Abstract
B-cell activating factor (BAFF) is critical for the survival and maturation of mature B-cells. BAFF, via the BAFF receptor (BAFFR), activates multiple signaling pathways in B-cells, including the alternative nuclear factor-κB (NF-κB) pathway. The transcription factors RELB and NF-κB2 (p100/p52) are the downstream mediators of the alternative pathway; however, the B-cell-intrinsic functions of these NF-κB subunits have not been studied in vivo using conditional alleles, either individually or in combination. We here report that B-cell-specific deletion of relb led to only a slight decrease in the fraction of mature splenic B cells, whereas deletion of nfkb2 caused a marked reduction. This phenotype was further exacerbated upon combined deletion of relb and nfkb2 and most dramatically affected the maintenance of marginal zone B-cells. BAFF-stimulation, in contrast to CD40-activation, was unable to rescue relb/nfkb2-deleted B-cells in vitro. RNA-sequencing analysis of BAFF-stimulated nfkb2-deleted vs. normal B-cells suggests that the alternative NF-κB pathway, in addition to its critical role in BAFF-mediated cell survival, may control the expression of genes involved in the positioning of B-cells within the lymphoid microenvironment and in the establishment of T-cell-B-cell interactions. Thus, by ablating the downstream transcription factors of the alternative NF-κB pathway specifically in B-cells, we here identify a critical role for the combined activity of the RELB and NF-κB2 subunits in B-cell homeostasis that cannot be compensated for by the canonical NF-κB pathway under physiological conditions.
Keywords: B-cell, follicular B-cell, marginal zone B-cell, NF-κB transcription factors
INTRODUCTION
The requirement of tonic signaling through the B-cell receptor (BCR) for the maintenance of resting, mature B-cells is well established 1,2. A second crucial survival signal for mature B-cells is mediated via the binding of B-cell-activating factor (BAFF) to the BAFF-receptor (BAFFR), which is expressed on B cells 3. Genetic ablation of either BAFF or BAFFR results in a dramatic reduction of peripheral B-cells 4–9, while BAFF overexpression causes B-cell hyperplasia 10,11. BAFF or BAFFR deficiencies lead to B-cell maturation arrest at the transitional-1 (T1) developmental stage 3. As a result, the survival of follicular B-cells is strongly impaired and marginal zone (MZ) B-cell development is ablated. BAFF activates several downstream signaling routes in B-cells. It stimulates the AKT, ERK, PKCβ and PI3K signaling axes which sustain B-cell survival and metabolic fitness 12–15. In addition, BAFF-mediated activation of the nuclear factor-κB (NF-κB) signaling pathway is a major regulator of B-cell maintenance 16,17.
The NF-κB signaling pathway can be divided into two major branches, a canonical and an alternative pathway that are activated through specific cell surface receptors 18,19. The downstream mediators of the canonical pathway are the transcription factors c-REL, RELA and p50 that mainly occur as heterodimers. In B-cells, these heterodimers translocate from the cytoplasm to the nucleus in response to BCR, Toll-like receptor and CD40-stimulation 20,21. The corresponding downstream mediators of the alternative pathway are RELB (encoded by relb) and p52 (generated by proteolytic cleavage of its precursor p100, encoded by nfkb2). These subunits mainly occur as heterodimers and in B-cells are activated by soluble BAFF or CD40-stimulation via interaction with T-cells expressing CD40 ligand 20–22. RELB, but not p52, contains a transactivation domain (TAD) and is thus capable of activating transcription. Receptor-mediated activation of the alternative pathway leads to the release of NF-κB-inducing kinase (NIK) from the TRAF2/TRAF3/cIAP1/2 complex, allowing its stabilization 23. NIK activates IκB kinase-α (IKKα), which phosphorylates and subsequently induces cleavage of p100 that is bound to RELB. This results in both the generation of p52 and the release of RELB, thereby allowing the nuclear translocation of RELB/p52 heterodimers 24.
In resting, mature B-cells, the predominant route of BAFF-mediated NF-κB activation is via the alternative pathway 25–27. There is however evidence for an additional contribution of the canonical pathway to BAFF-mediated pro-survival functions 28,29, and constitutive canonical NF-κB signaling can fully replace BAFFR signals 30. A role for the alternative pathway in B-cell homeostasis has previously been demonstrated based on the analysis of mice deficient in upstream components of the pathway 31–38. Whereas these studies have identified NIK and IKKα as critical factors in inducing the processing of p100 that facilitates nuclear translocation of RELB/p52 heterodimers, they can have additional functions. In certain cell systems, NIK has been found to also activate the canonical pathway 39–41, and IKKα has known NF-κB-independent roles 42. These alternative NF-κB pathway-independent functions of NIK and IKKα make it difficult to conclusively identify the biological role of RELB/p52-mediated target gene transcription from these studies.
The identification of the lymphocyte-intrinsic functions of RELB and NF-κB2 (that is, both p52 and p100) has been hampered by the fact that constitutional relb and nfkb2 knockout mice show severe defects in lymphoid organization due to the lack of RELB or NF-κB2 in stromal cells 43–45. Moreover, although the individual roles of RELB and NF-κB2 in mature B-cell development have been investigated with radiation-induced chimeras generated by transplanting hematopoietic cells from relb−/− mice or by adoptive transfer of bone marrow from nfkb2−/− mice into RAG1-deficient animals 44,46, similar experiments with hematopoietic cells from relb−/−nfkb2−/− mice to determine the biological consequences of the combined deletion of RELB and NF-κB2 in B-cells have not been conducted.
The studies outlined above have revealed much about the role of the alternative NF-κB pathway in BAFF-mediated B-cell homeostasis; however, several issues remain to be clarified. First, the cell-intrinsic requirement of the NF-κB2 or RELB subunits for mature B-cell maintenance has not been determined using conditional alleles that can be deleted specifically in B-cells. Second, knockout of either relb or nfkb2 alone does not allow complete ablation of the alternative NF-κB pathway. Indeed, in the absence of either relb or nfkb2, the remaining alternative NF-κB2 subunit may be able to direct transcription by binding to canonical NF-κB subunits. Furthermore, in nfkb2 knockout mice, lack of p100 not only prevents the generation of p52, but also eliminates the p100 inhibitor. p100 retains RELB in the cytoplasm, and in its absence, inappropriate translocation of RELB into the nucleus may occur, resulting in target gene transcription via the formation of heterodimers with other NF-κB subunits. Also, recent evidence suggests that RELB and p52 have common, but also distinct DNA binding sites in the genome 47; how these sites would be modulated upon binding of alternative NF-κB subunits dimerizing with canonical subunits is unclear. Therefore, identification of the biological consequences of complete inactivation of the alternative NF-κB pathway requires ablation of both RELB and NF-κB2 subunits. To address these issues, we have generated conditional relb and nfkb2 alleles. We found that combined deletion of relb and nfkb2 in B-cells had a markedly stronger effect on the survival of mature B-cells compared to the single gene deletions. These results for the first time reveal the extent to which the activity of the alternative NF-κB pathway controls B-cell homeostasis in vivo, and provide new insights into the biological program controlled by the alternative NF-κB pathway in response to BAFF.
MATERIALS AND METHODS
Generation of mice carrying a floxed relb or nfkb2 allele
The vector to target relb and nfkb2 has been described previously in the context of the targeting of the irf4 gene 48. The vector was constructed such that upon Cre-mediated deletion, the promoter regions and the exons comprising the first ATG of relb or nfkb2 were deleted with simultaneous activation of eGFP expression (Supplementary Fig. 1). Successively inserted into the cloning sites of the vector were each three DNA fragments of the relb and nfkb2 loci comprising the following: (1) relb, 2.2 kb of the region upstream of the relb promoter region; the relb promoter region, exon 1 which contains the translation start site and exon 2 (overall 2.8kb); and 3.8 kb of the region downstream of exon 2; (2) nfkb2, 2.0 kb of the region upstream of the nfkb2 promoter region; the nfkb2 promoter region, exon 1 and exon 2, which contains the translational start site (overall 2.4 kb); and 4.6 kb of the region downstream of exon 2. The linearized vectors were electroporated into KV1 embryonic stem (ES) cells (a 129:B6 hybrid ES cell line), and correctly targeted ES cell colonies were identified by Southern blot analysis after selection with gancyclovir and G418 (Supplementary Fig. 1). Chimeras were obtained after injection of targeted ES cell clones into blastocysts derived from C57BL/6 mice. From the chimeras bred with C57BL/6 females, we obtained mice with the ‘conditional’ relb and nfkb2 alleles in the germ-line. The conditional relb and nfkb2 alleles were backcrossed to C57BL/6 mice (n≥7). CD19-Cre mice have been described 49. Mice were housed and treated in compliance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals and according to the guidelines of the Institute of Comparative Medicine at Columbia University. The animal protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Columbia University.
B-cell isolation and culture
Single cell suspensions of mouse spleen were subjected to hypotonic lysis and B cells were purified by depletion of magnetically labeled non-B cells using the MACS B-cell isolation kit (Miltenyi Biotec). Purified B cells from the indicated genotypes were cultured in the presence of: 1 μg/ml anti-mouse CD40 (clone HM40-3; BD Pharmingen) or 25 ng/ml BAFF (R&D Systems). Cell density in CD40 and BAFF stimulation experiments was 1.5 × 106 cells/ml. For the RNA-seq analysis, cell pellets were lysed with TRIzol reagent (Life Technologies) for RNA isolation. For Western blot analysis, purified B cells were washed once with PBS and subjected to NP40-based lysis.
