Abstract
Heterologous immunity is recognized as a significant barrier to transplant tolerance. While pathogen elicited memory T cells can have high or low affinity for cross-reactive allogeneic peptide:MHC, the role T cell receptor (TCR) affinity during heterologous immunity has not been explored. We established a model with which to investigate the impact of TCR priming affinity on memory T cell populations following a graft rechallenge. In contrast to high affinity priming, low affinity priming elicited fully differentiated memory T cells with a CD45RBhi status. High CD45RB status enabled robust secondary responses in vivo, as demonstrated by faster graft rejection kinetics and greater proliferative responses. CD45RB blockade prolonged graft survival in low affinity, but not high affinity, primed mice. Mechanistically, low affinity primed memory CD8+ T cells produced more IL-2 and significantly upregulated IL-2Rα expression during rechallenge. We found that CD45RBhi status was also a stable marker of priming affinity within polyclonal CD8+ T cell populations. Following high affinity rechallenge, low affinity primed CD45RBhi cells became CD45RBlo, demonstrating that CD45RB status acts as an affinity-based differentiation switch on CD8+ T cells. Thus, these data establish a novel mechanism by which CD45 isoforms tune low affinity primed memory CD8+ T to become potent secondary effectors following heterologous rechallenge. These findings have direct implications for allogeneic heterologous immunity by demonstrating that despite a lower precursor frequency, low affinity priming is sufficient to generate memory cells that mediate potent secondary responses against a cross-reactive graft challenge.
Keywords: T cells, T cell receptors, transplantation
Introduction
Following encounter with microbial antigen, T cells can differentiate into memory cells with a multitude of phenotypic profiles in order to provide long-lasting protection against subsequent encounters with pathogens (1, 2). Memory T cells are recognized as a barrier to many immunomodulation strategies aimed at limiting alloreactive T cell responses (3). One reason is that microbe-elicited T cell memory can cross-react with allogeneic antigen and mediate graft rejection, a process termed allogeneic heterologous immunity (4, 5). In this scenario, memory CD8+ T cells that were primed with microbial antigen can recognize a unique cross-reactive allogeneic antigen. A number of studies have provided evidence of significant cross-reactivity of pathogen-elicited memory CD8+ T cells with allogeneic antigen in mice and humans (4–7).
The affinity of the TCR for antigen is also a critical facet of allogeneic heterologous immunity due to the recognition of TCR by unique microbial priming and allogeneic rechallenge antigens. Indeed, memory T cells can have high and low affinity for allogeneic antigen (5), and recent work has also demonstrated that allogeneic T cells are suited to recognize a greater number of unique peptide:MHC complexes than conventional T cells, providing strong evidence that allogeneic T cell interactions occur over a range of high and low TCR affinities (8–10). Recently, low affinity memory T cells have been studied in the context of protective and auto-immunity. However, a specific investigation of the impact of TCR priming affinity on subsequent phenotype and effector function of memory T cells during heterologous rechallenge has not been conducted.
CD45 is a transmembrane phosphatase that is known to tune proximal T cell signaling cascades via differential expression of multiple isoforms. However, the impact of T cell affinity on CD45 isoform expression and subsequent effector function has not been explored. Here we found that low affinity priming affinity dictates a distinct differentiation program compared to high affinity priming that is characterized by expression of large CD45 isoforms, denoted as CD45RBhi. A CD45RBhi status enabled low affinity primed memory CD8+ T cells to proliferate better than high affinity memory CD8+ T cells in response to a high affinity graft rechallenge. This study establishes a novel connection between the affinity of CD8+ T cell priming and CD45-mediated T cell tuning, and provides mechanistic insight into the functionality low affinity T cells in transplantation, protective immunity, and autoimmunity.
Materials & Methods
Mice
Male C57BL/6 Ly5.2-Cr (B6.SJL-Ptprca Pepcb/BoyJ; CD45.1+, H-2b) mice were obtained from the National Cancer Institute (Frederick, MD). OT-I (11) transgenic mice (C57BL/6-Tg(TcraTcrb)1100Mjb/J; H-2b), purchased from Taconic Farms, were bred to Thy1.1+ background at Emory University. mOVA mice (C57BL/6-Tg(CAG-OVA)916Jen/J; H-2b), which express membrane-bound N4 OVA under the control of the β-actin promoter, were a gift from Dr. Marc Jenkins ((12) University of Minnesota, Minneapolis, MN). All animals were maintained in accordance with Emory University Institutional Animal Care and Use Committee guidelines. All animals were housed in pathogen-free animal facilities at Emory University.
Generation of OT-I primary effectors, memory, and secondary effectors
For adoptive transfers of donor-reactive T cells, Thy1.1+OT-I mice were processed to single cell suspension and the frequency of OT-Is was determined by FACS analysis of CD8+Thy1.1+Vα2+ (BD Biosciences). Cells were resuspended in PBS and 1.0 × 104 cells were transferred intravenously into CD45.1+ male hosts. Listeria monocytogenes strains engineered to express the OVA APL epitope (LM-OVA APLs) were provided by Dr. Michael Bevan (University of Washington, Seattle, WA; (13)). Mice were infected with 104 CFU of LM-OVA APL strains intraperitoneally 24 hours after adoptive transfer. To generate secondary effectors, at 4 weeks following infection mice were immunized with 10 μg N4 OVA (SIINFEKL) peptide (GenScript, Inc) in each hind foot pad.
Assessment of in vivo OT-I populations
At primary effector time point day 7 post infection, CD8+CD44hiThy1.1+ OT-I cells were identified in the peripheral blood following collection in heparinized capillary tubes and red blood cells lysis. Primary and secondary effector OT-I cells were identified as CD8+CD19−CD44hiThy1.1+ from single cell suspensions. To assess resting OT-I memory cells, at week 4 post infection (day 28–35), spleen and lymph nodes (popliteal, inguinal, mesenteric, brachial, axial, and cervical) were pooled and enriched for Thy1.1 cells using magnetic beads (14). Briefly, single cell suspensions were incubated with anti-Thy1.1 PE and anti-PE microbeads (Miltenyi), following by enrichment over LS columns. The unbound column flow-through and wash fraction was routinely absent of OT-I cells. Memory OT-I cells were assessed as CD45.2+CD19−CD11c−CD4−CD8+CD44hiThy1.1+. In some experiments, 200 μL of 2 mg/mL BrdU was given intraperitoneally on day 4 post graft and splenic OT-I cells were enriched for analysis 18 h later. Absolute cell numbers were determined using AccuCheck beads (Invitrogen).
In vitro OVA APL OT-I stimulations
Spleen and mesenteric lymph node cells from OT-I mice were processed to single cell suspension and 3×106 splenocytes were plated in 24 well plates in complete RPMI supplemented with 0.1 μM OVA APL peptide, 0.1 μg/mL anti-CD28 (37.51, Biolegend), and 10 ng/mL IL-2 (Biolegend) for 3 days. Dead cells were removed using Lymphocyte Separation Medium (CellGro) and cells were cultured in media containing 10 ng/mL IL-15 (Biolegend) overnight, followed by flow cytometry. Cells were restimulated on day 4 following the addition of naïve B6 splenocytes at a 1:1 ratio for 5 hrs in the presence of 0.1 μM OVA APL peptide. For CD45RB cell sorting, Q4 OVA primed cells were isolated using Lymphocyte Separation Medium and stained with Live/Dead Aqua (Invitrogen), gated on Aqua−CD8+CD44hiThy1.1+, and sorted as CD45RBhi and CD45RBlo using a FACS Aria II (BD).
Assessment of polyclonal OVA specific CD8+ T cells
Mice were infected with 104 CFU of LM-OVA APL strains intraperitoneally and assessed on day 10–14 post infection. For N4 OVA-specific tetramer staining, monomers were obtained from the NIH Tetramer Core Facility and 180 μg of monomer (90% biotinylation) was tetramerized with streptavidin APC using standard techniques. Tetramer staining was performed on splenic CD3ε+CD19−CD11c−CD8+CD44hi cells for 20–30 min at room temperature at the indicated concentrations.
