Abstract
Since initially described in 1967, prostatic ductal adenocarcinoma (PDA) has engendered a series of controversies on its origin, histological features, and biological behavior. Owing to the improvement of molecular biological technique, there are some updated findings on the characteristics of PDA. In the current review, we will mainly analyze its origin, clinical manifestations, morphological features, differential diagnosis, immunophenotype and molecular genetics, with the purpose of enhancing recognition of this tumor and making a correct diagnosis and treatment choice.
Keywords: Prostatic cancer, ductal adenocarcinoma, clinicopathology, differential diagnosis
The prostatic ductal adenocarcinoma (PDA), a uncommon histological subtype of prostatic carcinoma, was first described as “endometrioid carcinoma” by Melicow and Patcher in 1967, due to its histological resemblance to endometrial carcinoma (1). In the following literatures, PDA was thought to arise from prostatic utricle that belongs to a remnant of müllerian duct structure, even to be an entity of prostatic acinar adenocarcinoma (PAA) (2,3). It is now clear that PDA is originated from the primary and secondary prostatic ducts and has been recognized as a variant of prostatic adenocarcinoma by both the Armed Forces Institute of Pathology (AFIP) fascicle and the World Health Organization (WHO) classification because of its distinct and unique origin, morphological and clinical features (4,5).
Epidemiology and clinical manifestations
Although rare, PDA is still the second common histological variant of prostatic carcinoma with diverse incidence in prostatectomy and biopsy specimens. Its incidence varies from 0.4% to 0.8% in a pure ductal form and up to 3% to 12.7% in a mixed ductal-acinar adenocarcinoma form (2,6-10). PDA mainly occurs in elderly men with the age of 63 to 72 years old (range from 41 to 89 years old). The tumors predominantly locate in the periurethral zone of prostate, but can be found in peripheral zone (4,11,12). The cystoscopy examination reveals that PDA is usually an exophytic, villous or polypoid mass with white fronds, or infiltrates into the prostatic urethra at or near the verumontanum (13). The patients with PDA in periurethral zone may present with urinary obstruction, urinary urgency, urinary frequency and hematuria, which is related to an exophytic growth of tumor into the urethra (14,15).
It is worth mentioning that the patients with PDA may have normal digital rectal examination (DRE), particularly when tumors originate from the larger periurethral prostatic ducts or at an earlier stage (13,16,17). Moreover, it is documented that most PDA patients have normal serum prostate-specific antigen (PSA) level (less than 4.0 ng/mL). In a study from 46 cases of metastatic prostate cancer, 20% cases of patients with serum PSA below 2 ng/mL have pure or mixed ductal adenocarcinoma (18). Therefore, PDA is less likely to be identified by DRE or PSA assessment, which may result in its delayed diagnosis or missed diagnosis (19).
Pathological characteristics
Microscopically, PDA usually exhibits a mixed growth pattern, including papillary, cribriform, glandular, solid, and prostatic intraepithelial neoplasia (PIN)-like architecture (Figure 1). Among them, large papillary and cribriform patterns are the most common (20). In addition, there are several rare histological variants, including micropapillary, mucinous, foamy gland, and cystic papillary pattern. The micropapillary variant is characterized by the detached fragments of micropapillary cores without central fibrovascular stalk. The foamy gland variant shows the pale foamy cytoplasm, overt nuclear enlargement and prominent nucleoli in some areas. The mucinous variant typically contains intracellular mucin or extracellular mucin extravasation. The cystic papillary variant is composed of cystic and dilated areas protruded by papillary and glandular structures as same as usual PDA (11). Intraductal spread of tumor cells can also occur in the cases of PDA (21).
