Abstract
Opioid abusers discount delayed reinforcers more rapidly than non-users; however, it is unclear whether chronic drug administration or its discontinuation impact discounting. This study examined daily morphine administration and its discontinuation on delay discounting of food in rhesus monkeys. Responding on one lever delivered 1 food pellet immediately; responding on another lever delivered 2 food pellets either immediately or after a delay (30–120 sec) that increased within the session. Monkeys (n=3) responded for the large reinforcer when both reinforcers were delivered immediately and more for the smaller, immediately available reinforcer as delay to delivery of the large reinforcer increased. When administered acutely, morphine (0.032–5.6 mg/kg) increased trial omissions and had variable effects on choice, with small doses decreasing and large doses increasing choice of the large delayed reinforcer. Chronic morphine administration (0.1 mg/kg/day to 3.2 mg/kg twice daily) reduced choice of the large delayed reinforcer in two monkeys while increasing choice in a third monkey. Despite the development of tolerance to some effects (i.e., rightward shifts in dose-effect curves for the number of trials omitted) and evidence of mild opioid dependence (e.g., decrease in the number of trials completed as well as body weight), discontinuation of treatment did not appear to systematically impact discounting. Overall, these results suggest that repeated opioid administration causes persistent effects on choice under a delay discounting procedure; however, differences in the direction of effect among individuals suggest factors other than, or in addition to, changes in discounting might play a role.
Keywords: delay discounting, dependence, withdrawal, morphine, opioid, rhesus monkey, impulsivity
Introduction
Delay discounting is a process whereby the effectiveness of a behavioral consequence decreases as a function of the delay to its presentation (e.g., Mazur, 1987). Discounting is thought to be relevant to socially important behavior, especially behavior that reflects greater impulsivity or a lack of self-control (Ainslie, 1974; Rachlin and Green, 1972; Logue, 1988; Evenden, 1999), including drug abuse (e.g., Bickel and Marsch, 2001; Reynolds, 2006; de Wit and Mitchell, 2010; MacKillop et al., 2011; Bickel et al., 2012). For example, opioid-dependent individuals discount the value of delayed reinforcers, including money or drugs, more rapidly than non-users or former users (e.g., Madden et al., 1997; Kirby et al., 1999) and discount the value of drugs and money more during acute periods of opioid withdrawal as compared to during opioid-maintenance therapy (Giordano et al., 2002). Enhanced discounting might promote drug-seeking and relapse by inducing an individual to choose the more immediately available effects of drug-taking rather than the delayed effects of remaining abstinent such as improved health, additional income, and positive social interactions.
Increased delay discounting and other forms of impulsive behavior can be a determinant or a consequence of drug abuse (e.g., Jentsch and Taylor, 1999; de Wit, 2009). Studies in humans (e.g., Dawes et al., 1997; Tarter et al., 2003; Ersche et al., 2012) indicate that individuals who exhibit greater impulsivity are at greater risk for drug abuse as compared to individuals who exhibit lower levels of impulsivity. Likewise, studies with nonhuman subjects indicate that delay discounting is predictive of drug-seeking and drug-taking (e.g., Perry et al., 2008; Dalley et al., 2007; Diergaarde et al., 2008; Marusich and Bardo, 2009). Conversely, drug use might enhance delay discounting through the direct effects of a drug (e.g., de Wit and Mitchell, 2010), through neurobiological changes that occur as a result of long-term exposure to a drug (e.g., Jentsch and Taylor, 1999), and during periods of withdrawal (e.g., Giordano et al., 2002).
Recent work suggests that repeated administration of mu opioid receptor agonists such as morphine increases delay discounting in rats responding for sucrose (Harvey-Lewis et al., 2012). Moreover, daily self-administration of the mu opioid receptor agonist heroin also appeared to enhance delay discounting in rats responding for food (Schippers et al., 2012). In pigeons, morphine either increased or decreased discounting of grain when administered repeatedly, with the direction of effects on choice varying across subjects; however, the effects of morphine on choice persisted during daily administration (i.e., there was no tolerance to effects on choice) despite significant tolerance to the rate-decreasing effects of morphine (Eppolito et al. 2013). Following discontinuation of morphine administration, choice of the large delayed reinforcer increased in most subjects within 1 to 3 days and, in some subjects, lasted for up to 5 weeks.
Opioid abuse remains a significant public health challenge (e.g., Alturi et al., 2014); determining whether factors such as delay discounting promote drug abuse will aid in the development of more effective prevention and treatment strategies. Although the association between enhanced delay discounting and drug abuse is apparent in clinical populations, the relationship between chronic drug use, discontinuation of drug use, and changes in delay discounting remain unclear. The current study examined the effects of daily administration of the mu opioid receptor agonist morphine and its discontinuation on delay discounting of food in rhesus monkeys.
Methods
Subjects
Three adult male rhesus monkeys (Macaca mulatta) served as subjects. Monkeys were fed primate chow (Harlan Teklad, High Protein Monkey Diet, Madison, WI, USA), fresh fruit, and peanuts daily after the session. Body weight increased from 5.7–7.0 to 7.6–8.2 kg during the course of the study. Water was continuously available in the home cage. Subjects were housed individually and were maintained under a 14/10-hour light/dark cycle (lights on at 0600 hr). Two monkeys (AN and GE) were previously trained to discriminate ketamine (unpublished data) and had received intermittent (less than daily) injections of opioid and non-opioid drugs; neither monkey received any drug for 12 months prior to beginning this study. Monkey BR was experimentally naïve. Animals used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio, and the 2011 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animals Resources on Life Sciences, National Research Council, National Academy of Sciences).
