Abstract
Malaria transmission is heterogeneous in the Greater Mekong Subregion with most of the cases occurring along international borders. Knowledge of transmission hotspots is essential for targeted malaria control and elimination in this region. This study aimed to determine the dynamics of malaria transmission and possible existence of transmission hotspots on a microgeographical scale along the China-Myanmar border. Microscopically confirmed clinical malaria cases were recorded in five border villages through a recently established surveillance system between January 2011 and December 2014. A total of 424 clinical cases with confirmed spatial and temporal information were analyzed, of which 330 (77.8%) were Plasmodium vivax and 88 (20.8%) were Plasmodium falciparum, respectively. The P. vivax and P. falciparum case ratio increased dramatically from 2.2 in 2011 to 4.7 in 2014, demonstrating that P. vivax malaria has become the predominant parasite species. Clinical infections showed a strong bimodal seasonality. There were significant differences in monthly average incidence rates among the study villages with rates in a village in China being 3-8 folds lower than those in nearby villages in Myanmar. Spatial analysis revealed the presence of clinical malaria hotspots in four villages. This information on malaria seasonal dynamics and transmission hotspots should be harnessed for planning targeted control.
Keywords: Clinical malaria, seasonality, hotspot, China-Myanmar border
Graphical Abstract
1. Introduction
Malaria is a serious vector-borne disease and a major public health problem in many tropical countries (WHO, 2014). Intensive intervention during the past two decades has led to a dramatic decrease in global malaria incidence. Countries like China and Thailand have entered the malaria elimination phase (Cui et al., 2012a; Cui et al., 2012b; Zhou et al., 2014a). As malaria transmission further declines, control measures will increasingly rely on accurate knowledge of risk factors as well as the ability to define high-risk areas and populations for targeted interventions (Bousema et al., 2010; Bousema et al., 2012; Marsh, 2010; Moonen et al., 2010; Mosha et al., 2014).
Intensified malaria control has also resulted in a major change in malaria epidemiology with an increasing proportion of Plasmodium vivax malaria. Of the four human malaria species, P. vivax is the most common and has the widest geographic range (Battle et al., 2012; Gething et al., 2012; Guerra et al., 2010). It is mostly found outside of Africa and is especially prevalent in Southeast Asia. This parasite is less responsive to control interventions and much more difficult to eliminate when compared to P. falciparum, partly due to the noted ability of P. vivax to form dormant liver stages that cause relapses (Chaves et al., 2008). The increased proportions of P. vivax malaria in Southeast Asia will be a formidable challenge for the malaria elimination course in these countries.
In the Greater Mekong Subregion (GMS), Myanmar has the highest regional malaria burden and is a key source of malaria exportation (Cui et al., 2012a; Cui et al., 2012b; Delacollette et al., 2009). As a consequence of shared borders, low malaria transmission countries such as China (Zhang et al., 2014b) and Thailand will continually face the challenges of managing cross-border malaria introduction (Parker et al., 2015; Wangdi et al., 2015; Zhou et al., 2005; Zhou et al., 2014a). China is entering the malaria elimination phase with a very low malaria incidence rate (< 0.3 cases per 1,000,000p erson year in 2013). However, the China-Myanmar border area has the highest malaria incidence rate (1.2 and ~10.3 cases per 1,000,000 person year in Yunnan Province and in four border counties, respectively). Therefore, border malaria control is crucial to malaria elimination in China (Zhang et al., 2014a). Southwestern Yunnan, which shares ~ 4,000 km of border with Myanmar, Laos and Vietnam, is the most malaria prevalent province in China (Feng et al., 2015; Zhang et al., 2014a). Border malaria – high malaria transmission along the international borders is a common phenomenon in all the GMS nations, which requires in-depth studies and coordinated international efforts. Since border regions represent the biggest reservoirs for malaria, and frequent malaria introductions by migratory human populations are extremely difficult to monitor, border malaria constitutes one of the biggest obstacles for malaria elimination in these countries. Some border regions in Myanmar were not reached by the national malaria control program, and internal political and military conflicts further exacerbate malaria situations in these border regions. Influx of refugees from areas of higher malaria endemicity as the result of military conflicts may inevitably accompany massive malaria introduction. Therefore, timely, detailed mapping of malaria epidemiology in the border regions is essential for identifying malaria transmission hotspots and guiding targeted control efforts. However, malaria epidemiology studies conducted so far in this region mostly focused on malaria in China on large scales (Bi et al., 2013a; Clements et al., 2009; Hui et al., 2009; Li et al., 2013; Lin et al., 2009; Xu et al., 2015; Zhou et al., 2014a). Though these studies depicted the overall trends of malaria situation, better understanding of malaria transmission on a microgeographic level (e.g., villages) would provide unprecedented opportunities for local health department officials to execute targeted control efforts. In this study, as our continued efforts to monitor malaria transmission in the China-Myanmar border area, we performed longitudinal malaria case detections in villages along the border in order to map malaria transmission on a much finer scale. It will offer more accurate information for local disease control organizations to carry out malaria control operations including mosquito control, drug distribution, and health education.