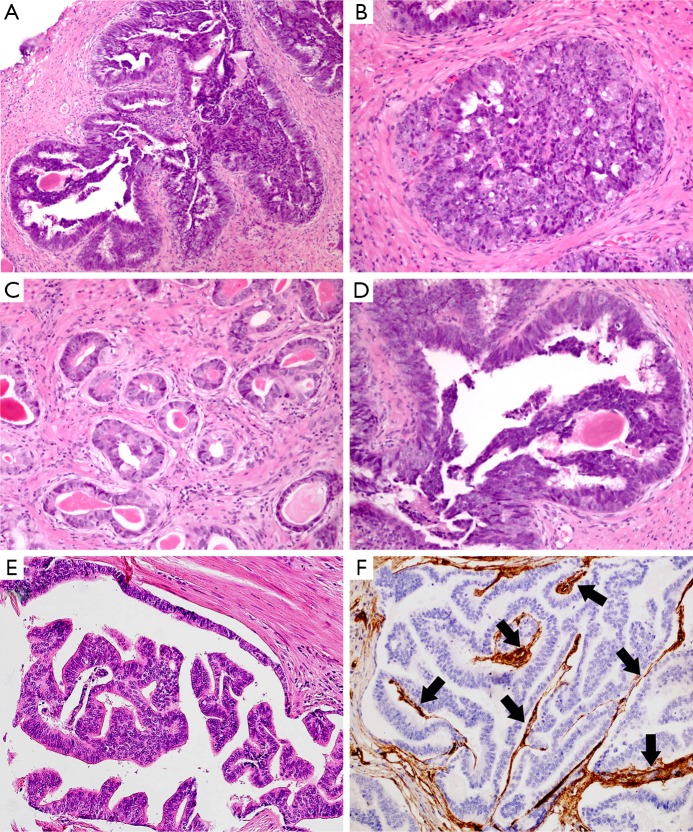
Figure 1.
PDA exhibits papillary (A), cribriform (B), and glandular (C) growth pattern. The tumor cells are pseudostratified columnar epithelium with amphophilic cytoplasm, elongated nuclei and prominent large nucleoli (D). This entity is characterized by the presence of tall, pseudostratified, columnar cells with abundant cytoplasm arranged in papillary pattern (E). The central fibrovascular cores in the papillae are highlighted by CD34 immunohistochemical staining (F, black arrows: blood vessels). (H&E: A, B, C&E, 200×, D, 400×; IHC, F, 200×).
In cytological terms, PDA exhibits various characteristics. Commonly, the tumor cells are tall, pseudostratified and columnar epithelium with high-grade nuclei. Those nuclei are elongated and oval shape with prominent nucleoli (Figure 1D). There are numerous mitoses and extensive necrosis. Rarely, the tumor cells display minimally cytological atypia. For example, those tumor cells in PIN-like PDA usually lack significant pleomorphism, high cellular proliferation activity, and prominent nucleoli. The cytoplasm of PDA is not usually amphophilic, occasionally it may be pale (4,15). If there are appearances of dense eosinophilic granules within columnar pseudostratified epithelial cells, the Paneth cell-like neuroendocrine differentiation should be taken into consideration (11).
Except pure ductal carcinoma form, PDA often presents as a heterogeneous disease, which mixed with other types of carcinoma occurred in the prostate. The most frequently coexisting histological type is acinar adenocarcinoma, the others include urothelial carcinoma, mucinous carcinoma, sarcomatoid carcinoma, and so on (22-26). Given their different biological behavior, it is necessary to identify all coexisting types of tumors, specify and estimate their proportions in the pathologic report.
