Abstract
Children with behavioral inhibition (BI), a temperament characterized by biologically-based hyper-vigilance to novelty, display threat-related attention biases (AB) that shape developmental trajectories of risk for anxiety. Here we explore the relations between BI, neural function, and anxiety. Fifty-six 9–12-year-olds (23 behaviorally inhibited) performed the dot-probe task while undergoing fMRI. AB scores were not associated with BI group or parent-rated anxiety symptoms. Trials requiring attention orienting away from threat engaged an executive and threat-attention network (dlPFC, vlPFC, mPFC, and amygdala). Within that network, behaviorally inhibited children showed greater activation in the right dlPFC. Heightened dlPFC activation related to increased anxiety, and BI levels accounted for the direct relation between dlPFC activation and anxiety. Behaviorally inhibited children may engage the executive attention system during threat-related processing as a compensatory mechanism. We provide preliminary evidence that the link between PFC functioning and anxiety might be attributed to early-emerging temperamental vulnerabilities present before the emergence of clinical anxiety.
Keywords: attention bias, fMRI, anxiety, temperament, dorsolateral prefrontal cortex
Introduction
Behavioral inhibition (BI) is a biologically-based, early-appearing temperament characterized by heightened vigilance and reactivity to novel sensory stimuli in infancy (Kagan et al., 1984), social reticence from toddlerhood to school age (Fox et al., 2001; 2005), and sensitivity to novel situations, particularly if social in nature. Stable BI across childhood is a risk factor for subsequent anxiety disorders (Chronis-Tuscano et al., 2009; Clauss & Blackford, 2012; Perez-Edgar & Fox, 2005). However, there are individual differences in the developmental trajectories of risk, such that not all children with BI develop or have concurrent anxiety problems (Degnan & Fox, 2007). Thus, examining variations in socioemotional information processing and its underlying neural correlates is important for identifying temperament-linked biological processes that potentiate vulnerability for anxiety among behaviorally inhibited children (Henderson, Pine, & Fox, 2015).
A growing number of functional neuroimaging studies (see Caouette & Guyer, 2014; Henderson et al., 2015, for reviews) have shown that adolescents and adults with a history of early BI, compared to non-inhibited individuals, display aberrant responding in neurocircuitries that overlap with networks associated with anxiety disorders (Blackford & Pine, 2012; Guyer, Masten, & Pine, 2013), even in the absence of the psychopathology. For example, young adults characterized with BI show atypical amygdala responses to novel faces (greater magnitude: Schwartz et al., 2003, faster latency: Blackford et al., 2009, and failure to habituate: Blackford et al., 2012; Schwartz et al., 2012). Among adolescents, a history of BI was associated with exaggerated amygdala activation when attending to their fear of emotional faces (Perez-Edgar et al., 2007). Considering the appetitive-motivational circuitry, adolescents with a childhood history of BI also displayed enhanced striatal reactivity during reward anticipation and receipt (Bar-Haim et al., 2009; Guyer et al., 2006; 2014; Helfinstein et al., 2011; Perez-Edgar et al., 2014a). Assessing neural functioning underlying cognitive control, young adults with early BI exhibited heightened activation of prefrontal and striatal regions when greater attention control was engaged in the presence of fearful faces (Jarcho et al., 2013; 2014).
Thus, early BI has been linked to distinct patterns of neural responses to emotionally- and motivationally-significant stimuli, relative to non-inhibited individuals. BI-related neural perturbations parallel perturbations evident in clinically anxious individuals, and are apparent even in the absence of current psychopathology. These studies have important implications for translational research in suggesting that there may be enduring neural mechanisms that serve as the foundation for the link between early BI and subsequent anxiety (Perez-Edgar & Guyer, 2014; Pine & Fox, 2015).
One information-processing mechanism that shapes the relation between BI and anxiety problems during development is threat-related attention bias (AB; Fox & Pine, 2012; Perez-Edgar et al., 2014b). Experimental evidence in adults suggests that clinical and subclinical anxiety is associated with biased attention orienting to threat (Bar-Haim et al., 2007; Cisler & Koster, 2010). Meta-analyses of studies administering attention bias modification training (ABMT), an anxiety intervention procedure that attempts to train attention away from threat (Bar-Haim, 2010), have reported that active ABMT with adults reduces AB and anxiety symptoms compared to control training (Beard, Sawyer, & Hofmann, 2012; Hakamata et al., 2010; Hallion & Ruscio, 2011; Mogoaşe, David, & Koster, 2014).
However, there is more heterogeneity in the link between AB and anxiety in youth (Puliafico & Kendall, 2006). While there is evidence supporting both the AB-anxiety link (Roy et al., 2008) and the effectiveness of ABMT on AB in anxious adolescents (Eldar et al., 2012), other studies have failed to find AB to threat in clinically anxious children (e.g. Kindt, Bogels, & Morren, 2003; Waters et al., 2008) or find attention avoidance (Salum et al., 2013). In line with the neural parallels between anxiety and BI, adolescents with stable childhood BI also display heightened AB (Perez-Edgar et al., 2010a). However, younger children characterized for BI did not display AB in other studies (Broeren et al., 2011; Perez-Edgar et al., 2011; White et al., in press). In summary, although there is relatively consistent evidence in adults suggesting that AB to threat appears to be causally implicated in the onset and maintenance of anxiety symptoms (Pine et al., 2009), inconsistencies in the youth literature require further research. Examining AB in temperamentally at-risk youth is particularly important for determining whether AB is a precursor or a symptom of anxiety disorders.
Biased attention allocation in childhood may elicit a cascade of effects on downstream information processing, including threat-related interpretation bias. These processes may influence each other through a feedback loop, contributing to the formation of a maladaptive response repertoire that perpetuates and strengthens anxious behaviors over time (Crick & Dodge, 1994). Indeed, longitudinal studies indicate that early BI predicted subsequent social withdrawal only in adolescents and children showing AB (Perez-Edgar et al., 2010a; 2011), underscoring that deeply-entrenched AB might bind at-risk children to a developmental trajectory marked by elevated anxiety symptoms (Perez-Edgar & Guyer, 2014; Perez-Edgar et al., 2014b). These data also support the presumption that BI may act as an endophenotypic marker of risk, aiding attempts to link variations in neural functioning with observed patterns of behavior (Perez-Edgar & Guyer, 2014).
Neurocognitive models delineate a frontolimbic neural network that mediates threat-related attention orienting (Bishop, 2008; Heeren et al., 2013; Shechner et al., 2012). This network encompasses: i) a bottom-up amygdala-based system that subserves salience detection, and engages other regions implicated in processing and responding to motivationally-relevant stimuli (Davis & Whalen, 2001; Phan et al., 2002) and ii) a top-down system that is comprised of regions in the prefrontal cortex (PFC). The medial PFC (mPFC), with direct reciprocal connections to the amygdala, plays a general role in processing emotion and self-relevant information (Phan et al., 2002; Schmitz & Johnson, 2007). Of the sub-regions, the dorsomedial PFC (dmPFC) is involved in monitoring and evaluating affective information, while ventromedial PFC (vmPFC) modulates and integrates limbic processing in support of context-appropriate emotion regulation (Etkin, 2010; Etkin, Egner, & Kalisch, 2011). The ventrolateral PFC (vlPFC) modulates amygdala responses to threat in order to maintain goal-directed behavior (Corbetta & Shulman, 2002; Fox & Pine, 2012). Additionally, the dorsolateral PFC (dlPFC) is implicated in ongoing maintenance of executive control, such as trial-by-trial voluntary attention allocation to support task-required performance in the presence of threat-related distractors (Bishop, 2008; 2009; Luks et al., 2007). Emerging research using transcranial direct current stimulation (tDCS) to increase excitability of the dlPFC suggests that dlPFC activity casually influences threat-related AB, likely by modulating attention to salient stimuli (Clarke et al., 2014; Ironside et al., 2015).
Studies examining the neural correlates of AB in relation to anxiety commonly employ the dot-probe task (Bar-Haim et al., 2007), in which each trial presents a pair of faces (either both neutral, or a neutral-threat pair) followed by a probe replacing one of the faces (youths: Britton et al., 2012; Monk et al., 2006; 2008; Price et al., 2014; Telzer et al., 2008; adults: Fani et al., 2012; Hardee et al., 2013). Biased attention toward threat is quantified behaviorally in the neutral-threat trials by slower reaction times (RTs) to the probes that replace the neutral face (incongruent trials) compared to the probes that replace the threat face (congruent trials).
Monk et al (2006) found that youth with generalized anxiety disorder (GAD) displayed greater vlPFC response to angry faces (500ms presentation) compared to healthy adolescents. However, amygdala activation did not differ between groups. Symptom severity was negatively correlated with vlPFC activation, suggesting that vlPFC recruitment may be a compensatory mechanism to regulate anxiety (Guyer et al; 2013; Shechner et al., 2012). With pre-conscious face presentation (17ms), adolescents with GAD showed greater amygdala response to angry faces, as well as a reduced negative amygdala-vlPFC coupling (Monk et al., 2008).
Additional studies have measured neural functioning associated with AB by comparing incongruent versus congruent neutral-threat trials. Here, youth with higher trait anxiety engaged greater dlPFC function when required to shift attention to the opposite location of threat compared to the same location (Telzer et al., 2008). Whereas healthy controls showed robust limbic deactivation when attention was shifted away from threat, deactivation was attenuated in clinically-anxious youth (Price et al., 2014). Using magnetoencephalography to allow for greater temporal resolution, Britton et al (2011) found that youth with GAD showed greater negative activation in vlPFC than controls, driven by greater vlPFC activation during the congruent trials. Taken together, current anxiety symptoms and vulnerability to anxiety are both associated with perturbed activation in the frontolimbic circuitries underlying threat-related attention processes.
