Abstract
Plantation forestry is expanding rapidly in China to meet an increasing demand for wood and pulp products globally. Fungal pathogens including species of Calonectria represent a serious threat to the growth and sustainability of this industry. Surveys were conducted in the Guangdong, Guangxi and Hainan Provinces of South China, where Eucalyptus trees in plantations or cuttings in nurseries displayed symptoms of leaf blight. Isolations from symptomatic leaves and soils collected close to infected trees resulted in a large collection of Calonectria isolates. These isolates were identified using the Consolidated Species Concept, employing morphological characters and DNA sequence comparisons for the β-tubulin, calmodulin, histone H3 and translation elongation factor 1-alpha gene regions. Twenty-one Calonectria species were identified of which 18 represented novel taxa. Of these, 12 novel taxa belonged to Sphaero-Naviculate Group and the remaining six to the Prolate Group. Southeast Asia appears to represent a centre of biodiversity for the Sphaero-Naviculate Group and this fact could be one of the important constraints to Eucalyptus forestry in China. The remarkable diversity of Calonectria species in a relatively small area of China and associated with a single tree species is surprising.
Key words: Calonectria, Cylindrocladium leaf blight, Eucalyptus, Soil, Taxonomy
Taxonomic novelties: New species: Calonectria aconidialis L. Lombard, Crous & S.F. Chen; C. arbusta L. Lombard, Crous & S.F. Chen; C. expansa L. Lombard, Crous & S.F. Chen; C. foliicola L. Lombard, Crous & S.F. Chen; C. guangxiensis L. Lombard, Crous & S.F. Chen; C. hainanensis L. Lombard, Crous & S.F. Chen; C. lateralis L. Lombard, Crous & S.F. Chen; C. magnispora L. Lombard, Crous & S.F. Chen; C. microconidialis L. Lombard, Crous & S.F. Chen; C. papillata L. Lombard, Crous & S.F. Chen; C. parakyotensis L. Lombard, Crous & S.F. Chen; C. pluriramosa L. Lombard, Crous & S.F. Chen; C. pseudokyotensis L. Lombard, Crous & S.F. Chen; C. seminaria L. Lombard, Crous & S.F. Chen; C. sphaeropedunculata L. Lombard, Crous & S.F. Chen; C. terrestris L. Lombard, Crous & S.F. Chen; C. tetraramosa L. Lombard, Crous & S.F. Chen; C. turangicola L. Lombard, Crous & S.F. Chen
Introduction
Eucalyptus plantation forestry has grown rapidly during the course of the past two decades in China. This is due to the country being the world's leading consumer of wood products (Turnbull 2007) and a growing global forest products market. In order to service this market, large-scale plantations of fast-growing trees and especially Eucalyptus spp. have been established in South and Central China. The area spans 19 provinces (Chen et al., 2011a, Chen et al., 2011b, Chen et al., 2011c, Chen et al., 2011d, Zhou and Wingfield, 2011) and the aim is to establish 13.3 M ha by 2015 (Turnbull 2007). As is true in other parts of the world, pests and diseases represent a significant challenge to reaching this goal (Zhou et al., 2008, Wingfield et al., 2010, Wingfield et al., 2013).
A recent survey of commercial Eucalyptus plantations and nurseries in the Guangdong, Guangxi, Yunnan and Hainan Provinces resulted in the identification of several important Eucalyptus pathogens. These included leaf pathogens belonging to the genera Mycosphaerella (Burgess et al. 2007), Quambalaria (Zhou et al. 2007) and Teratosphaeria (Burgess et al. 2006). Stem pathogens found included species of Botryosphaeriaceae (Chen et al. 2011a), Celoporthe (Chen et al. 2011b), Ceratocystis (Chen et al. 2013), Chrysoporthe (Chen et al. 2010) and Teratosphaeria (Chen et al. 2011c). In eucalypt nurseries, only isolates belonging to the genus Calonectria (as Cylindrocladium) were found and these were shown (Lombard et al. 2010d) to represent two novel taxa, C. cerciana and C. pseudoreteaudii, and the well-known Eucalyptus nursery pathogen, C. pauciramosa (Koike et al., 1999, Polizzi and Crous, 1999, Schoch et al., 1999, Crous, 2002, Lombard et al., 2010a, Lombard et al., 2010d). A more recent survey of Eucalyptus leaves showing symptoms of Calonectria Leaf Blight (CLB) in the Fujian Province resulted in the identification of three novel taxa, C. crousiana, C. fujianensis and C. pseudocolhounii, and the first record of C. pauciramosa as plantation pathogen (Chen et al. 2011d). Pathogenicity test showed that all four Calonectria species are aggressive pathogens of two important Eucalyptus hybrid clones extensively deployed in plantations (Chen et al. 2011d).
The genus Calonectria accommodates well-known pathogens of various agricultural, horticultural and forestry crops, worldwide (Crous, 2002, Lechat et al., 2010, Lombard et al., 2010a, Lombard et al., 2010b, Lombard et al., 2010c, Lombard et al., 2011). Diseases associated with these fungi include cutting and root rot, stem cankers as well as leaf and shoot blight (Crous, 2002, Lombard et al., 2010a, Lombard et al., 2010b, Lombard et al., 2011). In Asia, several Calonectria species have been reported on Eucalyptus trees grown in plantations with most species associated with CLB (Sharma et al., 1984, Booth et al., 2000, Kang et al., 2001a, Crous, 2002, Old et al., 2003, Crous et al., 2004b, Chen et al., 2011d). Of these species, members of the C. reteaudii complex (Lombard et al. 2010d) have most frequently been found on Eucalyptus trees, especially in tropical regions of Asia (Booth et al., 2000, Kang et al., 2001a, Kang et al., 2001b, Crous, 2002, Old et al., 2003, Lombard et al., 2010d).
Studies by Lombard et al. (2010d) and Chen et al. (2011d) suggested a high level of diversity of Calonectria species associated with Eucalyptus in plantations and nurseries in Southeast China. The aim of this study was to undertake surveys to further assess the limits of diversity of Calonectria in a relatively small area of China associated with Eucalyptus plantations.
Materials and methods
Isolates
An extensive survey for Calonectria species was conducted in Eucalyptus plantations in the Guangdong, Guangxi and Hainan Provinces, China in 2008 and 2009. Where present, leaves of Eucalyptus trees showing symptoms were collected in these plantations. In addition, soil samples were collected associated with the symptomatic trees and these baited with germinating Medicago sativa (alfalfa) seeds using the technique described by Crous (2002). Eucalyptus cuttings showing CLB symptoms were also collected in the nursery of the China Eucalypt Research Centre (CERC) in Guangdong Province.
Plant samples were incubated in moist chambers at room temperature for up to 14 d and inspected daily for fungal structures. Isolations were made directly from these structures onto malt extract agar (2 % w/v; MEA; Biolab, Midrand, South Africa) and incubated for 7 d at 24 °C under continuous near-ultraviolet light. From these primary isolations, single conidial cultures were prepared on MEA and these are maintained in the culture collection (CMW) of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa, the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands, the research collection of P.W. Crous (CPC) maintained at CBS, and the culture collection of CERC, Zhanjiang, Guangdong Province, China.
DNA sequence comparisons
Total genomic DNA was extracted from 7-d-old cultures established from single-conidial propagules, grown on MEA at room temperature, using the UltraClean™ Microbial DNA isolation kit (Mo Bio Laboratories, Inc., California, USA) following the protocols provided by the manufacturer. Partial gene sequences were determined for β-tubulin (tub2), calmodulin (cmdA), histone H3 (his3), and the translation elongation factor 1-alpha (tef1) regions using the primers and protocols described by Lombard et al. (2010b). To ensure the integrity of the sequences, the amplicons were sequenced in both directions using the same primers used for amplification. Consensus sequences for each locus were assembled in MEGA v. 5.1 (Tamura et al. 2011) and compared with representative sequences from Lombard et al. (2010b) and Alfenas et al. (2015). Subsequent alignments for each locus were generated in MAFFT v. 7.110 (Katoh & Standley 2013) and the ambiguously aligned regions of both ends were truncated.
Phylogenetic analyses were based on both Bayesian inference (BI) and Maximum Parsimony (MP). For BI, the best evolutionary model for each locus was determined using MrModeltest (Nylander 2004) and incorporated into the analyses. MrBayes v. 3.2.1 (Ronquist & Huelsenbeck 2003) was used to generate phylogenetic trees under optimal criteria for each locus. A Markov Chain Monte Carlo (MCMC) algorithm of four chains was initiated in parallel from a random tree topology with the heating parameter set at 0.3. The MCMC analysis lasted until the average standard deviation of split frequencies was below 0.01 with trees saved every 1 000 generations. The first 25 % of saved trees were discarded as the “burn-in” phase and posterior probabilities (PP) were determined from the remaining trees.
For MP, analyses were done using PAUP (Phylogenetic Analysis Using Parsimony, v. 4.0b10; Swofford 2003) with phylogenetic relationships estimated by heuristic searches with 1 000 random addition sequences. Tree-bisection-reconnection was used, with branch swapping option set on “best trees” only. All characters were weighted equally and alignment gaps treated as fifth state. Measures calculated for parsimony included tree length (TL), consistency index (CI), retention index (RI) and rescaled consistency index (RC). Bootstrap analyses (Hillis & Bull 1993) were based on 1 000 replications.
Phylogenetic analyses were conducted on two separate sequence datasets. Datasets were separated based on morphological characteristics into the Prolate Group and Sphaero-Naviculate Group as defined by Lombard et al. (2010b), making it possible to reduce the number of ambiguously aligned regions for the loci analysed. The dataset representing the Prolate Group of species was rooted to C. hongkongensis (CBS 114711 & CBS 114828) and the dataset representing the Sphaero-Naviculate Group was rooted to C. pauciramosa (CMW 5683 & CMW 30823).
Taxonomy
Axenic cultures were sub-cultured onto synthetic nutrient-poor agar (SNA; Nirenburg 1981) and incubated at room temperature for 7 d. Gross morphological characteristics of the asexual morphs were studied by mounting the structures in 85 % lactic acid and 30 measurements were made at ×1 000 magnification for all taxonomically informative characters.
Axenic cultures of Calonectria species of unknown identity and identified based on DNA sequence analyses were crossed among themselves in all possible combinations. Crosses were made on minimal salt agar (MN) with sterile toothpicks placed on the agar surface as described by Lombard et al., 2010b, Lombard et al., 2010d. Isolates were crossed with themselves as controls, thus making it possible to distinguish between heterothallic and homothallic mating systems of the isolates. The plates were stacked in plastic containers and incubated at 20 °C for 6–8 wk. Crosses were regarded as successful when isolate combinations produced ascomata extruding viable ascospores.
Morphological characteristics of the sexual morphs were studied by mounting ascomata in tissue freezing medium (Leica Biosystems, Nussloch, Germany) and cutting sections with a Leica CM1100 cryostate (Leica Biosystems, Nussloch, Germany). The 10 μm sections were mounted in 85 % lactic acid and 3 % KOH. The 95 % confidence levels were calculated for the conidia and ascospores with extremes provided in parentheses. For all other fungal structures measured, only the extremes are provided. Colony colour was assessed using 7-d-old cultures on MEA incubated at 25 °C and the colour charts of Rayner (1970). All descriptions, illustrations and nomenclatural data were deposited in MycoBank (Crous et al. 2004a).
Results
Isolates
A total of 278 isolates were collected of which 162 were from the Guangdong Province (44 isolates from soil; 45 isolates from Eucalyptus leaves on trees; 73 from cuttings in a single nursery), 87 isolates from Guangxi Province (63 from soil; 24 from Eucalyptus leaves in plantations), and 29 isolates from the Hainan Province (27 from soil; two from Eucalyptus leaves in plantations). One hundred and twenty of these isolates were selected for further study (Table 1) based on preliminary phylogenetic analysis of the cmdA and tub2 gene region sequences (results not shown).
Table 1.
Calonectria spp. used in phylogenetic analyses.
| Species | Isolate nr.1 | Substrate | Locality | GenBank Accession no.2 |
|||
|---|---|---|---|---|---|---|---|
| tub2 | cmdA | his3 | tef1 | ||||
| Calonectria aconidialis | CBS 136086; CMW 35174; CERC 1850 | Soil in Eucalyptus plantation | Hainan, China | – | KJ463017 | KJ463133 | KJ462785 |
| CBS 136091; CMW 35384; CERC1886 | Soil in Eucalyptus plantation | Hainan, China | – | – | KJ463134 | KJ462786 | |
| C. arbusta | CBS 136079; CMW 31370; CERC1705 | Soil in Eucalyptus plantation | Guangxi, China | KJ462904 | KJ463018 | KJ463135 | KJ462787 |
| CBS 136098; CPC 23519; CMW37981; CERC 1944 | Soil in Eucalyptus plantation | Guangxi, China | – | KJ463019 | KJ463136 | KJ462788 | |
| CPC 23481; CMW 31369; CERC1704 | Soil in Eucalyptus plantation | Guangxi, China | KJ462905 | KJ463020 | KJ463137 | KJ462789 | |
| CPC 23483; CMW 31371; CERC 1706 | Soil in Eucalyptus plantation | Guangxi, China | KJ462906 | KJ463021 | KJ463138 | KJ462790 | |
| CMW 31367; CERC 1702 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462907 | KJ463022 | KJ463139 | KJ462791 | |
| CMW 31368; CERC 1703 | Soil in Eucalyptus plantation | Guangxi, China | KJ462908 | KJ463023 | KJ463140 | KJ462792 | |
| C. asiatica | CBS 112711; CPC 3898 | Leaf litter | Thailand | AY725613 | AY725738 | AY725655 | AY725702 |
| CBS 114073; CPC 3900 | Leaf litter | Thailand | AY725616 | AY725741 | AY725658 | AY725705 | |
| C. brasiliensis | CBS 230.51; CPC 2390 | Anacardium sp. | Brazil | GQ267241 | GQ267421 | GQ267259 | GQ267328 |
| CBS 114257; CPC 1944 | Eucalyptus leaf | Brazil | GQ267242 | GQ267422 | GQ267260 | GQ267329 | |
| C. brassiana | CBS 134855 | Soil | Teresina, Piauí, Brazil | KM395969 | KM396056 | KM396139 | KM395882 |
| CBS 134856 | Soil | Teresina, Piauí, Brazil | KM395970 | KM396057 | KM396140 | KM395883 | |
| C. canadania | CBS 110817; CPC 499 | Canada | AF348212 | AY725743 | AF348228 | GQ267297 | |
| C. candelabra | CPC 1675; CMW 31000 | Eucalyptus sp. | Brazil | FJ972426 | GQ267367 | FJ972476 | FJ972525 |
| CMW 31001 | Eucalyptus sp. | Brazil | FJ972427 | GQ267368 | GQ267246 | GQ267246 | |
| C. cerciana | CBS 123693; CMW 25309 | Eucalyptus cutting | Zhanjiang, China | FJ918510 | GQ267369 | FJ918528 | FJ918559 |
| CBS 123695; CMW 25290 | Eucalyptus cutting | Zhanjiang, China | FJ918511 | GQ267370 | FJ918529 | FJ918560 | |
| C. chinensis | CBS 112744; CPC 4104 | Soil | Hong Kong, China | AY725618 | AY725746 | AY725660 | AY725709 |
| CBS 114827; CPC 4101 | Soil | Hong Kong, China | AY725619 | AY725747 | AY725661 | AY725710 | |
| CBS 136082; CMW 35367; CERC 1871 | Soil in Eucalyptus plantation | Guangdong, China | KJ462909 | KJ463024 | KJ463141 | KJ462793 | |
| CBS 136083; CMW 35179; CERC 1855 | Soil in Eucalyptus plantation | Guangdong | KJ462910 | KJ463025 | KJ463142 | KJ462794 | |
| CBS 136088; CMW 35376; CERC 1878 | Soil in Eucalyptus plantation | Hainan, China | KJ462911 | KJ463026 | KJ463143 | KJ462795 | |
| CBS 136090; CMW 35379; CERC 1881 | Soil in Eucalyptus plantation | Hainan, China | KJ462912 | KJ463027 | KJ463144 | KJ462796 | |
| C. colhounii | CBS 293.79 | Camellia sinensis | Bandung, Indonesia | DQ190564 | GQ267373 | DQ190639 | GQ267301 |
| CBS 114704 | Arachis pintoi | Australia | DQ190563 | GQ267372 | DQ190638 | GQ267300 | |
| C. colombiensis | CBS 112220; CPC 723 | Soil | La Selva, Brazil | GQ267207 | AY725748 | AY725662 | AY725711 |
| CBS 112221; CPC 724 | Eucalyptus grandis | La Selva, Brazil | AY725620 | AY725749 | AY725663 | AY725712 | |
| C. crousiana | CBS 127198; CMW 27249 | E. grandis | Fujian, China | HQ285794 | – | HQ285808 | HQ285822 |
| CBS 127199; CMW 27253 | E. grandis | Fujian, China | HQ285795 | – | HQ285809 | HQ285823 | |
| C. curvispora | CBS 116159; CPC 765 | Soil | Tamatave, Madagascar | AF333394 | GQ267374 | AY725664 | GQ267302 |
| C. cylindrospora | CBS 110666; CPC 496 | USA | FJ918509 | GQ267423 | FJ918527 | FJ918557 | |
| CBS 119670; CPC 12766 | Pistacia lentiscus | Italy | DQ521600 | – | DQ521602 | GQ421797 | |
| C. eucalypticola | CBS 134846 | Eucalyptus leaf | Eunápolis, Bahia, Brazil | KM395963 | KM396050 | KM396133 | KM395876 |
| CBS 134847 | Eucalyptus seedling | Santa Bárbara, Minas Gerais, Brazil | KM395964 | KM396051 | KM396134 | KM395877 | |
| C. expansa | CBS 136078; CMW 31441; CERC 1776 | Soil in Eucalyptus plantation | Guangdong, China | KJ462913 | KJ463028 | KJ463145 | KJ462797 |
| CBS 136247; CMW 31392; CERC 1727 | Soil in Eucalyptus plantation | Guangxi, China | KJ462914 | KJ463029 | KJ463146 | KJ462798 | |
| CMW 31413; CERC 1748 | Soil in Eucalyptus plantation | Guangxi, China | KJ462915 | KJ463030 | KJ463147 | KJ462799 | |
| C. foliicola | CBS 136641; CMW 31393; CERC 1728 | E. urophylla × E. grandis clone leaf | Guangxi, China | KJ462916 | KJ463031 | KJ463148 | KJ462800 |
| CMW 31394; CERC 1729 | E. urophylla × E. grandis clone leaf | Guangxi, China | KJ462917 | KJ463032 | KJ463149 | KJ462801 | |
| CMW 31395; CERC 1730 | E. urophylla × E. grandis clone leaf | Guangxi, China | KJ462918 | KJ463033 | KJ463150 | KJ462802 | |
| C. fujianensis | CBS 127200; CMW 27254 | E. grandis | Fujian, China | HQ285791 | – | HQ285805 | HQ285819 |
| CBS 127201; CMW 27257 | E. grandis | Fujian, China | HQ285792 | – | HQ285806 | HQ285820 | |
| C. glaeboicola | CBS 134852 | Soil | Martinho Campos, Minas Gerais, Brazil | KM395966 | KM396053 | KM396136 | KM395879 |
| CBS 134853 | Soil | Bico do Papagaio, Tocantins, Brazil | KM395967 | KM396054 | KM396137 | KM395880 | |
| C. guangxiensis | CBS 136092; CMW 35409; CERC 1900 | Soil in Eucalyptus plantation | Guangxi, China | KJ462919 | KJ463034 | KJ463151 | KJ462803 |
| CBS 136094; CMW 35411; CERC 1902 | Soil in Eucalyptus plantation | Guangxi, China | KJ462920 | KJ463035 | – | KJ462804 | |
| C. hainanensis | CBS 136248; CMW 35187; CERC 1863 | Soil in Eucalyptus plantation | Hainan, China | – | KJ463036 | KJ463152 | KJ462805 |
| C. hawksworthii | CBS 111870; CPC 2405; MUCL 30866 | Nelumbo nucifera | Mauritius | AF333407 | GQ267386 | DQ190649 | FJ918558 |
| C. hodgesii | CBS 133609; LPF 245 | Anadenanthera peregrina | Viçosa, Brazil | KC491228 | KC491222 | – | KC491225 |
| CBS 133610; LPF 261 | Azadirachta indica | Viçosa, Brazil | KC491229 | KC491223 | – | KC491226 | |
| C. hongkongensis | CBS 114711; CPC 686 | Soil | Hong Kong, China | AY725621 | AY725754 | AY725666 | AY725716 |
| CBS 114828; CPC 4670 | Soil | Hong Kong, China | AY725622 | AY725755 | AY725667 | AY725717 | |
| CBS 136080; CMW 31443; CERC 1778 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462921 | KJ463037 | KJ463153 | KJ462806 | |
| CBS 136246; CMW 31374; CERC 1709 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462922 | KJ463038 | KJ463154 | KJ462807 | |
| CPC 23478; CMW 31438; CERC 1773 | Soil in Eucalyptus plantation | Shiling, Zhanjiang, Guangdong, China | KJ462923 | KJ463039 | KJ463155 | KJ462808 | |
