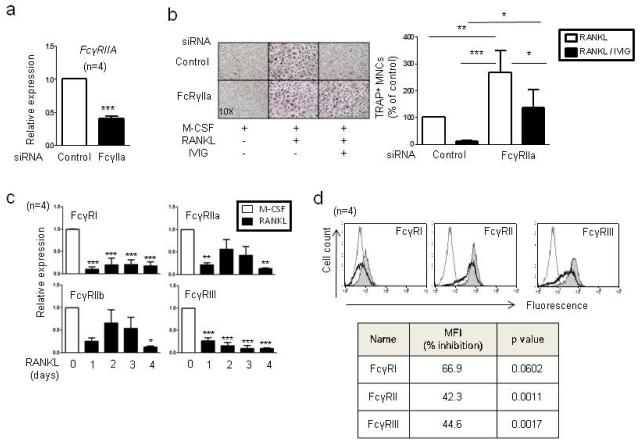
Major receptors for IVIG are Fcγ receptors (Schwab and Nimmerjahn, 2013). In human cells, three different classes of FcγRs (FcγRI, FcγRII and FcγRIII) have been described; FcγRII has an activating FcγRIIa and inhibitory FcγRIIb isoform. FcγRIV is only expressed in mouse cells and FcγRIIa is only expressed in human cells. In human OCPs, four Fcγ receptors (FcγRI, FcγRIIa, FcγRIIb, and FcγRIII) are expressed (19). To test the role of Fcγ receptors in IVIG-mediated inhibition on osteoclastogenesis, we knocked down the expression of individual Fcγ receptor using small interference RNAs (siRNAs). Knock-down of human specific FcγRIIa significantly reversed IVIG-mediated suppression of osteoclastogenesis (Fig. 2a and b). Other Fcγ receptors also played a role in IVIG’s inhibitory action but the contribution of these receptors was not statistically significant and was not sufficient to rescue IVIG-mediated inhibition of osteoclast differentiation (Supplementary Fig. 2). Decrease of FcγRIIa expression increased osteoclastogenesis in the control RANKL-stimulated condition, suggesting that immunoglobulin in serum can be involved in basal suppression in osteoclast differentiation in vitro. It has been shown that IVIG utilizes different Fcγ receptors in a disease-dependent manner and thus we measured surface expression of Fcγ receptors during osteoclastogenesis. Consistent with previous observations in murine cells (Seeling et al., 2013), RANKL treatment negatively regulated Fcγ receptor mRNA expression in a time-dependent manner (Fig. 2c). Accordingly, surface expression of Fcγ receptors diminished after RANKL stimulation (Fig. 2d). Our data show that RANKL stimulation decreases Fcγ receptor expression. Next we tested whether the suppressive effect of IVIG correlates with the level of expression of Fcγ receptors. IVIG lost its inhibitory effect on osteoclast differentiation when added after RANKL stimulation (Supplementary Fig. 3a and b). The size of osteoclasts appeared smaller in the IVIG conditions, although total osteoclast number actually increased (Supplementary Fig. 3b, lower panel). Corroborating a lack of suppressive effect on osteoclast differentiation, addition of IVIG after RANKL did not have a significant inhibitory effect on RANKL-induced osteoclast-specific gene expressions (Supplementary Fig. 3c), which contrasts with essentially complete suppression of these genes when IVIG was added prior to RANKL (Fig. 1). Our data suggest that low levels of Fcγ receptor expression may contribute to the inability of IVIG to suppress osteoclastogenesis in RANKL-pretreated cells. Taken together, our data demonstrate that IVIG treatment prior to RANKL suppresses osteoclastogenesis through FcγRIIa.
Fig.2. The role of FcγRIIa in IVIG-induced suppression of osteoclastogenesis.
(a and b) Human monocytes were nucleofected with control or FcγRIIa-specific small interfering RNAs (siRNAs). (a) FcγRIIa knock-down efficiency was measured by RT-qPCR and normalized relative to the expression of GAPDH. ***p < 0.001 by paired t-test. (b) TRAP-positive, multinucleated osteoclast formation was visualized by TRAP staining. Left panel, Representative results. Right panel, Values are the mean ± SEM from three experiments. The number of osteoclasts obtained from control siRNA is set as 100%. *p < 0.05, **p < 0.01, ***p < 0.001 by one-way ANOVA. (c-d) Down-regulation of mRNA and cell surface expression of Fcγ receptors by IVIG. (c and d) Human monocytes were cultured for one day with human M-CSF (20 ng/ml). (c) Cells were cultured with human RANKL (40ng/ml) for the indicated times and mRNA was measured by RT-qPCR. mRNA of Fcγ receptors was normalized relative to the expression of GAPDH. Values are the mean ± SEM. *;P=0.05, **: P < 0.01, *** ;P < 0.001. (d) RANKL (40ng/ml) was added to the culture for 36 hrs. Surface expression of Fcγ receptors was measured by flow cytometry. Dotted line represents an isotype control. Gray shaded are corresponds to control cells and the thick line represents IVIG treated cells. Left panel: Representative histograms from five experiments are shown. Right panel: MFI of control cells was set as 100% and mean % of inhibition of surface expression of proteins was shown.
