Abstract
Yin Yang 1 (YY1) is a zinc finger protein that functions as a transcriptional activator or repressor and participates in multiple biological processes, including development and tumorigenesis. To investigate the role of YY1 in developing T cells, we used mouse models that depleted YY1 at two distinct stages of thymocyte development. When YY1 was depleted in CD4−CD8− double negative (DN) thymocytes, development to the CD4+CD8+ double positive (DP) stage was impaired, due to increased apoptosis that prevented expansion of post–β-selection thymocytes. When YY1 was depleted in DP thymocytes, they underwent increased cell-autonomous apoptosis in vitro and displayed a shorter lifespan in vivo, as judged by their ability to undergo secondary Vα-to-Jα recombination. Mechanistically, we found that the increased apoptosis in YY1-deficient thymocytes was attributed to overexpression of p53, because concurrent loss of p53 completely rescued the developmental defects of YY1-deficient thymocytes. These results indicated that YY1 functions as a critical regulator of thymocyte survival and that it does so by suppressing the expression of p53.
Introduction
Effective T cell adaptive immunity depends upon efficient generation of T cells from intrathymic progenitors. CD4−CD8− double negative (DN) thymocytes serve as precursors for both αβ and γδ T cells. DN thymocytes can be subdivided into four stages: DN1 (CD25−CD44+, which includes pluripotent early thymic precursors), DN2 (CD25+CD44+), DN3 (CD25+CD44−), and DN4 (CD25−CD44−). DN1 and DN2 thymocytes proliferate extensively in a TCR-independent, Notch-dependent manner before progression to the DN3 stage (1). DN3 cells can be further divided into DN3a and DN3b based on cell size and CD27 expression (2). During the DN3a stage, most cells become quiescent and undergo V(D)J recombination at the Tcrg, Tcrd, and Tcrb loci (3, 4). Thymocytes with in-frame rearrangements of Tcrg and Tcrd express the γδ TCR, and may commit to the γδ lineage and maintain a DN phenotype. Cells that successfully rearrange Tcrb produce a functional TCRβ protein, which can assemble with pTα and CD3 proteins to form pre-TCRs (1, 5). Expression of the pre-TCR drives a burst of proliferation and allows cells to progress from DN3a to DN3b in a process called β-selection (5). Only cells that pass the β-selection checkpoint develop through the DN4 and immature single-positive stages into CD4+CD8+ double-positive (DP) thymocytes. This developmental progression represents the hallmark of αβ T cell lineage commitment.
Failure to assemble a functional pre-TCR complex, as occurs in mice that are deficient for RAG1, RAG2, pre-Tα or CD3γ, leads to a severe block of αβ T cell development at the DN stage (5–8). Signals that rescue thymocytes from death and promote their proliferation are critical for β-selection. Known trophic signals for thymocytes at the β-selection checkpoint include those generated by the pre-TCR, Notch and the IL-7 receptor (9, 10). Notch promotes thymocyte survival by regulating glucose metabolism (9). The pro-apoptotic factor p53 has been suggested to eliminate thymocytes that fail to pass β-selection, because the concurrent loss of p53 can rescue developmental defects in pre-TCR-deficient mice (6, 7, 11). However, the mechanisms underlying p53 regulation during thymocyte development are not fully understood.
Regulated cell survival and apoptosis are also critical for the proper development of DP thymocytes. As thymocytes develop to the DP stage, they stop proliferating and survive for an average of 3–4 days. During this time, DP thymocytes undergo multiple rounds of Vα-to-Jα rearrangements, with Jα segments used sequentially from the 5’ end to the 3’ end of the Jα array (12). Because the lifespan of DP thymocytes impacts the progression of Vα-to-Jα rearrangements and positive selection of T cells, factors that regulate the survival of DP thymocytes (e.g., RORγ, an orphan nuclear receptor, and Bcl-xL, an anti-apoptotic Bcl-2 family protein) are essential regulators of TCR repertoire diversity (13, 14). Depleting RORγ shortens the lifespan of DP thymocytes and limits Vα-to-Jα rearrangements to the most 5’Jα segments, whereas extending the lifespan of DP thymocytes with a Bcl-xL transgene skews Vα-to-Jα rearrangement towards 3’Jα segments (14).
Yin Yang 1 (YY1) is a ubiquitously expressed, multi-functional transcription factor, which can activate or repress transcription through interactions with other transcriptional regulators (15). YY1 has been shown to regulate multiple physiological processes including embryogenesis, differentiation and cellular proliferation (16–22). YY1 also functions as potential tumor suppressor, because it can negatively regulate p53 (23, 24). In this regard, YY1 expression is elevated in various types of cancer (25). Although numerous studies have been devoted to understanding the roles of YY1 in B cell development and V(D)J recombination of the Igh and Igk loci (18, 21, 26–29), studies of YY1 in T-lineage cells have been limited to its role in regulating Th2 cytokine production (30).
To investigate the role of YY1 in early T cell development, we conditionally deleted YY1 in developing thymocytes. We found that early ablation of YY1 caused severe developmental defects in the DN compartment due to a dramatic increase in DN thymocyte apoptosis. Furthermore, YY1 emerged as a novel regulator of the lifespan of DP thymocytes, because late ablation of YY1 resulted in increased apoptosis of DP thymocytes and a restricted TCRα repertoire. Mechanistically, we showed that p53 was upregulated in both DN and DP YY1-deficient thymocytes. Eliminating p53 in YY1-deficient thymocytes rescued the survival and developmental defects, indicating that these YY1-dependent defects were p53-mediated. We conclude that YY1 is required to maintain cell viability during thymocyte development by thwarting the accumulation of p53.
Materials and Methods
Mice
All mice were used in accordance with protocols approved by the Duke University Animal Care and Use Committee. Yy1f/f mice (B6;129S4-Yy1tm2Yshi/J) (16) obtained from The Jackson Laboratory were bred with Lck-Cre transgenic mice (B6.Cg-Tg(Lck-cre) 548Jxm/J), a gift from J. Rathmell (Duke University) (31), to generate Yy1f/f Lck-Cre mice and were further bred with Rag2−/− mice to generate Yy1f/f Lck-Cre Rag2−/−mice. Yy1f/f CD2-Cre mice were a gift from A. Feeney (The Scripps Research Institute) and were further bred with Rag2−/− mice to generate Yy1f/f CD2-Cre Rag2−/− mice. Trp53−/− mice (B6.129S2-Trp53tm1Tyj/J) were a gift from D. L. Silver (Duke University) and were bred with Yy1f/f CD2-Cre mice to generate Yy1f/f CD2-Cre Trp53−/− mice. The genetic background of mice used for experiments was a mixture of 129 and C57BL/6. Mice were analyzed at 4–5 weeks of age; those designated as wild-type carried floxed Yy1 alleles but lacked Cre recombinase expression.
Flow cytometry and cell sorting
All reagents were purchased from Biolegend unless otherwise indicated. To sort DN3 thymocytes, total thymocytes were stained with anti-CD4 (GK1.5) and anti-CD8 (53–6.7), and sheep anti-rat IgG Dynabeads (Life Technologies) were used to remove CD4+ and CD8+ thymocytes. DN cells were then stained with 7-aminoactinomycin D (7AAD) and antibodies against CD44 (IM7), CD25 (PC61), and lineage (Lin) markers Gr-1 (RB6-8C5), CD3ε (145-2C11), Ter119 (TER-119), and CD11b (M1/70). 7AAD−CD25+CD44−Lin− cells were isolated by cell sorting and used for further analysis. To separate DN3a from DN3b thymocytes, the DN thymocytes were also stained with anti-CD28 (37.51). For intracellular staining with anti-TCRβ (H57-597) or anti-YY1 (H-414), cells were first stained with antibodies against surface markers before fixation and permeabilization (BD Cytofix/Cytoperm™ Kit).
Anti-CD3ε treatment
Mice were injected i.p. with 150 μl of 1 mg/ml anti-CD3ε (145-2C11) or with an equal volume of PBS as previously described (32). Mice were euthanized 9 days after injection for isolation of DP thymocytes by cell sorting.
BrdU assay
Mice were injected i.p. with 1 mg BrdU at 2 or 4 hours prior to analysis. BrdU incorporation was detected by intracellular staining (FITC BrdU Flow kit; BD Pharmingen).
OP9-DL1 culture
OP9-DL1 co-cultures were carried out as previously described (33). DN3a (Lin−CD25+CD44loCD28lo forward scatterhi) or DN3b (Lin−CD25+CD44loCD28hi forward scatterlo) thymocytes were sorted and stained with Celltrace Violet (Life Technologies) and were then placed on OP9-DL1 monolayers with 5 ng/ml IL-7. Cells were harvested on days 2, 3 and 4 to measure dilution of Celltrace Violet and expression of CD4, CD8 and CD25. Apoptotic cells were assayed by staining with Annexin V (Biolegend) on day 4.