| CPC 23480; CMW 31414; CERC 1749 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462924 | KJ463040 | KJ463156 | KJ462809 | |
| CPC 23499; CMW 35175; CERC 1851 | Soil in Eucalyptus plantation | Hainan, China | KJ462925 | KJ463041 | KJ463157 | KJ462810 | |
| CPC 23877; CERC 1932 | Soil in Eucalyptus plantation | Hainan, China | KJ462926 | KJ463042 | KJ463158 | KJ462811 | |
| CPC 23878; CMW 37973; CERC 1936 | Soil in Eucalyptus plantation | Guangdong, China | KJ462927 | KJ463043 | KJ463159 | KJ462812 | |
| CMW 31375; CERC 1710 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462928 | KJ463044 | KJ463160 | KJ462813 | |
| CMW 31377; CERC 1712 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462929 | KJ463045 | KJ463161 | KJ462814 | |
| CMW 31382; CERC 1717 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462930 | KJ463046 | KJ463162 | KJ462815 | |
| CMW 31383; CERC 1718 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462931 | KJ463047 | KJ463163 | KJ462816 | |
| CMW 31384; CERC 1719 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462932 | KJ463048 | KJ463164 | KJ462817 | |
| CMW 31385; CERC 1720 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462933 | KJ463049 | KJ463165 | KJ462818 | |
| CMW 31387; CERC 1722 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462934 | KJ463050 | KJ463166 | KJ462819 | |
| CMW 31388; CERC 1723 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462935 | KJ463051 | KJ463167 | KJ462820 | |
| CMW 31399; CERC 1734 | Soil in Eucalyptus plantation | Shiling, Zhanjiang, Guangdong, China | KJ462936 | – | KJ463168 | KJ462821 | |
| CMW 31400; CERC 1735 | Soil in Eucalyptus plantation | Shiling, Zhanjiang, Guangdong, China | KJ462937 | KJ463052 | KJ463169 | KJ462822 | |
| CMW 31401; CERC 1736 | Soil in Eucalyptus plantation | Shiling, Zhanjiang, Guangdong, China | KJ462938 | KJ463053 | KJ463170 | KJ462823 | |
| CMW 31404; CERC1739 | Soil in Eucalyptus plantation | Shiling, Zhanjiang, Guangdong, China | KJ462939 | KJ463054 | KJ463171 | KJ462824 | |
| CMW 31432; CERC1767 | Soil in Eucalyptus plantation | Shiling, Zhanjiang, Guangdong, China | KJ462940 | KJ463055 | KJ463172 | KJ462825 | |
| CMW 31433; CERC1768 | Soil in Eucalyptus plantation | Shiling, Zhanjiang, Guangdong, China | KJ462941 | KJ463056 | KJ463173 | KJ462826 | |
| CMW 31434; CERC1769 | Soil in Eucalyptus plantation | Shiling, Zhanjiang, Guangdong, China | KJ462942 | KJ463057 | KJ463174 | KJ462827 | |
| CMW 31442; CERC1777 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462943 | KJ463058 | KJ463175 | KJ462828 | |
| CMW 35186; CERC1862 | Soil in Eucalyptus plantation | Hainan, China | KJ462944 | KJ463059 | KJ463176 | KJ462829 | |
| CMW 35188; CERC1864 | Soil in Eucalyptus plantation | Hainan, China | KJ462945 | KJ463060 | KJ463177 | KJ462830 | |
| CMW 35190; CERC1865 | Soil in Eucalyptus plantation | Guangxi, China | KJ462946 | KJ463061 | KJ463178 | KJ462831 | |
| CMW 35192; CERC1867 | Soil in Eucalyptus plantation | Guangxi, China | KJ462947 | KJ463062 | – | KJ462832 | |
| CMW 35371; CERC1874 | Soil in Eucalyptus plantation | Guangdong, China | KJ462948 | KJ463063 | KJ463179 | KJ462833 | |
| CMW 35378; CERC1880 | Soil in Eucalyptus plantation | Hainan, China | KJ462949 | KJ463064 | KJ463180 | KJ462834 | |
| CMW 35381; CERC1883 | Soil in Eucalyptus plantation | Hainan, China | KJ462950 | KJ463065 | KJ463181 | KJ462835 | |
| CMW 35401; CERC1892 | Soil in Eucalyptus plantation | Guangxi, China | KJ462951 | KJ463066 | KJ463182 | KJ462836 | |
| CMW 35404; CERC1895 | Soil in Eucalyptus plantation | Guangxi, China | KJ462952 | KJ463067 | KJ463183 | KJ462837 | |
| CMW 35414; CERC1905 | Soil in Eucalyptus plantation | Guangxi, China | KJ462953 | KJ463068 | KJ463184 | KJ462838 | |
| CMW 36270; CERC1928 | Soil in Eucalyptus plantation | Hainan, China | KJ462954 | KJ463069 | KJ463185 | KJ462839 | |
| C. ilicicola | CBS 190.50; CMW 30998; IMI 299389 | Solanum tuberosum | Bogor, Indonesia | AY725631 | AY725764 | AY725676 | AY725726 |
| CBS 115897; CPC 493; UFV 108 | Anacardium sp. | Brazil | AY725647 | GQ267403 | GQ267256 | AY725729 | |
| C. indonesiae | CBS 112823; CPC 4508 | Soil | Warambunga, Indonesia | AY725623 | AY725756 | AY725668 | AY725718 |
| CBS 112840; CPC 4554 | Syzygium aromaticum | Indonesia | AY725625 | AY725758 | AY725670 | AY725720 | |
| C. insularis | CBS 114558; CPC 768 | Soil | Tamatave, Madagascar | AF210861 | GQ267389 | FJ918526 | FJ918556 |
| CBS 114559; CPC 954 | Soil | Tamatave, Madagascar | AF210862 | GQ267390 | FJ918525 | FJ918555 | |
| C. kyotensis | CBS 413.67; CPC 2391; IMI 299577 | Paphiopedilum callosum | Celle, Germany | GQ267208 | GQ267379 | GQ267248 | GQ267307 |
| CBS 170.77; IMI 299388 | Idesia polycarpa | Auckland, New Zealand | GQ267209 | GQ267380 | GQ267249 | GQ267308 | |
| C. lateralis | CBS 136629; CMW 31412; CERC 1747 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462955 | KJ463070 | KJ463186 | KJ462840 |
| C. leucothoes | CBS 109166; CPC 2385; ATCC 64824 | Leucothoe axillaris | Gainsville, Florida, USA | FJ918508 | GQ267392 | FJ918523 | FJ918553 |
| C. magnispora | CBS 136249; CMW 35184; CERC 1860 | Soil in Eucalyptus plantation | Guangxi, China | KJ462956 | KJ463071 | KJ463187 | KJ462841 |
| C. malesiana | CBS 112710; CPC 3899 | Leaf litter | Thailand | AY725626 | AY725759 | AY725671 | AY725721 |
| CBS 112752; CPC 4223 | Soil | Sumatra, Indonesia | AY725627 | AY725760 | AY725672 | AY725722 | |
| C. maranhensis | CBS 134811 | Eucalyptus sp. | Açailândia, Maranhão, Brazil | KM395948 | KM396035 | KM396118 | KM395861 |
| CBS 134812 | Eucalyptus sp. | Açailândia, Maranhão, Brazil | KM395949 | KM396036 | KM396119 | KM395862 | |
| CBS 134858 | Soil | Urbano Santos, Maranhão, Brazil | KM395951 | KM396038 | KM396121 | KM395864 | |
| CBS 134829 | Soil | Urbano Santos, Maranhão, Brazil | KM395952 | KM396039 | KM396122 | KM395865 | |
| C. metrosideri | CBS 133604; LPF 103 | Metrosideros polymorpha | Viçosa, Brazil | KC294314 | KC294305 | KC294308 | KC294311 |
| CBS 133605; LPF 104 | M. polymorpha | Viçosa, Brazil | KC294315 | KC294306 | KC294309 | KC294312 | |
| C. microconidialis | CBS 136633; CMW 31471; CERC 1806 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462957 | KJ463072 | KJ463188 | KJ462842 |
| CBS 136634; CMW 31473; CERC 1808 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462958 | KJ463073 | KJ463189 | KJ462843 | |
| CBS 136636; CMW 31475; CERC 1810 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462959 | KJ463074 | KJ463190 | KJ462844 | |
| CBS 136638; CMW 31487; CERC 1822 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462960 | KJ463075 | KJ463191 | KJ462845 | |
| CBS 136640; CMW 31492; CERC 1827 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462961 | KJ463076 | KJ463192 | KJ462846 | |
| C. nemuricola | CBS 134837 | Soil | Araponga, Minas Gerais, Brazil | KM395979 | KM396066 | KM396149 | KM395892 |
| CBS 134838 | Soil | Araponga, Minas Gerais, Brazil | KM395980 | KM396067 | KM396150 | KM395893 | |
| C. nymphaeae | CBS 131802; HGUP 100003 | Nymphaea tetragona | Guizhou, China | JN984864 | – | – | KC555273 |
| C. pacifica | CBS 109063; CPC 2534; IMI 354528 | Araucaria heterophylla | Hawaii, USA | GQ267213 | AY725762 | GQ267255 | AY725724 |
| CBS 114038; CPC 10717 | Ipomoea aquatica | Auckland, New Zealand | AY725630 | GQ267402 | AY725675 | GQ267320 | |
| C. papillata | CBS 136084; CMW 35165; CERC 1841 | Soil in Eucalyptus plantation | Guangdong, China | KJ462962 | KJ463077 | KJ463193 | KJ462847 |
| CBS 136096; CMW 37972; CERC 1935 | Soil in Eucalyptus plantation | Guangdong, China | KJ462963 | KJ463078 | KJ463194 | KJ462848 | |
| CBS 136097; CMW 37976; CERC 1939 | Soil in Eucalyptus plantation | Guangdong, China | KJ462964 | KJ463079 | KJ463195 | KJ462849 | |
| CBS 136251; CMW 37971; CERC 1934 | Soil in Eucalyptus plantation | Guangxi, China | KJ462965 | KJ463080 | KJ463196 | KJ462850 | |
| C. parakyotensis | CBS 136085; CMW 35169; CERC 1845 | Soil in Eucalyptus plantation | Guangdong, China | – | KJ463081 | KJ463197 | KJ462851 |
| CBS 136095; CMW 35413; CERC 1904 | Soil in Eucalyptus plantation | Guangxi, China | – | KJ463082 | KJ463198 | KJ462852 | |
| C. pauciramosa | CMW 5683 | E. grandis | South Africa | FJ918514 | GQ267405 | FJ918531 | FJ918565 |
| CMW 30823 | E. grandis | South Africa | FJ918515 | GQ280404 | FJ918532 | FJ918566 | |
| C. pentaseptata | CBS 133349 | Eucalyptus hybrid | Bavi, Hanoi, Vietnam | JX855942 | – | JX855946 | JX855958 |
| CBS 133351 | Macadamia sp. | Bavi, Hanoi, Vietnam | JX855944 | – | JX855948 | JX855960 | |
| CBS 136087; CMW 35177; CERC 1853 | Eucalyptus leaf | Hainan, China | KJ462966 | KJ463083 | KJ463199 | KJ462853 | |
| CBS 136089; CMW 35377; CERC 1879 | Eucalyptus leaf | Hainan, China | KJ462967 | KJ463084 | KJ463200 | KJ462854 | |
| CBS 136250; CMW 35451; CERC 1923 | Eucalyptus leaf | Guangdong, China | KJ462968 | KJ463085 | KJ463201 | KJ462855 | |
| CBS 136646; CMW 35436; CERC 1908 | Eucalyptus leaf | Guangdong, China | KJ462969 | KJ463086 | KJ463202 | KJ462856 | |
| CMW 31332; CERC 1667 | Eucalyptus clone U6 leaf | Shiling, Zhanjiang, Guangdong, China | KJ462970 | KJ463087 | KJ463203 | KJ462857 | |
| CMW 31333; CERC 1668 | Eucalyptus clone U6 leaf | Shiling, Zhanjiang, Guangdong, China | KJ462971 | KJ463088 | KJ463204 | KJ462858 | |
| CMW 31336; CERC 1671 | Eucalyptus clone U6 leaf | Shiling, Zhanjiang, Guangdong, China | KJ462972 | KJ463089 | KJ463205 | KJ462859 | |
| CMW 31340; CERC 1675 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462973 | KJ463090 | KJ463206 | KJ462860 | |
| CMW 31343; CERC 1678 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462974 | KJ463091 | KJ463207 | KJ462861 | |
| CMW 31344; CERC 1679 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462975 | KJ463092 | KJ463208 | KJ462862 | |
| CMW 31345; CERC 1680 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462976 | KJ463093 | KJ463209 | KJ462863 | |
| CMW 31346; CERC 1681 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462977 | KJ463094 | KJ463210 | KJ462864 | |
| CMW 31347; CERC 1682 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462978 | KJ463095 | KJ463211 | KJ462865 | |
| CMW 31348; CERC 1683 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462979 | KJ463096 | KJ463212 | KJ462866 | |
| CMW 31355; CERC 1690 | E. urophylla × E. grandis leaf | Hepu, Guangxi, China | KJ462980 | KJ463097 | KJ463213 | KJ462867 | |
| CMW 31356; CERC 1691 | E. urophylla × E. grandis leaf | Hepu, Guangxi, China | KJ462981 | KJ463098 | KJ463214 | KJ462868 | |
| CMW 31357; CERC 1692 | E. urophylla × E. grandis leaf | Hepu, Guangxi, China | KJ462982 | KJ463099 | KJ463215 | KJ462869 | |
| CMW 31358; CERC 1693 | E. urophylla × E. grandis leaf | Hepu, Guangxi, China | KJ462983 | KJ463100 | KJ463216 | KJ462870 | |
| CMW 31359; CERC 1694 | E. urophylla × E. grandis leaf | Hepu, Guangxi, China | KJ462984 | KJ463101 | KJ463217 | KJ462871 | |
| CMW 31363; CERC 1698 | E. urophylla × E. grandis leaf | Hepu, Guangxi, China | KJ462985 | KJ463102 | KJ463218 | KJ462872 | |
| CMW 31422; CERC 1757 | Eucalyptus clone U6 leaf | Shiling, Zhanjiang, Guangdong, China | KJ462986 | KJ463103 | KJ463219 | KJ462873 | |
| CMW 31497; CERC 1832 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462987 | KJ463104 | KJ463220 | KJ462874 | |
| CMW 35385; CERC 1887 | Soil in Eucalyptus plantation | Hainan, China | KJ462988 | KJ463105 | KJ463221 | KJ462875 | |
| CMW 35437; CERC 1909 | Eucalyptus leaf | Guangdong, China | KJ462989 | KJ463106 | KJ463222 | KJ462876 | |
| CMW 35442; CERC 1914 | Eucalyptus leaf | Guangdong, China | KJ462990 | KJ463107 | KJ463223 | KJ462877 | |
| CMW 35452; CERC 1924 | Eucalyptus leaf | Guangdong, China | KJ462991 | KJ463108 | KJ463224 | KJ462878 | |
| CMW 35453; CERC 1925 | Eucalyptus leaf | Guangdong, China | KJ462992 | KJ463109 | KJ463225 | KJ462879 | |
| CMW 35454; CERC 1926 | Eucalyptus leaf | Guangdong, China | KJ462993 | KJ463110 | KJ463226 | KJ462880 | |
| C. piauiensis | CBS 134850 | Soil | Teresina, Piauí, Brazil | KM395973 | KM396060 | KM396143 | KM395886 |
| CBS 134851 | Soil | Teresina, Piauí, Brazil | KM395974 | KM396061 | KM396144 | KM395887 | |
| C. pluriramosa | CBS 136976; CMW 31440; CERC 1775 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462995 | KJ463112 | KJ463228 | KJ462882 |
| C. polizzi | CBS 125270; CMW 7804 | Callistemon citrinus | Messina, Sicily, Italy | FJ972417 | GQ267461 | FJ972436 | FJ972486 |
| CBS 125271; CMW 10151 | Arbustus unedo | Catania, Sicily, Italy | FJ972418 | GQ267462 | FJ972437 | FJ972487 | |
| C. propaginicola | CBS 134815 | Eucalyptus cutting | Santana, Pará, Brazil | KM395953 | KM396040 | KM396123 | KM395866 |
| CBS 134820 | Used planting substrate | Santana, Pará, Brazil | KM395956 | KM396043 | KM396126 | KM395869 | |
| CBS 134821 | Used planting substrate | Santana, Pará, Brazil | KM395957 | KM396044 | KM396127 | KM395870 | |
| C. pseudocerciana | CBS 134824 | Eucalyptus seedling | Santana, Pará, Brazil | KM395962 | KM396049 | KM396132 | KM395875 |
| C. pseudocolhounii | CBS 127195; CMW 27209 | E. dunnii | Fujian, China | HQ285788 | – | HQ285802 | HQ285816 |
| CBS 127196; CMW 27213 | E. dunnii | Fujian, China | HQ285789 | – | HQ285803 | HQ285817 | |
| C. pseudohodgesii | CBS 134818 | Azadirachta indica | Viçosa, Minas Gerais, Brazil | KM395905 | KM395991 | KM396079 | KM395817 |
| CBS 134819 | A. indica | Viçosa, Minas Gerais, Brazil | KM395906 | KM395992 | KM396080 | KM395818 | |
| C. pseudokyotensis | CBS 137332; CMW 31439; CERC 1774 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ462994 | KJ463111 | KJ463227 | KJ462881 |
| C. pseudometrosideri | CBS 134843 | Soil | Viçosa, Minas Gerais, Brazil | KM395907 | KM395993 | KM396081 | KM395819 |
| CBS 134845 | Soil | Maceió, Alagoas, Brazil | KM395909 | KM395995 | KM396083 | KM395821 | |
| C. pseudoreteaudii | CBS 123694; CMW 25310 | Eucalyptus hybrid cutting | Guangdong, China | FJ918504 | GQ267411 | FJ918519 | FJ918541 |
| CBS 123696; CMW 25292 | Eucalyptus hybrid cutting | Guangdong, China | FJ918505 | GQ267410 | FJ918520 | FJ918542 | |
| C. pseudoscoparia | CBS 125256; CMW 15216 | E. grandis | Pichincha, Ecuador | GQ267228 | GQ267440 | GQ267277 | GQ267348 |
| CBS 125257; CMW 15218 | E. grandis | Pichincha, Ecuador | GQ267229 | GQ267441 | GQ267278 | GQ267349 | |
| C. pseudospathulata | CBS 134840 | Soil | Araponga, Minas Gerais, Brazil | KM395982 | KM396069 | KM396152 | KM395895 |
| CBS 134841 | Soil | Araponga, Minas Gerais, Brazil | KM395983 | KM396070 | KM396153 | KM395896 | |
| C. queenslandica | CBS 112146; CPC 3213 | E. urophylla | Australia | AF389835 | GQ267415 | FJ918521 | FJ918543 |
| CBS 112155; CPC 3210 | E. pellita | Australia | AF389834 | GQ267416 | DQ190667 | FJ918544 | |
| C. reteaudii | CBS 112143; CPC 3200 | E. camaldulensis | Vietnam | GQ240642 | GQ267418 | DQ190660 | FJ918536 |
| CBS 112144; CPC 3201 | E. camaldulensis | Vietnam | AF389833 | GQ267417 | DQ190661 | FJ918537 | |
| C. seminaria | CBS 136630; CMW 31446; CERC 1781 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462996 | KJ463113 | KJ463229 | KJ462883 |
| CBS 136631; CMW 31449; CERC 1784 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462997 | KJ463114 | KJ463230 | KJ462884 | |
| CBS 136632; CMW 31450; CERC 1785 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462998 | KJ463115 | KJ463231 | KJ462885 | |
| CBS 136639; CMW 31489; CERC 1824 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ462999 | KJ463116 | KJ463232 | KJ462886 | |
| CBS 136648; CMW 37970; CERC 1933 | Eucalyptus leaf | Guangxi, China | KJ463000 | KJ463117 | KJ463233 | KJ462887 | |
| CPC 23486; CMW 31447; CERC 1782 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ463001 | KJ463118 | KJ463234 | KJ462888 | |
| CPC 23487; CMW 31448; CERC 1783 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ463002 | KJ463119 | KJ463235 | KJ462889 | |
| C. silvicola | CBS 134836 | Soil | Araponga, Minas Gerais, Brazil | KM395975 | KM396062 | KM396145 | KM395888 |
| CBS 135237 | Soil | Araponga, Minas Gerais, Brazil | KM395978 | KM396065 | KM396148 | KM395891 | |
| C. sphaeropedunculata | CBS 136081; CMW 31390; CERC 1725 | Soil in Eucalyptus plantation | Guangxi, China | KJ463003 | KJ463120 | KJ463236 | KJ462890 |
| C. sulawesiensis | CBS 125248; CMW 14857 | Eucalyptus sp. | Sulawesi, Indonesia | GQ267223 | GQ267435 | GQ267272 | GQ267343 |
| CBS 125253; CMW 14879 | Eucalyptus sp. | Sulawesi, Indonesia | GQ267220 | GQ267432 | GQ267269 | GQ267340 | |
| C. sumatrensis | CBS 112829; CPC 4518 | Soil | Sumatra, Indonesia | AY725649 | AY725771 | AY725696 | AY725733 |
| CBS 112934; CPC 4516 | Soil | Indonesia | AY725651 | AY725773 | AY725798 | AY725735 | |
| C. terrae-reginae | CBS 112151; CPC 3202 | E. urophylla | Queensland, Australia | FJ918506 | GQ267451 | FJ918522 | FJ918545 |
| CBS 112634; CPC 4233 | Xanthorrhoea australis | Victoria, Australia | FJ918507 | GQ267452 | DQ190668 | FJ918546 | |
| C. terrestris | CBS 136642; CMW 35180; CERC 1856 | Soil in Eucalyptus plantation | Guangdong, China | KJ463004 | KJ463121 | KJ463237 | KJ462891 |
| CBS 136643; CMW 35364; CERC 1868 | Soil in Eucalyptus plantation | Guangdong, China | KJ463005 | KJ463122 | KJ463238 | KJ462892 | |
| CBS 136644; CMW 35366; CERC 1870 | Soil in Eucalyptus plantation | Guangdong, China | KJ463006 | KJ463123 | KJ463239 | KJ462893 | |
| CBS 136645; CMW 35178; CERC 1854 | Soil in Eucalyptus plantation | Guangdong, China | KJ463007 | KJ463124 | KJ463240 | KJ462894 | |
| CBS 136647; CMW 35447; CERC 1919 | Eucalyptus leaf | Guangdong, China | KJ463008 | KJ463125 | KJ463241 | KJ462895 | |
| CBS 136651; CMW 37974; CERC 1937 | Soil | Guangdong, China | KJ463009 | KJ463126 | KJ463242 | KJ462896 | |
| CBS 136653; CMW 37980; CERC 1943 | Soil | Guangxi, China | KJ463010 | KJ463127 | KJ463243 | KJ462897 | |
| C. tetraramosa | CBS 136635; CMW 31474; CERC 1809 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ463011 | KJ463128 | KJ463244 | KJ462898 |
| CBS 136637; CMW 31476; CERC 1811 | E. urophylla × E. grandis clone seedling leaf | CERC Nursery, Zhanjiang, Guangdong, China | KJ463012 | KJ463129 | KJ463245 | KJ462899 | |
| C. turangicola | CBS 136077; CMW 31411; CERC 1746 | Soil in Eucalyptus plantation | Fangchenggang, Guangxi, China | KJ463013 | – | KJ463246 | KJ462900 |
| CBS 136093; CMW 35410; CERC 1901 | Soil in Eucalyptus plantation | Guangxi, China | KJ463014 | KJ463130 | KJ463247 | KJ462901 | |
| CBS 136652; CMW 37977; CERC 1940 | Soil | Guangxi, China | KJ463015 | KJ463131 | KJ463248 | KJ462902 | |
| CMW 35383; CERC 1885 | Soil in Eucalyptus plantation | Hainan, China | KJ463016 | KJ463132 | KJ463249 | KJ462903 | |
ATCC: American Type Culture Collection, Virginia, USA; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CERC: China Eucalypt Research Centre, Zhanjiang, Guangdong Province, China; CMW: culture collection of the Forestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa; CPC: Pedro Crous working collection housed at CBS; HGUP: Plant Pathology Herbarium of Guizhou University, Guiyang 550025, China; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, UK; LPF: Laboratório de Patologia Florestal, Universidade Federal de Viçosa, Viçosa, Brazil; MUCL: Mycothèque, Laboratoire de Mycologie Systématique st Appliqée, l’Université, Louvian-la-Neuve, Belgium; UFV: Universidade Federal de Viçosa, Viçosa, Brazil. Isolates obtained during the survey indicated in grey blocks.
