Abstract
Background
The establishment of the range of reference values and associated variations of two-dimensional speckle-tracking echocardiography (2DSTE) derived left ventricular (LV) strain is a prerequisite for its routine clinical adoption in pediatrics. The aims were to perform a meta-analysis of normal ranges of LV global longitudinal, circumferential, and radial strain (GLS, GCS, and GRS) measurements derived by 2DSTE in children and identify confounding factors that may contribute to variances in reported measures.
Methods
A systematic review was launched in Medline, Embase, Scopus, CINAHL, and Cochrane. Search hedges were created to cover the concepts of pediatrics, speckle-tracking echocardiography, and left heart ventricle. Two investigators independently identified and included studies if they reported the 2DSTE derived LV GLS, GCS or GRS. The weighted mean was estimated by using random-effects with 95% confidence interval (CI), heterogeneity was assessed by the Cochran's Q statistic and the inconsistency index (I2) and publication was evaluated using the Egger test. Effects of demographic (age), clinical, and vendor variables were assessed in a meta-regression.
Results
The search identified 2325 children from 43 data sets. The reported normal mean values of GLS among the studies varied from -16.7% to -23.6% (mean -20.2%, 95% CI -19.5% to -20.8%), GCS varied from -12.9% to -31.4% (mean -22.3%, 95% CI -19.9% to -24.6%) and GRS, varied from 33.9% to 54.5 % (mean 45.2 95% CI 38.3 to 51.7). 26 studies reported LS only from the apical 4-chamber view with a mean of -20.4%, (95% CI -19.8% to -21.7%). 23 studies reported CS (mean, -20.3%, 95% CI -19.4% to -21.2%) and RS (mean, 46.7%, 95% CI 42.3% to 51.1%) from the short axis view at the mid-ventricular level. A significant apex-to-base segmental longitudinal strain (SLS) gradient (P < .01) was observed in the LV free wall. There was significant between- study heterogeneity and inconsistency (I2 > 94% and P < .001 for each strain measure), which was not explained by age, gender, body surface area, blood pressure, heart rate, frame rate, FR/HR ratio tissue-tracking methodology, location of reported strain value along the strain curve, ultrasound equipment, or software. These metaregression showed that these effects were not significant determinants of variations among normal ranges of strain values. There was no evidence of publication bias (P = .40).
Conclusions
This study defined reference values of 2DSTE derived LV strain in children on the basis of a meta-analysis. In healthy children, the mean LV global longitudinal strain value is -20.2%, (95% CI -19.5% to -20.8%), mean global circumferential strain -22.3%, (95% CI -19.9% to -24.6%), and mean global radial strain is 45.2%, (95% CI 38.3% to 51.7%). LV segmental longitudinal strain has a stable apex-to-base gradient that is preserved throughout maturations. Although variations among different reference ranges in this meta-analysis were not dependent on differences in demographic, clinical, or vendor parameters, we established age- and vendor- specific referenced ranges as well.
Keywords: Left Ventricle, cardiac function, Global Strain, Speckle Tracking Echocardiography, Children
Introduction
Left ventricular (LV) function is an important prognostic determinant of cardiopulmonary pathologies in children.1-3 The LV myocardium has a complex architecture and consists of circumferential fibers in the mid-wall layer and longitudinal fibers in the endocardial and epicardial layers.4,5 This results in inhomogeneous and complex contraction patterns, as the myofiber orientation changes continuously from right-handed helix in subendocardium to left- handed helix in subepicardium.4-6 LV deformation comprises radial thickening, circumferential shortening, and longitudinal shortening and myocardial strain describes this deformation under an applied force.2,6 Specifically, two-dimensional speckle tracking echocardiography (2DSTE) is an angle-independent method for myocardial strain measurement that has been used to estimate deformation measures and quantitatively characterize LV function in children.7-71
Clinical application of cardiac strain by 2DSTE to measure LV function in children requires knowledge of the range of normal values.72 The use of strain imaging to assess LV systolic and diastolic function in healthy children and children with specific cardiac conditions have recently reported measures of normal global and segmental longitudinal, circumferential and radial strain and strain rate.7-71 Measurements of myocardial strain imaging are subject to “physiologic variation” depending on patient demographics (age, gender, race) clinical factors (HR, blood pressure, weight or body surface area), as well as equipment and image technique variables (ultrasound and vendor customized software, probe size, tissue tracking methodology, location of reported strain value along the strain curve, frame rate, and FR/HR ratio).1,73,74 Similar to Yingchoncharoen et al's. meta analysis on the normal ranges of LV strain in adults, and our own meta-analysis on normal ranges of RV strain in children, we sought to define a range of normal LV strain measures by utilizing a compilation of all studies that reported values for normal or control children cohorts.1,73 These reference values and associated variations of the deformation measures need to be “firmly established before routine clinical adoption” of LV strain measurements can be implemented in children.1,72,73
The objectives of this study were to perform a meta-analysis of normal ranges of LV global longitudinal, circumferential, and radial strain (GLS GCS, GRS) measurements derived by 2DSTE in children and identify factors that may contribute to differences in reported measures.
Methods
Search Strategy/Search Protocol
L.H.Y., A.H., and S.Y., the medical librarians at Washington University School of Medicine (Saint Louis, Missouri) trained in systematic reviews, created search hedges to cover the concepts of pediatrics/children, speckle tracking echocardiography, and left heart ventricles using terms harvested from standard term indices and on-topic articles (Appendix 1). To exclude animals, LHY used the Human filter for PubMed recommended in Cochrane Handbook for Systematic Reviews of Interventions, and then employed that as a model created by SY to create similar filters for the other searched databases.75 The search strategy was launched in Medline, Embase, Scopus, CINAHL (Cumulative Index of Nursing and Allied Health Literature), CENTRAL (Cochrane Central Register of Controlled Trials), and ClinicalTrials.gov. Searches were completed by November 2015. References of all selected manuscripts were screened to identify additional studies.
Study Selection/ Eligibility criteria
Studies were included if they reported using strain derived by 2DSTE to measure LV function in healthy pediatric normal or control subjects. Studies that exclusively included children < 21 years of age were considered eligible for the meta-analysis. The systematic review incorporated observational studies that used pediatric control groups with normal results on echocardiography (who were recruited for specific studies) or if healthy children were the primary objective.7-71 Studies were excluded if they were review articles or abstracts only without full text.
LV GLS and strain rate (GLSr) from a 17- or 18- segment model (calculated from segmental averaging of the three apical views, apical 4-, 3-, and 2- chambers) were included in this meta-analysis. Global CS and GRS, calculated from segmental averaging of the short axis views at the apical, mid-ventricular, and basal levels, were also included in the meta-analysis. In addition, we also evaluated the LV free wall longitudinal strain measures (FWLS), and included segmental longitudinal strain (SLS) at the apex, mid, and basal ventricular levels of the LV free wall from segmental averaging of the three apical views. Clinically, longitudinal strain (LS) is also reported from the weighted average of the six segments from the apical four-chamber view and circumferential and radial strain (CS and RS respectively) is reported from weighted average of the six segments from the mid-ventricular level at the papillary muscle.74,76 We therefore stratified our meta-analysis by the different methods, “Global” strain (GLS, GCS, GRS) and “Six-segment method” (LS, CS, RS) of reporting LV strain and incorporated the manuscripts that reported these different methods in the meta-analysis to account for the different techniques utilized between studies.
Data Collection
Each eligible article meeting the inclusion criteria was reviewed by two independent reviewers (P.T.L and A.M.), and the following data was extracted and entered into an electronic database: (1) Study: first and last authors, year of publication; (2) demographic: number of controls subjects, age, gender; (3) Clinical: (heart rate (HR), body surface area (BSA) or body metabolic index (BMI); and (4) echocardiographic parameters: (vendor customized ultrasound and model, vendor customized software and version, probe frequency, frame rate, frame rate to heart rate ratio (FR/HR),77 tissue tracking methodology, (endomyocardial, epicardium to endocardium), and reported location of the strain value along the stain curve (systolic strain, end systolic strain, or post-systolic strain).74 All the authors of the eligible studies were contacted by electronic mail to notify them of the meta-analysis and obtain any missing information not reported in their individual studies.
Quality Assessment
To assess the quality and reporting of studies, two reviewers (P.T.L. and A.M.) independently assessed 12 items that were considered relevant to this meta-analysis topic, based on the quality assessment methodology of Downs et al. and previously validated by our group, (Appendix 3).73,78
Statistical Analysis/Data Synthesis
Meta-analysis was performed using STATA version IC 13 (StataCorp LP, College Station, TX). The means and 95% confidence intervals (CI) of strain measures were computed using random-effects models weighted by inverse variance. Between-study statistical heterogeneity was assessed using the Cochran Q statistic and was quantified using the I2 method by measuring inconsistency (I2, the percentage of total variance across studies attributable to heterogeneity rather than chance). These results were presented as a forest plot, according to our previous described methodology.73,75 The forest plot was used as a graphical display of the relative strength of the effect estimates and CIs for each of the individual studies and the entire meta-analysis.73,75 Publication bias was assessed using funnel plots and the Egger test.75 The funnels plots are also presented according to our previously described methodology and combined with the Egger test to provide a quantitative evaluation of publication bias.73 The sources of the variation between the studies were sought using metaregression to estimate the percent of heterogeneity on the influence of the variation in normal strain measurements.1,73,75
Results
Eligibility Criteria
The search identified 603 articles. After excluding duplicates and triplicates articles (180), there were 423 studies screened for relevance. Articles not exclusively in children (73), articles unrelated to the topics (92), abstracts without text or reviews (178), and articles that did not have data on controls or normal children (15) were then excluded. Searching the bibliographies did not reveal any additional results. No ongoing studies were found in the clinical trials registries. Sixty-five published observational or case control studies met inclusion criteria (Figure 1). All the studies that met search criteria were in English, although the search criteria were not only limited to English manuscripts.
Figure 1. Process of inclusion of studies in the meta-analysis.
Electronic communication
The first or last authors of each of the eligible 65 studies were contacted by electronic mail. There were 20 manuscripts that used the same control dataset in multiple published studies,50-69 and two studies did not have the raw data available.70,71 In total, 43 datasets of strain measures from 63 manuscripts of strain measures with 2325 children were considered eligible for assessment in the meta-analysis (Figure 2).7-49 Forty out of 43 authors (93%) responded and provided either the raw strain data and/or filled in the missing information on study quality and potential sources of variability. The remaining three data sets (7%) from the authors that did not respond via electronic mail were included with the available information provided in their respective manuscripts. Authors that used the same control dataset in multiple published studies were consulted, and one control dataset was either provided or chosen from one manuscript based on author recommendation,50-69(Table 1 and Table 2). Specific correspondence regarding the handling of the use of the same control dataset in multiple published studies from the same authorship groups is described in Appendix 2.50-69
Figure 2.
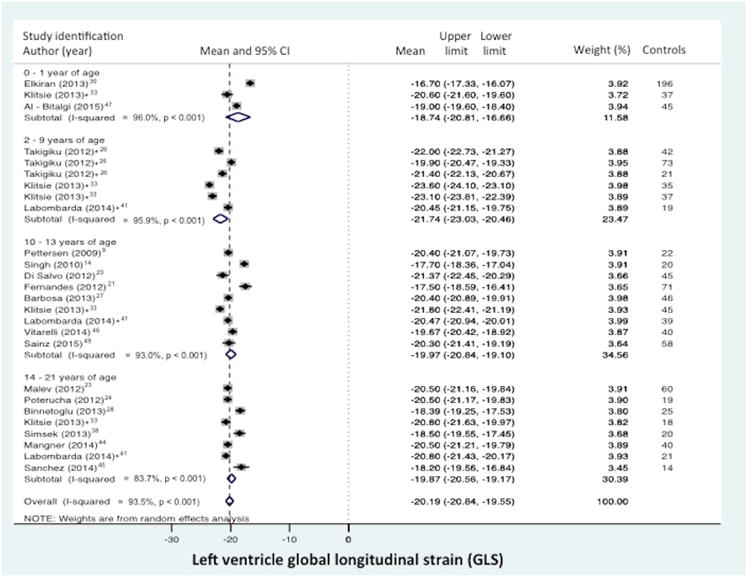
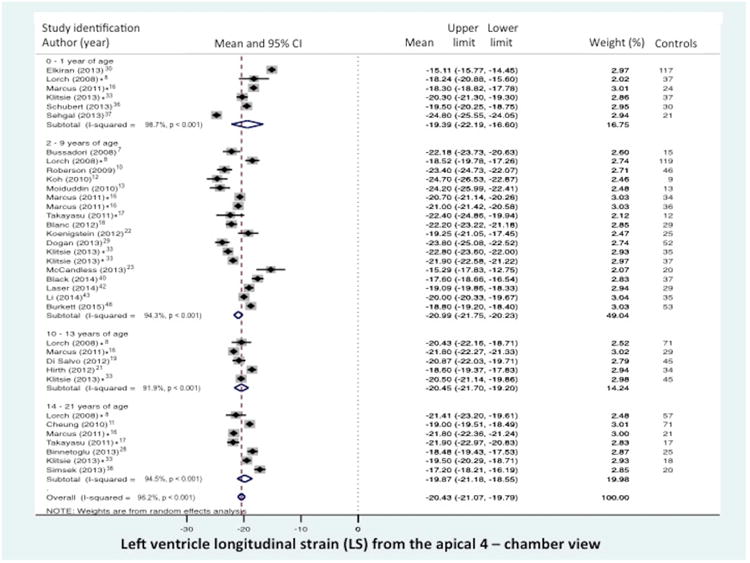
Normal value of LV global longitudinal strain (GLS) stratified by age distribution and view. (A) LV “global” LS derived from the segmental averaging of the three apical views and (B) LV LS from the apical 4-chamber view only. The forest plot lists the names of the included studies by age distribution and in chronological order, the mean and confidence intervals with the upper (95%) and lower (5%) limits. Each study is represented by a square that reflects the mean at the point estimate of effect and is proportional to the study's weight in the meta-analysis. A horizontal line extending from either side of the square reflects the 95% confidence interval. The overall meta-analysis measure of effect is plotted as a diamond with the lateral points of the diamond indicating confidence intervals for this mean estimate.73 *Klitsie et al., Labombarda et al., Lorch et al., Marcus et al., Takayasu et al., and Takigiku et al. all performed a cross-sectional study and reported strain values for multiple mean age groups from birth to 21 years of age.8,16,17,26,33,41
Table 1. Study Description and patient demographic and clinical characteristics.
