Abstract
Background
Altered lipid metabolism and plasma fatty acid (FA) levels are associated with colorectal cancer (CRC). Obesity and elevated waist circumference (WC) increase the likelihood of developing precancerous colon adenomas.
Methods
Venous blood was collected from 126 males, ages 48 to 65 years, who received routine colonoscopies. Plasma phospholipid (PPL) FAs were isolated, derivatized, and then analyzed using gas chromatography. Odds ratios (ORs) and 95% confidence intervals were determined using polytomous logistic regression after adjusting for confounding factors (i.e. age, smoking, WC, and BMI).
Results
PPL palmitic acid (PA) was inversely correlated with the presence of colon adenomas (p = 0.01). For each unit increase in palmitoleic acid (OR: 3.75, p = 0.04) or elaidic acid (OR: 2.92, p = 0.04) an individual was more likely to have adenomas relative to no colon polyps. Higher enzyme activity estimates (EAEs) of stearoyl-CoA desaturase-1 (SCD-1, p = 0.02) and elongation of very long chain-6 (Elovl-6, p = 0.03) were associated with an individual being approximately 1.5 times more likely to have an adenoma compared to no polyps.
Conclusions
PPL FAs and EAEs, which have previously been associated with CRC, are significantly different in those with adenomas when compared to those without polyps. PPL PA, elaidic acid, and SCD-1 and Elovl-6 EAEs are associated with adenomas independent of BMI and WC.
Impact
PPL PA, elaidic acid, and SCD-1 and Elovl-6 EAEs are associated with adenomas even after adjusting for obesity-related risk factors and may function as novel biomarkers of early CRC risk.
Keywords: Palmitic acid, Colon adenomas, Biomarker, Fatty acid metabolism, Fatty acid desaturation
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer among men and women in the US (1). Risk factors for CRC include obesity, waist circumference (WC), age, smoking, physical inactivity, inflammatory bowel disease, and a family history of CRC or adenomas (2). As much as 70% of the risk of developing CRC has been attributed to modifiable risk factors, including diet (3). Consequently, dietary intake of varying amounts of saturated fatty acids (SFA), monounsaturated fatty acids (MUFA), and polyunsaturated fatty acids (PUFA) has been an area of active research in the pathology and prevention of CRC.
Several specific FAs are associated with CRC. For example, a higher erythrocyte oleic acid (OA) to stearic acid (SA) ratio has been associated with CRC (4). Also, CRC is associated with higher levels of plasma phospholipid (PPL) SFAs, in particular palmitic acid (PA) (5). Among PUFAs, dietary consumption of greater amounts of omega-3 PUFAs and lesser amounts of omega-6 PUFAs is typically associated with a decreased risk for developing CRC (6). Diets higher in MUFAs and lower in SFAs also potentially prevent CRC (7). Blood FAs associated with CRC may originate from dietary intake as well as from endogenous synthesis though lipid metabolism.
Altered lipid metabolism also is suspected to play a role in colon carcinogenesis during the transformation of colorectal polyps to CRC (7–9). Dietary SFAs can be desaturated and elongated through the action of various enzymes. Stearoyl-CoA desaturase-1 (SCD-1) and elongation of very long chain fatty acid protein-6 (Elovl-6) are the rate-limiting enzymes controlling metabolic shifts towards production of long chain MUFAs. Upregulation of SCD-1, the desaturase responsible for converting PA and SA into MUFAs, has been linked to CRC (9). MUFAs influence cellular apoptosis and are believed to play a role in the mutagenesis of tumors in several types of cancer, including CRC (8, 10). However, FAs and the enzymes that regulate the endogenous production of long chain MUFAs have not been sufficiently investigated in relation to precancerous colon adenomas. Additionally, the complex mechanisms by which dietary FAs and lipid metabolism influence the development of CRC continue to be investigated.
The formation of adenomas precedes the onset of CRC, with removal of adenomas significantly decreasing the risk of developing CRC (11). Determining the levels of specific PPL FAs associated with the presence of adenomas could lead to the identification of blood-based biomarkers useful for early CRC screening, increasing opportunities for preventative interventions. PPLs are reflective of endogenous and exogenous sources of FAs and have been used to measure CRC risk in relation to FA intake (5, 12). Limitations in accurately measuring dietary intake combined with the need to assess endogenous lipid synthesis dictate that the direct analysis of PPLs is necessary in order to accurately determine the association between plasma FAs and colon carcinogenesis. Therefore, in this study we sought to identify specific PPL levels of SFAs, cis-MUFAs, and trans-MUFAs associated with the presence of colorectal adenomas.
Materials and Methods
Study Population and Clinical Parameters
Healthy male subjects (n = 126, > 96% Caucasian) 48 to 65 years of age were enrolled as previously reported (13). Individuals were excluded for medical conditions associated with increased CRC risk (13). Immediately after enrollment, trained staff collected anthropometric measurements and venous blood of study participants (13). Smoking status was assessed as “ever smoked” or “never smoked”. Each individual received a full colonoscopy as previously described (14). Serum and plasma fractions were separated from blood and stored at −80° C.
Plasma Phospholipid Extraction, Isolation and Analysis
In brief, approximately 200 mg plasma per subject was weighed and extracted using a modified Rose and Oaklander extraction (15). PPLs were isolated using Isolute-XL ® SPE aminopropyl columns (500 mg; Bioatage, Charlotte, NC) as described by Agren et al (16). Fatty acid methyl esters (FAMEs) were prepared as previously described (17, 18). PPL FAMEs were analyzed using HS-Omega-3 Index® methodology at OmegaQuant Analytics, LLC (Sioux Falls, SD) as previously described (19). The coefficient of variation for PPL extraction, isolation, and PPL FA analysis is less than 7% for the eleven FAs presented.
Statistical analyses
Frequencies, means, and standard deviations were calculated for descriptive analyses (Table 1). Each FA was expressed as a percentage of total PPL. Means were obtained for the PL FAs (Figure 1). PPL FA enzyme activity estimates (EAE) were calculated as the ratio of product-to-substrate. SCD-1 EAE was calculated in two ways (20): SCD n-7 index (SCD n-7) = palmitoleic (POA)/PA, and SCD n-9 index (SCD n-9) = OA/SA. A variation of the Elovl-6 EAE was calculated as Elovl-6 = Σ [SA + OA]/PA (21, 22). The total PPL SFA, cis-MUFA, trans-MUFA were calculated as follows: total PPL SFA was calculated as Σ PA + SA + arachidic + behenic + lignoceric; total PPL cis-MUFA was calculated as Σ POA + eicosaenoic + nervonic (NA); total PPL trans-MUFA was calculated as Σ palmitelaidic + elaidic. Spearman correlations were performed since several variables were not normally distributed. These correlations, presented in Table 2, were conducted using only the 106 individuals that had adenomas or no polyps.
