Abstract
Previously, we have reported that cefazolin and cefoperazone treatments attenuated ethanol consumption, at least in part, through upregulation of GLT-1 expression in male alcohol-preferring (P) rats. In this study, we determined the effects of these compounds on the expression of GLT-1 isoforms (GLT-1a and GLT-1b), cysteine/glutamate exchanger (xCT), which is another glial glutamate transporter co-localized with GLT-1, and glutamate/aspartate transporter (GLAST). We found that cefazolin and cefoperazone treatments decreased ethanol intake and upregulated both GLT-1 isoforms, GLT-1a and GLT-1b, in nucleus accumbens (NAc) and prefrontal cortex (PFC) compared to saline treated group. In addition, cefazolin increased the expression of xCT in NAc and PFC, while cefoperazone upregulated xCT expression only in NAc. However, we did not find any significant differences in GLAST expression between the treated and control groups. Overall, our findings suggest that cefazolin and cefoperazone may be considered as potential compounds for the treatment of ethanol dependence.
Keywords: Alcohol Intake, Glutamate, GLT-1a, GLT-1b, xCT, GLAST
1. Introduction
Impairment in synaptic glutamate reuptake has been linked to drug addiction (Shen et al., 2014). Glutamate receptors are known to modulate development of alcohol addiction (Backstrom and Hyytia, 2004; Besheer et al., 2010). A marked increase in the total extracellular glutamate concentration is associated with exposure to ethanol (Ding et al., 2012; Ding et al., 2013; Ward et al., 2009). In addition, studies have demonstrated the effect of ethanol on glutamate transporters (Abulseoud et al., 2014; Alhaddad et al., 2014b). Glutamate transporter 1 (GLT-1, its human homolog is excitatory amino acid transporter-2) regulates majority of total extracellular glutamate concentration (Jensen et al., 2015; Tanaka et al., 1997) and GLT-1 expression was found to be decreased following chronic exposure to ethanol (Aal-Aaboda et al., 2015; Alhaddad et al., 2014a; Alhaddad et al., 2014b). Therefore, GLT-1 upregulators are rationalized as potential treatment option for treating alcohol addiction. Following the discovery by Rothstein et al. reported β-lactam antibiotics as potent GLT-1 upregulators (Rothstein et al., 2005), we have shown that ceftriaxone, a β-lactam antibiotic, reduced ethanol intake and relapse to ethanol intake presumably by increasing the expression of GLT-1 in the mesocorticolimbic areas of the brain including NAc and PFC (Alhaddad et al., 2014a; Qrunfleh et al., 2013; Rao and Sari, 2014; Rao et al., 2015b). Similarly, other β-lactams, including cefazolin and cefoperazone at dose of 100 mg/kg/day for 5 consecutive days, were found to be effective in reducing ethanol intake in male P rats (Rao et al., 2015a). While GLT-1 upregulation by these compounds has been demonstrated, the effects of cefazolin and cefoperazone on modulating the expression of other transporters regulating extracellular glutamate concentrations have not been investigated.
GLT-1 is known to be expressed as three splice variants - GLT-1a, GLT-1b and GLT-1c - and these isoforms are expressed differentially in the central nervous system (CNS). GLT-1a is expressed in astrocytes as well as neurons, whereas GLT-1b is found to be expressed only in astrocytes (Berger et al., 2005; Holmseth et al., 2009). It has been reported that GLT-1c is localized mainly in the retina (Rauen et al., 2004). However, a difference in ability to transport glutamate amongst these isoforms has not been confirmed (Sullivan et al., 2004). Importantly, the expression of GLT-1 isoforms is known to change differentially depending on the disease model. For example, in motor cortex of patients with amyotrophic lateral sclerosis the expression of GLT-1a is downregulated while GLT-1b expression is upregulated (Maragakis et al., 2004). Previous studies from our laboratory have demonstrated that ceftriaxone-induced GLT-1a and GLT-1b expression in NAc and PFC, and this effect was found associated with a significant decrease in continuous ethanol intake and relapse-like ethanol drinking in male P rats (Alhaddad et al., 2014a; Rao et al., 2015b). In order to investigate differential effects on GLT-1a and GLT-1b expression, in this study, we tested the effects of both cefazolin and cefoperazone on GLT-1a and GLT-1b expression in P rats exposed to free choice ethanol (15% and 30%). We did not test GLT-1c isoform since it is mainly expressed in the retina (Rauen et al., 2004).
While GLT-1 is the major glutamate transporter in brain, the glutamate/aspartate transporter (GLAST, its human homolog is excitatory amino acid transporter-1, EAAT1) is also known to regulate synaptic glutamate homeostasis and is expressed in the cerebellum (Lehre and Danbolt, 1998). Although GLAST is distributed throughout the brain (Schmitt et al., 1997), the most common glutamate transporter in the inner ear and the retina is GLAST (Lehre and Danbolt, 1998; Takumi et al., 1997). In this study, we determined the effects of cefazolin and cefoperazone treatments on GLAST expression in NAc and PFC.