Attenuation of pathologic bone resorption by IVIG in the TNF-induced osteolysis model
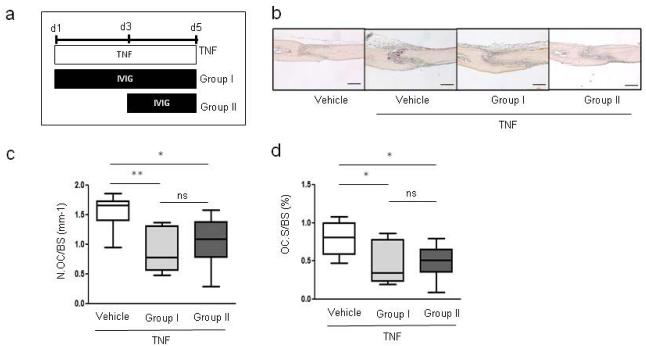
To address the biological importance of IVIG-mediated suppression of osteoclastogenesis, we tested whether IVIG could inhibit pathological bone resorption in vivo. To minimize the indirect effects that IVIG could exert on osteoclastogenesis by suppressing inflammation, we used the TNF-induced supracalvarial osteolysis model where administration of exogenous TNF allows testing of the effects of IVIG on downstream bone resorption. To test whether IVIG exerts time-dependent effects on in vivo osteoclastogenesis, IVIG was administered either at the same time as TNF or 2 days after initial TNF treatment to test preventive and therapeutic efficacy of IVIG on in vivo osteoclastogenesis (Fig. 3a, group I versus group II). IVIG attenuated TNF-mediated induction of TRAP-positive osteoclasts and associated bone resorption independent of treatment time (Fig. 3b). The reduction in in vivo osteoclastogenesis was corroborated using histomorphometric analysis to quantify osteoclast numbers and surface area; osteoclast numbers per bone surface (N.OC/BS) and osteoclast surface area per bone surface (OC.S/BS) were significantly lower in both IVIG-treated groups (Fig. 3c and 3d). These results show that IVIG effectively suppresses inflammatory bone resorption; the suppression of osteoclastogenesis when IVIG therapy was started after TNF is most likely explained by suppressive effects on osteoclast precursors before they are exposed to RANKL in vivo.
Fig.3. IVIG attenuates in vivo osteoclastogenesis in the TNF-induced supracalvarial osteolysis mouse model.
(a-d) TNF (1.5 µg of mouse TNF) was injected over calvarial periosterium of mice (n=6/group) for five consecutive days, and either saline control or IVIG (1 mg/kg body weight) was administered intraperitoneally (i.p.) once per day. (a) Experimental scheme. (b) Representative images of TRAP+ osteoclasts in calvarial sections. Scale bar = 240 µm (c and d) Histomorphometric analysis; osteoclast number per bone surface (N.Oc/BS) and osteoclast surface area per bone surface (OC.S/BS). *p < 0.05, **p < 0.01, ns = not significant by unpaired t-test.
IVIG suppress NFATC1 induction
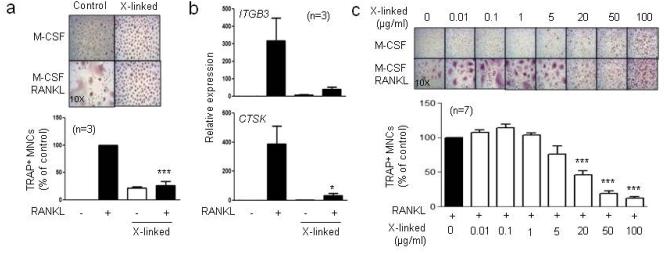
We previously showed that inhibition of distinct signaling pathways, such as Jak-STAT signaling, by IVIG is mediated by soluble polymeric IgGs contained within IVIG preparations (Park-Min et al., 2007). We tested whether IgG-mediated crosslinking of Fcγ receptors could inhibit osteoclast differentiation. We used plate-immobilized human IgG to model crosslinking Fcγ receptors on cells by soluble polymeric IgGs (Ravetch and Bolland, 2001). CD14+ cells were plated on IgG-precoated wells to crosslink Fcγ receptors, RANKL was added on the next day, and cells were cultured for five days. Crosslinking of Fcγ receptors (labeled X-linked) by 0.1 mg/ml of IgG strongly suppressed osteoclastogenesis (Fig. 4a) and osteoclast-associated gene expression (Fig. 4b). We next titrated the dose of IgG and tested the effects on osteoclastogenesis. Low avidity crosslinking by small amounts of IgG (0.1 – 1 μg/ml) slightly, albeit not significantly, increased osteoclastogenesis while the inhibitory effects of crosslinking Fcγ receptors only became clearly apparent at 50 µg/ml (Fig. 4c). Our data show that crosslinking Fcγ receptors inhibits osteoclastogenesis in a manner parallel to the suppressive effects of IVIG.
