Abstract
The skin is an important barrier organ and frequent target of autoimmunity and allergy. Here we found innate-like B cells that expressed the anti-inflammatory cytokine IL-10 in the skin of humans and mice. Unexpectedly, innate-like B1 and conventional B2 cells showed differential homing capacities with peritoneal B1 cells preferentially migrating into the inflamed skin of mice. Importantly, the skin-homing B1 cells included IL-10 secreting cells. B1 cell homing into the skin was independent of typical skin-homing trafficking receptors and instead required α4β1-integrin. Moreover, B1 cells constitutively expressed activated β1 integrin and relocated from the peritoneum to the inflamed skin and intestine upon innate stimulation, indicating an inherent propensity to extravasate into inflamed and barrier sites. We conclude that innate-like B cells migrate from central reservoirs into skin, adding an important cell type with regulatory and protective functions to the skin immune system.
Introduction
The skin is an important barrier organ that is constantly threatened by external insults but is also a frequent target of allergy and autoimmunity. Cells of the skin immune system provide regional immunity, tissue homeostasis and repair, and regulate cutaneous inflammation. While the function and migration of many cell types of the skin immune system, such as that of cutaneous T cell subsets, are well characterized, B cells were previously assumed to be absent from the uninflamed skin (1). In contrast to this assumption, we recently found that B cells exist in the dermis and skin-draining lymph of sheep (2). There is also a growing evidence that B cells are involved in the positive and negative regulation of various human skin pathologies, however, an analysis of skin B cell subsets as well as their trafficking and function has been lacking in humans and mice (reviewed in (3)).
B cells can be divided into conventional and innate-like B cell subsets. Conventional B2 cells recirculate between lymphoid tissues and blood and are essential for affinity-maturated long-lasting antibody responses. Innate-like B cell subsets encompass marginal zone B cells of the spleen and B1 cells residing primarily at mucosal sites and coelomic cavities (i.e. peritoneum and pleura; reviewed in (4, 5)). Innate-like B cells respond well to innate stimuli, such as Toll-like receptor activation, and they express B cell receptors that often recognize conserved pathogen patterns and are crossreactive with autoantigens (4, 5). Innate-like B cells, in particular B1 cells, bridge innate and adaptive immunity by efficiently mounting rapid T cell-independent antibody (IgM and IgA) responses, engaging in phagocytic and microbicidal activity, and by producing innate-stimulatory cytokines, such as GM-CSF (5-8).
While dysregulated B1 cells can be associated with autoimmunity and cutaneous hypersensitivity (5, 9), this cell type has potent anti-inflammatory properties that include the production of the immunosuppressive cytokine IL-10 and natural IgM (reviewed in (10, 11)). For example, IL-10+ peritoneal B1 cells suppress inflammation in mouse models of cutaneous hypersensitivity and colitis (12, 13). IL-10 producing B cells in general have recently received wide attention due to their ability to limit T cell-mediated inflammation in both the skin and non-cutaneous sites, such as the joints, central nervous system and colon, mainly by suppressing T cells and other cell types in lymphoid tissues (reviewed in (14, 15)). B cell-depleting therapies like the CD20-targeting antibody rituximab can exacerbate or induce the inflammatory skin disease psoriasis, supporting a protective role of B cells in skin inflammation also in humans (16-18). However the anti-inflammatory contributions of different B cell subsets and their anatomic locations are unclear in these human studies.
Mouse B1 cells recirculate homeostatically between the coelomic cavities and blood (19) and can be mobilized into mucosal sites (20, 21). Leukocyte migration from blood into tissues is mediated by a multistep-adhesion cascade requiring chemoattractant and adhesion receptors on the leukocyte that guide rolling, integrin activation, firm adhesion, and subsequent transendothelial migration through interaction with cognate endothelial ligands at each step (22). As an example, T cells require expression of ligands for E-selectin, CCR4, CCR8, and/or CCR10 as well as α4β1 or αLβ2 to efficiently migrate into the skin (23, 24). In contrast, the molecules that target B cells into the vast majority of extralymphoid organs, including the skin, are unknown.
In this study we found that B cells, including IL-10+ B1-like cells resided in the skin of humans and mice. IL-10+ peritoneal B1 cells migrated into the inflamed skin of mice in an α4β1 integrin-dependent manner. Moreover, B1 cells constitutively expressed activated β1 integrin and, following innate stimulation, rapidly relocated from the peritoneum to the inflamed skin. Our data establish a peritoneum – skin migratory axis for innate-like B cells and add an unexpected cell type to the skin immune system that is well equipped to limit skin inflammation and support tissue homeostasis and host defense.
Materials and Methods
Human specimens and mice
Peripheral blood mononuclear cells from healthy adult volunteers were received from the Human Immunology Core at the University of Pennsylvania. Normal adult human skin specimens were obtained fresh from skin surgery procedures through the University of Pennsylvania Skin Diseases Research Center. All human samples were de-identified prior to receipt.
All mice were on C57BL/6 background and between 8 and 16 weeks of age. Sex- and age-matched groups of male or female CD45.1 or CD45.2 congenic C57BL/6 mice were purchased from The Jackson Laboratory or bred in house. IL-10-GFP reporter mice (Vert-X (25)) and Rag1–/– mice (26) were kindly provided by Drs. Christopher Hunter and Serge Fuchs (both at the University of Pennsylvania), respectively. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Induction of skin inflammation and cell isolations
Similar as described (27), chronic skin inflammation in mice was induced by the subcutaneous injection of 50-100 μl of CFA (Sigma-Aldrich) emulsified with saline into the area of the flank. The chronically inflamed skin was analyzed 2-4 weeks later, when skin granulomas have formed (27).
Leukocytes were isolated from shaved human or mouse skin samples by mechanical disruption followed by two 30-minute enzymatic digestion steps in HBSS at 37°C with 0.1 mg/ml DNase I (Roche) and 12.5 μg/ml Liberase TM or 6.25-25 μg/ml Liberase TL (Roche), depending on the sensitivity of cell surface epitopes. Staining of digested and undigested lymph node or human peripheral blood mononuclear cells served to verify that stained epitopes were not cleaved during the cell-isolation process. Remaining tissue pieces were mashed through a 100 μm cell strainer (BD Bioscience) and released cells were washed in buffer containing 5% bovine serum (Hyclone Laboratories) or 0.5% BSA (Sigma-Aldrich). Lymphocytes were isolated from the small intestinal lamina propria as described (28). Peritoneal cavity cells were obtained by peritoneal lavage with 7-10 ml of PBS (Life Technologies). Cells were released from lymph nodes and spleens by passage through 70 μm cell strainers. Peripheral blood mononuclear cells were isolated from mouse blood by gradient centrifugation with Histopaque-1083 (Sigma-Aldrich).
Cell labeling, migration, relocation and chemotaxis assays
For radioactive homing experiments, B1 cells were isolated from peritoneal lymphocytes by negative selection with anti-biotin microbeads (Miltenyi Biotec) after labeling with biotinylated antibodies to CD23 (B3B4; eBioscience) and F4/80 (BM8; eBioscience), followed by positive selection with CD19 microbeads (Miltenyi Biotec), reaching a purity of ≥ 95% B1 cells. B1 cells were labeled with Indium-111 (Mallinckrodt Pharmaceuticals) and dead cells removed by Nycodenz gradient as described (29). 2.6-2.7×105 B1 cells were injected into the tail vein of each recipient mouse and 15 h later, radioactivity in indicated organs and the rest of the body was measured by gamma counter.
To label intravascular B cells in vivo, each mouse was injected intravenously with 1 μg phycoerythrin- (PE) labeled antibody to CD19 (1D3; eBioscience) five minutes prior to sacrifice as described (30). Subsequently, cells were isolated, stained for B cell subsets and analyzed by flow cytometry for in vivo labeled (PE+) intravascular vs. unlabeled extravascular B cells.
Peritoneal and splenic lymphocytes were differentially labeled with carboxyfluorescein succinimidyl ester (CFSE; Life Technologies) or Cell Proliferation Dye eFluor 670 (eBioscience) as described (31). A mixture of 1.5-4×106 peritoneal cells and splenocytes, adding up to 107 cells per recipient mouse, were injected intravenously. 12-15 h after transfer, the indicated organs of individual mice were analyzed for transferred cells by flow cytometry. Total numbers of cells were enumerated by hemocytometer or by flow cytometry using a bead standard (15 μm polystyrene beads; Polysciences). To calculate the homing index, the ratio of homed peritoneal B1 cells to splenic B2 cells was determined by flow cytometry and normalized to the input ratio. To control for potential effects of the cell labeling, the dyes were alternated between experiments. To block α4 integrin, a mix of differentially labeled peritoneal B1 cells and splenic B2 cells were resuspended in PBS containing 50 μg/mouse antibody to α4 integrin (PS/2; University of California San Francisco Monoclonal Antibody Core and Eugene Butcher at Stanford University) or an isotype control (rat IgG2b; University of California San Francisco Monoclonal Antibody Core) prior to injection, and each recipient mouse was additionally treated intraperitoneally with 300 μg PS/2 or isotype control in PBS.
To assess the long-term relocation of B cells from the peritoneum to the skin, peritoneal cells from CD45.1+ donor mice were intraperitoneally transferred into CD45.2+ congenic recipients. Cells from one donor mouse per recipient were used. Directly after transfer or up to three weeks later, cutaneous inflammation was induced with CFA. 3 weeks later, the percentage of transferred peritoneal cells among B1 cells residing in the inflamed skin, peritoneum, blood and spleen was analyzed.
To analyze the rapid relocation of peritoneal B1 cells from the peritoneal cavity to the inflamed skin following innate stimulation, CFSE and/or congenically (CD45.1+) labeled peritoneal cells combined from 2-3 donor mice per recipient were intraperitoneally transferred into WT recipients bearing CFA-induced chronically inflamed skin. 2 h later, similar as described (20), mice received 5-100 μg of LPS (from Salmonella enterica serotype minnesota; Sigma-Aldrich) intraperitoneally. 12 h after LPS challenge, the presence of CFSE+ transferred B1 cells was determined in the skin, small intestine, peritoneal cavity, blood as well as lymphoid tissues. For studies assessing adhesion molecules on B1 cells released from the peritoneal cavity, the same experiment was performed using naïve Rag1–/– mice as recipients (20), in which we observed a greater peritoneal release and easier tracking of donor B cells.