In addition to GLT-1 and GLAST, cystine-glutamate antiporter (xCT) is also known to regulate extracellular glutamate concentrations and plays a crucial role in neuroprotection (For review, see Albrecht et al., 2010). Importantly, depletion in glutathione content is associated with decreased xCT expression (Bell et al., 2011; Lewerenz et al., 2009). Ceftriaxone treatment was shown neuroprotection by inducing nuclear factor-erythroid 2-related factor (Nrf2)-mediated induction of xCT expression, leading to increase in intercellular glutathione levels (Bell et al., 2011; Lewerenz et al., 2009). A critical role of xCT has also been demonstrated in animal models of drug addiction. For instance, nicotine and cocaine self-administration decreased xCT expression in NAc (Knackstedt et al., 2009; Knackstedt et al., 2010). Similarly, xCT was found to be downregulated in NAc following chronic and relapse-like exposure to free choice ethanol (15% and 30%) as compared to alcohol naïve group (Alhaddad et al., 2014a; Alhaddad et al., 2014b). Interestingly, ceftriaxone treatment was found to upregulate xCT expression in NAc of rats exposed to both free choice ethanol (Alhaddad et al., 2014a; Rao et al., 2015b) and cocaine self-administration (Knackstedt et al., 2010). To explore if treatment with other cephalosporins can modulate xCT expression, in this study, the effect of cefazolin and cefoperazone treatments on xCT expression in NAc and PFC in P rats after chronic exposure to ethanol (15% and 30%) was determined.
2. Results
2.1 Effect of cefazolin and cefoperazone treatments on ethanol intake and water intake
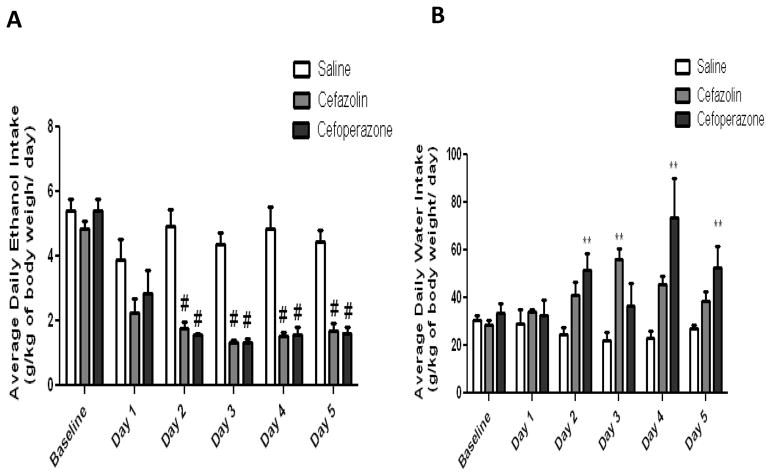
Mixed ANOVA demonstrated a significant main effect of day [F (1, 5) = 22.74, p< 0.0001] and a significant day x treatment interaction [F (2, 10) = 3.599, p= 0.0005] of ethanol intake. Moreover, one way ANOVA followed by Dunnett’s t-tests showed a significant decrease in ethanol intake with cefazolin (n=6) and cefoperazone (n=6) treated groups as compared to saline treated group (n=6) from Day 2 through Day 5 (p<0.001) (Figure 1). In addition, statistical analysis revealed a significant main effect of day [F (1, 5) = 2.782, p=0.0220] and a significant day x treatment interaction [F (2, 10) = 3.206, p= 0.0014] of water consumption. Dunnett’s t-tests followed by one way ANOVA showed a significant increase in water consumption in cefoperazone treated groups starting from Day 2 through Day 5 (except on Day 3) comparing to saline treated group (p<0.01),. However, cefazolin treatment increased water consumption only on Day 3 (p<0. 01) (Figure 1).
Figure 1.
Changes in ethanol and water intake following treatment of cefazolin and cefoperazone in male P rats exposed to five weeks of free choice of water and ethanol. A) Effects of cefazolin and cefoperazone treatments on ethanol intake (g/kg/day). B) Effects of cefazolin and cefoperazone treatments on water intake (g/kg/day). Data are represented as mean ± SEM; (**p < 0.01; #p < 0.001), (n = 6 for each group).