Flow cytometry
Spleen cell suspensions or cultured B cells were stained on ice in PBS/0.5% BSA with the following antibodies. From BD Pharmingen: APC-conjugated anti-IgM (clone II/41), PE-conjugated anti-IgD (clone 11-26c.2a) and PE-conjugated anti-CD23 (clone B3B4). From Biolegend: PerCP-conjugated anti-B220 (clone: RA3-6B2), APC-conjugated anti-CD21 (clone: 7E9), PE-conjugated anti-β2 (clone: M18/2), PE-conjugated anti-CD93 (clone: AA4.1), biotin-conjugated anti-ICOSL (clone: HK5.3) followed by streptavidin-PerCP (BD Pharmingen), PE-conjugated anti-BAFFR (clone: 7H22-E16) and PercP/Cy5.5-conjugated anti-CD40 (clone: 3/23). Annexin V/7-AAD stainings were conducted using the APC Annexin V Apoptosis Detection Kit with 7-AAD (Biolegend). For DNA content analysis, cells were lysed and stained with propidium iodide (PI). The cells or nuclei were analyzed on a FACSCalibur or LSRII (Becton Dickinson). Data were analyzed using FlowJo software.
Immunohistochemistry
Sections of 3 μm in thickness were cut from spleen tissue that was fixed overnight in 10% formalin and embedded in paraffin. Sections were stained with either H&E, unlabeled anti-CD3 rabbit antibody (clone: SP7; Thermo Scientific), or anti-GFP rabbit antibody (Molecular Probes, Invitrogen). Antibodies were incubated overnight at 4°C, stained with anti-rabbit HRP-labeled polymer (Dako) and developed in aminoethylcarbazole (AEC; Sigma). These slides were then counterstained for IgM by overnight incubation at 4°C with alkaline peroxidase (AP)-conjugated anti-IgM antibody (Southern Biotech). IgM stainings were developed in nitro blue tetrazolium chloride–5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP; Roche, New York, NY). The images were acquired by means of a Digital Sight camera (Nikon) mounted on a Nikon Eclipse E600 microscope (Nikon).
Immunoblot analysis
Purified B cells were subjected to NP40-based lysis, separated by SDS-PAGE and blotted on nitrocellulose membranes (GE Healthcare). Samples were incubated with the following primary antibodies overnight at 4°C: rabbit anti-RELB (Santa Cruz, clone: C-19), rabbit anti-p100/p52 (Cell Signaling Technologies, CST), mouse anti-β-actin (Sigma, clone: AC-15), rabbit anti-pAKT (Cell Signalling, clone: D9E) and rabbit anti-AKT (CST) Horseradish peroxidase-conjugated secondary antibodies and ECL Western Blotting Substrate or SuperSignal West Dura Extended Duration Substrate (Thermo Scientific) were used for detection.
RNA-seq
RNA was harvested from 3 culture replicates/genotype of BAFF-stimulated B cells and 3 culture replicates/genotype of CD40-stimulated B cells and submitted for RNA-sequencing at the Columbia University Genome Center. 30 million single-end, 100bp reads were sequenced per sample on the Illumina HiSeq2000/2500 V3 instrument. DESeq analysis was used to identify differentially expressed genes and determine fold expression changes between genotypes. RNA-seq data are available through the GEO database (http://www.ncbi.nlm.nih.gov/geo/) under accession numbers GSE75761 and GSE75762.
Statistical analysis
P values were obtained using unpaired Student’s t test or by one-way ANOVA with Tukey’s multiple comparisons (GraphPad Prism 5 software). P values < 0.05 were considered significant.
RESULTS
Conditional deletion of relb and nfkb2 in B-cells
To determine the B-cell-intrinsic functions of RELB and NF-κB2 in the maintenance of B-cells in vivo, we generated transgenic mouse strains carrying loxP-flanked alleles of relb and nfkb2 (encoding p100/p52), respectively (Fig. 1A,D). To enable B-cell-specific ablation of relb and nfkb2, these mice were crossed to mice expressing Cre recombinase specifically in B-cells 49. The region of the relb or nfkb2 genes flanked by loxP sites comprised the promoter region and the exon encoding the translational start site, thus allowing the creation of relb or nfkb2 null alleles upon Cre-mediated recombination of loxP sites. In addition, the conditional alleles were constructed such that the recombination of loxP sites is accompanied by the expression of an enhanced green fluorescent protein (eGFP) to enable tracking of relb or nfkb2-deleted cells in the tissues. To achieve this, an eGFP minigene was placed upstream of the relb or nfkb2 promoter regions in the opposite orientation; this minigene is silent in the targeted locus. However, following recombination of loxP sites, a phosphoglycerate kinase promoter (placed in intron 2 of relb or nfkb2) is placed proximal to the eGFP gene, allowing transcription. A similar strategy was previously used for the conditional deletion of irf4, rel and rela 48,50. In this system, gene deletion is directly linked to induction of eGFP expression, and therefore eGFP positivity is a marker for gene deletion. In addition, eGFP expression is quantitative, as B-cells from mice heterozygous or homozygous for the floxed allele can be distinguished by their mean eGFP fluorescence 48,50 (also see Fig. 2A,B lower panel). Correctly targeted relb or nfkb2 embryonic stem cell lines were identified by a PCR-based screening strategy followed by confirmation via Southern blot analysis (Supplementary Fig. 1A,B).
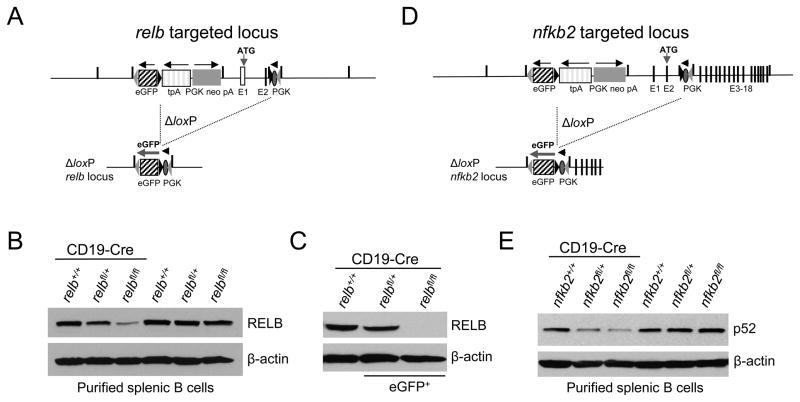
Figure 1. Generation of mice with conditional deletion of relb or nfkb2 in B-cells and simultaneous expression of eGFP.
(A and D) Targeting strategy showing the status of relb and nfkb2 before (top) and after (bottom) Cre-mediated recombination. Numbers indicate the respective exons. (B and E) Western blot analysis of RELB and p52 protein levels in purified splenic B cells of the indicated genotypes, and (C) of flow-sorted eGFP+ B-cells from relbfl/flCD19-Cre mice and the corresponding eGFP+ relfl/+CD19-Cre and eGFP− CD19-Cre control mice.
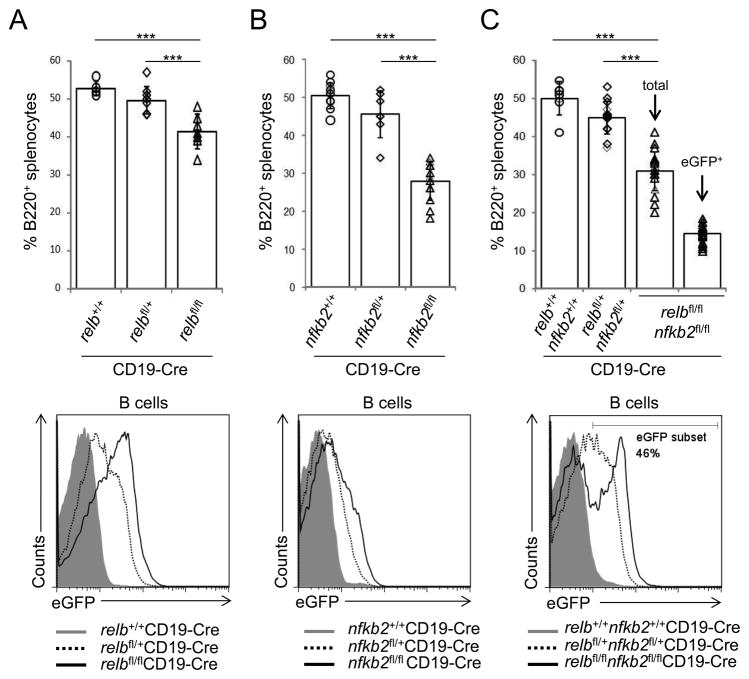
Figure 2. Reduced fractions of splenic B-cells in the absence of alternative NF-κB subunits.