Apparatus
Subjects were seated in commercially available chairs (Model R001; Primate Products, Miami, FL) located in ventilated, sound-attenuating operant conditioning chambers. Each chamber was equipped with a custom-made response panel containing two retractable levers (Model ENV-612M; Med Associates, Inc.; St. Albans, VT) aligned horizontally 32 cm apart and 15 cm below three horizontally aligned stimulus lights, approximately 10.5 cm apart (center to center), that could be illuminated white or green. Food pellets (300-mg banana flavored, Bio-Serv Dustless Precision Pellets, F0179, Frenchtown, NJ) were delivered from a pellet dispenser (Model ENV-203–300, Med Associates, Inc.) to a food receptacle located directly below the center light. An interface (Med Associates, Inc.) and a PC-compatible computer running MED-PC IV (Med Associates, Inc.) controlled experimental events and recorded data.
Procedure
Sessions were divided into 4 blocks, each comprising 2 forced trials followed by 6 choice trials. During a forced trial, one side light was illuminated green and the lever located directly below the light was extended. Thirty responses immediately retracted the lever, extinguished the green side light, illuminated the white center light, and delivered the reinforcer that was associated with responding on that lever: either one food pellet delivered immediately or two food pellets delivered immediately or after a delay that varied across blocks. During the second forced trial, the other side light was illuminated and the corresponding lever was extended; 30 responses immediately retracted the lever, extinguished the green side light, illuminated the white center light, and delivered the reinforcer that was associated with responding on that lever. The order of presentation of forced trials (left/right or right/left) was the same for each block within a session but changed quasi-randomly across sessions with the constraint that the same order did not occur for more than two consecutive sessions. There was no limited hold for forced trials; consequently, both forced trials had to be completed before choice trials were initiated. The time limit for each block was 25 min; failure to complete all trials in a block within that time initiated a 30-s timeout period during which all lights were extinguished and both levers were retracted, followed by the beginning of the next block.
During choice trials, both side lights were illuminated green and both levers were extended; the contingencies presented for responding on either lever were identical to those presented during the forced trials. In addition, responses on one lever reset the ratio requirement on the other lever; thus, 30 consecutive responses on either lever were required to satisfy the response requirement. Each choice trial lasted for a maximum of 30 s (limited hold). If the limited hold expired before the response requirement was satisfied, the green lights were extinguished, the levers were retracted, and a 140-s timeout was initiated.
Completion of the response requirement during forced and choice trials resulted in continuous illumination of the center white light until delivery of food; the white light was illuminated for 0.2 s when food was delivered immediately and for the duration of the delay when food was delayed. After the delay period expired, the white center light was extinguished and food was delivered. Each food delivery initiated a timeout during which all lights were extinguished and both levers remained retracted. The duration of each timeout was adjusted so that the time between the end of one trial, when the green lights were extinguished and levers retracted, and the beginning of the next trial was always 140 s. That is, timeouts lasted from 20 s when food delivery was delayed by 120 s to 140 s when food was delivered immediately.
Once responding under the choice procedure was established, sensitivity to reinforcer amount was first confirmed by alternating which lever was associated with the large reinforcer. Responding on one lever delivered 1 food pellet immediately and responding on the other lever delivered 2 food pellets immediately for all 4 blocks of the session. Once monkeys chose the large reinforcer on at least 80% of choice trials in each of the 4 blocks for 1 session, the levers associated with the small and large reinforcer were reversed. The location of the large reinforcer was alternated at least twice for each monkey prior to the introduction of delay. Once established, the lever delivering the large reinforcer remained constant for each monkey (right lever for monkeys AN and BR, left lever for monkey GE) for the remainder of the experiment. Delays were presented in ascending order (30, 60, and 120 s) in each session with one delay per block; during the first block both reinforcers were delivered immediately.
Early in training, sessions without delay were introduced every 5 to 7 sessions to confirm that changes in response allocation within a session were due to delay and not to other factors (e.g., passage of time within the session). Once implemented, no-delay sessions continued until the monkey chose the large reinforcer on at least 80% of choice trials in all 4 blocks of a session, with delays re-introduced in the next session. Periodically during the initial training period, sessions were conducted in which delivery of the large reinforcer was delayed by 120 s in all 4 blocks of the session until monkeys chose the large reinforcer on no more than 20% of choice trials for the entire session. Once the monkeys responded predominantly for the large reinforcer in all components, delay was removed for at least one session and the ascending delay sequence was reintroduced.
Monkeys received a saline injection (0.3 ml) daily, 15 min prior to the start of the first block of the session. Test sessions began when the following criteria were satisfied for 3 consecutive sessions. First, monkeys had to choose the large reinforcer on at least 80% of choice trials in the first (no-delay) block of the session and on no more than 20% of choice trials during the last block of the session. Second, at least 5 out of 6 choice trials were completed during each block. The acute effects of morphine were assessed first by substituting an injection of morphine for saline (i.e., 15-min pretreatment). Doses generally were tested in ascending order, ranging from a dose that did not substantially increase the number of trials omitted or impact the delay function to a dose that increased the number of trials omitted by at least 25%. Tests occurred every fourth session so long as responding during the immediately preceding 3 sessions satisfied the stability criteria.