The chemotaxis assay was performed and analyzed as previously described for T cells (32) using 5 μm Transwell inserts for 24-well plates (Corning) and a 90-minute migration period at 37°C. Recombinant mouse CXCL12, CXCL13, CCL1, CCL17, CCL28, CCL20 were obtained from R&D Systems and titrated in triplicate wells to include concentrations with confirmed activity to attract control cells (such as memory T cells).
Flow cytometry
Dead cells were excluded from the analysis after staining samples with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (L/D Aqua; Life Technologies) according to the manufacturer's instructions. To reduce non-specific staining, mouse cells were pre-incubated with rat IgG (Jackson ImmunoResearch), antibody to CD16/CD32 (2.4G2; UCSF Monoclonal Antibody Core), and if indicated Armenian hamster IgG (Innovative Research) and mouse IgG (Jackson ImmunoResearch); human cells were pre-incubated with mouse and/or rat IgG (Jackson ImmunoResearch Laboratories), and human FcR Binding Inhibitor (eBioscience). After blocking, mouse cells were labeled with the following biotinylated or fluorochrome- (fluorescein isothiocyanate, Pacific Blue, eFluor450, PE, Alexa Fluor 647, Alexa Fluor 700, allophycocyanin, allophycocyanin-Alexa Fluor 750, peridinin chlorophyll-eFluor 710, PE-cyanine 7, peridinin chlorophyll-cyanine 5.5) rat anti-mouse monoclonal antibodies: CD4 (RM4-5), CD5 (53-7.3), CD11b (M1/70), CD19 (1D3), CD44 (IM7), CD45 (30-F11), B220 (RA3-6B2), αL integrin (M17/4), α4 integrin (R1-2), and α4β7 (DATK-32) from eBioscience; CD43 (S7), CXCR4 (2B11), and activated β1 integrin (9EG7) from BD Biosciences; CCR10 (248918), CCR6 (140706) and CXCR3 (220803) from R&D Systems; and CD19 (6D5) from Biolegend; mouse anti-mouse monoclonal antibodies: CD45.1 (A20) and CD45.2 (104) from eBioscience; and Armenian hamster-anti mouse β1 integrin (HMB1-1; eBioscience) and CCR4 (2G12; Biolegend). Staining for activated β1 integrin and binding of an E-selectin-human IgG chimeric protein (R&D Systems) was performed in HBSS containing Ca2+ and Mg2+ for 30-45 minutes at room temperature and on ice, respectively. Staining for activated β1 integrin in HBSS containing 2 mM MnCl2 (Sigma-Aldrich) served as a positive control. Human cells were labeled with mouse anti-human monoclonal antibodies to CD3 (SK7), CD19 (HIB19), CD20 (2H7), and CD45 (2D1), from eBioscience; CD11b (ICRF44), CD27 (L128) and CD43 (1G10), from BD Bioscience; and IgM (MHM-88) from Biolegend. Streptavidin conjugated to PE-Texas Red- (Life Technologies), allophycocyanin, or PE (BD Bioscience) as well as PE-labeled multi-species adsorbed F(ab)2 donkey anti-human IgG (Jackson ImmunoResearch) were used as second step reagents.
To detect IL-10 competent cells, cells were stimulated with 10 μg/ml LPS, 10 ng/ml PMA, and 500 ng/ml ionomycin for 2 h, adding 10 μg/ml brefeldin A (all from Sigma Aldrich) and, to mouse cells also 2 μM monensin (eBioscience), during an additional 2-3 h incubation. Subsequently, the cells were stained for surface markers and analyzed without fixation when GFP was evaluated, or fixed with 2% paraformaldehyde, before staining in 0.5% saponin buffer for intracellular IL-10 (rat anti-mouse clone JES5-16E3, eBioscience; mouse anti-human clone JES3-9D7 Miltenyi Biotec). Samples were acquired on a BD LSRII or LSRFortessa using FACSDiVa software (BD Biosciences), and analyzed with FlowJo software (Tree Star). During analysis, all samples were gated on single lymphocytes by applying an appropriate side-scatter height vs. side-scatter width gate in combination with a large lymphocyte gate.
Immunofluorescence histology
Uninflamed control skin or chronically inflamed mouse skin was harvested 3 weeks after induction of inflammation with CFA, fixed for 6 hours in 4% PFA in PBS and incubated overnight in 30% sucrose before freezing in OCT. To reduce non-specific staining, 6-8 μm thick skin sections were blocked with 10% donkey and 40% goat serum. B cells were visualized with polyclonal goat anti-mouse CD20 (Santa Cruz Biotechnology), which was labeled with CF594 or CF488 Mix-n-Stain antibody labeling kits according to the manufacturer's instructions (Biotium). Tissue sections were additionally stained with rat anti-mouse CD31 (MEC13.3; BD Biosciences) or IL-10 (JES5-16E3; BD Biosciences). Multi-species adsorbed F(ab)2 donkey anti-rat IgG conjugated with Alexa Fluor 488 or 594 (Jackson ImmunoResearch) were used as secondary antibodies and DAPI (Invitrogen) to visualize nuclei. Specificity of the CD20 and IL-10 staining was confirmed by including tissues from Rag1–/– and Il10–/– mice, respectively (data not shown). Sections were mounted with Prolong Gold Antifade (Invitrogen) and images acquired on a Nikon Eclipse E600 microscope using a Photometrics CoolSNAP EZ camera and NIS-Elements BR 3.0 software.
Statistical analysis
For statistical analyses, the non-parametric Mann Whitney U test, parametric student's t test, and to compare migration to a hypothetical homing index of 1, the Wilcoxon signed rank test was employed using GraphPad Prism software. Statistical tests used are indicated in the respective figure legends. P < 0.05 was considered statistically significant.
Results
Innate-like B cells reside in mouse skin
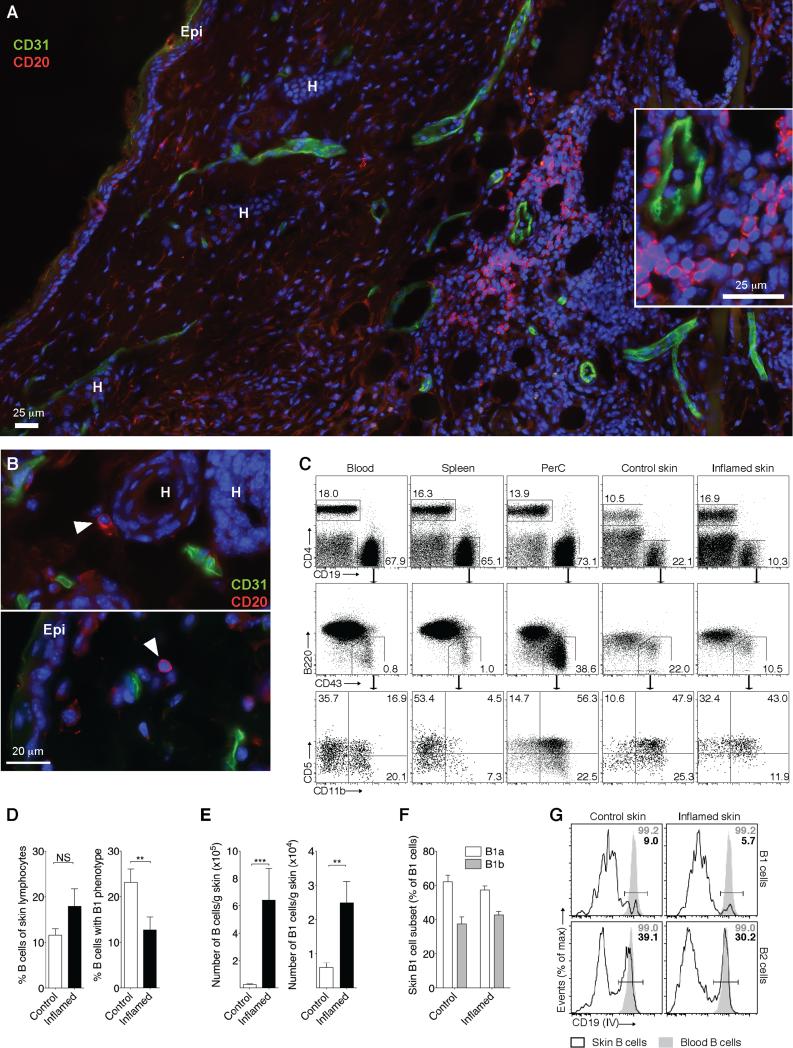
To determine whether B cells reside in mouse skin, we subcutaneously injected CFA, which induces chronic granulomatous skin inflammation characterized by mononuclear cell infiltrates (27). Strikingly, using immunofluorescence of frozen skin sections we found B cells in groups as well as individual cells in the chronically inflamed skin outside of CD31+ blood vessels (Fig 1A and inset). While B cells were very rare in the uninflamed skin, we clearly detected single B cells extravascular in the dermis, occasionally in the subcutis but never in the epidermis; B cells depicted are in proximity to epidermis (Epi) and hair follicle (H) (Fig. 1B, arrowheads). Using flow cytometry of enzymatically digested skin we found B cells in the inflamed and uninflamed skin (Fig. 1C-F). Surprisingly, 24.7 ± 12.8% (mean ± SD) of B cells isolated from the uninflamed skin resembled B1 cells (CD19+B220lo/– CD43+). While during inflammation the percentage of B cells with B1 phenotype decreased (P = 0.0091), the number of B cells, including that of B1 phenotype, increased (P = 0.0001 and P = 0.0034, respectively; Fig. 1C-E). This indicated stronger accumulation of B2 relative to B1 phenotype cells during chronic inflammation; however, B1 phenotype cells were still remarkably enriched compared with blood-borne B cells, of which only ~1% represent B1 cells (Fig. 1C). Cutaneous B1-phenotype cells consisted of both CD5+ B1a and CD5– B1b cells (on average 63.6% and 37.4%, respectively; Fig. 1C and F). Interestingly, while it was expected that only a small percentage of B1 cells in the spleen and blood expressed CD11b (33), cutaneous cells with B1 phenotype were enriched in CD11b expression similar to peritoneal B1 cells (Fig. 1C, bottom). As intravenous injection of antibody to CD19 labels all blood-borne B cells (30), we used this method to determine the extra- vs. intra-vascular localization of skin B cell subsets. We found that at least 90% of all B1 phenotype and 60-70 % of all B2 phenotype skin B cells were extravascular in the uninflamed and inflamed skin (Fig. 1G), confirming our histological findings for total B cells (Fig. 1A and B). We conclude that B cells, including innate-like B cells, are part of the skin immune system in mice.
