Abstract
Brain ribonuclease (BRB) is a member of the ribonuclease A superfamily that is constitutively expressed in a range of tissues, and is the functional homolog of human ribonuclease 1. This study was designed to characterize BRB gene expression in granulosa cells (GC) during development of bovine dominant ovarian follicles, and to determine the hormonal regulation of BRB in GC. Estrous cycles of Holstein cows (n = 18) were synchronized and cows were ovariectomized on either day 3 to 4 or day 5 to 6 post-ovulation during dominant follicle growth and selection. Ovaries were collected, follicular fluid (FFL) was aspirated, and GC were collected for RNA isolation and quantitative PCR. Follicles were categorized as small (1 to 5 mm; pooled per ovary), medium (5 to 8 mm; individually collected) or large (8.1 to 17 mm; individually collected) based on surface diameter. Estradiol (E2) and progesterone (P4) levels were measured by RIA in FFL. Abundance of BRB mRNA in GC was 8.6- to 11.8-fold greater (P < 0.05) in small (n = 31), medium (n = 66) and large (n = 33) subordinate E2-inactive (FFL E2 < P4) follicles than in large (n = 16) dominant E2-active (FFL E2 > P4) follicles. In the largest 4 follicles, GC BRB mRNA abundance was negatively correlated (P < 0.01) with FFL E2 (r = −0.65) and E2/P4 ratio (r = −0.46). In Exp. 2, GC from large (8 to 22 mm diameter) and small (1 to 5 mm diameter) follicles were treated with IGF1 (0 or 30 ng/mL), and/or tumor necrosis factor α (TNFα) (0 or 30 ng/mL); IGF1 increased (P < 0.05) BRB mRNA abundance and TNFα decreased (P < 0.001) the IGF1-induced BRB mRNA abundance in large-follicle GC. In Exp. 3 to 6, E2, FSH, fibroblast growth factor 9 (FGF9), cortisol, wingless 3A (WNT3A), or Sonic hedgehog (SHH) did not affect (P > 0.10) abundance of BRB mRNA in GC; thyroxine and LH increased (P < 0.05) whereas prostaglandin E2 (PGE2) decreased (P < 0.05) BRB mRNA abundance in small-follicle GC. Treatment of small-follicle GC with recombinant human RNase1 increased (P < 0.05) GC numbers and estradiol production. In conclusion, BRB is a hormonally and developmentally regulated gene in bovine GC and may regulate estradiol production during follicular growth in cattle.
Keywords: Brain ribonuclease (BRB), granulosa cells, insulin-like growth factor 1 (IGF1), tumor necrosis factor α (TNFα), follicular growth
1. Introduction
Brain ribonuclease (BRB) was first isolated from bovine brain [1–3] and later found to have widespread tissue expression in cattle [3, 4]. Brain ribonuclease is a member of the ribonuclease A superfamily of 10 to 28 kDa proteins [5–8] and was recently identified as the functional homolog of human ribonuclease-1 (RNase1) [9]. Ribonuclease A superfamily proteins are multifaceted and exhibit immuno-modulatory effects [9], antitumoral activity [10] and pro-apopototic activity [11]. Pancreatic RNase A is the better described RNase A superfamily member in cattle; this RNase is thought to function to breakdown the large amounts of RNA that accumulate in the ruminant gut [12, 13]. Another well-known RNase A superfamily member, seminal ribonuclease, is produced by bovine seminal vesicles, shares 80% identity with bovine pancreatic RNase A and has cytotoxic and immunosuppressive activity needed to protect spermatozoa from the female immune system [9, 14]. The ribonuclease A superfamily member, RNase5 (also called angiogenin), has been linked to morphological changes in the bovine ovary [15] and angiogenesis [16], but whether RNase1 homologs such as BRB change during follicular development or regulate follicular atresia is unknown. A previous study found that angiogenin (ANG) was the greatest up-regulated (by 20-fold) gene in granulosa cells of cystic vs. normal dominant follicles [17]. However, Affymetrix has revised the identity of their original ANG transcript on the microarray to be BRB. Therefore, we hypothesized that BRB expression in GC increases during normal follicular development in cattle. Other genes that were significantly different between cystic and normal follicles included the top 3 reproduction-related down-regulated genes: Indian hedgehog (IHH), fibroblast growth factor 9 (FGF9), secreted frizzled protein 4 (SFRP4), and the only other reproduction-related gene in the top 10 up-regulated genes was prostaglandin E2 receptor 4 (PTGER4) [17]. We further hypothesized that one or more of these intraovarian factors may regulate BRB mRNA in GC. Therefore, we evaluated the effects of IHH, FGF9, wingless 3A (WNT3A; a ligand for SFRP4) and PGE2 on BRB mRNA abundance in GC in the present study.
Hormones have been associated with production of other RNAse A superfamily members such as RNase1 in non-ovarian tissues. For example, tumor necrosis factor-α (TNFα) decreases RNase1 production by human umbilical vein endothelial cells [18]. Although TNFα inhibits basal and FSH-induced steroidogenesis in granulosa cells (GC) and is thought to play a role in the regulation of ovarian function [19, 20], the effect of TNFα on ovarian BRB production is unknown. Similarly, IGF1 is a major ovarian tropic hormone [21, 22], but whether IGF1 alters production of BRB in ovarian cells is unknown. Other hormones such as thyroxine (T4) have been implicated in regulating both reproduction [23] and angiogenesis [24]. Investigation of the hormonal control of BRB mRNA expression in large- and small-follicle GC may reveal possible regulatory mechanisms in the ovarian ribonuclease A superfamily system. The objectives of this study were to characterize BRB mRNA in GC during development of dominant follicles in cattle, and to evaluate the effect of ovarian trophic hormones LH, FSH, estradiol (E2) and IGF1, and other hormones and factors on ovarian BRB mRNA gene expression in cultured bovine GC.
2. Materials and methods
2.1. Hormones and reagents
The hormones and reagents used in cell culture were: ovine FSH (NIDDK-oFSH-20; activity: 175 X NIH-FSH-S1 U/mg) and ovine LH (NIDDK-oLH-26; activity: 1.0 X NIH-LH-S1 U/mg) from the National Hormone & Pituitary Program (Torrance, CA, USA); carrier-free recombinant human ANG, WNT3A, FGF9, Sonic hedgehog (SHH) and IGF1, recombinant bovine TNFα, and recombinant mouse IHH (amino terminal peptide C28II) from R&D Systems (Minneapolis, MN); recombinant human RNase 1 RNase1) from Novoprotein Scientific, Inc. (Summit, NJ); testosterone from Steraloids (Wilton NH); and cortisol, T4 and E2 from Sigma-Aldrich Corp. (St. Louis, MO, USA); and fetal calf serum (FCS) from EquiTech-Bio, Inc. (Kerrville, TX). Medium used for GC isolation and culture was Dulbecco’s modified Eagle’s medium and Ham’s F-12 (1:1) containing gentamicin (0.12 mM), glutamine (2.0 mM), and sodium bicarbonate (38.5 mM; Sigma-Aldrich Corp.).
2.2. Animals and in vivo Experimental Design- Exp. 1
This experiment was performed to determine if abundance of BRB mRNA in GC changes during folliculogenesis in cattle and compare GC BRB mRNA abundance in dominant and subordinate follicles. The animal experimentation described in this report was approved by the Oklahoma State University Institutional Animal Care and Use Committee.