Western blot
Antibodies specific for YY1 (H-414, Santa Cruz), p53 (1C12, Cell Signaling Technology), Bcl-xL (54H6, Cell Signaling Technology), Bim (559685, BD Biosciences), caspase-3 (8G10, Cell Signaling Technology), cleaved caspase-3 (5A1E, Cell Signaling Technology) and actin (I-19, Santa Cruz) were used according to the manufacturer's instructions.
PCR analysis of recombination and transcription
Tcra and Tcrb rearrangements were analyzed in genomic DNA isolated from sorted DP thymocytes and sorted intracellular (ic)TCRβ+ thymocytes, respectively. Analysis of Cd14 was used for normalization. PCR primers are listed in Supplemental Table 1 or were described previously (34, 35). Tcra rearrangements were quantified by SYBR Green real-time PCR and Tcrb rearrangements were quantified by Taqman real-time PCR; conditions for both PCR reactions were described previously (36). Jα usage was also analyzed in cDNA prepared from total thymocytes by PCR with Trav12 and Cα primers using the following program: 94°C for 2 min, 33–35 cycles of 92°C for 30 s, 55°C for 30 s and 72°C for 30 s, and 72°C for 4 minutes. PCR products were gel-purified, cloned with a TOPO TA Cloning Kit for Sequencing (Life Technologies), and sequenced using an internal Cα primer.
To analyze gene expression, total RNA was extracted with TRIzol reagent (Life Technologies) and reverse transcribed with SuperScript III First-Strand Synthesis System cDNA kit (Life Technologies) according to the manufacturer’s instruction. Amplification of Hprt or Gapdh was used for normalization. SYBR Green real-time PCR was conducted using PCR primers listed in Supplemental Table 1.
Statistics
Statistical analyses were performed using Graphpad Prism 6.0 software.
Results
Early ablation of YY1 severely blocks DN thymocyte development
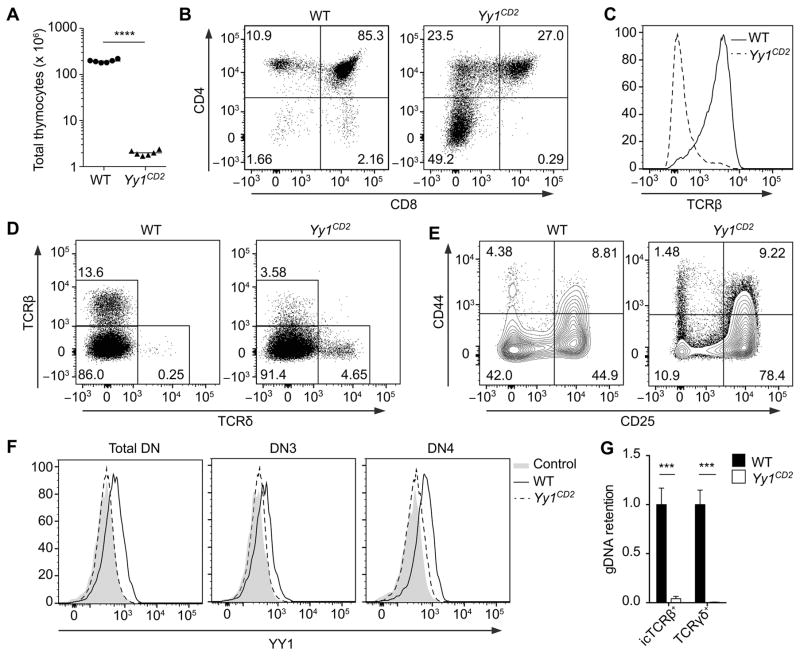
To elucidate the role of YY1 in early T cell development, we analyzed Yy1f/f mice expressing a human CD2-Cre transgene. The human CD2-Cre transgene is active in common lymphoid progenitors (37) and should promote Yy1 deletion in all T-lineage cells. These mice are hereafter referred to as Yy1CD2 mice. Yy1CD2 mice displayed a profound loss in thymocyte number, with thymus cellularity reduced to 1% of that of their wild-type (Yy1f/f) littermates (Fig. 1A). Although all thymocyte subsets were reduced in number (Supplemental Fig. 1A), there was a dramatic increase in the proportion of DN thymocytes relative to DP thymocytes (Fig. 1B, Supplemental Fig. 1A), suggesting that the progression from the DN to the DP stage was compromised. The proportion of CD4+ thymocytes was increased in Yy1CD2 mice (Fig. 1B). However, these thymocytes did not express a surface TCRβ chain, indicating that they were immature (Fig. 1C). Because a similar population of CD4+ thymocytes was not detected in Rag2−/−Yy1CD2 mice (Supplemental Fig. 1B), the population detected in Yy1CD2 mice must represent bonafide, post–β-selection immature single positive thymocytes, rather than pre-β-selection thymocytes with dysregulated CD4 expression.
Figure 1. Early ablation of Yy1 severely blocks T cell development.
(A) Number of total thymocytes in Yy1f/f (WT) and Yy1f/f CD2-Cre (Yy1CD2) mice. Each data point represents an individual mouse and the horizontal line indicates the mean. Statistical significance was evaluated by unpaired Student’s t-test. (B–F) Flow cytometry analysis of thymocytes from WT and Yy1CD2 littermates. (B) CD4 and CD8 staining is shown for total thymocytes. (C) TCRβ staining is shown for pre-gated CD4+CD8− thymocytes. (D) TCRβ and TCRδ staining is shown for total thymocytes. (E) CD44 and CD25 staining is shown for pre-gated CD4−CD8−Lin− thymocytes. (F) Intracellular staining of YY1 in pre-gated DN (CD4−CD8−Lin−), DN3 (CD4−CD8−Lin−CD25+CD44−) and DN4 (CD4−CD8−Lin−CD25−CD44−) thymocytes. The control consists of WT thymocytes incubated with anti-YY1 without fluorescent secondary antibody. Data are representative of three (B–E) or two (F) independent experiments. (G) Genomic DNA was extracted from sorted icTCRβ+ or TCRγδ+ thymocytes and deletion of Yy1 exon1 was measured by real-time PCR with normalization to Cd14. Data represent the mean ± SEM of 3 samples for each genotype. Statistical significance was evaluated by unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. ***P < 0.001, ****P < 0.0001.
Consistent with impaired development of αβ-lineage precursors to the more mature DP and single positive stages, TCRβ-expressing thymocytes were significantly reduced in Yy1CD2 mice (Fig. 1D). In contrast, the percentage of γδ T cells was substantially increased in Yy1CD2 mice compared with wild-type littermates, indicating that the developmental defect was restricted to the αβ-lineage (Fig. 1D). Further delineation of DN thymocyte populations showed that the percentage of DN3 (CD25+CD44−) thymocytes was increased in Yy1CD2 mice, whereas the percentage of DN4 (CD25−CD44−) thymocytes was markedly reduced (Fig. 1E). To exclude the possibility that the residual presence of DN4 thymocytes in Yy1CD2 mice was due to incomplete deletion of Yy1, YY1 expression was measured by intracellular (ic) staining in DN3 and DN4 thymocytes from wild-type and Yy1CD2 mice. YY1 protein was substantially reduced in both DN3 and DN4 thymocytes from Yy1CD2 mice (Fig. 1F). Consistent with this, only 4% of Yy1 alleles were intact in icTCRβ+ thymocytes (Fig.1G). Moreover, intact Yy1 alleles were essentially undetectable in TCRγδ + thymocytes (Fig. 1G). Hence, efficient γδ-lineage development and partial αβ-lineage development can occur in the absence of YY1. Taken together, our results demonstrated that Yy1CD2 thymocytes have a severe, αβ-lineage–specific developmental defect, which impairs the DN3-to-DN4-to-DP progression of thymocytes.
