Abstract
Live attenuated vaccines such as SIV with a deleted nef gene (SIVmac239Δnef) have provided the most robust protection against subsequent vaginal challenge with wild type (WT) SIV in the SIV-rhesus macaque (RM) model of HIV-1 transmission to women. Hence, identifying correlates of this protection could enable design of an effective HIV-1 vaccine. One such pre-challenge correlate of protection from vaginal challenge has recently been identified as a system with three components: 1) IgG antibodies reacting with the viral envelope glycoprotein, trimeric gp41 (gp41t); 2) produced by plasma cells in the submucosa and ectopic tertiary lymphoid follicles in the ectocervix and vagina; and 3) concentrated on the path of virus entry by the neonatal Fc receptor in the overlying epithelium. We now examine the mucosal production of antibody component of this system post-vaginal challenge. We show that vaginal challenge immediately elicits striking increases in plasma cells not only in the female reproductive tract but also at other mucosal sites, and that these increases correlate with low but persistent replication at mucosal sites. We describe vaginal ectopic follicles that are structurally and functionally organized like follicles in secondary lymphoid organs, and provide inferential evidence for a key role of the female reproductive tract epithelium in facilitating antibody production, affinity maturation and class switch recombination. Vaccination thus accesses an epithelial-immune system axis in the female reproductive tract to respond to exposure to mucosal pathogens. Designing strategies to mimic this system could advance development of an effective HIV-1 vaccine.
Introduction
The great advances in treating human immunodeficiency virus-1 (HIV-1) infections have reduced the morbidity and mortality HIV-1 infection causes, but there remains an urgent and continuing need to develop an effective vaccine to halt the progress of the epidemic, especially to stop transmission of HIV-1 to women who bear the brunt of infection in the pandemic’s epicenter in Africa (1,2). Toward that goal, we have been seeking correlates of the robust protection conferred by the live attenuated vaccine, SIVmac239Δnef (3–5), since these correlates could provide design principles for development of an effective HIV-1 vaccine. In these studies in the SIV-rhesus macaque model of HIV-1 transmission to women, we recently identified (6) IgG antibodies to trimeric gp41 (gp41t) prior to vaginal challenge as one correlate of the temporal maturation of protection (7) between 5 and 20 weeks post vaccination, times respectively when animals are not or are protected against high-dose vaginal challenge with WT SIV. We showed that these antibodies are locally produced by plasma cells and ectopic lymphoid follicles in the cervix and vagina, and concentrated by the neonatal Fc receptor (FcRn) (8) in the overlying epithelium, thus providing a mechanism for the antibodies to react with virus on the path of entry and thereby inhibit transmission.
The interaction just described was only one of the interactions identified at mucosal frontlines that point to a mucosal epithelium–immune system axis. Vaccination also induced: expression of CXCL10 in the female reproductive tract (FRT) epithelium as a chemotactic mechanism to recruit CXCR3+IgG+ plasma cells to the underlying submucosa (6); and expression of the inhibitory FcgRIIb inhibitory receptor in cervical epithelium to interact with immune complexes formed following vaginal challenge. This interaction then induced an inhibitory program (9) preventing the recruitment of CD4+ T cell targets that fuel local expansion in unvaccinated animals (10). In this report, we describe further evidence of a mucosal epithelial-immune system axis to facilitate antibody production, affinity maturation and class switch recombination (CSR) after vaginal challenge. This in situ rapid recall and sustained humoral immune response literally generates a “wall” of IgG antibodies at mucosal frontlines as defenses against exposure to mucosal pathogens as one concept and design principle for developing effective vaccines against HIV-1.
Materials and Methods
Animals, vaccination, and vaginal challenge
We examined tissues from 10 naïve animals archived from previously described (11) studies of transmission following high dose inoculation of WT SIV; tissues from 23 SIVmac239Δnef vaccinated female rhesus macaque monkeys (Macaca mulatta) at days 4 (n=1), 7 (n=1), 11(n=1), and 14 days (n=1) and at 5 (n=4) and 20 weeks (n=4) after vaccination; and at days 4 and 5 (n=5), 7 (n=3) and 14 days (n=3) after high dose vaginal challenge at 20 weeks post vaccination (6). The animals had been housed in accordance with the regulations of the American Association of Accreditation of Laboratory Animal Care and the standards of the Association for Assessment and Accreditation of Laboratory Animal Care International at the New England and California Primate Centers.
Tissue collection and processing
At the time of euthanasia, lung, jejunum, colon, rectum, cervix, vagina, spleen and various peripheral lymph nodes and other tissues were collected and fixed in 4% paraformaldehyde or SafeFix II and embedded in paraffin for later analyses, as described (6). Portions were also snap frozen for later extraction of RNA and DNA.
FRT mucosal epithelial in vitro model
The HEC-1A uterine epithelial cell culture system was used to examine responses to SIV, described in (6).
Immunohistochemistry and immunofluorescence
These methods were performed as described in (6, 12). Tissue sections (10-µm) mounted on glass slides were deparaffinized and rehydrated in deionized water. Heat-induced epitope retrieval was performed using a water bath (98°C) in EDTA Decloaker (Biocare Medical), followed by cooling to room temperature. Tissue sections were then blocked with SNIPER Blocking Reagent (Biocare Medical) for 30 minutes at room temperature. Primary antibodies were diluted in TNB (0.1M Tris-HCl, pH 7.5; 0.15M NaCl; 0.05% Tween 20 with Dupont blocking buffer) and incubated overnight at 4°C. After the primary antibody incubation, sections were washed with PBS and then incubated with biotin-conjugated secondary antibodies in TNB for 2 hr at room temperature, washed with PBS with 0.1% Tween 20 and then incubated with anti-biotin antibody conjugated to alkaline phosphatase in TNB for 2 hours at room temperature. After the incubation of anti-biotin antibody, the sections were washed with PBS with 0.1% Tween 20. Signal was detected with Warp red kit (Biocare Medical). The sections were counterstained with Harris Hematoxylin (Surgipath), dehydrated in rapidly in gradient ethanols, and mounted with Permount (Fisher Scientific). Stained sections were examined by light microscopy at ambient temperatures. Light micrographs were taken using an Olympus BX60 upright microscope with the following objectives: ×10 (0.3 NA), ×20 (0.5 NA), and ×40 (0.75 NA); images were acquired using a SPOT Color Mosaic camera (model 11.2; Diagnostic Instruments) and SPOT acquisition software (version 4.5.9; Diagnostic Instruments). Anti-biotin antibody conjugated to alkaline phosphatase was used as negative control antibodies in all instances and yielded negative staining results. For immunofluorescent staining, after the primary antibody incubation, sections were washed with PBS and then incubated with fluochrome-conjugated secondary antibodies in TNB for 2 hr at room temperature. Finally, sections were washed with PBS, nuclei counterstained blue with TOTO-3 or DAPI, and mounted using Aqua Poly/Mount (Polysciences Inc.). Immunofluorescent micrographs were taken using an Olympus BX61 Fluoview confocal microscope with the following objectives: ×20 (0.75 NA), ×40 (0.75 NA), and ×60 (1.42 NA); images were acquired and mean fluorescence intensity was analyzed by using Olympus Fluoview software (version 1.7a). Positive stained cells or follicles were enumerated in 20 randomly acquired, high-powered images (×200 or ×400 magnification) by manually counting in each image.
