Abstract
Background
Patients with advanced heart failure may persist for prolonged times with persistent hemodynamic abnormalities; intermediate and long-term outcomes of these patients are unknown.
Methods and Results
We used ESCAPE trial data to examine characteristics and outcomes of patients with invasive hemodynamic monitoring during an acute heart failure hospitalization. Patients were stratified by final measurement of cardiac index (CI; L/min/m2) and pulmonary capillary wedge pressure (PCWP; mmHg) before catheter removal. The study groups were CI ≥ 2/PCWP <20 (n = 74), CI ≥ 2/PCWP ≥ 20 (n = 37), CI < 2/PCWP < 20 (n = 23), and CI < 2/PCWP ≥ 20 (n = 17). Final CI was not associated with the combined risk of death, cardiovascular hospitalization, and transplantation (HR:1.03, 95% CI:0.96–1.11 per 0.2 L/min/m2 decrease, p=0.39), but final PCWP ≥ 20mmHg was associated with increased risk of these events (HR:2.03, 95% CI:1.31–3.15, p<0.01), as was higher final right atrial pressure (RAP; HR:1.09, 95% CI:1.06–1.12 per mmHg increase, p<0.01).
Conclusion
Final PCWP and final RAP were stronger predictors of post-discharge outcomes than CI in patients with advanced heart failure. The ability to lower filling pressures appears to be more prognostically important than improving CI in the management of patients with advanced heart failure.
ClinicalTrials.gov Identifier
Keywords: heart failure, edema, cardiogenic shock
Introduction
In the United States, heart failure affects over 5 million people and results in over 1 million hospitalizations per year.1 In patients age 65 and older, there are more hospitalizations for a primary diagnosis of heart failure than any other condition.2 While many patients have evidence of poor perfusion on admission,3 volume overload is the most common reason for hospitalization for heart failure.4–6 Even with in-patient treatment, many patients are discharged with signs and symptoms of persistent congestion.4 Despite optimal therapy for heart failure, morbidity and mortality following hospitalization remain high.6, 7
Invasive hemodynamic measurements of cardiac index (CI) and left ventricular filling pressure are commonly used to characterize the clinical phenotype of patients with advanced heart failure. Patients with heart failure may remain in a hemodynamic state consistent with cardiogenic shock and congestion for prolonged periods of time. However, data on the impact of persistent hemodynamic abnormalities are limited. The Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial enrolled patients hospitalized for acute heart failure, with at least one sign and one symptoms of congestion, and collected information from invasive hemodynamic assessments. The ESCAPE data provides an ideal population from which to assess associations between hemodynamic measurements and outcomes. Therefore, we examined morbidity and mortality outcomes of patients with advanced heart failure based upon hemodynamic variables obtained during an acute heart failure hospitalization.
Methods
ESCAPE Trial
The ESCAPE trial was a multicenter randomized controlled trial evaluating the effectiveness of pulmonary artery catheter (PAC) in the management of patients hospitalized with severe symptomatic heart failure with reduced ejection fraction. The trial was conducted at 26 sites from 2000 to 2003. Patients were eligible for the study if they had three months of symptoms despite treatment with an ACE inhibitor and diuretics and had at least one sign and one symptom of congestion. Patients were required to have a left ventricular ejection fraction ≤ 30% and systolic blood pressure ≤ 125mmHg. Exclusion criteria included creatinine level ≥ 3.5mg/dL, prior use of dobutamine or dopamine ≥3μg/kg/min, or prior use of milrinone during hospitalization. Four hundred thirty-three patients from 26 centers were randomized to receive therapy guided by clinical assessment alone or clinical assessment and data from a PAC. Of the 215 patients