Non-lactating Holstein cows (n = 18) were used for this experiment. These cows were identified to be culled for non-reproductive reasons from the Oklahoma State University herd, and were housed on pasture and group-fed a total mixed ration consisting of alfalfa hay, whole cottonseed, and concentrate ad libitum. Estrous cycles were synchronized using 2 injections (im) of PGF2α (Lutalyse®, 25 mg) with an interval of 11 d. From the first injection of prostaglandin F2α to the occurrence of ovulation after the second injection, follicle development was monitored daily via ultrasonography using an Aloka 500V with a 7.5 MHz probe. Following ovulation, cows continued to be monitored with daily ultrasonography and were assigned to be ovariectomized either at days 3 to 4 (early growing phase of the first dominant follicle; n = 9 cows) or days 5 to 6 (late growing phase of the first dominant follicle; n = 9 cows). From the 18 cows used in the synchronization program, 2 failed (one from day 3 and one from day 6 groups) to ovulate and were excluded from this experiment. Both ovaries from each cow were removed via lateral incision through the left paralumbar fossa area after local anesthesia (2% lidocaine; 60 to 80 mL sc and im). After each ovariectomy, ovaries were identified as right and left, put on ice, and transported to the laboratory where diameters of all follicles ≥ 5 mm (surface diameter) in diameter were recorded, and ovarian tissue and follicular fluid (FFL) collected as previously described [25–27].
For GC sample collection, follicles were categorized by surface diameter as small (1 to 5 mm), medium (5.1 to 8 mm) or large (8.1 to 17 mm) follicles. The FFL from medium and large follicles was aspirated individually and centrifuged to obtain GC, and FFL from small follicles was pooled within each ovary and then centrifuged to obtain GC as previously described [25, 28]. After centrifugation, FFL was aspirated and stored in another tube at −20 °C for measurement of E2 and progesterone (P4) via RIA. After aspiration of FFL, each medium and large follicle was bisected in situ, the inner wall was scraped, rinsed with cell culture medium to remove any remaining GC, and these GC were combined with GC collected from FFL as previously described [25, 29]. GC collected from small follicles were kept separate for each ovary. GC were lysed in 0.5 mL of TRIzol® reagent solution (Life Technologies, Inc., Grand Island, NY) and stored frozen at −80 °C until RNA extraction (see description below).
2.3. Cell culture and in vitro Experimental Design – Exp. 2 to 6
Ovaries from non-pregnant beef cows and heifers were collected from a local slaughterhouse, and based on surface diameter, GC were collected from small (1 to 5 mm) and large (8 to 22 mm) follicles as previously described [28–31]. Cells were re-suspended in medium containing collagenase and DNase (Sigma Chemical Co.) at 1.25 mg/mL and 0.5 mg/mL, respectively, to prevent cell clumping prior to plating.
Viable cells (2.0 x 105 in 20 to 80 μL of medium) were plated on 24-well Falcon multiwell plates (Becton Dickinson, Lincoln Park, NJ, USA) in 1.0 mL of medium containing 10% FCS (v/v). Cells were cultured at 38.5 °C in 10% FCS (v/v) for the first 48 h with a medium change at 24 h. Cells were then washed twice with serum-free medium and the various treatments (see below) were applied in serum-free medium for 24 h or 48 h after which medium was aspirated and TRIzol was added to the wells for collection of cellular RNA (see below).
Exp. 2 was designed to test the effect of IGF1 and TNFα on BRB mRNA in small- and large-follicle GC. Cells were cultured for 48 h in 10% FCS and then washed twice with serum free medium (0.5 mL) and treatments applied for 24 h. Treatments were as follows: Control (no additions), IGF1 (30 ng/mL), TNFα (30 ng/mL), and TNFα plus IGF1. Doses of IGF1 and TNFα were selected based on previous studies showing that these doses significantly alter steroidogenesis [19, 32, 33]. After 24 h of treatment, cells were lysed in 0.5 mL of TRIzol for RNA extraction (see below).
Exp. 3 was designed to test the effect of FSH, E2, FGF9, and/or IHH on BRB mRNA abundance in small- and large-follicle GC. Cells were cultured for 48 h as described in Exp. 1. All 6 treatments contained IGF1 and either: Control (no additions), FSH (30 ng/mL), E2 (300 ng/mL), FSH plus E2, FSH plus FGF9 (10 ng/mL), or FSH plus IHH (1 μg/mL); FSH, E2, FGF9 and IHH were tested because of their known effects within the ovary including effects on steroidogenesis [28, 29, 33, 34] and recent implication in cystic follicle development [17]. Doses of E2, FSH, FGF9 and IHH were selected based on previous studies showing that these doses significantly alter steroidogenesis [28, 29, 34–36]. After 24 h of treatment, cells were lysed in 0.5 mL of TRIzol for RNA extraction (see below).
Exp. 4 was designed to test the effect of ANG, cortisol, PGE2, SHH, and WNT3A on BRB mRNA abundance in small-follicle bovine GC. Cortisol and PGE2 were tested because of their effects on GC function [30, 37] and their reported effects on RNase5 mRNA in non-ovarian tissues [38, 39], and SHH, WNT3A and ANG were tested because of their implication in ovarian IGF1 stimulation [40] and follicle development [15, 34, 41]. Cells were cultured as described above with treatments applied for 24 h as follows (all treatments included 30 ng/mL of IGF1): Control, cortisol (300 ng/mL), PGE2 (300 ng/mL), SHH (500 ng/mL), WNT3A (300 ng/mL), and ANG (300 ng/mL). Doses of cortisol, IGF1, SHH, PGE2, and WNT3A were selected based on previous studies showing that these doses significantly alter GC function [15, 30, 34, 41]. The concentration of ANG used was selected based on studies indicating that average concentrations of ANG in bovine plasma [42, 43] and human FFL [44, 45] range between 3 and 300 ng/mL. After 24 h of treatment, medium was aspirated and cells were lysed in 0.5 mL of TRIzol for RNA extraction (see below).
Exp. 5 was designed to test the effect of PGE2 on BRB mRNA in large-follicle GC because of its effects in Exp. 4. Cells were cultured as described for Exp. 2 and 4. Both treatments contained IGF1 (30 ng/mL) and either: Control (no additions) or PGE2 (300 ng/mL). Medium was changed after 24 h. After 24 h of treatment, cells were lysed in 0.5 mL of TRIzol for RNA extraction (see below).
Exp. 6 was designed to test the effect of LH and T4 and their combination on BRB mRNA in small-follicle GC. Cells were cultured as described for Exp. 2 except that 4 treatments were applied in a 2 x 2 factorial arrangement for 48 h. All 4 treatments contained IGF1 (30 ng/mL) plus FSH (30 ng/mL) and either: LH (0 or 30 ng/mL), T4 (0 or 100 ng/mL), or both LH and T4. Medium was changed after 24 h. Doses of IGF1, FSH, LH and T4 were selected based on previous studies showing these doses affect steroidogenesis [23, 33]. After 48 h of treatment, cells were lysed in 0.5 mL of TRIzol for RNA extraction (see below).
Exp. 7 was designed to evaluate the effect of RNase1, a homolog of BRB, on steroidogenesis and cell proliferation of small-follicle GC. GC were cultured as described for Exp. 2 with the following treatments applied for 40 h in serum-free medium (containing 30 ng/mL of IGF1 and FSH, and 500 ng/mL of testosterone as an estrogen precursor) after a 6 h transfection with either control (plus lipofectomine medium) or RNase1 (300 ng/mL plus lipofectomine medium). Cells were treated with 300 ng/mL of recombinant human RNase1 per well using Lipofectamine 2000 in Opti-MEM-I (Invitrogen Corp.) to promote the entry of RNase1 into granulosa cells because the action of RNase1 is thought to be intracellular and RNase1 does not have membrane receptors [46]. Small-follicle GC were selected for this experiment rather than large-follicle GC because in vitro, small-follicle GC have less BRB mRNA and thus, presumably less endogenous BRB protein than in GC cultured from large follicles.