In the only published study linking BI and frontolimbic connectivity during the dot-probe paradigm, Hardee et al (2013) found that young adults with a history of stable BI showed stronger negative amygdala-dlPFC connectivity than individuals without childhood BI for the angry-versus-neutral face contrast. However, as with the other neuroimaging studies, neural activity and internalizing symptoms were assessed concurrently. As such, one cannot infer whether the altered connectivity was associated with early BI or was a result of socioemotional problems developed later in life. In the light of extant research, it is difficult to distinguish whether BI serves as a mediator that fuels the relations between neural perturbation and anxiety or a moderator, such that aberrant neural functioning triggers anxiety only in behaviorally inhibited individuals.
The present study aimed to provide additional insights into the link between BI, neural correlates of threat-related AB and anxiety by examining, for the first time, neural responses to the incongruent-versus-congruent contrast on the dot-probe task in young children (9 to 12-year-olds) characterized for level of BI. We also examined whether variation in neural response relates to anxiety symptoms, and whether BI mediates or moderates the relation between neural activation and anxiety. Investigating neural mechanisms associated with AB in children who are entering the developmental period of increased onset of anxiety disorders (Beesdo et al., 2009) is in line with the goals of the Research Domain Criteria (RDoC) to identify youth with at-risk neural profiles for early anxiety intervention (Pine & Fox, 2015). Our findings will also set the stage for identifying neural processes that mediate and/or moderate the effects of ABMT using the dot-probe paradigm (Bar-Haim, 2010).
We adopted an event-related fMRI dot-probe task involving angry and neutral faces as used in prior studies (e.g. Hardee et al., 2013; Monk et al., 2006) to assist comparison and interpretation of findings. The incongruent-versus-congruent contrast isolates neural responses to probes that appeared in the opposite location of the threat. We hypothesized that: 1) At the behavioral level, BI, AB to threat, and anxiety will all be positively correlated. 2) At the neural level, there will be greater amygdala and PFC response to incongruent relative to congruent trials across all participants. 3) Children in the BI group, compared to non-inhibited children, will display heightened PFC function to support voluntary attention allocation in the presence of a supraliminal (500ms) threat stimulus. 4) We tentatively hypothesized greater amygdala activation in the BI versus non-BI group. While the threat distractor might be more salient for behaviorally inhibited children, prior studies (Britton et al., 2011; Price et al., 2014; Telzer et al., 2008) did not find greater amygdala activation on the incongruent-versus-congruent contrast. 5) Given the lack of clear evidence on the impact BI may exert on neural perturbations and anxiety, we hypothesized that BI will influence (mediate or moderate) the association between activation in the frontolimbic network and trait anxiety in an exploratory analysis.
Method
Participants
Participants were eighty 9–12-year-olds, drawn from an ongoing study of AB and temperamental risk for anxiety. Families were recruited through a university database of families interested in participating in research studies, community outreach, and word-of-mouth. Participants were screened based on parent-report using the Behavioral Inhibition Questionnaire (BIQ; Bishop, Spence, & MacDonald, 2003) and designated as behaviorally inhibited if they scored high on either the social novelty subscale (≥60), the grand total score (≥119), or both (Table 1). Cut-off scores were based on previous studies of extreme temperament in children ages 4 to 15 years (Broeren & Muris, 2010). Of the 365 children characterized with the BIQ, 92 (25.2%) met criterion. Forty-six agreed to participate in the larger, multi-visit study (reasons for not participating include opting out, out of contact, and recruitment in progress). Of those, 36 participated in the fMRI session (reasons for not participating include orthodontics and opting out). Data presented here are from the baseline visit. Forty-four children who scored below the BI cut-offs participated as sex- and age-matched control participants (Table 1).
Table 1.
Demographic Characteristics and Descriptive Statistics (Mean and Standard Deviation).
| Included Participants | Excluded Participants | |||
|---|---|---|---|---|
| BI | Non-BI | BI | Non-BI | |
| Demographics | ||||
| N | 23 | 33 | 13 | 11 |
| Gender (M/F) | 11/12 | 14/19 | 4/9 | 4/7 |
| Age | 10.26 (0.92) |
10.30 (0.98) |
10.15 (0.99) |
10.18 (1.17) |
| IQ | 110.23 (14.12) |
113.21 (14.11) |
106.42 (14.99) |
107.36 (8.52) |
| BI Characterization | ||||
| Total BIQ |
126.46 (16.98) |
76.80** (20.48) |
128.31 (21.51) |
65.09** (20.01) |
| Number of children BIQ cut-off (≥119) only |
2 | NA | 1 | NA |
| Number of children novelty cut-off (≥60) only |
8 | NA | 3 | NA |
| Number of children on psychotropic medication |
1 | 2 | 2 | 0 |
| Symptom Characterization | ||||
| Total SCARED |
15.62 (8.25) |
7.38* (7.37) |
21.62 (13.64) |
4.56* (4.03) |
| Number of MDD symptoms | 4.09 (3.07) |
3.52 (4.03) |
4.25 (4.52) |
2.18 (3.06) |
| Dot-Probe Task Performance | ||||
| Valid Dot-probe Trials (%) | 90.08 (4.72) |
71.65 (24.60) |
||
| Congruent | 90.33 (5.74) |
90.00 (5.45) |
78.65 (17.91) |
64.50 (30.77) |
| Incongruent | 89.02 (4.18) |
90.27 (5.95) |
78.23 (18.55) |
62.63 (29.72) |
| Neutral-Neutral | 89.35 (5.25) |
91.06 (5.66) |
77.81 (16.95) |
64.13 (30.05) |
| RTs Valid Dot-probe Trials (ms) | 616.84 (78.20) |
616.99 (122.32) |
||
| Congruent | 624.52 (84.15) |
613.55 (73.36) |
622.24 (67.14) |
599.59 (200.47) |
| Incongruent | 623.90 (85.91) |
611.79 (76.64) |
622.40 (69.63) |
600.69 (190.13) |
| Neutral-Neutral | 620.27 (82.41) |
612.43 (78.36) |
615.37 (61.59) |
628.71 (155.88) |
| AB Score | −0.63 (15.64) |
−1.76 (17.22) |
0.16 (21.87) |
1.10 (19.97) |
Note: Raw values are presented to assist interpretation. IQ was measured using the vocabulary and block design subtests of the Wechsler Intelligence Scale for Children (WISC-IV, Wechsler, 2003) with scores computed from the sum of the scaled scores (Srauss, Sherman, Spreen, 2006). Total SCARED scores for included participants were log-transformed to correct for positive skewness. MDD was examined using the computerized Diagnostic Interview Schedule for Children – Fourth Edition (DISC-IV; Shaffer et al., 2000), administered to parents. BIQ = Behavioral Inhibition Questionnaire; SCARED = Screen for Child Anxiety Related Emotional Disorders; MDD = major depressive disorder; RTs = reaction times; AB = attention bias.
p<.001,
p=.001 for comparisons of BI group to Non-BI group.
Data were carefully inspected for quality assurance, with 21 participants (26.3%) excluded from fMRI analyses for meeting one of the exclusion criteria: exceeding movement thresholds (> 3mm, N=5), poor task performance (more than 25% of trials with missing responses, inaccurate responses or outlier RTs, N=10), and failure to detect visual cortex activation when task stimuli were present versus absent (N=6). Finally, in order to avoid genetic and shared environmental factors contributing to group-level variance (Gregory & Eley, 2007) that might confound the results, three participants were excluded due to being a member of a sibling pair participating in the study. The final sample consists of 56 children (Mage=10.3 years; SD=.95; 11 males; 23 BI group; 7 left-handed). Included and excluded participants did not differ in age, gender, IQ, BIQ scores, AB scores, levels of anxiety and depression symptoms, or number of children on psychotropic medications (p’s>.13, Table 1). Within the final sample, three children were on stimulants, and one was also prescribed an anti-panic medication. The anxiety measure was missing for 9 participants. They did not differ from the rest in total BIQ scores or AB (p’s>.36).
The study was approved by the institutional review board at the Pennsylvania State University. Parents and children provided written consent/assent.
Measures
Behavioral inhibition was assessed using the BIQ (Bishop et al., 2003), a 30-item instrument that measures the frequency of BI-linked behavior in the domains of social and situational novelty (plus a summed total score) on a seven-point scale ranging from 1 (“hardly ever”) to 7 (“almost always”). Four questions were edited to be more appropriate for the target age range in the current study (e.g. reference to preschool, kindergarten, and childcare was removed for the question: “Happily separates from parent(s) when left in new situations for the first time (e.g. kindergarten, preschool, childcare)”). The questionnaire has adequate internal consistency, construct validity, and validity in differentiating behaviorally inhibited from non-inhibited children (Bishop et al., 2003), parent reports on the BIQ correlate with laboratory observations of BI in social contexts (Dyson et al., 2011), and the BIQ had good internal consistency in the present study (Cronbach’s α = .91).
Anxiety symptoms were measured using the parent-report version the Screen for Child Anxiety Related Emotional Disorders (SCARED, Birmaher et al., 1999), a 41-item instrument assessing symptoms of panic disorder, generalized anxiety, separation anxiety, social phobia, and school phobia defined in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; American Psychiatric Association, 1994). Parents rated the frequency with which their children experience each symptom on three-point scales (0 = “almost never”, 1 = “sometimes”, and 2 = “often”). Subscale scores were summed to create the total score. The SCARED has satisfactory psychometric properties in both clinical (Birmaher et al., 1999) and community samples (Hale et al., 2005), and it offers a valuable tool to predict specific anxiety disorders in clinically-referred youths (Muris et al., 2004). It had good internal consistency in the present study (Cronbach’s α = .90).