Skin transplantation
Full-thickness tail and ear skins were transplanted onto the dorsal thorax of recipient mice and secured with adhesive bandages as previously described (15). In some experiments, mice were treated with 500 μg each hamster monoclonal anti–mouse CD154 (MR-1, BioXCell) and CTLA-4 Ig, or 250 μg anti-CD45RB (HB-220, BioXCell) on days 0, 2, 4, and 6 post transplant.
Flow cytometry and intracellular cytokine staining
Single cell suspensions were stained with anti-CD3, anti-CD8, anti-CD19, anti-CD25, anti-CD44, anti-CD45RB, anti-CD62L, anti-CD69, anti-CD122, anti-CD127, anti-CD11c, anti-PD-1, and anti-Thy1.1 or appropriate isotype control (BD Biosciences or Biolegend) for 15 min at room temperature. For intracellular marker and cytokine staining, cells were incubated for 5 h at 37 C in the presence of 1 μM N4 OVA peptide (GenScript) and 10 μg/ml GolgiPlug (BD Biosciences) and stained for intracellular IL-2, TNF, and IFN-γ following manufacturer’s instructions (BD Biosciences). Assessment of Nur77 (eBiosciences) and hnRNPLL (Clone TR75-89, Cell Signaling Technology) expression was performed using the FoxP3/Transcription Factor Staining Buffer Set (eBiosciences). hnRNPLL was detected with anti-rabbit F(ab)’2 secondary reagent (Cell Signaling Technology). Data were analyzed using FlowJo software (Tree Star).
Relative 2D affinity measurement of CD8+ T cells
Human RBCs were isolated in accordance with the Institutional Review Board at Emory University coated with Biotin-X-NHS (EMD) and 0.5 mg/ml streptavidin (Thermo Fisher Scientific) and 1–2 μg of N4 OVA or OVA APL H-2Kb monomers with mouse β-2 microglobulin (NIH Tetramer Core). Monomers cannot bind CD8 due to substitution of the mouse H-2Kb α3 domain with human HLA-A2 α3 domain. Monomer bound to RBCs was quantified with anti-N4 OVA Kb PE antibody (25-D1.16; ebioscience) and QuantiBrite Beads (BD Biosciences). Naïve splenic OT-I T cells for were purified using EasySep mouse CD8+ T cell negative selection kit (Stemcell Technologies), TCR was quantified by stained with anti-Vα2 PE antibody (B20.1; eBioscience) and QuantiBrite Beads. For the relative 2D affinity of polyclonal CD8+ T cells, splenic CD3ε+CD19−CD8+CD44hi cells from day 10 LM N4 OVA infected mice were sorted into CD45RBhi and CD45RBlo populations using a FACS Aria II (BD), and the TCR was quantified with anti-TCRβ (H57-597; BD Biosciences) and QuantiBrite beads. The micropipette adhesion frequency assay was then performed as previously described (16, 17). In brief, a pMHC-coated RBC and T cell were placed on apposing micropipettes and brought into contact by micromanipulation for a controlled contact area (Ac) and time (t). The T cell was retracted at the end of the contact period, and the presence of adhesion (indicating TCR:pMHC binding) was observed by elongation of the RBC membrane. This TCR-RBC contact was repeated 30 times and the adhesion frequency (Pa) was calculated. The relative 2D affinity (AcKm) of each cell that had a Pa of greater than 10% was calculated using the Pa at equilibrium (where t → ∞) using the following equation: AcKa = ln[1−Pa(∞)]/(mrml), where mr and ml reflect the receptor (TCR) and ligand (pMHC) densities, respectively.
Statistical analysis
Survival data were plotted on Kaplan-Meier curves and log-rank tests were performed. For analysis of absolute numbers and expression levels, paired or unpaired Student’s t-tests (two-tailed) were performed, where appropriate. Analysis of expression of markers on naïve and memory cells was performed using one-way ANOVA with Bonferroni post test. Analysis of expression of markers by CD45RB expression was performed using 2-way repeated measures ANOVA with Bonferroni post test. Fold expansion in proliferation of N4 OVA rechallenged secondary effectors was calculated as: Absolute number OT-I N4 OVA rechallenge/Average number resting OT-I. Fold change of CD45RB expression on resting memory vs. secondary effectors was calculated as (100 - (%CD45RBhi secondary effector/Average %CD45RBhi resting memory)). Percent maximum of N4 OVA tetramer binding was calculated as (Frequency N4 OVA Tet+)/(Frequency N4 OVA Tet+ 1:50 dilution) x 100. Comparisons of relative 2D affinity of OVA APLs compared to N4 OVA were performed using one-way ANOVA (Dunnett’s post test). Linear correlations of relative 2D affinity were evaluated with MST, CD45RB expression, or EC50 values (13). Results were considered significant if p<0.05. All analyses were done using GraphPad Prism software (GraphPad Software Inc). *p<0.05, **p<0.01, ***p<0.001.
Results
OVA APL relative 2D affinity correlates with functional avidity
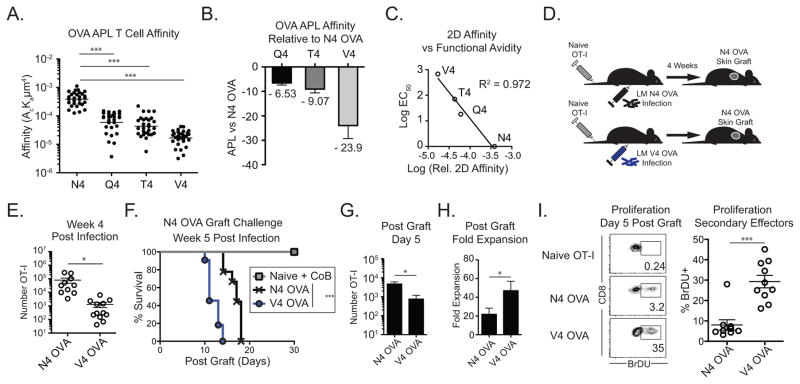
The affinity of TCR interactions during priming impacts naïve CD8+ T cell differentiation into effector and memory cells (1, 18, 19). While many studies have used the OVA-based altered peptide ligands (APLs) (13, 20–22), the affinity of the OT-I TCR for many OVA APLs has not been reported. Using a micropipette adhesion assay (17), we measured the relative 2D affinity of OT-I T cells to parental N4 OVA (SIINFEKL) as well as single amino acid substitution APLs Q4 OVA (SIIQFEKL), T4 OVA (SIITFEKL) and V4 OVA (SIIVFEKL). In contrast to functional avidity measurements (13, 22), this technique measures the isolated affinity of the TCR and peptide:MHC binding in the context of physiologic cellular membranes absent the contributions of other surface receptors, such as CD8 (16). We found that the 2D affinity of OVA APLs ranged from N4 OVA 3.8 x10−4 (high affinity) to V4 OVA 1.67 × 10−5 μm4 (low affinity) (Figure 1A), representing a 23.9-fold range of affinity (Figure 1B). This range between N4 and V4 OVA is more than 10-fold narrower than the reported 680-fold range in relative functional avidity (13). Importantly, we identified a linear relationship between relative functional avidity and 2D affinity (R2 = 0.972, Figure 1C).
Figure 1. Low affinity primed CD8+ T cells mount potent secondary responses against high affinity grafts.