(A–C, top) The fractions of splenic B-cells from relbfl/flCD19-Cre, nfkb2fl/flCD19-Cre or relbfl/flnfkb2fl/flCD19-Cre mice and the corresponding heterozygous and CD19-Cre control mice were determined by flow cytometry (for absolute B-cell numbers, see Supplementary Fig. 2). Each symbol represents a mouse. Data are shown as mean ± standard deviation. Statistical significance was determined by one-way ANOVA (***, P<0.001). (A–C, bottom) Flow cytometry of eGFP expression in splenic B-cells of the indicated genotypes. The number above the gate indicates the percentage of eGFP+ B-cells among B220+ B cells of relbfl/flnfkb2fl/flCD19-Cre mice.
Western blot analysis of B-cells isolated from relbfl/flCD19-Cre and nfkb2fl/flCD19-Cre mice demonstrated that the protein levels of RELB and p52, respectively, were strongly reduced compared to B-cells from littermate controls (Fig. 1B,E), confirming the loss of functionality of the newly generated loxP-flanked relb and nfkb2 alleles. The remaining protein is likely to be derived from eGFP− B-cells that have escaped Cre-mediated deletion. This possibility was confirmed by the complete absence of RELB protein in flow-sorted eGFP+ B-cells from relbfl/flCD19-Cre mice (Fig. 1C) (the low eGFP expression in B-cells of nfkb2fl/flCD19-Cre mice prevented us from unambiguously separating eGFP+ and eGFP− B-cells; we were therefore unable to perform a similar experiment for NF-κB2). B-cells from relbfl/+CD19-Cre and nfkb2fl/+CD19-Cre showed reduced protein expression of RELB and p52, respectively, relative to the CD19-Cre controls, indicating haploinsufficiency of RELB and p52 expression in B-cells (Fig. 1B,C,E). Importantly, in the absence of Cre, the unrearranged conditional relb and nfkb2 alleles produced physiological amounts of RELB and p52 protein, respectively (Fig. 1B,E).
Reduced fractions of splenic B-cells in the absence of alternative NF-κB subunits
To assess the B-cell-intrinsic functions of alternative NF-κB subunits in B-cell maintenance, we determined the fraction of mature splenic B-cells present in mice with B-cell-specific ablation of RELB or NF-κB2 individually, or combined RELB and NF-κB2 inactivation. Upon B-cell-specific ablation of RELB in relbfl/flCD19-Cre mice, we observed a slight but significantly reduced fraction of splenic B-cells compared to relbfl/+CD19-Cre and CD19-Cre control mice (Fig. 2A; for cell numbers, see Supplementary Fig. 2). B-cell-specific ablation of NF-κB2 was associated with a marked reduction in the fraction of splenic B-cells; B220+ cells comprised 28% of total splenocytes in nfkb2fl/flCD19-Cre mice compared to 46% and 50% in nfkb2fl/+CD19-Cre and CD19-Cre control mice, respectively (Fig. 2B; for cell numbers, see Supplementary Fig. 2). In order to obtain complete ablation of RELB/p52, the major heterodimer of the alternative pathway, the conditional relb and nfkb2 alleles were interbred to generate relbfl/flnfkb2fl/flCD19-Cre mice to allow simultaneous deletion of relb and nfkb2 in B cells. The fraction of splenic B-cells in these mice was similar to that observed following ablation of NF-κB2 alone (B220+ cells comprised 30% of total splenocytes in relbfl/flnfkb2fl/flCD19-Cre mice compared to 45% and 50% in relbfl/+nfkb2fl/+CD19-Cre and CD19-Cre control mice, respectively; Fig. 2C; for cell numbers, see Supplementary Fig. 2). However, analysis for eGFP expression among B-cells of relbfl/flnfkb2fl/flCD19-Cre mice identified two distinct eGFP+ and eGFP− peaks of approximately equal proportions (Fig. 2C; bottom panel). The presence of a sizable eGFP− population in mice with combined ablation of RELB and NF-κB2 indicates that eGFP+ B-cells deficient for both RELB and NF-κB2 are outcompeted by eGFP− B-cells that escaped Cre-mediated deletion. As a result, relb/nfkb2-deleted eGFP+ B-cells were present at a frequency of only 15% of total splenocytes in relbfl/flnfkb2fl/flCD19-Cre mice compared to the B-cell fraction of 50% observed in CD19-Cre control mice.
The unique eGFP-expression pattern in relbfl/flnfkb2fl/flCD19-Cre mice contrasts with the single eGFP+ peak observed in B-cells from relbfl/flCD19-Cre mice (Fig. 2A; bottom panel), suggesting that there is virtually no counter-selection against RELB-deficient B-cells in these mice. Due to the generally low eGFP-expression in B-cells from nfkb2fl/flCD19-Cre mice, a clear distinction between eGFP+ and eGFP− populations is not possible; however, eGFP expression of B-cells from nfkb2fl/flCD19-Cre mice is generally higher than that of B-cells from mice with heterozygous deletion of nfkb2 (nfkb2fl/+CD19-Cre mice) (Fig. 2B; bottom panel). In addition, Western blot analysis (Fig. 1E) demonstrated that the majority of B-cells from nfkb2fl/flCD19-Cre mice do not express NF-κB2 protein. Together, these observations argue against extensive counter-selection of NF-κB2-deficient B-cells in these mice, unlike what is observed in relbfl/flnfkb2fl/flCD19-Cre mice. Nonetheless, deletion of nfkb2 alone led to a strong reduction in the fraction of B-cells that was considerably higher than that observed in relbfl/flCD19-Cre mice, indicating that functionally, p52 appears to be the major subunit downstream of the alternative pathway in B cells.
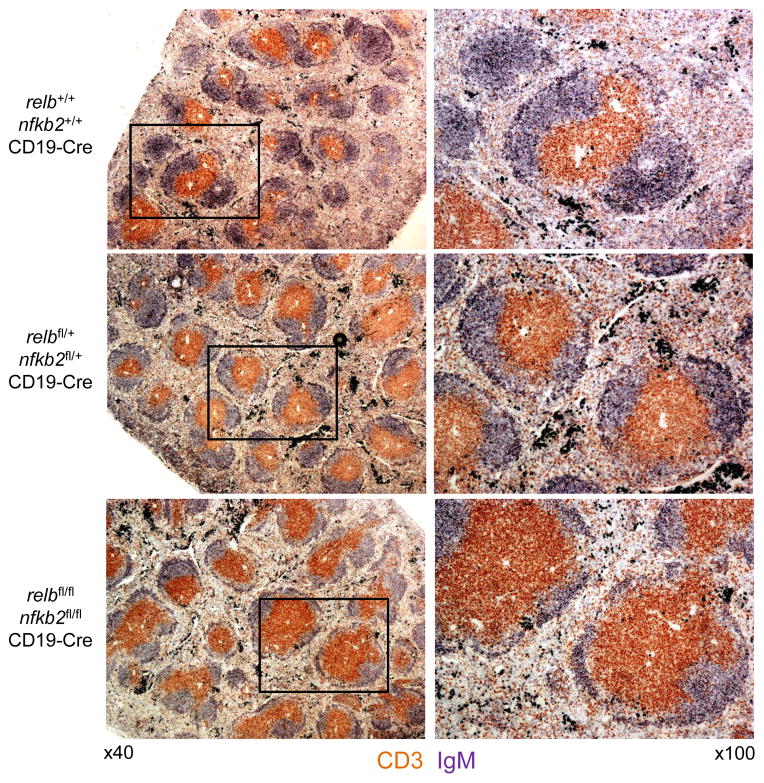
Immunohistochemical staining analysis of spleen sections for IgM and CD3 revealed that in accordance with the reduced B-cell fraction observed by flow cytometry, relbfl/flnfkb2fl/flCD19-Cre mice displayed fewer B-cell follicles within the white pulp compared to relbfl/+nfkb2fl/+CD19-Cre and CD19-Cre control mice (Fig. 3). The size of these B-cell follicles was also more heterogeneous in relbfl/flnfkb2fl/flCD19-Cre mice compared to the controls. In summary, combined B-cell-specific deletion of relb and nfkb2 results in a greatly reduced fraction of B-cells that is reflected by abnormalities in splenic white pulp architecture. Moreover, the severity of the B-cell phenotype upon relb/nfkb2 deletion appears to surpass that observed with the corresponding single gene deletions.
Figure 3. relbfl/flnfkb2fl/flCD19-Cre mice display fewer B-cell follicles within the white pulp, and exhibit more heterogeneity in the size of B-cell follicles, compared to control mice.
Spleen sections from mice of the indicated genotypes were analyzed via immunohistochemistry for the expression of CD3 and IgM. One representative mouse out of 3 per group is shown. 40x magnification (left) and 100x magnification (right).
Relb/nfkb2-deleted B-cells show a developmental block at the transitional stage 1 (T1)
In order to determine whether the alternative NF-κB subunits are involved in transmitting BAFF signals during the B-cell transitional phase (transitional stage 1–3; T1–T3) where BAFF signaling is known to be required 3, we analyzed splenic B-cells from relbfl/flnfkb2fl/flCD19-Cre and control mice for their expression of AA4.1+IgMhiCD23− (T1), AA4.1+IgMhiCD23+ (T2) and AA4.1+IgMlowCD23+ (T3) B-cells. We observed an accumulation of T1 B cells and a concomitant reduction in T3 B cells in relbfl/flnfkb2fl/flCD19-Cre mice in comparison to the CD19-Cre littermate controls (~25% vs. ~14% T1 B-cells and ~9% vs. 18% T3 B-cells) (Fig. 4). This finding is in agreement with published data demonstrating that BAFF signaling is required during the T1 to T2 transition 3 and suggests that the alternative NF-κB pathway has a role in this transition.
