Abstract
The dopamine transporter (DAT) inhibitor and nicotinic acetylcholine (nACh) receptor antagonist bupropion is being investigated as a candidate ‘agonist’ medication for methamphetamine addiction. In addition to its complex pharmacology, bupropion also has two distinct pharmacologically active metabolites. However, the mechanism by which bupropion produces methamphetamine-like ‘agonist’ effects remains unknown. The present aim was to determine the role of DAT inhibition, nACh receptor antagonism, and the hydroxybupropion metabolites in the methamphetamine-like discriminative stimulus effects of bupropion in rhesus monkeys. In addition, varenicline, a partial agonist at the nACh receptor, and risperidone, a dopamine antagonist, were tested as controls. Monkeys (n=4) were trained to discriminate 0.18 mg/kg intramuscular methamphetamine from saline in a two-key food-reinforced discrimination procedure. Potency and time course of methamphetamine-like discriminative stimulus effects were determined for all compounds. Bupropion, methylphenidate, and 2S,3S-hydroxybupropion produced full, ≥90%, methamphetamine-like effects. 2R,3R-hydroxybupropion, mecamylamine, and nicotine also produced full methamphetamine-like effects, but drug potency was more variable between monkeys. Varenicline produced partial methamphetamine-like effects, whereas risperidone did not. Overall, these results suggest DAT inhibition as the major mechanism of the methamphetamine-like ‘agonist’ effects of bupropion, although nACh receptor antagonism appeared, at least partially, to contribute. Furthermore, the contribution of the 2S,3S-hydroxybupropion metabolite could not be completely ruled out.
Keywords: methamphetamine, discrimination, bupropion, hydroxybupropion, mecamylamine, nicotine, methylphenidate, rhesus monkey
Introduction
Methamphetamine addiction remains a worldwide public health problem for which there is no Food and Drug Administration (FDA)-approved pharmacotherapy (Courtney and Ray, 2014). Furthermore, the 2014 United States National Forensic Laboratory Information System midyear report estimates that methamphetamine ranks second only behind cannabis/THC in both number and percentage of total drugs submitted for analysis (Drug Enforcement Administration, 2014). Preclinical drug discrimination procedures are hypothesized to model and be predictive of the subjective-like drug effects in humans (Fischman and Foltin, 1991; Horton et al., 2013; Schuster and Johanson, 1988). Furthermore, drug discrimination procedures have good resolution to interrogate the pharmacological mechanisms of action of central nervous system acting compounds and in particular, abused drugs (Grant, 1999; Lelas et al., 2000; Woods et al., 1988).
Bupropion is being examined as a candidate ‘agonist’ pharmacotherapy for methamphetamine addiction (Brensilver et al., 2013). Consistent with the pharmacological activity of bupropion as a dopamine transporter (DAT), but not norepinephrine transporter (NET) inhibitor (Czoty et al., 2004b; Tidey and Bergman, 1998), it produces methamphetamine-like discriminative stimulus effects in pigeons (Sasaki et al., 1995) and rats (Munzar and Goldberg, 2000). Furthermore, bupropion has been shown to antagonize in vitro methamphetamine-induced dopamine efflux and this effect was hypothesized to be mediated by bupropion inhibiting the DAT and preventing methamphetamine from entering the cell (Simmler et al., 2013). Moreover, human laboratory studies have suggested that bupropion pretreatment attenuated the subjective and other abuse-related effects of methamphetamine (Newton et al., 2006; Rau et al., 2005). Overall, this body of literature has been interpreted to suggest that DAT inhibition is major component of the pharmacological effects of bupropion related to its evaluation as a candidate medication for methamphetamine addiction.
However, the pharmacology of bupropion also includes antagonist activity at nicotinic acetylcholine (nACh) receptors, in addition to DAT and NET inhibition (Damaj et al., 2004; Fryer and Lukas, 1999; Lukas et al., 2010). These nACh receptor antagonist properties may have implications for bupropion as a pharmacotherapy for methamphetamine addiction. For example, both amphetamine and methamphetamine have been reported to function as nACh receptor agonists (Garcia-Ratés et al., 2007; Liu et al., 2003) or antagonists (Liu et al., 2003) depending upon the in vitro assay. Consistent with these agonist results, nicotine and methamphetamine cross-generalize in drug discrimination procedures, suggesting a potential shared pharmacological mechanism (Desai and Bergman, 2010, 2014; Gatch et al., 2008). However, whether the nACh receptor antagonist property of bupropion contributes to its methamphetamine-like effects in vivo has not been systematically explored.
The aim of the present study was to determine the pharmacological mechanism of the methamphetamine-like discriminative stimulus effects of bupropion in rhesus monkeys. To address this aim, we determined the substitution profile of the non-competitive nACh receptor antagonist mecamylamine and the DAT inhibitor methylphenidate, which does not possess in vitro nACh receptor antagonist properties (Besnard et al., 2012). In addition, we determined the substitution profile of two pharmacologically active metabolites of bupropion to further clarify the mechanisms of bupropion’s methamphetamine-like effects. For example, 2S,3S-hydroxybupropion produces both DAT and NET inhibition and nACh receptor antagonism effects similar to bupropion, whereas 2R,3R-hydroxybupropion primarily functions as a nACh receptor antagonist (Bondarev et al., 2003; Damaj et al., 2004; Lukas et al., 2010). Finally, the substitution profile of varenicline, a nACh receptor partial agonist, and the dopamine antagonist risperidone were tested as controls. The null hypothesis was that nACh receptor antagonism would not produce methamphetamine-like discriminative stimulus effects. We also hypothesized if either hydroxybupropion metabolite contributed to the methamphetamine-like discriminative stimulus effects of bupropion then bupropion might be more potent or display a longer time course compared to its metabolites.
