Abstract
The youngest peripheral T cells (recent thymic emigrants or RTEs) are functionally distinct from naïve T cells that have completed post-thymic maturation. We now assess the RTE memory response, and find that RTEs produced less granzyme B than their mature counterparts during infection, but proliferated more and therefore generated equivalent target killing in vivo. After infection, RTE numbers contracted less dramatically than those of mature T cells, but RTEs were delayed in their transition to central memory, displaying impaired expression of CD62L, IL-2, Eomesodermin, and CXCR4, which resulted in impaired bone marrow localization. RTE-derived and mature memory cells expanded equivalently during rechallenge, indicating the robust proliferative capacity of RTEs was maintained independently of central memory phenotype. Thus, the diminished effector function and delayed central memory differentiation of RTE-derived memory cells are counterbalanced by their increased proliferative capacity, driving the efficacy of the RTE response to that of mature T cells.
Introduction
Recent thymic emigrants (RTEs) are those T cells that have most recently left the thymus to enter the lymphoid periphery. RTEs are an important yet understudied population that not only helps seed the population of naïve peripheral T cells throughout life, but also comprises the bulk of T cells in neonates and individuals recovering from accidental or targeted lymphoablation. RTEs can be identified unambiguously as GFP+ peripheral T cells in mice carrying a GFP transgene driven by the RAG2 promoter (1). Analyzing RTEs from unmanipulated RAG2p-GFP transgenic (Tg) reporter mice has demonstrated that these youngest peripheral T cells are functionally distinct from their GFP− mature naïve (MN) counterparts (reviewed in 2, 3). CD4+ RTEs are Th2 skewed (4, 5), and in the context of infection, CD8+ RTEs exhibit increased responses to low affinity antigens, proliferate more robustly, and are skewed toward a short-lived effector phenotype (6–8). In addition, RTEs express elevated levels of homing molecules that allow increased entry into peripheral tissues and enhanced resistance to tissue infection (6). Although RTEs have a surface phenotype that is consistent with increased effector function, they produce lower levels of the effector cytokines IFNγ and TNFα (6, 7, 9). These data suggest that while RTEs respond more readily than mature T cells to a broader range of antigens in the context of a primary infection, their reduced cytokine production may help prevent excessive tissue damage. Understanding how this distinct antigen response profile translates into the generation of a memory T cell response was the goal of the current studies.
After infection is cleared, most responding CD8+ T cells die by apoptosis, but a small population converts to a central memory population that is characterized by increased expression of transcription factors such as Eomesodermin (Eomes), Id3, and Bcl-6, efficient localization to secondary lymphoid organs, and improved reexpansion during secondary infection (10). The failure of T cells to properly differentiate into central memory cells can lead to decreased T cell longevity and inability to control secondary infection (10). The current study addresses the capacity of RTEs to differentiate into central memory T cells and demonstrates that central memory conversion of RTEs is slowed relative to that of mature T cells after infection. This slower rate of conversion results in altered tissue localization, with more antigen-specific RTEs than mature T cells residing in the spleen and blood rather than in the lymph nodes. Contrary to expectation, RTE-derived memory cells do not mount a poor proliferative response to secondary infection, but instead reexpand as well as their mature counterparts. Our data indicate that while RTEs differentiate poorly into Tcm and show reduced effector cytokine production, these deficiencies are overcome by the enhanced proliferation of RTEs, a trait that persists through primary and secondary infection, well after RTEs have transitioned to the mature T cell compartment.
Materials and Methods
Mice
RAG2p-GFP transgenic (Tg) OT-I TCR Tg mice were backcrossed in our laboratory 12 generations onto the C57BL/6 (B6) background with both CD45.1 and CD45.2 congenic markers. B6xB6.SJL-PtprcaPepcb/BoyJ (CD45.2xCD45.1) adoptive recipients were bred in house. All mice were housed under specific pathogen free conditions and used in accordance with the University of Washington Institutional Animal Care and Use Committee.
Flow cytometry antibodies
Fluorochrome-conjugated antibodies against the following surface and intracellular molecules were purchased from eBioscience, BD Biosciences, or BioLegend: CD45.1 (A20), CD45.2 (104), NK1.1 (PK136), CD4 (RM4-5), CD8α (53-6.7), Ter119 (Ter119), CD11b (M1/70), B220 (RA3-6B2), CD44 (IM7), CD62L (MEL-14), CXCR4 (L276F12), IL-2 (JES6-5H4), IFNγ (XMG1.2), granzyme B (GB11), and Eomes (Dan11mag).