2. Materials and methods
2.1. Study area and population
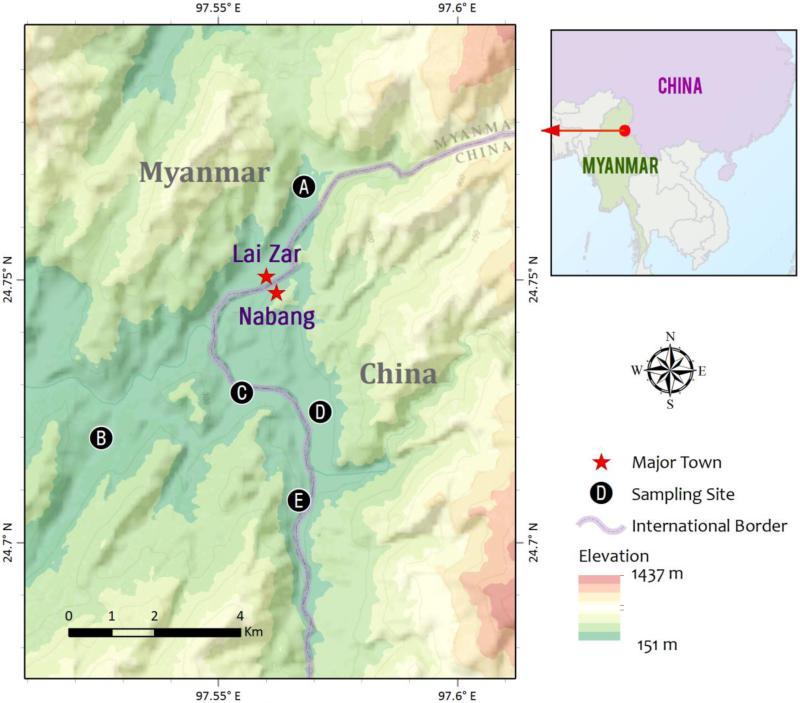
The total population (permanent residents) in Lai Zar and Na Bang (NB) is about 20,000 and 1,500 (from the 2012 census), respectively. Malaria surveillance was carried out between January 2011 and December 2014 in five closely located villages along the China-Myanmar border: JHK, MSY, SSL, SY in Kachin State, Myanmar, and NB in Yingjiang County, Yunnan Province, China (Fig. 1). JHK, MSY and SY are the rural Myanmar villages in the study area, and SSL is a randomly selected suburban area of Lai Zar town. NB consists of three natural villages, where malaria cases were analyzed in this study. The catchment population of each of the four Myanmar villages ranged from ~120 to ~480. The selected villages all had higher malaria incidence in the past. This region has a subtropical climate with January through March as the dry and cold months, and June to August as the rainy season. Annual rainfall is about 1500 mm, and average annual temperature is 22.7°C. Average monthly temperature is the lowest (~15°C) in January, which gradually rises to 24°C in May, and remains at that level through September before gradually declining in October to December.
Fig. 1.
Locations of study villages SSL (A), SY (B), MSY (C), NB (D), and JHK (E) along the China-Myanmar border.
2.2. Malaria surveillance
Demographic data of the study villages in Myanmar were surveyed and updated monthly through seasonal census surveys and weekly/biweekly household visits. Information gathered includes age, sex, onset date of a febrile illness, parasite species, final diagnosis, asexual stage parasitemia and gametocytemia. The latitude and longitude of each household was taken using a handset GPS device. Malaria infection data included clinical malaria infections both from passive case surveillance (PCS) and active case surveillance (ACS). PCS was conducted at five clinics, and only patients from the study villages were included in the final data. ACS was conducted through weekly (April to September) and biweekly (October to March) home visits to assess clinical malaria. During the survey, individuals who were diagnosed with malaria by clinicians (for PCS) or who showed malaria symptoms (for ACS), and who agreed to sign a consent and/or assent form (for minors under the age of 18) were included in the study. We collected all cases occurred during the survey period, and cases potentially resulted from relapses were treated as new cases. Trained nurses collected blood samples by a standard finger-prick method, and thick and thin smears were prepared on labeled slides for parasite species identification. A clinical malaria case was defined as an individual with malaria-related symptoms (fever, i.e., axillary temperature ≥ 37.5°C, chills, severe malaise, headache or vomiting) at the time of examination or 1-2 days prior to the examination with a Plasmodium-positive blood smear (Afrane et al., 2013; Zhou et al., 2015). Parasites were identified microscopically by two experienced technologists. For quality control purpose, a third microscopist confirmed parasite identification by random analysis of ~ 5% of the slides (Afrane et al., 2013; Zhou et al., 2015).
2.3. Statistical and spatial analysis
Clinical malaria incidence rates were calculated as cases per 1,000 populations per year (month) based on the populations from the August 2012 demographic survey. Differences in monthly malaria incidence rates among villages were compared using nonparametric Kruskal-Wallis ANOVA and Median test, while Duncan's range test was used to test the pair-wise difference between villages. Friedman's nonparametric ANOVA was used to test inter-year differences in malaria incidence in MSY. The odds ratio with a 95% CI was used.Analysis of spatial clustering in clinical malaria was performed by using hot spot analysis Getis-Ord'sGi*(d) statistics (Cartabia et al., 2012; Dhimal et al., 2014; Haque et al., 2014; Haque et al., 2012; Kelly-Hope et al., 2009; Ruktanonchai et al., 2014; Saxena et al., 2012). Statistical analysis and spatial clustering were conducted using STATISTICA 10.0 (StatSoft Inc., Tulsa, OK, USA) and ArcGIS 10.3 for Desktop (ESRI, Redlands, CA, USA), respectively.