Multiple imputation (seed = 20121119, imputations = 7) was used to impute all missing smoking data (23). The factors—smoking, PA, SA, arachidic, behenic, lignoceric, POA, OA, NA, palmitelaidic, elaidic—were used in the imputation algorithm of missing values. Eicosenoic acid was removed from the imputation algorithm due to a high correlation with elaidic acid.
The Wilcoxon-Mann-Whitney test was performed to compare the PPL FA composition of participants with adenomas to that of those with no polyps. Polytomous logistic regression models for categorical outcome data were used to determine odds ratios (OR) and 95% confidence intervals (CI) for the likelihood of having an adenoma relative to no polyps. Categories were defined as polyp severity: 1) Individuals with no colon polyps, and 2) Individuals with ≥ 1 adenoma. Individuals with polyps not classified as adenomas were excluded from statistical analyses. In all polytomous logistic regression models, polyp severity was analyzed categorically as the dependent variable with the reference category defined as individuals with no colon polyps. The odds ratios for Elovl-6, SCD n-7, and palmitelaidic acid have been calculated on the basis that there is a unit change of 0.01 for the respective beta coefficient for each given parameter. All models were adjusted for age and smoking status except where noted.
Due to high correlation (> 0.9, data not shown) between BMI and WC, these anthropometric measurements could not be analyzed in the same model. Two additional models were run, the first with the addition of BMI and the second with the addition of WC. These models are referred to as model 2 and model 3 respectively (Table 3). FAs were analyzed as continuous (Table 2 and 3, and Figure 1) and categorical independent variables (Figure 2). FAs were categorized into tertiles (with lowest tertile as reference) for adenomas relative to no polyps. Test for trend was carried out across tertiles for the FAs of interest. Because smoking data was imputed, multiple imputation analyze (Proc MI ANALYZE) was used to determine the results from analysis of the imputed datasets. P-values were considered statistically significant if p ≤ 0.05 and a statistical trend if 0.05 < p ≤ 0.09. Statistical analyses were conducted using SAS version 9.3 (Cary, NC).
Results
Participant characteristics are displayed in Table 1. As previously reported (13), 37 (29.4%) participants had adenomas while 69 (54.8%) had no polyps. Seventeen (13.5%) participants had ≥ 3 polyps including at least one adenoma. Both BMI and WC increased with polyp severity, as previously reported (13).
Table 1.
Characteristics of study population a
| Overall | No Polyp | Hyperplastic | Adenoma | |
|---|---|---|---|---|
| n = 126 | n = 69 | n = 20 | n = 37 | |
| Age (years) | 57 ± 5 | 57 ± 5 | 57 ± 4 | 57 ± 5 |
| Ever Smoked (% total) b | 31 | 15 | 4 | 12 |
| BMI (kg/m2) | 30 ± 5 | 28 ± 4 | 29 ± 5 | 32 ± 6 |
| WC (inches) | 41 ± 6 | 40 ± 6 | 42 ± 4 | 44 ± 6 |
Participants (n = 126) were male, > 96% Caucasian; Values expressed as mean ± standard deviation.
Data missing for 22 participants.
BMI: body mass index; WC: waist circumference.
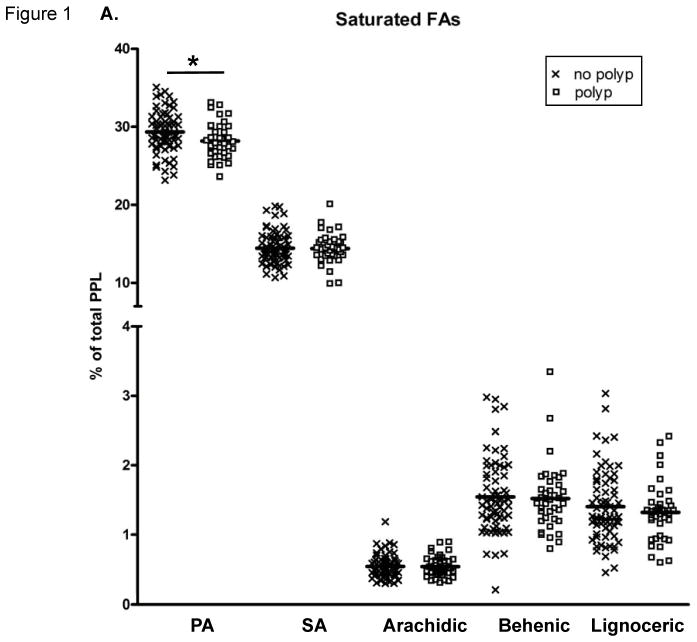
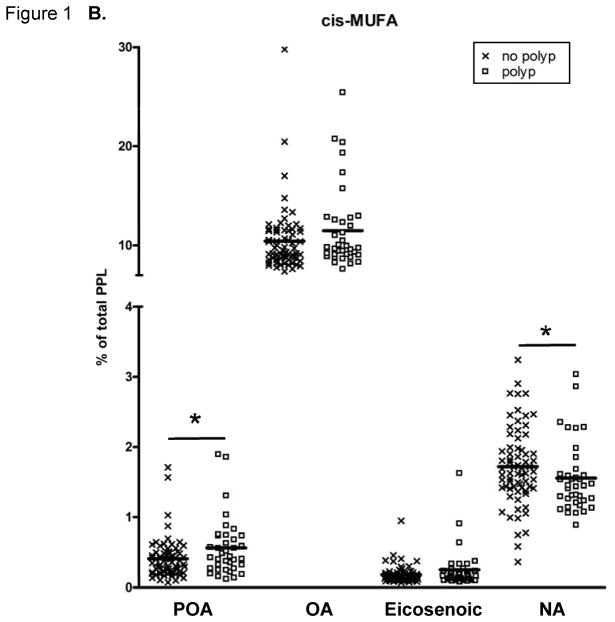
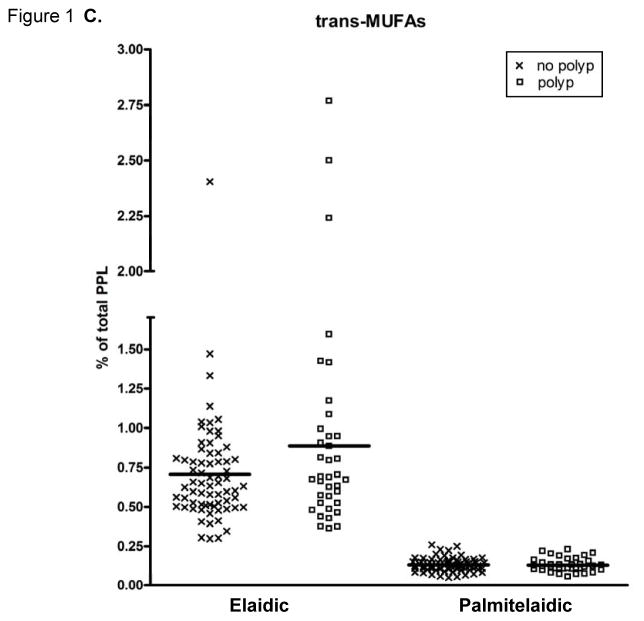
The PPL FA proportions are presented in Figure 1. PPL PA was significantly lower and total SFAs tended (p=0.0684) to be lower in those with adenomas compared to those without polyps. The PPL cis-MUFA POA was significantly higher in individuals with adenomas, while PPL cis-MUFA NA was significantly lower in the adenoma group compared to those with no colon polyps. The percentage of total trans-MUFAs in PPLs did not differ between the groups.
