Abstract
Currently, there is little consensus regarding the most appropriate animal model to study acute infection and the virus–specific CD8+ T cell (CTL) responses in neonates. TCRβ high-throughput sequencing in naïve CTL of differently aged neonatal mice was performed, which demonstrated differential Vβ family gene usage. Using an acute influenza infection model, we examined the TCR repertoire of the CTL response in neonatal and adult mice infected with influenza type A virus. Three-day old mice mounted a greatly reduced primary NP(366–374)-specific CTL response when compared to 7-day old and adult mice, while secondary CTL responses were normal. Analysis of NP(366–374)-specific CTL TCR repertoire revealed different Vβ gene usage and greatly reduced public clonotypes in 3-day old neonates. This could underlie the impaired CTL response in these neonates. To directly test this, we examined whether controlling the TCR would restore neonatal CTL responses. We performed adoptive transfers of both non-transgenic and TCR-transgenic OVA(257–264)-specific (OT-I) CD8+ T cells into influenza-infected hosts, which revealed that naïve neonatal and adult OT-I cells expand equally well in neonatal and adult hosts. In contrast, non-transgenic neonatal CD8+ T cells when transferred into adults failed to expand. We further demonstrate that differences in TCR avidity may contribute to decreased expansion of the endogenous neonatal CTL. These studies highlight the rapid evolution of the neonatal TCR repertoire during the first week of life and show that impaired neonatal CTL immunity results from an immature TCR repertoire, rather than intrinsic signaling defects or a suppressive environment.
Introduction
Although there is a tremendous need for a clinically-relevant neonatal animal model to mimic human infection, there is inconsistency in the literature about what constitutes a neonatal human equivalent in the mouse. TdT knockout mice have been used as a neonatal surrogate, although adult TdT knockout mice have a surprisingly intact immune response to various pathogens (1, 2). In contrast, human neonates fail to develop strong CTL responses to influenza virus and respiratory syncytial virus infection (RSV) (3); therefore, animals deficient in TdT are not a suitable model for a human infant. Studies have defined a neonatal mouse anywhere from an age of 1 day to 3 weeks of age (4–7), and have demonstrated that neonatal mice have blunted T cell responses to influenza virus (5, 8) and HSV (9). Differences in T cell hierarchy have been reported using a 7-day neonatal model of RSV (10). We were curious whether CTL immunity differences exist in neonatal mice before 7 days of age and whether the neonatal environment, intrinsic defects in neonatal CD8+ T cells or the TCR repertoire alone or together can contribute to the reduced CTL responses to pathogens in neonates.
To determine the evolution of the naïve TCRβ repertoire, TCRβ high-throughput sequencing in naïve CTL of differently aged neonatal mice was performed. We compared CTL responses to dominant and subdominant epitopes of influenza in 3-day old, 7-day old and adult mice. To dissect the mechanisms underlying reduced CTL responses of 3-day old neonatal mice, we used influenza virus infections and adoptive transfers of non-transgenic and TCR transgenic neonatal and adult T cells. We find that 3-day old neonatal mice have greatly reduced dominant and subdominant CTL responses compared to 7-day old and adult mice. TCR repertoire analysis of virus-specific CTL in 3-day old neonates revealed different and shorter TCRβ CDR3 sequences and Vβ family utilization, which reflected the naïve repertoire. Finally, we find that the neonatal environment does not inhibit CTL responses, and TCR-transgenic neonatal CD8+ T cells do not exhibit intrinsic defects. Our findings suggest that differences in TCR repertoire in the neonate are a major contributor to the defective neonatal CD8+ T cell response.
Materials and Methods
Mice and infections
Experienced timed pregnant female C57Bl/6 mice were purchased to generate neonatal mice and 8 week old adult C57Bl/6 mice were purchased from Charles River Laboratory. CD45.1+, and CD45.2+ mice were obtained from Charles River Laboratory. CD45.1+ OT-I and Thy1.1+ OT-I mice were generated through breeding. The mice were housed under specific-pathogen-free conditions in an American Association for the Accreditation of Laboratory Animal Care-certified barrier facility at the Drexel University College of Medicine Queen Lane Campus animal facility. Animal work was carried out according to approved Institutional Animal Care and Use Committee protocols.
Neonatal mice at 3 days of age (weight ~3g) were infected intranasally (i.n.) with 0.12 TCID50 (0.04 TCID50/g) of influenza virus H1N1 strain PR8 (A/Puerto Rico/8/34) (generous gift of Dr. W. Gerhard, Wistar Institute, Philadelphia, PA) in a 5μl volume. Neonatal mice at 7 days of age (weight ~5g) were infected intranasally (i.n.) with 0.20 TCID50 (0.04 TCID50/g) of influenza virus in a 7μl volume. Adult 8 week old C57Bl/6 mice (weight ~20g) were infected i.n. with a sublethal dose of 3 TCID50 in a 20μl volume (0.15 TCID50/g). The mice were anesthetized with inhaled isoflurane before intranasal inoculations. The primary response was examined by harvesting cells from lungs and spleens on various days post-infection.
For secondary infections, neonatal mice were infected i.n. with 0.08 TCID50 of influenza virus H1N1 strain PR8, a slightly reduced amount from the dose used for the primary response experiments. The adult mice were infected as above with H1N1 PR8 virus. Sixty days later, the mice were challenged with H3N2 X31 recombinant influenza virus strain. Seven days after this second infection, the mice were harvested and lungs, spleens, and mediastinal lymph nodes were processed to analyze cell populations.
For OT-I adoptive transfer experiments, 3-day old neonatal CD45.1+ OT-I and adult Thy 1.1 OT-I spleens on the C57 background were harvested and percentage of splenic OT-I CD8+ T cells was calculated using flow cytometry. 5x104 OT-I splenic CD8+ T cells were injected intraperitoneally into congenic mice. Mice were infected with WSN-OVA strain, a SIINFEKL peptide- (OVA257–264) expressing influenza A/WSN/33 virus strain (H1N1, kind gift from Dr. David Topham, University of Rochester) in a total volume of 20 μL on the same day as the adoptive transfer.
For wild-type naïve adoptive transfer experiments, 3-day old CD45.2+ C57Bl/6 and adult CD45.2+ C57Bl/6 spleens were harvested and percentage of splenic CD8+ T cells was calculated using flow cytometry. 1.2–1.5 x106 primary splenic CD8+ T cells were injected intravenously into CD45.1+ C57Bl/6 mice (Jackson Laboratories). Mice were infected with PR8 at the adult infecting dose, as above.
Isolation of pulmonary lymphocytes
Pulmonary lymphocytes were isolated from individual mice by removing lungs and mincing into smaller pieces. The tissue was then digested for 2 h at 37°C with 3.0 mg/ml collagenase A and 0.15 μg/ml DNase I (Roche) in RPMI 1640 (Mediatech) containing 5% heat-inactivated FBS (Life Technologies), 2 mM l-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin (Mediatech). The digested tissue was then run through a 40-μm cell strainer (Falcon) and washed in the same media as above. Cells were counted using trypan blue exclusion with light microscopy.
Adoptive Transfer Experiments
For the OT-I adoptive transfer experiments, on day 7 post-infection, lung lymphocytes were isolated, and flow cytometry was performed. Percentage of donor OT-I cells in recipients was determined using: APC-labeled MHC class I Kb tetramers loaded with OVA peptide, anti- CD45.1 conjugated to FITC (BD Biosciences), anti-Thy 1.1 conjugated to eFluor 450 (eBioscience) and anti-CD8α conjugated to PERCP (BD Biosciences).
For the non-transgenic adoptive transfer experiments, mice were harvested at day 10 post-infection, lung lymphocytes were isolated, and flow cytometry was performed. Percentage of donor versus recipient NP(366–374)-specific CD8+ T cells was detected using peptide-loaded MHC class I Db tetramers conjugated to APC. The donor and recipient cells were distinguished using the following antibodies: anti-CD45.2 conjugated to FITC, anti-CD45.1 conjugated to PE, and anti-CD8α conjugated to PerCP (all BD Biosciences).
Flow Cytometry
MHC class I tetramers were prepared with biotinylated monomeric H-2b class I MHC molecules refolded in the presence of equimolar amounts of β2-microglobulin and excess immunodominant NP(366–374) (ASNENMETM) peptide. Tetramers were prepared with APC-labeled streptavidin (Molecular Probes). Cells were co-stained with anti-mouse CD8α conjugated to PerCP (BD Biosciences) or PE (Tonbo Biosciences), and anti-mouse Vβ8.3 conjugated to FITC (Biolegend) monoclonal antibodies. Anti-CD16/32 (Fc Block, clone 2.4G2) (Tonbo Biosciences) was used in all stains. For the tetramer titration on 3-day old and adult mice, lung cell suspensions containing equal numbers (7.5x104) of CD8+ T cells (neonatal and adult) were mixed and then co-stained with NP(366–374) loaded MHC class I Db tetramer. Adult cells were Carboxyfluorescein Diacetate Succinimidyl Ester (CFSE, CellTrace, Thermofisher) labeled. All stains were completed on ice to prevent internalization. All absolute cell numbers are calculated per 100 mg of lung tissue. To assess cytokine production, the H2K or H2D associated influenza virus peptides NP(366–374), DbPA(224–233), and KbNS2(114–121) were used for stimulations. For intracellular staining, pulmonary lymphocytes were incubated for 5 hours at a concentration of 2 × 106 cells in 200 μl of RPMI 1640 medium containing 10% FBS, and 5 μg/ml Brefeldin A (Epicentre Technologies, Madison, WI), in the absence or presence of 1 μM viral-specific peptide, and then washed and stained with anti-mouse CD8α-PE Ab (Tonbo Biosciences). The cells were permeabilized in PBS/0.5% saponin for 20 min before staining with mouse IFN-γ-APC (Tonbo Biosciences). Cells were fixed in 1% paraformaldehyde (Fisher Scientific) before flow cytometric analysis. Data was collected on a FACS Calibur using Cell Quest software or a FACS Fortessa using FACS Diva software (BD Biosciences). Analysis was performed using Flow Jo software (Tree Star).