tub2 = β-tubulin, cmdA = calmodulin, his3 = histone H3, tef1 = translation elongation factor 1-alpha. Ex-type isolates indicated in bold. Sequences generated in this study indicated in italics.
DNA sequence comparisons
Approximately 500–550 bases were determined for the four gene regions used in this study. For the Bayesian analyses, a HKY+I+G model was selected for cmdA, tef1 and tub2 and the GTR+I+G model for his3. These models were incorporated for each of the datasets analysed. The Bayesian consensus trees for both datasets confirmed the tree topologies obtained from the MP analyses, and therefore, only the MP trees are presented with bootstrap support values (BS) and posterior probabilities (PP) shown for well-supported nodes.
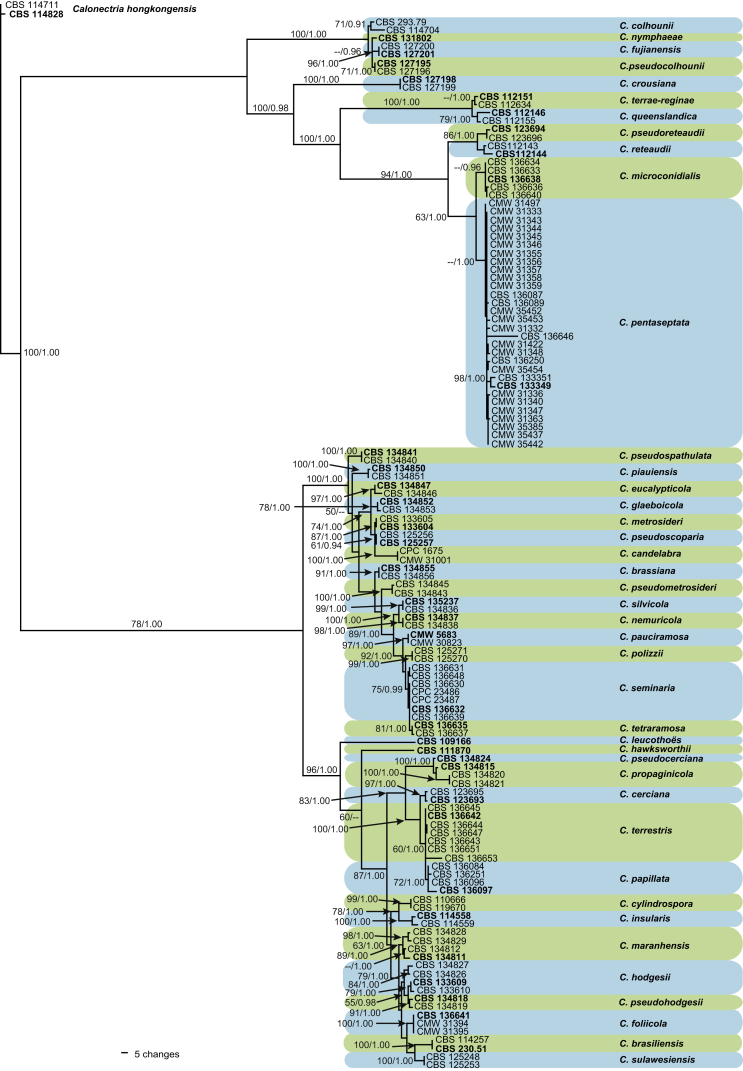
The dataset for the Prolate Group isolates included 127 ingroup taxa, with C. hongkongensis (CBS 114711 & CBS 114828) as the outgroup taxon. The sequence dataset consisted of 2 018 characters, including alignment gaps. Of these, 1 308 were constant, 73 were parsimony-uninformative and 637 parsimony-informative. The MP analysis yielded 1 000 trees (TL = 1 512; CI = 0.612; RC = 0.538; RI = 0.952) of which the first is presented (Fig. 1). The majority of the isolates included in this dataset clustered in the clade (BS < 50; PP = 1.00) representing C. pentaseptata (ex-type CBS 133349) with five isolates (CBS 136633, CBS 136634, CBS 136636, CBS 136638 & CBS 136640) forming a sister clade (BS < 50; PP = 0.96) to the C. pentaseptata clade. A clade (BS = 75; PP = 0.99) incorporating seven isolates (CBS 136630, CBS 136631, CBS 136632, CBS 133639, CBS 1336640, CPC 23486 & CPC 23487), with an additional two isolates (CBS 136635 & CBS 136637) forming a sister clade (BS = 81; PP = 1.00), clustered close but separate from C. pauciramosa (ex-type CMW 5683) and C. polizzii (CBS 125270 & CBS 125271). A further seven isolates (CBS 136642, CBS 136643, CBS 136644, CBS 136645, CBS 136647, CBS 136651 & CBS 136653) formed a clade (BS = 60; PP = 1.00) close but separate to C. cerciana (ex-type CBS 123693) with four isolates (CBS 136084, CBS 136096, CBS 136097 & CBS 136251) forming a sister clade (BS = 72; PP = 1.00) to these seven isolates. Three isolates (CBS 136641, CMW 31394 & CMW 31395) formed a clade (BS = 100; PP = 1.00) close but separate from C. brasiliensis (ex-type CBS 230.51) and C. sulawesiensis (CBS 125248 & CBS 125253).
Fig. 1.
One of 1 000 equally most parsimonious trees obtained from a heuristis search with 1 000 random taxon additions of the combined cmdA, his3, tef1 and tub2 sequence alignments of the Prolate group. Scale bar shows 5 changes. Bootstrap support values and Bayesian posterior probability values are shown at the nodes. The tree was rooted to C. hongkongensis (CBS 114711 & CBS 114878). Ex-type strains are indicated in bold.
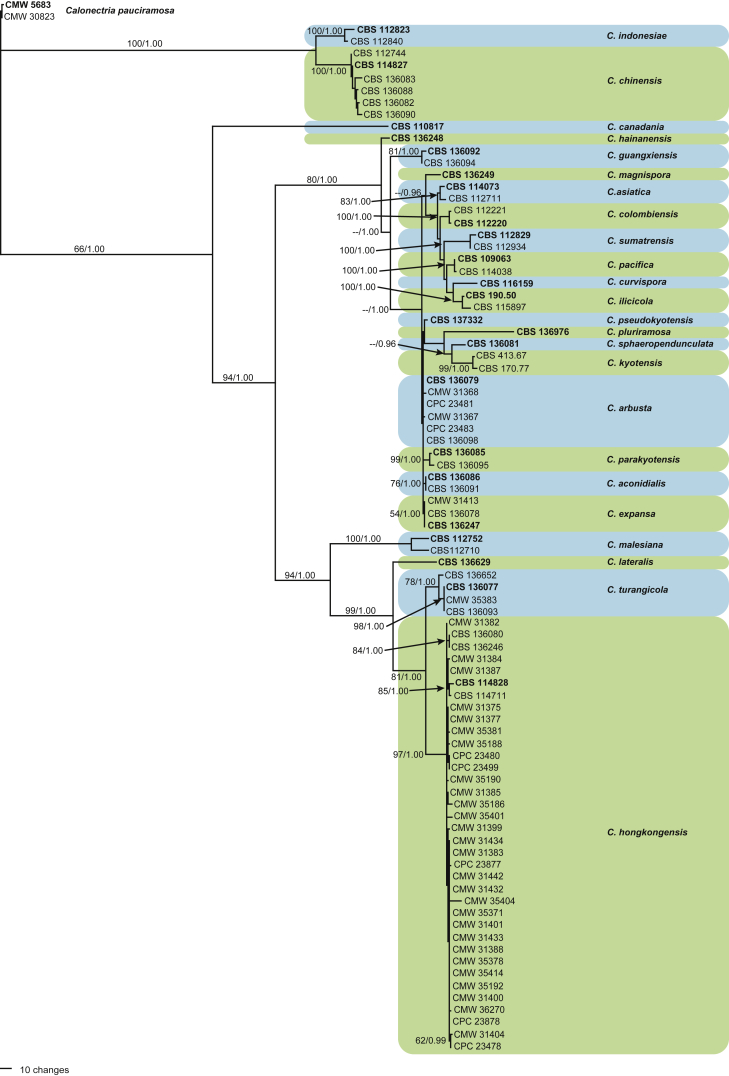
The dataset representing the Sphaero-Naviculate Group of isolates included 85 ingroup taxa, with C. pauciramosa (CMW 5683 & CMW 30823) as the outgroup taxon. This dataset consisted of 2 016 characters, of which 1 369 were constant, 127 were parsimony-uninformative and 520 were parsimony-informative. The MP analysis yielded 100 trees (TL = 1 264; CI = 0.672; RC = 0.633; RI = 0.942) of which the first is presented (Fig. 2). In this tree, 35 isolates clustered within the clade (BS = 97; PP = 1.00) representing C. hongkongensis (ex-type CBS 114828) with four isolates (CBS 136077, CBS 136093, CBS 136652 & CMW 35383) forming a sister clade (BS = 78; PP = 1.00) to the C. hongkongensis clade. A single isolate (CBS 136629) formed a basal sister lineage to both these clades. Four isolates (CBS 136082, CBS 136083, CBS 136088 & CBS 136090) clustered in a clade (BS = 100; PP = 1.00) with C. chinensis (ex-type CBS 114827). Sixteen isolates clustered near the C. kyotensis (ex-type CBS 413.67) clade (BS = 99; PP = 1.00) of which three isolates (CBS 136081, CBS 136976 & CMW 31439) formed single lineages. The remaining isolates clustered in four separate clades, three of which were well supported (BS = 99; PP = 1.00, BS = 76; PP = 1.00 & BS = 54; PP = 1.00, respectively). Of the remaining four isolates, two (CBS 136248 & CBS 136249) formed single lineages and two (CBS 136092 & CBS 136094) formed a unique clade (BS = 81; PP = 1.00).
Fig. 2.
One of 1 000 equally most parsimonious trees obtained from a heuristis search with 1 000 random taxon additions of the combined cmdA, his3, tef1 and tub2 sequence alignments of the Sphaero-Naviculate Group. Scale bar shows 10 changes. Bootstrap support values and Bayesian posterior probability values are shown at the nodes. The tree was rooted to C. pauciramosa (CMW 5683 & CMW 30823). Ex-type strains are indicated in bold.
Taxonomy
Morphological observation supported by phylogenetic inference showed that the majority of strains included in this study belonged to C. chinensis, C. hongkongensis and C. pentaseptata (Fig. 1, Fig. 2; Table 1). The remaining strains are shown to represent several distinct taxa that are provided names in Calonectria. Important morphological characters are summarised in Table 2.
Table 2.
Morphological characteristics of Calonectria spp. included in this study.