Figure 1. Innate-like B cells reside in mouse skin.
Chronic skin inflammation was induced in mice by subcutaneous injection of CFA. (A and B) Immunofluorescence of frozen skin sections revealing the cutaneous localization of CD20+ B cells three weeks after induction of inflammation (A) and in uninflamed control skin (B). (A) 12 adjacent visual fields were assembled into one image using NIS-Elements BR 3.0 software. Inset shows enlarged perivascular area from (A). Arrowheads in (B) mark B cells. One (A) or two (B) representative images from ≥ 6 analyzed mice are shown. DAPI was used to visualize nuclei. Epi, epidermis; H, hair follicle. Lymphocytes isolated from inflamed and uninflamed (Control) skin and indicated tissues were analyzed by flow cytometry. (C, top) Cells were gated on LIVE/DEAD Fixable Aqua Dead Cell Stain Kit– (L/D Aqua–), CD45+ single lymphocytes; (C, middle) expression of B1 and B2 cell markers by CD19+ B cells gated as shown; (C, bottom) phenotype of CD43+B220lo/– B1 cells, gated as shown. One example staining from 4-6 independent experiments with 3-5 mice each is shown. (D-F) Summary of the results in (C) for skin tissues from all analyzed mice and (E) corresponding B cell counts. (G) Staining of blood-borne B cells in vivo by intravenously (IV) injected antibody to CD19. Gated on B1 and B2 cells (in vitro stained) from uninflamed (Control) and inflamed skin. One representative staining from four experiments analyzing 3-4 mice each is shown. **, P < 0.01; ***, P < 0.001 using the Mann Whitney U test
Cutaneous B1 phenotype cells make IL-10
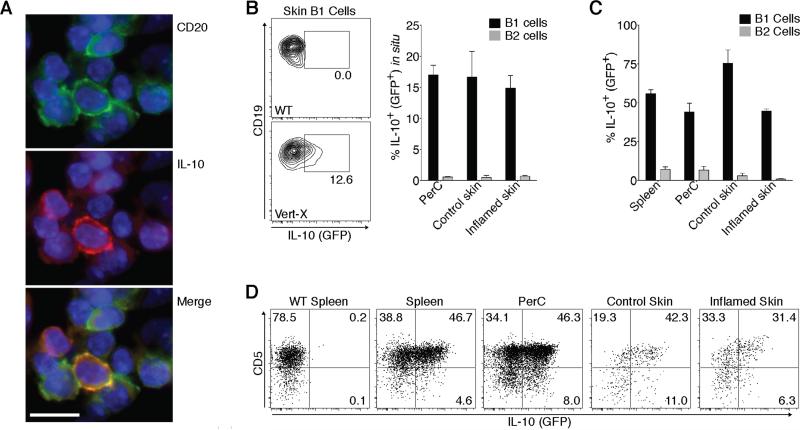
Many innate-like B cells are anti-inflammatory by virtue of producing IL-10 (10). To determine whether skin B cells make IL-10 we analyzed CFA-induced chronic skin inflammation by immunofluorescence histology. Importantly, we found that some (CD20+) B cells clearly made IL-10 at the site of inflammation (Fig. 2A). Next, we used IL-10-GFP reporter (Vert-X) mice, which allow for the detection of lymphocyte IL-10 production in vivo using flow cytometry (25). Importantly, on average 12.9% of all B1 phenotype cells but only 0.7% of the B2 phenotype cells in the inflamed skin of IL-10-GFP reporter mice produced IL-10 without exogenous stimulation (Fig. 2B), showing that skin B1 phenotype cells contribute to dermal IL-10 during chronic skin inflammation. Moreover, even in the absence of inflammation B1 but not B2 phenotype cells spontaneously produced IL-10 in the skin and the peritoneum (Fig. 2B). Next, we stimulated B cells from skin and other tissues of IL-10-reporter mice for 5 hours with PMA, ionomycin and LPS, a standard protocol to identify IL-10-competent B cells (34). Consistently, the majority of B1 cells, but only a small percentage of the B2 cells, in the spleen, peritoneal cavity as well as the uninflamed and inflamed skin produced IL-10 following stimulation (Fig. 2C). Similar results were obtained when staining IL-10 protein by intracellular staining in B cells from wildtype (WT) mice with chronic skin inflammation (Supplemental Fig. 1). While CD5 expression is characteristic of mouse B1a cells, in combination with CD1dhi expression it also serves as a marker for a potent subset of splenic IL-10+ regulatory B cells (34). While both CD5+ B1a and CD5– B1b phenotype cells in the skin and other sites were IL-10+; it was preferentially produced by CD5+ B1a cells (Fig. 2D), indicating potential overlap with the described (34) CD1dhiCD5+ IL-10+ regulatory B cell subset. In conclusion, our data show that B1 phenotype cells reside in the skin of mice, where they accumulate during inflammation and contribute to cutaneous IL-10.
Fig. 2. Cutaneous innate-like B cells make IL-10.
Chronic skin inflammation was induced in mice by subcutaneous injection of CFA 3 weeks prior to analysis. (A) Immunofluorescence histology of frozen skin sections detecting IL-10 production by CD20+ skin B cells. DAPI was used to visualize nuclei. One representative staining of > 6 analyzed mice. Scale bar, 10 μm. (B-D) B-cell IL-10 (GFP) expression in Vert-X IL-10-GFP reporter mice by flow cytometry. (B) Spontaneous IL-10 expression without in vitro stimulation showing (left) one representative staining and (right) summary of the results for all (≥6) mice analyzed in 3 independent experiments. (C and D) IL-10 expression after 5-h polyclonal stimulation of B cells from IL-10-GFP reporter mice. One representative experiment of 4 performed analyzing ≥4 mice each is shown. (B and C) Bars indicate the mean ± SEM of each group. NS, not significant. PerC, peritoneal cavity.
Human skin harbors IL-10+ innate-like B cells
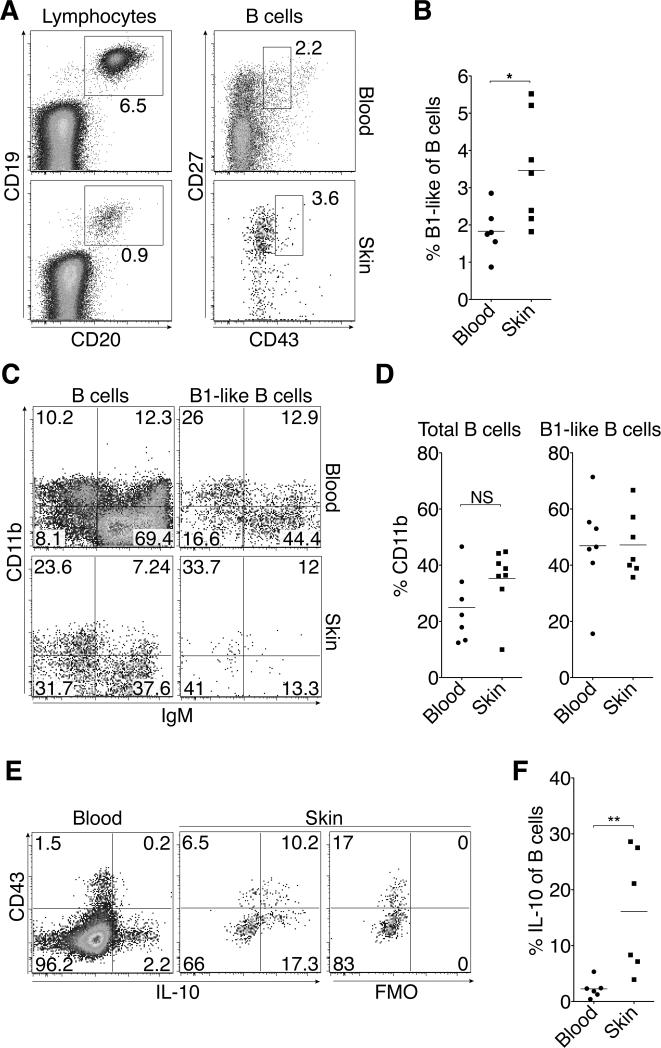
To address human relevance of our findings of skin B cells in mice, we analyzed cutaneous lymphocytes isolated from normal human skin of adults. This revealed a population of CD19 and CD20 double-positive skin B cells (Fig. 3A). While B1 cells in the mouse are well characterized, the existence and markers that delineate human counterparts are a matter of controversy (35-37). However, a population (3.5 ± 1.5 %, mean ± SD) of human skin B cells was of innate-like phenotype (CD3– CD19+CD20+CD27+CD43int; Fig. 3A and B) consistent with described human B1 cells (35) excluding CD43hi plasmablasts (36). Interestingly, this innate-like phenotype was more frequent among skin B cells relative to their blood counterparts (Fig. 3B, P = 0.022). Many skin B cells, including B1-like cells, expressed the adhesion molecule CD11b (Fig. 3C and D), whose expression is also associated with B1-like cells in several mammalian species including humans (38).
Figure 3. IL-10+ B cells are part of the human cutaneous immune system.