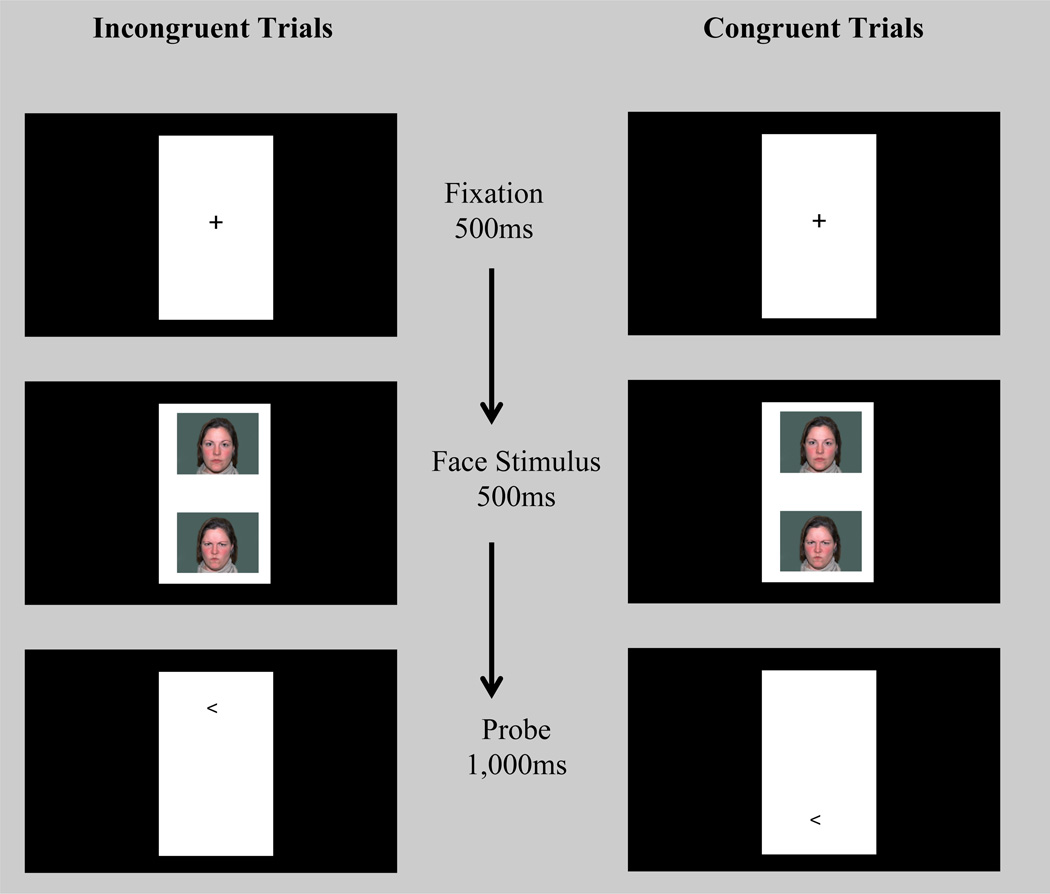
Threat-related AB was assessed in an event-related fMRI dot-probe task. The behavioral paradigm is well-validated in both adults (Mogg & Bradley, 1999) and youths (Monk et al., 2006). Stimuli were displayed on a projector with resolution of 1024(H) by 768(V) at 75Hz. At the viewing distance of 143cm, the display area was 20°(H)*16°(V). The visual angles for the face image are 1.80°(H)*1.36°(V). Each trial (2500ms) began with a 500ms fixation cross, followed by a face pair displayed on the top and bottom of the fixation point for 500ms (Figure 1). The faces and fixation point were replaced by an arrow-probe presented for 1000ms in the location of one of the preceding faces. Participants indicated whether the arrow pointed to left or right by pressing a button (response recorded for 2500ms). The inter-trial interval varied between 250ms to 750ms (average 500ms).
Figure 1.
Schematic of the dot-probe task illustrating congruent and incongruent trials. In incongruent trials, the probe appeared on the opposite location of the angry face. In congruent trials, the probe appeared on the same location as the angry face. The same actor appeared for both expressions within a trial. Each probe direction (< left or > right) appeared for half of the trials.
The task displayed face pairs of 20 actors (NimStim, Tottenham et al., 2009) across 320 trials divided into two task runs. There were 80 trials for each of 3 trial types in each run: 1) congruent trials in which the angry-neutral face pair was followed by an arrow in the same position as the angry face; 2) incongruent trials in which the angry-neutral face pair was replaced by the probe appeared on the opposite location of the angry face; and 3) neutral-neutral trials with the probe presented on either location. Eighty blank trials were added to each run to create jitter and serve as an additional baseline. Angry-face location, arrow-probe location, arrow-probe direction, and actors were counterbalanced for each participant.
Prior to fMRI acquisition, participants practiced the dot-probe task in a mock scanner. Participants repeated the 10 practice trials until achieving at least 80% accuracy. The mock scanner procedures were added to familiarize children with the fMRI environment, sounds, and task procedures, and train them to be as still as possible during scans. During mock and actual data acquisition, task stimuli were viewed with mirrors on the head coil. Padding was used to limit head and body movement.
fMRI Data Acquisition
Imaging data were acquired in two 170 volume runs (180 task trials/run) on a 3-Telsa MRI scanner (MAGNETOM Trio with Tim system, Siemens Medical Solutions, Erlangen, Germany), with descending acquisition of 43 continuous 3mm axial slices angled approximately 15 degrees above the AC-PC line. T2*-weighted echo-planar sequence was applied with a repetition time (TR) of 2500ms, echo time (TE) of 25ms, flip angle of 80 degrees, and field of view (FoV) of 192mm. The voxel size was 3×3×3 mm, and the image matrix was 64×64. High-resolution T1-weighted structural scans were also acquired using a magnetization prepared gradient echo sequence (MP-RAGE) (176 1mm slices, TR=1700, TE=2.01, flip angle=9 degrees, FoV=256mm, voxel size=1×1×1 mm; 256×256 matrix, T1=850ms).
fMRI Data Preprocessing
Data were preprocessed and analyzed using SPM8 (Wellcome Trust Center for Neuroimaging, London, UK) and MATLAB (Version 7.14.0; Mathworks, Inc., Natick, MA). Functional images were realigned to the first image of each run. The T1 was coregistered to the mean realigned functional image, and then normalized to the Cincinnati Children’s Hospital Pediatric Brain Template for 9–12 year olds (Wilke, Schmithorst, & Holland, 2002) using SPM’s unified segmentation. These normalization parameters were then applied to the functional time series, which were then spatially smoothed with a 6mm kernel.
Data Analysis
Behavioral data
RT data were cleaned based on the TAU-NIMH Toolbox (Abend, Pine, Bar-Haim, 2014). We excluded trials (9.92% total) with missing responses (2.55%), incorrect responses (2.79%), RTs outside a 150–2000 millisecond window post-probe presentation (0.03%), and RTs +/− 2 standard deviations of the individual child’s mean (derived from included trials; 4.55%). AB scores for each participant were calculated as mean RT to the probes on the incongruent trials minus mean RT to the probes on congruent trials.
fMRI data
At the first-level, fixed-effects analysis was conducted for each participant with four regressors created for the trial types: neutral-neutral, congruent, incongruent, and invalid (missing responses, incorrect responses and responses with outlier RTs), along with motion regressors specified based on the 24-parameter autoregressive model (Friston et al., 1996). Task-related regressors were modeled by convolving event onset times with a canonical hemodynamic response function. The t-contrast estimated for examining signal change corresponding to threat-related AB was incongruent-versus-congruent neutral-threat trials (Incongruent>Congruent).
The contrast images containing parameter estimates for individual participants were entered into two second-level, random-effects analyses: (1) whole-sample Incongruent>Congruent: to examine regions activated in the Incongruent>Congruent contrast across all participants in a one-sample t-test, and (2) between-group Incongruent>Congruent: to compare Incongruent>Congruent activation between the BI-group and non-BI group in a two-sample t-test. In addition to SPM’s implicit masking procedure set to include voxels with at least 20% of mean signal, an explicit brain mask was applied to limit the analyses to voxels with greater than 20% probability of being gray matter. For clusters exhibiting significant BI-group differences, subject-level percent signal-change values were extracted (MarsBaR; Brett et al., 2002) for secondary correlation analyses and model testing. As exploratory analyses, we also conducted an omnibus one-way repeated-measures ANOVA with trial type (incongruent, congruent, neutral-neutral) as the within-subjects factor, as well as a trial type (incongruent, congruent, neutral-neutral) × group (BI, non-BI) mixed design ANOVA. Details of the analyses and results are presented in the Supplemental Information.
For both one-sample and two-sample t-tests, to correct for multiple comparisons at the whole-brain cluster-wise alpha of 0.05 with a voxel-wise p-threshold of 0.005 (based on the average smoothness of our data: FWHM values of 11.2mm*11.4mm*10.2mm in x, y, and z dimensions), 10,000 Monte Carlo simulations (AFNI program 3dClustSim) found that a minimum cluster size of 85 contiguous voxels was necessary to achieve significance. Following prior literature (e.g. Hardee et al., 2013; Monk et al, 2006; 2008), to assess clusters of activation within the amygdala, small-volume correction (SVC) was applied based on a priori anatomical region of interest (ROI) including both left (79 voxels) and right (87 voxels) amygdala, defined using the AAL Atlas (Tzourio-Mazoyer et al., 2002) provided in the WFU Pick Atlas (Maldjian et al., 2003). Monte Carlo simulations (smoothness of 11.9*7.9*10.9 mm FWHM) determined that a minimum 2-voxel cluster size for both left and right amygdala was needed to achieve a cluster-wise p=.05 at voxel-wise p=.005.