2.2 Effects of cefazolin and cefoperazone treatments on GLT-1a and GLT-1b expression in NAc and PFC
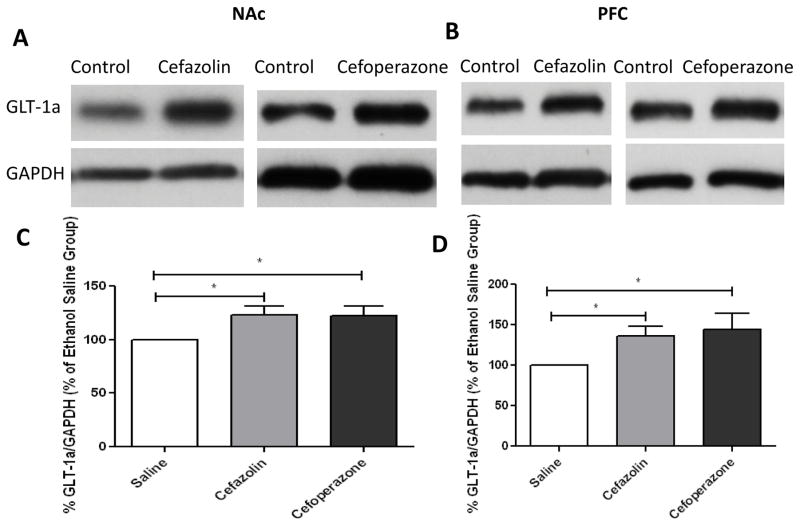
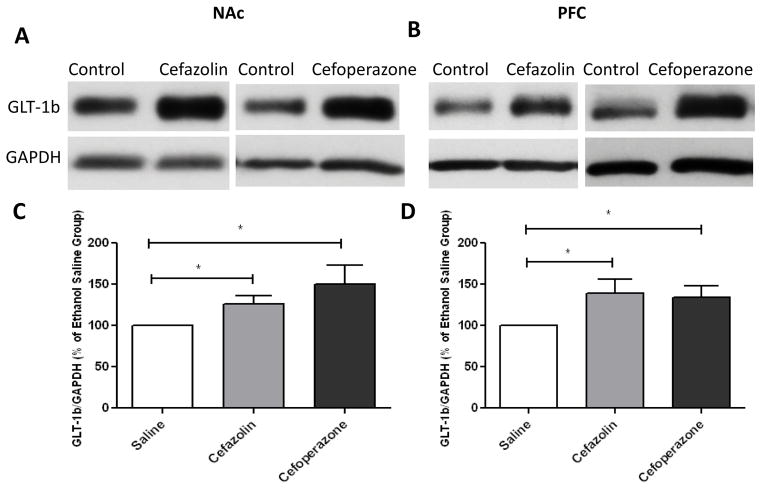
Independent t-test analyses of immunoblots demonstrated a significant increase in GLT-1a/GAPDH ratio in NAc and PFC with cefazolin- (p<0.05) and cefoperazone- (p<0.05) treated groups as compared to saline-treated groups (Figure 2). In addition, independent t-test analysis of the immunoblots demonstrated a significant increase in GLT-1b expression in NAc and PFC following treatment with cefazolin (p<0.05) and cefoperazone (p<0.05) as compared to saline-treated group (Figure 3).
Figure 2.
Changes in GLT-1a expression in NAc and PFC following treatment with cefazolin and cefoperazone in male P rats. A) Immunoblots for GLT-1a and control loading protein (GAPDH) expression in NAc. B) Immunoblots for GLT-1a and control loading protein (GAPDH) expression in PFC. C) Quantitative t-test analysis of immunoblots revealed a significant increase in GLT-1a/GAPDH ratio in NAc in cefazolin- and cefoperazone-treated groups compared with saline-treated group (control value is 100%). D) Quantitative t-test analysis of immunoblots revealed a significant increase in GLT-1a/GAPDH ratio in PFC in cefazolin- and cefoperazone- treated groups compared with saline-treated group (control value is 100%). Data are expressed as mean ± SEM. (*p<0.05). (n=6 for each group)
Figure 3.
GLT-1b expression following treatment of cefazolin and cefoperazone in NAc and PFC. A) Immunoblots for GLT-1b and control loading protein (GAPDH) expression in NAc. B) Immunoblots for GLT-1b and control loading protein (GAPDH) expression in PFC. C) Quantitative t-test analysis of immunoblots revealed a significant increase in GLT-1b/GAPDH ratio in NAc in cefazolin- and cefoperazone-treated groups compared with saline-treated group (control value is 100%). D) Quantitative t-test analysis of immunoblots revealed a significant increase in GLT-1b/GAPDH ratio in PFC in cefazolin- and cefoperazone-treated groups compared with saline-treated group (control value is 100%). Data are expressed as mean ± SEM. (*p<0.05). (n=6 for each group)
2.3 Effects of cefazolin and cefoperazone on xCT and GLAST expression in NAc and PFC
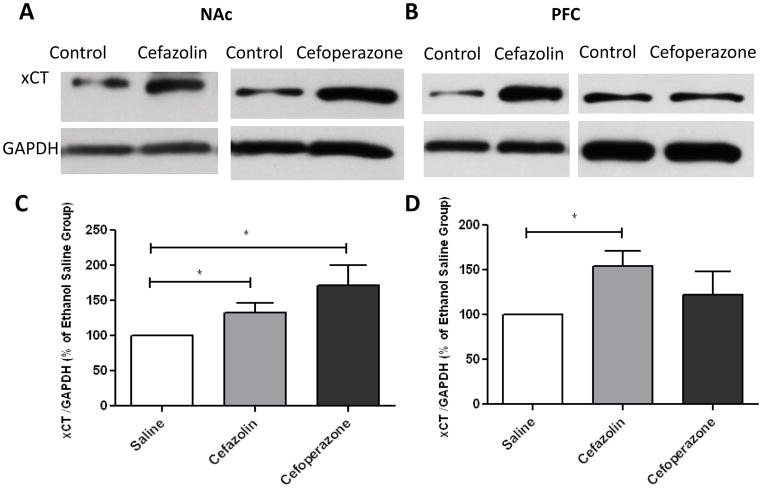
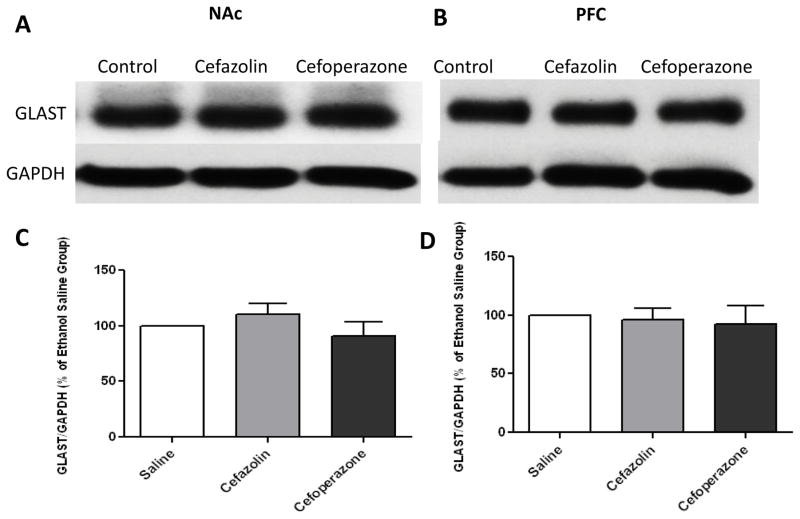
We further investigate the effect of cefazolin and cefoperazone treatments on xCT and GLAST expression. Quantitative t-test analyses of immunoblots showed a significant increase in xCT/GAPDH ratio in NAc after treatment of cefazolin (p<0.05) and cefoperazone (p<0.05) as compared to saline-treated group. However, only cefazolin treatment increased xCT/GAPDH ratio in PFC (p<0. 05) (Figure 4). Furthermore, an independent t-test analyses of the immunoblots did not reveal any significant increase in GLAST/GAPDH ratio in NAc and PFC in cefazolin- (p>0.05) or cefoperazone- (p>0.05) treated groups as compared to saline treated groups (Figure 5).