| Species | Perithecia |
Asci |
Ascospores |
Conidiogenous apparatus |
Stipe extension |
Vesicle |
Macroconidia |
Reference | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Size (μm) | Shape | Size (μm) | Size (μm) | Septation | Size (μm) | Branches | (μm) | Diam (μm) | Shape | Size (μm) | Septation | ||
| Calonectria reteaudii species complex | |||||||||||||
| C. microconidialis | 26–92 × 35–95 | 3 | 175–441 × 4–7 | 3–7 | Narrowly clavate | (69–)78–98(–113) × 7–9(10) | 4–6(7) | This study | |||||
| C. pentatseptata | 23–90 × 70–99 | 3 | 168–350 × 3–6 | 2–6 | Narrowly clavate | (75–)87–109(–115) × (5–)6–8(–10) | 5(–8) | Crous et al. (2012) | |||||
| C. pseudoreteaudii | 26–82 × 45–103 | 3 | 193–313 × 5–6 | 3–5 | Narrowly clavate | (61–)65–73(–78) × (4–)5–6(–7) | 4–6 | Lombard et al. (2010d) | |||||
| C. queenslandica | 27–68 × 39–64 | 3 | 105–156 × 4–5 | 3–4 | Narrowly clavate | (61–)65–73(–78) × (4–)5–6(–7) | 4–6 | Lombard et al. (2010d) | |||||
| C. reteaudii | 350–450 × 250–350 | Subglobose to ovoid | 70–150 × 7–20 | (50–)65–85(–100) × (4–)5–6(–7) | (1–)3(–5) | 20–70 × 80–100 | 6 | 150–380 × 2.5–3.5 | 3–6 | Clavate | (50–)75–95(–120) × (5–)6–7 | (1–)5(–6) | Crous (2002) |
| C. terrae-reginae | 33–48 × 35–54 | 4 | 127–235 × 4–6 | 3–5 | Narrowly clavate | 60–83(–87) × (4–)5–7(–8) | 4–6 | Lombard et al. (2010d) | |||||
| Calonectria candelabra species complex | |||||||||||||
| C. brassiana | 50–135 × 50–80 | 3 | 90–172 × 2–3 | 3–7 | Ellipsoid to narrowly obpyriform | (35–)50–56(–65) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. candelabra | 350–450 × 300–350 | Subglobose to ovoid | 70–130 × 7–15 | (40–)45–50(–60) × 5–6 | 1 | 30–70 × 50–80 | 5 | 100–220 × 3–3.5 | 5–8 | Ellipsoid to narrowly obpyriform | (45–)58–68(–80) × 4–5(–6) | 1 | Crous (2002) |
| C. eucalypticola | 45–75 × 35–62 | 3 | 145–170 × 2–4 | 5–7 | Ellipsoid to obpyriform | (43–)49–52(–55) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. glaeboicola | 25–40 × 27–45 | 2 | 100–165 × 2–4 | 3–5 | Ellipsoid to narrowly obpyriform | (45–)50–52(–55) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. metrosideri | 60–75 × 40–65 | 4 | 90–170 × 2–4 | 5–9 | Spathulate to obpyriform | (40–)44–46(–51) × 3–5 | 1 | Alfenas et al. (2013a) | |||||
| C. mossambicensis | 37–87 × 19–59 | 3 | 91–203 × 2–6 | 2–8 | Obpyriform to ellipsoidal | (35–)38–46(–50) × 3–6 | 1 | Crous et al. (2013) | |||||
| C. nemuricola | 50–80 × 40–60 | 4 | 150–205 × 6–12 | 7–13 | Obpyriform | (40–)44–46(–50) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. pauciramosa | 250–400 × 170–300 | Subglobose to ovoid | 70–140 × 8–25 | (30–)33–38(–40) × 6–7(–8) | 1 | 20–50 × 35–85 | 3 | 120–230 × 2–3 | 5–11 | Obpyriform to ellipsoidal | (30–)45–55(–60) × (3.5–)4–5 | 1 | Schoch et al. (1999) |
| C. piauiensis | 35–80 × 20–60 | 2 | 95–130 × 2–3 | 3–7 | Ellipsoid to narrowly obpyriform | (38–)47–52(–60) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. polizzii | 28–51 × 27–57 | 3 | 111–167 × 5–6 | 6–9 | Obpyriform to ellipsoidal | (31–)32–42(–49) × 3–5 | 1 | Lombard et al. (2010a) | |||||
| C. pseudoscoparia | 52–74 × 34–87 | 4 | 124–201 × 4–6 | 6–10 | Obpyriform to ellipsoidal | (41–)45–51(–52) × 3–3 | 1 | Lombard et al. (2010b) | |||||
| C. pseudospathulata | 60–100 × 30–70 | 3 | 145–190 × 2–4 | 7–10 | Obpyriform | (35–)41–44(–50) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. seminaria | 31–155 × 36–72 | 3 | 105–185 × 4–7 | 6–11 | Obpyriform to ellipsoidal | (42–)45–49(–52) × 3.5–4.5(–7) | 1 | This study | |||||
| C. silvicola | 45–105 × 35–90 | 3 | 130–195 × 3–4 | 7–10 | Obpyriform | (30–)40–42(–50) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. tetraramosa | 54–95 × 36–75 | 4 | 102–253 × 3–6 | 4–10 | Obpyriform | (45–)46.5–49.5(–51) × (4–)4.5–5.5(–6) | 1 | This study | |||||
| C. zuluensis | 292–394 × 170–285 | Subglobose to ovoid | 92–140 × 10–16 | (26–)29–34(–38) × 4–5 | 1 | 37–70 × 35–67 | 3 | 110–171 × 5–8 | 6–10 | Ellipsoid to obpyriform | (31–)34–38(–40) × 3–5 | 1 | Lombard et al. (2010a) |
| Calonectria cylindrospora species complex | |||||||||||||
| C. brasiliensis | 81–103 × 58–90 | 3 | 204–266 × 6–7 | 7–11 | Ellipsoid to obpyriform | (35–)36–40(–41) × 3–5 | 1 | Lombard et al. (2010a) | |||||
| C. cerciana | 62–113 × 70–98 | 4 | 148–222 × 5–6 | 8–13 | Fusiform to obpyriform | (37–)41–46(–49) × 5–6 | 1 | Lombard et al. (2010d) | |||||
| C. cylindrospora | 280–520 × 280–400 | Globose to subglobose | 75–100 × 8–15 | (24–)30–40(–49) × (4–)5–6(–8) | 1 | 60–100 × 60–110 | 6 | 150–200 × 3–4 | 6–8 | Ellipsoid to pyriform or clavate | (40–)42–50(–66) × 3–4(–5) | 1 | Crous (2002) |
| C. foliicola | 76–180 × 59–130 | 7 | 140–215 × 4–6 | 6–13 | Obpyriform to ellipsoidal | (41–)44–50(–52) × (3–)4–5(–6) | 1 | This study | |||||
| C. hawksworthii | 40–90 × 65–100 | 4 | 150–250 × 2–3 | 6–9 | Ellipsoid to clavate | (38–)50–60(–76) × 4(–5) | 1 | Crous (2002) | |||||
| C. hodgesii | 61–72 × 45–65 | 3 | 136–196 × 2–4 | 6–11 | Pyriform to ellipsoidal or ovoid to sphaeropedunculate | (44–)49–51(–55) × 3–5 | 1 | Alfenas et al. (2013b) | |||||
| C. insularis | 350–450 × 300–350 | Subglobose to ovoid | 70–125 × 7–18 | (27–)30–36(–42) × 5–6(–7) | 1 | 45–90 × 45–80 | 6 | 110–250 × 4–5 | 4–13 | Obpyriform to broadly ellipsoidal | (33–)40–50(–60) × 3.5–4 | 1 | Crous (2002) |
| C. leucothoës | 25–50 × 50–80 | 6 | 160–250 × 3–6 | 6–11.5 | Ellipsoid to obpyriform | (45–)68–78(–97) × (4–)5–5.5(–6.5) | (1–)3(–6) | Crous (2002) | |||||
| C. maranhensis | 45–65 × 45–71 | 3 | 125–190 × 3–5 | 7–11 | Ellipsoid, obpyriform to sphaeropedunculate | (50–)56–58(–65) × (3–)5(–6) | 1 | Alfenas et al. (2015) | |||||
| C. mexicana | 400–450 × 350–450 | Subglobose to ovoid | 70–120 × 10–20 | (35–)40–55(–65) × 5–6(–7) | 25–60 × 40–70 | 3 | 160–250 × 2–3 | 7–12 | Broadly ellipsoid with papillate apex | (35–)40–48(–52) × 3–4(–4.5) | 1 | Crous (2002) | |
| C .papillata | 425–455 × 345–395 | Subglobose to ovoid | 106–112 × 16–20 | (27–)32–40(–46) × 5–6(–7) | 1 | 45–114 × 33–82 | 4 | 163–218 × 4–7 | 8–14 | Obpyriform to ellipsoidal with papillate apex | (40–)43–47(–50) × (3–)4–5 | 1 | This study |
| C. propaginicola | 40–75 × 31–85 | 4 | 130–250 × 2–5 | 5–12 | Ellipsoid, obpyriform to sphaeropedunculate | (40–)48–51(–55) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. pseudocerciana | 50–90 × 40–95 | 3 | 130–190 × 2–5 | 7–12 | Obpyriform to sphaeropedunculate | (35–)43–46(–55) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. pseudohodgesii | 50–90 × 40–95 | 3 | 130–190 × 2–5 | 7–12 | Obpyriform to sphaeropedunculate | (35–)43–46(–55) × 3–5 | 1 | Alfenas et al. (2015) | |||||
| C. sulawesiensis | 43–81 × 41–79 | 5 | 113–262 × 5–7 | 5–7 | Broadly clavate to ellipsoid | (41–)45–51(–54) × (3–)4(–6) | 1 | Lombard et al. (2010b) | |||||
| C. terrestris | 35–89 × 35–102 | 4 | 147–228 × 4–7 | 5–12 | Obpyriform to pyriform to broadly clavate | (33–)36–40(–41) × (3–)4–5 | 1 | This study | |||||
| Calonectria kyotensis species complex | |||||||||||||
| C. aconidialis | 297–366 × 232–304 | Subglobose to ovoid | 111–113 × 15–18 | (28–)32–40(–44) × 5–7 | 1 | This study | |||||||
| C. arbusta | 357–444 × 276–391 | Subglobose to ovoid | 97–119 × 16–19 | (30–)35–41(–43) × 5–7(–8) | 1 | 58–151 × 54–108 | 5 | 134–196 × 3–6 | 7–13 | Sphaeropedunculate | (41–)42–48(–52) × 4–6 | 1 | This study |
| C. asiatica | 280–400 × 200–350 | Subglobose to ovoid | 70–120 × 12–20 | (28–)30–38(–40) × (5–)6(–7) | 1 | 40–80 × 40–90 | 5 | 200–280 × 3–7 | 12–17 | Sphaeropedunculate | (42–)48–55(–65) × (4–)5(–5.5) | 1 | Crous et al. (2004b) |
| C. canadania | 3 | 100–180 × 3–4 | 6–10 | Pyriform to sphaeropedunculate | (38–)48–55(–65) × 4(–5) | 1 | Kang et al. (2001b) | ||||||
| C. chinensis | 40–60 × 40–60 | 3 | 120–150 × 2.5–3.5 | 6–9 | Sphaeropedunculate | (38–)41–48(–56) × (3.5–)4(–4.5) | 1 | Crous et al. (2004b) | |||||
| C. colombiensis | 200–350 × 200–300 | Subglobose to ovoid | 90–150 × 11–23 | (28–)30–35(–40) × (4–)5(–6) | 1 | 25–60 × 40–60 | 5 | 130–200 × 3–4 | 7–12 | Sphaeropedunculate | (33–)48–58(–60) × (4–)4.5(–5) | 1(–3) | Crous et al. (2004b) |
| C. curvispora | 15–30 × 35–50 | 3 | 110–150 × 2–3 | 5–10 | Sphaeropedunculate | (45–)55–65(–70) × (4–)5–6 | 1(–3) | Crous (2002) | |||||
| C. expansa | 310–520 × 270–435 | Subglobose to ovoid | 107–146 × 16–21 | (33–)36–41(–44) × (4–)5–7 | 1 | 26–116 × 45–82 | 5 | 124–216 × 3–7 | 8–16 | Sphaeropedunculate | (44–)48–52(–57) × 4–6 | 1 | This study |
| C. guangxiensis | 295–435 × 265–355 | Subglobose to ovoid | 83–146 × 15–23 | (23–)32–40(–42) × 5–7(–8) | 1 | 31–95 × 55–85 | 4 | 175–193 × 5–7 | 11–14 | Sphaeropedunculate | (42–)45–49(–52) × 4–6 | 1 | This study |
| C. hainanensis | 300–455 × 230–385 | Subglobose to ovoid | 91–110 × 15–22 | (24–)30–38(–42) × (4–)5–7 | 1 | 54–119 × 41–80 | 5 | 112–186 × 5–9 | 7–14 | Sphaeropedunculate | (41–)43–49(–52) × 4–6 | 1 | This study |
| C. hongkongensis | 350–550 × 300–450 | Subglobose to ovoid | 80–140 × 14–20 | (25–)28–35(–40) × (4–)5–6(–7) | 1 | 70–120 × 70–100 | 8 | 100–200 × 3–4 | 8–14 | Sphaeropedunculate | (38–)45–48(–53) × 4(–4.5) | 1 | Crous et al. (2004b) |
| C. ilicicola | 300–550 × 280–400 | Subglobose to ovoid | 90–140 × 12–19 | (30–)37–50(–65) × (4–)5–6.5(–7) | 1(–3) | 25–100 × 55–100 | 3 | 120–140 × 3–4 | 6–12 | Sphaeropedunculate | (45–)70–82(–90) × (4–)5–6.5(–7) | (1–)3 | Crous (2002) |
| C. indonesiae | 60–80 × 40–60 | 5 | 110–160 × 2.5–3 | 7–9 | Sphaeropedunculate | (40–)45–55(–60) × (3–)4 | 1 | Crous et al. (2004b) | |||||
| C. kyotensis | 280–550 × 210–425 | Subglobose to ovoid | 70–140 × 13–22 | (18–)28–40(–48) × (4–)5–6(–7) | 1 | 40–100 × 40–90 | 5 | 100–200 × 3–4 | 6–12 | Sphaeropedunculate | (35–)45–50(–55) × 3–4(–5) | 1 | Crous (2002) |
| C. lateralis | 43–138 × 41–104 | 6 | 150–225 × 4–6 | 9–13 | Sphaeropedunculate | (35–)37–41(–44) × 4–5 | 1 | This study | |||||
| C. magnispora | 280–550 × 210–425 | Subglobose to ovoid | 91–125 × 14–17 | (33–)36–44(–49) × 5–7(–8) | 1 | 47–95 × 47–80 | 4 | 161–278 × 4–7 | 9–18 | Sphaeropedunculate | (46–)49–55(–60) × 4–6(–7) | 1 | This study |
| C. malesiana | 30–80 × 50–60 | 6 | 120–200 × 3–4 | 8–15 | Sphaeropedunculate to globose | (34–)45–52(–55) × (3–)4 | 1 | Crous et al. (2004b) | |||||
| C. pacifica | 20–60 × 30–80 | 3 | 150–250 × 3–4 | 7–15 | Sphaeropedunculate | (38–)45–65(–75) × 4–5 | 1 | Crous (2002) | |||||
| C. parakyotensis | 49–98 × 41–84 | 4 | 135–210 × 4–6 | 10–14 | Sphaeropedunculate | (39–)42–46(–49) × 4–5(–6) | 1 | This study | |||||
| C. pluriramosa | 76–177 × 59–127 | 7 | 140–215 × 4–6 | 6–13 | Sphaeropedunculate | (41–)44–50(–52) × (3–)4–5(–6) | 1 | This study | |||||
| C. pseudokyotensis | 43–103 × 76–109 | 4 | 145–320 × 5–7 | 10–13 | Pyriform to sphaeropedunculate | (43–)45–51(–53) × 5–7 | 1 | This study | |||||
| C. sphaeropedunculata | 470–575 × 345–465 | Subglobose to ovoid | 82–144 × 11–23 | (31–)33–40(–42) × 5–7(–8) | 1 | 63–144 × 40–111 | 6 | 152–253 × 4–8 | 10–14 | Sphaeropedunculate | (40–)43–47(–49) × 4–6 | 1 | This study |
| C. sumatrensis | 40–60 × 50–60 | 3 | 180–260 × 3–4 | 8–13 | Sphaeropedunculate | (45–)55–65(–70) × (4.5–)5(–6) | 1 | Crous et al. (2004b) | |||||
| C. turangicola | 48–110 × 35–86 | 5 | 133–195 × 4–6 | 8–12 | Sphaeropedunculate | (40–)42–46(–47) × 3–5 | 1 | This study | |||||
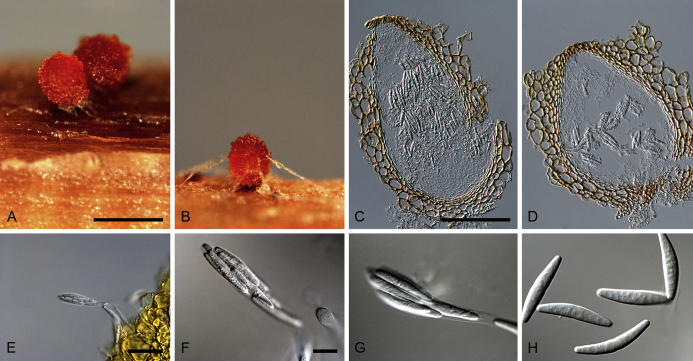
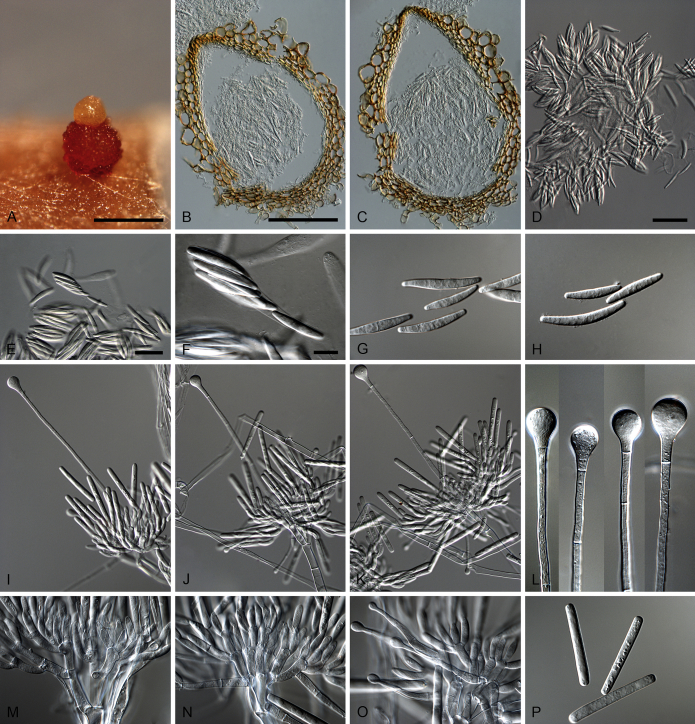
Calonectria aconidialis L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809043. Fig. 3.
Fig. 3.
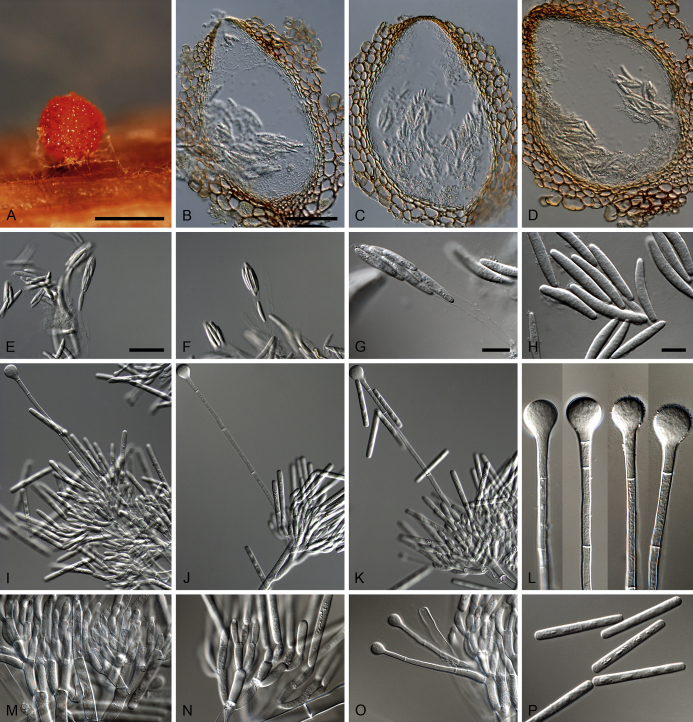
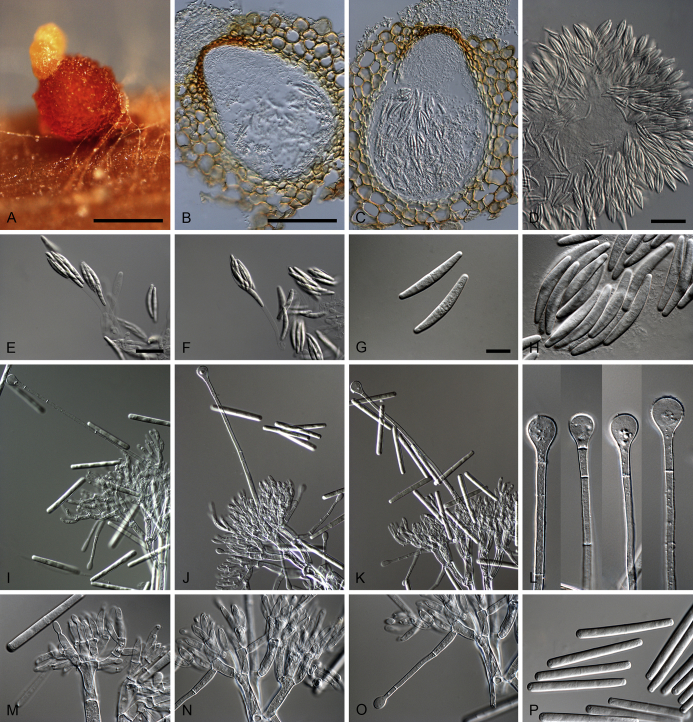
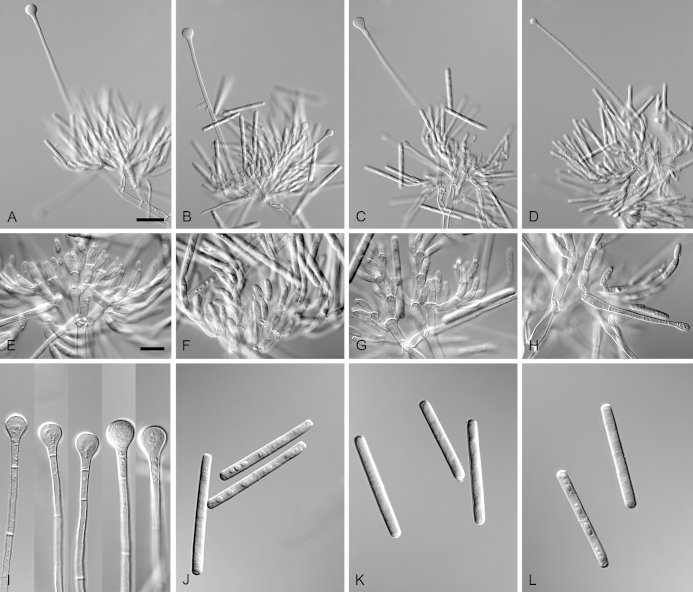
Calonectria aconidialis (ex-type CBS 136086). A–B. Ascomata. C–D. Vertical section through ascomata, showing wall structure. E–G. Asci. H. Ascospores. Scale bars: A = 500 μm; C = 100 μm (apply to D); E = 50 μm; F = 10 μm (apply to G–H).
Etymology: Name refers to an absence of macroconidia in the fungus.
Ascomata perithecial, solitary or in groups of two, orange, becoming orange-brown with age; in section, apex and body orange, base red-brown, subglobose to ovoid, 297–366 μm high, 232–304 μm diam, body turning dark orange to red, and base dark red-brown in 3 % KOH+; ascomatal wall rough, consisting of two thick-walled layers; outer layer of textura globulosa, 37–75 μm thick, cells becoming more compressed towards the inner layer of textura angularis, 16–23 μm thick, cells becoming thin-walled and hyaline towards the centre; outermost cells 14–45 × 12–35 μm, cells of inner layer 10–25 × 3–7 μm; ascomatal base up to 150 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuous with the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 111–113 × 15–18 μm, tapering into a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, 1-septate, sometimes constricted at the septum, (28–)32–40(–44) × 5–7 μm (av. 36 × 6 μm). Homothallic. Mega-, macro- and microconidia not observed.
Culture characteristics: Colonies moderately fast growing at 24 °C on MEA with mycelium immersed in medium with no sporulation on the medium surface; surface and reverse white to pale luteous after 7 d.
Specimens examined: China, Hainan Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & S.F. Chen (holotype CBS H-21481, culture ex-type CBS 136086 = CMW 35174 = CERC 1850), CBS 136091 = CPC 23504 = CMW 35384 = CERC 1886.