RNA extraction
Frozen tissue specimens were homogenized with a power homogenizer in TRIzol without thawing. Total RNA was isolated according to the manufacturer’s protocol, and further purified with an RNeasy mini kit.
Real-time RT-PCR to determine SIVmac239Δnef viral loads in tissues and plasma
RT-PCR assays were performed using primers specific for SIVmac239Δnef to determine the levels of SIVmac239Δnef in tissues and plasma, but with modifications for tissue RNA to accommodate the higher amounts and complexity of input RNA (13).
Soluble trimeric gp41 (sgp41t) and reverse immunohistochemistry (RIHC)
Cells with antibodies to gp41t were stained by RIHC, as described in (6).
Statistical analyses
Data represent means ± SEM in all graphs depicting error bars. The statistical significance of differences between experimental groups was determined using GraphPad Prism 6 and the indicated statistical tests. P values are indicated by * P ≤ 0.05; ** P ≤ 0.01; *** P ≤ 0.001; **** P ≤ 0.0001.
Results
Increased IgG+ plasma cells and ectopic follicles throughout the mucosal immune system
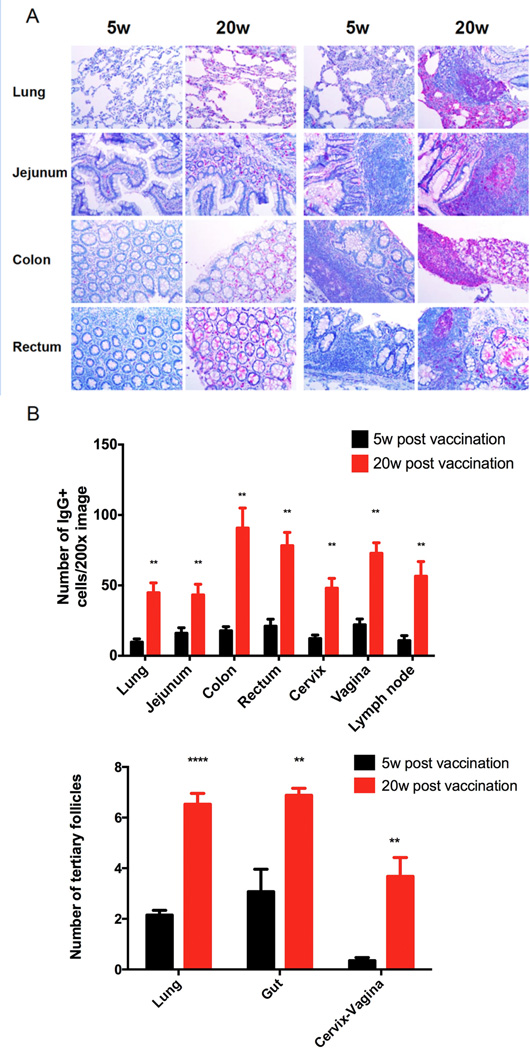
SIVmac239Δnef vaccination has previously been shown to elicit increased numbers of IgG+ plasma cells and ectopic lymphoid follicles beneath the cervical vaginal epithelium accompanying the maturation of protection between 5 and 20 weeks post vaccination (6). These increases, we now show, were not confined to the FRT, but were general throughout the mucosal immune system, including the lung and gut associated lymphoid tissue (Figure 1A, B).
FIGURE 1.
General increase in IgG+ cells and ectopic follicles at the listed mucosal sites associated with the maturation of protection between 5 and 20 weeks (w). A. The left panel comparisons of 5 and 20w show increased numbers of red-magenta staining IgG+ plasma cells in the lung and gut between 5 and 20w; the right panels show the increases in ectopic follicles with IgG+ plasma cells. Image magnification 200×. B. Quantification of the increases at all mucosal sites. Significant P values (indicated by *) were determined by Student’s t test.
SIVmac239Δnef antigen exposure in the mucosal immune system
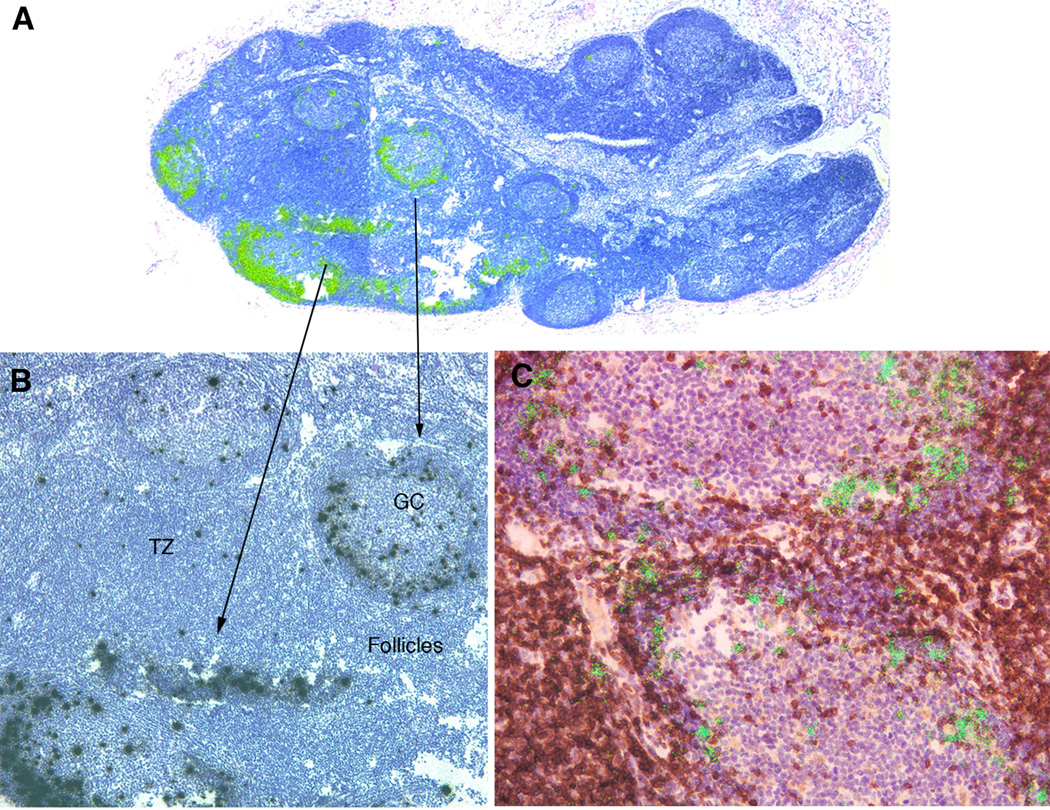
What might drive the increases in plasma cells and ectopic follicles producing IgG antibody at mucosal sites? One possibility we examined is that SIVmac239Δnef replicates at these sites to induce the mucosal IgG production. While this initially seemed unlikely, as we found as others have shown for SIV and HIV (14–16), that SIVmac239Δnef replicates predominantly in T follicular helper (TFH) cells (Figure 2) and was barely detectable at mucosal sites in the first two weeks following iv infection, and undetectable in cervix and vagina at 5 and 20 weeks post vaccination by assays available at the time (not shown). However, when there was sufficient tissue available for contemporary assays (13) of viral RNA in 108 cell equivalents, we found that although vRNA was most abundant in acute infection throughout lymphoid tissues, there were lower levels of in the FRT, lung and gut (Table 1). In the limited number of samples available for assay in the FRT at 5 and 20 weeks post vaccination, SIVmac239Δnef RNA was still detectable, albeit at even lower levels, through 20 weeks (Table 1). Thus, persistent exposure to virus at mucosal sites, low level inflammation and expression of IFN-γ and type I IFN (17, 18), could have generated expression of chemokines to recruit cells and induce ectopic follicles at mucosal surfaces.