Lymphocytes from human blood and normal skin were analyzed by flow cytometry and identified as L/D Aqua– CD45+ single cells with lymphocyte scatter and (A, left) gated on CD3–CD19+CD20+ total B cells (A, right) further distinguishing CD27hiCD43int B1-like cells. (A) One representative staining and (B) results for all analyzed samples. (C) One representative staining of CD11b and IgM by total and B1-like B cells as gated in (A), and (D) results for CD11b expression in all analyzed samples. (E) Representative staining and fluorescence minus one control (FMO) for IL-10 expression by total human B cells, gated as in (a), after stimulation with PMA and ionomycin and (F) percentage of B cells that express IL-10 for all samples analyzed. (B, D, F) Horizontal lines indicate the mean of each group, and data points show individual donors from 5-7 donors per group. NS, not significant; *, P < 0.05; **, P < 0.01 using the Mann Whitney U test.
We next addressed whether human skin B cells can produce IL-10 similar to their mouse counterparts. Strikingly, following 4-h stimulation with PMA, ionomycin and LPS, 3.9–28.6% of all skin B cells made IL-10, which was significantly higher compared with blood B cells (P = 0.0043; Fig. 3E and F). While most B-cell derived IL-10 was made by CD43– B cells, the percentage of IL-10+ cells among CD43int (innate-like) B cells was higher relative to CD43– B cells (Fig. 3E). We conclude that B cells, including B1-like cells and IL-10 secreting B cells, are part of the normal skin immune system of humans.
Peritoneal B1 cells traffic into the uninflamed and inflamed skin
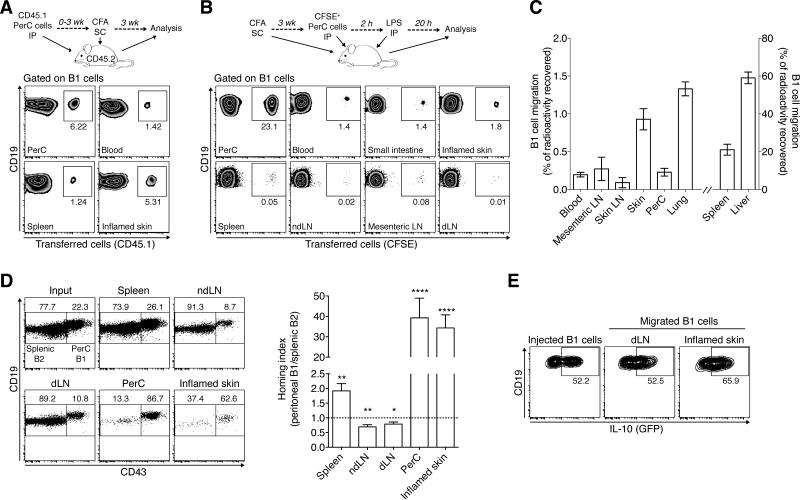
Mouse B1 cells recirculate homeostatically between the blood and body cavities (19). Thus, we wondered whether skin B1 phenotype cells originate from the peritoneal cavity. To address this, we returned to the mouse system and transferred congenically marked (CD45.1) WT peritoneal cells into the peritoneum of CD45.2 WT recipients before inducing chronic skin inflammation by subcutaneous injection of CFA (experimental outline in Fig. 4A). Three weeks after induction of skin inflammation, we were able to recover CD45.1+ B1 phenotype cells from the instillation site (peritoneum) as well as other organs. Strikingly, we found a higher percentage of donor-derived cells among B1 phenotype cells in the inflamed skin relative to spleen or blood (Fig. 4A). The data demonstrate that B cells leave the peritoneal cavity and give rise to B1 phenotype cells in the skin and other sites.
Figure 4. Peritoneal B1 cells home into uninflamed and inflamed skin.
(A) Long-term relocation of peritoneal B1 cells: experimental scheme (top) and flow cytometric analysis (bottom) of intraperitoneally (IP) transferred CD45.1+ unfractionated peritoneal cells recovered from different tissues 3 weeks after subcutaneous (SC) injection of CFA to induce chronic skin inflammation. Cells were gated on total B1 cells (L/D Aqua– CD45+CD19+CD43+B220lo/–) and gates show the percentage of (CD45.1+) donor origin. Representative plots from two experiments with similar results using 5-8 recipient mice each. (B) Short-term relocation of peritoneal B1 cells following innate stimulation: experimental scheme (top) and flow cytometric analysis (bottom) of intraperitoneally transferred CFSE-labeled unfractionated peritoneal cells recovered 20 h after intraperitoneal LPS challenge of recipient mice with chronic skin inflammation. Cells were gated on B1 cells as in (A), and gates show the percentage of B1 cells of (CFSE+) donor origin. One representative experiment out of three performed using 3 recipients each. (C) B1 cell homing into uninflamed skin and other tissues. 15 h after intravenous transfer of In-111-labeled purified peritoneal B1 cells, the distribution of radioactivity was measured. Combined analysis of 2 individual experiments with 6-8 mice each. (D) Competitive homing of peritoneal B1 vs. splenic B2 cells into the inflamed skin. Unfractionated peritoneal and splenic cells were differentially labeled with fluorescent dyes and transferred intravenously into recipient mice with chronically inflamed skin. 12-15 h later, tissues were analyzed by flow cytometry for transferred cells based on fluorescent labels (distinguishing splenic vs. peritoneal origin) and further gated on L/D Aqua–CD45+CD19+ B1 (CD43+B220lo/–) and B2 (CD43–B220hi) cells. These distinctly gated populations were plotted together to visualize their ratios in input and recovered samples. One representative staining (left) and the combined analysis of all (N=5) experiments analyzing ≥ 5 mice each (right). Homing index (right) was calculated as the ratio of recovered peritoneal B1 to splenic B2 cells, corrected for their injected ratio. The dotted line shows a homing index of 1, which indicates no migratory difference between groups. (E) IL-10 (GFP) expression by gated peritoneal B1 cells prior to injection and after homing into the inflamed skin and its draining lymph node (dLN). IL-10 expression was assessed after PMA, ionomycin, and LPS stimulation. One representative staining from a total of 7 analyzed mice is shown. (C and D) Bars indicate mean ± SEM of each group. *, P < 0.05; **, P < 0.01; ***, P < 0.0001 as determined by the Wilcoxon signed rank test comparing migration to a theoretical homing index of 1. PerC, peritoneal cavity; ndLN, non-draining lymph node.
Upon stimulation with LPS, peritoneal B1 cells rapidly leave the peritoneum and travel to the spleen and small intestine (20, 33). To determine whether bona fide peritoneal B1 cells also relocate to the skin, we transferred CFSE-labeled peritoneal cells into WT hosts with chronically inflamed skin. Two hours later, we challenged the recipient mice with LPS intraperitoneally (Fig. 4B). In line with Ha et al. (20), 20 h after LPS challenge a population of small intestinal lamina propria B1 cells were donor-derived (Fig. 4B). Importantly, a similar proportion of the B1 cells in the skin were also of donor origin (Fig. 4B), demonstrating that innate stimulation induces rapid B1 cell relocation from the peritoneum to the skin. Interestingly, B1 cells in lymphoid tissues were consistently to a lesser degree donor-derived compared with skin or intestinal B1 cells (Fig. 4B). The data support a model in which following inflammatory stimuli B1 cells are rapidly released from the peritoneum allowing for their deployment into barrier sites to provide innate and anti-inflammatory functions.
We next asked whether peritoneal B1 cells migrate into the uninflamed skin. Lymphocyte homing into the uninflamed skin is generally below the level of detection in flow cytometry-based homing assays. Thus, we chose a highly sensitive classical homing assay employing radioactive cell tracer In-111 (29). 15 hours after intravenous injection of purified microbead-sorted In-111-labeled peritoneal B1 cells, radioactivity could be detected in several organs with the highest signals in spleen and liver (Fig. 4C). Importantly, ~1% of the radioactivity could be recovered from the uninflamed skin (Fig. 4C), which is in a similar range as that of Th1 effector cells that have homed into the uninflamed skin (39). We conclude that innate-like B cells migrate into the uninflamed skin.
To determine how efficiently peritoneal B1 cells traffic into inflamed skin, we performed a competitive homing assay comparing the migration of differentially labeled peritoneal and splenic cells after intravenous co-transfer into recipient mice with chronic skin inflammation (Fig. 4D). The flow cytometric analysis of the ratios of splenic B2 and peritoneal B1 cell subsets recovered from different organs as compared to the input indicated a reduced capacity of peritoneal B1 cells to enter lymph nodes relative to splenic B2 cells (4D, left). In contrast, homed B1 cells were slightly enriched in spleen and strongly enriched in the peritoneal cavity and inflamed skin relative to homed splenic B2 cells (Fig. 4D, left). When determining the homing index (ratios of recovered population corrected by input ratio) to quantify migration capacities, peritoneal B1 cells showed a small, but statistically significant, reduced ability to enter lymph nodes (P < 0.05; compared to a theoretical homing index of 1, which indicates equal migration of B1 and B2 cells). Importantly, B1 cells possessed an on average 39.3-fold and 34.3-fold higher propensity relative to splenic B2 cells to enter the peritoneum and inflamed skin, respectively (P < 0.0001; Fig. 4D, right). After sorting and radioactive labeling, intravenously transferred peritoneal B1 cells also efficiently migrated into CFA-induced chronically inflamed skin (data not shown). Thus, the data reveal profound differences in the ability of B cell subsets to enter effector sites with peritoneal B1 cells showing an unexpected high propensity to home into the inflamed skin. These results also indicate that the increased accumulation of B2 cells in chronically inflamed skin (Fig. 1C and D) was due to recruitment-independent factors, such as enhanced retention, survival or local proliferation.