Notes: All attempts to induce the asexual morph of C. aconidialis failed. Ascomata formed readily within 16 d on MEA, SNA and MSA, either on the surface or immersed in the medium, exuding viable ascospores after 20 d.
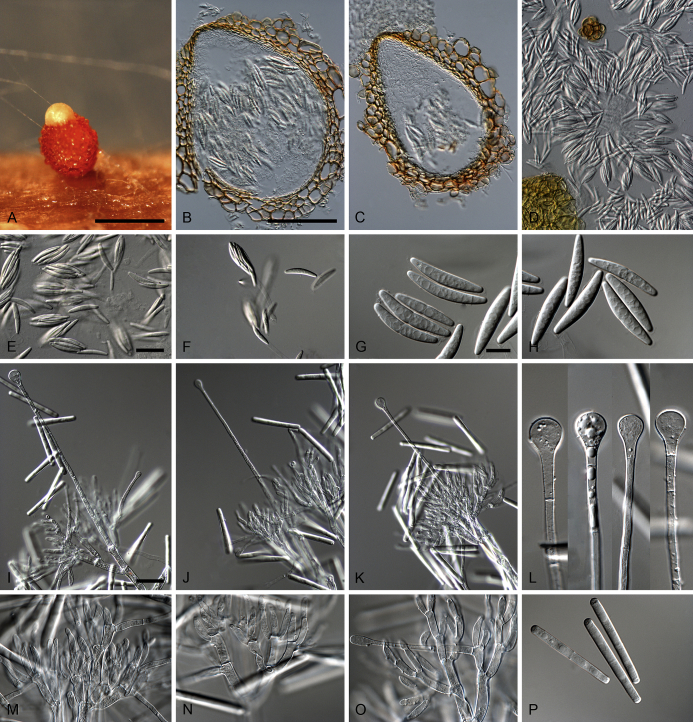
Calonectria arbusta L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809045. Fig. 4.
Fig. 4.
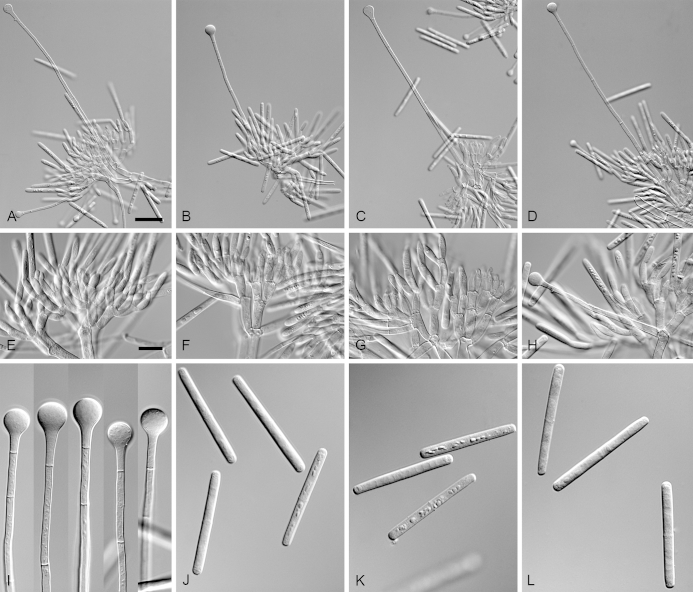
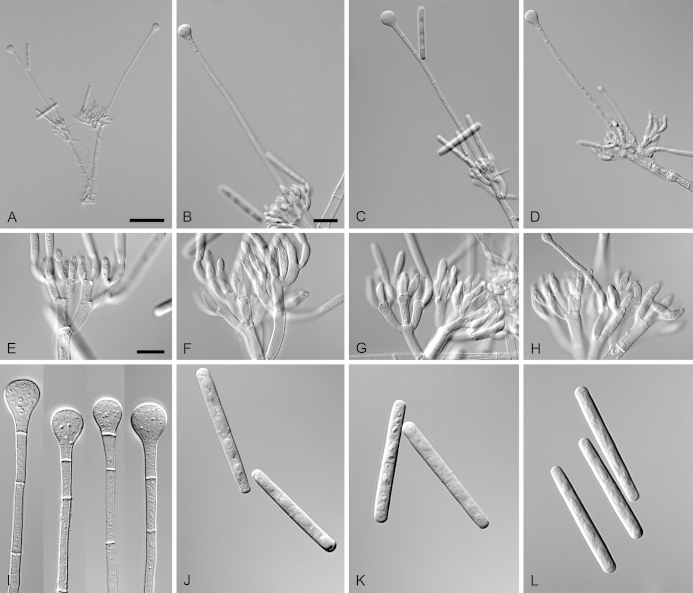
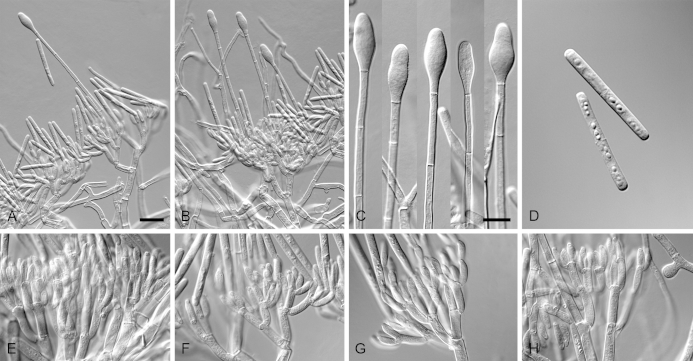
Calonectria arbusta (ex-type CBS 136079). A. Ascoma. B–C. Vertical section through ascomata, showing wall structure. D–F. Asci. G–H. Ascospores. I–K. Macroconidiophores. L. Sphaeropedunculate vesicles. M–N. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. O. Conidiogenous apparatus with lateral stipe extension. P. Macroconidia. Scale bars: A = 500 μm; B = 100 μm (apply to C–D); E = 50 μm (apply to F, I–K), G = 10 μm (apply to H, L–P).
Etymology: Name refers to a plantation and the environment from which this fungus was collected.
Ascomata perithecial, solitary, orange, becoming orange-brown with age; in section, apex and body orange, base red-brown, subglobose to ovoid, 357–444 μm high, 276–391 μm diam, body turning dark orange to red, and base dark red-brown in 3 % KOH+; ascomatal wall rough, consisting of two thick-walled layers; outer layer of textura globulosa, 37–66 μm thick, cells becoming more compressed towards the inner layer of textura angularis, 18–20 μm thick, cells becoming thin-walled and hyaline towards the centre; outermost cells 34–71 × 34–55 μm, cells of inner layer 23–32 × 6–9 μm; ascomatal base up to 137 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuous with the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 97–119 × 16–19 μm, tapering into a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, 1-septate, constricted at the septum, (30–)35–41(–43) × 5–7(–8) μm (av. 38 × 7 μm). Homothallic. Macroconidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 40–133 × 6–10 μm; stipe extension septate, straight to flexuous, 134–196 μm long, 3–6 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 7–13 μm diam; lateral stipe extensions (90° to main axis) abundant. Conidiogenous apparatus 58–151 μm wide, and 54–108 μm long; primary branches aseptate, 18–42 × 5–8 μm; secondary branches aseptate, 10–27 × 4–7 μm; tertiary branches aseptate, 9–18 × 3–6 μm; quaternary branches and additional branches (–5) aseptate, 10–20 × 3–6 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 9–15 × 2–4 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (41–)42–48(–52) × 4–6 μm (av. 45 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white to pale luteous aerial mycelium with profuse sporulation on the medium surface; reverse sienna to umber after 7 d; chlamydospores extensive throughout the medium forming microsclerotia.
Specimens examined: China, Guangxi Province, from soil collected in a Eucalyptus plantation, Mar. 2009, X. Zhou, G. Zhao & F. Han (holotype CBS H-21482, living ex-type culture CBS 136079 = CPC 23482 = CMW 31370 = CERC 1705), CPC 23481 = CMW 31369 = CERC 1704, CPC 23483 = CMW 31371 = CERC 1706, CMW 31368 = CERC 1703; Guangxi Province, Fangchenggang, from soil collected in a Eucalyptus plantation, Mar. 2009, X. Zhou, G. Zhao & F. Han, CMW 31367 = CERC 1702.
Note: Calonectria arbusta produces a larger conidiogenous apparatus than C. kyotensis and the ascospores and macroconidia of C. arbusta are also larger than those of C. kyotensis (Table 2).
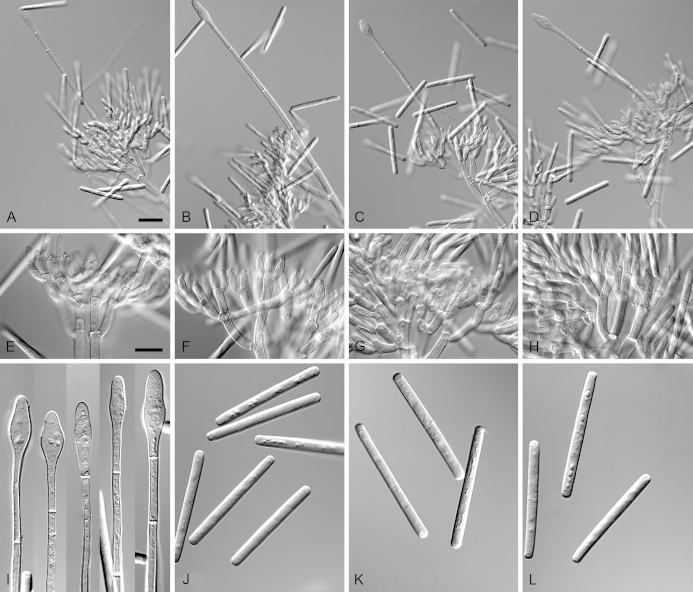
Calonectria expansa L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809046. Fig. 5.
Fig. 5.
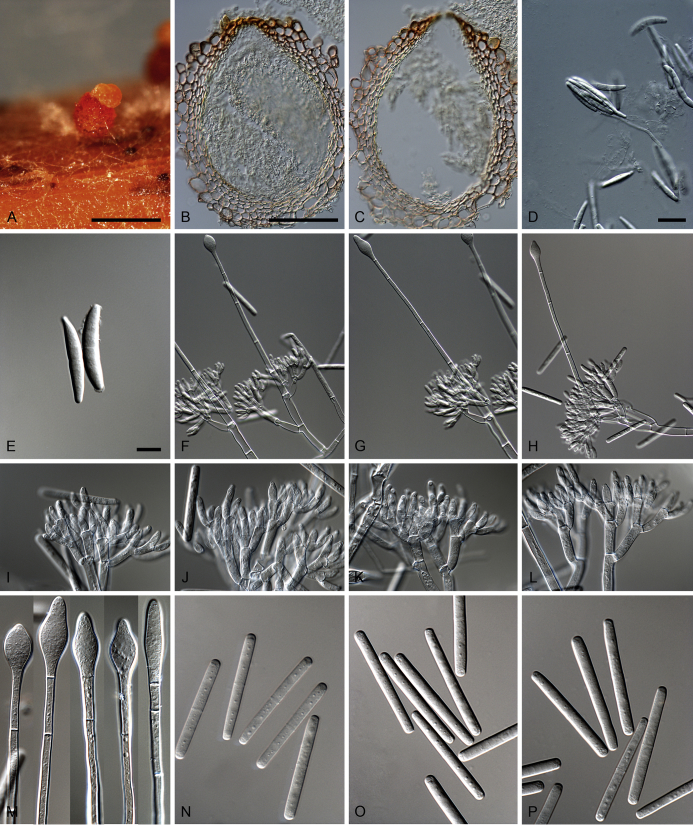
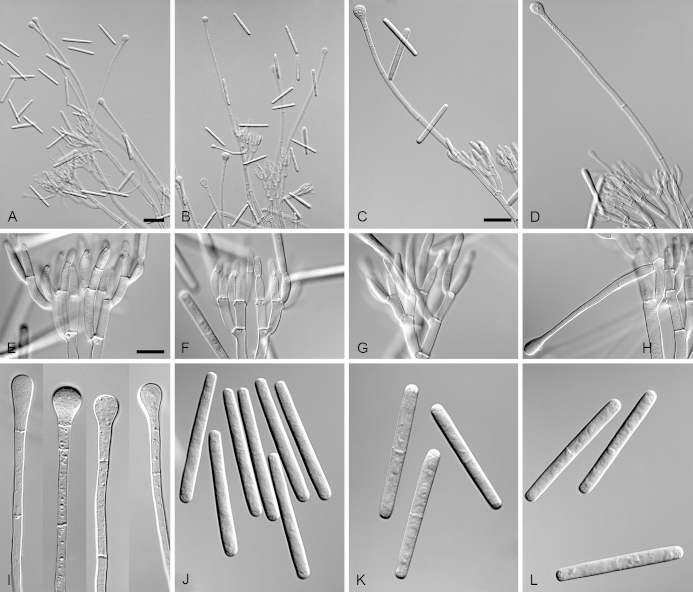
Calonectria expansa (ex-type CBS 136247). A. Ascoma. B–C. Vertical section through ascomata, showing wall structure. D–F. Asci. G–H. Ascospores. I–K. Macroconidiophores. L. Sphaeropedunculate vesicles. M–N. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. O. Conidiogenous apparatus with lateral stipe extension. P. Macroconidia. Scale bars: A = 500 μm; B, D = 100 μm (apply to C); E = 50 μm (apply to I–K), F = 10 μm (apply to G–H, L–P).
Etymology: Name refers to Guangxi Province, the “Western Expanse”, where this fungus was first collected.
Ascomata perithecial, solitary, orange, becoming orange-brown with age; in section, apex and body orange, base red-brown, subglobose to ovoid, 310–520 μm high, 270–435 μm diam, body turning dark orange to red, and base dark red-brown in 3 % KOH+; ascomatal wall rough, consisting of two thick-walled layers; outer layer of textura globulosa, 37–64 μm thick, cells becoming more compressed towards the inner layer of textura angularis, 13–25 μm thick, cells becoming thin-walled and hyaline towards the centre; outermost cells 13–31 × 9–20 μm, cells of inner layer 9–18 × 3–5 μm; ascomatal base up to 150 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuous with the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 107–146 × 16–21 μm, tapering into a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, 1-septate, sometimes constricted at the septum, (33–)36–41(–44) × (4–)5–7 μm (av. 39 × 6 μm). Homothallic. Macroconidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 61–169 × 5–10 μm; stipe extension septate, straight to flexuous, 124–216 μm long, 3–7 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 8–16 μm diam; lateral stipe extensions (90° to main axis) abundant. Conidiogenous apparatus 26–116 μm wide, and 45–82 μm long; primary branches aseptate, 18–29 × 5–7 μm; secondary branches aseptate, 12–22 × 4–7 μm; tertiary branches aseptate, 9–16 × 3–6 μm; quaternary branches and additional branches (–5) aseptate, 12–18 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 10–18 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (44–)48–52(–57) × 4–6 μm (av. 52 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white aerial mycelium with profuse sporulation on the medium surface; reverse sienna to umber after 7 d; chlamydospores extensive throughout the medium forming microsclerotia.
Specimens examined: China, Guangxi Province, from soil collected in a Eucalyptus plantation, Mar. 2009, X. Zhou, G. Zhao & F. Han (holotype CBS H-21483, living ex-type culture CBS 136247 = CPC 23485 = CMW 31392 = CERC 1727); Guangxi Province, Fangchenggang, from soil collected in a Eucalyptus plantation, Mar. 2009, X. Zhou, G. Zhao & F. Han, CBS 136078 = CMW 31441 = CERC 1776, CMW 31413 = CERC 1748.
Note: Calonectria expansa can be distinguished from C. arbusta and C. kyotensis by its larger macroconidia and longer stipe extension (Table 2).
Calonectria foliicola L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809047. Fig. 6.
Fig. 6.
Calonectria foliicola (ex-type 136641). A–D. Macroconidiophores. E–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Obpyriform to ellipsoidal vesicles. J–L. Macroconidia. Scale bars: A = 50 μm (apply to B–D); E = 10 μm (apply to F–L).
Etymology: Name refers to the natural habitat of this species, being a foliar pathogen.
Ascomata not observed. Macroconidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 47–190 × 6–12 μm; stipe extension septate, straight to flexuous, 140–215 μm long, 4–6 μm wide at the apical septum, terminating in a obpyrifrom to ellipsoidal vesicle, 6–13 μm diam. Conidiogenous apparatus 76–180 μm wide, and 59–130 μm long; primary branches aseptate, 17–37 × 5–8 μm; secondary branches aseptate, 16–30 × 4–7 μm; tertiary branches aseptate, 11–23 × 4–6 μm; quaternary and additional branches (–7) aseptate, 9–20 × 3–6 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 8–13 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (41–)44–50(–52) × (3–)4–5(–6) μm (av. 47 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white aerial mycelium and sporulating moderately on the medium surface; reverse sienna to umber after 7 d; chlamydospores formed abundantly throughout the medium, forming microsclerotia.
Specimen examined: China, Guangxi Province, from E. urophylla × E. grandis clone leaf, Mar. 2009, X. Zhou & G. Zhao (holotype CBS H-21472, living ex-type culture CBS 136641 = CPC 23491 = CMW 31393 = CERC 1728), CPC 23492 = CMW 31394 = CERC 1729, CMW 31395 = CERC 1730.
Notes: Calonectria foliicola is closely related to C. brasiliensis and C. sulawesiensis and can be distinguished from these species by the formation of up to seven levels of conidiophore branches. The macroconidia of C. foliicola are larger than those of C. brasiliensis but slightly smaller than those of C. sulawesiensis (Table 2).
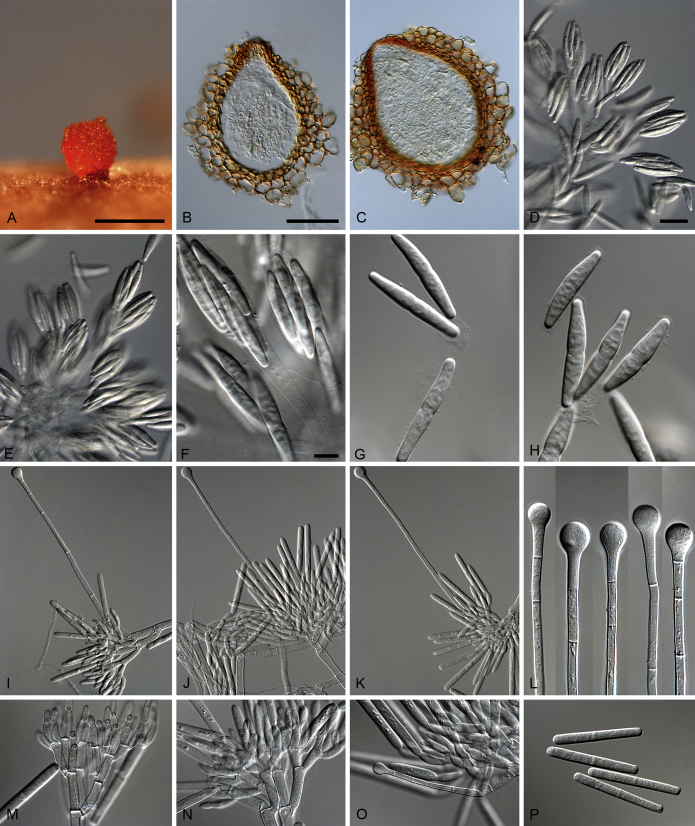
Calonectria guangxiensis L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809049. Fig. 7.
Fig. 7.
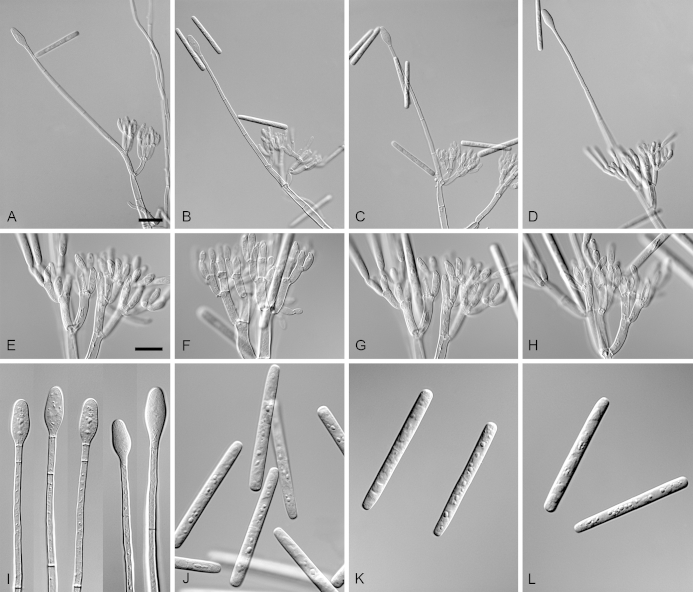
Calonectria guangxiensis (ex-type CBS 136092). A. Ascoma. B–C. Vertical section through ascomata, showing wall structure. D–F. Asci. G–H. Ascospores. I–K. Macroconidiophores. L. Sphaeropedunculate vesicles. M–N. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. O. Conidiogenous apparatus with lateral stipe extension. P. Macroconidia. Scale bars: A = 500 μm; B = 100 μm (apply to C); D = 50 μm (apply to E, I–K), F = 10 μm (apply to G–H, L–P).