FIGURE 2.
SIVmac239Δnef replicates initially in TFH. A. Green viral RNA+ cells in epifluorescent reflected light images at the margins of lymphoid follicles 7 days post i.v. infection. B. Black viral+ cells in transmitted light. C. Double-labeled green viral RNA+ brown stained PD-1+ TFH cells at these locations. Viral RNA+ cells were also CD3+ and CD4+ (not shown).
Table I.
SIVΔnef RNA in mucosal sites and lymphoid tissues1
| Days post vaccination |
Number of SIV RNA copies 108 CE | |||
|---|---|---|---|---|
| 4 | 7 | 11 | 14 | |
| Pelvic LN | NA | 4.7×107 | NA | NA |
| Inguinal LN | 5.8×105 | 7×106 | NA | 3.6×106 |
| Mesenteric LN | 2.3×105 | NA | 2×106 | 2.9×106 |
| Axillary LN | 4.7×104 | 2×106 | 3×105 | 3.5×105 |
| GALT | 1.1×106 | 1.7×106 | 1.1×105 | 1.9×106 |
| Spleen | 2.5×106 | 4.9×107 | 5.6×106 | 2.3×107 |
| Lung | 4×103 | 2×104 | 1.8×104 | 5.6×104 |
| Cervix | 7.7 × 102 | 1.5 ×106 | 9.3×103 | 7.4×105 |
| Vagina | 1.8×102 | 2.7×105 | 1.9×104 | 1.4×104 |
| Weeks post vaccination | 5 | 5 | 5 | |
| Cervix | 4×103 | 6.8×103 | 2.4×103 | |
| Vagina | NA | NA | 7.9×104 | |
| 20 | 20 | 20 | ||
| Cervix | 6.4×102 | 1.3×102 | 7.9×102 | |
| Vagina | NA | NA | 5.8×104 | |
SIV RNA copies per 108 cell equivalents (CE). When sufficient tissue was available for the assay, RNA was extracted from single animals from the tissue sites and days shown following SIVΔnef iv infection. There was sufficient cervical and vaginal tissue available from 3 animals each at 5 and 20 weeks for the assay. Copy numbers in extracted tissue RNA were determined by RT-PCR using specific primers, and expressed as number of copies of SIVΔnef RNA per 108 CE. The values shown for GALT are the highest respectively for jejunum, ileum, colon and rectum. NA, tissue samples of sufficient size for the assay were not available.
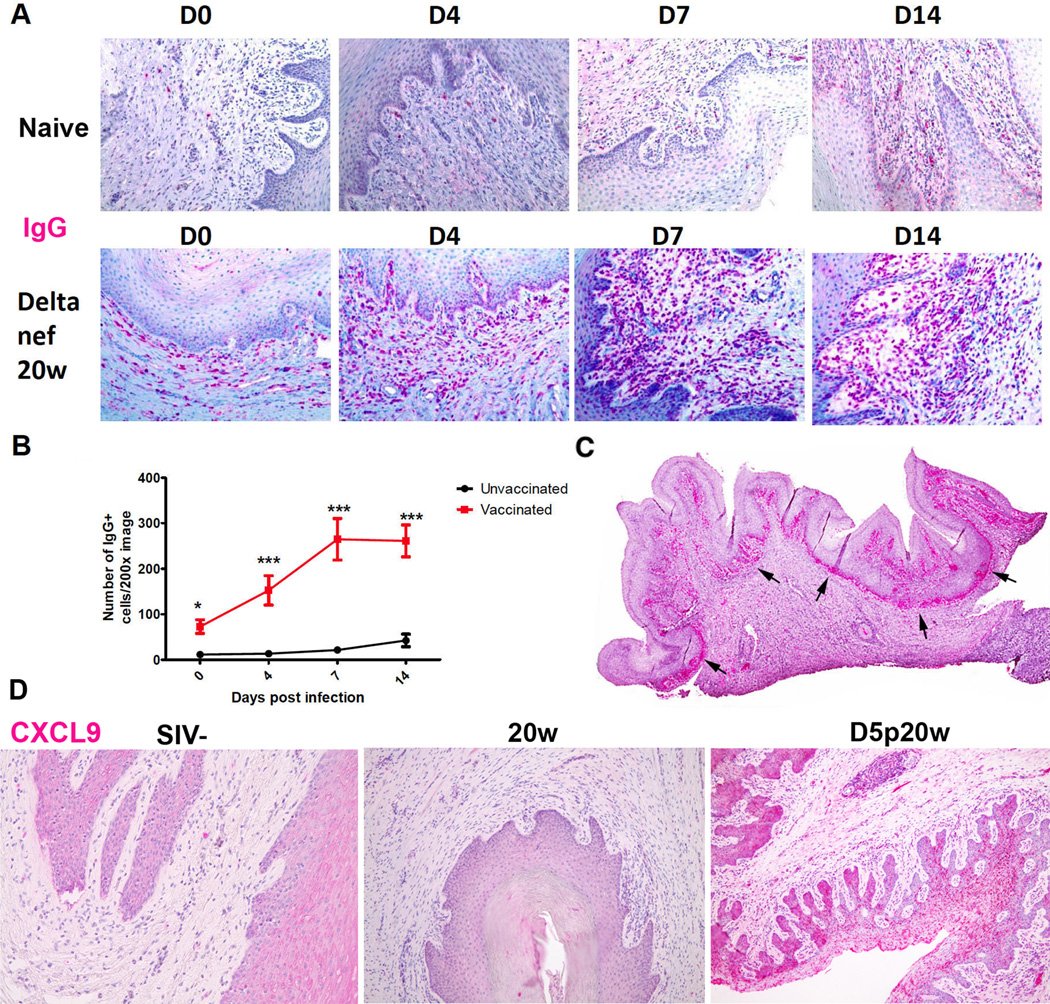
Vaginal plasma cell and IgG “wall” in vaccinated animals following challenge
Vaginal challenge increased the number of IgG+ plasma cells and ectopic follicles over the already increased number present pre-challenge in the in the FRT of vaccinated animals compared to unvaccinated controls (Figure 3). By day 4 following high dose WT SIV vaginal challenge, the increased density of IgG+ plasma cells in the vagina far exceeded the modest increases in unvaccinated animals inoculated vaginally with the same high doses of WT SIV (Figure 3A, B), and these increases in excess of naïve controls continued so that by day 14 nearly six times as many IgG+ cells in the submucosa of vaccinated animals as in the naïve controls. At this time, the conjunction of IgG+ plasma cells and IgG concentrated in the overlying FcRn+ basal vaginal epithelium (6) imparted the appearance of an IgG “wall” (Figure 3C). As surmised above, increased epithelial expression of CXCL9 (Figure 3D) and CXCL10 (previously shown in reference 6) in the vaccinated animals provides a mechanism for the continued recruitment of plasma cells into the FRT after vaginal challenge.