Cell preparation and flow cytometry
Red blood cells were water lysed from single cell suspensions of splenocytes, blood, and bone marrow (BM) harvested from femurs and tibias. These populations and those from lymph nodes (LNs) were surface stained for 20 minutes at 4°C. For CXCR4 staining, cells were stained first with anti-CXCR4 at 37°C for 30 minutes, washed, and stained for other surface molecules as above. For intracellular cytokine staining, 2–4x106 cells were incubated for 5 hours at 37°C with brefeldin A (GolgiPlug; BD Biosciences) and 10nM of the OVA-derived SIINFEKL peptide (GeneMed, Inc.) recognized by OT-I Tg T cells, surface stained, then fixed and permeabilized with BD Cytofix/Cytoperm according to the manufacturer’s instructions. For intranuclear staining, cells were fixed and permeabilized using Foxp3/Transcription Factor Staining Buffer Set (eBioscience) according to the manufacturer’s protocol. Single color compensation controls and experimental samples were identically fixed. All samples were run on an LSRII or FACSCanto (BD Biosciences) and live gated, singlet populations analyzed using FlowJo software (TreeStar).
Cell sorting, adoptive transfers, and infections
Splenocytes and LN cells from RAG2p-GFP Tg OT-I TCR Tg mice were first enriched with a no-touch method using a CD8 T cell isolation kit (StemCell Technologies), and Fc receptors were blocked with anti-CD16/32 (clone 2.4G2) before cells were sorted to >98% purity as GFP+ (RTEs) or GFP− (mature naïve or MN) NK1.1−CD4−Ter119−CD11b−B220− (dump gate) CD44loCD62Lhi cells. Sorted RTEs and MN T cells were counted, mixed 1:1 and unless otherwise noted, 104 total cells were injected i.v. into sex-matched congenic hosts. Where indicated, cells were labeled in the presence of 2.5–5µM CFSE for 10 min at 37°C to enable quantification of cell proliferation. The dim GFP signal from RTEs does not interfere with this analysis. One day after T cell transfer, adoptive hosts were infected i.v. with 2000 CFU of mid log phase (0.3–0.7 OD600) Listeria monocytogenes engineered to express chicken OVA (Lm.OVA), originally a gift from the Shen laboratory (11). For rechallenge experiments, 1–5x106 PFU of vesicular stomatitis virus engineered to express OVA (VSV.OVA) (12) were injected i.v. For secondary transfers, spleens were processed on d73 post-infection, analyzed for donor cell composition, and 9x106 cells injected into hosts that were infected 1d later with VSV.OVA. Donor cells were analyzed from secondary hosts 6 and 60 days after reinfection. In vivo killing assays were performed as described (13). Briefly, 3x103 RTEs or MN T cells were transferred into separate mice, and 7d days later, Lm.OVA infected or uninfected hosts received 106 SIINFEKL-pulsed splenocytes labeled with 2.5µM CFSE and 106 unpulsed targets labeled with 0.05µM CFSE. The percent killing at the 1 hour time point was calculated as: 100 − [(% peptide pulsed in infected/% unpulsed in infected)/(% peptide pulsed in uninfected/% unpulsed in uninfected)] × 100).
Statistics
As indicated, paired or unpaired 2-tailed Student’s t tests were used for comparisons and p<0.05 was considered statistically significant. Unless otherwise noted, * = p<0.05; ** = p<0.01; *** = p<0.001; **** = p<0.0001.
Results and Discussion
Greater burst size and prolonged blood residency characterize the RTE response to infection
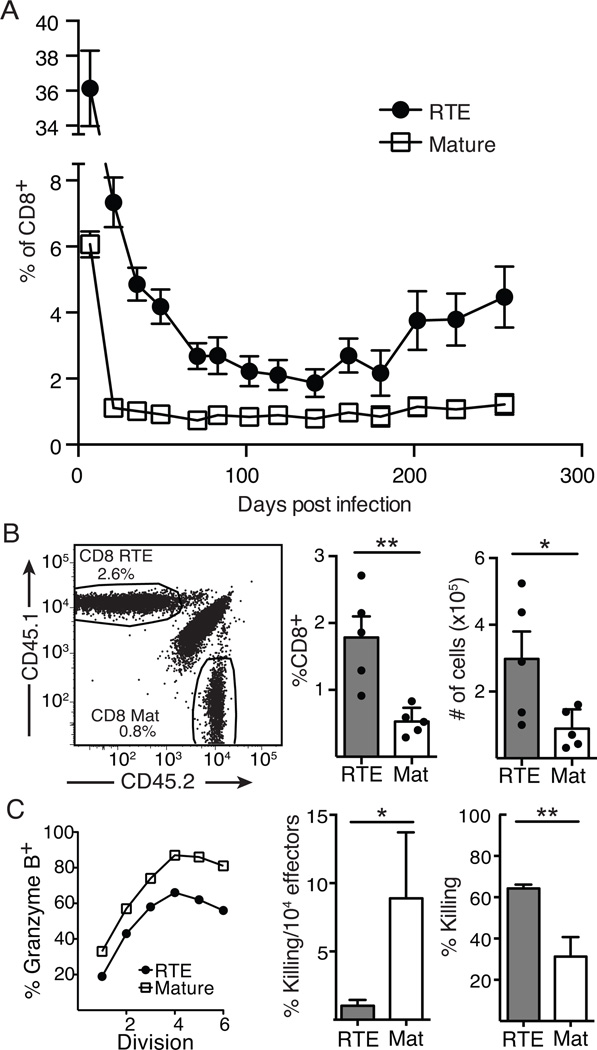