2.4. Ethical approval
Ethical clearance was granted by the institutional review boards of Kunming Medical University, China, University of California, Irvine, and Pennsylvania State University, USA, and the local Bureau of Health, Kachin State, Myanmar. Informed consent was obtained during the survey both orally and in writing from adult individuals, while for children it was obtained from their parents or guardians.
3. Results
3.1. Malaria incidence and parasite prevalence
A newly established malaria surveillance system was in place at the study villages in order to monitor malaria transmission on the international border. A total of 424 clinical malaria cases were detected by microscopy during the study period. Among them, 271 (64%) and 153 (36%) were from male and female patients, respectively, and the median age was 19. Of the 424 cases, 330 (77.8%) were P. vivax, 88 (20.8%) P. falciparum, and six (1.4%) mixed P. vivax and P. falciparum infections. More than 90% of malaria cases were diagnosed at the clinicians (for PCS), of which 340 cases had household locations. Since this was a retrospective study, household locations could not be obtained for 84 cases (19.8%, 84/424).
Clinical malaria incidence rates varied drastically among villages (Table 1) (Kruskal-Wallis ANOVA with the Median test χ2 = 26.57, d.f. = 4, P < 0.0001). JHK (96.0 cases per 1,000 person year) had a 7-fold higher malaria incidence rate than NB (18.1 cases per 1,000 person year). NB had the lowest malaria incidence rate amongst the study villages. Overall, villages in Myanmar had significantly higher incidence rates than NB in China (Duncan's range test, P < 0.05).
Table 1.
Clinical cases from 2011 to 2014 by village and parasite species.
| Site | Population† | Blood slides examined | Clinical cases |
Case rate‡ | |||
|---|---|---|---|---|---|---|---|
| Total | Pv | Pf | Pv+Pf | ||||
| JHK | 354 | 743 | 136 | 37 | 96 | 3 | 96.0 |
| MSY | 384 | 964 | 104 | 23 | 79 | 2 | 67.7 |
| SSL | 476 | 1713 | 84 | 8 | 76 | 0 | 44.1 |
| SY§ | 123 | 135 | 18 | 5 | 12 | 1 | 48.8 |
| NB$ | 1,500 | 170 | 82 | 15 | 67 | 0 | 13.7 |
Pv, P. vivax; Pf, P. falciparum, Pv+Pf, mixed infections of P. vivax and P. falciparum.
Population from a 2012 demographic survey.
Case rate: cases per 1,000 population per year.
2014 cases were not available.
Population included only permanent residents, while cases included all suspected malaria infections.
3.2. Temporal trend in clinical malaria
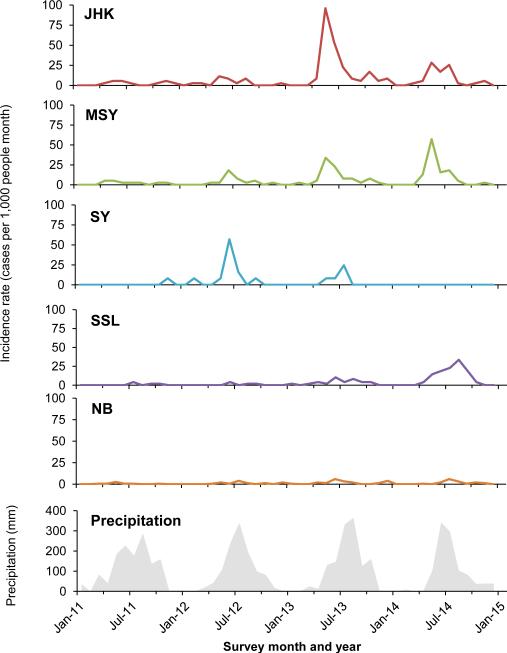
The overall clinical malaria incidence showed distinct seasonality with the peak season from April to October (Fig. 2). Despite that these villages are closely located from each other, the peak malaria month and year varied among villages. Specifically, the highest peak occurred in SY in 2012, JHK in 2013, and SSL in 2014, respectively (Fig. 2). MSY was the only village where incidence rates increased continuously from 2011 to 2014, with average incidence rates being 23.4, 41.7, 93.7, and 112.0 cases per 1,000 person years, respectively (Friedman ANOVA χ2 = 8.07, d.f. = 3, P < 0.05).
Fig. 2.
Temporal trends of malaria incidence rate (cases per 1,000 persons per year) in different villages from 2011 to 2014.
Changes in P. vivax/P. falciparum (Pv/Pf) ratio were significant over the four-year period (Table 2). In Myanmar, the Pv/Pf ratio increased from 1.6 in 2011 to 2.7, 3.6, and 4.91 in 2012, 2013 and 2014, respectively. The odds ratio also increased from 2.4 (95% CI [0.7, 7.9], P = 0.14) in 2011 to 23.3 (95% CI [12.2, 44.2], P < 0.0001) in 2014. In China, however, the Pv/Pf ratio decreased from 2011 to 2013 (Table 2).
Table 2.