Etymology: Name refers to the Guangxi Province of China where the fungus was first collected.
Ascomata perithecial, solitary or in groups of two, orange, becoming orange-brown with age; in section, apex and body orange, base red-brown, subglobose to ovoid, 295–435 μm high, 265–355 μm diam, body turning dark orange to red, and base dark red-brown in 3 % KOH+; ascomatal wall rough, consisting of two thick-walled layers; outer layer of textura globulosa, 32–80 μm thick, cells becoming more compressed towards the inner layer of textura angularis, 14–22 μm thick, cells becoming thin-walled and hyaline towards the centre; outermost cells 13–26 × 10–15 μm, cells of inner layer 11–15 × 4–5 μm; ascomatal base up to 175 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuous with the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 83–146 × 15–23 μm, tapering into a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, 1-septate, constricted at the septum, (23–)32–40(–42) × 5–7(–8) μm (av. 36 × 6 μm). Homothallic. Macroconidiophores consist of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 91–182 × 7–9 μm; stipe extension septate, straight to flexuous, 175–193 μm long, 5–7 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 11–14 μm diam; lateral stipe extensions (90° to main axis) rare. Conidiogenous apparatus 31–95 μm wide, and 55–85 μm long; primary branches aseptate, 17–26 × 4–7 μm; secondary branches aseptate, 10–19 × 3–6 μm; tertiary branches aseptate, 9–17 × 2–5 μm; quaternary branches aseptate, 12–16 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 8–19 × 3–7 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (42–)45–49(–52) × 4–6 μm (av. 47 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white to cream-coloured aerial mycelium and sporulating profusely on the medium surface at the edge of the colony; reverse sienna to umber after 7 d; chlamydospores formed abundantly throughout the medium, forming microsclerotia.
Specimen examined: China, Guangxi Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang (holotype CBS H-21484, culture ex-type CBS 136092 = CPC 23506 = CMW 35409 = CERC 1900), CBS 136094 = CPC 23507 = CMW 35411 = CERC 1902.
Notes: Calonectria guangxiensis can be distinguished from other species in the C. kyotensis complex by having fewer conidiophore branches and rarely forming lateral stipe extensions. The macroconidia of C. guangxiensis are slightly smaller than those of C. expansa and C. kyotensis and slightly larger than those of C. arbusta (Table 2).
Calonectria hainanensis L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809050. Fig. 8.
Fig. 8.
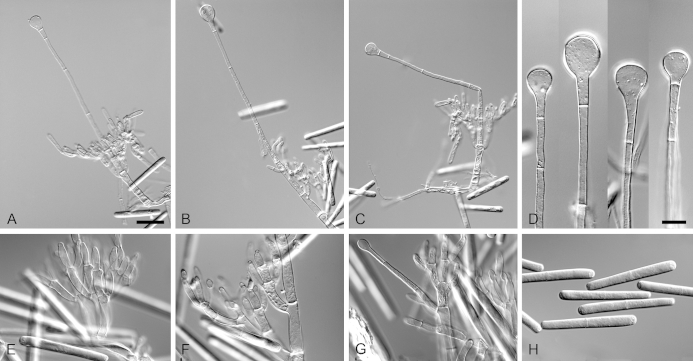
Calonectria hainanensis (ex-type CBS 136248). A. Ascoma. B–C. Vertical section through ascomata, showing wall structure. D–F. Asci. G–H. Ascospores. I–K. Macroconidiophores. L. Sphaeropedunculate vesicles. M–N. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. O. Conidiogenous apparatus with lateral stipe extension. P. Macroconidia. Scale bars: A = 500 μm; B = 100 μm (apply to C); D = 50 μm (apply to I–K), E = 10 μm (apply to F), G = 10 μm (apply to H, L–P).
Etymology: Name refers to the Hainan Province of China where the fungus was first collected.
Ascomata perithecial, solitary, orange, becoming orange-brown with age; in section, apex and body orange, base red-brown, subglobose to ovoid, 300–455 μm high, 230–385 μm diam, body turning dark orange to red, and base dark red-brown in 3 % KOH+; ascomatal wall rough, consisting of two thick-walled layers; outer layer of textura globulosa, 30–64 μm thick, cells becoming more compressed towards the inner layer of textura angularis, 10–16 μm thick, cells becoming thin-walled and hyaline towards the centre; outermost cells 16–42 × 13–42 μm, cells of inner layer 23–39 × 8–10 μm; ascomatal base up to 262 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuous with the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 91–110 × 15–22 μm, tapering into a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, 1-septate, sometimes constricted at the septum, (24–)30–38(–42) × (4–)5–7 μm (av. 34 × 6 μm). Homothallic. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 66–106 × 8–14 μm; stipe extension septate, straight to flexuous, 112–186 μm long, 4–11 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 7–14 μm diam; lateral stipe extensions (90° to main axis) abundant. Conidiogenous apparatus 54–119 μm wide, and 41–80 μm long; primary branches aseptate, 18–28 × 5–9 μm; secondary branches aseptate, 12–21 × 5–8 μm; tertiary branches aseptate, 10–19 × 3–6 μm; quaternary branches and additional branches (–5) aseptate, 9–15 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 10–17 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (41–)43–49(–52) × 4–6 μm (av. 46 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white to pale luteous aerial mycelium and sporulating profusely on the medium surface; reverse sienna to umber after 7 d; chlamydospores formed abundantly throughout the medium, forming microsclerotia.
Specimen examined: China, Hainan Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & S.F. Chen (holotype CBS H-21480, culture ex-type CBS 136248 = CPC 23505 = CMW 35187 = CERC 1863).
Notes: Based on morphological characteristics, C. hainanensis closely resembles C. malesiana. However, C. hainanensis readily produces fertile ascomata in culture, a feature not observed for C. malesiana (Crous et al. 2004b). Furthermore, C. hainanensis has fewer conidiophore branches than reported for C. malesiana, and the macroconidia of C. hainanensis are slightly smaller than those of C. malesiana (Table 2).
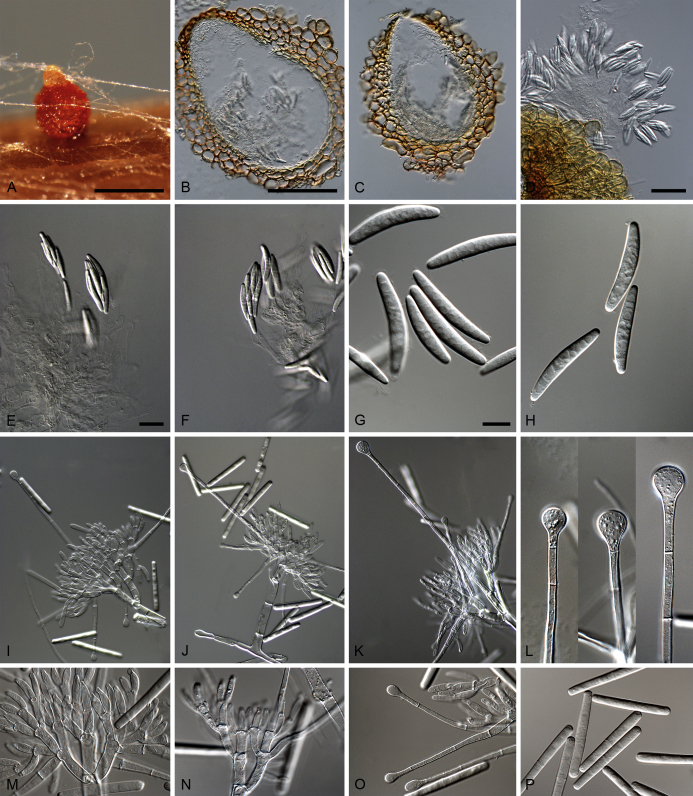
Calonectria lateralis L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809051. Fig. 9.
Fig. 9.
Calonectria lateralis (ex-type CBS 136629). A–D. Macroconidiophores. E–H. Conidiogenous apparatus with conidiophore branches, doliiform to reniform phialides and lateral stipe extensions. I. Sphaeropedunculate vesicles. J–L. Macroconidia. Scale bars: A = 50 μm (apply to B–D); E = 10 μm (apply to F–L).
Etymology: Name refers to the lateral stipe extensions on its macroconidiophores.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 55–185 × 4–8 μm; stipe extension septate, straight to flexuous, 150–225 μm long, 4–6 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 9–13 μm diam; lateral stipe extensions (90° to main axis) abundant. Conidiogenous apparatus 43–138 μm wide, and 41–104 μm long; primary branches aseptate, 17–28 × 4–7 μm; secondary branches aseptate, 11–26 × 3–7 μm; tertiary branches aseptate, 8–20 × 3–6 μm; quaternary and additional branches (–6) aseptate, 8–17 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–13 × 2–4 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (35–)37–41(–44) × 4–5 μm (av. 39 × 4 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white to sienna aerial mycelium with profuse sporulation on the medium surface; reverse sienna to umber after 7 d; chlamydospores extensive throughout the medium forming microsclerotia.
Specimen examined: China, Guangxi Province, Fangchenggang, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Zhou, G. Zhao & F. Han (holotype CBS H-21469, living ex-type CBS 136629 = CMW 31412 = CERC 1747).
Notes: Calonectria lateralis is closely related to C. hongkongensis and can be distinguished by having smaller macroconidia as compared to C. hongkongensis, and the stipe extensions of C. lateralis being longer than those of C. hongkongensis (Table 2).
Calonectria magnispora L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809052. Fig. 10.
Fig. 10.
Calonectria magnispora (ex-type CBS 136249). A. Ascoma. B–D. Vertical section through ascomata, showing wall structure. E–G. Asci. H. Ascospores. I–K. Macroconidiophores. L. Sphaeropedunculate vesicles. M–N. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. O. Conidiogenous apparatus with lateral stipe extension. P. Macroconidia. Scale bars: A = 500 μm; B = 100 μm (apply to C–D); E = 50 μm (apply to F, I–K), G = 20 μm, H = 10 μm (apply to L–P).
Etymology: Name reflects the characteristically large ascospores produced by this fungus.
Ascomata perithecial, solitary, orange, becoming orange-brown with age; in section, apex and body orange, base red-brown, subglobose to ovoid, 390–495 μm high, 315–410 μm diam, body turning dark orange to red, and base dark red-brown in 3 % KOH+; ascomatal wall rough, consisting of two thick-walled layers; outer layer of textura globulosa, 53–91 μm thick, cells becoming more compressed towards the inner layer of textura angularis, 16–20 μm thick, cells becoming thin-walled and hyaline towards the centre; outermost cells 16–28 × 10–18 μm, cells of inner layer 9–20 × 3–6 μm; ascomatal base up to 166 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuous with the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 91–125 × 14–17 μm, tapering into a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, 1-septate, not constricted at the septum, (33–)36–44(–49) × 5–7(–8) μm (av. 40 × 6 μm). Homothallic. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 57–139 × 7–11 μm; stipe extension septate, straight to flexuous, 161–278 μm long, 4–7 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 9–18 μm diam; lateral stipe extensions (90° to main axis) moderately formed. Conidiogenous apparatus 47–95 μm wide, and 47–80 μm long; primary branches aseptate, 18–35 × 5–9 μm; secondary branches aseptate, 13–23 × 3–7 μm; tertiary branches aseptate, 10–19 × 3–5 μm; quaternary branches aseptate, 12–16 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 8–16 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (46–)49–55(–60) × 4–6(–7) μm (av. 52 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white aerial mycelium and sporulating profusely on the medium surface; reverse sienna to umber after 7 d; chlamydospores formed abundant throughout the medium, forming microsclerotia.
Specimen examined: China, Guangxi Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang (holotype CBS H-21471, living ex-type culture CBS 136249 = CPC 23509 = CMW 35184 = CERC 1860).
Notes: Calonectria magnispora can be distinguished from C. arbusta, C. expansa, C. guangxiensis, C. hainanensis and C. kyotensis by having larger ascospores and macroconidia. The stipe extensions of C. magnispora are also longer than observed in these species (Table 2).
Calonectria microconidialis L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809053. Fig. 11.
Fig. 11.
Calonectria microconidialis (ex-type CBS 136638). A–D. Macroconidiophores. E–H. Conidiogenous apparatus with conidiophore branches and cylindrical to allantoid phialides. I. Narrowly clavate vesicles. J–L. Microconidiophores. M–O. Macro- and microconidia. Scale bars: A = 50 μm (apply to B, M); C = 20 μm (apply to D, L); E = 10 μm (apply to F–K, N–O).
Etymology: Name refers to the microconidial state that is readily produced by this species.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 59–195 × 7–11 μm; stipe extension septate, straight to flexuous, 175–441 μm long, 4–7 μm wide at the apical septum, terminating in a narrowly clavate vesicle, 3–7 μm diam. Conidiogenous apparatus 26–92 μm wide, and 35–95 μm long; primary branches aseptate or 1-septate, 23–34 × 5–7 μm; secondary branches aseptate, 16–28 × 3–6 μm; tertiary branches aseptate, 14–24 × 3–6 μm, each terminal branch producing 1–3 phialides; phialides cylindrical to allantoid, hyaline, aseptate, 12–25 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (69–)78–98(–113) × 7–9(–10) μm (av. 88 × 8 μm), 4–6(7)-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Microconidiophores simple with some lateral branching, comprising a stipe and a penicillate or subverticillate arrangement of fertile branches. Stipe septate, hyaline, smooth, 53–86 × 7–8 μm; primary branches aseptate, straight, 19–26 × 4–5 μm, terminating in 1–3 phialides that are cylindrical to allantoid, 12–27 × 4–5 μm; apex with minute periclinal thickening and collarette. Microconidia cylindrical, straight, rounded at the apex, flattened at the base, (23–)31–47(–58) × 4–6(–7) μm (av. 39 × 5 μm), 1–3-septate, held in fascicles by colourless slime. Megaconidia not observed.
Culture characteristics: Colonies slow growing at 24 °C on MEA with mycelia immersed in the media, sporulating profusely on the medium surface, forming white to amber colonies with irregular margins; reverse sienna to umber after 7 d. Chlamydospores not observed.
Specimens examined: China, Guangdong Province, Zhanjiang, CERC nursery, on E. urophylla × E. grandis clone seedling leaf, Mar. 2009, G. Zhao (holotype CBS H-21473, culture ex-type CBS 136638 = CMW 31487 = CERC 1822), CBS 136640 = CMW 31492 = CERC 1827 (Herb. CBS H-21474); Guangdong Province, Zhanjiang, CERC nursery, on E. urophylla × E. grandis clone seedling leaf, Mar. 2009, G. Zhao, CBS 136633 = CMW 31471 = CERC 1806, CBS 136634 = CMW 31473 = CERC 1808, CBS 136636 = CMW 31475 = CERC 1810.
Notes: Calonectria microconidialis resides in the C. reteaudii complex (Lombard et al., 2010d, Crous et al., 2012). The ability of C. microconidialis to produce microconidiophores and microconidia in culture distinguishes it from C. pentaseptata, C. queenslandica and C. terrae-reginae (Lombard et al., 2010d, Crous et al., 2012). The micro- and macroconidia of C. microconidialis are slightly larger than those of C. reteaudii but slightly smaller than those of C. pseudoreteaudii (Table 2).
Calonectria papillata L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809054. Fig. 12.
Fig. 12.
Calonectria papillata (ex-type CBS 136097). A. Ascoma. B–C. Vertical section through ascomata, showing wall structure. D. Asci. E. Ascospores. F–H. Macroconidiophores. I–L. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. M. Obpyriform to ellipsoid vesicles with papillate apex. N–P. Macroconidia. Scale bars: A = 500 μm; B = 100 μm (apply to C); D = 50 μm (apply to F–H); E = 10 μm (apply to I–P).
Etymology: Name refers to the papillate apices of the stipe vesicles.
Ascomata perithecial, solitary, orange, becoming orange-brown with age; in section, apex and body orange, base red-brown, subglobose to ovoid, 425–455 μm high, 345–395 μm diam, body turning dark orange to red, and base dark red-brown in 3 % KOH+; ascomatal wall rough, consisting of two thick-walled layers; outer layer of textura globulosa, 49–57 μm thick, cells becoming more compressed towards the inner layer of textura angularis, 21–22 μm thick, cells becoming thin-walled and hyaline towards the centre; outermost cells 25–52 × 21–38 μm, cells of inner layer 11–21 × 5–9 μm; ascomatal base up to 200 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuous with the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 106–112 × 16–20 μm, tapering into a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, 1-septate, constricted at the septum, (27–)32–40(–46) × 5–6(–7) μm (av. 36 × 6 μm). Homothallic. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 54–245 × 6–11 μm; stipe extension septate, straight to flexuous, 163–218 μm long, 4–7 μm wide at the apical septum, terminating in a obpyrifrom to ellipsoidal vesicle with a papillate apex, 8–14 μm diam. Conidiogenous apparatus 45–114 μm wide, and 33–82 μm long; primary branches aseptate, 18–32 × 5–9 μm; secondary branches aseptate, 11–25 × 4–7 μm; tertiary branches aseptate, 8–19 × 2–5 μm; quaternary branches aseptate, 9–12 × 3–4 μm, each terminal branch producing 2–4 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–16 × 3–4 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (40–)43–47(–50) × (3–)4–5 μm (av. 45 × 4 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white aerial mycelium and sporulating profusely on the medium surface; reverse sienna to umber after 7 d; chlamydospores abundant throughout the medium, forming microsclerotia.
Specimens examined: China, Guangdong Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang (holotype CBS H-21487, living ex-type culture CBS 136097 = CPC 23517 = CMW 37976 = CERC 1939), Guangdong Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang, CBS 136084 = CPC 23497 = CMW 35165 = CERC 1841, CBS 136096 = CPC 23515 = CMW 37972 = CERC 1935, Guangxi Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang, CBS 136251 = CPC 23514 = CMW 37971 = CERC 1934.
Notes: Calonectria papillata can be distinguished from both C. cerciana and C. terrestris by the papillate apices of the terminal vesicles on the stipe extension. This species is also homothallic, which is not the case for C. cerciana (Lombard et al. 2010d) or C. terrestris.
Calonectria parakyotensis L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809055. Fig. 13.
Fig. 13.
Calonectria parakyotensis (ex-type CBS 136085). A–C. Macroconidiophores. D. Sphaeropedunculate vesicles. E–G. Conidiogenous apparatus with conidiophore branches, doliiform to reniform phialides and lateral stipe extensions. H. Macroconidia. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–H).
Etymology: Name refers to fact that this species has an asexual morph that is very similar to that of C. kyotensis.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 42–125 × 5–9 μm; stipe extension septate, straight to flexuous, 135–210 μm long, 4–6 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 10–14 μm diam; lateral stipe extensions (90° to main axis) rare. Conidiogenous apparatus 49–98 μm wide, and 41–84 μm long; primary branches aseptate, 15–34 × 5–8 μm; secondary branches aseptate, 10–17 × 4–7 μm; tertiary branches aseptate, 9–17 × 3–6 μm; quaternary branches aseptate, 11–18 × 4–6 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 10–18 × 2–6 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (39–)42–46(–49) × 4–5(–6) μm (av. 44 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white aerial mycelium with profuse sporulation on the medium surface; reverse sienna to cinnamon after 7 d; chlamydospores extensive throughout the medium forming microsclerotia.
Specimens examined: China, Guangdong Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang (holotype CBS H-21470, living ex-type CBS 136085 = CPC 23498 = CMW 35169 = CERC 1845); Guangxi Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang, CBS 136095 = CPC 23508 = CMW 35413 = CERC 1904.
Note: Calonectria parakyotensis can be distinguished from other closely related species in the C. kyotensis complex by having fewer conidiophore branches and the fact that it rarely forms lateral stipe extensions.
Calonectria pluriramosa L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809056. Fig. 14.
Fig. 14.