Figure 3.
IgG+ cells beneath and expression of CXCL9 in vaginal epithelium following vaginal exposure to SIV in vaccinated animals. A. There are larger numbers of red-stained IgG+ plasma cells beneath vaginal epithelium at 20w in the vaccinated compared to the naive controls, and these differences widen in the first week following vagina exposure to WT SIV to the peak of replication day 14 (D14). Image magnification 200×. B. Quantification of the increases in 10 naïve and 11 vaccinated animals. C. Arrows point to a “wall” of IgG+ plasma cells and IgG in the overlying FcRn+ the vaginal epithelium at D14 in a scanned image of the whole section. D. Increased expression of CXCL9 in vaginal epithelium (magenta staining cells) in vaccinated animals after vaginal exposure to SIV compared to SIV− and vaccinated animal prior to vaginal challenge at 20w and day 5 post-20 week (D5p20w). Image magnification: 100×.
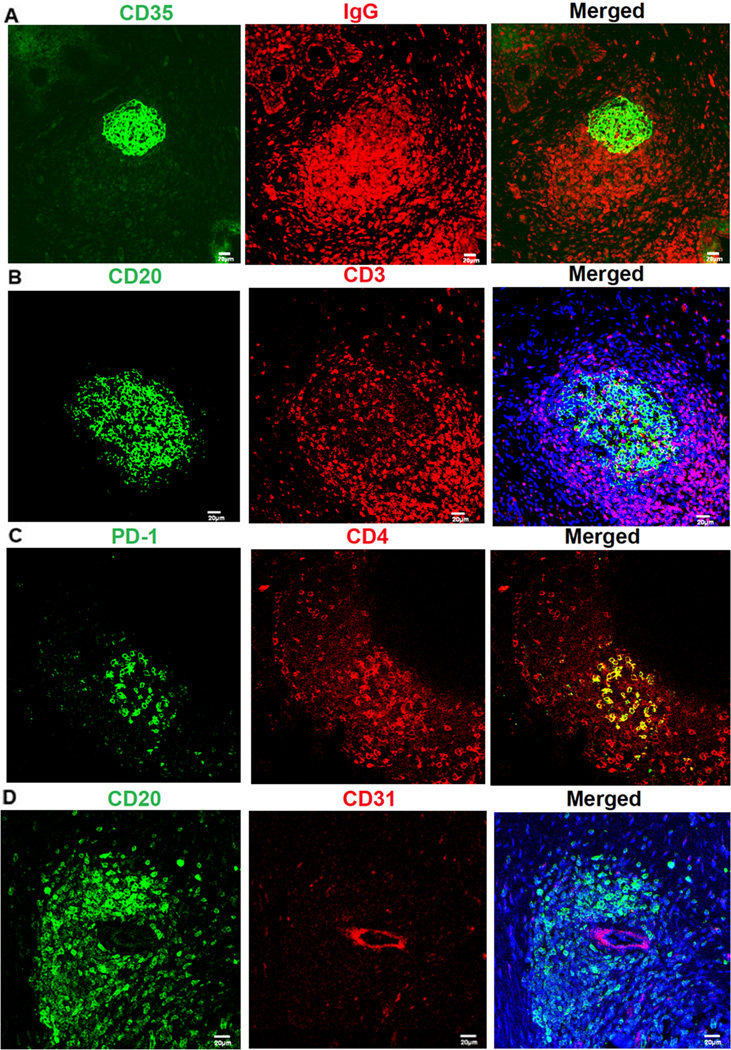
Follicle structural and functional organization for antibody production
In addition to the increased numbers of submucosal plasma cells for IgG production after vaginal challenge, there were also more ectopic follicles, structurally and functionally organized for antibody production, affinity maturation and CSR. The remarkable structural similarities the ectopic follicles in vagina and ectocervix share with the mature follicles and germinal centers (GC) characteristic of secondary lymphoid organs (19) included: a CD35+follicular dendritic cell (FDC) network in the center of the follicle (Figure 4A); IgG+ plasma cells, CD20+ B cells and PD-1+CD3+CD4+ T follicular helper cells in and around the follicle center, surrounded by CD3+ T cells at the periphery (Figure 4A–C); and CD31+ high endothelial venues (Figure 4D).
FIGURE 4.