Viral Loads
At various time points post-infection, the lungs of the mice were harvested, weighed, and frozen at −80°C in TRIzol (Invitrogen). RNA was isolated by the Qiagen RNeasy kit (Qiagen). The isolated RNA was then used for cDNA synthesis using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Virus was measured by real-time PCR using influenza specific primers as previously described (11). cDNA synthesis was performed using both a specific primer (5′-TCTAACCGAGGTCGAAACGTA-3′) and random hexamers. Real-time assays were performed in triplicate with 5 μl of cDNA, 12.5 μl of 2× TaqMan Universal PCR Master Mix (Applied Biosystems), 900 nM influenza A virus sense primer (5′-AAGACCAATCCTGTCACCTCTGA-3′), 900 nM influenza A virus antisense primer (5′-CAAAGCGTCTACGCTGCAGTCC-3′), and 200 nM influenza A virus probe (FAM-5′-TTTGTGTTCACGCTCACCGT-3′-TAMRA) (12). All primers were specific for the influenza A virus matrix protein. Amplification and detection were performed using an Applied Biosystems Prism 7900HT sequence detection system with SDS 2.2.1 software (Applied Biosystems) at the following conditions: 2 min at 50°C and 10 min at 95°C, then 45 cycles of 15 s at 95°C and 1 min at 60°C. For viral load measurement, a standard curve was developed with serial 10-fold dilutions of stock PR8 with known TCID50 concentration. Ct values were plotted against virus quantity in TCID50 per milliliter. This curve was used to convert the Ct values for viral loads to TCID50 equivalents. Virus RNA quantities in lungs were expressed as TCID50 equivalents/100 mg lung.
Cell Sorting and TCR sequencing
For the naïve CD8+ T cell sorting, spleens were harvested from 3-day old, 7-day old and 8 week old mice, and lymphocytes were isolated. Naïve CD8+ T cells were detected with CD8α conjugated to PE and CD44 conjugated to FITC (Tonbo Bioscience, San Diego CA). CD8+ T cells were then sorted on a FACSAria fluorescence-activated cell sorter (BD Biosciences). Lymphocytes were isolated from the lungs of influenza A infected newborn and adult mice on day 10 of infection. Influenza virus nuclear protein (NP)(366–374)-specific CD8+ T cells were detected using MHC class I tetramers. Cells were stained on ice for 40 min with anti-mouse CD8α conjugated to PE (Tonbo Bioscience, San Diego CA) and NP(366–374)-specific tetramer. Tetramers were prepared with APC-labeled streptavidin (Molecular Probes). Cells were washed once, counted and re-suspended in HBSS with 3% FBS at a concentration of 20 x 106 cells/ml. NP(366–374)-specific CD8+ T cells were then sorted on a FACSAria fluorescence-activated cell sorter (BD Biosciences). gDNA extraction was performed following the DNeasy Blood and Tissue Kit (Qiagen). Sorted cell numbers ranged from ~1,000 to ~100,000. Samples were analyzed with high-throughput sequencing at the survey level of the TCRβ CDR3 regions, which were amplified and sequenced using the Illumina Genome Analyzer using the ImmunoSeq® immune-profiling system (Adaptive Biotechnologies, Seattle, WA). In brief, bias-controlled V and J gene primers were used to amplify rearranged V(D)J segments for high-throughput sequencing designed to target an output of 200,000 assembled output sequences. CDR3 sequences were analyzed using the International ImMunoGeneTics (IMGT®)/V-QUEST tool (http://www.imgt.org), acknowledged as the international reference for immunoglobulin and TR sequence analysis, CSH Protocols, WHO/IUIS, to identify the V, D, and J genes that contributed to each rearrangement. Sequences were classified as nonproductive if it was determined that nontemplated insertions or deletions produced frameshifts or premature stop codons. Pielou’s evenness, a normalized Shannon’s H entropy, was calculated using the Adaptive Biotechnologies Immunoseq Analyzer software. Circos plots were generated with the Circos software package (13). The sequencing data for the 14 individual samples used for naive repertoire analysis and the 6 individual samples used for the primary response to influenza infection can be accessed from https://clients.adaptivebiotech.com/pub/Carey-2016-JI.
Statistical analysis
Statistical analysis was performed using the Shapiro-Wilk W test for normality, Student’s t-test and nonparametric Wilcoxon signed-rank test for paired and unpaired samples. Nonparametric (Spearman’s rho) statistics were used for measurements of correlation for data without a normal distribution and a linear regression was performed for data with a normal distribution. Analyses were performed with the JMP statistical analysis program (SAS, Cary, NC). P values<0.05 were considered to be statistically significant.
Results
The neonatal naïve CD8+ T cell receptor repertoire rapidly evolves during the first week of life
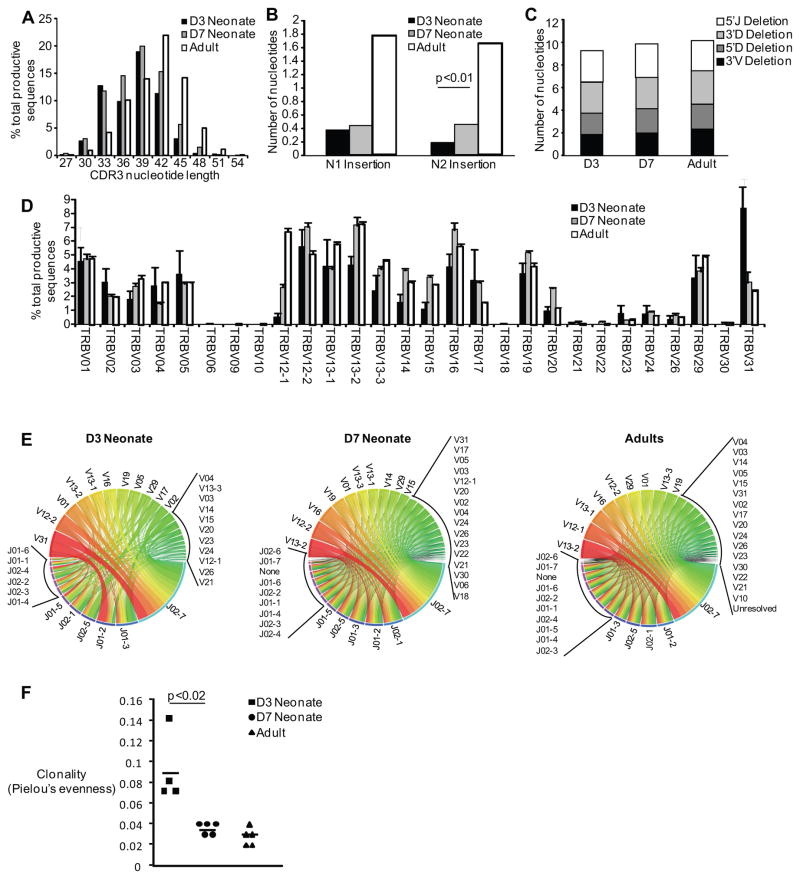
In order to investigate the mouse CD8+ T cell receptor repertoire in neonatal and adult mice, we performed high-throughput sequencing of the TCRβ CDR3 regions in sorted, naive CD8+ T cells from 3-day old, 7-day old, and adult mice. The CDR3 length of 3-day old mice was on average 37.8 nucleotides, as compared to 38.5 nucleotides for 7-day old mice and 41.5 for adult mice. Although the average weighted CDR3 length is essentially the same for 3-day old and 7-day old neonatal mice, the distribution of CDR3 lengths is different (Figure 1A), with 3-day old mice having fewer sequences with a length of 42 or 45 base pairs. Deconstruction analysis of the CDR3 region showed that the reduced length of the CDR3 lengths in neonatal samples was mostly due to reduced N nucleotide addition (Figure 1B), as previously reported (14). Of note, there is a statistically significant difference in the N2 additions between the 3-day and 7-day mice. Moreover, there was no increase in trimming at the 5′ and 3′ ends of the VDJ genes (Figure 1C), indicating that exonucleolytic activity does not contribute to generation of shorter CDR3 regions during neonatal life. Next, we looked at Vβ family usage among the samples. Our data demonstrate that the dominant Vβ usage in the 3-day old neonate is TRBV31, which is in direct contrast to the 7-day old and adult mouse usage of TRBV13-2 (Figure 1D and E). Finally, we calculated the Pielou’s evenness, which is a normalized Shannon’s entropy, to determine differences in clonality. Three-day old neonates have a higher clonality, indicating less diversity than their 7-day old and adult counterparts (p<0.02) in their naïve TCR repertoire (Figure 1F and Supplementary Table 1).
Figure 1. Comparison of CDR3 length, N1 and N2 insertions, junctional trimming, Vβ gene usage and V-J combination across the murine life span.
Naïve CD8+ T cells were sorted from 3-day old, 7-day old and adult mouse spleens and high-throughput sequencing was performed. (A) The CDR3 nucleotide lengths are depicted. (B) The number of nucleotides inserted into the N1 (region between the V and J gene segments) and N2 (region between the J and D gene segments) are shown. (C) 3′V and 3′D trimming is the nucleotide loss at the 3′of the V and D gene segments, respectively; 5′J and 5′D trimming is the nucleotide loss at the 5′ of the J and D gene segments, respectively. (D) The percentage of total Vβ gene usage (mean±SEM) of all productive sequences shown. (E) Circos plots of frequencies of age-specific Vβ and Jβ usage and combinations of productive sequences shown. The width of the band is proportional to the frequency. (F) The clonality of the naïve repertoire for both neonatal and adult animals, as determined by Pielou’s Evenness.