Calonectria pluriramosa (ex-type CBS 136976). A–D. Macroconidiophores. E–H. Conidiogenous apparatus with conidiophore branches, doliiform to reniform phialides and lateral stipe extensions. I. Sphaeropedunculate vesicles. J–L. Macroconidia. Scale bars: A = 100 μm; B = 20 μm (apply to C–D); E = 10 μm (apply to F–L).
Etymology: Name refers to the numerous conidiophore branches formed by this species.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 47–185 × 6–12 μm; stipe extension septate, straight to flexuous, 140–215 μm long, 4–6 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 6–13 μm diam; lateral stipe extensions (90° to main axis) rare. Conidiogenous apparatus 76–177 μm wide, and 59–127 μm long; primary branches aseptate or 1-septate, 17–37 × 5–8 μm; secondary branches aseptate, 16–30 × 4–7 μm; tertiary branches aseptate, 11–23 × 4–6 μm; quaternary branches and additional branches (–7) aseptate, 9–20 × 3–6 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 8–13 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (41–)44–50(–52) × (3–)4–5(–6) μm (av. 47 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white to sienna aerial mycelium with profuse sporulation on the medium surface; reverse sienna to umber after 7 d; chlamydospores extensive throughout the medium forming microsclerotia.
Specimen examined: China, Guangxi Province, Fangchenggang, from soil collected in a Eucalyptus plantation, Mar. 2009, X. Zhou, G. Zhao & F. Han (holotype CBS H-21485, living ex-type culture CBS 136976 = CMW 31440 = CERC 1775).
Notes: Calonectria pluriramosa is closely related to C. kyotensis and C. pseudokyotensis but can be distinguished by having a greater number of conidiophore branches. The macroconidia of C. pluriramosa are larger than those of C. kyotensis (Table 2). Unlike the latter two species, C. pluriramosa also failed to produce viable ascomata in culture.
Calonectria pseudokyotensis L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809057. Fig. 15.
Fig. 15.
Calonectria pseudokyotensis (ex-type CBS 137332). A–D. Macroconidiophores. E–H. Conidiogenous apparatus with conidiophore branches, doliiform to reniform phialides and lateral stipe extensions. I. Pyriform to sphaeropedunculate vesicles. J–L. Macroconidia. Scale bars: A = 50 μm (apply to B); C = 20 μm (apply to D); E = 10 μm (apply to F–L).
Etymology: Name refers to the morphological similarity to the asexual morph of C. kyotensis.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 85–205 × 6–10 μm; stipe extension septate, straight to flexuous, 145–320 μm long, 5–7 μm wide at the apical septum, terminating in a pyriform to sphaeropedunculate vesicle, 10–13 μm diam; lateral stipe extensions (90° to main axis) moderate. Conidiogenous apparatus 42–103 μm wide, and 76–109 μm long; primary branches aseptate or 1-septate, 24–40 × 5–8 μm; secondary branches aseptate, 14–32 × 5–7 μm; tertiary branches aseptate, 13–25 × 4–6 μm; quaternary branches aseptate, 14–24 × 4–6 μm, each terminal branch producing 2–6 phialides; phialides elongate doliiform to doliiform or reniform, hyaline, aseptate, 10–20 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (43–)45–51(–53) × 5–7 μm (av. 48 × 6 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white aerial mycelium with profuse sporulation on the medium surface; reverse sienna to umber after 7 d; chlamydospores extensive throughout the medium forming microsclerotia.
Specimen examined: China, Guangxi Province, Fangchenggang, from soil collected in a Eucalyptus plantation, Mar. 2009, X. Zhou, G. Zhao & F. Han (holotype CBS H-21774, living ex-type culture CBS 137332 = CMW 31439 = CERC 1774).
Notes: Calonectria pseudokyotensis has fewer fertile branches than C. kyotensis. Furthermore, the stipe extensions of C. pseudokyotensis are longer than those of C. kyotensis, terminating in pyriform to sphaeropedunculate vesicles, not observed in C. kyotensis (Table 2).
Calonectria seminaria L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809058. Fig. 16.
Fig. 16.
Calonectria seminaria (ex-type CBS 136632). A–D. Macroconidiophores. E–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Obpyriform to ellipsoid vesicles. J–L. Macroconidia. Scale bars: A = 100 μm (apply to B–D); E = 10 μm (apply to F–L).
Etymology: Name refers the fact that this species was collected in a nursery.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 39–101 × 6–10 μm; stipe extension septate, straight to flexuous, 105–185 μm long, 4–7 μm wide at the apical septum, terminating in an obpyrifrom to ellipsoid vesicle, 6–11 μm diam. Conidiogenous apparatus 31–155 μm wide, and 36–72 μm long; primary branches aseptate or 1-septate, 13–27 × 3–6 μm; secondary branches aseptate, 8–19 × 2–5 μm; tertiary branches aseptate, 10–20 × 2–6 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 7–14 × 2–4 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (42–)45–49(–52) × 3.5–4.5(–7) μm (av. 47 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white aerial mycelium and sporulating profusely on the medium surface; reverse amber to sepia-brown after 7 d; chlamydospores formed extensively in the media, forming microsclerotia.
Specimens examined: China, Guangdong Province, Zhanjiang, CERC nursery, on E. urophylla × E. grandis clone seedling leaf, Mar. 2009, G. Zhao (holotype CBS H-21475, living ex-type culture CBS 136632 = CPC 23488 = CMW 31450 = CERC 1785); Guangdong Province, Zhanjiang, CERC nursery, on E. urophylla × E. grandis clone seedling leaf, Mar. 2009, G. Zhao, CBS 136630 = CMW 31446 = CERC 1781, CBS 136631 = CMW 31449 = CERC 1784, CPC 23486 = CMW 31447 = CERC 1782, CPC 23487 = CMW 31448 = CERC 1783, CBS 136639 = CMW 31489 = CERC 1824; Guangxi Province, on leaf of Eucalyptus in plantation, Aug. 2009, X. Mou & R. Chang, CBS 136648 = CMW 37970 = CERC 1933.
Notes: Calonectria seminaria belongs to the C. candelabra species complex (Schoch et al., 1999, Lombard et al., 2010a; see Lombard et al. 2015), closely related to C. pauciramosa and C. polizzii. The macroconidia of C. seminaria are slightly smaller than those of C. pauciramosa, and larger than those of C. polizzii (Table 2).
Calonectria sphaeropedunculata L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809059. Fig. 17.
Fig. 17.
Calonectria sphaeropedunculata (ex-type CBS 136081). A. Ascoma. B–C. Vertical section through ascomata, showing wall structure. D–F. Asci. G–H. Ascospores. I–K. Macroconidiophores. L. Sphaeropedunculate vesicles. M–O. Conidiogenous apparatus with conidiophore branches, doliiform to reniform phialides and lateral stipe extension. P. Macroconidia. Scale bars: A = 500 μm; B = 100 μm (apply to C); D = 50 μm (apply to I–K), E = 20 μm (apply to F), G = 10 μm (apply to H, L–P).
Etymology: Name refers to the sphaeropedunculate vesicles produced by this species.
Ascomata perithecial, solitary or in groups of two, orange, becoming orange-brown with age; in section, apex and body orange, base red-brown, subglobose to ovoid, 470–575 μm high, 345–465 μm diam, body turning dark orange to red, and base dark red-brown in 3 % KOH+; ascomatal wall rough, consisting of two thick-walled layers; outer layer of textura globulosa, 40–80 μm thick, cells becoming more compressed towards the inner layer of textura angularis, 14–21 μm thick, cells becoming thin-walled and hyaline towards the centre; outermost cells 22–36 × 14–22 μm, cells of inner layer 13–32 × 6–8 μm; ascomatal base up to 216 μm wide, consisting of dark red, angular cells, merging with an erumpent stroma; cells of the outer wall layer continuous with the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, 82–144 × 11–23 μm, tapering into a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, guttulate, fusoid with rounded ends, straight to slightly curved, 1-septate, not constricted at the septum, (31–)33–40(–42) × 5–7(–8) μm (av. 37 × 6 μm). Homothallic. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 62–183 × 7–12 μm; stipe extension septate, straight to flexuous, 152–253 μm long, 4–8 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 10–14 μm diam; lateral stipe extensions (90° to main axis) formed moderately. Conidiogenous apparatus 63–144 μm wide, and 40–111 μm long; primary branches aseptate or 1-septate, 18–36 × 4–10 μm; secondary branches aseptate, 11–29 × 5–9 μm; tertiary branches aseptate, 14–23 × 5–8 μm; quaternary and additional branches (–6) aseptate, 9–19 × 4–7 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 9–17 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (40–)43–47(–49) × 4–6 μm (av. 46 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white to cinnamon aerial mycelium with profuse sporulation on the medium surface; reverse sienna to umber after 7 d; chlamydospores extensive throughout the medium forming microsclerotia.
Specimen examined: China, Guangxi Province, from soil collected in a Eucalyptus plantation, Mar. 2009, X. Zhou, G. Zhao & F. Han (holotype CBS H-21486, culture ex-type CBS 136081 = CPC 23484 = CMW 31390 = CERC 1725).
Notes: Calonectria sphaeropedunculata produces longer stipe extensions than those of C. kyotensis and C. pluriramosa, but shorter extensions than those of C. pseudokyotensis. The macroconidia of C. sphaeropedunculata are also smaller than those of C. kyotensis, C. pluriramosa and C. pseudokyotensis (Table 2).
Calonectria terrestris L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809060. Fig. 18.
Fig. 18.
Calonectria terrestris (ex-type CBS 136642). A–D. Macroconidiophores. E–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. I. Obpyriform to pyriform to broadly clavate vesicles. J–L. Macroconidia. Scale bars: A = 50 μm (apply to B–D); E = 10 μm (apply to F–L).
Etymology: Name refers to the fact that this fungus was isolated from soil.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 35–185 × 6–10 μm; stipe extension septate, straight to flexuous, 147–228 μm long, 4–7 μm wide at the apical septum, terminating in an obpyrifrom to pyriform to broadly clavate vesicle, 5–12 μm diam. Conidiogenous apparatus 35–89 μm wide, and 35–102 μm long; primary branches aseptate, 21–35 × 5–8 μm; secondary branches aseptate, 15–27 × 4–7 μm; tertiary branches aseptate, 10–18 × 4–6 μm; quaternary branches aseptate, 9–14 × 3–6 μm, each terminal branch producing 2–4 phialides; phialides doliiform to reniform, hyaline, aseptate, 8–12 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (33–)36–40(–41) × (3–)4–5 μm (av. 38.5 × 4.5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white to pale luteous aerial mycelium and sporulating profusely on the medium surface; reverse sienna to umber after 7 d; chlamydospores formed abundant throughout the medium, forming microsclerotia.
Specimens examined: China, Guangdong Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang (holotype CBS H-21478, culture ex-type CBS 136642 = CMW 35180 = CERC 1856), CBS 136643 = CPC 23493 = CMW 35364 = CERC 1868, CBS 136644 = CPC 23494 = CMW 35366 = CERC 1870, CBS 136645 = CPC 23496 = CMW 35178 = CERC 1854, CBS 136651 = CPC 23516 = CMW 37974 = CERC 1937 (CBS H-21479); Guangdong province, from leaf of Eucalyptus, Aug. 2009, X. Mou & R. Chang, CBS 136647 = CPC 23510 = CMW 35447 = CERC 1919; Guangxi Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang, CBS 136653 = CPC 23518 = CMW 37980 = CERC 1943.
Notes: Calonectria terrestris can be distinguished from C. cerciana and C. papillata by its obpyrifrom to pyriform to broadly clavate vesicles rather than the fusiform to obpyriform vesicles of C. cerciana, and obpyriform to ellipsoidal vesicles with papillate apex of C. papillata. The macroconidia of C. terrestris are also slightly smaller than those of C. cerciana and C. papillata (Table 2).
Calonectria tetraramosa L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809061. Fig. 19.
Fig. 19.
Calonectria tetraramosa (ex-type CBS 136635). A–B. Macroconidiophores. C. Obpyriform vesicles. D. Macroconidia. E–H. Conidiogenous apparatus with conidiophore branches and doliiform to reniform phialides. Scale bars: A = 50 μm (apply to B); C = 10 μm (apply to D–H).
Etymology: Name refers to the four levels of fertile branches produced by this species.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 47–109 × 6–9 μm; stipe extension septate, straight to flexuous, 102–253 μm long, 3–6 μm wide at the apical septum, terminating in a obpyrifrom vesicle, 4–10 μm diam. Conidiogenous apparatus 54–95 μm wide, and 36–75 μm long; primary branches aseptate, 15–29 × 4–7 μm; secondary branches aseptate, 10–20 × 3–6 μm; tertiary branches aseptate, 9–15 × 3–6 μm; quaternary branches aseptate, 10–13 × 3–4 μm, each terminal branch producing 2–6 phialides; phialides elongate doliiform to reniform, hyaline, aseptate, 8–14 × 3–5 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (45–)46.5–49.5(–51) × (4–)4.5–5.5(–6) μm (av. 48 × 5 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white aerial mycelium and sporulating profusely on the medium surface; reverse amber to sepia-brown after 7 d; chlamydospores formed extensively in the media, forming microsclerotia.
Specimen examined: China, Guangdong Province, Zhanjiang, CERC nursery, on E. urophylla × E. grandis clone seedling leaf, Mar. 2009, G. Zhao (holotype CBS H-21477, living ex-type culture CBS 136635 = CPC 23489 = CMW 31474 = CERC 1809), CBS 136637 = CMW 31476 = CERC 1811.
Notes: Calonectria tetraramosa is closely related to C. pauciramosa, C. polizzii and C. seminaria in the C. candelabra complex (Schoch et al., 1999, Lombard et al., 2010a). It can be distinguished from these three species by quaternary branches in the conidiogenous apparatus, which are not found in the other species. Furthermore, the macroconidia of C. tetraramosa are slightly smaller than those of C. pauciramosa, larger than those of C. polizzii, but similar to those of C. seminaria. The stipe extensions of C. tetraramosa are also longer than those of C. pauciramosa, C. polizzii and C. seminaria (Table 2).
Calonectria turangicola L. Lombard, Crous & S.F. Chen, sp. nov. MycoBank MB809062. Fig. 20.
Fig. 20.
Calonectria turangicola (ex-type CBS 136077). A–D. Macroconidiophores. E–H. Conidiogenous apparatus with conidiophore branches, doliiform to reniform phialides and lateral stipe extension. I. Sphaeropedunculate vesicles. J–L. Macroconidia. Scale bars: A = 50 μm (apply to B–D); E = 10 μm (apply to F–L).
Etymology: Name refers to the Chinese word for soil (Tŭrăng), the substrate from which this fungus was first isolated.
Ascomata not observed. Macroconidiophores consisting of a stipe bearing a penicillate arrangement of fertile branches, and a stipe extension terminating in a vesicle; stipe septate, hyaline, smooth, 45–122 × 6–9 μm; stipe extension septate, straight to flexuous, 133–195 μm long, 4–6 μm wide at the apical septum, terminating in a sphaeropedunculate vesicle, 8–12 μm diam; lateral stipe extensions (90° to main axis) abundant. Conidiogenous apparatus 48–110 μm wide, and 35–86 μm long; primary branches aseptate, 16–30 × 4–7 μm; secondary branches aseptate, 10–18 × 3–6 μm; tertiary branches aseptate, 9–17 × 3–5 μm; quaternary and additional branches (–5) aseptate, 10–16 × 3–5 μm, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, 8–16 × 3–7 μm, apex with minute periclinal thickening and inconspicuous collarette. Macroconidia cylindrical, rounded at both ends, straight, (40–)42–46(–47) × 3–5 μm (av. 44 × 4 μm), 1-septate, lacking a visible abscission scar, held in parallel cylindrical clusters by colourless slime. Mega- and microconidia not observed.
Culture characteristics: Colonies fast growing at 24 °C on MEA, producing abundant white to sienna aerial mycelium with profuse sporulation on the medium surface; reverse sienna to umber after 7 d; chlamydospores extensive throughout the medium forming microsclerotia.
Specimens examined: China, Guangxi Province, Fangchenggang, from soil collected in a Eucalyptus plantation, Mar. 2009, X. Zhou, G. Zhao & F. Han (holotype CBS H-21488, culture ex-type CBS 136077 = CPC 23479 = CMW 31411 = CERC 1746); Guangxi Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & R. Chang, CBS 136093 = CMW 35410 = CERC 1901, CBS 136652 = CMW 37977 = CERC 1940; Hainan Province, from soil collected in a Eucalyptus plantation, Aug. 2009, X. Mou & S.F. Chen, CMW 35383 = CERC 1885.
Note: The macroconidia of C. turangicola are slightly smaller than those of C. hongkongensis but larger than those of C. lateralis (Table 2).
Discussion
A surprisingly large number of Calonectria species were collected from soils and Eucalyptus tissue in a relatively small area of southern China. Phylogenetic inference was used to define the species boundaries but these were in most cases also well-supported by morphological features. The 18 new species described in this study add to the eleven species previously recognised in the Southern provinces of China (Crous et al., 2004b, Lombard et al., 2010d, Chen et al., 2011d, Xu et al., 2012).
Most of the isolates obtained from Eucalyptus leaves displaying symptoms of CLB were identified as C. pentaseptata, which was recently described in the C. reteaudii complex from Vietnam (Crous et al. 2012), making this the first report of the fungus from China. Calonectria pentaseptata was collected in all three provinces sampled, including the sampled nursery surveyed, with a single isolate obtained from soil collected in Hainan Province. The collection data suggest that this fungus could be amongst the more important Eucalyptus leaf and shoot pathogens but this hypothesis will need testing experimentally.
Calonectria microconidialis, which was collected from Eucalyptus leaves in the nursery, resides in the C. reteaudii species complex, which now includes six species (Lombard et al. 2010d). The only other species in this complex known from China is C. pseudoreteaudii (Lombard et al., 2010d, Chen et al., 2011d). Calonectria microconidialis produces microconidiophores in culture, a characteristic shared with C. reteaudii and C. pseudoreteaudii, but distinguishing it from C. pentaseptata, C. queenslandica and C. terrae-reginae (Lombard et al., 2010d, Crous et al., 2012). Species of the C. reteaudii complex are well-known causal agents of CLB in Australia, South America and Southeast Asia (Pitkethley, 1976, Bolland et al., 1985, Sharma and Mohanan, 1991, Sharma and Mohanan, 1992, Kang et al., 2001a, Crous, 2002, Rodas et al., 2005, Lombard et al., 2010d), but the pathogenicity of C. microconidialis and C. pentaseptata will need to be tested experimentally.
Calonectria seminaria and C. tetraramosa were found together with C. microconidialis in the nursery sampled in this study. These species represent new members of the C. candelabra complex (Schoch et al., 1999, Lombard et al., 2015), which includes several well-known nursery pathogens (Schoch et al., 1999, Koike et al., 1999, Polizzi and Crous, 1999, Polizzi, 2000, Koike and Crous, 2001, Polizzi and Catara, 2001, Polizzi and Vitale, 2001, Crous, 2002, Polizzi et al., 2006, Polizzi et al., 2007, Polizzi et al., 2009, Vitale et al., 2009, Lombard et al., 2010a, Lombard et al., 2010d, Vitale et al., 2013, Guarnaccia et al., 2014, Alfenas et al. 2015). The C. candelabra complex now includes 16 species (Schoch et al., 1999, Crous, 2002, Lombard et al., 2010a, Alfenas et al., 2013a, Alfenas et al., 2015) and has the highest diversity of species found in South America (Schoch et al., 1999, Schoch et al., 2001, Alfenas et al. 2015, see this volume). Although C. pauciramosa has been regarded as the dominant Eucalyptus nursery pathogen in previous studies (Schoch et al., 1999, Crous, 2002, Lombard et al., 2010a), it was not isolated here. Although it has previously also been found in China (Lombard et al. 2010d) it is clearly not as common as it is elsewhere in the world such as in South America and Southern Africa.