Figure 1.
Fatty acid (FA) content of plasma phospholipids (PPLs). (A) Saturated FAs (SatFAs), (B) cis-monounsaturated FAs (MUFAs), and (C) trans-MUFAs. The symbol “X” represents PPL FA levels of individuals with no polyps and “□” represents PPL FA levels of individuals with adenomas. The solid lines indicate the mean. FAs are expressed as a percent of total PPL FAs. A “*” indicates p ≤ 0.05, calculated by Wilcoxon-Mann-Whitney nonparametric U-test. NA, nervonic acid; OA, oleic acid; PA, palmitic acid; POA, palmitoleic acid; SA, stearic acid.
Elongating and desaturating EAEs were positively associated with polyp severity. SFAs are enzymatically desaturated to form cis-MUFAs. Both SFAs and cis-MUFAs can be enzymatically elongated to form longer chain products. SCD n-7, SCD n-9, and Elovl-6 EAEs are non-invasive methods to assess FA metabolism (24), calculated as the FA product-to-precursor ratio for respective EAE. We observed SCD n-7 was significantly elevated (p=0.0163) in those with adenomas compared to those with no colon polyps. However, SCD n-9 did not differ (p=0.5868) between individuals with no polyps and those with adenomas. Elovl-6 was significantly elevated (0.0105) in those with adenomas compared to those with no colon polyps.
Several PPL FAs measured were significantly correlated with polyp severity and with other SFAs and MUFAs (Table 2). Polyp severity was not correlated with PPL palmitelaidic, elaidic, or total trans-MUFA. Polyp severity was inversely correlated with PPL PA and NA (Table 2). Also, polyp severity was positively correlated with PPL POA, SCD n-7, and Elovl-6.
Table 2.
Spearman correlation between fatty acids and polyp severity a
| PA | SA | Arachidic | Behenic | Lignoceric | Total SFA b | POA | OA | Eicosenoic | NA | Total Cis-MUFA c | Palmitelaidic | Elaidic | Total Trans-MUFA d | SCD n-7 e | SCD n-9 f | Elovl-6 g | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyp severity | −0.245 | 0.042 | 0.003 | −0.033 | −0.051 | −0.185 | 0.195 | 0.124 | 0.094 | −0.205 | 0.102 | −0.033 | 0.127 | 0.110 | 0.228 | 0.048 | 0.255 |
| 0.011 | 0.665 | 0.973 | 0.740 | 0.603 | 0.058 | 0.045 | 0.207 | 0.340 | 0.035 | 0.296 | 0.738 | 0.195 | 0.260 | 0.019 | 0.624 | 0.008 | |
| PA | 0.023 | −0.005 | −0.042 | −0.035 | 0.752 | 0.337 | −0.041 | −0.352 | −0.238 | −0.087 | −0.083 | −0.193 | −0.190 | 0.216 | −0.044 | −0.768 | |
| 0.816 | 0.957 | 0.672 | 0.718 | <.0001 | 0.0004 | 0.675 | 0.0002 | 0.014 | 0.378 | 0.400 | 0.047 | 0.051 | 0.026 | 0.655 | <.0001 | ||
| SA | 0.078 | 0.150 | −0.053 | 0.551 | −0.277 | −0.526 | −0.149 | −0.346 | −0.584 | −0.028 | 0.180 | 0.156 | −0.282 | −0.783 | 0.209 | ||
| 0.428 | 0.125 | 0.587 | <.0001 | 0.004 | <.0001 | 0.127 | 0.0003 | <.0001 | 0.772 | 0.065 | 0.109 | 0.003 | <.0001 | 0.031 | |||
| Arachidic | 0.549 | 0.423 | 0.247 | −0.085 | −0.047 | 0.161 | 0.453 | 0.064 | −0.046 | 0.014 | 0.011 | −0.095 | −0.079 | 0.079 | |||
| <.0001 | <.0001 | 0.011 | 0.386 | 0.630 | 0.099 | <.0001 | 0.516 | 0.640 | 0.888 | 0.915 | 0.331 | 0.424 | 0.422 | ||||
| Behenic | 0.666 | 0.310 | −0.199 | −0.225 | 0.117 | 0.239 | −0.156 | 0.011 | 0.094 | 0.092 | −0.192 | −0.236 | −0.010 | ||||
| <.0001 | 0.001 | 0.041 | 0.021 | 0.231 | 0.013 | 0.111 | 0.914 | 0.340 | 0.348 | 0.048 | 0.015 | 0.918 | |||||
| Lignoceric | 0.192 | −0.083 | −0.008 | 0.157 | 0.274 | 0.025 | −0.032 | −0.008 | −0.015 | −0.073 | 0.005 | −0.007 | |||||
| 0.049 | 0.400 | 0.932 | 0.109 | 0.005 | 0.800 | 0.746 | 0.938 | 0.878 | 0.454 | 0.958 | 0.947 | ||||||
| Total SFA | 0.094 | −0.305 | −0.260 | −0.274 | −0.347 | −0.110 | −0.061 | −0.075 | 0.001 | −0.457 | −0.437 | ||||||
| 0.338 | 0.002 | 0.007 | 0.004 | 0.0003 | 0.262 | 0.531 | 0.445 | 0.991 | <.0001 | <.0001 | |||||||
| POA | 0.634 | −0.088 | −0.239 | 0.598 | −0.175 | −0.275 | −0.273 | 0.988 | 0.572 | −0.037 | |||||||
| <.0001 | 0.368 | 0.014 | <.0001 | 0.072 | 0.004 | 0.005 | <.0001 | <.0001 | 0.704 | ||||||||
| OA | 0.227 | 0.010 | 0.946 | −0.005 | −0.166 | −0.149 | 0.661 | 0.924 | 0.341 | ||||||||
| 0.019 | 0.917 | <.0001 | 0.957 | 0.089 | 0.127 | <.0001 | <.0001 | 0.0003 | |||||||||
| Eicosenoic | 0.255 | 0.336 | −0.151 | 0.191 | 0.166 | −0.044 | 0.205 | 0.424 | |||||||||
| 0.008 | 0.0004 | 0.122 | 0.050 | 0.089 | 0.657 | 0.035 | <.0001 | ||||||||||
| NA | 0.244 | 0.028 | −0.074 | −0.062 | −0.210 | 0.155 | 0.016 | ||||||||||
| 0.012 | 0.777 | 0.451 | 0.528 | 0.031 | 0.112 | 0.874 | |||||||||||
| Total Cis-MUFA | −0.050 | −0.167 | −0.152 | 0.626 | 0.918 | 0.330 | |||||||||||
| 0.614 | 0.086 | 0.120 | <.0001 | <.0001 | 0.001 | ||||||||||||
| Palmitelaidic | 0.338 | 0.463 | −0.173 | 0.003 | −0.016 | ||||||||||||
| 0.0004 | <.0001 | 0.076 | 0.977 | 0.868 | |||||||||||||