Given our a priori hypothesis of greater prefrontal activation in the Incongruent>Congruent contrast in BI-group versus non-BI group, we additionally created functionally-defined ROIs for significant prefrontal cortex clusters from the whole-sample Incongruent>Congruent analysis. Identifying regions that exhibited significant activation within one group of participants and using them as inclusive masks for correcting multiple comparisons in between-group analyses in the same sample is a commonly used procedure in multiple domains of research (e.g. Labuschagne, et al., 2010; Rizio & Dennis, 2014; Williams et al., 2015), and allows us to examine BI-group differences in task-related executive control regions.
Model testing
A moderated mediation model (Hayes, 2015; Preacher, Rucker, & Hayes, 2007) was tested to simultaneously examine whether BI levels (total BIQ scores, mean-centered) mediates or moderates the relation between neural activation (percent signal-change values extracted from ROIs, mean-centered) and levels of anxiety symptoms (total SCARED scores). For assessing whether BI is a mediator, the model estimates the effect of neural activation on BI (parameter a), the effect of BI on anxiety (parameter b1), the effect of neural activation on anxiety controlling for BI levels (direct effect, parameter c’), and the effect of neutral activation on anxiety through BI (indirect effect, parameter ab1). For examining whether BI is a moderator, the model estimates the interaction effect of neural activation and BI on anxiety (parameter b2). Additionally, index of moderated mediation (ab2) is estimated for assessing if the indirect effect varies depending on the level of neural activation. Significance was determined using 95% bootstrap bias corrected confidence intervals (CIs) for the indirect effect and index of moderated mediation generated based on 1,000 bootstrap samples (Hayes, 2013).
Results
Behavioral Results
One-sample t-tests examining AB to angry faces were non-significant for the whole sample, t(55)=−0.59, p=.56, d=−0.16, as well as separately for the BI group, t(22)=−0.19, p=.85, d=−0.08, and the non-BI group, t(32)=−0.59, p=.56, d=−0.21. The BI group vs. non-BI group comparison of AB was not significant, t(54)=0.25, p=.80, d=0.07. As shown in Table 2, AB did not correlate with total BIQ scores, r(54)=.06, p=.68, or total SCARED scores, r(45)=.24, p=.10. As expected, total BIQ scores positively correlated with total SCARED scores, r(45)=.54, p<.001, with behaviorally inhibited children scoring higher on anxiety symptoms than non-inhibited children, t(45)=3.93, p<0.001, d=1.16. However, the BI group did not significantly differ from the non-BI group in the number of depression symptoms, p=.57 (Table 1).
Table 2.
Bivariate Correlations for Study Variables.
| Variable | BIQ | AB score | Anxiety symptoms |
|---|---|---|---|
| BIQ | |||
| AB score | 0.06 | ||
| Anxiety symptoms | 0.54** | 0.24 | |
| dlPFC-cluster activation | 0.31* | 0.15 | 0.36* |
Note: dlPFC = dorsolateral prefrontal cortex; BIQ = Behavioral Inhibition Questionnaire. AB = attention bias. We selected the dlPFC-cluster where there was a BI-group difference in activation for the incongruent-versus-congruent contrast. Peak MNI coordinates for the dlPFC cluster: x, y, z=24, 62, 13; 9 voxels.
p<.001,
p<.05
fMRI Results
Whole sample Incongruent>Congruent
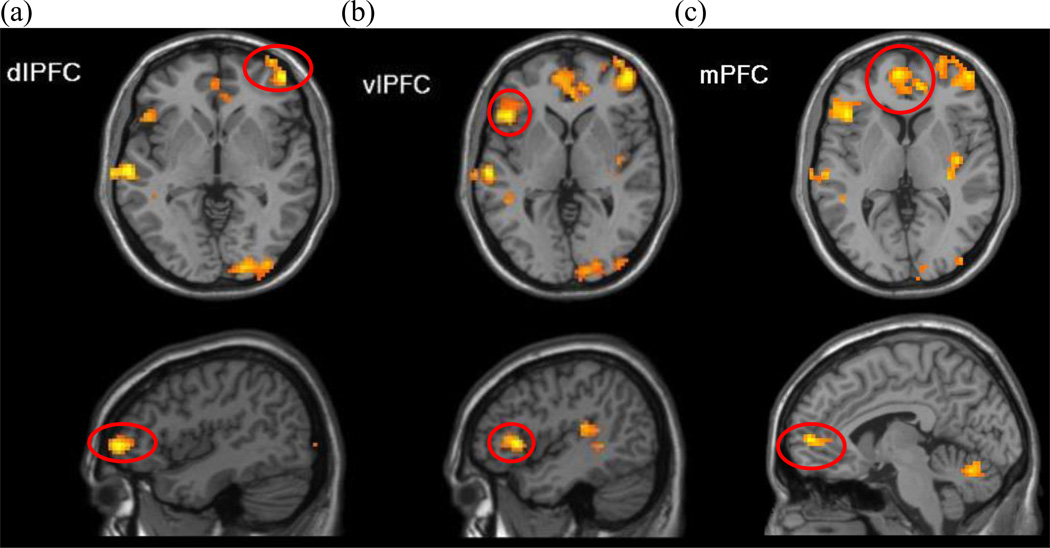
The whole-brain one-sample t-test identified 11 significant clusters in a contrast of incongruent versus congruent trials (Table 3). As hypothesized, dlPFC (peak MNI coordinates x, y, z= 45, 53, −2; encompassing inferior frontal gyrus pars triangularis, extending to the orbitofrontal cortex), vlPFC (peak MNI coordinates x, y, z= −45, 23, 1), and mPFC (peak MNI coordinates x, y, z = −6, 56, 4; overlapping with anterior cingulate cortex) showed significant activation for the incongruent-versus-congruent contrast (Figure 2).
Table 3.
Activation clusters in response to incongruent relative to congruent trials that survived the p<.05 cluster-corrected thresholds at voxel-wise threshold of p=.005 identified in the one-sample t-test, for both whole-brain (Figure 2) and amygdala ROI (Figure 3) analyses.
| Peak MNI coordinates |
|||||
|---|---|---|---|---|---|
| Region Name (AAL) | x | y | z | Cluster size (voxels) | t(55) |
| Whole-brain analysis | |||||
| Temporal_Mid_L | −69 | −22 | −8 | 392 | 4.90 |
| Cerebelum_Crus1_L | −18 | −79 | −20 | 345 | 4.23 |
| Occipital_Mid_L | −27 | −85 | 16 | 105 | 4.17 |
| Rolandic_Oper_R | 36 | −22 | 19 | 120 | 4.00 |
| Frontal_Mid_Orb_R | 45 | 53 | −2 | 260 | 3.98 |
| Frontal_Inf_Tri_L | −45 | 23 | 1 | 93 | 3.95 |
| Cerebelum_6_R | 18 | −79 | −20 | 124 | 3.87 |
| Parietal_Sup_R | 36 | −46 | 58 | 128 | 3.85 |
| Temporal_Mid_R | 57 | −49 | 13 | 90 | 3.83 |
| Lingual_R | 21 | −88 | −5 | 359 | 3.79 |
| Frontal_Sup_Medial_L | −6 | 56 | 4 | 151 | 3.74 |
| ROI analysis | |||||
| Amygdala_R | 18 | 5 | −17 | 2 | 3.03 |
| Amygdala_L | −27 | −1 | −29 | 2 | 2.80 |
Note: AAL = Automated Anatomical Labeling (Tzourio-Mazoyer et al., 2002).
Figure 2.
Whole sample Incongruent>Congruent. The whole-brain analysis comparing the incongruent to congruent trials revealed activation significantly greater than zero across all participants in the three prefrontal regions: a) right dlPFC, b) left vlPFC, and c) bilateral mPFC. Figures presented at p=.005 with a cluster extent threshold of 85 voxels.
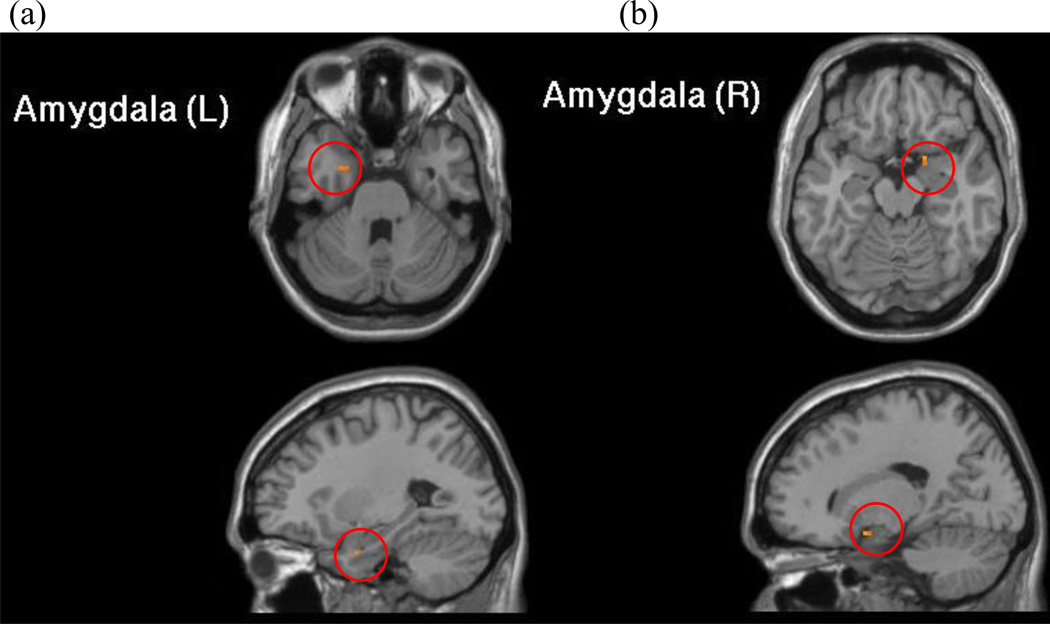
Whole sample Incongruent>Congruent amygdala SVC
The one-sample t-test indicated significant activation (p<.005) in both of the left and right amygdala ROIs for the sample as a whole in response to incongruent versus congruent trials (Figure 3; Table 3).