Expansion and memory formation by RTE- and MN-derived T cells were examined in recipient mice that were infected with Lm.OVA 1 day after co-transfer of equal numbers of CD8+ RTEs and MN T cells sorted from RAG2p-GFP Tg OT-I TCR Tg mice. At 7 days post infection, RTE-derived cells were present in 6-fold greater numbers than MN-derived donor T cells in the blood (Fig. 1A), consistent with previous data indicating RTE-derived CD8+ T cells have increased clonal expansion compared to their mature counterparts (6). MN-derived T cells represented about 1% of the total peripheral blood CD8+ T cells from 21 days post infection onward (Fig. 1A). RTE-derived cells remained in circulation for a longer period, reaching stable levels at about 60 days post infection, and gradually drift upward, outnumbering MN-derived T cells by a factor of 2–3 fold for >250 days after Lm.OVA infection (Fig. 1A). This increase in RTE-derived memory cells was also observed in the spleen, where RTEs outnumbered MN T cells by about 3-fold 60 days post infection (Fig. 1B). These data indicate that after infection, RTEs have a greater burst size than mature T cells and RTE-derived memory cells preferentially remain in the circulating and splenic memory pool.
Figure 1. Greater burst size, distinct contraction kinetics, and decreased per responder killing capacity characterize the RTE response to Listeria infection.
A and B: 5x103 each of OT-I TCR Tg RTEs and MN T cells were co-transferred into congenic hosts that were infected the following day with Lm.OVA. A) Blood was analyzed on days 7, 21, 28, 35, 71, 83, 102, 119, 141, 161, 180, 202, 225, and 254 post-infection to determine the percent of CD8+ T cells comprised of each donor population. Data are presented as mean ± SEM and compiled from 4 independent transfers; n=6–21. For all time points, p values vary from < 0.01 to <0.0001 by paired Student’s t test. B) Spleen cells were analyzed on d60 post-infection. Representative congenic marker gating on CD8+ T cells (left) and proportion (middle) and absolute number (right) of RTE-derived and mature (Mat) T cells. Data are presented as mean ± SEM and compiled from 2 independent experiments; n=5. C) Left panel: 106 each of OT-I TCR Tg RTEs and MN T cells were labeled with CFSE and co-transferred into congenic hosts that were infected the next day with Lm.OVA and 5 days later, granzyme B expression in donor-derived splenocytes was determined as a function of the number of divisions. Data are from cells from 1 of 3 representative mice. Middle and right panels: 3x103 OT-I TCR Tg RTEs or MN T cells were transferred into separate congenic hosts that were infected the following day with Lm.OVA, and 7 days later, 106 CFSEhi peptide-pulsed and CFSElo unpulsed splenocytes were transferred i.v. Percent target cell killing was determined 1 hour later in the spleen for RTE and mature effectors (right). At the same time point, target cell killing was normalized to the number of splenic OT-I Tg effectors (middle). Data are presented as mean ± SD from one of two representative experiments, analyzed using unpaired Student’s t test.
The large RTE burst compensates for their defective per responder killing capacity
We next determined whether the increased numbers of RTEs generated during a primary infection were sufficient to reverse the effects of their diminished effector cytokine production (6, 7). To address this, CFSE-labeled OT-I Tg RTEs and MN T cells were transferred into mice that were then infected with Lm.OVA. Five days later, a lower proportion of RTEs were granzyme B+ than mature T cells at each division (Fig. 1C, left). Representative flow plots (Supplemental Fig 1) reveal minimal differences in the mean fluorescence intensity (MFI) of granzyme B staining in RTEs and mature T cells, suggesting that any influence on staining levels caused by degranulation are minimal at this time point. On a per responder cell basis, RTE-derived effector cells lysed fewer OVA peptide-pulsed splenocytes (Fig. 1C, middle). However, the per host complement of RTEs killed the bolus of transferred target cells even more efficiently than their mature counterparts (Fig. 1C, right). Thus, RTEs have reduced levels of granzyme B and are poor at lysing target cells, but their abundance allows them to maintain a population level effector function that is equal to or better than that of their mature counterparts.