Figure 4.
xCT expression in NAc and PFC following treatment with cefazolin and cefoperazone. A) Immunoblots for xCT and control loading protein (GAPDH) expression in NAc. B) Immunoblots for xCT and control loading protein (GAPDH) expression in PFC. C) Quantitative t-test analysis of immunoblots revealed a significant increase in xCT/GAPDH ratio in NAc in cefazolin- and cefoperazone-treated groups compared with saline-treated group (control value is 100%). D) Quantitative t-test analysis of immunoblots revealed a significant increase in xCT/GAPDH ratio in PFC in cefazolin-treated group and not significant in cefoperazone-treated group compared with saline-treated group (control value is 100%). Data are expressed as mean ± SEM. (*p<0.05). (n=6 for each group)
Figure 5.
GLAST expression following treatment with cefazolin and cefoperazone in NAc and PFC. A) Immunoblots for GLAST and control loading protein (GAPDH) expression in NAc. B) Immunoblots for GLAST and control loading protein (GAPDH) expression in PFC. C) Quantitative t-test analysis of immunoblots revealed a non-significant increase in GLAST/GAPDH ratio in NAc in cefazolin- and cefoperazone-treated groups compared with saline-treated group (control value is 100%). D) Quantitative t-test analysis of immunoblots revealed a non-significant increase in GLAST/GAPDH ratio in PFC in cefazolin- and cefoperazone-treated groups compared with saline-treated group (control value is 100%). Data are expressed as mean ± SEM. (n=6 for each group)
3. Discussion
Previous studies from our laboratory have demonstrated that ceftriaxone, β-lactam antibiotic, increased GLT-1 and xCT expression in NAc and PFC regions and that these changes are associated with reduced ethanol intake in male P rats (Alhaddad et al., 2014a; Qrunfleh et al., 2013; Rao et al., 2015b; Sari et al., 2011). In addition, we have also shown that other β-lactams, including cefazolin and cefoperazone, are equally effective in reducing ethanol consumption in P rats (Rao et al., 2015a). In this study, for the first time, we report that both cefazolin, a first generation cephalosporin β-lactam antibiotic, and cefoperazone, a third generation cephalosporin β-lactam, treatments at dose of 100 mg/kg/day for 5 consecutive days have significant upregulatory effect on the expression of major GLT-1 isoforms (GLT-1a and GLT-1b) and xCT in NAc and PFC in male P rats. It is important to note that both cefazolin and cefoperazone were found in the cerebrospinal fluid (Rao et al., 2015a). Therefore, we believe that these compounds act centrally to reduce ethanol drinking. Additionally, cefoperazone was found to inhibit the activity of liver alcohol dehydrogenase enzyme, suggesting a possible disulfiram-like effect in modulating ethanol intake (Rao et al., 2015a).
