Abstract
Nontypeable Haemophilus influenzae (NTHi) is a gram-negative, opportunistic pathogen that frequently causes ear infections, bronchitis, pneumonia, and exacerbations in patients with underlying inflammatory diseases, such as chronic obstructive pulmonary disease. In mice, NTHi is rapidly cleared, but a strong inflammatory response persists, underscoring the concept that NTHi induces dysregulation of normal inflammatory responses and causes a failure to resolve. Lipid-derived specialized pro-resolving mediators (SPMs) play a critical role in the active resolution of inflammation by both suppressing pro-inflammatory actions and promoting resolution pathways. Importantly, SPMs lack the immune suppressive properties of classical anti-inflammatory therapies. Based on these characteristics, we hypothesized that aspirin-triggered resolvin D1 (AT-RvD1) would dampen NTHi-induced inflammation while still enhancing bacterial clearance. C57BL/6 mice were treated with AT-RvD1 and infected with live NTHi. AT-RvD1 treated mice had lower total cell counts and neutrophils in bronchoalveolar lavage fluid (BALF), and had earlier influx of macrophages. Additionally, AT-RvD1 treated mice showed changes in temporal regulation of inflammatory cytokines and enzymes, with decreased KC at 6hrs and decreased IL-6, TNFα, and Cox-2 expression at 24hrs post-infection. Despite reduced inflammation, AT-RvD1 treated mice had reduced NTHi bacterial load, mediated by enhanced clearance by macrophages and a skewing towards an M2 phenotype. Finally, AT-RvD1 protected NTHi-infected mice from weight loss, hypothermia, hypoxemia and respiratory compromise. This research highlights the beneficial role of SPMs in pulmonary bacterial infections, and provides the groundwork for further investigation into SPMs as alternatives to immunosuppressive therapies like steroids.
Introduction
Nontypeable Haemophilus influenzae (NTHi) is a gram-negative, opportunistic bacterial pathogen found in the upper respiratory tract. NTHi is the most common colonizing bacterium and causes ear infections, bronchitis, and more invasive diseases such as bacteremia and pneumonia (1, 2). People with underlying lung inflammation, such as chronic obstructive pulmonary disease (COPD), cystic fibrosis, or a preexisting infection, are particularly susceptible to infectious exacerbations, which lead to high rates of hospitalization and worsening of symptoms; NTHi is a major cause of exacerbation (3-6). NTHi is indeed the most common colonizing bacteria found in COPD patients, with the bacterial load correlating to airway inflammation, symptoms, and exacerbations (6-8). These infections are persistent and recurring, and a history of exacerbations is the single best predictor of further exacerbations (9). While a vaccine is available for the commonly infectious Haemophilus influenzae type B, it does not protect against NTHi, which continues to increase in incidence (10). Furthermore, there is increased prevalence of strains with antibiotic resistance (10-12). These infections are typically persistent; with some strains acquiring drug resistance or the ability to avoid opsonization and thereby “trick” macrophages to avoid phagocytosis, resulting in bacteria propagating and colonizing in the airways (13, 14). Multiple studies have shown that NTHi infections induce a strong and prolonged inflammatory response, characterized by influx of inflammatory cells, release of cytokines, activation of the NF-κB pathway and initiation of toll-like receptor (TLR) signaling pathways (15-18). In mice, NTHi is rapidly cleared, but inflammation persists, providing evidence for how this bacteria might induce a dysregulation of normal inflammatory responses and a failure to resolve.
Recently, endogenous lipid derived mediators termed specialized pro-resolving mediators (SPMs) have been identified as key players in the resolution of inflammation (19). SPMs are derivatives of polyunsaturated fatty acids and are divided into subclasses based on their biosynthetic pathways and structures, including lipoxins, resolvins, protectins, and maresins (19). SPMs have both anti-inflammatory and pro-resolving actions, and it is clear that they are not immunosuppressive. These molecules act to promote a paradigm shift in immune cell function, enhancing apoptotic cell clearance, promoting production of pro-resolving cytokines, and inducing an alternative M2 macrophage phenotype (19-22). SPMs are effective at reducing inflammation and promoting resolution in a variety of non-microbial models, including lung related diseases such as COPD, asthma, and fibrosis (20, 21, 23).
Along with these pro-inflammatory non-microbial insults, there is a growing interest in evaluating the efficacy of SPMs against infectious agents. Lipopolysaccharide (LPS) is often used as a surrogate for gram-negative bacteria, and certain SPMs, namely lipoxins and D-series resolvins, dampen LPS-induced inflammatory cell influx and pro-inflammatory cytokine production (24-26). SPMs have also shown efficacy in sepsis models by promoting resolution while enhancing bacterial clearance, and ligands for the lipoxin A4 receptor (FPR2/ALX) have a protective effect against experimental sepsis (27-32). Indeed, SPMs can be used as an adjunct to antibiotics, and thereby lower antibiotic requirements or act synergistically with antibiotics for improved host responses (33, 34). There still exists, though, a major knowledge gap regarding the efficacy of SPMs in resolving pulmonary bacterial infections; this is especially critical given the susceptibility of the lung to infection. Based on the non-immunosuppressive nature of SPMs and their efficacy in other inflammatory lung models, we hypothesized that aspirin-triggered resolvin D1 (AT-RvD1) would attenuate NTHi-induced lung inflammation without impairing bacterial clearance. Given the high incidence of NTHi and other bacterial lung infections and their role in promoting exacerbations, this represents an important area of translational research with high clinical impact.
Materials & Methods
Materials
Mouse IL-6 (431401), IL-10 (431411) and TNFα (430901) ELISA kits and Anti-F4/80 mouse BV421 antibody (123131) were purchased from BioLegend (San Diego, CA). Mouse CXCL1/KC ELISA kit (DY453-05) was purchased from R&D Systems (Minneapolis, MN). Collagenase (234155) was purchased from EMD Millipore (Billerica, MA). Anti-CD206 mouse Alexa Fluor 647 antibody (MCA2235A647T) was purchased from AbD Serotec (Raleigh, NC). Anti-Ly-6G (Gr-1) mouse PE antibody (553128) was purchased from BD Pharmingen (San Jose, CA). Blood agar base (70133), Hemin (H9039), and b-Nicotinamide Adenine Dinucleotide hydrate (NAD) (N3014) were purchased from Sigma (St. Louis, MO). Brain Heart Infusion (237500) and anti-CD80 mouse PerCP-Cy5.5 antibody (560526) were purchased from BD Bioscience (San Jose, CA). PGE2 and AT-RvD1 (7S,8R,17R-trihydroxy-4Z,9E,11E,13Z,15E, 19Z-docosahexaenoic acid) were purchased from Cayman Chemical (Ann Arbor, MI). Secondary Western blot antibodies (115-035-146, 111-035-144) were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). PBS (14200-075) was purchased from Gibco (Waltham, MA).
NTHi culture and growth
NTHi strain 12 (clinical isolate) was used for all infections. To culture bacteria, NTHi glycerol stock was streaked on chocolate agar plates and grown at 37°C, 5% CO2 for 24hrs. A single colony was inoculated into supplemented brain heart infusion broth (BHI, hemin, NAD) overnight. The bacterial suspension was subcultured and then centrifuged to form a bacterial pellet. The NTHi pellet was resuspended in 1× PBS and the optical density measured via spectrophotometer at a 600nM wavelength, using OD to determine colony forming units (CFUs) (1.00 OD= 1 × 109 CFUs).
In vivo treatment and exposures
Adult female C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME) and used at 8–10 weeks of age. All animal procedures were approved and supervised by the University of Rochester University Committee on Animal Resources (UCAR protocol number 2007-127). For initial dosing experiments, mice were infected by oropharyngeal aspiration (35) with 1 × 105-108 CFUs of NTHi in 40uL 1× PBS or PBS vehicle and euthanized at 6-120hrs following infection. Mice were euthanized with IP injection of 100mg/kg pentobarbital sodium plus 12.5mg/kg phenytoin sodium (Euthasol, Virbac AH Inc., Fort Worth, TX).