randomized to PAC, 141 (65.6%) had complete hemodynamic and follow-up data at 6 months, and were included in this analysis. Ten patients who were not randomized to PAC had hemodynamic data and were included in this analysis. Hemodynamic measurements from the PAC were recorded at baseline and serially at least twice daily until the catheter was removed (median 48 hours). All hemodynamic measurements were performed at rest. Follow-up occurred after hospital discharge at 1–2 weeks, then at 1, 2, 3, and 6 months. The primary endpoint was days to death, cardiac transplantation, or cardiac hospitalization in the 6 months following randomization. Results of the ESCAPE trial have been published previously.8
Classification and Definitions
For the present study, we included patients with complete hemodynamic data and follow up (N=151). Treatment goals in the PAC group included resolution of signs and symptoms of congestion, pulmonary capillary wedge pressure (PCWP) ≤ 15mmHg, and right atrial pressure ≤ 8mmHg. Final measurements were defined as the last recorded measurements prior to PAC removal. Patients were stratified by final measurements of CI (CI < 2, CI ≥ 2 L/min/m2) and pulmonary capillary wedge pressure (PCWP < 20, PCWP ≥ 20 mmHg). The cutoffs for CI and PCWP were chosen to reflect the severity of poor perfusion and congestion in this patient population, and have been used previously to define shock or the need for invasive hemodynamic monitoring.9, 10
Statistics
Demographics, physical and laboratory findings, medical history, and therapies were summarized as frequencies and percentages for categorical variables and by the medians with 25th and 75th percentiles for continuous variables. Baseline characteristics were compared using the Kruskal-Wallis test for continuous variables, and chi-square or Fisher’s exact tests for categorical variables. Event rate curves for the primary endpoint in the four hemodynamic groups were shown using unadjusted Kaplan-Meier estimates and compared with log-rank tests. Relationships between baseline characteristics or hemodynamic measurements and 6-month mortality, cardiovascular hospitalization, or transplant were tested with univariable Cox proportional hazards regression models. Hazard ratios (HRs) with corresponding 95% confidence intervals (CIs) are presented for baseline and final hemodynamic measures as well as significant baseline patient characteristics. Statistical significance was assessed using 2-sided P values. A P value <0.05 was considered statistically significant. All statistical computations were generated using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
Table 1 shows the baseline characteristics of the study population. Of 151 patients, 74 (49.0%) had final CI ≥ 2/PCWP <20 (warm and dry), 37 (24.5%) had final CI ≥ 2/PCWP ≥ 20 (warm and wet), 23 (15.2%) had final CI < 2/PCWP < 20 (cold and dry), and 17 (11.3%) had final CI < 2/PCWP ≥ 20 (cold and wet). Patients with the most abnormal final hemodynamic measurements (low CI and high PCWP) were more likely to have ischemic etiology and other comorbidities including peripheral vascular disease, cerebrovascular disease, and diabetes. They also had the shortest baseline 6 minute walk distance compared with the other groups. Those with a persistently reduced CI were older, and there was a higher percentage of female patients with persistently reduced CI than with a normal final CI. Patients with a final PCWP <20mmHg were more likely to be female, non-white, and have higher baseline blood pressure and lower baseline creatinine.
Table 1.
Baseline Characteristics of the Study Population*
| Variable | Preserved Cardiac Index (≥2 L/min/m2) | Reduced Cardiac Index (<2 L/min/m2) | p | ||
|---|---|---|---|---|---|
| PCWP < 20 (N=74) | PCWP ≥ 20 (N=37) | PCWP < 20 (N=23) | PCWP ≥ 20 (N=17) | ||
| Age, years | 56 (47 – 66) | 54 (49 – 66) | 67 (49 – 71) | 60 (49 – 64) | 0.45 |
| Gender, female | 23 (31.1) | 5 (13.5) | 12 (52.2) | 5 (29.4) | 0.02 |
