Abstract
Under normal physiological conditions, Slo2.1 K+ channels are in a closed state unless activated by an elevation in [Na+]i. Fenamates such as niflumic acid also activate Slo2.1. Previous studies suggest that activation of Slo2.1 channels is mediated by a conformational change in the selectivity filter, and not a widening of the aperture formed by the S6 segment bundle crossing as occurs in voltage-gated K+ channels. It is unclear how binding of Na+ or fenamates is allosterically linked to opening of the presumed selectivity filter activation gate in Slo2.1. Here we examined the role of the S5 transmembrane segment in the activation of Slo2.1. Channels were heterologously expressed in Xenopus laevis oocytes and whole cell currents measured with the voltage-clamp technique. Ala substitution of five residues located on a single face of the S5 α-helical segment induced constitutive channel activity. Leu-209, predicted to face towards Phe-240 in the pore helix was investigated by further mutagenesis. Mutation of Leu-209 to Glu or Gln induced maximal channel activation as did the combined mutation to Ala of all three hydrophobic S5 residues predicted to be adjacent to Phe-240. Together these results suggest that hydrophobic interactions between residues in S5 and the C-terminal end of the pore helix stabilize Slo2.1 channels in a closed state.
Keywords: biophysics, potassium channel, Xenopus, gating, voltage clamp, Slo2.1
Graphical abstract
1. Introduction
Na+-activated K+ (KNa) channels were initially identified in mammalian cardiomyocytes [2] and avian neurons [3]. Two genes encoding KNa channels were cloned 2003 and named Slo2.1 (or “Slick” for “sequence like an intermediate conductance K+”) and Slo2.2 (or “Slack” for “sequence like a calcium-activated K+”) [5, 6]. Slo2.1 channels are normally in a closed state and are naturally activated by an elevation in [Na+]i. Activation of Slo2.1 is very weakly voltage-dependent (effective valence, z = 0.48 e) and neutralization of all the charged residues present in the S4 segment does not further reduce its indifference to transmembrane voltage [7]. Once opened, the activity of Slo2.1 channels can be modulated by intracellular Cl− ions and NAD+ [8], but apparently not by ATP [9, 10] as previously reported [5]. Selective pharmacological agents for Slo2.1 have not yet been described. Fenamates such as niflumic acid (NFA) activate Slo2.1 more effectively than intracellular Na+ [7], but have low potency [11] and either block [12] or alter the gating [13] of many other channels. The putative binding site for intracellular Na+, but not NFA, has been defined. A charge-reversing mutation of Asp-757 completely abolishes the ability of intracellular Na+ to activate Slo2.1 [14]. Asp-757 is located in the C-terminal domain of the channel subunit at the interface of the RCK2 domain of one subunit and the RCK1 domain of an adjacent subunit. This places the Asp at the inner perimeter of the intracellular gating ring formed by all four subunits.
Similar to Kv channels, Slo2.1 channels are tetrameric complexes. Each subunit contains six transmembrane segments (S1-S6). The pore domain is composed of a pore helix and the selectivity filter region, book-ended by the S5 and S6 transmembrane segments. Unlike Kv channels, the selectivity filter, and not the intracellular S6 bundle crossing, has been proposed to perform the duties of the activation gate of Slo2.1 [7, 11], KCa3.1 [15, 16], Slo1 [17] and KCa2 [18] K+ channels. Scanning mutagenesis analysis of the S6 segment in Slo2.1 indicated that Pro-271 and Glu-275 are required to maintain the channel in an open configuration [19]. Pro-271 adds a kink in the otherwise straight S6 α-helix, whereas electrostatic repulsion between Glu-275 residues in all four subunits was proposed to prevent formation of a tight S6 bundle crossing typical of a closed Kv channel. Further mutagenesis of Slo2.1 suggested that intrasubunit dynamic coupling between the pore helix and nearby residues in the S5 and S6 segments mediates activation of the selectivity filter activation gate. Specifically, of the four pore helix residues that face towards the S5 and S6 segments of the same subunit, Ala substitution of only one of these residues (Phe-240) enhanced constitutive channel activity, and F240C channels were maximally activated as indicated by whole cell conductance and kinetics that were independent of NFA, time or voltage [19]. Phe-240 in Slo2.1 is located within position 2 of the 8 amino acid “K+ channel signature sequence” (TXXTXGYG) first described in 1994 by Heginbotham et al [20]. A few years later, x-ray crystal structures of KcsA bacterial K+ channels [21–24] indicated that K+ ions within the narrow selectivity filter were bound to oxygen atoms (backbone carbonyl or hydroxyl groups) supplied by the TXGYG residues and that position 2 of the K+ channel signature sequence (Met in most K+ channels, Phe in Slo2.1) was located at the C-terminal end of the pore helix, immediately outside the selectivity filter. Our previous work also showed that volume-reducing mutations in some, but not all of the residues in S5 or S6 predicted to face the pore helix in Slo2.1 induced modest constitutive channel activity [19]. Leu-209 in S5 was of special interest because homology modeling (based on the bacterial MthK K+ channel) indicated that it was in close contact with Phe-240, and substitution of Leu-209 with a polar residue (Thr) induced more constitutive activity than did an Ala substitution. Together these findings led to our previous hypothesis that a hydrophobic interaction between Leu-209 in S5 and Phe-240 in the pore helix stabilizes the closed state of the Slo2.1 channel [19]. Presumably intracellular Na+ binding to the cytoplasmic RCK1-RCK2 interfaces leads to a change in configuration of the gating ring that is allosterically coupled via the S5/S6 transmembrane segments to the selectivity filter gate.
In the present study, we first perform a full Ala-scan of the S5 α-helix, unbiased by molecular models of Slo2.1, to identify specific residues in this transmembrane segment that may be important for channel gating. Further mutagenesis was employed to test the simple hypothesis that mutations that reduced the hydrophobic volume of side chain residues in the region of the S5 in close proximity to Phe-240 in the pore helix would induce opening of Slo2.1 channels that otherwise exhibit a very low probability of opening.
2. Materials and Methods
2.1. Molecular Biology
Human Slo2.1 (KCNT2; NCBI Genbank accession no. NM_198503) cDNA in pTRACER plasmid (provided by L. Kaczmarek, Yale University, New Haven, CT) was isolated by EcoRV and SpeI digestion and subcloned into the psGEM oocyte expression vector. Point and multiple mutations in wild-type (wt) Slo2.1 in psGEM were introduced via primers containing the desired nucleotide substitutions using a standard PCR approach and confirmed by sequencing at the University of Utah Core Sequencing Facility. Plasmids were linearized with SfiI, and cRNA was prepared using the mMessage mMachine T7 kit (Ambion).
2.2. Oocyte Isolation and cRNA Injection
Protocols for isolation of oocytes from Xenopus laevis were approved by the Institutional Animal Care and Use Committee of the University of Utah. To isolate individual oocytes free of their follicle cells, dispersed ovarian lobes were placed into a Ca2+-free saline solution containing 1 mg/ml each of type 1 and 2 collagenase (Worthington Biochemical Corp.) and gently shaken for 1–1.5 h. The Ca2+-free saline solution contained (in mM): 96 NaCl, 2 KCl, 1 MgCl2, and 5 HEPES; pH was adjusted to 7.6 with NaOH. Single stage 4 and 5 oocytes were injected with a variable amount (0.4–10 ng) of wt or mutant Slo2.1 cRNA, then incubated at 17 °C for 1–8 days in Barth’s solution that contained (in mM): 88 NaCl, 1 KCl, 0.41 CaCl2, 0.33 Ca(NO3)2, 1 MgSO4, 2.4 NaHCO3, 10 HEPES, and 1 pyruvate, plus gentamycin (50 mg/liter), amikacin (100 mg/liter) and ciprofloxacin (25 mg/liter); pH of the solution was adjusted to 7.4 with NaOH.