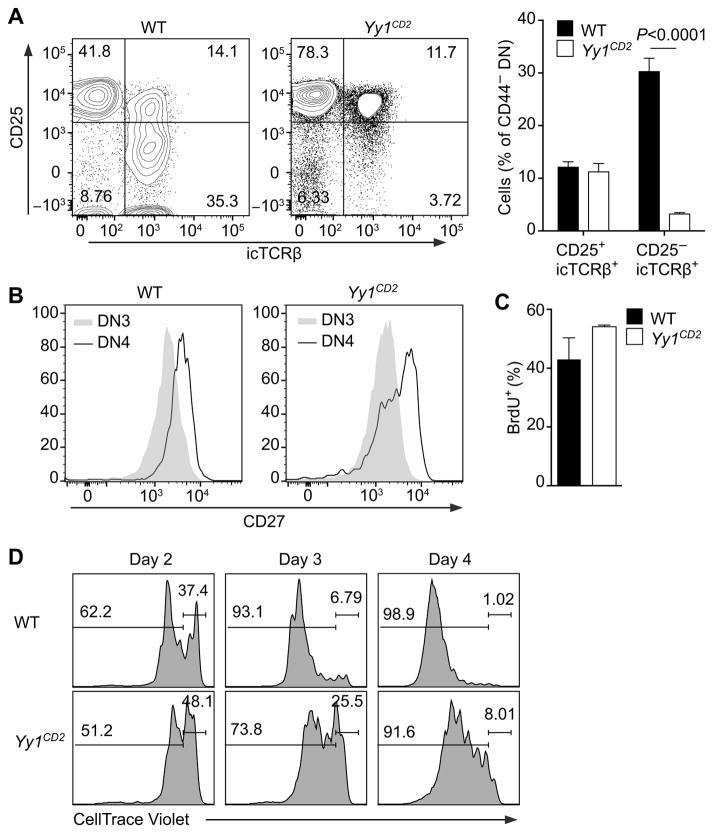
A developmental defect at the β-selection checkpoint could reflect impaired Tcrb rearrangement in Yy1CD2 mice. However, a substantial proportion of DN3 thymocytes expressed icTCRβ protein in Yy1CD2 mice (Fig. 2A) and the TCRβ repertoire was minimally altered in these mice (Supplemental Fig. 1C). We then asked whether DN4 thymocytes in Yy1CD2 mice had undergone a normal process of β-selection. We tested CD27 expression in DN3 and DN4 thymocytes, because upregulation of CD27 during the DN3-to-DN4 transition marks cells that pass the β-selection checkpoint (2). Although the mean fluorescence intensity of CD27 in Yy1CD2 DN4 thymocytes was lower than in wild-type thymocytes (Fig. 2B), a substantial portion of Yy1CD2 DN4 thymocytes had appropriately upregulated CD27, indicating that they represented bona-fide post–β-selection thymocytes. To rule out a possible defect in pre-TCR–driven proliferation, Yy1CD2 mice and their wild-type littermates were pulsed with BrdU for 2 hours, and incorporation of BrdU into proliferating cells was measured by flow cytometry. Proliferating icTCRβ+ DN thymocytes were slightly more abundant in Yy1CD2 mice than in wild-type mice (Fig. 2C), perhaps reflecting a compensatory mechanism in the face of reduced cellularity. Nevertheless, this result indicated that the proliferative capacity of pre-TCR competent thymocytes was not impaired by loss of YY1. To further examine the dynamics of cell proliferation, purified DN3 thymocytes were stained with Celltrace Violet and co-cultured with OP9-Delta-like 1 (OP9-DL1) stromal cells, which provided the Notch signaling required for thymocyte development (38). Yy1CD2 DN3 thymocytes proliferated with slower dynamics as compared to wild-type DN3 thymocytes (Fig. 2D). This could reflect a primary defect in proliferation, or alternatively, higher cell death during proliferation, resulting in lower cell numbers in successive generations.
Figure 2. β-selection in YY1-deficient mice.
(A) icTCRβ staining is shown for pregated CD4−CD8−Lin−CD44− (DN3 and DN4) thymocytes of Yy1f/f (WT) and Yy1f/f CD2-Cre (Yy1CD2) mice (left panels). Mean ± SEM of three independent experiments (right panel). Statistical significance was evaluated by two-way ANOVA with Sidak’s multiple-comparison test. (B) Cell surface expression of CD27 analyzed in pregated DN3 and DN4 thymocytes. Data are representative of two independent experiments. (C) WT and Yy1CD2 mice were pulsed with BrdU for 2 h and the percentage of BrdU+ cells was measured in icTCRβ+ DN thymocytes. Data are presented as the mean ± SEM of two independent experiments. (D) sorted DN3a thymocytes were stained with Celltrace Violet and cultured on OP9-DL1 stromal cells (Day 0). The dilution of Celltrace Violet was measured at the indicated time points. Data are representative of three independent experiments.
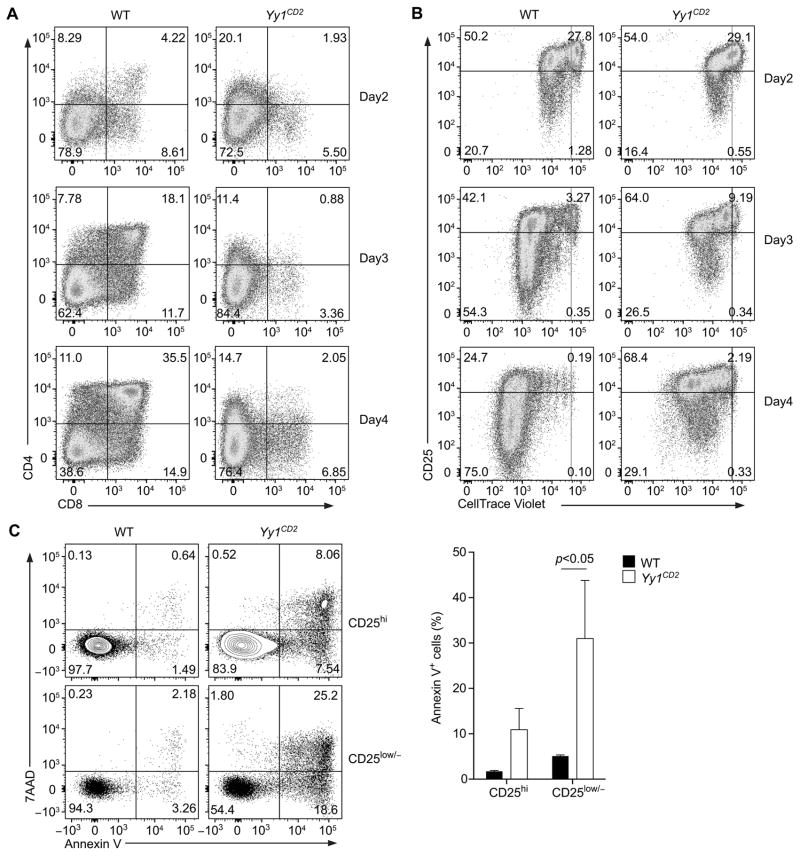
To test whether increased cell death was responsible for the developmental defect in Yy1CD2 mice, we analyzed the viability of YY1-deficient thymocytes in vitro, because apoptotic thymocytes are often undetected in vivo due to clearance by phagocytosis (39). Pre-β-selection DN3a thymocytes (7AAD−CD25+CD44−Lin−CD28−) were sorted as previously described (40,41), stained with Celltrace Violet, and co-cultured with OP9-DL1 cells. After 4 days in culture, DN3a cells from wild-type mice developed into DP cells (Fig. 3A). In contrast, DN3a cells from Yy1CD2 mice failed to generate DP cells (Fig. 3A), in accord with the developmental defects observed in vivo (Fig. 1B). During the same time-frame, wild-type DN3a thymocytes proliferated vigorously, as determined by dilution of Celltrace Violet, and gradually downregulated CD25 expression as they proliferated (Fig. 3B), consistent with previous analysis (40). By day 4, 75% of the cells had downregulated CD25 expression, indicating that these cells had adopted a DN4-DP phenotype (Fig. 3B). In contrast, although DN3a thymocytes from Yy1CD2 mice proliferated, fewer CD25low/− cells were generated (Fig. 3B).
Figure 3. Increased cell death in YY1-deficient DN thymocytes.
Sorted DN3a thymocytes from Yy1f/f (WT) and Yy1f/f CD2-Cre (Yy1CD2) mice were labeled with Celltrace Violet and placed in OP9-DL1 co-cultures. CD4 and CD8 expression (A) and CD25 expression and dilution of Celltrace Violet (B) were analyzed at the indicated time points. The results are representative of three independent experiments. (C) Annexin V and 7AAD staining of CD25hi and CD25low/− proliferating cells (a combination of the left upper and lower quadrants in (B)) at day 4 of culture (left). Mean ± SEM of two independent experiments analyzing the results of three WT and two Yy1CD2 cultures (right). Statistical significance was evaluated by two-way ANOVA with Sidak’s multiple-comparison test.
To further analyze population dynamics, thymocytes were harvest from culture on day 4, and apoptosis was assayed in gated proliferating cells. The percentages of early apoptotic cells (Annexin V+7AAD−) and late apoptotic cells (Annexin V+7AAD+) were determined in both the CD25hi and CD25low/− populations. Apoptosis was higher in Yy1CD2 than in wild-type thymocytes, particularly in the CD25low/− population (Fig. 3C). To rule out the possibility that defective differentiation and survival in these assay was an artifact of β-selection in vitro, we sorted post–β-selection DN3b thymocytes (7AAD−CD25+CD44−Lin−CD28+) and subjected them to the same OP9-DL1 co-culture conditions. As expected (40), wild-type DN3b cells differentiated into DP thymocytes more rapidly and proliferated more vigorously in the co-cultures than did DN3a cells (Supplemental Fig. 2A,B). However, similar to Yy1CD2 DN3a thymocytes, Yy1CD2 DN3b thymocytes desplayed defects in DN-to-DP differentiation and increased apoptosis (Supplemental Fig. 2A-C). These results suggested that the developmental defects of Yy1CD2 DN thymocytes were instrinsic properties of post–β-selection thymocytes. Together, these data indicate that YY1 is required for normal thymocyte development because it protects proliferating DN4 thymocytes from apoptosis.