Fig.4. Crosslinking of Fcγ receptors suppresses osteoclastogenesis.
(a and b) Human monocytes were cultured for one day with human M-CSF (20 ng/ml) in control wells or wells coated with 100 µg/ml of IgG (labeled X-linked). After 24 hours, 40 ng/ml RANKL was added for 5 additional days. (a) TRAP+ multinucleated cells were counted five days after the addition of RANKL. The number of osteoclasts in control conditions is set at 100%. Values are the mean ± SEM. (b) Cells were cultured as in (a), and mRNA was measured using RT-qPCR and normalized relative to the expression of GAPDH. (c) Cells were plated in the wells coated with the indicated concentrations of IgG with human M-CSF for 24 hours, and 40 ng/ml RANKL was added for 5 additional days. The number of TRAP+ osteoclasts in control condition is set as 100%. Values are the mean ± SEM. *p < 0.05, ***p < 0.001 by one-way ANOVA.
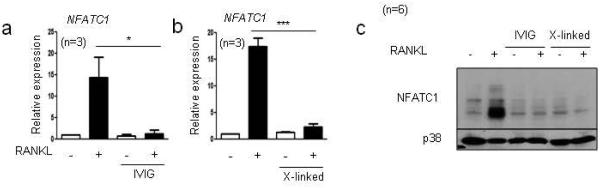
We then investigated mechanisms by which IVIG and crosslinking of Fcγ receptors inhibit osteoclastogenesis. A key event in osteoclastogenesis is the induction of the transcription factor NFATc1, a master regulator of osteoclast differentiation. RANKL induced NFATc1 expression, but NFATc1 expression was inhibited by IVIG at both mRNA and protein levels (Fig. 5a and b). Similarly, crosslinking Fcγ receptors suppressed RANKL-induced NFATc1 expression at both mRNA and protein levels (Fig. 5a and b). These results suggest that IVIG and Fcγ receptor crosslinking suppress osteoclastogenesis by inhibiting RANKL-induced NFATc1 induction.
Fig.5. IVIG suppresses RANKL-induced NFATc1 expression.
(a-c) Human monocytes were cultured for one day with human M-CSF (20 ng/ml) in control wells, wells with 1 mg/ml of IVIG, or wells coated with 100 µg/ml of IgG (labeled X-linked). Cells were subsequently cultured with RANKL for 24 hours. (a-b) mRNA was measured using RT-qPCR and normalized relative to the expression of GAPDH. *p < 0.05, ***p < 0.001 by one-way ANOVA. (b) Immunoblot of whole cell lysates using NFATc1 and p38 antibodies.
A20 induction by IVIG
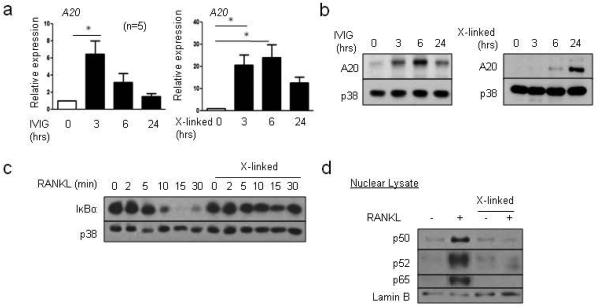
Induction of NFATc1 expression requires RANK signaling and concomitant downregulation of transcriptional repressors such as BCL6, IRF8 and MafB that render the NFATC1 gene unresponsive to upstream signaling (Ivashkiv, 2008; Zhao and Ivashkiv, 2011). We tested the effects of Fcγ receptor crosslinking on expression of transcriptional repressors of osteoclastogenesis whose deficiency in mice accelerates osteoclastogenesis (Zhao and Ivashkiv, 2011) and on RANK signaling. As expected, the transcriptional repressors of osteoclastogenesis BCL6, IRF8 and MafB were downregulated by RANKL (Supplementary Fig. 4). Crosslinking of Fcγ receptors prevented RANKL-induced downregulation of BCL6 and IRF8 (Supplementary Fig. 4). B lymphocyte-induced matulation protein 1 (Blimp1) is a negative regulator of BCL6 and IRF8 (Miyauchi et al., 2012; Miyauchi et al., 2010) and was induced by RANKL (Supplementary Fig. 5). Crosslinking of Fcγ receptors suppressed RANKL-induced BLIMP1 expression (Supplementary Fig. 5), suggesting that RANK signals could be affected by crosslinking of Fcγ receptors. We performed unbiased transcriptomic analysis to identify IgG-induced negative regulators and found that A20 is induced by IgG (data not shown). We investigated whether A20 is induced by IVIG and crosslinking of Fcγ receptors by IVIG using real-time PCR. IVIG and crosslinking of Fcγ receptors induced the expression of A20 mRNA and protein in a time-dependent manner (Fig. 6a and b), which corroborated previous work (Wang et al., 2010). The deubiquitinating enzyme A20 has been shown to attenuate NF-κB signaling by suppressing signaling upstream of IKKs (Shembade et al., 2010). We then tested if crosslinking of Fcγ receptors regulates NF-κB pathways downstream of RANK signaling pathways. RANKL stimulation of human OCPs activated downstream signaling via NF-κB pathways with robust degradation of I-κBα (Fig. 6c). Strikingly, prior crosslinking of Fcγ receptors suppressed RANKL-induced degradation of I-κBα (Fig. 6c). We further analyzed the effect of crosslinking of Fcγ receptors on RANKL-induced NF-κB signaling. RANKL increased nuclear localization of p50, p52 and p65, whereas crosslinking of Fcγ receptors suppressed p50, p52 and p65 translocation upon RANKL stimulation (Fig. 6d). Taken together, our results show that pretreatment of OCPs with IVIG induces A20 expression and suppresses degradation of I-κBα and NF-κB signaling in response to RANKL stimulation.