| Study | Year | n | Mean age (y) | Female (%) | HR Mean ± SD | SBP | BSA Mean | GLS(%) | GCS(%) | GRS(%) | Control Selection | Disease studied |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bussadori et al.7 | 2008 | 15 | 8.2 | 53 | NR | NR | NR | Yes | Yes | No | Normal | Healthy children |
| Lorch et al.8 | 2009 | 284 | 8.7 | 44 | 88 ± 28 | Yes | 1.07 | Yes | No | No | Normal | Healthy children |
| Pettersen et al.9 | 2009 | 22 | 12.7 | 36 | NR | Yes | 1.44 | Yes | Yes | No | Normal | TGA |
| Roberson et al.10 | 2009 | 46 | 8.5 | 57 | 96 ± 34 | NR | 1.09 | Yes | No | No | Normal | Systolic dysfunction |
| Cheung et al. 11 | 2010 | 44 | 16.4 | 53 | NR | NR | NR | Yes | Yes | Yes | Normal | Anthracyclin therapy |
| Koh et al. 12 | 2010 | 9 | 5.5 | 78 | NR | NR | 0.81 | Yes | Yes | No | Normal | LVNC |
| Moiduddin et al.13 | 2010 | 13 | 5.7 | 46 | 88 ± 11 | NR | NR | Yes | No | No | Normal | LV function |
| Singh et al.14 | 2010 | 20 | 12.9 | 55 | 75 ± 15 | Yes | 1.22 | Yes | Yes | No | Normal | Healthy children |
| Yu et al.15 | 2010 | 35 | 2.64 | 51 | 107 ± 14 | NR | 0.59 | Yes | Yes | Yes | Normal | Kawasaki |
| Marcus et al.16 | 2011 | 139 | 7.5 | 41 | 90 ± 12 | Yes | 0.99 | Yes | Yes | Yes | Normal | Healthy children |
| Takayasu et al.17 | 2011 | 27 | 7.4 | 15 | NR | NR | NR | Yes | Yes | No | Normal | TOF |
| Blanc et al.18 | 2012 | 29 | 8.8 | 28 | 79 ± 11 | NR | 1.07 | Yes | Yes | No | Normal | Sickle cell disease |
| Di Salvo et al.19 | 2012 | 45 | 11 | 36 | 78 ± 16 | Yes | 1.32 | Yes | Yes | Yes | Normal | FH |
| Fernandes et al.20 | 2012 | 71 | 10 | NR | NR | No | NR | Yes | No | Yes | Normal | TOF |
| Hirth et al.21 | 2012 | 34 | 11.7 | 29 | 70 ± 15 | Yes | 1.41 | Yes | No | No | Normal | Renal transplant |
| Koenigstein et al.22 | 2012 | 25 | 8.8 | NR | NR | NR | NR | Yes | No | No | Normal | TOF |
| Malev et al.23 | 2012 | 60 | 19.9 | 37 | 74 ± 16 | Yes | 1.78 | Yes | Yes | Yes | Normal | MVP |
| Poterucha et al.24 | 2012 | 19 | 15.3 | 42 | 62 ± 10 | Yes | 1.7 | Yes | No | No | Normal | Anthracyclin therapy |
| Sato et al.25 | 2012 | 180 | 10 | 57 | 80 ± 20 | NR | NA | NO | Yes | Yes | Normal | Healthy children |
| Takigiku et al.26 | 2012 | 100 | 8 | NR | NR | Yes | NR | Yes | Yes | Yes | Normal | Healthy children |
| Barbosa et al.27 | 2013 | 46 | 11.5 | NR | 74 ± 8 | Yes | NR | Yes | No | No | Normal | Obesity |
| Binnetoglu et al.28 | 2013 | 25 | 14.7 | 28 | 75 ± 11 | Yes | 1.5 | Yes | Yes | Yes | Athletes | Athletes |
| Dogan et al.29 | 2013 | 52 | 3.2 | 25 | 102 ± 26 | NR | 0.87 | Yes | Yes | Yes | Normal | CAS |
| Elkiran et al.30 | 2013 | 117 | 0.1 | 46 | 46 | NR | NR | Yes | Yes | No | Normal | Healthy neonates |
| Gziri et al.31 | 2013 | 62 | 2 | 42 | 106 ± 14 | Yes | 0.56 | Yes | Yes | No | Normal | Maternal chemotherapy |
| Hauser et al.32 | 2013 | 26 | 12.61 | 31 | 71 ± 16 | NR | NR | Yes | No | No | Normal | Athletes |
| Klitsie et al.33 | 2013 | 172 | 8 | 12 | 97 ± 13 | NR | 0.3 | Yes | Yes | Yes | Normal | Healthy children |
| McCandless et al.34 | 2013 | 20 | 2 | 45 | NA | NR | Yes | Yes | Yes | No | Normal | Kawasaki |
| Ryan et al.35 | 2013 | 61 | 5.2 | 0 | 111 ± 2 | Yes | Yes | No | Yes | No | Normal | DMD |
| Schubert et al.36 | 2013 | 30 | 0.1 | 63 | NR | NR | NR | Yes | No | No | Normal | Healthy Infants |
| Sehgal et al.37 | 2013 | 21 | 0.1 | NR | NR | NR | NR | Yes | No | No | Normal | Asphyxia |
| Simsek et al.38 | 2013 | 20 | 16.4 | 0 | 66 ± 15 | Yes | NR | Yes | No | No | Athletes | Athletes |
| Van der Ende et al.39 | 2013 | 40 | 8.4 | 48 | NR | NR | 1.07 | Yes | No | No | Normal | LVOTO |
| Black et al.40 | 2014 | 37 | 9.2 | 0 | 70 ± 8 | Yes | NR | Yes | No | No | Normal | Obesity |
| Labombarda et al.41 | 2014 | 79 | 11.8 | 47 | 76 ± 11 | Yes | 1.27 | Yes | Yes | Yes | Normal | DM I |
| Laser et al.42 | 2014 | 15 | 7.4 | 73 | 100 ± 23 | Yes | 0.94 | Yes | Yes | Yes | Normal | CHD |
| Li et al. 43 | 2014 | 35 | 5.7 | 63 | 85 ± 20 | NR | NR | Yes | Yes | Yes | Normal | TOF |
| Mangner et al.44 | 2014 | 40 | 14.1 | 50 | 70 ± 12 | Yes | 1.54 | Yes | Yes | No | Normal | Obesity |
| Sanchez et al.45 | 2014 | 14 | 15 | 43 | NR | Yes | NR | Yes | No | No | Normal | Obesity |
| Vitarelli et al.46 | 2014 | 40 | 11.28 | 55 | 76 ± 12 | Yes | NR | Yes | Yes | Yes | Normal | Obesity |
| Al-Biltagi et al.47 | 2015 | 45 | 0.1 | 51 | 124 ± 17 | Yes | NR | Yes | No | No | Normal | Gestational DM |
| Burkett et al.48 | 2015 | 53 | 8 | 36 | 79 ± 14 | Yes | 1.10 | Yes | Yes | No | Normal | Pulmonary hypertension |
| Sainz et al.49 | 2015 | 58 | 13.3 | 62 | 79 ± 13 | Yes | NR | Yes | No | No | Normal | HIV |
NR, Not recorded; GLS, Global longitudinal strain; GCS, Global circumferential strain; GRS, Global radial strain;
HR, Heart rate; SBP, Systolic blood pressure; BSA, Body surface area
TGA, transposition of the great arteries; LVNC, Left ventricle non-compaction; TOF, Tetralogy of Fallot, FH, Familial hypercholesterolemia; MVP, Mitral valve prolapse;
CAS, Congenital aortic stenosis; LVOTO, Left ventricle outflow tract obstruction; DM, Diabetes mellitus; CHD, Congenital heart disease; DMD, Duchene Muscular Dystrophy
Table 2. Echocardiographic imaging and data analysis parameters.
| Study | Year | n | Vendor (Machine) | Software (version) | Probe | Frame rate (FR) (Range) |
FR/HR | Tissue tracking | Location of strain valuealong strain curve | Apical views (Segments) |
Short axis views (Segments) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bussadori et al.7 | 2008 | 15 | Esaote (MyLab 50) | XStrain | NS | 30 | NS | Endomyocardial | NS | Apical 4-chamber (6) | NA |
| Lorch et al.8 | 2009 | 284 | Siemens (NS) | VVI | 5-10 | 69 - 112 | > 0.7 | Endomyocardial | Systolic | Apical 4-chamber (6) | NA |
| Pettersen et al.9 | 2009 | 22 | GE (Vivid 7) | GE EP (NS) | NS | 69 - 112 | NS | Endomyocardial | Systolic | Three Apical views (18) | A,M,B (18) |
| Roberson et al.10 | 2009 | 46 | Philips (iE33) | Philips (Q-lab) | NS | 100 | > 0.7 | NS | NS | Apical 4-chamber (6) | NA |
| Cheung et al. 11 | 2010 | 44 | GE (Vivid 7) | GE EP (NS) | NS | NS | NS | Epi-endoc | Systolic | Apical 4-chamber (6) | M (6) |
| Koh et al. 12 | 2010 | 9 | GE (Vivid 7) | GE EP (NS) | 4.4 - 10 | 60 - 80 | NS | NS | NS | Apical 4-chamber (6) | A,M,B (18) |
| Moiduddin et al.13 | 2010 | 13 | GE (Vivid 7/Vivid I) | GE EP (108.1.0) | 5- 7 | 60 - 100 | > 0.7 | Epi-endoc | Systolic | Apical 4-chamber (6) | NA |
| Singh et al.14 | 2010 | 20 | GE (Vivid 7) | GE EP (NS) | 5 | 60 - 90 | > 0.7 | Endomyocardial | Systolic | Three Apical views (18) | A,M,B (18) |
| Yu et al.15 | 2010 | 35 | GE (Vivid 7) | GE EP (7.0) | 5- 7 | 40 - 100 | 0.5 - 0.9 | Epi-endoc | NS | Three Apical views (18) | M,B (12) |
| Marcus et al.16 | 2011 | 139 | GE (Vivid 7) | GE EP (6.1.0) | 3- 5 | 40 - 80 | 0.5 - 0.9 | Endomyocardial | ESS | Apical 4-chamber (6) | M,B (12) |
| Takayasu et al.17 | 2011 | 27 | GE (Vivid 7) | GE EP (108.1.4) | 4 - 5 | 70 - 90 | NS | Endomyocardial | ESS | Apical 4-chamber (6) | M,B (12) |
| Blanc et al.18 | 2012 | 29 | GE (Vivid 7) | GE EP (6.1.0) | 5 | 75 - 125 | > 0.7 | Endomyocardial | NS | Apical 4-chamber (6) | M,B (12) |
| Di Salvo et al.19 | 2012 | 45 | GE (Vivid 7) | GE EP (6.0) | 5 | 70 - 100 | > 0.7 | Epi-endoc | Systolic | Three Apical views (18) ** | M,B (12) |
| Fernandes et al.20 | 2012 | 71 | GE (Vivid 7) | GE EP (6.0.1) | NS | 59 - 91 | NR | Endomyocardial | Peak | Three Apical views (18) | NA |
| Hirth et al.21 | 2012 | 34 | GE (Vivid 7) | GE EP (NS) | 3 - 5 | 60 - 90 | > 0.7 | Endomyocardial | Systolic | Apical 4-chamber (6) | NA |
| Koenigstein et al.22 | 2012 | 25 | GE (Vivid 7) | GE EP (NS) | 2.5 - 3.5 | 60 | NS | NS | NS | Apical 4-chamber (6) | NA |
| Malev et al.23 | 2012 | 60 | GE (Vivid 7) | GE EP (BT 08) | 3.5 | 50 | > 0.7 | NS | ESS | Three Apical views (18) | A,M,B (18) |
| Poterucha et al.24 | 2012 | 19 | GE (Vivid 7) | GE EP (NS) | 5 | 50 -85 | > 0.7 | NS | ESS | Three apical views (18) | NA |
| Sato et al.25 | 2012 | 180 | GE (Vivid 7) | GE EP (NS) | NS | 80 | > 0.7 | Epi-endoc | NS | NA | M (6) |
| Takigiku et al.26 | 2012 | 100 | GE (Vivid 7 or 9) / Philips (iE33) / Toshiba (Artida or Aplio) | GE EP (110.13) / Philips Q-lab (7.1) / Toshiba (UE) | NS | 52-65 | NS | Epi-endoc | NS | Three apical views (18) | A,M,B (18) |
| Barbosa et al.27 | 2013 | 46 | GE (Vivid 7) | GE EP (NS) | 3 - 7 | 44 | 0.5 | Endomyocardial | Systolic | Three apical views (18) | NA |
| Binnetoglu et al.28 | 2013 | 25 | GE (Vivid 7) | GE EP (NS) | NS | 60 - 90 | > 0.7 | Endomyocardial | Systolic | Three apical views (18) ** | A,M,B (18) |
| Dogan et al.29 | 2013 | 52 | GE (Vivid 7) | GE EP (6.3.6) | 3 - 7 | 40 | < 0.5 | Endomyocardial | NS | Apical 4-chamber (6) | A,B (12) |
| Elkiran et al.30 | 2008 | 117 | Esaote (MyLab 50) | XStrain | NS | 50-70 | NS | Endomyocardial | NS | Three Apical views (18) | M (6) |
| Gziri et al.31 | 2013 | 62 | GE (Vivid 7) | GE EP (7.0) | 4 - 7 | 50 - 90 | > 0.5 | Endomyocardial | Systolic | Apical 4-chamber (6) | M (6) |
| Hauser et al.32 | 2013 | 26 | GE (Vivid 7) | GE EP (BT 08) | 4- 5 | 50 - 90 | > 0.7 | NS | NS | Apical 4-chamber (6) | NA |
| Klitsie et al.33 | 2013 | 172 | GE (Vivid 7) | GE EP (11.1.8) | 5- 10 | 44 - 155 | > 0.5 | Endomyocardial | Peak | Three apical views (18) ** | M (6) |
| McCandless et al.34 | 2013 | 20 | Siemens (NS) | Syngo (VVI) | NS | NS | NS | Endomyocardial | NS | Apical 4-chamber (6) | M (6) |
| Ryan et al.35 | 2013 | 61 | GE (Vivid 7) / Phillips (iE33) | TomTec | NS | 50 - 100 | > 0.5 | Endomyocardial | NS | NA | M (6) |
| Schubert et al.36 | 2013 | 30 | GE (Vivid 7) | GE EP (NS) | 1.5 - 10.5 | 176 - 200 | NS | Endomyocardial | Systolic | Apical 4-chamber (6) | NA |
| Sehgal et al.37 | 2013 | 21 | GE (Vivid 7) | GE EP (NS) | 10 | > 80 | NS | Endomyocardial | NS | Apical 4-chamber (6) | NA |
| Simsek et al.38 | 2013 | 20 | GE (Vivid 7) | GE EP (NS) | 2.5 | 60 - 100 | > 0.7 | Endomyocardial | Systolic | Three apical views (18) ** | NA |
| Van der Ende et al.39 | 2013 | 40 | GE (Vivid 7) | GE EP (06) | NS | > 45 | NS | Endomyocardial | NS | Three Apical views (18) | NA |
| Black et al.40 | 2014 | 37 | Phillips (iE33) | Philips QLAB (8.1) | 5 - 7 | NS | NS | NS | NS | Apical 4-chamber (6) | NA |
| Labombarda et al.41 | 2014 | 79 | Phillips (iE33) | Philips QLAB (6.0) | 5 | > 50 | > 0.7 | Endomyocardial | Systolic | Three Apical views (18) | M (6) |
| Laser et al.42 | 2014 | 29 | GE (Vivid 7) | GE EP (6.1.2) | 5 | 55 - 90 | 0.5-0.9 | NS | NS | Apical 4-chamber (6) | M (6) |
| Li et al.43 | 2014 | 35 | GE (Vivid 7) | GE EP (BT06) | 3 - 7 | 60 - 90 | 0.7-0.9 | Endomyocardial | NS | Apical 4-chamber (6) | M (6) |
| Mangner et al.44 | 2014 | 40 | GE (Vivid 7) | GE EP (113) | NS | 40 - 80 | > 0.6 | NS | NS | Three Apical views (18) | M (6) |
| Sanchez et al.45 | 2014 | 14 | GE (Vivid 7) | GE EP (6.0.1) | 5 | 60 - 90 | NS | Endomyocardial | Systolic | Three Apical views (18) | NA |
| Vitarelli et al.46 | 2014 | 40 | GE (Vivid 9) | GE EP (BT12) | 5 | > 50 | > 0.7 | NS | Systolic | Three Apical views (17) | A,M,B (18) |
| Al-Biltagi et al.47 | 2015 | 45 | GE (Vivid 7) | GE EP (NS) | 5 | > 65 | > 0.5 | NS | Systolic | Three Apical views (18) | NA |
| Burkett et al.48 | 2015 | 53 | GE (Vivid 7 or 9) | GE EP (113) | 3-5 | NS | NS | Endomyocardial | Systolic/ESS | Apical 4-chamber (6) | M (6) |
| Sainz et al.49 | 2015 | 58 | Phillips (CX50) | Philips QLAB (7.1) | 5 | 30 - 60 | 0.4 – 0.8 | Endomyocardial | NS | Three Apical views (18) | NA |
NS, Not specified, NA, Not applicable; FR/H, frame rate to heart rate ratio;
GE, General electric; EP, Echo PAC; UE, Ultra-Extend Toshiba; VVI, vector velocity imaging; AS, Acuson Sequoia,
A, apex; M, mid-ventricular; B, base
Epi-endoc, Epicardial-endocardial
Peak strain point; The peak strain may coincide with the A) Systolic (s), before the closure of the AV valve; B) End-systolic peak (ESS), at the closure of the AV valve, or C) ‘Post-systolic strain’ (PSS), after aortic valve closure. Manuscripts either (1) described the strain measurement point as one of the three locations along the curve, (2) failed to specify, or (3) only mentioned the use of “peak” strain, without specification of location.