Methods
Subjects
Four adult male rhesus monkeys (Macaca mulatta) weighing between 6-11 kg served as research subjects. Three monkeys (M1510, M1511, M1512) were experimental naïve at the start of methamphetamine discrimination training and one monkey (M1479) had a previous history of responding under a two-key food-reinforced cocaine vs. saline discrimination procedure. However, this monkey had not participated in cocaine discrimination studies for 3 months before initiating methamphetamine discrimination training. Although there are reports of retained drug discriminations following sequential training (Li and McMillan, 2003; McMillan et al., 1996), the influence of this previous cocaine discrimination should be minimal for the proposed experiments given the shared pharmacological discriminative stimulus mechanisms between cocaine and methamphetamine or amphetamine (Kamien and Woolverton, 1989; Kleven et al., 1990; Negus et al., 2007). The monkeys’ diet consisted of food biscuits (Lab Diet® High Protein Monkey Biscuits, PMI Feeds, Inc. St. Louis, MO) supplemented with fresh fruit. Water was continuously available in the home chamber. Additionally, monkeys could earn 1-g banana-flavored food pellets (5TUR grain-based precision primate tablets, Test Diets, Richmond, IL) during the experimental session (described below). A 12h light-dark cycle was in effect (lights on from 06.00 to 19.00h), and temperature and humidity levels were controlled and monitored daily. Environmental enrichment consisting of various puzzles and foraging devices in addition to videos or radio was provided during the week at the conclusion of the behavioral sessions. Animal research facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee approved both experimental and environmental enrichment protocols.
Methamphetamine Discrimination Procedure
Experimental behavioral sessions were conducted in each monkey’s home chamber. On the front wall of each chamber was an operant response panel that included three square response keys arranged horizontally, only the left and right keys were used in the present studies. Attached to the back of each panel was a pellet dispenser (Med Associates, ENV-203-1000, St. Albans, VT). Equipment operation and data collection were accomplished with a Windows-based computer and MED-PC IV software (Med Associates).
During initial training sessions, 0.1 mg/kg intramuscular (+)-methamphetamine was administered in a two-key, food-reinforced drug discrimination procedure. Discrimination training was conducted 5 days per week and consisted of two phases. In Phase 1, daily experimental sessions consisted of a single response component. Each component consisted of a 5-minute response period, during which the right and left response keys were transilluminated red and green, respectively, and monkeys could earn up to 10 pellets by responding under a fixed-ratio (FR) schedule of food presentation. The FR value was initially set at one and was independently increased on the saline- (mean 25; range 16 – 30 training sessions) and methamphetamine- (mean 35; range 17 – 53 training sessions) associated keys to the terminal FR30 schedule of food presentation. Either saline or (0.1 mg/kg methamphetamine) was administered i.m. approximately 15 min prior to the start of the component and saline- or methamphetamine-appropriate training sessions were conducted in a double-alternating pattern. Following administration of saline, only responding on the green key (the saline-appropriate key) produced food, whereas following administration of 0.1 mg/kg methamphetamine, only responding on the red key (the methamphetamine-appropriate key) produced food. Responses on the inappropriate key reset the FR requirement on the appropriate key. Once subjects reached the terminal FR30 schedule for both training conditions, subjects progressed to phase 2.
In Phase 2, daily experimental sessions were composed of two components presented at 3-h intervals, and either saline or 0.1 mg/kg methamphetamine was administered i.m. approximately 15 min prior to the start of each component. Thus, on training days, monkeys would receive a sequence of saline (S) and methamphetamine (M) injections in the order SS, SM, MS, or MM. These training sequences were randomly presented. The goal of this training regimen was to engender daily experience with randomized sequences of saline- and methamphetamine-appropriate response components. The 3h duration of inter-component intervals was selected to exceed the time course of discriminative stimulus effects produced by the methamphetamine-training dose in rhesus monkeys and thereby to minimize effects of methamphetamine administered in earlier trials on performance during later trials on the same day. The criterion for accurate discrimination was ≥85% injection-appropriate responding before delivery of the first reinforcer, ≥90% injection-appropriate responding for the entire component, and response rates ≥0.1 responses/s (sufficient to earn at least one pellet) for all components during 7 of 8 consecutive sessions. However, none of the monkeys met accurate discrimination criterion after 136 training sessions and thus the methamphetamine-training dose was increased to 0.18 mg/kg. All monkeys met discrimination criterion within 73 sessions (range 30-73) after increasing the methamphetamine dose.