To determine the efficacy of AT-RvD1, mice were treated with 20ng or 100ng/mouse of AT-RvD1 in 40uL 1× PBS by oropharyngeal aspiration and given a second dose 24hrs later, prior to infection. As the average starting weight of the mice was 18.1±1.5 g (mean ± S.D.), this corresponded to doses of 1.1±0.1 mg/kg and 5.5±0.4 mg/kg. Mice were then infected with 1 × 106 CFUs of NTHi in 40uL 1× PBS by oropharyngeal aspiration; 1× PBS was used as a vehicle control. Mice were euthanized 6-24hrs following NTHi infection. Mice were weighed immediately before NTHi infection and again immediately before euthanasia. Immediately prior to euthanasia, temperature readings were also taken using a rectal thermometer. Respiratory physiology was assessed using the Harvard Apparatus Small Rodent Plethysmograph. Oxygen saturation of hemoglobin was measured using a tail clip and the Starr Life Science Corporations Mouse Oximeter according to manufacturer’s protocols. After all biometric readings were obtained, the mice were euthanized and tissue harvested as described below.
Analysis of bronchoalveolar lavage fluid and lung tissue
Bronchoalveolar lavage fluid (BALF) was collected as previously described (21). The cranial lobe of the right lung was removed to assess bacterial load. The remainder of the right lung was frozen for further analysis. Differential BAL cell counts were obtained using Richard Allen 3-step staining according to manufacturer’s protocol. Total protein in BALF was determined by the bicinchoninic acid (BCA) colorimetric assay (Thermo-Sci, Waltham, MA, 23225). Levels of IL-6, TNFα, KC, and IL-10 cytokines in BALF were determined by ELISA. The right lung was homogenized in CW buffer (50mM Tris-HCL, 2% SDS) using a glass bead homogenizer (Next Advance Bullet Blender, Averill Park, NY) and Cox-2 protein was analyzed by Western blotting (36). In some experiments, the lungs were inflated and fixed with 10% neutral buffered formalin without undergoing lavage. Tissues were embedded with paraffin, sectioned (5 μm), and stained for neutrophils as described previously (21).
Assessment of bacterial load
The cranial lung lobe was homogenized in 1mL of 1× PBS. Serial dilutions were plated onto chocolate agar plates and NTHi grown at 37°C, 5% CO2. Colonies were counted 24hrs after plating.
Assessment of bacterial clearance, efferocytosis, and macrophage phenotype
Efferocytosis was performed as previously described 6 and 24hrs after NTHi infection (21). To assess bacterial clearance and macrophage phenotype, mice were sacrificed 8hrs after NTHi infection. BALF was obtained or the right lung lobes were removed and digested with collagenase for 30min. Cells were collected through a cell strainer and centrifuged at 7000 × g for 10min. Red blood cells were removed using 1× ACK lysis buffer. Cells from BALF and digested lung were stained with antibodies against CD80, CD206, F4/80, CD11b, and Gr-1 for 30mins to identify cellular populations, macrophage phenotype, and bacterial clearance. For these experiments, NTHi was labeled with FITC antibody; 10uL of a 10mg/mL solution of FITC in DMSO was added to a suspension of NTHi and incubated in the dark for 30min, followed by centrifugation and resuspension of the bacteria in 1× PBS as above. Following staining, cells were fixed with 4% paraformaldehyde (EMS, 15710) for 20min. Fixed cells were then permeabilized and stained for intracellular Gr-1. Endpoints were assessed by flow cytometry.
Statistical Analysis
All experiments were performed on 4-9 mice/group and were repeated in a minimum of 2-4 independent experiments. Results are expressed as mean ± standard error (SEM). All data was normally distributed and parametric statistical analyses were performed using a t-test or one- or two-way analysis of variance (ANOVA) with Bonferroni’s posttest correction for multiple comparisons (indicated on figures) using GraphPad Prism Software (San Diego, CA). No statistical significance was found between veh/veh vs. AT-RvD1 unless indicated. Additionally, AT-RvD1/veh vs. NTHi groups yielded a similar level of statistical significance as veh/veh vs. NTHi groups, and veh/veh vs. AT-RvD1/NTHi yielded a similar level of statistical significance as veh/veh vs. veh/NTHi comparisons unless otherwise indicated. A lack of statistical bar indicates that no significance was found.
Results
NTHi dose dependently induces persistent inflammation despite rapid bacterial clearance
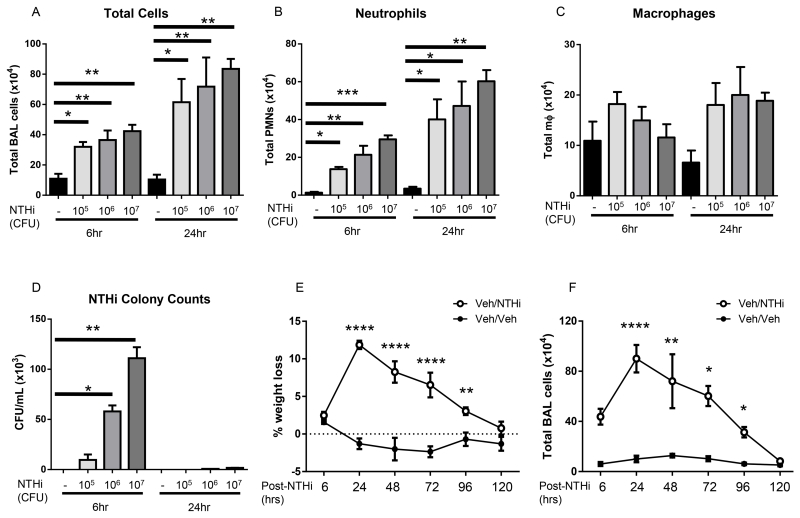
To characterize the timing and magnitude of NTHi-induced inflammation and infection, we first infected mice with 1 × 105-108 CFUs NTHi and evaluated several key markers of inflammation. Instillation of 1 × 108 CFUs caused significant tissue damage and morbidity (data not shown). At lower doses (1 × 105- 1 × 107), NTHi increased the total number of inflammatory cells, neutrophils, and macrophages in bronchoalveolar lavage fluid (BALF) at both 6 and 24hrs post-infection in a dose-dependent manner (Fig. 1A-C). We additionally determined bacterial colony counts to evaluate clearance. In pilot testing, bacteria were distributed throughout the lung lobes in equal ratio to the lobe size (data not shown), and the right cranial (upper) lobe was used in future experiments to maintain consistency. Bacterial CFUs reflected the infective dose at 6hrs; >98% of NTHi was cleared by 24hrs (Fig. 1D). NTHi was never detected in the blood, spleen, or liver of infected mice (data not shown). Despite this rapid bacterial clearance and lack of bacteremia, mice infected with 1× 106 CFUs of NTHi exhibited significant weight loss up to 96hrs post-infection, with recovery beginning at 48hrs (Fig. 1E). Mice additionally had increasing total inflammatory cell counts 48hrs post-infection; by 72hrs mice these elevated cell counts began to decrease, indicative of the initiation of resolution (Fig. 1F). These data confirm that NTHi induces persistent and prolonged lung inflammation, despite rapid bacterial clearance.
Fig. 1. NTHi dose-dependently induces inflammation in C57BL/6 mice.
Mice infected with 1 × 105-107 CFUs NTHi had dose-dependently increased numbers of total cells, neutrophils, and macrophages at 6hrs and 24hrs (A-C) post-infection. NTHi infected mice also had dose-dependent levels of bacteria present in the lung at 6hrs, with >90% of bacteria cleared by 24hrs for all doses (D). NTHi infected mice had prolonged weight loss (E) and prolonged influx of inflammatory cells (F). Statistical significance was determined by one-way ANOVA (overall p=0.0014 [A 6hr], p=0.0041 [A 24hr], p=0.0002 [B 6hr], p=0.0022 [B 24hr], p=0.06 [C 24hr] p<0.0001 [D, E, F]) with Bonferroni’s posttest for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001) n=5 mice/group.