Decreased Tcm generation by RTE-derived memory T cells
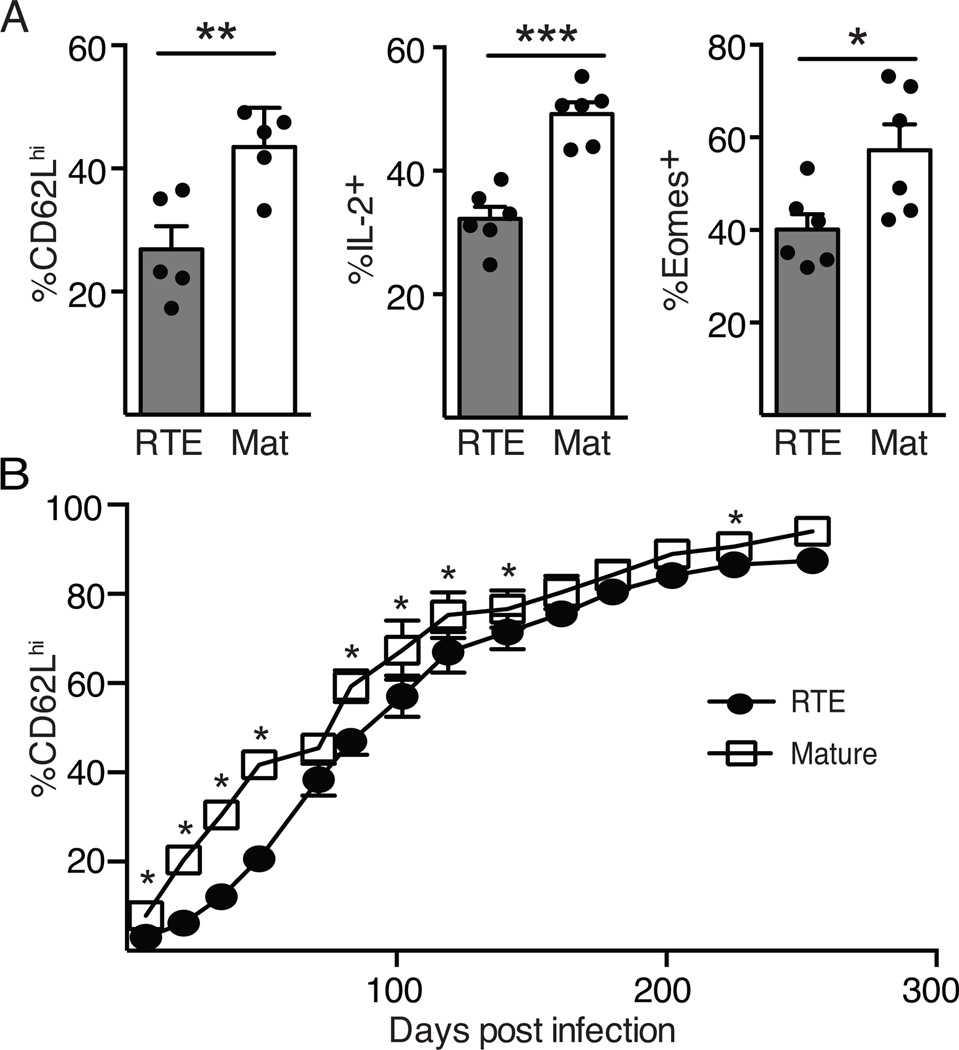
After infection, a subset of CD8+ T cells develops a central memory (Tcm) phenotype, characterized by increased expression of the LN homing molecules CD62L and CCR7, increased production of IL-2, and enhanced reexpansion potential (10). The increased number of RTEs in the circulation suggests these cells may be slower to acquire a Tcm phenotype and localize to LNs. This hypothesis is supported by previous data demonstrating that fewer RTEs acquire a memory precursor phenotype during acute infection (6, 7). To measure conversion to a Tcm phenotype, expression of CD62L was analyzed on RTE- and MN-derived splenocytes 60 days after infection. As T cells entered the memory phase, RTE-derived cells were slower to upregulate CD62L than MN-derived cells (Fig. 2A, left; see Supplemental Fig 2A for representative flow plots). CD62L expression was diminished in RTEs both in the proportion of positive cells (Fig 2A) and in the per cell expression levels (Supplemental Fig. 2B). Further phenotypic comparison of RTE- and MN-derived memory CD8+ T cells indicated that the former were reduced in their ability to produce IL-2 after in vitro stimulation (Fig. 2A, middle). Expression of Eomes, a transcription factor required for Tcm development (14), was also reduced in RTEs by proportion (Fig. 2A, right) and by MFI (Supplemental Fig. 2B). Analysis of T cells in the blood indicated that it took 85 days after infection for 40% of RTE-derived memory cells to convert to a CD62Lhi phenotype, while mature T cells reached this point at 50 days (Fig. 2C). The failure of RTE-derived memory cells to efficiently reexpress CD62L persisted for greater than 120 days even after infection with Lm.OVA expressing low affinity altered peptide ligands (data not shown), to which RTEs respond relatively better than mature T cells (6). These data indicate RTEs are slower than mature T cells to acquire a Tcm phenotype. This slow Tcm conversion parallels our earlier findings that naïve CD8+ RTEs are IL-7Rαlo (1) and that in Listeria infected hosts, responding RTEs are more prone than mature T cells to differentiate into KLRG1hiIL-7Rαlo short-lived effectors (6,7). It would be informative to determine whether forced expression of IL-7R through transgenesis alters the kinetics of Tcm conversion in RTE-derived effector cells.