Time course test sessions were identical to training sessions except (1) completing the response requirement on either key produced food and (2) 5-min response components began 10, 30, 56, 100, 180 and 300 min after injection to assess the time course of drug effects. (+)-Methamphetamine (0.01 – 0.18 mg/kg), (±)-methylphenidate (0.032 – 0.32 mg/kg), (±)-bupropion (0.32 – 3.2 mg/kg), 2S,3S-hydroxybupropion (0.32 – 3.2 mg/kg), 2R,3R-hydroxybupropion (1.0 – 10.0 mg/kg), (±)-mecamylamine (0.32 – 1.8 mg/kg), (−)-nicotine (0.1 – 1.0 mg/kg), varenicline (0.1 – 1.0 mg/kg), and risperidone (0.01 – 0.1 mg/kg) were tested up to doses that either produced full substitution for the methamphetamine training dose or disrupted rates of operant responding in at least two monkeys. Test sessions were separated by at least 3 days, and were usually conducted on Tuesdays and Fridays, with training sessions conducted on other weekdays. Test sessions were conducted only if performance during the two preceding training sessions met the criteria for accurate discrimination described above. All doses from one test drug were determined in one monkey before testing the next drug. Drug doses were counterbalanced between monkeys. Methamphetamine studies were conducted first and methamphetamine doses were determined twice. All other drug test sessions were determined once.
Drugs
(+)-Methamphetamine HCl, (±)-methylphenidate HCl, and (±)-mecamylamine HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). Bruce Blough (RTI) synthesized (±)-bupropion HCl, 2S,3S-hydroxybupropion HCl and 2R,3R-hydroxybupropion HCl, using previously described methods (Lukas et al., 2010). (−)-Nicotine hydrogen bitartrate salt (Sigma Aldrich, St. Louis, MO) was dissolved in sterile water and sodium hydroxide was added to the solution to reach a pH of approximately 5-7. Risperidone (Sigma Aldrich) was dissolved in 2% lactic acid (85% w/w; Sigma Aldrich) and sodium hydroxide was added to reach a pH of approximately 5-6. All other drugs, including varenicline tartrate (Sigma Aldrich) were dissolved in sterile water. All drug doses are expressed as the salt forms listed above, except for nicotine and risperidone, which were expressed as the base. Nicotine doses were expressed as the free base by multiplying the bitartrate dose by 0.325 (Matta et al., 2007).
Data Analysis
The primary dependent measures were (1) percent methamphetamine-appropriate responding (%MAR) {defined as (number of responses on the methamphetamine-associated key divided by the total number of responses on both the methamphetamine-and saline-associated keys)*100}, and (2) response rates during each component. These dependent measures were then plotted as a function of time after drug or saline administration. Percent MAR and response rates were analyzed using linear mixed-model analysis with drug dose and time as the main fixed effects and subjects as the random effect (JMP Pro 11.1.1, SAS, Cary, NC). A significant drug×time interaction or significant effect of drug dose was followed by the Dunnett’s post-hoc test for comparison to vehicle (saline) conditions.
Results
Discriminative stimulus effects of methamphetamine, risperidone, methylphenidate, and bupropion
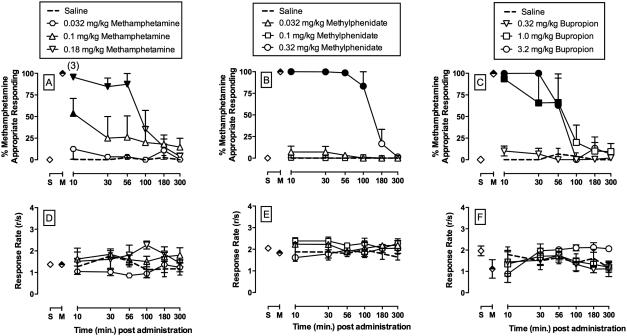
On all training days that preceded test days, monkeys responded almost exclusively on the methamphetamine-appropriate key during methamphetamine training components (mean ± SEM: 99.9 ± 0.1) and almost exclusively on the saline-appropriate key during saline training components (99.9 ± 0.1). Mean (± SEM) response rates were 1.7 (± 0.3) and 2.0 (± 0.3) responses per second during methamphetamine and saline training components, respectively. Figure 1A shows potency and time course of saline and methamphetamine to produce methamphetamine-appropriate responding. Saline administration produced ≤ 10% MAR at all time points and in all test sessions. 0.1 mg/kg methamphetamine produced full substitution, ≥90% MAR, in one monkey and 0.18 mg/kg methamphetamine produced full substitution in all 4 monkeys. Methamphetamine produced a dose- and time-dependent increase in %MAR (dose: F3,68.2=11.8, p<0.001; dose×time: F15,68=2.7, p<0.025). Methamphetamine had no significant effects on rates of operant responding at any time point (Figure 1D). In contrast to methamphetamine, 0.1 mg/kg risperidone produced < 10% MAR (data not shown) and significantly decreased rates of operant responding (risperidone dose: F2,34=4.2, p<0.025; data not shown).
Figure 1.
Potency and time course of the discriminative stimulus effects of (A, D) (+)-methamphetamine (0.01 – 0.18 mg/kg, i.m.), (B, E) (±)-methylphenidate (0.032 – 0.32 mg/kg, i.m.), and (C, F) (±)-bupropion (0.32 – 3.2 mg/kg, i.m.) in rhesus monkeys (n=3-4) trained to discrimination methamphetamine (0.18 mg/kg, i.m.) from saline. Upper vertical axes: percent methamphetamine-appropriate responding. Lower vertical axes : rates of responding in responses per second. Horizontal axes: time in min after injection. Symbols above “S” and “M” represent the group averages for all training sessions preceding test sessions when the saline- and methamphetamine-associated keys were correct, respectively. Filled symbols indicate statistical significance compared to saline within a given time point (p < 0.05). Numbers in parentheses indicate the number of subjects contributing to that data point if < 3 (methylphenidate or bupropion) or 4 (methamphetamine) subjects and indicative of a time point where a monkey failed to complete at least one ratio requirement during the response period.