2.4. RNA extraction and quantification
Total RNA was extracted using TRIzol reagent protocol (Life Technologies), and RNA was quantitated by spectrophotometry at 260 nm using a NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE) as previously described [31, 47]. Quantification of BRB gene expression was conducted by fluorescent real-time PCR using an ABI Prism® 7500 sequence detection system as previously described [47]. The sequences for bovine BRB (accession no. NM_173891.2) primers and probe were: forward: CTGCTACCAGAGCAAATCTACC, reverse: CTAGTCTTGTAGGCACAGTTGG, and probe: TGCCGCGAGACAGGCAGCTCTAAGTA. The internal standard was 18S rRNA. A target gene dual labeled probe (FAM-TAMRA) and an 18S probe (VIC) for TaqMan were obtained from Applied Biosystems. Data analysis was done using the comparative threshold cycle (Ct) method as previously described [31, 47]. Fold changes in BRB mRNA abundance were calculated as being equal to 2−ΔΔCt.
2.5. Radioimmunoassays (RIA)
The P4 and E2 RIA were performed as previously described [23, 25]. All samples for each experiment were run in a single assay for each of the steroid RIA. The intra-assay coefficient of variation averaged 11.6 % for the P4 RIA and 10.6% for the E2 RIA.
2.6. Statistical analysis
The analysis of Exp. 1 data aimed to determine if BRB mRNA abundance changes in GC during different periods of follicular development and to detect its relationship with steroidogenesis. Data were analyzed via factorial ANOVA with MIXED procedures of SAS for Windows (version 9.2, SAS Institute Inc., Cary, NC) and are presented as means (± SEM) of measurements. Main factors in this split unit model were days post-ovulation (main unit factor; early, days 3 to 4, and late, days 5 to 6, growing phase of the first dominant follicle), follicle group (split unit factor; n = 4) based on follicle size (small, medium, or large) and their follicle estrogenic status (E2 active: E2 > P4 concentrations or E2 inactive: E2 < P4 concentrations), and their interactions. Random effect of cow nested in day was used as the error term for day effect, and follicle group by cow nested in day was used as the error term for follicle group effect and the follicle group by day interaction. To correct for heterogeneity of variance, abundance of BRB mRNA and E2 concentrations in FFL were analyzed after transformation natural log (x + 1). Mean differences were determined by Fisher’s protected least significant differences test [48] only if significant main effects in the ANOVA were detected. To evaluate the relationships among variables measured, Pierson correlation coefficients were generated using CORR procedure of SAS (SAS Institute, Cary, NC). Significance was declared at P < 0.05.
For in vitro experiments, data are presented as the least squares means (± SEM) of measurements from 3 or more individual pools of large- and small-follicle GC used as experimental replicates. Each replicate experiment was conducted on cells collected from at least 3 animals. Each of the large-follicle GC pools was obtained from 5 to 10 follicles. Small-follicle GC were obtained from 6 to 20 ovaries within each experimental replicate. For RNA experiments (Exp. 2 to 6), treatments were applied to 4 different wells on 24-well plates, and duplicate samples for each pool and treatment were obtained by combining RNA from 2 wells. For Exp. 7, each treatment was applied to 3 different wells on 24-well plates, and medium and cells were collected from individual wells. Pool (i.e., experimental replicate) and its interaction with treatments were included in each ANOVA. Specific differences in relative fold mRNA abundance, steroid production or cell numbers among treatments were determined via ANOVA using GLM procedure of SAS (Statistical Analysis System, Cary, NC) and Fisher’s protected least significant difference procedure if significant main effects were observed [48]. Significance was declared at P < 0.05.
3. Results
3.1. Exp. 1: E2 and P4 in FFL and BRB mRNA relative abundance in GC
3.1.1. Size, E2 and P4 concentrations in FFL
Follicle estrogenic status (i.e., size and E2-active or E2-inactive; P < 0.001) but not day (P > 0.10) or their interaction (P > 0.10) influenced follicle size. Diameter of the large dominant E2-active follicles averaged 12.0 ± 0.4 mm on days 3 to 4, and 13.8 ± 0.6 mm on days 5 to 6. Diameter of large subordinate E2-inactive and medium subordinate E2-inactive follicles averaged 9.81 ± 0.36 and 6.36 ± 0.23 mm, respectively, on days 3 to 4, and 9.14 ± 0.35 and 6.38 ± 0.27 mm, respectively, on days 5 to 6. Because FFL was pooled among small follicles for each ovary, no individual diameters were recorded.
Concentrations of E2 were influenced (P < 0.001) by follicle status (i.e., size and E2-active or E2-inactive) but not day or their interaction (P > 0.10). Concentrations of E2 averaged 186.5 ± 29.5, 8.45 ± 3.7, 2.3 ± 0.8, and 2.0 ± 0.2 ng/mL in large dominant E2-active, large subordinate E2-inactive, medium E2-inactive, and small E2-inactive follicles, respectively. In an analysis of the 4 largest follicles with ‘rank’ and ‘day’ as main effects, rank was significant but day and their interaction was not. Averaged across days, F1 follicles had greater (P < 0.001) concentration of E2 than F2, F3 and F4 follicles (169, 57, 6.3, and 0.5 ± 18 ng/mL, respectively).
Concentrations of P4 were not influenced (P > 0.10) by day, follicle status (i.e., size and E2-active or E2-inactive) or their interaction. Concentrations of P4 averaged 61 ± 7, 160 ± 36, 236 ± 42, and 162 ± 26 ng/mL in large dominant E2-active, large subordinate E2-inactive, medium E2-inactive, and small E2-inactive follicles, respectively. In an analysis of the 4 largest follicles with ‘rank’ and ‘day’ as main effects, rank, day and their interaction were not significant. Concentrations of P4 in F1, F2, F3 and F4 follicles averaged across days were 127, 136, 214, and 139 ± 49 ng/mL, respectively.
3.1.2. BRB mRNA relative abundance in GC
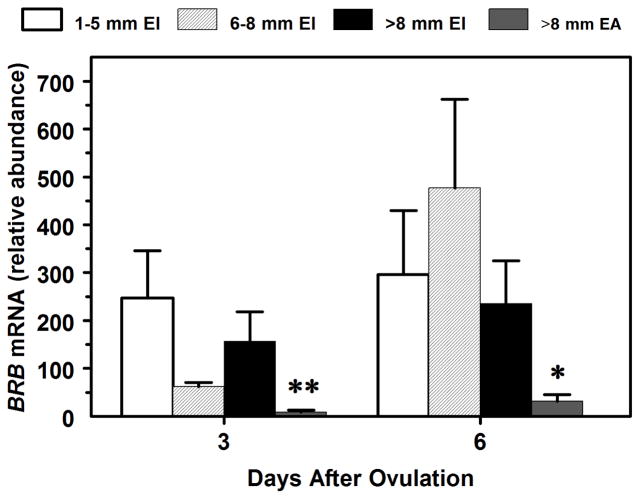
Follicle status but not day or their interaction affected abundance of BRB mRNA such that BRB mRNA abundance was 8.6- to 11.8-fold greater (P < 0.01) in subordinate large, medium and small E2-inactive (E2/P4 ratio < 1) follicles than in large dominant E2-active (E2/P4 ratio > 1) follicles on both days 3 and 6 (Fig. 1). In an analysis of the 4 largest follicles with ‘rank’ and ‘day’ as main effects, rank was significant but day or their interaction was not. Averaged across days, F1 follicles had 57% to 69% lower (P < 0.05) BRB mRNA abundance than F2, F3 and F4 follicles (data not shown). In the largest 4 follicles (n = 64) among cows, BRB mRNA abundance in GC was negatively correlated with FFL E2 (r = −0.65, P < 0.01), E2/P4 ratio (r = −0.46, P < 0.01), and diameter (r = −0.30, P < 0.05) whereas BRB mRNA abundance in GC was positively correlated with FFL P4 (r = 0.29, P < 0.05). In small-follicles (n = 31), BRB mRNA was not significantly correlated with either FFL E2 (r = 0.04) or P4 (r = 0.13).
Fig. 1.