For at least 8 sessions prior to the beginning of daily morphine administration, monkeys received two saline injections daily, one 3 h prior to the session and another 15 min prior to the session (Figure 1, phase A). Once responding was stable, morphine was administered 3 h prior to the session and saline was administered 15 min prior to the session. This dosing regimen was repeated daily for at least 14 days (phase B) and until responding was stable; then the first injection of morphine was replaced with saline and a test dose of morphine was injected 15 min prior to one session (during phase C). On the day immediately following a test dose, daily administration of morphine resumed and continued until responding was stable again, at which point an additional test dose of morphine was administered as before. Once responding was stable after the second test dose and monkeys had received morphine for at least 21 days, morphine administration was discontinued (phase D). Monkeys received saline for both daily injections for at least 3 days and until responding was stable, at which point no-delay sessions were implemented (as described above) to reassess control by delay. When no-delay test sessions were complete and responding was again stable, daily administration of another dose of morphine began (phase B). Daily treatment doses of morphine were tested in ascending order (Table 1). The first dose tested (0.1 mg/kg) was chosen because it was 3-fold smaller than a dose which enhanced discounting for juice in a previous study (Maguire et al. 2012). When monkeys received twice-daily injections of morphine, the morning and pre-session injections did not change, and a third injection was given at 17.00 h, 5 h after the previous session and 17 h prior to the next session.
Figure 1.
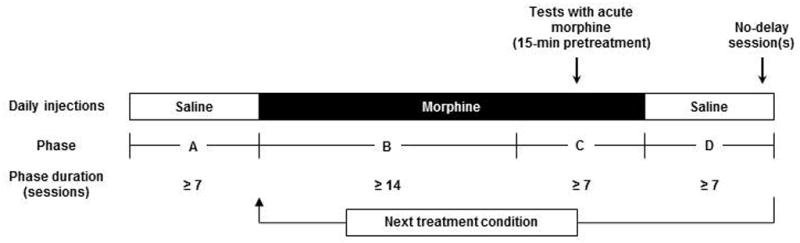
Timeline of experimental phases. Monkeys received daily injections of saline (open boxes; Phases A and D) or morphine (filled box; Phases B and C). Values shown in the lower row are the minimum number of sessions for each phase. The entire timeline was repeated for each treatment condition (i.e., dose of morphine). Other details (daily treatment doses, order of conditions, and exact treatment durations) are provided in Table 1.
Table 1.
Order of treatment conditions and number of sessions under each treatment
| Treatment condition | Monkey
|
||
|---|---|---|---|
| AN | BR | GE | |
| 1 | 0.1a (21)b | 0.1 (25) | 0.1 (21) |
| 2 | 0.32 (21) | 0.32 (27) | 0.32 (21) |
| 3 | 1.0 (21) | 1.0 (33) | 1.0 (21) |
| 4 | 3.2 (25) | 3.2 (24) | 3.2 (25) |
| 5 | 5.6 (25) | 1.0x2 (31) | 5.6 (26) |
| 6 | 3.2x2c (42) | 3.2x2 (43) | 3.2x2 (42) |
Daily treatment dose of morphine (mg/kg)
Days of administration of each dose are shown in parentheses
”x2” indicates twice daily treatment
Drugs
Morphine sulfate was provided by the Research Technology Branch, National Institute on Drug Abuse (Rockville, MD), dissolved in sterile water, and injected s.c. in the back in a volume of 0.2 to 0.8 ml.
Data Analyses
Delay functions for each session were obtained by plotting the proportion of choices for the large reinforcer as a function of delay; for each block, the number of choices of the large reinforcer was divided by the total number of choice trials completed. In addition, proportional choice of the large reinforcer was calculated for each session by dividing the total number of choices of the large reinforcer by the total number of choice trials completed for an entire session. This measure provides an index of choice that could be tracked across sessions; a leftward shift in the delay function would be represented by a decrease in proportional choice of the large reinforcer for the entire session.
A change in the potency of morphine to increase the number of choice trials omitted was quantified by calculating ED50 values using linear interpolation. A line that spanned the 50% effect level was fit to the dose-effect curve, including data from the largest dose that produced fewer than 12 omissions to the smallest dose that produced more than 12 omissions (half of the maximum number of omissions possible). In the event that the doses tested did not increase the number of choice trials omitted to more than 12, it was assumed that the next larger dose (quarter-log unit increments) would produce a full effect (i.e., 24 omissions). Changes in the potency of morphine to increase the number of choice trials omitted was determined by dividing the ED50 of the morphine dose-effect curve obtained during daily administration by the ED50 of the morphine dose-effect curve obtained before daily administration. Ratios greater than 1.0 indicate a rightward shift in the morphine dose-effect curve and a decrease in potency (i.e. tolerance).