2.3. TEVC Methods
Ionic currents were recorded at room temperature (22–24 °C) using standard TEVC techniques. Oocytes were placed in a 0.3-ml chamber (RC-IZ; Warner Instrument) and superfused at a rate of ~2 ml/min with KCM211 solution that contained (in mM): 98 NaCl, 2 KCl, 1 CaCl2, 1 MgCl2, and 5 HEPES; pH adjusted to 7.6 with NaOH. In some experiments, this solution was modified to make K104 solution by increasing [KCl] to 104 mM and omitting NaCl. To reduce leakage of KCl from recording pipettes into an oocyte, 1% agarose-cushion microelectrodes were fabricated as described [25]. The tip resistances of these microelectrodes varied from 0.3–0.8 MΩ after back-filling with 3 M KCl. A Dell personal computer, GeneClamp 500 amplifier (Molecular Devices, Inc.), Digidata 1322A data acquisition system (Molecular Devices, Inc.), and pClamp 8.2 software (Molecular Devices, Inc.) were used for data acquisition. In most experiments, Slo2.1 current (ISlo2.1) was activated by superfusing the chamber with a solution containing 1–6 mM NFA (Sigma-Aldrich). The peak increase of ISlo2.1 in response to NFA was attained within 6 min for 1 mM NFA and 3 min for 6 mM NFA. To record ISlo2.1, voltage pulses were usually applied from a holding potential of −80 mV to a test potential (Vt) that was varied in 10-mV increments from +80 to −140 mV. Unless specified otherwise, the interpulse interval was 10 s.
Slo2.1 channels can also be activated by increasing [NaCl]i For these experiments, recording micropipettes were filled with a 2 M NaCl solution and their tips were not plugged with agarose. Na+-loading micropipettes had a resistance of 0.4–1.0 MΩ, and leakage of NaCl out of the pipette and into the oocytes quickly activated ISlo2.1, reaching a peak effect in 5–10 min. I-Vt relationships were recorded immediately after impalement of an oocyte with the pipettes and again after 15 min. This method of activating S1o2.1 channels proved useful as a non-quantitative assay for functional expression of mutant channels, but has several obvious limitations, including uncertainty regarding [NaCl]i achieved and presumed preferential activation of channels located in the region of plasma membrane nearest to the electrode tips.
2.4. Homology model
Our study was initially guided by the crystal structure of the bacterial cyclic-nucleotide regulated channel MlotiK1 because recent cryo-electron microscopy structures of the closed and open states of this channel suggest that ion conductance is gated by the selectivity filter and not the S6 helix bundle crossing [26], similar to what we have proposed for the gating mechanism of Slo2.1. After completion of our experiments, the cryo-electron microscopy structure of the Slo2.2 channel was published [27] and this structure (PDB ID: 5A6G) was used as a template to construct a homology model of the Slo2.1 pore region (S5-S6) using YASARA [28].
2.5 Data Analysis
Under normal conditions, wt Slo2.1 channels have an extremely low open probability (Po) unless activated by an increase in [Na+]i or by extracellular application of a fenamate such as niflumic acid. Fenamates are more efficacious activators than Na+ [14], and 10 mM niflumic acid induces maximal activation of Slo2.1 channels. However, this high concentration of NFA is poorly tolerated, with most oocytes exhibiting a rapid increase in background leak current. Niflumic acid at 6 mM rarely caused an increase in leak currents, yet achieved 90–95% of maximum activation of whole cell current [7, 11, 19]. Thus, the relative magnitude of constitutive current (Ic-rel) for wt or mutant channels was defined as peak ISlo2.1 (at 0 mV) in the absence of NFA divided by ISlo2.1 after maximal channel activation by 6 mM NFA. For wt Slo2.1 channels expressed in Xenopus oocytes, Ic-rel is very low (<0.005 when leak and endogenous currents are accounted for), whereas some mutant channels such as R190E [7] or F240C [19] have an Ic-rel of 1.0. Gain of function mutations in Slo2.1 that increase Ic-rel, but to a level < 1.0 (e.g., E275D [19]) also exhibit an increased sensitivity to activation by NFA, defined by a lower value of the EC50 value (the [NFA] that produced a half maximal effect). Thus, to quantify both constitutive channel activity under control conditions (Ic-rel) and estimate changes in the efficacy of NFA, we use the more general term Irel, defined as ISlo2.1 under control conditions or in the presence of 1 mM NFA divided by ISlo2.1 in the presence of 6 mM NFA.
To determine the cumulative [NFA]-response relationship, ISlo2.1 was elicited with repetitive test pulses to 0 mV until the current (I) reached a steady-state level at each concentration of NFA. I/Imax was plotted as a function of [NFA] and the data were fitted with the logistic equation:
| (Eq. 1) |
where nH is the Hill coefficient.
pClamp 8.2 software (Molecular Devices, Inc.) was used to analyze digitized data. Origin 8.6 (OriginLab) was used for curve fitting and to prepare figures. All data are expressed as mean ± SEM (n = number of oocytes), and statistical significance was evaluated by a Student’s t test where appropriate. Differences between mean values were considered significant at p < 0.01.
3. Results
3.1. Activation of ISlo2.1 by NFA
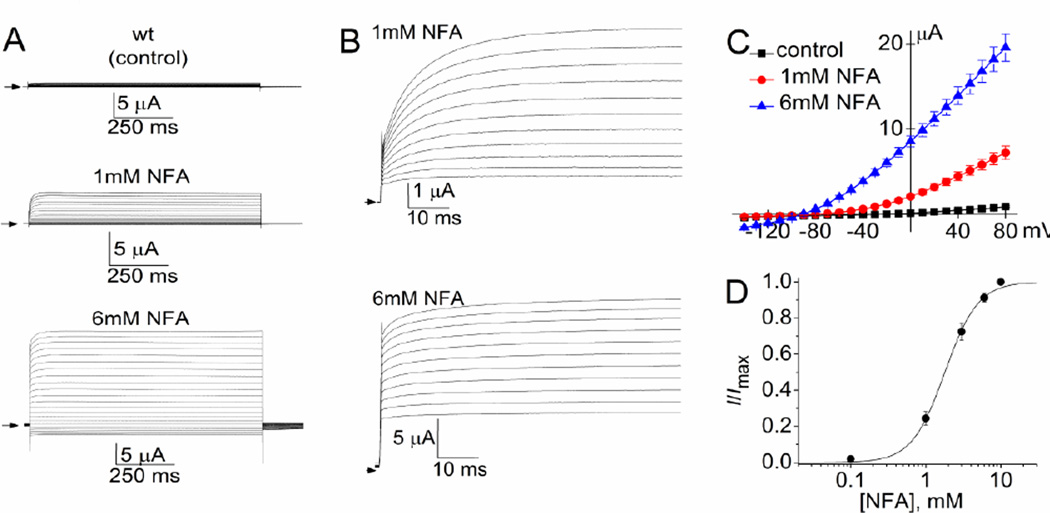
An example of currents recorded from an oocyte expressing wt Slo2.1 channels is presented in Fig. 1. Currents elicited by 1-s pulses applied to a Vt ranging from +80 to −140 mV were recorded under control conditions and after achieving a steady-state increase in response to 1 mM NFA and 6 mM NFA (Fig. 1A). The initial 75 ms of currents activated by pulses applied to a Vt ≥ −20 mV are shown in Fig. 1B. Note that the relative amplitude of instantaneous current is larger and the rate of activation faster for currents measured in response to 6 mM NFA compared to 1 mM NFA. When fully-activated by 10 mM NFA, ISlo2.1 is time independent (currents activate instantaneously) and whole-cell conductance is voltage independent [7]. The averaged I–Vt relationships for ISlo2.1 recorded from multiple oocytes expressing wt channels are plotted in Fig. 1C. The average current at 0 mV was 85 ± 14 nA for uninjected cells and 137 ± 9 nA for cells expressing wt Slo2.1 (n = 14). The response of wt channels to 1 mM NFA (ISlo2.1: 2.09 ± 0.27 µA) was 24% of that achieved with 6 mM NFA (ISlo2.1: 8.56 ± 0.65 µA, n = 14). Normalized current (I/Imax) at 0 mV was plotted as a function of [NFA] and fitted with the logistic equation (Fig. 1D). The EC50 for NFA activation of Slo2.1 channel currents was 2.2 ± 0.3 mM (nH = 2.1 ± 0.2; n = 11).
Fig. 1.
Activation of wt Slo2.1 channels by NFA. (A) Representative currents measured by TEVC in a Xenopus oocyte expressing wt Slo2.1 channels under control conditions and after achieving a steady-state increase in response to extracellular application of 1 and 6 mM NFA. Currents were measured in response to 1-s pulses to a Vt ranging from +80 mV to −140 mV, applied in 10 mV increments. Small arrows indicate 0 current level. (B) Expanded view of currents shown in panel A. The initial 75 ms for currents measured at a Vt ≥ −20 mV are shown. (C) Average I–Vt relationship for whole cell Slo2.1 channel currents measured in Xenopus oocytes (n = 14). (D) [NFA]-response relationships for wt Slo2.1 channels. The peak currents measured at 0 mV were normalized to peak response (I/Imax) and plotted as a function of [NFA]. The relationship was fitted with a logistic equation (smooth curve). EC50 was 2.2 ± 0.3 mM, and nH was 2.1 ± 0.2 (n = 11).