| Race, non white | 34 (45.9) | 10 (27.0) | 13 (56.5) | 5 (29.4) | 0.07 |
| Ischemic Etiology | 35 (47.3) | 20 (54.1) | 12 (52.2) | 13 (76.5) | 0.19 |
| Past Medical History | |||||
| Angina pectoris | 28 (37.8) | 10 (27.0) | 3 (13.0) | 8 (47.1) | 0.07 |
| Myocardial infarction | 32 (43.2) | 22 (59.5) | 8 (34.8) | 12 (70.6) | 0.05 |
| PTCI‡ | 16 (21.6) | 11 (29.7) | 5 (21.7) | 8 (47.1) | 0.17 |
| CABG | 19 (25.7) | 14 (37.8) | 5 (21.7) | 5 (29.4) | 0.49 |
| Peripheral vascular disease | 7 (9.5) | 5 (13.5) | 2 (8.7) | 6 (35.3) | 0.06 |
| COPD | 11 (14.9) | 5 (13.5) | 5 (21.7) | 4 (23.5) | 0.67 |
| Diabetes | 21 (29.2) | 12 (33.3) | 7 (30.4) | 7 (41.2) | 0.81 |
| Hypertension | 37 (50.0) | 18 (48.6) | 11 (47.8) | 7 (41.2) | 0.93 |
| ICD | 21 (28.4) | 11 (29.7) | 4 (17.4) | 4 (23.5) | 0.71 |
| Atrial fibrillation | 17 (23.0) | 17 (45.9) | 8 (34.8) | 5 (29.4) | 0.10 |
| Ventricular tachycardia/fibrillation | 11 (14.9) | 8 (21.6) | 3 (13.0) | 3 (17.6) | 0.79 |
| Cerebrovascular disease | 8 (10.8) | 7 (18.9) | 5 (21.7) | 4 (23.5) | 0.31 |
| Renal insufficiency† | 5 (6.8) | 3 (8.1) | 0 (0) | 1 (6.7) | 0.68 |
| Physical Exam | |||||
| BMI, kg/m2 | 27.9 (23.9 – 33.5) | 27.7 (24.1 – 33.4) | 24.4 (21.3 – 28.4) | 28.4 (24.2 – 32.1) | 0.12 |
| Baseline heart rate, bpm | 81 (70 – 93.5) | 79 (72 – 88) | 84 (76 – 93) | 74.5 (64.5 – 93) | 0.37 |
| Baseline SBP, mmHg | 109 (95 – 120) | 98 (94 – 108) | 110 (99 – 125) | 98 (90 –116) | 0.05 |
| Baseline DBP, mmHg | 68 (60 – 76) | 64 (57 – 70) | 70 (61 – 84) | 64 (58 – 70) | 0.17 |
| Baseline Testing | |||||
| Baseline EF, % | 20 (15 – 25) | 20 (15 – 22) | 15 (15 – 20) | 19 (15 – 20) | 0.07 |
| 6 min walk distance, feet | 249 (0 – 650) | 390 (0 – 650) | 360 (0 – 650) | 50 (0 – 725) | 0.80 |
| Sodium, mEq/L | 137 (136 – 140) | 136 (134 – 139) | 138 (136 – 141) | 136 (131 – 138) | 0.05 |
| Creatinine, mg/dL | 1.4 (1 – 1.8) | 1.6 (1.3 – 2) | 1.3 (1 – 1.5) | 1.5 (1.2 – 2) | 0.12 |
| BUN, mg/dL | 27 (17 – 41) | 33 (24 – 51) | 25 (20 – 31) | 31 (28 – 43) | 0.12 |
| ALT, units/L | 25.5 (18 – 37.5) | 24 (18 – 34) | 34 (22 – 59) | 24 (22 – 38) | 0.43 |
| AST, units/L | 29 (21 – 41) | 27 (22 – 34) | 30 (24 – 49) | 29 (25 – 31) | 0.61 |
| Albumin, g/dL | 3.5 (3.3 – 3.8) | 3.8 (3.30 – 4.10) | 3.4 (3.1 – 3.7) | 3.7 (3.5 – 3.9) | 0.18 |
| Total bilirubin, mg/dL | 0.7 (0.40 – 1.10) | 1.1 (0.6 – 1.4) | 1.1 (0.70 – 1.40) | 0.4 (0.3 – 1.1) | 0.02 |
Presented as N (%) or median (25th, 75th percentile).
Past medical history of renal insufficiency defined as history of creatinine >3.5mg/dL or history of chronic dialysis. Current creatinine >3.5mg/dL was an exclusion criterion for the study.
Abbreviations: ALT: alanine aminotransferase, AST: aspartate aminotransferase, BMI: body mass index, CABG: coronary artery bypass graft, COPD: chronic obstructive pulmonary disease, DBP: diastolic blood pressure, EF: ejection fraction, ICD: implantable cardioverter defibrillator, PCI: percutaneous coronary intervention, PVD: peripheral vascular disease, SBP: systolic blood pressure
Patients with a low CI and high PCWP at the end of the study had the highest right atrial pressure, pulmonary artery pressure, and PCWP at baseline. Conversely, patients with the most favorable final hemodynamic measurements (higher CI and lower PCWP) were most likely to have a lower right atrial pressure, pulmonary artery pressure, and PCWP at baseline. (Figure 1, Table 2) Patients with an elevated final PCWP had higher baseline right atrial pressure, pulmonary artery pressure, and PCWP compared to patients with a lower final PCWP. Patients with residual low CI had a higher baseline right atrial pressure and PCWP, and lower baseline CI, regardless of final PCWP.
Figure 1.
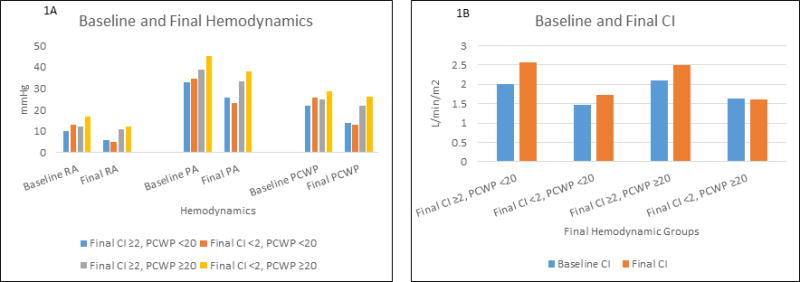
Bar Graph of Baseline and Final Median Hemodynamic Pressure and Cardiac Index Measurements by Group
Panel A shows the median baseline and final hemodyamic pressure measurements for patients stratified by final hemodynamic measurements.