Results
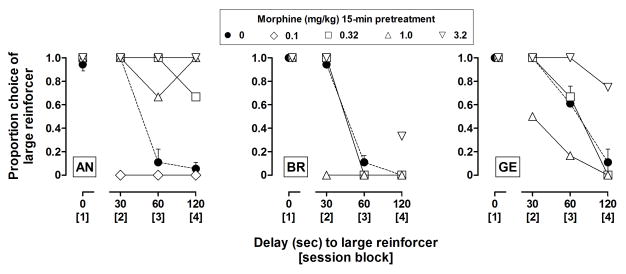
Proportional choice of the large reinforcer exceeded 0.9 when both reinforcers were delivered immediately (data over “0”, Figure 2). Proportional choice of the large reinforcer decreased as a function of delay, to less than 0.2 by the third block of the session (60-sec delay) for monkeys AN and BE and by the fourth block of the session (120-sec delay) for monkey GE. For the entire session, proportional choice of the large reinforcer (averaged for each monkey) was 0.56 (AN), 0.54 (BR), and 0.68 (GE). In sessions without delay, monkeys chose the large reinforcer exclusively during all blocks (i.e., proportional choice of the large reinforcer for the entire session was 1.0), and in sessions during which the delivery of the large reinforcer was delayed by 120 s for all blocks, the average proportional choice of the large reinforcer (data not shown) was 0.07 (AN), 0.11 (BR), and 0.07 (GE).
Figure 2.
Effects of acutely administered morphine on delay discounting of food. Proportional choice of the large reinforcer is plotted as a function of delay (s) to the large reinforcer for individual monkeys; delay increased across blocks (indicated by brackets) for each session. Filled circles indicate the mean (± SEM) for 3 baseline sessions immediately prior to administration of the first dose of morphine. Open symbols indicate the effects of different doses of morphine administered s.c. 15-min prior to the session. See “Data Analyses” for further details.
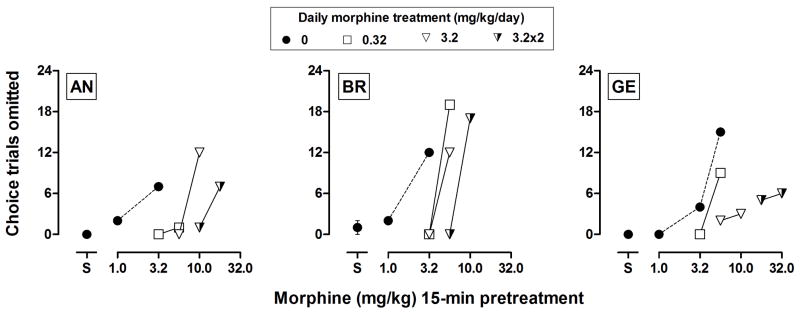
When administered 15 min prior to the session, the smallest doses of morphine tested in each monkey (0.032 mg/kg for AN [data not shown] and 0.32 mg/kg for GE and BR) did not substantially alter the delay function or the number of choice trials completed. A 3-fold larger dose of morphine (0.1 mg/kg for AN and 1.0 mg/kg for BR and GE) did not impact choice of the large reinforcer in the first component (no delay; Figure 2) or substantially increase trial omissions (Figure 4); however, choice of the large reinforcer was decreased when its delivery was delayed by 30 s (all monkeys) or 60 s (monkey GE). Larger doses of morphine (0.32 mg/kg and larger for AN; 3.2 mg/kg for GE) increased choice of the larger reinforcer when its delivery was delayed by 60 and 120 s (Figure 2), and doses of 3.2 mg/kg and larger increased the number of choice trials omitted (Figure 4).
Figure 4.
Effects of acutely administered morphine (15-min pretreatment) on omissions in choice trials before and during morphine treatment. The number of choice trial omissions during the session (maximum of 24) is plotted as a function of dose of morphine. Filled circles above “S” show the mean (± SEM) for 3 baseline sessions immediately prior to administration of the first dose of morphine. Open and half-filled symbols indicate effects of morphine determined during daily morphine treatment.
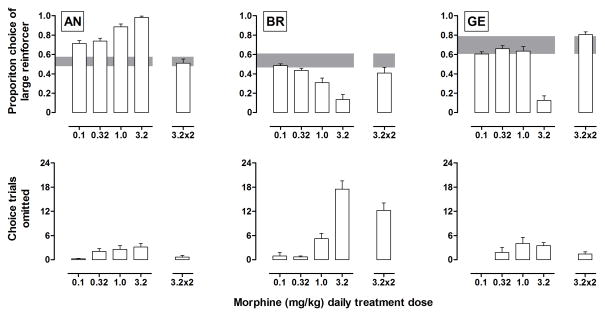
For monkey AN, daily administration of morphine dose dependently increased choice of the large delayed reinforcer, with 3.2 mg/kg of morphine producing near exclusive choice of the large reinforcer (Figure 3, top) and modestly increasing the number of choice trials omitted (bottom). For BR and GE, daily administration also increased the number of choice trials omitted but decreased proportion choice of the large delayed reinforcer. Increasing the frequency of drug administration to twice daily attenuated effects of morphine on choice and on omissions (Figure 3, bars above “3.2x2”). Before daily administration, morphine dose dependently increased omissions (filled circles, Figure 4) with ED50s ranging from 2.5 to 4.8 mg/kg (Table 2). The potency of morphine to increase omissions decreased as the dose of morphine administered daily increased. ED50s increased by as much as 8-fold following twice daily administration of 3.2 mg/kg of morphine (half-filled triangles, Figure 4; Table 2).