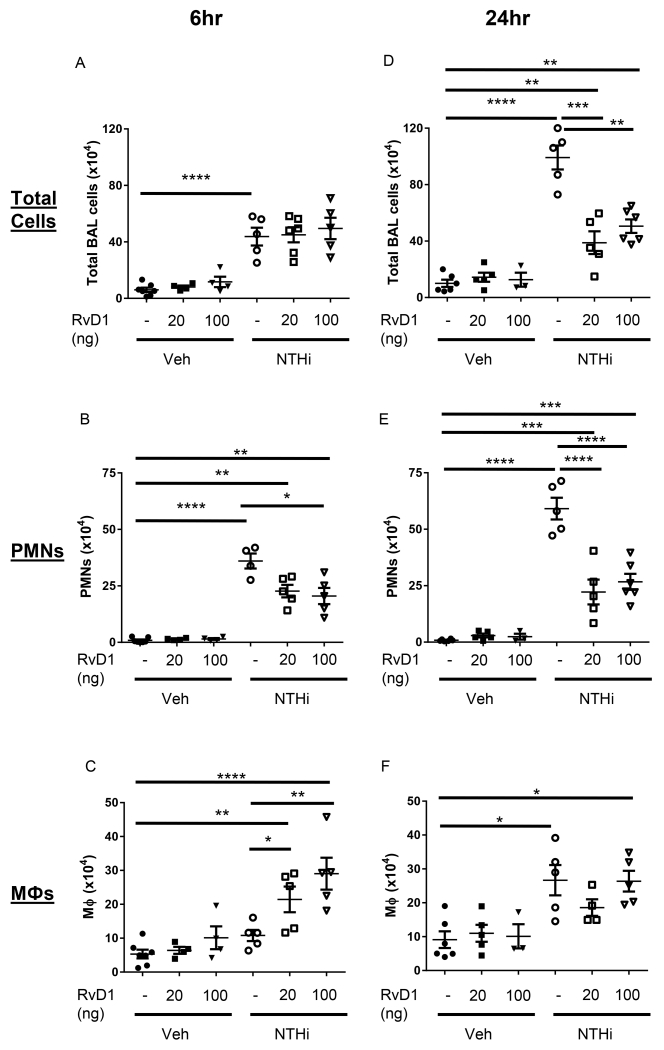
AT-RvD1 alters the NTHi-induced inflammatory cell profile and promotes efferocytosis
We next evaluated whether AT-RvD1 could attenuate excessive NTHi-induced inflammation. AT-RvD1 did not dampen initial NTHi-induced cell infiltration at 6hrs, but AT-RvD1 treated mice had significantly reduced total BAL cell counts at 24hrs post-infection (Fig. 2A, D). AT-RvD1 treated mice also had decreased total neutrophil (PMN) numbers at both 6 and 24hrs (Fig. 2B, E). In contrast, AT-RvD1 increased the total number of macrophages at 6hrs (Fig. 2C). However, at 24hrs NTHi infected mice showed increased total levels of macrophages; AT-RvD1 treated mice had no difference in total macrophage counts compared to NTHi infected and vehicle treated mice by 24hrs (Fig. 2F).
Fig. 2. AT-RvD1 shifts the profile of inflammatory cell influx.
Differential cell counts were determined from bronchoalveolar lavage of AT-RvD1 (20 or 100ng/mouse, OA) and/or NTHi (1 × 106 CFUs, OA) inoculated mice. Total cell (A, D), neutrophil (B, E), and macrophage (C, F) counts were evaluated at 6hrs (A-C) and 24hrs (D-F). Statistical significance was determined by two-way ANOVA (overall p<0.0001 NTHi [all panels], p= 0.0055 AT-RvD1 [B], p=0.0021 AT-RvD1 [C], p<0.0001 AT-RvD1 [D, E]) with Bonferroni’s posttest for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001), n=5-8 mice/group.
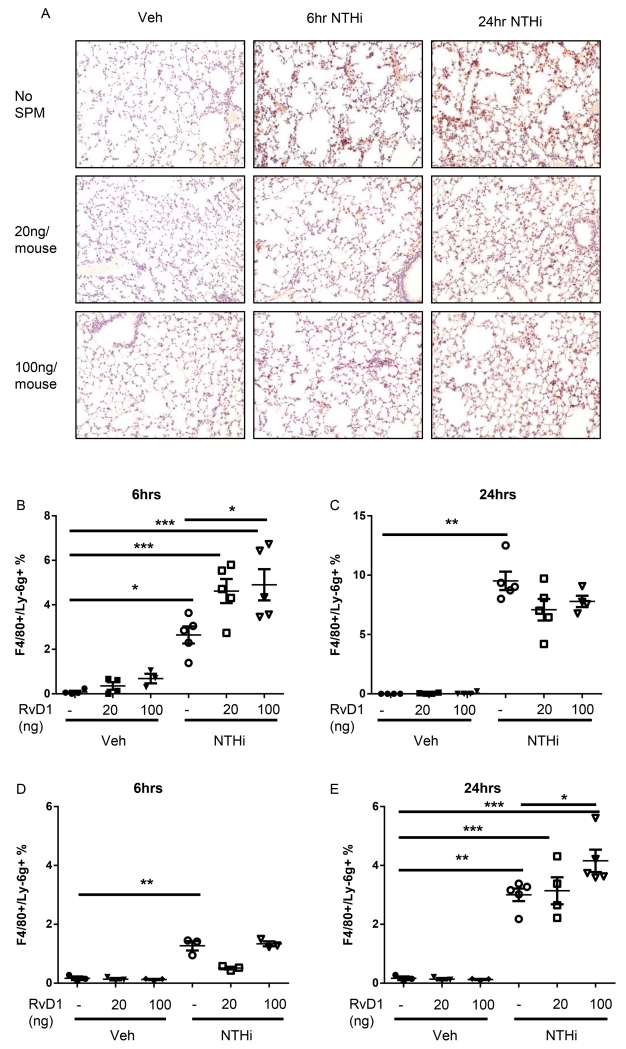
Histological sections of lung tissue were also evaluated for neutrophil influx. NTHi infected mice had increased neutrophil staining at 6 and 24hrs after infection (Fig. 3A). These increases were strongly attenuated by AT-RvD1 treatment at both time points (Fig. 3A). Alveolar macrophages were additionally evaluated by flow cytometry to determine if enhanced efferocytosis accounted for this difference. AT-RvD1 treated mice had increased uptake of apoptotic neutrophils (early in the inflammatory response at 6hrs) in macrophages from BALF, and enhanced neutrophil uptake at 24hrs by macrophages from collagenase digested lungs (Fig. 3B-E).
Fig. 3. AT-RvD1 decreases neutrophil influx and promotes enhanced efferocytosis.
Histological sections were taken from AT-RvD1 (20 or 100ng/mouse, OA) and/or NTHi (1 × 106 CFUs, OA) inoculated mice at 6hrs and 24hrs and stained for neutrophils (brown stain) and counterstained with hemotoxylin (A). Efferocytosis from bronchoalveolar lavage (B-C) and collagenase digestion (D-E) at 6hrs (B, D) and 24hrs (C, E) were also evaluated by flow cytometry. F4/80+/Ly6G+ cells represent macrophages with ingested neutrophils. Statistical significance was determined by two-way ANOVA (overall p<0.0001 NTHi [all panels], p=0.0178 AT-RvD1 [B]) with Bonferroni’s posttest for multiple comparisons (*p<0.05, **p<0.01), n=5-8 mice/group.
AT-RvD1 temporally dampens pro-inflammatory cytokines and Cox-2
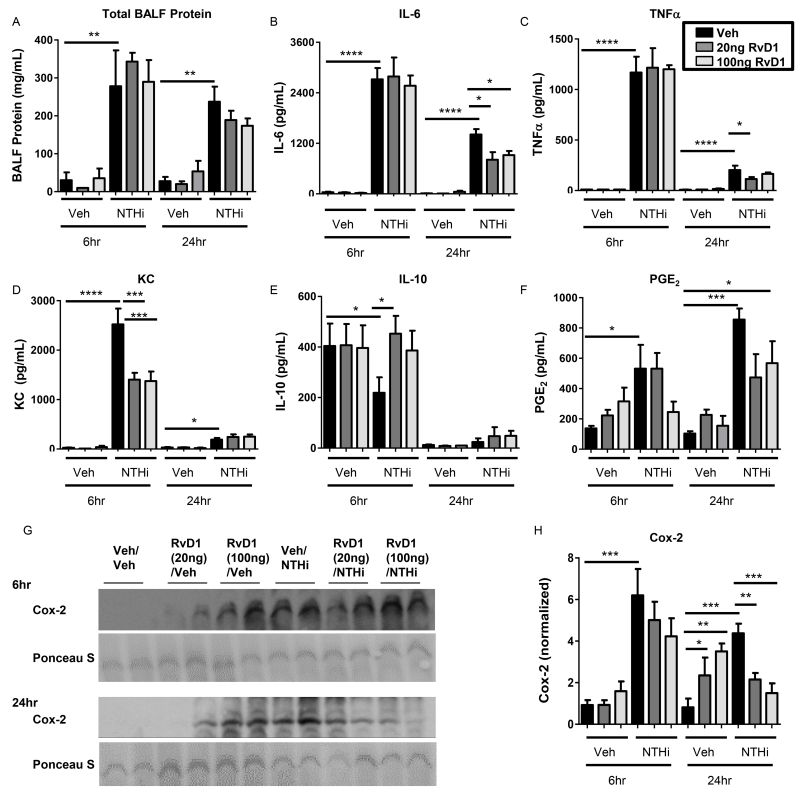
In addition to inflammatory cell influx, we also measured the effect of AT-RvD1 on NTHi-induced production of inflammatory cytokines in BALF. NTHi infected mice had significantly more protein in their BALF at both 6 and 24hrs, indicative of edema and inflammation (Fig. 4A). NTHi also promoted induction of pro-inflammatory cytokines IL-6, TNFα, and KC (Fig. 4B-D). AT-RvD1 did not alter IL-6 or TNFα production at 6hrs, but did reduce the level of KC (Fig. 4B-D). At 24hrs, AT-RvD1 attenuated production of both TNFα and IL-6 levels (Fig. 4B-C). KC had returned to near-background levels at 24hr and there were no further reductions with AT-RvD1 (Fig. 4D). Along with pro-inflammatory cytokines, we evaluated the effect of NTHi and AT-RvD1 on the anti-inflammatory cytokine IL-10. At 6hrs, NTHi reduced IL-10 expression, which was restored by AT-RvD1 treatment (Fig. 4E).