Immunophenotype and molecular genetics
Similar to the immunophenotype of PAA, PDA usually expresses PSA and prostatic specific acid phosphatase (PSAP) in both primary and metastatic tumor tissues. However, it should be noted that distribution and intensity of staining for PSA and PSAP are often weak and/or focal, in concordance with high-grade PAA (26,27). The majority of tumor cells show strong expression for alpha-methylacyl coenzyme A racemase (AMACR). In one third cases of PDA, remnants of basal cells that can be identified by p63 or high molecular weight cytokeratin (HMWCK) in a rather weak and patchy pattern, which most likely represents intraductal extension of tumor cells (28). Being an independent prognostic marker of prostate cancer, nuclear expression of Ki-67 labeling index appears to be significantly higher in PDA than PAA. The roles of p53 and EGFR in distinguishing PDA from PAA are still controversial (29-31). Furthermore, PDA positively expresses androgen receptor and negatively expresses estrogen receptor (29). Occasionally, PDA may positively express the markers of gastrointestinal adenocarcinoma (e.g, CK7, CK20, CDX2, villin and monoclonal CEA), which makes it necessary to stain prostatic markers (PAP and PSAP) for ruling out metastatic tumors (32). Immunoreactivity of thyroid transcription factor-1 (TTF-1) in PDA could be a diagnostic pitfall, particularly in lung or prostatic biopsy specimens without detailed clinical history (33).
Numerous studies have sought to understand molecular genetics differences between PDA and PAA. The results of gene expression profiles suggest that these two tumors are strikingly similar. Only no more than 30 characterized gene transcripts in the entire assayed transcriptome are identified to be significantly different in protein expression level. Among them, prolactin receptor is more frequently expressed in the cases of PDA than PAA at both transcript and translation level (34). Likewise, the analysis of DNA ploidy profiles shows there is no obvious difference between those two entities. The androgen-regulated fusion gene TMPRSS2-ERG is regarded as a useful biomarker for revealing the evolution of prostate cancer stem cells. ERG, TMPRSS2-ERG gene fusion product, may induce maturation arrest in early prostate stem cells or cause late prostate progenitor or differentiated cells to gain the stem cell properties, eventually leading to carcinogenesis (35).Compared with the matched cases of acinar adenocarcinoma, the TMPRSS2-ERG gene fusion is significantly less frequent in PDA by an analysis of fluorescence in situ hybridization (45% vs. 11% of cases) (36). However, ERG is found to be positive expression in 38.3% cases of PDA and 31.2% cases of PAA (29). Hence, the presences of the TMPRSS2-ERG gene fusion in some cases of PDA reveal the relevance between PDA and PAA in genetics (36).
Differential diagnosis
Morphologically, conventional PAA typically has cuboidal or low columnar epithelium arranged in more or less well-defined acinar structure without true papillary architecture and nuclear stratification. It seems like easily to be distinguished from PDA. However, some PAA may share certain pathological features with PDA, such as elongated nuclei or tall columnar epithelium, pronounced nuclear atypia, intraluminal necrosis, cribriform growth, and location of the peripheral zone (37). Therefore, the delicate differences between these two tumors should not be ignored by the observers. The papillary architecture lined by columnar cells with central fibrovascular cores is considered to be the most useful clue to diagnose the PDA, which do not exist in the conventional PAA. Besides that, the irregular slit-like lumen in the cribriform configuration prefers to the diagnosis of PDA (Figure 2A), while characteristic of punched-out round lumen with cuboidal or low columnar epithelium is in favor of the diagnosis of PAA with cribriform pattern (Figure 2B). Although solid variant with delicate vessels and comedo-necrosis in PDA are very similar to those poorly differentiated PAA, it is not possible to make a diagnosis of PDA only by above-mentioned patterns without typical papillary or cribriform components. Also, stromal desmoplastic reaction and hemosiderin deposition are less frequently found in PAA than PDA (15). Of note, immunohistochemical (IHC) staining is not very helpful in differential diagnosis. The main differential points between PDA and PAA are summarized in Table 1.