Figure 4. Relb/nfkb2-deleted B-cells show a developmental block at the T1 stage.
IgM and CD23 expression of AA4.1+ splenic B cells from mice of the indicated genotypes were analyzed by flow cytometry. Numbers besides gates indicate the percentage of T1 (AA4.1+IgMhiCD23−), T2 (AA4.1+IgMhiCD23+) and T3 (AA4.1+IgMlowCD23+) B-cells (left). Summary of the frequencies of T1–T3 B-cells (right). Each symbol represents a mouse. Data are shown as mean ± standard deviation. Statistical significance was determined by one-way ANOVA (**, P<0.01; ***, P<0.001).
Marked counter-selection against relb/nfkb2-deleted MZ B-cells
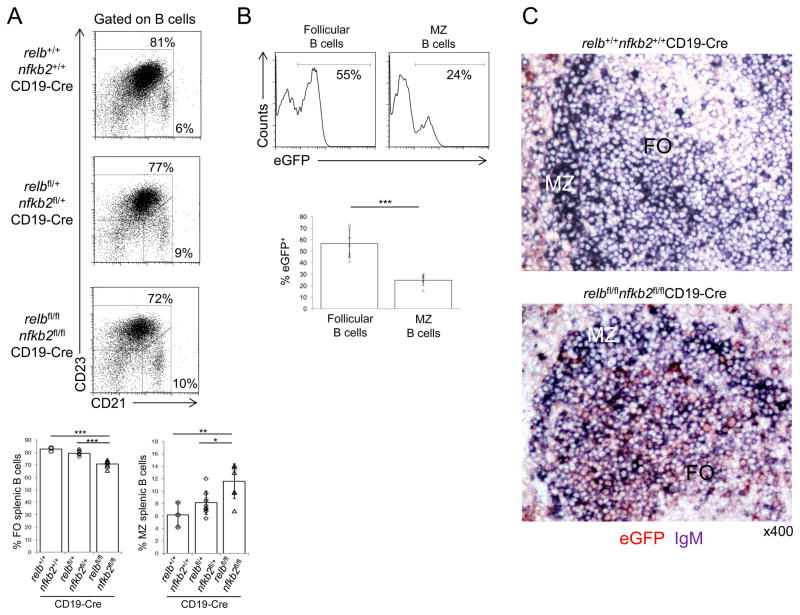
Among the splenic B-cell subsets in relbfl/flnfkb2fl/flCD19-Cre mice, a slightly reduced fraction of follicular B-cells (CD23+CD21int) and an increased fraction of MZ B-cells (CD21hiCD23−) was observed compared to CD19-Cre mice (~70% vs. ~83% follicular B-cells and ~11% vs. ~6% MZ B-cells) (Fig. 5A). Similar observations were made when analyzing IgMhiIgDlow and IgM+IgD+ B cell subpopulations as well as CD1d+ and CD9+ B-cell subsets 51,52 (data not shown). However, the extent of counter-selection against relb/nfkb2-deleted B-cells differed considerably between the follicular and MZ B-cell fractions (Fig. 5B), with the strongest counter-selection observed in the MZ B-cell compartment. Analysis of eGFP expression within MZ B-cells (Fig. 5B) demonstrated that only ~25% of MZ B-cells were eGFP+ compared to ~57% of follicular B-cells. The observed dramatic counter-selection against relb/nfkb2-deleted MZ B-cells is consistent with previous publications that invoked roles for RELB or NF-κB2 in the development of MZ B-cells from the study of constitutional knockout mice 29,46. Curiously, morphologic evaluation of hematoxylin & eosin stained splenic sections from relbfl/flnfkb2fl/flCD19-Cre mice demonstrated focal MZ enlargement in these mice compared to the controls (data not shown). The prominent MZs presumably reflect the higher fractions of MZ vs. follicular B-cells that were observed among splenic B-cells by flow cytometric analysis (see Fig. 5A). Importantly, immunohistochemical staining analysis of splenic sections revealed that the majority of the B-cells in the enlarged MZs of relbfl/flnfkb2fl/flCD19-Cre mice did not stain for eGFP, whereas eGFP+ follicular B cells were readily observed (Fig. 5C), a finding which is in accordance with the flow cytometry results (Fig. 5B). In summary, relbfl/flnfkb2fl/flCD19-Cre mice show strong counter-selection against relb/nfkb2-deleted MZ B-cells.
Figure 5. Counter selection against relb/nfkb2-deleted follicular and MZ B-cells in relbfl/flnfkb2fl/flCD19-Cre mice.
(A) CD21 and CD23 expression of splenic B cells from mice of the indicated genotypes were analyzed by flow cytometry. Numbers besides gates indicate the percentage of follicular (CD23+CD21int) and MZ (CD21hiCD23−) B-cells (top). Summary of the frequencies of follicular and MZ B-cells (bottom). Each symbol represents a mouse. Data are shown as mean ± standard deviation. Statistical significance was determined by one-way ANOVA (*, P<0.05; **, P<0.01; ***, P<0.001). (B) The fractions of eGFP+ cells among splenic follicular (CD23+CD21int) and MZ (CD21hiCD23−) B-cells in relbfl/flnfkb2fl/flCD19-Cre mice were determined by flow cytometry. Numbers above gates indicate the percentage of eGFP+ B-cells among the indicated B-cell subsets (top). Summary of the frequency of eGFP+ cells among the corresponding B-cell subsets (bottom). (C) Spleen sections from mice of the indicated genotypes were analyzed for the expression of eGFP and IgM by immunohistochemistry. FO, follicular area; MZ, marginal zone area. One representative mouse out of 3 per group is shown. 400x. Each symbol represents a mouse. Data are shown as mean ± standard deviation. Statistical significance was determined by Student’s t test (***, P<0.001).
Identification of BAFF-responsive genes controlled by the alternative NF-κB pathway
The data outlined above demonstrate that the alternative NF-κB pathway controls a biological program that promotes B-cell maintenance. In order to gain insight into this program, we sought to identify genes that are transcriptionally regulated by the alternative NF-κB subunits in B-cells stimulated with BAFF in vitro. A time-course analysis of BAFF stimulation on normal B-cells revealed that at 6h, the majority of p100 was processed to p52, demonstrating robust activation of the alternative NF-κB pathway (Supplementary Fig. 3A). Phosphorylation of AKT, an additional event known to occur downstream of BAFF stimulation 12, was observed after 24h of stimulation (Supplementary Fig. 3A). We chose to stimulate B cells for 6h in order to identify early BAFF-responsive genes. B cells purified by magnetic cell separation from nfkb2fl/flCD19-Cre mice were used for this transcriptional profiling experiment as these mice showed a marked reduction in mature B-cells without evidence of strong counter-selection against nfkb2-deleted B-cells, unlike what is observed in relbfl/flnfkb2fl/flCD19-Cre mice. B-cells isolated from 3 nfkb2fl/flCD19-Cre and CD19-Cre mice each were stimulated with BAFF for 6 hours in vitro and RNA harvested from the cultures was subjected to RNA sequencing.
Differentially expressed sequence analysis (DE-SEQ) of NF-κB2-proficient vs. NF-κB2-deficient BAFF-stimulated B-cells identified 150 genes with reduced and 174 genes with increased (Supplementary Table I) expression in the absence of NF-κB2 at a significance threshold of padj<0.05. The fold changes were mostly small, but highly significant. Genes were assigned to putative functional categories (Supplementary Fig. 3B and Supplementary Table I), including B-cell markers, apoptosis, cellular trafficking, and immune response–T-dependent (Table 1). A transcriptional profile analysis of BAFF-responsive genes controlled by the alternative NF-κB pathway has not previously been undertaken. However, cd23 (encoding FcεRII) is a known BAFF-controlled gene in B-cells 53, and the gene encoding inducible costimulator ligand (ICOSL) has been identified as a bona fide target of the alternative subunits in response to BAFFR stimulation in murine B-cells 54. In the RNA-seq analysis, we observed reduced expression of mRNA encoding cd23 and icosl in NF-κB2-deficient vs. control B-cells (Table 1). Accordingly, following BAFF or CD40 stimulation, ICOSL protein was expressed at significantly reduced levels on eGFP+ (i.e. relb/nfkb2-deleted) B-cells derived from relbfl/flnfkb2fl/flCD19-Cre mice, whereas eGFP− B-cells from the same mice displayed surface levels of ICOSL similar to those observed on WT B-cells (Fig. 6A). These results validate our strategy for identifying BAFF-responsive targets. In the following sections, we present major findings of the RNA-seq analysis.
Table 1.