Fig. 4. AT-RvD1 dampens production of pro-inflammatory cytokines.
Levels of pro-inflammatory cytokines were measured in bronchoalveolar lavage fluid of AT-RvD1 (veh- black bars, 20ng/mouse- dark grey bars, or 100ng/mouse- light grey bars, OA) and/or NTHi (1 × 106 CFUs, OA) inoculated mice at 6hrs and 24hrs. Total protein was determined by BCA (A). BALF was assessed for IL-6 (B), TNFα (C), KC (D), and IL-10 (E). PGE2 (F) and Cox-2 (G, H) expression were also evaluated in lung homogenates by EIA and Western Blot, respectively (representative blots shown, densitometry on n=4-5 mice/group). Statistical significance was determined by two-way ANOVA (overall p<0.0001 NTHi [A, B, C, D, F, H], p=0.030 AT-RvD1 [B 6hr], p=0.0257 AT-RvD1 [D 24hr], p=0.0131 AT-RvD1 [H 6hr]) with Bonferroni’s posttest for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001), n= 4-6 mice/group.
We further evaluated the effect of AT-RvD1 on prostaglandin E2 (PGE2) and cyclooxygenase-2 (Cox-2) expression. Cox-2 plays multiple roles in the inflammatory process and catalyzes production of PGE2, a prostaglandin with important roles in bacterial infections. NTHi induced strong Cox-2 expression. AT-RvD1 did not attenuate NTHi induced Cox-2 expression at 6hrs, but dose-dependently dampened expression at 24hrs. (Fig. 4G-H). PGE2 was also increased by NTHi, with trending decreases with AT-RvD1 treatment (Fig. 4F). Taken together, these data reflect the temporal regulation abilities of AT-RvD1 in reducing inflammation and promoting resolution without being overtly immunosuppressive.
AT-RvD1 promotes bacterial clearance
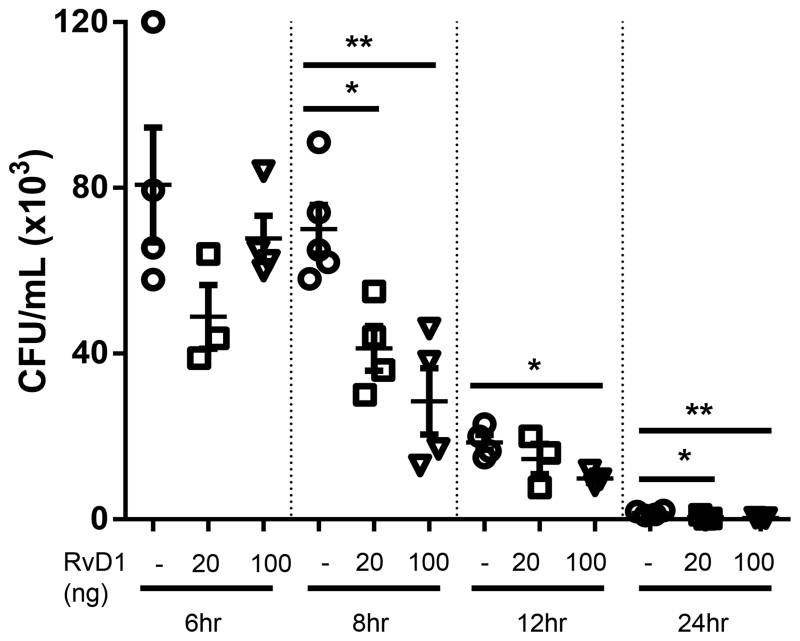
A defining characteristic of AT-RvD1 is its ability to dampen inflammation and promote resolution without being immunosuppressive. To evaluate this property, we examined the effect of AT-RvD1 treatment on pulmonary bacteria levels. AT-RvD1 treated mice displayed dose-dependent decreases in bacterial colonies, consistent with bacterial clearance; this effect was evident as early as 6hrs and was statistically significant by 8, 12, and 24hrs (Fig. 5). AT-RvD1 did not directly act to kill NTHi, as colony counts on chocolate agar plates streaked with an NTHi or NTHi plus AT-RvD1 suspension were not significantly different (data not shown).
Fig. 5. AT-RvD1 treated mice have decreased NTHi bacterial burden.
Colony counts were assessed in lung homogenates 6-24hrs post-infection with 1 × 106 CFUs of NTHi. Statistical significance was determined at each time point by one-way ANOVA with Bonferroni’s posttest for multiple comparisons (*p<0.05, **p<0.01), n=3-6mice/group.
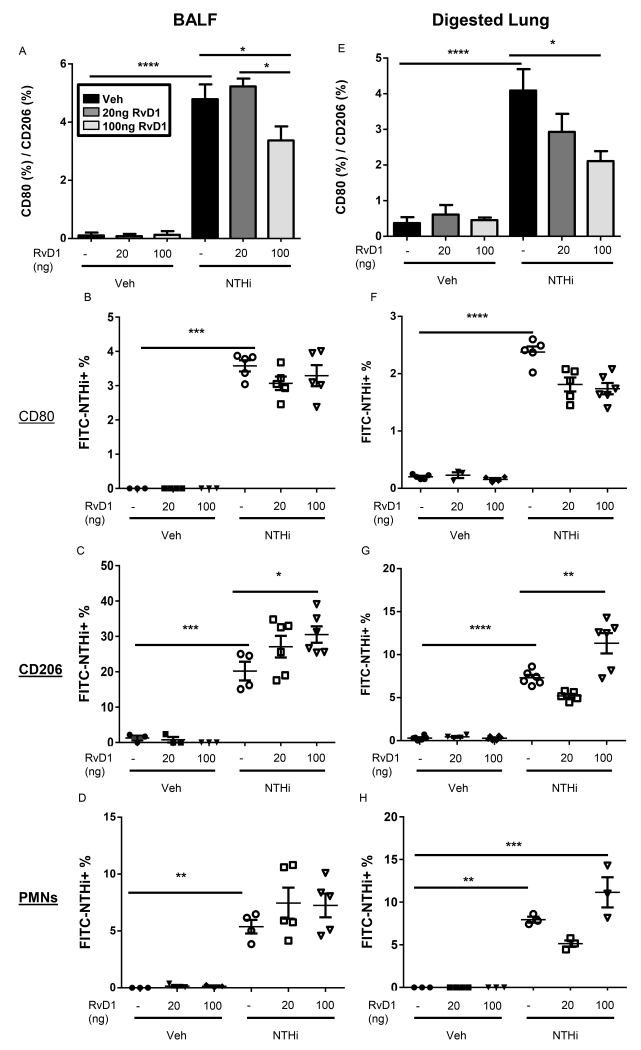
SPMs have been shown in non-microbial models of inflammation to increase phagocytic abilities of innate immune cells (20, 22, 37, 38). In particular, SPMs promote alternative M2 macrophages to mediate this enhanced phagocytosis. We hypothesized that AT-RvD1 induced decreases in bacterial CFUs were therefore due to polarization of macrophages to an M2 phenotype which thereby mediated enhanced clearance of NTHi. Macrophages obtained by both bronchoalveolar lavage and lung collagenase digestion from AT-RvD1 treated mice had a reduced ratio of pro-inflammatory M1 macrophages (CD80) to pro-resolving M2 macrophages (CD206) macrophages compared to NTHi-infected mouse macrophages (Fig. 6A, E). This indicates a shift towards alternative M2 macrophages in AT-RvD1 treated mice. We further evaluated phagocytosis of NTHi by M1 macrophages, M2 macrophages, and neutrophils. Mice were instilled with fluorescently labeled NTHi and double stained macrophages and neutrophils were analyzed by flow cytometry. AT-RvD1 treatment induced no difference in NTHi phagocytosis by CD80+ macrophages or by neutrophils, but CD206+ macrophages had a dose-dependent increase in NTHi uptake with AT-RvD1 treatment (Fig. 6B-D, F-H).
