Abstract
Arachidonic acid (AA) is metabolized by cyclooxygenase (COX) and cytochrome P450 (CYP) enzymes into eicosanoids, which are involved in cardiovascular diseases and stroke. Evidence has demonstrated the important functions of these eicosanoids in regulating cerebral vascular tone, cerebral blood flow, and autoregulation of cerebral circulation. Although COX-2 inhibitors have been suggested as potential treatments for stroke, adverse events, including an increased risk of stroke, occur following long-term use of coxibs. It is important to note that prolonged treatment with rofecoxib increased circulating levels of 20-hydroxyeicosatetraenoic acid (20-HETE), and 20-HETE blockade is a possible strategy to prevent coxib-induced stroke events. It appears that 20-HETE has detrimental effects in the brain, and that its blockade exerts cerebroprotection against ischemic stroke and subarachnoid hemorrhage (SAH). There is clear evidence that activation of EP2 and EP4 receptors exerts cerebroprotection against ischemic stroke. Several elegant studies have contributed to defining the importance of stabilizing the levels of epoxyeicosatrienoic acids (EETs), by inhibiting or deleting soluble epoxide hydrolase (sEH), in stroke research. These reports support the notion that sEH blockade is cerebroprotective against ischemic stroke and SAH. Here, we summarize recent findings implicating these eicosanoid pathways in cerebral vascular function and stroke. We also discuss the development of animal models with targeted gene deletion and specific enzymatic inhibitors in each pathway to identify potential targets for the treatment of ischemic stroke and SAH.
Keywords: Cyclooxygenase, cytochrome P450, eicosanoids, soluble epoxide hydrolase, stroke
1. Introduction
Stroke, the fifth leading cause of death, is one of the most life-threatening cerebrovascular disorders in the U.S. [1, 2]. According to a 2015 report from the CDC, every year more than 795,000 people in the U.S. have a stroke. Also, strokes kill almost 130,000 Americans each year, accounting for about 1 out of every 20 deaths. There are two main types of stroke: ischemic and hemorrhagic. It has been estimated that ischemic stroke accounts for about 80%–85% of all stroke incidents, while hemorrhagic stroke accounts for the remaining 15%–20% of stroke incidents [1, 2]. Ischemic stroke occurs when a thrombus or embolus blocks cerebrovascular circulation, resulting in irreversible damage to the ischemic core and its surrounding region. Hemorrhagic stroke is mainly due to the rupture of cerebral aneurysms, resulting in subarachnoid hemorrhage (SAH) and intracranial hemorrhage [3].
The use of recombinant tissue plasminogen activator (rtPA) has been the standard of care for treatment of acute ischemic stroke. However, patients with ischemic stroke need to receive this drug within therapeutic window of four-and-a-half hours. Also, there is increasing concern that treatment of rtPA may cause side effects, including the disruption of the blood brain barrier, as well as seizures and progressive neuronal damage. Although rtPA treatment provides significant benefits for stroke patients, this therapy did not show significant benefits for patients with large artery occlusions [4]. To resolve this issue, five clinical trials, including MR CLEAN, ESCAPE, EXTEND IA, SWIFT PRIME, and REVASCAT, were conducted to evaluate the use of endovascular thrombectomy in stroke patients [4, 5]. The results from these clinical trials show that this therapy provides consistent benefits for stroke patients even when it was performed beyond 4.5 h in patients who had already received rtPA [5, 6]. Thus, although more clinical studies are needed to validate the outcomes of these studies, endovascular thrombectomy could be a promising therapy for the treatment of ischemic stroke in the near future. Although there have been significant advances in understanding the pathophysiology following stroke, current treatments for stroke are limited in both their utility (e.g., rtPA) and effectiveness (e.g., aspirin). Therefore, there is a critical need for basic and clinical research to investigate potential therapeutic targets for the treatment of stroke.
Since cerebral vascular function and dysfunction are the key factors in the onset and progression of stroke, this review aims to provide important information about how cyclooxygenase (COX) and cytochrome P450 (CYP)- derived eicosanoids affect cerebral vascular function, as well as the role of these lipid effector molecules in ischemic stroke and SAH.
2. Regulation of cerebral vascular function by COX- and CYP-eicosanoids
2.1. Role of COX-eicosanoids in cerebral vascular function
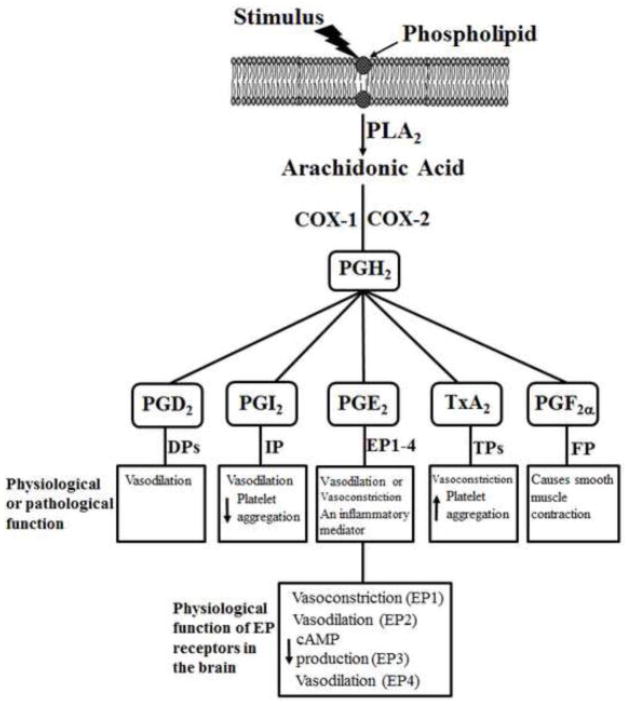
Arachidonic acid (AA) is a 20-carbon polyunsaturated fatty acid that is usually esterified to the second carbon in membrane phospholipids. The release of AA from phospholipids is achieved through the activity of phospholipase A2 (PLA2), which specifically recognizes the sn-2 acyl bond of phospholipids and catalytically hydrolyzes the bond releasing AA and lysophospholipids. AA is metabolized to prostaglandin H2 (PGH2) by COX-1 or COX-2. COX-1 is constitutively expressed in most cells and is involved in normal physiologic functions [7]. COX-2 is expressed in many organs, such as the brain, and it is highly inducible by pro-inflammatory cytokines [7]. PGH2 is the substrate for the activities of tissue-specific isomerases and synthases that synthesize PG2 and TX (Figure 1) [8]. The major PGs are PGD2, PGE2, PGI2, and PGF2α; the major TX is TXA2. The action of these PGs and TXA2 is mediated through the binding of these products into their membrane-bound receptors, including DP, EPs (EP1 to EP4), IP, FP, and TP receptors (Figure 1) [9, 10]. For an overview and function of COXs, there are several excellent reviews regarding to COX-1 and inducible COX-2 in the brain [11–13].
Figure 1.
Cyclooxygenase (COX)-derived eicosanoids from the arachidonic acid (AA) cascade. After trigging by inflammatory conditions such as the presence of cytokines and growth factors, AA-containing phospholipids are hydrolyzed by phospholipase A2 (PLA2) resulting in the release of free AA. AA can be further metabolized by COX-1 or COX-2 to PGH2. The generation of PGD2, PGI2, PGE2, TxA2, and PGF2α is mediated through tissue-specific isomerases and synthases. The action of these PGs and TXA2 is mediated via DPs, EPs (EP1 to EP4), IP, FP, and TPs receptors. The physiological or pathological function of these eicosanoids is also summarized in Figure 1. We also indicate the physiological function of EP receptors in the brain in Figure 1.