Figure 3.
Effects of daily morphine treatment on choice of the large reinforcer (upper panels) and trial omissions (lower panels). Each bar indicates the mean (+ SEM) of the first 14 days of daily administration of morphine. The shaded region indicates the mean (± 2 SD) for 3 baseline sessions. The “x2” next to a symbol indicates a condition in which that dose of morphine was administered twice daily (see “Methods” for details).
Table 2.
ED50s (mg/kg) and dose ratios for the effects of morphine on trial omissions
| Monkey | Before daily treatmenta | Daily treatment dose of morphine (mg/kg/day)
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 0.32 | 1.0 | 3.2 | 5.6c | 1.0x2d | 3.2x2 | |||
| AN | ED50 | 3.7 | 4.0 | 7.4 | 12.4 | 10.0 | 7.8 | 21.1 | |
| Dose ratiob | 1.1 | 2.0 | 3.3 | 2.7 | 2.1 | 5.6 | |||
| BR | ED50 | 2.5 | 2.9 | 4.5 | 4.3 | 5.6 | 9.8 | 8.4 | |
| Dose ratio | 1.2 | 1.8 | 1.7 | 2.3 | 4.0 | 3.4 | |||
| GE | ED50 | 4.8 | 6.7 | 6.3 | 5.0 | 12.8 | 17.8 | 38.3 | |
| Dose ratio | 1.4 | 1.3 | 1.0 | 2.7 | 3.7 | 8.0 | |||
ED50 values determined prior to daily administration of morphine
Dose ratio (ED50 value during daily treatment divided by the ED50 value before treatment)
Only GE and AN were treated with 5.6 mg/kg once daily
Only BR was treated with 1.0 mg/kg twice daily
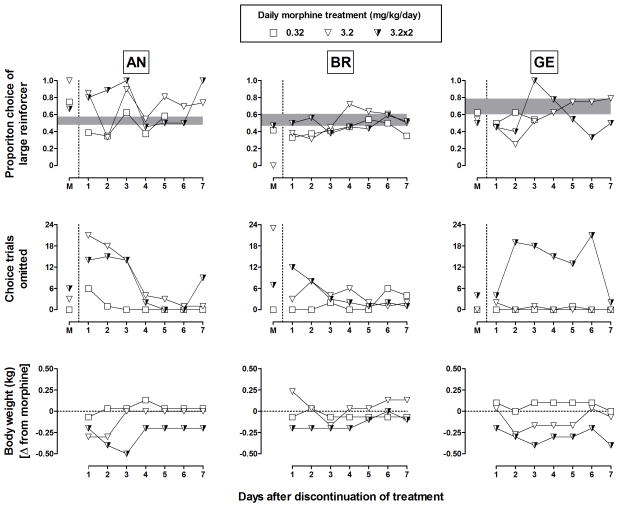
Discontinuation of once-daily administration of 0.32 mg/kg of morphine (Figure 5, squares) resulted in a return to baseline choice for AN and a small increase in trial omissions (middle row), while having no obvious impact on choice or omissions for BR or GE. Discontinuation of 0.32 mg/kg/day of morphine did not impact body weight in any monkey (Figure 5, lower row). Discontinuation of once-daily administration of 3.2 mg/kg of morphine (open triangles) decreased proportional choice of the large reinforcer on day 2 in monkey AN but overall resulted in a gradual decrease in choice of the large reinforcer from near-exclusive choice of the large reinforcer to baseline levels. For monkey BR, discontinuation increased choice of the large reinforcer from 0 during daily morphine administration to baseline levels (open triangles) and dramatically reduced the number of trial omissions. For monkey GE, discontinuation of 3.2 mg/kg/day of morphine decreased choice of the large reinforcer on the second day, followed by a return to baseline. Upon discontinuation of daily morphine, body weight decreased by 0.25 kg in AN and GE, an effect that lasted for up to 5 days in GE, and increased BR. Discontinuation of twice-daily administration of 3.2 mg/kg of morphine (half-filled triangles) substantially increased choice of the large delayed reinforcer in AN, lasting 3 days, had no impact in BR, and had variable effects on choice in GE (choice of the large reinforcer increased and then decreased). Discontinuation of twice daily administration of morphine increased omissions and decreased body weight in all three monkeys, with body weight not recovering for up to 7 days.
Figure 5.
Effects of discontinuing daily morphine treatment on choice of the large reinforcer (top), trial omissions (middle), and body weight (bottom). Data are shown for the last session of daily morphine treatment (above “M”) and for up to the first 7 sessions after discontinuation. The shaded region indicates the mean (± 2 SD) for 3 baseline sessions. Body weight is expressed as a difference (kg) from body weight on the last session of treatment, calculated individually for each daily treatment dose of morphine.
Discussion
Opioid abuse is associated with enhanced delay discounting in so far as current users discount the value of delayed consequences more rapidly than non-users or former users, and opioid-dependent individuals in treatment discount more rapidly during periods of abstinence as compared to during treatment (e.g., Madden et al., 1997; Giordano et al., 2002). A growing body of research suggests that repeated opioid administration and its discontinuation (i.e. withdrawal) might impact delay discounting in a manner that promotes continued drug use or relapse to drug use in abstinent individuals. However, the relationships among impulsivity, treatment dose, discontinuation of treatment, type of reinforcer used to assess impulsivity, and specific measures of impulsivity (e.g., delay discounting) have not been studied extensively. The current study examined the effects of daily administration of the mu opioid receptor agonist morphine and its discontinuation on delay discounting of food in rhesus monkeys.