Fig. 6. AT-RvD1 promotes M2 macrophages to mediate NTHi clearance.
Mice were treated with AT-RvD1 (veh- black bars, 20ng/mouse- dark grey bars, or 100ng/mouse- light grey bars, OA) and/or NTHi (1 × 106 CFUs, OA) for 8hrs. Macrophages and neutrophils were obtained from bronchoalveolar lavage (A-D) and collagenase digestion (E-H). Macrophage phenotype was assessed by flow cytometry (A, E). Uptake of NTHi by CD80+ macrophages, CD206+ macrophages, and neutrophils was also assessed by flow cytometry in bronchoalveolar lavage (B-D) and collagenase digested lungs (F-H). Statistical significance was determined by two-way ANOVA (overall p<0.0001 NTHi [all panels], p=0.0288 AT-RvD1 [A], p=0.0018 AT-RvD1 [F], p<0.0001 AT-RvD1 [G], p=0.0032 AT-RvD1 [H]) with Bonferroni’s posttest for multiple comparisons (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001), n=3-6 mice/group.
AT-RvD1 protects health outcomes in NTHi-infected mice
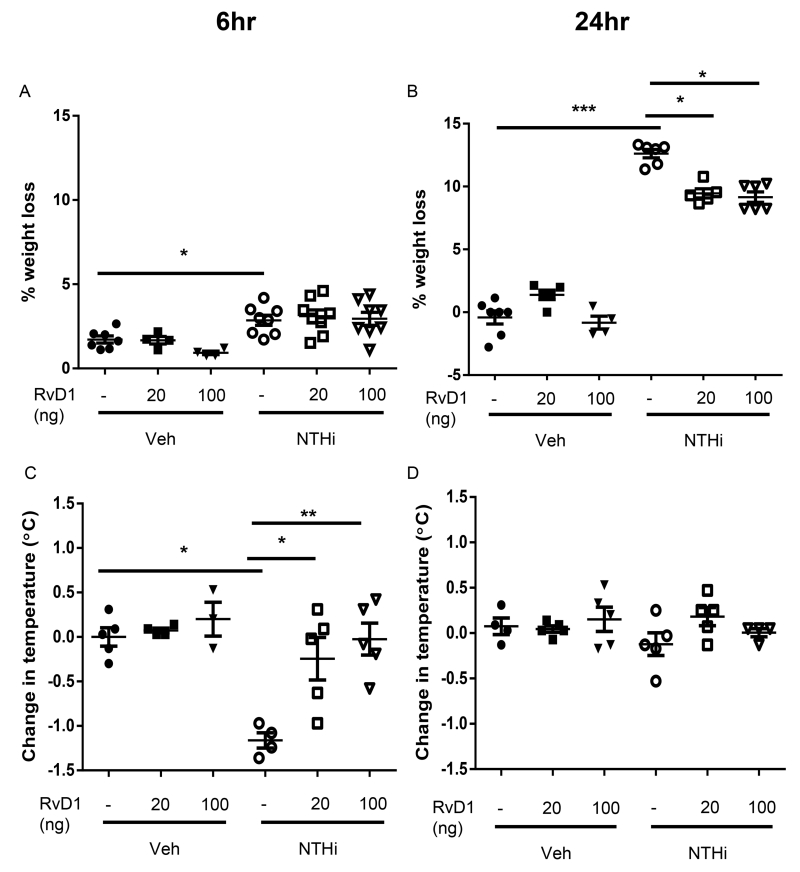
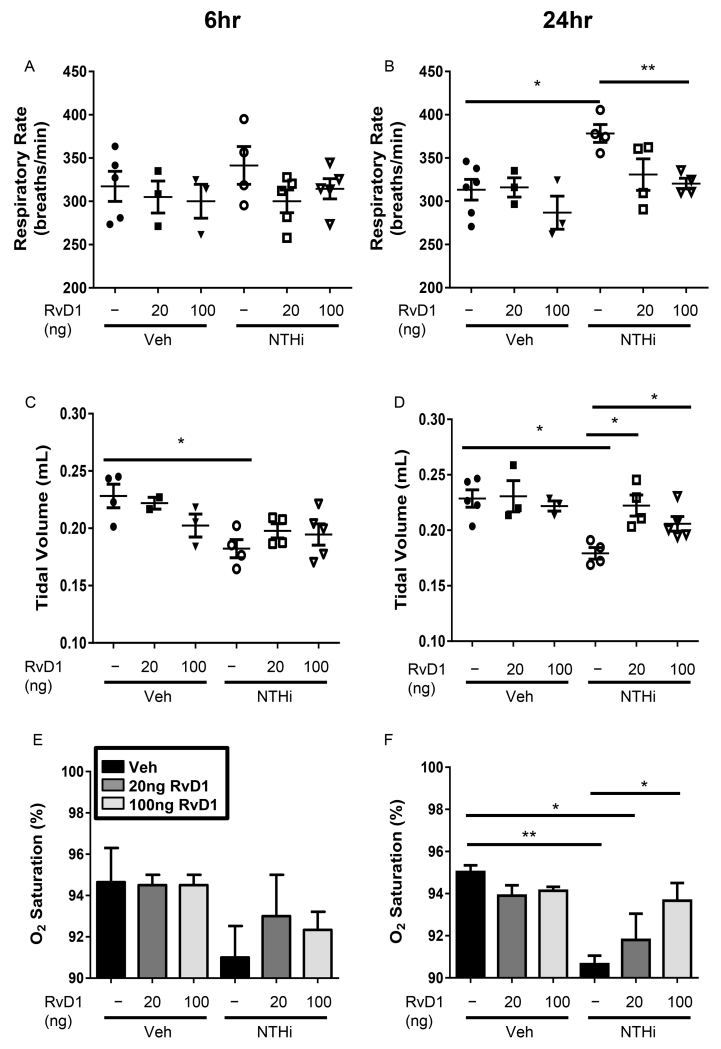
Along with dampened inflammation and enhanced bacterial clearance, we investigated whether AT-RvD1 could improve the general health of infected mice. NTHi-infected mice exhibit a trend toward weight loss as early as 6hr after infection, with significant (up to 15%) weight loss at 24hr (Fig. 7A-B). The mice also exhibit a significant 1.5°C drop in body temperature 6hrs post-infection; normal body temperature was restored by 24hrs (Fig. 7C-D). AT-RvD1 treatment protected the mice both from weight loss and hypothermia, thereby preventing them from going into shock (Fig. 7A-D). NTHi-infected mice also had altered respiratory physiology, with increased respiratory rates and decreased tidal volume at 24hrs, indicative of respiratory distress (Fig. 8A-D). NTHi infected mice further exhibit a dramatic drop in the O2 saturation of hemoglobin comparable to that seen in humans who require mechanical ventilation (Fig. 8E-F). These alterations to respiratory physiology and O2 saturation were dose-dependently rescued by AT-RvD1 treatment, restoring respiratory health (Fig. 8A-F). These results demonstrate the strong therapeutic potential of AT-RvD1 against NTHi infection.
Fig. 7. AT-RvD1 dampens NTHi-induced weight loss and hypothermia.
Mice inoculated with AT-RvD1 (20 or 100ng/mouse, OA) and/or NTHi (1 × 106 CFUs, OA) were weighed immediately before infection and at time of sacrifice at 6 and 24hrs (A-B). Additionally, the core temperature was recorded prior to euthanasia at 6 and 24hrs (C-D). Statistical significance was determined by two-way ANOVA (overall p<0.0001 NTHi [A, B, C], p=0.0006 AT-RvD1 [B], p=0.0019 AT-RvD1 [C]) with Bonferroni’s posttest for multiple comparisons (*p<0.05, **p<0.01), n=3-6 mice/group.