In the cerebral vascular system, COX-1 and COX-2 are important in the modulation of cerebral blood flow [14]. For example, indomethacin, a nonselective inhibitor of COX-1 and COX-2, reduced resting cerebral blood flow and attenuated elevations in cerebral blood flow produced by endothelium-dependent vasodilators [15, 16]. However, previous studies [17, 18] demonstrated that indomethacin has off-target effects that are unrelated to COX inhibition, including inhibition of IP receptor and cAMP-dependent protein kinase activity. Interestingly, Niwa et al. [19] have found that COX-1 knockout (KO) and SC-560 (a selective COX-1 inhibitor) significantly attenuated resting cerebral blood flow by 13% to 20%, respectively. Further investigation showed that SC-560 attenuated the cerebral blood flow induced by hypercapnia, bradykinin, calcium ionophore A23187, and AA in wild-type mice but not COX-1 KO mice [19]. These findings demonstrate that COX-1 has a critical role in maintaining resting vascular tone and in selective vasodilator responses in cerebral circulation. To determine the importance of COX-2 in cerebral circulation, Niwa et al. [20] showed that NS-398, a selective inhibitor of COX-2, attenuated the increase of somatosensory cortex blood flow induced by vibrissal stimulation. However, neither NS-398 nor COX-2 KO affected increases in cerebral blood flow induced by hypercapnia, acetylcholine, or bradykinin. These results provide solid evidence that COX-2 is important to increase cortex blood flow that accompanies neural activity.
2.2. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in cerebral vascular function
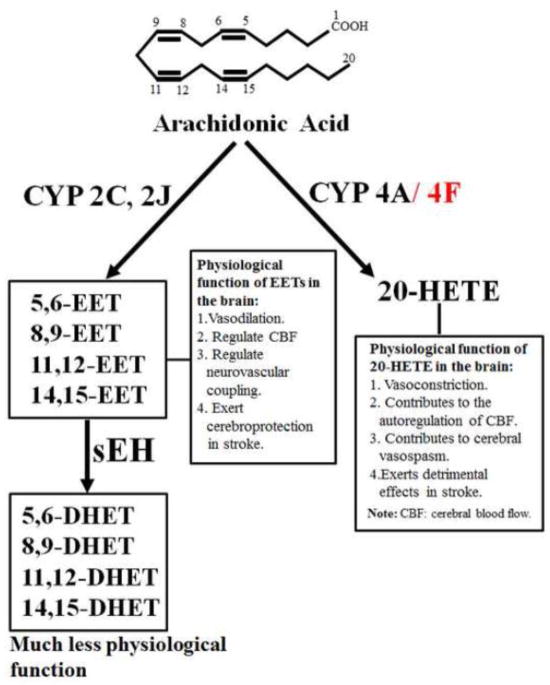
20-HETE, the ω-hydroxylation product of AA, is the principal AA metabolite of CYP enzymes in vascular smooth muscle [21] and kidney [22]. Synthesis of 20-HETE is catalyzed by the CYP4A gene family [21] (Figure 2). This subfamily encodes several CYP enzymes in different species. In the rat, four CYP4A enzymes have been identified: CYP4A1, CYP4A2, CYP4A3, and CYP4A8 [23]. These isoforms, although sharing 66%–98% homology and common catalytic activity, are expressed in the liver, kidney, and brain [24]. The recombinant CYP4A1, CYP4A2, and CYP4A3, but not CYP4A8, catalyzed AA ω-hydroxylation to 20-HETE with the highest catalytic efficiency (Vmax/Km) for CYP4A1, followed by CYP4A2 and CYP4A3 [25]. In the mouse, four Cyp4a enzymes have been identified: Cyp4a10, Cyp4a12a, Cyp4a12b, and Cyp4a14. Muller et al. [26] have demonstrated that AA ω-hydroxylation is catalyzed by Cyp4a10, Cyp4a12a, and Cyp4a12b. Cyp4a12a and Cyp4a12b have similar catalytic activity for 20-HETE production, with a Vmax value of about 10/min and a Km value of about 20–40 μM [26]. The ω-hydroxylase activity of AA for Cyp4a10 is about 25 to 75-fold lower than that of Cyp4a12 isoforms. These results suggest that Cyp4a12 isoforms constitute the major source of 20-HETE synthesis. Notably, besides CYP4A enzymes, CYP4F isoforms are also important for 20-HETE production [27].
Figure 2.
The metabolic pathway of arachidonic acid by cytochrome P450 (CYP) enzymes. 20-HETE, 20-hydroxyeicosatetraenoic acid; EET, epoxyeicosatrienoic acid; DHET, dihydroxyeicosatrienoic acid; sEH: soluble epoxide hydrolase. The physiological function of 20-HETE and EETs in the brain is also summarized in Figure 2.
In the cerebral vascular system, 20-HETE production was first identified in 1994 [28] and a study by Gebremedhin et al. [29] has demonstrated that CYP4A1, CYP4A2, CYP4A3, and CYP4A8 are expressed in rat cerebral microvessels. 20-HETE is a potent vasoconstrictor that depolarizes vascular smooth muscle cells by inhibiting K+ channel activity and is important in regulating renal hemodynamics and renal function [30]. In cerebral microcirculation, Gebremedhin et al. [29] showed that an elevation in transmural pressure, from 20 to 140 mm Hg, increased 20-HETE levels by 6-fold, which was determined by GC/MS, in cerebral arteries. Moreover, they also showed that 20-HETE blockade by DDMS and 20-HETE antagonists attenuated autoregulation of CBF to elevations of arterial pressure [29]. In pressurized cerebral arterial segments, 20-HETE elicits vascular contraction that is triggered by inhibition of the activity of the large conductance Ca2+-activated K+ channel (Kca) and increasing influx of Ca2+ through the activation of L-type Ca2+ channels [28]. Taken together, these results support the notion that 20-HETE has vital function in autoregulation of cerebral blood flow.
2.3 Role of epoxyeicosatrienoic acids (EETs) in cerebral vascular function
AA is epoxidized by the CYP enzymes into four epoxyeicosatrienoic acids, 5,6-EET, 8,9-EET, 11,12-EET, and 14,15-EET (Figure 2). EETs are further metabolized by soluble epoxide hydrolase (sEH) to the corresponding vic-dihydroxyeicosatrienoic acids (DHETs) (Figure 2) [31]. EETs production has been attributed to the CYP 2C and 2J isoforms [32]. For example, Capdevila and colleagues [33] have shown that recombinant CYP2C11, CYP2C23, and CYP2C24, in that order, have the highest to lowest epoxygenase activity. Similarly, recombinant rat CYP2J3 and CYP2J4 are active for EETs production [34, 35]. EETs are metabolized by sEH into DHETs [36]. Thus, the action of sEH is to limit the physiological effects of EETs because EETs are generally more biologically active than DHETs (Figure 2). For example, 11,12-DHET, a sEH product, has no vasodilatory action, whereas 11,12-EET produces dilation of renal microvessels [37, 38]. However, in some vascular beds, including canine coronary [39], human coronary arterioles [40], and murine mesenteric arteries [41], both EETs and DHETs cause vasodilation. The rapid development of selective inhibitors of sEH in the past decade is noteworthy; sEH inhibitors have been consistently demonstrated to exhibit protective effects in cardiovascular [42, 43] and renal [44, 45] diseases.
In the brain, EETs production is found in numerous sites, including neurons, astrocytes, and cerebral blood vessels. These lipid effector molecules are important in coupling neuronal activity and astrocytes to induce dilatory responses in cerebral arterioles [46]. For example, in astrocytes, CYP2C11, the major rat epoxygenase enzyme, can produce EETs in the astrocytes [47]. Previous studies have demonstrated that EETs produced in the astrocytes promote opening of astrocyte KCa channels [48–50], which can generate sufficient K+ to open inward-rectifier K+ channels in vascular smooth muscle and thereby elicit hyperpolarization and dilation in cerebral arterioles. Moreover, EETs can be released from astrocytes, and they act in a paracrine manner to open Ca2+-sensitive K+ (KCa) channels, hyperpolarize vascular smooth muscle cells, and cause vasodilation in cerebral arterioles [46, 51]. To determine the importance of EETs in cerebral circulation, one interesting study [52] showed that superfusion with MS-PPOH, a selective epoxygenase inhibitor, and miconazole, a reversible CYP inhibitor, significantly attenuated cerebral blood flow during whisker stimulation. However, neither MS-PPOH nor miconazole affected baseline cerebral blood flow. Similarly, Peng et al. [53] have found that MS-PPOH attenuated cerebral blood flow during forepaw stimulation. Taken together, these findings establish a role of EETs in regulating cerebral blood flow to neural activation.