| Elaidic | 0.987 | −0.259 | −0.208 | 0.128 | |||||||||||||
| <.0001 | 0.007 | 0.033 | 0.192 | ||||||||||||||
| Total Trans-MUFA | −0.257 | −0.188 | 0.113 | ||||||||||||||
| 0.008 | 0.054 | 0.250 | |||||||||||||||
| SCD n-7 | 0.592 | 0.066 | |||||||||||||||
| <.0001 | 0.503 | ||||||||||||||||
| SCD n-9 | 0.159 | ||||||||||||||||
| 0.103 |
Correlations were conducted using only the 106 individuals that had no polyps or adenomas. Numbers in gray rows indicate spearman correlation coefficient, and numbers listed directly below, in white rows, indicate corresponding p-value. P-values bolded if significant (p ≤ 0.05) and italicized if 0.05 > p ≤ 0.09. EAE, enzyme activity estimate; NA, nervonic acid; OA, oleic acid; PA, palmitic acid; SA, stearic acid; SFA, saturated fatty acids; SCD n-7, stearoyl-CoA desaturase n-7 EAE; SCDn-9, stearoyl-CoA desaturase n-9 EAE. Elovl-6; elongation of very long chain fatty acids-6 EAE.
Total SFA calculated as the Σ PA+ SA + arachidic + behenic + lignoceric.
Total Cis-MUFA calculated as the Σ POA+ OA + eicosenoic + NA.
Total Trans-MUFA calculated as the Σ palmitelaidic + elaidic.
SCDn-7 calculated as the ratio of POA/PA.
SCD n-9 calculated as the ratio of OA/SA
Elovl-6 calculated as the ratio of Σ [ SA+ OA]/PA.
Colon polyps and several PPL FAs were correlated with confounding factors such as age, smoking status, BMI, and WC (data not shown). Polytomous logistic regression was performed to determine which PPL FAs and EAEs were significantly associated with adenomas after adjusting for these confounding factors (Table 3). Model 1 included PPL FA, and was adjusted for age and smoking. To account for the potential contribution of BMI or visceral adiposity (WC) to the likelihood of having an adenoma, two additional models were tested. Model 2 included PPL FA and was adjusted for BMI, in addition to age and smoking. Model 3 included PPL FA and was adjusted for WC, in addition to age and smoking.
Table 3.
Association of fatty acids and enzyme activity estimates, as continuous variables, with having adenomas relative to no colon polyps a
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | OR (95%CI) | P-value | OR (95%CI) | P-value | OR (95%CI) | P-value |
| C16:0 Palmitic (PA) | 0.830 (0.701, 0.982) | 0.0303 | 0.718 (0.582, 0.886) | 0.0020 | 0.756 (0.623, 0.917) | 0.0045 |
| C18:0 Stearic (SA) | 0.973 (0.806, 1.174) | 0.7737 | 0.917 (0.748, 1.123) | 0.4008 | 0.914 (0.747, 1.119) | 0.3851 |
| C20:0 Arachidic | 0.901 (0.058, 13.943) | 0.9403 | 1.053 (0.058, 19.211) | 0.9721 | 0.978 (0.059, 16.274) | 0.9877 |
| C21:0 Behenic | 0.923 (0.400, 2.131) | 0.8504 | 0.916 (0.383, 2.196) | 0.8449 | 0.936 (0.400, 2.188) | 0.8778 |
| C24:0 Lignoceric | 0.732 (0.306, 1.754) | 0.4842 | 0.925 (0.366, 2.335) | 0.8686 | 0.848 (0.345, 2.085 | 0.7192 |
| Total SFA b | 0.902 (0.803, 1.013) | 0.0811 | 0.837 (0.730, 0.960) | 0.0112 | 0.847 (0.742, 0.967) | 0.0144 |
| C16:1 Palmitoleic (POA) | 3.750 (1.079, 13.036) | 0.0376 | 2.442 (0.679, 8.783) | 0.1716 | 2.652 (0.739, 9.519) | 0.1347 |
| C18:1 Oleic (OA) | 1.097 (0.981, 1.226) | 0.1048 | 1.106 (0.983, 1.245) | 0.0932 | 1.103 (0.981, 1.239) | 0.1002 |
| C20:1 Eicosenoic | 1.020 (0.995, 1.045) | 0.1161 | 1.023 (0.997, 1.050) | 0.0794 | 1.024 (0.998, 1.051) | 0.0741 |
| C24:1 Nervonic (NA) | 0.568 (0.260, 1.243) | 0.1570 | 0.743 (0.327, 1.690) | 0.4792 | 0.745 (0.329, 1.686) | 0.4794 |
| Total Cis-MUFA c | 1.088 (0.981, 1.206) | 0.1103 | 1.099 (0.985, 1.226) | 0.0901 | 1.100 (0.987, 1.226) | 0.0864 |
| C16:1 Palmitelaidic d | 0.982 (0.894, 1.078) | 0.7017 | 1.013 (0.916, 1.119) | 0.8073 | 1.006 (0.912, 1.108) | 0.9116 |
| C18:1 Elaidic | 2.915 (1.030, 8.246) | 0.0438 | 3.111 (1.031, 9.388) | 0.0440 | 3.224 (1.060, 9.801) | 0.0391 |
| Total Trans-MUFA e | 2.708 (1.000, 7.337) | 0.0501 | 2.990 (1.029, 8.687) | 0.0441 | 3.066 (1.050, 8.955) | 0.0405 |
| SCD n-7 d,f | 1.538 (1.068, 2.215) | 0.0207 | 1.383 (0.960, 1.992) | 0.0819 | 1.410 (0.977, 2.035) | 0.0664 |
| SCD n-9 g | 2.229 (0.724, 6.864) | 0.1623 | 2.846 (0.850, 9.534) | 0.0899 | 2.739 (0.822, 9.132) | 0.1010 |
| Elovl-6 d,h | 1.358 (1.039, 1.775) | 0.0250 | 1.467 (1.090, 1.973) | 0.0114 | 1.405 (1.059, 1.865) | 0.0184 |
Models defined as: Model 1: adenoma = fatty acid + age + smoking. Model 2: adenoma = fatty acid + age + smoking + BMI. Model 3: adenoma = fatty acid + age + smoking + waist circumference. Fatty acids expressed as percent of total phospholipids. P-values bolded if significant (p ≤ 0.05) and italicized if 0.05 > p ≤ 0.09. EAE, enzyme activity estimate; MUFA, monounsaturated fatty acid; SFA, saturated fatty acids; SCDn-7, stearoyl-CoA desaturase n-7 EAE; SCDn-9, stearoyl-CoA desaturase n-9 EAE; Elovl-6, elongation of very long chain fatty acids-6 EAE.