Mesocorticolimbic pathway plays an important role in mediating the rewarding effects of drugs of abuse, including ethanol. It is important to note that NAc receives glutamatergic projections from amygdala, PFC and hippocampus [For review see (Tye, 2012)]. PFC has been shown to send and receive glutamatergic projections into NAc as well as from other brain regions, including amygdala (McDonald, 1996). It is well known that ethanol consumption is associated with a marked increase in extracellular glutamate concentration (Ding et al., 2012; Ding et al., 2013; Kapasova and Szumlinski, 2008; Ward et al., 2009). Furthermore, β-lactams antibiotics, including ceftriaxone, have been effective in reducing ethanol intake in male P rats and attenuating relapse-like ethanol intake behavior (Rao and Sari, 2014; Sari et al., 2011). This effect was associated in part with upregulation of the two principal C-terminal splice variants of GLT-1 (GLT-1a and GLT-1b) and xCT in NAc and PFC (Alhaddad et al., 2014a; Qrunfleh et al., 2013; Rao et al., 2015b; Sari et al., 2011). Furthermore, recent study from our laboratory has confirmed the ceftriaxone-induced reduction in extracellular glutamate concentration as a consequence of increased GLT-1 expression in NAc (Das et al., 2015). Results from the present study, for the first time, demonstrated that changes in expression of GLT-1 isoforms and xCT extend beyond ceftriaxone to other cephalosporins, including cefazolin and cefoperazone.
In our earlier study, we found that cefazolin and cefoperazone treatments at dose of 100 mg/kg for 5 consecutive days successfully reduced ethanol consumption in male P rats, presumably through upregulation of GLT-1 expression in NAc and PFC (Rao et al., 2015a). As an extension of our previous work, in the present study, we have found that cefazolin and cefoperazone treatments maintained comparable induction of expression for the two major GLT-1 isoforms - GLT-1a and GLT-1b - in NAc and PFC. Although GLT-1a and GLT-1b are not expressed similarly across brain regions (Berger et al., 2005; Holmseth et al., 2009), the lack of selectively towards upregulatory effect of GLT-1 isoforms by the tested cephalosporins indicates possible regulation via common cellular mechanisms.
In addition to modulation of GLT-1 expression, ceftriaxone treatment-induced reduction in ethanol intake is also associated with upregulation of xCT expression, an important transporter in glutamate homeostasis, in NAc, PFC and amygdala in male P rats (Alhaddad et al., 2014a; Rao and Sari, 2014). The xCT system exchanges intracellular glutamate with extracellular cystine, involved in the biosynthesis of neuroprotective molecule glutathione (Baker et al., 2002; Shih et al., 2006), and changes in the expression of this system have been implicated in the development of drug addiction (Knackstedt et al., 2010, Alhaddad et al., 2014a). Moreover, xCT is known to influence synaptic glutamate release via activation of presynaptic mGluR2/3 metabotropic receptors, which regulate the negative feedback for synaptic glutamate release. Therefore, the effect of cefazolin and cefoperazone treatments on xCT expression in NAc and PFC was also determined in this study. Compared to control animals, cefazolin treatment was found to upregulate xCT expression in NAc and PFC. However, cefoperazone treatment was able to induce xCT expression only in NAc but not in PFC. These results suggest that in addition to modulating synaptic glutamate concentration, these cephalosporins can influence the presynaptic release of glutamate as well.
In addition to influencing the expression of glutamate transporters in mesocorticolimbic pathways, cefoperazone is known to display disulfiram-like effects (Fromtling and Gadebusch, 1983; Rao et al., 2015a). Cefoperazone, and not cefazolin, was found to significantly decrease the activity of hepatic mitochondrial enzyme ALDH2 in treated P rats thereby offering another possible mechanism for the effects of cefoperazone treatment on ethanol drinking in P rats (Rao et al., 2015a). Reduced CNS bioavailability of cefoperazone, perhaps owing to significant peripheral actions, was also confirmed in a previous analysis of CSF concentrations for this drug (Rao et al., 2015a). A reduced concentration in brain, and therefore PFC, might be responsible for the unchanged expression of xCT in PFC in cefoperazone-treated rats compared to vehicle-treated control animals.
We further investigated the effects of cefazolin and cefoperazone treatments on GLAST expression in NAc and PFC, which is co-localized with GLT-1 in astrocytes. We did not find any significant effects on GLAST expression with cefazolin and cefoperazone treatments. This effect is in agreement with our previous studies revealing that ceftriaxone treatment did not increase GLAST expression (Alhaddad et al., 2014a; Alhaddad et al., 2014b; Rao et al., 2015b). These findings suggest a more specific role for xCT and GLT-1 isoforms in regulating ethanol consumption behavior.