Consistent with earlier studies (e.g. Evenden and Ryan, 1996), responding was sensitive to reinforcer amount and delay. When given a choice between 1 and 2 food pellets, both delivered immediately, monkeys responded predominantly for the larger amount of food. Choice of the large reinforcer decreased as the delay to its delivery increased. When administered acutely, intermediate doses of morphine (0.1–1.0 mg/kg) decreased choice of the large reinforcer when its delivery was delayed but not when it was delivered immediately. In contrast, larger doses of morphine increased choice of the large delayed reinforcer, particularly in monkeys AN and GE. The tendency for intermediate doses of morphine to decrease choice of the larger reinforcer in some subjects is consistent with previous studies (Kieres et al., 2004; Pitts and McKinney, 2005; Pattij et al., 2009; Maguire et al., 2012; Tanno et al., 2014). Increased choice of the large reinforcer following acute administration of larger doses of morphine has been reported less frequently (e.g., Eppolito et al. 2013) and occurred concomitantly with increased trial omissions. The effect of larger doses of morphine on choice might reflect the involvement of factors other than or in addition to changes in discounting. For example, other effects might have overshadowed effects on discounting, such as changes in sensitivity to reinforcer amount or increased perseveration, causing increased choice of the large delayed reinforcer. As is the case with stimulant drugs (e.g., Cardinal et al., 2000; Tanno et al., 2014; Maguire et al. 2014), the effects of opioids on discounting might vary markedly across different experimental conditions; however, effects of opioids on discounting have been studied under a very narrow range of conditions compared with studies using stimulant drugs.
Based on studies with opioid-dependent individuals (e.g., Madden et al., 1997; Giordano et al., 2002) and with opioid-treated, non-human subjects (e.g., Harvey-Lewis et al., 2012; Maguire et al., 2012; Schippers et al., 2012), it was hypothesized that repeated administration of a mu opioid receptor agonist such as morphine would decrease choice of the large delayed reinforcer (i.e., enhance delay discounting) and that changes in choice would persist for the duration of treatment. In the current study, daily morphine administration decreased choice of the large delayed reinforcer in 2 monkeys (BR and GE), consistent with the view that repeated administration of morphine enhances delay discounting; however, for a third monkey (AN), daily administration of morphine increased choice of the large reinforcer. It is unclear whether increased choice of the large reinforcer represents reduced discounting or the development of a bias for the lever associated with the large reinforcer (see above). Increasing the delays might have revealed a switch in choice from the large delayed to the small immediately available alternative.
Differences among individuals in the effects of opioids on delay discounting have been reported in non-human studies (Pitts and McKinney, 2005; Eppolito et al., 2013) but have not been thoroughly examined. Individuals can vary markedly in their sensitivity to the effects of drugs on delay discounting and these differences might reflect important factors relevant to vulnerability for drug abuse. The role of individual differences in how delay discounting is related to abuse-related drug effects (e.g., self-administration) has been studied (e.g., see Perry and Carrol, 2008 for a review), although individual differences in sensitivity to drug effects on delay discounting has received less attention, perhaps because many studies report group data which masks inter-subject variability. The importance of individual differences in measures of impulsivity with regard to predicting vulnerability to abuse drugs remains to be determined.
Notwithstanding differential effects on choice, in all monkeys the effects of daily morphine administration were dose-related and persisted for at least 14 days of treatment, consistent with previous studies using pigeons (e.g., Eppolito et al. 2013) and suggesting that tolerance to the effects of morphine on choice does not necessarily develop despite the development of tolerance to its effects on omissions (Figure 4; Table 1). Increasing the frequency of treatment from once to twice daily produced tolerance to the effects of morphine on choice (compare 3.2 to 3.2 x2; Figure 3).
One potential consequence of repeated administration of a mu opioid receptor agonist is the development of physical dependence which is revealed by the emergence of withdrawal upon discontinuation of treatment. Enhanced delay discounting has been reported for persons undergoing acute withdrawal from opioids as compared to discounting when the same individuals are maintained on daily buprenorphine treatment (Giordano et al., 2002). Similarly, delay discounting in morphine-treated rats responding for sucrose is enhanced following injection of the opioid receptor antagonist naloxone (i.e., during antagonist-precipitated withdrawal; Harvey-Lewis et al., 2014). Enhancement of delay discounting upon discontinuation or interruption of treatment might be one sign of withdrawal that could increase the likelihood of continued drug use. In the current study, discontinuation of daily morphine administration decreased food-maintained responding and decreased body weight (Figure 5) in two monkeys, consistent with the emergence of mild opioid withdrawal (e.g., Holtzman and Villarreal, 1973; Gellert and Sparber, 1977). However, discontinuation of treatment did not consistently decrease choice of the large delayed reinforcer. Rather, discontinuation of treatment increased session-to-session variability in choice; increased variation in choice often coincided with increased trial omissions. Increased variability in choice might reflect a loss of sensitivity of responding to reinforcer amount and delay, owing in part to decreased reinforcing effectiveness of food during opioid withdrawal. This loss of sensitivity might allow for other factors (e.g., response bias) to impact responding.