FRT ectopic follicles resemble follicles in secondary lymphoid tissues (all images are FRT ectopic follicles). A. IgG+ cells (red) surround a (green) CD35+ FDC network. B, C. CD20+ B cells (green) and PD-1+CD4+ TFH (yellow) in the follicles with CD3+ T cells (red) at the periphery. D. CD31+ high endothelial venules (red) in ectopic follicles. Representative images were taken from animals at 20 weeks post vaccination
The ectopic follicles were also functionally organized to support proliferation and survival of B cells and plasma blasts, and affinity maturation and CSR of antibodies:
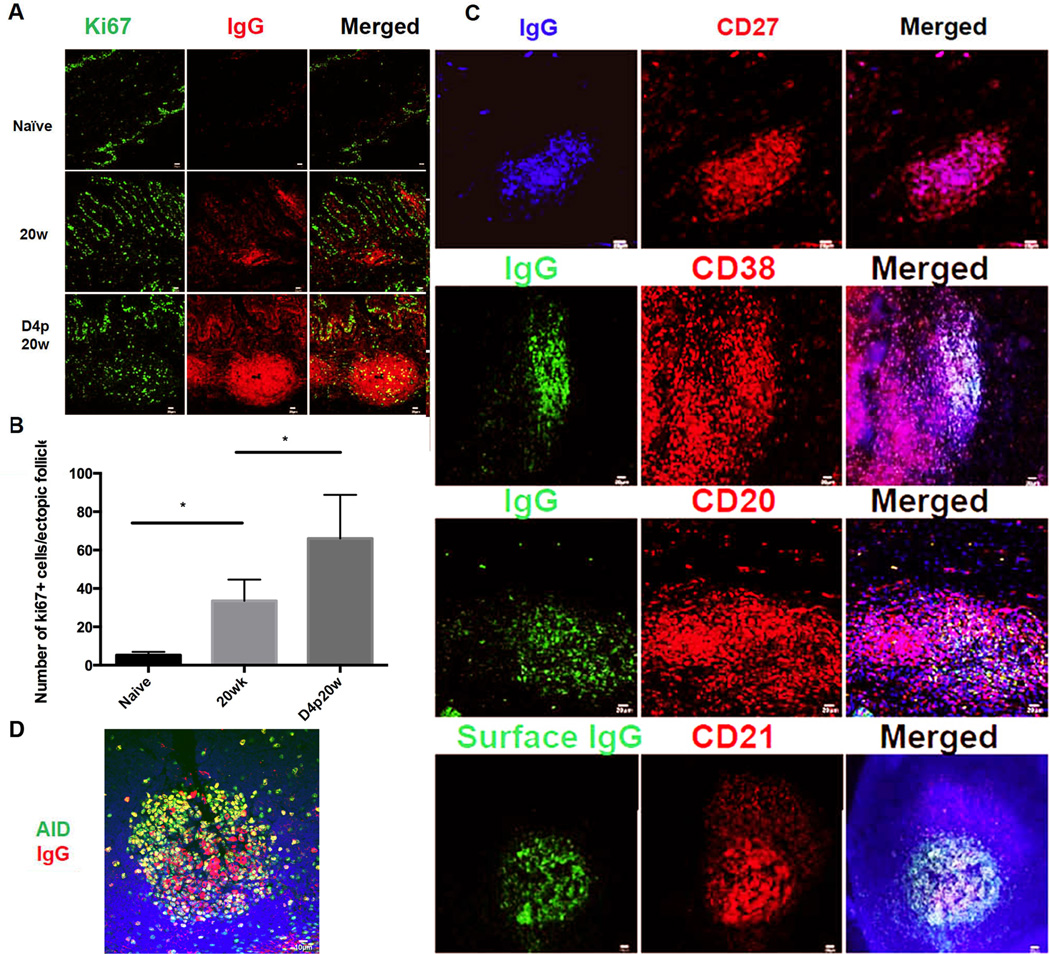
In naïve animals, there is little expression Ki67 in the lamina propria in FRT tissues (the majority of Ki67+ cells in Figure 5A are epithelial cells), whereas at 20 weeks post vaccination, while there are Ki67+ proliferating IgG+ cells, most are Ki67-terminally differentiated plasma cells (Figure 5A). Following vaginal challenge, the number of both Ki67+ and Ki67-IgG+ cells significantly increased four days after vaginal challenge, in parallel with enlargement of the ectopic follicles in the vaccinated animals (Figure 5B).
Many of the IgG+ cells in the ectopic follicles are CD27+CD38+CD20+ surface IgG+ memory B cells (20) or early plasmablasts (Figure 5C).
The expression of AID (activation-induced cytidine deaminase), a DNA-editing enzyme essential for immunoglobulin gene diversification by somatic hypermutation, and for CSR (21–24), also increased following vaginal challenge (Figure 5D).
FIGURE 5.
Rapid induction of SIV-specific humoral immune response within vaginal ectopic follicles after WT SIVmac251 challenge. A. Increased red-stained IgG+ cells and follicles at 20w in vaccinated compared to unvaccinated animals. Most of the IgG+ cells are Ki67− (the green stained Ki67+ cells are primarily epithelial cells). Following vaginal challenge, Ki67+IgG+ cells increase beneath epithelium and particularly in the ectopic follicles. Representative images from n=4 for SIV-animals, n=4 for 20w and 3 for D4p20w animals. B. Quantification of increases in Ki67-cells at 2ow and D4p20w. Asterisks indicate significant increases. C. Phenotypic analysis of the IgG+ cells within ectopic follicles shows that they are CD38+CD27+CD20+ surface IgG+ plasmablasts. D. Fluorescent staining shows increased expression of activation-induced cytidine deaminase (AID) in the ectopic follicles after WT SIVmac251 challenge. Representative images were taken from animals at day 4 post-20 week vaccination.