Study Selection Based on Strain Measures
Forty-one datasets with 2084 patients were eligible for the meta-analysis of GLS or LS;7-24,26-34,36-49 26 datasets with 1506 patients were eligible for the meta-analysis of GCS or CS,7,9,11,12,14-20,23,25,26,28-31,33,34,41-44,46,48 and 15 datasets with 1092 patients were eligible for the meta-analysis of GRS or RS.11,15,16,19,20,23,25,26,28,29,33,41-43,46 The patient characteristics of the included studies are listed in Table 1. The echocardiographic variables included from the studies are listed in Table 2.
Study Quality Assessment
Critical appraisal of the studies demonstrated moderate quality in all the studies included. The eligible datasets met > 75% of the quality checklist items (Appendix 3). Specifically, all studies clearly defined the objectives, the primary outcomes that were measured, and the main findings. All the studies used a deformation imaging acquisition and post-processing protocol. Reproducibility analysis was performed in 32/437-10,14-21,23-27,33,35-46,48,49 datasets and referenced in 4/43.11,30,31,47
Normal Ranges of Longitudinal Strain
Global Longitudinal Strain Measures from the apical 4-, 3-, 2- chamber views
Global LS from the combined apical 4-, 3- and 2- chamber views was reported in 19 of the 43 data sets (n=1183 children).9,14,15,19,20,23,24,26-28,30,33,38,39,41,44-47,49 The normal mean values of GLS varied from -16.7% to -23.6% (mean -20.2%, 95% CI -19.5% to -20.8%), (Figure 2a). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 561 (P < 0.0001) and inconsistency by an I2 value of 95.5%. LV FWLS from the combined 4-, 3-, and 2- chamber views was reported in 7 of 43 data sets (n=352).14,20,23,24,28,30,39 The normal mean values of FWLS varied from -17.0% to -24.0% (mean -19.6%, 95% CI -17.5 to -21.7). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 177 (P < 0.0001) and inconsistency by an I2 value of 96.6%. The mean values and 95% CI are listed in Table 3.
Table 3. Reference mean values of left ventricle strain measures.
| Age distribution | Global longitudinal strain Mean GLS (CI) | Longitudinal strain - 4CH Mean LS (CI) | Global circumferential strain Mean GCS (CI) | Circumferential strain - Mid Mean CS (CI) | Global radial strain Mean GRS (CI) | Radial strain - Mid Mean RS (CI) |
|---|---|---|---|---|---|---|
| 0 - 1 | -18.7% (-20.8, -16.7) | -19.4% (-22.2, -16.6) | NA | -18.2% (-22.6, -13.7) | NA | 44.4% (36.6, 52.1) |
| 2 - 9 | -21.7% (-23.0, -20.5) | -21.0% (-21.8, -20.2) | -24.5% (-27.2, -21.7) | -20.3% (-21.4, -19.1) | 48.0% (33.3, 62.8) | 50.8% (47.4, 54.1) |
| 10 - 13 | -20.0% (-20.8, -19.1) | -20.5% (-21.7, -19.2) | -21.9% (-26.5, -17.4) | -21.5% (-23.1, -19.8) | 43.7% (33.0, 54.5) | 52.1% (48.5, 55.8) |
| 14 - 21 | -19.9% (-20.6, -19.2) | -19.9% (-21.2, -18.6) | -16.4% (-23.3, -9.6) | -19.7% (-22.1, -17.4) | 44.0% (41.6, 46.4) | 46.4% (39.7, 53.1) |
| Overall | -20.2% (-20.8, -19.6) | -20.4%(-21.1, -19.8) | -22.3% (-19.9, -24.6) | -21.4% (-20.6, -22.4) | 45.2% (38.8, 51.7) | 49.4% (47.2, 51.6) |
Data presented as mean (confidence interval)
CI, 95% confidence interval
GLS, Global longitudinal strain; LS, Longitudinal strain from the apical 4-chamber view
GCS, Global circumferential strain; CS, Circumferential strain at the mid-ventricular level (papillary muscle)
GRS, Global radial strain; RS, Radial strain at the mid-ventricular level (papillary muscle)
Global Longitudinal Strain Rate Measures from the combined apical 4-, 3-, 2- chamber views
Global LSr from the combined apical 4-, 3- and 2- chamber views was reported in 9 of the 43 data sets (n=403 children).9,20,23,24,30,38,44-46 The normal mean values of systolic GLSr varied from -1.08 to -1.32 (mean -1.18, 95% CI -1.10 to -1.25) Between-study heterogeneity was evidenced by a Cochran's Q statistic of 83 (P < 0.0001) and inconsistency by an I2 value of 94.0%. The normal mean values of early diastolic GLSr varied from 1.40 to 1.85 (mean 1.62, 95% CI 1.31 to 1.92). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 53 (P < 0.0001) and inconsistency by an I2 value of 96.2%. The normal mean values of late diastolic GLSr varied from 0.60 to 0.74 (mean 0.67, 95% CI 0.54 to 0.81). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 4.38 (P < 0.0001) and inconsistency by an I2 value of 87.2%.
Longitudinal Strain Measures from the apical 4-chamber view
Longitudinal strain (LS) from the apical 4-chamber view was reported in 26 of the 43 data sets (n=1443 children).7,8,10-13,16-19,21,22,28-34,36-38,40,42,43,48 (There were four datasets that reported both the GLS and the LS from the apical 4-chamber view only.19,28,30,33) The normal mean values of LS varied from -15.1% to -24.8% (mean -20.4, 95% CI -19.8% to -21.7%), (Figure 2b). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 910 (P < 0.0001) and inconsistency by an I2 value of 96.2%. LV free wall from the apical 4- chamber views was reported in 9 of 43 data sets (n=716 children).7,8,16,17,28-30,34,36 The normal mean values of FWSL varied from -17.00% to -23.4 % (mean -20.2%, 95% CI -19.2% to -22.2%). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 458 (P < 0.0001) and inconsistency by an I2 value of 96.3%. The mean values and 95% CI are listed in Table 3.
Longitudinal Strain Rate Measures from the apical 4-chamber view
Longitudinal Sr from the apical 4-chamber view was reported in 19 of the 43 data sets (n=889 children). 7,8,11-13,17,18,21,29-31,34,36-38,40,42,43,48 The normal mean values of systolic LSr varied from -0.41 to -2.59 (mean -1.20, 95% CI -0.96 to -1.44). The normal mean values of early diastolic LSr varied from 1.60 to 3.15 (mean 2.23, 95% CI 1.89 to 2.53), and the normal mean values of late diastolic LSr for varied from 0.40 to 2.41 (mean 0.80, 95% CI 0.64 to 0.95). Between-study heterogeneity for strain rate was evidenced by Cochran's Q statistic ranging between 153 and 700 (P < 0.0001) and inconsistency by an I2 value > 94.8%.
Regional longitudinal strain measures
LV regional or SLS is assessed at the apical, mid, and basal ventricular levels of the LV free wall and has been clinically used to assess left ventricle function in both adult and pediatric disease. Eight out of the 43 eligible datasets (n=387 children) in this meta-analysis reported LV SLS from the segmental averaging from apical 4-, 3-, and 2- chambers views at all three levels of the LV free wall (8 out of 19 datasets that reported GLS).14,15,20,23,24,28,30,39 Nine of the 44 datasets (n=716 children) reported LV SLS from the apical 4-chamber view only (9 out of 26 that reported LS from the apical 4-chamber view only).7,8,16,17,28-30,34,36 The meta-analysis demonstrated a significant (P < 0.001) apex-to-base (highest-to-lowest) gradient for the mean values of normal LV SLS from the three apical views (-19.9% -19.2%, -18.7%), respectively and from the apical 4-chamber alone (-20.6%, -19.9%, -19.5%, respectively). Between-study heterogeneity was evidenced by a Cochran's Q statistic ranging from 312 to 555 (P < 0.001) and inconsistency by an I2 value ranging from 97.2% to 98.9%. The mean values for the SLS are listed in Appendix 4.
The heterogeneity for GLS, GRSL, SL, LSr, and SLS was not explained by age, gender, BSA, heart rate, blood pressure, tissue tracking methodology, reporting of strain value along the strain curve, frame rate, FR/HR or probe size.
Normal Ranges of Circumferential and Radial Strain
Circumferential strain
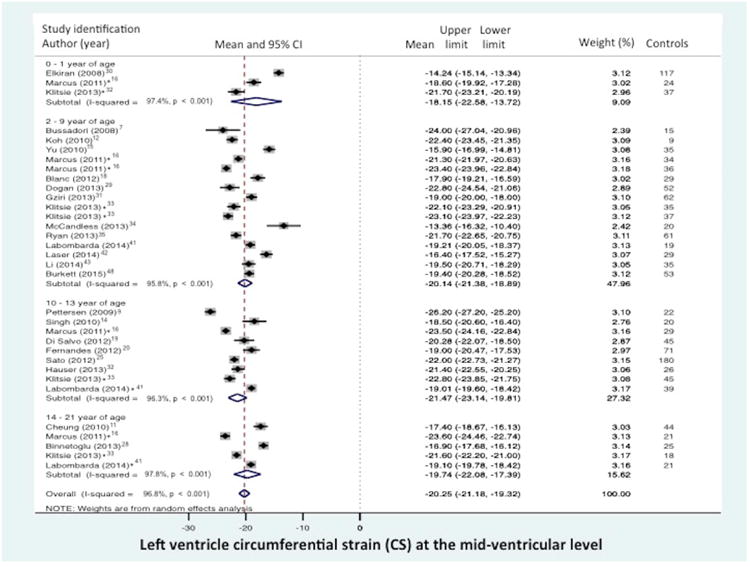
Global CS from the combined short axis views at the base (level of the mitral valve), mid-ventricular (level of the papillary muscle), and the apex was reported in 10 of the 43 data sets (n=474 children).7,12,14,19,20,23,26,28,46,48 The normal mean values of GCS varied from -12.9% to -31.4% (mean -22.3, 95% CI -19.9% to -24.6%), (Figure 3a). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 569 (P < 0.0001) and inconsistency by an I2 value of 98.1%. Circumferential strain (CS) from the mid-ventricular level (papillary muscle) was reported in 23 of the 43 data sets (n=1380 children).7,9,11,12,14-20,23,25,26,28-31,33,34,41-44,46,48 (There were seven studies that presented both GCS and CS).7,12,14,19,20,28,48 The normal mean values of CS varied from -14.2% to -26.2% (mean -20.3%, 95% CI -19.4% to -21.2%), (Figure 3b). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 777 (P < 0.0001) and inconsistency by an I2 value of 96.8%.
Figure 3.