Effects of follicular size (Large are > 8 mm diameter; Medium are 5.1 to 8 mm (6–8 mm); Small are 1 to 5 mm in diameter) and E2 status (EA = estrogen active; EI = estrogen inactive) (Panel A) and day post-ovulation (Panel B) on BRB mRNA by bovine GC. Values (n = 6 to 38 follicles per group) are normalized to constitutively expressed 18S ribosomal RNA and are least squares means ± SEM. **For day 3, mean differs (P < 0.01) from all other means. *For day 6, mean differs (P < 0.05) from small- and medium-sized follicle means.
3.2. Exp. 2: Effect of IGF1 and TNFα on small- and large-follicle GC BRB mRNA abundance
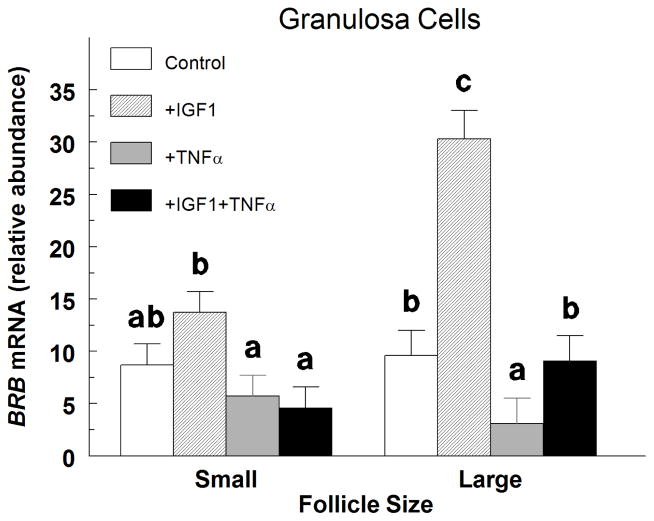
In small-follicle GC treated with IGF1, TNFα decreased (P < 0.05) the abundance of BRB mRNA by 66%, but TNFα alone did not (P > 0.10) alter BRB mRNA abundance (Fig. 2). In large-follicle GC, IGF1 increased (P < 0.0001) BRB mRNA abundance by 3.2-fold, and this increase was completely blocked with the addition of TNFα (Fig. 2). Furthermore, TNFα decreased (P < 0.05) BRB mRNA abundance by 68% compared to controls (Fig. 2).
Fig. 2.
Effect of insulin-like growth factor 1 (IGF1) and tumor necrosis factor α (TNFα) on small- and large-follicle GC BRB mRNA abundance in Exp. 2. Cells were cultured for 48 h as described in Materials and Methods, and then treated for an additional 24 h with: Control (no additions), IGF1 (30 ng/mL), TNFα (30 ng/mL) or TNFα plus IGF1. Values are means ± SEM of 3 separate experiments. abcWithin follicle size group, means without a common letter differ (P < 0.05).
3.3 Exp. 3: Effect of IGF1, FSH, E2, FGF9, and IHH on small- and large-follicle GC BRB mRNA abundance
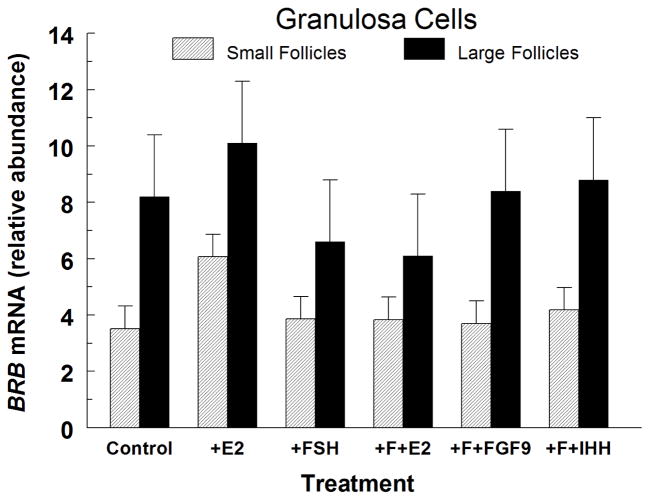
Treatment of small- and large-follicle GC with E2 or FSH alone had no effect (P > 0.15) on BRB mRNA abundance (Fig. 3). Also, E2, FGF9 and IHH had no effect (P > 0.10) on BRB mRNA abundance in FSH-treated GC from small and large follicles (Fig. 3).
Fig. 3.
Effect of estradiol (300 ng/mL; E2), FSH (30 ng/mL), FSH plus E2 (F+E2), FSH plus 30 ng/mL of FGF9 (F+FGF9), and FSH plus 1 μg/mL of IHH (F+IHH) on BRB mRNA abundance in IGF1-treated (Control) small-follicle GC of Exp. 3. Cells were cultured for 48 h as described in Materials and Methods, and then treated for 24 h with 100 ng/mL of IGF1 and the indicated hormones for 24 h. Values are means ± SEM of 3 separate experiments.
3.4. Exp. 4: Effect of ANG, cortisol, PGE2, SHH and WNT3A on small-follicle GC BRB mRNA abundance
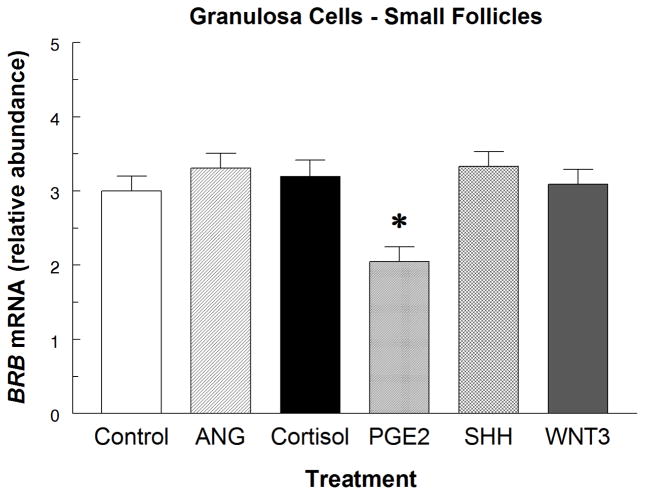
PGE2 reduced (P < 0.05) BRB mRNA abundance by 32% in IGF1-treated small-follicle GC, but there was no effect of ANG, cortisol, SHH or WNT3A (P > 0.50) on BRB mRNA abundance after 24 h of treatment (Fig. 4).
Fig. 4.
Effect of angiogenin (300 ng/mL; ANG), cortisol (300 ng/mL), prostaglandin E2 (PGE2; 300 ng/mL), Sonic hedgehog (SHH; 500 ng/mL), and wingless-3A (WNT3; 300 ng/mL) on BRB mRNA abundance in IGF1-treated (Control) small-follicle GC of Exp. 4. Cells were cultured for 48 h as described in Materials and Methods, and then treated for 24 h with 30 ng/mL of IGF1 and the indicated hormones for 24 h. Values are means ± SEM of 3 separate experiments. *Asterisk indicates mean differs (P < 0.05) from Control.
3.5. Exp. 5: Effect of PGE2 on large-follicle GC BRB mRNA abundance
In contrast to Exp. 4, there was no effect of PGE2 (P > 0.50) on BRB mRNA abundance in IGF1-treated large-follicle GC; relative BRB mRNA abundance averaged 10.1 and 8.77 ± 1.5 for Control and PGE2-treated cultures, respectively.