3.2. Ala Scanning of S5 Segment
We previously Ala-scanned the S6 segment to identify residues critical for gating of Slo2.1 channels [19]. Here we extended the scan to the S5 segment. The amino acid sequence of the S4-S5 linker and S5 transmembrane segment of Slo2.1 is aligned with other K+ channels in Fig. 2A. Each of the 25 residues in the S5 segment, plus two additional residues just outside the S5, of Slo2.1 was individually mutated to Ala and the resulting mutant channels heterologously expressed in Xenopus oocytes. ISlo2.1 was activated by bath application of NFA at concentrations of 1 and 6 mM. The relative Po of channels under various conditions can be estimated by comparison to the peak outward current measured in the presence of 6 mM NFA. As illustrated in Fig. 2B, the constitutive activity of wt channels was very small. The amplitude of ISlo2.1 measured at a Vt of 0 mV under control conditions and after addition of 1 and 6 mM NFA for the 27 mutant channels are presented in Fig. 2C. In Fig. 2D, data are plotted as normalized current (Irel), defined as the current magnitude under control conditions, or in the presence of 1 mM NFA, relative to current measured in the presence of 6 mM NFA. Irel in the presence of 1 mM NFA was increased in 17 of the 27 mutant channels. Irel recorded under control conditions, a measure of constitutive channel activity, and referred to here as Ic-rel, was enhanced by Ala substitution of Asn-197, Leu-202, Thr-205, Leu-209, Thr-212, Gly-216 and Ile-217. These 7 mutations are indicated by red text on the x-axis of the plot in Fig. 2D. We did not determine the [NFA]-response relationships for the mutant channels, but it is important to note that we have found that Slo2.1 mutations that increase Ic-rel also reduce the EC50 for NFA [7, 19]. The side chain of one of these residues (Asn-197) is predicted to contact the N-terminal end of the S4-S5 linker of an adjacent subunit (not shown), while I217A expressed very poorly. Neither one of these two mutations were studied further. The other five residues that significantly increased Ic-rel align together on a single face of a helical wheel projection (Fig. 2E).
Fig. 2.
Alanine scan of S5 segment of Slo2.1. (A) Amino acid sequence alignment for the S4-S5 linker (S45L) and the S5 transmembrane segment (S5) of Slo2.1 and other K+ channels. The name of each channel is indicated to the left of each sequence. The position of the terminal residue in the channel subunit is indicated on the right of the sequence. Ala substitution of 6 residues (indicated by red text) caused a gain of function. Residues indicated by green text (Trp-211 in KCa3.1, Leu-273 in KCNQ1) have been reported to interact with the base of the pore helix, as described in the Discussion. (B) Representative traces of wt Slo2.1 channel currents. Top panel shows voltage pulse protocol. Currents were applied once every 10 s. Bottom panel shows Slo2.1 channel current traces recorded under control conditions and after peak responses to application of a solution containing 1 mM and 6 mM NFA. (C) Average current amplitudes (in µA) recorded at a Vt of 0 mV is plotted on the y-axis. The x-axis indicates the point mutation in Slo2.1. Currents recorded from uninjected oocytes (Uninj) and cells expressing wild-type channels (wt) are also plotted. The 25 residues within the S5 segment are underlined. Oocytes were injected with 0.4–10 ng cRNA and recorded by TEVC 1–4 days later (n = 4–14). (D) Data from panel C expressed as normalized current (Irel), defined as current magnitude under control conditions, or in the presence of 1 mM NFA, relative to current measured in the presence of 6 mM NFA. *, p < 0.01 compared to wt channels. (E) Helical wheel plot of the S5 segment of Slo2.1 from residue Val-199 to Gly-216 (indicated by single letter amino acid code), constructed using Wheel.pl program (http://rzlab.ucr.edu/scripts/wheel/wheel.cgi ). Asterisks mark those residues that when mutated to Ala had both constitutive channel activity and responses to 1 mM NFA (dark and light bars, respectively in panel D) that were significantly larger (p < 0.01) than wt channels. (F) Homology model of the S5 segment, pore helix (PH) and S6 segment of Slo2.1 based on the structure of Slo2.2 channel. The side chains of Leu-202, Thr-205, Leu-209, Thr-212 and Gly-216 are labeled. Error bars in panels C and D indicate + S.E.
In a homology model of the pore region of Slo2.1 based on the Slo2.2 K+ channel in a closed state [27], the side chains of the five key residues, spanning four helical turns of the S5 α-helix, are oriented towards and interact with residues in the S6 segment of the same subunit (Fig. 2F). A minor rotation of the S5 helix (e.g., in response to activation of the channel by intracellular Na+) could alter the specific interactions between one or more of these five residues and residues in the pore helix and S6 segment. However, in the static, closed state homology model of Slo2.1 only Leu-209 is predicted to face towards the pore helix.
3.3. Characterization of Leu-209 Mutant Channels
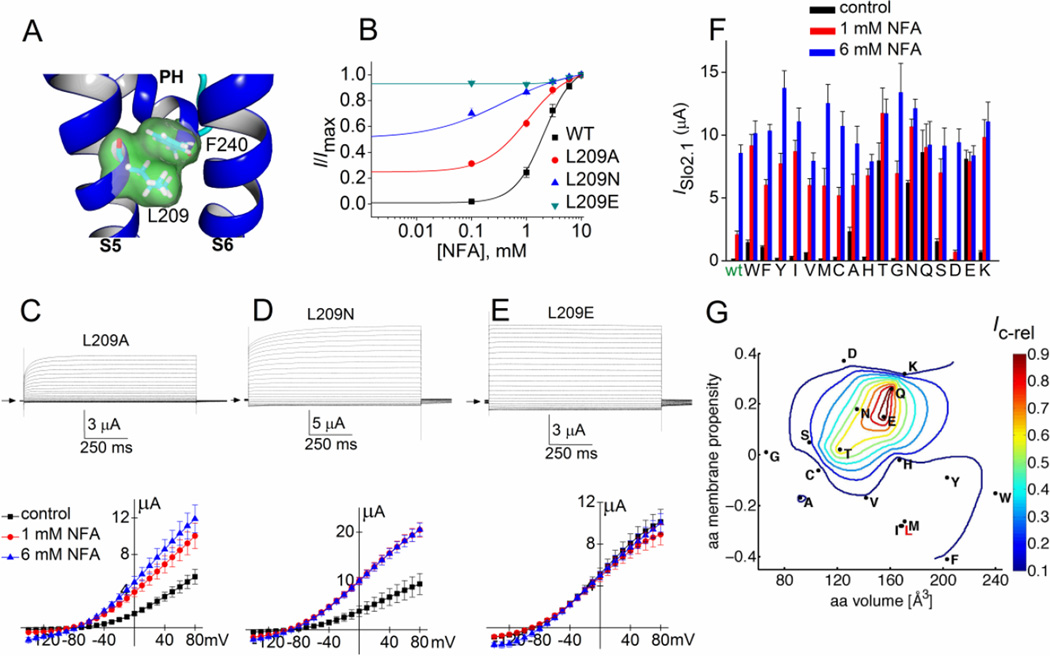
In our homology model, the side chain of Leu-209 makes direct contact with the phenyl group of Phe-240 located at the C-terminal end of the pore helix (Fig. 3A). We previously reported that L209A and L209T Slo2.1 channels exhibited much greater constitutive activity than wt channels [19]. Here we extend the analysis of Leu-209 by examining the effects of 15 additional amino acid substitutions. We first compared the [NFA]-response relationship for wt, L209A, L209N and L209E channels. All three mutations induced constitutive channel activity in the absence of an activator. Ic-rel, not corrected for endogenous currents, of wt channels was 0.012 ± 0.001 (n = 14), 0.25 ± 0.016 for L209A (n = 5), 0.52 ± 0.04 for L209N (n = 6) and 0.95 ± 0.02 for L209E channels (n = 5). The EC50 values for NFA activation of ISlo2.1 were reduced in accordance with the increase in Ic-rel (Fig. 3B). NFA at 6 and 10 mM enhanced outward current by ~5% in oocytes expressing L209E channels, but this was judged to be an effect on endogenous currents as a similar effect was observed in uninjected oocytes.
Fig. 3.