Total SFA calculated as the Σ PA+ SA + arachidic + behenic + lignoceric.
Total Cis-MUFA calculated as the Σ POA+ OA + eicosenoic + NA.
Odds ratios for Palmitoelaidic, SCD n-7, and ELOVL-6 have been calculated on the basis that there is a unit change of 0.01 for the respective beta coefficient for each given parameter.
Trans-MUFA calculated as the Σ palmitelaidic + elaidic.
SCDn-7 calculated as the ratio of POA/PA.
SCD n-9 calculated as the ratio of OA/SA.
Elovl-6 calculated as the ratio of Σ [SA+ OA]/PA.
The odds that an individual whose PPL contained high levels of PA would have an adenoma were significantly lower than those of an individual whose PPL contained low levels of PA. This was consistent across all three models (Table 3). Specifically, for each unit increase in PPL PA individuals were 0.83 (95% CI: 0.70 – 0.98) times as likely in model 1, 0.72 (95% CI: 0.58 – 0.89) times as likely in model 2, and 0.76 (95% CI: 0.62 – 0.92) times as likely in model 3 to have adenomas rather than no colon polyps. PPL SA, arachidic, behenic, and lignoceric acid showed no association with adenomas in these 3 models. However, for each unit increase in total PPL SFAs, individuals tended to be 0.90 (95% CI: 0.80 – 1.01) times as likely to have adenomas in model 1, and individuals were 0.84 (95% CI: 0.73 – 0.96) and 0.85 (95% CI: 0.74 – 0.97) times as likely to have adenomas compared to no colon polyps when adjusted for BMI or WC, respectively.
Some MUFAs were significantly associated with the presence of adenomas (Table 3). In model 1, for each unit increase in PPL POA, an individual was 3.75 (95% CI: 1.08 – 13.04) times more likely to have an adenoma compared to no colon polyps, but there were no significant associations after adjusting for BMI (model 2) or WC (model 3). PPL elaidic acid, a C18:1 trans-MUFA, was highly associated with an increased likelihood of adenoma presence in all 3 models analyzed. Specifically, for each unit increase in PPL elaidic acid individuals were 2.92 (95% CI: 1.03 – 8.25) times more likely in model 1, 3.11 (95% CI: 1.03 – 9.39) times more likely in model 2, and 3.22 (95% CI: 1.06 – 9.80) times more likely in model 3, to have adenomas relative to no colon polyps (Table 3). PPL palmitelaidic acid, a C16:1 trans-MUFA, was not significantly associated with adenomas. For each unit increase in PPL total trans-MUFA, calculated as the Σ elaidic + palmitelaidic, individuals tended be 2.71 (95% CI: 1.00 – 7.34) times more likely to have adenomas in model 1, and individuals were 2.99 (95% CI: 1.03 – 8.69) and 3.07 (95% CI: 1.05 – 8.96) times more likely to have adenomas rather than no colon polyps in model 2 and model 3 respectively (Table 3).
Each unit increase in SCD n-7 was associated with individuals being 1.54 (95% CI: 1.07 – 2.22) times more likely to have adenomas than no polyps, and individuals with high SCD n-7 tended to be 1.38 (95% CI: 0.96 – 1.99) and 1.41 (95% CI: 0.98 – 2.04) times more likely to have adenomas rather than no polyps in models 2 and 3, respectively (Table 3). Unit increases in Elovl-6 were associated with adenomas in all 3 models analyzed. Specifically, for each unit increase in Elovl-6, individuals were 1.36 (95% CI: 1.04 – 1.78) times more likely in model 1, 1.47 (95% CI: 1.09 – 1.97) times more likely in model 2, and 1.41 (95% CI: 1.06 – 1.87) times more likely in model 3 to have adenomas relative to no colon polyps (Table 3).
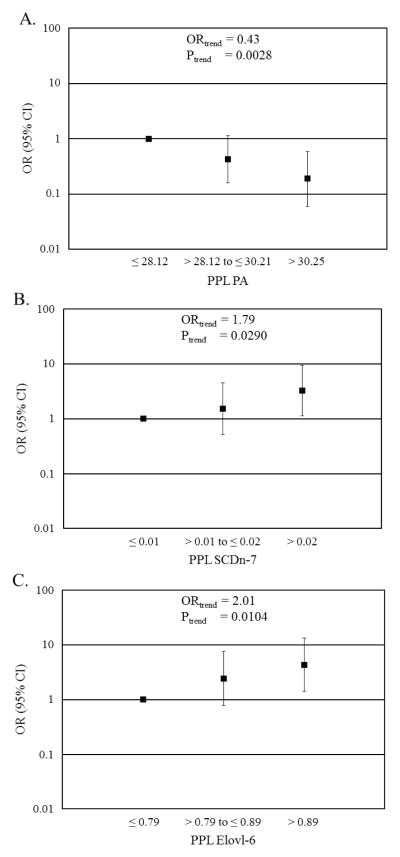
Next, we separated several of our highly significant FAs and EAEs into tertiles, providing insight into the specific PPL FA ranges that were most likely to be associated with the presence of adenomas (Figure 2). For each tertile increase in PPL PA, individuals were 0.43 (95% CI: 0.25 – 0.75) times as likely to have adenomas rather than no colon polyps (Figure 2A). Categorical increases for POA, total SFA, elaidic, and total trans-MUFA showed no significant association with adenomas. However, for each tertile increase in SCD n-7, the calculated ratio of POA/PA, an individual was 1.79 (95% CI: 1.06 – 3.03) times more likely to have at least one adenoma rather than no polyps (Figure 2B). The association of Elovl-6 with colon adenomas was similar to the association of SCD n-7 with colon adenomas. For each tertile increase in Elovl-6, individuals were 2.01 (95% CI: 1.18 – 3.42) times more likely to have an adenoma rather than no polyps (Figure 2C).