YY1 is required for the normal life span of DP thymocytes
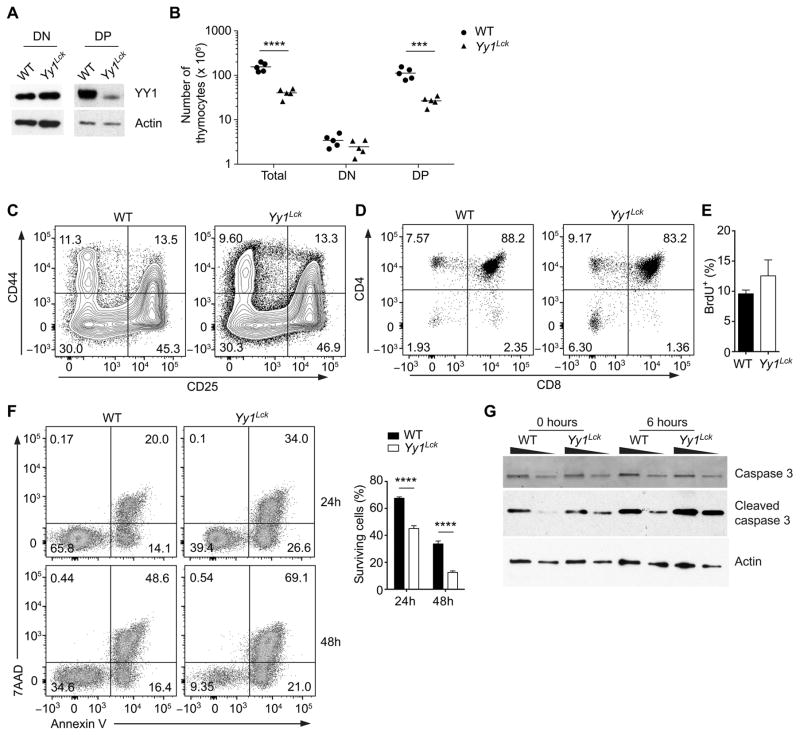
We next investigated whether YY1 regulates cell death in DP thymocytes. Yy1f/f mice were crossed with Lck-Cre transgenic mice to generate Yy1Lck mice. Although Lck-Cre has been reported to be active in DN2 and DN3 thymocytes (42), YY1 protein was not substantially depleted until the DP stage in Yy1Lck mice (Fig. 4A). We found that Yy1Lck mice possessed 30% of the total thymocytes detected in their wild-type littermates (Fig. 4B), with normal numbers of DN thymocytes and a normal DN1-to-DN4 progression (Fig. 4B, C). However, the percentage of DN thymocytes was higher in Yy1Lck mice than in wild-type mice (Fig. 4D), and the absolute number of DP thymocytes was significantly reduced (Fig. 4B). To address whether the loss of DP thymocytes was due to a lower replenishment from proliferating precursors, we tracked DP thymocytes with a recent history of proliferation by labeling with BrdU in vivo. Four hours after BrdU injection, the percentage of BrdU+ DP thymocytes in Yy1Lck mice was comparable to that in wild-type mice (Fig. 4E). Thus, YY1 deficiency significantly decreased the number of DP thymocytes but did not affect the generation of DP thymocytes from proliferating precursors. To elucidate whether the DP thymocytes in Yy1Lck mice were reduced because of a survival defect, we cultured wild-type and Yy1Lck DP thymocytes in vitro to assess cell-autonomous apoptosis as previously described (13). After 24 and 48 hours of culture, there were far fewer viable cells (Annexin V−7AAD−) in cultures from Yy1Lck mice than from wild-type mice (Fig. 4F). Reduced viability of Yy1Lck DP thymocytes was due to increased apoptosis, because these cells exhibited higher levels of caspase 3 cleavage after 6 hours of in vitro culture (Fig. 4G).
Figure 4. YY1 regulates the survival of DP thymocytes.
(A) Western blot of YY1 and actin in purified DN (CD4−CD8−Lin−) and DP thymocytes of Yy1f/f (WT) and Yy1f/f Lck-Cre (Yy1Lck) mice. Results are representative of two independent experiments. (B) Numbers of total, DN and DP thymocytes in WT and Yy1Lck mice. Each data point represents an individual mouse and the horizontal line indicates the mean. Statistical significance was evaluated by unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. (C) CD44 and CD25 staining of WT and Yy1Lck thymocytes pre-gated as CD4−CD8−Lin−. Results are representative of three independent experiments. (D) CD4 and CD8 staining of total thymocytes of WT and Yy1Lck mice. (E) Frequency of BrdU+ WT and Yy1Lck DP thymocytes following a 4 h pulse with BrdU. The mean ± SEM of three independent experiments is shown. (F) Sorted DP thymocytes were cultured in vitro for 24 or 48 h and stained with Annexin V and 7AAD (left). Mean ± SEM survival is presented for three WT and four Yy1Lck cultures (right). Statistical significance was evaluated by two-way ANOVA with Sidak’s multiple-comparison test. (G) Sorted DP thymocytes were analyzed for caspase 3 and cleaved caspase 3 by western blot either immediately ex vivo or after 6 h of in vitro culture. The wedges indicate 2-fold dilutions of cell extract. Data are representative of two independent experiments. ***P < 0.001, ****P < 0.0001.
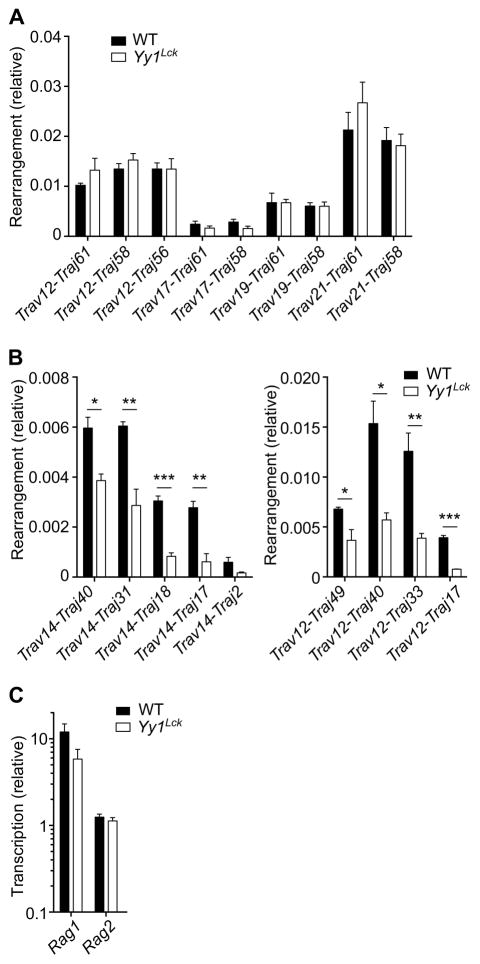
Reduced survival of DP thymocytes in vivo should be apparent as a bias in the TCRα repertoire, since Jα usage follows a temporal progression during DP thymocyte development (14). Indeed, although rearrangements of Vα segments to 5’Jα segments were comparable in wild-type and Yy1Lck DP thymocytes (Fig. 5A), rearrangements of Vα segments to more 3’Jα segments were underrepresented (Fig. 5B). Impaired Tcra rearrangement was not due to defective RAG expression (43), because Rag1 and Rag2 gene expression was normal in Yy1Lck DP thymocytes (Fig. 5C). Therefore, we concluded that YY1 is required to protect DP thymocytes from apoptosis in order to generate a normal TCRα repertoire.
Figure 5. YY1 regulates the TCRα repertoire.
Genomic DNA extracted from DP thymocytes from Yy1f/f (WT) and Yy1f/f Lck-Cre (Yy1Lck) mice was analyzed for rearrangement of Vα segments to 5’Jα segments (A) and to 3’Jα segments (B) by real-time PCR with normalization to Cd14. Data represent the mean ± SEM of three DNA preparations for each genotype, each preparation representing a different mouse. (C) Rag1 and Rag2 transcription in DP thymocytes was analyzed by real-time PCR with normalization to Hprt. Data represent the mean ± SEM of three cDNA preparations for each genotype, each preparation representing a different mouse. Statistical significance was evaluated by unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. *P < 0.05, **P < 0.01, ***P < 0.001.