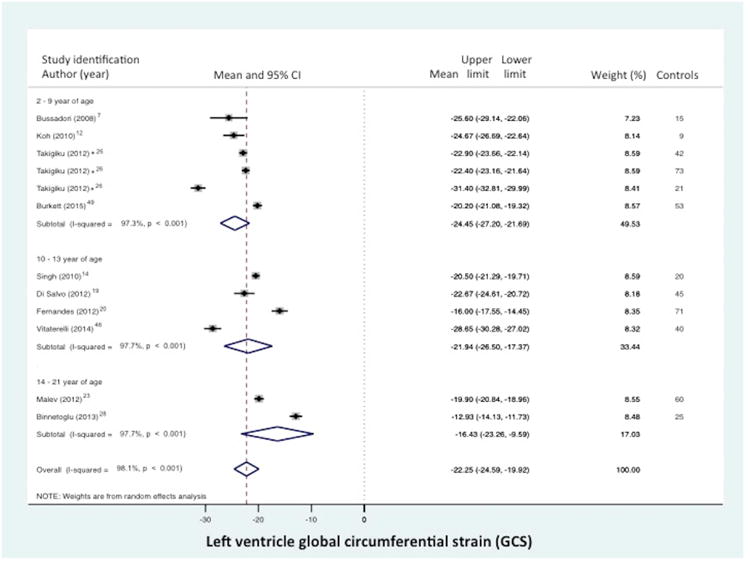
Normal value of LV global circumferential strain (GCS) stratified by age distribution and view. (A) LV “global” CS derived from the segmental averaging of the three short axis views at the base (mitral valve), mid-ventricular (papillary muscle), and apical levels views and (B) LV CS at the level of the papillary muscle only. The forest plot lists the names of the included studies by age distribution and in chronological order, the mean and confidence intervals with the upper (95%) and lower (5%) limits. Each study is represented by a square that reflects the mean at the point estimate of effect and is proportional to the study's weight in the meta-analysis. A horizontal line extending from either side of the square reflects the 95% confidence interval. The overall meta-analysis measure of effect is plotted as a diamond with the lateral points of the diamond indicating confidence intervals for this mean estimate.73 *Klitsie et al., Labombarda et al., Lorch et al., Marcus et al., Takayasu et al., and Takigiku et al. all performed a cross-sectional study and reported strain values for multiple mean age groups from birth to 21 years of age.8,16,17,26,33,41
Radial strain
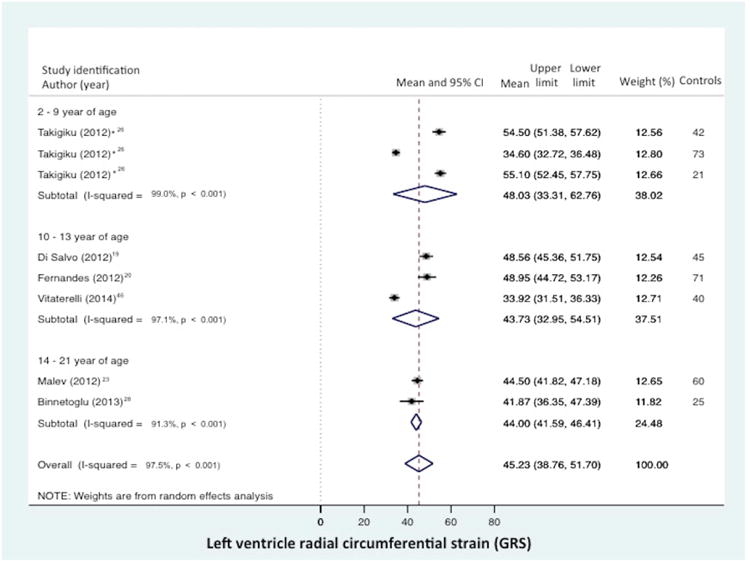
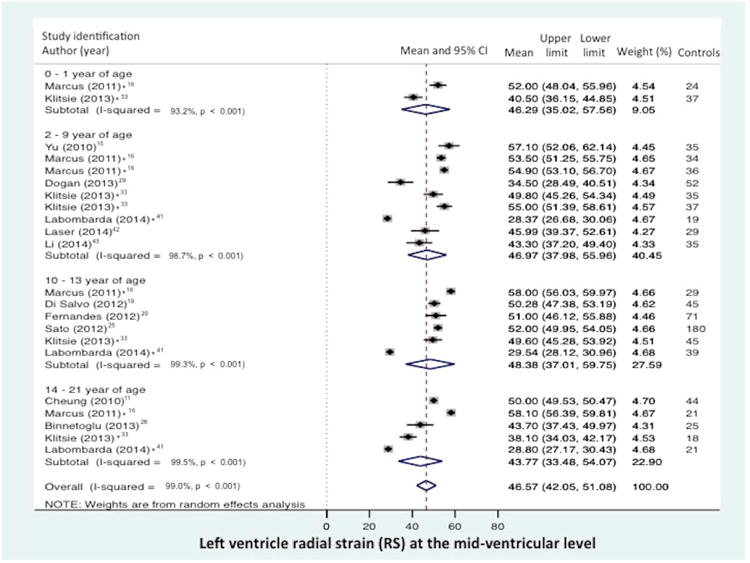
Global RS from the combined short axis views at the base (level of the mitral valve), mid-ventricular (level of the papillary muscle), and the apex was reported in 6 of the 43 data sets (n=377 children).19,20,23,26,28,46 The normal mean values of GRS varies from 33.9% to 54.5 % (mean 45.2%, 95% CI 38.8% to 51.7%), (Figure 4a).19,20,23,26,28,46 Between-study heterogeneity was evidenced by a Cochran's Q statistic of 283 (P < 0.0001) and inconsistency by an I2 value of 97.5%. Radial strain (RS) from the mid-ventricular level (papillary muscle) was reported in 12 of the 43 data sets (n=946 children).11,15,16,19,20,25,28,29,33,41-43 (There were three studies that presented both GRS and RS).19,20,28 The normal mean values of RS for varied from 28.8% to 58.1% (mean 46.7%, 95% CI 42.3% to 51.1%), (Figure 4b). Between-study heterogeneity was evidenced by a Cochran's Q statistic of 1811 (P < 0.0001) and inconsistency by an I2 value of 99.0%.
Figure 4.
Normal value of LV global radial strain (GRS) stratified by age distribution and view. (A) LV “global” RS derived from the segmental averaging of the three short axis views at the base (mitral valve), mid-ventricular (papillary muscle), and apical levels views and (B) LV RSat the level of the papillary muscle only. The forest plot lists the names of the included studies by age distribution and in chronological order, the mean and confidence intervals with the upper (95%) and lower (5%) limits. Each study is represented by a square that reflects the mean at the point estimate of effect and is proportional to the study's weight in the meta-analysis. A horizontal line extending from either side of the square reflects the 95% confidence interval. The overall meta-analysis measure of effect is plotted as a diamond with the lateral points of the diamond indicating confidence intervals for this mean estimate.73 *Klitsie et al., Labombarda et al., Lorch et al., Marcus et al., Takayasu et al., and Takigiku et al. all performed a cross-sectional study and reported strain values for multiple mean age groups from birth to 21 years of age.8,16,17,26,33,41
The heterogeneity for GCS, CS, GRS, and RS is not explained by the different methods, or the age, gender, BSA, heart rate, tissue tracking methodology, reporting of strain value along the strain curve, frame rate, FR/HR or probe size.
Age and Global Strain (%)
Age did not explain the heterogeneity of the reported normal ranges of values for GLS, GCS, or GRS. Lorch et al.,8 Marcus et al.,16 Klitsie et al.,33 and Labombarda et al.41 performed a crossed sectional study with patients from birth to 21 years of age. Takayasu et al generated strain measures in two separate cohorts of children and adolescents.17 The breakdown of the age distribution for the remaining 38 datasets was: four data-sets recruited patients 0-1 years of age,30,36,37,47 18 datasets had patients with age ranges of 2 and 9 years of age,7,10,12,13,15,18,22,26,29,31,34,35,39,40,42,43,48 10 datasets had patients with age ranges of 10 and 13 years of age,9,14,19-21,25,27,32,41,46 6 datasets examined patients with age ranges 14-21 years of age.11,23,24,28,38,44 We performed a separate meta analysis for LV strain measures stratified by age distribution using the mean age from each study as a continuous variable and also by categorizing each study into one of the four age distribution categories, 0-1, 2-9, 10-13, and 14-21 (Figures 2-4). The Cochran Q statistic ranged from 39 to 370 (P < 0.0001) and the I2 value remained the same in both methods and ranged from 82.19% - 98.0%. The means and 95%CI for the GLS, GCS, GRS strain values and LS from the apical 4-chamber view, and CS and RS at the mid-ventricular level are listed in Table 3. A similar analysis stratified by age was done for segmental longitudinal strain from the segmental averaging of the three apical views and from the apical 4- chamber view only. An apex-to-base gradient existed for all ages and the results are listed in the Appendix 4.
Publication Bias
Both visual inspection of the funnel plot and the non-significant results of the Egger test for the GLS, GCS, GRS measures (p=0.40) suggest the absence of publication bias (Figure 5).75
Figure 5.
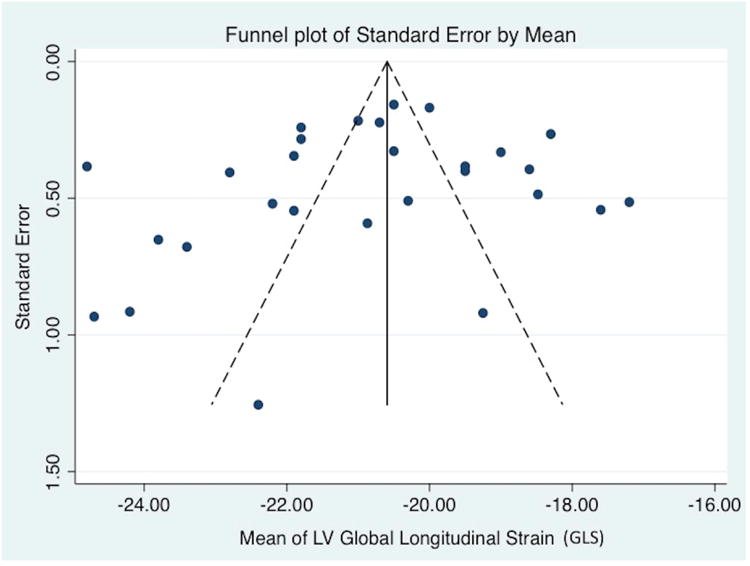
Publication bias. Funnel plot for studies of left ventricle global longitudinal strain. The standard error of the effect estimate is plotted on the vertical axis. The mean of the LV GLS is plotted on the horizontal axis. Visual inspection shows symmetry in the distribution of the studies that suggests the absence of publication bias (P=0.40 from the Egger test for statistical funnel plot symmetry.
Sources of Variability
In this meta-analysis age, gender, body surface area, heart rate, frame rate, FR/HR ratio, tissue-tracking method, location of reported strain value along the strain curve, ultrasound vendor (model), and software (version) were tested to determine if any of these parameters influenced the variability in reporting of normal strain and strain rate measures in children (Table 2). We stratified the meta-analysis by the method of generating the strain measurements: “six segment” method vs. Global (17-, 18- segmental average) method. To account for maturational changes in hemodynamic parameters from infancy to adolescence, we stratified the meta-analysis by age distribution to determine its contribution to the reported ranges of normal values.73
Individual meta-regression analysis on each dependent strain measure and each independent variable was performed to examine which parameter might statistically influence the variation in strain measures in this meta-analysis. None of the demographic, clinical, or echocardiographic variables were significantly associated with the mean values for any of the strain measures (Table 4). Inter vendor-equipment and software was independently assessed; Nine (21%) of the 43 datasets7,8,10,26,30,34,40,41,49 utilized non-GE equipment and all but one of the studies35 acquired and then analyzed the strain imaging with the same vendor and software package. Six data sets used Philips equipment and software10,26,35,40,41,49, two utilized Siemen's products,8,34 and two used Esaote (Mylab 50/XStrain).7,30 One study used both Philips and GE35, and one study used Philips, GE, and Toshiba ultrasound machines26. We created vendor specific normal ranges of values for GE, Philips, Siemens, and Esaote, (Appendix 5).
Table 4. Meta-regression results for LV global strain.
| P value (GLS) | P value (GCS) | P value (GRS) | |
|---|---|---|---|
| Age | 0.56 | 0.38 | 0.19 |
| Gender | 0.67 | 0.56 | 0.56 |
| Body surface area | 0.67 | NA | 0.34 |
| Heart rate | 0.34 | 0.78 | 0.22 |
| Frame Rate | 0.14 | 0.47 | 0.48 |
| FR/HR ratio | 0.23 | 0.56 | 0.14 |
| Ultrasound Scanner* | 0.19 | 0.62 | 0.47 |
| Model** | 0.43 | 0.12 | 0.17 |
| Vendor Software* | 0.22 | 0.35 | 0.36 |
| Version*** | 0.23 | 0.37 | 0.69 |
| Probe Size | 0.26 | 0.43 | 0.32 |
| Tissue tacking methodology | 0.54 | 0.34 | 0.19 |
| Location of strain value | 0.14 | 0.47 | 0.48 |
NA, not analyzed because there were not enough variables
36 of the 43 eligible data sets in this meta-analysis used machines and software from one manufacturer (GE).
Different models of GE machines (GE Vivid E7, E9, I), Philips (iE33, CX50), and Esaote (MyLab 50) ultrasound machines were used. Siemens and Toshiba models were also utilized, but the model was not specified.
Different version of the GE EchoPAC software (6.0, 6.01., 6.3.6, 7.0, 108.1.4, 108.1.5 11.1.8, and BT 08), Philips QLAB software (6.0, 7.1, 8.0), Esoate (Xstrain), Toshiba (ultra extended), and Siemens (Syngo) were employed in the image acquisition and data analysis.
Discussion
Deformation imaging is used as measure of LV function in the diagnosis and management of several cardiopulmonary diseases in children.7-71 Defining the reference range of values for 2DSTE derived LV longitudinal, circumferential, and radial strain and their variance is an important step in using them as “echocardiographic end points and surrogates for theses outcomes”.73 This study establishes a reference range of values of LV global and regional longitudinal, circumferential, and radial strain measures in healthy children on the basis of a meta-analysis and assesses the contribution of the potential cofounders (demographic, clinical, and imaging) to the variation in the reported ranges.
This is the second study to utilize a meta-analysis approach to define reference values of LV longitudinal, circumferential, and radial strain derived by 2DTSE in children.79 Our study is a comprehensive systematic review and meta-analysis that complements and expands the recent meta-analysis of Jashari et al.79 They used five search engines, but only applied “key terms” and identified 1,192 children from 28 articles.79 We utilized trained librarians to create “search hedges” to cover “concepts” (pediatrics/children, speckle tracking, and left heart ventricles) using phrases harvested from standard word indices and on-topic articles and identified 2325 children from 63 articles (43 data sets), (Appendix 1). Jashari et al. elected not to incorporate studies with missing data raising the possibility that the observed effect estimate is biased.79 To overcome this, we contacted all the authors of the eligible studies by electronic mail to fill in the missing gaps in data in an attempt to decrease heterogeneity between studies and to publically notify them of the meta-analysis.73 Due to the rapid rate of strain articles being published (one a day by some accounts), we “re-ran” our meta-analysis four times during the writing and review process to maximize inclusion.73 We hope that these approaches will now serve as a template to replicate, update, and define reference ranges on the basis on a systemic review and meta-analysis with other novel cardiac measures in children and neonate.
There is an expanding literature of deformation imaging meta-analyses in children and adults that was started by Yingchoncharoen and Marwick et al's. meta- analysis of the normal ranges of left ventricular strain in adults in 2012.1 Kalam and Marwick et al. used a meta-analysis approach to demonstrate the prognostic value of GLS appears to have superior prognostic value to ejection fraction (EF) for predicting major adverse cardiac events.80 Fine et al. recently published a partial nested meta analysis on reference ranges of RV strain values in adult patients.81 In addition to the recent work by Jashari et al.79 and our original meta-analysis on normal ranges of RV Strain in children,73 Cantinotti et al performed a “systematic search” in PubMed only to review the published nomograms of LV strain derived by TDI, 2DSTE, and 3D echocardiography in children, and concluded that there is a need for comprehensive nomograms of strain involving a large population of healthy children obtained using standardized methodology.82 Our study attempts to answer that question with 2DSTE-derived strain, but does not address strain derived by TDI or MRI. All of these systematic reviews and meta-analysis in adults and children highlight the growing recognition that strain is an invaluable tool for the assessment of cardiac function in a wide range of diseases, and determining reference ranges of both LV and RV strain values and identifying factors that contribute to the reported variations is the first step in introducing deformation imaging into clinical guidelines.