(A–C) The relative 2D affinity of naïve OT-I T cells to N4 OVA, Q4 OVA, T4 OVA, and V4 OVA H-2b were measured using a micropipette. (A) Relative 2D affinity of OT-I T cells to N4 OVA and OVA APLs (B) Fold difference in 2D affinity between N4 OVA and OVA APLs. (C) Linear association between relative 2D affinity and reported relative functional avidity. (D–H) Naïve mice adoptively transferred with 104 OT-I T cells were infected the following day with either LM-N4 OVA or LM-V4 OVA. Naïve mice or mice containing N4 OVA or V4 OVA primed memory OT-I cells were transplanted with N4 OVA skin grafts 5 weeks post infection. (E) Frequency of memory N4-OVA and V4 OVA primed memory OT-I cells in pooled secondary lymphoid organs (N4 OVA vs V4 OVA p<0.001). (F) N4 OVA skin graft survival in LM-N4 OVA or LM-V4 OVA infected mice. Naïve mice were treated with CTLA-4 Ig and anti-CD154 costimulation blockade (N4 OVA vs V4 OVA groups p<0.0003). (G) Number of N4 OVA or V4 OVA primed OT-I cells recovered in the draining LNs on day 5 post-graft. (H) Fold expansion of N4 OVA and V4 OVA primed memory cells from day 0 to day 5 post graft. (I) Frequency of BrDU+ cells among N4 OVA or V4 OVA primed secondary effectors on day 5 following skin graft, or in ungrafted naïve OT-I controls. Data compiled from at least 3 independent experiments. *p<0.05, ***p<0.0001. LM, Listeria monocytogenes. CoB, costimulation blockade.
Low affinity primed memory CD8+ T cells are tuned to generate robust secondary recall responses
During heterologous immunity, T cells primed with high or low affinity for the priming pathogen antigen can cross-react with allogeneic antigen and mediate graft rejection. Thus, we developed an in vivo model to investigate the role that TCR affinity plays in heterologous immunity. We used Listeria monocytogenes strains engineered to express the OT-I epitope N4 OVA or the low affinity V4 OVA epitopes to generate high or low affinity primed CD8+ T cell memory, respectively and then challenged these memory populations with an N4 OVA expressing skin graft (Figure 1D). Compared to N4 OVA priming, V4 OVA priming led to ~80-fold lower frequency of CD8+ OT-I effectors in the blood on day 7 (Supplemental Figure 1A). Using an enrichment technique to reliably detect and quantify very low frequency OT-I memory T cells (14), we found that high affinity N4 and low affinity V4 OVA primed cells formed memory populations in secondary lymphoid organs (Figure 1E, Supplemental Figure 1B). Low affinity primed V4 OVA cells were found at 61-fold lower frequency than those primed by N4 OVA (N4 OVA 8.0×104±3.3×104, V4 OVA 1.3×103±5.3×102, Figure 1E). Consistent with the phenotype of high quality CD8+ memory T cells, both N4 OVA and V4 OVA primed memory cells expressed high levels of CD44 and CD127, and low levels of CD69 and Granzyme B (Supplemental Figure 1C). Thus, while primary effector proliferation correlates with TCR signal strength, low affinity priming leads to memory cell formation.
Following challenge with high affinity N4 OVA skin grafts, high affinity primed N4 OVA memory elicited rejection of grafts with an MST = 17 days (Figure 1F). Surprisingly, despite a 61-fold lower precursor frequency, low affinity primed V4 OVA memory cross-reacting with graft-expressed high affinity N4 OVA mediated faster graft rejection (MST 11 days, p < 0.0001, Figure 1F). Challenge of mice with memory primed with either of two additional intermediate-affinity OVA APLs Q4 and T4 resulted in graft rejection kinetics that were faster than high affinity N4 OVA (Q4 OVA MST = 15.5 days, T4 OVA MST = 14.0 days, Supplemental Figure 1D). The mean survival time of high affinity N4 OVA skin graft challenge correlated with the priming affinity ((13), Supplemental Figure 1D).
We investigated the relative differences in graft rejection kinetics were the result of differential qualitative TCR signaling or proliferative potential between high and low affinity primed memory CD8+ T cells. In response to high affinity N4 OVA rechallenge, high and low affinity primed memory CD8+ T cells upregulated similar levels of CD69 and Nur77, which report qualitative TCR-mediated signal strength at early timepoints (22, 23) (Supplemental Figure 2A–B).
On day 5 following N4 OVA skin graft, fewer low affinity V4 OVA primed cells were recovered in the draining lymph nodes compared to N4 OVA primed cells (Figure 1G). However, low affinity V4 OVA primed cells underwent a greater fold expansion (Figure 1H) and a significantly higher frequency of V4 OVA primed cells incorporated BrdU on day 5 post graft (p < 0.0001, Figure 1I). These results could not be attributed to different rates of apoptosis between memory populations (Supplemental Figure 2C), nor due to differences in OVA-specific endogenous CD8+ populations, as similar frequency and absolute number of endogenous CD8+ T cells were N4 OVA-specific (Supplemental Figure 2D). Thus, in response to high affinity rechallenge, low affinity primed memory CD8+ T cells can receive strong TCR-mediated activation signals and are poised to undergo greater proliferative responses compared to high affinity primed memory T cells.
CD45RB is a stable marker of the affinity experience of memory CD8+ T cells
We investigated the mechanism underlying the ability of low affinity primed V4 OVA memory cells to mediate faster graft rejection and undergo greater proliferation than high affinity primed N4 OVA memory in response to high affinity graft challenge. CD45 is a transmembrane phosphatase that is critical for TCR signaling. Interestingly, the relative abundance of CD45 isoforms can be used to distinguish T cell differentiation status in mice, as naïve CD8+ T cells are CD45RBhi and effector/memory CD8+ T cells downregulate CD45RB to become predominantly CD45RBlo (24).
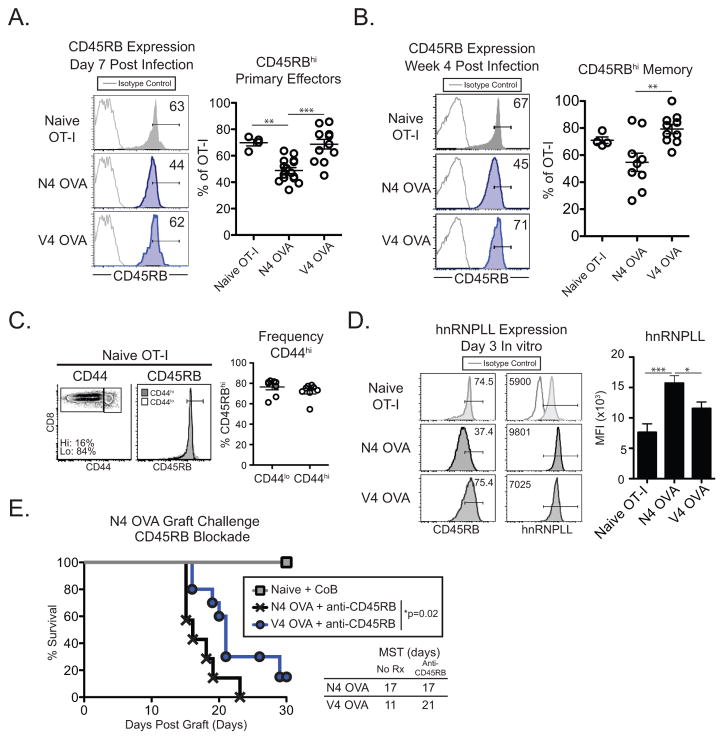
Given their overall memory cell phenotype compared to high affinity primed memory cell (Supplemental Figure 1C and (13)), we questioned whether low affinity primed memory CD8+ T cells became CD45RBlo during effector and memory differentiation. During the primary effector response we found that high affinity primed N4 OVA effectors downregulated CD45RB (48.9±2.2% CD45RBhi, Figure 2A). Surprisingly, V4 primed OT-I cells in the blood maintained a predominantly CD45RBhi status compared to high affinity primed cells (V4 OVA 68.7±3.6% CD45RBhi, p<0.001, Figure 2A). At memory, the divergent frequency of CD45RBhi cells was maintained in high and low affinity primed OT-I cells residing in secondary lymphoid tissue (N4 OVA 54.7±6.76%, V4 OVA 79.3±3.4%, p=0.0064, Figure 2B).