Changes in P. vivax and P. falciparum (Pv/Pf) incidence ratio over time.
| Site | Year | Pv/Pf | Odds ratio [95% CI] | P value |
|---|---|---|---|---|
| Overall | ||||
| 2011 | 2.20 | 4.84 [1.68, 13.93] | 0.0027 | |
| 2012 | 3.21 | 8.75 [3.90, 19.61] | <0.0001 | |
| 2013 | 3.47 | 11.64 [7.02, 19.28] | <0.0001 | |
| 2014 | 4.66 | 21.16 [12.38, 36.19] | <0.0001 | |
| Total | 3.70 | 14.35 [10.47, 19.69] | <0.0001 | |
| Myanmar | ||||
| 2011 | 1.56 | 2.42 [0.74, 7.91] | 0.1407 | |
| 2012 | 2.73 | 6.18 [2.46, 15.51] | <0.0001 | |
| 2013 | 3.63 | 12.60 [7.17, 22.15] | <0.0001 | |
| 2014 | 4.91 | 23.26 [12.24, 44.21] | <0.0001 | |
| Total | 3.63 | 12.30 [8.56, 17.66] | <0.0001 | |
| China | ||||
| 2011 | 8.00 | 64.00 [3.38, 1210.61] | 0.0034 | |
| 2012 | 5.00 | 25.00 [4.33, 144.31] | <0.0001 | |
| 2013 | 2.88 | 8.27 [2.65, 25.79] | 0.0001 | |
| 2014 | 4.10 | 16.81 [6.32, 44.68] | <0.0001 | |
| Total | 3.95 | 15.64 [8.07, 30.30] | <0.0001 |
3.3. Hotspots of clinical malaria
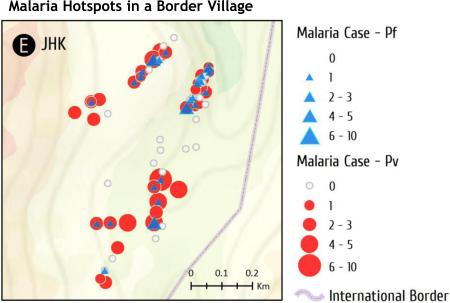
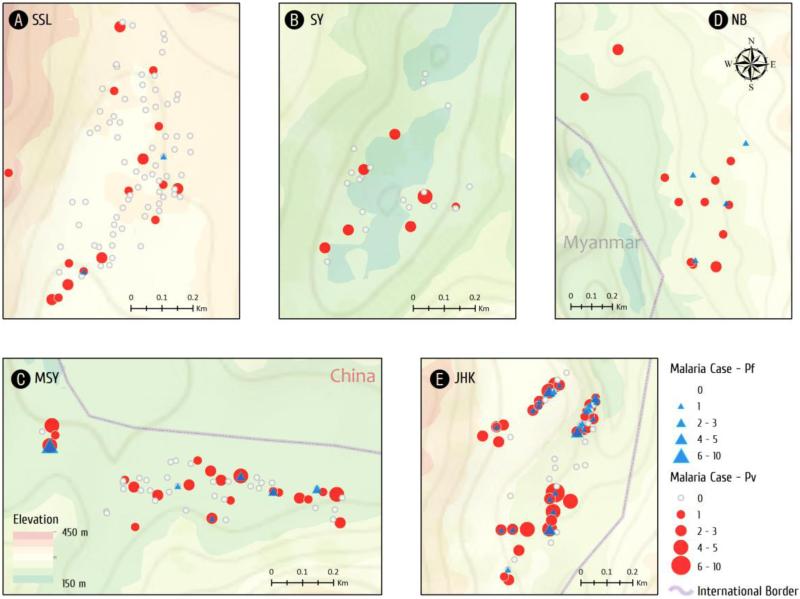
Spatial distribution of malaria cases varied significantly both among villages and within a particular village (Fig. 3). The locations of P. falciparum and P. vivax cases are mapped in Fig. 3. The median number of cases in each village was JHK 1.5, MSY 0, SY 0, SSL 0, and NB 0, respectively. In NB, SY, and SSL, there was at most one case per household, while most households had no malaria. In contrast, in both JHK and MSY, malaria cases at the household level ranged from zero to more than 10 cases during the study period. Furthermore, most households in JHK had experienced at least one episode of malaria during the study period.
Fig. 3.
Spatial distribution of malaria cases in each village at the household level. Malaria cases were pooled from 2011 to 2014. Symbol sizes correspond to the numbers of malaria cases. P. falciparum and P. vivax cases are shown as blue triangles and red circles, respectively.
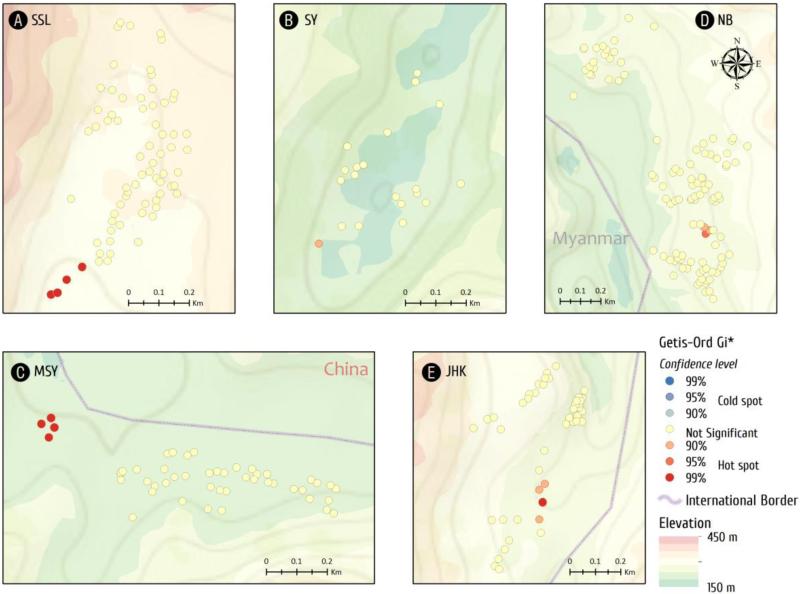
Getis-Ord's analysis showed the presence of evident malaria hotspots (z > 2.58, P < 0.01) in four villages, JHK, MSY, SSL, and NB, whereas clustering of malaria cases was marginal (1.65<z <1.96, P < 0.10) in SY (Fig. 4). Interestingly, malaria cases clustered in the village center of JHK, whereas the clusters were located at the gateways of the remaining four villages.
