Abstract
The first large-scale survey of sexual and asexual Trichoderma morphs collected from plant and fungal materials conducted in Southern Europe and Macaronesia including a few collections from French islands east of Africa yielded more than 650 specimens identified to the species level. Routine sequencing of tef1 revealed a genetic variation among these isolates that exceeds previous experience and ca. 90 species were recognized, of which 74 are named and 17 species newly described. Aphysiostroma stercorarium is combined in Trichoderma. For the first time a sexual morph is described for T. hamatum. The hitherto most complete phylogenetic tree is presented for the entire genus Trichoderma, based on rpb2 sequences. For the first time also a genus-wide phylogenetic tree based on acl1 sequences is shown. Detailed phylogenetic analyses using tef1 sequences are presented in four separate trees representing major clades of Trichoderma. Discussions involve species composition of clades and ecological and biogeographic considerations including distribution of species.
Key words: acl1, Hypocrea, Hypocreaceae, Hypocreales, New species, Phylogenetic analysis, rpb2, Systematics, tef1
Taxonomic novelties: new species: Trichoderma balearicum Jaklitsch & Voglmayr, T. ceciliae Jaklitsch & Voglmayr, T. christiani Jaklitsch & Voglmayr, T. cremeoides Jaklitsch & Voglmayr, T. europaeum Jaklitsch & Voglmayr, T. euskadiense Jaklitsch & Voglmayr, T. gliocladium Jaklitsch & Voglmayr, T. hausknechtii Jaklitsch & Voglmayr, T. helicolixii Jaklitsch & Voglmayr, T. istrianum Jaklitsch & Voglmayr, T. italicum Jaklitsch & Voglmayr, T. leguminosarum Jaklitsch & Voglmayr, T. mediterraneum Jaklitsch & Voglmayr, T. pararogersonii Jaklitsch & Voglmayr, T. paratroviride Jaklitsch & Voglmayr, T. priscilae Jaklitsch & Voglmayr, T. rubi Jaklitsch & Voglmayr
New combination: Trichoderma stercorarium (Barrasa, A.T. Martínez & G. Moreno) Jaklitsch & Voglmayr
Introduction
Much has been published about applied aspects of the economically important genus Trichoderma, Hypocreaceae, Hypocreales (see Mukherjee et al. 2013 and Schuster & Schmoll 2010 for recent reviews). For the past nearly 200 years the genera Trichoderma and Hypocrea have been treated as separate genera, with many species linked as, respectively, asexual (anamorph) and sexual (teleomorph) morphs of one and the same species. Taxonomy of Trichoderma leaped substantially behind that of Hypocrea, for which several hundred epithets already existed by the end of the 19th century. After some pioneering work in the 20th century (see Jaklitsch 2009 for a historical overview) this changed rapidly, when molecular phylogeny enabled rapid distinction of Trichoderma species. This method provided certainty in the connection and congenericity of the different morphs.
Beginning in 2013, the revised International Code of Nomenclature for algae, fungi and plants (ICN) stipulated that individual species of pleomorphic fungi, such as Trichoderma/Hypocrea, would no longer bear more than one name. Trichoderma is older and has therefore priority over Hypocrea and following a poll by the International Subcommission on Trichoderma and Hypocrea (ISTH), Rossman et al. (2013) proposed this generic name for acceptance by the Nomenclature Committee for Fungi (NCF) and the General Committee (GC) of the International Association for Plant Taxonomy (IAPT). In line with this proposal, Jaklitsch & Voglmayr (2014) combined 46 Hypocrea species, for which some molecular data are available, in Trichoderma.
Within the last 15 years the taxonomy and phylogeny of the genus have experienced tremendous refinement at the species level. This refinement is owed to the development of the phylogenetic markers used for Trichoderma. The initially used markers of the ribosomal cluster, especially the ITS region, soon proved to be of little use for satisfactory resolution and in following years rpb2 and tef1 exon (Chaverri and Samuels, 2004, Overton et al., 2006a, Overton et al., 2006b) or the tef1 intron 5 (Lu et al. 2004) were used exclusively or in combination. Finally, the tef1 intron 4, usually used in combination with intron 5, proved to provide highest resolution for species of the genus and was of particular aid in the distinction of species within the section Trichoderma, addressed below as Viride Clade (Jaklitsch et al., 2006a, Samuels et al., 2006). To fulfil criteria of the genealogical concordance phylogenetic species recognition (GCPSR) concept (Taylor et al. 2000), other, less variable genes were added such as cal1 or chi18-5 (see e.g., Druzhinina et al. 2012).
Trichoderma is a hyperdiverse genus. The latest inventory of nearly 200 named species was presented on an rpb2-based phylogenetic tree (Atanasova et al. 2013). Jaklitsch (2009) ascribed the remarkable genetic variation to the mycoparasitic habit of many species. This observation was confirmed by an extensive study combining multigene phylogenetic analysis of 143 species along with ancestral character reconstructions and diversification analysis (Chaverri & Samuels 2013). Genetic diversification following host shifts proposed by Chaverri & Samuels (2013) was supported by the fact that the genomes of two of the species that they proposed to be mycoparasites include mycoparasitism-specific genes while a third species (T. reesei) that was considered to be saprobic lacked those mycoparasitism genes (Kubicek et al. 2011).
Most earlier studies on the taxonomy, diversity and phylogeny of Trichoderma (e.g. Chaverri and Samuels, 2004, Overton et al., 2006a, Overton et al., 2006b) were based on random collections, particularly on the numerous sexual morph specimens collected by Gary J. Samuels (USDA-ARS, retired). However, designed diversity studies concentrated on soil-inhabiting species in geographically limited areas; e.g. Hoyos-Carvajal et al. (2009) distinguished 29 species among 183 isolates (see also Hoyos-Carvajal & Bissett 2011) and Smith et al. (2013) seven species among 21 isolates in Colombia; Mulaw et al. (2010) reported eight named and eight putatively new species from 134 isolates from the Coffea rhizosphere in Ethiopia; Naeimi et al. (2011) found six species among 201 isolates from rice fields in Iran, and Sun et al. (2012) reported 23 species from the impressive number of 1 910 soil isolates in China. In Europe, Wuczkowski et al. (2003) detected eight species in 46 isolates in a relatively small area in the Donau-Auen National Park near Vienna, Austria, while Migheli et al. (2009) detected 15 species in Sardinia in a voluminous number of 482 isolates, and Zachow et al. (2009) found eight species in 42 isolates from Tenerife, all obtained from soil. Błaszczyk et al. (2011), who expanded the soil studies by including cereal grains, compost and wood, detected 14 species in 170 isolates in Poland. Many of these studies relied on ITS and the identification routines on the ISTH webpage (http://www.isth.info/), implicating that some detected species were species clusters. Soil studies generally indicated that T. harzianum s.l. is the predominant species cluster in that habitat. The largest species diversity study of Trichoderma based on sexual morph specimens collected predominantly from dead wood and bark was carried out by Jaklitsch, 2009, Jaklitsch, 2011, who reported 75 species among 620 Hypocrea specimens in Central and Northern Europe. Southern Europe was not investigated in that work, therefore a separate project was designed for this task, differing from the earlier work in that asexual morphs were collected in addition to sexual morphs. Here we report the diversity of Trichoderma in Southern Europe and Macaronesia including a few collections from two French islands east of Africa. We also present the hitherto most complete phylogenetic tree of Trichoderma based on rpb2 sequences, a genus-wide tree based on acl1 sequences produced in this study with a reduced number of species mostly originating from the lab of the authors plus some additional strains received from the CBS-KNAW Fungal Biodiversity Centre, Utrecht, the Netherlands (CBS), the United States Department of Agriculture in Beltsville (USDA-ARS) and Agriculture and Agri-Food Canada (AAFC).
Materials and methods
The studied region
In the present large-scale assessment of species diversity of Trichoderma we studied six countries of Southern Europe. This region basically comprises the European countries of the Mediterranean Basin and the northern part of Macaronesia, with some exceptions. Although this area is amongst the main biodiversity hotspots of the world (Myers et al. 2000), biodiversity of most fungal lineages, including Trichoderma, is still very insufficiently studied.
According to the Köppen climate classification (Köppen, 1936, Peel et al., 2007, Pidwirny, 2011), the climate of large areas of Southern Europe is Mediterranean, defined as a mid-latitude temperate climate with mild, humid winters and warm to hot, arid summers; it is further subdivided into Interior Mediterranean (Csa) and Coastal Mediterranean climate (Csb). The Csa is characterised by distinctly dry hot summers caused by continental high-pressure influence, whilst Csb also has dry but cooler summers caused by maritime high-pressure influence, the latter therefore being mainly confined to the western Mediterranean. Csa and Csb climates also dominate the western Canary Islands (Tenerife, La Palma and La Gomera) as well as Madeira (AEMET 2012). However, the Csa and Csb classifications apply only to a part of the Mediterranean region, as other temperate, arid, and even snow climate types are present due to the complex geography and orography of the region. Especially in the northern parts of the Mediterranean region large areas are dominated by a mid-latitude temperate climate without dry summers (Cfa and Cfb). These complex climatic patterns as well as geography and orography are key factors for the high species biodiversity observed within the Mediterranean region.
The vegetation in the Mediterranean area is highly diverse and, depending on climate, geology as well as anthropogenic influence, ranges from oak and mixed sclerophyll forests, pine woodlands, over shrublands characterised by dense thickets of evergreen sclerophyll shrubs and small trees called maquis or macchia, coastal scrublands known as garrigue, mixed deciduous forests dominated by oaks, montane beech-fir forests to (agricultural) grasslands. Several zones of Mediterranean vegetation can be distinguished (Reisigl 2001), depending mainly on the occurrence and duration of frost in winter and of extent of heat and drought in summer. In the following, only the zones that have been sampled during the present study are briefly listed. The thermo-mediterranean is the warmest zone without winter frost, characterised by evergreen trees and shrubs like Olea europaea subsp. oleaster, Ceratonia siliqua, Pistacia lentiscus, Phillyrea latifolia and Laurus nobilis; it is confined to coastal areas of Spain, Sardinia, southern Italy and southern Greece. The meso-mediterranean (= eumediterranean) zone is less warm and may occasionally face some short periods of frost in winter. It dominates large areas of the Mediterranean and is characterised by the evergreen oak Quercus ilex and Pinus halepensis; however, to a large extent it has been deforested and converted to agricultural land, degraded by human impact to macchia or garrigues or re-forested, mainly with various pines. The supra-mediterranean zone is dominated by submediterranean deciduous trees like Quercus pubescens and related oaks, Fraxinus ornus and Ostrya carpinifolia, and finally the oro-mediterranean zone, dominated by oak-beech-fir forests, corresponds to the montane zone of Central Europe.
In the current study, collecting focussed on the meso- and supra-mediterranean zones, with a few extensions to the oro-mediterranean zone especially in Italy. In the eastern part of the Mediterranean we confined collection to Croatia, predominantly the meso-mediterranean coastal area of Istria between Vrsar and Pula and some submediterranean parts of the peninsula and the islands Cres and Lošinj, and the Mediterranean Greek islands Corfu (Kerkyra) in the Ionian Sea and Crete. We concentrated collecting in the western Mediterranean, particularly the Italian and Iberian Peninsulae and the Canary Islands. In Italy, the climate of which differs considerably from other countries of the Mediterranean, we examined the meso-mediterranean zone of Sardinia, a part of the north (Lombardia, South Tyrol, Trentino, Veneto) predominantly characterised by Central European climate, a central part (Lazio, Abruzzo) and the southern regions Apulia, Basilicata, Calabria and Campania. In the southern regions we focused collecting in the oro-mediterranean mountainous areas dominated by beech forests as well as the submediterranean vegetation of the lowlands, whereas in the central part we sampled only submediterranean vegetation. The meso-mediterranean zone of the Italian peninsula is fragmentary and only narrowly present at the coast, a few such habitats were briefly examined in the Gargano peninsula. Most of the latter, however, has a submediterranean to montane vegetation, especially in the ancient forest Foresta Umbra. In Spain we collected in the northern regions Asturias and the Basque Country, and extended the range to the north-east, the French Basque Country, the region Midi-Pyrénées and there particularly the departments Aquitaine and Ariège, which are mainly characterised by wet Oceanic (Atlantic; Köppen: Cfb) climate. The second region that we studied in Spain is the south, viz. Andalucía and the Balearic island Mallorca, both of which have a Mediterranean climate; here we investigated areas with meso- and supra-mediterranean vegetation. Finally, we studied a part of Macaronesia, particularly the considerably forested Canary Islands Tenerife and La Palma, with a short visit to La Gomera, all belonging to Spain, and a single excursion in Madeira (Portugal). Although basically comparable to the Mediterranean zone, positions and altitudinal profiles of these volcanic islands show considerable climate variations in the different parts of the islands and thus e.g., in Tenerife up to seven different vegetation zones, generally with a high percentage of endemic plants, are recognised. To the specimens collected in the regions described above, a few specimens were added from the tropical French islands La Réunion and Mayotte east of Africa.
Isolates and specimens
Sexual and asexual morphs were collected on dead plant and fungal material, particularly on dead branches or twigs lying on the ground or fungi growing on them, except where noted. The isolates originated from ascospores or conidia. Some strains were received from the CBS, USDA-ARS or Agriculture and Agri-Food Canada (AAFC). Strain numbers including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms are taken from GenBank and taxonomic papers; the prefix S refers to our own strains from the current work (maintained at the Division of Systematic and Evolutionary Botany, University of Vienna), and Hypo and C.P.K. are specimens and strains from earlier works of W. Jaklitsch and some of other workers (maintained at the University of Technology Vienna). For explanation of some other acronyms, see Jaklitsch et al. (2013). The collector W. Jaklitsch is abbreviated as W.J. and H. Voglmayr as H.V. in specimen data. Representative isolates have been deposited at the CBS. Specimens have been deposited in the Herbarium of the Institute of Botany, University of Vienna (WU).
Table 1.
Strains and NCBI GenBank accessions used in the phylogenetic analyses. GenBank accession numbers starting with KJ were newly generated during this study. Taxonomic novelties in bold print. (T = ex-type).