Figure 2. Low affinity memory CD8+ T cells retain a CD45RBhi status.
Naïve mice adoptively transferred with 104 OT-I T cells were infected the following day with either LM-N4 OVA or LM-V4 OVA and memory OT-I T cells and assessed (A) in the blood on day 7 or (B) in the secondary lymphoid organs 4 weeks post infection. (A) Frequency of CD45RBhi cells on naïve or primary effector N4 OVA or V4 OVA T cells. (B) Frequency of CD45RBhi cells among naïve OT-I or N4 OVA or V4 OVA memory OT-I cells. (C) Frequency of CD45RBhi cells among naïve OT-I CD44hi and CD44lo cells. (D) hnRNPLL expression in OT-I T cells primed with N4 OVA or Q4 OVA peptide for 3 days in vitro. (E) Naïve mice or mice containing N4 OVA or V4 OVA primed memory OT-I cells were transplanted with N4 OVA skin grafts 5 weeks post infection and treated with anti-CD45RB. Naïve mice were treated with CTLA-4 Ig and anti-CD154 costimulation blockade. Data compiled from 2–4 independent experiments. *p<0.05, **p<0.01, ***p<0.001.
A small fraction of naïve OT-I cells are CD44hi and have a memory-like phenotype (25). We found that both CD44hi and CD44lo naïve OT-I T cell populations were CD45RBhi, indicating that the divergent CD45RB status of OT-I effectors following high and low affinity priming was not due to differential expression of CD45RB on naïve OT-I T cells prior to adoptive transfer (Figure 2C). We also investigated the expression of several other molecules that have been shown to modulate TCR signaling. We found no significant difference in expression of Vα2, CD8, or CD5 expression on high and low affinity primed OT-I cells (Supplemental Figure 2E).
The mRNA for the gene PTPRC undergoes alternative splicing of exons 3, 4, and 5 to generate mature transcripts that encode the extracellular CD45 A, B, and C domains, respectively (24). The heteronuclear ribonuclear protein L-like (hnRNPLL) has been shown to control inclusion of exons A and C in the PTPRC transcript in peripheral CD8+ T cells (26, 27). We questioned whether hnRNPLL was differentially expressed following high and low affinity priming, which could reflect the presence of larger CD45 transcripts that contain domains A and/or C. We found that hnRNPLL expression increased following stimulation of OT-I cells with N4 OVA, consistent with a previous report (26). However, hnRNPLL expression was not induced following low affinity V4 OVA priming (Figure 2D). Thus, the CD45RBhi status of low affinity primed cells likely reflects the expression of a greater frequency of large CD45 isoforms that also contain domains A and C.
In order to determine if CD45RB expression on low affinity primed memory CD8+ T cells was functionally important in mediating graft rejection, we treated N4 OVA and V4 OVA memory mice with anti-CD45RB mAb during skin graft rechallenge. Blockade of CD45RB during N4 OVA skin graft challenge did not significantly prolong graft survival in mice containing N4 OVA primed cells compared to untreated mice (Untreated MST 17 d, Figure 1F; anti-CD45RB MST 17 d, Figure 2E). However, treatment of mice containing V4 OVA primed memory CD8+ T cells with anti-CD45RB led to significant prolongation of graft survival (Untreated MST 11 d, Figure 1F; anti-CD45RB MST 21 d, Figure 2E), demonstrating that CD45RB is functionally important specifically in context of a secondary recall response mediated by low affinity primed memory CD8+ T cells. These data are the first demonstration that that long-lived CD44hi memory T cells can remain predominantly CD45RBhi and that this CD45RBhi status is functionally important during rechallenge.
Low affinity primed secondary effector cells downregulate CD45RB and produce high levels of IL-2
In light of the finding that low affinity primed CD8+ memory T cells mount robust secondary recall responses against high affinity antigen, we investigated the phenotype of high and low affinity primed secondary effectors. Because skin grafts elicit relatively little expansion of antigen-specific T cells, we rechallenged memory populations with peptide in vivo and assessed T cells in the draining lymph node. Similar to post graft secondary responses, low affinity primed secondary effectors underwent a 5.3-fold greater fold-expansion than high affinity primed secondary populations (p<0.0001, Supplemental Figure 3A–B).
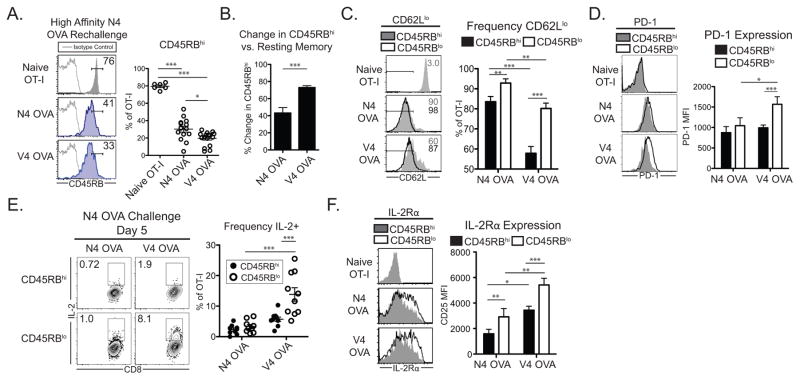
High affinity N4 OVA peptide rechallenge of N4 OVA primed memory cells slightly downregulated CD45RB expression (30.2±3.39% CD45RBhi, Figure 3A). In contrast, V4 OVA primed memory cells significantly downregulated CD45RB expression during the secondary proliferative response to high affinity antigen (19.6±1.96% CD45RBhi, Figure 3A). The loss of CD45RBhi cells was significantly greater in V4 OVA primed secondary effectors compared to N4 OVA primed secondary effectors (p<0.001, Figure 3B). Low affinity primed secondary effectors also downregulated CD45RB following N4 OVA skin graft challenge (Supplemental Figure 3C). A large proportion of both secondary effector populations were CD62Llo (Figure 3C). However, a greater frequency of both N4 OVA and V4 OVA CD45RBlo secondary effectors had a CD62Llo effector phenotype compared to their respective CD45RBhi populations (N4 OVA and V4 OVA p<0.001, respectively, Figure 3C). Interestingly, a slightly smaller frequency of CD62Llo cells was contained within the V4 OVA CD45RBlo population compared to the N4 OVA CD45RBlo population (p<0.01, Figure 3C). The cosignaling receptor PD-1 has been shown to be a marker of cumulative effector signal strength on effector CD8+ T cells and to correlate with CD62L expression (28). PD-1 was significantly upregulated in CD45RBlo fraction of V4 OVA primed cells (Figure 3D), demonstrating that this population perceives strong TCR signals.
Figure 3. Low affinity primed secondary effectors downregulate CD45RB and have a distinct IL-2 producing effector phenotype.
Naïve mice adoptively transferred with 104 OT-I T cells were infected the following day with either LM-N4 OVA or LM-V4 OVA. Four weeks post infection, mice were challenged with N4 OVA peptide in the foot pad and the draining popliteal lymph nodes were assessed 5 days later. (A) Frequency of CD45RBhi cells among naïve and secondary effector OT-I T cells. (B) Relative reduction in CD45RBhi frequency of secondary effector populations compared to resting memory cells. (C) Frequency of CD62Llo cells in CD45RBhi and CD45RBlo fractions of secondary effector OT-I cells. (D) PD-1 expression in CD45RBhi and CD45RBlo fractions of secondary effector OT-I cells. (E) IL-2 expression by secondary effectors following brief N4 peptide restimulation in CD45RBhi and CD45RBlow fractions. (F) IL-2Rα (CD25) expression in CD45RBhi and CD45RBlo fractions of secondary effector OT-I cells. Data compiled from 2–3 independent experiments. *p<0.05, **p<0.01, ***p<0.001.