Figure 1 also shows the potency and time course of methylphenidate (B, E) and bupropion (C, F) to produce methamphetamine-like discriminative stimulus effects. 0.32 mg/kg methylphenidate produced full methamphetamine-like effects and in all three monkeys and these methamphetamine-like effects were significant from 10-100 min (dose: F3,54 = 99.3, p < 0.001; dose×time: F18,54 = 23.7, p < 0.001). For bupropion, both 1.0 and 3.2 mg/kg produced full substitution in all 3 monkeys tested. Both bupropion doses produced a dose- and time-dependent increase in %MAR with significant effects up to 56 min (dose: F3,46=18.8, p<0.001; dose×time: F15,46=3.7, p<0.001). Figures 1E and 1F show that neither methylphenidate nor bupropion significantly altered rates of operant responding.
Discriminative stimulus effects of 2S-3S-hydroxybupropion and 2R,3R-hydroxybupropion
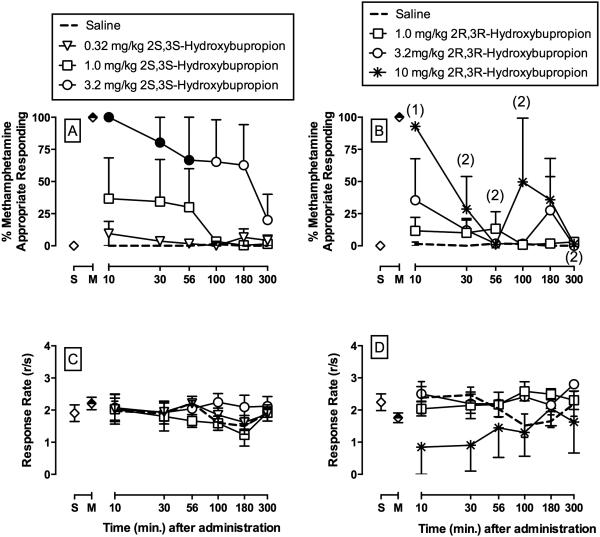
Figure 2 shows the potency and time course of 2S,3S–hydroxybupropion (A, C) and 2R,3R–hydroxybupropion (B, D) to produce methamphetamine-like discriminative stimulus effects. 1.0 mg/kg 2S,3S–hydroxybupropion produced full substitution in one monkey and 3.2 mg/kg 2S,3S–hydroxybupropion produced full substitution in all 3 monkeys. 2S,3S–hydroxybupropion produced a dose-dependent increase in %MAR with significant effects up to 56 min (dose: F3,46.8=8.5, p<0.001). For 2R,3R–hydroxybupropion, 3.2 mg/kg produced full substitution in one monkey and 10 mg/kg 2R,3R–hydroxybupropion produced full substitution in all 3 monkeys, although at different time points and perhaps as a consequence of rate decreasing effects in two out of three monkeys. Figure 2C shows 2S,3S –hydroxybupropion did not significantly alter rates of operant responding, whereas Figure 2D shows that 10 mg/kg 2R,3R–hydroxybupropion significantly (dose: F3,46=3.7, p<0.02) decreased rates of operant responding compared to saline.
Figure 2.
Potency and time course of the methamphetamine-like discriminative stimulus effects of (A, C) 2S,3S-hydroxybupropion (0.32 – 3.2 mg/kg, i.m.), and (B, D) 2R,3R-hydroxybupropion (1.0 – 10.0 mg/kg, i.m.) in rhesus monkeys (n=3) trained to discriminate methamphetamine (0.18 mg/kg, i.m.) from saline. Upper vertical axes : percent methamphetamine-appropriate responding. Lower vertical axes : rates of responding in responses per second. Horizontal axes: time in min after injection. Symbols above “S” and “M” represent the group averages for all training sessions preceding test sessions when the saline- and methamphetamine-associated keys were correct, respectively. Filled symbols indicate statistical significance compared to saline within a given time point (p < 0.05). Numbers in parentheses indicate the number of subjects contributing to that data point if < 3 subjects and indicative of a time point where a monkey failed to complete at least one ratio requirement during the response period.