3. Role of COX- and CYP-eicosanoids in stroke
3.1. Role of COX-eicosanoids in ischemic stroke
Numerous studies have demonstrated that inflammation is a major contributor to the pathological damage that occurs after ischemic stroke. Following cerebral ischemia, inflammatory cells such as neutrophils infiltrate into the ischemic brain, which triggers the release of chemokines and pro-inflammatory cytokines [7]. It is well recognized that the inflammatory response following cerebral ischemia causes the upregulation of COX-2 expression and increased synthesis of inflammatory PGs [7]. Thus, major research in this area has focused on whether COX-2 blockade or KO is protective against ischemic brain damage. Nagawa et al. [54] showed that the expression of COX-2 is upregulated in the injured hemisphere after cerebral ischemia and that administration of NS-398, a selective COX-2 inhibitor, attenuated the elevation of PGE2 synthesis in the post-ischemic brain and reduced infarct volume by 29% in rats. Another study [55] showed that treatment with NS-398 after ischemic stroke reduces infarct volume and improves neurologic deficits in mice. Notably, COX-2 KO has beneficial effects in the brain injury produced by MCAO, and the protection by COX-2 KO is attributed to the reduction of glutamate neurotoxicity [56]. These findings suggest that COX-2 inhibitors or COX-2 KO exert beneficial effects in ischemic stroke.
3.2. Role of selective COX-2 inhibitors (coxibs) in adverse stroke events
It is estimated that doses of more than 30 billion nonsteroidal anti-inflammatory drugs (NSAIDs) are purchased over-the-counter annually in the U.S. [57]. However, use of conventional NSAIDs is often associated with significant gastrointestinal complications such as ulcers and bleeding. Based on strong data that COX-2 is the major enzyme for the production of inflammatory PGE2 and that COX-1 is a key enzyme in the production of cytoprotective PGs in the stomach [58], the Food and Drug Administration (FDA) has approved the use of three coxibs (selective COX-2 inhibitors), rofecoxib, celecoxib, and valdecoxib. Coxibs were developed to reduce the side effects of NSAIDs [58]. While substantial evidence from clinical trials (Adenoma Prevention with Celecoxib (APC), Adenomatous Polyp Prevention on Vioxx (APPROVe), and Prevention of Colorectal Sporadic Adenomatous Polyps (PreSAP) trial) demonstrate that coxibs reduce or prevent the incidence of colorectal cancer [58], long-term use of rofecoxib is also associated with an increased risk of side effects, including increasing incidence of stroke [59]. Because of these unfavorable side effects, rofecoxib and valdecoxib were withdrawn from the market and NIH halted clinical trials of the use of coxibs in 2004. Currently, celecoxib (Celebrex), which is less potent than rofecoxib, is the only coxib on the market, but the black-box warning states that celecoxib may cause risk of stroke.
Numerous reports [60–70] indicate that coxib-induced stroke events are a major obstacle to prolonged use of these drugs. The prevailing theory to explain the adverse cardiovascular effects of coxibs is that they reduce the production of PGI2, a potent inhibitor of platelet aggregation (Figure 1), but do not affect the production of TXA2, a potent platelet-aggregating agent (Figure 1) [71]. Thus, stroke complications caused by coxibs might be a consequence of a shifting balance between the levels of PGI2 and TXA2 [72]. Although this theory is attractive, it cannot fully explain why other nonselective COX inhibitors, including diclofenac, ibuprofen, naproxen, and indomethacin, also significantly increase the risk of side effects [73]. Hence, more complex mechanisms may be responsible for the coxib-induced stroke events.
Recent work by Zhang et al. [58] has provided the first evidence that anti-tumor therapy with rofecoxib induces circulating 20-HETE levels. One could predict that the increased 20-HETE levels are caused by elevation of the substrate, AA, after blocking COX-2. However, rofecoxib did not affect the levels of EETs/DHETs, 5-HETE, 8-HETE, 11-HETE, or 15-HETE. These results are consistent with the previous finding [73] that rofecoxib administration markedly increased 20-HETE levels, but it did not affect circulating levels of EETs, HETEs, LXA4, and PGD2. Intriguingly, Zhang et al. [58] further showed that 20-HETE blockade by HET0016 prevented stroke event, hemorrhagic transformation, induced by rofecoxib. Since 20-HETE significantly increases platelet aggregation [73] and has detrimental effects on cerebral circulation, it is possible that accumulation of 20-HETE levels after prolonged treatment with rofecoxib contributes to coxib-induced stroke events. Therefore, these findings support the concept that 20-HETE blockade could be a promising approach to prevents coxib-induced cerebral ischemic damage and stroke events.
3.3. Role of EP receptors in ischemic stroke
As mentioned previously, inflammation is a major contributor to the pathological damage occurring following ischemic stroke. PGs are important inflammatory mediators produced in the brain during cerebral ischemia. The deleterious effects of COX-2 activation following stroke have been confirmed in ischemic stroke model. In addition, substantial evidence has demonstrated that inhibition or deletion of COX-2 is cerebroprotective against ischemic stroke [74]. Unfortunately, long-term clinical trials have reported adverse effects following use of coxibs, including an increased risk of stroke and myocardial infarction [59]. Therefore, extensive research has focused on whether downstream EP receptors exert cerebroprotective effects against ischemic stroke.
It is well known that the action of PGE2 in the brain is mediated by specific G-protein-coupled EP1 to EP4 receptors (Figure 1), which are involved in various physiologic and pathophysiologic conditions [11]. In peripheral blood vessels, the activation of EP1 receptor causes vasoconstriction, leading to the hypothesis that EP1 exerts deleterious effects in ischemic stroke. To test this hypothesis, Saleem et al. [75] used MCAO model to study the role of EP1 KO in cerebral blood flow and neuronal cell death during ischemic stroke. They showed that after ischemic stroke cerebral blood flow in EP1 KO mice was significantly elevated relative to wild-type mice. This suggests that EP1 is important in regulating cerebral vascular tone during cerebral ischemia. Moreover, neuronal cultures derived from EP1 KO were more resistant to tert-butyl hydroperoxide-induced injury than were neurons from wild-type mice. Another study [76] demonstrated that pretreatment with ONO-8713, an EP1 receptor antagonist, showed notable benefit by reducing infarct size as compared with vehicle group. Additionally, a previous study [77] showed that EP1 receptor blockade improved the survival of hippocampal slices by preventing the attenuation in AKT activity induced by oxygen glucose deprivation (OGD). These findings support the notion that EP1 receptor has a significant function in regulating cerebral blood flow and neuronal cell death, and that EP1 blockade may improve cerebral blood flow and prevent neuronal survival in ischemic stroke.
It is well established that the activation of EP2 receptor triggers cAMP production. Also, EP2 is expressed in neurons of the cerebral cortex, striatum, and hippocampus as well as in the cerebral vasculature [11]. Several studies have demonstrated that activation of EP2 has notable benefit in paradigms on NMDA toxicity and oxygen glucose deprivation [11, 60]. In peripheral blood vessels, the activation of EP2 receptor causes vasodilation, leading to the hypothesis that EP2 exerts cerebroprotective effects against ischemic stroke. To test this, an interesting study by McCullough et al. [78] showed that using MCAO model, EP2 KO led to increased cerebral infarction in the cerebral cortex and subcortical structures. This study also showed that EP2 KO did not alter cerebral blood flow after ischemic stroke, which suggests that EP2 KO does not alter the severity of ischemic insult. Another interesting study [79] showed that EP2 KO caused significant increase in stroke volume. Moreover, Li et al. [80] demonstrate that treatment with misoprostol, which activates both EP2 and EP4 receptors, caused significant reduce of infarct volume at 24 and 72 h after MCAO. Taken together, these results support the notion that activation of EP2 receptor is cerebroprotective against ischemic stroke.
It is well established that the activation of EP3 receptor decreases cAMP production and that EP3 is expressed mainly in the hypothalamus [11]. To investigate the role of EP3 receptor in ischemic stroke, Saleem et al. [81] used MCAO in EP3 KO and wild-type mice. Although EP3 KO led to a decrease in infarct volume at 48 h after ischemic stroke, it did not affect infarct volume at 96 h after ischemic stroke. In contrast, Ahmad et al. [82] showed that treatment with ONO-AE-248, a selective EP3 agonist, dose-dependently increased infarct size in MCAO model, suggesting that EP3 activation exerts injurious effects in ischemic stroke. Given these conflicting findings, the question of whether activation of EP3 has cerebroprotective or injurious effects in ischemic stroke remain unsolved.