In conclusion, the results from this study indicate that cefazolin and cefoperazone treatments decrease ethanol consumption in P rats possibly by increasing GLT-1a, GLT-1b and xCT expression in NAc and PFC. In conjunction with our previous findings, the present study bolsters the rationale that both cefazolin and cefoperazone treatments reduced ethanol-consumption via resumption of glutamate homeostasis in the mesocorticolimbic regions. As established in previous study from our laboratory (Das et al., 2015), we rationalize a direct translation of β-lactam-induced GLT-1 upregulation to restoration of extracellular glutamate concentration and decreased ethanol intake in P rats, and intend to establish this in our future studies. Further studies are also required to obtain a dose response effect of these drugs on ethanol dependence for their clinical effectiveness.
4. Experimental Procedure
4.1 Animals
Alcohol-preferring male (P) rats were provided by Indiana University, School of Medicine (Indianapolis, IN, USA). It is noteworthy to mention that P rat is an accepted animal model that meet the criteria for studying ethanol dependence (Bell et al., 2006). In addition, P rat model has been found to have several physiological and behavioral alcoholics’ characteristics (Murphy et al., 2002). The rats were individually housed in bedded plastic tubs at 21°C and 50% humidity in the Department of Laboratory Animal Resource at The University of Toledo, Health Science Campus. The Institutional Animal Care and Use Committee of The University of Toledo approved all animal housing and experimental procedures in accordance with guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996). We divided P rats into three experimental groups: a) cefazolin group was treated with 100 mg/kg of the drug (i.p) (n=6), b) cefoperazone group was treated with 100 mg/kg of the drug (i.p) (n=6), and c) saline (control) vehicle treated group (n=6). β-lactam drugs were dissolved in 0.9 % saline. All animals had ad libitum access to food and water throughout the study.
4.2 Behavioral drinking paradigms
At the age of three months, all treatment and control groups were given free choice to food, water and two concentrations of ethanol (15% and 30%, v/v) over a period of five weeks. Water intake and ethanol intake were measured three times per week during week 4 and week 5. Densitometry formula was used to convert ethanol intake readings to gram per kilogram of body weight per day. Animals used in this study were required to consume more than 4 g/kg/day of ethanol intake during last two weeks. Water intake and ethanol intake were evaluated during last two weeks and served as baseline values. In week 6, all P rats were treated once per day (i.p. injection) with either 0.9% saline solution (control group) or 100 mg/kg body weight of β-lactam antibiotics (cefazolin or cefoperazone) for five consecutive days. During these five days, water and ethanol intake were measured every day. All animals were euthanized 24h after the last treatment by exposure to CO2. Brain were removed, frozen on dry ice and then stored at −80°C. Both NAc and PFC tissues were microdissected at −20°C using cryostat machine (Leica) followed the Paxinos and Watson Atlas for the rat brain regions (Paxinos, 2007) and stored at −80°C for immunoblotting assay.
4.3 Western blot protocol for detection of GLT-1a, GLT-1b, xCT and GLAST
Expression of GLT-1a, GLT-1b, xCT, GLAST and GAPDH for all groups in NAc and PFC tissues were determined using Western blot assay as described previously (Alhaddad et al., 2014a; Alhaddad et al., 2014b; Rao and Sari, 2014). Tissues (NAc and PFC) were homogenized in lysis buffer supplemented with protease inhibitor and total protein in samples was quantified (BioRad, USA). Equal amount of lysed NAc or PFC tissue from all groups were loaded on 10–20% polyacrylamide gel. Subsequently, proteins were transferred on a PVDF membrane and blocked with 3% milk in Tris-buffered saline Tween-20 (TBST) for 30 minutes at room temperature. Membranes were then incubated overnight at 4°C with one of the following antibodies: rabbit anti-GLT-1a (1:5,000 gift from Dr. Jeffery Rothstein at Johns Hopkins University), rabbit anti-GLT-1b (1:5,000 gift from Dr. Paul Rosenberg at Harvard Medical School University), rabbit anti-xCT (1:1,000 Abcam), and rabbit anti- GLAST (1:5,000 Abcam). The specificity of antibodies for GLT-1 isoforms has been established by the respective groups in their previous publications (Chen et al., 2002; Rothstein et al., 1994). Mouse anti-GAPDH (1:5,000, Millipore) was used as the loading control marker. On the second day, the membranes were washed with TBST and then blocked with 3% percent milk in TBST for 30 minutes at room temperature. Following incubation with secondary antibody (anti-rabbit IgG or anti-mouse IgG) at 1:3000 dilution for 90 minutes, the membranes were washed three timed with TBST, dried and then developed using the chemiluminescent kit (Super Signal West Pico, Pierce Inc.) for protein detection. HyBlot CL Film (Thermo Fisher Scientific) was exposed to the membranes and then developed using SRX-101A processor. MCID machine was used to quantify and analyze the digitized band images. GLT-1a, GLT-1b, xCT and GLAST levels were normalized against GAPDH, a control loading protein. The data from saline-treated animals were reported as 100% to evaluate the changes in protein expression in brain regions collected from β-lactam treated groups.