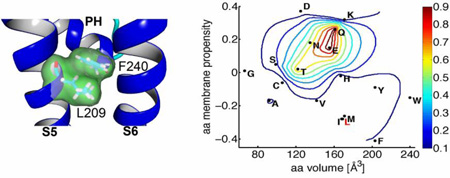
Point mutations of Leu-209 alter constitutive activity of Slo2.1 channels. (A) Slo2.2-based homology model of the S5-S6 region of a Slo2.1 subunit, highlighting the close proximity of Leu-209 in S5 and Phe-240 at the base of the pore helix (PH). (B) [NFA]-response relationships for wt (same data as in Fig. 1D) and three Leu-209 mutant channels. For L209A channels, EC50 = 1.4 ± 0.3 mM, nH = 1.6 ± 0.5 (n = 5). For L209N channels, EC50 = 0.28 ± 0.04 mM, nH = 0.60 ± 0.09 (n = 6). L209E channels do not respond to 1 or 6 mM NFA (n = 5), indicating that these mutant channels are maximally activated. (C–E) Representative currents (upper panels) recorded from individual oocytes expressing indicated mutant Slo2.1 channels under control conditions and I–Vt relationships (lower panels) for multiple oocytes before and after application of 1 and 6 mM NFA (L209A, n = 8; L209N, n = 5; L209E, n = 6). (F) Peak outward current measured at 0 mV in the absence (control) and presence of 1 and 6 mM NFA for channels containing Leu-209 substitutions as indicated on x-axis (n = 5–10). Amino acid substitutions are listed from left to right in order of decreasing hydrophobicity. (G) The variation in normalized constitutive current (Ic-rel) is contour plotted (z-axis) as a function of volume and membrane propensity of the residue substituted for Leu-209. The aa volume (x-axis) represents the average volume of a residue buried in a protein [1]. The amino acid membrane propensity (y-axis) is a knowledge-based hydropathy scale derived from an extensive transmembrane helix database and optimization algorithm described in detail by Punta and Maritan [4], where a negative value indicates a high membrane propensity. The native residue, Leu-209 (L) is indicated in red text. Error bars indicate ± S.E. (panels C–E) or + S.E. (panel F).
Representative current traces measured under control conditions for L209A, L209N and L209E channels are presented in the top panels of Fig. 3, C, D and E, respectively. The corresponding I–Vt relationships recorded from multiple oocytes under control conditions and after addition of 1 and 6 mM NFA are plotted in the bottom panels of Fig. 3C–E. Note that L209A and L209N Slo2.1 channel currents elicited with the more positive test potentials were time-dependent, whereas L209E channel currents were time-independent at all test potentials. Thus, the relative contribution of instantaneous current was increased as a function of the constitutive activity of the mutant channels. ISlo2.1 for 14 additional Leu-209 mutant channels was recorded at 0 mV (Fig. 3F). The relationship between constitutive channel activity (i.e., Ic-rel) and two physical properties of the amino acid substituted for Leu-209 are presented as a contour plot in Fig. 3G. In this plot, amino acid volume, plotted on the x-axis represents the average volume of a residue buried in a protein, calculated from the surface area of the side chain [1]. The amino acid membrane propensity scale plotted on the y1-axis is a hydropathy scale derived from transmembrane helices [4], while the normalized constitutive channel activity (Ic-rel) is plotted on the z-axis. The most striking finding from this analysis is that substitution of Leu-209 with Glu, Gln, Asn or Thr induced strong constitutive channel activity, whereas substitution with Asp or Ser had little effect on channel activity. Thus, a very specific combination of side chain volume and hydropathy of the amino acid in position 209 of the S5 segment was required to alter the equilibrium between the closed and open state of Slo2.1 channels.
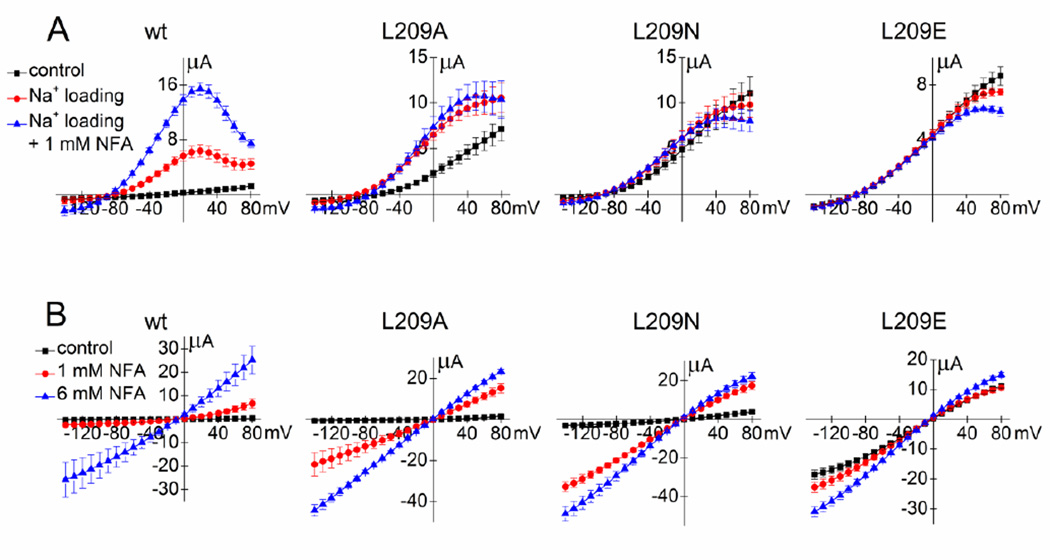
Slo2.1 channels are naturally activated by elevation of [Na+]i. Therefore, we determined whether changes in [Na+]i affected the relative Po of select Leu-209 mutant channels. As described in Experimental Procedures, [NaCl]i of oocytes was increased by diffusion of NaCl from recording micropipettes that did not contain an agarose plug. Using this technique, ISlo2.1 was rapidly induced in oocytes expressing wt Slo2.1 channels. Subsequent addition of 1 mM NFA to the bathing solution caused a further enhancement of ISlo2.1. I–Vt relationships recorded under all three conditions are plotted in Fig. 4A along with the results of similar experiments performed with oocytes expressing L209A, L209N and L209E channels. In contrast to wt channels, 1 mM NFA did not enhance L209A currents beyond the 2–3 fold increase obtained by NaCl loading alone, L209N channels were only slightly increased by NaCl loading without additional effects by NFA, and fully activated L209E mutant channels were not further activated by Na+ loading or addition of NFA. Note that in all cells, NaCl loading reduced the slope conductance of the I–Vt relationship at positive potentials, a result of voltage-dependent block of outward currents by the elevated [Na+]i [14]. Together, these findings confirm that L209A/N/E channels have an increased relative Po under normal physiological conditions compared to wt Slo2.1 channels.
Fig. 4.
The effects of elevated [Na+]i or high [K+]e on Leu-209 mutant channel activity. (A) I–Vt relationships for oocytes expressing either wt (n = 5) or mutant Slo2.1 channels (L209A, n = 4; L209N, n = 4; L209E, n = 7). Currents were recorded immediately after impalement of an oocyte with 2M NaCl-containing electrodes (control), after maximum response to intracellular Na+ loading and again after addition of 1 mM NFA to the extracellular solution. (B) I–Vt relationships for wt (n = 6), L209A (n = 3), L209N (n = 4) and L209E (n = 7) Slo2.1 channels measured from oocytes bathed in K104 extracellular solution in the absence (control) and presence of 1 and 6 mM NFA. Error bars indicate ± S.E.
The Po of constitutively active Slo2.1 channels is reduced in the presence of high [K+]o and low [Na+]o [7]. The constitutive activity of L209A and L209N channels was low, similar to wt channels, when oocytes were bathed in K104 solution (Fig. 4B). However, the increase in current magnitude induced by 1 mM NFA relative to 6 mM NFA was greater for these two mutant channels than for wt channels. In contrast, the constitutive activity of L209E channels was only slightly inhibited by the high [K+]o solution. The observation that high [K+]o strongly inhibits constitutive activity of mutant channels unless the mutation increased Ic-rel to a value near 1.0 (i.e., L209E) was previously reported for point mutations of Arg-190 in Slo2.1 [7].