It is well established that EP4 activation increases cAMP production. Moreover, EP4 activation causes vasodilation in vascular beds [83], which is associated with activation of endothelial NOS and NO-mediated relaxation of smooth muscle [11, 84]. These findings have led to the hypothesis that EP4 activation is cerebroprotective against ischemic stroke. To test this, an interesting study by Liang et al. [85] showed that both endothelial- and neuronal-specific KO of EP4 exacerbated stroke injury and decreased cerebral reperfusion in MCAO model. Moreover, treatment with AE1-329, an EP4 agonist, reduced infarct volume and improved behavioral performance 7 days after ischemic stroke. Also, the activation of EP4 by AE1-329 is associated with elevation of the expression of eNOS in cerebral microvessels. Similarly, treatment with L-902688, an EP4 agonist, caused reduction of infarct volume at 48 h post stroke [86]. Interestingly, a previous study [87] demonstrated that conditional KO of EP4 in macrophages and microglia increased lipid peroxidation and pro-inflammatory gene expression in brain, whereas the treatment with an EP4 agonist decreased LPS-induced pro-inflammatory gene expression in hippocampus and in isolated adult microglia, suggesting the beneficial effect of EP4 receptor signaling in suppressing brain inflammation. Taken together, these novel findings provide the first evidence that EP4 receptor exerts neuronal and vascular protection in ischemic stroke.
3.4. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in ischemic stroke and subarachnoid hemorrhage
Substantial evidence demonstrates that the impairment in microcirculation significantly contributes to cerebral ischemic damage, and that microcirculation in the peri-infarct region is an important target for the treatment of cerebral ischemia [88]. It is widely accepted that 20-HETE has detrimental effects [89–92] on cerebral circulation, including that it causes vasoconstriction of cerebral arteries; it contributes to the development of cerebral vasospasm; and it increases neuronal excitotoxicity in vivo (Figure 2). Extensive research has focused on whether 20-HETE blockade has any beneficial effects against ischemic stroke. Tanaka et al. [93], in an early study, made the noteworthy finding that an elevation of 20-HETE levels occurred in the plasma and brain after cerebral ischemia in rats. It is possible that such increased levels were associated with the impairment of cerebral autoregulation after stroke. Importantly, other studies have demonstrated that 20-HETE blockade by TS-011 reduced infarct volume and improved the neurological outcome in rat and monkey stroke models [88, 93, 94].
To further investigate the effects of 20-HETE blockade on cerebral microcirculatory regulation, Marumo et al. [90] used an in-vivo two-photon imaging system to determine cerebral blood flow after ischemic stroke. They showed that in a vehicle-treated control group, blood flow velocity was significantly decreased at 1 and 2 h after reperfusion, at 4 h after reperfusion, blood flow velocity returned to preocclusion values, but fell again at 7 h after reperfusion. Interestingly, in the TS-011-treated group, blood flow velocities at all time-points after reperfusion were almost identical to the preocclusion value. Although these findings support the notion that 20-HETE blockade improves defects in the regulation of peri-infarct microcirculation, other studies have shown that 20-HETE blockade does not affect cerebral blood flow. For example, Renic et al. [89] showed that although 20-HETE blockade by TS-011 reduced cortical infarct volume, this treatment did not affect CBF during or up to 30 mins after the ischemic period. Similarly, a previous study [95] reported that 20-HETE blockade by HET0016 did not affect pial arteriolar dilation during transient MCAO, suggestion that 20-HETE blockade does not affect cerebral microcirculation after ischemic stroke. Thus, the contribution of 20-HETE in regulating cerebral blood flow after ischemic stroke remains unresolved, and this subject requires further investigation.
It has been postulated that the beneficial effects of 20-HETE blockade in cerebral ischemia are the result of a reduction in oxidative stress and inhibition of apoptosis because in ischemic renal injury 20-HETE increases the generation of superoxide, resulting in apoptosis and cell death of renal cells [96], and 20-HETE blockade attenuated apoptosis after myocardial ischemia reperfusion injury [97]. To support this hypothesis, Renic et al. [98], using organotypic hippocampal slices after oxygen-glucose deprivation, have demonstrated that 20-HETE contributes to neuronal death through the generation of reactive oxygen species and activation of caspase-3.
As noted earlier, hemorrhagic stroke accounts for 15%-20% of all stroke incidents. Cerebral vasospasm is an important pathological event that occurs in the early phase of hemorrhagic stroke. Interestingly, substantial evidence suggests that 20-HETE constricts cerebral arteries, regulates new blood vessel growth, augments vascular remodeling, and contributes to the development of acute and delayed cerebral vasospasm [99–101]. Thus, many research has focused on the role of 20-HETE in SAH. By injecting arterial blood into the cisterna magna to mimic SAH, Kehl et al. [3] showed that 20-HETE blockade by HET0016 reduced the initial fall in cerebral blood flow by 40% after SAH. Moreover, they further showed that the concentration of 20-HETE in the cerebrospinal fluid rose from 12 to 199 ng/ml after SAH. These results demonstrate that 20-HETE plays an important role in SAH. Another study [102] has demonstrated that the development of delayed vasospasm after SAH in dogs was associated with the elevation of 20-HETE levels in cerebrospinal fluid and that 20-HETE blockade by TS-011 reversed delayed vasospasm in this model. Interestingly, a recent study by Crago et al. [99] determined whether 20-HETE levels in the cerebrospinal fluid are associated with delayed cerebral ischemia in patients with SAH. This report [99] showed that 20-HETE levels were observed in 31% of patients with SAH; also, the levels of 20-HETE were associated with delayed cerebral ischemia. These findings provide important clinical evidence that inhibiting 20-HETE synthesis could be a useful therapeutic intervention in patients with SAH.
3.5. Role of soluble epoxide hydrolase (sEH) in ischemic stroke and subarachnoid hemorrhage
Substantial evidence shows that EETs are important mediators that cause vasodilation in cerebral arterioles, regulate cerebral blood flow and neurovascular coupling, promote angiogenesis, and have protective effects against ischemia [2, 46, 51, 103]. However, chronic treatment with exogenous EETs in in-vivo experiments is impractical because EETs are rapidly degraded by sEH (Figure 2) [32]. Therefore, researchers have used sEH blockade or KO to increase EETs systemically and to investigate their effects in ischemic stroke. To examine the role of EETs in hypertension-induced ischemic stroke, Dorrance et al. [104] demonstrated that treatment with AUDA, a selective sEH blocker, significantly reduced infarct size in stroke-prone spontaneously hypertensive rats (SHRSP) with cerebral ischemia, but it did not affect the blood pressure of SHRSP. Thus, sEH blockade is protective against cerebral ischemia in a blood-pressure-independent manner in SHRSP. Moreover, Simpkins et al. [105] reported that using MCAO model, sEH blockade reduced hemispheric infarct size, reduced wall-to-lumen ratio and collagen deposition, increased cerebral microvessel density, and reduced the expression of apoptotic factors in SHPSR. These findings support the notion that sEH blockade against cerebral ischemia via vascular and neural protection in SHRSP rats. Notably, several other interesting studies have demonstrated that sEH blockade is cerebroprotective in mouse model of ischemic stroke [106], rat model of ischemic stroke [107], type-1 diabetic stroke model [108], and type-2 diabetic stroke model [109].
To further investigate the role of sEH in cerebral circulation during ischemic stroke, Zhang et al. [110] determined the impact of sEH KO with 2-hour MCAO, showing that sEH KO significantly reduced infarct size and improved regional cerebral blood flow rates after cerebral ischemia. Importantly, they further showed that 14,15-EET infusion reduced infarct size. These findings demonstrate that sEH KO is protective against ischemic stroke by a cerebrovascular mechanism. Furthermore, a recent study [111] has determined the role of sEH in the endothelium using transgenic mice with endothelial-specific expression of human sEH (Tie2-hsEH) against cerebral ischemia. This study showed that following 1 μM acetylcholine administration, cerebral blood flow was significantly reduced in Tie2-hsEH mice as compared to male wild-type mice [111]. Although no difference in infarct size was observed in male Tie2-hsEH and wild-type mice, female Tie2-hsEH mice exhibited larger infarct volumes than male in the striatum after ischemic stroke. These findings provide solid evidence that endothelial sEH is a key player in regulating cerebrovascular endothelial function as well as an important contributor to the sexually dimorphic response to cerebral ischemia.