Figure 3.
Whole sample Incongruent>Congruent amygdala small-volume correction (SVC). In response to incongruent versus congruent trials, activation in a priori anatomically-defined ROIs of left (a) and right amygdala (b) were significantly greater than zero across all children. Figures presented at p=.005 with cluster corrected to 2 voxels.
BI-group differences in Incongruent>Congruent
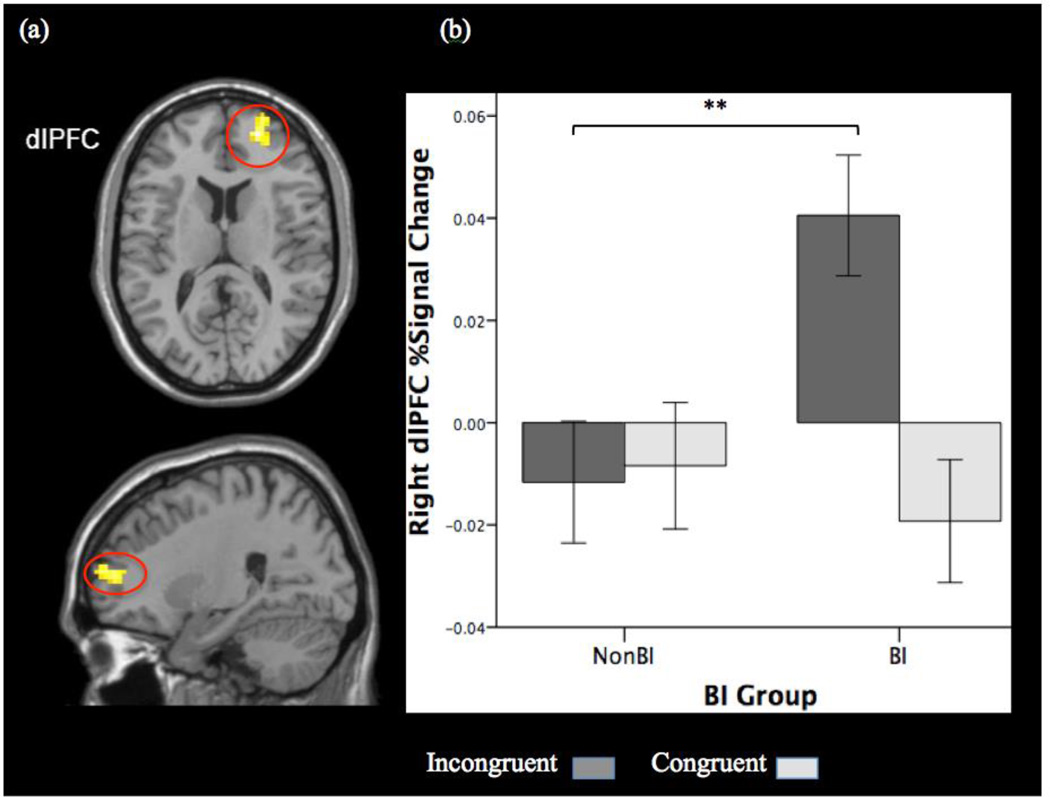
BI-group comparisons were restricted to the three task-related prefrontal regions. The mean FWHM values estimated based on each ROI mask, in the respective x, y, and z dimensions, were 10.48mm*8.44mm*9.08mm for dlPFC, 10.44mm*8.78mm*9.29mm for vlPFC, and 6.05mm*9.55*10.64mm for mPFC. Monte Carlo simulations determined the minimum cluster size needed to identify significant activation at voxel-wise threshold of p<.005 with cluster threshold α<.05 in dlPFC (6 voxels, 162mm3), vlPFC (3 voxels, 81mm3), and mPFC (4 voxels, 108mm3). The two-sample t-test revealed two clusters in the right dlPFC (peak MNI coordinates for cluster one: x, y, z=24, 62, 13; 9 voxels; cluster two: x, y, z=24, 53, 10; 6 voxels). Given the preliminary analysis indicating that percent signal-change values extracted from the two clusters did not differ (M=.02, SD=.07, for both clusters), we present results of analyses that included the percent signal-change values extracted from the larger cluster. Greater activation in the right dlPFC cluster was observed for BI-group versus non-BI group on the incongruent-versus-congruent contrast (Figure 4a), which was driven by BI group showing greater activation than non-BI group in response to incongruent trials (Figure 4b). There was no significant group difference in the dlPFC-cluster activation for the congruent trials (p=.55).
Figure 4.
BI-group comparison on Incongruent>Congruent in dlPFC. (a) BI children showed greater activation in a right dlPFC-cluster (small volume corrected for task-related dlPFC region; peak MNI coordinates x, y, and z=24, 62, 13; 9 voxels) than non-BI children in response to threat-incongruent probes relative to threat-congruent probes. (b) The BI group’s greater Incongruent>Congruent activation was driven by greater activation for the incongruent trials. Activation is presented at p=.005 with cluster corrected to 6 voxels. Error bars: ±1 standard error. **p<.01
No between-group differences were evident in either the amygdala anatomical ROIs or the vlPFC and mPFC functional ROIs. The two-sample t-test showed no regions that surpassed the whole-brain-corrected threshold for the congruent-versus-incongruent contrast.
The relation between dlPFC activation, BI, and anxiety symptoms
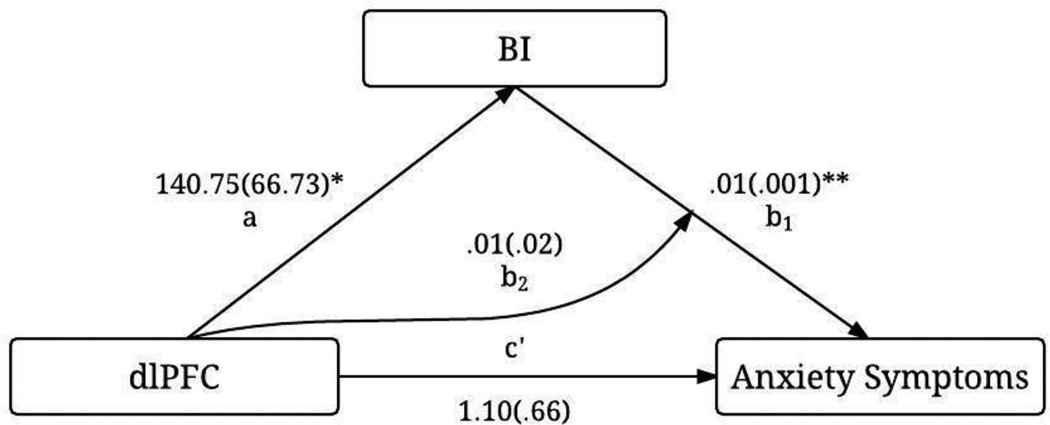
The moderated mediation model revealed that the significant association between dlPFC activation and anxiety is explained through BI. Zero-order correlations between the percent signal change in the right dlPFC extracted for the incongruent-versus-congruent contrast, total BIQ scores, and total SCARED scores are presented in Table 2. In the model (Figure 5), the paths were significant from dlPFC activation to BI (a), t=2.11, p=.04 (as expected, since the extracted dlPFC-cluster was based on the BI-group comparison), and from BI to anxiety symptoms (b1), t=3.60, p<.001. The direct effect of dlPFC activation on anxiety symptoms (c’) was not significant, t=1.66, p=.10. The indirect effect of dlPFC activation on anxiety through BI was significant, ab1=.71, CI=0.17–1.58. The interaction between dlPFC activation and BI score (b2) was not significant, t=0.22, p=.83. The indirect effect did not vary as a function of dlPFC activation, based on the moderated mediation index (ab2)=.66, CI=−5.51–6.77.
Figure 5.
Path results for the moderated mediation model depicting the relation between the right dlPFC activation, BIQ scores, and anxiety symptoms. Noted are the effect coefficients with standard errors in parentheses. *p<.05; **p<.001
To examine whether AB affects the relations between dlPFC activation, BI, and anxiety, the model was repeated with AB included as a covariate. Path a, t=2.12, p=.04, and b1, t=3.69, p<.001, as well as the indirect effect, ab1=.81, CI=0.17–1.79, were still significant. Moreover, AB neither mediated the link between dlPFC activation and anxiety, CI=−0.25–1.03, nor moderated paths from dlPFC activation to BI, t=−0.47, p=.64, from BI to anxiety, t=0.17, p=.86, or from dlPFC activation to anxiety, t=−0.09, p=.93.