4.4 Statistical analyses
Two way (mixed) ANOVA with repeated measures was performed to analyze behavioral statistical data, including average daily ethanol intake and average daily water intake. We also used one way ANOVA followed by Dunnett’s post hoc test to determine the effects of cefazolin and cefoperazone treatments on each day. An unpaired t-test was used to analyze Western blot data obtained for proteins of interest between treatments (cefazolin or cefoperazone) and saline groups in NAc and PFC. To determine the changes in expression of proteins for cefazolin and cefoperazone treated groups, western blot band density from saline-treated group (control) was converted to 100%. All statistical analyses were based on a p<0.05 level of significance.
Highlights.
cefazolin and cefoperazone treatments decreased ethanol intake.
cefazolin and cefoperazone upregulated GLT-1a and GLT-1b in NAc and PFC.
Cefazolin increased xCT expression in NAc and PFC.
Cefoperazone upregulated xCT expression in NAc.
Acknowledgments
This work was supported by Award Number R01AA019458 (Y.S.) from the National Institutes on Alcohol Abuse and Alcoholism. The authors thank Dr. J. Rothstein and Dr. P. Rosenberg for providing us with GLT-1a and GLT-1b antibodies, respectively.
Abbreviations
- GLT-1
Glutamate transporter 1
- NAc
nucleus accumbens
- PFC
prefrontal cortex
- CEF
ceftriaxone
- P rats
alcohol-preferring
- CSF
cerebrospinal fluid
- EAAT
Excitatory amino-acid transporters
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aal-Aaboda M, et al. Effects of (R)-(−)-5-methyl-1-nicotinoyl-2-pyrazoline on glutamate transporter 1 and cysteine/glutamate exchanger as well as ethanol drinking behavior in male, alcohol-preferring rats. J Neurosci Res. 2015 doi: 10.1002/jnr.23554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulseoud OA, et al. Attenuation of ethanol withdrawal by ceftriaxone-induced upregulation of glutamate transporter EAAT2. Neuropsychopharmacology. 2014;39:1674–84. doi: 10.1038/npp.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht P, et al. Mechanisms of oxidative glutamate toxicity: the glutamate/cystine antiporter system xc- as a neuroprotective drug target. CNS Neurol Disord Drug Targets. 2010;9:373–82. doi: 10.2174/187152710791292567. [DOI] [PubMed] [Google Scholar]
- Alhaddad H, Das SC, Sari Y. Effects of ceftriaxone on ethanol intake: a possible role for xCT and GLT-1 isoforms modulation of glutamate levels in P rats. Psychopharmacology (Berl) 2014a;231:4049–57. doi: 10.1007/s00213-014-3545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhaddad H, et al. Effects of MS-153 on chronic ethanol consumption and GLT1 modulation of glutamate levels in male alcohol-preferring rats. Front Behav Neurosci. 2014b;8:366. doi: 10.3389/fnbeh.2014.00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom P, Hyytia P. Ionotropic glutamate receptor antagonists modulate cue-induced reinstatement of ethanol-seeking behavior. Alcohol Clin Exp Res. 2004;28:558–65. doi: 10.1097/01.alc.0000122101.13164.21. [DOI] [PubMed] [Google Scholar]
- Baker DA, et al. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci. 2002;22:9134–41. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell KF, et al. Activation of Nrf2-regulated glutathione pathway genes by ischemic preconditioning. Oxid Med Cell Longev. 2011;2011:689524. doi: 10.1155/2011/689524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, et al. The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol. 2006;11:270–88. doi: 10.1111/j.1369-1600.2005.00029.x. [DOI] [PubMed] [Google Scholar]
- Berger UV, et al. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J Comp Neurol. 2005;492:78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besheer J, et al. Metabotropic glutamate receptor 5 activity in the nucleus accumbens is required for the maintenance of ethanol self-administration in a rat genetic model of high alcohol intake. Biol Psychiatry. 2010;67:812–22. doi: 10.1016/j.biopsych.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, et al. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci. 2002;22:2142–52. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SC, et al. Ceftriaxone attenuates ethanol drinking and restores extracellular glutamate concentration through normalization of GLT-1 in nucleus accumbens of male alcohol-preferring rats. Neuropharmacology. 2015;97:67–74. doi: 10.1016/j.neuropharm.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, et al. Ethanol increases glutamate neurotransmission in the posterior ventral tegmental area of female wistar rats. Alcohol Clin Exp Res. 2012;36:633–40. doi: 10.1111/j.1530-0277.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZM, et al. Alcohol drinking and deprivation alter basal extracellular glutamate concentrations and clearance in the mesolimbic system of alcohol-preferring (P) rats. Addict Biol. 2013;18:297–306. doi: 10.1111/adb.12018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromtling RA, Gadebusch HH. Ethanol-cephalosporin antibiotic interactions: an animal model for the detection of disulfiram (Antabuse)-like effects. Methods Find Exp Clin Pharmacol. 1983;5:595–600. [PubMed] [Google Scholar]