CTL immunity of 3-day old neonatal mice is less robust than 7-day old mice
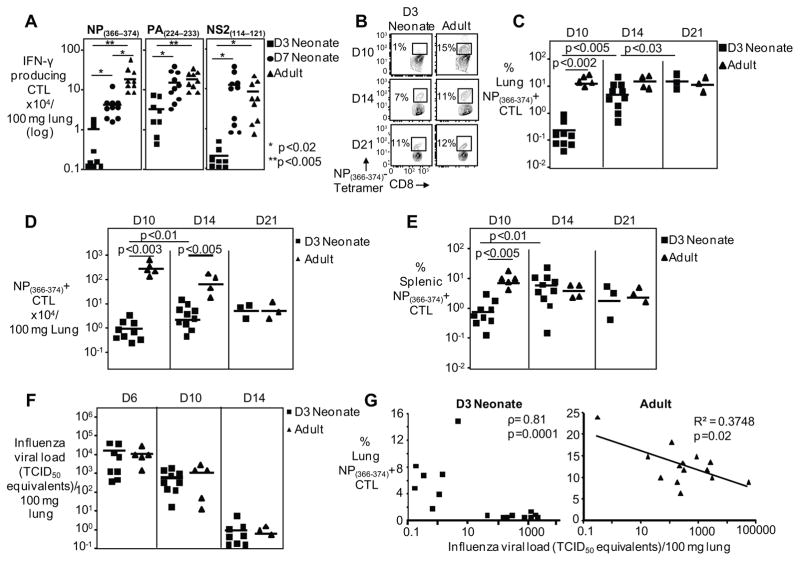
High-throughput sequencing of the naïve CTL TCR repertoire identifies a shift in Vβ family usage and longer CDR3 lengths from 3 days of life to 7 days of life suggesting that 7-day old mice may be more similar to adult mice than previously thought (Figure 1E). Based on the above, we hypothesized that the 7-day old mouse may have a more adult-like CTL response to influenza infection. We infected 3-day old, 7-day old, and adult mice with H1N1 PR8 influenza virus and measured virus-specific CTL responses against dominant and subdominant epitopes (15) on day 10 post-infection, the peak of the CTL response (16). Both groups of neonates had a depressed NP(366–374)-specific CTL response in terms of absolute numbers (1.3±0.6 x104 and 4.6±1.1 x104 per 100 mg lung tissue for 3-day and 7-day old mice, respectively) versus the adult controls (21.9x104±5.4 per 100 mg lung tissue). However, 7-day old neonates have an intermediate response when compared to 3-day old neonates and adult controls indicating that the T cell repertoire evolves over the first week of life (Figure 2A). We also examined immunity to the PA(224–233) epitope, another dominant CTL epitope in the adult C57Bl/6 mouse. For the PA(224–233) epitope, 3-day old neonates have a depressed CTL response (2.9±1.0 x104 per 100 mg lung tissue) versus the other two groups (15.3±4.3 x104 and 21.2±4.0 x104 cells per 100 mg lung tissue for 7-day and adult mice, respectively) (Figure 2A). Finally, the NS2(114–121) subdominant epitope response in 3-day old neonates is also reduced in terms of absolute numbers (0.2±0.08 x104per 100 mg lung tissue) versus the other two groups (14.4±5.3 x104 and 7.8±3.4 x104 per 100 mg lung tissue for 7-day and adult mice, respectively) (Figure 2A). These results demonstrate two points: first, 3-day old neonates have reduced CTL responses to both dominant and subdominant epitopes, and second, although 7-day old mice have lower CTL responses to the NP(366–374) dominant peptide than adults, it still is significantly higher than in 3-day old neonates. Despite their lower NP(366–374) response, 7-day old mice are able to develop an adult-equivalent CTL response to the second dominant PA(224–233) epitope and the NS2(114–121) subdominant epitope, confirming previous studies that showed changes in epitope dominance in 7-day old mice compared to adult mice (10). Together, these data demonstrate that the CTL response to influenza virus dynamically evolves over the first week of life.
Figure 2. Diminished and delayed primary CD8+ T cell response in Influenza virus infected neonates.
(A) IFN-γ producing CTL numbers for the NP366–374, PA224–233 and NS2114–121 peptide stimulation of d10 post infection pulmonary lymphocytes are depicted. Symbols represent individual animals, with the horizontal line marking the mean of each group (n= 8–10 mice per group, 2 independent experiments). Representative flow cytometry plots (B) and pooled data (C) showing frequencies of pulmonary CTLs 10,14, and 21 days after PR8 infection. Symbols represent individual animals, horizontal line marking mean of each group (n= 3–10 mice per group, at least two independent experiments). (D) NP(366–374)-specific CTL numbers per 100 mg of lung shown from the lungs day 10,14, and 21 post-infection. (E) Percentage of splenic NP(366–374)-specific CTLs are shown day 10,14, and 21 post-infection. (F) Neonates have similar viral loads as compared to the adults during influenza infection. Viral loads were measured by real-time PCR and normalized per 100 mg of lung tissue. Symbols represent individual animals, horizontal line marking mean of each group (n= 3–9 mice per group, at least two independent experiments). (G) Viral load and NP(366–374)-specific CTL frequency inversely correlate in neonates and adults. Nonparametric (Spearman’s rho) statistics were used for measurements of correlation for data without a normal distribution (neonates) and a linear regression was performed for data with a normal distribution (adults). Each symbol depicts one animal (neonates=16, adults=14).
The primary CD8+ T cell response in influenza virus infected neonates is diminished and delayed
We next sought to further characterize the influenza -specific CTL response in the 3-day old neonatal mouse. The immunodominant NP(366–374)-specific primary CD8+ T cell response in adult mice peaks on days 10–11 (16). Therefore, a shift in the kinetics of the response could account for the reduced CTL response in 3-day old mice. To exclude this, we infected 3-day old and adult mice with PR8 virus and harvested animals at days 10, 14 and 21 post-infection and stained with peptide-loaded MHC class I tetramers. Confirming our results above, at day 10 post-influenza virus infection, neonates have a significantly reduced frequency of lung NP(366–374)-specific CTL when compared to adult mice (0.3% ± 0.1 versus 16.4% ± 2.3 of CD8+ T cells, respectively) (Figure 2B and C). This was accompanied by a 2-log reduction in the numbers of lung NP(366–374)-specific CTL in neonates relative to adult mice (0.9±0.3 x104 versus 219±65 x104 per 100 mg of lung tissue, respectively) (Figure 2D). This reduction of neonatal NP(366–374)-specific CTL was also seen in the spleen (Figure 2E), suggesting that the reduction in lung numbers was not due to altered migration. By day 14 post-infection, however, while the adult NP(366–374)-specific CTL response was already contracting (13.7%±1.6 of CD8+ T cells; 55±16.4 x104 per 100 mg of lung tissue), the neonatal NP(366–374)-specific CTL response continued to increase (5.4%±1.3 of CD8+ T cells; 4.5±1.4 x104 per 100 mg of lung tissue) (Figure 2C and D). This results in a comparable frequency of virus-specific CD8+ T cells in the lungs of neonates and adult mice at day 14. However, the absolute numbers of virus-specific CTLs remain 10-fold reduced in the neonatal lungs. Finally, at day 21 post infection, the frequency of the neonatal NP(366–374)-specific CTL response continued to increase (14.0% ± 2.0) (Figure 2C and D) and the absolute numbers of neonatal NP(366–374)-specific CTLs remained essentially the same (5.7±1.0 x104 per 100 mg of lung tissue) as compared to day 14, while the adult absolute numbers continued to contract and were comparable with the neonatal numbers (5.8±1.3 x104 per 100 mg of lung tissue for adult mice). Therefore, despite the fact that the neonates reach adult-level frequencies at a delayed time point, they never attain the peak adult-level of absolute numbers of NP(366–374)-specific CTLs. The neonatal day 14 numbers were nearly 50-fold lower than the day 10 peak of the adult animals (4.5±1.4 x104 versus 219±65 x104 per 100 mg of lung tissue, neonate day 14 post-infection and adult day 10 post-infection, respectively) (Figure 2D). From these studies, it is evident that neonatal mice mount a diminished NP(366–374)-specific CTL response against influenza virus infection which remains low in magnitude and exhibits delayed kinetics compared to adult mice.
Impaired CTL responses in neonates are not due to diminished antigenic load
To understand why 3-day old neonates mount such diminished primary CTL responses, we first asked whether neonates had lower lung viral titers and the associated reduced antigenic load resulted in reduced neonatal virus-specific CTLs. We find that 3-day old mice have a similar viral load kinetic as compared to their adult controls at days 6, 10, and 14 post-infection (Figure 2F). This demonstrates that the neonates are productively infected and their reduced numbers of virus-specific CTLs cannot be attributed to reduced antigen loads. The CTL response plays a critical role in the control and clearance of virally-infected cells (17). Therefore, we correlated the frequency of virus-specific CTLs with viral loads in both the neonates and the adults (Figure 2G). The viral load decreases with increasing numbers of NP(366–374)-specific CTLs, demonstrating that although it is a diminished and delayed response in neonates, these virus-specific CTLs do contribute to viral control.
Mice infected during the neonatal period have a robust secondary CTL response
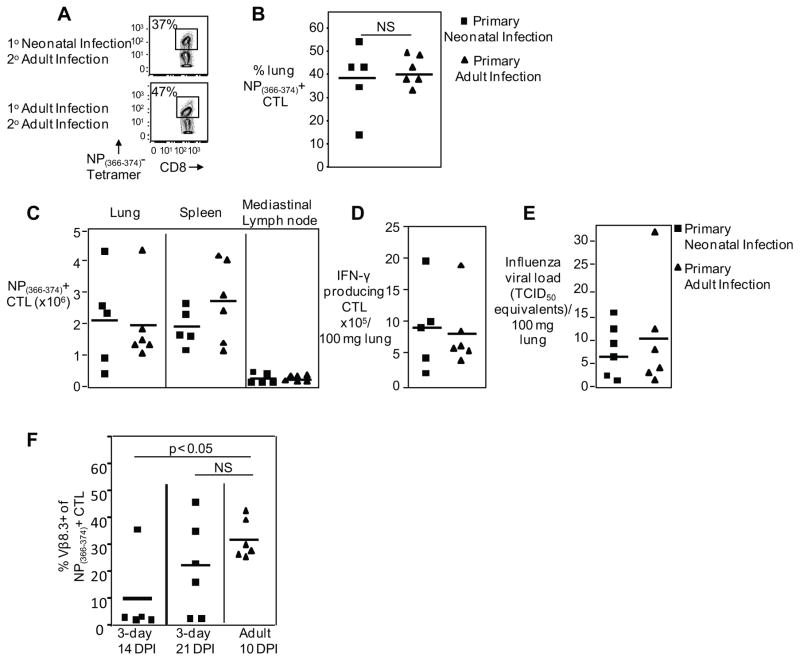
Because neonatal mice had an impaired primary virus-specific CTL response, we investigated whether memory and secondary responses to influenza virus were also affected. To perform these studies, we infected 3-day old neonates and adults with H1N1 PR8 virus and re-challenged these mice at 60 days post-infection with heterosubtypic H3N2 X31 influenza virus. In adult mice, the secondary NP(366–374)-specific CTL response to influenza virus in the lung peaks on day 7–8 with a frequency of ~30% of the CD8+ T cells. We found that secondary lung NP(366–374)-specific CTL responses in mice primed as neonates were similar in magnitude to those of mice primed as adults in both frequency (38.5%±8.0 versus 40.9%±2.5 of lung CD8+ T cells, respectively) (Figure 3A and B) and numbers (2.2±0.6 x106 versus 2.0±0.6 x106 NP(366–374)-specific CTL, respectively) (Figure 3C). In addition, the absolute numbers of NP(366–374)-specific CTL in the spleen (1.9±0.3 x106 versus 2.8±0.6 x106, in neonates and adults respectively) and the mediastinal lymph nodes (0.2±0.05 x106 versus 0.1±0.02 x106, in neonates and adults respectively) were similar (Figure 3C).