Panel B shows the median baseline and final cardiac index measurements for patients stratified by final hemodynamic measurements.
Table 2.
Baseline and Final Hemodynamics of the Study Population*
| Variable | Preserved Cardiac Index (≥2 L/min/m2) | Reduced Cardiac Index (<2 L/min/m2) | p | ||
|---|---|---|---|---|---|
| PCWP < 20 (N=74) | PCWP ≥ 20 (N=37) | PCWP < 20 (N=23) | PCWP ≥ 20 (N=17) | ||
| Baseline Hemodynamics | |||||
| RAP, mmHg | 10 (6 – 15) | 12 (8 – 20) | 13 (8 – 18) | 17 (12.5 – 20) | 0.01 |
| PA mean, mmHg | 33 (26 – 41) | 39 (33 – 46) | 34.5 (32 – 44) | 45 (31.5 – 49) | 0.04 |
| CI, L/min/m2 | 2.0 (1.8 – 2.4) | 2.1 (1.7 – 2.4) | 1.5 (1.2 – 1.8) | 1.6 (1.4 – 1.9) | <0.001 |
| CO, L/min | 3.9 (3.1 – 4.7) | 4 (3.6 – 5.1) | 2.9 (2.4 – 3.2) | 3.23 (2.7 – 4.0) | <0.001 |
| SVR, dynes x sec/cm2 | 1322 (1116–1631) | 1162 (921–1440) | 1923 (1350–2088) | 1546 (1464–2003) | <0.001 |
| PCWP, mmHg | 22 (16 – 27) | 25 (21 – 36) | 25.5 (20 – 30) | 28.5 (24 – 33.5) | <0.001 |
| Final Hemodynamics | |||||
| RAP, mmHg | 6 (4 – 10) | 11 (9 – 15) | 5 (4 – 8) | 12 (9 – 20) | <0.001 |
| PA mean, mmHg | 25.5 (22 – 30) | 33.5 (30 – 39) | 23 (20 – 32) | 38 (33 – 40) | <0.001 |
| CI, L/min/m2 | 2.6 (2.2 – 2.8) | 2.5 (2.3 – 2.9) | 1.73 (1.5 – 1.9) | 1.60 (1.5 – 1.9) | by definition |
| CO, L/min | 4.81 (4.3 – 5.6) | 5.10 (4.58 – 5.9) | 3.18 (2.61 – 3.6) | 3.3 (2.8 – 3.9) | <0.001 |
| SVR, dynes x sec/cm2 | 1083 (813 – 1207) | 867 (568 – 1022) | 1735 (1490–1903) | 1446 (1213–1748) | <0.001 |
| PCWP, mmHg | 14 (10 – 17) | 22 (21 – 24) | 13 (11 – 15) | 26 (23 – 30) | by definition |
Presented as median (25th, 75th percentile).
Abbreviations: CI: cardiac index, CO: cardiac output, PA: pulmonary artery, PCWP: pulmonary capillary wedge pressure, RAP: right atrial pressure, SVR: systemic venous resistance
Supplemental Tables 1 and 2 show pairwise comparisons between those with CI < 2 and CI ≥ 2 and those with PCWP < 20 and PCWP ≥ 20 for baseline characteristics and hemodynamic measurements, and medication use, respectively. Supplemental Table 3 presents medication use in the patient groups stratified by hemodynamic profiles. The hemodynamic profile was not significantly associated with baseline medications, drugs used during the hospitalization, or discharge medications.
Supplemental Table 4 presents in-hospital complications and procedures. Few patients experienced in-hospital complications or underwent cardiac procedures. While patients with a high PCWP and normal CI were more likely to have ventricular tachyarrhythmias and receive cardiopulmonary resuscitation and cardioversion, those with a high PCWP and low CI were most likely to have ischemia or angina and receive mechanical circulatory support with intra-aortic balloon pump or left ventricular assist device.
In follow-up, 34 patients died, 60 were rehospitalized, and 9 underwent cardiac transplantation (Table 3). Variables associated with increased risk of mortality, cardiovascular hospitalization, or cardiac transplant included abnormal baseline and final right- and left-sided filling pressures, abnormal renal function, and COPD, while variables associated with decreased risk of adverse events included higher baseline sodium, higher baseline blood pressure, and ACE inhibitor use (Figure 2). Final CI was not associated with the combined risk of death, cardiovascular hospitalization, or cardiac transplantation (HR 1.03, 95% CI 0.96–1.11 per 0.2 L/min/m2 decrease, p=0.39). Conversely, final PCWP ≥ 20 mmHg was univariably associated with increased morbidity and mortality (HR 2.03, 95% CI 1.31–3.15, p<0.01), as was final right atrial pressure (HR 1.09, 95% CI 1.06–1.12 per mmHg increase, p<0.01). Figure 3 presents the unadjusted association between final hemodynamic measurements and the combined outcomes of death, cardiac hospitalization, and cardiac transplantation.