IL-10+ peritoneal B1 cells suppress skin inflammation (12) and we found IL-10 producing B1-phenotype cells in human and mouse skin (Figs. 2B-D and 3E and F). We therefore wondered whether peritoneal B1 cells with the potential to produce IL-10 home into the inflamed skin. To address this, we intravenously transferred peritoneal cells from IL-10-GFP reporter mice into WT recipients with CFA-induced chronic skin inflammation and used 4-h stimulation with PMA, ionomycin and LPS (as in Figs. 2 and 3) to reveal IL-10 competence. Importantly, the percentage of IL-10+ B1 cells that had homed into the inflamed skin and its draining lymph node was similar to that of injected peritoneal cells (>50% on average) (Fig. 4E). Thus, IL-10 producing peritoneal B1 cells are recruited into both the lymph nodes draining the inflamed skin and the inflamed skin itself, where they are well positioned to suppress cutaneous inflammation.
In conclusion, our data establish a novel migratory pathway for B1 cells by demonstrating that peritoneal B1 cells, including IL-10+ B1 cells with known anti-inflammatory potential, home into the skin, where they contribute to a cutaneous B cell pool.
B1 cells do not express typical skin homing chemokine receptors
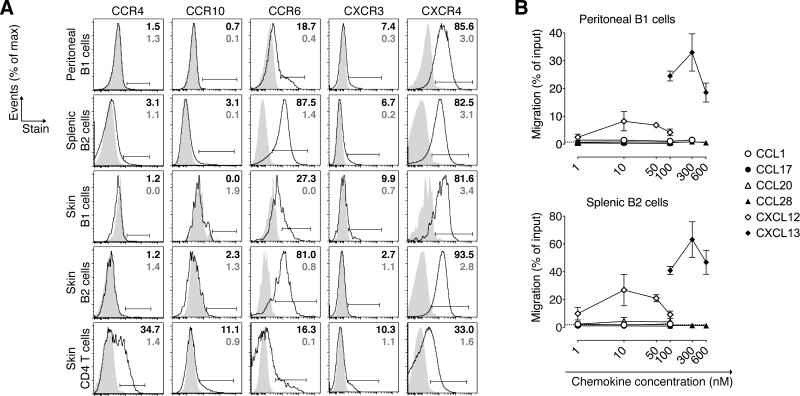
The molecules that mediate B cell migration into skin and the vast majority of other extralymphoid tissues are unknown. To identify potential trafficking receptors that target B cells into the skin and to determine the molecular basis for the observed differential skin migration between B1 and B2 cells (Fig. 4D), we examined peritoneal B1 and splenic B2 cells as well as their skin counterparts for their expression of typical skin-homing signatures (23, 24). Despite their skin-homing potential, peritoneal B1 cells did not express skin-homing chemokine receptors CCR4 and CCR10 (Fig. 5A) or migrate toward the respective ligands CCL17 and CCL28 in a chemotaxis assay (Fig. 5B). While, as expected, cutaneous CD4 T cells expressed CCR4 and CCR10, only a negligible fraction of skin B1 and B2 cells expressed these receptors (Fig. 5A). Peritoneal B1 cells were unresponsive to CCL1, which recruits skin homing T cells via CCR8 (24). Thus, even though peritoneal B1 cells home efficiently into the skin (Fig. 4C and D), they do so independently of chemokine receptors that target T cells into skin.
Figure 5. Chemokine receptor expression by B cell subsets.
(A) Flow cytometric analysis of the indicated B and T cell subsets from mice with chronically inflamed skin. L/D Aqua– CD45+ lymphocytes were gated on CD4+ or CD19+ B cells further distinguishing B1 (CD43+B220lo/–) and B2 (CD43– B220hi) cells. Shaded areas depict isotype controls of the respective subsets and organ. Percent of receptor+ cells (black) and isotype staining (grey) of indicated gates is shown in one representative staining from ≥8 analyzed mice in ≥ 2 experiments. (B) Chemotaxis of B cell subsets toward the indicated chemokines was tested ex vivo in a Transwell chemotaxis assay. Data are expressed as the percentage of cells of the respective subset that migrated to the lower chamber, and is represented as the mean ± SD of triplicate wells at each concentration. Horizontal dashed lines indicate migration to media alone. One of two experiments with similar results is shown.
In contrast, CCR6, which attracts Langerhans cells into the epidermis (40) was expressed by most splenic and cutaneous B2 cells and by a population of peritoneal and skin B1 cells (Fig. 5A). However, unlike skin recirculating B cells in sheep (2), neither splenic B2 nor peritoneal B1 cells migrated in response to the CCR6 ligand CCL20 (Fig. 5B). In a mixed bone-marrow chimera approach, in which lethally irradiated Rag1–/– recipient mice were reconstituted with an equal mixture of congenically marked bone marrow cells from Ccr6–/– and WT mice, we did not observe differences in the numbers or percentages of Ccr6–/– and WT B1 and B2 cells residing in uninflamed or inflamed skin (data not shown). Thus, CCR6 does not appear essential for B cell localization to mouse skin. CXCR4, whose ligand CXCL12 is constitutively expressed in the skin vasculature (41) and many other sites (42), was expressed by almost all analyzed B1 and B2 cells. Congruently, peritoneal B1 and splenic B2 cells migrated in response to CXCL12 although at lower levels when compared to their chemotaxis to the B-cell attracting ligand CXCL13, which served as a positive control (Fig. 5A and B). Additionally, CXCR3, whose ligands can be induced in skin during inflammation (43), was found on a small population of all B cell subsets analyzed (Fig. 5A). While CXCR4 and/or CXCR3 might be able to guide B cells into the skin, they are unlikely responsible for the differential ability of peritoneal B1 cells vs. splenic B cells to migrate into skin, as we found similar expression on these B cell subsets.
B1 cells require α4β1 integrin to home into inflamed skin
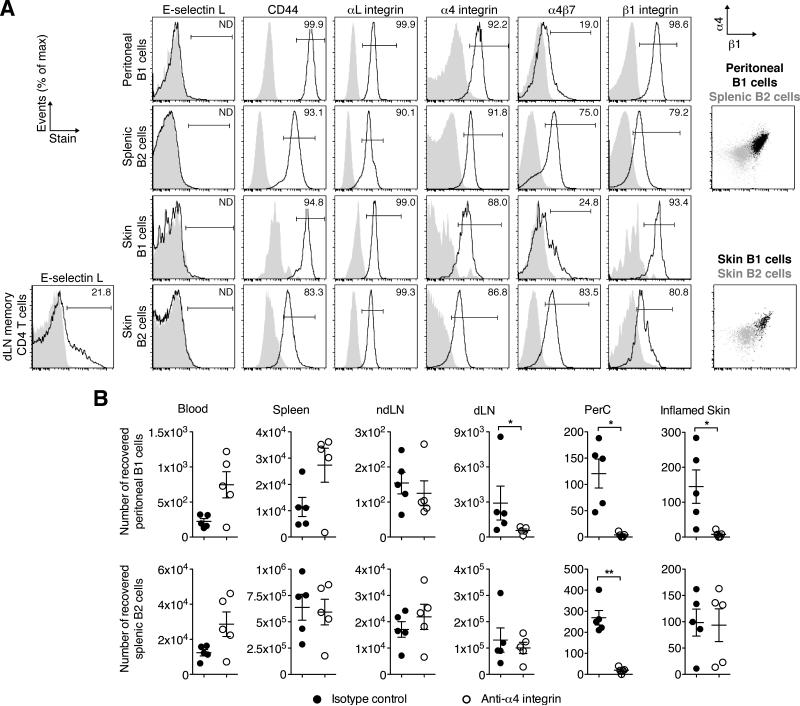
We next determined the expression of adhesion molecules that could potentially mediate skin homing of B cells. Expression of ligands binding E-selectin (largely overlapping with an epitope called CLA, cutaneous lymphocyte antigen) is a hallmark of skin homing T cells. However, while E-selectin was bound by a population of memory (CD44hi) CD4 T cells in skin draining lymph nodes, we could not detect binding by B1 or B2 cells (Fig. 6A). There are varying contributions of CD44, integrins α4β1 (VLA-4) and αLβ2 (LFA-1) to T-cell migration into inflamed skin, and no known relevance of intestinal homing receptor α4β7 integrin in this process. B1 and B2 cells at all analyzed sites expressed similar levels of αLβ2 (Fig. 6A). In contrast, while most skin and spleen B2 cells were α4β7+, expression on peritoneal and cutaneous B1 cells was on average 3- and 4.1-fold lower by comparison, respectively (differences in the geometric mean fluorescence intensity of the staining and that of the isotype (ΔMFI) 148 ± 20 (mean ± SD) vs. 440 ± 48 for peritoneal B1 vs. splenic B2 cells, and 135 ± 16 vs. 552 ± 91 for skin B1 vs. B2 cells, respectively; Fig. 6A). However, as described for peritoneal and splenic B cells (20, 44, 45), we found that both peritoneal and cutaneous B1 cells expressed on average between 3- and 8-fold higher levels of CD44 and integrin α4β1 compared with splenic and cutaneous B2 cells (ΔMFI for CD44: 36,138 ± 4,302 vs. 4,172 ± 1,008 for peritoneal B1 vs. splenic B2 cells, and 19,502 ± 1,513 vs. 2,410 ± 598 for skin B1 vs. B2 cells, respectively; ΔMFI for β1 integrin: 3,714 ± 161 and 669 ± 114 for peritoneal B1 vs. splenic B2 cells and 3,116 ± 616 vs. 1,011 ± 631 for skin B1 vs. B2 cells, respectively; Fig. 6A). As α4β1 binds VCAM-1, which is constitutively expressed at low levels by cutaneous vascular endothelial cells and further upregulated in inflammation (46), we addressed whether α4β1 mediates B1 cell migration into skin. Specifically, we neutralized α4 integrin with a blocking monoclonal antibody (PS/2) and tested migration of intravenously transferred fluorescently labeled peritoneal and splenic cells in recipient mice with chronic skin inflammation. In this 12-h homing assay, α4 blockade, but not an isotype control antibody, completely abrogated B1 cell migration into the peritoneal cavity and the inflamed skin and reduced migration into the inflammation-draining lymph node (Fig. 6B). Importantly, while blocking α4-integrin abrogated migration of B2 cells into the peritoneum as described (45), the treatment did not affect the migration of splenic B2 cells into the inflamed skin (Fig. 6B). These data demonstrate that α4β1 is selectively required for B1 cell migration into the inflamed skin.