Figure 3. Mice infected during the neonatal period have a robust secondary CTL response.
Primary infection was performed in 3-day old neonatal or adult mice with PR8 influenza, 60 days later mice were challenged with H3N2 X31 recombinant strain and virus-specific CTLs were analyzed day 7 post-infection. Representative flow cytometry plots (A) and pooled data (B) showing percentage of pulmonary NP(366–374)-specific CTLs from mice either infected as neonates or adults and challenged as adults. (C) Pooled data showing NP(366–374)-specific CTL numbers in the lung, spleen and mediastinal lymph nodes from mice either infected as neonates or adults and challenged as adults. (D) IFN-γ producing CTL numbers shown in response to peptide stimulation after challenge. (E) Viral loads of neonates and adults after challenge were measured by real-time PCR and normalized per 100 mg of lung tissue. (F) Pooled data showing percentage of pulmonary NP(366–374)-specific CTLs which were Vβ 8.3 positive from mice either infected as neonates or adults and harvested at various time points. Symbols represent individual animals, horizontal line marking mean of each group (n= 5–6 mice per group, 2–3 independent experiments).
We also performed peptide stimulations and found that the absolute numbers of NP(366–374) peptide-induced IFNγ-producing CTLs are similar between animals infected the first time as a neonate versus those animals infected for the first time as adults (8.9±2.9 x105 versus 8.2±2.2 x105 in neonates and adults respectively) (Figure 3D). Additionally, viral loads did not differ in these animals (Figure 3E), further indicating the memory population function normally in animals infected as a neonate. Thus, in spite of a defective primary response in 3-day old neonates, these animals can establish memory and mount an intact secondary response.
To further investigate the intact secondary response in those animals with primary infection in the neonatal period, we infected 3-day old neonates and adults with influenza and then harvested the adult animals at their peak virus-specific CTL response on day 10 post infection, and the neonates at days 14 and 21 post infection, and found that there is an increase in the frequency of the adult dominant Vβ.3 family (TRBV 13.1) in the neonatal NP(366–374)-specific CTL (adult day 10: 31.6%±7.5, neonate day 14: 9.6%±14.6 and day 21: 24.5%±24) (Figure 3F). These results suggest that 3-day old neonates recruit “adult-like” Vβ TCR over time, which may explain the intact secondary response in these mice.
Neonatal virus-specific CTLs have a different, unfocused TCR repertoire that lacks public TCR clonotypes seen in adults
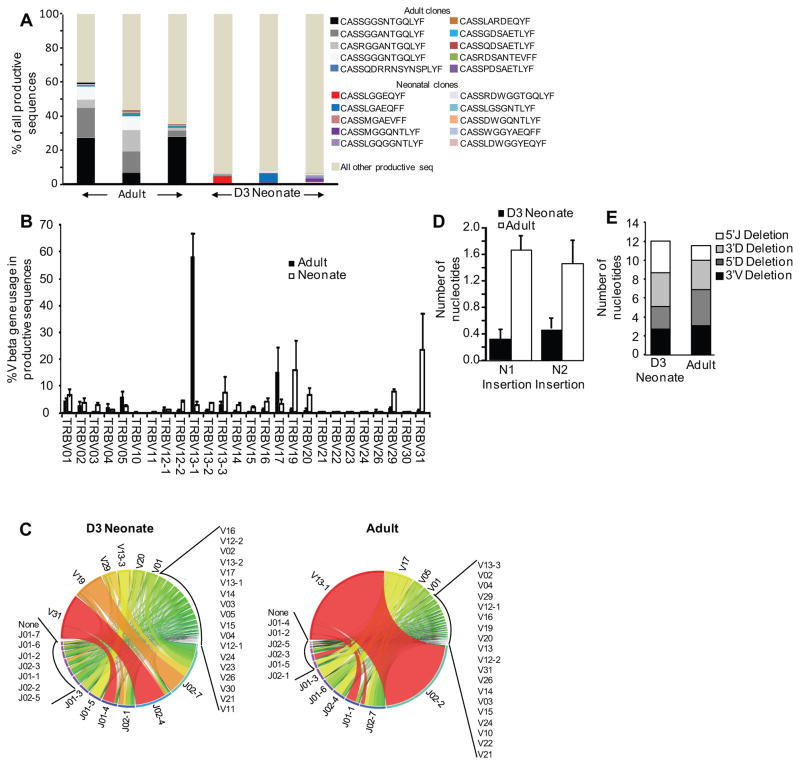
Because our naïve CD8+ T cell TCR repertoire analysis showed major differences in 3-day old mice, we hypothesized that TCR utilization by virus-specific CTL may be dissimilar in neonatal mice and may explain, at least in part, CTL response differences in adult mice. To examine this we performed next generation sequencing of sorted, NP(366–374)- specific CTLs from animals on day 10 post-infection. We found that the top shared adult clones to the immunodominant NP(366–374) epitope used the CDR3 sequences: CASSGGSNTGQLYF, CASSGGANTGQLYF, CASSGGGNTGQLYF, and CASRGGANTGQLYF, all sequences that have been previously established as public clones in the mouse NP(366–374)-specific CTL response to influenza infection (1, 18, 19). These public clonotypes, however, were infrequent in the neonates. The ten most frequent shared clones in adults comprised 46%±7% of all clones while the ten most frequent shared clones in neonates only made up 6%±0.5% of all clones (Fig. 4A, p< 0.03, Supplementary Tables 1 and 2). Importantly, adult mice have a high frequency of these shared clones, in contrast to the neonates who each had a different dominant clone (Figure 4A). When the Pielou’s evenness was calculated for the adult and neonatal samples, the adults had a higher average clonality (0.55) versus the neonates (0.41), indicating that the neonates have a less focused NP(366–374)-specific response. This is particularly interesting in comparison to the naïve data (Figure 1F), which showed that 3-day old neonates have a more focused naïve repertoire. Together, these data indicate that the 3-day old neonate has a less diverse naïve repertoire, and when challenged with antigen, is unable to focus like their adult counterparts.
Figure 4. High-throughput sequencing of the neonatal versus adult mouse T cell receptor repertoire of virus-specific CTLs.
High-throughput sequencing was performed on sorted NP(366–374)- specific CTLs. (A) The CDR3 sequence frequency for all productive sequences for both the neonates and adults is shown, with the ten most common shared clones among the adults and neonates highlighted. (B) The percentage of total Vβ gene family usage (mean±SEM) of all productive sequences shown. (C) Circos plots of frequencies of Vβ and Jβ usage and combinations of productive sequences shown. The width of the band is proportional to the frequency. (D) The number of nucleotides inserted into the N1 (region between the V and J gene segments) and N2 (region between the J and D gene segments) are shown. (E) 3′V and 3′D trimming is the nucleotide loss at the 3′of the V and D gene segments, respectively; 5′J and 5′D trimming is the nucleotide loss at the 5′ of the J and D gene segments, respectively.
In addition to utilizing private clonotypes, the neonates also have a less focused use of Vβ families. The number of Vβ families in the top 50% of all TCR sequences ranged from 4 to 11 in each of the neonates, as opposed to 1 to 2 in the adults, highlighting again the preferential use of Vβ and the much more focused response in adults. The predominant Vβ used in neonates is also different, as neonates utilized TRBV31 and TRBV19 while the adult TCRs used predominantly the TRBV13-1 and TRBV17 families (Figure 4B and C). Neonates used primarily TRBJ2-4 and TRBJ2-7 while adults used TRBJ2-2 (Figure 4C). The neonates also have a shorter CDR3 region (37.8 ±0.6 versus 42.7 ±1.0 bases, for neonates and adults respectively, p=0.015), which inherently leads to less diversity. This was most likely due to fewer N1 and N2 nucleotide insertions in neonates (Figure 4D), indicating reduced TdT activity. Junctional trimming was not different (Figure 4E). The above indicate that the virus-specific CTL response in neonates utilizes a very different and less focused TCR repertoire as compared to adult animals, and lacks the characteristic public clonotypes found in adult mice.
Adult public NP(366–374)- specific TCR clonotypes are rare in neonates
Based on the above, we questioned whether the reduced use of public TCR clonotypes for their NP(366–374)- specific CTL response by the 3-day old neonatal mice was due to these clonotypes being absent from their naïve TCR repertoire. To test this, we queried the CDR3 sequences in the naïve CD8+ T cell TCR repertoire of 3-day old, 7-day old and adult mice for the presence of adult shared public NP(366–374)- specific clonotypes. Nine out of 10 of these adult shared, public NP(366–374)- specific clonotypes (adult clones in Figure 4A) are found in the naïve adult TCR repertoire, 5 out of 10 clones are present in the 7-day old neonates and only 2 out of 10 clones are present in the 3-day old neonates. These findings suggest that 3-day old mice use less public NP(366–374)- specific clonotypes due to the greatly reduced frequency of these clonotypes in their naïve TCR repertoire. These public clonotypes clearly increase during the first week of life as they are found in 7-day old mice at a higher frequency, again confirming the rapid maturation of the TCR repertoire during the first week of life.