| Taxon | Clade | Strain | Country | GenBank accessions |
||
|---|---|---|---|---|---|---|
| tef1 | rpb2 | acl1 | ||||
| Trichoderma aeroaquaticum | Viride | NBRC 108031 | Japan | AB646533 | AB646529 | – |
| NBRC 108034 (T) | Thailand | AB646530 | AB646526 | – | ||
| T. aerugineum | Green | Hypo 414 = CBS 120541 (T) | Germany | FJ860608 | FJ860516 | KJ664938 |
| T. aethiopicum | Longibrachiatum | C.P.K. 1837 = CBS 130628 (T) | Ethiopia | – | HM182986 | – |
| PPRC H5 | Ethiopia | EU401616 | – | – | ||
| T. aggressivum | Green/harzianum | G.J.S. 99-29 | USA | – | FJ442770 | – |
| CBS 100526 (T), CBS 100525; CBS 100525 | Ireland; UK: England | AF348096 + AF534614 | AF545541 | – | ||
| T. albocorneum | Green | G.J.S. 97-28 | Japan | AY937440 | – | – |
| T. albofulvum | Viride | GJS 01-265 | Thailand | DQ835494 | DQ835524 | – |
| T. albolutescens | Basal | CBS 119286 = Hypo 235 (T) | Germany | FJ860609 | FJ860517 | KJ664939 |
| S396 = CBS 131489 | Spain | KJ665354 | KJ665240 | KJ664940 | ||
| T. alcalifuscescens | Basal | CBS 122303 = TFC 00-36 (T) | Estonia | FJ860610 | DQ834462 | KJ664941 |
| T. alni | Green/harzianum | Hypo 254 = CBS 120633 (T) | UK: England | EU498312 | EU498349 | KJ664942 |
| Hypo 468 | Austria | – | – | KJ664943 | ||
| S344 | Spain | KJ665355 | – | – | ||
| S365 | France | KJ665356 | – | – | ||
| T. alutaceum | Polysporum | CBS 120535 = Hypo 252 (T) | UK: England | FJ179567 | FJ179600 | KJ664944 |
| CBS 199.73 | Germany | – | – | KJ664945 | ||
| T. amazonicum | Green/harzianum | IB 95 | Peru | HM142377 | HM142368 | – |
| T. americanum | Hypocreanum | G.J.S. 92-93 | USA | – | DQ835455 | – |
| AFTOL-ID 52 | USA | DQ471043 | – | – | ||
| T. andinense | Longibrachiatum | G.J.S. 90-140 = CBS 354.97 = ATCC 208857 (T) | Venezuela | AY956321 | JN175531 | – |
| T. appalachiense | Viride | G.J.S. 00-67 | USA | DQ307502 | – | KC285743 |
| CBS 133558 = G.J.S. 97-243 (T) | USA | DQ307503 | – | KC285744 | ||
| T. arundinaceum | Brevicompactum | CBS 119576 = ATCC 90237 | Namibia | EU338291 | EU338326 | – |
| T. asperelloides | Viride | G.J.S. 04-116 | Vietnam | GU248412 | GU248411 | – |
| T. asperellum | Viride | CBS 433.97 = TR3 (T) | USA | AF456907 + AF401000 | EU248617 | – |
| T. atlanticum | Polysporum | CBS 120632 = Hypo 238 (T) | France | FJ860649 | FJ860546 | KJ664947 |
| Hypo 214 = C.P.K. 1896 | Austria | JQ685864 | – | KJ664946 | ||
| T. atrobrunneum | Green/harzianum | G.J.S. 90-254 | Germany | AF443943 | FJ442735 | – |
| Hypo 4 | Germany | KJ665365 | – | KJ664949 | ||
| Hypo 19 | Austria | KJ665358 | – | – | ||
| Hypo 25 | Austria | KJ665359 | – | – | ||
| Hypo 148 = C.P.K. 1934 | Austria | FJ179573 | FJ179608 | – | ||
| Hypo 182 | Germany | KJ665357 | – | KJ664948 | ||
| Hypo 268 | UK: England | KJ665360 | – | – | ||
| Hypo 272 | UK: England | KJ665361 | – | – | ||
| Hypo 275 | UK: England | KJ665362 | – | – | ||
| Hypo 313 | Austria | KJ665363 | – | – | ||
| Hypo 370 | Austria | KJ665364 | – | – | ||
| Hypo 427 | Austria | KJ665366 | – | – | ||
| Hypo 428 | Denmark | KJ665367 | – | – | ||
| S3 | Italy | KJ665376 | KJ665241 | KJ664950 | ||
| S4 | Italy | KJ665392 | – | – | ||
| S10 | Italy | KJ665368 | – | – | ||
| S53 | Italy | KJ665402 | – | – | ||
| S66 | Italy | KJ665412 | – | – | ||
| S82 | Italy | KJ665414 | – | – | ||
| S147 | Italy | KJ665369 | – | – | ||
| S153 | Italy | KJ665370 | – | – | ||
| S165 | Spain | KJ665371 | – | – | ||
| S180 | Spain | KJ665372 | – | – | ||
| S228 | Spain | KJ665373 | – | – | ||
| S235 | Spain | KJ665374 | – | – | ||
| S266 | Croatia | KJ665375 | – | – | ||
| S300 | Croatia | KJ665377 | – | – | ||
| S317 | Spain | KJ665378 | – | – | ||
| S320 | Spain | KJ665379 | – | – | ||
| S321 | Spain | KJ665380 | – | – | ||
| S338 | Spain | KJ665381 | – | – | ||
| S340 | Spain | KJ665382 | – | – | ||
| S343 | Spain | KJ665383 | – | – | ||
| S369 | France | KJ665384 | – | – | ||
| S371 | France | KJ665385 | – | – | ||
| S386 | Spain | KJ665386 | – | – | ||
| S387 | Spain | KJ665387 | – | – | ||
| S389 | Spain | KJ665388 | – | – | ||
| S392 | Spain | KJ665389 | – | – | ||
| S394 | Spain | KJ665390 | – | – | ||
| S397 | Spain | KJ665391 | – | – | ||
| S414 | Spain | KJ665393 | – | – | ||
| S420 | Spain | KJ665394 | – | – | ||
| S443 | Spain | KJ665395 | – | – | ||
| S447 | Spain | KJ665396 | – | KJ664951 | ||
| S485 | Spain | KJ665397 | – | – | ||
| S502 | Spain | KJ665398 | – | – | ||
| S507 | Spain | KJ665399 | – | – | ||
| S516 | Spain | KJ665400 | – | – | ||
| S525 | Spain | KJ665401 | – | – | ||
| S534 | Spain | KJ665403 | – | – | ||
| S556 | Greece | KJ665404 | – | – | ||
| S561 | France | KJ665405 | – | – | ||
| S576 | Italy | KJ665406 | – | – | ||
| S578 | Italy | KJ665407 | – | – | ||
| S583 | Greece | KJ665408 | – | – | ||
| S618 | Greece | KJ665409 | – | – | ||
| S625 | Greece | KJ665410 | – | – | ||
| S638 | Greece | KJ665411 | – | – | ||
| S662 | Spain | KJ665413 | – | – | ||
| T. atroviride | Viride | CBS 119499 = Hypo 326 | Austria | FJ860611 | FJ860518 | KJ664952 |
| S127 | Italy | KJ665415 | – | – | ||
| S141 | Italy | KJ665416 | – | – | ||
| S264 | Croatia | KJ665417 | – | – | ||
| S356 | France | KJ665418 | – | – | ||
| S360 | France | KJ665419 | – | KJ664953 | ||
| S363 | France | KJ665420 | – | – | ||
| S367 | France | KJ665421 | – | – | ||
| S383 | Spain | KJ665422 | – | – | ||
| S384 | Spain | KJ665423 | – | – | ||
| S504 | Spain | KJ665424 | – | – | ||
| S508 | Spain | KJ665425 | – | – | ||
| S545 | Spain | KJ665426 | – | – | ||
| S646a | Italy | KJ665427 | – | – | ||
| T. auranteffusum | Brevicompactum | Hypo 5 | Austria | – | – | KJ664955 |
| CBS 119284 = Hypo 145 (T) | Austria | FJ860613 | FJ860520 | – | ||
| Hypo 432 | Austria | – | – | KJ664954 | ||
| S48 | Italy | KJ665429 | – | – | ||
| S283 | Croatia | KJ665428 | – | KJ664956 | ||
| S565 | Italy | KJ665430 | – | – | ||
| T. aureoviride | Green | Hypo 260 = CBS 120536 | UK: England | – | FJ179602 | KJ664957 |
| Hypo 473 = C.P.K. 2848 | Netherlands | FJ860615 | JQ685882 | – | ||
| Hypo 474 = C.P.K. 2849 | Netherlands | – | – | KJ664958 | ||
| S21 | Italy | KJ665431 | – | – | ||
| S550 | Spain | KJ665432 | – | – | ||
| T. austriacum | Hypocreanum | CBS 122770 = Hypo 508 | Austria | – | – | KJ664959 |
| CBS 122494 = Hypo 580 (T) | Austria | FJ860619 | FJ860525 | KJ664960 | ||
| T. austrokoningii | Viride | CBS 247.63 | New Zealand | DQ307568 | FJ442772 | – |
| G.J.S. 99-146 = CBS 119092 (T) | Australia | DQ307561 | – | – | ||
| Hypo 498 = C.P.K. 2865 | Ukraine | KJ665433 | – | – | ||
| T. avellaneum | Basal | CTR 77-155 | USA | AY225857 | AF545562 | – |
| T. balearicum | Psychrophilum | S402 = CBS 133222 (T) | Spain | KJ665434 | KJ665242 | KJ664961 |
| T. barbatum | Stromaticum | G.J.S. 04-308 = CBS 125733 (T) | USA | HQ342223 | HQ342286 | – |
| T. bavaricum | Polysporum | Hypo 319 = C.P.K. 2021 | Germany | FJ860620 | FJ860526 | – |
| CBS 120538 = Hypo 342 (T) | Germany | FJ860621 | – | KJ664962 | ||
| S49 = CBS 136988 | Italy | KJ665435 | – | KJ664963 | ||
| T. brevicompactum | Brevicompactum | CBS 112443 = IBT 40867 | Papua New Guinea | – | EU338319 | – |
| G.J.S. 05-178 | Iran | EU338293 | – | – | ||
| T. britannicum | Green | SB1 = CBS 253.62 (T) | UK: England | KF134796 | KF134787 | – |
| SB | Germany | KF134795 | – | – | ||
| T. britdaniae | aff. Longibrachiatum | WU 31610 = Hypo 637 | Denmark | JQ685866 | JQ685880 | KJ664967 |
| K 89878 = Hypo 646 (T) | UK: England | JQ685865 | JQ685881 | KJ664968 | ||
| T. brunneoviride | Green/harzianum | Hypo 170 = CBS 121130 (T) | Germany | EU498316 | – | KJ664969 |
| Hypo 363 = C.P.K. 2425 | Austria | – | – | KJ664970 | ||
| Hypo 442 = CBS 120928 | Austria | EU498318 | EU498358 | – | ||
| T. caerulescens | Viride | S195 = CBS 130011 (T) | Spain | JN715621 | JN715604 | KC285710 |
| S206 = CBS 130012 | Portugal | JN715624 | JN715605 | KC285711 | ||
| S232 | Spain | JN715631 | JN715606 | KC285712 | ||
| S252 | Croatia | – | – | KJ664971 | ||
| T. caesareum | Stromaticum | G.J.S. 01-225 = CBS 124369 (T) | Thailand | HQ342216 | HQ342279 | – |
| T. calamagrostidis | Psychrophilum | CBS 121133 = Hypo 401 (T) | Denmark | FJ860622 | FJ860528 | KJ664972 |
| T. capillare | Longibrachiatum | G.J.S. 06-66 | Vietnam | JN175585 | JN175530 | – |
| C.P.K. 885 = MA3642 = G.J.S. 10-169 | Austria | JN182277 | – | – | ||
| T. caribbaeum | Viride | CBS 119093 = G.J.S. 97-3 (T) | Guadeloupe | KJ665443 | KJ665246 | KJ664973 |
| G.J.S. 98-43 | Puerto Rico | – | FJ442723 | – | ||
| Dis 320c | Ecuador | DQ289010 | – | – | ||
| T. catoptron | Green/harzianum | G.J.S. 02-76 = CBS 114232 (T) | Sri Lanka | AY737726 + AY391963 | AY391900 | – |
| T. ceciliae | Lone lineage | S164 = CBS 130010 (T) | Italy | KJ665444 | KJ665247 | KJ664974 |