Mucosal epithelial-humoral immune axis
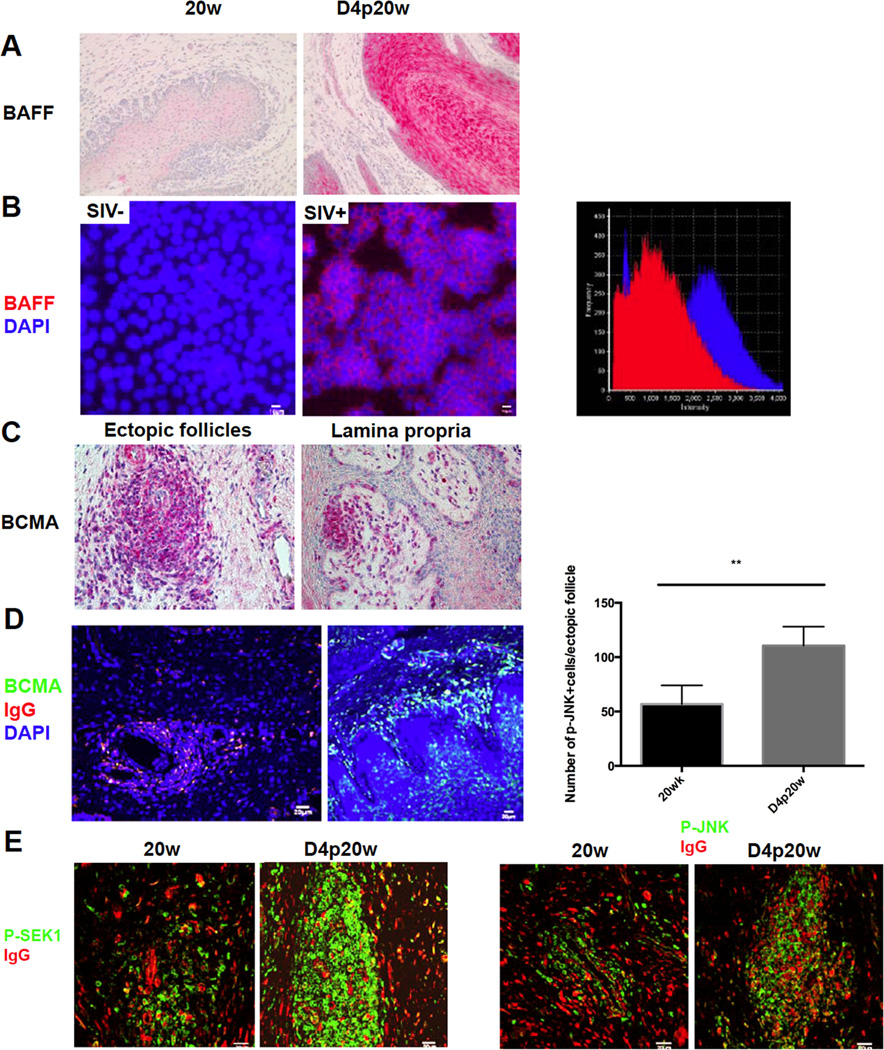
The vaginal epithelium not only can actively recruit plasma cells via CXCL9 and CXCL10 expression, but also expresses cytokines that create a favorable niche for the B cells and plasma cells to proliferate, survive, and differentiate. Because of the importance of B-cell activating factor (BAFF) in B and plasma cell survival and proliferation, antibody production, maturation and CSR (25–33), we stained sections to see if there might be a source of BAFF to induce the changes in the follicles just described. We indeed found that the epithelium could be one source of BAFF after vaginal challenge, since there were striking increases in BAFF staining in vaginal epithelium after challenge (Figure 6A). One hypothetical mechanism driving increased vaginal epithelial expression of BAFF could be exposure to SIV in the inoculum, and we did find that exposing an FRT epithelial cell line, HEC-1A cells, to WT SIV significantly increased BAFF expression (Figure 6B) consistent with this hypothesis. We have also previously shown that SIV in immune complexes is taken up by epithelium and interacts with the Fcg2Rb inhibitory receptor to block CD4 T cell recruitment following vaginal exposure, and in future work plan to determine whether viral binding might intersect with additional signaling pathways to elicit increased expression in BAFF.
FIGURE 6.
Epithelial cells responses to promote the humoral immune response in the FRT. A, Increased BAFF expression in (red-stained) vaginal epithelium after WT SIVmac251 challenge. Image magnification: 200×. B. Fluorescent staining of BAFF (red) and DAPI (blue) shows that exposure to SIVmac251 for 2 days leads to up-regulation of BAFF in the HEC-1A epithelial cell line. Image on the far right shows the quantification by fluorescence intensity of BAFF staining in vehicle treated (SIV-) HEC-1A cells (red) and in SIV (SIV+) treated HEC-1A cells (blue). Result represents two independent experiments with duplicates of each treatment C, BCMA expression in magenta-stained cells within ectopic follicles and beneath the vaginal epithelium after WT SIVmac251 challenge. Representative images were taken from animals at day 5 and 7 post-20 week vaccination. Image magnification 400×. D, BMCA+ cells after challenge are IgG+ (yellow to yellow green). E. Fluorescent staining of phosphorylated-JNK and phosphorylated SEK1 (green) and IgG (red) shows that phosphorylated-JNK and phosphorylated SEK1, two key molecules in BAFF-BCMA signaling pathway, are both up-regulated after WT SIVmac251 challenge. Quantification in the panel above p-JNK staining shows the significant increases indicated by asterisks.
We also documented potential functional interactions between BAFF and one of its receptors, receptor, B cell maturation antigen (BCMA) (29, 30), within ectopic follicles and plasma cells in the lamina propria (Figure 6C, D); and that two key molecules involved in BCMA signaling, phosphorylated JNK (Jun N-terminal Kinases) and phosphorylated SEK1 (SAPK/Erk kinase) (34–36) were also up-regulated within follicles in the vaccinated animals (Figure 6E). Thus, there is a spatially proximate axis between the vaginal epithelium and underlying plasma cells and ectopic follicles to facilitate antibody production and maturation in response to vaginal challenge.
Production of SIV-specific antibodies in ectopic follicles and vaginal submucosa
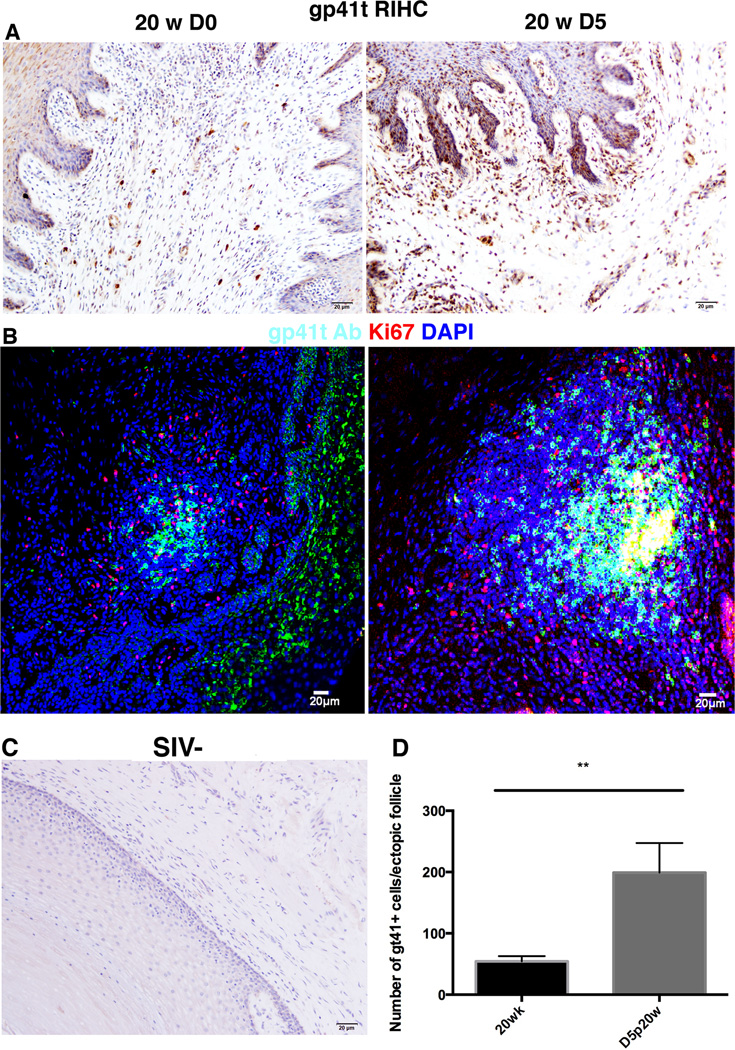
We had thus far visualized and characterized a general system for production of IgG antibodies in the FRT in response to vaginal challenge, and now show that the B cells and plasma cells in the submucosa and ectopic follicles were producing SIV-specific IgG antibodies to gp41t, which we had previously shown to be one correlate of the maturation of SIVmac239δnef mediated protection against vaginal challenge (6). We detected these antibodies by reverse immunohistochemistry (RIHC), using a labeled soluble gp41t construct (6). Four and five days following vaginal challenge, we found an increased number of trimeric gp41t+ plasma cells in the submucosa and ectopic follicles, consistent with expression of antibody specific for this antigen (Figure 7A, B). Some of the IgG+ cells in the follicles at day 4-post challenge were also Ki67+ (Figure 7B), consistent with gp41t-specific plasmablasts generated in situ in response to vaginal exposure to cognate antigen. The RIHC-staining was specific (Figure 7C) and the ~ 4-fold increases in gp41t+ cells in the ectopic follicles was significant at a P < 0.01 (Figure 7D).