Discussion
The present study presents the first investigation of neural responses to congruent and incongruent threat cues among young children varying in levels of BI. BIQ scores positively correlated with concurrent anxiety levels; however, neither BI nor anxiety scores correlated with behavioral AB to threat. Across all children, there was greater activation in the frontolimbic network that supports threat-related attention processing when the task demanded attending to the location opposite to threat (the incongruent-versus-congruent contrast), namely, bilateral amygdala, right dlPFC, left vlPFC, and bilateral mPFC. Within this network, behaviorally inhibited children deployed greater dlPFC activation relative to their non-inhibited peers in response to threat-incongruent trials. Lastly, dlPFC activation related to trait anxiety, and BI mediated the relation between dlPFC activation and anxiety. Behavioral AB levels did not impact our findings.
Together, our results add to growing evidence indicating that BI is associated with distinct neural responses linked to threat-related information processing, and neural processes, in turn, are associated with elevated anxiety even before reaching established clinical cut-offs. Extending from findings examining the link between neural correlates of AB and anxiety, this study suggests that the association between elevated dlPFC function and heightened anxiety can be attributed to early-emerging biologically-based temperamental vulnerability, which shapes the development of threat-related attention processes and anxiety over time (Fox & Pine, 2012).
The absence of significant relations between BI and behavioral AB to threat is not uncommon in behavioral (Broeren et al., 2011; Perez-Edgar et al., 2011; White et al., in press) and neuroimaging (Hardee et al., 2013) studies adopting the dot-probe paradigm. Several neuroimaging studies with clinically affected youth also found no associations between behavioral AB and anxiety (Britton et al., 2011; Monk et al., 2008; Price et al., 2014). Aside from variations in task designs, these inconsistencies in the literature might be due to limitations of dot-probe paradigms in measuring threat-related AB. Using difference scores of RTs on incongruent versus congruent trials may not be a reliable or stable measure of AB as they show poor internal consistency and temporal stability, particularly in children (Brown et al., 2014).
In our data, for example, there was no significant correlation between AB scores in the first and second run (r=.09, p=.53). However, Britton et al (2013) found that while AB scores in healthy youths were not stable across two assessment points, participants reliably recruited greater vlPFC function in the incongruent relative to congruent trials across testing sessions. Hence, the lack of BI-group differences in behavioral AB in this study does not suggest that attention processes measured in the dot-probe task are irrelevant to the BI phenotype per se. Rather, neural measures might prove more reliable and sensitive in tapping into the threat-related attentional mechanisms that distinguish behaviorally inhibited children from non-inhibited peers (Henderson et al., 2015; Shechner et al., 2012).
Relative to congruent trials, responding to incongruent trials activated the frontolimbic network across all participants. The demand of attending to the opposite location of threat (as oppose to attending to the threat location) may have potentiated the salience of the probe in incongruent trials, which has been associated with greater amygdala engagement (Britton et al., 2014). The concomitant mPFC, vlPFC, and dlPFC activations may reflect the deployment of top-down executive control processes to monitor and regulate threat reactivity and allocate attention to support performance (Bishop, 2009; Etkin, 2010; Heeren et al., 2013; Shechner et al., 2012) in incongruent trials.
Frontolimbic functioning supports threat-related attention processing and may also underpin the effects of ABMT, an intervention that aims to reduce anxiety symptoms by targeting threat-related AB. Active ABMT reduces AB to threat and anxiety symptoms relative to control training in both adults (Beard, et al., 2012; Hakamata et al., 2010; Hallion & Ruscio, 2011; Mogoaşe, et al., 2014) and children (Eldar et al., 2012), although the effect sizes for anxiety symptom reduction in adults are small-to-medium (e.g. Beard et al., 2012; Mogoaşe, et al., 2014). Moreover, active ABMT delivered in clinic settings ameliorates AB and clinician-reported anxiety but not self-reported symptoms in adult anxiety patients (Linetzky et al., 2015). Neuroimaging studies indicate that active training enhanced activation in vlPFC and dlPFC during emotional face processing (Browning et al., 2010; Taylor et al., 2014). Adults who displayed training-induced increases in vmPFC function showed reduced AB to threat and anxiety reactivity to stressors (Taylor et al., 2014). Britton et al (2014) found increased bilateral amygdala activation to the incongruent-versus-congruent contrast in the dot-probe task administered after active training compared to baseline. The greatest anxiety reduction was found in adults who displayed greater left amygdala activation to the same contrast at baseline. Studying the neural basis of ABMT-induced effects on anxiety symptoms provides an important avenue for novel interventions for anxiety.
The present study is the first to show greater dlPFC activation in behaviorally inhibited versus non-inhibited children in response to threat-incongruent trials. In the absence of differences in behavioral AB, the BI-group differences in dlPFC responses cannot be attributed to a performance confound. Rather, this pattern of activation may reflect compensatory processes behaviorally inhibited children deploy in order to reach comparable levels of behavioral performance. Tellingly, increased dlPFC activation was related to greater anxiety. Our findings are in line with Telzer et al (2008), which indicated that trait anxiety in youths was associated with greater dlPFC engagement on the same contrast presented here using a comparable dot-probe task. Moreover, using an emotional conflict task, Jarcho et al (2014) found that adults with stable early BI displayed greater dlPFC activation to fearful faces in incongruent (the face and the superimposed word suggested different genders) versus congruent trials (the face and word suggested the same gender). Hence, trials containing task-irrelevant threat distractors might increase attention-control demands and associated dlPFC function especially for behaviorally inhibited children who are thought to have a biologically-rooted hypervigilance to threat.
As the core dot-probe task captures multiple attention processes during stimuli processing (Shechner et al., 2012), the threat-related attention mechanism associated with enhanced dlPFC activation in behaviorally inhibited children is not entirely clear. The trial type × group mixed design ANOVA (Supplemental Information) was designed to investigate whether dlPFC activation was associated with facilitated threat engagement or difficulty in disengagement (Koster et al., 2004). However, no dlPFC cluster showed a significant interaction. Hence, one cannot draw definitive conclusions of whether the greater dlPFC function in the BI group for the incongruent-versus-congruent contrast modulates threat vigilance or disengagement. Additionally, while tDCS stimulation applied to the bilateral dlPFC reduced AB to fear faces in healthy adults (Ironside et al., 2015), the causal relation between dlPFC activation and specific component of AB remains unclear.
Although speculative, the engagement of compensatory attention control processes might have masked any underlying group differences in amygdala activation. Anxious adolescents display a hyperactive amygdala response to brief (17ms) face presentation times, marking an initial pre-conscious response to threat presentation, that may be dampened when regulatory mechanisms can be invoked at longer (500ms) stimulus presentation times (Monk et al, 2006; 2008). Adults with a history of BI show more negative amygdala-dlPFC connectivity both during responses to threat-versus-angry faces (Hardee et al., 2013) and in resting state (Roy et al., 2014), suggesting that atypical dynamics in this circuitry might be a marker of temperamental risk for anxiety. Future research is needed to compare the magnitude and directionality of amygdala-dlPFC functional connectivity between behaviorally inhibited and non-inhibited children.
The exploratory moderated mediation analysis underscores the argument that the previously found association between dlPFC perturbations and anxiety (Blackford & Pine, 2012; Fani et al., 2012; Guyer et al., 2013; Telzer et al., 2008) may be attributed to an early-emerging temperamental vulnerability. It is important to note that due to the cross-sectional design, we cannot infer the directionality of the effects or how these relations unfold over the course of development (Maxwell & Cole, 2007). By the same token, the finding cannot rule out the alternative causal relation, in which BI acts as a moderator, shaping the link between early neural risk-marker and later anxiety (Perez-Edgar & Guyer, 2014). Notwithstanding this limitation, our data extend longitudinal studies examining the relation between threat-related attention processes and internalizing problems in adolescents (Perez-Edgar et al., 2010a) and adults (Hardee et al., 2013) to childhood BI by indicating that the link between threat-attention neural patterns, BI levels, and anxiety are evident in childhood, preceding the emergence of socioemotional maladjustment and full-fledged anxiety disorders later in life (Beesdo et al., 2007).
Within the framework of a risk potentiation model of control (Henderson et al., 2015), the temperamental proclivity towards hyperresponsivity to novelty and potential threat might trigger compensatory attention control processes in order to support goal-directed behavior in the context of threat. These reactive and regulatory activities could form a positive feedback loop: heightened deployment of attention control potentiates vigilance to threat, which in turn amplifies attention control. Children who cannot marshal compensatory responses to both automatic (e.g. Hardee et al., 2013) and controlled (e.g. Lamm et al., 2014) processes may be bound to early temperament risk and the later development of anxiety problems (Caouette & Guyer, 2014; Pérez-Edgar et al, 2014b).
The current findings should be interpreted in the light of several limitations. First, our cross-sectional study involving children with a narrow age range does not address whether enhanced dlPFC recruitment is causal versus consequential of early BI, or whether the interrelations of neural activities, BI, and trait anxiety change with age. Future longitudinal studies employing multiple waves of repeated assessments are required to provide strong evidence on how early BI accounts for the causal effects of exaggerated neural responses underlying attention control on anxiety problems and whether there is age specificity for the associations.
Second, our BI categorization based on parents’ reports might be less optimal compared to early identification based on laboratory observations (Kagan, 2003). Nevertheless, the BIQ is a reliable and valid instrument that has been used to identify BI in children (e.g. Coplan et al., 2009; 2010). It should also be noted that four questions from the BIQ were edited to make the questions more appropriate for the age range of our target population. While the BIQ has satisfactory internal consistency in the present study, one cannot assume that the current BIQ maintained similar psychometric properties versus the original version.