| T. ceraceum | Green/harzianum | G.J.S. 95-159, G.J.S. 88-28 | USA | AY937437 + AY391964 | – | – |
| G.J.S. 95-159 (T) | USA | – | AF545508 | – | ||
| T. ceramicum | Green | CBS 114576 | USA | FJ860628 | FJ860531 | – |
| S353 | France | KJ665445 | KJ665248 | KJ664975 | ||
| S366 | France | KJ665446 | KJ665249 | KJ664976 | ||
| S370 | France | KJ665447 | – | – | ||
| S373 | France | KJ665448 | – | – | ||
| T. cerinum | Green/harzianum | DAOM 230012 (T) | Nepal | AY605802 + AY937443 | – | – |
| CBS 136992 = S357 | France | KF134797 | KF134788 | KJ664977 | ||
| T. chlorosporum | Green | G.J.S. 88-33 (T) | USA | – | AY391903 | – |
| G.J.S. 98-1 | Costa Rica | AY737737 + AY391968 | AY391906 | – | ||
| T. christiani | Green/harzianum | S43 | Italy | KJ665438 | KJ665243 | KJ664964 |
| S93 | Italy | KJ665442 | KJ665245 | KJ664966 | ||
| S179 | Spain | KJ665436 | – | – | ||
| S189 | Spain | KJ665437 | – | – | ||
| CBS 132572 = S442 (T) | Spain | KJ665439 | KJ665244 | KJ664965 | ||
| S474 | Spain | KJ665440 | – | – | ||
| S639 | Greece | KJ665441 | – | – | ||
| T. chromospermum | Green | G.J.S. 94-67 | USA | AY737728 + AY391973 | – | – |
| G.J.S. 94-68 (T) | USA | – | AY391913 | – | ||
| T. cinnamomeum | Green/harzianum | G.J.S. 97-230 = CBS 114235 (T) | USA | – | AY391918 | – |
| G.J.S. 97-237 | USA | AY737732 + AY391979 | – | – | ||
| T. citrinoviride | Longibrachiatum | G.J.S. 92-8 = CBS 636.92 = IMI 352472 | France | – | JN175544 | – |
| CBS 121275 = Hypo 162 | Germany | – | FJ860586 | KJ664978 | ||
| Hypo 247 = C.P.K. 2004 | UK: England | – | – | KJ664979 | ||
| Hypo 290 = C.P.K. 2005 | Austria | FJ860694 | – | – | ||
| S20 | Italy | KJ665449 | KJ665250 | KJ664980 | ||
| S27 | Italy | KJ665450 | KJ665251 | KJ664981 | ||
| S379 | Spain | KJ665451 | – | – | ||
| S430 | Spain | KJ665452 | – | – | ||
| S567 | Italy | KJ665453 | – | – | ||
| S659 | Spain | KJ665454 | – | – | ||
| T. citrinum | Hypocreanum | CBS 894.85 (T) | Belgium | – | AF545561 | – |
| CBS 121278 = Hypo 50 | Austria | – | – | KJ664982 | ||
| Hypo 54 = C.P.K. 960 | Austria | FJ860631 | FJ179603 | – | ||
| Hypo 55 = C.P.K. 961 | Czech Republic | – | – | KJ664983 | ||
| T. compactum | Green/harzianum | CBS 121218 (T) | China | KF134798 | KF134789 | KJ664984 |
| T. composticola | Viride | CBS 439.95 | Northern Ireland | AY937413 | – | KC285720 |
| CBS 133497 = S590 (T) | Greece | KC285631 | KC285754 | KC285721 | ||
| T. corneum | Green/harzianum | G.J.S. 97-82 | Thailand | KJ665455 | KJ665252 | KJ664985 |
| T. costaricense | Green | P.C. 21 (T) | Costa Rica | AY737741 + AY391980 | AY391921 | – |
| T. crassum | Green | DAOM 164916 = CBS 336.93 = C.P.K. 63 (T ana) | Canada | EU280048 + AF534615 | AF545542 | KJ664986 |
| G.J.S. 01-227 = CBS 114230 (T teleo) | Thailand | – | AY481587 | – | ||
| G.J.S. 95-157 | USA | – | AF545543 | – | ||
| T. cremeoides | Green | S98 | Italy | KJ665463 | – | KJ664989 |
| S112 (T) | Italy | KJ665456 | KF134790 | KJ664987 | ||
| S113 | Italy | KJ665457 | – | – | ||
| S117 | Italy | KJ665458 | – | – | ||
| S191 | Spain | KJ665459 | – | KJ664988 | ||
| S192 | Spain | KJ665460 | KJ665254 | – | ||
| S207 | Portugal | KJ665461 | – | – | ||
| S431 | Spain | KJ665462 | – | – | ||
| T. cremeum | Green | G.J.S. 91-125 = CBS 111146 (T) | USA | AY737736 + AF534598 | AF545511 | – |
| T. croceum | Polysporum | DAOM 167068 (T) | Canada | AY750879 | – | – |
| T. crystalligenum | Psychrophilum | Hypo 32 = C.P.K. 943 | Austria | – | – | KJ664990 |
| Hypo 167 = C.P.K. 1911 | Germany | DQ345344 | DQ345348 | – | ||
| S38 | Italy | – | – | KJ664992 | ||
| S286 | Croatia | KJ665464 | – | KJ664991 | ||
| T. cuneisporum | Green | G.J.S. 91-93 = CBS 111148 (T) | USA | AY737727 + AF534600 | AF545512 | – |
| T. dacrymycellum | Green/harzianum | Hypo 233 = WU 29044 | Germany | FJ860633 | FJ860533 | KJ664993 |
| T. danicum | Green | Hypo 402 = CBS 121273 (T) | Denmark | FJ860634 | FJ860534 | – |
| S553 | Spain | KJ665465 | KJ665255 | – | ||
| T. decipiens | Hypocreanum | G.J.S. 91-101 | USA | – | DQ835520 | – |
| CBS 121307 = G.J.S. 97-207 (T) | France | FJ860635 | – | – | ||
| S372 | France | KJ665466 | KJ665256 | KJ664994 | ||
| T. delicatulum | Basal | CBS 120631 = Hypo 47 (T) | Austria | FJ860636 | FJ860535 | KJ664995 |
| T. deliquescens | Deliquescens | CBS 121131 = Hypo 267 (T) | UK: England | – | FJ179609 | KJ664996 |
| CBS 121132 = Hypo 391 | Germany | FJ860644 | – | KJ664997 | ||
| T. dingleyae | Viride | G.J.S. 99-105 | New Zealand | DQ289008 | EU341803 | – |
| CBS 119056 = G.J.S. 02-50 (T) | New Zealand | KJ665467 | KJ665257 | KJ664998 | ||
| T. dorotheae | Viride | G.J.S. 99-202 (T) | New Zealand | DQ307536 | EU248602 | – |
| G.J.S. 99-97 | New Zealand | DQ288990 | – | – | ||
| S231 | Spain | KJ665468 | KJ665258 | KJ664999 | ||
| S444 | Spain | KJ665469 | KJ665259 | – | ||
| S480 | Spain | KJ665470 | – | – | ||
| S482 | Spain | KJ665471 | – | – | ||
| S549 | Spain | KJ665472 | – | – | ||
| T. effusum | Longibrachiatum | C.P.K. 254 = DAOM 230007 (T) | India | KJ665473 | KJ665260 | KJ665000 |
| T. eijii | Viride | TUFC 100002 = CBS 133190 (T) | Japan | JX684011 | JX238484 | – |
| T. epimyces | Green/harzianum | Hypo 175 = C.P.K. 1980 | Germany | – | – | KJ665001 |
| Hypo 194 = CBS 120534 (T) | Austria | EU498320 | EU498360 | KJ665002 | ||
| Hypo 460 = C.P.K. 2487 | Austria | – | – | KJ665003 | ||
| T. erinaceus | Viride | C.P.K. 427 = DAOM 230019 | Thailand | – | – | KJ665004 |
| DIS 7 | Peru | DQ109547 | EU248604 | – | ||
| T. estonicum | Green | Hypo 456 = C.P.K. 2484 | Sweden | – | – | KJ665005 |
| Hypo 501 = CBS 121556 | Sweden | FJ860637 | FJ860536 | – | ||
| Hypo 548 = C.P.K. 3149 | UK: England | – | – | KJ665006 | ||
| T. eucorticioides | Hypocreanum | G.J.S. 99-61 | Costa Rica | DQ835502 + DQ835474 | DQ835518 | – |
| T. europaeum | Polysporum | CBS 901.72 | Germany | – | AY481588 | – |
| CBS 121276 =C.P.K. 1609 = Hypo 117 (T) | Austria | FJ179574 | FJ179610 | KJ665008 | ||
| Hypo 14 | Austria | – | – | KJ665009 | ||
| Hypo 42 | Austria | – | KJ665263 | KJ665012 | ||
| Hypo 64 | Czech Republic | KJ665476 | KJ665264 | KJ665013 | ||
| Hypo 102 | Austria | – | – | KJ665007 | ||
| Hypo 183 | Germany | KJ665474 | KJ665261 | KJ665010 | ||
| Hypo 300 | Austria | KJ665475 | KJ665262 | KJ665011 | ||
| S37 | Italy | KJ665484 | KJ665266 | KJ665016 | ||
| S50 | Italy | KJ665486 | – | – | ||
| S58 | Italy | KJ665488 | – | – | ||
| S60 | Italy | – | KJ665267 | KJ665017 | ||
| S64 | Italy | KJ665490 | – | – | ||
| S99 | Italy | KJ665491 | – | – | ||
| S100 | Italy | KJ665477 | – | – | ||
| S114 | Italy | KJ665478 | – | – | ||
| S125 | Italy | KJ665479 | – | – | ||
| S133 | Italy | KJ665480 | – | – | ||
| S134 | Italy | KJ665481 | KJ665265 | KJ665014 | ||
| S331 | Spain | KJ665482 | – | – | ||
| S332 | Spain | KJ665483 | – | KJ665015 | ||
| S381 | Spain | KJ665485 | – | – | ||
| S569 | Italy | KJ665487 | – | – | ||
| S611 | Greece | KJ665489 | KJ665268 | KJ665018 | ||
| T. euskadiense | Longibrachiatum | S377 = CBS 130013 (T) | Spain | KJ665492 | KJ665269 | KJ665019 |
| T. evansii | Viride | DIS341hi = CBS 123079 (T) | Ecuador | EU883566 | EU883558 | – |
| T. fertile | Semiorbis | DAOM 167070 | Canada | AY605801 + AF534617 | AF545545 | – |
| DAOM 167161 (T) | Canada | – | AF545546 | – | ||
| T. cf. fertile | Semiorbis | S606 = CBS 137003 | Greece | KJ665493 | – | KJ665020 |
| T. flagellatum | Longibrachiatum | C.P.K. 3525 = G.J.S. 10-164 = CBS 130626 = PPRC–ET58 (T) | Ethiopia | FJ763184 | – | – |
| G.J.S. 10-156 = C.P.K. 3334 = PPRC–ET7 | Ethiopia | FJ763149 | JN258688 | – | ||
| T. flaviconidium | Viride | G.J.S. 99-49 | Costa Rica | DQ020001 | EU883557 | – |
| C.P.K. 455 | Costa Rica | AY665711 | – | – | ||
| T. flavipes (= H. cinereoflava) | aff. Longibrachiatum | G.J.S. 92-102 (T) | USA | DQ834454 | DQ834461 | – |
| T. floccosum | Stromaticum | G.J.S. 01-238 = CBS 124372 (T) | Thailand | HQ342218 | HQ342281 | – |
| T. foliicola | Polysporum | Hypo 645 = CBS 130008 (T) | Germany | JQ685862 | JQ685876 | KJ665021 |
| Hypo 650 = CBS 131939 | Denmark | JQ685863 | JQ685877 | KJ665022 | ||
| T. fomiticola | Semiorbis | CBS 121136 = Hypo 439 (T) | Austria | FJ860639 | FJ860538 | KJ665023 |
| Hypo 530 = C.P.K. 3137 | Austria | – | – | KJ665024 | ||
| T. gamsii | Viride | G.J.S. 05-111 = CBS 120072 | Italy | DQ841722 | – | – |
| G.J.S. 04-09 | USA | DQ307541 | JN133561 | – | ||
| S488 | Spain | JN715613 | KJ665270 | KJ665025 | ||
| S496 | Spain | KJ665494 | – | – | ||
| S582 | Italy | KJ665495 | – | – | ||
| S595 | Greece | KJ665496 | – | – | ||
| S643 | Italy | KJ665497 | – | – | ||
| T. gelatinosum | Green | Hypo 139 = C.P.K. 1618 | Austria | FJ179569 | FJ179604 | – |
| Hypo 154 = C.P.K. 1919 | Austria | – | – | KJ665026 | ||