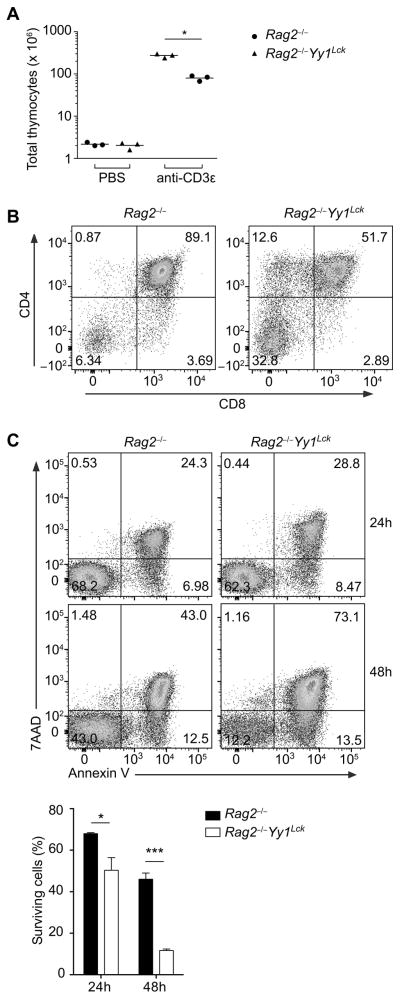
To assess whether increased death of DP thymocytes depended on the process of Tcra gene rearrangement or αβ TCR-dependent selection events, we crossed Yy1Lck mice onto a Rag2−/− background to prevent the generation of αβ TCRs or RAG-dependent DNA breaks. Rag2−/− background mice were treated with anti-CD3ε to mimic pre-TCR signaling and drive DN-to-DP development in the absence of TCRβ (32). Nine days after injection of anti-CD3ε, YY1-sufficient Rag2−/− thymocytes expanded by over 100-fold, whereas YY1-deficient Rag2−/− thymocytes expanded only 30-40-fold (Fig. 6A), with fewer DP thymocytes (Fig. 6B). Moreover, YY1-deficient Rag2−/− thymocytes were more prone to apoptosis when cultured in vitro (Fig. 6C). Therefore, increased apoptosis of Yy1Lck DP thymocytes was not caused by an altered response to V(D)J recombination-induced double-strand breaks or TCR-dependent selection signals.
Figure 6. YY1 regulates of DP thymocyte survival independent of V(D)J recombination or TCR expression.
Rag2−/− Yy1f/f (Rag2−/−) or Rag2−/− Yy1f/f Lck-Cre (Rag2−/− Yy1Lck) mice were injected with anti-CD3ε or PBS and thymocytes were analyzed 9 d later. (A) Total number of thymocytes. Each data point represents an individual mouse and the horizontal line indicates the mean. Statistical significance was evaluated by unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. (B) CD4 and CD8 staining. Results are representative of three independent experiments. (C) Annexin V and 7AAD staining of DP thymocytes cultured in vitro for the indicated time points (top). Mean ± SEM survival (Annexin V−7AAD+) is shown for three WT and three Yy1Lck cultures, each from a different mouse (bottom). Statistical significance was evaluated by two-way ANOVA with Sidak’s multiple-comparison test. *P < 0.05, ***P < 0.001.
YY1 regulates the p53-dependent apoptosis pathway
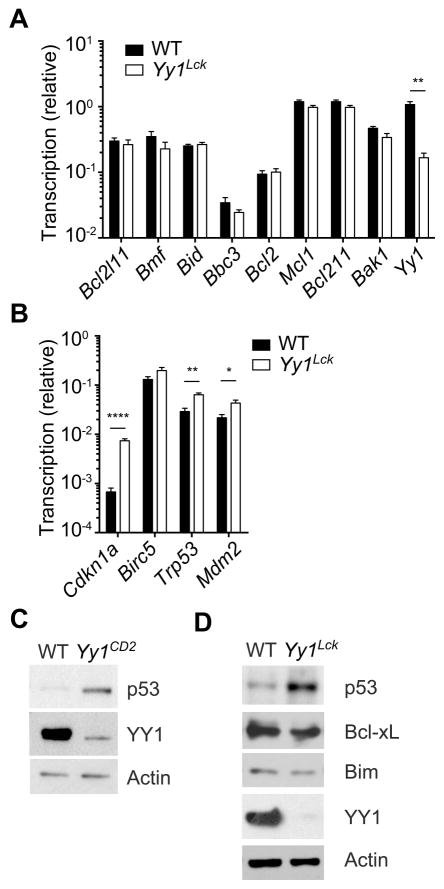
Because YY1-deficient thymocytes underwent cell-autonomous apoptosis in the absence of death receptor stimulation, we assessed involvement of the mitochondrial intrinsic apoptosis pathway. This pathway is primarily regulated by three groups of Bcl-2 family proteins: Bcl-2 homology 3-only apoptosis initiator proteins including PUMA and Bid; pro-survival cell guardians including Bcl-2, Bcl-xL and Mcl-1; and pro-apoptotic effector proteins including Bax and Bak (44). Additionally, p53, which is negatively regulated by YY1 (23, 24), has been connected to the intrinsic apoptosis pathway. p53 not only transactivates several genes encoding Bcl-2 family proteins but also antagonizes Bcl-2 and Bcl-xL and activates Bax and Bak through protein-protein interactions (45-49). We found that the transcription of Bcl2l11 (encoding Bim), Bmf, Bid, Bbc3 (encoding PUMA), Bcl2, Mcl1, Bcl2l1 (encoding Bcl-xL) and Bak1 (encoding Bak) was comparable in wild-type and Yy1Lck DP thymocytes (Fig. 7A). Transcription of Birc5 (which encodes Survivin, another pro-survival factor in thymocytes), was also unchanged in Yy1Lck DP thymocytes (Fig. 7B), even though a previous report showed that YY1 represses Birc5 transcription (50). However, the transcription of Trp53 (encoding p53), Cdkn1a (encoding p21) and Mdm2 was significantly upregulated in Yy1Lck DP thymocytes (Fig. 7B). These data were consistent with observations in other mammalian cells showing that Cdkn1a and Mdm2 are direct targets of YY1 (24, 51). Furthermore, we found that the abundance of p53 protein was significantly increased in DN3 thymocytes from Yy1CD2 mice (Fig. 7C), and in DP thymocytes from Yy1Lck mice (Fig. 7D), as compared to wild-type controls. However, expression of Bim and Bcl-xL proteins was normal in Yy1Lck DP thymocytes (Fig. 7D). These results indicated that YY1 is a negative regulator of p53 abundance in thymocytes.
Figure 7. YY1 negatively regulates p53.
The abundance of transcripts encoding pro- and anti-apoptotic proteins, YY1, and p21 were analyzed in DP thymocytes of Yy1f/f (WT) and Yy1f/f Lck-Cre (Yy1Lck) mice, with normalization to Hprt (A) or Gapdh (B). Data represent the mean ± SEM of three WT and three Yy1Lck preparations, each from a different mouse. Statistical significance was evaluated by unpaired Student’s t-test with Holm-Sidak correction for multiple comparisons. Sorted DN3 thymocytes from WT or Yy1CD2 mice (C) or DP thymocytes from WT or Yy1Lck mice (D) were analyzed by western blot. Data are representative of three independent experiments. *P < 0.05, **P < 0.01, ****P < 0.0001.