Fig. 8. AT-RvD1 improves respiratory health in NTHi infected mice.
Respiratory outcomes were measured for mice inoculated with AT-RvD1 (veh- black bars, 20ng/mouse- dark grey bars, or 100ng/mouse- light grey bars, OA) and/or NTHi (1 × 106 CFUs, OA) at 6 and 24hrs post-infection. Respiratory rates (A, B), tidal volume (C, D) and O2 saturation of hemoglobin (E, F) were assessed. Statistical significance was determined by two-way ANOVA (overall p=0.0044 NTHi [B], p=0.004 NTHi [C], p=0.002 [D], p=0.0005 NTHi [F], p=0.0069 AT-RvD1 [B], p=0.0452 AT-RvD1 [D]) with Bonferroni’s posttest for multiple comparisons (*p<0.05, **p<0.01), n=3-6 mice/group.
Discussion
SPMs represent a novel class of non-immunosuppressive small lipid molecules with high clinical potential for treatment of inflammatory diseases. Here, we have shown that AT-RvD1 is effective at dampening NTHi-induced lung inflammation, while still promoting bacterial clearance. AT-RvD1 treated mice had reduced inflammatory cell influx, with a higher macrophage:neutrophil ratio and increased efferocytosis compared to NTHi infected mice with vehicle treatment. AT-RvD1 attenuated levels of pro-inflammatory cytokines and Cox-2 expression while preventing decreases in anti-inflammatory cytokines. Critically, these anti-inflammatory effects did not impair bacterial clearance, and AT-RvD1 treated mice had fewer NTHi CFUs. This decreased bacterial burden was mediated by an increase in M2 macrophages, which had enhanced bacterial phagocytosis. Overall, AT-RvD1 treated mice had less weight loss, less hypothermia, improved respiratory physiology, and a smaller drop in O2 saturation, indicating less hypoxemia. These effects underscore the clinical therapeutic potential of AT-RvD1 for NTHi infection.
In each of our observed endpoints, all NTHi infected mice mounted an initial inflammatory response, but the AT-RvD1 treated mice had more rapid clearance of NTHi and resolution of inflammation. At 6hr post-infection, in the early stages of bacterial clearance, AT-RvD1 dampened KC, a cytokine that promotes neutrophil recruitment, contributing to decreased levels of neutrophils in AT-RvD1 treated lungs. However, AT-RvD1 did not dampen levels of IL-6 and TNFα until 24hrs. These cytokines are important for initial inflammatory responses, with TNFα particularly contributing to bacterial phagocytosis and killing. This same effect was seen in inflammatory cell influx, wherein AT-RvD1 did not halt inflammatory cell influx, but shifted the cellular profile to promote an early increase in macrophages and a faster clearance of neutrophils. This regulation of cytokines at different time points is key for several reasons. First, AT-RvD1 does not broadly block all cytokine signaling, distinguishing SPMs from other strictly anti-inflammatory therapies. This non-immunosuppressive nature is further seen as SPMs increase macrophage influx, enhance efferocytosis, and increase the phagocytic abilities of macrophages (20-22, 25, 33, 39). Second, AT-RvD1 decreases in KC levels early on demonstrate that SPMs can preferentially regulate early cytokine release to jump-start resolution. Third, endogenous resolution of NTHi-induced inflammation is slow, occurring days after bacterial clearance. AT-RvD1, however, enhances natural resolution, more rapidly clearing apoptotic cells and dampening pro-inflammatory cytokine release. These data highlight that SPMs do not suppress immune cell function, but instead promote a shift in macrophage functional activities to speed the resolution process.
Along with temporal regulation of pro-inflammatory cell influx and cytokines, SPMs are also capable of shifting cytokine production to promote enhanced levels of anti-inflammatory or pro-resolving cytokines. SPMs are derived from omega three and omega six fatty acids, precursors that can undergo lipid class switching to produce both pro- and anti-inflammatory molecules in a temporal manner and under different stimuli (40). This is highlighted by our results with Cox-2; while AT-RvD1 partially inhibited the increased expression of Cox-2 in NTHi-treated mice at 24hr, it paradoxically increased Cox-2 expression in vehicle-treated mice. Cox-2 is capable of producing both pro- and anti-inflammatory mediators and AT-RvD1 has been shown to increase Cox-2 under homeostatic conditions in human macrophages (20, 41). Therefore, AT-RvD1 may be acting to promote other SPMs or prostaglandins to maintain homeostatic conditions in the absence of an inflammatory response, but dampening NTHi-induced Cox-2 expression to prevent production of pro-inflammatory mediators. Furthermore, PGE2 continues to increase at 24hrs, in contrast to other cytokines, despite no change in mPGES1 (data not shown). While Cox-2 expression is lower at 24hrs than at 6hrs, Cox-2 in veh/NTHi mice is still significantly elevated about veh/veh mice, which could account for the increased PGE2. Furthermore, PGE2 has been shown to have both pro- and anti-inflammatory actions. Thus, late stage PGE2 could be acting in an anti-inflammatory manner or may simply be the result of accumulated prostaglandins. The connection between Cox-2 and SPM production is a large area of study and one that bears further investigation in this model. Overall, regulation of lipid mediator class switching is an important characteristic of SPMs, as is the shift to promote anti-inflammatory cytokines. Eicosanoids, including both SPMs and pro-inflammatory mediators, produced in the early stages of inflammation can form feedback loops to promote the shift to anti-inflammatory lipids (40, 42). While further in depth investigations are needed to fully elucidate the role of Cox-2 in inflammation and SPM mediated resolution, there is evidence that these distinct lipid mediator profiles produced at different stages of inflammation can mediate the progression from initiation of inflammation through to resolution and can reprogram immune cells to promote resolution.
In our studies, some actions of AT-RvD1 are dose-dependent (such as phagocytosis and efferocytosis), while other effects are similar at the 20ng and 100ng doses (such as reductions in inflammatory cells and cytokines). Different components of the resolution process may be more sensitive to AT-RvD1’s actions (such as dampening of inflammatory cytokines compared to pro-resolving phagocytosis), and it is possible that a dose-dependent changes in cytokines and inflammatory cells would have been seen at doses below 20 ng. Because different SPMs act through different receptors (43, 44), it is also possible that a combination of SPMs would have stronger pro-resolving effects than higher doses of single SPMs. Such combination studies will be an important next step toward development of SPMs as clinical therapies.
The non-immunosuppressive properties of SPMs are most clearly demonstrated by their ability to mediate macrophage phenotypic switching. We observed an increase in the percentage of M2 macrophages and a decrease in the percentage of M1 macrophages, culminating in an overall shift in the ratio of these phenotypes with AT-RvD1 treatment. M2 macrophages are known to promote resolution, and a number of studies have demonstrated their enhanced phagocytic capabilities (39, 45-47). Moreover, multiple chronic inflammatory diseases have been linked to a skewed M1/M2 profile, with a deficiency in M2 macrophages presumably causing phagocytic and resolution deficiencies (45, 48).
Chronic obstructive pulmonary disease patients in particular have deficient phagocytosis, and NTHi is a common pathogen in COPD exacerbations and other underlying inflammatory conditions (3). NTHi can avoid opsonization and clearance, allowing for persistent colonization in the human lung. In our study, AT-RvD1 increased bacterial clearance from the lung through enhanced macrophage phagocytosis. While the specific mechanisms by which AT-RvD1 acts to enhance phagocytosis are unknown, this ability has been observed in other models (20-22, 38). Additionally, AT-RvD1 treated mice may have decreased bacterial burden because of enhanced macrophage killing. While SPMs do not have direct antibacterial properties, they can enhance internalization of bacteria, TNFα expression, and ROS production to mediate killing (25, 33). Although we have not yet demonstrated that AT-RvD1 or other SPMs can improve phagocytosis and bacterial killing in the context of chronic lung inflammatory disease, we recently reported that AT-RvD1 attenuates cigarette smoke-induced emphysema in a mouse model, with reductions in inflammatory signaling and oxidative stress (49). Future studies that evaluate SPMs in a setting of infection following chronic lung disease will be important to understand the translation potential of these compounds. We will also be very interested to determine if SPMs are equally efficacious in a model of chronic recurrent infection; i.e., would SPMs be effective if given during a third or fourth infection after several rounds of untreated infection.