The neonatal environment does not suppress CTL responses to influenza virus
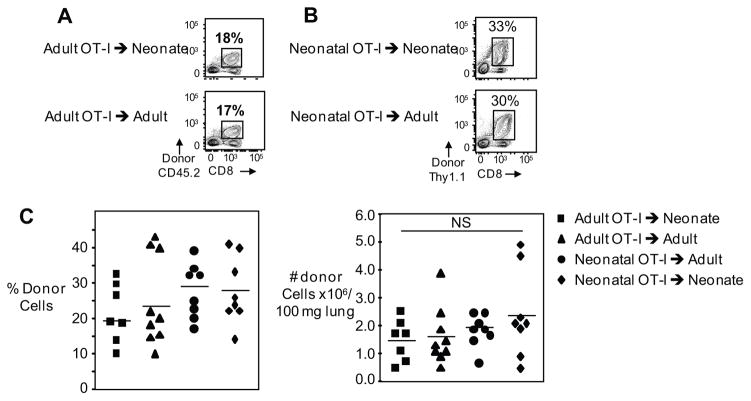
Despite the marked difference in the neonate’s TCR utilization, the reduced primary CTL responses of neonates could be, in part, attributed to a suppressive neonatal environment (20–22). To test this directly, we performed adoptive transfer experiments of congenically mismatched CD45.2+ TCR transgenic OT-I cells from adult mice into CD45.1+ 3-day old neonates and adult mice, and infected these mice with the OVA(257–264)-expressing influenza A/WSN/33 (WSN-OVA) virus. Previous studies have indicated that TCR transgenic CD8+ T cells behave most like endogenous T cells when low numbers of cells are transferred by intravenous injections (23). Therefore, we performed a dose response of transferred cells and found that the lowest number of intraperitoneally injected cells into neonates and adults that resulted in a detectable expansion post infection was 50,000. Based on these results, all adoptive transfer experiments were done with 5x104 OT-I cells. Adult OT-I cells expanded to the same degree in neonates and adult hosts in terms of both frequencies (19.4%±3.3 versus 22.8%±4.6 of lung CD8+ T cells, respectively) (Figure 5A) and numbers (1.5±0.3 x106 versus 1.6±0.3 x106 donor OT-I cells per 100mg of lung, respectively) (Figure 5C). At 7 days post-infection with WSN-OVA, both neonates and adults have an average viral load of 103 TCID50 equivalents/100 mg lung. These findings show that adult CD8+ T cells can expand normally in the neonatal environment and exclude that a suppressive neonatal environment is responsible for the reduced primary CTL responses we find in neonates.
Figure 5. Expansion of neonatal and adult OT-I CD8+ T-cells after adoptive transfer into neonatal and adult mice is comparable.
Splenocytes from neonatal and adult mice were adoptively transferred into neonates or adults and the expansion of OT-I CD8+ cells were analyzed on day 7 post-infection. Representative flow cytometry plots of pulmonary lymphocytes depict percentage of adult (A) and neonatal (B) donor OT-I CD8+ T cells on day 7 post-infection. Pooled data showing percentage (C left) and numbers (C right) of pulmonary donor OT-I CTLs. Symbols represent individual animals, horizontal line marking mean of each group (n= 7–9 mice per group, two independent experiments). Statistical analysis was performed using the Shapiro-Wilk W test for normality, Student’s t-test for paired and unpaired samples.
TCR-transgenic neonatal CD8+ T cells expand normally
Potentially, there could be an intrinsic defect in the neonatal CD8+ T cells’ ability to proliferate or survive, such as signaling defects in T cells due to their immaturity (24) or to the reduced diversity or makeup of the neonatal TCR repertoire (25–28). To address the question of an intrinsic defect of neonatal CD8+ T cells while circumventing differences of TCR repertoire in neonates, we transferred congenically mismatched neonatal OT-I cells into neonates and adults and compared them to adult OT-I cell transfers. We found that 3-day old neonatal OT-I cells expanded to the same degree as adult OT-I cells when transferred into adult host mice both in terms of frequency (26.6% ± 2.7 versus 22.8% ± 4.6 of lung CD8+ T cells in neonatal and adult hosts respectively) (Figure 5B) and numbers (2.5±0.6 x106 versus 1.6±0.3 x106 donor OT-I cells per 100 mg of lung in neonatal and adult hosts respectively) (Figure 5C). Potentially, neonatal CD8+ T cells could specifically express a receptor for a suppressive cytokine present in the neonatal milieu. To investigate this, neonatal OT-I cells were transferred into neonatal hosts, and they expanded similar to transferred adult cells both in terms of frequency (26.5% ± 3.8 of lung CD8+ T cells) and numbers (2.4±0.6 x106 donor OT-I cells per 100 mg of lung) (Figure 5B and C). Therefore, the neonatal environment did not selectively suppress neonatal CD8+ T cells. The above data demonstrate that neonatal cells can expand within the adult environment, which excludes an intrinsic neonatal T cell signaling defect. In addition, if one circumvents the TCR repertoire of the neonatal T cells with a high affinity TCR, neonatal CD8+ T cells can mount robust primary responses to viral infection in both neonatal and adult hosts.
Non-transgenic neonatal CD8+ T cells in an adult environment have reduced expansion
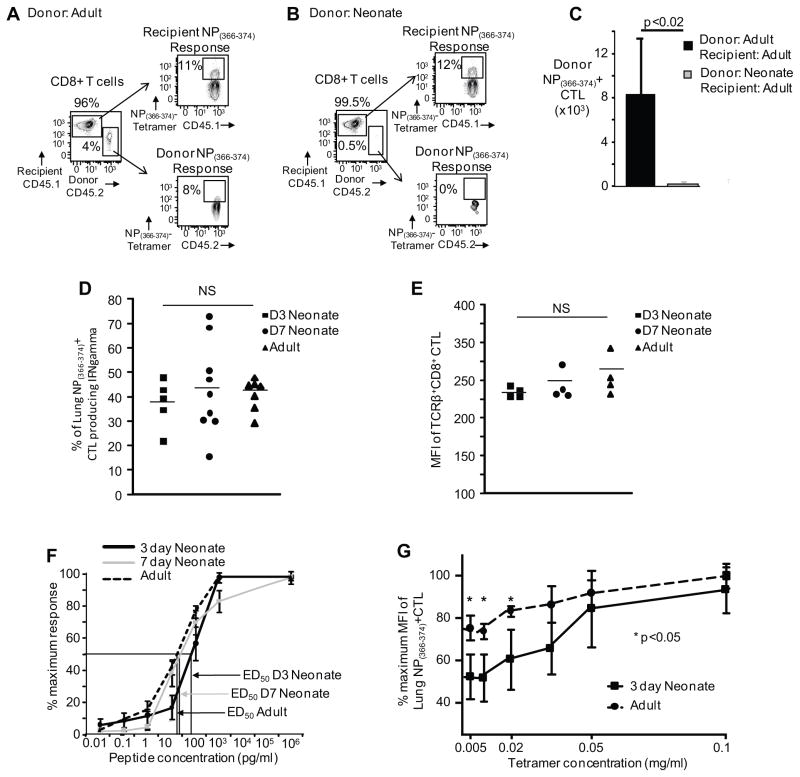
Next, we investigated whether endogenous neonatal T cells without a transgenic TCR could expand in the adult host to clearly exclude a suppressive neonatal environment. Equal numbers (1.5x106 CD8+ T cells) of congenically mismatched CD45.2+ naïve CD8+ T cells from neonatal and adult mice were injected intravenously into adult CD45.1+ mice, which were then infected with influenza virus. Ten days after adoptive transfer and infection, the frequency and absolute numbers of lung neonatal NP(366–374)-specific CTLs was significantly lower than the adult response (0.3±0.2 x103 versus 8.2±4.9 x103 donor NP(366–374)-specific CTL) (Figure 6A, B and C). Together, these data indicate that equal numbers of non-transgenic neonatal CD8+ T cells are not able to expand in the adult host as well as non-transgenic adult CD8+ T cells. This argues against the neonates diminished expansion of NP(366–374)-specific CTLs being solely due to a smaller pool of CD8+ T cells in the neonate. Taken together with the adoptive transfers of OT-I cells, these findings argue that TCR differences are a major contributor to the reduced ability of neonates to mount an efficient virus-specific CTL response.
Figure 6. Expansion of neonatal non-transgenic CD8+ T-cells after adoptive transfer into adult mice is diminished as compared to adult non-transgenic CD8+ T cells.
Splenocytes from neonatal and adult mice with matched numbers of CD8+ lymphocytes were adoptively transferred into adults, mice were infected with influenza virus and the expansion of donor CD8+ cells were analyzed on day 10. Representative flow cytometry plots from pulmonary lymphocytes depict percentage of donor NP(366–374)-specific CTLs from adult (A) and neonatal mice (B) versus the recipient’s endogenous response. (C) Pooled data showing absolute numbers of the donor NP(366–374)-specific CTLs from the lungs 10 days post-infection (n= 4–6 mice per group, four independent experiments). (D) Similar IFNγ production after NP(366–374)-peptide stimulation. Percentage of NP(366–374)-specific CTLs (based on MHC I tetramer staining) producing IFNγ is similar among 3-day old, 7-day old and adult mice. (E) Similar MFI of TCRβ+CD8+ CTL among 3-day old, 7-day old and adult mice. (F) Reduced binding affinity of the 3-day old TCR. NP(366–374)-peptide responses (0.01 pg – 1 μg) CTLs 14 days post-infection is depicted as percentage maximum response determined by IFNγ production (n= 4–9 mice per group, 2–3 independent experiments). Arrows indicate ED50 for the different groups. (G) Tetramer titration assay. Equal numbers of adult CD8+ CTL at day 10 post infection and 3-day old CD8+ CTL at day 14 post infection were co-stained with decreasing concentrations of NP(366–374) loaded MHC class I Db tetramer (n= 4 mice per group). Statistical analysis was performed using the Student’s t-test for unpaired samples.