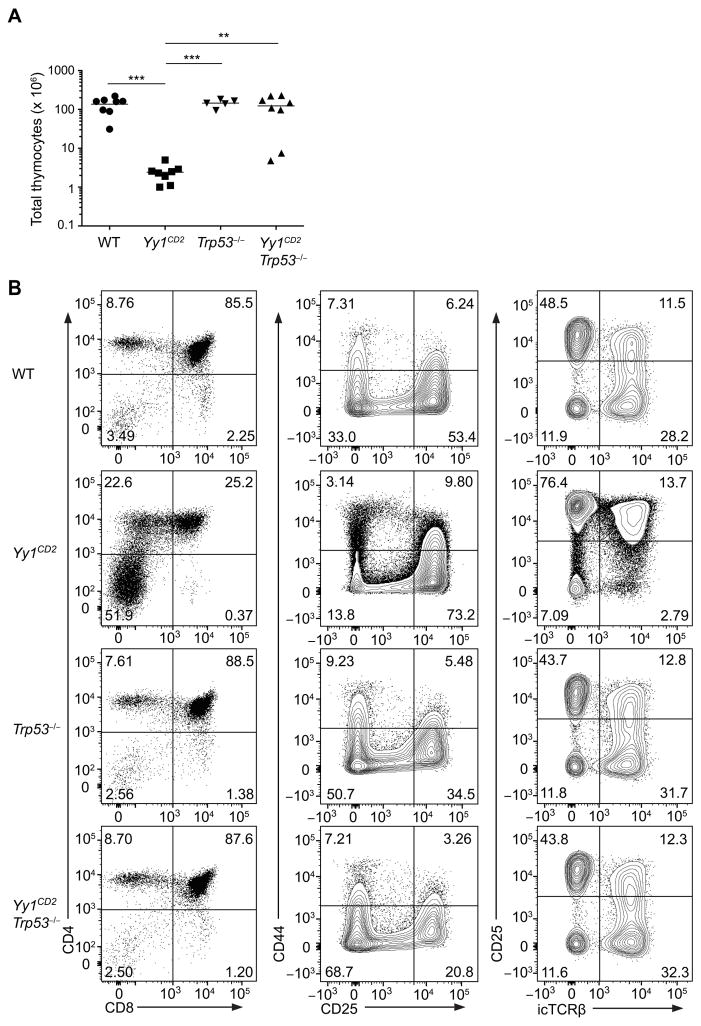
To evaluate whether YY1 regulation of p53 expression could account for impaired thymocyte development in the YY1-deficient mice, we crossed Yy1CD2 mice with Trp53−/− mice to generate Yy1CD2Trp53−/− double-knockout mice. Absence of p53 complemented the defect in thymus cellularity in Yy1CD2 mice (Fig. 8A), as well as the losses of DP thymocytes (Fig. 8B; left column) and DN4 thymocytes (Fig. 8B; middle and right columns). Absence of p53 also complemented the defect in Jα usage in YY1-deficient thymocytes (Supplemental Fig. 3). Together, these data demonstrated that overexpression of p53 is responsible for impaired development of YY1-deficient thymocytes.
Figure 8. Absence of p53 rescues the developmental defect in YY1CD2 mice.
(A) Number of total thymocytes in Yy1f/f (WT), Yy1f/f CD2-Cre (Yy1CD2), Trp53−/− and Yy1f/f CD2-Cre Trp53−/− (Yy1CD2 Trp53−/−) mice. Each data point represents an individual mouse and horizontal lines indicate the mean. Statistical significance was evaluated by one-way ANOVA with Tukey’s multiple-comparison test. (B) Staining of CD4 and CD8 in total thymocytes (left column), of CD44 and CD25 in DN thymocytes (CD4−CD8−Lin−) (middle column), and of CD25 and icTCRβ in DN thymocytes (CD4−CD8−Lin−) (right column) are shown. The results are representative of two independent experiments. **P < 0.01, ***P < 0.001.
Discussion
By disrupting Yy1 gene expression at two distinct stages of thymocyte development, we identified a novel function for YY1 in the generation of αβT lymphocytes. Yy1CD2 mice displayed increased apoptosis of post–β-selection DN4 thymocytes, leading to severe developmental arrest at the DN4 stage. Yy1Lck mice had fewer DP thymocytes due to reduced DP thymocyte lifespan in vivo; consistent with this, YY1-deficient DP thymocytes were prone to cell-autonomous apoptosis when cultured in vitro. Further analysis revealed elevated levels of p53 protein in both Yy1CD2 DN and Yy1Lck DP thymocytes, and Trp53 gene deletion corrected the blockade of T cell development in Yy1CD2 mice. Taken together, these data suggested that YY1 plays a critical cell-intrinsic role in thymocyte development by suppressing the level of p53. This function of YY1 is specific to the αβ T cell lineage, because γδ T cell development was normal in Yy1CD2 mice.
YY1 is likely to regulate the accumulation of p53 protein in two ways. First, we showed that YY1 suppresses Trp53 transcription. In addition, YY1 has been shown to negatively regulate p53 post-transcriptionally by stabilizing the interaction of p53 with Mdm2, an E3 ubiquitin ligase (23, 24). This interaction is required for ubiquitination and proteasomal degradation of cytoplasmic p53 (52, 53). YY1 has also been documented to inhibit transcriptional activation by p53 by blocking its interaction with co-activator p300 (24). However, we do not believe that this mechanism contributes significantly to the phenotype of YY1-deficient thymocytes, since we found no change in the transcription of a number of genes that are direct transcriptional targets of p53 (e.g., Bbc3, Bak1, Mdm2, Bcl2, and Birc5) (45, 54–59). Thus, transcriptional activation by p53 is largely unchanged in YY1-deficient thymocytes. We did detect increased transcription of one p53 target, Cdkn1a, in Yy1Lck DP thymocytes. However, YY1 has been shown to repress Cdkn1a transcription by blocking Sp1 binding to the Cdkn1a promoter in smooth muscle cells (51). Thus, we speculate that Cdkn1a expression may be regulated by YY1 directly, rather than by p53. However, we think it unlikely that p21 contributes to the developmental defect in Yy1Lck DP thymocytes because most DP thymocytes are quiescent, non-cycling cells.
Pre-TCR signals promote the differentiation, proliferation and survival of thymocytes at the β-selection checkpoint (2, 5). Previous studies have addressed the role of p53 at this stage. RAG2-deficient thymocytes do not differentiate to the DP stage because they fail to assemble a pre-TCR. In these mice, Trp53 gene deletion suppressed apoptosis to reveal limited DP differentiation, but in the absence of pre-TCR signals, these cells did not expand (7). CD3γ-deficient mice have small numbers of DP thymocytes due to compromised pre-TCR signaling. In these mice, loss of p53 fully restored the DP compartment by suppressing apoptosis in cells that were differentiating and proliferating in response to CD3γ-independent pre-TCR signals (6). Together, these studies suggest that p53 normally enforces β-selection by inducing apoptosis in pre-TCR negative thymocytes, and that pre-TCR signaling promotes cell survival by downregulating p53 (6, 7). With this in mind, quantitative rescue of the DP compartment by p53-deficiency in YY1-deficient thymocytes (Fig. 8A, B) implies that pre-TCR signaling pathways that promote differentiation and proliferation are largely intact in YY1-deficient thymocytes. We note that YY1-deficient thymocytes closely phenocopy Rpl22-deficient thymocytes (60). This makes sense, because YY1 and Rpl22 both function to suppress the accumulation of p53 in post–β-selection thymocytes.
Because transcription of pro- and anti-apoptotic genes was normal, increased apoptosis of DN and DP thymocytes from Yy1CD2 and Yy1Lck mice, respectively, was likely due to mitochondrial damage induced by accumulated cytoplasmic p53 (61, 62). p53 can rapidly associate with the mitochondrial outer membrane upon cell death signaling and form a complex with Bcl-2 and Bcl-xL (63). The Bcl-xL-p53 complex is not apoptotic; rather, apoptosis is induced only after PUMA releases p53 from Bcl-xL (45). Free p53 protein can then further interact with and activate Bak and Bax, thus inducing mitochondrial outer membrane permeabilization and the release of cytochrome c and other pro-apoptotic factors (45, 46). Recently, p53 has been shown to activate programmed necrosis (or necroptosis) in response to oxidative stress and ischemia, which involve opening the mitochondrial permeability transition pore (64). However, increased cleavage of caspase 3 in cultured Yy1Lck thymocytes indicated that YY1-deficient thymocytes underwent apoptosis rather than necroptosis (Fig. 4G).
It has been shown that YY1 plays critical roles during B cell development (18). YY1 binds to multiple sites in the Igh locus and regulates Igh recombination through effects on locus transcription, conformation and long-distance chromatin interactions (18, 26, 27, 29). YY1 also binds to multiple sites across the Igk locus and regulates Igk recombination (28). Based on these results, the ubiquitously expressed YY1 protein might also have been considered a candidate regulator of TCR loci. However, we detected no substantial changes in TCR locus rearrangement in our study. Although there was a modest reduction in the proportion of DN3 thymocytes that were icTCRβ+ in Yy1CD2 mice (Fig. 2A), we observed normal numbers of icTCRβ+ DN3 thymocytes in Yy1CD2 Trp53−/− mice (Fig. 8B), suggesting that any reduction in Tcrb rearrangement may reflect increased p53-dependent sensitivity to V(D)J recombination-induced double strand breaks (11). Moreover, we discerned, at best, only a minor effect on the TCRβ repertoire in Yy1CD2 mice (Supplemental Fig. 1C). We similarly observed no effect of YY1 on Jα usage when YY1-deficiency was analyzed on a Trp53−/− background (Supplemental Fig. 3). Further, we detected no obvious effect on γδ T cell development (Fig. 1D). Although influences of YY1 on TCR repertoires may have gone undetected in our assays, we suggest that any direct effects of YY1 on TCR loci are likely to be relatively subtle as compared to those at Ig loci.