Figure 2. Decreased Tcm generation by RTE-derived memory T cells.
5x103 each of OT-I TCR Tg RTEs and MN T cells were co-transferred into congenic hosts that were infected the following day with Lm.OVA. A) Splenocytes were analyzed on d60 post-infection. Shown are the percent of RTE and mature CD8+ T cells expressing CD62L and Eomes directly ex vivo and IL-2 production after peptide restimulation. Data are presented as mean ± SEM and are compiled from 2–4 independent experiments. B) Blood was analyzed at the same time points as in Figure 1A to determine the percent of RTE-derived and mature T cells that were CD62Lhi. Data are presented as mean ± SEM and are compiled from 4 independent transfers; n=6–21. * p values vary from <0.05 to < 0.0001 by paired Student’s t test.
Reduced BM localization of RTE-derived memory T cells
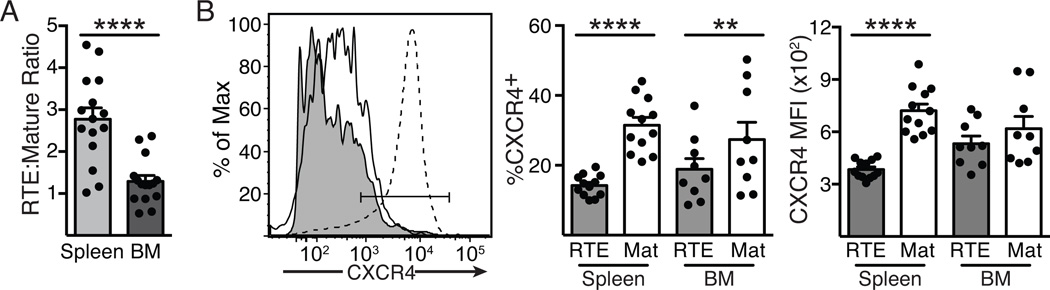
One feature of Tcm is their ability to preferentially home to the BM, where they access homeostatic cytokines such as IL-7 and IL-15 and undergo increased proliferation compared to cells in other lymphoid organs (15, 16). Loss of Eomes is associated with reduced CXCR4 expression and reduced BM localization (14). We assessed the BM homing capabilities of RTE- and MN-derived memory CD8+ T cells 60 days after infection, and found that both populations were present at similar frequencies, in contrast to the spleen, in which RTE-derived T cells outnumber their MN counterparts by approximately 2.8-fold (Fig. 3A). We next measured the expression on RTE- and MN-derived memory T cells of the chemokine receptor CXCR4, which is upregulated on memory T cells and plays an important role in recruiting CD8+ T cells into the BM (17). The percentage of RTEs expressing CXCR4 in the spleen and even the BM was significantly reduced when compared to their mature counterparts (Fig. 3B). Furthermore, the level of CXCR4 expression per cell, as measured by MFI, was also significantly reduced in splenic RTEs, though not in those in the BM, perhaps a result of that chemokine receptor’s role in cellular recruitment to this site. These data demonstrate that RTE-derived memory cells express lower levels of CXCR4 and accordingly, show reduced recruitment to or retention within the BM. It therefore became important to test whether reduced BM residency impacted the maintenance of long-term memory among RTE-derived T cells.
Figure 3. Impaired BM localization by RTE-derived memory T cells.
5x103 each of OT-I TCR Tg RTEs and MN T cells were co-transferred into congenic hosts that were infected the following day with Lm.OVA; spleen and BM were analyzed 60 days post-infection. A) The ratio of RTE- and mature donor cell-derived CD8+ T cells in the spleen and BM. B) The left panel depicts representative CXCR4 staining of RTE-derived (filled histogram) and mature (open histogram) donor T cells from the BM, using neutrophils (dotted histogram) as a positive control. Data in the right panels show the mean ± SEM of the %CXCR4+ and the MFI of CXCR4 staining, compiled from 3 independent experiments; n=9–12.