BAFF and CD40-responsive genes controlled by the alternative NF-κB pathway as identified by RNA-seq analysis of BAFF and CD40-stimulated nfkb2-deleted and WT B-cells.
| 6h BAFF | 6h CD40 | ||||
|---|---|---|---|---|---|
| Functional category | Gene | Fold change | padj value | Fold change | padj value |
| - | Nfkb2 | −2.53 | 3.72E-88 | −3.23 | 0 |
| IMMUNE RESPONSE – B CELL | Fcer2a (CD23) | −1.33 | 0.018 | −1.39 | 7.03E-58 |
| APOPTOSIS | Bcl2 | −1.33 | 0.029 | unchanged | - |
| TRAFFICKING | Sell | −1.47 | 1.49E-15 | unchanged | - |
| Gpr183 (Ebi2) | −1.49 | 1.27E-12 | −1.35 | 2.05E-20 | |
| Ccr7 | −1.61 | 3.95E-10 | −1.22 | 1.71E-12 | |
| Cxcr5 | −1.19 | 0.0099 | unchanged | - | |
| IMMUNE RESPONSE – T-DEPENDENT | H2-DMb2 | −1.32 | 8.73E-07 | unchanged | - |
| Icosl | −1.28 | 5.89E-06 | unchanged | - | |
| Ctss | −1.27 | 0.00013 | unchanged | - | |
| H2-Oa | −1.25 | 0.0038 | unchanged | - | |
| Cd83 | −1.92 | 0.0083 | unchanged | - | |
| H2-Aa | −1.28 | 0.014 | unchanged | - | |
Figure 6. Differential expression of BAFF-responsive genes controlled by the alternative NF-κB pathway.
(A) Flow cytometric analysis of BAFF or CD40-stimulated B-cells from relbfl/flnfkb2fl/flCD19-Cre and CD19-Cre mice at d3 for the expression of ICOSL. Staining of eGFP+ and eGFP− B-cells from relbfl/flnfkb2fl/flCD19-Cre mice and B-cells from CD19-Cre mice (top). Summary of the corresponding median fluorescence intensities (MFI) (bottom). eGFP+ and eGFP− identifies relb/nfkb2-deleted and non-deleted B-cells from relbfl/flnfkb2fl/flCD19-Cre mice, respectively. Each symbol represents a mouse. Data are shown as mean ± standard deviation. Statistical significance was determined by one-way ANOVA (*, P<0.05; **, P<0.01; ***, P<0.001). (B) Flow cytometric analysis of BAFF or CD40-stimulated purified B-cells from relbfl/flnfkb2fl/flCD19-Cre and CD19-Cre mice at d3 for DNA content by propidium iodide (PI) staining (top) and summary of the corresponding percentage Sub-G1 (bottom). (B) Flow cytometric analysis of BAFF or CD40-stimulated purified B-cells from relbfl/flnfkb2fl/flCD19-Cre and CD19-Cre mice at d3 for apoptotic/dead cells by annexin V/7AAD staining (top) and summary of the corresponding percentage of annexin V/7AAD cells (bottom). (D) Flow cytometric analysis of ex vivo B-cells from relbfl/flnfkb2fl/flCD19-Cre and CD19-Cre mice for BAFFR expression (right) and summary of the corresponding MFIs (right). eGFP+ identifies relb/nfkb2-deleted B-cells from relbfl/flnfkb2fl/flCD19-Cre mice. (E) Flow cytometric analysis of ex vivo B-cells from relbfl/flnfkb2fl/flCD19-Cre and CD19-Cre mice for β2 expression (left) and summary of the corresponding MFIs (right). eGFP+ identifies relb/nfkb2-deleted B-cells from relbfl/flnfkb2fl/flCD19-Cre mice. (F) Flow cytometric analysis of ex vivo B-cells from relbfl/flnfkb2fl/flCD19-Cre and CD19-Cre mice for CD21 expression (left) and summary of the corresponding MFIs (right). eGFP+ identifies relb/nfkb2-deleted B-cells from relbfl/flnfkb2fl/flCD19-Cre mice. (B–F) Each symbol represents a mouse. Data are shown as mean ± standard deviation. Statistical significance was determined by Student’s t test (*, P<0.05; **, P<0.01; ***, P<0.001).
Control of BAFF-mediated survival by the alternative NF-κB pathway
We observed a slightly reduced expression of mRNA encoding the anti-apoptotic gene bcl2 in NF-κB2-deficient vs. control B-cells (Table 1). In addition, the pro-apoptotic genes Bmf and Bbc3 (PUMA) were expressed at higher levels in BAFF-stimulated relb/nfkb2-deleted vs. WT B-cells (Supplementary Table I). Together, these findings suggest that B-cells with impaired signaling through the alternative pathway have an increased propensity to undergo apoptosis. In agreement, increased cell death of BAFF-stimulated B-cells that express a RELB mutant protein that is unable to bind DNA compared to WT controls has previously been demonstrated 55. To assess the survival capacity of RELB/NF-κB2-deficient B-cells in vitro, we stimulated B cells from relbfl/flnfkb2fl/flCD19-Cre or CD19-Cre control mice with either BAFF or CD40 for three days, as both BAFF and CD40 are strong activators of the alternative pathway. The results showed significantly enhanced cell death in the cultures of RELB/NF-κB2-deficient vs. control B-cells in response to BAFF stimulation (~63% vs. ~23% by propidium iodide (PI) staining and ~21% vs. ~66% by annexin V/7AAD staining) (Fig. 6B,C). Of note, we also observed reduced expression of BAFFR on RELB/NF-κB2-deficient vs WT B-cells (Fig. 6D), which may contribute to the observed enhanced cellular death. In contrast, cell viability was comparable among the genotypes in response to CD40 stimulation (cell death ~14% vs. ~22% by PI staining and ~17% vs. ~20% by annexin V/7AAD staining), although the small difference observed in the PI staining reached significance (Fig. 6B,C). These findings suggest that CD40 stimulation activates a transcriptional program in RELB/NF-κB2-deficient B-cells that is able to overcome the viability defect observed in response to BAFF. In agreement with this finding, RNA-seq analysis of NF-κB2-deficient B-cells and wild-type (WT) controls upon 6h of CD40 stimulation in vitro revealed that there was little overlap in the genes controlled by NF-κB2 following BAFF vs. CD40 stimulation (Supplementary Table I, Supplementary Fig. 3C). A Venn diagram demonstrates the overlap in genes with reduced expression in NF-κB2-deficient B cells upon BAFF vs. CD40 stimulation (Supplementary Fig. 3D). Listed in Table 1, we determined the expression of the genes which showed differential expression following 6h of BAFF stimulation with the expression levels following 6h of CD40 stimulation. Finally, genes were assigned to putative functional categories and a pie chart summarizing these findings is shown in Supplementary Fig. 3C.
Control of B-cell trafficking by the alternative NF-κB pathway
Both relb−/− and nfkb2−/− mice show altered lymphoid organization 43–45 and analysis of total splenic mRNA revealed reduced expression of the chemokines CXCL13, CCL19 and CCL21 compared to controls 46. Mildly reduced expression of the chemokine receptors CXCR5 and CCR7 was also observed in splenic mRNA from relb−/− mice 46. These findings provided the first suggestion that the alternative pathway may be required for proper lymphoid organization by controlling the expression of chemokines or chemokine receptors in stromal and/or other cell types. In support of this notion, RNA-seq analysis revealed reduced mRNA expression of the chemokine receptors CXCR5, CCR7 and EBI2 (Gpr183), as well as the adhesion molecule L-selectin (Sell, CD62L), in NF-κB2-deficient B-cells compared to controls following BAFF stimulation (Table 1). These findings suggests that in B-cells, the alternative pathway controls a gene expression program that permits homing to and positioning within B-cell follicles in response to cues from stromal cells. We would like to note that staining B-cells from relbfl/flnfkb2fl/flCD19-Cre mice did not reveal significant differences in the expression levels of CXCR5, CCR7 and CD62L (data not shown); similarly, transwell migration assays using CXCL13, the ligand for CXCR5, on RELB/NF-κB2-deficient and WT B-cells did not detect significant differences in the migration potential among the genotypes (data not shown). We attribute these observations to the possibility that the RELB/NF-κB2-deficient B-cells which have reached maturity in relbfl/flnfkb2fl/flCD19-Cre mice may represent a selected population of B-cells that have survived and migrated to the spleen and lodged in the follicles due to near normal expression of these genes. Future experiments aimed at inducibly deleting relb/nfkb2 specifically in B-cells may help to clarify this matter.
Since the absence of alternative NF-κB subunits correlated with a reduction in the fraction of MZ B-cells (ref. 29,46, and Fig. 5B,C), we determined the surface protein expression of the integrin chains αL and β2, that together form the LFA-1 complex, on relb/nfkb2-deleted and WT control B-cells. LFA-1 is important for the retention of B-cells within the splenic MZ 56. Whereas we detected no significant differences in the expression level of αL between eGFP+ (i.e. relb/nfkb2-deleted) B-cells from relbfl/flnfkb2fl/flCD19-Cre and CD19-Cre control mice, relb/nfkb2-deleted B-cells showed strongly reduced surface expression of β2 compared to B-cells from CD19-Cre control mice (Fig. 6E). This observation is likely to be relevant to the strong counter-selection against eGFP+ MZ B-cells observed in relbfl/flnfkb2fl/flCD19-Cre mice (Fig. 5B,C), and may suggest an impaired retention of these cells in the MZ compartment (see discussion).