Table 3.
Follow-up Outcomes of Study Population
| Final Hemodynamics | Death | Cardiovascular hospitalization | Heart transplant |
|---|---|---|---|
| CI ≥ 2 L/min/m2, PCWP <20 mmHg (N=74) | 10 (13.5%) | 23 (31.1%) | 3 (4.1%) |
| CI ≥ 2 L/min/m2, PCWP ≥ 20 mmHg (N=37) | 12 (32.4%) | 20 (54.1%) | 4 (1.1%) |
| CI <2 L/min/m2, PCWP <20 mmHg (N=23) | 5 (21.7%) | 11 (47.8%) | 1 (4.3%) |
| CI <2 L/min/m2, PCWP ≥ 20 mmHg (N=17) | 7 (41.2%) | 6 (35.3%) | 1 (5.9%) |
|
| |||
| ALL PCWP <20mmHg (N=97) | 15 (15.5%) | 34 (35.1%) | 4 (4.1%) |
| ALL PCWP ≥ 20mmHg (N=54) | 19 (35.2%) | 26 (48.1%) | 5 (9.3%) |
| ALL CI ≥ 2 L/min/m2 (N=111) | 22 (19.8%) | 43 (38.7%) | 7 (6.3%) |
| ALL CI <2 L/min/m2 (N=40) | 12 (30.0%) | 17 (42.5%) | 2 (5.0%) |
Abbreviations: CI: cardiac index, PCWP: pulmonary capillary wedge pressure
Figure 2.
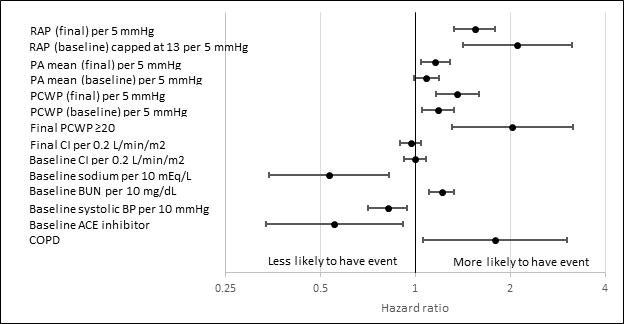
Univariate Associations with Death or Cardiac Hospitalization or Cardiac Transplant
Figure 3.
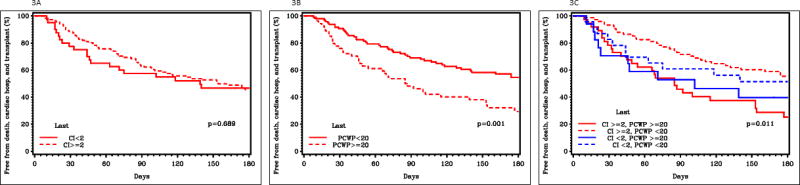
Kaplan-Meier estimates of freedom from death, cardiac hospitalization, and cardiac transplantation.
Panel A shows the Kaplan-Meier estimates of freedom from death, cardiac hospitalization, and cardiac transplantation for patients with final CI < 2 L/min/m2 and final CI ≥ 2 L/min/m2.
Panel B shows the Kaplan-Meier estimates of freedom from death, cardiac hospitalization, and cardiac transplantation for patients with final PCWP < 20 mmHg and final PCWP ≤ 20 mmHg.
Panel C shows the Kaplan-Meier estimates of freedom from death, cardiac hospitalization, and cardiac transplantation for patients with final CI ≥ 2 L/min/m2 and PCWP ≥ 20 mmHg, CI ≥ 2 L/min/m2 and PCWP < 20 mmHg, CI < 2 L/min/m2 and PCWP ≥ 20 mmHg, and CI < 2 L/min/m2 and PCWP < 20 mmHg.
Discussion
The role of hemodynamic perturbation is central to our understanding of heart failure physiology. Reduced contractility leads to reduced stroke volume, which in turn leads to increased heart rate, increased filling pressures, and increased vasoconstriction. These compensatory mechanisms become maladaptive and ultimately lead to increased myocardial oxygen demand and worsening cardiac function.11 In its most advanced stages, heart failure is characterized by elevated intracardiac filling pressures, peripheral vasoconstriction, and decreased cardiac output. These hemodynamic alterations indirectly form the basis of targeted pharmacotherapy. While hemodynamic abnormalities in heart failure may persist despite optimal medical treatment, data on the impact of persistent hemodynamic abnormalities on intermediate-term morbidity and mortality outcomes are limited.5, 12, 13 We demonstrate that baseline hemodynamics tend to predict the hemodynamic profile following medical therapy. More importantly, persistently elevated right- and left-sided filling pressures in patients with heart failure during a heart failure hospitalization is predictive of the combined risk of death, cardiovascular hospitalization, and heart transplantation whereas resting CI has less prognostic utility.