AT-RvD1 may be acting through a number of signaling pathways to mediate its effects. As described above, Cox-2 can regulate the production of pro- and anti-inflammatory signals, including SPM production. NTHi has been shown to activate the NF-κB and MAPK pathways to promote inflammation (17). SPMs have also been shown in multiple studies to act on the NF-κB pathway to mediate their effects (20, 24, 27, 33). Moreover, NTHi induces expression of TAK-1, which leads to enhanced NF-κB expression; TAK-1 is negatively regulated by cylindromatosis (CYLD), a primary negative regulator for NTHi induced inflammation overall ((50, 51). Our lab has shown in epithelial cells that AT-RvD1 can dampen TAK-1, highlighting another potential target in NTHi infections (37). Many different signaling molecules exist which are regulated by both NTHi and SPMs, and these candidate markers represent an interesting area for future mechanistic studies.
The pro-resolving actions of AT-RvD1 yielded markedly improved respiratory physiology in treated mice in this study. NTHi-infected mice demonstrated elevated respiratory rates and decreased tidal volumes; importantly, these alterations were accompanied by dramatic decreases in oxygen saturation of hemoglobin, highlighting an important clinical consequence of impaired lung physiology. Remarkably, AT-RvD1 treatment significantly improved lung physiology, resulting in improved oxygen saturation of hemoglobin in NTHi-infected mice. The ability of AT-RvD1 to protect against NTHi-induced weight loss was likely multifactorial; since treated mice had improved respiratory physiology and thermal homeostasis, and thus lower metabolic demand for basic functions such as breathing and maintaining body temperature, this contributed to preventing weight loss. These are the first observed effects of NTHi on respiratory rates, oxygen saturation, and temperature in mice, and the first evidence that SPMs can act to improve these health markers in a live bacteria, pulmonary infection model.
These data represent the first evidence in a pulmonary model that SPMs can act in a pro-resolving manner to dampen inflammation and improve lung physiology while still enhancing bacterial clearance. These unique properties open up a wide range of therapeutic opportunities for AT-RvD1, particularly as an alternative for chronic inflammatory conditions such as COPD, where infective exacerbations are common. AT-RvD1 has high therapeutic potential for such patients, and these studies are a first step into investigating this novel and critical area of resolution research.
Acknowledgements
We would like to thank the URMC Vivarium for providing instruments for several animal procedures; the URMC flow core for their flow cytometry assistance; the Jian-Dong Li lab for their generous donation of NTHi; Kristina Owens, Claire McCarthy, and Parker Duffney for technical assistance; and Samir Bhagwat, Frank Gigliotti, and Terry Wright for their technical expertise and use of equipment in measuring respiratory outcomes and O2 saturation.
Sources of Support: This work was supported in part by NIEHS T32ES007026, NIH P30ES01247, NIH R01HL120908, NIH T32HL066988 and the PhRMA Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work was supported in part by Awards UL1RR024160 and 8UL1TR000042 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health. P.J.S. was supported in part by the C. Jane Davis and C. Robert Davis Fund.
Abbreviations
- AT-RvD1
aspirin-triggered Resolvin D1
- Cox-2
cyclooxygenase-2
- LPS
lipopolysaccharide
- NTHi
Nontypeable Haemophilus influenzae
- PGE2
prostaglandin E2
- SPMs
specialized pro-resolving mediators
Footnotes
Disclaimers: SL declares that the views expressed herein are those of the author and do not reflect the official policy or position of the Department of the Army, Department of Defense, or the U.S. Government. The other authors have no conflicts of interest to declare.
References
- 1.Murphy TF. Haemophilus Infections. In: Braunwald F, Kaspar DL, Hauser SL, Longo DL, Jameson L, editors. Harrisons Principles of Internal Medicine. McGraw Hill; New York, NY: 2005. pp. 824–229. [Google Scholar]
- 2.King PT, Sharma R. The Lung Immune Response to Nontypeable Haemophilus influenzae (Lung Immunity to NTHi) Journal of immunology research. 2015;2015:706376. doi: 10.1155/2015/706376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moghaddam SJ, Ochoa CE, Sethi S, Dickey BF. Nontypeable Haemophilus influenzae in chronic obstructive pulmonary disease and lung cancer. International journal of chronic obstructive pulmonary disease. 2011;6:113–123. doi: 10.2147/COPD.S15417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Microbiologic follow-up study in adult bronchiectasis. Respiratory medicine. 2007;101:1633–1638. doi: 10.1016/j.rmed.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Fitzpatrick ME, Sethi S, Daley CL, Ray P, Beck JM, Gingo MR. Infections in “noninfectious” lung diseases. Annals of the American Thoracic Society. 2014;11(Suppl 4):S221–226. doi: 10.1513/AnnalsATS.201401-041PL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bandi V, Apicella MA, Mason E, Murphy TF, Siddiqi A, Atmar RL, Greenberg SB. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. American journal of respiratory and critical care medicine. 2001;164:2114–2119. doi: 10.1164/ajrccm.164.11.2104093. [DOI] [PubMed] [Google Scholar]
- 7.Desai H, Eschberger K, Wrona C, Grove L, Agrawal A, Grant B, Yin J, Parameswaran GI, Murphy T, Sethi S. Bacterial colonization increases daily symptoms in patients with chronic obstructive pulmonary disease. Annals of the American Thoracic Society. 2014;11:303–309. doi: 10.1513/AnnalsATS.201310-350OC. [DOI] [PubMed] [Google Scholar]
- 8.Bandi V, Jakubowycz M, Kinyon C, Mason EO, Atmar RL, Greenberg SB, Murphy TF. Infectious exacerbations of chronic obstructive pulmonary disease associated with respiratory viruses and non-typeable Haemophilus influenzae. FEMS immunology and medical microbiology. 2003;37:69–75. doi: 10.1016/S0928-8244(03)00100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurst JR, Vestbo J, Anzueto A, Locantore N, Mullerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, Macnee W, Calverley P, Rennard S, Wouters EF, Wedzicha JA. Susceptibility to exacerbation in chronic obstructive pulmonary disease. The New England journal of medicine. 2010;363:1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 10.CDC . Active Bacterial Core Surveillance Report, Haemophilus Influenzae. In: E. I. P. Network, editor. The Journal of pharmacology and experimental therapeutics. 2013. [Google Scholar]
- 11.Resman F, Ristovski M, Forsgren A, Kaijser B, Kronvall G, Medstrand P, Melander E, Odenholt I, Riesbeck K. Increase of beta-lactam-resistant invasive Haemophilus influenzae in Sweden, 1997 to 2010. Antimicrobial agents and chemotherapy. 2012;56:4408–4415. doi: 10.1128/AAC.00415-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wan Sai Cheong J, Smith H, Heney C, Robson J, Schlebusch S, Fu J, Nourse C. Trends in the epidemiology of invasive Haemophilus influenzae disease in Queensland, Australia from 2000 to 2013: what is the impact of an increase in invasive non-typable H. influenzae (NTHi)? Epidemiology and infection. 2015;143:2993–3000. doi: 10.1017/S0950268815000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clementi CF, Murphy TF. Non-typeable Haemophilus influenzae invasion and persistence in the human respiratory tract. Frontiers in cellular and infection microbiology. 2011;1:1. doi: 10.3389/fcimb.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallstrom T, Riesbeck K. Haemophilus influenzae and the complement system. Trends in microbiology. 2010;18:258–265. doi: 10.1016/j.tim.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Lugade AA, Bogner PN, Murphy TF, Thanavala Y. The role of TLR2 and bacterial lipoprotein in enhancing airway inflammation and immunity. Frontiers in immunology. 2011;2:10. doi: 10.3389/fimmu.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lugade AA, Vethanayagam RR, Nasirikenari M, Bogner PN, Segal BH, Thanavala Y. Nrf2 regulates chronic lung inflammation and B-cell responses to nontypeable Haemophilus influenzae. American journal of respiratory cell and molecular biology. 2011;45:557–565. doi: 10.1165/rcmb.2010-0321OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu F, Xu Z, Zhang R, Wu Z, Lim JH, Koga T, Li JD, Shen H. Nontypeable Haemophilus influenzae induces COX-2 and PGE2 expression in lung epithelial cells via activation of p38 MAPK and NF-kappa B. Respiratory research. 