CD21 has been identified as a target of BAFF receptor signaling 53. We observed lower protein levels on the surface of eGFP+ relb/nfkb2-deleted B-cells in comparison to CD19-Cre control mice (Fig. 6F), suggesting that BAFF mediates expression of CD21 via the alternative NF-κB pathway. High expression of CD21 is a marker of MZ B-cells 57. It is possible that reduced expression of CD21 on RELB/NF-κB2-deficient MZ B-cells impairs the antigen-stimulation properties of those cells as the CD19/CD21 co-receptor complex has been implicated in lowering the threshold of BCR signaling 58.
Genes involved in T-cell B-cell interactions regulated by the alternative NF-κB pathway
As mentioned above, the alternative NF-κB pathway has been implicated in the regulation of ICOSL expression on B-cells. ICOSL is involved in facilitating interactions with T cells and its expression on B-cells is required for the development of T-follicular helper cells 54. In addition to decreased expression of icosl in BAFF-stimulated NF-κB2-deficient vs. control B-cells, our RNA-seq analysis revealed reduced expression of several genes involved in MHC class-II (MHCII) antigen presentation (Table 1). These included the genes ctss, H2-DMb2 and H2-Oa, which respectively encode cathepsin S, H2-DMb2 and H2-Oa and are involved in the processing and removal of invariant chain peptide (CLIP) from MHCII molecules to allow loading of antigenic peptides. In addition, Cd83 was expressed at lower levels in NF-κB2-deficient B-cells compared to WT B-cells (Table 1). CD83 is a member of the Ig superfamily that is associated with B-cell activation, and CD83-deficient splenic B-cells also have reduced MHCII antigen presentation 59. Thus, it appears that besides regulating ICOSL expression, the alternative NF-κB pathway may have a more substantial role in activating the expression of genes required for optimal T cell-dependent B-cell responses in response to BAFF.
DISCUSSION
BAFF-mediated activation of the alternative NF-κB pathway has long been implicated in the survival of mature B-cells. However, the B-cell-intrinsic roles of transcription factors downstream of the pathway, RELB or NF-κB2, have not as yet been identified. Perhaps most importantly, it is unknown how the combined deletion of relb and nfkb2 affects the development and function of B-cells. We here crossed newly generated conditional relb and nfkb2 alleles, either separately or combined, to CD19-Cre mice in order ablate these subunits specifically in B-cells. We found that the individual gene deletions led to a decrease in the fraction of mature B-cells; however, the most dramatic reduction in peripheral B-cells was observed upon simultaneously ablating RELB and NF-κB2. Thus, by ablating the downstream transcription factors of the alternative NF-κB pathway in B-cells, our results for the first time define the extent to which this pathway regulates mature B-cell homeostasis.
Similar to what has been described in mice with B-cell-conditional deletion of a major regulator of the canonical pathway, ikkβ60,61, we observed a marked counter-selection of relb/nfkb2-deleted B-cells in mice with B-cell-specific RELB/NF-κB2 deficiency, which was strongest in the MZ B-cell compartment. Nevertheless, neither of these mouse models showed a complete disappearance of ikkβ or relb/nfkb2-deleted B-cells, indicating that the B-cell phenotypes are less severe than those observed in mice deficient in BCR or BAFF signaling that show a virtually complete absence of B-cells. One possibility to explain these findings is that the canonical and alternative NF-κB pathways may complement each other to a certain extent during B-cell development and maintenance, as suggested by the observation that adoptive transfer of bone marrow from nfkb1−/−nfkb2−/− mice into RAG1-deficient animals resulted in the generation of only few mature B-cells 62. It is however clear that non-NF-κB-mediated effects of BAFFR or BCR stimulation also contribute to the survival of mature B-cells 12–15,63.
In in vitro-cultures, relb/nfkb2-deleted B-cells were sensitive to apoptosis when stimulated with BAFF, suggesting that the alternative NF-κB pathway controls an anti-apoptotic program in normal B-cells that is activated in response to BAFF stimulation. BAFF signaling has long been implicated in controlling B-cell survival 64 and in agreement with this notion, ectopic expression of BCL2 led to the accumulation of B-cells in BAFFR-deficient mice 9,65,66. It is interesting to note that activation of relb/nfkb2-deleted B-cells with CD40, also a strong inducer of p100 processing 67, did not lead to increased cell death, indicating that CD40 signaling can transmit survival signals by a different route, presumably via the canonical NF-κB pathway 68. Interestingly, RNA-seq analysis revealed limited overlap between the genes controlled by NF-κB2 in response to BAFF vs. CD40 stimulation. This includes BCL2, whose mRNA expression changed slightly upon BAFF stimulation while it remained unaffected upon CD40 stimulation.
BCL2 overexpression does not fully rescue the loss of B-cells in BAFFR-deficient mice 9,65,66, indicating that BAFF stimulation has additional functions besides providing pro-survival signals. In accordance, our RNA-seq analysis identified several BAFF responsive genes controlled by the alternative pathway, either directly or indirectly, that are involved in lymphocyte trafficking, including adhesion molecules and chemokine receptors. Two previous studies have implicated the alternative pathway in the regulation of B-cell homing to the lymphoid follicles and the MZ, respectively. Based on an RT-PCR analysis of mRNA derived from whole spleen of relb−/− mice, Weih et al. reported slightly reduced mRNA levels of the chemokine receptors CXCR5 and CCR7 46. Expression of CXCR5 is required for homing of B-cells to the follicle, while CCR7 directs positioning at the B-T-cell boundary 69. We found both receptors to be expressed at slightly lower mRNA levels in BAFF-stimulated NF-κB2-deficient B-cells compared to controls. In addition, we observed reduced expression of the chemokine receptor EBI2, which guides B-cell localization in the lymphoid microenvironment 70, and the adhesion molecule L-selectin (CD62L), which mediates the entry of lymphocytes from the blood into lymphoid tissue. These results may suggest that RELB/NF-κB2-deficient B-cells that fail to express chemokine receptors and adhesion molecules at physiological levels may be impaired in their homing to the follicle. In agreement with this notion, mice with B-cell-specific deletion of both relb and nfkb2 have a decreased number of splenic B-cells and fewer B-cell follicles.
A critical role for the alternative NF-κB pathway in the development of MZ B-cells has been suggested based on two observations: First, relb−/−→WT chimeras show a significant reduction of MZ B-cells compared to the controls 46. Second, BAFF-transgenic mice and mice with inactivation of upstream components of the alternative pathway (that induce processing of p100), as well as mice that lack p100 but still express p52, are characterized by MZ hyperplasia 10,32–35,71. As reported by Enzler et al., the latter phenotype is likely due to the upregulation of the integrin chains αLβ2 (LFA-1) and α4β1 (VLA-4) 29 that facilitate homing to the MZ 56. The expression of these genes was found to be at least partially impaired in BAFF-Tg/nfkb2−/− mice that presented with a strongly reduced fraction of MZ B-cells relative to BAFF-Tg mice 29. In light of these findings, our observation that, compared to the controls, relb/nfkb2-deleted B-cells had significantly reduced surface-expression of the β2 integrin chain suggests that these cells might have defects in homing to and retention within the MZ. In turn, this may contribute to the strong reduction in RELB/NF-κB2-deficient MZ B-cells observed in the spleens of relbfl/flnfkb2fl/flCD19-Cre mice. It is possible that relb/nfkb2-deleted MZ B-cells that fail to be retained by the stroma are flushed out into the blood and eventually disappear due to the lack of stroma-associated survival signals, similar to what has been observed in various other experimental systems 29,56,72.
Adding to a previous report 54 (see results), we observed reduced protein expression of ICOSL on the cell surface of B-cells upon BAFF stimulation in relb/nfkb2-deleted B-cells. Our RNA-seq analysis in addition revealed that several genes involved in MHC class II-antigen presentation failed to be expressed at physiological levels in BAFF-stimulated NF-κB2-deficient B-cells. These findings further suggest that the alternative NF-κB pathway appears to control the expression of genes involved in facilitating optimal T-B interactions.
Our findings may have implications for diseases manifesting with chronic B-cell activation, as BAFF has been implicated in promoting autoimmunity, including SLE 73. Also, BIRC3 (cIAP-2), an upstream regulator of the alternative pathway, is frequently mutated in chronic lymphocytic leukemia 74, a malignancy of mature, resting B-cells, and in splenic MZ lymphoma 75. Our results confirm an important role for the alternative NF-κB transcription factors RELB and NF-κB2 in mature B-cell homeostasis. The constitutive activation of these transcription factors may promote cell survival and distort trafficking within the lymphoid tissue, and potentially lead to sustained activation of B-cells, which may contribute to disease pathogenesis.
Supplementary Material
Acknowledgments
We thank Thomas Ludwig and Evangelos Pefanis for advice on the targeting vectors, and the members of the Klein lab for insights and discussion. We also thank Victor Lin of the Transgenic Mouse and the Molecular Pathology and Flow Cytometry Shared Resources of the HICCC.
Abbreviations
- BCR
B-cell receptor
- NF-κB
nuclear factor-κB
- MZ
marginal zone
Footnotes
GRANT SUPPORT
This work was supported by NCI/NIH grant R01-CA157660 to U.K., a grant from the Stewart Trust Foundation (USA), the HICCC, and a Cancer Biology Training Program fellowship (NCI/NIH 19 grant 5T32-CA009503-26) to N.S.D.