Third, the present paradigm did not include other emotional face stimuli (e.g., happy faces). While our design allows for increased power with comparable number of trials, we cannot determine whether the enhanced neural activation to incongruent relative to congruent trials were specific to threat (angry) faces or would also be observed for other affective stimuli. However, Telzer et al (2008) indicated that increased dlPFC activation on the incongruent-versus-congruent contrast positively related to trait anxiety only for angry faces.
The current study indicates greater recruitment of the frontolimbic system, including the amygdala, dlPFC, vlPFC, and mPFC in response to targets in the opposite versus same location of threat. Behaviorally inhibited children, in particular, show exaggerated neural responses relative to non-inhibited children in the dlPFC, which may be associated with enhanced engagement of attention control. These data set the foundation for examining how the interplay between automatic and controlled processes influences the development of sustained BI and anxiety problems over time and understanding whether and how early preventive interventions, such as ABMT, can influence the trajectory from temperamental vulnerability to anxiety (Fox & Pine, 2012, Henderson et al, 2015; Perez-Edgar et al., 2014b). Here, we provide initial evidence that BI accounts, in part, for the relation between increased dlPFC activation and anxiety found in the literature, and that these associations are present relatively early in development prior to the developmental window for the emergence of clinical anxiety.
Supplementary Material
Acknowledgement
This work is supported by a grant from the National Institutes of Health [BRAINS R01 MH094633] to KPE. The authors would like to thank the Penn State Social, Life, & Engineering Sciences Imaging Center (SLEIC) 3T MRI Facility, the TAU / NIMH ABMT Initiative for providing the toolkit, and the many individuals who contributed to the data collection and data processing. We would especially like to thank the parents of the children who participated and continue to participate in our studies.
References
- Abend R, Pine DS, Bar-Haim Y. The TAU-NIMH Attention Bias Measurement Toolbox. 2014 Retrieved from http://people.socsci.tau.ac.il/mu/anxietytrauma/research/.
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition (DSM-IV) Washington: American Psychiatric Association; 1994. [Google Scholar]
- Bar-Haim Y. Research review: attention bias modification (ABM): a novel treatment for anxiety disorders. Journal of Child Psychology and Psychiatry. 2010;51(8):859–870. doi: 10.1111/j.1469-7610.2010.02251.x. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychological Science. 2009;20(8):1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behavior Therapy. 2012;43(4):724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K, Bittner A, Pine DS, Stein MB, Höfler M, Lieb R, Wittchen HU. Incidence of social anxiety disorder and the consistent risk for secondary depression in the first three decades of life. Archives of General Psychiatry. 2007;64(8):903–912. doi: 10.1001/archpsyc.64.8.903. [DOI] [PubMed] [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatric Clinics of North America. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmaher B, Brent DA, Chiappetta L, Bridge J, Monga S, Baugher M. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. Journal of the American Academy of Child & Adolescent Psychiatry. 1999;38(10):1230–1236. doi: 10.1097/00004583-199910000-00011. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Neural mechanisms underlying selective attention to threat. Annals of the New York Academy of Sciences. 2008;1129(1):141–152. doi: 10.1196/annals.1417.016. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12(1):92–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Bishop G, Spence SH, McDonald C. Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Development. 2003;74(6):1899–1917. doi: 10.1046/j.1467-8624.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Social Cognitive and Affective Neuroscience. 2012;8:143–150. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neuroscience. 2009;10(1):145–154. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child and Adolescent Psychiatric Clinics of North America. 2012;21(3):501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16(2):S497. [Google Scholar]
- Britton JC, Bar-Haim Y, Carver FW, Holroyd T, Norcross MA, Detloff A, Pine DS. Isolating neural components of threat bias in pediatric anxiety. Journal of Child Psychology and Psychiatry. 2012;53(6):678–686. doi: 10.1111/j.1469-7610.2011.02503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Bar-Haim Y, Clementi MA, Sankin LS, Chen G, Shechner T, Pine DS. Training-associated changes and stability of attention bias in youth: implications for attention bias modification treatment for pediatric anxiety. Developmental Cognitive Neuroscience. 2013;4:52–64. doi: 10.1016/j.dcn.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Suway JG, Clementi MA, Fox NA, Pine DS, Bar-Haim Y. Neural changes with Attention Bias Modification (ABM) for anxiety: A randomized trial. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeren S, Muris P. A psychometric evaluation of the behavioral inhibition questionnaire in a non-clinical sample of Dutch children and adolescents. Child Psychiatry & Human Development. 2010;41(2):214–229. doi: 10.1007/s10578-009-0162-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broeren S, Muris P, Bouwmeester S, Field A, Voerman J. Processing biases for emotional faces in 4- to 12-year-old non-clinical children: An exploratory study of developmental patterns and relationships with social anxiety and behavioral inhibition. Journal of Experimental Psychopathology. 2011;2(4):454–474. [Google Scholar]
- Brown HM, Eley TC, Broeren S, Macleod C, Rinck M, Hadwin JA, Lester KJ. Psychometric properties of reaction time based experimental paradigms measuring anxiety-related information-processing biases in children. Journal of Anxiety Disorders. 2014;28(1):97–107. doi: 10.1016/j.janxdis.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer CJ. Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biological Psychiatry. 2010;67(10):919–925. doi: 10.1016/j.biopsych.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caouette JD, Guyer AE. Gaining insight into adolescent vulnerability for social anxiety from developmental cognitive neuroscience. Developmental Cognitive Neuroscience. 2014;8:65–76. doi: 10.1016/j.dcn.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Raggi VL, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. Journal of the American Academy of Child & Adolescent Psychiatry. 2009;48(9):928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler JM, Koster EH. Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review. 2010;30(2):203–216. doi: 10.1016/j.cpr.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PJ, Browning M, Hammond G, Notebaert L, MacLeod C. The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: Evidence from transcranial direct current stimulation. Biological Psychiatry. 2014;76(12):946–952. doi: 10.1016/j.biopsych.2014.03.003. [DOI] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, Blackford JU. The nature of individual differences in inhibited temperament and risk for psychiatric disease: a review and meta-analysis. Progress in Neurobiology. 2015;1364:1–22. doi: 10.1016/j.pneurobio.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. Journal of the American Academy of Child & Adolescent Psychiatry. 2012;51(10):1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan RJ, DeBow A, Schneider BH, Graham AA. The social behaviours of inhibited children in and out of preschool. British Journal of Developmental Psychology. 2009;27(4):891–905. doi: 10.1348/026151008x396153. [DOI] [PubMed] [Google Scholar]
- Coplan RJ, Schneider BH, Matheson A, Graham A. ‘Play skills’ for shy children: development of a Social Skills Facilitated Play early intervention program for extremely inhibited preschoolers. Infant and Child Development. 2010;19(3):223–237. [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crick NR, Dodge KA. A review and reformulation of social information-processing mechanisms in children's social adjustment. Psychological Bulletin. 1994;115(1):74–101. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6(1):13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Fox NA. Behavioral inhibition and anxiety disorders: Multiple levels of a resilience process. Development and Psychopathology. 2007;19(03):729–746. doi: 10.1017/S0954579407000363. [DOI] [PubMed] [Google Scholar]
- Dyson MW, Klein DN, Olino TM, Dougherty LR, Durbin CE. Social and non-social behavioral inhibition in preschool-age children: differential associations with parent-reports of temperament and anxiety. Child Psychiatry & Human Development. 2011;42(4):390–405. doi: 10.1007/s10578-011-0225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar S, Apter A, Lotan D, Edgar KP, Naim R, Fox NA, Bar-Haim Y. Attention Bias Modification Treatment for Pediatric Anxiety Disorders: A Randomized Controlled Trial. The American Journal of Psychiatry. 2012;169(2):213–220. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. In: Stein MB, Steckler T, editors. Behavioral Neurobiology of Anxiety and Its Treatment. Berlin Heidelberg: Springer; 2010. pp. 251–277. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15(2):85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fani N, Jovanovic T, Ely TD, Bradley B, Gutman D, Tone EB, Ressler KJ. Neural correlates of attention bias to threat in post-traumatic stress disorder. Biological Psychology. 2012;90(2):134–142. doi: 10.1016/j.biopsycho.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annual Review of Psychology. 2005;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: Psychophysiological and behavioral influences across the first four years of life. Child Development. 2001;72(1):1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Pine DS. Temperament and the emergence of anxiety disorders. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51(2):125–128. doi: 10.1016/j.jaac.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magnetic Resonance in Medicine. 1996;35(3):346–355. doi: 10.1002/mrm.1910350312. [DOI] [PubMed] [Google Scholar]
- Gregory AM, Eley TC. Genetic influences on anxiety in children: What we’ve learned and where we’re heading. Clinical Child and Family Psychology Review. 2007;10(3):199–212. doi: 10.1007/s10567-007-0022-8. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, Nelson EE. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology. 2014;26(01):229–243. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Masten CL, Pine DS. Neurobiology of pediatric anxiety disorders. In: Vasa RA, Roy AK, editors. Pediatric Anxiety Disorders: A Clinical Guide. Current Clinical Psychiatry. New York: Springer; 2013. pp. 23–46. [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. The Journal of Neuroscience. 2006;26(24):6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Pine DS. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale WW, Raaijmakers Q, Muris P, Meeus WIM. Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED) in the general adolescent population. Journal of the American Academy of Child & Adolescent Psychiatry. 2005;44(3):283–290. doi: 10.1097/00004583-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137(6):940–958. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, Pérez-Edgar K. Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biological Psychiatry. 2013;74(4):273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. Guilford Press; 2013. [Google Scholar]