IL-2 is a critical cytokine for CD8+ T cell secondary responses (29). Consistent with previous published results (30, 31), a low frequency of N4 OVA primed secondary effectors produced IL-2 (Figure 3E). In contrast, we found that a significantly greater proportion of V4 OVA primed CD45RBlo cells produced IL-2 (Figure 3E). High IL-2 production was specific to low affinity primed CD45RBlo secondary effectors during N4 OVA rechallenge, as high and low affinity primed day 7 effectors produced similarly low levels of IL-2 (Supplemental Figure 3D). In contrast, V4 OVA primed CD45RBlo populations did not possess greater frequencies of IFN-γ+ or TNF+ cells compared to CD45RBhi populations following high affinity rechallenge (Supplemental Figure 3E).
IL-2 signaling can induce the expression of the high affinity IL-2Rα chain (CD25, (32)), and high IL-2Rα expression is associated with differentiated effector cells (32–34). We found that V4 OVA primed CD45RBlo cells expressed significantly higher levels of IL-2Rα compared to both CD45RBhi cells and N4 OVA primed CD45RBlo cells (N4 OVA CD45RBhi vs CD45RBlo p<0.001, CD45RBlo N4 OVA vs V4 OVA p<0.01, Figure 3F). High expression of the IL-2Rβ chain, CD122, can confer sensitivity to IL-15 signaling in memory T cells, while lower expression is associated with IL-2 sensitivity and effector T cells (34, 35). We found that high affinity N4 OVA primed secondary effectors expressed similar levels of IL-2Rβ (Supplemental Figure 3F). Among low affinity V4 OVA primed secondary effectors, however, CD45RBlo cells expressed significantly lower IL-2Rβ levels than CD45RBhi cells (p<0.01, Supplemental Figure 3F). Together, these results demonstrate that low affinity primed CD45RBlo secondary effectors effectively switch into a distinct effector-like phenotype characterized by high IL-2 production, high expression of IL-2Rα, and effector IL-2Rβ expression.
High CD45RB expression tunes CD8+ T cells to respond to heterologous antigen rechallenge
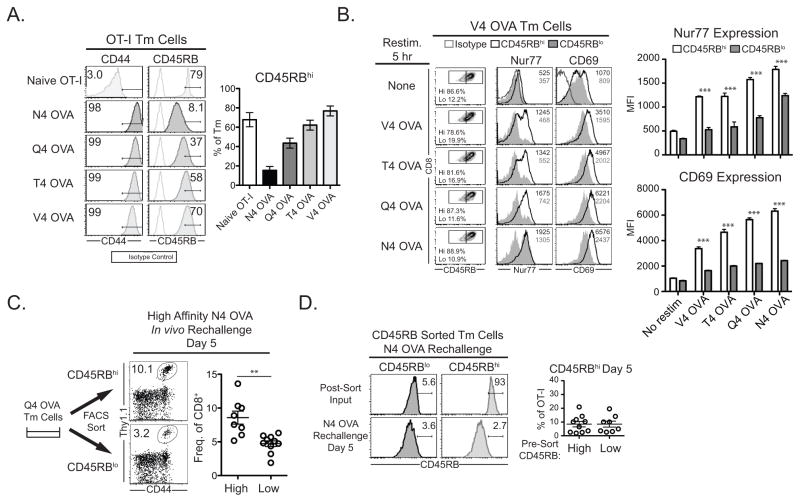
In order to control for priming conditions and cell numbers encountered after priming with Listeria strains in vivo, we investigated CD45RB expression on in vitro primed OT-I cells (Tm cells). Following in vitro priming of OT-I cells with OVA APLs, we found that Tm cells similarly upregulated CD44 expression but that CD45RB expression correlated inversely with priming affinity (Figure 4A), consistent with in vivo derived effector and memory CD8+ T cells (Figure 2A–B).
Figure 4. High CD45RB expression tunes low affinity primed memory CD8+ T cells for heterologous rechallenge responses.
OT-I T cells were activated in vitro with N4 OVA, Q4 OVA, T4 OVA, or V4 OVA peptide for 4 days. (A) Expression of CD44 and CD45RB on OVA APL Tm cells. (B) V4 OVA APL Tm cells were restimulated for 5 h with N4 OVA, Q4 OVA, T4 OVA, or V4 OVA peptide and Nur77 and CD69 expression was assessed. (C–D) Q4 OVA primed OT-I cells were sorted into CD45RBhi and CD45RBlo populations and adoptively transferred into naïve congenic hosts and rechallenged with N4 OVA peptide (C) The frequency of Q4 OVA cells was assessed 5 days following rechallenge. (D) Expression of CD45RB on Q4 OVA Tm cells before and after rechallenge with N4 OVA peptide for 5 days. Data compiled from 2–3 independent experiments. **p<0.01.
We investigated whether the differential expression of CD45 isoforms conferred a qualitative TCR signaling advantage by assessing the expression of CD69 and Nur77 in CD45RBhi V4 OVA primed Tm cells restimulated with OVA APL ligands. After a brief 5 hour restimulation, CD45RB expression did not change in these cells (Figure 4B). We found that the expression of both Nur77 and CD69 was significantly greater in CD45RBhi cells compared to CD45RBlo cells following stimulation with N4 OVA or each OVA APL (Figure 4B), demonstrating that CD45RBhi cells received qualitatively stronger TCR activation signals compared to CD45RBlo cells.
To investigate whether high CD45RB status is sufficient to confer enhanced recall potential during secondary challenge, we sorted CD45RBhi and CD45RBlo OT-I cells elicited by Q4 OVA priming and rechallenged them with N4 OVA peptide in naïve congenic hosts. With this experimental setup, CD45RBhi and CD45RBlo cells encounter identical priming conditions and are rechallenged at identical precursor frequencies. CD45RBhi cells proliferated to a significantly greater number than CD45RBlo cells (p=0.0012, Figure 4C). Similar to in vivo low affinity primed CD45RBhi memory T cells that become CD45RBlo following secondary rechallenge with high affinity antigen (Figure 3A), both CD45RBhi and CD45RBlo input populations were predominantly CD45RBlo five days following restimulation (Figure 4D), demonstrating that CD45RBhi cells downregulate expression of CD45RB on a per cell basis in response to high affinity rechallenge. Taken together, these data demonstrate that a CD45RBhi status is sufficient to confer a qualitatively greater activation signal and a proliferative advantage on low affinity primed CD8+ T cells and that CD45RB status is a marker of the affinity experience of memory and secondary effector cells.
Polyclonal low affinity CD8+ T cells express high levels of CD45RB
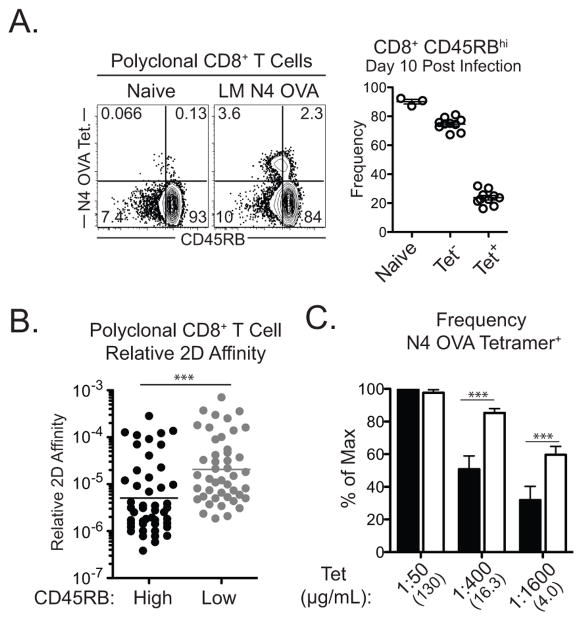
To determine if these findings in TCR transgenic CD8+ T cells apply to polyclonal CD8+ T cell populations, we investigated the ability of CD45RB to distinguish the affinity experience of polyclonal CD8+ T cells. In mice infected with LM-OVA, polyclonal populations of naïve CD8+ T cells and activated non-N4 OVA specific CD8+ effectors were predominantly CD45RBhi (90.1±1.5% and 74.6±1.36% CD45RBhi, respectively), while polyclonal antigen-specific CD8+ T cell effectors display a range of CD45RB expression (23.8±1.55% CD45RBhi, Figure 5A). This data suggests that CD45RB expression reflects the range of priming affinities of polyclonal CD8+ T cells for a given antigen.