Reference ranges of global LV longitudinal, circumferential, and radial strain measures
This study defines reference values for LV GLS, GCS, and GRS. All 43 eligible datasets from 63 studies reported normal values of strain measures from small cohorts of healthy children. Forty studies recruited healthy children as a control population to compare their strain measures to a diseased population in case/control observational study format.7-27,29-31,33-37,39-49 The remaining three studies recruited healthy athletes and the strain values at rest were incorporated in this meta-analysis.28,32,38 By combining data from all these different studies in a meta-analysis format, this systematic review offers a more “representative estimate of the range of normal strain values than are possible with individual studies.” 73,75
In clinical and research practice “Global” LV strain has been defined by different LV segmentation models. The 18- and 17- segment model is recommended to assess myocardial perfusion with echocardiography and other imaging techniques.3,74,76 The 16- segment model is recommended for routine studies assessing wall motion, because endocardial excursion and thickening of the tip of the apex are often imperceptible.74,76 Regardless of which segment model is utilized in practice; each segment should be evaluated in multiple views to assess wall motion. LV longitudinal strain should be acquired from the averaging of the three apical views, and LV circumferential and radial strain should be acquired from averaging of the three short axis views. Despite these recommendations, deformation imaging is still reported only from the apical 4- chamber view for LS and from the mid-ventricular level at the papillary muscle for RS and CS in a majority of studies.3 Specifically, in this meta-analysis, LS was reported in 59% and GLS was reported in 44% of the eligible studies. Similarly CS was reported in 52% and GCS was reported in 23% of the eligible studies. Strain is still reported from one short axis view most likely because in some children it is sometimes difficult to show an apex clearly as it exists near the surface and it is not always contained in the view.25 Although there is no consensus on which approach is more accurate or correlates more efficiently with health and disease outcomes, this meta-analysis expands the recent study by Jashari et al79 and now provides reference values for a) GLS and GLSr from the segmental averaging of the three apical views, b) LS and LSr from the apical 4-chamber view only, c) GCS and GRS from the three short axis views, d) CS and RS at the mid-ventricular level only, e) and regional SLS from the LV free wall at the apical, mid-ventricular, and base levels.
Reference ranges of segmental longitudinal strain measures
Recent recommendations suggest that that despite “promising data”, quantitative assessment of the regional LV deformation could not be recommended because of lack of reference values from small individual studies.3,74,76 This meta-analysis defined normal ranges for segmental longitudinal strain (SLS) at the apical, mid-ventricular, and basal levels of the left ventricular free wall (SLS--Apex, SLS -Mid, SLS -Base, respectively), (Appendix 4). Previous individual studies have demonstrated an apex-to-base (highest-to-lowest) SLS gradient for the LV in children and adults, and this apex-to-base gradient is reflective in this SLS meta-analysis (Appendix 4).16 The apex-to-base gradient occurs because of two primary reasons: (1) Torsional mechanisms of LV deformation is greatest toward the apex, as the right handed helix in the subendocardium and the left -handed helix in the subepicardium converge toward the apex to form the “vortex of the double helical loop;”2,5,6,8,16 and (2) the electric excitation of cardiac motion begins in the apex and travels to the base.6 This apex-to-base gradient remains relatively unchanged with growth and may reflect the relative constant geometry of healthy heart with maturation.8 Alteration of this “physiological” apex-to-base gradient has the potential to discern clinical changes in myocardial function in patients with different disease processes.
Clinical impact of reference Strain values
Non-invasive strain imaging of the LV has been primarily utilized in research studies in children, but with an accepted reference range of normal values of LV strain, we strongly feel that these myocardial deformation parameters can now be properly included into pediatric recommendations to assess LV function in children. Strain imaging has recently been incorporated in several consensus guidelines for recommendation for monitoring cardiotoxicity of cancer therapeutic drugs and early detection of LV dysfunction in adults and children,83,84 and was also included in Lang et al.'s 2015 update on the recommendations for chamber quantification.76 There has also been an explosion of clinical research and application of two-dimensional strain parameters to diagnose acute rejection after heart transplantation.85 Furthermore, several studies have attempted to show LV strain may be more feasible and reliable than the traditional measures of LV function, shortening and ejection fraction.80 Reference ranges of strain values established with meta-analyses and validated with specific image acquisition and data analysis, coupled with forth coming work from the European Association of Cardiovascular Imaging (EACVI) and the American Society Echocardiography (ASE) Industry Task Force to standardize strain imaging and reduce inter-vendor differences and ambiguities in the strain algorithms, will allow deformation imaging to be used more routinely to assess clinical changes in myocardial function “across a broad range of physiologic and pathologic conditions in children.” and provide a valid basis that allows comparison between studies.3,73,74
Source of Bias
Demographics and clinical variables
There are demographic and clinical confounders related to maturation that may have an impact on LV strain in children.8,16,82 The meta-regression in our study showed that the effects of age, gender, body surface area, blood pressure, HR, frame rate, FR/HR ratio, tissue tracking methodology, location of reported strain value along the strain curve, and probe size were not significant determinants of variations among normal ranges of reported LV strain measurements in children. The lack of explanation of these variables in causing heterogeneity between studies should “not be misconstrued to mean that these features” do not influence strain.1,73
Age and Strain values
The affect of age on LV strain during growth remains unclear from individual studies. Marcus et al. observed a statistically significant “second-order polynomial relation” between global peak systolic strain parameters and age in that deformation patterns were lowest in the youngest and oldest age groups.16 Zhang et al. (using 3D echocardiography) demonstrated that there were small maturational changes in GLS and GCS, but not in GRS and GS, that are “statistically significant but probably clinically irrelevant.”86 Similarly, Kaku et al. showed minimal change from birth to adolescence in GLS, GCS, and GRS using three-dimensional echocardiography.87 Lorch et al. demonstrated that GLS strain did not change significantly with maturation and declining heart rate from birth to 18 years of age, but LSr changed with age.8 Klitsie et al., showed no linear relation between age and most global peak strain parameters derived by 2DSTE.33 Finally, Labombarda et al. demonstrated that in healthy controls GLS, GCS, and GRS were preserved throughout maturation irrespective of age or gender.41 To assess the contribution of age to the variation in the reported reference values, we stratified by the age distribution (years) in children: infancy (0-1), pre-puberty (2-9), puberty (10-13), and late adolescents (14-21).73 Age did not explain the between-study heterogeneity of the reported reference ranges of values for GLS, GRS or GCS. In this meta-analysis LV strain is preserved from birth to adolescence. The reference mean values of LV strain by age distribution are listed in Table 3.
Vendor hardware and software
The “Achilles heel” of strain imaging remains the role of inter-vendor ultrasound machines and vendor-customized software. The EACVI/ASE/Industry task force to standardize deformation imaging recently tested the variability of 2DTSE derived LV GLS and LS from the 4-chamber view among different vendors and demonstrated a small difference that rarely exceeded 10% and “might therefore have no major impact on clinical interpretation.”3 In this meta-analysis, 36 out of the 43 of the eligible data sets used equipment and software from one manufacturer, 9,11-29,31-33,36-40,42-48 General Electric (GE), (Table 2), and all but one data set acquired and then analyzed the strain imaging with the same vendor and software package.35 Six data sets used Philips equipment and software,10,26,35,40,41,49 two utilized Siemen's products,8,34 and two used Esaote.7,30 This is similar to the meta-analysis in adults by Yingchoncharoen et al. where they found that in 28 eligible data sets, only 5 used non-GE equipment, and the use of different vendors was not an explanation of between-study differences in the reported values.1 Takigiko et al performed a cross sectional study in three different groups of healthy children and provided reference values of normal 2-D strain for three different ultrasound vendors (and software platforms).26 Although there was low inter-vendor agreement, the variability was not tested as each of the three groups acquired and generated strain values with one of the three specified vendors and software platforms. Jashari et al. concluded from their meta-analysis that the vendor significantly determined the variations in radial strain values in children, however this conclusion may be limited by the smaller number of datasets included.79 Although in this meta-analysis the differences in imaging vendor and software platforms did not explain the heterogeneity between the studies and were not significant variables in the meta-regression, it should not to be misconstrued to mean that vendor and software variable “do not influence strain or the reporting of strain reference values.” Until the EACVI/ASE/Industry task force analysis the role of the vendor (models) and software (versions) with respects to GCS and GRS (as they did with GLS)3, it is still important to interpret the reference ranges with respects to the vendor. We created vendor-specific normal ranges in this meta-analysis (Appendix 5).
Limitations
This meta-analysis did not provide reference values for circumferential or radial strain rate. Less than 25% of the eligible studies recorded these strain rate indices and most authors could not demonstrate significant reliability.22 There is still a paucity of studies that used radial or circumferential strain rate measurements in clinical practice to measure LV function in children. Furthermore, this meta-analysis showed that some of the ranges for CS and RS are wide and should be used with caution.
In this meta-analysis 84% (36) of the eligible data sets either performed or referenced “reproducibility analysis”. Reproducibility is one item that is combined with 11 other tools (Appendix 3) to give a summary score or checklist to assess the quality of a deformation imaging study in the context of a systematic review.73 Quality scoring with a checklist is utilized to limit the extent of bias in a given study, but the assessment of the validity of any study involves a degree of subjectivity.75 The quality of a study is based on three categories: a) quality of reporting, b) external validity, and c) internal validation. The failure to meet one or more of the checklist items does not imply that the manuscript should be excluded from the meta-analysis, but rather that quality assessment is diminished for that specific category.75,78 The eligible datasets met > 75% of the quality checklist items, which is similar to other deformation meta-analysis studies.73 There is ongoing work focused on the reproducibility of strain measures using different software analysis packages in this post-standardization era of deformation imaging.3,88
The “Peak” strain may coincide with one of three clinically significant time points: A) Systolic, before the closure of the AV valve; B) End-systolic peak (ESS), at the closure of the AV valve, or C) Post-systolic strain (PSS), after aortic valve closure.74 In this meta-analysis, the location of reported strain value along the strain curve from each eligible study was not a significant determinant of the variation among normal ranges of strain values. Eighteen out of 43 eligible data sets reported the strain time point as “systolic” strain,8,9,11,13,14,19,21,23,27,28,31,36,38,41,45-48 five reported the strain time point as ESS,16,17,23,24,48 two reported the “Peak” as the highest strain value at any time point during one cardiac cycle for each patient, irrespective of its location,20,33 and 20 did not specify the time point location along the strain curve (Table 2).7,10,12,15,18,22,25,26,29,30,32,34,35,37,39,40,42-44,49 The lack of consistency in reporting of the strain value time point along the strain curve is due in part because only three47-49 of the eligible studies were published after the recommendations from Voigt et al to report ESS as the “default parameter for the description of myocardial deformation.”74 In healthy children (with higher heart rates than adults) the systolic and ESS points along the curve may or may not be visually distinguishable, but is most likely clinically insignificant. Systolic and ESS both describe the strain within the period during which the ventricle is ejecting. Further studies are needed to interpret the clinical significance of “Systolic” vs. “ESS” value in healthy children.
Although feasibility and reproducibility of deformation imaging has recently been established in premature infants,89 we did not include any studies that had premature infants (< 37 weeks gestational age at birth) because anthropometric parameters, blood pressure, heart rate, and pulmonary hemodynamics considerably change with each passing month of post-menstrual age. Future work will focus on the understanding the maturational patterns of strain in this age group.
Conclusions
In healthy children, the mean peak left ventricle global longitudinal strain value is -20.2%, (95% CI -19.5% to -20.8%), mean global circumferential strain -22.3%, (95% CI -19.9% to -24.6%), and mean global radial strain is 45.2%, (95% CI 38.3% to 51.7%). A significant apex-to-base gradient of the LV lateral wall longitudinal strain in healthy children was observed from the meta-analysis. Variations among different reference ranges do not appear to be dependent on differences in demographic, clinical, or equipment or vendor parameters in this meta-analysis.
Highlights.
A systematic review and meta-analysis was performed to establish the range ofreference values of two-dimensional speckle-tracking echocardiography (2DSTE) derived left ventricular (LV) strain is children.
The search identified 2325 children from 43 data sets.
The mean LV global longitudinal strain value is -20.2%, mean global circumferentialstrain -22.3% and mean global radial strain is 45.2%. LV strain does not vary by age.
LV segmental longitudinal strain has a stable apex-to-base gradient that is preservedthroughout maturation.
Acknowledgments
This study was supported by grants from Premature and Respiratory Outcomes Program (NIH 1U01 HL1014650, U01 HL101794).
Abbreviations
- LV
Left Ventricle
- 2DSTE
Two-dimensional speckle tracking echocardiography
- GLS
Global longitudinal strain
- GCS
Global circumferential strain
- GRS
Global radial strain
- Sr
Strain rate
- SLS
Segmental longitudinal strain
- FW
free wall
- I2
inconsistency index
- CI
confidence intervals
Appendix 1
Electronic Database Search Hedges
Five search engines were used to identify eligible articles in this review. The search strategies are listed below by their name (results): date of search.