2008;9:16. doi: 10.1186/1465-9921-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Steere RR, Fedorchuk CA, Pang J, Lee JY, Lim JH, Xu H, Pan ZK, Maggirwar SB, Li JD. Activation of epidermal growth factor receptor is required for NTHi-induced NF-kappaB-dependent inflammation. PloS one. 2011;6:e28216. doi: 10.1371/journal.pone.0028216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buckley CD, Gilroy DW, Serhan CN. Proresolving Lipid Mediators and Mechanisms in the Resolution of Acute Inflammation. Immunity. 2014;40:315–327. doi: 10.1016/j.immuni.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP. Resolvins Attenuate Inflammation and Promote Resolution in Cigarette Smoke-Exposed Human Macrophages. American journal of physiology. Lung cellular and molecular physiology. 2015 doi: 10.1152/ajplung.00125.2015. ajplung 00125 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ. A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PloS one. 2013;8:e58258. doi: 10.1371/journal.pone.0058258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalli J, Serhan CN. Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood. 2012;120:e60–72. doi: 10.1182/blood-2012-04-423525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogerio AP, Haworth O, Croze R, Oh SF, Uddin M, Carlo T, Pfeffer MA, Priluck R, Serhan CN, Levy BD. Resolvin D1 and aspirin-triggered resolvin D1 promote resolution of allergic airways responses. Journal of immunology. 2012;189:1983–1991. doi: 10.4049/jimmunol.1101665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liao Z, Dong J, Wu W, Yang T, Wang T, Guo L, Chen L, Xu D, Wen F. Resolvin D1 attenuates inflammation in lipopolysaccharide-induced acute lung injury through a process involving the PPARgamma/NF-kappaB pathway. Respiratory research. 2012;13:110. doi: 10.1186/1465-9921-13-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer CD, Mancuso CJ, Weiss JP, Serhan CN, Guinan EC, Levy O. 17(R)-Resolvin D1 differentially regulates TLR4-mediated responses of primary human macrophages to purified LPS and live E. coli. Journal of leukocyte biology. 2011;90:459–470. doi: 10.1189/jlb.0311145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kure I, Nishiumi S, Nishitani Y, Tanoue T, Ishida T, Mizuno M, Fujita T, Kutsumi H, Arita M, Azuma T, Yoshida M. Lipoxin A(4) reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-kappaB activation. The Journal of pharmacology and experimental therapeutics. 2010;332:541–548. doi: 10.1124/jpet.109.159046. [DOI] [PubMed] [Google Scholar]
- 27.Chen F, Fan XH, Wu YP, Zhu JL, Wang F, Bo LL, Li JB, Bao R, Deng XM. Resolvin D1 improves survival in experimental sepsis through reducing bacterial load and preventing excessive activation of inflammatory response. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2013 doi: 10.1007/s10096-013-1978-6. [DOI] [PubMed] [Google Scholar]
- 28.Walker J, Dichter E, Lacorte G, Kerner D, Spur B, Rodriguez A, Yin K. Lipoxin a4 increases survival by decreasing systemic inflammation and bacterial load in sepsis. Shock. 2011;36:410–416. doi: 10.1097/SHK.0b013e31822798c1. [DOI] [PubMed] [Google Scholar]
- 29.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, Flower RJ, Perretti M, Serhan CN. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdulnour RE, Sham HP, Douda DN, Colas RA, Dalli J, Bai Y, Ai X, Serhan CN, Levy BD. Aspirin-triggered resolvin D1 is produced during self-resolving gram-negative bacterial pneumonia and regulates host immune responses for the resolution of lung inflammation. Mucosal immunology. 2015 doi: 10.1038/mi.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gobbetti T, Coldewey SM, Chen J, McArthur S, le Faouder P, Cenac N, Flower RJ, Thiemermann C, Perretti M. Nonredundant protective properties of FPR2/ALX in polymicrobial murine sepsis. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:18685–18690. doi: 10.1073/pnas.1410938111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu B, Walker JA, Temmermand D, Mian K, Spur B, Rodriguez A, Stein TP, Banerjee P, Yin K. Lipoxin A(4) promotes more complete inflammation resolution in sepsis compared to stable lipoxin A(4) analog. Prostaglandins, leukotrienes, and essential fatty acids. 2013;89:47–53. doi: 10.1016/j.plefa.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature. 2012;484:524–528. doi: 10.1038/nature11042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ueda T, Fukunaga K, Seki H, Miyata J, Arita M, Miyasho T, Obata T, Asano K, Betsuyaku T, Takeda J. Combination therapy of 15-epi-lipoxin A4 with antibiotics protects mice from Escherichia coli-induced sepsis*. Critical care medicine. 2014;42:e288–295. doi: 10.1097/CCM.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 35.Lakatos HF, Burgess HA, Thatcher TH, Redonnet MR, Hernady E, Williams JP, Sime PJ. Oropharyngeal aspiration of a silica suspension produces a superior model of silicosis in the mouse when compared to intratracheal instillation. Experimental lung research. 2006;32:181–199. doi: 10.1080/01902140600817465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McMillan DH, Baglole CJ, Thatcher TH, Maggirwar S, Sime PJ, Phipps RP. Lung-targeted overexpression of the NF-kappaB member RelB inhibits cigarette smoke-induced inflammation. The American journal of pathology. 2011;179:125–133. doi: 10.1016/j.ajpath.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsiao HM, Thatcher TH, Levy EP, Fulton RA, Owens KM, Phipps RP, Sime PJ. Resolvin D1 Attenuates Polyinosinic-Polycytidylic Acid-Induced Inflammatory Signaling in Human Airway Epithelial Cells via TAK1. Journal of immunology. 2014;193:4980–4987. doi: 10.4049/jimmunol.1400313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN. Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. American journal of physiology. Cell physiology. 2014;307:C39–54. doi: 10.1152/ajpcell.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ariel A, Serhan CN. New Lives Given by Cell Death: Macrophage Differentiation Following Their Encounter with Apoptotic Leukocytes during the Resolution of Inflammation. Frontiers in immunology. 2012;3:4. doi: 10.3389/fimmu.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN. Lipid mediator class switching during acute inflammation: signals in resolution. Nature immunology. 2001;2:612–619. doi: 10.1038/89759. [DOI] [PubMed] [Google Scholar]
- 41.Gilroy DW, Colville-Nash PR, Willis D, Chivers J, Paul-Clark MJ, Willoughby DA. Inducible cyclooxygenase may have anti-inflammatory properties. Nature medicine. 1999;5:698–701. doi: 10.1038/9550. [DOI] [PubMed] [Google Scholar]
- 42.Wu D, Zheng S, Li W, Yang L, Liu Y, Zheng X, Yang Y, Yang L, Wang Q, Smith FG, Jin S. Novel biphasic role of resolvin D1 on expression of cyclooxygenase-2 in lipopolysaccharide-stimulated lung fibroblasts is partly through PI3K/AKT and ERK2 pathways. Mediators of inflammation. 2013;2013:964012. doi: 10.1155/2013/964012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee CH, Yang R, Petasis NA, Serhan CN. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE, Shimizu T, Yokomizo T, Brink C. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58:463–487. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 45.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime reports. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. Journal of immunology. 2011;187:5408–5418. doi: 10.4049/jimmunol.1100225. [DOI] [PubMed] [Google Scholar]
- 47.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 48.Hodge S, Matthews G, Mukaro V, Ahern J, Shivam A, Hodge G, Holmes M, Jersmann H, Reynolds PN. Cigarette smoke-induced changes to alveolar macrophage phenotype and function are improved by treatment with procysteine. American journal of respiratory cell and molecular biology. 2011;44:673–681. doi: 10.1165/rcmb.2009-0459OC. [DOI] [PubMed] [Google Scholar]
- 49.Hsiao HM, Thatcher TH, Colas RA, Serhan CN, Phipps RP, Sime PJ. Resolvin D1 Reduces Emphysema and Chronic Inflammation. The American journal of pathology. 2015;185:3189–3201. doi: 10.1016/j.ajpath.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim JH, Jono H, Koga T, Woo CH, Ishinaga H, Bourne P, Xu H, Ha UH, Xu H, Li JD. Tumor suppressor CYLD acts as a negative regulator for non-typeable Haemophilus influenza-induced inflammation in the middle ear and lung of mice. PloS one. 2007;2:e1032. doi: 10.1371/journal.pone.0001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]