Figure 5. Polyclonal CD8+ T cells express are CD45RBhi.
Naïve B6 mice were infected with LM-N4 OVA and splenic CD8+ T cells were assessed 10–14 days later. (A) CD45RB expression on tetramer stained N4-OVA specific CD8+ T cells. (B) Relative 2D affinity of CD45RBhi and CD45RBlo CD8+CD44hi T cells. (C) Frequency of tetramer staining among CD45RBhi and CD45RBlo fractions of CD8+CD44hi T cells. Data compiled from 2–3 independent experiments. *p<0.05, ***p<0.001.
We investigated the affinity of activated CD8+ T cells based on CD45RB status using 2D affinity measurements. We measured the relative 2D affinity of sorted OVA-specific CD44hi CD45RBhi and CD45RBlo populations from LM-OVA infected mice (Supplemental Figure 4A). We found that CD45RBhi cells had ~10-fold lower 2D relative affinity compared to CD45RBlo cells (p = 0.0001, Figure 5B). Tetramer binding can also be used to delineate relative affinity of T cell populations, as low affinity cells do not efficiently bind tetramer compared to high affinity cells (17). We found that compared to CD8+CD44hiCD45RBhi cells, CD44hiCD45RBlo cells bound tetramer more effectively at lower concentrations (Figure 5C and Supplemental Figure 4B). Compared to CD45RBlo cells, CD45RBhi cells expressed similar levels of CD3ε and slightly higher CD8 coreceptor (Supplemental Figure 4C), thus demonstrating that differential expression of TCR or CD8 coreceptor do not account for tetramer binding avidity of CD45RBhi and CD45RBlo cells. Thus, these data demonstrate that in a polyclonal population of CD44hiCD8+ T cells, a CD45RBhi status denotes cells primed with lower affinity than CD45RBlo cells.
Discussion
Here we establish a novel role for CD45 in tuning low affinity primed memory CD8+ T cells to become potent secondary effectors during heterologous rechallenge. While it is appreciated that high affinity memory CD8+ T cells can mediate graft rejection (36, 37), this study specifically addresses the contribution of TCR priming affinity to the function of memory T cells that are directed against a graft. In general, the affinity for allogeneic antigen and foreign microbial antigen are recognized to have a similar range of affinities that is greater than 10-fold (5). In the context of heterologous immunity, a recent study that measured the affinity of a human TCR for both its viral cognate antigen and a cross-reactive alloantigen reported a ~15-fold higher affinity for allogeneic antigen than for the viral epitope (38). In our model, we found a 23.9-fold difference in affinity between N4 OVA and V4 OVA for the OT-I TCR, a magnitude that is in line with both the known range of T cell recognition of allogeneic antigen as well as with microbe-specific T cells that cross-react with allogeneic antigen. Thus, this model recapitulates a critical facet of heterologous immunity by eliciting memory CD8+ T cells with distinct priming affinities in the context of a pathogen that subsequently recognize a related, but not identical, antigen presented in the context of a transplant.
While the importance of CD45 for immune homeostasis and signaling has been well-studied, this study provides a novel physiologic context for CD45 mediated tuning by linking CD8+ TCR priming affinity and CD45RB expression on memory and secondary effector T cells. We found that low affinity primed cells maintain a CD45RBhi status, which has previously been described only on naïve CD8+ T cells. CD45RB was stably expressed at high levels on fully differentiated CD44hi TCR transgenic and polyclonal memory CD8+ T cells following low affinity priming. The maintenance of a CD45RBhi status was associated with lower expression of hnRNPLL, which suggests that low affinity primed CD45RBhi CD8+ T cells may express a high frequency of multiple large CD45 isoforms. This difference in hnRNPLL expression, however, is likely not the only mechanism of differential CD45 isoform usage following low affinity priming, as hnRNPLL does not control the inclusion of the B domain in peripheral CD8+ T cells (26). Expression of other molecules that are known to tune T cell signaling, including CD8 and CD5, were similarly expressed on high and low affinity primed memory cells. Expression of Nur77 and CD69, respectively, have been shown to correlate with priming affinity (13, 22, 23). However, the expression of these markers, as well as functional avidity measured by IFN-γ production, rapidly changes following stimulation (13, 22, 23). Thus, in conjunction with CD44 expression, high CD45RB expression is a novel and stable marker by which low affinity primed CD8+ memory T cells can be identified.
Previous in vitro work has demonstrated that large CD45 isoforms tune T cells for stronger proximal TCR signaling by undergoing less inhibitory dimerization than smaller isoforms (24, 39). Consistent with the ability of larger CD45RABC isoform to mediate greater calcium flux compared to CD45RO (39), we demonstrate that low affinity primed memory and CD45RBhi cells receive greater cumulative TCR signal strength (as assessed by upregulation of Nur77 and CD69) and are poised to undergo greater proliferative and effector responses compared to CD45RBlo cells. Many investigations of the consequences of altered T cell receptor signaling potency have focused on antigen density or genetically attenuated TCR signaling (18, 40–44). However, a recent study elegantly demonstrated that TCR binding affinity and antigen density induce broadly distinct gene expression profiles (45). Thus, the role of CD45 tuning represents a previously unappreciated mechanism by which TCR priming affinity dictates the differentiation program of memory CD8+ T cells during heterologous rechallenge.
These data suggest a model in which CD45RB defines an affinity-based differentiation switch on CD8+ T cells, as low affinity priming enables proliferation and effector functions along with a CD45RBhi status similar to naïve T cells. Following high affinity rechallenge, low affinity primed CD45RBhi memory cells differentiate into CD45RBlo secondary effectors with a distinct phenotype, characterized by high PD-1 and CD25 expression, increased IL-2 production, and lower CD122 expression. While PD-1 is a marker of exhaustion on CD8+ memory T cells following chronic antigen exposure, its expression is also associated with recent antigen experience in effector CD8+ T cells (28). It remains to be seen whether PD-1 is functioning in a costimulatory or coinhibitory capacity on secondary effectors under these conditions. This prominent IL-2 signaling phenotype was not a general characteristic of high affinity priming, as N4 OVA and V4 OVA primary effectors expressed similarly low levels of IL-2. Recent work revealed that IL-2 signals are critical for secondary effector CD8+ responses (29, 46) and that CD25 is a marker of terminally differentiated effectors (32, 33). This study is the first to identify TCR affinity as a driver of an IL-2 dependent memory CD8+ phenotype and extends previous work establishing the requirement of IL-2 for secondary recall responses by revealing that low affinity primed CD8+ secondary effectors produce significant amounts of IL-2.