The type of commodity being discounted can significantly impact rates of delay discounting and could impact interactions between drug use and discounting. For example, drug abusers discount drug reinforcers more steeply than money (Bickel et al., 1999; Coffey et al., 2003; Madden et al., 1997; Petry, 2001), and differences in discounting across reinforcers appear to be modulated by the level of drug use and degree of deprivation. For example, 24-h nicotine deprivation in cigarette smokers increases preference for immediately available cigarettes when the alternative is delayed money; however, when subjects were given a choice between different amounts of money there was no effect of nicotine deprivation (Mitchell, 2004). Thus, interactions among drug dependence, withdrawal, and delay discounting likely depend upon the type of reinforcer. It is possible that delay discounting procedures in which subjects choose between different reinforcers, such as different doses of a mu opioid receptor agonist (e.g., remifentanil; Maguire et al., 2013a) or between a drug and food (Maguire et al., 2013b), might be more affected by repeated opioid treatment and/or withdrawal, compared with the procedure used in the current study in which subjects chose between different amounts of food.
In summary, this study examined the effects of acute and chronic morphine administration and its discontinuation on delay discounting of food in rhesus monkeys. Under baseline conditions, responding was sensitive to reinforcer amount and delay. When administered acutely, intermediate doses of morphine decreased, whereas larger doses increased, choice of the larger delayed amount of food. Daily morphine administration decreased choice of the large delayed reinforcer in two monkeys and increased choice in a third monkey. Despite the development of tolerance to some effects of morphine (e.g., rightward shifts in dose-effect curves for the number of trials omitted) and evidence for mild opioid dependence (e.g., decreased number of trials completed and decreased body weight), discontinuation of daily treatment did not appear to systematically impact discounting. Overall, these results suggest that repeated opioid administration produces persistent effects on choice under a delay discounting procedure; however, differences in the direction of effect among individuals suggest factors other than, or in addition to, changes in discounting might play a role. Individual differences both in sensitivity to delay and in response to drug effects on delay discounting might be related to and predictive of vulnerability. Discontinuation of daily morphine administration did not systematically impact delay discounting of food; it remains to be determined whether repeated administration of an opioid receptor agonist, or its discontinuation, modifies discounting of other types of reinforcers (e.g., drugs).
Acknowledgments
The work was supported, in part, by the National Institutes of Health National Institute on Drug Abuse (Grants R01DA005018, T32DA031115, F32DA035605, and K05DA17918). The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institute on Drug Abuse or the National Institutes of Health.
The authors thank the following individuals for excellent technical assistance: Toshi Kuroda, Ian McGraw, Shannon Malesky, Mark Garza, Crystal Taylor, Andrew Lisenby, and Marlisa Burton.
Footnotes
The authors have no conflict of interest.
References
- Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–96. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain physician. 2014;17:E119–E128. [PubMed] [Google Scholar]
- Bickel WK, Odum AL, Madden GJ. Impulsivity and cigarette smoking: Delay discounting in current, never, and ex-smokers. Psychopharmacology (Berl) 1999;146:447–54. doi: 10.1007/pl00005490. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96:73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012;134:287–97. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, α-flupenthixol and behavioural manipulations on choice of signalled and unsignalled delayed reinforcement in rats. Psychopharmacology (Berl) 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- Coffey SF, Gudleski GD, Saladin ME, Brady KT. Impulsivity and rapid discounting of delayed hypothetical rewards in cocaine-dependent individuals. Exp Clin Psychopharmacol. 2003;11:18–25. doi: 10.1037//1064-1297.11.1.18. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DEH, Laane D, Pena Y. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–70. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawes MA, Tarter RE, Kirisci L. Behavioral self-regulation: correlates and 2 year follow- ups for boys at risk for substance abuse. Drug Alcohol Depend. 1997;45:165–76. doi: 10.1016/s0376-8716(97)01359-8. [DOI] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addiction Biology. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Mitchell SH. Drug effects on delay discounting. In: Madden GJ, Bickel WK, editors. Impulsivity: the behavioral and neurological science of discounting. American Psychological Association; Washington: 2010. pp. 213–241. [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer ANM, de Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biological Psychiatry. 2008;63:301–8. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Eppolito AK, France CP, Gerak LR. Effects of acute and chronic morphine on delay discounting in pigeons. J Exp Anal Beh. 2013;99:277–89. doi: 10.1002/jeab.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science. 2012;335:601–4. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146:348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- Evenden JL, Ryan CN. The pharmacology of impulsive behaviour in rats: the effects of drugs on response choice with varying delays of reinforcement. Psychopharmacology (Berl) 1996;128:161–70. doi: 10.1007/s002130050121. [DOI] [PubMed] [Google Scholar]