Figure 6. α4β1 integrin mediates B1 cell migration into the inflamed skin.
(A, left) Flow cytometric analysis of adhesion molecule expression on peritoneal B1 cells, splenic B2 cells, and B1 and B2 cells from chronically inflamed skin or memory CD4 T cells from skin draining lymph nodes. Shaded areas depict isotype controls of the respective subsets and organs. Percent of positive staining above isotype is indicated. (A, right) Overlay of α4 and β1 integrin expression on B1 vs. B2 cell subsets. One representative staining from ≥ 4 experiments analyzing 3-5 mice each is shown. (B) Homing of peritoneal B1 cells (top panel) and splenic B2 cells (bottom panel) was tested in recipient mice with chronic skin inflammation. Recipient mice were treated with a blocking antibody to α4-integrin or an isotype control antibody. 12 h after cell transfer of unfractionated fluorescently labeled splenic and peritoneal cells, donor B cells in the specified organs were analyzed and enumerated by flow cytometry. One representative of three experiments is shown. Data points indicate individual recipient mice and the mean ± SEM for each group. *, P < 0.05; **, P < 0.01 using the Mann Whitney U test. dLN, inflammation draining lymph node; ndLN, non-draining lymph node; PerC, peritoneal cavity.
B1 cells constitutively express activated β1 integrin
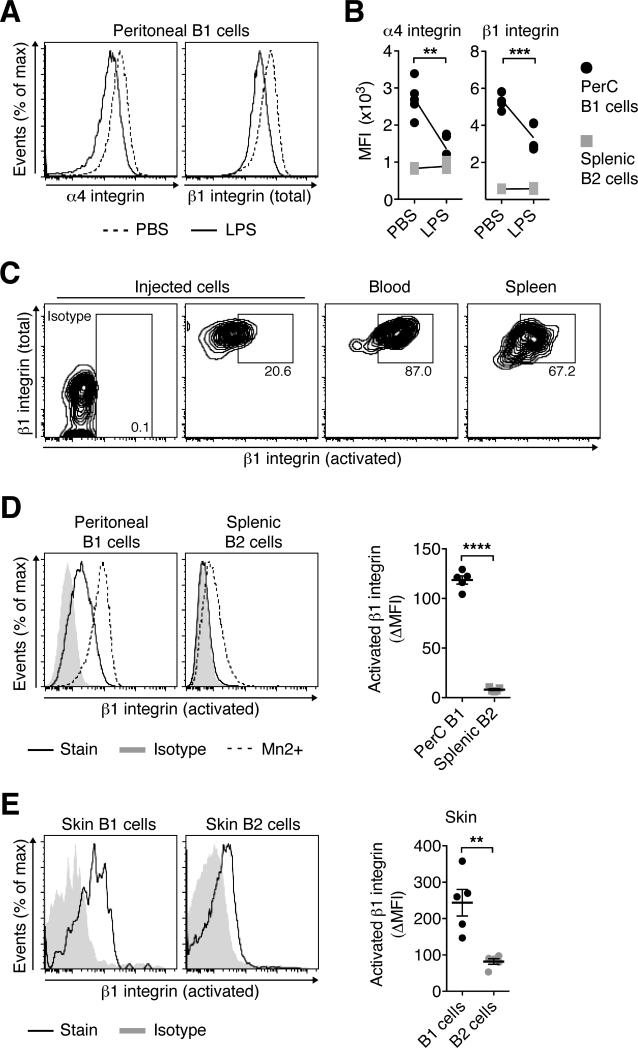
Ha et al. showed previously that upon innate stimulation with LPS, peritoneal B1 cells downregulate α4β1 integrin, allowing for release from the peritoneum (20). Having established that B1 cells rely on α4β1 integrin for their migration into the inflamed skin (Fig. 6B), we encountered an apparent contradiction: how can the same molecule be downregulated on B1 cells to facilitate release from the peritoneum subsequently be used for skin homing? Consistent with Ha et al. (20), we saw a moderate down-regulation of α4β1-integrin on peritoneal B1 cells, but not splenic B2 cells, 6 h after intraperitoneal injection of WT mice with LPS (P < 0.01; Fig. 7A and B). However, as much of the function of integrins is regulated via their affinity regulation and less so through surface expression levels, we wondered whether B1 that were released from the peritoneum would still be capable of activating α4β1. To address this question, we transferred CFSE-labeled peritoneal cells into Rag1–/– recipient mice prior to intraperitoneal challenge with LPS as described (20). 12 h later, we analyzed B1 cells that were released from the peritoneum and had entered the blood and spleen and stained them with antibody 9EG7, which recognizes a high affinity site of β1-integrin that is only accessible following integrin activation and conformational change (47). Strikingly, most B1 cells that were released from the peritoneum and had entered the blood circulation or spleen expressed activated β1 integrin (Fig. 7C). The data suggest that while total levels of α4β1 are downregulated on peritoneal B1 cells after LPS stimulation, presumably allowing for release from low affinity interaction with extracellular matrix, high affinity α4β1 remains inducible on these cells, targeting released B1 cells into the inflamed skin.
Figure 7. B1 cells constitutively express activated β1 integrin.
(A and B) Two groups of WT mice received LPS or PBS intraperitoneally. 6 h later, peritoneal B1 and splenic B2 cells were analyzed by flow cytometry. (A) One representative staining and (B) the geometric mean fluorescence intensity (MFI) for individual mice and the mean of each group, indicated as connecting lines, in one out of two similar experiments with 5 mice per group are shown. (C) CD45.1+ peritoneal cells were transferred into congenic CD45.2+ Rag1–/– recipients before inducing B1 cell relocation by intraperitoneal injection of LPS. 12 h later, expression of activated β1 integrin was determined on B1 cells that had left the peritoneum and the input population by flow cytometry using antibody 9EG7. One representative staining of 4 experiments analyzing 3-5 mice each is shown. (D and E) Expression levels of activated β1 integrin on B1 and B2 cell subsets in (D) naïve mice and (E) mice with CFA-induced chronically inflamed skin. One representative out of ≥ 3 experiment analyzing 3-5 mice each, summarized as the differences in the MFI of the staining and the MFI of the isotype (ΔMFI) for each B cell subset. Data points indicate individual mice and the mean ± SEM of each group. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 using student's t test.
We noted that activated β1 integrin was not only expressed by B1 cells that had left the peritoneum, but also by peritoneal B1 cells in the steady state. Specifically, in naïve mice activated β1 integrin was clearly found on peritoneal B1 cells, while expression was barely detectable in splenic B2 cells (P < 0.0001 when comparing ΔMFIs; Fig. 7D). Staining in the presence of Mn2+ cations, which force an activated conformation of lymphocyte-expressed integrins (47, 48), demonstrated a lower inducibility of activated β1 integrin in splenic B2 cells (Fig. 7D, left). Furthermore, activated β1 expression was on average 3-fold higher expressed on cutaneous B1 cells compared with skin B2 cells (P < 0.001; Fig. 7E). The data suggest that expression of activated β1-integrin is a hallmark of B1 cells, revealing them as tissue-targeting effector cells.
Discussion
In this study we establish that B cells, including IL-10+ innate-like B cells and conventional B cells, reside in the skin of mice and humans. Formally adding B cells to the skin immune system opens the door to revealing the specialized roles of skin B cell subsets in cutaneous host defense and inflammation as well as in tissue homeostasis and repair.
Our study shows that innate-like B cells in the skin of mice and humans secrete IL-10. Additional cell types in the skin make IL-10, such as dendritic cells, keratinocytes, and T cells (reviewed in (49)) as well as some B2 cells (Fig. 2). However, anatomical localization within the skin (i.e. epidermis vs. dermis), stimulation requirements (innate vs. antigenic receptor signals) and cell mobility (i.e. sessile vs. migratory) differ for each cell type, thus, assigning distinct roles in the provision of cutaneous IL-10. For example, B cells are mobile cells that localize to the dermis (Fig. 1). Additionally, unlike conventional lymphocytes, innate-like B cells rapidly respond with IL-10 production to innate stimulation (10). As a result, cutaneous innate-like B cells can readily respond to various external and immune insults of the skin.
Our findings are consistent with the non-redundant role of B cells and/or B cell-derived IL-10 in limiting skin inflammation in human psoriasis (16-18) and mouse models of cutaneous hypersensitivity and psoriasis-like inflammation (12, 34, 50). Importantly, human psoriasis is associated with low levels of cutaneous IL-10 and clinically responsive to treatment with dermal IL-10 (51), stressing the importance of cutaneously produced IL-10 in limiting skin inflammation. While studies suggest that B cells suppress skin inflammation extracutaneously (e.g. in lymphoid tissues) (12, 50), we provide evidence that at least innate-like regulatory B cells additionally act in the skin itself and make IL-10 during inflammation. Importantly, peritoneal IL-10+ B1 cells, which suppress cutaneous inflammation (12), preferentially migrate into the inflamed skin, and inflammatory signals stimulate relocation of B1 cells from the peritoneum into the inflamed skin (Fig. 4). Thus, anti-inflammatory B cells reside in the skin during the steady state and larger numbers are rapidly mobilized from central reservoirs and recruited into the skin during inflammation. Notably, immunosuppressive therapy with an antibody to α4 integrin (natalizumab), which we have shown blocks (IL-10+) B1 but not B2 cell migration into inflamed skin, is able to “paradoxically” exacerbate psoriasis (52). Thus, it is possible that impairment of the recruitment and/or function of IL-10+ skin B cells promotes skin inflammation in psoriasis and other inflammatory skin diseases.