FIGURE 7.
Increased gp41t-antibody producing plasma cells and ectopic follicles after vaginal challenge shown by RIHC. A. RIHC-staining shows increased gp41t+IgG+ (red-brown) plasma cells in the vaginal submucosa 5 days after vaginal challenge at 20w. B. Some of the gp41t+ cells (green) in follicles are also KI67+ (red) and have a white color in the merged confocal micrograph of follicles after vaginal challenge at 20w, consistent with rapid proliferative response following vaginal exposure. C. Representative image of RIHC control staining in an SIV-negative uninfected unvaccinated animal. D. Quantification of increased gp41t+ cells in follicles after vaginal challenge.
Discussion
In vaccines evaluated in NHP models, SIVmac239Δnef and other live attenuated vaccines have consistently provided the most robust protection against acquisition on subsequent WT SIV challenge by parenteral and mucosal routes (3–6), and thus their study provides an opportunity to identify correlates of protection and design principles for developing an effective HIV-1 vaccine. This approach has thus far identified as one correlate of protection prior to vaginal challenge, IgG antibodies reactive with trimeric gp41, (6, 9). These antibodies are produced locally as well as systemically, and concentrated by the FcRn receptor in cervical reserve and basal vaginal epithelium, thereby optimally localizing the antibodies to intercept virus and block access to target cells. In this way, they could prevent establishment and local expansion of infected founder populations at the portal of entry. The concentrated antibodies also play a critical role in the second correlate of protection by generating immune complexes generated after vaginal challenge. These immune complexes interact with the inhibitory FcgRIIb receptor in cervical epithelium to suppress recruitment of CD4 T cell targets to thereby prevent local expansion of infection (9).
Here we characterize the response after vaginal challenge in SIVmac239Δnef-vaccinated animals. We first show that vaccination induces general increases in plasma cells and ectopic follicles at mucosal sites that are not principal sites of viral replication. SIVmac239Δnef, other live attenuated SIV strains, WT SIV and HIV infect T follicular helper and other CD4+ T cells in lymphoid tissues (13–16). Nonetheless, we document low levels of replication at mucosal sites, and speculate that it is the persistent low level of SIVmac239Δnef-replication and expression of IFNs (17,18) that induce expression of CXCL9 and CXCL10 responsible for the influx of B cells and plasma cells and induction of ectopic lymphoid follicles at mucosal sites prior to challenge. In the FRT, we speculate that vaginal exposure to WT SIVmac251 might act as a “pull” as has been described for T cells (37) to increase epithelial expression of these chemokines and rapidly augment the recruitment of plasma cells over and above the larger number already recruited there by 20 weeks compared to 5 weeks post vaccination (6). These plasma cells and ectopic follicles then produce a “wall” of IgG in vaginal epithelium as striking evidence of the recall response in the FRT.
The ectopic follicles are structurally and functionally organized in a remarkably similar way to follicles in secondary lymphoid organs (19). The common features include: follicles with an FDC network and HEVs in GCs with the necessary cellular constituents of B cells, plasma cells and TFH for antibody production; and cells expressing AID and other proteins that mediate B cell proliferation and affinity maturation and CSR.
Our findings provide further evidence of a mucosal epithelium-immune system axis and a role for this axis in the response to vaginal challenge. The now epithelial components of this axis include: 1) expression of CCL20 in cervical epithelium associated with recruitment of CD4+ T cells to fuel local expansion of small founder populations of infected cells following vaginal challenge (10); 2) cervical vaginal epithelial expression of CXCL10 and FcRn to recruit plasma cells to produce and concentrate IgG antibodies at mucosal frontlines to intercept virus at entry (6); and epithelial expression of FcgRIIb, SPRED1, COMMD1 and RORa to block target cell recruitment (9). We show here that vaginal exposure to SIV rapidly induces BAFF expression in epithelium where interaction with BMCA could promote antibody production and maturation at the site of exposure. Thus, the FRT mucosal epithelium is literally the frontline of a system that on exposure to pathogens can initiate a rapid recall response at the portal of entry through interactions with the mucosal humoral immune system. Recreating this axis and organized system for rapid antibody production and maturation with safer alternatives to live attenuated SIV infection (38, 39) is both a design principle and goal for developing an effective vaccine against HIV-1.
Acknowledgments
We thank Ron Desrosiers and Chris Miller for virus stocks, Angela Carville for expert veterinary care, Elizabeth Curran and Andrew Miller for assistance with tissue processing and analysis, Susan Westmoreland for review of the manuscript, and Colleen O’Neill and Tim Leonard for preparation of the manuscript and figures.