Fig. 4.
Hot and cold spots of malaria incidents detected by Getis-OrdGi* analysis at the household level. Dot colors indicate different significance levels. Reddish dots indicate hot spots, bluish dots indicate cold spots, and yellow dots represent “not significant”.
4. Discussion
Motivated by recent achievements in malaria control, several nations in the GMS are pursuing malaria elimination with China taking the lead and aiming to achieve this goal by 2020. Malaria morbidity has continued to decline over the past two decades in China, with the majority of malaria concentrated in western Yunnan (Bi et al., 2013a). International border areas have become key control points for malaria elimination in China (Cui et al., 2012a; Cui et al., 2012b; Zhang et al., 2014a; Zhou et al., 2014a; Zhou et al., 2008). Investigation of malaria incidence in border counties of Yunnan province, China revealed that clinical P. falciparum malaria was closely associated with travel to Myanmar, whereas local P. vivax transmission remained (Xu et al., 2015; Zhou et al., 2014a).This highlights the need for both strengthened local control efforts as well as tightened monitoring near international borders to prevent malaria introduction (Mosha et al., 2014; Wangdi et al., 2015).
At the last stage of malaria elimination, control programs must shift focus from high coverage of interventions to seeking out infections and interrupting transmission. Targeted intervention is probably the most cost-effective way to rid of the source of parasites (Bousema et al., 2010; Bousema et al., 2012; Cotter et al., 2013; Mosha et al., 2014). However, targeted intervention requires accurate and timely information for identifying the target areas (Bousema et al., 2010; Bousema et al., 2012; Cotter et al., 2013). In China, a malaria elimination surveillance system was set up with a “1-3-7” approach to meet the challenges in communicating and monitoring surveillance and response activities (Cao et al., 2014).The disease control programs will have massive data gathering and dissemination responsibilities, and timely analysis and communications are essential (Cao et al., 2013; Zhou et al., 2014b). In this aspect, spatial and temporal maps to track populations at risk of malaria at local levels would be of great value.
Our analysis of malaria epidemiology on a micro-geographical scale revealed significant malaria transmission heterogeneity in both time and space. First, malaria in this area has clear seasonality with the peak malaria season coincident with the rainy season, which is likely a reflection of the climatic pattern. Yet, it is surprising that peak malaria incidence also had considerable variations among different villages and in different years and months. This may be attributed to an insufficient malaria management system that focuses on epidemics rather than systematic interventions. In several villages, annual malaria incidence in 2012-2014 was much higher than 2011, suggesting of insufficient control efforts, probably as a consequence of military conflicts taken place in recent years between the local and central governments. Second, our spatial analysis clearly showed the presence of malaria transmission hotspots in most of the villages. The strongly aggregated distribution of malaria in JHK and MSY may be attributed to the existence of mosquito breeding habitats, which were associated with high Anopheles mosquito abundance as detected in our mosquito surveys in 2011 to 2013. In this area, twenty species of Anopheles mosquitoes were identified, with An. minimus s.l. accounting for 85% of the total collections (54–91 % of total captures in different villages). Mosquito densities varied from 0.05 to 3.00 females per trap per night, with strong seasonality in all sites and a density peak from June to August. Species richness peaked from April to August according to our previously reports (Bi et al., 2013b; Wang et al., 2015; Yu et al., 2013).
Interestingly, most of the hotspots were located in the crossroad or junctions areas. For example, the hotspot in JHK coincides with the major junction of roads to China, the camps for internally displaced people, and inland of Myanmar. It is possible that such hotspots may facilitate local and distant spread of malaria. Finally, the increase of malaria incidence in these villages in recent years accompanied the increase of Pv/Pf ratio, indicating increased transmission of vivax malaria in these villages. The emergence of drug-resistant parasites in this region (Yuan et al., 2014) and less stringent uses of primaquine for radical care because the presence of a significant portion of the local ethnic population with glucose-6-phosphate dehydrogenase deficiency (Li et al., 2015) may be partially responsible for the increased vivax cases in this region, which underlines the urgency for implementation of more effective P. vivax control measures (Waltmann et al., 2015; Zhou et al., 2014a). Furthermore, current treatment of P. falciparum was effective, which may contribute to the decrease in falciparum malaria, similar to what has been found in Thailand (Phimpraphi et al., 2008; Wang et al., 2015; Zhou et al., 2005).The migration flow between two countries is huge, estimated to be at least 20-fold higher than Lai Zar's residents, and 300-fold higher than NB's residents based on local records from the border control (unpublished). Our study is limited in that we did not collect data on cross-border movement of the study participants, and therefore could not differentiate indigenous from imported cases. Such information is important for a better understanding of malaria transmission at the border region.