References
- 1.Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death. Cell. 1997;90:1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- 2.Kraus M, Alimzhanov MB, Rajewsky N, Rajewsky K. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer. Cell. 2004;117:787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 3.Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 4.Gross JA, Dillon SR, Mudri S, Johnston J, Littau A, Roque R, Rixon M, Schou O, Foley KP, Haugen H, McMillen S, Waggie K, Schreckhise RW, Shoemaker K, Vu T, Moore M, Grossman A, Clegg CH. TACI-Ig neutralizes molecules critical for B cell development and autoimmune disease. impaired B cell maturation in mice lacking BLyS. Immunity. 2001;15:289–302. doi: 10.1016/s1074-7613(01)00183-2. [DOI] [PubMed] [Google Scholar]
- 5.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 6.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2108–2111. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 7.Yan M, Brady JR, Chan B, Lee WP, Hsu B, Harless S, Cancro M, Grewal IS, Dixit VM. Identification of a novel receptor for B lymphocyte stimulator that is mutated in a mouse strain with severe B cell deficiency. Curr Biol. 2001;11:1547–1552. doi: 10.1016/s0960-9822(01)00481-x. [DOI] [PubMed] [Google Scholar]
- 8.Shulga-Morskaya S, Dobles M, Walsh ME, Ng LG, MacKay F, Rao SP, Kalled SL, Scott ML. B cell-activating factor belonging to the TNF family acts through separate receptors to support B cell survival and T cell-independent antibody formation. J Immunol. 2004;173:2331–2341. doi: 10.4049/jimmunol.173.4.2331. [DOI] [PubMed] [Google Scholar]
- 9.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 10.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, Ross L, Delaney J, Wang L, Lacey D, Boyle WJ, Hsu H. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patke A, Mecklenbrauker I, Erdjument-Bromage H, Tempst P, Tarakhovsky A. BAFF controls B cell metabolic fitness through a PKC beta- and Akt-dependent mechanism. J Exp Med. 2006;203:2551–2562. doi: 10.1084/jem.20060990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Otipoby KL, Sasaki Y, Schmidt-Supprian M, Patke A, Gareus R, Pasparakis M, Tarakhovsky A, Rajewsky K. BAFF activates Akt and Erk through BAFF-R in an IKK1-dependent manner in primary mouse B cells. Proc Natl Acad Sci U S A. 2008;105:12435–12438. doi: 10.1073/pnas.0805460105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jellusova J, Miletic AV, Cato MH, Lin WW, Hu Y, Bishop GA, Shlomchik MJ, Rickert RC. Context-specific BAFF-R signaling by the NF-kappaB and PI3K pathways. Cell Rep. 2013;5:1022–1035. doi: 10.1016/j.celrep.2013.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweighoffer E, Vanes L, Nys J, Cantrell D, McCleary S, Smithers N, Tybulewicz VL. The BAFF receptor transduces survival signals by co-opting the B cell receptor signaling pathway. Immunity. 2013;38:475–488. doi: 10.1016/j.immuni.2012.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siebenlist U, Brown K, Claudio E. Control of lymphocyte development by nuclear factor-kappaB. Nat Rev Immunol. 2005;5:435–445. doi: 10.1038/nri1629. [DOI] [PubMed] [Google Scholar]
- 17.Gardam S, Brink R. Non-Canonical NF-kappaB Signaling Initiated by BAFF Influences B Cell Biology at Multiple Junctures. Front Immunol. 2014;4:509. doi: 10.3389/fimmu.2013.00509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 19.Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733. doi: 10.1146/annurev.immunol.021908.132641. [DOI] [PubMed] [Google Scholar]
- 20.Gerondakis S, Siebenlist U. Roles of the NF-kappaB pathway in lymphocyte development and function. Cold Spring Harb Perspect Biol. 2010;2:a000182. doi: 10.1101/cshperspect.a000182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaileh M, Sen R. NF-kappaB function in B lymphocytes. Immunol Rev. 2012;246:254–271. doi: 10.1111/j.1600-065X.2012.01106.x. [DOI] [PubMed] [Google Scholar]
- 22.Beinke S, Ley SC. Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology. Biochem J. 2004;382:393–409. doi: 10.1042/BJ20040544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He JQ, Saha SK, Kang JR, Zarnegar B, Cheng G. Specificity of TRAF3 in its negative regulation of the noncanonical NF-kappa B pathway. J Biol Chem. 2007;282:3688–3694. doi: 10.1074/jbc.M610271200. [DOI] [PubMed] [Google Scholar]
- 24.Sun SC. The noncanonical NF-kappaB pathway. Immunol Rev. 2012;246:125–140. doi: 10.1111/j.1600-065X.2011.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nat Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 26.Kayagaki N, Yan M, Seshasayee D, Wang H, Lee W, French DM, Grewal IS, Cochran AG, Gordon NC, Yin J, Starovasnik MA, Dixit VM. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity. 2002;17:515–524. doi: 10.1016/s1074-7613(02)00425-9. [DOI] [PubMed] [Google Scholar]
- 27.Morrison MD, Reiley W, Zhang M, Sun SC. An atypical tumor necrosis factor (TNF) receptor-associated factor-binding motif of B cell-activating factor belonging to the TNF family (BAFF) receptor mediates induction of the noncanonical NF-kappaB signaling pathway. J Biol Chem. 2005;280:10018–10024. doi: 10.1074/jbc.M413634200. [DOI] [PubMed] [Google Scholar]
- 28.Hatada EN, Do RK, Orlofsky A, Liou HC, Prystowsky M, MacLennan IC, Caamano J, Chen-Kiang S. NF-kappa B1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-kappa B2 p100 to p52 in quiescent mature B cells. J Immunol. 2003;171:761–768. doi: 10.4049/jimmunol.171.2.761. [DOI] [PubMed] [Google Scholar]
- 29.Enzler T, Bonizzi G, Silverman GJ, Otero DC, Widhopf GF, Anzelon-Mills A, Rickert RC, Karin M. Alternative and classical NF-kappa B signaling retain autoreactive B cells in the splenic marginal zone and result in lupus-like disease. Immunity. 2006;25:403–415. doi: 10.1016/j.immuni.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki Y, Derudder E, Hobeika E, Pelanda R, Reth M, Rajewsky K, Schmidt-Supprian M. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Yamada T, Mitani T, Yorita K, Uchida D, Matsushima A, Iwamasa K, Fujita S, Matsumoto M. Abnormal immune function of hemopoietic cells from alymphoplasia (aly) mice, a natural strain with mutant NF-kappa B-inducing kinase. J Immunol. 2000;165:804–812. doi: 10.4049/jimmunol.165.2.804. [DOI] [PubMed] [Google Scholar]
- 32.Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 33.Xie P, Stunz LL, Larison KD, Yang B, Bishop GA. Tumor necrosis factor receptor-associated factor 3 is a critical regulator of B cell homeostasis in secondary lymphoid organs. Immunity. 2007;27:253–267. doi: 10.1016/j.immuni.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Gardam S, V, Turner M, Anderton H, Limaye S, Basten A, Koentgen F, Vaux DL, Silke J, Brink R. Deletion of cIAP1 and cIAP2 in murine B lymphocytes constitutively activates cell survival pathways and inactivates the germinal center response. Blood. 2011;117:4041–4051. doi: 10.1182/blood-2010-10-312793. [DOI] [PubMed] [Google Scholar]
- 36.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 37.Kaisho T, Takeda K, Tsujimura T, Kawai T, Nomura F, Terada N, Akira S. IkappaB kinase alpha is essential for mature B cell development and function. J Exp Med. 2001;193:417–426. doi: 10.1084/jem.193.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brightbill HD, Jackman JK, Suto E, Kennedy H, Jones C, 3rd, Chalasani S, Lin Z, Tam L, Roose-Girma M, Balazs M, Austin CD, Lee WP, Wu LC. Conditional Deletion of NF-kappaB-Inducing Kinase (NIK) in Adult Mice Disrupts Mature B Cell Survival and Activation. J Immunol. 2015;195:953–964. doi: 10.4049/jimmunol.1401514. [DOI] [PubMed] [Google Scholar]
- 39.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-kappaB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 40.Woronicz JD, Gao X, Cao Z, Rothe M, Goeddel DV. IkappaB kinase-beta: NF-kappaB activation and complex formation with IkappaB kinase-alpha and NIK. Science. 1997;278:866–869. doi: 10.1126/science.278.5339.866. [DOI] [PubMed] [Google Scholar]