Figure 2.
Associations of plasma phospholipid (PPL) fatty acid and enzyme activity estimates (EAEs), as tertiles, with having adenomas relative to no colon polyps. (A) Palmitic acid (PA), (B) SCDn-7, and (C) Elovl-6. The symbol “■” represent the odds ratio and error bars indicate lower and upper confidence intervals, respectively. Both test for exposure and test for trend models adjusted for age and smoking. PA is expressed as a percent of total PPL FAs. Elovl-6, elongation of very long chain fatty acids-6 EAE, PA, palmitic acid; SCDn-7, stearoyl-CoA desaturase n-7 EAE.
Discussion
This study characterized PPL FA profiles associated with the presence of adenomas in adult males. Specifically, we report adenomas are positively associated with PPL elaidic, POA, total trans-MUFAs, as well as SCD n-7 and Elovl-6 EAEs. PPL PA was inversely associated with the presence of adenomas. These data indicate specific PPL FAs and EAEs are associated with adenomas even after adjusting for obesity, smoking, age, and elevated WC, which are factors known to increase CRC risk (2).
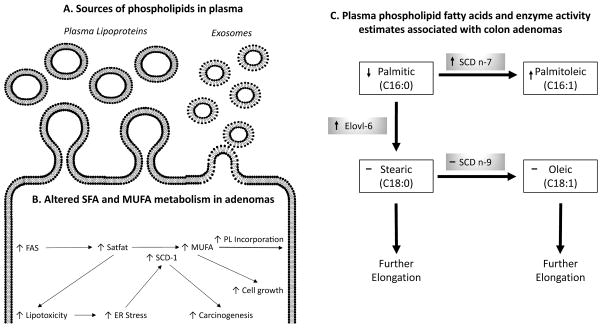
The PPL FA compartment is an ideal location for biomarker identification. Not only is the PPL FA compartment easily accessible to clinicians through a blood draw or simple blood spot using cards treated to prevent oxidation, but the PPL FA compartment also contains PL from sources such as plasma lipoproteins (25) and plasma microvesicle exosomes (26) (Figure 3A). Since PLs are endogenously synthesized, proportional differences in PPL FAs likely reflect cellular FA metabolism (27) (Figure 3B). If cellular FA metabolism is changed during the formation of adenomas, then new FA metabolites would be detectable in the PPL fraction. However, PL FA proportions in individuals also may reflect dietary FA intake (28), in addition to altered lipid metabolism associated (28) with colon carcinogenesis (8).
Figure 3.
Relationship between cellular fatty acid (FA) metabolic pathways and observed associations of FAs and enzyme acitivity estimates (EAEs) with colorectal adenomas. (A) Lipoproteins and exosomes are the most abundant sources of plasma phospholipid (PPL) FAs. (B) Increased fatty acid synthase (FAS) increases intracellular saturated fatty acids (SFAs). These SFAs have lipotoxic effects causing stearoyl-COA desaturase-1 (SCD-1) expression to increase. Higher concentrations of SFAs and expression SCD-1, increase monounsaturated FA (MUFA) production leading to increased PL MUFA incorporation and cellular enlargement. (C) Visual representation of PA metabolic pathway. PPL FAs appear in white boxes and EAEs appear in gray boxes. The arrow in each box indicates the direction of the association between the substrate and the likelihood of having an adenoma compared to having no colon polyps. A “–“ indicates no observed association. Elovl-6, elongation of very long chain fatty acids-6 EAE; ER, endoplasmic reticulum; SCDn-7, stearoyl-CoA desaturase n-7 EAE; SCDn-9, stearoyl-CoA desaturase n-9 EAE.
The ability to easily measure changes in cellular FA metabolism is important in the identification of biomarkers of colorectal polyp formation because colon adenomas are associated with changes in FA metabolism. For instance, colon adenomas are positively associated with fatty acid synthase (FAS) expression (29), which increases SFA synthesis, in particular PA synthesis (30). Endogenous FA synthesis occurs in the smooth ER, where the enzymes Elovl-6 and SCD-1 enzymes are located (31). Elevated intracellular concentrations of SFAs are associated with increased lipotoxicity and endoplasmic reticulum (ER) stress (32–34). The positive association of cellular stress responses and carcinogenesis are well documented [reviewed in detail (35)]. Thus, our observation that higher SCD n-7 and Elovl-6 EAEs are associated with the presence of adenomas may be indicative of a cellular stress response to the process of carcinogenesis.
Aside from cellular stress, FA metabolism also increases during mitogenesis. Mitogenic factors associated with adenomas increase SCD-1 expression (36), which in turn increases de novo production of MUFAs such as POA (37). In order for cell division to occur, cells must double their membrane FA content (38). In particular, there is an increased demand for MUFA incorporation into PL membranes (8, 37). Therefore, changes in FA metabolism (i.e. FAS, Elovl-6, and SCD-1) associated with increased cellular proliferation (i.e. adenomas), may be detectable by identifying specific proportions of PPL FAs and EAEs. Higher plasma SCD EAEs are associated with an increased risk of several cancers (39–41). What remains unclear, is whether the resulting metabolites specifically participate in the process of carcinogenesis or if they are merely a by-product of the metabolism of abnormal cells. The visual representation of the PA pathway in Figure 3C incorporates results from our logistic regressions that demonstrate significant associations between the presence of adenomas and the PPL FAs and EAEs associated with PA metabolism. Taken together, our data suggests the observed associations are likely the result of altered desaturation and elongation of PA during carcinogenesis.
PA is desaturated by SCD-1 to form the cis-MUFA POA. We used two separate estimates of SCD-1 activity, SCD n-7 and SCD n-9. We observed SCD n-7 EAE was positively associated with adenomas, and there was no association of SCD n-9 with adenomas. An increase in the proportion of plasma POA is positively associated with risk of future all-cause cancer mortality (42). Higher levels of PPL POA are indicative of increased de novo synthesis, because dietary POA is rapidly oxidized after absorption resulting in negligible effects of dietary POA on the lipid profile (43). Plasma SCD n-7 EAE positively correlates with SCD-1 enzyme activity measured in biopsied tissues, but SCD n-9 EAE does not (44). Aside from PA, no other SFAs analyzed (SA, arachidic, behenic, or lignoceric) had significant associations with adenomas. We speculate the inverse association between PPL PA and adenomas reflects underlying changes in PA metabolism such as increased desaturation.