In this study the combined primary endpoint was driven by rehospitalizations, which accounted for more than half of the events. Furthermore, the mortality rate for those with persistent congestion was more than double that of patients who achieved adequate congestion. Persistent congestion and symptoms may have been the basis for the rehospitalizations, given that most patients hospitalized with heart failure present with dyspnea.7 Taken in the context of prior studies that have shown that hospitalizations are associated with increased mortality in the heart failure population and that the risk of death increases with repeated hospitalizations, these findings highlight the importance assessing for and managing congestion in patients with acute heart failure.14–16
Prior studies that have shown that the presence of congestion is associated with adverse outcomes, including heart failure hospitalization and death.3, 10, 17–20 It is also recognized that a significant proportion of patients hospitalized for volume overload are inadequately decongested at the time of discharge, and persistent congestion is associated with worse outcomes.12, 21 In addition, prior work has shown that a change in cardiac index with treatment is not predictive of poor outcomes.10, 13 Our findings confirm these prior findings using invasive hemodynamic data. Furthermore, by categorizing patients by both PCWP and CI, we extend the prior findings by showing that congestion is associated with worse outcomes independent of CI.
While we found that resting CI is not associated with outcomes, prior work has shown that using resting CI in conjunction with exercise testing is predictive of outcomes.22–24 In our study, it appears that congestion is the driver of adverse outcomes in this patient population; however, low CI likely contributes in that it may be more difficult to achieve adequate diuresis in patients with a low CI. Notably, patients with persistent congestion had lower blood pressure and worse renal function at baseline. Poor perfusion may lead to impaired renal function which limits the bioavailability of diuretics; furthermore, hypoperfusion resulting from low blood pressure often reduces the tolerability of decongestion and vasodilator strategies.
The downstream effects of congestion on other organs may be another mechanism by which persistently congested patients have worse outcomes. Several studies have shown interactions between renal function and congestion. Prior work from Metra and colleagues showed persistent congestion in the setting of worsening renal function in acute heart failure was associated with worse outcomes compared to worsening renal function alone.25 Additionally, in a prior analysis from ESCAPE, renal insufficiency at baseline and discharge were associated with increased risk of death and rehospitalization. The results could not be explained by low cardiac output; however, a correlation between right atrial pressure and renal function was noted, suggesting that elevated filling pressures may have played a role.26
Despite the differences, patients with low final PCWP and high final PCWP were treated similarly with regard to baseline, in-hospital, and discharge medications. Relatively few patients experienced inhospital complications or underwent cardiac procedures to treat low CI or elevated intracardiac filling pressures. This may reflect the lack of supportive treatments that result in sustained improvements in CI during the time period the ESCAPE study was conducted. While inotropes can temporarily augment cardiac output, they provide no long term positive effects on cardiac recovery or remodeling, and are associated with increased mortality.27–29 And while temporary mechanical circulatory support (MCS) can help sustain a patient in the short-term, the benefits do not persist once the device is removed.30, 31 Furthermore, availability of durable MCS as a long term therapy did not develop until after completion of ESCAPE.32–35 While there is a paucity of short-term treatment strategies to improve long-term CI, it appears, based on this study, that the driver of outcomes is not in the ability to improve CI, but to improve filling pressures.
Initiation of inotropic support and referral for consideration of advanced heart failure therapies is often driven by low CI and advanced therapies may be withheld in the setting of preserved CI. However, congestion, regardless of CI, may be an additional target for agents that increase contractility or devices that directly unload the left ventricle to lower PCWP.
Clinical Implications
Results of this analysis confirm that many patients have persistent hemodynamic abnormalities despite treatment aimed at reversing these abnormalities. While persistently low CI and persistently high PCWP or high right atrial pressure are all associated with poor outcomes, it appears that persistent volume overload is a stronger predictor of worse outcomes in a heart failure population compared with CI.
Importantly, though invasive hemodynamic testing was used to determine hemodynamic profiles in this study, clinician assessments of hemodynamics based on history and physical exam findings have also been shown to predict outcomes.3, 17, 36 Therefore, these results may be able to be extended to patients without invasive hemodynamic measurements.
In the care of patients with advanced heart failure, choosing when to abort temporary measures, such as inotropes or temporary mechanical support, for more permanent solutions, like durable LVADs or transplantation, can be a difficult decision. This study suggests that the inability to effectively achieve a more normal intravascular volume status may be a harbinger of poor outcomes; therefore persistent congestion may represent an important clinical sign that in addition to other clinical characteristics may help to inform the decision on when to move forward with advanced heart failure therapies.