EMBASE Search (314 results): November 3, 2015
(‘heart left ventricle’/exp OR ‘left cardiac ventricle’ OR ‘left heart ventricle’ OR ‘left ventricle’ OR ‘ventriculusi sinister’) AND (‘speckle tracking’ OR ‘speckle-tracking’ OR ‘STE-resolution’ OR ‘2D-STE’ OR ‘2DSTE’ OR ‘STE-Derived’ OR ‘2D STE’ OR ‘3D STE’ OR ‘two dimensional STE’ OR ‘Three dimensional STE’ OR ‘2D-strain echocardiography’ OR ((‘echocardiography’/exp OR ‘Echocardiography’ OR ‘tracking’ OR ‘imaging’) AND (‘speckles’ OR ‘speckle’ OR ‘STE’:ti OR ‘STE’:ab))) AND (‘pediatrics’/exp OR ‘child’/exp OR ‘adolescent’/exp OR ‘Child’ OR ‘Children’ OR ‘Children’ OR ‘toddler’ OR ‘toddlers’ OR ‘Infant’ OR ‘Infants’ OR ‘Newborn Infant’ OR ‘Newborn Infants’ OR ‘Newborns’ OR ‘Newborn’ OR ‘Neonate’ OR ‘Neonates’ OR ‘Adolescent’ OR ‘Adolescents’ OR Teen* OR ‘Youth’ OR ‘Youths’ OR ‘Adolescence’ OR ‘girl’ OR ‘girls’ OR ‘boy’ OR ‘boys’ OR ‘juvenile’ OR ‘juveniles’ OR ‘Pediatrics’ OR ‘pediatric’ OR ‘pediatry’ OR ‘section 7’) NOT ([animals]/lim NOT [humans]/lim)
MEDLINE Search (99 results): November 3, 2015
((((“Heart Ventricles”[Mesh] OR “Left Ventricle” OR “Left Ventricles” OR “left cardiac ventricle” OR “ventriculusi sinister”)) AND ((“speckle tracking”[All Fields] OR “speckletracking”[All Fields] OR “STE-resolution”[All Fields] OR “2D-STE”[All Fields] OR “2DSTE”[All Fields] OR “STE-Derived”[All Fields] OR “2D STE”[All Fields] OR “3D STE”[All Fields] OR “two dimensional STE”[All Fields] OR “Three dimensional STE”[All Fields] OR “2D-strain echocardiography”[All Fields] OR ((“Echocardiography”[Mesh] OR “Echocardiography”[All Fields] OR “tracking”[All Fields] OR “imaging”[All Fields]) AND (“speckles”[All Fields] OR “speckle”[All Fields] OR “STE”[tiab]))))) AND (“Child”[Mesh] OR “Infant”[Mesh] OR “Adolescent”[Mesh] OR “Pediatrics”[Mesh] OR “Child” OR “Children” OR “Children” OR “toddler” OR “toddlers” OR “Infant” OR “Infants” OR “Newborn Infant” OR “Newborn Infants” OR “Newborns” OR “Newborn” OR “Neonate” OR “Neonates” OR “Adolescent” OR “Adolescents” OR Teen* OR “Youth” OR “Youths” OR “Adolescence” OR “girl” OR “girls” OR “boy” OR “boys” OR “juvenile” OR “juveniles” OR “Pediatrics” OR “pediatric” OR “pediatry” OR “section 7”)) NOT ((“Animals”[Mesh] NOT (“Animals”[Mesh] AND “Humans”[Mesh])))
Cumulative Index of Nursing and Allied Health Literature, CINAHL search (30 results): November 3, 2015
((MH “Hypertrophy, Right Ventricular”) OR (MH “Hypertrophy, Left Ventricular”) OR (MH “Heart Hypertrophy”) OR (ventric?l* N3 overload) OR (cardiac N3 overload) OR (diastolic N3 overload) OR (heart N3 overload) OR (systolic N3 overload) OR (myocardi* N3 overload) OR (LV N3 overload) OR (ventric?l* N3 strain) OR (cardiac N3 strain) OR (diastolic N3 strain) OR (heart N3 strain) OR (systolic N3 strain) OR (myocardi* N3 strain) OR (LV N3 strain) OR (ventric?l* N3 deform*) OR (cardiac N3 deform*) OR (diastolic N3 deform*) OR (heart N3 deform*) OR (systolic N3 deform*) OR (myocardi* N3 deform*) OR (LV N3 Deform*) OR (ventric?l* N3 hypertrophy) OR (cardiac N3 hypertrophy) OR (heart N3 hypertrophy) OR (myocardi* N3 hypertrophy) OR (LV N3 hypertrophy) OR (heart N3 hyperplasia) OR (ventric?l* N3 “wall thickness”) OR (cardiac N3 “wall thickness”) OR (heart N3 “wall thickness”) OR (myocardi* N3 “wall thickness”) OR (LV N3 “wall thickness”) OR (ventric?l* N3 enlargement) OR (heart N3 enlargement) OR LV N3 enlargement)) AND ((MH “Infant”) OR (MH “Infant, Premature”) OR (MH “Infant, Low Birth Weight”) OR (MH “Infant, Very Low Birth Weight”) OR infant* OR newborn* OR preterm OR pre-term OR premature* OR neonat* OR (new N1 born) OR (low birth weight) OR (LBW N2 (infant OR neonate OR newborn)) OR (low birthweight)) AND ((speckle tracking) OR (speckle-tracking) OR (STE-resolution) OR (2D-STE) OR (2DSTE) OR (STE-Derived) OR (2D STE) OR (3D STE) OR (two dimensional STE) OR (Three dimensional STE) OR (2D-strain echocardiography) OR (((MH “Echocardiography”) OR “Echocardiography” OR “tracking” OR “imaging”) AND (“speckles” OR “speckle” OR (TI “STE”) OR (AB “STE”)))) NOT ((MH “Animals+”) NOT (MH “Animals+” AND MH “Human”))
SCOPUS search (157 results): November 3, 2015
(TITLE-ABS-KEY(“heart left ventricle” OR “left cardiac ventricle” OR “left heart ventricle” OR “left ventricle” OR “ventriculusi sinister”)) AND (TITLE-ABS-KEY(“speckle tracking” OR “speckle-tracking” OR “STE-resolution” OR “2D-STE” OR “2DSTE” OR “STE-Derived” OR “2D STE” OR “3D STE” OR “two dimensional STE” OR “Three dimensional STE” OR “2D-strain echocardiography” OR ((“Echocardiography” OR “Echocardiography” OR “tracking” OR “imaging”) AND (“speckles” OR “speckle” OR “STE”)))) AND (TITLE-ABS-KEY(“Child” OR “Infant” OR “Adolescent” OR “Pediatrics” OR “Child” OR “Children” OR “Children” OR “toddler” OR “toddlers” OR “Infant” OR “Infants” OR “Newborn Infant” OR “Newborn Infants” OR “Newborns” OR “Newborn” OR “Neonate” OR “Neonates” OR “Adolescent” OR “Adolescents” OR teen* OR “Youth” OR “Youths” OR “Adolescence” OR “girl” OR “girls” OR “boy” OR “boys” OR “juvenile” OR “juveniles” OR “Pediatrics” OR “pediatric” OR “pediatry” OR “section 7”)) AND (LIMIT-TO(EXACTKEYWORD, “Human”) OR LIMIT-TO(EXACTKEYWORD, “Humans”))
COCHRANE Library (3 results): November 3, 2015
Cochrane Database of Systematic Reviews (CDSR): 0
Database of Abstracts of Reviews of Effects (DARE): 0
Cochrane Central Register of Controlled Trials (CENTRAL): 3
(“Heart Ventricles” OR “heart left ventricle” OR “left cardiac ventricle” OR “left heart ventricle” OR “left ventricle” OR “ventriculusi sinister”) AND (“speckle tracking” OR “speckle-tracking” OR “STE-resolution” OR “2D-STE” OR “2DSTE” OR “STE-Derived” OR “2D STE” OR “3D STE” OR “two dimensional STE” OR “Three dimensional STE” OR “2D-strain echocardiography” OR ((“Echocardiography” OR “Echocardiography” OR “tracking” OR “imaging”) AND (“speckles” OR “speckle” OR “STE”))) AND (“Child” OR “Infant” OR “Adolescent” OR “Pediatrics” OR “Child” OR “Children” OR “Children” OR “toddler” OR “toddlers” OR “Infant” OR “Infants” OR “Newborn Infant” OR “Newborn Infants” OR “Newborns” OR “Newborn” OR “Neonate” OR “Neonates” OR “Adolescent” OR “Adolescents” OR Teen* OR “Youth” OR “Youths” OR “Adolescence” OR “girl” OR “girls” OR “boy” OR “boys” OR “juvenile” OR “juveniles” OR “Pediatrics” OR “pediatric” OR “pediatry” OR “section 7”)
Appendix 2
Handling of manuscripts that used the same control dataset in multiple published studies
The first or last authors of each of the multiple published studies that used the same control dataset were contacted by electronic mail, and one control dataset or manuscripts was either provided or chosen based on author recommendation.50-62,64-69 There were eight manuscripts eligible from Friedberg and Mertens et al. (Toronto)20,50-56 and six manuscripts eligible from Marcus et al. (Netherlands)16,58-62 that compared strain from a large database of overlapping pediatric controls to different disease outcomes in children, or healthy children. In consultation with the authors, the datasets from Fernandes et al.20 (Toronto) and Marcus et al.16 (Netherlands) were chosen as they contained the largest amount of control patients. (Reference values for LV strain imaging from a large pediatric and neonatal cohort from Toronto has been presented in abstract form and a manuscript, similar to their work on reference values of tissue Doppler imaging derived measures, is forthcoming).90-92 Gziri et al. recruited 34 control patients in Toronto and 28 patients in Belgium and these patients were included separately in the meta-analysis.31 There were six manuscripts from Klitsie et al. that utilized the same control populations;33,63-67 and Klitsie et al. 201333 was chosen because it included the most patients. Labombarda et al. also published two manuscripts42,69 that used overlapping control datasets and the larger data-set42 was used in the meta-analysis. Binnetoglu et al. used the same datasets in their two publications28,69, and Binnetoglu et al. 201328 was chosen because it included data on both “global” and “six-segment” strain values. Finally, Sanchez et al. 201445 and Singh et al. 201368 used the same control data set and the authors suggested using the manuscript by Sanchez et al. 45 in the analysis.
Appendix 3
Qualitative data eligible for data sets.
| Study | Year | Objective defined? | Outcome described? | Characteristics described? | Confounders described? | Main findings outlined? | Heterogeneous population | Strain imaging protocol | Individuals generating data blinded to outcomes | Sonographers blinded to outcome | Reproducibility analysis performed | Case/controls recruited over same time period? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bussadori et al.7 | 2008 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | Yes | Yes | Yes |
| Lorch et al.8 | 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Pettersen et al.9 | 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | Yes | NS |
| Roberson et al.10 | 2009 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Cheung et al. 11 | 2010 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes* | Yes |
| Koh et al. 12 | 2010 | Yes | Yes | Yes | Yes | Yes | No | Yes | No | No | No | No |
| Moiduddin et al.13 | 2010 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | No | No |
| Singh et al.14 | 2010 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Yu et al.15 | 2010 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Marcus et al.16 | 2011 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | Yes | Yes | Yes |
| Takayasu et al.17 | 2011 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Blanc et al.18 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Di Salvo et al.19 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Fernandes et al.20 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Hirth et al.21 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Koenigstein et al.22 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | No | Yes |
| Malev et al.23 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Poterucha et al.24 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Sato et al.25 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Takigiku et al.26 | 2012 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Barbosa et al.27 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Binnetoglu et al.28 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | No | Yes |
| Dogan et al.29 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | No | Yes |
| Elkiran et al.30 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes* | NS |
| Gziri et al.31 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes* | Yes |
| Hauser et al.32 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | No | Yes |
| Klitsie et al.33 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| McCandless et al.34 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | No | Yes |
| Ryan et al.35 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Schubert et al.36 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Sehgal et al.37 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Simsek et al.38 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Van der Ende et al.39 | 2013 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Black et al.40 | 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Labombarda et al.41 | 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Laser et al.42 | 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Li et al. 43 | 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | No | Yes | Yes |
| Mangner et al.44 | 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Sanchez et al.45 | 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Vitarelli et al.46 | 2014 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
| Al-Biltagi et al.47 | 2015 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes* | Yes |
| Burkett et al.48 | 2015 | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| Sainz et al.49 | 2015 | Yes | Yes | Yes | Yes | Yes | No | Yes | NS | NS | Yes | Yes |
NR, not recorded; GSl, Global longitudinal strain; GSc, Global circumferential strain; GSr, Global radial strain;
HR, Heart rate; SBP, Systolic blood pressure; BSA, Body surface area
TGA, transposition of the great arteries; LVNC, Left ventricle non-compaction; TOF, Tetralogy of Fallot, FH, Familial hypercholesterolemia; MVP, Mitral valve prolapse;
CAS, Congenital aortic stenosis; LVOTO, Left ventricle outflow tract obstruction; DM, Diabetes mellitus; CHD, Congenital heart disease; DMD, Duchenne Muscular Dystrophy
Appendix 4
Segmental longitudinal strain patterns in children.
| Age distribution (years) | Studies | Patients | SSl - Apex | SSl - Mid-ventricular | SSl - Base |
|---|---|---|---|---|---|
| “Global” SLS (%) | |||||
| 0 – 1 | 1 | 196 | -15.9% (-13.6, -16.5) | -14.9% (-14.2, -15.5) | -14.5% (-14.1 -15.3) |
| 2 - 9 | 2 | 75 | -25.2% (-21.10, -28.9) | -23.9% (-23.3, -24.5) | -20.9% (-20.3, -21.5) |
| 10 - 13 | 2 | 91 | -21.4% (-17.6, -25.2) | -19.9% (-16.0, -23.8) | -18.2% (-14.6, -21.7) |
| 14 - 21 | 4 | 123 | -20.4 % (-16.7, -22.0) | -19.2% (-16.2, -20.7) | -18.3% (-16.5, -21.1) |
| “Six segment” SLS (%) | |||||
| 0 - 1 | 3 | 250 | -18.3% (-15.8, -20.8) | -16.9% (-15.1, -18.6) | -16.8% (-14.7, -18.9) |
| 2 - 9 | 8 | 271 | -21.0% (-19.7, -21.7) | -19.8% (-18.4, -21.3) | -20.3% (-18.9, -21.8) |
| 10 - 13 | 1 | 29 | -23.9% (-23.3, -24.5) | -21.7% (-21.1, -22.3) | -20.0% (-19.6, -20.4) |
| 14 - 21 | 2 | 104 | -20.6% (-11.8,- 2.0) | -19.5% (-13.6,-25.5) | -19.7% (-18.3,-21.5) |
Data is presented as mean (95% Confidence interval)
“Global” SLS, Global segmental longitudinal strain refers to segmental strain calculated from segmental averaging of the three apical views, apical 4-, 3-, and 2- chambers.
“Six Segment” SLS, Six-segment longitudinal strain refers to segmental strain from the apical 4-chamber view only.
Note: An apex-to-base gradient of segmental longitudinal strain (SLS) exists throughout maturation from birth to 21 years of age.