In conclusion, this study investigated temporal and spatial patterns of malaria transmission in villages along the China-Myanmar border. While malaria here has an overall seasonal trend, there were considerable variations in the peak transmission month between the villages. Spatial analysis identified clear hotspots of malaria clinical cases within most studied villages. Malaria incidence rates were significantly higher in the Myanmar villages than in the neighboring village in China. These findings further depicted a scenario of border malaria in the GMS. Difference in malaria transmission levels between the two sides of the border emphasizes the threat of cross-border malaria introduction. These findings are vital for health workers on both sides of the border to implement targeted malaria intervention and to develop a strategy for controlling and eliminating “border malaria” in the GMS countries.
Highlights.
Malaria cases in 5 villages along the China-Myanmar border were mapped.
P. vivax/P. falciparum ratio increased dramatically during the four study years.
Monthly incidence rates differed greatly among the study villages.
Myanmar villages had much higher malaria incidence rates than a village in China.
Spatial analysis revealed hotspots of clinical malaria incidences in four villages.
Acknowledgements
We thank the local communities for willingness to participate in this research. This project was funded by grants from the National Institutes of Health (U19 AI089672), the National Natural Science Foundation of China (U1202226 and 31260508 to ZY). Yanrui Wu was supported by grants (2014YNPHXT05 and 2014FB005) from the Yunnan province.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afrane YA, Zhou G, Githeko AK, Yan G. Utility of health facility-based malaria data for malaria surveillance. PLoS One. 2013;8:e54305. doi: 10.1371/journal.pone.0054305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battle KE, Gething PW, Elyazar IR, Moyes CL, Sinka ME, Howes RE, Guerra CA, Price RN, Baird KJ, Hay SI. The global public health significance of Plasmodium vivax. Adv. Parasitol. 2012;80:1–111. doi: 10.1016/B978-0-12-397900-1.00001-3. [DOI] [PubMed] [Google Scholar]
- Bi Y, Hu W, Yang H, Zhou XN, Yu W, Guo Y, Tong S. Spatial patterns of malaria reported deaths in Yunnan Province. China. Am. J. Trop. Med. Hyg. 2013a;88:526–535. doi: 10.4269/ajtmh.2012.12-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Y, Yu W, Hu W, Lin H, Guo Y, Zhou XN, Tong S. Impact of climate variability on Plasmodium vivax and Plasmodium falciparum malaria in Yunnan Province, China. Parasit. Vectors. 2013b;6:357. doi: 10.1186/1756-3305-6-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousema T, Drakeley C, Gesase S, Hashim R, Magesa S, Mosha F, Otieno S, Carneiro I, Cox J, Msuya E, Kleinschmidt I, Maxwell C, Greenwood B, Riley E, Sauerwein R, Chandramohan D, Gosling R. Identification of hot spots of malaria transmission for targeted malaria control. J. Infect. Dis. 2010;201:1764–1774. doi: 10.1086/652456. [DOI] [PubMed] [Google Scholar]
- Bousema T, Griffin JT, Sauerwein RW, Smith DL, Churcher TS, Takken W, Ghani A, Drakeley C, Gosling R. Hitting hotspots: spatial targeting of malaria for control and elimination. PLoS Med. 2012;9:e1001165. doi: 10.1371/journal.pmed.1001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Sturrock HJ, Cotter C, Zhou S, Zhou H, Liu Y, Tang L, Gosling RD, Feachem RG, Gao Q. Communicating and monitoring surveillance and response activities for malaria elimination: China's “1-3-7” strategy. PLoS Med. 2014;11:e1001642. doi: 10.1371/journal.pmed.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Zhou SS, Zhou HY, Yu YB, Tang LH, Gao Q. Malaria from control to elimination in China: transition of goal, strategy and interventions. Chinese Journal of Schistosomiasis Control. 2013;25:439–443. [PubMed] [Google Scholar]
- Cartabia M, Campi R, Clavenna A, Bortolotti A, Fortino I, Merlino L, Bonati M. Geographical epidemiology of antibacterials in the preschool age. Int. J. Health. Geogr. 2012;11:52. doi: 10.1186/1476-072X-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves LF, Kaneko A, Taleo G, Pascual M, Wilson ML. Malaria transmission pattern resilience to climatic variability is mediated by insecticide-treated nets. Malar. J. 2008;7:100. doi: 10.1186/1475-2875-7-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements AC, Barnett AG, Cheng ZW, Snow RW, Zhou HN. Space-time variation of malaria incidence in Yunnan province. China. Malar. J. 2009;8:180. doi: 10.1186/1475-2875-8-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter C, Sturrock HJ, Hsiang MS, Liu J, Phillips AA, Hwang J, Gueye CS, Fullman N, Gosling RD, Feachem RG. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet. 2013;382:900–911. doi: 10.1016/S0140-6736(13)60310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Yan G, Sattabongkot J, Cao Y, Chen B, Chen X, Fan Q, Fang Q, Jongwutiwes S, Parker D, Sirichaisinthop J, Kyaw MP, Su XZ, Yang H, Yang Z, Wang B, Xu J, Zheng B, Zhong D, Zhou G. Malaria in the Greater Mekong Subregion: heterogeneity and complexity. Acta. Trop. 2012a;121:227–239. doi: 10.1016/j.actatropica.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Yan G, Sattabongkot J, Chen B, Cao Y, Fan Q, Parker D, Sirichaisinthop J, Su XZ, Yang H, Yang Z, Wang B, Zhou G. Challenges and prospects for malaria elimination in the Greater Mekong Subregion. Acta. Trop. 2012b;121:240–245. doi: 10.1016/j.actatropica.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacollette C, D'Souza C, Christophel E, Thimasarn K, Abdur R, Bell D, Dai TC, Gopinath D, Lu S, Mendoza R, Ortega L, Rastogi R, Tantinimitkul C, Ehrenberg J. Malaria trends and challenges in the Greater Mekong Subregion. Southeast Asian. J. Trop. Med. Public. Health. 2009;40:674–691. [PubMed] [Google Scholar]
- Dhimal M, O'Hara RB, Karki R, Thakur GD, Kuch U, Ahrens B. Spatio-temporal distribution of malaria and its association with climatic factors and vector-control interventions in two high-risk districts of Nepal. Malar. J. 2014;13:457. doi: 10.1186/1475-2875-13-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Xiao H, Xia Z, Zhang L, Xiao N. Analysis of Malaria Epidemiological Characteristics in the People's Republic of China, 2004-2013. Am. J. Trop. Med. Hyg. 2015;93:293–299. doi: 10.4269/ajtmh.14-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Mueller I, Baird JK, Hay SI. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS. Negl. Trop. Dis. 2012;6:e1814. doi: 10.1371/journal.pntd.0001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra CA, Howes RE, Patil AP, Gething PW, Van Boeckel TP, Temperley WH, Kabaria CW, Tatem AJ, Manh BH, Elyazar IR, Baird JK, Snow RW, Hay SI. The international limits and population at risk of Plasmodium vivax transmission in 2009. PLoS. Negl. Trop. Dis. 2010;4:e774. doi: 10.1371/journal.pntd.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque U, Overgaard HJ, Clements AC, Norris DE, Islam N, Karim J, Roy S, Haque W, Kabir M, Smith DL, Glass GE. Malaria burden and control in Bangladesh and prospects for elimination: an epidemiological and economic assessment. Lancet. Glob. Health. 2014;2:e98–105. doi: 10.1016/S2214-109X(13)70176-1. [DOI] [PubMed] [Google Scholar]
- Haque U, Scott LM, Hashizume M, Fisher E, Haque R, Yamamoto T, Glass GE. Modelling malaria treatment practices in Bangladesh using spatial statistics. Malar. J. 2012;11:63. doi: 10.1186/1475-2875-11-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui FM, Xu B, Chen ZW, Cheng X, Liang L, Huang HB, Fang LQ, Yang H, Zhou HN, Yang HL, Zhou XN, Cao WC, Gong P. Spatio-temporal distribution of malaria in Yunnan Province, China. Am. J. Trop. Med. Hyg. 2009;81:503–509. [PubMed] [Google Scholar]
- Kelly-Hope LA, Hemingway J, McKenzie FE. Environmental factors associated with the malaria vectors Anopheles gambiae and Anopheles funestus in Kenya. Malar. J. 2009;8:268. doi: 10.1186/1475-2875-8-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Parker DM, Yang Z, Fan Q, Zhou G, Ai G, Duan J, Lee MC, Yan G, Matthews SA, Cui L, Wang Y. Risk factors associated with slide positivity among febrile patients in a conflict zone of north-eastern Myanmar along the China-Myanmar border. Malar. J. 2013;12:361. doi: 10.1186/1475-2875-12-361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yang F, Liu R, Luo L, Yang Y, Zhang L, Liu H, Zhang W, Fan Z, Yang Z, Cui L, He Y. Prevalence and Molecular Characterization of Glucose-6-Phosphate Dehydrogenase Deficiency at the China-Myanmar Border. PloS one. 2015 doi: 10.1371/journal.pone.0134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Lu L, Tian L, Zhou S, Wu H, Bi Y, Ho SC, Liu Q. Spatial and temporal distribution of falciparum malaria in China. Malar. J. 2009;8:130. doi: 10.1186/1475-2875-8-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh K. Research priorities for malaria elimination. Lancet. 2010;376:1626–1627. doi: 10.1016/S0140-6736(10)61499-7. [DOI] [PubMed] [Google Scholar]