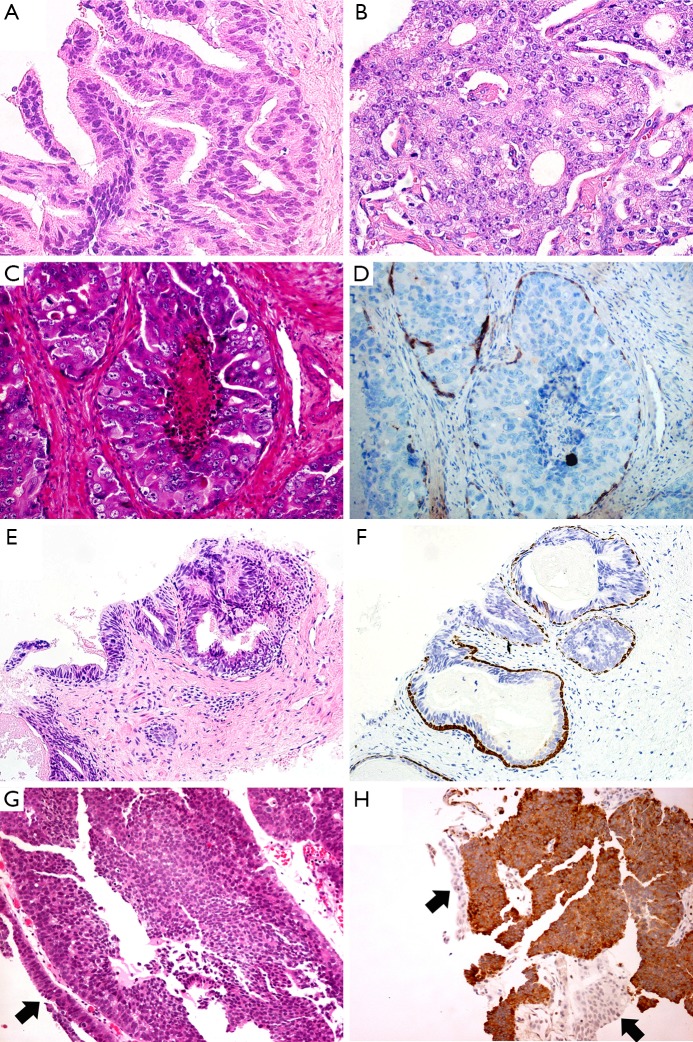
Figure 2.
The cribriform pattern of PDA exhibits the irregular slit-like lumens with tall pseudostratified columnar neoplastic cells (A). The cribriform characteristic of PAA is punched-out round lumens with cuboidal tumor cells (B). IDAC-P exhibits comedo-necrosis in the lumen. The tumor cells are cuboidal, with very large and atypical round or oval nuclei with prominent nucleoli (C). The basal cells present at the periphery in a patchy pattern (D). The columnar tumor cells in HGPIN mimic PDA (E). Basal cells outlined by HMWCK and P63 suggest the diagnosis of HGPIN (F). One case of PDA was misdiagnosed as urothelial carcinoma in core needle biopsy specimen. The tumor cells infiltrate into prostatic urethra (G). Expression of PSA favors the diagnosis of PDA (H). (H&E: A&B, 400×, C, D&G, 200×; IHC: D, F&H, 200×; black arrows: normal urothelium).
Table 1. Key pathologic and immunohistochemical features contrasting PDA, PAA, IDAC-P and HGPIN.
| Characteristics | PDA | PAA | IDAC-P | HGPIN |
|---|---|---|---|---|
| Cellular shape | Columnar | Cuboidal | Cuboidal | Cuboidal |
| Nuclear shape | Elongated & oval | Round | Round | Round |
| Nuclear size | 2–3× normal acinar cell | Enlarged, usually 2× normal acinar cell | At least 6× normal acinar cell | Enlarged, usually 2× normal acinar cell |
| Cribriform pattern | +, slit-like lumens | +, round lumens | +, round lumens | rare + |
| True papillae with fibrovascular cores | usually + | −, tufts of cells | −, tufts of cells | −, tufts of cells |
| Basal cells | −/+, few | − | +, at least focal | + |
| IHC for p63 & HMWCK | −/+, weak & patchy | − | +, patchy | + |
PDA, prostatic ductal adenocarcinoma; PAA, prostatic acinar adenocarcinoma; IDAC-P, intraductal adenocarcinoma of prostate; HGPIN, high-grade prostatic intraepithelial neoplasia; IHC, immunohistochemistry; HMWCK, high molecular weight cytokeratin.