In summary, we identified YY1 as a critical regulator of thymocyte development, and showed that YY1 regulates thymocyte development by setting the threshold of p53 expression and p53-dependent apoptosis. The influence of YY1 is apparent during αβ T cell development, but is not mirrored in γδ T cells. This may reflect a more limited role for p53 in γδ T cells, given that they undergo limited proliferation as compared to αβ T cells (2, 65).
Supplementary Material
Acknowledgments
We thank L. Martinek, N. Martin and B. Li of the Duke Cancer Institute Flow Cytometry Shared Resource for help with cell sorting and analysis, S. Langdon of the DNA Analysis Facility for help with DNA sequencing. Z. Huang for technical support, P-Y Yang and J. Liang for technical advice, G. Hammer and Y. Zhuang for reagents, and Y.W. He for comments on the manuscript.
Abbreviations used in this paper
- 7AAD
7-aminoactinomycin-D
- DN
double negative
- DP
double positive
- ic
intracellular
- Lin
lineage
- YY1
Yin Yang 1
Footnotes
This work was supported by National Institutes of Health Grants R37 GM41052 (to M.S.K.), RO1 AI083988 (to D.B.S.) and by the Robert Wood Johnson Foundation (grant #67038 to the Child Health Institute of New Jersey).
References
- 1.Yui MA, Rothenberg EV. Developmental gene networks: a triathlon on the course to T cell identity. Nat Rev Immunol. 2014;14:529–545. doi: 10.1038/nri3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taghon T, Yui MA, Pant R, Diamond RA, Rothenberg EV. Developmental and molecular characterization of emerging β- and γδ-selected pre-T cells in the adult mouse thymus. Immunity. 2006;24:53–64. doi: 10.1016/j.immuni.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 3.Livak F, Tourigny M, Schatz DG, Petrie HT. Characterization of TCR gene rearrangements during adult murine T cell development. J Immunol. 1999;162:2575–2580. [PubMed] [Google Scholar]
- 4.Capone M, Hockett RD, Jr, Zlotnik A. Kinetics of T cell receptor beta, gamma, and delta rearrangements during adult thymic development: T cell receptor rearrangements are present in CD44+CD25+ Pro-T thymocytes. Proc Natl Acad Sci USA. 1998;95:12522–12527. doi: 10.1073/pnas.95.21.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michie AM, Zuniga-Pflucker JC. Regulation of thymocyte differentiation: pre-TCR signals and β-selection. Semin Immunol. 2002;14:311–323. doi: 10.1016/s1044-5323(02)00064-7. [DOI] [PubMed] [Google Scholar]
- 6.Haks MC, Krimpenfort P, van den Brakel JH, Kruisbeek AM. Pre-TCR signaling and inactivation of p53 induces crucial cell survival pathways in pre-T cells. Immunity. 1999;11:91–101. doi: 10.1016/s1074-7613(00)80084-9. [DOI] [PubMed] [Google Scholar]
- 7.Jiang D, Lenardo MJ, Zuniga-Pflucker JC. p53 prevents maturation to the CD4+CD8+ stage of thymocyte differentiation in the absence of T cell receptor rearrangement. J Exp Med. 1996;183:1923–1928. doi: 10.1084/jem.183.4.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 9.Ciofani M, Zuniga-Pflucker JC. Notch promotes survival of pre-T cells at the β-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 10.Boudil A, I, Matei R, Shih HY, Bogdanoski G, Yuan JS, Chang SG, Montpellier B, Kowalski PE, Voisin V, Bashir S, Bader GD, Krangel MS, Guidos CJ. IL-7 coordinates proliferation, differentiation and Tcra recombination during thymocyte β-selection. Nat Immunol. 2015;16:397–405. doi: 10.1038/ni.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guidos CJ, Williams CJ, Grandal I, Knowles G, Huang MT, Danska JS. V(D)J recombination activates a p53-dependent DNA damage checkpoint in scid lymphocyte precursors. Genes Dev. 1996;10:2038–2054. doi: 10.1101/gad.10.16.2038. [DOI] [PubMed] [Google Scholar]
- 12.Carico Z, Krangel MS. Chromatin dynamics and the development of the TCRα and TCRδ repertoires. Adv Immunol. 2015;128:307–361. doi: 10.1016/bs.ai.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, Mebius RE, Littman DR. Requirement for RORγ in thymocyte survival and lymphoid organ development. Science. 2000;288:2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 14.Guo J, Hawwari A, Li H, Sun Z, Mahanta SK, Littman DR, Krangel MS, He YW. Regulation of the TCRα repertoire by the survival window of CD4+CD8+ thymocytes. Nat Immunol. 2002;3:469–476. doi: 10.1038/ni791. [DOI] [PubMed] [Google Scholar]
- 15.Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. BBA Rev Cancer. 1997;1332:F49–F66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- 16.Affar el B, Gay F, Shi Y, Liu H, Huarte M, Wu S, Collins T, Li E, Shi Y. Essential dosage-dependent functions of the transcription factor yin yang 1 in late embryonic development and cell cycle progression. Mol Cell Biol. 2006;26:3565–3581. doi: 10.1128/MCB.26.9.3565-3581.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramkumar C, Cui H, Kong Y, Jones SN, Gerstein RM, Zhang H. Smurf2 suppresses B-cell proliferation and lymphomagenesis by mediating ubiquitination and degradation of YY1. Nat Commun. 2013;4:2598. doi: 10.1038/ncomms3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Schmidt-Supprian M, Shi Y, Hobeika E, Barteneva N, Jumaa H, Pelanda R, Reth M, Skok J, Rajewsky K, Shi Y. Yin Yang 1 is a critical regulator of B-cell development. Genes Dev. 2007;21:1179–1189. doi: 10.1101/gad.1529307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang N, Li X, Wu CW, Dong Y, Cai M, Mok MT, Wang H, Chen J, Ng SS, Chen M, Sung JJ, Yu J. microRNA-7 is a novel inhibitor of YY1 contributing to colorectal tumorigenesis. Oncogene. 2013;32:5078–5088. doi: 10.1038/onc.2012.526. [DOI] [PubMed] [Google Scholar]
- 20.Perekatt AO, Valdez MJ, Davila M, Hoffman A, Bonder EM, Gao N, Verzi MP. YY1 is indispensable for Lgr5+ intestinal stem cell renewal. Proc Natl Acad Sci USA. 2014;111:7695–7700. doi: 10.1073/pnas.1400128111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan X, Jones M, Jiang J, Zaprazna K, Yu D, Pear W, Maillard I, Atchison ML. Increased expression of PcG protein YY1 negatively regulates B cell development while allowing accumulation of myeloid cells and LT-HSC cells. PLoS One. 2012;7:e30656. doi: 10.1371/journal.pone.0030656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vella P, Barozzi I, Cuomo A, Bonaldi T, Pasini D. Yin Yang 1 extends the Myc-related transcription factors network in embryonic stem cells. Nucleic Acids Res. 2012;40:3403–3418. doi: 10.1093/nar/gkr1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui GC, El Bachir A, Shi YJ, Brignone C, Wall NR, Yin P, Donohoe M, Luke MP, Calvo D, Grossman SR, Shi Y. Yin Yang 1 is a negative regulator of p53. Cell. 2004;117:859–872. doi: 10.1016/j.cell.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Gronroos E, Terentiev AA, Punga T, Ericsson J. YY1 inhibits the activation of the p53 tumor suppressor in response to genotoxic stress. Proc Natl Acad Sci USA. 2004;101:12165–12170. doi: 10.1073/pnas.0402283101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castellano G, Torrisi E, Ligresti G, Malaponte G, Militello L, Russo AE, McCubrey JA, Canevari S, Libra M. The involvement of the transcription factor Yin Yang 1 in cancer development and progression. Cell Cycle. 2009;8:1367–1372. doi: 10.4161/cc.8.9.8314. [DOI] [PubMed] [Google Scholar]