Of course, skin B cells are a heterogeneous population and some of these cells likely fulfill pro-inflammatory functions similar to the pro- and anti-inflammatory B cell subsets that reside at other extralymphoid sites, such as adipose tissue (53-55). In our human skin samples, IL-10 production by B cells appeared less strictly associated with an innate-like (CD43+) phenotype compared to cutaneous mouse B cells. Additionally, the recruitment of IL-10 producing B cells into skin is not always desirable as IL-10 can impede pathogen clearance in infection (56) and potentially play a pathogenic role in the cutaneous autoimmune disease pemphigus vulgaris by promoting immunoglobulin class switch to disease-perpetuating IgG4 (49). Therefore, future studies are needed to reveal the various subsets and functions of cutaneous and other extralymphoid tissue B cells in human disease settings.
Confirming the results by others (9, 20, 33, 57), inflammatory challenge (i.e. LPS) stimulates relocation of peritoneal B1 cells into activated lymphoid tissues (Fig. 4B). However, upon peritoneal release and in short-term homing assays, B1 cells preferentially relocate to extralymphoid tissues that are considered barrier sites, such as the peritoneum, small intestine and inflamed skin (Fig. 4B and D). Given the critical role B1 cells play in the early phase of non-cutaneous infections (5), they are expected to be beneficial responders also following skin injury and infection. Together, this suggests an innate mechanism of host defense that limits immunopathology by the rapid deployment of B1 cells from central reservoirs into inflamed or threatened barrier tissues.
B1 cells that had left the peritoneal cavity and entered the blood almost uniformly express activated β1 integrin, allowing for their migration into skin. To our surprise, B1 cells express activated β1 integrin already at steady-state and bind VCAM-1 (Fig. 7D and not shown). While α4β1 integrin supports endothelial rolling (58) and can likely substitute for the expression of selectin ligands during skin homing, activation of integrins during extravasation from the blood is usually accomplished by recognition of endothelial chemoattractants. In contrast, constitutive expression of activated α4β1 integrin and β1 integrins is unusual and has been described for a limited number of effector cells, such as effector T cells (59-61), NK cells (59) as well as metastatic tumor cells (62). As constitutively activated α4β1 integrin mediates adhesion to VCAM-1 in the absence of chemoattractant signals (60, 61), it potentially explains why B1 cells migrate into skin despite their lack of responsiveness to ligands for skin-associated chemokine receptors (Fig. 5B). Moreover, it is thought that expression of activated α4β1 on circulating cells enhances their binding to low levels of endothelial VCAM-1 and facilitates transendothelial migration early in inflammation (59). Collectively, these findings suggest that expression of activated β1 integrin endows B1 cells with tissue-seeking properties required for effectors despite their lack of a typical skin homing signature.
Surprisingly, B1 cells that are released from the peritoneum home into both the inflamed skin and the small intestine (Fig. 4 and (20)), even though skin and gut homing signatures are distinct and induced in T cells during antigenic responses in a mutually exclusive manner (reviewed in (23, 63). However, it is unclear in our studies if skin and gut homing B1 cells are discrete or overlapping populations. Additionally, endothelial VCAM-1 is upregulated by inflammatory stimuli throughout the body, raising the question if (activated) α4β1 enables B1 cells to ubiquitously home into inflamed tissues similar to the inflammation-seeking migration of innate leukocytes, such as neutrophils (64). Alternatively, B1 cells might initially home into multiple sites prior to further differentiation and acquisition of tissue specificity. Thus, it will be important to determine whether the differentiation into B1 cells with specialized effector phenotypes e.g. class switch to IgA (65) or GM-CSF production (8) is paralleled by the acquisition of organ-selective homing.
In conclusion, our study reveals innate-like B cells as novel tissue-targeting effectors that follow a unique peritoneum–skin migratory axis to provide cutaneous immunosurveillance and anti-inflammatory functions. We lay the foundation for future studies determining additional roles of B cell subsets in extralymphoid tissues during autoimmunity, inflammation, cancer, and infection.
Supplementary Material
Acknowledgements
The authors thank the University of Pennsylvania Skin Disease Research Center for human skin samples, Jiang Tianying at the Abramson Cancer Center Histology Core for tissue sectioning, Uta Lauer for excellent technical assistance with the radioactive homing assay, Paul Wilson for help with sample processing, Jean Jang and Eugene Butcher for PS/2, and Tzvete Dentchev for invaluable assistance with skin histology. We are indebted to Aimee Payne, Damian Maseda and Mike Cancro for helpful discussions and critical comments on the manuscript.
Footnotes
This work was supported in part by NIH/NIAMS grants R01-AR056730 (to GFD) and P30-AR057217 (Penn Skin Disease Research Center), an AAI Careers in Immunology Fellowship (to DG and GFD), and DFG grant SFB650 TP1 (to AH).
References
- 1.Bos JD, Teunissen MB. Innate and Adaptive Immunity. In: Gaspari AA, Tyring SK, editors. Clinical and Basic Immunodermatolgy. Springer; London: 2008. pp. 17–30. [Google Scholar]
- 2.Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, Young AJ, Hay JB, Debes GF. The skin, a novel niche for recirculating B cells. J. Immunol. 2012;188:6027–6035. doi: 10.4049/jimmunol.1102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egbuniwe IU, Karagiannis SN, Nestle FO, Lacy KE. Revisiting the role of B cells in skin immune surveillance. Trends Immunol. 2015;36:102–111. doi: 10.1016/j.it.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 4.Kearney JF. Innate-like B cells. Springer Semin. Immunopathol. 2005;26:377–383. doi: 10.1007/s00281-004-0184-0. [DOI] [PubMed] [Google Scholar]
- 5.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011;11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 6.Parra D, Rieger AM, Li J, Zhang YA, Randall LM, Hunter CA, Barreda DR, Sunyer JO. Pivotal advance: peritoneal cavity B-1 B cells have phagocytic and microbicidal capacities and present phagocytosed antigen to CD4+ T cells. J. Leukoc. Biol. 2012;91:525–536. doi: 10.1189/jlb.0711372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakashima M, Kinoshita M, Nakashima H, Habu Y, Miyazaki H, Shono S, Hiroi S, Shinomiya N, Nakanishi K, Seki S. Pivotal advance: characterization of mouse liver phagocytic B cells in innate immunity. J. Leukoc. Biol. 2012;91:537–546. doi: 10.1189/jlb.0411214. [DOI] [PubMed] [Google Scholar]
- 8.Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, Hilgendorf I, Tiglao E, Figueiredo JL, Iwamoto Y, Theurl I, Gorbatov R, Waring MT, Chicoine AT, Mouded M, Pittet MJ, Nahrendorf M, Weissleder R, Swirski FK. Innate response activator B cells protect against microbial sepsis. Science. 2012;335:597–601. doi: 10.1126/science.1215173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Itakura A, Szczepanik M, Campos RA, Paliwal V, Majewska M, Matsuda H, Takatsu K, Askenase PW. An hour after immunization peritoneal B-1 cells are activated to migrate to lymphoid organs where within 1 day they produce IgM antibodies that initiate elicitation of contact sensitivity. J. Immunol. 2005;175:7170–7178. doi: 10.4049/jimmunol.175.11.7170. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X. Regulatory functions of innate-like B cells. Cell. Mol. Immunol. 2013;10:113–121. doi: 10.1038/cmi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gronwall C, Silverman GJ. Natural IgM: beneficial autoantibodies for the control of inflammatory and autoimmune disease. J. Clin. Immunol. 2014;34(Suppl 1):S12–21. doi: 10.1007/s10875-014-0025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, Takahashi Y, Tamaki K, Sato S, Tedder TF, Fujimoto M. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J. Immunol. 2010;184:4637–4645. doi: 10.4049/jimmunol.0901719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maseda D, Candando KM, Smith SH, Kalampokis I, Weaver CT, Plevy SE, Poe JC, Tedder TF. Peritoneal cavity regulatory B cells (B10 cells) modulate IFN-gamma+CD4+ T cell numbers during colitis development in mice. J. Immunol. 2013;191:2780–2795. doi: 10.4049/jimmunol.1300649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Candando KM, Lykken JM, Tedder TF. B10 cell regulation of health and disease. Immunol. Rev. 2014;259:259–272. doi: 10.1111/imr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilgenberg E, Shen P, Dang VD, Ries S, Sakwa I, Fillatreau S. Interleukin-10-producing B cells and the regulation of immunity. Curr. Top. Microbiol. Immunol. 2014;380:69–92. doi: 10.1007/978-3-662-43492-5_4. [DOI] [PubMed] [Google Scholar]
- 16.Dass S, Vital EM, Emery P. Development of psoriasis after B cell depletion with rituximab. Arthritis Rheum. 2007;56:2715–2718. doi: 10.1002/art.22811. [DOI] [PubMed] [Google Scholar]
- 17.Mielke F, Schneider-Obermeyer J, Dorner T. Onset of psoriasis with psoriatic arthropathy during rituximab treatment of non-Hodgkin lymphoma. Ann. Rheum. Dis. 2008;67:1056–1057. doi: 10.1136/ard.2007.080929. [DOI] [PubMed] [Google Scholar]
- 18.Guidelli GM, Fioravanti A, Rubegni P, Feci L. Induced psoriasis after rituximab therapy for rheumatoid arthritis: a case report and review of the literature. Rheumatol. Int. 2013;33:2927–2930. doi: 10.1007/s00296-012-2581-3. [DOI] [PubMed] [Google Scholar]
- 19.Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002;16:67–76. doi: 10.1016/s1074-7613(01)00257-6. [DOI] [PubMed] [Google Scholar]