Footnotes
This work was supported by the International AIDS Vaccine Initiative, NIH grants AI071306 and U19 AI095985 and OD01110, and in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
References
- 1.Haase AT. Targeting early infection to prevent HIV-1 mucosal transmission. Nature. 2010;464:217–223. doi: 10.1038/nature08757. [DOI] [PubMed] [Google Scholar]
- 2.Quinn TC, Overbaugh J. HIV/AIDS in women: an expanding epidemic. Science. 2005;308:1582–1583. doi: 10.1126/science.1112489. [DOI] [PubMed] [Google Scholar]
- 3.Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RP. Live attenuated AIDS vaccines: hazards and hopes. Nat. Med. 1999;5:154–155. doi: 10.1038/5515. [DOI] [PubMed] [Google Scholar]
- 5.Koff WC, Johnson RP, Watkins DW, Burton DR, Lifson JD, Hasenkrug KJ, McDermott AB, Schultz A, Zamb TJ, Boyle R, Desrosiers RC. HIV vaccine design: insights from live atenuated SIV vaccines. Nat. Immunol. 2006;7:19–23. doi: 10.1038/ni1296. [DOI] [PubMed] [Google Scholar]
- 6.Li Q, Zeng M, Duan L, Voss JE, Smith AJ, Pambuccian S, Shang L, Wietgrefe S, Southern PJ, Reilly CS, Skinner PJ, Zupancic ML, Carlis JV, Piatak M, Jr, Waterman D, Reeves RK, Masek-Hammerman K, Derdeyn CA, Alpert MD, Evans DT, Kohler H, Müller S, Robinson J, Lifson JD, Burton DR, Johnson RP, Haase AT. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol. 2014;193:3113–3125. doi: 10.4049/jimmunol.1400820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connor RI, Montefiori DC, Binley JM, Moore JP, Bonhoeffer S, Gettie A, Fenamore EA, Sheridan KE, Ho DD, Dailey PJ, Marx PA. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Virol. 1998;72:7501–7509. doi: 10.1128/jvi.72.9.7501-7509.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Imm. 2007;7:716–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 9.Smith AJ, Wietgrefe SW, Shang L, Reilly CS, Southern PJ, Perkey KE, Duan L, Kohler H, Müller S, Robinson J, Carlis JV, Li Q, Johnson RP, Haase AT. Live simian immunodeficiency virus vaccine correlation of protection: immune complex-inhibitory Fc receptor interactions that reduce target cell availability. J Immunol. 2014;193:3126–3133. doi: 10.4049/jimmunol.1400822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller CJ, Li Q, Abel K, Kim E-Y, Ma Z-M, Wietgrefe S, LaFranco-Scheuch L, Compton L, Duan L, Shore MD, Zupancic M, Busch M, Carlis J, Wolinsky S, Haase AT. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, Estes JD, Burton GF, Silvestri G, Lifson JD, Carlis JV, Haase AT. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin. Invest. 2011;121:998–1008. doi: 10.1172/JCI45157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salisch NC, Kaufmann DE, Awad AS, Reeves RK, Tighe DP, Li Y, Piatak M, Jr, Lifson JD, Evans DT, Pereyra F, Freeman GJ, Johnson RP. Inhibitory TCR coreceptor PD-1 is a sensitive indicator of low-level replication of SIV and HIV-1. J Immunol. 2010;184:476–487. doi: 10.4049/jimmunol.0902781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukazawa Y, Park H, Cameron MJ, Lefebvre F, Lum R, Coombes N, Mahyari E, Hagen SI, Bae JY, Reyes MD, 3rd, Swanson T, Legasse AW, Sylvester A, Hansen SG, Smith AT, Stafova P, Shoemaker R, Li Y, Oswald K, Axthelm MK, McDermott A, Ferrari G, Montefiori DC, Edlefsen PT, Piatak M, Jr, Lifson JD, Sékaly RP, Picker LJ. Lymph node T cell responses predict the efficacy of live attenuated SIV vaccines. Nat. Med. 2012;18:1673–1681. doi: 10.1038/nm.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, Pan L, Poholek A, Rao SS, Brenchley JM, Alam SM, Tomaras GD, Roederer M, Douek DC, Seder RA, Germain RN, Haddad EK, Koup RA. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp. Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi Y, Bao L, Gerard C, Iwasaki A. CD8+ T lymphocyte mobilization to virus infected tissue requires CD4+ T-cell help. Nature. 2009;462:510–514. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drayton DL, Liaio S, Mounzer RH, Ruddle NH. Lymphoid organ development: from ontogeny to neogenesis. Nat. Immunolog. 2006;7:344–353. doi: 10.1038/ni1330. [DOI] [PubMed] [Google Scholar]
- 20.Klei U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood cells expressing the CD27 surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp. Med. 1998;188:1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 23.Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, Scharff MD. The bio-chemistry of somatic hypermutation. Annu. Rev. Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 24.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moore PA, Belvedere O, Orr A, Pieri K, LaFleur DW, Feng P, Soppet D, Charters M, Gentz R, Parmelee D, Li Y, Galperina O, Giri J, Roschke V, Nardelli B, Carrell J, Sosnovtseva S, Greenfield W, Ruben SM, Olsen HS, Fikes J, Hilbert DM. BLyS: member of the tumor necrosis factor family and B lymphocyte stimulator. Science. 1999;285:260–263. doi: 10.1126/science.285.5425.260. [DOI] [PubMed] [Google Scholar]
- 26.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp. Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Avery DT, Kalled SL, Ellyard JI, Ambrose C, Bixler SA, Thien M, Brink R, MacKay F, Hodgkin PD, Tangye SG. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin. Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X, Park CS, Yoon SO, Li L, Hsu YM, Ambrose C, Choi YS. BAFF supports human B cell differentiation in the lymphoid follicles through distinct receptors. Int. Immunol. 2005;17:779–788. doi: 10.1093/intimm/dxh259. [DOI] [PubMed] [Google Scholar]
- 29.Thompson JS, Schneider P, Kalled SL, Wang L, Lefevre EA, Cachero TG, MacKay F, Bixler SA, Zafari M, Liu ZY, Woodcock SA, Qian F, Batten M, Madry C, Richard Y, Benjamin CD, Browning JL, Tsapis A, Tschopp J, Ambrose C. BAFF binds to the tumor necrosis factor receptor-like molecule B cell maturation antigen and is important for maintaining the peripheral B cell population. J Exp. Med. 2000;192:129–135. doi: 10.1084/jem.192.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin L-L, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bossen C, Tardivel A, Willen L, Fletcher CA, Perroud M, Beermann F, Rolink AG, Scott ML, MacKay F, Schneider P. Mutation of the BAFF furin cleavage site impairs B-cell homeostasis and antibody responses. Eur. J. Immunol. 2011;41:787–797. doi: 10.1002/eji.201040591. [DOI] [PubMed] [Google Scholar]
- 32.Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P, Cerutti A. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, Hochman PS, Scott ML, Kalled SL. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003;171:547–551. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- 34.Lin A, Minden A, Martinetto H, Claret FX, Lange-Carter C, Mercurio F, Johnson GL, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 35.Swat W, Fujikawa K, Ganiatsas S, Yang D, Xavier RJ, Harris NL, Davidson L, Ferrini R, Davis RJ, Labow MA, Flavell RA, Zon LI, Alt FW. SEK1/MKK4 is required for maintenance of a normal peripheral lymphoid compartment but not for lymphocyte development. Immunity. 1998;8:625–634. doi: 10.1016/s1074-7613(00)80567-1. [DOI] [PubMed] [Google Scholar]
- 36.Yang M, Hase H, Legarda-Addison D, Varughese L, Seed B, Ting AT. B cell maturation antigen, the receptor for a proliferation-inducing ligand and B cell activating factor of the TNF family, induces antigen presentation in B cells. J Immunol. 2005;175:2814–2824. doi: 10.4049/jimmunol.175.5.2814. [DOI] [PubMed] [Google Scholar]
- 37.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baba T, Jeong YS, Penninck D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- 39.Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, Bronson R, Greene MF, McClure HM, Martin LN, Ruprecht RM. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]