Our cross-sectional study was conducted in a population of males (n=126, > 96% Caucasian, ages 48–65) to identify associations between colon polyps and PPL FAs or EAEs. We recognize that the generalizability of these observations is limited. Therefore studies need to be conducted prospectively in larger, more diverse populations. In addition, we report PPL FA-based EAEs of Elovl-6 and SCD n-7 are associated with adenomas. These EAEs have yet to be extensively validated and may not fully represent enzyme kinetics in adenomas. Thus, reported differences in EAEs could be related to other factors (i.e. diet, preferential FA uptake, etc.) rather than enzyme activities as we did not directly collect or assess dietary intake in this study. To our knowledge, no research group has sought to establish a preliminary range of PPL FA or EAE levels associated with colorectal adenomas. Our research suggests specific levels of PPL FAs and EAEs may be useful as novel biomarkers of colon carcinogenesis.
Acknowledgments
Financial support: Research supported in part by the National Cancer Institute 1R03CA142000 (J. I. Fenton) and the Clinical and Translational Sciences Institute at MSU.
We thank Catherine Belcher for assistance with sample processing, and Emily Davidson for figure editing.
Abbreviations
- BHT
Butylated hydroxytoluene
- BMI
Body mass index
- CC
Colon cancer
- CI
Confidence interval
- CRC
Colorectal cancer
- EAE
Enzyme activity estimate
- Elovl
Elongation of very long chain fatty acid
- FA
Fatty acid
- FAME
Fatty acid methyl ester
- FAS
Fatty acid synthase
- MUFA
Monounsaturated fatty acid
- NA
nervonic acid
- OA
Oleic acid
- OR
Odds ratio
- PA
Palmitic acid
- PL
phospholipid
- POA
Palmitoleic acid
- PPL
Plasma phospholipid
- PUFA
Polyunsaturated fatty acid
- SA
Stearic acid
- SCD
Stearoyl-CoA desaturase
- SFA
Saturated fatty acid
- SNP
Single nucleotide polymorphism
- WC
Waist circumference
Footnotes
Disclosure: No authors report a conflict of interest.
References
- 1.Group USCSW. United States Cancer Statistics: 1999–2011 Incidence and Mortality Web-based Report. 2014 cited 2015 May 29; Available from: www.cdc.gov/uscs.
- 2.Haggar FA, Boushey RP. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clinics in colon and rectal surgery. 2009;22:191–7. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willett WC. Diet and cancer: an evolving picture. JAMA : the journal of the American Medical Association. 2005;293:233–4. doi: 10.1001/jama.293.2.233. [DOI] [PubMed] [Google Scholar]
- 4.Kelly SB, Miller J, Wood CB, Williamson RC, Habib NA. Erythrocyte stearic acid desaturation in patients with colorectal carcinoma. Diseases of the colon and rectum. 1990;33:1026–30. doi: 10.1007/BF02139217. [DOI] [PubMed] [Google Scholar]
- 5.Hodge AM, Williamson EJ, Bassett JK, MacInnis RJ, Giles GG, English DR. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. International journal of cancer Journal international du cancer. 2015 doi: 10.1002/ijc.29479. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Martinez LR, Ray S. Phospholipid remodeling and eicosanoid signaling in colon cancer cells. Indian journal of biochemistry & biophysics. 2014;51:512–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Bamia C, Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. European journal of epidemiology. 2013;28:317–28. doi: 10.1007/s10654-013-9795-x. [DOI] [PubMed] [Google Scholar]
- 8.Igal RA. Stearoyl-CoA desaturase-1: a novel key player in the mechanisms of cell proliferation, programmed cell death and transformation to cancer. Carcinogenesis. 2010;31:1509–15. doi: 10.1093/carcin/bgq131. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Strable MS, Ntambi JM. Stearoyl CoA desaturase 1: role in cellular inflammation and stress. Advances in nutrition. 2011;2:15–22. doi: 10.3945/an.110.000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Progress in lipid research. 2004;43:91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 11.Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. The New England journal of medicine. 2012;366:687–96. doi: 10.1056/NEJMoa1100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arab L. Biomarkers of fat and fatty acid intake. The Journal of nutrition. 2003;133(Suppl 3):925S–32S. doi: 10.1093/jn/133.3.925S. [DOI] [PubMed] [Google Scholar]
- 13.Comstock SS, Hortos K, Kovan B, McCaskey S, Pathak DR, Fenton JI. Adipokines and obesity are associated with colorectal polyps in adult males: a cross-sectional study. PloS one. 2014;9:e85939. doi: 10.1371/journal.pone.0085939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comstock SS, Xu D, Hortos K, Kovan B, McCaskey S, Pathak DR, et al. Association of insulin-related serum factors with colorectal polyp number and type in adult males. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2014;23:1843–51. doi: 10.1158/1055-9965.EPI-14-0249-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rose HG, Oklander M. Improved Procedure for the Extraction of Lipids from Human Erythrocytes. Journal of lipid research. 1965;6:428–31. [PubMed] [Google Scholar]
- 16.Agren JJ, Julkunen A, Penttila I. Rapid separation of serum lipids for fatty acid analysis by a single aminopropyl column. Journal of lipid research. 1992;33:1871–6. [PubMed] [Google Scholar]
- 17.Burdge GC, Wright P, Jones AE, Wootton SA. A method for separation of phosphatidylcholine, triacylglycerol, non-esterified fatty acids and cholesterol esters from plasma by solid-phase extraction. The British journal of nutrition. 2000;84:781–7. [PubMed] [Google Scholar]
- 18.Pickens CA, Sordillo LM, Comstock SS, Harris WS, Hortos K, Kovan B, et al. Plasma phospholipids, non-esterified plasma polyunsaturated fatty acids and oxylipids are associated with BMI. Prostaglandins, leukotrienes, and essential fatty acids. 2014 doi: 10.1016/j.plefa.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gurzell EA, Wiesinger JA, Morkam C, Hemmrich S, Harris WS, Fenton JI. Is the omega-3 index a valid marker of intestinal membrane phospholipid EPA+DHA content? Prostaglandins, leukotrienes, and essential fatty acids. 2014 doi: 10.1016/j.plefa.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vessby B, Gustafsson IB, Tengblad S, Berglund L. Indices of fatty acid desaturase activity in healthy human subjects: effects of different types of dietary fat. The British journal of nutrition. 2013;110:871–9. doi: 10.1017/S0007114512005934. [DOI] [PubMed] [Google Scholar]