Fig.6. IVIG and plate-bound IgG regulate RANKL-induced signaling pathways, in part, by inducing A20 expression.
(a-b) Human monocytes were cultured for one day with human M-CSF (20 ng/ml) in control wells, wells with 1 mg/ml of IVIG, or wells coated with 100 µg/ml of IgG (labeled X-linked). Cells were harvested at 0, 3, 6 and 24 hours after stimulation. (a) mRNA was measured using RT-qPCR and normalized relative to the expression of GAPDH. (b) Immunoblot of whole cell lysates with A20 and p38 antibodies. Representative images from three independent donors. (c) 40 ng/ml RANKL was added for the indicated times, and cells were harvested. Immunoblot of whole cell lysates using I-κBα and p38 antibodies. Representative data from at least three independent experiments are shown. (d) OCPs were treated with 40 ng/ml RANKL for one hour and nuclear lysates were immunoblotted with p50/p105, p52/p100, p65, and lamin B antibodies. Representative data from four independent donors.
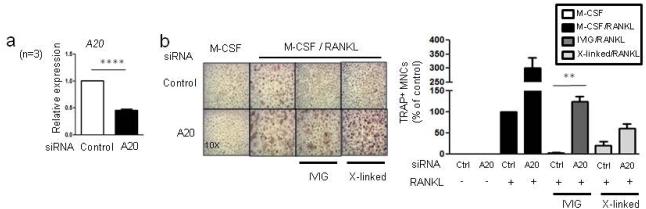
To test the role of A20 in the inhibitory action of IVIG on osteoclastogenesis, we next knocked down A20 expression using small interfering RNA (siRNA) (Fig. 7a). siRNA-mediated knockdown of A20 increased osteoclastogenesis and partially reversed the suppressive effects of IVIG and crosslinking of Fcγ receptors on osteoclastogenesis (Fig. 7b). Thus, our results show that A20 expression, in part, mediated the suppressive effect of IVIG or crosslinking Fcγ receptors on osteoclastogenesis.
Fig.7. The role of A20 in the inhibitory effect of IVIG on human osteoclastogenesis.
(a and b) Human monocytes were nucleofected with control or A20 specific siRNAs. (a) A20 knock-down efficiency was measured by RT-qPCR. ****p < 0.0001 by paired t-test. (b) TRAP-positive, multinucleated osteoclast formation was visualized by TRAP staining. Left panel, Representative results. Right panel, Values are the mean ± SEM from three experiments. The number of osteoclasts obtained from control siRNA is set as 100%. *p < 0.05, **p < 0.01 by one-way ANOVA.
Discussion
IVIG has been used as an effective therapy for a wide range of autoimmune diseases and immunodeficiency disorders (Kazatchkine and Kaveri, 2001; Schwab and Nimmerjahn, 2013). Several mechanisms have been proposed for the immunomodulatory and anti-inflammatory action of IVIG in autoimmune diseases. However, the direct effect of IVIG on osteoclastogenesis has not been mechanistically investigated and signaling pathways that are important for IVIG’s suppressive effect on osteoclast differentiation are not known. In this study, we found that IVIG suppresses osteoclast differentiation and confers partial protection from TNF-induced osteolysis in vivo. In our study, IVIG induced a refractoriness of human OCPs to RANKL stimulation, which resulted in diminished NFATc1 induction that prevented osteoclast precursor cells from differentiating into mature osteoclasts. The decrease in NFATc1 expression was explained by attenuated RANKL-induced NF-κB signaling. Both IVIG and crosslinking of Fcγ receptors induced the expression of A20, an inhibitor of NF-κB signaling, and knock-down of A20 expression partially reversed IVIG’s inhibitory action. Thus, we have identified an inhibitory pathway that plays an important role in the regulation of osteoclast differentiation.