- Gellert VF, Sparber SB. A comparison of the effects of naloxone upon body weight loss and suppression of fixed-ratio operant behavior in morphine-dependent rats. J Pharm Exp Ther. 1977;137:44–54. [PubMed] [Google Scholar]
- Giordano LA, Bickel WA, Loewenstein G, Jacobs EA, Marsch L, Badger GJ. Mild opioid deprivation increases the degree that opioid-dependent outpatients discount delayed heroin and money. Psychopharmacology (Berl) 2002;163:174–82. doi: 10.1007/s00213-002-1159-2. [DOI] [PubMed] [Google Scholar]
- Harvey-Lewis C, Perdrizet J, Franklin KBJ. The effect of morphine dependence on impulsive choice in rats. Psychopharmacology (Berl) 2012;223:477–87. doi: 10.1007/s00213-012-2738-5. [DOI] [PubMed] [Google Scholar]
- Harvey-Lewis C, Brisebois AD, Yong H, Franklin KBJ. Naloxone-precipitated withdrawal causes an increase in impulsivity in morphine-dependent rats. Behav Pharmacol. 2014;26:326–329. doi: 10.1097/FBP.0000000000000106. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Villarreal JE. Operant behavior in the morphine-dependent rhesus monkey. J Pharm Exp Ther. 1973;184:528–41. [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- Kieres AK, Hausknecht KA, Farrar AM, Acheson A, de Wit H, Richards JB. Effects of morphine and naltrexone on impulsive decision making in rats. Psychopharmacology (Berl) 2004;173:167–74. doi: 10.1007/s00213-003-1697-2. [DOI] [PubMed] [Google Scholar]
- Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- Logue AW. Research on self-control: an integrating framework. Beh Brain Sci. 1988;11:665–79. [Google Scholar]
- MacKillop J, Amlung MT, Rew LR, Ray LA, Sweet LH, Munafo MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) 2011;216:305–21. doi: 10.1007/s00213-011-2229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5:256–62. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Maguire DR, Li J-X, France CP. Impulsivity and drugs of abuse: a juice-reinforced operant procedure for determining within-session delay discounting functions in rhesus monkeys. J Pharm Tox Methods. 2012;66:264–9. doi: 10.1016/j.vascn.2012.08.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP. Effect of delay on self-administration of remifentanil under a drug versus drug choice procedure in rhesus monkeys. J Phar Exp Ther. 2013a;347:557–63. doi: 10.1124/jpet.113.208355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Gerak LR, France CP. Delay discounting of food and remifentanil in rhesus monkeys. Psychopharmacology (Berl) 2013b;229:323–30. doi: 10.1007/s00213-013-3121-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire DR, Henson C, France CP. Effects of amphetamine on delay discounting in rats depend upon the manner in which delay is varied. Neuropharmacology. 2014;87:173–9. doi: 10.1016/j.neuropharm.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–54. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin, editors. Quantitative analyses of behavior. Vol. 5. Erlbaum; Hillsdale, NJ: 1987. pp. 55–73. [Google Scholar]
- Mitchell SH. Effects of short-term nicotine deprivation on decision-making: delay, uncertainty and effort discounting. Nicotine Tob Res. 2004;6:819–28. doi: 10.1080/14622200412331296002. [DOI] [PubMed] [Google Scholar]
- Odum AL, Madden GJ, Badger GJ, Bickel WK. Needle sharing in opioid-dependent outpatients: psychological processes underlying risk. Drug Alcohol Depend. 2000;60:259–266. doi: 10.1016/s0376-8716(00)00111-3. [DOI] [PubMed] [Google Scholar]
- Pattij T, Schetters D, Janssen MCW, Wiskerke J, Schoffelmeer ANM. Acute effects of morphine on distinct forms of impulsive behavior in rats. Psychopharmacology (Berl) 2009;205:489–502. doi: 10.1007/s00213-009-1558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology. 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Nelson SE, Carroll ME. Impulsive choice as a predictor of acquisition of IV cocaine self-administration and reinstatement of cocaine-seeking behavior in male and female rats. Exp Clin Psychopharmacol. 2008;16:165–77. doi: 10.1037/1064-1297.16.2.165. [DOI] [PubMed] [Google Scholar]
- Petry NM. Pathological gamblers, with and without substance use disorders, discount delayed rewards at high rates. J Abnorm Psychol. 2001;110:482–7. doi: 10.1037//0021-843x.110.3.482. [DOI] [PubMed] [Google Scholar]
- Pitts RC, McKinney AP. Effects of methylphenidate and morphine on delay-discount functions obtained within sessions. J Exp Anal Behav. 2005;83:297–314. doi: 10.1901/jeab.2005.47-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachlin H, Green L. Commitment, choice, and self-control. J Exp Anal Behav. 1972;17:15–22. doi: 10.1901/jeab.1972.17-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds B. A review of delay-discounting research with humans: relations to drug use and gambling. Behav Pharmacol. 2006;17:651–67. doi: 10.1097/FBP.0b013e3280115f99. [DOI] [PubMed] [Google Scholar]
- Schippers MC, Binnekade R, Schoffelmeer ANM, Pattij T, De Vries TJ. Unidirectional relationship between heroin self-administration and impulsive decision-making in rats. Psychopharmacology (Berl) 2012;219:443–52. doi: 10.1007/s00213-011-2444-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanno T, Maguire DR, Henson C, France CP. Effects of amphetamine and methylphenidate on delay discounting in rats: interactions with order of delay presentation. Psychopharmacology (Berl) 2014;231:85–95. doi: 10.1007/s00213-013-3209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. American J Psychiatry. 2003;160:1078–85. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]