Discriminative stimulus effects of mecamylamine, nicotine, and varenicline
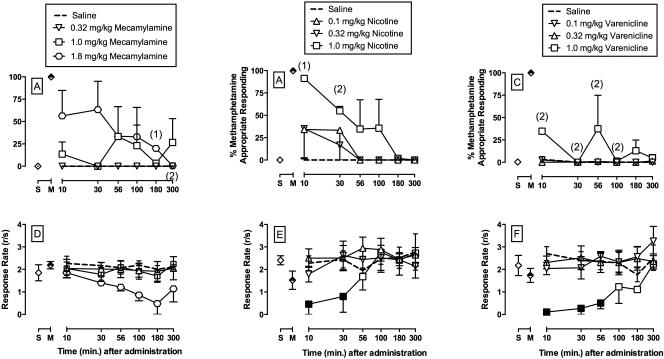
Figure 3 shows the potency and time course of (±)-mecamylamine (A, D), (−)-nicotine (B, E), and varenicline to produce methamphetamine-like discriminative-stimulus effects. 1.0 mg/kg mecamylamine produced full substitution in 1 out of 3 monkeys and 1.8 mg/kg produced full substitution in 2 out of 3 monkeys and partial substitution (73% MAR) in the third monkey. For nicotine, both 0.1 and 0.32 mg/kg produced full substitution for methamphetamine in 1 out of 3 monkeys and 1.0 mg/kg nicotine produced full methamphetamine-like discriminative stimulus effects in 2 out of 3 monkeys and partial substitution (50% MAR) in the third monkey. In addition, 1.0 mg/kg nicotine produced methamphetamine-appropriate responding that was significantly different from saline (dose: F3,45.6=4.3, p<0.01). In contrast to mecamylamine and nicotine, varenicline failed to produce full substitution at any dose, but 1.0 mg/kg did produce partial substitution in all three monkeys (maximum %MARs of 75, 36, and 37) and this varenicline effect was significantly different from saline (dose: F3,40.9=3.2, p<0.05). Figure 3D shows that lower, but not significant, rates of operant responding after 1.8 mg/kg mecamylamine. Figure 3E shows that 1.0 mg/kg nicotine significantly decreased rates of operant responding at 10 and 30 min compared to saline (dose: F3,46=12.9, p<0.001; dose×time: F15,46=3.9, p<0.001). Figure 3F shows that 1.0 mg/kg varenicline significantly decreased rates of operant responding from 10 to 56 min compared to saline (dose: F3,46=14.5, p<0.001; dose×time: F15,46=2.2, p<0.025).
Figure 3.
Potency and time course of the discriminative stimulus effects of and (A, D) (±)-mecamylamine (0.32 – 1.8 mg/kg, i.m.), (B, E) (−)-nicotine (0.1 – 1.0 mg/kg, i.m.), and (C, F) varenicline (0.1 – 1.0 mg/kg, IM) in rhesus monkeys (n=3) trained to discriminate methamphetamine (0.18 mg/kg, i.m.) from saline. Upper vertical axes : percent methamphetamine-appropriate responding. Lower vertical axes : rates of responding in responses per second. Horizontal axes: time in min after injection. Symbols above “S” and “M” represent the group averages for all training sessions preceding test sessions when the saline- and methamphetamine-associated keys were correct, respectively. Filled symbols indicate statistical significance compared to saline within a given time point (p < 0.05). Numbers in parentheses indicate the number of subjects contributing to that data point if < 3 subjects and indicative of a time point where a monkey failed to complete at least one ratio requirement during the response period.
Discussion
The aim of the present study was to determine the pharmacological mechanisms of the methamphetamine-like discriminative stimulus effects of bupropion in rhesus monkeys. There were two main findings. First, drugs that possessed DAT inhibition produced consistent, dose- and time-dependent methamphetamine-like discriminative stimulus effects. In contrast, compounds that only functioned as nACh receptor antagonists produced less consistent methamphetamine-like discriminative stimulus effects, and at doses that also generally decreased rates of operant responding. Consequently, these results cannot rule out a contributing role of nACh receptor antagonism in the methamphetamine-like discriminative stimulus effects of bupropion. A second main finding was that mecamylamine and nicotine produced qualitatively similar methamphetamine-like discriminative stimulus effects. Although the present results are inconsistent with previous methamphetamine discrimination results with mecamylamine and nicotine in rats (Desai and Bergman, 2010; Gatch et al., 2008), they are consistent with previous mecamylamine and nicotine results in rhesus monkeys trained to discriminate cocaine (Banks, 2014; Gould et al., 2011). Overall, these behavioral data suggest potential species differences in the shared pharmacological mechanisms between nACh receptor compounds and monoaminergic compounds, that remain to be fully elucidated.
The present study demonstrating that methamphetamine can be trained as a discriminative stimulus in rhesus monkeys confirms and extends previous results in pigeons (Sasaki et al., 1995), rodents (French and Witkin, 1993), squirrel monkeys (Desai and Bergman, 2014; Tidey and Bergman, 1998), and humans (Sevak et al., 2011). The time course of the methamphetamine-training dose in the present study is similar to previous time course results of a slightly larger methamphetamine-training dose in squirrel monkeys (Czoty et al., 2004a). Furthermore, the present risperidone (negative control) results are consistent with a previous human data demonstrating that risperidone does not produce methamphetamine-like subjective effects (Wachtel et al., 2002). Overall, these results provide an empirical framework for evaluation of the substitution profile of bupropion, its hydroxybupropion metabolites, and the relative contribution of DAT inhibition compared to nACh receptor antagonism to bupropion’s methamphetamine-like effects.
The present study was designed to examine mechanisms by which bupropion may produce an “agonist-like” methamphetamine effect. First, bupropion metabolism produces two hydroxybupropion metabolites that have distinct pharmacological activity to inhibit monoamine transporters and/or antagonize nACh receptors (Damaj et al., 2004; Lukas et al., 2010). If either bupropion metabolite contributed to the methamphetamine-like stimulus effects of bupropion, then we would predict that the metabolite would also produce methamphetamine-like effects and bupropion would potentially have a longer duration of action compared to the metabolite. Although both bupropion and 2S,3S–hydroxybupropion produced similar time courses of methamphetamine-like effects, suggesting that the contribution of 2S,3S–hydroxybupropion may be minimal, we cannot completely rule out a potential role of 2S,3S–hydroxybupropion, as the time to maximum concentration of hydroxybupropion following bupropion administration in nonhuman primates was approximately 30 min (Rytting et al., 2014). Future pharmacokinetic studies will be required to clarify the role of hydroxybupropion enantiomers in the behavioral effects of bupropion.