| S35 | Italy | – | – | KJ665028 | ||
| S51 | Italy | KJ665500 | – | – | ||
| S162 | Italy | KJ665498 | – | KJ665027 | ||
| S456 | Spain | KJ665499 | – | – | ||
| S663 | Spain | KJ665501 | – | – | ||
| T. ghanense | Longibrachiatum | G.J.S. 95-137 = IAM 13109 (T) | Ghana | AY937423 | JN175559 | – |
| DAOM 165776 | USA | JN175610 | JN175560 | – | ||
| T. gillesii | Longibrachiatum | CBS 130435 = G.J.S. 00-72 (T) | France | JN175583 | JN175527 | – |
| T. gliocladium | Green | S81 = CBS 130009 (T) | Italy | KJ665502 | KJ665271 | KJ665029 |
| S83 | Italy | KJ665503 | KJ665272 | – | ||
| S89a | Italy | KJ665504 | – | – | ||
| T. gracile | Longibrachiatum | CBS 130714 = G.J.S. 10-263 (T) | Malaysia | JN175598 | JN175547 | – |
| T. guizhouense | Green/harzianum | HGUP0039 | China | JX089585 | – | – |
| S278 | Croatia | KF134799 | KF134791 | KJ665030 | ||
| S279 | Croatia | KJ665505 | – | – | ||
| S393 | Spain | KJ665506 | – | – | ||
| S548 | Spain | KJ665507 | – | KJ665031 | ||
| S579 | Italy | KJ665508 | – | – | ||
| S581 | Italy | KJ665509 | – | – | ||
| S597 | Greece | KJ665510 | – | – | ||
| S628 | Greece | KJ665511 | KJ665273 | KJ665032 | ||
| S642 | Italy | KJ665512 | – | – | ||
| T. hamatum | Viride | DAOM 167057 (T) | Canada | EU279965 + AF534620 | AF545548 | – |
| Hypo 647 | France | KJ665513 | KJ665274 | KJ665033 | ||
| Hypo 648 = CBS 132565 | France | KJ665514 | KJ665275 | – | ||
| T. harzianum | Green/harzianum | CBS 226.95 (T neo) | UK: England | AF348101 + AF534621 | AF545549 | – |
| T. hausknechtii | Green/harzianum | Hypo 649 = CBS 133493 (T) | France | KJ665515 | KJ665276 | KJ665034 |
| T. helicolixii | Green/harzianum | S640 = CBS 133499 (T) | Greece | KJ665517 | KJ665278 | KJ665036 |
| S515 = CBS 135583 | Spain | KJ665516 | KJ665277 | KJ665035 | ||
| T. helicum | Helicum | DAOM 230016 | Malaysia | EU280055 | – | – |
| DAOM 230021 | Thailand | – | DQ087239 | – | ||
| DAOM 230022 = C.P.K. 431 (T) | Thailand | – | – | KJ665037 | ||
| T. aff. helicum | Helicum | S446 | Spain | KJ665518 | – | KJ665038 |
| T. hispanicum | Viride | S172 = CBS 130538 | Spain | JN715655 | – | KJ665039 |
| S453 = CBS 130540 (T) | Spain | JN715659 | JN715600 | KJ665040 | ||
| T. hunua | Semiorbis | CBS 238.63 | New Zealand | KJ665519 | KJ665279 | KJ665041 |
| T. inhamatum | Green/harzianum | CBS 273.78 (T) | Colombia | AF348099 | FJ442725 | – |
| T. intricatum | Viride | G.J.S. 97-88 (T) | Thailand | AY376060 | AY376060 | – |
| T. istrianum | Viride | S120 | Italy | KJ665520 | – | KJ665042 |
| S123 | Italy | KJ665521 | KJ665280 | KJ665043 | ||
| S272 | Croatia | KJ665522 | – | – | ||
| S310 = CBS 130539 (T) | Croatia | KJ665523 | KJ665281 | KJ665044 | ||
| T. italicum | Green/harzianum | S15 | Italy | KJ665526 | KJ665283 | KJ665046 |
| S128 | Italy | KJ665524 | – | – | ||
| S131 = CBS 132567 (T) | Italy | KJ665525 | KJ665282 | KJ665045 | ||
| T. ivoriense | Stromaticum | G.J.S. 01-312 = CBS 125734 (T) | Ivory Coast | HQ342217 | HQ342280 | – |
| T. junci | Viride | CBS 120926 = Hypo 399 (T) | Denmark | FJ860641 | FJ860540 | KJ665047 |
| T. konilangbra | Longibrachiatum | CBS 100808 = G.J.S. 96-145 = ATCC 208860 = IMI 378807 = C.P.K. 132 (T) | Uganda | JN258681 | KJ665284 | KJ665048 |
| G.J.S. 96-147 | Uganda | AY937425 | – | – | ||
| T. koningii | Viride | Hypo 51 = CBS 119500 = C.P.K. 957 | Austria | KC285594 | FJ860541 | KC285713 |
| Hypo 242 | France | – | – | KJ665049 | ||
| Hypo 315 = C.P.K. 3564 | Germany | FJ860642 | – | – | ||
| S22 | Italy | KC285595 | KC285749 | KC285714 | ||
| S28 | Italy | KJ665532 | – | – | ||
| S79 | Italy | KJ665545 | – | – | ||
| S204 | Portugal | KJ665527 | – | – | ||
| S227 | Spain | KC285596 | JN715609 | – | ||
| S260 | Croatia | KJ665528 | – | – | ||
| S267 | Croatia | KJ665529 | – | – | ||
| S268 | Croatia | KJ665530 | – | – | ||
| S273 | Croatia | KJ665531 | – | – | ||
| S288 | Croatia | KJ665533 | – | – | ||
| S291 | Croatia | KJ665534 | – | – | ||
| S298 | Croatia | KJ665535 | – | – | ||
| S346 | Spain | KJ665536 | – | – | ||
| S358 | France | KJ665537 | – | – | ||
| S376 | Spain | KJ665538 | – | – | ||
| S380 | Spain | KJ665539 | – | – | ||
| S415 | Spain | KJ665540 | – | – | ||
| S511 | Spain | KJ665541 | – | – | ||
| S512 | Spain | KJ665542 | – | – | ||
| S528 | Spain | KJ665543 | – | – | ||
| S566 | Italy | KJ665544 | – | – | ||
| T. koningiopsis | Viride | G.J.S. 93-20 (T) | Cuba | DQ284966 | EU241506 | – |
| G.J.S. 06-263 | Ecuador | FJ467647 | – | – | ||
| S359 | France | KJ665546 | KJ665285 | KJ665050 | ||
| T. lacuwombatense | Polysporum | CBS 112266 = G.J.S. 99-198 (T) | New Zealand | KJ665547 | KJ665286 | KJ665051 |
| T. lanuginosum | Stromaticum | G.J.S. 01-176 = CBS 125718 (T) | Cameroon | HQ342221 | HQ342284 | – |
| T. leguminosarum | aff. Longibrachiatum | S391 | Spain | KJ665548 | KJ665287 | KJ665052 |
| S399 | Spain | KJ665549 | – | – | ||
| S487 | Spain | KJ665550 | – | – | ||
| S494 = CBS 130014 (T) | Spain | KJ665551 | KJ665288 | KJ665053 | ||
| S503 | Spain | KJ665552 | KJ665289 | KJ665054 | ||
| S518 | Spain | KJ665553 | – | – | ||
| S536 | Spain | KJ665554 | – | – | ||
| S559 | France | KJ665555 | – | – | ||
| T. leucopus | Polysporum | CBS 122499 = Hypo 574 (T) | Finland | FJ179571 | FJ179605 | KJ665055 |
| CBS 122495 = Hypo 578 | Finland | – | – | KJ665056 | ||
| T. lieckfeldtiae | Viride | G.J.S. 00-14 = CBS 123049 (T) | Colombia | EU856326 | EU883562 | – |
| T. lixii | Green/harzianum | G.J.S. 97-96 = CBS 110080 = C.P.K. 2784 (T epi) | Thailand | FJ716622 | KJ665290 | – |
| T. longibrachiatum | Longibrachiatum | CBS 816.68 = ATCC 18648 (T) | USA | EU401591 | DQ087242 | – |
| G.J.S. 04-31 = CBS 118640 = ATCC MYA–3642 | Mexico | – | JN175509 | – | ||
| S328 | Spain | JQ685867 | JQ685883 | KJ665057 | ||
| T. longipile | Green | DAOM 177227 (T) | Canada | EU280051 | AF545550 | – |
| Hypo 80 = CBS 120953 | Sweden | FJ860643 | FJ860542 | KJ665058 | ||
| S40 | Italy | KJ665556 | KJ665292 | KJ665059 | ||
| S514 | Spain | KJ665557 | – | – | ||
| S658 | Spain | KJ665558 | – | – | ||
| T. luteffusum | Polysporum | CBS 120537 = Hypo 279 | Germany | FJ860645 | FJ860543 | KJ665060 |
| T. luteocrystallinum | Deliquescens | CBS 123828 = Hypo 598 (T) | Germany | FJ860646 | FJ860544 | KJ665061 |
| Hypo 636 | Denmark | – | – | KJ665062 | ||
| T. lycogaloides | Green | CBS 123493 = SL | French Guiana | KF134800 | KF134792 | – |
| T. margaretense | Brevicompactum | CBS 120540 = Hypo 361 (T) | Austria | – | – | KJ665063 |
| Hypo 513 = C.P.K. 3127 | Austria | FJ860625 | FJ860529 | – | ||
| Hypo 518 = C.P.K. 3129 | Austria | – | – | KJ665064 | ||
| S106 | Italy | KJ665559 | – | – | ||
| S368 | France | KJ665560 | – | – | ||
| S544 | Spain | KJ665561 | – | – | ||
| T. martiale | Viride | G.J.S. 04-40 = CBS 123052 (T) | Brazil | EU248618 | EU248597 | – |
| T. matsushimae | Viride | IMI266915 cloneA1 | Scotland | AB646534 | – | – |
| T. medusae | Stromaticum | G.J.S. 01-171 = CBS 125719 (T) | Cameroon | HQ342214 | HQ342277 | – |
| T. megalocitrinum | Psychrophilum | BEO 00-09 | USA | AY225855 | AF545563 | – |
| T. melanomagnum | Deliquescens | G.J.S. 99-153 = CBS 114236 (T) | Australia | AY737751 + AY391985 | AY391926 | – |
| T. microcitrinum | Hypocreanum | G.J.S. 91-61 | USA | DQ835478 | DQ835460 | – |
| T. mienum | Semiorbis | TUFC 61533 = CBS 132690 (T) | Japan | JQ621978 | JQ621968 | – |
| T. mediterraneum | Polysporum | S6 | Italy | KJ665608 | – | – |
| S12 | Italy | KJ665562 | KJ665293 | KJ665065 | ||
| S13 | Italy | KJ665563 | KJ665294 | KJ665066 | ||
| S25 | Italy | KJ665575 | – | – | ||
| S26 | Italy | KJ665576 | – | – | ||
| S29 | Italy | KJ665578 | KJ665298 | KJ665073 | ||
| S30 | Italy | KJ665580 | KJ665300 | KJ665075 | ||
| S171 | Spain | KJ665564 | – | KJ665067 | ||
| S174 | Spain | KJ665565 | – | – | ||
| S175 | Spain | KJ665566 | – | – | ||
| S184 | Spain | KJ665567 | KJ665295 | KJ665068 | ||
| S190 | Spain | KJ665568 | KJ665296 | KJ665069 | ||
| S213 | Spain | KJ665569 | – | KJ665070 | ||
| S239 | Spain | KJ665570 | – | – | ||
| S240 | Spain | KJ665571 | KJ665297 | KJ665071 | ||
| S241 | Spain | KJ665572 | – | – | ||
| S242 | Spain | KJ665573 | – | KJ665072 | ||
| S247 | Spain | KJ665574 | – | – | ||
| S287 | Croatia | KJ665577 | – | – | ||
| S292 | Croatia | KJ665579 | KJ665299 | KJ665074 | ||
| S312 | Croatia | KJ665581 | – | KJ665076 | ||
| S403 | Spain | KJ665583 | – | – | ||
| S408 | Spain | KJ665584 | – | – | ||
| S409 | Spain | KJ665585 | – | – | ||
| S410 | Spain | KJ665586 | KJ665302 | KJ665078 | ||
| S413 | Spain | KJ665588 | – | – | ||
| S425 | Spain | KJ665589 | – | – | ||
| S440 | Spain | KJ665590 | – | – | ||
| S461 | Spain | KJ665594 | – | – | ||
| S463 | Spain | KJ665595 | – | – | ||