Neonatal virus-specific CTLs have reduced peptide affinity
Because TCR usage was a major determinant of the reduced CTL response in the neonate, we hypothesized that these CTLs may have decreased TCR binding affinity. Several studies have shown that influenza and other respiratory viruses induce functionally impaired CD8+ T cells in the lung (29). Therefore, it was important to determine that the function of the virus-specific CTLs was similar in the pulmonary environment at all three of these ages. In fact, we found that regardless of age, virus-specific CTLs had similar function (Figure 6D), whereby only ~50% of the lung NP(366–374) tetramer-positive cells make cytokine or degranulate after peptide stimulation. After we had established equivalent function, we sought to determine that there are equal levels of TCRs on the surface of the CD8+ in both the neonate and adult. Therefore, we stained 3-day old, 7-day old and adult CD8+ CTL with a pan-TCR antibody, and found that the MFI of TCR expression of CD8+ cells is equivalent (Figure 6E). Next, we performed NP(366–374) peptide dose responses 14 days post-infection from 3-day old, 7-day old and adult mice and intracellular IFNγ staining was performed. We chose to look at 14 days post-infection because prior to this point there were insufficient virus-specific CTLs to obtain accurate results. These studies revealed that the 3-day neonatal mice had an ED50 that was 4-fold lower than 7-day old mice and 6-fold lower than adult mice (ED50: 91.6±1.3pg/ml, 21.6±1.5pg/ml, and 15.4±1.4pg/ml, 3-day, 7-day and adult mice respectively) (Figure 6F). This indicates that the 3-day old neonatal TCRs have decreased affinity for peptide, and this affinity increases by 4-fold over the first week of life.
To further address the question of TCR avidity, we performed a tetramer titration experiment, where we mixed and co-stained equal numbers of adult CD8+ CTL at day 10 post infection and 3-day old CD8+ CTL at day 14 post infection with decreasing concentrations of NP(366–374) loaded MHC class I Db tetramer. Adult cells were labeled with CFSE to distinguish the adult cells in the co-stain from neonatal cells. We found that the 3-day old neonates had a more rapid decline in Mean Fluorescence Intensity (MFI) as the concentration of tetramer decreases, as compared to the adults (Figure 6G). Based on the above, lower TCR affinity could explain the decreased expansion of virus-specific CTLs during acute influenza infection in neonatal mice.
Discussion
Although neonatal and adult CTL responses have been compared in the setting of DNA vaccines (30, 31), virus-like particles (32), and adjuvants (33), little is known about the neonatal CTL response to acute viral infection. To dissect the CTL response in neonates, we first examined the TCR repertoire of naïve CD8+ T cells in 3-day, 7-day and adult mice. We find that the Vβ family usage was different in 3-day old mice, while 7-day old mice had adult-like usage. We also find that 3-day mice have shorter CDR3 and the CDR3 length distribution shifts to longer CDR3 lengths from 3 days of age to 7 days of age to adult mice. This shift in CDR3 length appears to be due to the lack of N1 and N2 nucleotide additions, as opposed to VDJ junctional trimming. These N nucleotides are added by the enzyme TdT (34) suggesting that this activity is not fully functional during the first weeks of life, consistent with previous work (28). More junctional trimming is found in the human fetus as compared to infant control samples (35) potentially indicating that our 3-day and 7-day neonates correspond to human neonates. Our analysis points to a rapid evolution of the naïve TCR repertoire during the first week of birth with an increase in TdT activity and changes in availability of Vβ families shaping this evolution towards an adult-type repertoire.
To investigate the neonatal CTL response to a viral infection and understand the mechanisms that shape this response, we used influenza virus infections of 3-day old, 7-day old and adult mice. We find that 3-day old mice have greatly diminished CTL responses to both dominant and subdominant epitopes. In contrast, 7-day old mice have normal CTL responses to the dominant PA(224–233) and subdominant NS2(114–121) epitopes. These 7-day old mice mounted a NP(366–374) response that was intermediate between that of 3-day old and adult mice. Such altered patterns of neonatal CTL hierarchy have also been demonstrated by others in a 7-day old mouse model of RSV infection (10). Our study suggests that the CTL response is rapidly evolving during the first week of mouse life. Previous work has demonstrated that when 2-day old mice are challenged with influenza virus, there are reduced numbers of T cells in the lungs (5); however, these studies did not examine the influenza virus-specific CTL response. Our current study therefore reports for the first time the dominant and subdominant epitope-specific CTL response in such young neonates and demonstrates that they are very different from 7-day old and adult mice.
Our findings of greatly reduced CTL responses in neonatal mice raise the question of what is the responsible mechanism. Because we had determined that the naïve CD8+ T cell TCR repertoire was different in 3-day old mice, we postulated that the TCR usage of the immunodominant NP(366–374) –specific CTL responses may reflect biases found in the naïve TCR repertoire. Our study suggests that the major cause of a neonatal mouse’s failure to mount robust CTL responses against influenza virus is the reduced frequency of public, high affinity clonotypes in their TCR repertoire. Adult NP(366–374) –specific CTLs demonstrated the well-established, characteristic public clonotypes shared between different C57Bl/6 mice (1, 18, 19). In contrast, neonates use private clonotypes of NP(366–374) –specific CTLs, which utilize TRBV31 and TRBV19 while the adult CTLs use predominantly TRBV13-1 and TRBV17. Our data suggests that alternate TCR Vβ family usage in the naïve neonatal repertoire may be responsible for their lack of public clonotypes. Different TCR Vβ families are used in the naïve TCR repertoire of 3-day mice as compared to adults, which is consistent with the evolution of the BCR VH utilization. During fetal development, D proximal VH genes are preferentially used in the BCR repertoire and VH usage spreads with gestational age (36–38). Interestingly, 3-day old neonatal mice use predominantly TRBV31 in both their naïve and NP(366–374) –specific repertoire. TRBV31 has some unique features, as it is the most 3′ TRBV gene and has opposite polarity than that of the D-J-C cluster (39). The TRBV31 usage by neonates may be controlled in part by RAG activity as mice expressing core, but not full-length RAG1 show a 5-fold increase in TRBV31expressing thymocytes (40). In humans, TRBV30 is the homologue of mouse TRBV31 and also has a similar 3′position in the TRB locus and opposite polarity as mouse TRBV31. Human TRBV30 is expressed highest in neonates and decreases with age (41). Future studies are needed to examine whether these 3′positioned TRBV genes are expressed early in development and how they may affect CTL immunity.
TCR repertoire analysis revealed different TCR usage in neonates and the use of private instead of public clonotypes, but this does not address the quality of the TCR. To test this, we determined the affinity of 3-day old neonatal CTL to influenza virus NP(366–374) dominant epitope and compared it to 7-day and adult mice. We find that the TCRs utilized by 3-day old neonatal mice have much lower affinity than adult CTL TCRs, while 7-day old CTL TCRs have an affinity similar to adults. This lower affinity could explain the failure of neonatal mice to mount robust CTL responses, particularly when coupled with our finding of the lack of adult public clones in the neonatal naïve repertoire, and as others have shown with an initially small naïve repertoire in the neonatal CD4+ T cell pool (42). Specifically, the differences in TCR repertoire in neonates underlie the lack of high affinity precursors.
Although we demonstrated that TCRs used by neonatal mice in their virus-specific CTL response was different from adults and of lower affinity, there still remained the possibility that the neonatal environment (43, 44), or intrinsic defects in neonatal CD8+ T cells (45, 46) contributed to the diminished CTL response of neonates. To test directly if controlling the TCR would restore neonatal CTL responses, we performed a series of careful adoptive transfer experiments where we showed that neonatal high affinity TCR transgenic OT-I cells can expand normally in neonatal and adult animals (excludes intrinsic defects of neonatal CTL, neonatal environment and their combination as factors) and that non-transgenic neonatal CD8+ T cells fail to expand in adult mice (excludes the neonatal environment and the interaction of the neonatal T cells with the neonatal environment being factors). A caveat is that the adoptive transfer of 5x104 cells could have circumvented possible environmental effects because the animal was receiving a higher precursor frequency, in addition to the fact that the repertoire was changed with transgenic TCR CD8+ T cells. However, transfers of less cells were attempted and were not successful because of the limitation of doing adoptive transfers through the intraperitoneal route in neonatal mice.
Our findings argue that if one corrects the TCR on neonatal CD8+ T cells, a neonate can mount a normal CTL response. Although our neonatal OT-I transfer into neonatal recipients argues that the TCR can correct the CTL defect, we cannot exclude that the NP(366–374)-specific CTL response may be more susceptible to neonatal dendritic cell functional differences than OT-I cells. Neonatal dendritic cells may preferentially recruit lower avidity or more promiscuous TCR for the NP(366–374)-specific response, and these outcompete public idiotypes.
There is little data describing neonatal memory development after neonatal acute viral infection. Some previous investigations into the neonatal memory response were performed using models of chronic infection. These studies found that neonate-primed CD8+ T cells develop into cells that rival adult-primed cells in proliferation and effector function in a murine leukemia virus model of chronic viral infection (47, 48). However, such chronic infections due to the sustained presence of high antigen loads do not reflect true memory, and may not be informative on memory generation after an acute infection that is followed by antigen clearance. Others have examined priming of virus-specific memory T cells in 1-day old mice injected intravenously with irradiated influenza virus (49); these studies found that there was a secondary cytotoxic T lymphocyte response when splenocytes were stimulated in vitro as adults. Rudd and colleagues investigated the memory recall response of neonatally vaccinated versus adult vaccinated mice, and found that neonatal memory clonotypes are not efficiently recruited into the proliferative recall response to secondary challenge (50). For our studies, we used the natural infection route of influenza virus and examined the secondary expansion of memory CD8+ T cell responses primed on the third day of life. Given that the primary CD8+ T cell response was impaired in the neonates, it is possible that the primary CD4+ T cell response may also be affected. This could set the stage for the generation of “helpless” CD8+ T cell memory that is characterized by defective expansion during secondary responses, often termed as reduced quality of memory (51–53). It was thus important to determine whether CD8+ T cell memory generated after neonatal infection could expand during a secondary challenge. Despite the reduced and delayed primary CD8+ T cell response in neonates, our studies have shown that secondary responses against the immunodominant NP(366–374) epitope for PR8 influenza virus, are normal, as others have shown with vaccinia virus (50). Similarly, Kollmann and colleagues using older (day 5–7) mice have shown that after only one immunization with an attenuated strain of Listeria monocytogenes, neonatally immunized mice generate memory CD8+ T cells with kinetics and frequencies similar to adult-immunized mice (54). Here, we demonstrate that when 3-day old neonates are exposed to an acute infection, clear the virus, and are then rechallenged, despite their reduced primary responses, they mount a robust secondary response equivalent to adult primed mice. One explanation for the robust secondary response in neonates could be persistent antigen that is presented at later times in the primary response, which allows for the recruitment of new TCR that are generated in the repertoire and emigrate from the thymus after 7–10 days of age (55). In response to this possibility, we devised a time course experiment where we infected animals at 3 days of life and 8 weeks of life, harvested animals at different time points post infection, and stained pulmonary lymphocytes with NP(366–374)-specific tetramer and Vβ8.3 (TRBV13-1, the dominant Vβ family in the public NP(366–374)-specific response in the adult C57Bl/6 mouse). We found that at later time points post-infection, the 3 day old neonate started to develop a more “adult-like” response to influenza, as shown by a greater percentage of NP specific CD8+ T cells, which co-stained for Vβ 8.3. However, to fully answer this question, future studies need address the kinetics and identify the TCR sequences at several time points post-infection of infected 3-day old mice, as well as performing RNA sequencing of virus-specific CTL’s after a secondary challenge.