Limitations
There are several limitations of this study. First, this study was a retrospective analysis. Second, only 151 patients in the ESCAPE trial had complete hemodynamic data and thorough follow-up, limiting the sample size for the study. Given the overall limited sample size, the number of patients in each group was small. Furthermore, due to the small sample size and few number of events, a multivariable analysis could not be done. Third, while most patients hospitalized for heart failure have congestion, the entry criteria for this trial required it, so patients were only included in this study if they had one sign and one symptom of congestion, potentially influencing the importance of congestion for prognosis in this cohort. Furthermore, patients with worse final hemodyanmics may have been more likely to be referred to transplant or had a lower threshould for rehospitalization given that it was known that they were sicker. Fourth, the ESCAPE trial was designed to evaluate an acute heart failure population in which there was clinical equipoise with regard to PAC use. Therefore, patients deemed “too sick” or “too well” were not included. It is possible that persistent hemodynamic derangements have different effects on outcomes for those patients not captured in the trial. Also, treatment strategies were not specified in the trial. While all centers participating in the ESCAPE trial were experienced in the management of advanced heart failure, patients may have received different treatments for similar hemodynamic profiles. Finally, treatment options for the advanced heart failure population has changed in the time period between the ESCAPE trial and this analysis, specifically with the increased use of durable MCS devices.
Conclusion
Time to death, cardiovascular hospitalization, or transplant was not influenced by CI whereas elevated right- and left-sided filling pressures were associated with this endpoint. PCWP was a stronger predictor of worse outcomes than CI in patients with advanced heart failure within six months of hospitalization. Our study suggests the ability to lower filling pressures appears to be more prognostically important than improving CI in the management of patients with advanced heart failure.
Supplementary Material
Highlights.
We performed an analysis using ESCAPE data of patients with invasive hemodynamic data.
We examined outcomes of HF patients with persistent hemodynamic abnormalities.
Final PCWP was associated with adverse outcomes, but final CI was not.
Acknowledgments
Funding Source:
ESCAPE was funded by contract N01-HV-98177 from the NHLBI to Duke University Medical Center. Dr. Cooper is supported by grant T32HL069749-11A1 from the National Institute of Health.
Footnotes
Disclaimer:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures:
The authors report no relevant conflicts of interest related to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. National Hospital Discharge Survey. Natl Health Stat Report. 2006;2008:1–20. [PubMed] [Google Scholar]
- 3.Nohria A, Tsang SW, Fang JC, Lewis EF, Jarcho JA, Mudge GH, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–804. doi: 10.1016/s0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 4.O’Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Card Fail. 2005;11:200–5. doi: 10.1016/j.cardfail.2004.08.160. [DOI] [PubMed] [Google Scholar]
- 5.Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119:S3–s10. doi: 10.1016/j.amjmed.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. The EuroHeart Failure survey programme-- a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24:442–63. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 7.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, et al. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE) Am Heart J. 2005;149:209–16. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, et al. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625–33. doi: 10.1001/jama.294.13.1625. [DOI] [PubMed] [Google Scholar]
- 9.Landry DW, Levin HR, Gallant EM, Ashton RC, Jr, Seo S, D’Alessandro D, et al. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122–5. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson LW, Tillisch JH, Hamilton M, Luu M, Chelimsky-Fallick C, Moriguchi J, et al. Importance of hemodynamic response to therapy in predicting survival with ejection fraction less than or equal to 20% secondary to ischemic or nonischemic dilated cardiomyopathy. Am J Cardiol. 1990;66:1348–54. doi: 10.1016/0002-9149(90)91166-4. [DOI] [PubMed] [Google Scholar]
- 11.Hollenberg SM, Kavinsky CJ, Parrillo JE. Cardiogenic shock. Ann Intern Med. 1999;131:47–59. doi: 10.7326/0003-4819-131-1-199907060-00010. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J. 2013;34:835–43. doi: 10.1093/eurheartj/ehs444. [DOI] [PubMed] [Google Scholar]
- 13.Fonarow GC. The treatment targets in acute decompensated heart failure. Rev Cardiovasc Med. 2001;2(Suppl 2):S7–s12. [PubMed] [Google Scholar]