Rechallenged RTE-derived memory T cells recapitulate the primary response
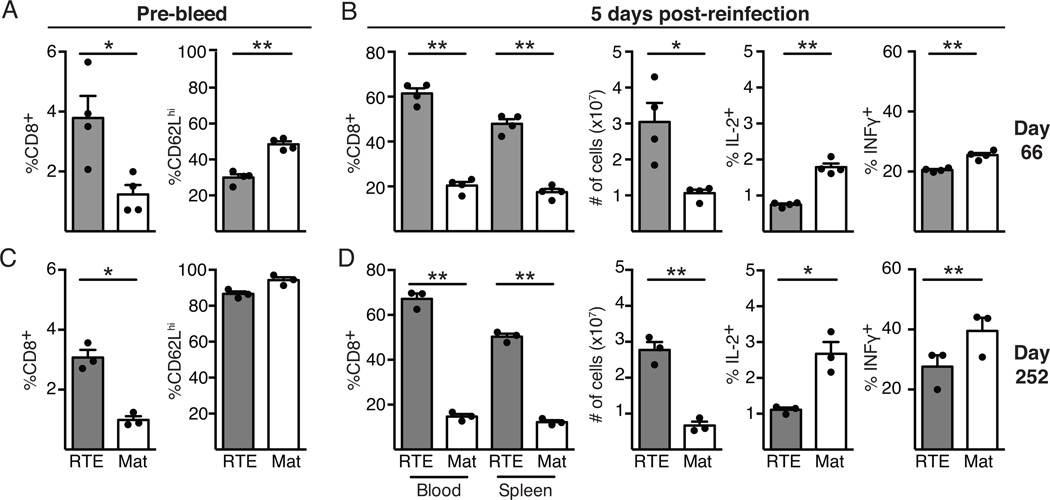
CD8+ Tcm cells are thought to be superior in their ability to reexpand upon secondary challenge, whereas effector memory T cells (Tem) are capable of exhibiting more immediate effector function (10). The developing RTE-derived memory pool is defective in its ability to generate Tcm when compared to MN-derived memory T cells, suggesting that upon rechallenge, RTE-derived memory cells might be hindered in their reexpansion. To examine the recall potential of RTE- and MN-derived memory T cells, mice that had received OT-I T cells and were primed with Lm.OVA 66 days earlier (and in which RTEs outnumber their mature counterparts but express less CD62L, Fig. 4A) were infected with VSV.OVA. Surprisingly, the reexpansion of RTE-derived memory T cells was similar to that of MN-derived T cells 5 days after VSV.OVA challenge (Fig. 4B, left panels). Functionally, RTE-derived memory T cells were imprinted with the same deficiencies observed upon initial antigen encounter, including reduced production of IL-2 and IFNγ (Fig. 4B, right panels). The MFI for cytokine (IL-2, IFNγ, and TNFα) production was also lower for RTEs, indicating the defect was measurable both in terms of the proportion of cytokine producing cells and in the amount of cytokine produced per cell (data not shown). Other mice were rechallenged with VSV.OVA at 252 days after primary infection, when RTE-derived memory T cells still outnumber their MN-derived counterparts but have similar expression of CD62L in the blood (Fig. 4C). Again, RTE-derived memory cells expanded at least as well as MN-derived T cells (Fig. 4D, left panels) but continued to display defects in cytokine production, with a lower percentage of RTEs producing IFNγ and IL-2 (and TNFα, not shown) compared to their mature counterparts (Fig. 4D, right panels). These data indicate the impaired transition of RTEs to Tcm and their diminished BM localization do not trigger a corresponding defect in the magnitude of their recall response, but that reduced cytokine production by RTE-derived T cells persists during secondary expansion. The fact that RTE-derived memory cells show reduced cytokine production >250 days after initial priming is striking, given that RTEs transition into the mature T cell pool after ~3 weeks of residence in the lymphoid periphery. Clearly, the stage of maturation at which peripheral T cells first meet antigen has a lasting impact on their immune function.
Figure 4. Rechallenged RTE-derived memory T cells recapitulate the primary response.
5x103 each of OT-I TCR Tg RTEs and MN T cells were co-transferred into congenic hosts that were infected the following day with Lm.OVA. Mice were then infected with VSV.OVA 66 or 252 days later, as indicated. A) and C) Blood was analyzed prior to VSV.OVA infection. B) and D) At 5 days post secondary infection, blood and splenocytes were analyzed to determine the proportion of RTE- and mature-derived donor cells (left panel), and splenocytes were analyzed for the absolute number (2nd panel) of RTE- and mature-derived T cells and for their IL-2 and IFNγ production after in vitro restimulation (right 2 panels). Data are presented as mean ± SEM and statistical significance assessed using a paired Student’s t test.