| S469 | Spain | KJ665596 | – | – | ||
| S470 | Spain | KJ665597 | – | – | ||
| S473 | Spain | KJ665598 | – | – | ||
| S481 | Spain | KJ665599 | KJ665306 | KJ665082 | ||
| S495 | Spain | KJ665600 | KJ665307 | KJ665083 | ||
| S522 | Spain | KJ665601 | KJ665308 | KJ665084 | ||
| S523 | Spain | KJ665602 | KJ665309 | – | ||
| S524 | Spain | KJ665603 | – | – | ||
| S526 | Spain | KJ665604 | KJ665310 | KJ665085 | ||
| S541 | Spain | KJ665605 | – | – | ||
| S554 | Spain | KJ665606 | – | – | ||
| S594 | Greece | KJ665607 | KJ665311 | KJ665086 | ||
| S600 | Greece | KJ665609 | KJ665312 | KJ665087 | ||
| S621 | Greece | KJ665610 | KJ665313 | KJ665088 | ||
| S665 | Spain | KJ665611 | – | – | ||
| T. mediterraneum 1 | Polysporum | S347 | Spain | KJ665582 | KJ665301 | KJ665077 |
| S411 | Spain | KJ665587 | KJ665303 | KJ665079 | ||
| T. mediterraneum 2 | Polysporum | S451 | Spain | KJ665591 | KJ665304 | KJ665080 |
| S454 | Spain | KJ665592 | KJ665305 | KJ665081 | ||
| S455 | Spain | KJ665593 | – | – | ||
| T. minutisporum | Polysporum | DAOM 167069 = CBS 341.93 (T) | Canada | KJ665612 | KJ665314 | KJ665089 |
| CBS 112255 = G.J.S. 90-82 | USA | KJ665618 | KJ665316 | KJ665095 | ||
| CBS 124756 = G.J.S. 04-163 | USA | KJ665615 | – | KJ665092 | ||
| DAOM 178046 | Canada | KJ665613 | – | KJ665090 | ||
| DAOM 179894 | Canada | KJ665614 | – | KJ665091 | ||
| DAOM 216516 = G.J.S. 91-94 | USA | KJ665619 | KJ665317 | KJ665096 | ||
| CBS 112253 = G.J.S. 90-115 | USA | KJ665617 | KJ665315 | KJ665094 | ||
| CBS 112254 = G.J.S. 90-112 | USA | KJ665616 | – | KJ665093 | ||
| T. moravicum | Semiorbis | Hypo 46 = C.P.K. 954 | Austria | – | – | KJ665098 |
| Hypo 334 = C.P.K. 2411 | Austria | FJ860650 | – | KJ665097 | ||
| Hypo 462 = C.P.K. 2489 | Austria | – | FJ860549 | – | ||
| T. neokoningii | Viride | G.J.S. 04-216 = CBS 120070 (T) | Peru | KJ665620 | KJ665318 | KJ665099 |
| T. neorufoides | Viride | Hypo 112 = CBS 119506 (T) | Austria | FJ860657 | – | KJ665100 |
| Hypo 261 = C.P.K. 1900 | UK: England | – | FJ860553 | – | ||
| Hypo 265 = C.P.K. 2357 | UK: England | – | – | KJ665101 | ||
| S59 | Italy | – | KJ665319 | KJ665102 | ||
| S63 | Italy | KJ665623 | – | – | ||
| S306 | Croatia | KJ665621 | – | – | ||
| S568 | Italy | KJ665622 | – | – | ||
| T. neorufum | Viride | CBS 119498 = Hypo 41 | Austria | FJ860653 | FJ860550 | KJ665104 |
| Hypo 210 = C.P.K. 2016 | Austria | – | – | KJ665103 | ||
| T. neosinense | Viride | CBS 134884 = G.J.S. 94-11 (T) | Taiwan | KJ665624 | KC285777 | KC285746 |
| T. nothescens | Viride | CBS 134882 = G.J.S. 99-142 (T) | Australia | DQ307512 | EU241498 | KC285722 |
| T. novae–zelandiae | Longibrachiatum | G.J.S. 81-265 = CBS 639.92 = CBS 496.97 = ATCC 28856 (T) | New Zealand | AY937448 | JN133563 | – |
| CBS 472.97 = G.J.S. 81-264 | New Zealand | AY865639 | – | – | ||
| T. nybergianum | Polysporum | CBS 122500 = Hypo 572 | Finland | FJ179575 | FJ179611 | KJ665105 |
| CBS 122496 = Hypo 577 | Finland | – | – | KJ665106 | ||
| T. oblongisporum | Semiorbis | DAOM 167085 | Canada | AY750884 + AF534623 | AF545551 | – |
| T. ochroleucum | Viride | CBS 119502 = Hypo 274 | UK: England | FJ860659 | FJ860556 | KJ665107 |
| T. olivascens | Viride | Hypo 273 = CBS 119322 = C.P.K. 2047 | UK: England | DQ672609 | KC285750 | KC285717 |
| S34 | Italy | KC285615 | KC285751 | KC285718 | ||
| S475 = CBS 132574 (T) | Spain | KC285624 | KC285752 | KC285719 | ||
| T. orientale | Longibrachiatum | DIS 270f | Ecuador | – | JN175521 | – |
| S187 = CBS 131488 | Spain | JQ685868 | JQ685884 | KJ665108 | ||
| G.J.S. 88-81 = CBS 130428 (T) | China | EU401581 | – | – | ||
| T. ovalisporum | Viride | DIS 70a = CBS 113299 (T) | Ecuador | – | FJ442742 | – |
| DIS 172i | Brazil | DQ288999 | – | – | ||
| T. pachypallidum | Polysporum | CBS 122126 = Hypo 62 (T) | Czech Republic | FJ860662 | JQ685879 | KJ665110 |
| Hypo 317 = C.P.K. 2790 | Germany | – | – | KJ665109 | ||
| CBS 120921 = Hypo 298 | Czech Republic | FJ179578 | FJ179614 | KJ665111 | ||
| T. parareesei | Longibrachiatum | CBS 125925 = C.P.K. 717 = TUB F–1066 (T) | Argentina | GQ354353 | HM182963 | KJ665112 |
| T. pararogersonii | Viride | S301 = CBS 133496 (T) | Croatia | KJ665625 | KJ665320 | KJ665113 |
| S584 | Greece | KJ665626 | – | – | ||
| T. paratroviride | Viride | S385 = CBS 136489 (T) | Spain | KJ665627 | KJ665321 | KJ665114 |
| S489 | Spain | KJ665628 | KJ665322 | KJ665115 | ||
| T. paraviridescens | Viride | Hypo 372 = CBS 119321 = C.P.K. 2140 (T) | Austria | DQ672610 | KC285763 | KC285730 |
| S122 = CBS 132566 | Italy | KC285671 | KC285764 | KC285731 | ||
| S664 | Spain | KJ665629 | – | – | ||
| T. parepimyces | Green/harzianum | Hypo 357 = CBS 122769 (T) | Austria | FJ860664 | FJ860562 | KJ665116 |
| Hypo 521 = CBS 122768 | Austria | – | – | KJ665117 | ||
| T. parestonicum | Green | Hypo 366 = C.P.K. 2427 | Austria | – | – | KJ665118 |
| Hypo 437 = CBS 120636 (T) | Austria | FJ860667 | FJ860565 | KJ665119 | ||
| Hypo 583 = C.P.K. 3167 | Germany | – | – | KJ665120 | ||
| T. parmastoi | Basal | CBS 121139 = Hypo 455 | Austria | FJ860668 | FJ860567 | KJ665121 |
| T. patella | aff. Longibrachiatum | G.J.S. 91-141 | USA | KJ665630 | KJ665323 | KJ665122 |
| G.J.S. 95-173 | USA | – | KJ665324 | KJ665123 | ||
| T. paucisporum | Viride | G.J.S. 01-13 = CBS 118645 (T) | Ecuador | DQ109540 | FJ150787 | – |
| T. peltatum | Basal | G.J.S. 08-207 | USA | – | HQ260610 | – |
| T. cf. peltatum | Basal | J.D.Rogers 1_new | USA | EF392731 | – | – |
| T. petersenii | Viride | G.J.S. 04-164 | USA | DQ289004 | FJ442783 | – |
| DAOM 165782 | USA | DQ289000 | – | – | ||
| CBS 119507 = Hypo 45 | Austria | FJ860670 | FJ860568 | KJ665125 | ||
| Hypo 341 = C.P.K. 2413 | Germany | – | – | KJ665124 | ||
| S109 | Italy | KJ665631 | KJ665325 | KJ665126 | ||
| S167 | Spain | KJ665632 | KJ665326 | – | ||
| S170 | Spain | JN715612 | – | KJ665127 | ||
| S178 | Spain | KJ665633 | – | – | ||
| S182 | Spain | KJ665634 | – | KJ665128 | ||
| S197 | Portugal | KJ665635 | – | – | ||
| S200 | Portugal | KJ665636 | KJ665327 | – | ||
| S202 | Portugal | KJ665637 | – | – | ||
| S209 | Spain | KJ665638 | – | – | ||
| S210 | Spain | KJ665639 | – | – | ||
| S211 | Spain | KJ665640 | – | – | ||
| S223 | Spain | KJ665641 | – | – | ||
| S224 | Spain | KJ665642 | – | – | ||
| S236 | Spain | KJ665643 | – | – | ||
| S325 | Spain | KJ665644 | – | – | ||
| S342 | Spain | KJ665645 | – | – | ||
| S388 | Spain | KJ665646 | – | – | ||
| S390 | Spain | KJ665647 | – | – | ||
| S395 | Spain | KJ665648 | – | – | ||
| S400 | Spain | KJ665649 | – | – | ||
| S417 | Spain | KJ665650 | – | – | ||
| S418 | Spain | KJ665651 | – | – | ||
| S442a | Spain | KJ665652 | – | – | ||
| S448 | Spain | KJ665653 | – | – | ||
| S476 | Spain | KJ665654 | – | – | ||
| S477 | Spain | KJ665655 | – | – | ||
| S478 | Spain | KJ665656 | – | – | ||
| S483 | Spain | KJ665657 | – | – | ||
| S484 | Spain | KJ665658 | – | – | ||
| S493 | Spain | KJ665659 | – | – | ||
| S500 | Spain | KJ665660 | – | – | ||
| S509 | Spain | KJ665661 | – | – | ||
| S510 | Spain | KJ665662 | – | – | ||
| S527 | Spain | KJ665663 | – | – | ||
| S542 | Spain | KJ665664 | – | – | ||
| S543 | Spain | KJ665665 | – | – | ||
| S551 | Spain | KJ665666 | – | – | ||
| S555 | Greece | KJ665667 | – | – | ||
| S558 | Spain | KJ665668 | – | – | ||
| S615 | Greece | KJ665669 | – | – | ||
| S633 | Greece | KJ665670 | – | – | ||
| S636 | Greece | KJ665671 | – | – | ||
| T. pezizoides | Viride | C.P.K. 775 = G.J.S. 97-83 = CBS 101131 | Thailand | – | JN715610 | KJ665129 |
| G.J.S. 01-257, G.J.S. 01-231 | Thailand | AY937438 + AY225859 | – | – | ||
| T. phellinicola | Hypocreanum | CBS 119283 = Hypo 353 (T) | Austria | FJ860672 | FJ860569 | KJ665130 |
| T. phyllostachydis | Green | CBS 114071 | France | FJ860673 | FJ860570 | KJ665131 |
| S564 | Italy | KJ665672 | – | KJ665132 | ||
| T. piluliferum | Polysporum | CBS 120927 = Hypo 413 (T) | Germany | FJ860674 | FJ179615 | KJ665133 |
| Hypo 537 = C.P.K. 3143 | UK: England | – | – | KJ665134 | ||
| T. pinnatum | Longibrachiatum | G.J.S. 04-100 = CBS 131292 (T) | Vietnam | JN175571 | JN175515 | – |
| T. placentula | Polysporum | CBS 120924 = Hypo 249 (T) | UK: England | FJ179580 | FJ179616 | KJ665135 |
| CBS 121134 = Hypo 407 | Germany | – | – | KJ665136 | ||
| T. pleuroti | Green/harzianum | CBS 124387 (T) | Korea | HM142382 | HM142372 | – |
| C.P.K. 2117 | Hungary | EU279975 | – | – | ||
| T. pleuroticola | Green/harzianum | CBS 124383 (T) | Korea | HM142381 | HM142371 | – |
| C.P.K. 3196 | Hungary | EU918160 | – | – | ||
| T. polysporum | Polysporum | Hypo 299 = C.P.K. 1988 | Czech Republic | – | – | KJ665137 |
| Hypo 422 = C.P.K. 2461 | Austria | – | FJ179613 | – | ||