Intraductal adenocarcinoma of prostate (IDAC-P) represents a unique form of high-grade prostatic carcinoma with a peculiar propensity for intraductal extension and growth with at least focal residual of basal cell layer (Figure 2C). It nearly always associated with high-grade acinar carcinoma. It is proposed to be the most difficult differential diagnosis to distinguish from PDA because nearly one third cases of PDA show intraductal growth by IHC staining (28). Generally, IDAC-P is lined by cuboidal cells with rounded lumens in its dense or loose cribriform pattern, and without true fibrovascular stalks in its micropapillary pattern. The basal cells always present at the periphery although they may be patchy by IHC staining (Figure 2D). The nuclear size of some tumor cells is at least 6 times larger than normal acinar cells (38). In contrast, PDA is characterized by columnar epithelium with stratified elongated high-grade nuclei. It usually lacks basal cells although basal cells in some cases can be identified in a rather weak and patchy pattern. The nuclear size of PDA is the same as twice to three times of normal acinar cells, much smaller than that of IDAC-P. Seipel et al. (37) emphasized that only papillary architecture with true fibrovascular stalks is the most useful diagnostic feature of PDA. And the presence of nuclear elongation may also help for distinguishing PDA from IDAC-P, as shown in Table 1.
High-grade prostatic intraepithelial neoplasia (HGPIN), a precancerous lesion of PAA, often contains tufts of cells without true papillae (Figure 2E). Conversely, PDA usually shows true papillary projections with well-established fibrovascular cores (Table 1). The glands of HGPIN are normal size and their distribution is similar to normal duct-lobule architecture, whereas the neoplastic glands of PDA may be very large or arranged back to back in a complex architecture. Besides, PDA usually has more significant nuclear atypia and higher mitotic activity than HGPIN. Comedo-necrosis, perineural invasion and hemosiderin deposition are more commonly seen in PDA than HGPIN (38). As a special subtype, PIN-like PDA consists of stratified columnar epithelium with a flat or tufting or micropapillary architecture formed in simple glands, exactly resembling to HGPIN. However, PIN-like PDA has inconspicuous nucleoli, whereas the diagnosis of HGPIN requires the presence of prominent nucleoli (39,40). PDA usually lacks of basal cells, although sometimes it may displays weak and patchy IHC staining for basal cells (29,38). In contrast, HGPIN have diffuse basal cell layer in all atypical glands (Figure 2F).
Due to sharing with tall columnar epithelium, papillary or cribriform architecture, primary and metastatic carcinomas from urinary bladder, lung or the gastrointestinal tract, may be confused with PDA, especially in an initial transrectal ultrasound (TRUS)-guided biopsy of the prostate. Panels of IHC markers should be used for differential diagnoses. For instance, PSA and PSAP are helpful to rule out pulmonary and colorectal adenocarcinoma, even though TTF-1 might be positive in both PDA and pulmonary adenocarcinoma, and CDX-2 and villin might be positive in either colorectal adenocarcinoma or PDA. Similarly, the positive expression of PSA or PSAP and negative expression of uroplakin or thrombomodulin can identify PDA and rule out high-grade urothelial carcinoma (Figure 2G,H). It is noteworthy that half of PDC may stain only focally or weakly for PSA or PSAP, particularly after androgen-deprivation therapy (21). The coexistence of acinar adenocarcinoma, when present, can support to the diagnosis of PDA (15).
Grading and prognosis
So far, there is no unique grading system for PDA. In a recent update of Gleason scoring system, classic PDAs (e.g., cribriform and papillary pattern) are classified to Gleason score 4 (39). The presence of comedo-necrosis in PDA warrants assignment of Gleason pattern 5 (40). The PIN-like PDA is considered to behave similarly to Gleason pattern 3 (41).