- 26.Verma-Gaur J, Torkamani A, Schaffer L, Head SR, Schork NJ, Feeney AJ. Noncoding transcription within the Igh distal VH region at PAIR elements affects the 3D structure of the Igh locus in pro-B cells. Proc Natl Acad Sci USA. 2012;109:17004–17009. doi: 10.1073/pnas.1208398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medvedovic J, Ebert A, Tagoh H, Tamir IM, Schwickert TA, Novatchkova M, Sun Q, Huis In 't Veld PJ, Guo C, Yoon HS, Denizot Y, Holwerda SJ, de Laat W, Cogne M, Shi Y, Alt FW, Busslinger M. Flexible long-range loops in the VH gene region of the Igh locus facilitate the generation of a diverse antibody repertoire. Immunity. 2013;39:229–244. doi: 10.1016/j.immuni.2013.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan X, Papasani M, Hao Y, Calamito M, Wei F, Quinn WJ, Iii, Basu A, Wang J, Hodawadekar S, Zaprazna K, Liu H, Shi Y, Allman D, Cancro M, Atchison ML. YY1 controls Igκ repertoire and B-cell development, and localizes with condensin on the Igappa; locus. EMBO J. 2013;32:1168–1182. doi: 10.1038/emboj.2013.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerasimova T, Guo C, Ghosh A, Qiu X, Montefiori L, Verma-Gaur J, Choi NM, Feeney AJ, Sen R. A structural hierarchy mediated by multiple nuclear factors establishes Igh locus conformation. Genes Dev. 2015;29:1683–1695. doi: 10.1101/gad.263871.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang SS, Kim YU, Lee S, Jang SW, Kim MK, Koh BH, Lee W, Kim J, Souabni A, Busslinger M, Lee GR. Transcription factor YY1 is essential for regulation of the Th2 cytokine locus and for Th2 cell differentiation. Proc Natl Acad Sci USA. 2013;110:276–281. doi: 10.1073/pnas.1214682110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hennet T, Hagen FK, Tabak LA, Marth JD. T-cell-specific deletion of a polypeptide N-acetylgalactosaminyl-transferase gene by site-directed recombination. Proc Natl Acad Sci USA. 1995;92:12070–12074. doi: 10.1073/pnas.92.26.12070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shinkai Y, Alt FW. CD3ε-mediated signals rescue the development of CD4+CD8+ thymocytes in RAG-2−/− mice in the absence of TCRβ chain expression. Int Immunol. 1994;6:995–1001. doi: 10.1093/intimm/6.7.995. [DOI] [PubMed] [Google Scholar]
- 33.Ciofani M, Schmitt TM, Ciofani A, Michie AM, Cuburu N, Aublin A, Maryanski JL, Zuniga-Pflucker JC. Obligatory role for cooperative signaling by pre-TCR and notch during thymocyte differentiation. J Immunol. 2004;172:5230–5239. doi: 10.4049/jimmunol.172.9.5230. [DOI] [PubMed] [Google Scholar]
- 34.Chen L, Carico Z, Shih HY, Krangel MS. A discrete chromatin loop in the mouse Tcra-Tcrd locus shapes the TCRβ and TCRβ repertoires. Nat Immunol. 2015;16:1085–1093. doi: 10.1038/ni.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gopalakrishnan S, Majumder K, Predeus A, Huang Y, Koues OI, Verma-Gaur J, Loguercio S, Su AI, Feeney AJ, Artyomov MN, Oltz EM. Unifying model for molecular determinants of the preselection Vβ repertoire. Proc Natl Acad Sci USA. 2013;110:E3202–E3215. doi: 10.1073/pnas.1304048110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shih HY, Verma-Gaur J, Torkamani A, Feeney AJ, Galjart N, Krangel MS. Tcra gene recombination is supported by a Tcra enhancer- and CTCF-dependent chromatin hub. Proc Natl Acad Sci USA. 2012;109:E3493–E3502. doi: 10.1073/pnas.1214131109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 38.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 39.Schlegel RA, Callahan MK, Williamson P. The central role of phosphatidylserine in the phagocytosis of apoptotic thymocytes. Ann N Y Acad Sci. 2000;926:217–225. doi: 10.1111/j.1749-6632.2000.tb05614.x. [DOI] [PubMed] [Google Scholar]
- 40.Yui MA, Feng N, Rothenberg EV. Fine-scale staging of T cell lineage commitment in adult mouse thymus. J Immunol. 2010;185:284–293. doi: 10.4049/jimmunol.1000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teague TK, Tan C, Marino JH, Davis BK, Taylor AA, Huey RW, Van De Wiele CJ. CD28 expression redefines thymocyte development during the pre-T to DP transition. Int Immunol. 2010;22:387–397. doi: 10.1093/intimm/dxq020. [DOI] [PubMed] [Google Scholar]
- 42.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S, Cherry SR, Tsai JH, Tucker SM, Weaver WM, Kelso A, Jaenisch R, Wilson CB. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 43.Yannoutsos N, Wilson P, Yu W, Chen HT, Nussenzweig A, Petrie H, Nussenzweig MC. The role of recombination activating gene (RAG) reinduction in thymocyte development in vivo. J Exp Med. 2001;194:471–480. doi: 10.1084/jem.194.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. doi: 10.1038/nrm3722. [DOI] [PubMed] [Google Scholar]
- 45.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 46.Leu JI, Dumont P, Hafey M, Murphy ME, George DL. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat Cell Biol. 2004;6:443–450. doi: 10.1038/ncb1123. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Zhang L. The transcriptional targets of p53 in apoptosis control. Biochem Biophys Res Commun. 2005;331:851–858. doi: 10.1016/j.bbrc.2005.03.189. [DOI] [PubMed] [Google Scholar]
- 48.Hemann MT, Lowe SW. The p53-Bcl-2 connection. Cell Death Differ. 2006;13:1256–1259. doi: 10.1038/sj.cdd.4401962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hagn F, Klein C, Demmer O, Marchenko N, Vaseva A, Moll UM, Kessler H. BclxL changes conformation upon binding to wild-type but not mutant p53 DNA binding domain. J Biol Chem. 2010;285:3439–3450. doi: 10.1074/jbc.M109.065391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galloway NR, Diaz Osterman CJ, Reiber K, Jutzy JM, Li F, Sui G, Soto U, Wall NR. Yin Yang 1 regulates the transcriptional repression of Survivin. Biochem Biophys Res Commun. 2014;445:208–213. doi: 10.1016/j.bbrc.2014.01.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santiago FS, Ishii H, Shafi S, Khurana R, Kanellakis P, Bhindi R, Ramirez MJ, Bobik A, Martin JF, Chesterman CN, Zachary IC, Khachigian LM. Yin Yang-1 inhibits vascular smooth muscle cell growth and intimal thickening by repressing p21WAF1/Cip1 transcription and p21WAF1/Cip1-Cdk4-cyclin D1 assembly. Circ Res. 2007;101:146–155. doi: 10.1161/CIRCRESAHA.106.145235. [DOI] [PubMed] [Google Scholar]
- 52.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 53.Marchenko ND, Hanel W, Li D, Becker K, Reich N, Moll UM. Stress-mediated nuclear stabilization of p53 is regulated by ubiquitination and importin-alpha3 binding. Cell Death Differ. 2010;17:255–267. doi: 10.1038/cdd.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakano K, Vousden KH. PUMA, a novel proapoptotic gene, is induced by p53. Mol Cell. 2001;7:683–694. doi: 10.1016/s1097-2765(01)00214-3. [DOI] [PubMed] [Google Scholar]
- 55.Yu J, Zhang L, Hwang PM, Kinzler KW, Vogelstein B. PUMA induces the rapid apoptosis of colorectal cancer cells. Mol Cell. 2001;7:673–682. doi: 10.1016/s1097-2765(01)00213-1. [DOI] [PubMed] [Google Scholar]
- 56.Mirza A, McGuirk M, Hockenberry TN, Wu Q, Ashar H, Black S, Wen SF, Wang L, Kirschmeier P, Bishop WR, Nielsen LL, Pickett CB, Liu S. Human survivin is negatively regulated by wild-type p53 and participates in p53-dependent apoptotic pathway. Oncogene. 2002;21:2613–2622. doi: 10.1038/sj.onc.1205353. [DOI] [PubMed] [Google Scholar]
- 57.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 58.Villunger A, Michalak EM, Coultas L, Mullauer F, Bock G, Ausserlechner MJ, Adams JM, Strasser A. p53- and drug-induced apoptotic responses mediated by BH3-only proteins puma and noxa. Science. 2003;302:1036–1038. doi: 10.1126/science.1090072. [DOI] [PubMed] [Google Scholar]
- 59.Graupner V, Alexander E, Overkamp T, Rothfuss O, De Laurenzi V, Gillissen BF, Daniel PT, Schulze-Osthoff K, Essmann F. Differential regulation of the proapoptotic multidomain protein Bak by p53 and p73 at the promoter level. Cell Death Differ. 2011;18:1130–1139. doi: 10.1038/cdd.2010.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson SJ, Lauritsen JPH, Hartman MG, Foushee AMD, Lefebvre JM, Shinton SA, Gerhardt B, Hardy RR, Oravecz T, Wiest DL. Ablation of ribosomal protein L22 selectively impairs alpha beta T cell development by activation of a p53-dependent checkpoint. Immunity. 2007;26:759–772. doi: 10.1016/j.immuni.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Riley T, Sontag E, Chen P, Levine A. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–412. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 62.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 64.Vaseva AV, Marchenko ND, Ji K, Tsirka SE, Holzmann S, Moll UM. p53 opens the mitochondrial permeability transition pore to trigger necrosis. Cell. 2012;149:1536–1548. doi: 10.1016/j.cell.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Komarov PG, Komarova EA, Kondratov RV, Christov-Tselkov K, Coon JS, Chernov MV, Gudkov AV. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–1737. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.