Recently, considerable evidence has been presented that pathogen specific T cells can cross-react with allogeneic antigen. In humans, one recent study found that nearly half of viral specific T cell clones were cross-reactive with allogeneic antigen (6) and fifteen individual TCRs that recognize pathogen-allogeneic antigen combinations have been characterized (5, 38). In mice, LCMV infection generates memory CD8+ T cells that cross-react with allogeneic antigen and mediate graft rejection (4, 7, 47). However, in addition to single TCR cross-reactivity with allogeneic antigen, dual TCR T cells may also play a role in heterologous immunity against allogeneic antigen (48–51), and it is likely that both cross-reactivity and dual TCR expression contribute to heterologous immunity in polyclonal populations. In this model, we can assess the relative contribution of TCR affinity to memory T cell function in the context of a skin graft, but we cannot assess the relative contribution of additional mechanisms of heterologous immunity within polyclonal T cell populations.
In the context of protective immunity, the ability of low affinity priming to form memory and undergo robust secondary responses to heterologous rechallenge represents a means of maintaining clonal diversity and protection against encounter with diverse pathogens. However, following transplantation this phenomenon represents a potentially potent driver of allogeneic T cell responses and a barrier to successful immunomodulation. The contribution of low affinity memory T cells to heterologous immunity provides new rationale for the therapeutic targeting of CD45RB to prevent pathogenic T cell responses, a strategy that has shown promise in pre-clinical murine (52–54) and non-human primate transplantation models (55–57). Together, these data highlight the importance of the pathogen priming history of an individual in shaping the potentially pathogenic memory T cell repertoire in settings of allogeneic heterologous immunity and autoimmunity.
Supplementary Material
Acknowledgments
This work was supported by R01 AI073707 (M.L.F.), R01 AI104699 (M.L.F.), R37 AI040519 (C.P.L.), T32 AI007610-11 (S.M.K.), T32 GM08169-23 (S.M.K.), T32 A1070081 (S.M.K.), and F30DK098928-01 (S.M.K.).
The authors would like to thank Dr. Michael Bevan for the contribution of LM-OVA APL strains, Aaron Rae and the Emory+Children’s Pediatric Research Flow Cytometry Core for FACS sorting, and Dr. Jonathan Maltzman for critical reading of the manuscript.
Abbreviations
- LM
Listeria monocytogenes
- OVA
ovalbumin
- APL
altered peptide ligand
- TCR
T cell receptor
- MHC
major histocompatibility complex
- MST
mean survival time
- CoB
costimulation blockade
References
- 1.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat Rev Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jameson SC, Masopust D. Diversity in T cell memory: an embarrassment of riches. Immunity. 2009;31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ML, Larsen CP. Overcoming the memory barrier in tolerance induction: molecular mimicry and functional heterogeneity among pathogen-specific T-cell populations. Curr Opin Organ Transplant. 2010;15:405–410. doi: 10.1097/MOT.0b013e32833b7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, Wherry EJ, Onami T, Lanier JG, Kokko KE, Pearson TC, Ahmed R, Larsen CP. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith C, Miles JJ, Khanna R. Advances in direct T-cell alloreactivity: function, avidity, biophysics and structure. Am J Transplant. 2012;12:15–26. doi: 10.1111/j.1600-6143.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 6.Amir AL, D’Orsogna LJ, Roelen DL, van Loenen MM, Hagedoorn RS, de Boer R, van der Hoorn MA, Kester MG, Doxiadis, Falkenburg JH, Claas FH, Heemskerk MH. Allo-HLA reactivity of virus-specific memory T cells is common. Blood. 2010;115:3146–3157. doi: 10.1182/blood-2009-07-234906. [DOI] [PubMed] [Google Scholar]
- 7.Brehm MA, Daniels KA, Priyadharshini B, Thornley TB, Greiner DL, Rossini AA, Welsh RM. Allografts stimulate cross-reactive virus-specific memory CD8 T cells with private specificity. Am J Transplant. 2010;10:1738–1748. doi: 10.1111/j.1600-6143.2010.03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felix NJ, Donermeyer DL, Horvath S, Walters JJ, Gross ML, Suri A, Allen PM. Alloreactive T cells respond specifically to multiple distinct peptide-MHC complexes. Nat Immunol. 2007;8:388–397. doi: 10.1038/ni1446. [DOI] [PubMed] [Google Scholar]
- 9.Morris GP, Ni PP, Allen PM. Alloreactivity is limited by the endogenous peptide repertoire. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:3695–3700. doi: 10.1073/pnas.1017015108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Falkenburg WJ, Melenhorst JJ, van de Meent M, Kester MG, Hombrink P, Heemskerk MH, Hagedoorn RS, Gostick E, Price DA, Falkenburg JH, Barrett AJ, Jedema I. Allogeneic HLA-A*02-Restricted WT1-Specific T Cells from Mismatched Donors Are Highly Reactive but Show Off-Target Promiscuity. Journal of immunology. 2011;187:2824–2833. doi: 10.4049/jimmunol.1100852. [DOI] [PubMed] [Google Scholar]
- 11.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 12.Ehst BD, Ingulli E, Jenkins MK. Development of a novel transgenic mouse for the study of interactions between CD4 and CD8 T cells during graft rejection. Am J Transplant. 2003;3:1355–1362. doi: 10.1046/j.1600-6135.2003.00246.x. [DOI] [PubMed] [Google Scholar]
- 13.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009;458:211–214. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JJ, Chu HH, Hataye J, Pagan AJ, Pepper M, McLachlan JB, Zell T, Jenkins MK. Tracking epitope-specific T cells. Nat Protoc. 2009;4:565–581. doi: 10.1038/nprot.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang J, V, Zarnitsyna I, Liu B, Edwards LJ, Jiang N, Evavold BD, Zhu C. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–936. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabatino JJ, Jr, Huang J, Zhu C, Evavold BD. High prevalence of low affinity peptide-MHC II tetramer-negative effectors during polyclonal CD4+ T cell responses. J Exp Med. 2010;208:81–90. doi: 10.1084/jem.20101574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corse E, Gottschalk RA, Allison JP. Strength of TCR-peptide/MHC interactions and in vivo T cell responses. Journal of immunology. 2011;186:5039–5045. doi: 10.4049/jimmunol.1003650. [DOI] [PubMed] [Google Scholar]
- 19.Zehn D, King C, Bevan MJ, Palmer E. TCR signaling requirements for activating T cells and for generating memory. Cell Mol Life Sci. 2012;69:1565–1575. doi: 10.1007/s00018-012-0965-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King CG, Koehli S, Hausmann B, Schmaler M, Zehn D, Palmer E. T cell affinity regulates asymmetric division, effector cell differentiation, and tissue pathology. Immunity. 2012;37:709–720. doi: 10.1016/j.immuni.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knudson KM, Goplen NP, Cunningham CA, Daniels MA, Teixeiro E. Low-affinity T cells are programmed to maintain normal primary responses but are impaired in their recall to low-affinity ligands. Cell Rep. 2013;4:554–565. doi: 10.1016/j.celrep.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Daniels MA, Teixeiro E, Gill J, Hausmann B, Roubaty D, Holmberg K, Werlen G, Hollander GA, Gascoigne NR, Palmer E. Thymic selection threshold defined by compartmentalization of Ras/MAPK signalling. Nature. 2006;444:724–729. doi: 10.1038/nature05269. [DOI] [PubMed] [Google Scholar]
- 23.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, Hogquist KA. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J Exp Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hermiston ML, Xu Z, Weiss A. CD45: a critical regulator of signaling thresholds in immune cells. Annu Rev Immunol. 2003;21:107–137. doi: 10.1146/annurev.immunol.21.120601.140946. [DOI] [PubMed] [Google Scholar]