PDA is considered to be an aggressive subtype of prostate cancer with higher risk of disease progression than PAA, except PIN-like subtype (42). A number of literatures report that it is associated with a more aggressive clinical course than PAA (36,41). PDA is prone to have extracapsular extension, positive margins, seminal vesicle involvement, and pelvic lymph node metastasis (21). It appears to have a propensity to metastasize to other distant sites, including lung, axial skeleton, liver, rectum, testis, brain and penis (19,43,44). Pure ductal adenocarcinoma may be a different biologic and clinical entity from mixed PDA. In contrast to mixed PDA, the patients who had pure ductal adenocarcinoma (more than 75% of the tumor contained a ductal component) have an increasing risk for local recurrence after radical prostatectomy (21). Clinical biological behavior of mixed ductal and acinar adenocarcinoma is considered to be depended on the proportion of ductal component as well as the Gleason score of acinar component. The conventional therapies, including hormonal therapy and radiotherapy, have been verified to be less responsive to the patients with PDA by a handful of prior studies (19). In this regard, that local control (particularly prostatectomy) should be very important to improve the prognosis of PDA patients (10). Moreover, serum PSA level is not associated with tumor staging, recurrence and metastasis, and might not be an ideal prognostic indicator for risk assessment and prediction of recurrence in PDA (18).
In summary, PDA is a rare, but the second common histological variants of prostatic carcinoma. It has unique origin, histological features, and biological behavior. Due to its aggressive clinical course and high risk of disease progression, it is important for us to differentiate PDA from other mimickers.
Acknowledgements
Funding: This work was supported by National Natural Science Foundation of China (NSFC 81070582, 81372783 & 81572545).
Footnotes
Conflict of Interest: The authors have no conflicts of interest to declare.
References
- 1.Melicow MM, Pachter MR. Endometrial carcinoma of proxtatic utricle (uterus masculinus). Cancer 1967;20:1715-22. [DOI] [PubMed] [Google Scholar]
- 2.Bock BJ, Bostwick DG. Does prostatic ductal adenocarcinoma exist? Am J Surg Pathol 1999;23:781-5. [DOI] [PubMed] [Google Scholar]
- 3.Grignon DJ. Unusual subtypes of prostate cancer. Mod Pathol 2004;17:316-27. [DOI] [PubMed] [Google Scholar]
- 4.Yang X, Cheng L, Helpap B, et al. Ductal adenocarcinoma. In: Eble JN, Sauter G, Epstein JI, et al., editors. Tumours of the urinary system and male genital organs. Lyon: IARC Press, 2004:199-210. [Google Scholar]
- 5.Young RH, Srigley JR, Amin MB, et al. Variants of prostatic adenocarcinoma, other primary carcinomas of prostate, and secondary carcinomas (Chapter 5) and miscellaneous tumors of the prostate (Chapter 6). In: Tumors of the Prostate Gland, Seminal Vesicles, Male Urethra, and Penis, Third Series, Fascicle 28. Washington DC: Armed Forces Institute of Pathology, 2000;217-88. [Google Scholar]
- 6.Greene LF, Farrow GM, Ravits JM, et al. Prostatic adenocarcinoma of ductal origin. J Urol 1979;121:303-5. [DOI] [PubMed] [Google Scholar]
- 7.Bostwick DG, Kindrachuk RW, Rouse RV. Prostatic adenocarcinoma with endometrioid features. Clinical, pathologic, and ultrastructural findings. Am J Surg Pathol 1985;9:595-609. [DOI] [PubMed] [Google Scholar]