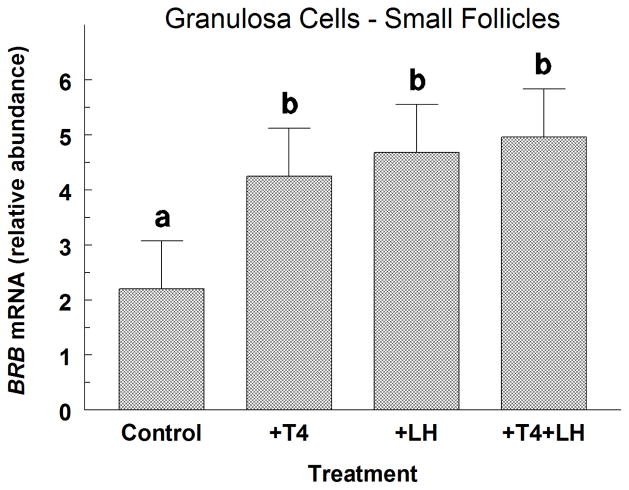
3.6. Exp. 6: Effect of LH and T4 on small-follicle GC BRB mRNA abundance
Treatment with LH alone and T4 alone increased (P < 0.05) BRB mRNA abundance by 2.1-fold and 1.9-fold, respectively in small-follicle GC treated with FSH plus IGF1 (Fig. 5). Combined treatments of LH and T4 also increased (P < 0.05) BRB mRNA abundance above controls, but these levels did not differ (P > 0.10) from levels found in GC treated with either LH alone or T4 alone (Fig. 5).
Fig. 5.
Effect of thyroxine (T4; 100 ng/mL) and LH (30 ng/mL) on BRB mRNA abundance in FSH plus IGF1-treated (Control) small-follicle GC of Exp. 6. Cells were cultured for 48 h as described in Materials and Methods, and then treated for 48 h with 30 ng/mL of FSH and IGF1 and either T4 or LH. Values are means ± SEM of 3 separate experiments. abMeans without a common letter differ (P < 0.05).
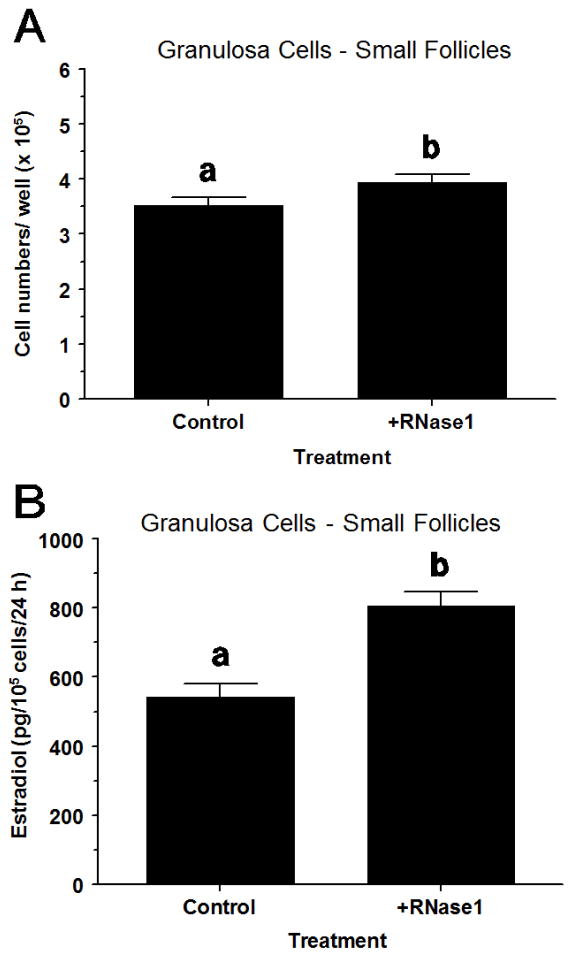
3.7. Exp. 7: Effect of RNase1 on small-follicle GC steroidogenesis and cell numbers
In small-follicle GC, RNase1 increased (P < 0.05) cell numbers by 12% (Fig. 6A) and increased (P < 0.05) E2 production by 49% (Fig. 6B) compared to vehicle-treated controls. However, RNase1 did not (P > 0.10) alter progesterone production (data not shown).
Fig. 6.
Effect of recombinant human RNase1 on proliferation and estradiol production in FSH plus IGF1-treated small-follicle GC of Exp. 7. Cells were cultured for 48 h as described in Materials and Methods, and then treated for 40 h with RNase1 (0 or 300 ng/mL), FSH (30 ng/mL) and IGF1 (30 ng/mL). Values are means ± SEM of 3 separate experiments. abWithin a panel, means without a common letter differ (P < 0.05).
4. Discussion
The current study was conducted to determine if BRB mRNA abundance in GC changes during follicle development, and to determine if various hormones alter ovarian BRB mRNA gene expression in bovine GC in vitro. Results revealed that in GC: 1) BRB mRNA abundance was less in E2-active dominant follicles than E2-inactive subordinate follicles; 2) IGF1, LH and T4 increased BRB mRNA abundance, 3) TNFα and PGE2 inhibited IGF1-induced BRB mRNA abundance; 4) FSH, FGF9, E2, cortisol, and IHH/SHH had no effect on IGF1-induced BRB mRNA abundance; and 5) exogenous RNase1 increased numbers of GC and increased GC E2 production.
The abundance of BRB mRNA in GC was less in large E2-active dominant follicles at early and late growing phases of the first follicular wave than in small, medium and large E2-inactive subordinate follicles, indicating that BRB production likely decreases as the follicle becomes dominant and increased steroidogenesis is required for its further differentiation. Interestingly, treatment of small-follicle GC with RNase1, a homologue to BRB, increased E2 production in the present study. Perhaps BRB stimulates E2 production in small follicles but inhibits E2 production in large follicles. The negative correlation between BRB mRNA and E2 in large follicles but not small follicles supports this suggestion. If BRB is acting as a pro-apoptotic factor as suggested for some RNases [10], decreases in BRB would be needed during follicle growth and dominance. Another possibility is that if BRB has cytotoxic effects similar to bovine seminal RNase [6, 14] then BRB activity would have to be low in the oocyte-cumulus cell complexes when ovulated so the oocyte and sperm are allowed to survive during the fertilization process. However, our in vitro study indicated that RNase1 increases cell numbers, and thus would be considered anti-apoptotic, at least in small-follicle GC. Further research will be required to further clarify the functional role of BRB in follicular development of cattle.
Levels of E2 in FFL and E2:P4 ratio were negatively correlated with GC BRB mRNA abundance in the present study. The possibility that increased E2 may directly inhibit BRB mRNA expression was discounted because in vitro, E2 had no significant effect on bovine GC BRB mRNA abundance in Exp. 3. Similarly, based on results from Exp. 3, FSH is also not likely involved with changes in BRB expression in GC. Consistent with our findings that E2 had no effect on BRB mRNA abundance, Koga et al. [49] found no differences in RNase5 mRNA expression in human endometrial stromal cells treated with E2 alone for 4 to 18 d. The absence of any change in FFL P4 levels during significant changes in GC BRB mRNA abundance and its low positive correlation with FFL P4 indicates that P4 likely does not regulate BRB mRNA. Conversely, because treatment of GC with RNase1 had no effect on P4 production in the present study, it is likely that BRB does not regulate progesterone production in bovine granulosa cells. Further research will be required to verify these suggestions.
Consistent with findings of the present study (where LH increased GC BRB mRNA in Exp. 6), Koga et al. [44] found that RNase5 mRNA was upregulated by hCG in cultured human GC. Because RNase A family members exhibit pro-apoptotic activity [10], BRB may be involved in initiating atresia at the end of the first follicular wave in a monovulatory species such as the bovine. In addition to LH, IGF1 increased BRB mRNA abundance in large-follicle GC by several fold in the present study. IGF1 has been shown to be positively correlated with RNase5 levels in blood serum in humans [50] and further supports a role for IGF1 in regulating ribonucleases. Because dominant follicles have greater free IGF1 than subordinate follicles [22, 51] and greater LH receptors in GC [52, 53] but less BRB mRNA in GC, the hormonal regulation of decreased BRB mRNA in E2-active dominant follicles will require further elucidation.