Our findings raise the question of how predictive is the primary response in neonates for long term protection. Measuring the neonatal primary responses to a vaccine or a primary infection may not appropriately evaluate the efficacy of a vaccine or be predictive of long-term protection to the infectious agent. Neonates display a suboptimal response to many vaccines, impairing the ability to protect this large segment of the population (56). It will be important to determine whether using adjuvants that simulate the effect of acute infection in terms of inflammation or pattern recognition receptor triggering improve neonatal vaccine efficacy, or whether vaccines with increased antigen persistence or shorter boosting intervals may overcome neonatal vaccine failure. Several models of experimental respiratory tract viral infection (Vesicular Stomatitis Virus, RSV and influenza) have demonstrated that viral antigen in the form of processed peptide persists in the draining lymph nodes at the site of initial pathogen entry (57–59). This might explain the delayed expansion of Vβ8.3 family clones within the virus-specific CTL response and the robust secondary responses to influenza in our neonatal animal model.
Neonatal mice have proved useful to model human infant viral infections (60). However, the age of the mouse that most closely approximates a human infant has not been established. A neonatal mouse is defined in the literature anywhere from an age of 1 day to 3 weeks of age, which can lead to inconsistency in the development of clinically-relevant animal models to mimic human infection (4–7). Our study shows a rapid evolution of the TCR repertoire over the first week of life; 7-day old mice have an intermediate anti-viral CTL response and TCR repertoire, between 3-day old and adult mice. Our studies suggest caution when assuming that the 7-day old mouse most closely reflects a full-term human neonate. Alternatively, 3-day old mice may not reflect a full term human neonate but rather reflect a premature human neonate, for which an animal model is lacking. Future studies, comparing human and mouse TCR Vβ repertoires using high-throughput sequencing, as we employed here, could determine the exact mouse neonatal age that is more relevant to human full-term and premature neonates.
Our studies using a neonatal acute infection model demonstrate that the critical component underlying the neonate’s faulty primary CTL response is the neonatal TCR repertoire. Despite their impaired primary CTL response, however, neonates can mount normal secondary responses suggesting that appropriate vaccine strategies can result in protective immunity in this vulnerable population.
Supplementary Material
Acknowledgments
Funding Sources: Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number K08AI108791 to AC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. In addition, funding was provided by the Margaret Q. Landenberger Foundation, Commonwealth Universal Research Enrichment Grant (Commonwealth of Pennsylvania, Department of Health) and the Professional Enrichment and Growth grant (Drexel University College of Medicine) to AC.
Footnotes
The authors declare no competing financial interests.
References
- 1.Gavin MA, Bevan MJ. Increased peptide promiscuity provides a rationale for the lack of N regions in the neonatal T cell repertoire. Immunity. 1995;3:793–800. doi: 10.1016/1074-7613(95)90068-3. [DOI] [PubMed] [Google Scholar]
- 2.Gilfillan S, Bachmann M, Trembleau S, Adorini L, Kalinke U, Zinkernagel R, Benoist C, Mathis D. Efficient immune responses in mice lacking N-region diversity. Eur J Immunol. 1995;25:3115–3122. doi: 10.1002/eji.1830251119. [DOI] [PubMed] [Google Scholar]
- 3.Welliver TP, Garofalo RP, Hosakote Y, Hintz KH, Avendano L, Sanchez K, Velozo L, Jafri H, Chavez-Bueno S, Ogra PL, McKinney L, Reed JL, Welliver RC., Sr Severe human lower respiratory tract illness caused by respiratory syncytial virus and influenza virus is characterized by the absence of pulmonary cytotoxic lymphocyte responses. J Infect Dis. 2007;195:1126–1136. doi: 10.1086/512615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamath AT, Rochat AF, Christensen D, Agger EM, Andersen P, Lambert PH, Siegrist CA. A liposome-based mycobacterial vaccine induces potent adult and neonatal multifunctional T cells through the exquisite targeting of dendritic cells. PLoS One. 2009;4:e5771. doi: 10.1371/journal.pone.0005771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lines JL, Hoskins S, Hollifield M, Cauley LS, Garvy BA. The migration of T cells in response to influenza virus is altered in neonatal mice. J Immunol. 2010;185:2980–2988. doi: 10.4049/jimmunol.0903075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gareau MG, Wine E, Reardon C, Sherman PM. Probiotics prevent death caused by Citrobacter rodentium infection in neonatal mice. J Infect Dis. 2010;201:81–91. doi: 10.1086/648614. [DOI] [PubMed] [Google Scholar]
- 7.Yasui H, Kiyoshima J, Hori T. Reduction of influenza virus titer and protection against influenza virus infection in infant mice fed Lactobacillus casei Shirota. Clin Diagn Lab Immunol. 2004;11:675–679. doi: 10.1128/CDLI.11.4.675-679.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.You D, Ripple M, Balakrishna S, Troxclair D, Sandquist D, Ding L, Ahlert TA, Cormier SA. Inchoate CD8+ T cell responses in neonatal mice permit influenza-induced persistent pulmonary dysfunction. J Immunol. 2008;181:3486–3494. doi: 10.4049/jimmunol.181.5.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez MA, I, Evans A, Hassan EH, Carbone FR, Jones CA. Neonatal CD8+ T cells are slow to develop into lytic effectors after HSV infection in vivo. Eur J Immunol. 2008;38:102–113. doi: 10.1002/eji.200636945. [DOI] [PubMed] [Google Scholar]
- 10.Ruckwardt TJ, Malloy AM, Gostick E, Price DA, Dash P, McClaren JL, Thomas PG, Graham BS. Neonatal CD8+ T-cell hierarchy is distinct from adults and is influenced by intrinsic T cell properties in respiratory syncytial virus infected mice. PLoS Pathog. 2011;7:e1002377. doi: 10.1371/journal.ppat.1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borowski AB, Boesteanu AC, Mueller YM, Carafides C, Topham DJ, Altman JD, Jennings SR, Katsikis PD. Memory CD8+ T cells require CD28 costimulation. J Immunol. 2007;179:6494–6503. doi: 10.4049/jimmunol.179.10.6494. [DOI] [PubMed] [Google Scholar]
- 12.Ward CL, Dempsey MH, Ring CJ, Kempson RE, Zhang L, Gor D, Snowden BW, Tisdale M. Design and performance testing of quantitative real time PCR assays for influenza A and B viral load measurement. J Clin Virol. 2004;29:179–188. doi: 10.1016/S1386-6532(03)00122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, Horsman D, Jones SJ, Marra MA. Circos: an information aesthetic for comparative genomics. Genome Res. 2009;19:1639–1645. doi: 10.1101/gr.092759.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zemlin M, Schelonka RL, Bauer K, Schroeder HW., Jr Regulation and chance in the ontogeny of B and T cell antigen receptor repertoires. Immunol Res. 2002;26:265–278. doi: 10.1385/IR:26:1-3:265. [DOI] [PubMed] [Google Scholar]
- 15.Belz GT, Stevenson PG, Doherty PC. Contemporary analysis of MHC-related immunodominance hierarchies in the CD8+ T cell response to influenza A viruses. J Immunol. 2000;165:2404–2409. doi: 10.4049/jimmunol.165.5.2404. [DOI] [PubMed] [Google Scholar]
- 16.Flynn KJ, Belz GT, Altman JD, Ahmed R, Woodland DL, Doherty PC. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 17.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. Cell-mediated protection in influenza infection. Emerg Infect Dis. 2006;12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kedzierska K, Turner SJ, Doherty PC. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proc Natl Acad Sci U S A. 2004;101:4942–4947. doi: 10.1073/pnas.0401279101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Gisbergen KP, Klarenbeek PL, Kragten NA, Unger PP, Nieuwenhuis MB, Wensveen FM, ten Brinke A, Tak PP, Eldering E, Nolte MA, van Lier RA. The costimulatory molecule CD27 maintains clonally diverse CD8+ T cell responses of low antigen affinity to protect against viral variants. Immunity. 2011;35:97–108. doi: 10.1016/j.immuni.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Adkins B, Bu Y, Cepero E, Perez R. Exclusive Th2 primary effector function in spleens but mixed Th1/Th2 function in lymph nodes of murine neonates. J Immunol. 2000;164:2347–2353. doi: 10.4049/jimmunol.164.5.2347. [DOI] [PubMed] [Google Scholar]
- 21.Barrios C, Brawand P, Berney M, Brandt C, Lambert PH, Siegrist CA. Neonatal and early life immune responses to various forms of vaccine antigens qualitatively differ from adult responses: predominance of a Th2-biased pattern which persists after adult boosting. Eur J Immunol. 1996;26:1489–1496. doi: 10.1002/eji.1830260713. [DOI] [PubMed] [Google Scholar]
- 22.Ridge JP, Fuchs EJ, Matzinger P. Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science. 1996;271:1723–1726. doi: 10.1126/science.271.5256.1723. [DOI] [PubMed] [Google Scholar]