A second potential mechanism relates to the complex pharmacological profile of bupropion with affinity to inhibit DAT and NET, and to antagonize nACh receptors (Carroll et al., 2009). Although DAT inhibition is the presumed primary mechanism of the methamphetamine-like effects of bupropion, the relative contribution of nACh receptor antagonism to those effects has not been determined. Previous nonhuman primate methamphetamine discrimination studies have consistently demonstrated that NET inhibitors do not produce full substitution (Czoty et al., 2004b; Tidey and Bergman, 1998), and as a result of this literature, NET inhibitors were not examined in the present study. The substitution profile of the DAT inhibitors bupropion, 2S,3S–hydroxybupropion, and methylphenidate in the present study extends previous studies with other DAT inhibitors in both rodents (Desai et al., 2010) and monkeys (Czoty et al., 2004a,b; Desai and Bergman, 2014; Tidey and Bergman, 1998). The present results also extend previous results demonstrating that bupropion and 2S,3S–hydroxybupropion produced full substitution in amphetamine-trained rats (Bondarev et al., 2003) to methamphetamine-trained monkeys. Overall, the present results and the broader scientific literature cited support DAT inhibitors as candidate ‘agonist’ medications for methamphetamine addiction, based on shared discriminative stimulus effects. However, subchronic DAT inhibitor treatments have so far failed to attenuate methamphetamine vs. food choice in monkeys (Banks and Blough, 2015; Schwienteck and Banks, 2015) or human laboratory methamphetamine self-administration (Stoops et al., 2015), and do not produce reliable and robust decreases in methamphetamine use in double blind, placebo-controlled clinical trials (Elkashef et al., 2008; Miles et al., 2013). Thus, the utility of ‘agonist-based’ DAT inhibitor medications for methamphetamine addiction remains to be fully elucidated.
Unexpectedly, both mecamylamine and 2R,3R–hydroxybupropion produced full methamphetamine-like discriminative stimulus effects in at least two out of three monkeys. Although there was considerable individual subject variability in the time course of both compounds, most of the variability in %MAR could be accounted for by individual subjects’ sensitivity to the rate-decreasing effects produced by mecamylamine and 2R,3R–hydroxybupropion. The substitution profile of mecamylamine in the present study was inconsistent with previous mecamylamine results in methamphetamine-trained rats where mecamylamine produced saline-appropriate responding (Desai and Bergman, 2010). Furthermore, the substitution profile of 2R,3R–hydroxybupropion has not been examined in amphetamine- or methamphetamine-trained animals, to the best of our knowledge, and this prevents a comparison with the literature. However, the methamphetamine substitution profile of nACh receptor antagonists in the present study is consistent the substitution profile in rhesus monkeys trained to discriminate cocaine (Banks, 2014; Gould et al., 2011) and suggests potential species differences in the pharmacological activity of nicotinic compounds.
Also consistent with the hypothesis of potential species differences in the pharmacological activity of nicotinic compounds, differences were also noted with the present nicotine and varenicline results compared to previous results in rats (Desai and Bergman, 2010; Gatch et al., 2008) and squirrel monkeys (Desai and Bergman, 2014). In general, the substitution profile of nicotine and varenicline for the methamphetamine discriminative stimulus in rhesus monkeys was weaker compared to previous rat and squirrel monkey results. Similar species differences between rats (Desai et al., 2003) and rhesus monkeys (Banks, 2014; Gould et al., 2011; Mello and Newman, 2011) have also been reported for cocaine and nicotinic compounds. Overall, these behavioral results suggest that rhesus monkeys may exhibit decreased expression or function of nicotinic receptors compared to rats, which results in a more variable and weaker substitution profile of nicotinic agonists in monoaminergic discrimination procedures.
Acknowledgments
We acknowledge the technical assistance of Crystal Reyns and Kevin Costa for coding the original version of the behavioral program.
Funding Sources: Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Numbers R01DA031718 and R01DA012970. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: There are no existing or perceived conflicts for any author.