- Hayes AF. An index and test of linear moderated mediation. Multivariate Behavioral Research. 2015;50(1):1–22. doi: 10.1080/00273171.2014.962683. [DOI] [PubMed] [Google Scholar]
- Heeren A, De Raedt R, Koster EH, Philippot P. The (neuro) cognitive mechanisms behind attention bias modification in anxiety: proposals based on theoretical accounts of attentional bias. Frontiers in Human Neuroscience. 2013;7(119):1–6. doi: 10.3389/fnhum.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Perez-Edgar K, Bar-Haim Y, Detloff A, Pine DS, Ernst M. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49(3):479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, Fox NA. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2015;40:207–224. doi: 10.1038/npp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironside MM, O’Shea J, Cowen PJ, Harmer CJ. Frontal cortex stimulation reduces vigilance to threat: implications for the treatment of depression and anxiety. Biological Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.06.012. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, Ernst M. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biological Psychology. 2013;92(2):306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Leibenluft E, Shechner T, Degnan KA, Ernst M. Enduring influence of early temperament on neural mechanisms mediating attention-emotion conflict in adults. Depression and Anxiety. 2014;31(1):53–62. doi: 10.1002/da.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan J. Behavioral inhibition as a temperamental category. In: Davidson RJ, Scherer KR, Goldsmith HH, editors. Handbook of Affective Sciences. New York: Oxford University Press; 2003. pp. 320–331. [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Development. 1984;55(6):2212–2225. [Google Scholar]
- Kindt M, Bögels S, Morren M. Processing bias in children with separation anxiety disorder, social phobia and generalized anxiety disorder. Behavior Change. 2003;20(03):143–150. [Google Scholar]
- Koster EH, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: Differentiating vigilance and difficulty to disengage. Behavior Research and Therapy. 2004;42(10):1183–1192. doi: 10.1016/j.brat.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Labuschagne I, Phan KL, Wood A, Angstadt M, Chua P, Heinrichs M, Stout JC, Nathan PJ. Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology. 2010;35(12):2403–2413. doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM, Fox NA. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Developmental Science. 2014;17(5):667–681. doi: 10.1111/desc.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, Bar-Haim Y. Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depression and Anxiety. 2015 doi: 10.1002/da.22344. [DOI] [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Dale CL, Hough MG. Preparatory allocation of attention and adjustments in conflict processing. Neuroimage. 2007;35(2):949–958. doi: 10.1016/j.neuroimage.2006.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annual Review of Clinical Psychology. 2005;1:167–195. doi: 10.1146/annurev.clinpsy.1.102803.143916. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA. Bias in cross-sectional analyses of longitudinal mediation. Psychological Methods. 2007;12(1):23–44. doi: 10.1037/1082-989X.12.1.23. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Some methodological issues in assessing attentional biases for threatening faces in anxiety: A replication study using a modified version of the probe detection task. Behaviour Research and Therapy. 1999;37(6):595–604. doi: 10.1016/s0005-7967(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Mogoaşe C, David D, Koster EH. Clinical Efficacy of Attentional Bias Modification Procedures: An Updated Meta-Analysis. Journal of Clinical Psychology. 2014;70(12):1133–1157. doi: 10.1002/jclp.22081. [DOI] [PubMed] [Google Scholar]
- Monk CS, Nelson EE, McClure EB, Mogg K, Bradley BP, Leibenluft E, Pine DS. Ventrolateral prefrontal cortex activation and attentional bias in response to angry faces in adolescents with generalized anxiety disorder. The American Journal of Psychiatry. 2006;163(6):1091–1097. doi: 10.1176/ajp.2006.163.6.1091. [DOI] [PubMed] [Google Scholar]
- Monk CS, Telzer EH, Mogg K, Bradley BP, Mai X, Louro HM, Pine DS. Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Archives of General Psychiatry. 2008;65(5):568–576. doi: 10.1001/archpsyc.65.5.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muris P, Dreessen L, Bögels S, Weckx M, Melick M. A questionnaire for screening a broad range of DSM-defined anxiety disorder symptoms in clinically referred children and adolescents. Journal of Child Psychology and Psychiatry. 2004;45(4):813–820. doi: 10.1111/j.1469-7610.2004.00274.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, McDermott JNM, Chronis-Tuscano A, Pine DS, Fox NA. Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion. 2010a;10(3):349–357. doi: 10.1037/a0018486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Fox NA. Temperament and anxiety disorders. Child and Adolescent Psychiatric Clinics of North America. 2005;14(4):681–706. doi: 10.1016/j.chc.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K, Guyer AE. Behavioral Inhibition: Temperament or Prodrome? Current Behavioral Neuroscience Reports. 2014;1:182–190. doi: 10.1007/s40473-014-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Hardee JE, Guyer AE, Benson BE, Nelson EE, Gorodetsky E, Ernst M. DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Social Cognitive and Affective Neuroscience. 2014a;9(4):445–453. doi: 10.1093/scan/nst001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Reeb-Sutherland BC, McDermott JM, White LK, Henderson HA, Degnan KA, Hane AA, Pine DS, Fox NA. Attention biases to threat link behavioral inhibition to social withdrawal over time in very young children. Journal of Abnormal Child Psychology. 2011;39:885–895. doi: 10.1007/s10802-011-9495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. Neuroimage. 2007;35(4):1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Taber-Thomas B, Auday E, Morales S. Temperament and attention as core mechanisms in the early emergence of anxiety. In: Lagattuta K, editor. Children and Emotion: New Insights into Developmental Affective Science. Vol. 26. Karger Publishing; 2014b. pp. 42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Pine DS, Fox NA. Childhood antecedents and risk for adult mental disorders. Annual Review of Psychology. 2015;66:459–485. doi: 10.1146/annurev-psych-010814-015038. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, Fox NA. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology. 2009;34(1):213–228. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research. 2007;42(1):185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur CD, McFarland A, Dahl RE, Ryan ND. Looking under the hood of the dot-probe task: an fMRI study in anxious youth. Depression and Anxiety. 2014;31(3):178–187. doi: 10.1002/da.22255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puliafico AC, Kendall PC. Threat-related attentional bias in anxious youth: A review. Clinical Child and Family Psychology Review. 2006;9(3–4):162–180. doi: 10.1007/s10567-006-0009-x. [DOI] [PubMed] [Google Scholar]
- Rizio AA, Dennis NA. The Cognitive Control of Memory: Age Differences in the Neural Correlates of Successful Remembering and Intentional Forgetting. PLoS ONE. 2014;9(1):e87010. doi: 10.1371/journal.pone.0087010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Benson BE, Degnan KA, Perez-Edgar K, Pine DS, Fox NA, Ernst M. Alterations in amygdala functional connectivity reflect early temperament. Biological Psychology. 2014;103:248–254. doi: 10.1016/j.biopsycho.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Vasa RA, Bruck M, Mogg K, Bradley BP, Sweeney M the CAMS Team. Attention bias toward threat in pediatric anxiety disorders. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47(10):1189–1196. doi: 10.1097/CHI.0b013e3181825ace. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salum GA, Mogg K, Bradley BP, Gadelha A, Pan P, Tamanaha AC, Pine DS. Threat bias in attention orienting: evidence of specificity in a large community-based study. Psychological Medicine. 2013;43(04):733–745. doi: 10.1017/S0033291712001651. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neuroscience & Biobehavioral Reviews. 2007;31(4):585–596. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, Bloch RB. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Molecular Psychiatry. 2012;17(10):1042–1050. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Rauch SL. Inhibited and uninhibited infants" grown up": adult amygdalar response to novelty. Science. 2003;300(5627):1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child & Adolescent Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Pérez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, Pine DS. Attention biases, anxiety, and development: toward or away from threats or rewards? Depression and Anxiety. 2012;29(4):282–294. doi: 10.1002/da.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E, Sherman EM, Spreen O. A compendium of neuropsychological tests: Administration, norms, and commentary. USA: Oxford University Press; 2006. [Google Scholar]
- Taylor CT, Aupperle RL, Flagan T, Simmons AN, Amir N, Stein MB, Paulus MP. Neural correlates of a computerized attention modification program in anxious subjects. Social Cognitive and Affective Neuroscience. 2013;9(9):1379–1387. doi: 10.1093/scan/nst128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer EH, Mogg K, Bradley BP, Mai X, Ernst M, Pine DS, Monk CS. Relationship between trait anxiety, prefrontal cortex, and attention bias to angry faces in children and adolescents. Biological Psychology. 2008;79(2):216–222. doi: 10.1016/j.biopsycho.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Waters AM, Wharton TA, Zimmer-Gembeck MJ, Craske MG. Threat-based cognitive biases in anxious children: comparison with non-anxious children before and after cognitive behavioral treatment. Behavior Research and Therapy. 2008;46(3):358–374. doi: 10.1016/j.brat.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children-WISC-IV. Psychological Corporation; 2003. [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of spatial normalization of whole-brain magnetic resonance images in children. Human Brain Mapping. 2002;17(1):48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, Oler JA, Fox AS, McFarlin DR, Rogers GM, Jesson MA, Kalin NH. Fear of the Unknown: Uncertain Anticipation Reveals Amygdala Alterations in Childhood Anxiety Disorders. Neuropsychopharmacology. 2015:1–8. doi: 10.1038/npp.2014.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, Henderson HA, Pérez-Edgar KE, Walker OL, Degnan KA, Shechner T, Fox NA. Developmental relations between behavioral inhibition, anxiety, and attention biases to threat and positive information. Child Development. doi: 10.1111/cdev.12696. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.