| Hypo 522 = C.P.K. 3131 | Austria | FJ860661 | JQ685878 | KJ665138 | ||
| S307 | Croatia | KJ665678 | – | – | ||
| S308 | Croatia | KJ665679 | – | – | ||
| S315 | Croatia | KJ665680 | – | – | ||
| S45 | Italy | KJ665681 | – | KJ665144 | ||
| S56 | Italy | KJ665683 | – | KJ665146 | ||
| S72 | Italy | KJ665685 | – | KJ665148 | ||
| S77 | Italy | KJ665686 | – | KJ665149 | ||
| S103 | Italy | KJ665673 | KJ665328 | KJ665139 | ||
| S121 | Italy | KJ665674 | KJ665329 | KJ665140 | ||
| S124 | Italy | KJ665675 | – | KJ665141 | ||
| S176 | Spain | KJ665676 | – | KJ665142 | ||
| S258 | Croatia | KJ665677 | KJ665330 | KJ665143 | ||
| S458 | Spain | KJ665682 | KJ665331 | KJ665145 | ||
| S608 | Greece | KJ665684 | – | KJ665147 | ||
| T. priscilae | Green/harzianum | Hypo 657 | Austria | KJ665687 | – | – |
| S118 | Italy | KJ665688 | – | – | ||
| S129 | Italy | KJ665689 | KJ665332 | KJ665150 | ||
| S144 | Italy | KJ665690 | – | – | ||
| S168 = CBS 131487 (T) | Spain | KJ665691 | KJ665333 | KJ665151 | ||
| S416 | Spain | KJ665692 | – | – | ||
| S449 | Spain | KJ665693 | – | – | ||
| S580 | Italy | KJ665694 | – | – | ||
| T. protopulvinatum | Hypocreanum | CBS 121274 = Hypo 373 | Austria | – | – | KJ665152 |
| Hypo 378 = C.P.K. 2434 | Switzerland | FJ860677 | FJ860574 | – | ||
| Hypo 440 = C.P.K. 2476 | Austria | – | – | KJ665153 | ||
| T. protrudens | Brevicompactum | DIS 119F = CBS 121320 (T) | India | EU338289 | EU338322 | – |
| T. pseudocandidum | Green | P.C. 59 = CBS 114249 (T) | Costa Rica | AY737742 + AY391962 | AY391899 | – |
| T. pseudogelatinosum | Green/harzianum | CNU N309 | Korea | HM920202 | HM920173 | – |
| T. pseudokoningii | Longibrachiatum | NS 19 = DAOM 167678 = CBS 408.91 = ATCC 298861 (T) | Australia | JN175588 | JN175535 | – |
| T. pseudolacteum | Lone lineage | TUFC 61490 = CBS 133191 (T) | Japan | JX238493 | JX238478 | – |
| T. pseudonigrovirens | Helicum | G.J.S. 99-64 = CBS 114330 (T) | Costa Rica | AY737744 + AF534582 | AF545518 | – |
| T. pseudostramineum sensu Overton | Hypocreanum | G.J.S. 90-74 | USA | – | DQ835454 | – |
| G.J.S. 95-189 | USA | DQ005521 + DQ835446 + DQ835480 | – | – | ||
| T. pseudostramineum | Hypocreanum | TUFC 60104 | Japan | JQ797400 | JQ797408 | – |
| T. psychrophilum | Psychrophilum | Hypo 37 = C.P.K. 1602 | Austria | FJ860680 | FJ860575 | KJ665154 |
| Hypo 379 | Germany | – | – | KJ665155 | ||
| S647 | Spain | KJ665695 | – | KJ665156 | ||
| T. pubescens | Viride | DAOM 166162 (T) | USA | AY750887 + AF534624 | EU248613 | – |
| T. pulvinatum | Hypocreanum | Hypo 7 = C.P.K. 2385 | Austria | – | – | KJ665160 |
| CBS 121279 = Hypo 36 | Austria | FJ860683 | FJ860577 | KJ665159 | ||
| Hypo 82 = C.P.K. 2395 | Sweden | – | – | KJ665161 | ||
| Hypo 121 = C.P.K. 1991 | Czech Republic | – | – | KJ665157 | ||
| Hypo 122 = C.P.K. 1992 | Germany | – | – | KJ665158 | ||
| T. pyramidale | Green/harzianum | S73 = CBS 135574 (T) | Italy | KJ665699 | KJ665334 | KJ665163 |
| S119 | Italy | KJ665696 | – | – | ||
| S533 | Spain | KJ665697 | – | KJ665162 | ||
| S573 | Italy | KJ665698 | – | – | ||
| T. reesei | Longibrachiatum | QM 6a = CBS 383.78 (T) | New Guinea | – | HM182969 | – |
| QM 6a genome | New Guinea | – | – | KJ665164 | ||
| T. rhododendri | Psychrophilum | CBS 119288 = Hypo 209 (T) | Austria | FJ860685 | FJ860578 | KJ665165 |
| T. rodmanii | Brevicompactum | CBS 120895 = G.J.S. 91-88 (T) | USA | – | EU338324 | – |
| CBS 121553 = Hypo 390 | Germany | – | – | KJ665166 | ||
| Hypo 478 = C.P.K. 2852 | Austria | FJ860688 | FJ860581 | KJ665167 | ||
| T. rogersonii | Viride | G.J.S. 04-157 | USA | DQ307558 | JN133566 | – |
| CBS 119503 = Hypo 310 | Austria | FJ860690 | FJ860583 | KJ665168 | ||
| Hypo 331 = C.P.K. 2410 | Austria | – | – | KJ665169 | ||
| T. rossicum | Stromaticum | DAOM 230011 (T) | Russia | AY937441 | HQ342288 | – |
| DAOM 233977 | Peru | EU280062 | – | – | ||
| T. cf. rossicum | Stromaticum | S334 | Spain | KJ665700 | KJ665335 | KJ665170 |
| S501 | Spain | KJ665701 | – | – | ||
| S505 | Spain | KJ665702 | – | KJ665171 | ||
| S586 | Greece | KJ665703 | – | KJ665172 | ||
| T. rubi | Lone lineage | S146 = CBS 127380 (T) | Italy | KJ665704 | KJ665336 | KJ665173 |
| T. sambuci | aff. Longibrachiatum | WU 29467 = Hypo 426 | Austria | FJ860693 | FJ860585 | KJ665174 |
| T. samuelsii | Viride | S5 = CBS 130537 (T) | Italy | JN715655 | JN715599 | KC285715 |
| S42 | Italy | JN715652 | JN715598 | – | ||
| S398 | Spain | – | – | KJ665175 | ||
| S537 | Spain | KC285597 | – | KC285716 | ||
| T. saturnisporopsis | Longibrachiatum | TR 175 = C.P.K. 1356 (T) | USA | – | DQ857348 | – |
| S19 = CBS 128829 | Italy | JQ685869 | JQ685885 | KJ665176 | ||
| T. saturnisporum | Longibrachiatum | ATCC 18903 = CBS 330.70 = C.P.K. 1266 (T) | USA | EU280044 | DQ087243, JN182309 | – |
| T. scalesiae | Viride | G.J.S. 03-74 (T) | Ecuador | DQ841726 | EU252007 | – |
| T. semiorbis | Semiorbis | DAOM 167636 = CBS 244.63 = C.P.K. 452 | New Zealand | AF545568 | AF545522 | KJ665177 |
| T. sempervirentis | Viride | CBS 133498 = S599 (T) | Greece | KC285755 | KC285632 | KC285723 |
| S601 | Greece | KC285633 | KC285756 | KC285724 | ||
| T. seppoi | Polysporum | CBS 122498 = Hypo 575 (T) | Finland | FJ179581 | FJ179617 | KJ665178 |
| CBS 122497 = Hypo 576 | Finland | – | – | KJ665179 | ||
| T. silvae–virgineae | Helicum | Hypo 71 = C.P.K. 974 | Czech Republic | – | – | KJ665181 |
| CBS 120922 = Hypo 101 (T) | Austria | FJ860696 | FJ860587 | KJ665180 | ||
| Hypo 658 | Sweden | KJ665705 | – | – | ||
| T. simmonsii | Green/harzianum | Hypo 15 = C.P.K. 1596 | Austria | KJ665706 | – | – |
| Hypo 30 = C.P.K. 2391 | Austria | KJ665707 | – | KJ665182 | ||
| S7 | Italy | KJ665719 | KJ665337 | KJ665185 | ||
| S85 | Italy | KJ665720 | – | – | ||
| S86 | Italy | KJ665721 | – | – | ||
| S271 | Croatia | KJ665708 | – | – | ||
| S280 | Croatia | KJ665709 | – | – | ||
| S282 | Croatia | KJ665710 | – | – | ||
| S297 | Croatia | KJ665711 | – | – | ||
| S303 | Croatia | KJ665712 | – | – | ||
| S311 | Croatia | KJ665713 | – | – | ||
| S314 | Croatia | KJ665714 | – | – | ||
| S355 | France | KJ665715 | – | KJ665183 | ||
| S547 | Spain | KJ665716 | – | KJ665184 | ||
| S571 | Italy | KJ665717 | – | – | ||
| S627 | Greece | KJ665718 | – | – | ||
| T. sinense | Longibrachiatum | DAOM 230004 | Taiwan | AY750889 | JN175528 | – |
| T. sinuosum | Green | PC 8 = CBS 114247 = DAOM 232839 (T) | USA | AY737743 + AY391997 | – | – |
| Hypo 13 = C.P.K. 1595 | Austria | FJ860697 | FJ179619 | KJ665186 | ||
| Hypo 232 = C.P.K. 2010 | Germany | – | – | KJ665187 | ||
| S333 | Spain | KJ665727 | – | – | ||
| T. sinuosum 2 | Green | S158 | Italy | KJ665722 | KJ665338 | KJ665188 |
| S274 | Croatia | KJ665724 | – | – | ||
| S276 | Croatia | KJ665725 | KJ665340 | KJ665190 | ||
| S295 | Croatia | KJ665726 | – | – | ||
| T. sinuosum 3 | Green | S270 | Croatia | KJ665723 | KJ665339 | KJ665189 |
| S349 | Spain | KJ665728 | KJ665341 | – | ||
| T. sinuosum 4 | Green | S378 | Spain | KJ665729 | KJ665342 | KJ665191 |
| T. solani | Longibrachiatum | G.J.S. 08-81 = CBS 130506 (T) | Mexico | JN175597 | JN175546 | – |
| T. spinulosum | Green | Hypo 424 = CBS 121280 | Denmark | FJ860699 | FJ860589 | KJ665201 |
| Hypo 425 = CBS 121272 | Germany | FJ860700 | – | KJ665202 | ||
| CBS 311.50 = C.P.K. 1510 | UK: England | FJ860701 | FJ860591 | – | ||
| T. spirale | Green | DAOM 183974 (T) | Thailand | EU280049 + AF534626 | – | – |
| DIS 311D | Cameroon | – | FJ442694 | – | ||
| S212 | Spain | KJ665740 | KJ665348 | KJ665203 | ||
| T. stellatum | Polysporum | CBS 112265 = G.J.S. 99-222 (T) | New Zealand | KJ665741 | KJ665349 | KJ665204 |
| T. stercorarium | Hypocreanum | CBS 148.85 = ATCC 62321 (T) | Spain | FJ860607 | EF469103 | KJ665205 |
| T. stilbohypoxyli | Viride | G.J.S. 96-30 = CBS 992.97 (T) | Puerto Rico | DQ109546 | – | – |
| G.J.S. 96-32 | Puerto Rico | – | EU341805 | – | ||
| Hypo 256 = C.P.K. 1977 | UK: England | FJ860702 | FJ860592 | KJ665206 | ||
| S24 | Italy | KJ665742 | KJ665350 | KJ665207 | ||
| S75 | Italy | KJ665743 | – | – | ||
| T. stramineum | Green/harzianum | G.J.S. 02-84 = CBS 114248 (T) | Sri Lanka | AY737746 + AY391999 | AY391945 | – |
| T. strictipile | Green | Hypo 24 = C.P.K. 1601 | Austria | FJ860704 | FJ860594 | KJ665209 |
| Hypo 72 = C.P.K. 975 | Czech Republic | – | – | KJ665210 | ||
| Hypo 137 = C.P.K. 1616 | Austria | – | – | KJ665208 | ||
| S574 | Italy | KJ665744 | – | – | ||
| S575 | Italy | KJ665745 | – | – | ||
| T. strigosellum | Viride | G.J.S. 05-02 | Cameroon | EU248631 | EU248607 | – |
| CBS 102817 = C.P.K. 3604 (T) | Colombia | JQ425705 | – | – | ||
| T. strigosum | Viride | DAOM 166121 (T) | USA | AY937442 + AF534629 | AF545556 | – |
| T. stromaticum | Stromaticum | GJS 97-183 + P.C. 209 | Brazil | AY937418 + AF534613 | – | – |