- Moonen B, Cohen JM, Snow RW, Slutsker L, Drakeley C, Smith DL, Abeyasinghe RR, Rodriguez MH, Maharaj R, Tanner M, Targett G. Operational strategies to achieve and maintain malaria elimination. Lancet. 2010;376:1592–1603. doi: 10.1016/S0140-6736(10)61269-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosha JF, Sturrock HJ, Brown JM, Hashim R, Kibiki G, Chandramohan D, Gosling RD. The independent effect of living in malaria hotspots on future malaria infection: an observational study from Misungwi, Tanzania. Malar. J. 2014;13:445. doi: 10.1186/1475-2875-13-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker DM, Matthews SA, Yan G, Zhou G, Lee MC, Sirichaisinthop J, Kiattibutr K, Fan Q, Li P, Sattabongkot J, Cui L. Microgeography and molecular epidemiology of malaria at the Thailand-Myanmar border in the malaria pre-elimination phase. Malar. J. 2015;14:198. doi: 10.1186/s12936-015-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phimpraphi W, Paul RE, Yimsamran S, Puangsa-art S, Thanyavanich N, Maneeboonyang W, Prommongkol S, Sornklom S, Chaimungkun W, Chavez IF, Blanc H, Looareesuwan S, Sakuntabhai A, Singhasivanon P. Longitudinal study of Plasmodium falciparum and Plasmodium vivax in a Karen population in Thailand. Malar. J. 2008;7:99. doi: 10.1186/1475-2875-7-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruktanonchai CW, Pindolia DK, Striley CW, Odedina FT, Cottler LB. Utilizing spatial statistics to identify cancer hot spots: a surveillance strategy to inform community-engaged outreach efforts. Int. J. Health. Geogr. 2014;13:39. doi: 10.1186/1476-072X-13-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena R, Nagpal BN, Das MK, Srivastava A, Gupta SK, Kumar A, Jeyaseelan AT, Baraik VK. A spatial statistical approach to analyze malaria situation at micro level for priority control in Ranchi district, Jharkhand. Indian. J. Med. Res. 2012;136:776–782. [PMC free article] [PubMed] [Google Scholar]
- Waltmann A, Darcy AW, Harris I, Koepfli C, Lodo J, Vahi V, Piziki D, Shanks GD, Barry AE, Whittaker M, Kazura JW, Mueller I. High Rates of Asymptomatic, Sub-microscopic Plasmodium vivax Infection and Disappearing Plasmodium falciparum Malaria in an Area of Low Transmission in Solomon Islands. PLoS. Negl. Trop. Dis. 2015;9:e0003758. doi: 10.1371/journal.pntd.0003758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhong D, Cui L, Lee MC, Yang Z, Yan G, Zhou G. Population dynamics and community structure of Anopheles mosquitoes along the China-Myanmar border. Parasit. Vectors. 2015;8:445. doi: 10.1186/s13071-015-1057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangdi K, Gatton ML, Kelly GC, Clements AC. Cross-border malaria: a major obstacle for malaria elimination. Adv. Parasitol. 2015;89:79–107. doi: 10.1016/bs.apar.2015.04.002. [DOI] [PubMed] [Google Scholar]
- WHO World Malaria Report 2014. 2014 [Google Scholar]
- Xu JW, Liu H, Zhang Y, Guo XR, Wang JZ. Risk factors for border malaria in a malaria elimination setting: a retrospective case-control study in Yunnan, China. Am. J. Trop. Med. Hyg. 2015;92:546–551. doi: 10.4269/ajtmh.14-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G, Yan G, Zhang N, Zhong D, Wang Y, He Z, Yan Z, Fu W, Yang F, Chen B. The Anopheles community and the role of Anopheles minimus on malaria transmission on the China-Myanmar border. Parasit. Vectors. 2013;6:264. doi: 10.1186/1756-3305-6-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Wang Y, Parker DM, Gupta B, Yang Z, Liu H, Fan Q, Cao Y, Xiao Y, Lee MC, Zhou G, Yan G, Baird JK, Cui L. Therapeutic Responses of Plasmodium vivax Malaria to Chloroquine and Primaquine Treatment in Northeastern Myanmar. Antimicrob. Agents. Chemother. 2014;59:1230–1235. doi: 10.1128/AAC.04270-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Feng J, Xia ZG. Malaria situation in the People's Republic of China in 2013. Chinese Journal of Parasitology & Parasitic Diseases. 2014a;32:407–413. [PubMed] [Google Scholar]
- Zhang Q, Lai S, Zheng C, Zhang H, Zhou S, Hu W, Clements AC, Zhou XN, Yang W, Hay SI, Yu H, Li Z. The epidemiology of Plasmodium vivax and Plasmodium falciparum malaria in China, 2004-2012: from intensified control to elimination. Malar. J. 2014b;13:419. doi: 10.1186/1475-2875-13-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Afrane YA, Malla S, Githeko AK, Yan G. Active case surveillance, passive case surveillance and asymptomatic malaria parasite screening illustrate different age distribution, spatial clustering and seasonality in western Kenya. Malar. J. 2015;14:41. doi: 10.1186/s12936-015-0551-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Sirichaisinthop J, Sattabongkot J, Jones J, Bjornstad ON, Yan G, Cui L. Spatio-temporal distribution of Plasmodium falciparum and p. Vivax malaria in Thailand. Am. J. Trop. Med. Hyg. 2005;72:256–262. [PubMed] [Google Scholar]
- Zhou G, Sun L, Xia R, Duan Y, Xu J, Yang H, Wang Y, Lee MC, Xiang Z, Yan G, Cui L, Yang Z. Clinical malaria along the China-Myanmar border, Yunnan Province, China, January 2011-August 2012. Emerg. Infect. Dis. 2014a;20:675–678. doi: 10.3201/eid2004.130647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou SS, Wang Y, Fang W, Tang LH. Malaria situation in the People's Republic Of China in 2007. Chinese Journal of Parasitology & Parasitic Diseases. 2008;26:401–403. [PubMed] [Google Scholar]
- Zhou WG, Qu Y, Wang WG, Tang SY. Application of health education of house-to-house visit in malaria prevention and control. Chinese Journal of Schistosomiasis Control. 2014b;26:517–521. [PubMed] [Google Scholar]