Cerebral edema, which occurs as a result of a disruption of the blood-brain barrier, is associated with the activation of vascular inflammatory cascade, and it is a common and serious complication of hemorrhagic stroke [112]. Since EETs decrease VCAM-1 expression in cerebral microvessels and regulate dilatory responses in cerebral arterioles, recent studies [112, 113] have focused on the role of sEH in SAH. Using endovascular puncture to mimic SAH, Siler et al. [112] recently reported that wild-type mice exhibited tissue edema within 6 hours and the peaked at 24 hours, which was determined by T2-weighted MRI images, after SAH. They also demonstrated that sEH KO significantly reduced edema, reduced overall VCAM-1 uptake, and improved functional outcome after SAH as compared with wild-type mice. These findings demonstrate that sEH KO reduces vascular inflammation and edema and improves outcome after SAH. To further define the role of EETs in patients with SAH, Siler et al. [113] recently documented that 14,15-EET levels were significantly elevated in the cerebrospinal fluid of patients who had suffered SAH as compared to control subjects. This study [113] also showed that sEH KO caused higher levels of 14,15-EET relative to control mice in the whole brain and had the cerebroprotective effects from the delayed decrease in microvascular cortical perfusion after SAH. These findings demonstrate that increasing EETs levels may act as an endogenous protective response against delayed cerebral ischemia after SAH. Together, these findings provide important evidence that either inhibiting sEH or increasing EETs levels could serve as a comprehensive approach to preventing the development of delayed cerebral ischemia in SAH.
1. Conclusion
Substantial evidence has demonstrated that cerebral vascular function and dysfunction are the key factors in the onset and progression of ischemic and hemorrhagic stroke. COX and CYP-derived eicosanoids are important lipid mediators that mediate cerebral vascular tone, cerebral blood flow, and autoregulation of cerebral circulation. These lipid mediators have important functions in ischemia-induced damage. In this review, we have outlined the role of COX- and CYP-derived eicosanoids in cerebral vascular function as well as their role in ischemic stroke and SAH.
Two COXs isoforms, COX-1 and COX-2, are expressed in the brain. COX-1 is constitutively expressed, and COX-2 is highly inducible after ischemic stroke. It appears that COX-1 is involved in regulating resting cerebral blood flow, whereas COX-2 is involved in the increase of cortex blood flow that accompanies neural activity. Although animal studies have shown that inhibition or deletion of COX-2 is cerebroprotective against ischemic stroke, many clinical data demonstrate that long-term treatment with coxibs is associated with increased risk of side effects, including stroke and myocardial infarction. For this reason, research has begun to be focused on delineating the role of downstream EP receptors in ischemic stroke. Accumulating evidence has demonstrated that activation of EP2 and EP4 receptors exerts cerebroprotection in ischemic stroke. Therefore, the development of novel pharmacological agents targeting EP2 and EP4 receptors could be an important area for clinical studies in patients with ischemic stroke.
20-HETE is the major CYP product in the cerebral circulation. It is well known that CYP4A and CYP4F isoforms are the major enzymes for 20-HETE synthesis and that these enzymes are expressed in cerebral microvessels. Substantial evidence demonstrates that 20-HETE, a highly potent vasoconstrictor in cerebral arteries, depolarizes vascular smooth muscle cells by inhibiting K+ channel activity and is active in the autoregulation of cerebral blood flow. It appears that 20-HETE has detrimental effects in the brain. Also, growing evidence demonstrates that 20-HETE blockade improves defects in the autoregulation of peri-infarct microcirculation and is cerebroprotective against ischemic stroke and SAH. Although some evidence suggests that the action of 20-HETE is mediated through a 20-HETE receptor, that receptor has yet to be identified. Additional studies are needed to identify 20-HETE receptor, which will facilitate future studies in the role of 20-HETE in stroke research.
EETs, which are CYP2C and CYP2J-derived eicosanoids, are important lipid mediators in cerebral circulation. Substantial evidence demonstrates that EETs cause vasodilation in cerebral arterioles, regulate cerebral blood flow and neurovascular coupling, and have protective effects against ischemia. Since EETs are rapidly degraded by sEH, much research has focused on the role of sEH blockade and KO to increase systematic EETs levels. Evidence is accumulating that endothelial sEH is a key player in regulating cerebrovascular endothelial function and contributes to the sexually dimorphic response to cerebral ischemia. sEH blockade provides cerebroprotection in SAH. It is important to note that a recent study revealed that EET analog has beneficial effects in hypertension and renal protection [114]. Thus, the development of novel EET analogs for in-vivo studies will be an interesting approach in investigating the role of EETs in ischemic stroke and SAH.
Highlights.
Long-term use of COX-2 inhibitors (coxibs) increase risk of stroke, and 20-HETE blockade is a possible strategy to prevent coxib-induced stroke events.
Activation of EP2 and EP4 receptors exerts cerebroprotection against ischemic stroke.
20-HETE has detrimental effects in the brain, and that its blockade exerts cerebroprotection against ischemic stroke and subarachnoid hemorrhage (SAH).
sEH blockade is cerebroprotective against ischemic stroke and SAH.
Acknowledgments
Funding
Part of the work described in this review was supported by NSFC (81422011, 81370837, and 81170647) to H. Huang, and National Eye Institute R01 grant (EY023315) to M. Al-Shabrawey, and an American Heart Association Grant-in-Aid Grant (AHASE 00090) to M. H. Wang.
Footnotes
Conflict of Interest
There are no conflicts to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shu S, Pei L, Lu Y. Promising targets of cell death signaling of NR2B receptor subunit in stroke pathogenesis. Regen Med Res. 2014;2:8. doi: 10.1186/2050-490X-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis CM, Fairbanks SL, Alkayed NJ. Mechanism of the sex difference in endothelial dysfunction after stroke. Transl Stroke Res. 2013;4:381–9. doi: 10.1007/s12975-012-0227-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kehl F, Cambj-Sapunar L, Maier KG, et al. 20-HETE contributes to the acute fall in cerebral blood flow after subarachnoid hemorrhage in the rat. Am J Physiol Physiol. 2002;282:H1556–H1565. doi: 10.1152/ajpheart.00924.2001. [DOI] [PubMed] [Google Scholar]
- 4.Grotta JC, Hacke W. Stroke Neurologist's Perspective on the New Endovascular Trials. Stroke. 2015;46:1447–52. doi: 10.1161/STROKEAHA.115.008384. [DOI] [PubMed] [Google Scholar]
- 5.Pierot L, Derdeyn C. Interventionalist perspective on the new endovascular trials. Stroke. 2015;46:1440–6. doi: 10.1161/STROKEAHA.115.008416. [DOI] [PubMed] [Google Scholar]
- 6.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 7.Jadhav V, Ostrowski RP, Tong W, et al. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning-induced neuroprotection in the mouse model of surgical brain injury. Stroke. 2009;40:3139–42. doi: 10.1161/STROKEAHA.109.549774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris RC. COX-2 and the kidney. J Cardiovasc Pharmacol. 2006;47(Suppl 1):S37–S42. doi: 10.1097/00005344-200605001-00007. [DOI] [PubMed] [Google Scholar]
- 9.Hao CM, Breyer MD. Roles of lipid mediators in kidney injury. Semin Nephrol. 2007;27:338–51. doi: 10.1016/j.semnephrol.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Robertson RP. Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes. 1998;47:1379–83. doi: 10.2337/diabetes.47.9.1379. [DOI] [PubMed] [Google Scholar]