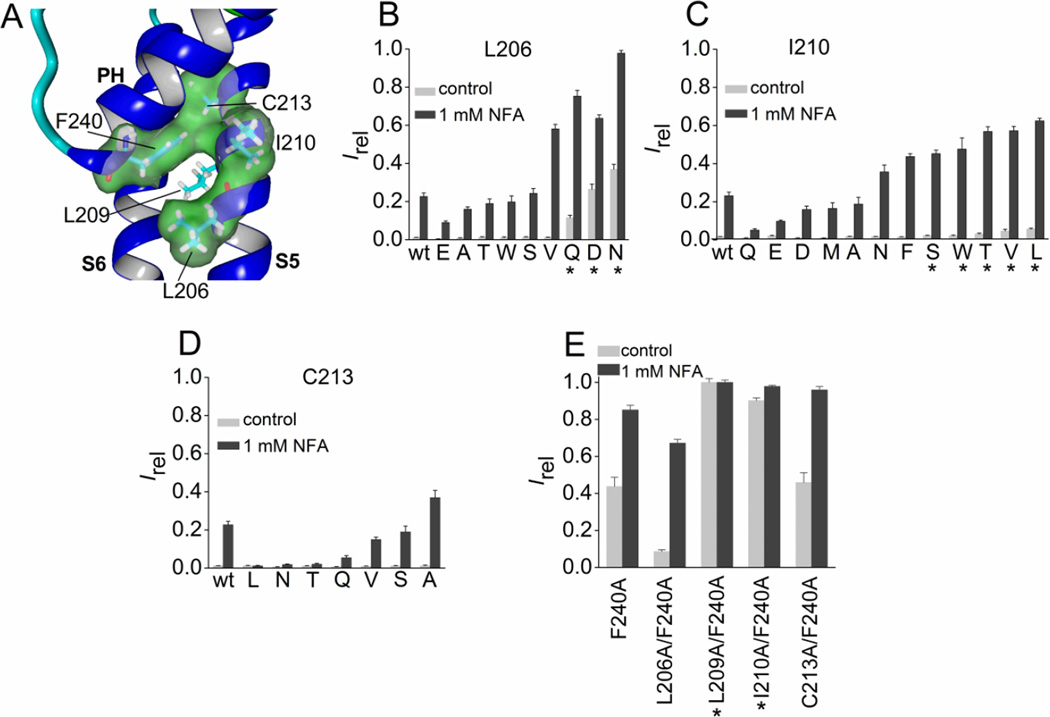
3.4. Point mutations of S5 residues surrounding Phe-240
In addition to Leu-209, three additional residues in the S5 segment (Leu-206, Ile-210 and Cys-213) are predicted to be in close proximity to Phe-240 (Fig. 5A). Substitution of Leu-206 with Asn, Gln or Asp increased channel activity, whereas polar residues with a shorter side chain (Thr and Ser) did not (Fig. 5B), suggesting that a specific H-bonding interaction between position 206 in S5 with some other unidentified residue can stabilize the open state of the channel. Substitution of Ile-210 with aromatic, other hydrophobic, or polar residues had relatively minor effects on channel activity (Fig. 5C), and mutation of Cys-213 to residues other than Ala reduced Irel in response to 1 mM NFA (Fig. 5D). Together these findings confirm the special importance of Leu-209 in modulating the relative Po of Slo2.1 channels.
Fig. 5.
Mutation analysis of three residues in the S5 segment of Slo2.1 predicted to be in close proximity to Phe-240. (A) Slo2.2-based homology model of a Slo2.1 subunit, highlighting the close proximity of three residues in the S5 segment to Phe-240 located at the base of the pore helix. For clarity, the molecular surface for Leu-209 in S5 is not shown. (B–D) Irel measured at 0 mV in the absence (control) or presence of 1 mM NFA for wt channels (n = 14), Leu-206 mutant channels (B, n = 5–8), Ile-210 mutant channels (C, n = 5–10) and Cys-213 mutant channels (D, n = 5–8). (E) Combined Ala substitutions of Phe-240 and a single nearby S5 residue. Irel was measured at 0 mV in the absence (control) or presence of 1 mM NFA for Phe-240 Slo2.1 channels containing an additional Ala substitution of the indicated S5 residue (n = 5–8). For panels B–E, amino acid substitutions of the indicated native residue are indicated by single letter code and are ordered by increasing Irel. *, indicates an increase (p < 0.01) in both measures of Irel compared to wt channels. Error bars indicate + S.E.
Do Phe-240 and Leu-209 directly interact with one another? Attempts to characterize the effects of cross-linking Cys-209 and Cys-240 were not successful. Although L209C/F240C channels were constitutively active similar to F240C channels [19], treatment of both wt and these mutant channels with a reducing agent (10 – 20 mM dithiothreitol) or an oxidant (0.5 – 1 mM t-butyl HO2) caused current inhibition (data not shown), likely due to modification of one or more of the many native Cys residues in the channel. Interaction between Phe-240 and Leu-209 was suggested by comparison of the effects of single and double Ala mutations on channel activity under basal conditions. The F240A mutation alone increased Ic-rel to 0.44 ± 0.05 (n = 7; Fig. 5E). Single Ala substitutions of three of the four S5 residues with side chains predicted to be close to Phe-240 (L206A, I210A and C213A) had almost no effect on constitutive channel activity (Ic-rel ≤ 0.013) whereas Ic-rel for L209A channels was 0.25 (Fig. 2C). Combining F240A with C213A had no effect on Ic-rel, strongly suggesting a lack of interaction between these residues. By contrast, Ic-rel for L206A/F240A channels was reduced by > 4-fold, and was more than the additive effects of single mutations for I210A/F240A (Ic-rel = 0.9), and L209A/F240A channels (Ic-rel = 1.0) (Fig. 5E). A more quantitative, energetics based analysis of these double mutations [29] was not practical for several technical reasons. ΔG values for ion channels can be calculated based on V0.5, the half-point of voltage dependent activation (ΔG = zFV0.5). However, while V0.5 for activation can be measured for constitutively active mutant channels, V0.5 for wt channels is variable because they are closed under basal conditions, display only a very weak voltage dependence when partially activated by NFA, and are voltage independent when fully activated with >6 mM NFA [7]. ΔG can also be estimated from the maximum single channel open probability, Po (ΔG = kTln(Po-max)). However, accurate measurement of single channel Po-max in excised patches is complicated by severe run-down of excised patches [14].
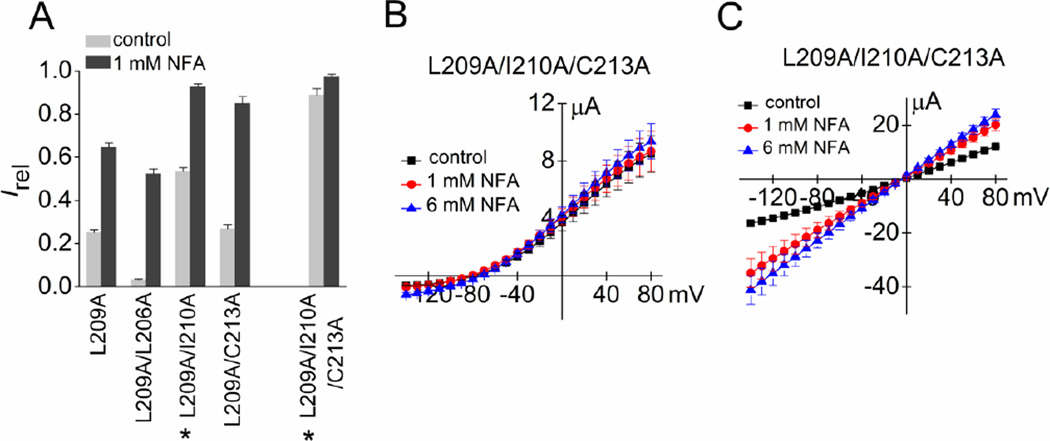
As a second approach to examine the importance of hydrophobic volume of S5 residues in the vicinity of the pore helix, the effects of combined Ala substitutions of Leu-209 and a second S5 residue predicted to be near Phe-240 were evaluated. Reducing the hydrophobic volume of Leu-209 and Ile-210 together increased Ic-rel of the channels to 0.54 ± 0.02 (n = 8, Fig. 6A), more than the sum of Ic-rel for the two single mutations. In contrast, the Ic-rel of L209A/L206A channels (0.03 ± 0.002, n = 8) was less than that of L209A alone (0.25 ± 0.01, n = 7) and Ic-rel of L209A/C213A channels was the same (0.27 ± 0.02, n = 7) as L209A alone. Combined Ala substitutions of three S5 residues (L209A/I210A/C213A) increased Ic-rel to 0.89 ± 0.03 (n = 7, Fig. 6A) and whole cell currents conducted by this triple mutant channel were not significantly increased by 1 or 6 mM NFA over a full range of Vt when measured using KCM211 solution (Fig. 6B). However, similar to L209E channels (Fig. 4B), the constitutive activity of L209A/I210A/C213A channels was partially inhibited by bathing oocytes in K104 solution (Fig. 6C). In summary, reducing the hydrophobic volume of three of the four side chains of S5 segment residues predicted to be in close proximity to Phe-240 induced a high level of constitutive channel activity nearly equivalent to that achieved by the L209A/F240A double mutation or the single mutation L209E (Fig. 3E). This finding suggests that hydrophobic interactions between the S5 segment and the base of the pore helix (perhaps specifically Phe-240) are responsible for maintaining wt channels in a closed conformation under normal physiological conditions.