- 21.Shimamura K, Kitazawa H, Miyamoto Y, Kanesaka M, Nagumo A, Yoshimoto R, et al. 5,5-Dimethyl-3-(5-methyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-1-phenyl-3-( trifluoromethyl)-3,5,6,7-tetrahydro-1H-indole-2,4-dione, a potent inhibitor for mammalian elongase of long-chain fatty acids family 6: examination of its potential utility as a pharmacological tool. The Journal of pharmacology and experimental therapeutics. 2009;330:249–56. doi: 10.1124/jpet.109.150854. [DOI] [PubMed] [Google Scholar]
- 22.Zarrouk A, Riedinger JM, Ahmed SH, Hammami S, Chaabane W, Debbabi M, et al. Fatty acid profiles in demented patients: identification of hexacosanoic acid (C26:0) as a blood lipid biomarker of dementia. Journal of Alzheimer’s disease : JAD. 2015;44:1349–59. doi: 10.3233/JAD-142046. [DOI] [PubMed] [Google Scholar]
- 23.Y y. Multiple Imputation Using SAS Software. Journal of Statistical Software. 2011;45 doi: 10.18637/jss.v045.i01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cormier H, Rudkowska I, Lemieux S, Couture P, Julien P, Vohl MC. Effects of FADS and ELOVL polymorphisms on indexes of desaturase and elongase activities: results from a pre-post fish oil supplementation. Genes & nutrition. 2014;9:437. doi: 10.1007/s12263-014-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS medicine. 2012;9:e1001255. doi: 10.1371/journal.pmed.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosseini-Beheshti E, Pham S, Adomat H, Li N, Tomlinson Guns ES. Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Molecular & cellular proteomics : MCP. 2012;11:863–85. doi: 10.1074/mcp.M111.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swinnen JV, Vanderhoydonc F, Elgamal AA, Eelen M, Vercaeren I, Joniau S, et al. Selective activation of the fatty acid synthesis pathway in human prostate cancer. International journal of cancer Journal international du cancer. 2000;88:176–9. doi: 10.1002/1097-0215(20001015)88:2<176::aid-ijc5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Harris WS, Pottala JV, Sands SA, Jones PG. Comparison of the effects of fish and fish-oil capsules on the n 3 fatty acid content of blood cells and plasma phospholipids. The American journal of clinical nutrition. 2007;86:1621–5. doi: 10.1093/ajcn/86.5.1621. [DOI] [PubMed] [Google Scholar]
- 29.Rashid A, Pizer ES, Moga M, Milgraum LZ, Zahurak M, Pasternack GR, et al. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. The American journal of pathology. 1997;150:201–8. [PMC free article] [PubMed] [Google Scholar]
- 30.Visca P, Alo PL, Del Nonno F, Botti C, Trombetta G, Marandino F, et al. Immunohistochemical expression of fatty acid synthase, apoptotic-regulating genes, proliferating factors, and ras protein product in colorectal adenomas, carcinomas, and adjacent nonneoplastic mucosa. Clinical cancer research : an official journal of the American Association for Cancer Research. 1999;5:4111–8. [PubMed] [Google Scholar]
- 31.Enoch HG, Catala A, Strittmatter P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. The Journal of biological chemistry. 1976;251:5095–103. [PubMed] [Google Scholar]
- 32.Weigert C, Brodbeck K, Staiger H, Kausch C, Machicao F, Haring HU, et al. Palmitate, but not unsaturated fatty acids, induces the expression of interleukin-6 in human myotubes through proteasome-dependent activation of nuclear factor-kappaB. The Journal of biological chemistry. 2004;279:23942–52. doi: 10.1074/jbc.M312692200. [DOI] [PubMed] [Google Scholar]
- 33.Staiger H, Staiger K, Stefan N, Wahl HG, Machicao F, Kellerer M, et al. Palmitate-induced interleukin-6 expression in human coronary artery endothelial cells. Diabetes. 2004;53:3209–16. doi: 10.2337/diabetes.53.12.3209. [DOI] [PubMed] [Google Scholar]
- 34.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. Journal of lipid research. 2006;47:2726–37. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Fulda S. Evasion of apoptosis as a cellular stress response in cancer. International journal of cell biology. 2010;2010:370835. doi: 10.1155/2010/370835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuel W, Nagineni CN, Kutty RK, Parks WT, Gordon JS, Prouty SM, et al. Transforming growth factor-beta regulates stearoyl coenzyme A desaturase expression through a Smad signaling pathway. The Journal of biological chemistry. 2002;277:59–66. doi: 10.1074/jbc.M108730200. [DOI] [PubMed] [Google Scholar]
- 37.Simonsen NR, Fernandez-Crehuet Navajas J, Martin-Moreno JM, Strain JJ, Huttunen JK, Martin BC, et al. Tissue stores of individual monounsaturated fatty acids and breast cancer: the EURAMIC study. European Community Multicenter Study on Antioxidants, Myocardial Infarction, and Breast Cancer. The American journal of clinical nutrition. 1998;68:134–41. doi: 10.1093/ajcn/68.1.134. [DOI] [PubMed] [Google Scholar]
- 38.Jackowski S. Cell cycle regulation of membrane phospholipid metabolism. The Journal of biological chemistry. 1996;271:20219–22. doi: 10.1074/jbc.271.34.20219. [DOI] [PubMed] [Google Scholar]
- 39.Chajes V, Joulin V, Clavel-Chapelon F. The fatty acid desaturation index of blood lipids, as a biomarker of hepatic stearoyl-CoA desaturase expression, is a predictive factor of breast cancer risk. Current opinion in lipidology. 2011;22:6–10. doi: 10.1097/MOL.0b013e3283404552. [DOI] [PubMed] [Google Scholar]
- 40.Chajes V, Jenab M, Romieu I, Ferrari P, Dahm CC, Overvad K, et al. Plasma phospholipid fatty acid concentrations and risk of gastric adenocarcinomas in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) The American journal of clinical nutrition. 2011;94:1304–13. doi: 10.3945/ajcn.110.005892. [DOI] [PubMed] [Google Scholar]
- 41.Macasek J, Vecka M, Zak A, Urbanek M, Krechler T, Petruzelka L, et al. Plasma fatty acid composition in patients with pancreatic cancer: correlations to clinical parameters. Nutrition and cancer. 2012;64:946–55. doi: 10.1080/01635581.2012.716138. [DOI] [PubMed] [Google Scholar]
- 42.Byberg L, Kilander L, Warensjo Lemming E, Michaelsson K, Vessby B. Cancer death is related to high palmitoleic acid in serum and to polymorphisms in the SCD-1 gene in healthy Swedish men. The American journal of clinical nutrition. 2014;99:551–8. doi: 10.3945/ajcn.113.065714. [DOI] [PubMed] [Google Scholar]
- 43.Zong G, Ye X, Sun L, Li H, Yu Z, Hu FB, et al. Associations of erythrocyte palmitoleic acid with adipokines, inflammatory markers, and the metabolic syndrome in middle-aged and older Chinese. The American journal of clinical nutrition. 2012;96:970–6. doi: 10.3945/ajcn.112.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hodson L, Fielding BA. Stearoyl-CoA desaturase: rogue or innocent bystander? Progress in lipid research. 2013;52:15–42. doi: 10.1016/j.plipres.2012.08.002. [DOI] [PubMed] [Google Scholar]