The expansion driven by reinfection is similar in RTE- and MN-derived memory populations
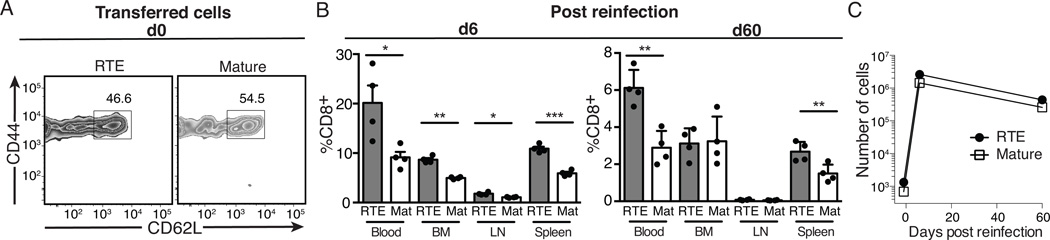
To definitively determine if the skewed Tcm phenotype of RTEs alters expansion and cytokine production after secondary infection, we isolated RTE- and MN-derived memory CD8+ T cells from mice at 73 days post infection and transferred them into naïve animals. At this time point, RTE-derived memory cells displayed reduced expression of CD62L (Fig. 5A) when compared to mature T cells. After T cell transfer, mice were infected with VSV.OVA and T cells were analyzed at 6 and 60 days post secondary infection. At day 6, RTEs outnumbered mature donor cells in the blood, spleen, LN, and BM (Fig. 5B, left) and both RTE and MN-derived memory cells expanded approximately 2000-fold (Fig. 5C). At 60 days post infection, RTEs continued to outnumber MN-derived secondary memory cells in the blood and spleen, but both were present at equal numbers in the LN and BM (Fig. 5B, right), as seen during primary memory.
Figure 5. The clonal expansion driven by reinfection is similar in RTE- and MN-derived memory T cell populations.
5x103 each of OT-I TCR Tg RTEs and MN T cells were co-transferred into congenic hosts that were infected the following day with Lm.OVA. A) At d73 post infection, spleen cells were enriched for CD8 T cells and the 2 donor cell types analyzed for their memory cell phenotype. 9.2x106 splenocytes (including 7x103 mature- and 1.3x104 RTE-derived memory T cells) were transferred into naïve recipients that were infected with VSV.OVA 1d later. B) At days 6 and 60 post secondary infection, RTE- and mature T cell-derived memory T cells were quantified in recipient blood, BM, LN, and spleen. Data are presented as mean ± SEM and statistical significance assessed using a paired Student’s t test. C) The total number of donor cells in the spleen, plus peripheral LNs, plus 1 ml of blood was calculated at the indicated time points. The fold increase from d0 to d6, assuming 10% original engraftment, was approximately 1970 for RTEs and 2100 for mature T cells.
We have demonstrated that compared to mature T cells, CD8+ RTEs proliferate more readily, remain in the circulation for an extended period of time, and exhibit a short-lived effector phenotype. However, this is at the expense of their conversion into Tcm, as RTEs fail to efficiently express Eomes, produce less IL-2, and home to the BM poorly when compared to mature T cells. These data would predict that upon secondary challenge, RTEs would be outcompeted by MN-derived memory T cells; however, RTEs proliferated at least as well as mature T cells during secondary challenge. The ability of RTEs to proliferate more robustly than MN T cells has been attributed to increased surface expression of the TCR and CD3 and enhanced TCR signal transduction (6, 18); this could persist into the memory phase, allowing RTE-derived memory cells that are skewed toward a Tem phenotype to proliferate as well as MN-derived memory cells that are Tcm skewed. Hypermethylation of the Il2 promoter is observed in CD4+ RTEs and may limit IL-2 production (19), epigenetic alterations that likely persist through multiple rounds of stimulation and may also regulate cytokine production by CD8+ RTEs. Overall, these data suggest RTEs maintain an effector phenotype and patrol the body for a prolonged period, which would provide a substantial benefit in neonatal and other lymphopenic settings to ensure pathogen clearance and a more immediate response to reinfection. The unique biology of RTEs reflects a program that unfolds when the cells initially encounter antigen and extends for >8 months thereafter, allowing this distinct cellular compartment to maintain enhanced readiness without a concomitant loss in proliferative capacity.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by NIH Grant R01 AI 064318 (to P.J.F.) and Predoctoral Training Grant AI 106677 (to A.M.B.)