Fig. 6.
Reduction in the hydrophobic volume of all three S5 residues located near Phe-240 fully activates Slo2.1 channels. (A) Irel measured at 0 mV in the absence (control) or presence of 1 mM NFA for L209A channels containing additional Ala substitutions of other S5 residues also predicted to be in close proximity to Phe-240 (n = 7–10). *, indicates an increase (p < 0.01) in both measures of Irel compared to L209A channels. (B) I–Vt relationships for L209A/I210A/C213A Slo2.1 channels in the absence and presence of 1 and 6 mM NFA (n = 6). Oocytes were bathed in KCM211 extracellular solution. (C) I–Vt relationships for L209A/I210A/C213A Slo2.1 channel currents measured for oocytes bathed in K104 extracellular solution in the absence and presence of 1 and 6 mM NFA (n = 4). Error bars indicate + S.E. (A) or ± S.E. (B, C).
3.5. S6 segment
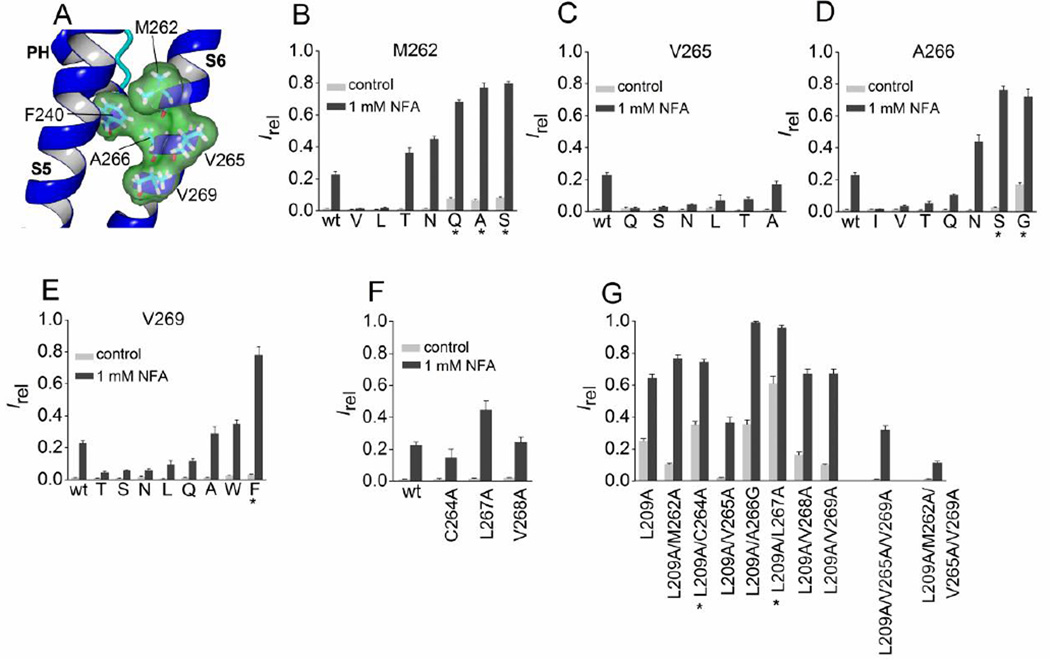
We next investigated whether S6 residues near the pore helix contribute to stabilizing the closed state of Slo2.1 channels. In the S6 segment, four residues (Met-262, Val-265, Ala-266, Val-269) are predicted by the homology model to be in close proximity to Phe-240 (Fig. 7A). Substitution of Met-262 with Gln, Ala or Ser induced greater constitutive channel activity, but mutation to Val, Leu, Thr, or Asn did not appreciably alter channel activity (Fig. 7B). All of the Val-265 substituted channels that were investigated exhibited reduced Irel in response to 1 mM NFA when compared to wt channels (Fig. 7C), suggesting that this residue does not contribute to stabilizing the closed state. Mutation of Ala-266 to Ser or Gly increased Ic-rel and Irel for 1 mM NFA, but substitution with Gln and Thr did not (Fig. 7D). Both Gly and Ser can disrupt the normal conformation of an α-helix and this may be the cause of the increased channel activity of these two mutant channels. The relative response to 1 mM NFA was decreased for most Val-269 substitutions examined, with the exception of Ala and Trp that were similar to wt, and Phe that showed a small increase in Ic-rel and a large increase in Irel in response to 1 mM NFA (Fig. 7E).
Fig. 7.
Mutation analysis of S6 segment residues predicted to be in close proximity to Phe-240. (A) Slo2.2-based homology model of the pore helix and S6 segment of a Slo2.1 subunit, highlighting the close proximity of four residues in the S6 segment to Phe-240 in the pore helix. (B–F) Irel measured at 0 mV in the absence (control) or presence of 1 mM NFA for wt channels (n = 14), Met-262 mutant channels (B, n = 5–9), Val-265 mutant channels (C, n = 5–9), Ala-266 mutant channels (D, n = 3–8), Val-269 mutant channels (E, n = 3–7), and point mutations in S6 as indicated (F, n = 5–9). (G) Irel measured at 0 mV in the absence (control) and presence of 1 mM NFA for L209A channels containing an additional one or more Ala substitution of the indicated S6 residue(s) (n = 7–8). For panels B–E, amino acid substitutions of the indicated native residue are indicated by single letter code. *, indicates an increase (p < 0.01) in both measures of Irel compared to wt channels, or L209A in panel G. Error bars indicate + S.E.
Next we examined whether reduction in the volume of hydrophobic side chains of residues located in the S6 segment predicted to be in close proximity to Phe-240 altered constitutive channel activity when combined with the L209A substitution in S5. In addition to the four residues predicted to be close to Phe-240 (Met-262, Val-265, Ala-266, Val-269; Fig. 7A), we also mutated the intervening residues Cys-264, Leu-267 and Val-268. Ala substitution of these later three residues did not significantly alter Irel of the mutant channels (Fig. 7F). S6 residues were mutated to Ala, except for Ala-266 that was mutated to Gly. Irel values for all seven of the S6 Ala-substituted channels were similar to wt channels, with the exception of M262A (Fig. 7B) and A266G (Fig. 7D) – these channels exhibited higher than normal constitutive activity. As summarized in Fig. 7G, pairing the single S6 Ala substitutions with L209A led to variable changes in Irel. Compared to that observed for L209A alone, combining L209A with M262A, V265A, V268A or V269A reduced Ic-rel, whereas pairing with C264A or L267A increased both measures of Irel. Finally, two (V265A/V269A) or three (M262A/V265A/V269A) of the S6 Ala mutations were combined with L209A to achieve an even greater reduction in the hydrophobic volume of the residue side chains in this restricted region of the S6 segment. Both the triple (L209A/V265A/V269A) and quadruple (L209A/M262A/V265A/V269A) mutant channels exhibited a low Ic-rel compared to L209A alone (Fig. 7G). Together, the results presented in Fig. 5 – Fig. 7 suggest that hydrophobic residues in the S5 segment, but not the S6 segment, interact with Phe-240 to stabilize the closed state of Slo2.1 channels.
4. Discussion
A hallmark feature of ligand-gated channels is their low level of constitutive activity in the absence of an activating ligand. The Po of human Slo2.1 channels expressed in Xenopus oocytes is extremely low when measured under normal physiological conditions. When maximally activated by NFA, the onset of ISlo2.1 in response to changes in membrane potential is time- and voltage-independent [7] and its magnitude is increased by ~400-fold (Fig. 3B). Thus, in the absence of an activator, the relative Po of Slo2.1 channels in oocytes is < 0.0025. Single channel recordings indicate that even when maximally activated by NFA, Po < 0.5 [7], indicating that the absolute Po value for Slo2.1 channels under basal conditions is even lower (~0.001). As in our previous studies [7, 19], the ratio of whole cell ISlo2.1 measured under basal conditions and after maximal activation with NFA was used to provide an estimate of the relative Po for mutant Slo2.1 channels under basal conditions (i.e., Ic-rel) and after submaximal activation by NFA or elevated [Na+]i. Single channel recordings of inside-out patches (to facilitate application of a variable [Na+]i) or outside-out patches (for application of variable [NFA]o) are required to precisely measure channel Po. However, this approach is very difficult for Slo2.1 channels that, unlike Slo2.2 [30], run down very quickly after excision of a membrane patch from the cell. Although run-down can be slowed by including phosphatidylinositol 4,5-bisphosphate in the intracellular solution [9], we have found that in the majority of inside-out macropatch recordings of wt Slo2.1 channels, elevating [Na+]i from 3 to 100 mM did not activate channels that could subsequently be readily induced to open by NFA [14], indicating that other unknown intracellular factor(s) are required for stable channel activity.