Our study investigated the effect of IVIG on human osteoclast differentiation and supports a new mechanism for the inhibitory action of IVIG. Although IVIG has been used as a beneficial therapy in various inflammatory and autoimmune diseases, the cost, time, and shortage of supply of IVIG poses potential problems for patients (Vaitla and McDermott, 2010). To overcome the limitations of IVIG therapy, several studies have focused on identifying the active component(s) in IVIG preparations, including sialylated IgG and immune complexes (Schwab and Nimmerjahn, 2013). We showed that crosslinking of Fcγ receptors by IVIG inhibits human osteoclastogenesis similarly to the effect of IVIG, suggesting polymeric IgGs serve as an active component for IVIG’s inhibitory effect on osteoclastogenesis. In addition, the corresponding receptor for IVIG’s inhibitory action has been intensively studied. However, different Fcγ receptors have been implicated in the effect of IVIG, and the role of Fcγ receptors in different diseases is not well understood. Our findings have established that IVIG elicits a suppressive effect on in vitro osteoclastogenesis mainly dependent on FcγRIIa. FcγRIIa contains immunoreceptor tyrosine-based activating motif (ITAM) and has been considered an activating Fcγ receptor. The importance of FcγRIIa has been demonstrated in several autoimmune diseases (Tan Sardjono et al., 2005). The classical paradigm of ITAM motifs being activating and ITIM (immunoreceptor tyrosine-based inhibitory motif) motifs being inhibitory has evolved, and it is increasingly appreciated that ITAM motifs can have inhibitory functions as well (Ivashkiv, 2009). This is supported by recent studies demonstrating the inhibitory functions of FcγRIIa-ITAM and crosslinking of FcγRIIa and IVIG have been shown to ameliorate arthritis in FcγRIIa transgenic mice (Ben Mkaddem et al., 2014). In addition, allelic variants in FcγRIIa have been associated with differential responsiveness of patients with Kawasaki diseases to IVIG therapy (Shrestha et al., 2012). These studies and our data argue that assessing genetic variation or level of expression of FcγRIIa may be useful in evaluating the clinical response to IVIG. However, further study will be needed to demonstrate a conclusive correlation between differences in FcγRIIa expression and IVIG treatment in bone diseases.
Previous studies show that ligation of activating Fcγ receptors by immune complexes can generate either negative or positive effects on osteoclastogenesis (Grevers et al., 2013; Negishi-Koga et al., 2015; Seeling et al., 2013). Although the contribution of Fcγ receptors to bone erosion during inflammatory arthritis varies among different models of arthritis (Negishi-Koga et al., 2015; Seeling et al., 2013; van Lent et al., 2006), it has been shown that serum of arthritic mice containing pathological immune complexes directly promotes in vitro osteoclastogenesis (Negishi-Koga et al., 2015), suggesting the diverse function of IgGs in bone metabolism. Depending on the intensity of signals and timing of the ligation, ITAM-mediated signals that are associated with integrin receptors also have a negative role in osteoclast differentiation (Park-Min et al., 2013). Consistently, our findings have established that IVIG elicits a bimodal effect on in vitro osteoclastogenesis depending on OCPs’ differentiation status – the suppressive effects of IVIG are abrogated by prior RANKL stimulation. One explanation for this time dependent effect of IVIG is likely that downregulation of Fcγ receptor expression by RANK signaling diminishes the ability of IVIG to generate inhibitory signals. This may occur because lower level Fcγ receptor activation may not reach a threshold required to generate an inhibitory signal (for example, a minimal level of A20 required to suppress signaling), or because occupancy of different Fcγ receptors may generate a qualitatively different signal. Because the inhibitory effect of IVIG on osteoclastogenesis, in part, comes from immune-complexes, our findings help to explain the discrepancy between previous studies showing that ligation of activating Fcγ receptors by immune complexes can generate either negative or positive effects on osteoclastogenesis (Grevers et al., 2013; Seeling et al., 2013). However, in the TNF-induced osteolysis model IVIG attenuated in vivo osteoclastogenesis regardless of timing of the treatment, suggesting that in this model the response to IVIG of OCPs prior to their arrival at target sites where they are exposed to RANKL plays an important role in suppression of in vivo bone destruction.
Although inhibitory functions for Fcγ receptors on osteoclasts have been previously described, signaling pathways that are targeted for suppression are not known. Here, we show that crosslinking of Fcγ receptors mainly targets NF-κB activation downstream of RANK. NF-κB signaling is rapidly induced after exposure of cells to various factors including TLR ligands, TNF and RANKL (Ghosh and Hayden, 2008). Both canonical and non-canonical NF-κB pathways play an important role in osteoclast differentiation, function, and survival (Soysa and Alles, 2009). NF-κB pathways are tightly regulated by multiple negative feedback mechanisms at different stages of activation to limit detrimental and excessive activation. Two key factors that negatively regulate NF-κB activation are A20 and I-κBα (Werner et al., 2008), and IgG crosslinking regulates both A20 and I-κBα. We have provided evidence supporting the role of IVIG-induced A20 in mediating the suppression of osteoclastogenesis by IVIG, but it is likely that the integrated functions of other signaling pathways downstream of IVIG as well as the maintenance of expression of transcriptional repressors contribute to the IVIG-induced suppression of osteoclast differentiation.