- 14.Solomon SD, Dobson J, Pocock S, Skali H, McMurray JJ, Granger CB, et al. Influence of nonfatal hospitalization for heart failure on subsequent mortality in patients with chronic heart failure. Circulation. 2007;116:1482–7. doi: 10.1161/CIRCULATIONAHA.107.696906. [DOI] [PubMed] [Google Scholar]
- 15.Lum HD, Studenski SA, Degenholtz HB, Hardy SE. Early hospital readmission is a predictor of one-year mortality in community-dwelling older Medicare beneficiaries. J Gen Intern Med. 2012;27:1467–74. doi: 10.1007/s11606-012-2116-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Setoguchi S, Stevenson LW, Schneeweiss S. Repeated hospitalizations predict mortality in the community population with heart failure. Am Heart J. 2007;154:260–6. doi: 10.1016/j.ahj.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 17.Drazner MH, Rame JE, Stevenson LW, Dries DL. Prognostic importance of elevated jugular venous pressure and a third heart sound in patients with heart failure. N Engl J Med. 2001;345:574–81. doi: 10.1056/NEJMoa010641. [DOI] [PubMed] [Google Scholar]
- 18.Sachdeva A, Horwich TB, Fonarow GC. Comparison of usefulness of each of five predictors of mortality and urgent transplantation in patients with advanced heart failure. Am J Cardiol. 2010;106:830–5. doi: 10.1016/j.amjcard.2010.04.045. [DOI] [PubMed] [Google Scholar]
- 19.Fonarow GC, Stevenson LW, Steimle AE, Hamilton MA, Moriguchi JD, Walden JA, et al. Persistently high left-ventricular filling pressures predict mortality despite angiotensin-converting enzyme-inhibition in advanced heart failure. Circulation. 1994;90:488–488. [Google Scholar]
- 20.Fonarow G, Hamilton M, Moriguchi J, Creaser J, et al. Hemodynamic predictors of clinical outcomes in decompensated advanced heart failure. J Card Fail. 2001;3:13. [Google Scholar]
- 21.Lucas C, Johnson W, Hamilton MA, Fonarow GC, Woo MA, Flavell CM, et al. Freedom from congestion predicts good survival despite previous class IV symptoms of heart failure. Am Heart J. 2000;140:840–7. doi: 10.1067/mhj.2000.110933. [DOI] [PubMed] [Google Scholar]
- 22.Patel CB, DeVore AD, Felker GM, Wojdyla DM, Hernandez AF, Milano CA, et al. Characteristics and outcomes of patients with heart failure and discordant findings by right-sided heart catheterization and cardiopulmonary exercise testing. Am J Cardiol. 2014;114:1059–64. doi: 10.1016/j.amjcard.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Methvin A, Georgiopoulou VV, Kalogeropoulos AP, Malik A, Anarado P, Chowdhury M, et al. Usefulness of Cardiac Index and Peak Exercise Oxygen Consumption for Determining Priority for Cardiac Transplantation. Am J Cardiol. 2010;105:1353–1355. doi: 10.1016/j.amjcard.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 24.Myers J, Wong M, Adhikarla C, Boga M, Challa S, Abella J, et al. Cardiopulmonary and noninvasive hemodynamic responses to exercise predict outcomes in heart failure. J Card Fail. 2013;19:101–7. doi: 10.1016/j.cardfail.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 25.Metra M, Davison B, Bettari L, Sun H, Edwards C, Lazzarini V, et al. Is worsening renal function an ominous prognostic sign in patients with acute heart failure? The role of congestion and its interaction with renal function. Circ Heart Fail. 2012;5:54–62. doi: 10.1161/CIRCHEARTFAILURE.111.963413. [DOI] [PubMed] [Google Scholar]
- 26.Nohria A, Hasselblad V, Stebbins A, Pauly DF, Fonarow GC, Shah M, et al. Cardiorenal interactions: insights from the ESCAPE trial. J Am Coll Cardiol. 2008;51:1268–74. doi: 10.1016/j.jacc.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 27.Cuffe MS, Califf RM, Adams KF, Jr, Benza R, Bourge R, Colucci WS, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–7. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 28.Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM, et al. Effect of oral milrinone on mortality in severe chronic heart failure. N Engl J Med. 1991;325:1468–75. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 29.Felker GM, O’Connor CM. Inotropic therapy for heart failure: An evidence-based approach. Am Heart J. 2001;142:393–401. doi: 10.1067/mhj.2001.117606. [DOI] [PubMed] [Google Scholar]
- 30.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–96. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- 31.Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, et al. Intra-aortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock (IABP-SHOCK II): final 12 month results of a randomised, open-label trial. Lancet. 2013;382:1638–45. doi: 10.1016/S0140-6736(13)61783-3. [DOI] [PubMed] [Google Scholar]
- 32.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–51. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 33.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–96. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 34.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–43. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 35.Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 36.Drazner MH, Hellkamp AS, Leier CV, Shah MR, Miller LW, Russell SD, et al. Value of clinician assessment of hemodynamics in advanced heart failure: the ESCAPE trial. Circ Heart Fail. 2008;1:170–7. doi: 10.1161/CIRCHEARTFAILURE.108.769778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