Ala-scanning mutagenesis identified six residues in the S5 segment of Slo2.1 that are of potential importance for stabilizing channels in a closed state. In the static homology model of the Slo2.1 pore domain, the side chains of five of these S5 residues face towards the S6 segment and thus, may couple conformational changes in the S6 movement in response to ligand binding. Leu-209 is the only one of these S5 residues predicted by the homology model to be in close proximity to the side chain of Phe-240, located at the C-terminal end of the pore helix. We previously proposed that a hydrophobic interaction between these two residues could contribute to the low Po of Slo2.1 channels [19]. In the present study we explored further the importance of residue 209. Ic-rel was enhanced by substitution of Leu-209 with an acidic residue (Glu) or several polar residues (Asn, Gln, Thr, Ser). However, substitution of Leu-209 with other charged residues did not alter (Asp, His) or only slightly increased (Lys) Ic-rel. These findings indicate that hydrophobicity of Leu-209 is not the sole determinant of the very low Po of wt Slo2.1 channels under normal physiological conditions. The contrast in effects between substitution of Leu-209 with two similar acidic residues that differ by only one carbon (Asp and Glu) was dramatic and indicates that a very specific interaction between Glu-209 and another native residue induces channel opening. In our homology model, the side chain of residue 209, regardless of identity, is in closest proximity to Phe-240; however, the uncertainties inherent in a static homology model preclude identification of specific atomic interactions that mediate the observed effects of Leu-209 mutations.
It is unlikely that a single pair of residues (e.g., Leu-209 and Phe-240) is responsible for determining the Po of a channel. Indeed our extensive mutagenesis approach identified multiple residues in S5 that collectively appear to modulate basal channel activity. Reduction in the side chain volume by combined substitution with Ala of three S5 residues (Leu-209, Ile-210, Cys-213) predicted to be in close proximity to Phe-240 induced maximum constitutive activity of Slo2.1, rivaling that achieved by the more perturbing L209E mutation. As discussed previously, the gating of ligand-gated, voltage-independent K+ channels such as Slo2.1 [19] or KCa3.1 [16, 31] is likely to involve subtle movements of the S5/S6 segments. Thus, it is plausible that the specific residues in S5 that interact with the pore helix in the channel could vary depending on the extent of activation by intracellular Na+ or NFA.
Previous studies of other K+ channels, KCa3.1 and KCNQ1 in particular, have demonstrated that specific interactions between single residues in the S5 transmembrane segment and the base of the pore helix can modulate channel opening. Garneau et al [16, 31] have studied the structural basis of KCa3.1 channel gating. These channels have a low Po-max (~0.2) even after maximal activation with saturating concentrations of intracellular Ca2+. However, specific point mutations of Phe-248 or Trp-216 can increase Po-max to above 0.8 [16]. Based on extensive modeling and analysis of mutant channels, it was proposed that an aromatic-aromatic interaction between Phe-248 located at the base of the pore helix and Trp-216 in the S5 segment stabilizes the closed state of KCa3.1 channels and [16]. Phe-248 and Trp-216 of KCa3.1 are equivalent to Phe-240 and Ile-210 of Slo2.1 (Fig. 2A). While mutations of Ile-210 clearly affect channel activity (Figs. 5–7), the effects are not as dramatic as that observed for Leu-209 mutations. It is likely that both residues interact with Phe-240 as suggested by our finding that L209A/F240A and I210A/F240A channels both appeared to be maximally activated under basal conditions (Fig. 6A). Mutation of Phe-248 to a Thr had almost no effect on Po-max of single KCa3.1 channels [16], whereas the equivalent mutation in Slo2.1 (F240T) induced maximal channel activation. Such differences can be accounted for if the hydroxyl group of Thr formed a polar H-pi interaction with Trp-216 in KCa3.1, whereas in Slo2.1 the Thr hydroxyl group was repelled by the hydrophobic residues Leu-209 and Ile-210.
In KCNQ1 channels, mutation of Val-310 (equivalent to Phe-240 in Slo2.1) to the smaller residues Ala or Gly induced strong inactivation and prevented channels from fully closing at negative potentials [32]. Modeling suggests that Val-310 interacts with Leu-273 in S5 (equivalent to Ile-210 in Slo2.1) and Phe-340 in S6. Mutation of either the S5 or S6 residues in KCNQ1 induces the same effects as the V310A/G mutations [32], suggesting that an interaction between Val-310 and the two closest hydrophobic residues in the S5 and S6 segments modulates the selectivity filter inactivation gate. Thus, interactions between the pore helix and S5 (and S6 to a lesser extent) modify channel gating mediated by the selectivity filter in KCa3.1, KCNQ1 and Slo2.1 channels and perhaps in other K+ channels that have yet to be investigated in a similar fashion.
While the crystal structure of MlotiK1 was used to guide mutagenesis in this study, it must be emphasized that the process was initiated with a model-independent Ala-scanning of the S5 segment, followed by multiple mutations of single residues of special interest (e.g., Leu-209) and combined substitutions of multiple residues. This approach allowed us to identify residues in S5 and S6 that were of potential importance in determining the very low basal activity of wt Slo2.1 channels. A homology model for KCa3.1 based on MlotiK best predicted meaningful, experimentally verified interactions between Phe-248 and residues in S5 [16]. Moreover, the structural basis of channel activation, deduced from analysis of recent cryo-EM structures of the resting state and active (cAMP bound to the CNBD) state, makes MlotiK1 a more relevant point of reference than voltage-gated bacterial or vertebrate K+ channels. In MlotiK1 the aperture formed by the S6 bundle crossing is 8.5–10 Å in both the closed and open state, wide enough for passage of hydrated K+ ions, ligand (cAMP) binding to a cytoplasmic C-terminal domain (the CNBD) causes only a subtle twisting of the S5 and S6 segments, and ion conductance is gated by the selectivity filter [26]. All these features of channel gating are similar to what has been proposed for KCa3.1, Slo1 and Slo2.1. We previously suggested that the opening of the putative selectivity filter gate of Slo2.1 is allosterically coupled to Na+ binding via a conformational change (twisting?) of the S6 segments [19]. Our new findings highlight the important role of a specific and dynamic interaction between the S5 segment and the pore helix in modulating channel activity. Based on our present and previous findings we suggest the following model for Slo2.1 channel activation by intracellular Na+: Na+ binds to the C-terminus at four equivalent sites located at the interface of the RCK2 domain of one subunit and the RCK1 domain of an adjacent subunit [14]. Na+ binding causes a subtle twist or displacement in the S6 segments that are connected to the RCK1 domains of each subunit via C-linkers. The conformational change in S6 segments in turn induces a twist/displacement of the S5 segments either directly, as suggested by the results of our Ala-scanning of the S5 segment (Fig. 2C), or via the S4-S5 linker as has been proposed for KCa3.1 channels [16]. Finally, the movement of S5 disrupts the hydrophobic interaction between the pore helix residue Phe-240 and the S5 residues Leu-209 and Ile-210, causing an undefined change in the conformation of the selectivity filter that favors the open, ion conducting state of the channel. In contrast to this model for Slo2.1 channel activation, the recently published structure of Slo2.2 (in a Na+-free state) indicates that a barrier to ion conduction is formed near the C-terminal ends of the S6 segments, specifically Met-333, that constricts the pore to an estimated diameter (4–6 Å) that is too narrow to permit diffusion of a hydrated K+ ion [27]. Thus, an alternate interpretation of all our experimental findings would be that mutations of Phe-240, Leu-209 and other interacting residues cause Slo2.1 channels to open exerting a long-range allosteric effect on the cytoplasmic gate formed by the S6 inner helix. However, it is difficult to imagine how such a gating mechanism could account for the dramatic differences in Ic-rel induced by substitution of Leu-209 with such similar residues as Glu and Asp (Fig. 3).
There are several limitations of our study. First, Ala scanning is a biased method for identification of residues that have an important role in channel gating and cannot distinguish between direct effects of the mutation and induced allosteric effects. Substitution with a different residue could have produced a different result. Second, activation of Slo2.1 by NFA is unnatural and may proceed by protein conformational changes that do not mimic normal channel activation by cytoplasmic Na+. Third, quantifying the constitutive activity of mutant channels by comparing whole cell ISlo2.1 before and after application of NFA is imprecise compared to Po estimates based on single channel recordings.