In the present study, TNFα decreased IGF1-induced BRB mRNA abundance in small- and large-follicle GC implicating the immune system in regulating ovarian ribonucleases. Similarly, Gansler et al. [18] found that TNFα decreases RNase1 production by human umbilical vein endothelial cells. In contrast, Etoh et al. [54] found that TNFα induced RNase5 mRNA expression in human colon cancer cells. Perhaps differences in the specific RNase, species and/or tissue exist in their response to TNFα, and will require further elucidation. The present finding that the inhibitory effect of TNFα and stimulatory effect of IGF1 on BRB mRNA abundance was more pronounced in large- than small-follicle GC indicates that BRB gene expression is under developmental and opposing control by TNFα and IGF1. Developmental differences in the steroidogenic response to TNFα in bovine GC [19, 55] has also been reported. In another physiological context, TNFα increases within the corpus luteum during luteal regression [20] and increases during infections [56–58] in cattle. Thus, our studies support the hypothesis that increased TNFα either during normal luteal regression or during acute-phase responses (i.e., disease) may impact ovarian follicular function via altered follicular BRB production. Because there were developmental differences in the BRB mRNA response to TNFα, changes in this response during follicular growth should be explored in further detail in cattle and other species.
In the present study, ANG, cortisol, FGF9, IHH/SHH, and WNT3A had no effect on BRB mRNA abundance in GC, whereas T4 increased and PGE2 decreased BRB mRNA expression in GC, a novel finding. It should be cautioned that the present study evaluated only a single dose of these intraovarian factors and thus, further work will be required to ascertain whether lower or higher doses of these factors affect BRB expression in GC. The inhibitory effect of PGE2 on BRB mRNA was observed in small-follicle GC but not in large-follicle GC. In cattle, FFL levels of PGE2 increase dramatically after the LH surge/GnRH treatment [59, 60], LH induces PGE2 production by GC [61], and PGE2 amplifies insulin-induced oxytocin release by GC [37]. Perhaps increased PGE2 released by the ovulatory follicle acts as a paracrine factor inhibiting small-follicle BRB production. The novel finding that T4 increased BRB mRNA further links the thyroid gland to ovarian function in cattle. In bovine theca cells, T4 stimulates LH-induced steroidogenesis [23] and is thought to play a role in the regulation of angiogenesis in several tissues [24] including the ovary [62]. The present study further expands the role of PGE2 and T4 to that of regulating production of BRB within the follicle.
Conclusions
In conclusion, BRB mRNA expression in GC is less in E2-active dominant follicles than E2-inactive subordinate follicles and is positively regulated by IGF1, T4 and LH and negatively regulated by PGE2 and TNFα. Because BRB mRNA is decreased in dominant follicles and is increased in cystic and E2-inactive follicles, BRB could be preventing differentiation and/or ovulation in bovine follicles. Based on studies with RNase1, a BRB homologue, indicating that BRB may stimulate E2 production and proliferation of small-follicle GC, we hypothesize that inhibition of BRB may be more important for successful ovulation rather than for differentiation. Additional research is needed to further elucidate the physiological functions of BRB and its interactions with IGF1, LH, T4, PGE2 and TNFα during growth and atresia of ovarian follicles in cattle.
Highlights.
Brain ribonuclease (BRB) mRNA is lower in dominant vs. subordinate follicles.
We examine which hormones regulate BRB mRNA in granulosa cells.
IGF1, LH and thyroxine increase whereas TNFalpha and PGE2 decrease BRB mRNA.
RNase1, a BRB homologue, stimulates granulosa cell estradiol production.
Acknowledgments
The authors thank: J. Evans, J. Williams, and A. Burress for technical assistance; Cristina Cortinovis for assistance with ultrasound work; David Jones, Jeff Davis and other members of the OSU Dairy Cattle Center for care and management of the cows; the OSU Microarray Core Facility and OSU Recombinant DNA/Protein Resource Facility for use of equipment; the Bill & Melinda Gates Foundation for financial support of J. Dentis; Dr. A. F. Parlow, National Hormone & Pituitary Program, (Torrance, CA) for purified LH and FSH; and Creekstone Farms (Arkansas City, KS) for their generous donations of bovine ovaries. Approved for publication by the Director, Oklahoma Agric. Exp. Sta., and supported in part by: the NICHD, National Institutes of Health, through Agreement R15-HD-066302, and the Oklahoma State University Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elson M, Glitz DG. Characterization of a ribonuclease from bovine brain. Biochemistry. 1975;14:1471–6. doi: 10.1021/bi00678a019. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe H, Katoh H, Ishii M, Komoda Y, Sanda A, Takizawa Y, Ohgi K, Irie M. Primary structure of a ribonuclease from bovine brain. J Biochem. 1988;104:939–45. doi: 10.1093/oxfordjournals.jbchem.a122587. [DOI] [PubMed] [Google Scholar]
- 3.Sasso MP, Lombardi M, Confalone E, Carsana A, Palmieri M, Furia A. The differential pattern of tissue-specific expression of ruminant pancreatic type ribonucleases may help to understand the evolutionary history of their genes. Gene. 1999;227:205–12. doi: 10.1016/s0378-1119(98)00586-1. [DOI] [PubMed] [Google Scholar]
- 4.Wheeler TT, Maqbool NJ, Gupta SK. Mapping, phylogenetic and expression analysis of the RNase (RNase A) locus in cattle. J Mol Evol. 2012;74:237–48. doi: 10.1007/s00239-012-9502-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Confalone E, Breukelman HJ, Sasso MP, Jekel PA, Hodge E, Furia A, Beintema JJ. Ruminant brain ribonucleases: expression and evolution. Biochim Biophys Acta. 2001;1547:95–103. doi: 10.1016/s0167-4838(01)00173-x. [DOI] [PubMed] [Google Scholar]
- 6.Gupta SK, Haigh BJ, Griffin FJ, Wheeler TT. The mammalian secreted RNases: mechanisms of action in host defence. Innate Immun. 2013;19:86–97. doi: 10.1177/1753425912446955. [DOI] [PubMed] [Google Scholar]
- 7.Goo SM, Cho S. The expansion and functional diversification of the mammalian ribonuclease a superfamily epitomizes the efficiency of multigene families at generating biological novelty. Genome Biol Evol. 2013;5:2124–40. doi: 10.1093/gbe/evt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Premzl M. Comparative genomic analysis of eutherian ribonuclease A genes. Mol Genet Genomics. 2014;289:161–7. doi: 10.1007/s00438-013-0801-5. [DOI] [PubMed] [Google Scholar]
- 9.Eller CH, Lomax JE, Raines RT. Bovine brain ribonuclease is the functional homolog of human ribonuclease 1. J Biol Chem. 2014;289:25996–6006. doi: 10.1074/jbc.M114.566166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ardelt W, Ardelt B, Darzynkiewicz Z. Ribonucleases as potential modalities in anticancer therapy. Eur J Pharmacol. 2009;625:181–9. doi: 10.1016/j.ejphar.2009.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gotte G, Laurents DV, Merlino A, Picone D, Spadaccini R. Structural and functional relationships of natural and artificial dimeric bovine ribonucleases: new scaffolds for potential antitumor drugs. FEBS Lett. 2013;587:3601–8. doi: 10.1016/j.febslet.2013.09.038. [DOI] [PubMed] [Google Scholar]