- Holmseth S, et al. The concentrations and distributions of three C-terminal variants of the GLT1 (EAAT2; slc1a2) glutamate transporter protein in rat brain tissue suggest differential regulation. Neuroscience. 2009;162:1055–71. doi: 10.1016/j.neuroscience.2009.03.048. [DOI] [PubMed] [Google Scholar]
- Jensen AA, et al. Excitatory amino acid transporters: recent insights into molecular mechanisms, novel modes of modulation and new therapeutic possibilities. Curr Opin Pharmacol. 2015;20C:116–123. doi: 10.1016/j.coph.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–31. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Knackstedt LA, et al. The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry. 2009;65:841–5. doi: 10.1016/j.biopsych.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Melendez RI, Kalivas PW. Ceftriaxone restores glutamate homeostasis and prevents relapse to cocaine seeking. Biol Psychiatry. 2010;67:81–4. doi: 10.1016/j.biopsych.2009.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–7. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewerenz J, et al. Induction of Nrf2 and xCT are involved in the action of the neuroprotective antibiotic ceftriaxone in vitro. J Neurochem. 2009;111:332–43. doi: 10.1111/j.1471-4159.2009.06347.x. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Dykes-Hoberg M, Rothstein JD. Altered expression of the glutamate transporter EAAT2b in neurological disease. Ann Neurol. 2004;55:469–77. doi: 10.1002/ana.20003. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Glutamate and aspartate immunoreactive neurons of the rat basolateral amygdala: colocalization of excitatory amino acids and projections to the limbic circuit. J Comp Neurol. 1996;365:367–79. doi: 10.1002/(SICI)1096-9861(19960212)365:3<367::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Murphy JM, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–88. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Paxinos G. Atlas of the developing mouse brain at E17.5, P0 and P6. Elsevier; Amsterdam; Boston: 2007. [Google Scholar]
- Qrunfleh AM, Alazizi A, Sari Y. Ceftriaxone, a beta-lactam antibiotic, attenuates relapse-like ethanol-drinking behavior in alcohol-preferring rats. J Psychopharmacol. 2013;27:541–9. doi: 10.1177/0269881113482529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, Sari Y. Effects of ceftriaxone on chronic ethanol consumption: a potential role for xCT and GLT1 modulation of glutamate levels in male P rats. J Mol Neurosci. 2014;54:71–7. doi: 10.1007/s12031-014-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, et al. Effects of ampicillin, cefazolin and cefoperazone treatments on GLT-1 expressions in the mesocorticolimbic system and ethanol intake in alcohol-preferring rats. Neuroscience. 2015a;295:164–74. doi: 10.1016/j.neuroscience.2015.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao PS, et al. Effects of ceftriaxone on GLT1 isoforms, xCT and associated signaling pathways in P rats exposed to ethanol. Psychopharmacology (Berl) 2015b doi: 10.1007/s00213-015-3868-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen T, et al. A new GLT1 splice variant: cloning and immunolocalization of GLT1c in the mammalian retina and brain. Neurochem Int. 2004;45:1095–106. doi: 10.1016/j.neuint.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–25. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–7. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Sari Y, et al. Ceftriaxone, a beta-lactam antibiotic, reduces ethanol consumption in alcohol-preferring rats. Alcohol Alcohol. 2011;46:239–46. doi: 10.1093/alcalc/agr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt A, et al. Cellular and regional distribution of the glutamate transporter GLAST in the CNS of rats: nonradioactive in situ hybridization and comparative immunocytochemistry. J Neurosci. 1997;17:1–10. doi: 10.1523/JNEUROSCI.17-01-00001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, et al. Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci. 2014;34:5649–57. doi: 10.1523/JNEUROSCI.4564-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih AY, et al. Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. J Neurosci. 2006;26:10514–23. doi: 10.1523/JNEUROSCI.3178-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, et al. Cloning, transport properties, and differential localization of two splice variants of GLT-1 in the rat CNS: implications for CNS glutamate homeostasis. Glia. 2004;45:155–69. doi: 10.1002/glia.10317. [DOI] [PubMed] [Google Scholar]
- Takumi Y, et al. Discrete cellular and subcellular localization of glutamine synthetase and the glutamate transporter GLAST in the rat vestibular end organ. Neuroscience. 1997;79:1137–44. doi: 10.1016/s0306-4522(97)00025-0. [DOI] [PubMed] [Google Scholar]
- Tanaka K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tye KM. Glutamate inputs to the nucleus accumbens: does source matter? Neuron. 2012;76:671–3. doi: 10.1016/j.neuron.2012.11.008. [DOI] [PubMed] [Google Scholar]
- Ward RJ, et al. Neuro-inflammation induced in the hippocampus of ‘binge drinking’ rats may be mediated by elevated extracellular glutamate content. J Neurochem. 2009;111:1119–28. doi: 10.1111/j.1471-4159.2009.06389.x. [DOI] [PubMed] [Google Scholar]