References
- Banks ML. Effects of the nicotinic acetylcholine receptor antagonist mecamylamine on the discriminative stimulus effects of cocaine in male rhesus monkeys. Exp Clin Psychopharmacol. 2014;22:266–273. doi: 10.1037/a0035274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE. Effects of environmental maniuplations and bupropion and risperidone treatments on choice between methamphetamine and food in rhesus monkeys. Neuropsychoharmacology. 2015;40:198–206. doi: 10.1038/npp.2015.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Smith DA, Kisor DF, Poklis JL. Relationship between discriminative stimulus effects and plasma methamphetamine and amphetamine levels of intramuscular methamphetamine in male rhesus monkeys. Pharmacol Biochem Behav. 2015 doi: 10.1016/j.pbb.2015.12.001. doi:10.1016/j.pbb.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besnard J, Ruda GF, Setola V, Abecassis K, Rodriguiz RM, Huang XP, et al. Automated design of ligands to polypharmacological profiles. Nature. 2012;492:215–220. doi: 10.1038/nature11691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarev ML, Bondareva TS, Young R, Glennon RA. Behavioral and biochemical investigations of bupropion metabolites. Eur J Pharmacol. 2003;474:85–93. doi: 10.1016/s0014-2999(03)02010-7. [DOI] [PubMed] [Google Scholar]
- Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine-type stimulant dependence: An update. Drug and Alcohol Rev. 2013;32:449–460. doi: 10.1111/dar.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll FI, Blough BE, Abraham P, Mills AC, Holleman JA, Wolckenhauer SA, et al. Synthesis and biological evaluation of bupropion analogues as potential pharmacotherapies for cocaine addiction. J Med Chem. 2009;52:6768–6781. doi: 10.1021/jm901189z. [DOI] [PubMed] [Google Scholar]
- Courtney KE, Ray LA. Methamphetamine: An update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend. 2014;143:11–21. doi: 10.1016/j.drugalcdep.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Makriyannis A, Bergman J. Methamphetamine discrimination and in vivo microdialysis in squirrel monkeys. Psychopharmacology. 2004a;175:170–178. doi: 10.1007/s00213-004-1798-6. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ramanathan CR, Mutschler NH, Makriyannis A, Bergman J. Drug discrimination in methamphetamine-trained monkeys: Effects of monoamine transporter inhibitors. J Pharmacol Exp Ther. 2004b;311:720–727. doi: 10.1124/jpet.104.071035. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Carroll FI, Eaton JB, Navarro HA, Blough BE, Mirza S, et al. Enantioselective effects of hydroxy metabolites of bupropion on behavior and on function of monoamine transporters and nicotinic receptors. Mol Pharmacol. 2004;66:675–682. doi: 10.1124/mol.104.001313. [DOI] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Drug discrimination in methamphetamine-trained rats: Effects of cholinergic nicotinic compounds. J Pharmacol Exp Ther. 2010;335:807–816. doi: 10.1124/jpet.110.173773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Bergman J. Methamphetamine-like discriminative-stimulus effects of nicotinic agonists. J Pharmacol Exp Ther. 2014;348:478–488. doi: 10.1124/jpet.113.211235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai R, Barber D, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology. 2003;167:335–343. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, Desai R, Bergman J. Monoaminergic psychomotor stimulants: Discriminative stimulus effects and dopamine efflux. J Pharmacol Exp Ther. 2010;333:834–843. doi: 10.1124/jpet.110.165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drug Enforcement Administration . National Forensic Laboratory Information System Special Report: Synthetic cannabinoids and synthetic cathinones reported in NFLIS, 2010-2013. Office of Diversion Control, Department of Justice. Drug Enforcement Administration; Springfiled, VA: 2014. [Google Scholar]
- Elkashef AM, Rawson RA, Anderson AL, Li SH, Holmes T, Smith EV, et al. Bupropion for the Treatment of Methamphetamine Dependence. Neuropsychopharmacology. 2008;33:1162–1170. doi: 10.1038/sj.npp.1301481. [DOI] [PubMed] [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- French D, Witkin JM. Effects of the dopamine release inhibitor, CGS 10746B, on the locomotor stimulant and discriminative stimulus effects of cocaine and methamphetamine. Pharmacol Biochem Behav. 1993;46:989–993. doi: 10.1016/0091-3057(93)90233-j. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Lukas RJ. Noncompetitive functional inhibition at diverse, human nicotinic acetylcholine receptor subtypes by bupropion, phencyclidine, and ibogaine. J Pharmacol Exp Ther. 1999;288:88–92. [PubMed] [Google Scholar]
- Garcia-Ratés S, Camarasa J, Escubedo E, Pubill D. Methamphetamine and 3,4-methylenedioxymethamphetamine interact with central nicotinic receptors and induce their up-regulation. Toxicol Appl Pharmacol. 2007;223:195–205. doi: 10.1016/j.taap.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Flores E, Forster MJ. Nicotine and methamphetamine share discriminative stimulus effects. Drug Alcohol Depend. 2008;93:63–71. doi: 10.1016/j.drugalcdep.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Nader SH, Nader MA. Effects of varenicline on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 2011;339:678–686. doi: 10.1124/jpet.111.185538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant KA. Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:261–267. doi: 10.1016/s0091-3057(99)00075-1. [DOI] [PubMed] [Google Scholar]
- Horton DB, Potter DM, Mead AN. A translational pharmacology approach to understanding the predictive value of abuse potential assessments. Behav Pharmacol. 2013;24:410–436. doi: 10.1097/FBP.0b013e3283644d2e. [DOI] [PubMed] [Google Scholar]
