Abstract
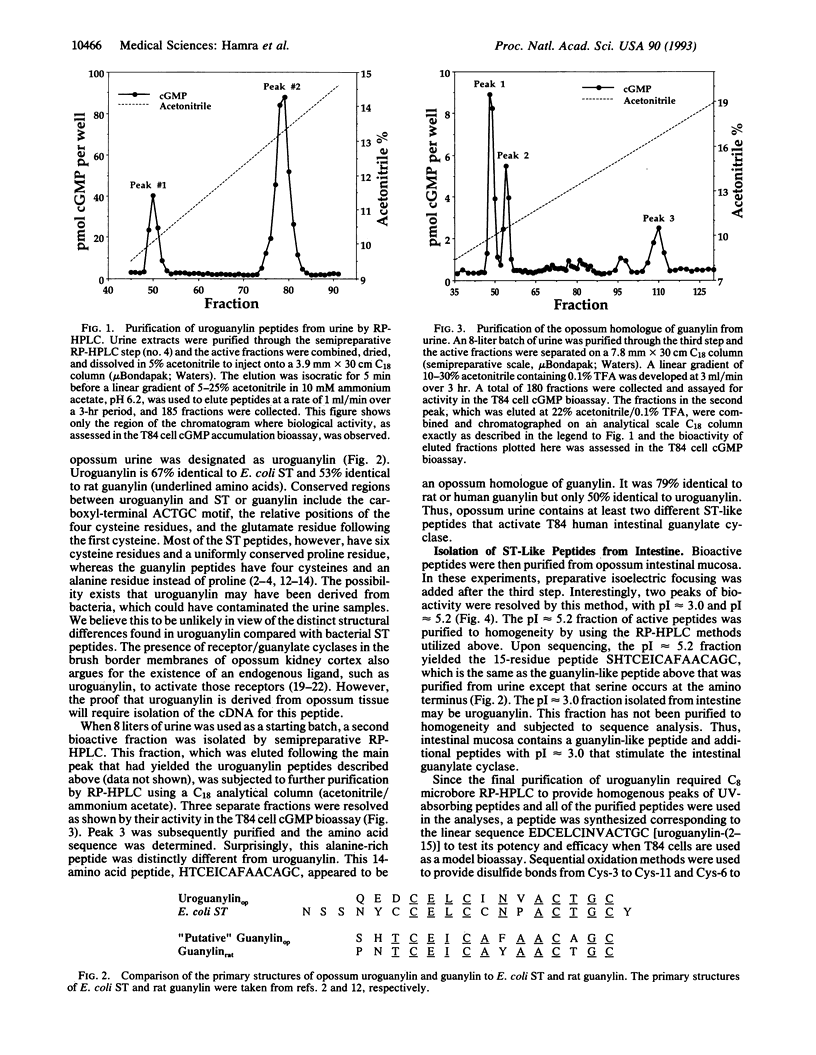
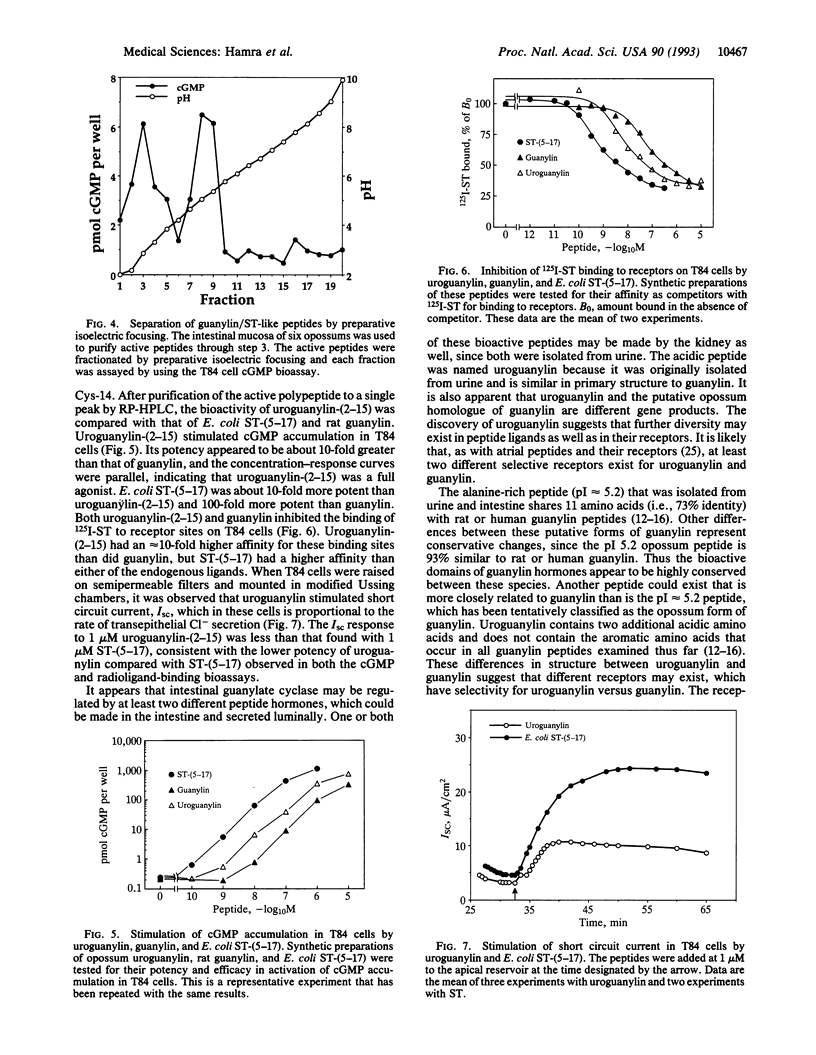
The intestinal hormone guanylin and bacterial heat-stable enterotoxins (STs) are members of a peptide family that activates intestinal membrane guanylate cyclase. Two different peptides that activate the human intestinal T84 cell guanylate cyclase have been purified from urine and intestinal mucosa of opossums (Didelphis virginiana). The highly acidic peptide, QEDCELCINVACTGC, was named uroguanylin because it was isolated from urine and shares 53% identity with guanylin. A second peptide, SHTCEICAFAACAGC, was purified from urine and intestinal mucosa. This alanine-rich peptide was 47% identical to uroguanylin and 73% identical to human guanylin, suggesting that it may be an opossum homologue of guanylin. Synthetic uroguanylin-(2-15) (i.e., EDCELCINVACTGC) was 10-fold more potent than synthetic rat guanylin, but both peptides were less potent than Escherichia coli ST in the T84 cell cGMP bioassay. Uroguanylin-(2-15) and guanylin inhibited 125I-ST binding to T84 cell receptors in competitive radioligand binding assays. Transepithelial Cl- secretion was stimulated by 1 microM uroguanylin, indicated by an increase in the short circuit current of T84 cells. Thus, uroguanylin is another paracrine hormone in the emerging peptide family that activates intestinal membrane guanylate cyclase. The second peptide may be the opossum form of guanylin, or perhaps, it is still another member of this peptide family. The presence of uroguanylin and guanylin in urine and receptors in proximal tubules suggests that these peptides may also originate from renal tissue and may regulate kidney function.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aimoto S., Takao T., Shimonishi Y., Hara S., Takeda T., Takeda Y., Miwatani T. Amino-acid sequence of a heat-stable enterotoxin produced by human enterotoxigenic Escherichia coli. Eur J Biochem. 1982 Dec 15;129(2):257–263. doi: 10.1111/j.1432-1033.1982.tb07047.x. [DOI] [PubMed] [Google Scholar]
- Baxter P. S., Goldhill J., Hardcastle J., Hardcastle P. T., Taylor C. J. Accounting for cystic fibrosis. Nature. 1988 Sep 15;335(6187):211–211. doi: 10.1038/335211a0. [DOI] [PubMed] [Google Scholar]