This study included the description of three new species, C. foliicola, C. papillata and C. terrestris in the C. cylindrospora complex (Crous et al., 1993, Schoch et al., 1999, Schoch et al., 2001, Lombard et al., 2010d, Alfenas et al., 2013b, Alfenas et al., 2015; see Lombard et al. 2015), which displays a similarly high level of species diversity in South America (Alfenas et al., 2013b, Alfenas et al., 2015). Calonectria papillata and C. terrestris are sibling species of C. cerciana, but can be distinguished by their characteristic terminal vesicles and the morphology of their macroconidia. Calonectria papillata is also homothallic, a feature not known in C. cerciana (Lombard et al. 2010d) nor in C. terrestris described in this study. Both C. papillata and C. terrestris were isolated from soils collected in Guangdong Province, but only a single isolate of C. terrestris was obtained from a Eucalyptus leaf collected from the same province. Calonectria foliicola, isolated from Eucalyptus leaves collected in Guangxi Province, is closely related to C. brasiliensis and C. sulawesiensis, but can be distinguished from those species based on its macroconidiophore morphology. Although some members of the C. cylindrospora complex are well-known pathogens (Crous, 2002, Lombard et al., 2010c), nothing is known regarding the pathogenicity of C. foliicola, C. papillata and C. terrestris.
Most isolates of Calonectria spp. baited from soils in this study belonged to C. hongkongensis, a member of the C. kyotensis complex (Crous et al. 2004b) and the Sphaero-Naviculate Group (Lombard et al. 2010b). This fungus is characterised by its sphaeropedunculate terminal vesicles, a common feature for all members of the C. kyotensis complex (Crous et al. 2004b), and they also all have up to eight conidiophore branches (Crous et al. 2004b). Like C. hongkongensis, its sibling species C. turangicola and C. lateralis described here, were also isolated exclusively from soil. These species can be distinguished from C. hongkongensis by having fewer conidiophore branches and from each other based on the morphology of their macroconidia.
Results of this study add 10 species to the C. kyotensis complex, which includes C. aconidialis, C. arbusta, C. expansa, C. guangxiensis, C. hainanensis, C. magnispora, C. parakyotensis, C. pseudokyotensis, C. pluriramosa and C. sphaeropedunculata. All 10 species were isolated from soils collected in all three provinces, although nothing is thus far known regarding their pathogenicity.
Calonectria aconidialis produced only its sexual morph in this study, despite many attempts to stimulate the production of conidiophores and conidia. However, sibling species such as C. arbusta and C. expansa formed both morphs in cultures derived from single conidia, and were thus homothallic. Calonectria parakyotensis, also a sibling species of C. aconidialis, failed to produce a sexual morph during this study. Different mating systems in species within related groups of Calonectria spp. are well-known and have been reported for members of the C. candelabra (Lombard et al. 2010a) and C. kyotensis complexes (Crous et al. 2004b). Calonectria aconidialis was found only in samples from the Hainan Province, and C. arbusta only in Guangxi, whereas both C. expansa and C. parakyotensis were found in soils collected in Guangdong and Guangxi provinces. Whether these species are restricted geographically would be interesting but more intensive and structured sampling would be needed to resolve this question.
Calonectria pseudokyotensis, C. pluriramosa and C. sphaeropedunculata are closely related to C. kyotensis and are easily distinguished from each other and C. kyotensis based on morphological features and phylogenetic inference. All of these novel species were isolated from soils collected in the Guangxi Province. Only C. sphaeropedunculata displayed a homothallic mating system, a feature shared with C. kyotensis (Crous, 2002, Crous et al., 2004b), whereas C. pseudokyotensis and C. pluriramosa did not produce any sexual morphs in culture during this study.
The large ascospores and macroconidia of C. magnispora distinguish this novel species from the other members of the C. kyotensis complex. This species, along with C. guangxiensis, was isolated from soils collected in the Guangxi Province. Together with C. hainanensis, isolated from soil collected in the Hainan Province, these novel species readily formed their sexual morphs in culture, and are homothallic. Several isolates were also identified as C. chinensis based on phylogenetic inference and morphological features. This species, known only from China (Crous et al. 2004b), belongs to the C. kyotensis complex, and nothing is known regarding its ability to infect plants.
The greatest diversity of species found in this study came from baiting of soils collected in the Guangxi Province, followed by the Guangdong and Hainan Provinces. Of the 29 Calonectria species now known from China, 16 belong to the Sphaero-Naviculate Group, and 13 to the Prolate Group as defined by Lombard et al. (2010b). Ten species in the former group have a homothallic mating system and the remaining six species are more likely to be heterothallic because single conidial isolates mated in culture did not produce ascomata. Interestingly, all homothallic species originated exclusively from a soil habitat, while those thought to be heterothallic were from both soil and plant material. It is possible that homothallism in these Calonectria species represents an adaptation to the soil environment where only short-distance spread is required, as ascospores are extremely susceptible to desiccation (Rowe & Beute 1975). The majority of the putative heterothallic Calonectria species in this study were isolated from leaves of different Eucalyptus clones displaying CLB symptoms. Since heterothallism results in sexual outcrossing and the generation of genetic diversity (Billiard et al., 2012, Heitman et al., 2013), such a mating system would be beneficial to fungi that infect plants where sexual outcrossing would facilitate the process of overcoming host resistance.
To better understand the genetic variation within these homothallic and putative heterothallic Calonectria species more knowledge of the population structure of these species is required. Relatively few studies have focused on the population dynamics of Calonectria species (Wright et al., 2006, Wright et al., 2007, Wright et al., 2010), and therefore limited knowledge is available on the population structure, distribution of genetic diversity, gene flow, centres of origin and the role of mating strategies for these fungi. Population studies on these fungi, especially those associated with CLB in China would better facilitate our understanding of the epidemiology, and in turn, the management of CLB in Eucalyptus plantations in China. These studies would also allow the prediction of efficacy of host plant resistance to these fungi, necessary for the establishment of future commercial plantations in China.
Although several Calonectria species were isolated from Eucalyptus leaves displaying symptoms of CLB in this study, relatively little is known about their pathogenicity, and their roles as potential pathogens can only be assumed based on the symptoms they are associated with. Therefore, pathogenicity tests need to be done experimentally to determine whether these species are pathogenic to Eucalyptus and if they are host specific. These studies would help identify which Calonectria species are important to commercial Eucalyptus forestry in China. The high diversity of Calonectria species in a relatively small area of southern China, and especially in virgin soils, implies that more Calonectria species remain to be discovered as sampling is extended to more provinces in China, which would also have to be tested as possible threats to Eucalyptus production in China.
Acknowledgements
This study was initiated through the bilateral agreement between the Governments South Africa and China, and we are grateful for the funding via projects 2010KJCX015-03 (Forestry Science and Technology Innovation Project of Guangdong Province of China), 2012DFG31830 (International Science & Technology Cooperation Program of China), 31400546 (National Natural Science Foundation of China) and the NWO Joint Scientific Thematic Research Programme – Joint Research Projects 2012 ALW file number 833.13.005 “Building the fungal quarantine & quality barcode of life database to ensure plant health”. We also appreciate the financial support of members of the Tree Protection Co-operative Programme (TPCP). We thank Arien van Iperen, Trix Merkx and Dr Seonju Marincowitz for their invaluable assistance with cultures.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- Alfenas R.F., Pereira O.L., Ferreira M.A. Calonectria metrosideri, a highly aggressive pathogen causing leaf blight, root rot, and wilt of Metrosideros spp. in Brazil. Forest Pathology. 2013;43:257–265. [Google Scholar]
- Alfenas R.F., Pereira O.L., Jorge V.L. A new species of Calonectria causing leaf blight and cutting rot of three forest tree species in Brazil. Tropical Plant Pathology. 2013;38:513–521. [Google Scholar]
- Alfenas R.F., Lombard L., Pereira O.L. Diversity and potential impact of Calonectria species in Eucalyptus plantations in Brazil. Studies in Mycology. 2015;80:89–130. doi: 10.1016/j.simyco.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billiard S., López-Villavicencio M., Hood M.E. Sex, outcrossing and mating types: unsolved questions in fungi and beyond. Journal of Evolutionary Biology. 2012;25:1020–1038. doi: 10.1111/j.1420-9101.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- Bolland L., Tierney J.W., Tierney B.J. Studies on leaf spot and shoot blight of Eucalyptus caused by Cylindrocladium quinqueseptatum. European Journal of Forest Pathology. 1985;15:385–397. [Google Scholar]
- Booth T.H., Jovanovic T., Old K.M. Climatic mapping to identify high-risk areas for Cylindrocladium quinqueseptatum leaf blight on eucalypts in mainland South East Asia and around the world. Environmental Pollution. 2000;108:365–372. doi: 10.1016/s0269-7491(99)00215-8. [DOI] [PubMed] [Google Scholar]
- Burgess T.I., Andjic V., Hardy G.E.S.J. First report of Phaeophleospora destructans in China. Journal of Tropical Forest Science. 2006;18:144–146. [Google Scholar]
- Burgess T.I., Barber P.A., Sufaati S. Mycosphaerella spp. on Eucalyptus in Asia: new species, new hosts and new records. Fungal Diversity. 2007;24:135–157. [Google Scholar]
- Chen S.F., Gryzenhout M., Roux J. Identification and Pathogenicity of Chrysoporthe cubensis on Eucalyptus and Syzygium spp. in South China. Plant Disease. 2010;94:1143–1150. doi: 10.1094/PDIS-94-9-1143. [DOI] [PubMed] [Google Scholar]
- Chen S.F., Pavlic D., Roux J. Characterization of Botryosphaeriaceae from plantation-grown Eucalyptus species in South China. Plant Pathology. 2011;60:739–751. [Google Scholar]
- Chen S.F., Gryzenhout M., Roux J. Novel species of Celoporthe from Eucalyptus and Syzygium trees in China and Indonesia. Mycologia. 2011;103:1384–1410. doi: 10.3852/11-006. [DOI] [PubMed] [Google Scholar]
- Chen S.F., Barnes I., Chungu D. High population diversity and increasing importance of the Eucalyptus stem canker pathogen, Teratosphaeria zuluensis, in South China. Australasian Plant Pathology. 2011;40:407–415. [Google Scholar]
- Chen S.F., Lombard L., Roux J. Novel species of Calonectria associated with Eucalyptus leaf blight in Southeast China. Persoonia. 2011;26:1–12. doi: 10.3767/003158511X555236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.F., Van Wyk M., Roux J. Taxonomy and pathogenicity of Ceratocystis species on Eucalyptus trees in South China, including C. chinaeucensis sp. nov. Fungal Diversity. 2013;58:267–279. [Google Scholar]
- Crous P.W. APS Press; St. Paul, Minnesota, USA: 2002. Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. [Google Scholar]
- Crous P.W., Alfenas A.C., Wingfield M.J. Calonectria scoparia and Calonectria morganii sp. nov., and variation among isolates of their Cylindrocladium anamorphs. Mycological Research. 1993;97:701–708. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Groenewald J.Z., Risède J.-M. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology. 2004;50:415–430. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Shivas R.G., Wingfield M.J. Fungal Planet description sheets: 128–153. Persoonia. 2012;29:146–201. doi: 10.3767/003158512X661589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Guarro J. Fungal Planet description sheets: 154–213. Persoonia. 2013;31:188–296. doi: 10.3767/003158513X675925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarnaccia V., Aiello D., Polizzi G. Emergence of plochloraz-resistant populations of Calonectria pauciramosa and Calonectria polizzii in ornamental nurseries of southern Italy. Plant Disease. 2014;98:344–350. doi: 10.1094/PDIS-04-13-0425-RE. [DOI] [PubMed] [Google Scholar]
- Heitman J., Sun S., James T.Y. Evolution of fungal sexual reproduction. Mycologia. 2013;105:1–27. doi: 10.3852/12-253. [DOI] [PubMed] [Google Scholar]
- Hillis D.M., Bull J.J. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Systematic Biology. 1993;42:182–192. [Google Scholar]
- Kang J.-C., Crous P.W., Old K.M. Non-conspecificity of Cylindrocladium quinqueseptatum and Calonectria quinqueseptata based on beta-tubulin gene phylogeny and morphology. Canadian Journal of Botany. 2001;79:1241–1247. [Google Scholar]
- Kang J.-C., Crous P.W., Schoch C.L. Species concepts in the Cylindrocladium floridanum and Cy. spathiphylli complexes (Hypocreaceae) based on multi-allelic sequence data, sexual compatibility and morphology. Systematic and Applied Microbiology. 2001;24:206–217. doi: 10.1078/0723-2020-00026. [DOI] [PubMed] [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S.T., Crous P.W. First report of a root and crown rot disease of myrtle in California caused by Cylindrocladium pauciramosum. Plant Disease. 2001;85:448. doi: 10.1094/PDIS.1999.83.6.589D. [DOI] [PubMed] [Google Scholar]
- Koike S.T., Henderson D.M., Crous P.W. A new root and crown rot disease of heath in California caused by Cylindrocladium pauciramosum. Plant Disease. 1999;83:589. doi: 10.1094/PDIS.1999.83.6.589D. [DOI] [PubMed] [Google Scholar]
- Lechat C., Crous P.W., Groenewald J.Z. The enigma of Calonectria species occurring on leaves of Ilex aquifolium in Europe. IMA Fungus. 2010;1:101–108. doi: 10.5598/imafungus.2010.01.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D. Multigene phylogeny and mating tests reveal three cryptic species related to Calonectria pauciramosa. Studies in Mycology. 2010;66:15–30. doi: 10.3114/sim.2010.66.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D. Phylogeny and systematics of the genus Calonectria. Studies in Mycology. 2010;66:31–69. doi: 10.3114/sim.2010.66.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D. Species concepts in Calonectria (Cylindrocladium) Studies in Mycology. 2010;66:1–14. doi: 10.3114/sim.2010.66.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Merwe N.A. van der, Groenewald J.Z. Generic concepts in Nectriaceae. Studies in Mycology. 2015;80:189–245. doi: 10.1016/j.simyco.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Polizzi G., Guarnaccia V. Calonectria spp. causing leaf spot, crown and root rot of ornamental plants in Tunisia. Persoonia. 2011;27:73–79. doi: 10.3767/003158511X615086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Zhou X.D., Crous P.W. Calonectria species associated with cutting rot of Eucalyptus. Persoonia. 2010;24:1–11. doi: 10.3767/003158510X486568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirenburg H.I. A simplified method for identifying Fusarium spp. occurring on wheat. Canadian Journal of Botany. 1981;59:1599–1609. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; 2004. MrModeltest v. 2. Programme distributed by the author. [Google Scholar]
- Old K.M., Wingfield M.J., Yuan Z.Q. Center for International Forestry Research; Jakarta, Indonesia: 2003. A manual of diseases of eucalypts in South-East Asia. [Google Scholar]
- Pitkethley R.N. Cylindrocladium quinqueseptatum on myrtaceous tree seedlings. Australian Plant Pathology Society Newsletter. 1976;5:57. [Google Scholar]
- Polizzi G. Prime esperience di lotta chimica nei confrontidel marciume del colletto e delle radici di Polygala myrtifolia causato da Cylindrocladium pauciramosum. Informatore Fitopatologico. 2000;11:39–47. [Google Scholar]
- Polizzi G., Catara V. First report of leaf spot caused by Cylindrocladium pauciramosum on Acacia retinodes, Arbutus unedo, Feijoa sellowiana and Dodenaea viscosa in Southern Italy. Plant Disease. 2001;85:803. doi: 10.1094/PDIS.2001.85.7.803C. [DOI] [PubMed] [Google Scholar]
- Polizzi G., Crous P.W. Root and collar of milkwort caused by Cylindrocladium pauciramosum, a new record for Europe. European Journal of Plant Pathology. 1999;105:407–411. [Google Scholar]
- Polizzi G., Grasso F.M., Vitale A. First occurrence of Calonectria Leaf Spot on Mexican Blue Palm in Italy. Plant Disease. 2007;91:1057. doi: 10.1094/PDIS-91-8-1052A. [DOI] [PubMed] [Google Scholar]
- Polizzi G., Vitale A. First report of the prevalence of benzimidazole-resistant isolates in a population of Cylindrocladium pauciramosum in Italy. Plant Disease. 2001;85:1210. doi: 10.1094/PDIS.2001.85.11.1210B. [DOI] [PubMed] [Google Scholar]
- Polizzi G., Vitale A., Aiello D. First record of crown and root rot caused by Cylindrocladium pauciramosum on California Lilac in Italy. Plant Disease. 2006;90:1459. doi: 10.1094/PD-90-1459B. [DOI] [PubMed] [Google Scholar]
- Polizzi G., Vitale A., Aiello D. First record of crown and root rot caused by Cylindrocladium pauciramosum on bush cherry in Italy. Plant Disease. 2009;93:547. doi: 10.1094/PDIS-93-5-0547A. [DOI] [PubMed] [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute, Kew, Surrey. British Mycological Society; 1970. A mycological colour chart. [Google Scholar]
- Rodas C.A., Lombard L., Gryzenhout M. Cylindrocladium blight of Eucalyptus grandis in Colombia. Australasian Plant Pathology. 2005;34:134–149. [Google Scholar]
- Ronquist F., Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rowe R.C., Beute M.K. Ascospore formation and discharge by Calonectria crotalariae. Phytopathology. 1975;65:393–398. [Google Scholar]
- Schoch C.L., Crous P.W., Polizzi G. Female fertility and single nucleotide polymorphism comparisons in Cylindrocladium pauciramosum. Plant Disease. 2001;85:941–946. doi: 10.1094/PDIS.2001.85.9.941. [DOI] [PubMed] [Google Scholar]
- Schoch C.L., Crous P.W., Wingfield B.D. The Cylindrocladium candelabrum species complex includes four distinct mating populations. Mycologia. 1999;91:286–298. [Google Scholar]
- Sharma J.K., Mohanan C. Pathogenic variation in Cylindrocladium quinqueseptatum causing leaf blight of Eucalyptus. European Journal of Forest Pathology. 1991;21:210–217. [Google Scholar]
- Sharma J.K., Mohanan C. Relative susceptibility of Eucalyptus provenances to Cylindrocladium leaf blight in Kerala, India. European Journal of Forest Pathology. 1992;22:257–265. [Google Scholar]
- Sharma J.K., Mohanan C., Maria Florence E.J. Nursery diseases of Eucalyptus in Kerala. European Journal of Forest Pathology. 1984;14:77–89. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts, USA: 2003. PAUP*. Phylogenetic analysis using parsimony (*and other methods), v. 4.0b10. Computer programme. [Google Scholar]
- Tamura K., Peterson D., Peterson N. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull J.W. Australian Centre for International Agricultural Research; Canberra, Australia: 2007. Development of sustainable forestry plantations in China: a review. (Impact assessment series Report No. 45). [Google Scholar]
- Vitale A., Aiello D., Castello I. Severe outbreak of crown rot and root rot caused by Cylindrocladium pauciramosum on strawberry tree in Italy. Plant Disease. 2009;93:842. doi: 10.1094/PDIS-93-8-0842B. [DOI] [PubMed] [Google Scholar]
- Vitale A., Crous P.W., Lombard L. Calonectria diseases on ornamental plants in Europe and the Mediterranean basin: an overview. Journal of Plant Pathology. 2013;95:463–476. [Google Scholar]
- Wingfield M.J., Roux J., Slippers B. Established and new technologies reduce increasing pests and pathogen threats to eucalypt plantations. Forest Ecology and Management. 2013;301:35–42. [Google Scholar]
- Wingfield M.J., Slippers B., Wingfield B.D. Novel associations between pathogens, insects and tree species threaten world forests. New Zealand Journal of Forestry Science. 2010;40(Suppl.):S95–S103. [Google Scholar]
- Wright L.P., Davis A.J., Wingfield B.D. Population structure of Cylindrocladium parasiticum infecting peanuts (Arachis hypogaea) in Georgia, USA. European Journal of Plant Pathology. 2010;127:199–206. [Google Scholar]
- Wright L.P., Wingfield B.D., Crous P.W. Isolation and characterization of microsatellite loci in Cylindrocladium parasiticum. Molecular Ecology Notes. 2006;6:110–112. [Google Scholar]
- Wright L.P., Wingfield B.D., Crous P.W. Isolation and characterization of microsatellite loci in Cylindrocladium pauciramosum. Molecular Ecology Notes. 2007;7:343–345. [Google Scholar]
- Xu J.-J., Qin S.-Y., Hao Y.-Y. A new species of Calonectria causing leaf disease of water lily in China. Mycotaxon. 2012;122:177–185. [Google Scholar]
- Zhou X.D., Beer Z.W. De, Xie Y. DNA-based identification of Quambalaria piterka causing severe leaf blight of Corymbia citriodora in China. Fungal Diversity. 2007;25:245–254. [Google Scholar]
- Zhou X.D., Xie Y.J., Chen S.F. Diseases of eucalypt plantation in China: challenges and opportunities. Fungal Diversity. 2008;32:1–7. [Google Scholar]
- Zhou X., Wingfield M.J. Eucalypt diseases and their management in China. Australasian Plant Pathology. 2011;40:339–345. [Google Scholar]