- Kamien JB, Woolverton WL. A pharmacological analysis of the discriminative stimulus properties of d-amphetamine in rhesus monkeys. J Pharmacol Exp Ther. 1989;248:938–946. [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990;254:312–317. [PubMed] [Google Scholar]
- Lelas S, Spealman RD, Rowlett JK. Using behavior to elucidate receptor mechanisms: A review of the discriminative stimulus effects of benzodiazepines. Exp Clin Psychopharmacol. 2000;8:294–311. doi: 10.1037//1064-1297.8.3.294. [DOI] [PubMed] [Google Scholar]
- Li M, McMillan DE. Retention of sequential drug discriminations under fixed-interval schedules for long time periods without training. Eur J Pharmacol. 2003;476:79–85. doi: 10.1016/s0014-2999(03)02150-2. [DOI] [PubMed] [Google Scholar]
- Liu PS, Liaw CT, Lin MK, Shin SH, Kao LS, Lin LS. Amphetamine enhances Ca2+ entry and catecholamine release via nicotinic receptor activation in bovine adrenal chromaffin cells. Eur J Pharmacol. 2003;460:9–17. doi: 10.1016/s0014-2999(02)02870-4. [DOI] [PubMed] [Google Scholar]
- Lukas RJ, Muresan AZ, Damaj MI, Blough BE, Huang X, Navarro HA, et al. Synthesis and characterization of in vitro and in vivo profiles of hydroxybupropion analogues: Aids to smoking cessation. J Med Chem. 2010;53:4731–4748. doi: 10.1021/jm1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology. 2007;190:269–319. doi: 10.1007/s00213-006-0441-0. [DOI] [PubMed] [Google Scholar]
- McMillan DE, Sun WL, Hardwick WC. Effects of drug discrimination history on the generalization of pentobarbital to other drugs. J Pharmacol Exp Ther. 1996;278:50–61. [PubMed] [Google Scholar]
- Mello NK, Newman JL. Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine + nicotine combinations in rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:203–214. doi: 10.1037/a0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles SW, Sheridan J, Russell B, Kydd R, Wheeler A, Walters C, et al. Extended-release methylphenidate for treatment of amphetamine/methamphetamine dependence: a randomized, double-blind, placebo-controlled trial. Addiction. 2013;108:1279–1286. doi: 10.1111/add.12109. [DOI] [PubMed] [Google Scholar]
- Munzar P, Goldberg SR. Dopaminergic involvement in the discriminative-stimulus effects of methamphetamine in rats. Psychopharmacology. 2000;148:209–216. doi: 10.1007/s002130050044. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Blough BE, Baumann MH, Rothman RB. Monoamine releasers with varying selectivity for dopamine/norepinephrine versus serotonin release as candidate "agonist" medications for cocaine dependence: Studies in assays of cocaine discrimination and cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:627–636. doi: 10.1124/jpet.106.107383. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, III, Fong T, Wallace CL, Li SH, et al. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–1544. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Rau KS, Birdsall E, Hanson JE, Johnson-Davis KL, Carroll FI, Wilkins DG, et al. Bupropion increases striatal vesicular monoamine transport. Neuropharmacology. 2005;49:820–830. doi: 10.1016/j.neuropharm.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Rytting E, Wang X, Vernikovskaya DI, Zhan Y, Bauer C, Abdel-Rahman SM, et al. Metabolism and disposition of bupropion in pregnant baboons (Papio cynocephalus) Drug Metab Dispos. 2014;42:1773–1779. doi: 10.1124/dmd.114.058255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki JE, Tatham TA, Barrett JE. The discriminative stimulus effects of methamphetamine in pigeons. Psychopharmacology. 1995;120:303–310. doi: 10.1007/BF02311178. [DOI] [PubMed] [Google Scholar]
- Schuster CR, Johanson CE. Relationship between the discriminative stimulus properties and subjective effects of drugs. Psychopharmacol Ser. 1988;4:161–175. doi: 10.1007/978-3-642-73223-2_13. [DOI] [PubMed] [Google Scholar]
- Schwienteck KL, Banks ML. Effects of continuous 7-day d-amphetamine, methylphenidate, and cocaine treatment on choice between methamphetamine and food in male rhesus monkeys. Drug Alcohol Depend. 2015;155:16–23. doi: 10.1016/j.drugalcdep.2015.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevak RJ, Vansickel AR, Stoops WW, Glaser PE, Hays LR, Rush CR. Discriminative-stimulus, subject-rated, and physiological effects of methamphetamine in humans pretreated with aripiprazole. J Clin Psychopharmacol. 2011;31:470–480. doi: 10.1097/JCP.0b013e318221b2db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmler LD, Wandeler R, Liechti ME. Bupropion, methylphenidate, and 3,4-methylenedioxypyrovalerone antagonize methamphetamine-induced efflux of dopamine according to their potencies as dopamine uptake inhibitors: implications for the treatment of methamphetamine dependence. BMC Research Notes. 2013;6:1–5. doi: 10.1186/1756-0500-6-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Pike E, Hays LR, Glaser PE, Rush CR. Naltrexone and bupropion, alone or combined, do not alter the reinforcing effects of intranasal methamphetamine. Pharmacol Biochem Behav. 2015;129:45–50. doi: 10.1016/j.pbb.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Bergman J. Drug discrimination in methamphetamine-trained monkeys: Agonist and antagonist effects of dopaminergic drugs. J Pharmacol Exp Ther. 1998;285:1163–1174. [PubMed] [Google Scholar]
- Wachtel SR, Ortengren A, de Wit H. The effects of acute haloperidol or risperidone on subjective responses to methamphetamine in healthy volunteers. Drug Alcohol Depend. 2002;68:23–33. doi: 10.1016/s0376-8716(02)00104-7. [DOI] [PubMed] [Google Scholar]
- Woods JH, Bertalmio AJ, Young AM, Essman WD, Winger G. Receptor mechanisms of opioid drug discrimination. Psychopharmacol Ser. 1988;4:95–106. doi: 10.1007/978-3-642-73223-2_8. [DOI] [PubMed] [Google Scholar]