Appendix 5
Reference mean values of left ventricle strain measures by vendor.
| Age distribution | Global longitudinal strain Mean GLS (CI) | Longitudinal strain - 4CH Mean LS (CI) | Global circumferential strain Mean GCS (CI) | Circumferential strain - Mid Mean CS (CI) | Global radial strain Mean GRS (CI) | Radial strain - Mid Mean RS (CI) |
|---|---|---|---|---|---|---|
| General Electric (GE) | ||||||
| 0 - 1 | -19.7% (-21.3, -18.2) | -20.7% (-23.7, -17.8) | NA | -20.1% (-23.2, -17.1) | NA | 46.3% (35.0, 57.6) |
| 2 - 9 | -22.9% (-23.9 -22.0) | -21.5% (-22.3, -20.6) | -22.5% (-24.8, -20.1) | -20.3% (-21.6, -19.0) | 54.5% (51.4.3,57.6) | 47.3% (39.0, 55.5) |
| 10 - 13 | -19.8% (-21.0, -18.7) | -20.5% (-21.9, -19.0) | -21.9% (-26.5, -17.4) | -21.8% (-23.3, -20.4) | 43.7% (33.0, 54.5) | 52.4% (48.8, 56.0) |
| 14 - 21 | -19.7% (-20.5, -18.9) | -19.9% (-21.2, -18.6) | -16.4% (-23.3, -9.6) | -19.9% (-23.0, -16.8) | 44.0% (41.6, 46.4) | 47.9% (41.5, 54.2) |
| Overall | -20.3% (-21.1, -19.4) | -20.,% (-21.4, -20.2) | -20.9% (-23.3, -18.5) | -20.6% (-21.6, -19.7) | 45.4% (38.8, 52.0) | 48.5% (45.0, 51.9) |
| Philips | ||||||
| 0 - 1 | NA | -NA | NA | NA | NA | NA |
| 2 - 9 | -20.1% (-20.7, -19.6) | -20.5% (-26.2, -14.8) | -22.4% (-23.2, -21.6) | NA | 34.6% (32.7, 36.5) | NA |
| 10 - 13 | -20.6% (-21.1, -20.1) | NA | NA | -19.0% (-19.6, -18.4) | NA | 29.5% (28.1, 31.0) |
| 14 - 21 | -20.8 %(-21.4, -20.2) | NA | NA | -18.0% (-20.2, -15.9) | NA | 35.9% (21.3, 50.5) |
| Overall | -20.5% (-20.8, -20.1) | NA | NA | -18.4% (-19.7, -17.1) | NA | 32.2% (28.2, 36.3) |
| Siemens | ||||||
| 0 - 1 | NA | -18.2 (-20.9, -16.0) | NA | -18.2% (-22.6, -13.7) | NA | 44.4% (36.6, 52.1) |
| 2 - 9 | NA | -18.5 (-19.8, -17.3) | -13.4%(-16.2, -10.4) | -20.3% (-21.4, -19.1) | NA | 50.8% (47.4,54.1) |
| 10 - 13 | NA | -20.4 (-22.2, -18.7) | NA | -21.5% (-23.1, -19.8) | NA | 52.1% (48.5,55.8) |
| 14 - 21 | NA | NA | NA | -19.7% (-22.1, -17.4) | NA | 46.4% (39.7,53.1) |
| Overall | NA | -19.1% (-20.5, -17.8) | NA | -21.4% (-20.6, -22.4) | NA | 49.4% (47.2,51.6) |
| Esaote | ||||||
| 0 - 1 | -16.7% (-17.3, -11.2) | -15.1% (-15.8, -14.5) | NA | -14.2% (-15.1, -13.3) | NA | NA |
| 2 - 9 | NA | -22.2% (-23.7, -20.6) | -25.6% (-29.1, -22.1) | -24.0% (-27.0, -21.0) | NA | NA |
| 10 - 13 | NA | -NA | NA | NA | NA | NA |
| 14 - 21 | NA | -NA | NA | NA | NA | NA |
| Overall | NA | -18.6% (-25.5, -11.7) | NA | -19.0% (-28.6, -9.4) | NA | NA |
Data presented as mean (confidence interval); NA, not applicable (no studies in this age range)
CI, 95% confidence interval
GLS, Global longitudinal strain; LS, Longitudinal strain from the apical 4-chamber view
GCS, Global circumferential strain; CS, Circumferential strain at the mid-ventricular level (papillary muscle)
GRS, Global radial strain; RS, Radial strain at the mid-ventricular level (papillary muscle)
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yingchoncharoen T, Agarwal S, Popovic ZB, Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–91. doi: 10.1016/j.echo.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–69. doi: 10.1016/j.echo.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Farsalinos KE, Daraban AM, Ünlü S, Thomas JD, Badano LP, Voigt JU. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors. J Am Soc Echocardiogr. 2015;28:1171–81. doi: 10.1016/j.echo.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Greenbaum RA, Ho SY, Gibson DG, Becker AE, Anderson RH. Left ventricular fibre architecture in man. Br Heart J. 1981;45:248–63. doi: 10.1136/hrt.45.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Streeter DDJ, Spotnitz HM, Patel DP, Ross JJ, Sonnenblick EH. Fiber orientation in the canine left ventricle during diastole and systole. Circulation Research. 1969;24:339–47. doi: 10.1161/01.res.24.3.339. [DOI] [PubMed] [Google Scholar]
- 6.Buckberg G, Hoffman JIE, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118:2571–87. doi: 10.1161/CIRCULATIONAHA.107.754424. [DOI] [PubMed] [Google Scholar]
- 7.Bussadori C, Moreo A, Di Donato M, De Chiara B, Negura D, Dall'Aglio E, et al. A new 2D-based method for myocardial velocity strain and strain rate quantification in a normal adult and paediatric population: assessment of reference values. Cardiovascular ultrasound. 2009;7:1–11. doi: 10.1186/1476-7120-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lorch SM, Ludomirsky A, Singh GK. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J Am Soc Echocardiogr. 2008;21:1207–15. doi: 10.1016/j.echo.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Pettersen E, Fredriksen PM, Urheim S, Thaulow E, Smith HJ, Smevik B, et al. Ventricular function in patients with transposition of the great arteries operated with arterial switch. Am J Cardiol. 2009;104:583–9. doi: 10.1016/j.amjcard.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 10.Roberson DA, Cui W. Tissue Doppler Imaging Measurement of Left Ventricular Systolic Function in Children: Mitral Annular Displacement Index Is Superior to Peak Velocity. J Am Soc Echocardiogr. 2009;22:376–82. doi: 10.1016/j.echo.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Cheung EWY, Liang XC, Lam WWM, Cheung YF. Impact of right ventricular dilation on left ventricular myocardial deformation in patients after surgical repair of tetralogy of fallot. Am J Cardiol. 2009;104:1264–70. doi: 10.1016/j.amjcard.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Koh C, Hong WJ, Cheung YF. Systolic-diastolic coupling of myocardial deformation of the left ventricle in children with left ventricular noncompaction. Heart Vessels. 2010;25:493–9. doi: 10.1007/s00380-010-0001-8. [DOI] [PubMed] [Google Scholar]
- 13.Moiduddin N, Texter KM, Zaidi AN, Herskenson JA, Stefaniak CA, Hayes J, et al. Two-dimensional speckle strain and dyssynchrony in single right ventricles versus normal right ventricles. J Am Soc Echocardiogr. 2010;23:673–97. doi: 10.1016/j.echo.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 14.Singh GK, Cupps B, Pasque M, Woodard PK, Holland MR, Ludomirsky A. Accuracy and reproducibility of strain by speckle tracking in pediatric subjects with normal heart and single ventricular physiology: a two- dimensional speckle-tracking echocardiography and magnetic resonance imaging correlative study. J Am Soc Echocardiogr. 2010;23:1143–52. doi: 10.1016/j.echo.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Hulst AE, Delgado V, Holman ER, Kroft LJM, de Roos A, Hazekamp MG, et al. Relation of left ventricular twist and global strain with right ventricular dysfunction in patients after operative “correction” of tetralogy of fallot. Am J Cardiol. 2010;106:723–9. doi: 10.1016/j.amjcard.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Yu JJ, Choi HS, Kim YB, Son JS, Kim YH, Ko JK, et al. Analyses of left ventricular myocardial deformation by speckle-tracking imaging during the acute phase of Kawasaki disease. Pediatr Cardiol. 2010;31:807–12. doi: 10.1007/s00246-010-9708-7. [DOI] [PubMed] [Google Scholar]
- 17.Takayasu H, Takahashi K, Takigiku K, Yasukochi S, Furukawa T, Akimoto K, et al. Left ventricular torsion and strain in patients with repaired tetralogy of Fallot assessed by speckle tracking imaging. Echocardiography. 2011;28:720–9. doi: 10.1111/j.1540-8175.2011.01417.x. [DOI] [PubMed] [Google Scholar]
- 18.Marcus KA, Mavinkurve-Groothuis AMC, Barends M, van Dijk A, Feuth T, de Korte C, et al. Reference Values for Myocardial Two-Dimensional Strain Echocardiography in a Healthy Pediatric and Young Adult Cohort. J Am Soc Echocardiogr. 2011;24:625–36. doi: 10.1016/j.echo.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Blanc J, Stos B, de Montalembert M, Bonnet D, Boudjemline Y. Right ventricular systolic strain is altered in children with sickle cell disease. J Am Soc Echocardiogr. 2012;25:511–7. doi: 10.1016/j.echo.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 20.Di Salvo G, D'Aiello AF, Castaldi B, Fadel B, Limongelli G, D'Andrea A, et al. Early left ventricular abnormalities in children with heterozygous familial hypercholesterolemia. J Am Soc Echocardiogr. 2012;25:1075–82. doi: 10.1016/j.echo.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes FP, Manlhiot C, Roche SL, Grosse-Wortmann L, Slorach C, McCrindle BW, et al. Impaired left ventricular myocardial mechanics and their relation to pulmonary regurgitation, right ventricular enlargement and exercise capacity in asymptomatic children after repair of tetralogy of Fallot. J Am Soc Echocardiogr. 2012;25:494–503. doi: 10.1016/j.echo.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Hirth A, Edwards NC, Greve G, Tangeraas T, Gerdts E, Lenes K, et al. Left ventricular function in children and adults after renal transplantation in childhood. Pediatr Nephrol. 2012;27:1565–74. doi: 10.1007/s00467-012-2167-z. [DOI] [PubMed] [Google Scholar]
- 23.Koenigstein K, Raedle-Hurst T, Hosse M, Hauser M, Abdul-Khaliq H. Altered Diastolic Left Atrial and Ventricular Performance in Asymptomatic Patients After Repair of Tetralogy of Fallot. Pediatr Cardiol. 2012:1–6. doi: 10.1007/s00246-012-0584-1. [DOI] [PubMed] [Google Scholar]
- 24.Malev E, Zemtsovsky E, Pshepiy A, Timofeev E, Reeva S, Prokudina M. Evaluation of left ventricular systolic function in young adults with mitral valve prolapse. Exp Clin Cardiol. 2012;17:165–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:733–40. doi: 10.1016/j.echo.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Sato Y, Maruyama A, Ichihashi K. Myocardial strain of the left ventricle in normal children. Journal of Cardiology J Cardiol. 2012;60:145–9. doi: 10.1016/j.jjcc.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 27.Takigiku K, Takeuchi M, Izumi C, Yuda S, Sakata K, Ohte N, et al. Normal range of left ventricular 2-dimensional strain: Japanese Ultrasound Speckle Tracking of the Left Ventricle (JUSTICE) study. Circ J. 2012;76:2623–32. doi: 10.1253/circj.cj-12-0264. [DOI] [PubMed] [Google Scholar]
- 28.Barbosa JAA, Mota CCC, Simoes E, Silva AC, Nunes MDCP, Barbosa MM. Assessing pre-clinical ventricular dysfunction in obese children and adolescents: the value of speckle tracking imaging. Eur Heart J Cardiovasc Imaging. 2013;14:882–9. doi: 10.1093/ehjci/jes294. [DOI] [PubMed] [Google Scholar]
- 29.Binnetoğlu FK, Babaoğlu K, Altun G, Kayabey Ö. Effects That Different Types of Sports Have on the Hearts of Children and Adolescents and the Value of Two-Dimensional Strain-Strain-Rate Echocardiography. Pediatr Cardiol. 2013;35:126–39. doi: 10.1007/s00246-013-0751-z. [DOI] [PubMed] [Google Scholar]
- 30.Dogan V, Öcal B, Orun UA, Ozgur S, Yılmaz O, Keskin M, et al. Strain and strain rate echocardiography findings in children with asymptomatic congenital aortic stenosis. Pediatr Cardiol. 2013;34:1152–8. doi: 10.1007/s00246-012-0619-7. [DOI] [PubMed] [Google Scholar]
- 31.Elkiran O, Karakurt C, Kocak G, Karadag A. Tissue Doppler, strain, and strain rate measurements assessed by two-dimensional speckle-tracking echocardiography in healthy newborns and infants. Cardiol Young. 2014;24:201–11. doi: 10.1017/S1047951112002284. [DOI] [PubMed] [Google Scholar]
- 32.Gziri MM, Hui W, Amant F, Van Calsteren K, Ottevanger N, Kapusta L, et al. Myocardial function in children after fetal chemotherapy exposure. A tissue Doppler and myocardial deformation imaging study. Eur J Pediatr. 2013;172:163–70. doi: 10.1007/s00431-012-1849-7. [DOI] [PubMed] [Google Scholar]
- 33.Hauser M, Kuehn A, Petzuch C, Schoen P, Elmenhorst J, Schoenfelder M, et al. The Munich Triathlon Heart Study: ventricular function, myocardial velocities and 2-D strain in healthy children before and after endurance stress. Pediatr Cardiol. 2013;34:576–82. doi: 10.1007/s00246-012-0500-8. [DOI] [PubMed] [Google Scholar]
- 34.Klitsie LM, Roest AAW, Van der Hulst AE, Stijnen T, Blom NA, Harkel Ten ADJ. Assessment of intraventricular time differences in healthy children using two-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2013;26:629–39. doi: 10.1016/j.echo.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 35.McCandless RT, Minich LL, Wilkinson SE, McFadden ML, Tani LY, Menon SC. Myocardial strain and strain rate in Kawasaki disease. Eur Heart J Cardiovasc Imaging. 2013;14:1061–8. doi: 10.1093/ehjci/jet041. [DOI] [PubMed] [Google Scholar]
- 36.Ryan TD, Taylor MD, Mazur W, Cripe LH, Pratt J, King EC, et al. Abnormal Circumferential Strain is Present in Young Duchenne Muscular Dystrophy Patients. Pediatr Cardiology. 2013;34:1159–65. doi: 10.1007/s00246-012-0622-z. [DOI] [PubMed] [Google Scholar]
- 37.Schubert U, Muller M, Norman M, Abdul-Khaliq H. Transition from fetal to neonatal life: Changes in cardiac function assessed by speckle-tracking echocardiography. Early Hum Dev. 2013;89:803–8. doi: 10.1016/j.earlhumdev.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Sehgal A, Wong F, Menahem S. Speckle tracking derived strain in infants with severe perinatal asphyxia: a comparative case control study. Cardiovascular ultrasound Cardiovascular Ultrasound. 2013;11:1–8. doi: 10.1186/1476-7120-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simsek Z, Hakan Tas M, Degirmenci H, Gokhan Yazıcı A, Ipek E, Duman H, et al. Speckle Tracking Echocardiographic Analysis of Left Ventricular Systolic and Diastolic Functions of Young Elite Athletes with Eccentric and Concentric Type of Cardiac Remodeling. Echocardiography. 2013;30:1202–8. doi: 10.1111/echo.12263. [DOI] [PubMed] [Google Scholar]
- 40.Van der Ende J, Vázquez Antona CA, Erdmenger Orellana J, Romero Cárdenas Á, Roldan FJ, Vargas Barrón J. Left ventricular longitudinal strain measured by speckle tracking as a predictor of the decrease in left ventricular deformation in children with congenital stenosis of the aorta or coarctation of the aorta. Ultrasound Med Biol. 2012;39:1207–14. doi: 10.1016/j.ultrasmedbio.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Black D, Bryant J, Peebles C, Davies L, Inskip H, Godfrey K, et al. Increased regional deformation of the left ventricle in normal children with increased body mass index: Implications for future cardiovascular health. Pediatr Cardiol. 2014;35:315–22. doi: 10.1007/s00246-013-0778-1. [DOI] [PubMed] [Google Scholar]
- 42.Labombarda F, Leport M, Morello R, Ribault V, Kauffman D, Brouard J, et al. Longitudinal left ventricular strain impairment in type 1 diabetes children and adolescents: A 2D speckle strain imaging study. Diabetes Metab. 2014;40:292–8. doi: 10.1016/j.diabet.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 43.Laser KT, Haas NA, Fischer M, Habash S, Degener F, Prinz C, et al. Left ventricular rotation and right–left ventricular interaction in congenital heart disease: the acute effects of interventional closure of patent arterial ducts and atrial septal defects. Cardiol Young. 2014;24:661–74. doi: 10.1017/S1047951113000978. [DOI] [PubMed] [Google Scholar]