- 8.Epstein JI, Woodruff JM. Adenocarcinoma of the prostate with endometrioid features. A light microscopic and immunohistochemical study of ten cases. Cancer 1986;57:111-9. [DOI] [PubMed] [Google Scholar]
- 9.Pickup M, Van der Kwast TH. My approach to intraductal lesions of the prostate gland. J Clin Pathol 2007;60:856-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Samaratunga H, Duffy D, Yaxley J, et al. Any proportion of ductal adenocarcinoma in radical prostatectomy specimens predicts extraprostatic extension. Hum Pathol 2010;41:281-5. [DOI] [PubMed] [Google Scholar]
- 11.Lee TK, Miller JS, Epstein JI. Rare histological patterns of prostatic ductal adenocarcinoma. Pathology 2010;42:319-24. [DOI] [PubMed] [Google Scholar]
- 12.Seipel AH, Wiklund F, Wiklund NP, et al. Histopathological features of ductal adenocarcinoma of the prostate in 1,051 radical prostatectomy specimens. Virchows Arch 2013;462:429-36. [DOI] [PubMed] [Google Scholar]
- 13.Kan RW, Kan CF, Wong JH, et al. Ductal adenocarcinoma of the prostate: a Hong Kong case series. Int Urol Nephrol 2014;46:2133-7. [DOI] [PubMed] [Google Scholar]
- 14.Bostwick DG, Meiers I. Neoplasms of the prostate. In: Bostwick DG, Cheng L, eds. Urologic Surgical Pathology. 2nd ed. Philadelphia: Mosby Elsevier; 2008:462-8. [Google Scholar]
- 15.Epstein JI. Prostatic ductal adenocarcinoma: a mini review. Med Princ Pract 2010;19:82-5. [DOI] [PubMed] [Google Scholar]
- 16.Hertel JD, Humphrey PA. Ductal adenocarcinoma of the prostate. J Urol 2011;186:277-8. [DOI] [PubMed] [Google Scholar]
- 17.Stajno P, Kalinowski T, Ligaj M, et al. An incidentally diagnosed prostatic ductal adenocarcinoma. Cent European J Urol 2013;66:164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leibovici D, Spiess PE, Agarwal PK, et al. Prostate cancer progression in the presence of undetectable or low serum prostate-specific antigen level. Cancer 2007;109:198-204. [DOI] [PubMed] [Google Scholar]
- 19.Morgan TM, Welty CJ, Vakar-Lopez F, et al. Ductal adenocarcinoma of the prostate: increased mortality risk and decreased serum prostate specific antigen. J Urol 2010;184:2303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ro JY, Ayala AG, Wishnow KI, et al. Prostatic duct adenocarcinoma with endometrioid features: immunohistochemical and electron microscopic study. Semin Diagn Pathol 1988;5:301-11. [PubMed] [Google Scholar]
- 21.Tu SM, Lopez A, Leibovici D, et al. Ductal adenocarcinoma of the prostate: clinical features and implications after local therapy. Cancer 2009;115:2872-80. [DOI] [PubMed] [Google Scholar]
- 22.Lemberger RJ, Bishop MC, Bates CP, et al. Carcinoma of the prostate of ductal origin. Br J Urol 1984;56:706-9. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura K, Higashino M, Hara T, et al. Prostatic adenocarcinoma showing features of endometrioid and mucinous carcinomas: a case report. Hinyokika Kiyo 1995;41:805-7. [PubMed] [Google Scholar]
- 24.Kopelson G, Harisiadis L, Romas NA, et al. Periurethral prostatic duct carcinoma: clinical features and treatment results. Cancer 1978;42:2894-902. [DOI] [PubMed] [Google Scholar]
- 25.Pacchioni D, Casetta G, Piovano M, et al. Prostatic duct carcinoma with combined prostatic duct adenocarcinoma and urothelial carcinoma features: report of a case. Int J Surg Pathol 2004;12:293-7. [DOI] [PubMed] [Google Scholar]
- 26.Millar EK, Sharma NK, Lessells AM. Ductal (endometrioid) adenocarcinoma of the prostate: a clinicopathological study of 16 cases. Histopathology 1996;29:11-9. [DOI] [PubMed] [Google Scholar]