| G.J.S. 97-180 | Brazil | – | – | – | ||
| CBS 101875 = G.J.S. 97-183 = C.P.K. 386 (T) | Brazil | – | HQ342245 | KJ665211 | ||
| T. subalpinum | aff. Longibrachiatum | CBS 119128 = Hypo 226 (T) | Austria | FJ860705 | FJ860595 | KJ665212 |
| Hypo 512 | Austria | – | – | KJ665213 | ||
| T. subeffusum | Viride | CBS 120929 = Hypo 447 (T) | Austria | FJ860707 | FJ860597 | – |
| Hypo 497 = C.P.K. 2864 | Ukraine | – | – | KJ665214 | ||
| S116 | Italy | KJ665746 | – | – | ||
| S149 | Italy | KJ665747 | – | – | ||
| S234 | Spain | KJ665748 | – | KJ665215 | ||
| S238 | Spain | KJ665749 | – | KJ665216 | ||
| S592 | Greece | KJ665750 | – | – | ||
| S602 | Greece | KJ665751 | – | – | ||
| T. subsulphureum | Hypocreanum | M-141 | Japan | DQ835492 | DQ835522 | – |
| T. sulawesense | Green | G.J.S. 85-228 | Indonesia | AY737730 + AY392002 | AY391954 | – |
| T. sulphureum | Hypocreanum | Hypo 2 = C.P.K. 1593 | Austria | FJ860709 | FJ860599 | KJ665218 |
| Hypo 126 = C.P.K. 2040 | Ukraine | – | – | KJ665217 | ||
| T. surrotundum | Green | G.J.S. 88-73 = CBS 111145 (T) | USA | AY737734 + AF534594 | AF545540 | – |
| T. taiwanense | Viride | C.P.K. 416 = TUB F–597 | Singapore | – | JN715608 | KJ665219 |
| G.J.S. 95-93 (T) | Taiwan | DQ284973 | – | – | ||
| T. tawa | Green/harzianum | DAOM 232841 | New Zealand | EU279972 + AY392004 | – | – |
| G.J.S. 97-174 = CBS 114233 (T) | Thailand | – | AY391956 | – | ||
| T. taxi | Lone lineage | ZJUF0986 (T) | China | DQ859029 | DQ859032 | – |
| T. thailandicum | Green | G.J.S. 97-61 = CBS 114234 (T) | Thailand | AY737748 + AY392005 | AY391957 | – |
| T. thelephoricola | Green | Hypo 344 = CBS 120925 | Austria | FJ860711 | JQ685886 | KJ665220 |
| Hypo 454 | Austria | – | – | KJ665221 | ||
| S572 | Italy | KJ665752 | – | – | ||
| S577 | Italy | KJ665753 | – | – | ||
| T. theobromicola | Viride | DIS 85f (T) | Peru | EU856321 | FJ007374 | – |
| T. tomentosum | Green/harzianum | DAOM 178713a (T) | Canada | AF534630 | AF545557 | – |
| CBS 120637 = C.P.K. 2498 | Austria | FJ860629 | FJ860532 | KJ665222 | ||
| Hypo 608 = C.P.K. 2563 | Austria | KJ665754 | – | – | ||
| S23 | Italy | KJ665759 | KJ665351 | KJ665223 | ||
| S33 | Italy | KF134801 | KF134793 | KJ665224 | ||
| S193 | Spain | KJ665755 | – | – | ||
| S208 | Spain | KJ665756 | – | – | ||
| S215 | Spain | KJ665757 | – | – | ||
| S229 | Spain | KJ665758 | – | – | ||
| S230 | Spain | KJ665760 | – | – | ||
| S318 | Spain | KJ665761 | – | – | ||
| S319 | Spain | KJ665762 | – | – | ||
| S435 | Spain | KJ665763 | – | – | ||
| S468 | Spain | KJ665764 | – | – | ||
| S513 | Spain | KJ665765 | – | – | ||
| S521 | Spain | KJ665766 | – | – | ||
| S530 | Spain | KJ665767 | – | – | ||
| S563 | Italy | KJ665768 | – | – | ||
| T. tremelloides | aff. Longibrachiatum | CBS 120634 = Hypo 308 | Germany | FJ860713 | FJ860602 | KJ665225 |
| Hypo 469 = C.P.K. 2495 | Austria | – | – | KJ665226 | ||
| T. trixiae | Viride | ATCC 32630 = Tr 26 | Sweden | DQ307526 | KC285770 | KC285736 |
| CBS 134702 = C.P.K. 2138 = Hypo 228 (T) | Germany | DQ672606 | – | KC285738 | ||
| T. turrialbense | Brevicompactum | CBS 112445 (T) | Costa Rica | EU338284 | EU338321 | – |
| T. valdunense | Viride | CBS 120923 = Hypo 222 | Austria | FJ860717 | FJ860605 | KJ665227 |
| T. velutinum | Green/harzianum | DAOM 230013 = C.P.K. 298 (T) | Nepal | KJ665769 | KF134794 | KJ665228 |
| T. vermipilum | Stromaticum | PPRI 3359 = CBS 127103 (T) | South Africa | HQ342219 | HQ342282 | – |
| T. victoriense | Hypocreanum | G.J.S. 99-200 (T) | Australia | – | DQ835517 | – |
| C.P.K. 3565 | Australia | FJ860718 | – | KJ665229 | ||
| T. vinosum | Viride | G.J.S. 99-156 = ICMP 16293 | Australia | DQ307527 | KC285778 | KC285747 |
| G.J.S. 99-158 = ICMP 16294 = CBS 119087 (T) | New Zealand | – | KC285779 | KC285748 | ||
| G.J.S. 99-183 | Australia | DQ841719 | – | – | ||
| T. virens | Green | DAOM 167652 + Gli39 | USA | AY750891 + AF534631 | – | – |
| Gli39 = CBS 249.59 (T) | USA | – | AF545558 | – | ||
| DAOM 167651 | USA | – | – | – | ||
| Gv29-8 genome | – | – | – | KJ665230 | ||
| T. virescentiflavum | Green | P.C. 278 | Costa Rica | AY737749 + AY392007 | AY391959 | – |
| T. viridarium | Viride | S136 = CBS 132568 (T) | Italy | KC285658 | KC285760 | KC285728 |
| Hypo 246 = C.P.K. 2046 | UK: England | DQ672608 | – | KJ665231 | ||
| T. viride | Viride | CBS 119325 = Hypo 292 (T) | Czech Republic | DQ672615 | EU711362 | – |
| Hypo 239 = C.P.K. 1995 | France | – | – | KJ665232 | ||
| Hypo 306 = C.P.K. 2000 | Austria | – | – | KJ665233 | ||
| S472 | Spain | KJ665770 | – | – | ||
| S552 | Spain | KJ665771 | – | – | ||
| T. viridescens | Viride | S1 | Italy | KC285634 | KC285757 | KC285725 |
| S452 = CBS 132573 (T) | Spain | KC285646 | KC285758 | KC285726 | ||
| S471 | Spain | – | – | KJ665234 | ||
| T. viridialbum | Viride | S177 | Spain | KC285705 | – | – |
| S250 = CBS 133495 (T) | Spain | KC285706 | KC285774 | KC285741 | ||
| S429 | Spain | – | – | KJ665235 | ||
| T. virilente | Viride | S281 = CBS 132569 (T) | Croatia | KC285692 | KC285767 | KC285733 |
| S520 | Spain | – | – | KJ665236 | ||
| S661 | Spain | KJ665772 | – | – | ||
| T. voglmayrii | Lone lineage | CBS 117710 = C.P.K. 1592 = Hypo 8 | Austria | – | – | KJ665238 |
| CBS 117711 = Hypo 40 (T) | Austria | DQ086146 | FJ179622 | KJ665237 | ||
| T. yunnanense | Viride | CBS 121219 = YMF 1.01694 (T) | China | AY941825 | GU198274 | – |
| T. sp. S138 | Green/harzianum | S138 | Italy | KJ665730 | KJ665343 | KJ665192 |
| T. sp. S169 | Green | S169 | Spain | KJ665731 | KJ665344 | KJ665193 |
| T. sp. S222 | Green/harzianum | S222 | Spain | KJ665732 | KJ665345 | KJ665194 |
| T. sp. S404 | Green/harzianum | S404 | Spain | KJ665733 | KJ665346 | KJ665195 |
| T. sp. S466 | Green/harzianum | S466 | Spain | KJ665734 | – | KJ665196 |
| T. sp. S467 | Green/harzianum | S467 | Spain | KJ665735 | – | KJ665197 |
| T. sp. S605 | Green | S605 | Greece | KJ665736 | – | KJ665198 |
| T. sp. S610 | Green/harzianum | S610 | Greece | KJ665737 | – | – |
| T. sp. S624 | Green | S624 | Greece | KJ665738 | KJ665347 | KJ665199 |
| T. sp. S637 | Polysporum | S637 = CBS 137007 | Greece | KJ665739 | – | KJ665200 |
| Protocrea farinosa | Outgroup | Hypo 327 = C.P.K. 2408 | Denmark | – | – | KJ664936 |
| CBS 121551 = Hypo 371 (T) | Austria | – | EU703935 | – | ||
| Hypo 409 = C.P.K. 2453 | Germany | – | – | KJ664937 | ||
| Hypo 434 = C.P.K. 2472 | Austria | EU703892 | – | – | ||
| S18 | Italy | KJ665352 | – | – | ||
| S243 | Spain | – | KJ665239 | – | ||
| S401 | Spain | KJ665353 | – | – | ||
| Protocrea pallida | Outgroup | CBS 121552 = Hypo 376 | Denmark | – | EU703944 | – |
| CBS 299.78 (T) | USA | EU703900 | – | – | ||
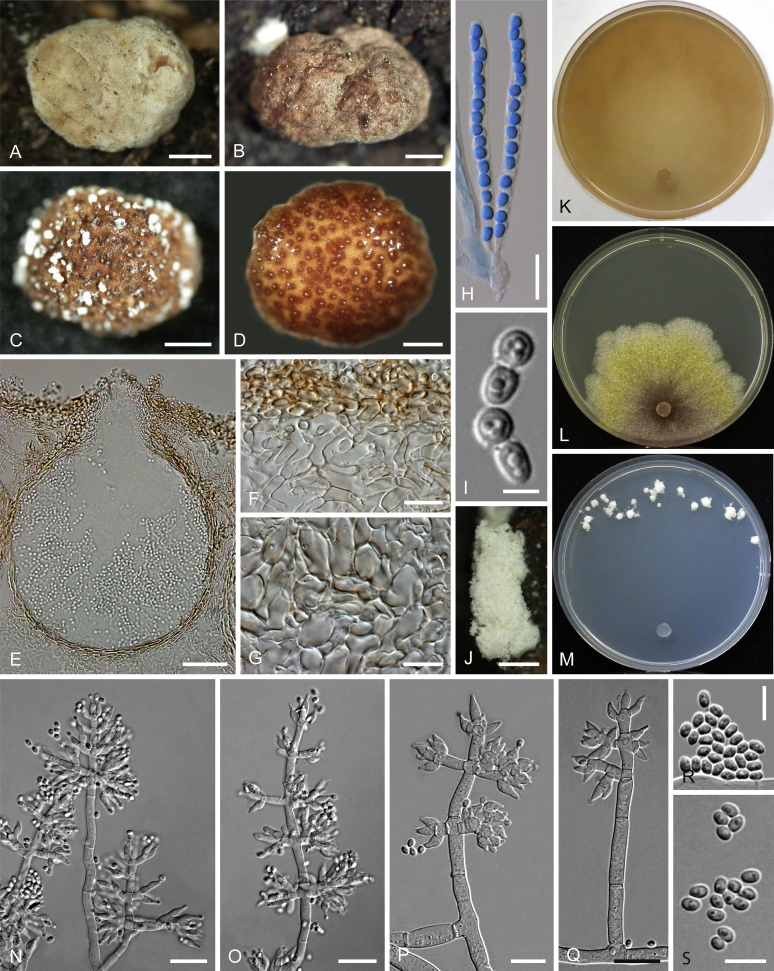
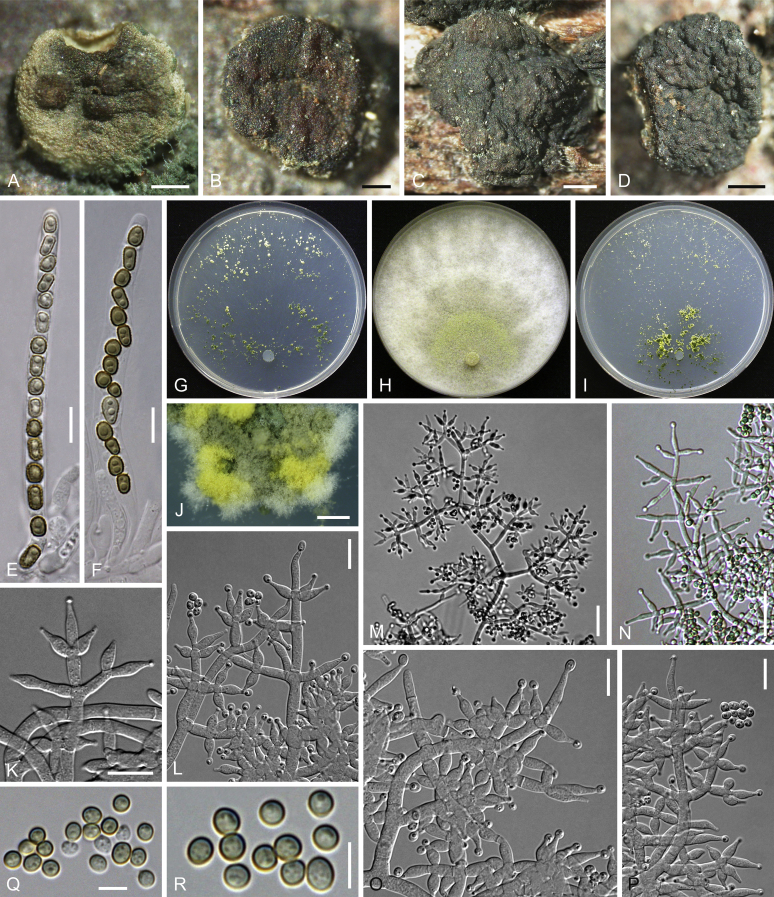
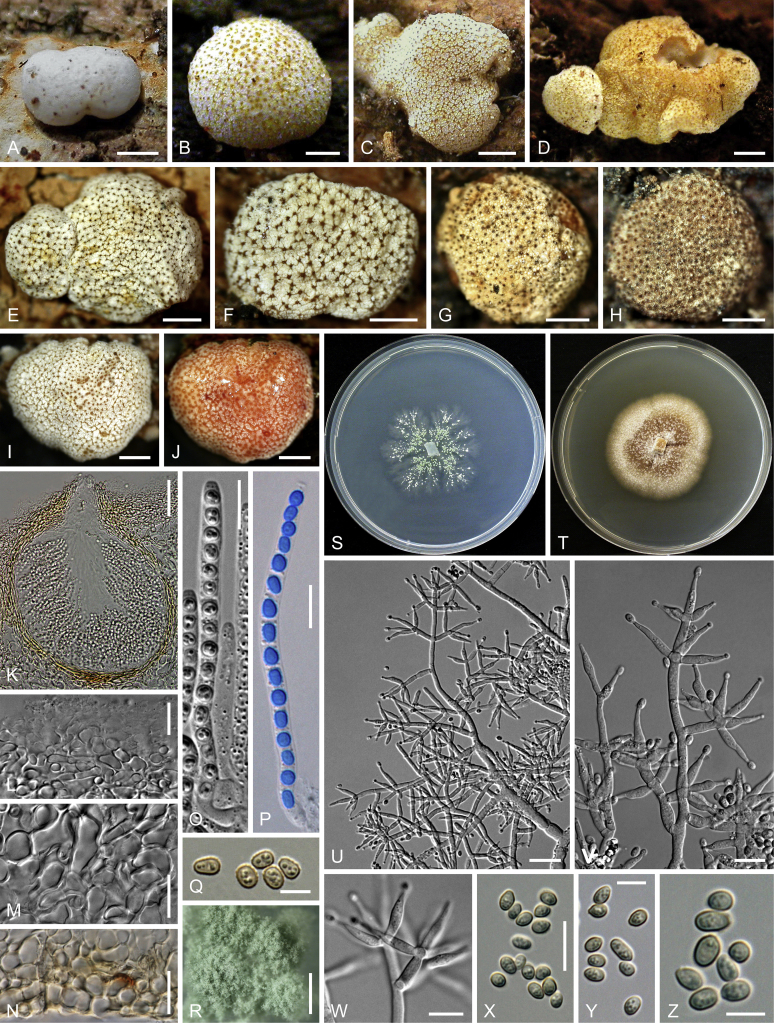
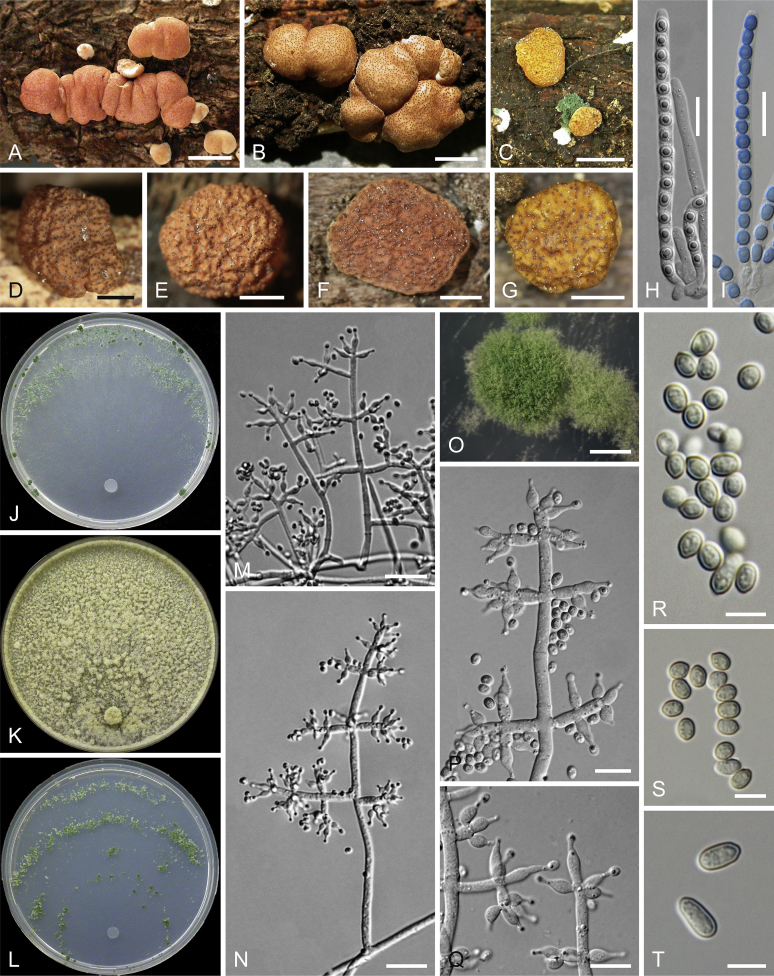
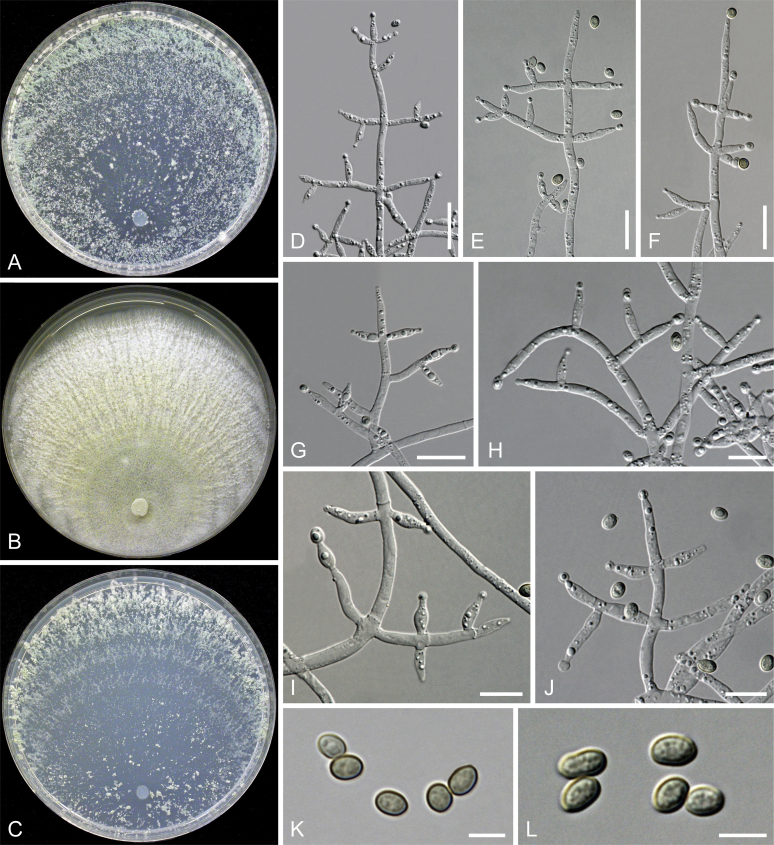
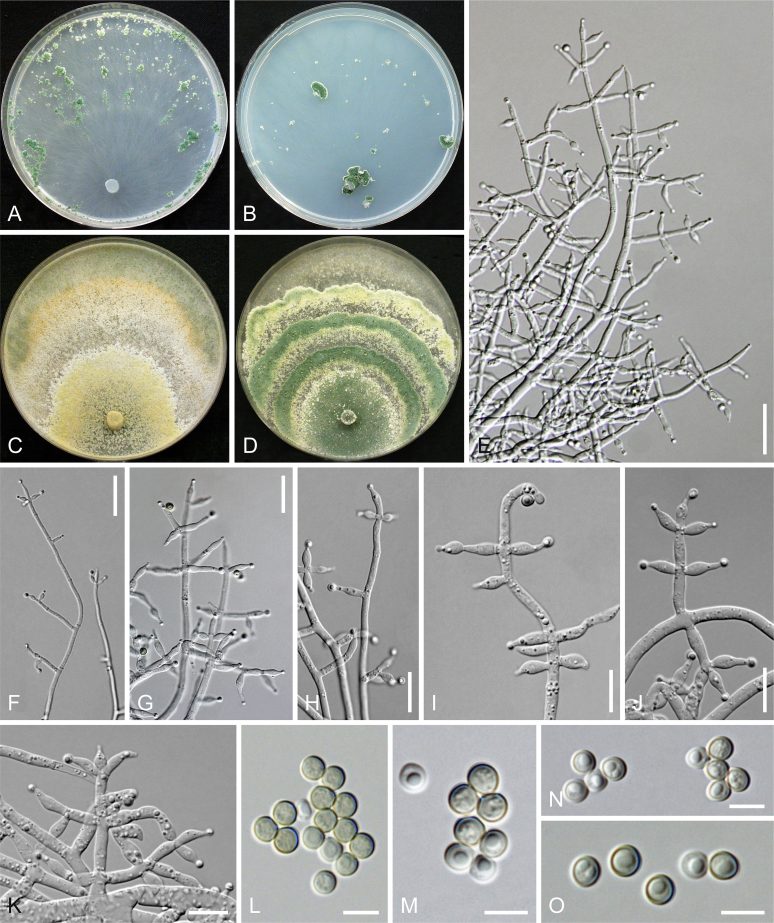
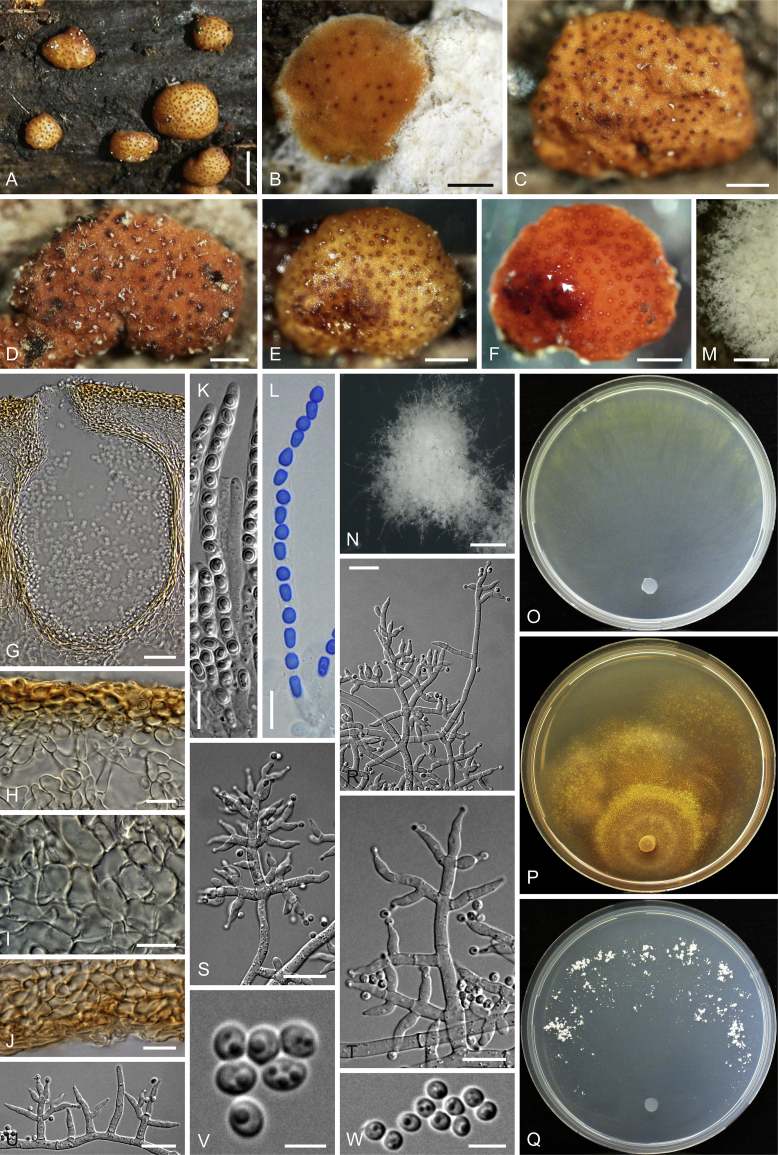
Culture preparation, growth rate determination and morphology
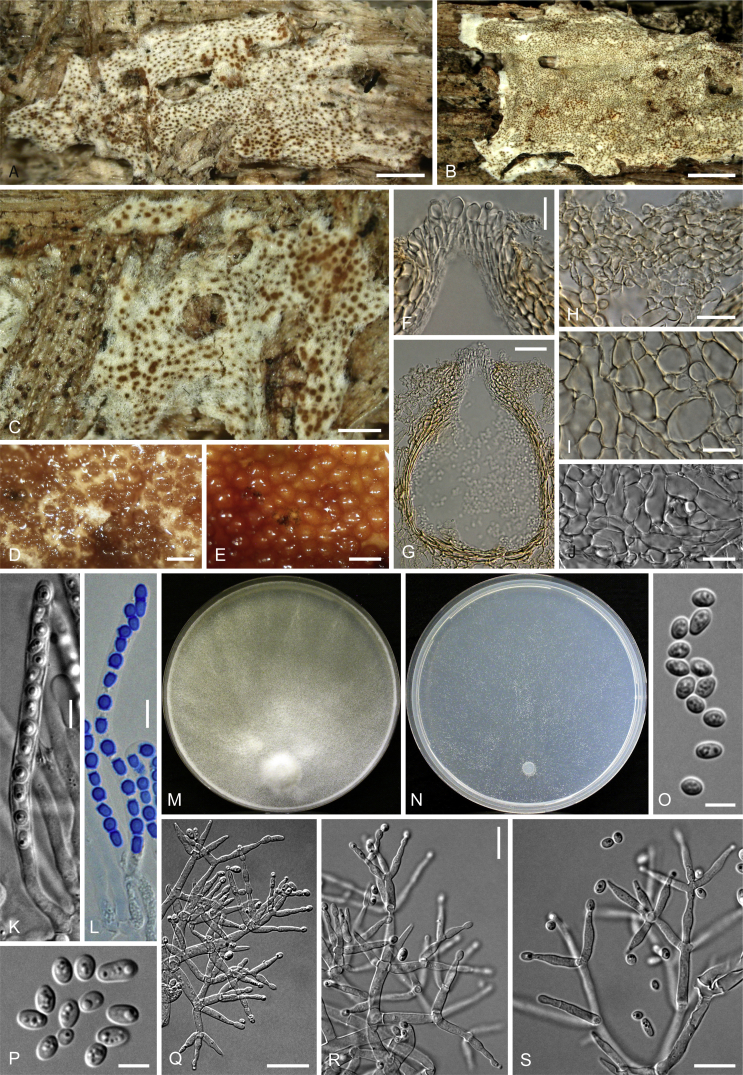
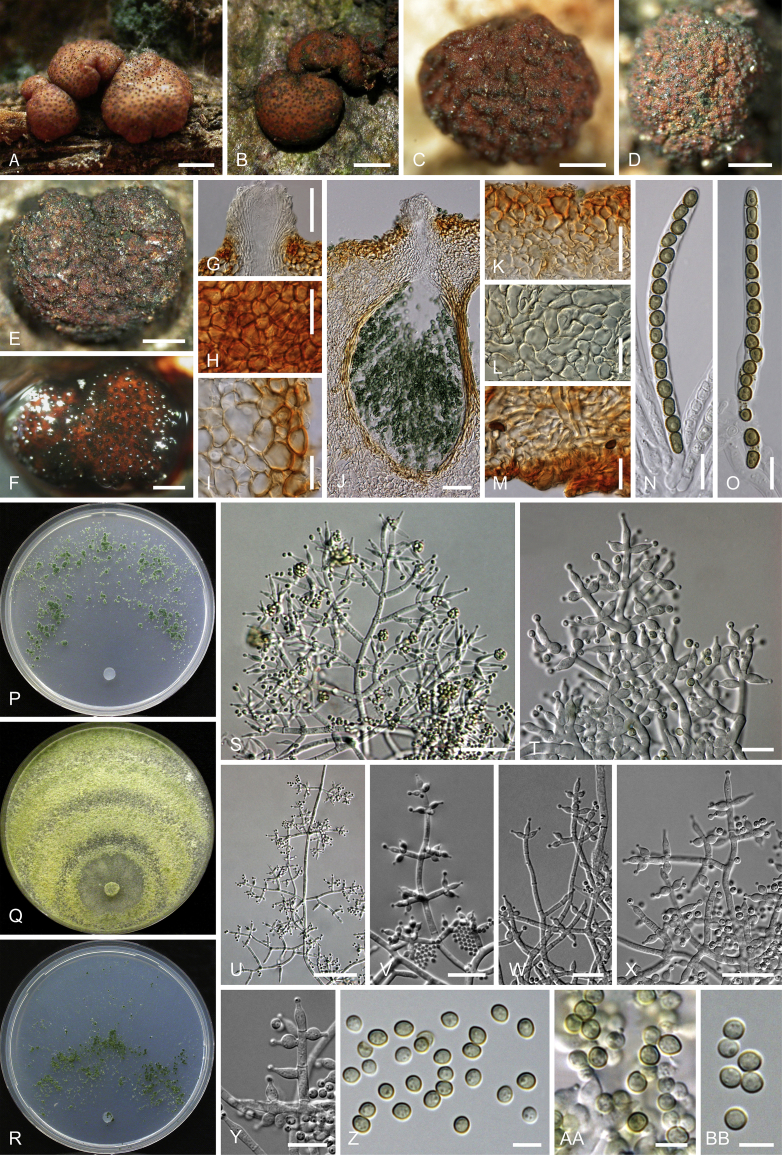
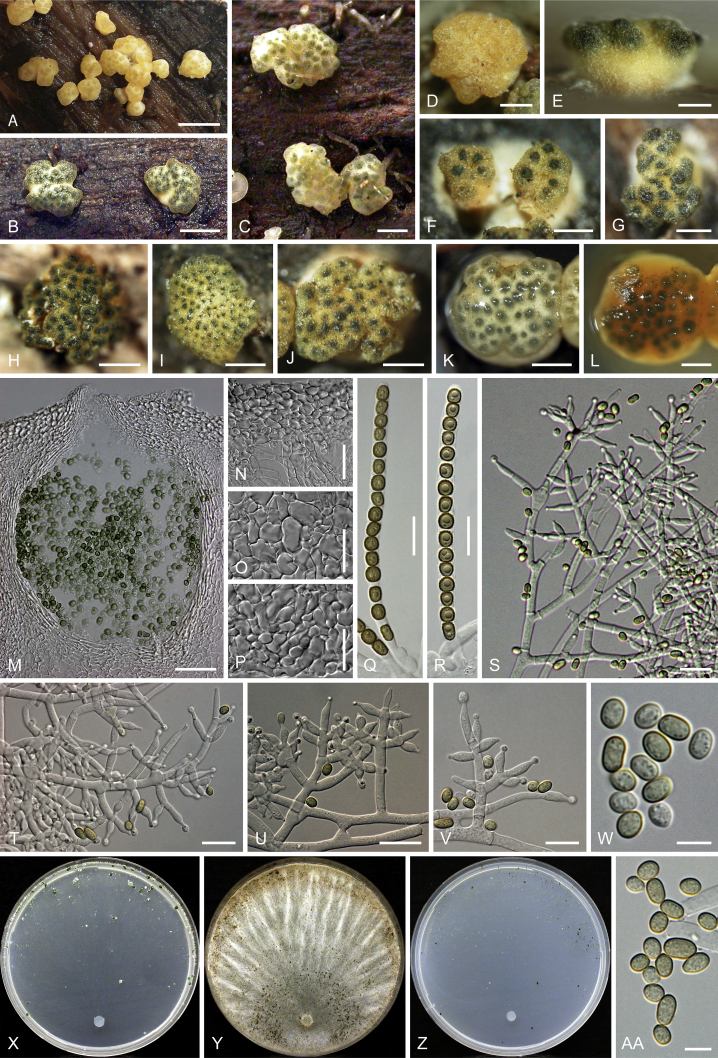
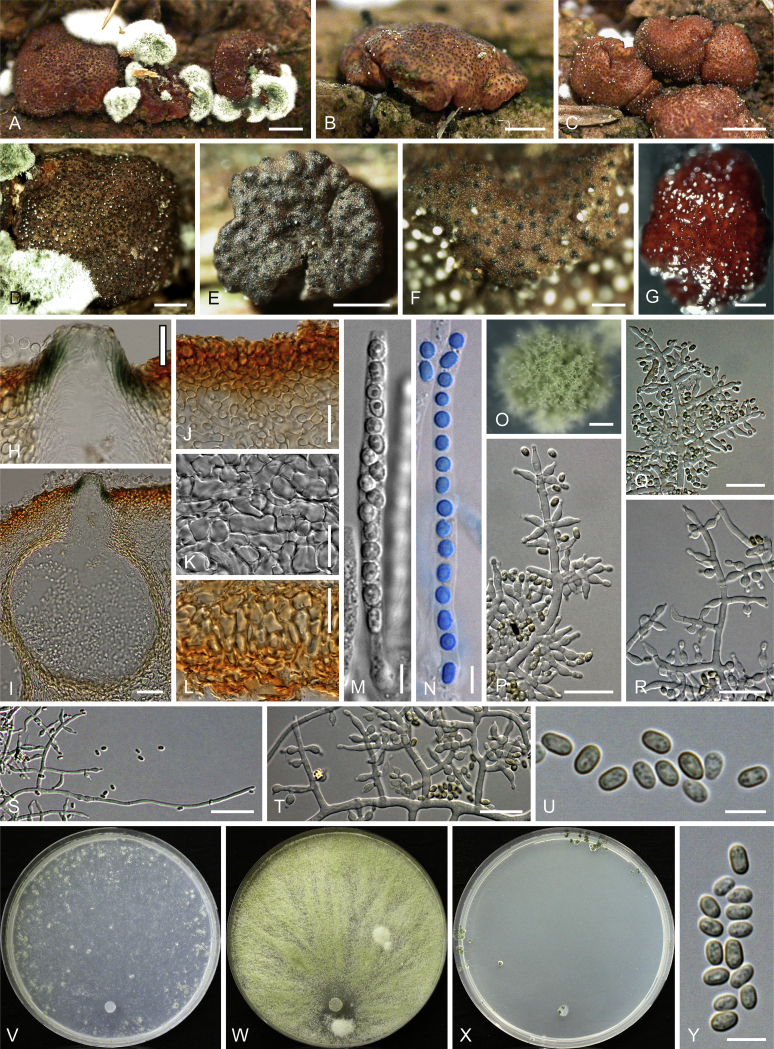
Cultures were prepared and maintained as described previously (Jaklitsch et al., 2005, Jaklitsch, 2009). Cultures used for study of asexual morph micro-morphology were grown on CMD (cornmeal agar from Sigma, St. Louis, Missouri, supplemented with 2 % (w/v) D(+)-glucose-monohydrate) containing 0.02 % (w/v) streptomycin sulfate (Sigma) and 0.02 % (w/v) neomycin sulfate (Sigma), PDA (potato dextrose agar, Merck, Darmstadt, Germany) and low nutrient agar (SNA, Nirenberg 1976) or exceptionally MEA (2 % malt extract, 2 % agar-agar, both from Merck) at 25 °C under alternating 12 h cool white fluorescent light and 12 h darkness. Growth rate experiments, recording of culture characteristics and morphological analyses of microscopic characters were carried out as described earlier (Jaklitsch et al., 2005, Jaklitsch, 2009). These papers should also be consulted for the descriptive terminology used here. Freezing microtome sections were prepared at (8–)10–12 μm. Microscopic observations were made in 3 % KOH, except for microtome sections that were examined in lactic acid. Chlamydospores were measured from 7–30-d-old cultures on CMD or SNA plates under a compound microscope using a 40× objective. Data were gathered using a Nikon Coolpix 4500 or a Nikon DS-U2 digital camera and measured by using NIS-Elements D v. 3.0 software. Methods of microscopy included stereo-microscopy (stereo) and Nomarski differential interference contrast (DIC). The Methuen Code by Kornerup & Wanscher (1978) was used as the colour standard.