- 11.Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat. 2010;91:104–12. doi: 10.1016/j.prostaglandins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candelario-Jalil E, Fiebich BL. Cyclooxygenase inhibition in ischemic brain injury. Curr Pharm Des. 2008;14:1401–18. doi: 10.2174/138161208784480216. [DOI] [PubMed] [Google Scholar]
- 13.Hewett SJ, Bell SC, Hewett JA. Contributions of cyclooxygenase-2 to neuroplasticity and neuropathology of the central nervous system. Pharmacol Ther. 2006;112:335–57. doi: 10.1016/j.pharmthera.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 14.Davidge ST. Prostaglandin H synthase and vascular function. Circ Res. 2001;89:650–60. doi: 10.1161/hh2001.098351. [DOI] [PubMed] [Google Scholar]
- 15.Sakabe T, Siesjo BK. The effect of indomethacin on the blood flow-metabolism couple in the brain under normal, hypercapnic and hypoxic conditions. Acta Physiol Scand. 1979;107:283–4. doi: 10.1111/j.1748-1716.1979.tb06476.x. [DOI] [PubMed] [Google Scholar]
- 16.Leffler CW, Busija DW, Fletcher AM, et al. Effects of indomethacin upon cerebral hemodynamics of newborn pigs. Pediatr Res. 1985;19:1160–4. doi: 10.1203/00006450-198511000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Parfenova H, Zuckerman S, Leffler CW. Inhibitory effect of indomethacin on prostacyclin receptor-mediated cerebral vascular responses. Am J Physiol. 1995;268:H1884–H1890. doi: 10.1152/ajpheart.1995.268.5.H1884. [DOI] [PubMed] [Google Scholar]
- 18.Kantor HS, Hampton M. Indomethacin in submicromolar concentrations inhibits cyclic AMP-dependent protein kinase. Nature. 1978;276:841–2. doi: 10.1038/276841a0. [DOI] [PubMed] [Google Scholar]
- 19.Niwa K, Haensel C, Ross ME, et al. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–8. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- 20.Niwa K, Araki E, Morham SG, et al. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–70. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang JS, Zhang F, Jiang M, et al. Transfection and functional expression of CYP4A1 and CYP4A2 using bicistronic vectors in vascular cells and tissues. J Pharmacol Exp Ther. 2004;311:913–20. doi: 10.1124/jpet.104.070979. [DOI] [PubMed] [Google Scholar]
- 22.Wang MH, Smith A, Zhou Y, et al. Downregulation of renal CYP-derived eicosanoid synthesis in rats with diet-induced hypertension. Hypertension. 2003;42:594–9. doi: 10.1161/01.HYP.0000090123.55365.BA. [DOI] [PubMed] [Google Scholar]
- 23.Wang MH, Guan H, Nguyen X, et al. Contribution of cytochrome P-450 4A1 and 4A2 to vascular 20-hydroxyeicosatetraenoic acid synthesis in rat kidneys. Am J Physiol. 1999;276:F246–F253. doi: 10.1152/ajprenal.1999.276.2.F246. [DOI] [PubMed] [Google Scholar]
- 24.Wang MH, Wang J, Chang HH, et al. Regulation of renal CYP4A expression and 20-HETE synthesis by nitric oxide in pregnant rats. Am J Physiol. 2003;285:F295–F302. doi: 10.1152/ajprenal.00065.2003. [DOI] [PubMed] [Google Scholar]
- 25.Nguyen X, Wang MH, Reddy KM, et al. Kinetic profile of the rat CYP4A isoforms: arachidonic acid metabolism and isoform-specific inhibitors. Am J Physiol. 1999;276:R1691–R1700. doi: 10.1152/ajpregu.1999.276.6.R1691. [DOI] [PubMed] [Google Scholar]
- 26.Muller DN, Schmidt C, Barbosa-Sicard E, et al. Mouse Cyp4a isoforms: enzymatic properties, gender- and strain-specific expression, and role in renal 20-hydroxyeicosatetraenoic acid formation. Biochem J. 2007;403:109–18. doi: 10.1042/BJ20061328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoopes SL, Garcia V, Edin ML, et al. Vascular actions of 20-HETE. Prostaglandins Other Lipid Mediat. 2015 doi: 10.1016/j.prostaglandins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harder DR, Gebremedhin D, Narayanan J, et al. Formation and action of a P-450 4A metabolite of arachidonic acid in cat cerebral microvessels. Am J Physiol. 1994;266:H2098–H2107. doi: 10.1152/ajpheart.1994.266.5.H2098. [DOI] [PubMed] [Google Scholar]
- 29.Gebremedhin D, Lange AR, Lowry TF, et al. Production of 20-HETE and its role in autoregulation of cerebral blood flow. Circ Res. 2000;87:60–5. doi: 10.1161/01.res.87.1.60. [DOI] [PubMed] [Google Scholar]
- 30.Zhou Y, Luo p, Chang HH, et al. Colfibrate attenuates blood pressure and sodium retention in DOCA-salt hypertension. Kidney Int. 2008;74:1040–8. doi: 10.1038/ki.2008.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu Z, Xu F, Huse LM, Morisseau C, et al. Soluble epoxide hydrolase regulates hydrolysis of vasoactive epoxyeicosatrienoic acids. Circ Res. 2000;87:992–8. doi: 10.1161/01.res.87.11.992. [DOI] [PubMed] [Google Scholar]
- 32.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holla VR, Makita K, Zaphiropoulos PG, et al. The kidney cytochrome P-450 2C23 arachidonic acid epoxygenase is upregulated during dietary salt loading. J Clin Invest. 1999;104:751–60. doi: 10.1172/JCI7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang QY, Ding X, Kaminsky LS. CDNA cloning, heterologous expression, and characterization of rat intestinal CYP2J4. Arch Biochem Biophys. 1997;340:270–8. doi: 10.1006/abbi.1997.9922. [DOI] [PubMed] [Google Scholar]
- 35.Zhang QY, Dunbar D, Kaminsky LS. Characterization of mouse small intestinal cytochrome P450 expression. Drug Metab Dispos. 2003;31:1346–51. doi: 10.1124/dmd.31.11.1346. [DOI] [PubMed] [Google Scholar]
- 36.Spector AA, Fang X, Snyder GD, et al. Epoxyeicosatrienoic acids (EETs): metabolism and biochemical function. Prog Lipid Res. 2004;43:55–90. doi: 10.1016/s0163-7827(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 37.Imig JD. Epoxide hydrolase and epoxygenase metabolites as therapeutic targets for renal diseases. Am J Physiol. 2005;289:F496–F503. doi: 10.1152/ajprenal.00350.2004. [DOI] [PubMed] [Google Scholar]
- 38.Imig JD. Eicosanoid regulation of the renal vasculature. Am J Physiol Renal Physiol. 2000;279:F965–F981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 39.Oltman CL, Weintraub NL, VanRollins M, et al. Epoxyeicosatrienoic acids and dihydroxyeicosatrienoic acids are potent vasodilators in the canine coronary microcirculation. Circ Res. 1998;83:932–9. doi: 10.1161/01.res.83.9.932. [DOI] [PubMed] [Google Scholar]
- 40.Larsen BT, Miura H, Hatoum OA, et al. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. Am J Physiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hercule HC, Schunck WH, Gross V, et al. Interaction between P450 eicosanoids and nitric oxide in the control of arterial tone in mice. Arterioscler Thromb Vasc Biol. 2009;29:54–60. doi: 10.1161/ATVBAHA.108.171298. [DOI] [PubMed] [Google Scholar]
- 42.Manhiani M, Quigley JE, Knight SF, et al. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol. 2009;297:F740–8. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Imig JD, Zhao X, Capdevila JH, et al. Soluble epoxide hydrolase inhibition lowers arterial blood pressure in angiotensin II hypertension. Hypertension. 2002;39:690–4. doi: 10.1161/hy0202.103788. [DOI] [PubMed] [Google Scholar]