- 23.Badovinac VP, Haring JS, Harty JT. Initial T cell receptor transgenic cell precursor frequency dictates critical aspects of the CD8(+) T cell response to infection. Immunity. 2007;26:827–841. doi: 10.1016/j.immuni.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boursalian TE, Golob J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 25.Rudd BD, Venturi V, Davenport MP, Nikolich-Zugich J. Evolution of the antigen-specific CD8+ TCR repertoire across the life span: evidence for clonal homogenization of the old TCR repertoire. J Immunol. 2011;186:2056–2064. doi: 10.4049/jimmunol.1003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothenberg E, Triglia D. Clonal proliferation unlinked to terminal deoxynucleotidyl transferase synthesis in thymocytes of young mice. J Immunol. 1983;130:1627–1633. [PubMed] [Google Scholar]
- 27.Borkowsky W, Chen SH, Belitskaya-Levy I. Distribution and evolution of T-cell receptor Vbeta repertoire on peripheral blood lymphocytes of newborn infants of human immunodeficiency virus (HIV)-infected mothers: differential display on CD4 and CD8 T cells and effect of HIV infection. Clin Vaccine Immunol. 2007;14:1215–1222. doi: 10.1128/CVI.00092-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feeney AJ. Junctional sequences of fetal T cell receptor beta chains have few N regions. J Exp Med. 1991;174:115–124. doi: 10.1084/jem.174.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang J, Braciale TJ. Respiratory syncytial virus infection suppresses lung CD8+ T-cell effector activity and peripheral CD8+ T-cell memory in the respiratory tract. Nat Med. 2002;8:54–60. doi: 10.1038/nm0102-54. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Silvestri N, Whitton JL, Hassett DE. Neonates mount robust and protective adult-like CD8+ T-cell responses to DNA vaccines. J Virol. 2002;76:11911–11919. doi: 10.1128/JVI.76.23.11911-11919.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez X, Brandt C, Saddallah F, Tougne C, Barrios C, Wild F, Dougan G, Lambert PH, Siegrist CA. DNA immunization circumvents deficient induction of T helper type 1 and cytotoxic T lymphocyte responses in neonates and during early life. Proc Natl Acad Sci U S A. 1997;94:8726–8731. doi: 10.1073/pnas.94.16.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martinez X, Regner M, Kovarik J, Zarei S, Hauser C, Lambert PH, Leclerc C, Siegrist CA. CD4+-independent protective cytotoxic T cells induced in early life by a non-replicative delivery system based on virus-like particles. Virology. 2003;305:428–435. doi: 10.1006/viro.2002.1775. [DOI] [PubMed] [Google Scholar]
- 33.Kovarik J, Bozzotti P, Love-Homan L, Pihlgren M, Davis HL, Lambert PH, Krieg AM, Siegrist CA. CpG oligodeoxynucleotides can circumvent the Th2 polarization of neonatal responses to vaccines but may fail to fully redirect Th2 responses established by neonatal priming. J Immunol. 1999;162:1611–1617. [PubMed] [Google Scholar]
- 34.Desiderio SV, Yancopoulos GD, Paskind M, Thomas E, Boss MA, Landau N, Alt FW, Baltimore D. Insertion of N regions into heavy-chain genes is correlated with expression of terminal deoxytransferase in B cells. Nature. 1984;311:752–755. doi: 10.1038/311752a0. [DOI] [PubMed] [Google Scholar]
- 35.Rechavi E, Lev A, Lee YN, Simon AJ, Yinon Y, Lipitz S, Amariglio N, Weisz B, Notarangelo LD, Somech R. Timely and spatially regulated maturation of B and T cell repertoire during human fetal development. Sci Transl Med. 2015;7:276ra225. doi: 10.1126/scitranslmed.aaa0072. [DOI] [PubMed] [Google Scholar]
- 36.Alt FW, Blackwell TK, Yancopoulos GD. Development of the primary antibody repertoire. Science. 1987;238:1079–1087. doi: 10.1126/science.3317825. [DOI] [PubMed] [Google Scholar]
- 37.Schroeder HW, Jr, Hillson JL, Perlmutter RM. Early restriction of the human antibody repertoire. Science. 1987;238:791–793. doi: 10.1126/science.3118465. [DOI] [PubMed] [Google Scholar]
- 38.Casali P, Schettino EW. Structure and function of natural antibodies. Curr Top Microbiol Immunol. 1996;210:167–179. doi: 10.1007/978-3-642-85226-8_17. [DOI] [PubMed] [Google Scholar]
- 39.Bosc N, Lefranc M, Ginestoux C. Locus representation: House mouse (Mus musculus) TRB, IMGT Repertoire. IMGT®, the international ImMunoGenetics information system®. 2001 http://www.imgt.org, Created: 15/01/2001. Version: 15/04/2015.
- 40.Horowitz JE, Bassing CH. Noncore RAG1 regions promote Vbeta rearrangements and alphabeta T cell development by overcoming inherent inefficiency of Vbeta recombination signal sequences. J Immunol. 2014;192:1609–1619. doi: 10.4049/jimmunol.1301599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Beemd R, Boor PP, van Lochem EG, Hop WC, Langerak AW, Wolvers-Tettero IL, Hooijkaas H, van Dongen JJ. Flow cytometric analysis of the Vbeta repertoire in healthy controls. Cytometry. 2000;40:336–345. doi: 10.1002/1097-0320(20000801)40:4<336::aid-cyto9>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 42.Nelson RW, Rajpal MN, Jenkins MK. The Neonatal CD4+ T Cell Response to a Single Epitope Varies in Genetically Identical Mice. J Immunol. 2015;195:2115–2121. doi: 10.4049/jimmunol.1500405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pettengill M, Robson S, Tresenriter M, Millan JL, Usheva A, Bingham T, Belderbos M, Bergelson I, Burl S, Kampmann B, Gelinas L, Kollmann T, Bont L, Levy O. Soluble ecto-5′-nucleotidase (5′-NT), alkaline phosphatase, and adenosine deaminase (ADA1) activities in neonatal blood favor elevated extracellular adenosine. J Biol Chem. 2013;288:27315–27326. doi: 10.1074/jbc.M113.484212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith NL, Wissink E, Wang J, Pinello JF, Davenport MP, Grimson A, Rudd BD. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J Immunol. 2014;193:177–184. doi: 10.4049/jimmunol.1400553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mastelic B, Kamath AT, Fontannaz P, Tougne C, Rochat AF, Belnoue E, Combescure C, Auderset F, Lambert PH, Tacchini-Cottier F, Siegrist CA. Environmental and T cell-intrinsic factors limit the expansion of neonatal follicular T helper cells but may be circumvented by specific adjuvants. J Immunol. 2012;189:5764–5772. doi: 10.4049/jimmunol.1201143. [DOI] [PubMed] [Google Scholar]
- 47.Fadel SA, Cowell LG, Cao S, Ozaki DA, Kepler TB, Steeber DA, Sarzotti M. Neonate-primed CD8+ memory cells rival adult-primed memory cells in antigen-driven expansion and anti-viral protection. Int Immunol. 2006;18:249–257. doi: 10.1093/intimm/dxh360. [DOI] [PubMed] [Google Scholar]
- 48.Fadel SA, Ozaki DA, Sarzotti M. Enhanced type 1 immunity after secondary viral challenge in mice primed as neonates. J Immunol. 2002;169:3293–3300. doi: 10.4049/jimmunol.169.6.3293. [DOI] [PubMed] [Google Scholar]
- 49.Schwartz DH, Hurwitz JL, Greenspan NS, Doherty PC. Priming of virus-immune memory T cells in newborn mice. Infect Immun. 1984;43:202–205. doi: 10.1128/iai.43.1.202-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudd BD, Venturi V, Smith NL, Nzingha K, Goldberg EL, Li G, Nikolich-Zugich J, Davenport MP. Acute neonatal infections ‘lock-in’ a suboptimal CD8+ T cell repertoire with impaired recall responses. PLoS Pathog. 2013;9:e1003572. doi: 10.1371/journal.ppat.1003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 52.Shedlock DJ, Shen H. Requirement for CD4+ T cell help in generating functional CD8+ T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 53.Sun JC, Bevan MJ. Defective CD8+ T cell memory following acute infection without CD4+ T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kollmann TR, Reikie B, Blimkie D, Way SS, Hajjar AM, Arispe K, Shaulov A, Wilson CB. Induction of protective immunity to Listeria monocytogenes in neonates. J Immunol. 2007;178:3695–3701. doi: 10.4049/jimmunol.178.6.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ballesteros-Tato A, Leon B, Lee BO, Lund FE, Randall TD. Epitope-specific regulation of memory programming by differential duration of antigen presentation to influenza-specific CD8(+) T cells. Immunity. 2014;41:127–140. doi: 10.1016/j.immuni.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mortellaro A, Ricciardi-Castagnoli P. From vaccine practice to vaccine science: the contribution of human immunology to the prevention of infectious disease. Immunol Cell Biol. 2011;89:332–339. doi: 10.1038/icb.2010.152. [DOI] [PubMed] [Google Scholar]
- 57.Schwarze J, O’Donnell DR, Rohwedder A, Openshaw PJ. Latency and persistence of respiratory syncytial virus despite T cell immunity. Am J Respir Crit Care Med. 2004;169:801–805. doi: 10.1164/rccm.200308-1203OC. [DOI] [PubMed] [Google Scholar]
- 58.Kim TS, Hufford MM, Sun J, Fu YX, Braciale TJ. Antigen persistence and the control of local T cell memory by migrant respiratory dendritic cells after acute virus infection. J Exp Med. 2010;207:1161–1172. doi: 10.1084/jem.20092017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turner DL, Cauley LS, Khanna KM, Lefrancois L. Persistent antigen presentation after acute vesicular stomatitis virus infection. J Virol. 2007;81:2039–2046. doi: 10.1128/JVI.02167-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cormier SA, You D, Honnegowda S. The use of a neonatal mouse model to study respiratory syncytial virus infections. Expert Rev Anti Infect Ther. 2010;8:1371–1380. doi: 10.1586/eri.10.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.