- 25.Lee YJ, Jameson SC, Hogquist KA. Alternative memory in the CD8 T cell lineage. Trends in immunology. 2011;32:50–56. doi: 10.1016/j.it.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Jia X, de la Cruz L, Su XC, Marzolf B, Troisch P, Zak D, Hamilton A, Whittle B, Yu D, Sheahan D, Bertram E, Aderem A, Otting G, Goodnow CC, Hoyne GF. Memory T cell RNA rearrangement programmed by heterogeneous nuclear ribonucleoprotein hnRNPLL. Immunity. 2008;29:863–875. doi: 10.1016/j.immuni.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oberdoerffer S, Moita LF, Neems D, Freitas RP, Hacohen N, Rao A. Regulation of CD45 alternative splicing by heterogeneous ribonucleoprotein, hnRNPLL. Science. 2008;321:686–691. doi: 10.1126/science.1157610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obar JJ, Lefrancois L. Early signals during CD8 T cell priming regulate the generation of central memory cells. Journal of immunology. 2010;185:263–272. doi: 10.4049/jimmunol.1000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 31.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, Jacob J, Calame K, Kaech SM. Transcriptional repressor Blimp-1 promotes CD8(+) T cell terminal differentiation and represses the acquisition of central memory T cell properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Ralpha expression on virus-specific CD8+ T cells favors terminal-effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 34.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12:180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 35.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 36.Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, Ford ML. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant. 2012;12:69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ford ML, Koehn BH, Wagener ME, Jiang W, Gangappa S, Pearson TC, Larsen CP. Antigen-specific precursor frequency impacts T cell proliferation, differentiation, and requirement for costimulation. J Exp Med. 2007;204:299–309. doi: 10.1084/jem.20062319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macdonald WA, Chen Z, Gras S, Archbold JK, Tynan FE, Clements CS, Bharadwaj M, Kjer-Nielsen L, Saunders PM, Wilce MC, Crawford F, Stadinsky B, Jackson D, Brooks AG, Purcell AW, Kappler JW, Burrows SR, Rossjohn J, McCluskey J. T cell allorecognition via molecular mimicry. Immunity. 2009;31:897–908. doi: 10.1016/j.immuni.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 39.Xu Z, Weiss A. Negative regulation of CD45 by differential homodimerization of the alternatively spliced isoforms. Nat Immunol. 2002;3:764–771. doi: 10.1038/ni822. [DOI] [PubMed] [Google Scholar]
- 40.Teixeiro E, Daniels MA, Hamilton SE, Schrum AG, Bragado R, Jameson SC, Palmer E. Different T cell receptor signals determine CD8+ memory versus effector development. Science. 2009;323:502–505. doi: 10.1126/science.1163612. [DOI] [PubMed] [Google Scholar]
- 41.Wherry EJ, Puorro KA, Porgador A, Eisenlohr LC. The induction of virus-specific CTL as a function of increasing epitope expression: responses rise steadily until excessively high levels of epitope are attained. Journal of immunology. 1999;163:3735–3745. [PubMed] [Google Scholar]
- 42.Smith-Garvin JE, Burns JC, Gohil M, Zou T, Kim JS, Maltzman JS, Wherry EJ, Koretzky GA, Jordan MS. T-cell receptor signals direct the composition and function of the memory CD8+ T-cell pool. Blood. 2010;116:5548–5559. doi: 10.1182/blood-2010-06-292748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiehagen KR, Corbo E, Schmidt M, Shin H, Wherry EJ, Maltzman JS. Loss of tonic T-cell receptor signals alters the generation but not the persistence of CD8+ memory T cells. Blood. 2010;116:5560–5570. doi: 10.1182/blood-2010-06-292458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wherry EJ, McElhaugh MJ, Eisenlohr LC. Generation of CD8(+) T cell memory in response to low, high, and excessive levels of epitope. Journal of immunology. 2002;168:4455–4461. doi: 10.4049/jimmunol.168.9.4455. [DOI] [PubMed] [Google Scholar]
- 45.Gottschalk RA, Hathorn MM, Beuneu H, Corse E, Dustin ML, Altan-Bonnet G, Allison JP. Distinct influences of peptide-MHC quality and quantity on in vivo T-cell responses. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:881–886. doi: 10.1073/pnas.1119763109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feau S, Arens R, Togher S, Schoenberger SP. Autocrine IL-2 is required for secondary population expansion of CD8(+) memory T cells. Nat Immunol. 2011;12:908–913. doi: 10.1038/ni.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brehm MA, Markees TG, Daniels KA, Greiner DL, Rossini AA, Welsh RM. Direct visualization of cross-reactive effector and memory allo-specific CD8 T cells generated in response to viral infections. Journal of immunology. 2003;170:4077–4086. doi: 10.4049/jimmunol.170.8.4077. [DOI] [PubMed] [Google Scholar]
- 48.Ni PP, Solomon B, Hsieh CS, Allen PM, Morris GP. The ability to rearrange dual TCRs enhances positive selection, leading to increased Allo- and Autoreactive T cell repertoires. Journal of immunology. 2014;193:1778–1786. doi: 10.4049/jimmunol.1400532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morris GP, Uy GL, Donermeyer D, Dipersio JF, Allen PM. Dual receptor T cells mediate pathologic alloreactivity in patients with acute graft-versus-host disease. Sci Transl Med. 2013;5:188ra174. doi: 10.1126/scitranslmed.3005452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris GP, Allen PM. Cutting edge: Highly alloreactive dual TCR T cells play a dominant role in graft-versus-host disease. Journal of immunology. 2009;182:6639–6643. doi: 10.4049/jimmunol.0900638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Padovan E, Casorati G, Dellabona P, Meyer S, Brockhaus M, Lanzavecchia A. Expression of two T cell receptor alpha chains: dual receptor T cells. Science. 1993;262:422–424. doi: 10.1126/science.8211163. [DOI] [PubMed] [Google Scholar]
- 52.Gagliani N, Gregori S, Jofra T, Valle A, Stabilini A, Rothstein DM, Atkinson M, Roncarolo MG, Battaglia M. Rapamycin combined with anti-CD45RB mAb and IL-10 or with G-CSF induces tolerance in a stringent mouse model of islet transplantation. PLoS One. 2011;6:e28434. doi: 10.1371/journal.pone.0028434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gagliani N, Jofra T, Valle A, Stabilini A, Morsiani C, Gregori S, Deng S, Rothstein DM, Atkinson M, Kamanaka M, Flavell RA, Roncarolo MG, Battaglia M. Transplant tolerance to pancreatic islets is initiated in the graft and sustained in the spleen. Am J Transplant. 2013;13:1963–1975. doi: 10.1111/ajt.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fecteau S, Basadonna GP, Freitas A, Ariyan C, Sayegh MH, Rothstein DM. CTLA-4 up-regulation plays a role in tolerance mediated by CD45. Nat Immunol. 2001;2:58–63. doi: 10.1038/83175. [DOI] [PubMed] [Google Scholar]
- 55.Chen G, Luke PP, Yang H, Visser L, Sun H, Garcia B, Qian H, Xiang Y, Huang X, Liu W, Senaldi G, Schneider A, Poppema S, Wang H, Jevnikar AM, Zhong R. Anti-CD45RB monoclonal antibody prolongs renal allograft survival in cynomolgus monkeys. Am J Transplant. 2007;7:27–37. doi: 10.1111/j.1600-6143.2006.01598.x. [DOI] [PubMed] [Google Scholar]
- 56.Yang H, Arp J, Ma Y, Welch I, Haig A, Liu W, Reimann K, Jevnikar A, Rothstein D. Combination of Novel Anti-CD45RB and Anti-CD40 Chimeric Antibodies Promotes Operational Tolerance and Induction of T Regulatory Cells in Cynomolgus Monkey Renal Allograft Recipients. World Transplant Congress; San Francisco, CA. 2014. [Google Scholar]
- 57.Luke PP, Deng JP, O’Brien CA, Everest M, Hall AV, Chakrabarti S, O’Connell PJ, Zhong R, Jevnikar AM. Alteration in CD45RBhi/CD45RBlo T-cell ratio following CD45RB monoclonal-antibody therapy occurs by selective deletion of CD45RBhi effector cells. Transplantation. 2003;76:400–409. doi: 10.1097/01.TP.0000072373.77323.D4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.