- 12.Barnard EA. Biological function of pancreatic ribonuclease. Nature. 1969;221:340–4. doi: 10.1038/221340a0. [DOI] [PubMed] [Google Scholar]
- 13.Rutkoski TJ, Raines RT. Evasion of ribonuclease inhibitor as a determinant of ribonuclease cytotoxicity. Curr Pharm Biotechnol. 2008;9:185–9. doi: 10.2174/138920108784567344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Alessio G, Di Donato A, Parente A, Piccoli R. Seminal RNase: a unique member of the ribonuclease superfamily. Trends Biochem Sci. 1991;16:104–6. doi: 10.1016/0968-0004(91)90042-t. [DOI] [PubMed] [Google Scholar]
- 15.Lee HS, Lee I-S, Kang T-C, Jeong GB, Chang S-I. Angiogenin is involved in morphological changes and angiogenesis in the ovary. Biochem Biophys Res Commun. 1999;257:182–6. doi: 10.1006/bbrc.1999.0359. [DOI] [PubMed] [Google Scholar]
- 16.Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin. 2008;40:619–24. doi: 10.1111/j.1745-7270.2008.00442.x. [DOI] [PubMed] [Google Scholar]
- 17.Grado-Ahuir JA, Aad PY, Spicer LJ. New insights into the pathogenesis of cystic follicles in cattle: microarray analysis of gene expression in GC. J Anim Sci. 2011;89:1769–86. doi: 10.2527/jas.2010-3463. [DOI] [PubMed] [Google Scholar]
- 18.Gansler J, Preissner KT, Fischer S. Influence of proinflammatory stimuli on the expression of vascular ribonuclease 1 in endothelial cells. FASEB J. 2014;28:752–60. doi: 10.1096/fj.13-238600. [DOI] [PubMed] [Google Scholar]
- 19.Spicer LJ, Alpizar E. Effects of cytokines on FSH-induced estradiol production by bovine GC in vitro: dependence on size of follicle. Domest Anim Endocrinol. 1994;11:25–34. doi: 10.1016/0739-7240(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 20.Sakumoto R, Berisha B, Kawate N, Schams D, Okuda K. TNFα and its receptors in bovine corpus luteum sampled by continuous-flow microdialysis during luteolysis in vivo. Biol Reprod. 2003;62:192–9. doi: 10.1095/biolreprod62.1.192. [DOI] [PubMed] [Google Scholar]
- 21.Spicer LJ, Echternkamp SE. The ovarian insulin and insulin-like growth factor system with an emphasis on domestic animals. Domest Anim Endocrinol. 1995;12:223–45. doi: 10.1016/0739-7240(95)00021-6. [DOI] [PubMed] [Google Scholar]
- 22.Spicer LJ. Proteolytic degradation of insulin-like growth factor binding proteins by ovarian follicles: a control mechanism for selection of dominant follicles. Biol Reprod. 2004;70:1223–30. doi: 10.1095/biolreprod.103.021006. [DOI] [PubMed] [Google Scholar]
- 23.Spicer LJ, Alonso J, Chamberlain CS. Effects of thyroid hormones on bovine granulosa and thecal cell function in vitro: dependence on insulin and gonadotropins. J Dairy Sci. 2001;84:1069–76. doi: 10.3168/jds.S0022-0302(01)74567-5. [DOI] [PubMed] [Google Scholar]
- 24.Pinto M, Soares P, Ribatti D. Thyroid hormone as a regulator of tumor induced angiogenesis. Cancer Lett. 2011;301:119–26. doi: 10.1016/j.canlet.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 25.Stewart RE, Spicer LJ, Hamilton TD, Keefer BE, Dawson LJ, Morgan GL, Echternkamp SE. Levels of insulin-like growth factor (IGF) binding proteins, luteinizing hormone and IGF-I receptors, and steroids in dominant follicles during the first follicular wave in cattle exhibiting regular estrous cycles. Endocrinology. 1996;137:2842–50. doi: 10.1210/endo.137.7.8770905. [DOI] [PubMed] [Google Scholar]
- 26.Spicer LJ, Chamberlain CS, Morgan GL. Proteolysis of insulin-like growth factor binding proteins during preovulatory follicular development in cattle. Domest Anim Endocrinol. 2001;21:1–15. doi: 10.1016/s0739-7240(01)00103-5. [DOI] [PubMed] [Google Scholar]
- 27.Aad PY, Echternkamp SE, Sypherd DD, Schreiber NB, Spicer LJ. The hedgehog system in ovarian follicles of cattle selected for twin ovulations and births: evidence of a link between the IGF and hedgehog systems. Biol Reprod. 2012;87:79. doi: 10.1095/biolreprod.111.096735. [DOI] [PubMed] [Google Scholar]
- 28.Langhout DJ, Spicer LJ, Geisert RD. Development of a culture system for bovine GC: effects of growth hormone, estradiol, and gonadotrophins on cell proliferation, steroidogenesis, and protein synthesis. J Anim Sci. 1991;69:3321–34. doi: 10.2527/1991.6983321x. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber NB, Spicer LJ. Effects of fibroblast growth factor 9 (FGF9) on steroidogenesis and gene expression and control of FGF9 mRNA in bovine granulosa cells. Endocrinology. 2012;153:4491–4501. doi: 10.1210/en.2012-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spicer LJ, Chamberlain CS. Influence of cortisol on insulin- and insulin-like growth factor 1 (IGF-1)-induced steroid production and on IGF-1 receptors in cultured bovine GC and thecal cells. Endocrine. 1998;9:153–61. doi: 10.1385/ENDO:9:2:153. [DOI] [PubMed] [Google Scholar]
- 31.Aad PY, Voge JL, Santiago CA, Malayer JR, Spicer LJ. Real-time RT-PCR quantification of pregnancy-associated plasma protein-A mRNA abundance in bovine granulosa and theca cells: effects of hormones in vitro. Domest Anim Endocrinol. 2006;31:357–372. doi: 10.1016/j.domaniend.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 32.Spicer LJ, Hamilton TD, Keefer BE. Insulin-like growth factor-I enhancement of steroidogenesis by bovine GC and thecal cells: Dependence on de novo cholesterol synthesis. J Endocrinol. 1996;151:365–73. doi: 10.1677/joe.0.1510365. [DOI] [PubMed] [Google Scholar]
- 33.Spicer LJ, Chamberlain CS, Maciel SM. Influence of gonadotropins on insulin- and insulin-like growth factor-I (IGF-I)-induced steroid production by bovine GC. Domest Anim Endocrinol. 2002;22:237–54. doi: 10.1016/s0739-7240(02)00125-x. [DOI] [PubMed] [Google Scholar]
- 34.Spicer LJ, Sudo S, Aad PY, Wang LS, Chun S-Y, Ben-Shlomo I, Klein C, Hsueh AJW. The hedgehog-patched signaling pathway and function in the mammalian ovary: a novel role for hedgehog proteins in stimulating proliferation and steroidogenesis of theca cells. Reproduction. 2009;138:329–39. doi: 10.1530/REP-08-0317. [DOI] [PubMed] [Google Scholar]
- 35.Schreiber NB, Totty ML, Spicer LJ. Expression and effect of fibroblast growth factor 9 in bovine theca cells. J Endocrinol. 2012;215:167–75. doi: 10.1530/JOE-12-0293. [DOI] [PubMed] [Google Scholar]
- 36.Spicer LJ. Effects of estradiol on bovine thecal cell function in vitro: dependence on insulin and gonadotropins. J Dairy Sci. 2005;88:2412–21. doi: 10.3168/jds.S0022-0302(05)72919-2. [DOI] [PubMed] [Google Scholar]
- 37.McArdle CA. Chronic regulation of ovarian oxytocin and progesterone release by prostaglandins: opposite effects in bovine granulosa and early luteal cells. J Endocrinol. 1990;126:245–53. doi: 10.1677/joe.0.1260245. [DOI] [PubMed] [Google Scholar]