Abbreviations
- B6
C57Bl/6
- BM
bone marrow
- Eomes
eomesodermin
- Lm.OVA
Listeria monocytogenes engineered to express chicken ovalbumin
- LN
lymph node
- MFI
mean fluorescence intensity
- MN
mature naïve
- RTE
recent thymic emigrant
- Tcm
central memory T cell
- Tem
effector memory T cell
- Tg
transgenic
- VSV.OVA
vesicular stomatitis virus engineered to express chicken OVA
References
- 1.Boursalian TE, Golub J, Soper DM, Cooper CJ, Fink PJ. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 2004;5:418–425. doi: 10.1038/ni1049. [DOI] [PubMed] [Google Scholar]
- 2.Hogquist KA, Xing Y, Hsu FC, Shapiro VS. T cell adolescence: Maturation events beyond positive selection. J. Immunol. 2015;195:1351–1357. doi: 10.4049/jimmunol.1501050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fink PJ. The biology of recent thymic emigrants. Ann. Rev. Immunol. 2013;31:31–50. doi: 10.1146/annurev-immunol-032712-100010. [DOI] [PubMed] [Google Scholar]
- 4.Hendricks DW, Fink PJ. Recent thymic emigrants are biased against the T-helper type 1 and toward the T-helper type 2 effector lineage. Blood. 2011;117:1239–1249. doi: 10.1182/blood-2010-07-299263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opiela SJ, Koru-Sengul T, Adkins B. Murine neonatal recent thymic emigrants (RTE) are phenotypically and functionally distinct from adult RTE. Blood. 2009;113:5635–5643. doi: 10.1182/blood-2008-08-173658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berkley AM, Fink PJ. Cutting Edge: CD8+ recent thymic emigrants exhibit increased responses to low-affinity ligands and improved access to peripheral sites of inflammation. J. Immunol. 2014;193:3262–3266. doi: 10.4049/jimmunol.1401870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makaroff LE, Hendricks DW, Niec RE, Fink PJ. Postthymic maturation influences the CD8 T cell response to antigen. Proc. Natl. Acad. Sci. USA. 2009;106:4799–4804. doi: 10.1073/pnas.0812354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith NL, Wissink E, Wang J, Pinello JF, Davenport MP, Grimson A, Rudd BD. Rapid proliferation and differentiation impairs the development of memory CD8+ T cells in early life. J. Immunol. 2014;193:177–184. doi: 10.4049/jimmunol.1400553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priyadharshini B, Welsh RM, Greiner DL, Gerstein RM, Brehm MA. Maturation-dependent licensing of naive T cells for rapid TNF production. PLoS One. 2010;5:e15038. doi: 10.1371/journal.pone.0015038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaech SM, Cui W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 2012;12:749–761. doi: 10.1038/nri3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope C, Kim SK, Marzo A, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J. Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 12.Turner MJ, Jellison ER, Lingenheld EG, Puddington L, Lefrancois L. Avidity maturation of memory CD8 T cells is limited by self-antigen expression. J. Exp. Med. 2008;205:1859–1868. doi: 10.1084/jem.20072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber D, Wherry E, Ahmed R. Cutting Edge: Rapid in vivo killing by memory CD8 T cells. J. Immunol. 2003;171:27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 14.Banerjee A, Gordon S, Intlekofer A, Paley M, Mooney E, Lindsten T, Wherry E, Reiner S. Cutting Edge: The transcription factor eomesodermin enables CD8+ T cells to compete for the memory cell niche. J. Immunol. 2010;185:4988–4992. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker T, Coley S, Wherry E, Ahmed R. Bone marrow is a preferred site for homeostatic proliferation of memory CD8 T cells. J. Immunol. 2005;174:1269–1273. doi: 10.4049/jimmunol.174.3.1269. [DOI] [PubMed] [Google Scholar]
- 16.Parretta E, Cassese G, Barba P, Santoni A, Guardiola J, Di Rosa F. CD8 cell division maintaining cytotoxic memory occurs predominantly in the bone marrow. J. Immunol. 2005;174:7654–7664. doi: 10.4049/jimmunol.174.12.7654. [DOI] [PubMed] [Google Scholar]
- 17.Mazo IB, Honczrenko M, Leung H, Cavanagh LL, Bonasio R, Weninger W, Engelke K, Xia L, McEver RP, Koni PA, Silberstein LE, von Andrian UH. Bone marrow is a major reservoir and site of recruitment for central memory CD8 T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Fink PJ, Hendricks DW. Post-thymic maturation: young T cells assert their individuality. Nat. Rev. Immunol. 2011;11:544–548. doi: 10.1038/nri3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berkley AM, Hendricks DW, Simmons KB, Fink PJ. Recent thymic emigrants and mature naïve T cells exhibit differential DNA methylation at key cytokine loci. J. Immunol. 2013;190:6180–6186. doi: 10.4049/jimmunol.1300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.