- 44.Elmarakby AA, Faulkner J, Al-Shabrawey M, et al. Deletion of soluble epoxide hydrolase gene improves renal endothelial function and reduces renal inflammation and injury in streptozotocin-induced type 1 diabetes. Am J Physiol. 2011;301:R1307–R1317. doi: 10.1152/ajpregu.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parrish AR, Chen G, Burghardt RC, et al. Attenuation of cisplatin nephrotoxicity by inhibition of soluble epoxide hydrolase. Cell Biol Toxicol. 2009;25:217–25. doi: 10.1007/s10565-008-9071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imig JD, Simpkins AN, Renic M, et al. Cytochrome P450 eicosanoids and cerebral vascular function. Expert Rev Mol Med. 2011;13:e7. doi: 10.1017/S1462399411001773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alkayed NJ, Narayanan J, Gebremedhin D, et al. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–9. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- 48.Gebremedhin D, Yamaura K, Zhang C, et al. Metabotropic glutamate receptor activation enhances the activities of two types of Ca2+-activated K+ channels in rat hippocampal astrocytes. J Neurosci. 2003;23:1678–87. doi: 10.1523/JNEUROSCI.23-05-01678.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamaura K, Gebremedhin D, Zhang C, et al. Contribution of epoxyeicosatrienoic acids to the hypoxia-induced activation of Ca2+ -activated K+ channel current in cultured rat hippocampal astrocytes. Neuroscience. 2006;143:703–16. doi: 10.1016/j.neuroscience.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 50.Liu X, Li C, Gebremedhin D, Hwang SH, et al. Epoxyeicosatrienoic acid-dependent cerebral vasodilation evoked by metabotropic glutamate receptor activation in vivo. Am J Physiol. 2011;301:H373–H381. doi: 10.1152/ajpheart.00745.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koehler RC, Roman RJ, Harder DR. Astrocytes and the regulation of cerebral blood flow. Trends Neurosci. 2009;32:160–9. doi: 10.1016/j.tins.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 52.Peng X, Carhuapoma JR, Bhardwaj A, et al. Suppression of cortical functional hyperemia to vibrissal stimulation in the rat by epoxygenase inhibitors. Am J Physiol. 2002;283:H2029–H2037. doi: 10.1152/ajpheart.01130.2000. [DOI] [PubMed] [Google Scholar]
- 53.Peng X, Zhang C, Alkayed NJ, et al. Dependency of cortical functional hyperemia to forepaw stimulation on epoxygenase and nitric oxide synthase activities in rats. J Cereb Blood Flow Metab. 2004;24:509–17. doi: 10.1097/00004647-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 54.Nogawa S, Zhang F, Ross ME, et al. Cyclooxygenase-2 gene expression in neurons contributes to ischemic brain damage. J Neurosci. 1997;17:2746–55. doi: 10.1523/JNEUROSCI.17-08-02746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sugimoto K, Iadecola C. Delayed effect of administration of COX-2 inhibitor in mice with acute cerebral ischemia. Brain Res. 2003;960:273–6. doi: 10.1016/s0006-8993(02)03805-2. [DOI] [PubMed] [Google Scholar]
- 56.Iadecola C, Niwa K, Nogawa S, et al. Reduced susceptibility to ischemic brain injury and N-methyl-D-aspartate-mediated neurotoxicity in cyclooxygenase-2-deficient mice. Proc Natl Acad Sci. 2001;98:1294–9. doi: 10.1073/pnas.98.3.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peura DA, Goldkind L. Balancing the gastrointestinal benefits and risks of nonselective NSAIDs. Arthritis Res Ther. 2005;7:S7–13. doi: 10.1186/ar1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Hoda MN, Zheng X, et al. Combined therapy with COX-2 inhibitor and 20-HETE inhibitor reduces colon tumor growth and the adverse effects of ischemic stroke associated with COX-2 inhibition. Am J Physiol. 2014;307:R693–R703. doi: 10.1152/ajpregu.00422.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bresalier RS, Sandler RS, Quan H, et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N Engl J Med. 2005;352:1092–102. doi: 10.1056/NEJMoa050493. [DOI] [PubMed] [Google Scholar]
- 60.Wang D, DuBois RN. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene. 2010;29:781–8. doi: 10.1038/onc.2009.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greene ER, Huang S, Serhan CN, et al. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011;96:27–36. doi: 10.1016/j.prostaglandins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang D, DuBois RN. Eicosanoids and cancer. Nat Rev Cancer. 2010;10:181–93. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cathcart MC, Lysaght J, Pidgeon GP. Eicosanoid signalling pathways in the development and progression of colorectal cancer: novel approaches for prevention/intervention. Cancer Metastasis Rev. 2011;30:363–85. doi: 10.1007/s10555-011-9324-x. [DOI] [PubMed] [Google Scholar]
- 64.Dixon DA, Blanco FF, Bruno A, et al. Mechanistic aspects of COX-2 expression in colorectal neoplasia. Recent Results Cancer Res. 2013;191:7–37. doi: 10.1007/978-3-642-30331-9_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim B, Giardiello FM. Chemoprevention in familial adenomatous polyposis. Best Pract Res Clin Gastroenterol. 2011;25:607–22. doi: 10.1016/j.bpg.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mohammed A, Janakiram NB, Li Q, et al. Chemoprevention of colon and small intestinal tumorigenesis in APC(Min/+) mice by licofelone, a novel dual 5-LOX/COX inhibitor: potential implications for human colon cancer prevention. Cancer Prev Res. 2011;4:2015–26. doi: 10.1158/1940-6207.CAPR-11-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Atukorala I, Hunter DJ. Valdecoxib : the rise and fall of a COX-2 inhibitor. Expert Opin Pharmacother. 2013;14:1077–86. doi: 10.1517/14656566.2013.783568. [DOI] [PubMed] [Google Scholar]
- 68.Warner TD, Mitchell JA. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet. 2008;371:270–3. doi: 10.1016/S0140-6736(08)60137-3. [DOI] [PubMed] [Google Scholar]
- 69.Scheiman JM, Hindley CE. Strategies to optimize treatment with NSAIDs in patients at risk for gastrointestinal and cardiovascular adverse events. Clin Ther. 2010;32:667–77. doi: 10.1016/j.clinthera.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 70.Roumie CL, Mitchel EF, Jr, Kaltenbach L, et al. Nonaspirin NSAIDs, cyclooxygenase 2 inhibitors, and the risk for stroke. Stroke. 2008;39:2037–45. doi: 10.1161/STROKEAHA.107.508549. [DOI] [PubMed] [Google Scholar]
- 71.FitzGerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–11. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 72.Funk CD, FitzGerald GA. COX-2 inhibitors and cardiovascular risk. J Cardiovasc Pharmacol. 2007;50:470–9. doi: 10.1097/FJC.0b013e318157f72d. [DOI] [PubMed] [Google Scholar]
- 73.Liu JY, Li N, Yang J, Li N, et al. Metabolic profiling of murine plasma reveals an unexpected biomarker in rofecoxib-mediated cardiovascular events. Proc Natl Acad Sci. 2010;107:17017–22. doi: 10.1073/pnas.1011278107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iadecola C, Gorelick PB. The Janus face of cyclooxygenase-2 in ischemic stroke: shifting toward downstream targets. Stroke. 2005;36:182–5. doi: 10.1161/01.STR.0000153797.33611.d8. [DOI] [PubMed] [Google Scholar]
- 75.Saleem S, Li RC, Wei G, et al. Effects of EP1 receptor on cerebral blood flow in the middle cerebral artery occlusion model of stroke in mice. J Neurosci Res. 2007;85:2433–40. doi: 10.1002/jnr.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahmad AS, Yun YT, Ahmad M, et al. Selective blockade of PGE2 EP1 receptor protects brain against experimental ischemia and excitotoxicity, and hippocampal slice cultures against oxygen-glucose deprivation. Neurotox Res. 2008;14:343–51. doi: 10.1007/BF03033858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou P, Qian L, Chou T, et al. Neuroprotection by PGE2 receptor EP1 inhibition involves the PTEN/AKT pathway. Neurobiol Dis. 2008;29:543–51. doi: 10.1016/j.nbd.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.McCullough L, Wu L, Haughey N, et al. Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci. 2004;24:257–68. doi: 10.1523/JNEUROSCI.4485-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu D, Wu L, Breyer R, et al. Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol. 2005;57:758–61. doi: 10.1002/ana.20461. [DOI] [PubMed] [Google Scholar]
- 80.Li J, Liang X, Wang Q, et al. Misoprostol, an anti-ulcer agent and PGE2 receptor agonist, protects against cerebral ischemia. Neurosci Lett. 2008;438:210–5. doi: 10.1016/j.neulet.2008.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saleem S, Kim YT, Maruyama T, et al. Reduced acute brain injury in PGE2 EP3 receptor-deficient mice after cerebral ischemia. J Neuroimmunol. 2009;208:87–93. doi: 10.1016/j.jneuroim.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahmad M, Ahmad AS, Zhuang H, et al. Stimulation of prostaglandin E2-EP3 receptors exacerbates stroke and excitotoxic injury. J Neuroimmunol. 2007;184:172–9. doi: 10.1016/j.jneuroim.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108:25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dumont I, Hou X, Hardy P, et al. Developmental regulation of endothelial nitric oxide synthase in cerebral vessels of newborn pig by prostaglandin E2. J Pharmacol Exp Ther. 1999;291:627–33. [PubMed] [Google Scholar]