- 38.Lonnroth C, Svaninger G, Gelin J, Cahlin C, Iresjo B, Cvetkovska E, Edstrom S, Andersson M, Svanberg E, Lundholm K. Effects related to indomethacin prolonged survival and decreased tumor-growth in a mouse-tumor model with cytokine dependent cancer cachexia. Int J Oncol. 1995;7:1405–13. doi: 10.3892/ijo.7.6.1405. [DOI] [PubMed] [Google Scholar]
- 39.Verselis SJ, Olson AO, Fett JW. Regulation of angiogenin expression in human HepG2 hepatoma cells by mediators of the acute-phase response. Biochem Biophys Res Commun. 1999;259:178–84. doi: 10.1006/bbrc.1999.0744. [DOI] [PubMed] [Google Scholar]
- 40.Grado-Ahuir JA, Aad PY, Ranzenigo G, Caloni F, Cremonesi F, Spicer LJ. Microarray analysis of insulin-like growth factor-I-induced changes in messenger ribonucleic acid expression in cultured porcine GC: possible role of insulin-like growth factor-I in angiogenesis. J Anim Sci. 2009;87:1921–33. doi: 10.2527/jas.2008-1222. [DOI] [PubMed] [Google Scholar]
- 41.Lapointe E, Boerboom D. WNT signaling and the regulation of ovarian steroidogenesis. Front Biosci (Schol Ed) 2011;3:276–85. doi: 10.2741/s151. [DOI] [PubMed] [Google Scholar]
- 42.Bond MD, Vallee BL. Isolation of bovine angiogenin using a placental ribonuclease inhibitor binding assay. Biochemistry. 1988;27:6282–7. doi: 10.1021/bi00417a013. [DOI] [PubMed] [Google Scholar]
- 43.Chang S-I, Jeong G-B, Park S-H, Ahn B-C, Choi J-D, Chae Q, Namgoong SK, Chung S-I. Detection, quantitation, and localization of bovine angiogenin by immunological assays. Biochem Biophys Res Commun. 1997;232:323–7. doi: 10.1006/bbrc.1997.6280. [DOI] [PubMed] [Google Scholar]
- 44.Koga K, Osuga Y, Tsutsumi O, Momoeda M, Suenaga A, Kuga K, Fujiwara T, Takai Y, Yano T, Taketani Y. Evidence for the presence of angiogenin in human follicular fluid and the up-regulation of its production by human chorionic gonadotropin and hypoxia. J Clin Endocrinol Metab. 2000;85:3352–5. doi: 10.1210/jcem.85.9.6837. [DOI] [PubMed] [Google Scholar]
- 45.Malamitsi-Puchner A, Sarandakou A, Baka S, Hasiakos D, Kouskouni E, Creatsas G. In vitro fertilization: angiogenic, proliferative, and apoptotic factors in the follicular fluid. Ann NY Acad Sci. 2003;997:124–8. doi: 10.1196/annals.1290.043. [DOI] [PubMed] [Google Scholar]
- 46.Psarras K, Ueda M, Yamamura T, Ozawa S, Kitajima M, Aiso S, Komatsu S, Seno M. Human pancreatic RNase1-human epidermal growth factor fusion: an entirely human ‘immunotoxin analog’ with cytotoxic properties against squamous cell carcinomas. Protein Eng. 1998;11:1285–92. doi: 10.1093/protein/11.12.1285. [DOI] [PubMed] [Google Scholar]
- 47.Lagaly DV, Aad PY, Grado-Ahuir JA, Hulsey LB, Spicer LJ. Role of adiponectin in regulating ovarian granulosa and theca cell function. Mol Cell Endocrinol. 2008;284:38–45. doi: 10.1016/j.mce.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 48.Ott L. An Introduction to Statistical Methods and Data Analysis. North Scituate, MA: Duxbury Press; 1977. [Google Scholar]
- 49.Koga K, Osuga Y, Tsutsumi O, Yano T, Yoshino O, Takai Y, Matsumi H, Hiroi H, Kugu K, Momoeda M, Fujiwara T, Taketani Y. Demonstration of mangiogenin in human endometrium and its enhanced expression in endometrial tissues in the secretory phase and decidua. J Clin Endocrinol Metab. 2001;86:5609–14. doi: 10.1210/jcem.86.11.8038. [DOI] [PubMed] [Google Scholar]
- 50.Silha JV, Krsek M, Hana V, Marek J, Weiss V, Jezkova J, Rosicka M, Jarkovska Z, Murphy LJ. The effects of growth hormone status on circulating levels of vascular growth factors. Clin Endocrinol. 2005;63:79–86. doi: 10.1111/j.1365-2265.2005.02303.x. [DOI] [PubMed] [Google Scholar]
- 51.Santiago CA, Voge JL, Aad PY, Allen DT, Stein DR, Malayer JR, Spicer LJ. Pregnancy- associated plasma protein-A and insulin-like growth factor binding protein mRNAs in granulosa cells of dominant and subordinate follicles of preovulatory cattle. Domest Anim Endocrinol. 2005;28:46–63. doi: 10.1016/j.domaniend.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Ginther OJ, Beg MA, Donadeu FX, Bergfelt DR. Mechanism of follicle deviation in monovular farm species. Anim Reprod Sci. 2003;78:239–257. doi: 10.1016/s0378-4320(03)00093-9. [DOI] [PubMed] [Google Scholar]
- 53.Fortune JE, Rivera GM, Yang MY. Follicular development: the role of the follicular microenvironment in selection of the dominant follicle. Anim Reprod Sci. 2004;83:109–126. doi: 10.1016/j.anireprosci.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 54.Etoh T, Kenji S, Barnard GF, Kitano S, Mori M. Angiogenin expression in human colorectal cancer: the role of focal macrophage infiltration. Clin Cancer Res. 2000;6:3545–51. [PubMed] [Google Scholar]
- 55.Basini G, Mainardi GL, Bussolati S, Tamanini C. Steroidogenesis, proliferation and apoptosis in bovine granulosa cells: role of tumour necrosis factor-α and its possible signalling mechanisms. Reprod Fertil Dev. 2002;14:141–50. doi: 10.1071/rd01049. [DOI] [PubMed] [Google Scholar]
- 56.Blum JW, Dosogne H, Hoeben D, Vangroenweghe F, Hammon HM, Bruckmaier RM, Burvenich C. Tumor necrosis factor-α and nitrite/nitrate responses during acute mastitis induced by Escherichia coli infection and endotoxin in dairy cows. Domest Anim Endocrinol. 2000;19:223–35. doi: 10.1016/s0739-7240(00)00079-5. [DOI] [PubMed] [Google Scholar]
- 57.Burciaga-Robles LO, Step DL, Krehbiel CR, Holland BP, Richards CJ, Montelongo MA, Confer AW, Fulton RW. Effects of exposure to calves persistently infected with bovine viral diarrhea virus type 1b and subsequent infection with Mannheima haemolytica on clinical signs and immune variables: model for bovine respiratory disease via viral and bacterial interaction. J Anim Sci. 2010;88:2166–78. doi: 10.2527/jas.2009-2005. [DOI] [PubMed] [Google Scholar]
- 58.Sacchini F, Luciani M, Salini R, Scacchia M, Pini A, Lelli R, Naessens J, Poole J, Jores J. Plasma levels of TNF-α, IFN-γ, IL-4 and IL-10 during a course of experimental contagious bovine pleuropneumonia. BMC Vet Res. 2012;8:44. doi: 10.1186/1746-6148-8-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Algire JE, Srikandakumar A, Guilbault LA, Downey BR. Preovulatory changes in follicular prostaglandins and their role in ovulation in cattle. Canadian J Vet Res. 1992;56:67–69. [PMC free article] [PubMed] [Google Scholar]
- 60.Peters MW, Pursley JR, Smith GW. Inhibition of intrafollicular PGE2 synthesis and ovulation following ultrasound-mediated intrafollicular injection of the selective cyclooxygenase-2 inhibitor NS-398 in cattle. J Anim Sci. 2004;82:1656–62. doi: 10.2527/2004.8261656x. [DOI] [PubMed] [Google Scholar]
- 61.Fortune JE, Willis EL, Bridges PJ, Yang CS. The periovulatory period in cattle: progesterone, prostaglandins, oxytocin and ADAMTS proteases. Anim Reprod. 2009;6:60–71. [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang JY, Miyabayashi K, Nottola SA, Umezu M, Cecconi S, Sato E, Macchiarelli G. Thyroxine treatment stimulated ovarian follicular angiogenesis in immature hypothyroid rats. Histol Histopath. 2008;23:1387–98. doi: 10.14670/HH-23.1387. [DOI] [PubMed] [Google Scholar]