- Crawford I., Maloney P. C., Zeitlin P. L., Guggino W. B., Hyde S. C., Turley H., Gatter K. C., Harris A., Higgins C. F. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9262–9266. doi: 10.1073/pnas.88.20.9262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currie M. G., Fok K. F., Kato J., Moore R. J., Hamra F. K., Duffin K. L., Smith C. E. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning G. M., Anderson M. P., Amara J. F., Marshall J., Smith A. E., Welsh M. J. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992 Aug 27;358(6389):761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- Denning G. M., Ostedgaard L. S., Cheng S. H., Smith A. E., Welsh M. J. Localization of cystic fibrosis transmembrane conductance regulator in chloride secretory epithelia. J Clin Invest. 1992 Jan;89(1):339–349. doi: 10.1172/JCI115582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Rao M. C., Chang E. B. Intestinal electrolyte transport and diarrheal disease (1). N Engl J Med. 1989 Sep 21;321(12):800–806. doi: 10.1056/NEJM198909213211206. [DOI] [PubMed] [Google Scholar]
- Forte L. R., Eber S. L., Turner J. T., Freeman R. H., Fok K. F., Currie M. G. Guanylin stimulation of Cl- secretion in human intestinal T84 cells via cyclic guanosine monophosphate. J Clin Invest. 1993 Jun;91(6):2423–2428. doi: 10.1172/JCI116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte L. R., Krause W. J., Freeman R. H. Escherichia coli enterotoxin receptors: localization in opossum kidney, intestine, and testis. Am J Physiol. 1989 Nov;257(5 Pt 2):F874–F881. doi: 10.1152/ajprenal.1989.257.5.F874. [DOI] [PubMed] [Google Scholar]
- Forte L. R., Krause W. J., Freeman R. H. Receptors and cGMP signalling mechanism for E. coli enterotoxin in opossum kidney. Am J Physiol. 1988 Nov;255(5 Pt 2):F1040–F1046. doi: 10.1152/ajprenal.1988.255.5.F1040. [DOI] [PubMed] [Google Scholar]
- Forte L. R., Thorne P. K., Eber S. L., Krause W. J., Freeman R. H., Francis S. H., Corbin J. D. Stimulation of intestinal Cl- transport by heat-stable enterotoxin: activation of cAMP-dependent protein kinase by cGMP. Am J Physiol. 1992 Sep;263(3 Pt 1):C607–C615. doi: 10.1152/ajpcell.1992.263.3.C607. [DOI] [PubMed] [Google Scholar]