In summary, hydrophobic interaction between Phe-240 in the pore helix and nearby residues in the S5 segment stabilize the closed state of Slo2.1. As previously suggested for KCa3.1 channels, pharmacological modulation of this interaction provides an interesting approach for activation of Slo2.1 channels. Future studies are needed to determine if fenamates activate Slo channels by disruption of interactions between the pore helix and the S5 segment.
Highlights.
Intracellular Na+-activated Slo2.1 K+ channels have a very low open probability.
S5 segment-pore helix hydrophobic interactions stabilize Slo2.1 in a closed state.
The selectivity filter acts as the activation gate in Slo2.1 channels.
Acknowledgments
This work was supported by NIH NHLBI grant R01 HL103877 and a grant from the Nora Eccles Treadwell Foundation to MCS.
Abbreviations
- Ic-rel
constitutive Irel
- Irel
current normalized to that induced by 6 mM NFA
- ISlo2.1
Slo2.1 current
- KNa
Na+-activated K+
- NFA
niflumic acid
- Po
open probability
- TEVC
two-electrode voltage clamp
- Vt
test voltage
- wt
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Richards FM. Areas, volumes, packing and protein structure. Ann Rev Biophys Bioengin. 1977;6:151–176. doi: 10.1146/annurev.bb.06.060177.001055. [DOI] [PubMed] [Google Scholar]
- 2.Kameyama M, Kakei M, Sato R. Intracellular Na+ activates a K+ channel in mammalian cardiac cells. Nature. 1984;309:354–356. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- 3.Bader CR, Bernheim L, Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985;317:540–542. doi: 10.1038/317540a0. [DOI] [PubMed] [Google Scholar]
- 4.Punta M, Maritan A. A knowledge-based scale for amino acid membrane propensity. Proteins. 2003;50:114–121. doi: 10.1002/prot.10247. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci. 2003;23:11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan A, Santi CM, Wei A, Wang ZW, Pollak K, Nonet M, Kaczmarek L, Crowder CM, Salkoff L. The sodium-activated potassium channel is encoded by a member of the Slo gene family. Neuron. 2003;37:765–773. doi: 10.1016/s0896-6273(03)00096-5. [DOI] [PubMed] [Google Scholar]
- 7.Dai L, Garg V, Sanguinetti MC. Activation of Slo2.1 channels by niflumic acid. J Gen Physiol. 2010;135:275–295. doi: 10.1085/jgp.200910316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamsett TJ, Picchione KE. A. Bhattacharjee, NAD+ activates KNa channels in dorsal root ganglion neurons. J Neurosci. 2009;29:5127–5134. doi: 10.1523/JNEUROSCI.0859-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg P, Sanguinetti MC. Intracellular ATP does not inhibit Slo2.1 K+ channels. Physiological reports. 2014;2:e12118. doi: 10.14814/phy2.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berg AP, Sen N, Bayliss DA. TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J Neurosci. 2007;27:8845–8856. doi: 10.1523/JNEUROSCI.0551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg P, Sanguinetti MC. Structure-activity relationship of fenamates as Slo2.1 channel activators. Mol Pharmacol. 2012;82:795–802. doi: 10.1124/mol.112.079194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White MM, Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2+-activated Cl channels in Xenopus oocytes. Mol Pharmacol. 1990;37:720–724. [PubMed] [Google Scholar]
- 13.Wang HS, Dixon JE, McKinnon D. Unexpected and differential effects of Cl channel blockers on the Kv4.3 and Kv4.2 K+ channels. Implications for the study of the Ito2 current. Circ Res. 1997;81:711–718. doi: 10.1161/01.res.81.5.711. [DOI] [PubMed] [Google Scholar]
- 14.Thomson SJ, Hansen A, Sanguinetti MC. Identification of the intracellular Na+ sensor in Slo2.1 potassium channels. J Biol Chem. 2015;290:14528–14535. doi: 10.1074/jbc.M115.653089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein H, Garneau L, Banderali U, Simoes M, Parent L, Sauve R. Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: results from a substituted cysteine accessibility analysis. J Gen Physiol. 2007;129:299–315. doi: 10.1085/jgp.200609726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garneau L, Klein H, Lavoie MF, Brochiero E, Parent L, Sauve R. Aromatic-aromatic interactions between residues in KCa3.1 pore helix and S5 transmembrane segment control the channel gating process. J Gen Physiol. 2014;143:289–307. doi: 10.1085/jgp.201311097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Aldrich RW. Charge substitution for a deep-pore residue reveals structural dynamics during BK channel gating. J Gen Physiol. 2011;138:137–154. doi: 10.1085/jgp.201110632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jenkins DP, Strobaek D, Hougaard C, Jensen ML, Hummel R, Sorensen US, Christophersen P, Wulff H. Negative gating modulation by (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphthylamine (NS8593) depends on residues in the inner pore vestibule: pharmacological evidence of deep-pore gating of KCa2 channels. Mol Pharmacol. 2011;79:899–909. doi: 10.1124/mol.110.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garg P, Gardner A, Garg V, Sanguinetti MC. Structural basis of ion permeation gating in Slo2.1 K+ channels. J Gen Physiol. 2013;142:523–542. doi: 10.1085/jgp.201311064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+ channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacKinnon R, Cohen SL, Kuo A, Lee A, Chait BT. Structural conservation in prokaryotic and eukaryotic potassium channels. Science. 1998;280:106–109. doi: 10.1126/science.280.5360.106. [DOI] [PubMed] [Google Scholar]
- 22.Roux B, MacKinnon R. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science. 1999;285:100–102. doi: 10.1126/science.285.5424.100. [DOI] [PubMed] [Google Scholar]
- 23.Morais-Cabral JH, Zhou Y, MacKinnon R. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 2001;414:37–42. doi: 10.1038/35102000. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Morais-Cabral JH, Kaufman A, MacKinnon R. Chemistry of ion coordination and hydration revealed by a K+ channel- Fab complex at 2.0 A resolution. Nature. 2001;414:43–48. doi: 10.1038/35102009. [DOI] [PubMed] [Google Scholar]
- 25.Schreibmayer W, Lester HA, Dascal N. Voltage clamping of Xenopus laevis oocytes utilizing agarose-cushion electrodes. Pflugers Arch. 1994;426:453–458. doi: 10.1007/BF00388310. [DOI] [PubMed] [Google Scholar]
- 26.Kowal J, Chami M, Baumgartner P, Arheit M, Chiu PL, Rangl M, Scheuring S, Schroder GF, Nimigean CM, Stahlberg H. Ligand-induced structural changes in the cyclic nucleotide-modulated potassium channel MloK1. Nature communications. 2014;5:3106. doi: 10.1038/ncomms4106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hite RK, Yuan P, Li Z, Hsuing Y, Walz T, MacKinnon R. Cryo-electron microscopy structure of the Slo2.2 Na+-activated K+ channel. Nature. 2015;527:198–203. doi: 10.1038/nature14958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krieger E, Koraimann G, Vriend G. Increasing the precision of comparative models with YASARA NOVA--a self-parameterizing force field. Proteins. 2002;47:393–402. doi: 10.1002/prot.10104. [DOI] [PubMed] [Google Scholar]
- 29.Horovitz A. Double-mutant cycles: a powerful tool for analyzing protein structure and function. Folding & design. 1996;1:R121–R126. doi: 10.1016/S1359-0278(96)00056-9. [DOI] [PubMed] [Google Scholar]
- 30.Yan Y, Yang Y, Bian S, Sigworth FJ. Expression, purification and functional reconstitution of slack sodium-activated potassium channels. J Membr Biol. 2012;245:667–674. doi: 10.1007/s00232-012-9425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garneau L, Klein H, Banderali U, Longpre-Lauzon A, Parent L, Sauve R. Hydrophobic interactions as key determinants to the KCa3.1 channel closed configuration. An analysis of KCa3.1 mutants constitutively active in zero Ca2+ . J Biol Chem. 2009;284:389–403. doi: 10.1074/jbc.M805700200. [DOI] [PubMed] [Google Scholar]
- 32.Seebohm G, Westenskow P, Lang F, Sanguinetti MC. Mutation of colocalized residues of the pore helix and transmembrane segments S5 and S6 disrupt deactivation and modify inactivation of KCNQ1 K+ channels. J Physiol. 2005;563:359–368. doi: 10.1113/jphysiol.2004.080887. [DOI] [PMC free article] [PubMed] [Google Scholar]