It has been suggested that defective A20 expression or activity is associated with rheumatoid arthritis (Vereecke et al., 2009). A20 deficiency in myeloid cells induces erosive polyarthritis (Matmati et al., 2011), and this induction of arthritis is dependent on Nlrp3 inflammasome (Vande Walle et al., 2014), suggesting that increasing A20 expression would have beneficial effects on inflammatory arthritis. In addition, allelic variants (SNPs) associated with increased risk for rheumatoid arthritis are located in the A20 (TNFAIP3) gene locus (Ma and Malynn, 2012; Matmati et al., 2011) . In myeloid cells, we have been shown that A20 expression is regulated by ITAM signaling (Wang et al., 2010). Our study demonstrates that A20 is increased by IVIG treatment and plays an important role in the suppressive effect of IVIG on osteoclast differentiation. We speculate that induction of A20 may have a dual role in erosive arthritis by suppressing both inflammation and osteoclastogenesis. Thus, IVIG would be an efficient tool to induce A20 expression and to provide therapy for pathogenic bone destruction.
Our findings also highlight the importance of negative regulators such as A20 in osteoclastogenesis. Our study and previous reports reveal the negative role of A20 in osteoclast differentiation. Myeloid specific deletion of A20 increased murine osteoclastogenesis (Matmati et al., 2011) and here, we show that increased expression of A20 suppresses human osteoclastogenesis. OCPs express high levels of transcriptional repressors that negatively regulate osteoclast differentiation. RANK signaling decreases these transcriptional repressors, permitting the expression of NFATc1 and osteoclast differentiation. A20 expression is not suppressed by RANK signaling (data not shown) unlike transcriptional repressors in OCPs. Our data suggest that A20 functions as a barrier for uncontrolled activation during osteoclast differentiation but exact mechanisms need to be studied further. Overall, our results support the idea that IVIG changes the balance of negative regulators during osteoclast differentiation towards promoting A20 expression and subsequently suppressing osteoclastogenesis to decrease RANK signaling below the threshold required to induce osteoclast differentiation. In summary, we demonstrate that IVIG has inhibitory effects on osteoclastogenesis and identify the inhibitory signaling pathway that is induced by IVIG. Thus, IVIG may have a beneficial effect on inflammatory bone destruction.
Supplementary Material
Acknowledgements
We thank Drs. Sung Ho Park and George Kalliolias for critical review of the manuscript.
This work was supported by grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) grant AR061430 (K.-H.P.-M.) and NIH grants (L.B.I.)
Footnotes
Conflict of interest
The authors declare no financial conflicts of interest.
References
- Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475(7354):110–113. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Mkaddem S, Hayem G, Jonsson F, Rossato E, Boedec E, Boussetta T, El Benna J, Launay P, Goujon JM, Benhamou M, Bruhns P, Monteiro RC. Shifting FcgammaRIIA-ITAM from activation to inhibitory configuration ameliorates arthritis. The Journal of clinical investigation. 2014;124(9):3945–3959. doi: 10.1172/JCI74572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce BF, Yao Z, Xing L. Functions of nuclear factor kappaB in bone. Annals of the New York Academy of Sciences. 2010;1192:367–375. doi: 10.1111/j.1749-6632.2009.05315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IK, Miescher S, Branch DR, Mott PJ, Lazarus AH, Han D, Maraskovsky E, Zuercher AW, Neschadim A, Leontyev D, McKenzie BS, Kasermann F. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. Journal of immunology. 2014;192(11):5031–5038. doi: 10.4049/jimmunol.1301611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Hayden MS. New regulators of NF-kappaB in inflammation. Nature reviews Immunology. 2008;8(11):837–848. doi: 10.1038/nri2423. [DOI] [PubMed] [Google Scholar]
- Grevers LC, de Vries TJ, Everts V, Verbeek JS, van den Berg WB, van Lent PL. Immune complex-induced inhibition of osteoclastogenesis is mediated via activating but not inhibitory Fcgamma receptors on myeloid precursor cells. Annals of the rheumatic diseases. 2013;72(2):278–285. doi: 10.1136/annrheumdis-2012-201568. [DOI] [PubMed] [Google Scholar]
- Harre U, Georgess D, Bang H, Bozec A, Axmann R, Ossipova E, Jakobsson PJ, Baum W, Nimmerjahn F, Szarka E, Sarmay G, Krumbholz G, Neumann E, Toes R, Scherer HU, Catrina AI, Klareskog L, Jurdic P, Schett G. Induction of osteoclastogenesis and bone loss by human autoantibodies against citrullinated vimentin. The Journal of clinical investigation. 2012;122(5):1791–1802. doi: 10.1172/JCI60975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiby N, Doring G, Schiotz PO. The role of immune complexes in the pathogenesis of bacterial infections. Annual review of microbiology. 1986;40:29–53. doi: 10.1146/annurev.mi.40.100186.000333. [DOI] [PubMed] [Google Scholar]