- 27.Samaratunga H, Delahunt B. Ductal adenocarcinoma of the prostate: current opinion and controversies. Anal Quant Cytol Histol 2008;30:237-46. [PubMed] [Google Scholar]
- 28.Herawi M, Epstein JI. Immunohistochemical antibody cocktail staining (p63/HMWCK/AMACR) of ductal adenocarcinoma and Gleason pattern 4 cribriform and noncribriform acinar adenocarcinomas of the prostate. Am J Surg Pathol 2007;31:889-94. [DOI] [PubMed] [Google Scholar]
- 29.Seipel AH, Samaratunga H, Delahunt B, et al. Immunohistochemical profile of ductal adenocarcinoma of the prostate. Virchows Arch 2014;465:559-65. [DOI] [PubMed] [Google Scholar]
- 30.Tarján M, Lenngren A, Hellberg D, et al. Immunohistochemical verification of ductal differentiation in prostate cancer. APMIS 2012;120:510-8. [DOI] [PubMed] [Google Scholar]
- 31.Tulunay O, Orhan D, Baltaci S, et al. Prostatic ductal adenocarcinoma showing Bcl-2 expression. Int J Urol 2004;11:805-8. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein NS. Immunophenotypic characterization of 225 prostate adenocarcinomas with intermediate or high Gleason scores. Am J Clin Pathol 2002;117:471-7. [DOI] [PubMed] [Google Scholar]
- 33.Lim TK, Teo C, Giron DM, et al. Thyroid transcription factor-1 may be expressed in ductal adenocarcinoma of the prostate:a potential pitfall. J Clin Pathol 2007;60:941-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanati S, Watson MA, Salavaggione AL, et al. Gene expression profiles of ductal versus acinar adenocarcinoma of the prostate. Mod Pathol 2009;22:1273-9. [DOI] [PubMed] [Google Scholar]
- 35.Tu SM, Lin SH. Prostate cancer stem cells. Clin Genitourin Cancer 2012;10:69-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lotan TL, Toubaji A, Albadine R, et al. TMPRSS2-ERG gene fusions are infrequent in prostatic ductal adenocarcinomas. Mod Pathol 2009;22:359-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seipel AH, Delahunt B, Samaratunga H, et al. Diagnostic criteria for ductal adenocarcinoma of the prostate: interobserver variability among 20 expert uropathologists. Histopathology 2014;65:216-27. [DOI] [PubMed] [Google Scholar]
- 38.Cohen RJ, Wheeler TM, Bonkhoff H, et al. A proposal on the identification, histologic reporting, and implications of intraductal prostatic carcinoma. Arch Pathol Lab Med 2007;131:1103-9. [DOI] [PubMed] [Google Scholar]
- 39.Epstein JI. An update of the Gleason grading system. J Urol 2010;183:433-40. [DOI] [PubMed] [Google Scholar]
- 40.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. Update on the Gleason grading system for prostate cancer: results of an international consensus conference of urologic pathologists. Adv Anat Pathol 2006;13:57-9. [DOI] [PubMed] [Google Scholar]
- 41.Tavora F, Epstein JI. High-grade prostatic intraepithelial neoplasialike ductal adenocarcinoma of the prostate: a clinicopathologic study of 28 cases. Am J Surg Pathol 2008;32:1060-7. [DOI] [PubMed] [Google Scholar]
- 42.Hameed O, Humphrey PA. Stratified epithelium in prostatic adenocarcinoma: a mimic of high-grade prostatic intraepithelial neoplasia. Mod Pathol 2006;19:899-906. [DOI] [PubMed] [Google Scholar]
- 43.Copeland JN, Amin MB, Humphrey PA, et al. The morphologic spectrum of metastatic prostatic adenocarcinoma to the lung: special emphasis on histologic features overlapping with other pulmonary neoplasms. Am J Clin Pathol 2002;117:552-7. [DOI] [PubMed] [Google Scholar]
- 44.Tu SM, Reyes A, Maa A, et al. Prostate carcinoma with testicular or penile metastases. Clinical, pathologic, and immunohistochemical features. Cancer 2002;94:2610-7. [DOI] [PubMed] [Google Scholar]