- Garbers D. L. Guanylyl cyclase receptors and their endocrine, paracrine, and autocrine ligands. Cell. 1992 Oct 2;71(1):1–4. doi: 10.1016/0092-8674(92)90258-e. [DOI] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Krause W. J., Freeman R. H., Fort L. R. Autoradiographic demonstration of specific binding sites for E. coli enterotoxin in various epithelia of the North American opossum. Cell Tissue Res. 1990 May;260(2):387–394. doi: 10.1007/BF00318641. [DOI] [PubMed] [Google Scholar]
- Schulz S., Chrisman T. D., Garbers D. L. Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem. 1992 Aug 15;267(23):16019–16021. [PubMed] [Google Scholar]
- Takao T., Shimonishi Y., Kobayashi M., Nishimura O., Arita M., Takeda T., Honda T., Miwatani T. Amino acid sequence of heat-stable enterotoxin produced by Vibrio cholerae non-01. FEBS Lett. 1985 Dec 2;193(2):250–254. doi: 10.1016/0014-5793(85)80163-0. [DOI] [PubMed] [Google Scholar]
- Takao T., Tominaga N., Shimonishi Y., Hara S., Inoue T., Miyama A. Primary structure of heat-stable enterotoxin produced by Yersinia enterocolitica. Biochem Biophys Res Commun. 1984 Dec 28;125(3):845–851. doi: 10.1016/0006-291x(84)91360-3. [DOI] [PubMed] [Google Scholar]
- White A. A., Krause W. J., Turner J. T., Forte L. R. Opossum kidney contains a functional receptor for the Escherichia coli heat-stable enterotoxin. Biochem Biophys Res Commun. 1989 Feb 28;159(1):363–367. doi: 10.1016/0006-291x(89)92447-9. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Kato J., Currie M. G. Rat guanylin cDNA: characterization of the precursor of an endogenous activator of intestinal guanylate cyclase. Biochem Biophys Res Commun. 1992 Jun 30;185(3):812–817. doi: 10.1016/0006-291x(92)91699-q. [DOI] [PubMed] [Google Scholar]
- Wiegand R. C., Kato J., Huang M. D., Fok K. F., Kachur J. F., Currie M. G. Human guanylin: cDNA isolation, structure, and activity. FEBS Lett. 1992 Oct 19;311(2):150–154. doi: 10.1016/0014-5793(92)81387-2. [DOI] [PubMed] [Google Scholar]
- de Sauvage F. J., Keshav S., Kuang W. J., Gillett N., Henzel W., Goeddel D. V. Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9089–9093. doi: 10.1073/pnas.89.19.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]