- Ivashkiv LB. A signal-switch hypothesis for cross-regulation of cytokine and TLR signalling pathways. Nat Rev Immunol. 2008;8(10):816–822. doi: 10.1038/nri2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nature immunology. 2009;10(4):340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Ohmura K, Mahmood U, Lee DM, Hofhuis FM, Boackle SA, Takahashi K, Holers VM, Walport M, Gerard C, Ezekowitz A, Carroll MC, Brenner M, Weissleder R, Verbeek JS, Duchatelle V, Degott C, Benoist C, Mathis D. Arthritis critically dependent on innate immune system players. Immunity. 2002;16(2):157–168. doi: 10.1016/s1074-7613(02)00275-3. [DOI] [PubMed] [Google Scholar]
- Kazatchkine MD, Kaveri SV. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. The New England journal of medicine. 2001;345(10):747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest. 2005;115(12):3418–3427. doi: 10.1172/JCI26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Jung YO, Ryu JG, Kang CM, Kim EK, Son HJ, Yang EJ, Ju JH, Kang YS, Park SH, Kim HY, Cho ML. Intravenous immunoglobulin attenuates experimental autoimmune arthritis by inducing reciprocal regulation of th17 and treg cells in an interleukin-10-dependent manner. Arthritis & rheumatology. 2014;66(7):1768–1778. doi: 10.1002/art.38627. [DOI] [PubMed] [Google Scholar]
- Lorenzo J, Horowitz M, Choi Y. Osteoimmunology: interactions of the bone and immune system. Endocrine reviews. 2008;29(4):403–440. doi: 10.1210/er.2007-0038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nature reviews Immunology. 2012;12(11):774–785. doi: 10.1038/nri3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLellan LM, Montgomery J, Sugiyama F, Kitson SM, Thummler K, Silverman GJ, Beers SA, Nibbs RJ, McInnes IB, Goodyear CS. Co-opting endogenous immunoglobulin for the regulation of inflammation and osteoclastogenesis in humans and mice. Arthritis and rheumatism. 2011;63(12):3897–3907. doi: 10.1002/art.30629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancardi DA, Jonsson F, Iannascoli B, Khun H, Van Rooijen N, Huerre M, Daeron M, Bruhns P. Cutting Edge: The murine high-affinity IgG receptor FcgammaRIV is sufficient for autoantibody-induced arthritis. Journal of immunology. 2011;186(4):1899–1903. doi: 10.4049/jimmunol.1003642. [DOI] [PubMed] [Google Scholar]
- Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Mc Guire C, Vereecke L, Chu Y, Boon L, Staelens S, Matthys P, Lambrecht BN, Schmidt-Supprian M, Pasparakis M, Elewaut D, Beyaert R, van Loo G. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nature genetics. 2011;43(9):908–912. doi: 10.1038/ng.874. [DOI] [PubMed] [Google Scholar]
- May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunology today. 1998;19(2):80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England journal of medicine. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- Miyauchi Y, Miyamoto H, Yoshida S, Mori T, Kanagawa H, Katsuyama E, Fujie A, Hao W, Hoshi H, Miyamoto K, Sato Y, Kobayashi T, Akiyama H, Morioka H, Matsumoto M, Toyama Y, Miyamoto T. Conditional inactivation of Blimp1 in adult mice promotes increased bone mass. The Journal of biological chemistry. 2012;287(34):28508–28517. doi: 10.1074/jbc.M112.356634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi Y, Ninomiya K, Miyamoto H, Sakamoto A, Iwasaki R, Hoshi H, Miyamoto K, Hao W, Yoshida S, Morioka H, Chiba K, Kato S, Tokuhisa T, Saitou M, Toyama Y, Suda T, Miyamoto T. The Blimp1-Bcl6 axis is critical to regulate osteoclast differentiation and bone homeostasis. J Exp Med. 2010;207(4):751–762. doi: 10.1084/jem.20091957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat C, Bertotto A, Ercolani R, Bistoni O, Agea E, Cesarotti M, Fiorucci G, Spinozzi F, Gerli R. Long term treatment of rheumatoid arthritis with high doses of intravenous immunoglobulins: effects on disease activity and serum cytokines. Annals of the rheumatic diseases. 1995;54(5):382–385. doi: 10.1136/ard.54.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi-Koga T, Gober HJ, Sumiya E, Komatsu N, Okamoto K, Sawa S, Suematsu A, Suda T, Sato K, Takai T, Takayanagi H. Immune complexes regulate bone metabolism through FcRgamma signalling. Nature communications. 2015;6:6637. doi: 10.1038/ncomms7637. [DOI] [PubMed] [Google Scholar]
- Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annual review of immunology. 2008;26:513–533. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- Novack DV. Role of NF-kappaB in the skeleton. Cell research. 2011;21(1):169–182. doi: 10.1038/cr.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annual review of pathology. 2008;3:457–484. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- Ochi S, Shinohara M, Sato K, Gober HJ, Koga T, Kodama T, Takai T, Miyasaka N, Takayanagi H. Pathological role of osteoclast costimulation in arthritis-induced bone loss. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(27):11394–11399. doi: 10.1073/pnas.0701971104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Park-Min KH, Lee EY, Moskowitz NK, Lim E, Lee SK, Lorenzo JA, Huang C, Melnick AM, Purdue PE, Goldring SR, Ivashkiv LB. Negative regulation of osteoclast precursor differentiation by CD11b and beta2 integrin-B-cell lymphoma 6 signaling. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28(1):135–149. doi: 10.1002/jbmr.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Min KH, Serbina NV, Yang W, Ma X, Krystal G, Neel BG, Nutt SL, Hu X, Ivashkiv LB. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26(1):67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Annual review of immunology. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Schett G, Gravallese E. Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nature reviews Rheumatology. 2012;8(11):656–664. doi: 10.1038/nrrheum.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nature reviews Immunology. 2013;13(3):176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- Seeling M, Hillenhoff U, David JP, Schett G, Tuckermann J, Lux A, Nimmerjahn F. Inflammatory monocytes and Fcgamma receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(26):10729–10734. doi: 10.1073/pnas.1301001110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327(5969):1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrestha S, Wiener H, Shendre A, Kaslow RA, Wu J, Olson A, Bowles NE, Patel H, Edberg JC, Portman MA. Role of activating FcgammaR gene polymorphisms in Kawasaki disease susceptibility and intravenous immunoglobulin response. Circulation Cardiovascular genetics. 2012;5(3):309–316. doi: 10.1161/CIRCGENETICS.111.962464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siragam V, Crow AR, Brinc D, Song S, Freedman J, Lazarus AH. Intravenous immunoglobulin ameliorates ITP via activating Fc gamma receptors on dendritic cells. Nature medicine. 2006;12(6):688–692. doi: 10.1038/nm1416. [DOI] [PubMed] [Google Scholar]
- Soysa NS, Alles N. NF-kappaB functions in osteoclasts. Biochemical and biophysical research communications. 2009;378(1):1–5. doi: 10.1016/j.bbrc.2008.10.146. [DOI] [PubMed] [Google Scholar]
- Tak PP, Firestein GS. NF-kappaB: a key role in inflammatory diseases. The Journal of clinical investigation. 2001;107(1):7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nature reviews Immunology. 2007;7(4):292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- Tan Sardjono C, Mottram PL, van de Velde NC, Powell MS, Power D, Slocombe RF, Wicks IP, Campbell IK, McKenzie SE, Brooks M, Stevenson AW, Hogarth PM. Development of spontaneous multisystem autoimmune disease and hypersensitivity to antibody-induced inflammation in Fcgamma receptor IIa-transgenic mice. Arthritis and rheumatism. 2005;52(10):3220–3229. doi: 10.1002/art.21344. [DOI] [PubMed] [Google Scholar]
- Vaitla PM, McDermott EM. The role of high-dose intravenous immunoglobulin in rheumatology. Rheumatology. 2010;49(6):1040–1048. doi: 10.1093/rheumatology/keq021. [DOI] [PubMed] [Google Scholar]
- van Lent PL, Grevers L, Lubberts E, de Vries TJ, Nabbe KC, Verbeek S, Oppers B, Sloetjes A, Blom AB, van den Berg WB. Fcgamma receptors directly mediate cartilage, but not bone, destruction in murine antigen-induced arthritis: uncoupling of cartilage damage from bone erosion and joint inflammation. Arthritis and rheumatism. 2006;54(12):3868–3877. doi: 10.1002/art.22253. [DOI] [PubMed] [Google Scholar]
- Vande Walle L, Van Opdenbosch N, Jacques P, Fossoul A, Verheugen E, Vogel P, Beyaert R, Elewaut D, Kanneganti TD, van Loo G, Lamkanfi M. Negative regulation of the NLRP3 inflammasome by A20 protects against arthritis. Nature. 2014;512(7512):69–73. doi: 10.1038/nature13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends in immunology. 2009;30(8):383–391. doi: 10.1016/j.it.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Wada T, Nakashima T, Hiroshi N, Penninger JM. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends in molecular medicine. 2006;12(1):17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- Waldman M, Madaio MP. Pathogenic autoantibodies in lupus nephritis. Lupus. 2005;14(1):19–24. doi: 10.1191/0961203305lu2054oa. [DOI] [PubMed] [Google Scholar]
- Wang L, Gordon RA, Huynh L, Su X, Park Min KH, Han J, Arthur JS, Kalliolias GD, Ivashkiv LB. Indirect inhibition of Toll-like receptor and type I interferon responses by ITAM-coupled receptors and integrins. Immunity. 2010;32(4):518–530. doi: 10.1016/j.immuni.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner SL, Kearns JD, Zadorozhnaya V, Lynch C, O'Dea E, Boldin MP, Ma A, Baltimore D, Hoffmann A. Encoding NF-kappaB temporal control in response to TNF: distinct roles for the negative regulators IkappaBalpha and A20. Genes & development. 2008;22(15):2093–2101. doi: 10.1101/gad.1680708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Ivashkiv LB. Negative regulation of osteoclastogenesis and bone resorption by cytokines and transcriptional repressors. Arthritis Res Ther. 2011;13(4):234. doi: 10.1186/ar3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler NJ. The immunopathology of joint inflammation in rheumatoid arthritis. Advances in immunology. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.