- 20.Ha SA, Tsuji M, Suzuki K, Meek B, Yasuda N, Kaisho T, Fagarasan S. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 2006;203:2541–2550. doi: 10.1084/jem.20061041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber GF, Chousterman BG, Hilgendorf I, Robbins CS, Theurl I, Gerhardt LM, Iwamoto Y, Quach TD, Ali M, Chen JW, Rothstein TL, Nahrendorf M, Weissleder R, Swirski FK. Pleural innate response activator B cells protect against pneumonia via a GM-CSF-IgM axis. J. Exp. Med. 2014;211:1243–1256. doi: 10.1084/jem.20131471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 23.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat. Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Islam SA, Luster AD. T cell homing to epithelial barriers in allergic disease. Nat. Med. 2012;18:705–715. doi: 10.1038/nm.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madan R, Demircik F, Surianarayanan S, Allen JL, Divanovic S, Trompette A, Yogev N, Gu Y, Khodoun M, Hildeman D, Boespflug N, Fogolin MB, Grobe L, Greweling M, Finkelman FD, Cardin R, Mohrs M, Muller W, Waisman A, Roers A, Karp CL. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J. Immunol. 2009;183:2312–2320. doi: 10.4049/jimmunol.0900185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 27.Brown MN, Fintushel SR, Lee MH, Jennrich S, Geherin SA, Hay JB, Butcher EC, Debes GF. Chemoattractant receptors and lymphocyte egress from extralymphoid tissue: changing requirements during the course of inflammation. J. Immunol. 2010;185:4873–4882. doi: 10.4049/jimmunol.1000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J. Exp. Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegmund K, Hamann A. Use of Labeled Lymphocytes to Analyze Trafficking In Vivo. In: Hamann A, Engelhardt B, editors. Leukocyte Trafficking. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim: 2005. pp. 497–508. [Google Scholar]
- 30.Muppidi JR, Arnon TI, Bronevetsky Y, Veerapen N, Tanaka M, Besra GS, Cyster JG. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J. Exp. Med. 2011;208:1941–1948. doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Geherin SA, Wilson RP, Jennrich S, Debes GF. CXCR4 Is dispensable for T Cell egress from chronically inflamed skin via the afferent lymph. PLoS One. 2014;9:e95626. doi: 10.1371/journal.pone.0095626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Debes GF, Dahl ME, Mahiny AJ, Bonhagen K, Campbell DJ, Siegmund K, Erb KJ, Lewis DB, Kamradt T, Hamann A. Chemotactic responses of IL-4-, IL-10-, and IFN-gamma-producing CD4+ T cells depend on tissue origin and microbial stimulus. J. Immunol. 2006;176:557–566. doi: 10.4049/jimmunol.176.1.557. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y, Tung JW, Ghosn EE, Herzenberg LA. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl. Acad. Sci. U. S. A. 2007;104:4542–4546. doi: 10.1073/pnas.0700001104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J. Exp. Med. 2011;208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Descatoire M, Weill JC, Reynaud CA, Weller S. A human equivalent of mouse B-1 cells? J. Exp. Med. 2011;208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tangye SG. To B1 or not to B1: that really is still the question! Blood. 2013;121:5109–5110. doi: 10.1182/blood-2013-05-500074. [DOI] [PubMed] [Google Scholar]
- 38.Rothstein TL, Griffin DO, Holodick NE, Quach TD, Kaku H. Human B-1 cells take the stage. Ann. N. Y. Acad. Sci. 2013;1285:97–114. doi: 10.1111/nyas.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 40.Charbonnier AS, Kohrgruber N, Kriehuber E, Stingl G, Rot A, Maurer D. Macrophage inflammatory protein 3alpha is involved in the constitutive trafficking of epidermal langerhans cells. J. Exp. Med. 1999;190:1755–1768. doi: 10.1084/jem.190.12.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Avniel S, Arik Z, Maly A, Sagie A, Basst HB, Yahana MD, Weiss ID, Pal B, Wald O, Ad-El D, Fujii N, Arenzana-Seisdedos F, Jung S, Galun E, Gur E, Peled A. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J. Invest. Dermatol. 2006;126:468–476. doi: 10.1038/sj.jid.5700069. [DOI] [PubMed] [Google Scholar]
- 42.Nagasawa T. CXC chemokine ligand 12 (CXCL12) and its receptor CXCR4. J. Mol. Med. (Berl.) 2014;92:433–439. doi: 10.1007/s00109-014-1123-8. [DOI] [PubMed] [Google Scholar]
- 43.Flier J, Boorsma DM, van Beek PJ, Nieboer C, Stoof TJ, Willemze R, Tensen CP. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J. Pathol. 2001;194:398–405. doi: 10.1002/1096-9896(200108)194:4<397::aid-path899>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 44.Murphy TP, Kolber DL, Rothstein TL. Elevated expression of Pgp-1 (Ly-24) by murine peritoneal B lymphocytes. Eur. J. Immunol. 1990;20:1137–1142. doi: 10.1002/eji.1830200529. [DOI] [PubMed] [Google Scholar]
- 45.Berberich S, Dahne S, Schippers A, Peters T, Muller W, Kremmer E, Forster R, Pabst O. Differential molecular and anatomical basis for B cell migration into the peritoneal cavity and omental milky spots. J. Immunol. 2008;180:2196–2203. doi: 10.4049/jimmunol.180.4.2196. [DOI] [PubMed] [Google Scholar]
- 46.Quinlan KL, Song IS, Naik SM, Letran EL, Olerud JE, Bunnett NW, Armstrong CA, Caughman SW, Ansel JC. VCAM-1 expression on human dermal microvascular endothelial cells is directly and specifically up-regulated by substance P. J. Immunol. 1999;162:1656–1661. [PubMed] [Google Scholar]
- 47.Lenter M, Uhlig H, Hamann A, Jeno P, Imhof B, Vestweber D. A monoclonal antibody against an activation epitope on mouse integrin chain beta 1 blocks adhesion of lymphocytes to the endothelial integrin alpha 6 beta 1. Proc. Natl. Acad. Sci. U. S. A. 1993;90:9051–9055. doi: 10.1073/pnas.90.19.9051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dransfield I, Cabanas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho MJ, Ellebrecht CT, Payne AS. The dual nature of interleukin-10 in pemphigus vulgaris. Cytokine. 2015 Jun;73(2):335–41. doi: 10.1016/j.cyto.2014.11.002. doi 10.1016/j.cyto.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yanaba K, Kamata M, Ishiura N, Shibata S, Asano Y, Tada Y, Sugaya M, Kadono T, Tedder TF, Sato S. Regulatory B cells suppress imiquimod-induced, psoriasis-like skin inflammation. J. Leukoc. Biol. 2013;94:563–573. doi: 10.1189/jlb.1112562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asadullah K, Sterry W, Stephanek K, Jasulaitis D, Leupold M, Audring H, Volk HD, Docke WD. IL-10 is a key cytokine in psoriasis. Proof of principle by IL-10 therapy: a new therapeutic approach. J. Clin. Invest. 1998;101:783–794. doi: 10.1172/JCI1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millan-Pascual J, Turpin-Fenoll L, Del Saz-Saucedo P, Rueda-Medina I, Navarro-Munoz S. Psoriasis during natalizumab treatment for multiple sclerosis. J. Neurol. 2012;259:2758–2760. doi: 10.1007/s00415-012-6713-1. [DOI] [PubMed] [Google Scholar]
- 53.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, Tsui H, Wu P, Davidson MG, Alonso MN, Leong HX, Glassford A, Caimol M, Kenkel JA, Tedder TF, McLaughlin T, Miklos DB, Dosch HM, Engleman EG. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat. Med. 2011;17:610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura S, Manabe I, Takaki S, Nagasaki M, Otsu M, Yamashita H, Sugita J, Yoshimura K, Eto K, Komuro I, Kadowaki T, Nagai R. Adipose Natural Regulatory B Cells Negatively Control Adipose Tissue Inflammation. Cell Metab. 2013;18:759–766. doi: 10.1016/j.cmet.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Wu L, Parekh VV, Hsiao J, Kitamura D, Van Kaer L. Spleen supports a pool of innate-like B cells in white adipose tissue that protects against obesity-associated insulin resistance. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E4638–4647. doi: 10.1073/pnas.1324052111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect. Dis. 2006;6:557–569. doi: 10.1016/S1473-3099(06)70577-1. [DOI] [PubMed] [Google Scholar]
- 57.Choi YS, Baumgarth N. Dual role for B-1a cells in immunity to influenza virus infection. J. Exp. Med. 2008;205:3053–3064. doi: 10.1084/jem.20080979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J. Cell Biol. 1995;128:1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rose DM, Cardarelli PM, Cobb RR, Ginsberg MH. Soluble VCAM-1 binding to alpha4 integrins is cell-type specific and activation dependent and is disrupted during apoptosis in T cells. Blood. 2000;95:602–609. [PubMed] [Google Scholar]
- 60.Lim YC, Wakelin MW, Henault L, Goetz DJ, Yednock T, Cabanas C, Sanchez-Madrid F, Lichtman AH, Luscinskas FW. Alpha4beta1-integrin activation is necessary for high-efficiency T-cell subset interactions with VCAM-1 under flow. Microcirculation. 2000;7:201–214. [PubMed] [Google Scholar]
- 61.Shulman Z, Cohen SJ, Roediger B, Kalchenko V, Jain R, Grabovsky V, Klein E, Shinder V, Stoler-Barak L, Feigelson SW, Meshel T, Nurmi SM, Goldstein I, Hartley O, Gahmberg CG, Etzioni A, Weninger W, Ben-Baruch A, Alon R. Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat. Immunol. 2012;13:67–76. doi: 10.1038/ni.2173. [DOI] [PubMed] [Google Scholar]
- 62.Kato H, Liao Z, Mitsios JV, Wang HY, Deryugina EI, Varner JA, Quigley JP, Shattil SJ. The primacy of beta1 integrin activation in the metastatic cascade. PLoS One. 2012;7:e46576. doi: 10.1371/journal.pone.0046576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: new concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 64.McDonald B, Kubes P. Cellular and molecular choreography of neutrophil recruitment to sites of sterile inflammation. J. Mol. Med. (Berl.) 2011;89:1079–1088. doi: 10.1007/s00109-011-0784-9. [DOI] [PubMed] [Google Scholar]
- 65.Fagarasan S, Kawamoto S, Kanagawa O, Suzuki K. Adaptive immune regulation in the gut: T cell-dependent and T cell-independent IgA synthesis. Annu. Rev. Immunol. 2010;28:243–273. doi: 10.1146/annurev-immunol-030409-101314. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.