- 41.Ramakrishnan P, Wang W, Wallach D. Receptor-specific signaling for both the alternative and the canonical NF-kappaB activation pathways by NF-kappaB-inducing kinase. Immunity. 2004;21:477–489. doi: 10.1016/j.immuni.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 42.Mills DM, Bonizzi G, Karin M, Rickert RC. Regulation of late B cell differentiation by intrinsic IKKalpha-dependent signals. Proc Natl Acad Sci U S A. 2007;104:6359–6364. doi: 10.1073/pnas.0700296104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 44.Franzoso G, Carlson L, Poljak L, Shores EW, Epstein S, Leonardi A, Grinberg A, Tran T, Scharton-Kersten T, Anver M, Love P, Brown K, Siebenlist U. Mice deficient in nuclear factor (NF)-kappa B/p52 present with defects in humoral responses, germinal center reactions, and splenic microarchitecture. J Exp Med. 1998;187:147–159. doi: 10.1084/jem.187.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caamano JH, Rizzo CA, Durham SK, Barton DS, Raventos-Suarez C, Snapper CM, Bravo R. Nuclear factor (NF)-kappa B2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998;187:185–196. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weih DS, Yilmaz ZB, Weih F. Essential role of RelB in germinal center and marginal zone formation and proper expression of homing chemokines. J Immunol. 2001;167:1909–1919. doi: 10.4049/jimmunol.167.4.1909. [DOI] [PubMed] [Google Scholar]
- 47.Zhao B, Barrera LA, Ersing I, Willox B, Schmidt SC, Greenfeld H, Zhou H, Mollo SB, Shi TT, Takasaki K, Jiang S, Cahir-McFarland E, Kellis M, Bulyk ML, Kieff E, Gewurz BE. The NF-kappaB genomic landscape in lymphoblastoid B cells. Cell Rep. 2014;8:1595–1606. doi: 10.1016/j.celrep.2014.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein U, Casola S, Cattoretti G, Shen Q, Lia M, Mo T, Ludwig T, Rajewsky K, Dalla-Favera R. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat Immunol. 2006;7:773–782. doi: 10.1038/ni1357. [DOI] [PubMed] [Google Scholar]
- 49.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res. 1997;25:1317–1318. doi: 10.1093/nar/25.6.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heise N, De Silva NS, Silva K, Carette A, Simonetti G, Pasparakis M, Klein U. Germinal center B cell maintenance and differentiation are controlled by distinct NF-kappaB transcription factor subunits. J Exp Med. 2014;211:2103–2118. doi: 10.1084/jem.20132613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guinamard R, Okigaki M, Schlessinger J, Ravetch JV. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 52.Won WJ, Kearney JF. CD9 is a unique marker for marginal zone B cells, B1 cells, and plasma cells in mice. J Immunol. 2002;168:5605–5611. doi: 10.4049/jimmunol.168.11.5605. [DOI] [PubMed] [Google Scholar]
- 53.Gorelik L, Cutler AH, Thill G, Miklasz SD, Shea DE, Ambrose C, Bixler SA, Su L, Scott ML, Kalled SL. Cutting edge: BAFF regulates CD21/35 and CD23 expression independent of its B cell survival function. J Immunol. 2004;172:762–766. doi: 10.4049/jimmunol.172.2.762. [DOI] [PubMed] [Google Scholar]
- 54.Hu H, Wu X, Jin W, Chang M, Cheng X, Sun SC. Noncanonical NF-kappaB regulates inducible costimulator (ICOS) ligand expression and T follicular helper cell development. P Proc Natl Acad Sci U S A. 2011;108:12827–12832. doi: 10.1073/pnas.1105774108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almaden JV, Tsui R, Liu YC, Birnbaum H, Shokhirev MN, Ngo KA, Davis-Turak JC, Otero D, Basak S, Rickert RC, Hoffmann A. A pathway switch directs BAFF signaling to distinct NFkappaB transcription factors in maturing and proliferating B cells. Cell Rep. 2014;9:2098–2111. doi: 10.1016/j.celrep.2014.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lu TT, Cyster JG. Integrin-mediated long-term B cell retention in the splenic marginal zone. Science. 2002;297:409–412. doi: 10.1126/science.1071632. [DOI] [PubMed] [Google Scholar]
- 57.Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision. Nat Rev Immunol. 2009;9:767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- 58.Rickert RC. Regulation of B lymphocyte activation by complement C3 and the B cell coreceptor complex. Curr Opin Immunol. 2005;17:237–243. doi: 10.1016/j.coi.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 59.Kuwano Y, Prazma CM, Yazawa N, Watanabe R, Ishiura N, Kumanogoh A, Okochi H, Tamaki K, Fujimoto M, Tedder TF. CD83 influences cell-surface MHC class II expression on B cells and other antigen-presenting cells. Int Immunol. 2007;19:977–992. doi: 10.1093/intimm/dxm067. [DOI] [PubMed] [Google Scholar]
- 60.Pasparakis M, Schmidt-Supprian M, Rajewsky K. IkappaB kinase signaling is essential for maintenance of mature B cells. J Exp Med. 2002;196:743–752. doi: 10.1084/jem.20020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li ZW, Omori SA, Labuda T, Karin M, Rickert RC. IKK beta is required for peripheral B cell survival and proliferation. J Immunol. 2003;170:4630–4637. doi: 10.4049/jimmunol.170.9.4630. [DOI] [PubMed] [Google Scholar]
- 62.Franzoso G, Carlson L, Xing L, Poljak L, Shores EW, Brown KD, Leonardi A, Tran T, Boyce BF, Siebenlist U. Requirement for NF-kappaB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Srinivasan L, Sasaki Y, Calado DP, Zhang B, Paik JH, DePinho RA, Kutok JL, Kearney JF, Otipoby KL, Rajewsky K. PI3 kinase signals BCR-dependent mature B cell survival. Cell. 2009;139:573–586. doi: 10.1016/j.cell.2009.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tardivel A, Tinel A, Lens S, Steiner QG, Sauberli E, Wilson A, Mackay F, Rolink AG, Beermann F, Tschopp J, Schneider P. The anti-apoptotic factor Bcl-2 can functionally substitute for the B cell survival but not for the marginal zone B cell differentiation activity of BAFF. Eur J Immunol. 2004;34:509–518. doi: 10.1002/eji.200324692. [DOI] [PubMed] [Google Scholar]
- 66.Rahman ZS, Manser T. B cells expressing Bcl-2 and a signaling-impaired BAFF-specific receptor fail to mature and are deficient in the formation of lymphoid follicles and germinal centers. J Immunol. 2004;173:6179–6188. doi: 10.4049/jimmunol.173.10.6179. [DOI] [PubMed] [Google Scholar]
- 67.Coope HJ, Atkinson PG, Huhse B, Belich M, Janzen J, Holman MJ, Klaus GG, Johnston LH, Ley SC. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 2002;21:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zarnegar B, He JQ, Oganesyan G, Hoffmann A, Baltimore D, Cheng G. Unique CD40-mediated biological program in B cell activation requires both type 1 and type 2 NF-kappaB activation pathways. Proc Natl Acad Sci U S A. 2004;101:8108–8113. doi: 10.1073/pnas.0402629101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cyster JG. Chemokines, sphingosine-1-phosphate, and cell migration in secondary lymphoid organs. Annu Rev Immunol. 2005;23:127–159. doi: 10.1146/annurev.immunol.23.021704.115628. [DOI] [PubMed] [Google Scholar]
- 70.Gatto D, Brink R. B cell localization: regulation by EBI2 and its oxysterol ligand. Trends Immunol. 2013;34:336–341. doi: 10.1016/j.it.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Guo F, Weih D, Meier E, Weih F. Constitutive alternative NF-kappaB signaling promotes marginal zone B-cell development but disrupts the marginal sinus and induces HEV-like structures in the spleen. Blood. 2007;110:2381–2389. doi: 10.1182/blood-2007-02-075143. [DOI] [PubMed] [Google Scholar]
- 72.Simonetti G, Carette A, Silva K, Wang H, De Silva NS, Heise N, Siebel CW, Shlomchik MJ, Klein U. IRF4 controls the positioning of mature B cells in the lymphoid microenvironments by regulating NOTCH2 expression and activity. J Exp Med. 2013;210:2887–2902. doi: 10.1084/jem.20131026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol. 2014;10:365–373. doi: 10.1038/nrrheum.2014.33. [DOI] [PubMed] [Google Scholar]
- 74.Rossi D, Fangazio M, Rasi S, Vaisitti T, Monti S, Cresta S, Chiaretti S, Del Giudice I, Fabbri G, Bruscaggin A, Spina V, Deambrogi C, Marinelli M, Fama R, Greco M, Daniele G, Forconi F, Gattei V, Bertoni F, Deaglio S, Pasqualucci L, Guarini A, Dalla-Favera R, Foa R, Gaidano G. Disruption of BIRC3 associates with fludarabine chemorefractoriness in TP53 wild-type chronic lymphocytic leukemia. Blood. 2012;119:2854–2862. doi: 10.1182/blood-2011-12-395673. [DOI] [PubMed] [Google Scholar]
- 75.Rossi D, Deaglio S, Dominguez-Sola D, Rasi S, Vaisitti T, Agostinelli C, Spina V, Bruscaggin A, Monti S, Cerri M, Cresta S, Fangazio M, Arcaini L, Lucioni M, Marasca R, Thieblemont C, Capello D, Facchetti F, Kwee I, Pileri SA, Foa R, Bertoni F, Dalla-Favera R, Pasqualucci L, Gaidano G. Alteration of BIRC3 and multiple other NF-kappaB pathway genes in splenic marginal zone lymphoma. Blood. 2011;118:4930–4934. doi: 10.1182/blood-2011-06-359166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.