- 85.Liang X, Lin L, Woodling NS, et al. Signaling via the prostaglandin E(2) receptor EP4 exerts neuronal and vascular protection in a mouse model of cerebral ischemia. J Clin Invest. 2011;121:4362–71. doi: 10.1172/JCI46279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Akram A, Gibson CL, Grubb BD. Neuroprotection mediated by the EP(4) receptor avoids the detrimental side effects of COX-2 inhibitors following ischaemic injury. Neuropharmacology. 2013;65:165–72. doi: 10.1016/j.neuropharm.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 87.Shi J, Johansson J, Woodling NS, et al. The prostaglandin E2 E-prostanoid 4 receptor exerts anti-inflammatory effects in brain innate immunity. J Immunol. 2010;184:7207–18. doi: 10.4049/jimmunol.0903487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miyata N, Seki T, Tanaka Y, et al. Beneficial effects of a new 20-hydroxyeicosatetraenoic acid synthesis inhibitor, TS-011 [N-(3-chloro-4-morpholin-4-yl) phenyl-N'-hydroxyimido formamide], on hemorrhagic and ischemic stroke. J Pharmacol Exp Ther. 2005;314:77–85. doi: 10.1124/jpet.105.083964. [DOI] [PubMed] [Google Scholar]
- 89.Renic M, Klaus JA, Omura T, et al. Effect of 20-HETE inhibition on infarct volume and cerebral blood flow after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2009;29:629–39. doi: 10.1038/jcbfm.2008.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Marumo T, Eto K, Wake H, et al. The inhibitor of 20-HETE synthesis, TS-011, improves cerebral microcirculatory autoregulation impaired by middle cerebral artery occlusion in mice. Br J Pharmacol. 2010;161:1391–402. doi: 10.1111/j.1476-5381.2010.00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Poloyac SM, Zhang Y, Bies RR, et al. Protective effect of the 20-HETE inhibitor HET0016 on brain damage after temporary focal ischemia. J Cereb Blood Flow Metab. 2006;26:1551–61. doi: 10.1038/sj.jcbfm.9600309. [DOI] [PubMed] [Google Scholar]
- 92.Yang ZJ, Carter EL, Kibler KK, et al. Attenuation of neonatal ischemic brain damage using a 20-HETE synthesis inhibitor. J Neurochem. 2012;121:168–79. doi: 10.1111/j.1471-4159.2012.07666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tanaka Y, Omura T, Fukasawa M, et al. Continuous inhibition of 20-HETE synthesis by TS-011 improves neurological and functional outcomes after transient focal cerebral ischemia in rats. Neurosci Res. 2007;59:475–80. doi: 10.1016/j.neures.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 94.Omura T, Tanaka Y, Miyata N, et al. Effect of a new inhibitor of the synthesis of 20-HETE on cerebral ischemia reperfusion injury. Stroke. 2006;37:1307–13. doi: 10.1161/01.STR.0000217398.37075.07. [DOI] [PubMed] [Google Scholar]
- 95.Cao S, Wang LC, Kwansa H, et al. Endothelin rather than 20-HETE contributes to loss of pial arteriolar dilation during focal cerebral ischemia with and without polymeric hemoglobin transfusion. Am J Physiol. 2009;296:R1412–R1418. doi: 10.1152/ajpregu.00003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nilakantan V, Maenpaa C, Jia G, et al. 20-HETE-mediated cytotoxicity and apoptosis in ischemic kidney epithelial cells. Am J Physiol. 2008;294:F562–F570. doi: 10.1152/ajprenal.00387.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lv X, Wan J, Yang J, et al. Cytochrome P450 omega-hydroxylase inhibition reduces cardiomyocyte apoptosis via activation of ERK1/2 signaling in rat myocardial ischemia-reperfusion. Eur J Pharmacol. 2008;596:118–26. doi: 10.1016/j.ejphar.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 98.Renic M, Kumar SN, Gebremedhin D, et al. Protective effect of 20-HETE inhibition in a model of oxygen-glucose deprivation in hippocampal slice cultures. Am J Physiol. 2012;302:H1285–H1293. doi: 10.1152/ajpheart.00340.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crago EA, Thampatty BP, Sherwood PR, et al. Cerebrospinal fluid 20-HETE is associated with delayed cerebral ischemia and poor outcomes after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42:1872–7. doi: 10.1161/STROKEAHA.110.605816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Roman RJ, Renic M, Dunn KM, et al. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurol Res. 2006;28:738–49. doi: 10.1179/016164106X152016. [DOI] [PubMed] [Google Scholar]
- 101.Donnelly MK, Crago EA, Conley YP, et al. 20-HETE is associated with unfavorable outcomes in subarachnoid hemorrhage patients. J Cereb Blood Flow Metab. 2015 doi: 10.1038/jcbfm.2015.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hacein-Bey L, Harder DR, Meier HT, et al. Reversal of delayed vasospasm by TS-011 in the dual hemorrhage dog model of subarachnoid hemorrhage. Am J Neuroradiol. 2006;27:1350–4. [PMC free article] [PubMed] [Google Scholar]
- 103.Iliff JJ, Jia J, Nelson J, et al. Epoxyeicosanoid signaling in CNS function and disease. Prostaglandins Other Lipid Mediat. 2010;91:68–84. doi: 10.1016/j.prostaglandins.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dorrance AM, Rupp N, Pollock DM, et al. An epoxide hydrolase inhibitor, 12-(3-adamantan-1-yl-ureido)dodecanoic acid (AUDA), reduces ischemic cerebral infarct size in stroke-prone spontaneously hypertensive rats. J Cardiovasc Pharmacol. 2005;46:842–8. doi: 10.1097/01.fjc.0000189600.74157.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simpkins AN, Rudic RD, Schreihofer DA, et al. Soluble epoxide inhibition is protective against cerebral ischemia via vascular and neural protection. Am J Pathol. 2009;174:2086–95. doi: 10.2353/ajpath.2009.080544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang W, Koerner IP, Noppens R, et al. Soluble epoxide hydrolase: a novel therapeutic target in stroke. J Cereb Blood Flow Metab. 2007;27:1931–40. doi: 10.1038/sj.jcbfm.9600494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shaik JS, Ahmad M, Li W, et al. Soluble epoxide hydrolase inhibitor trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is neuroprotective in rat model of ischemic stroke. Am J Physiol. 2013;305:H1605–H1613. doi: 10.1152/ajpheart.00471.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jouihan SA, Zuloaga KL, Zhang W, et al. Role of soluble epoxide hydrolase in exacerbation of stroke by streptozotocin-induced type 1 diabetes mellitus. J Cereb Blood Flow Metab. 2013;33:1650–6. doi: 10.1038/jcbfm.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zuloaga KL, Krasnow SM, Zhu X, et al. Mechanism of protection by soluble epoxide hydrolase inhibition in type 2 diabetic stroke. PLoS One. 2014;9:e97529. doi: 10.1371/journal.pone.0097529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang W, Otsuka T, Sugo N, et al. Soluble epoxide hydrolase gene deletion is protective against experimental cerebral ischemia. Stroke. 2008;39:2073–8. doi: 10.1161/STROKEAHA.107.508325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang W, Davis CM, Edin ML, et al. Role of endothelial soluble epoxide hydrolase in cerebrovascular function and ischemic injury. PLoS One. 2013;8:e61244. doi: 10.1371/journal.pone.0061244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Siler DA, Berlow YA, Kukino A, et al. Soluble epoxide hydrolase in hydrocephalus, cerebral edema, and vascular inflammation after subarachnoid hemorrhage. Stroke. 2015;46:1916–22. doi: 10.1161/STROKEAHA.114.008560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Siler DA, Martini RP, Ward JP, et al. Protective role of P450 epoxyeicosanoids in subarachnoid hemorrhage. Neurocrit Care. 2015;22:306–19. doi: 10.1007/s12028-014-0011-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khan AH, Falck JR, Manthati VL, et al. Epoxyeicosatrienoic acid analog attenuates angiotensin II hypertension and kidney injury. Front Pharmacol. 2014;5:216. doi: 10.3389/fphar.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]