- 44.Li Y, Xie M, Wang X, Lü Q, Zhang L, Ren P. Impaired right and left ventricular function in asymptomatic children with repaired tetralogy of fallot by two-dimensional speckle tracking echocardiography study. Echocardiography. 2015;32:135–43. doi: 10.1111/echo.12581. [DOI] [PubMed] [Google Scholar]
- 45.Mangner N, Scheuermann K, Winzer E, Wagner I, Hoellriegel R, Sandri M, et al. Childhood Obesity Impact on Cardiac Geometry and Function. JACC Cardiovasc Imaging. 2014;7:1198–1205. doi: 10.1016/j.jcmg.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Sanchez AA, Levy PT, Sekarski TJ, Arbelaez AM, Hildrebolt CF, Holland MR, et al. Markers of Cardiovascular Risk and Insulin Resistance are Associated with Subclinical Ventricular Dysfunction and Remodeling in Obese Adolescents. J Pediatr. 2015;166:660–5. doi: 10.1016/j.jpeds.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vitarelli A, Martino F, Capotosto L, Martino E, Colantoni C, Ashurov R, et al. Early myocardial deformation changes in hypercholesterolemic and obese children and adolescents: a 2D and 3D speckle tracking echocardiography study. Medicine. 2014;93(1-10):e71. doi: 10.1097/MD.0000000000000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al-Biltag M, Rab OA, Tolba E, Rowisha MA, El-Sayed Mahfouz A, Elewa MA. Speckle Tracking and Myocardial Tissue Imaging in Infant of Diabetic Mother with Gestational and Pregestational Diabetes. Pediatr Cardiol. 2014;36:445–53. doi: 10.1007/s00246-014-1033-0. [DOI] [PubMed] [Google Scholar]
- 49.Burkett DA, Slorach C, Patel SS, Redington AN, Ivy DD, Mertens L, et al. Pulmonary Arterial HypertensionLeft Ventricular Myocardial Function in Children With Pulmonary Hypertension. Circ Cardiovasc Imaging. 2015;8:1–10. doi: 10.1161/CIRCIMAGING.115.003260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sainz T, Álvarez-Fuente M, Fernández-Jiménez R, González-Tomé MI, de José MI, Ramos JT, et al. Cardiac Function in Vertically HIV-infected Children and Adolescents in the Era of Highly Active Antiretroviral Therapy. Pediatr Infect Dis J. 2015;34:e125–31. doi: 10.1097/INF.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 51.Friedberg MK, Slorach C. Relation between left ventricular regional radial function and radial wall motion abnormalities using two-dimensional speckle tracking in children with idiopathic dilated cardiomyopathy. Am J Cardiol. 2008;102:335–9. doi: 10.1016/j.amjcard.2008.03.064. [DOI] [PubMed] [Google Scholar]
- 52.Dragulescu A, Friedberg MK, Grosse-Wortmann L, Redington A, Mertens L. Effect of chronic right ventricular volume overload on ventricular interaction in patients after Tetralogy of Fallot repair. J Am Soc Echocardiogr. 2014;27:896–902. doi: 10.1016/j.echo.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 53.Dragulescu A, Grosse-Wortmann L, Redington A, Friedberg MK, Mertens L. Differential effect of right ventricular dilatation on myocardial deformation in patients with atrial septal defects and patients after tetralogy of Fallot repair. Int J Cardiol. 2012;168:803–10. doi: 10.1016/j.ijcard.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 54.Forsey J, Benson L, Rozenblyum E, Friedberg MK, Mertens L. Early changes in apical rotation in genotype positive children with hypertrophic cardiomyopathy mutations without hypertrophic changes on 2D imaging. J Am Soc Echocardiogr. 2014;27:215–21. doi: 10.1016/j.echo.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 55.Koopman LP, Slorach C, Hui W, Manlhiot C, McCrindle BW, Friedberg MK, et al. Comparison between different speckle tracking and color tissue Doppler techniques to measure global and regional myocardial deformation in children. J Am Soc Echocardiogr. 2010;23:919–28. doi: 10.1016/j.echo.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 56.Koopman LP, Slorach C, Manlhiot C, McCrindle BW, Jaeggi ET, Mertens L, et al. Assessment of myocardial deformation in children using Digital Imag- ing and Communications in Medicine (DICOM) data and vendor indepen- dent speckle tracking software. J Am Soc Echocardiogr. 2011;24:37–44. doi: 10.1016/j.echo.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Koopman LP, McCrindle BW, Slorach C, Chahal N, Hui W, Sarkola T, et al. Interaction between myocardial and vascular changes in obese children: A pilot study. J Am Soc Echocardiogr. 2012;25:401–11. doi: 10.1016/j.echo.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 58.Labombarda F, Zangl E, Dugue AE, Bougle D, Pellissier A, Ribault V, et al. Alterations of left ventricular myocardial strain in obese children. Eur Heart J Cardiovasc Imaging. 2013;14:668–76. doi: 10.1093/ehjci/jes238. [DOI] [PubMed] [Google Scholar]
- 59.Mavinkurve-Groothuis AM, Weijers G, Groot-Loonen J, Pourier M, Feuth T, de Korte CL, et al. Interobserver, intraobserver and intrapatient reliability scores of myocardial strain imaging with 2-d echocardiography in patients treated with anthracyclines. Ultrasound Med Biol. 2009;35:697–704. doi: 10.1016/j.ultrasmedbio.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 60.Marcus KA, Barends M, Morava-Kozicz E, Feuth T, de Korte CL, Kapusta L. Early detection of myocardial dysfunction in children with mitochondrial disease: An ultrasound and two-dimensional strain echocardiography study. Mitochondrion. 2011;11:405–12. doi: 10.1016/j.mito.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 61.Marcus KA, Janousek J, Barends ME, Weijers G, de Korte CL, Kapusta L. Synchronicity of systolic deformation in healthy pediatric and young adult subjects: a two-dimensional strain echocardiography study. Am J Physiol Heart Circ Physiol. 2012;302:196–205. doi: 10.1152/ajpheart.00740.2011. [DOI] [PubMed] [Google Scholar]
- 62.Marcus KA, de Korte CL, Feuth T, Thijssen JM, van Oort AM, Tanke RB, Kapusta L. Persistent reduction in left ventricular strain using two-dimensional speckle-tracking echocardiography after balloon valvuloplasty in children with congenital valvular aortic stenosis. J Am Soc Echocardiogr. 2012;25:473–85. doi: 10.1016/j.echo.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Mavinkurve-Groothuis AMl, Marcus KA, Pourier M, Loonen J, Feuth T, Hoogerbrugge PM, de Korte CL, et al. Myocardial 2D strain echocardiography and cardiac biomarkers in children during and shortly after anthracycline therapy for acute lymphoblastic leukaemia (ALL): A prospective study. Eur Heart J Cardiovasc Imaging. 2013;14:562–9. doi: 10.1093/ehjci/jes217. [DOI] [PubMed] [Google Scholar]
- 64.Klitsie LM, Kuipers IM, Roest AA, Van der Hulst AE, Stijnen T, Hazekamp MG, et al. Disparity in right vs left ventricular recovery during follow-up after ventricular septal defect correction in children. Eur J Cardiothorac Surg. 2013;44:269–74. doi: 10.1093/ejcts/ezt003. [DOI] [PubMed] [Google Scholar]
- 65.Klitsie LM, Roest AA, Haak MC, Blom NA, Ten Harkel AD. Longitudinal follow-up of ventricular performance in healthy neonates. Early Hum Dev. 2013;89:993–7. doi: 10.1016/j.earlhumdev.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Klitsie LM, Roest AA, Kuipers IM, Van der Hulst AE, Hazekamp MG, Blom NA, et al. Enhanced characterization of ventricular performance after coarctation repair in neonates and young children. Ann Thorac Surg. 2013;96:629–36. doi: 10.1016/j.athoracsur.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 67.Klitsie LM, Roest AA, Kuipers IM, Hazekamp MG, Blom NA, Ten Harkel AD. Left and right ventricular performance after arterial switch operation. J Thorac Cardiovasc Surg. 2014;147:1561–7. doi: 10.1016/j.jtcvs.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 68.Singh GK, Vitola BE, Holland MR, Sekarski T, Patterson BW, Magkos F, et al. Alterations in ventricular structure and function in obese adolescents with nonalcoholic fatty liver disease. J Pediatr. 2013;16:1160–8. doi: 10.1016/j.jpeds.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Binnetoğlu FK, Yıldırım §, Topaloğlu N, Tekin M, Kaymaz N, Aylanç H, et al. Early detection of myocardial deformation by 2D speckle tracking echocardiography in normotensive obese children and adolescents. Anatol J Cardiol. 2015;15:151–7. doi: 10.5152/akd.2014.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Akao M, Katsube Y, Kamisago M, Watanabe M, Abe M, Fukazawa R, et al. Developmental changes in left and right ventricular function evaluated with color tissue doppler imaging and strain echocardiography. J Nippon Med Sch. 2013;80:260–7. doi: 10.1272/jnms.80.260. [DOI] [PubMed] [Google Scholar]
- 71.Tham EB, Smallhorn JF, Kaneko S, Valiani S, Myers KA, Colen TM, et al. Insights into the evolution of myocardial dysfunction in the functionally single right ventricle between staged palliations using speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27:314–22. doi: 10.1016/j.echo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 72.Kaul S, Miller JG, Grayburn PA, Hashimoto S, Hibberd M, Holland MR, et al. A suggested roadmap for cardiovascular ultrasound research for the future. J Am Soc Echocardiogr. 2011;24:455–64. doi: 10.1016/j.echo.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 73.Levy PT, Sanchez A, Machefsky A, Fowler S, Holland MR, Singh GK. Normal ranges of right ventricular systolic and diastolic strain measures in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2014;27:549–60. doi: 10.1016/j.echo.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Voigt JU, Pedrizzetti G, Lysyansky P, Marwick TH, Houle H, Baumann R, et al. Definitions for a Common Standard for 2D Speckle Tracking Echocardiography: Consensus Document of the EACVI/ASE/Industry Task Force to Standardize Deformation Imaging. J Am Soc Echocardiogr. 2015;28:183–93. doi: 10.1016/j.echo.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 75.Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions: Cochrane book series. Copenhagen, Denmark: Cochrane Collaboration; 2008. [Google Scholar]
- 76.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez AA, Levy PT, Sekarski TJ, Hamvas A, Holland MR, Singh GK. Effects of Frame Rate on Two-Dimensional Speckle Tracking–Derived Measurements of Myocardial Deformation in Premature Infants. Echocardiography. 2015;32:839–47. doi: 10.1111/echo.12716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Downs SH, Black N. The feasibility of creating a checklist for the assess- ment of the methodological quality both of randomised and non- randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jashari H, Rydberg A, Ibrahimi P, Bajraktari G, Kryeziu L, Jashari F, et al. Normal ranges of left ventricular strain in children: a meta-analysis. Cardiovascular Ultrasound. 2015;13:37. doi: 10.1186/s12947-015-0029-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fine NM, Chen L, Bastiansen PM, Frantz RP, Pellikka PA, Oh JK, et al. Reference Values for Right Ventricular Strain in Patients without Cardiopulmonary Disease: A Prospective Evaluation and Meta-Analysis. Echocardiography. 2015;32:787–96. doi: 10.1111/echo.12806. [DOI] [PubMed] [Google Scholar]
- 81.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100:1673–80. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 82.Cantinotti M, Kutty S, Giordano R, Assanta N, Murzi B, Crocetti M, et al. Review and status report of pediatric left ventricular systolic strain and strain rate nomograms. Heart Fail Rev. 2015:601–12. doi: 10.1007/s10741-015-9492-9. [DOI] [PubMed] [Google Scholar]
- 83.Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert Consensus for Multimodality Imaging Evaluation of Adult Patients during and after Cancer Therapy: A Report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–39. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 84.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of Myocardial Strain Imaging by Echocardiography for the Early Detection of Cardiotoxicity in Patients During and After Cancer Chemotherapy. J Am Coll Cardiol. 2014;63:2751–68. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 85.Mingo-Santos S, Moñivas-Palomero V, Garcia-Lunar I, Mitroi CD, Goirigolzarri-Artaza J, Rivero B, et al. Usefulness of Two-Dimensional Strain Parameters to Diagnose Acute Rejection after Heart Transplantation. J Am Soc Echocardiogr. 2015;28:1149–56. doi: 10.1016/j.echo.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 86.Zhang L, Gao J, Xie M, Yin P, Liu W, Li Y, et al. Left ventricular three- dimensional global systolic strain by real-time three-dimensional speckle-tracking in children: feasibility, reproducibility, maturational changes, and normal ranges. J Am Soc Echocardiogr. 2013;26:853–9. doi: 10.1016/j.echo.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 87.Kaku K, Takeuchi M, Tsang W, Takigiku K, Yasukochi S, Patel AR, et al. Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27:55–64. doi: 10.1016/j.echo.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Yang H, Marwick TH, Fukuda N, Oe H, Saito M, Thomas JD, et al. Improvement in Strain Concordance between Two Major Vendors after the Strain Standardization Initiative. J Am Soc Echocardiogr. 2015;28:642–8. doi: 10.1016/j.echo.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 89.Levy PT, Holland MR, Sekarski T, Hamvas A, Singh GK. Feasibility and reproducibility of right ventricular strain measurements by speckle tracking echocardiography. J Am Soc Echocardiogr. 2013;26:1201–13. doi: 10.1016/j.echo.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cifra B, Dragulescu A, Slorach C, Manlhiot C, Friedberg MK, Mertens L. Reference values for myocardial two-dimensional strain echocardiography during exercise in children. J Am Soc Echocardiogr. 2014;27:B79. Abstract. [Google Scholar]
- 91.Dallaire F, Slorach C, Hui W, Sarkola T, Friedberg MK, Bradley TJ, et al. Reference values for pulse wave Doppler and tissue Doppler imaging in pediatric echocardiography. Circ Cardiovasc Imaging. 2015;8:1–9. doi: 10.1161/CIRCIMAGING.114.002167. [DOI] [PubMed] [Google Scholar]
- 92.Hauck A, Landeck B, Manlhiot C, Dragulescu A, Friedberg M, Younoszai A, et al. Global Longitudinal Strain in Different Right Ventricular Walls Using Alternate Right Ventricular Apical Views: Validation and Establishment of Normal Values in Children. Circulation. 2014;130:A19604. Absract. [Google Scholar]