DNA isolation and sequencing
The extraction of genomic DNA from mycelium grown in 1 % liquid malt extract was performed as reported previously (Jaklitsch et al. 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany). A ca. 1.2 kb fragment of the translation elongation factor 1 alpha (tef1) was amplified using the primers EF1-728F (Carbone & Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005). A ca. 1.1 kb fragment of RNA polymerase II subunit B (rpb2) was amplified using the primer pair fRPB2-5f and fRPB2-7cr (Liu et al. 1999). A 0.9 kb fragment of the larger subunit of ATP citrate lyase (acl1) was amplified using the primers acl1-230up and acl1-1220low (Gräfenhan et al. 2011). DNA sequences were obtained after purification of the amplicons with an enzymatic PCR clean-up as described by Jaklitsch (2009) using the BigDye Terminator v. 3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, California) and an automated DNA sequencer (3730xl DNA Analyzer, Applied Biosystems) with the same primers as in PCR or with the internal primers TEF1_INTF and TEF1_INTR (Voglmayr & Jaklitsch 2011) for tef1.
Phylogenetic analyses
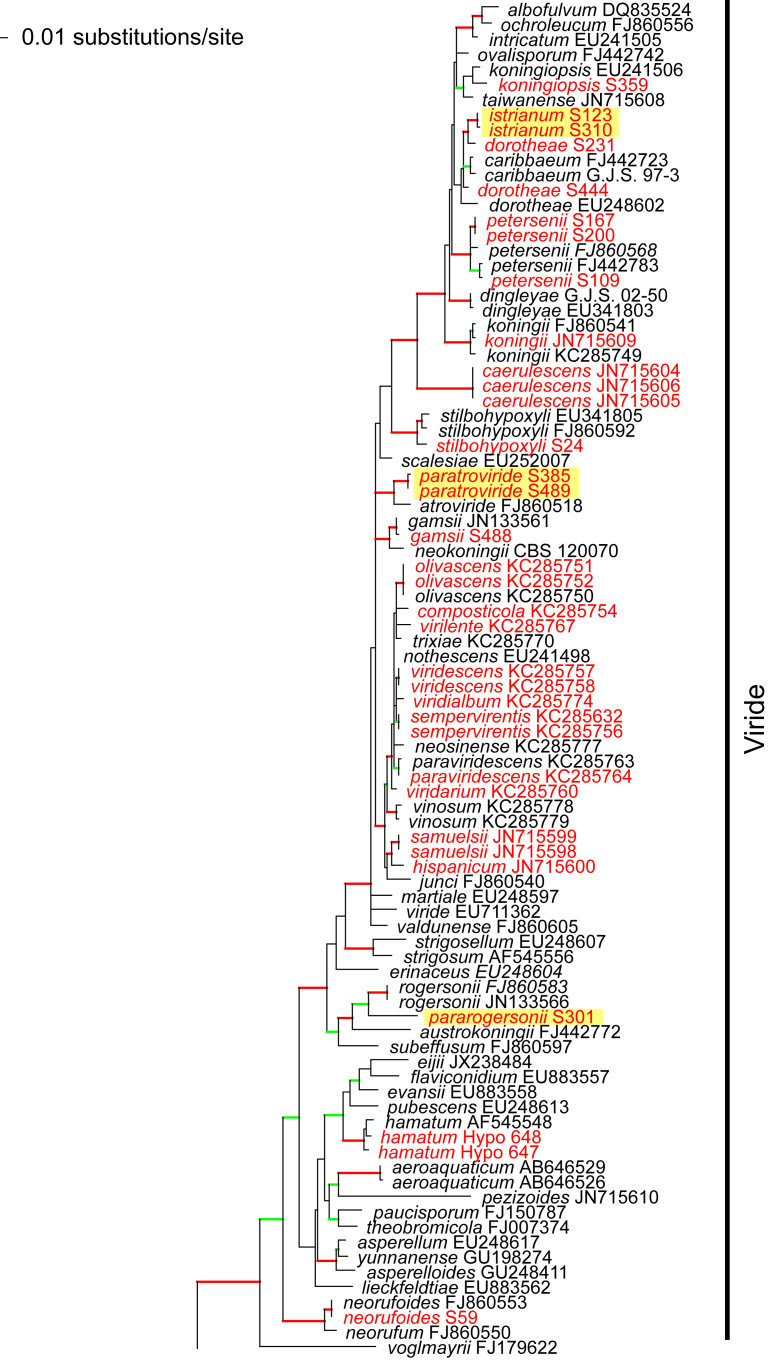
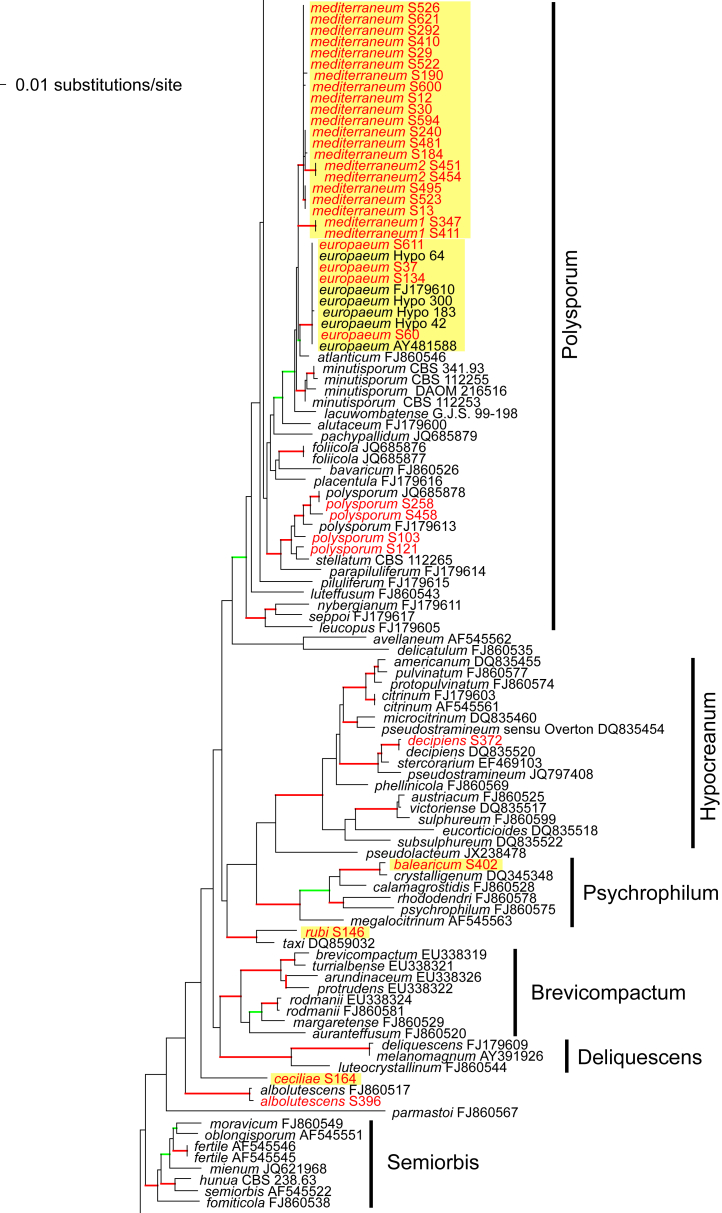
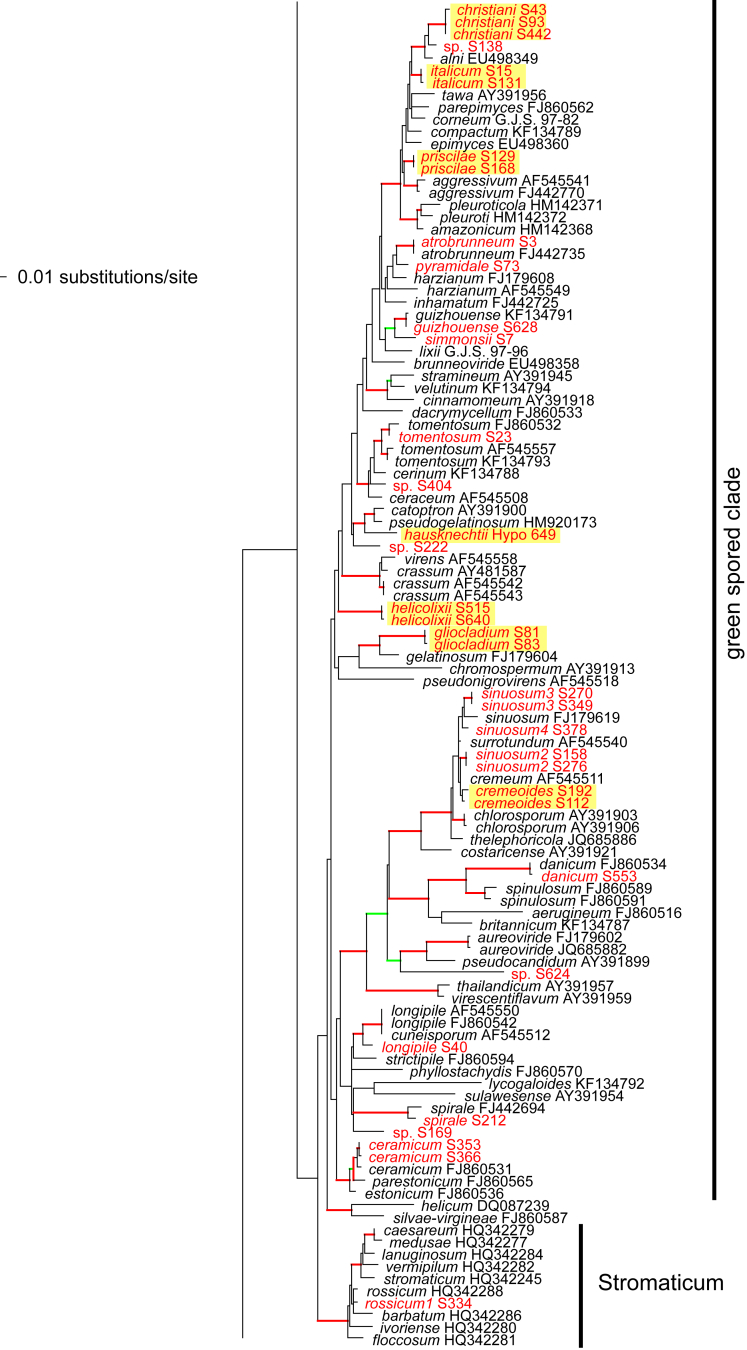
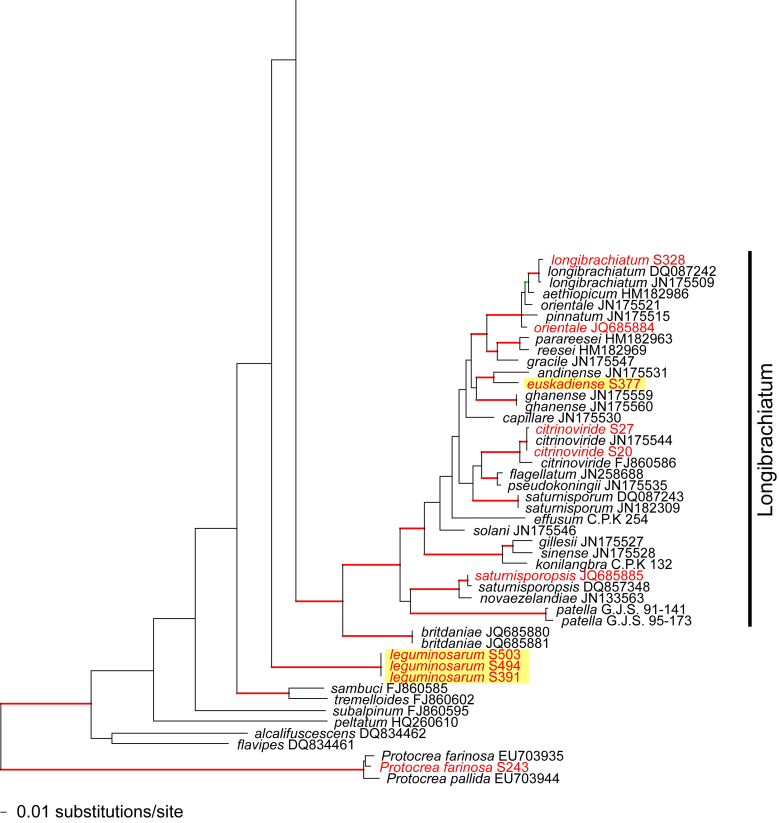
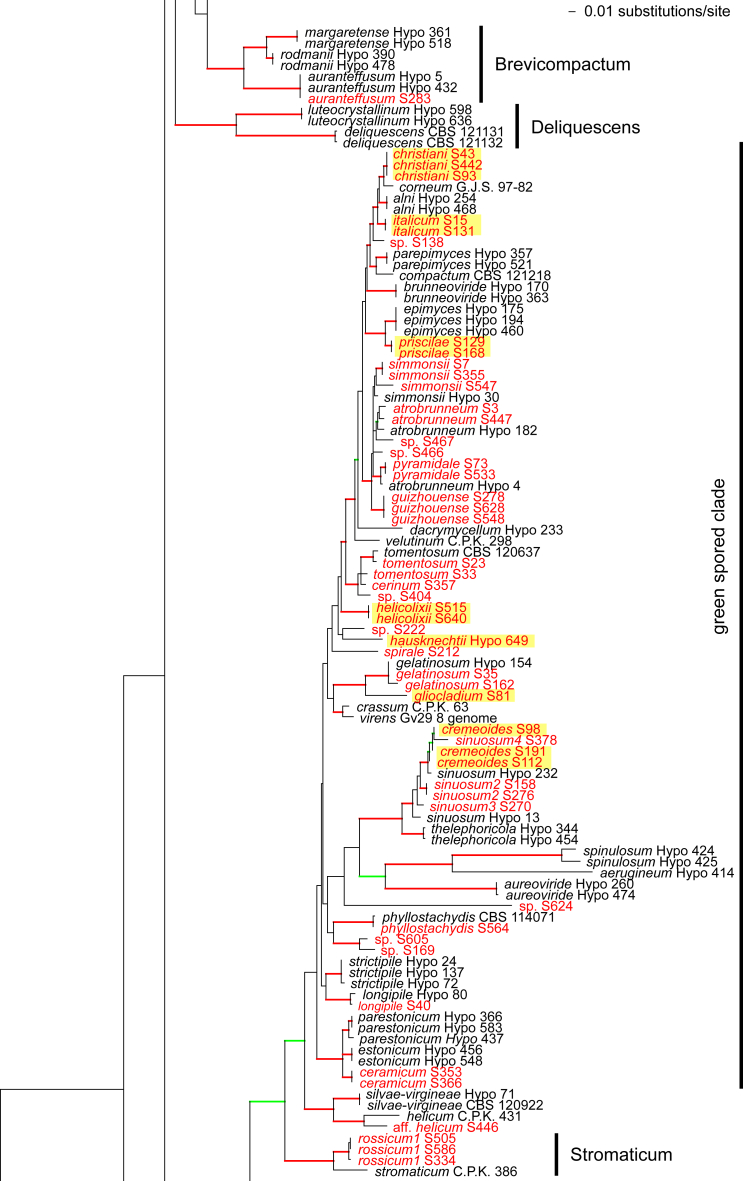
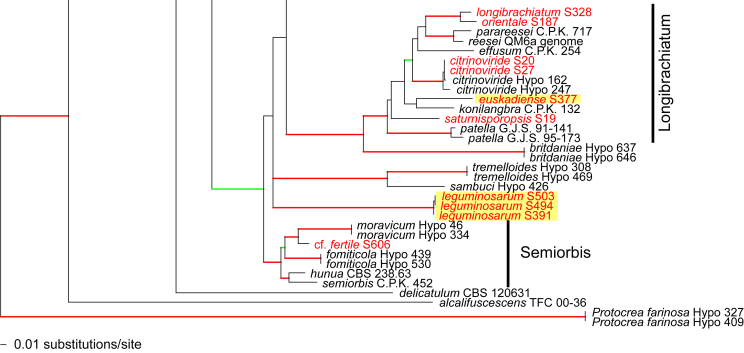
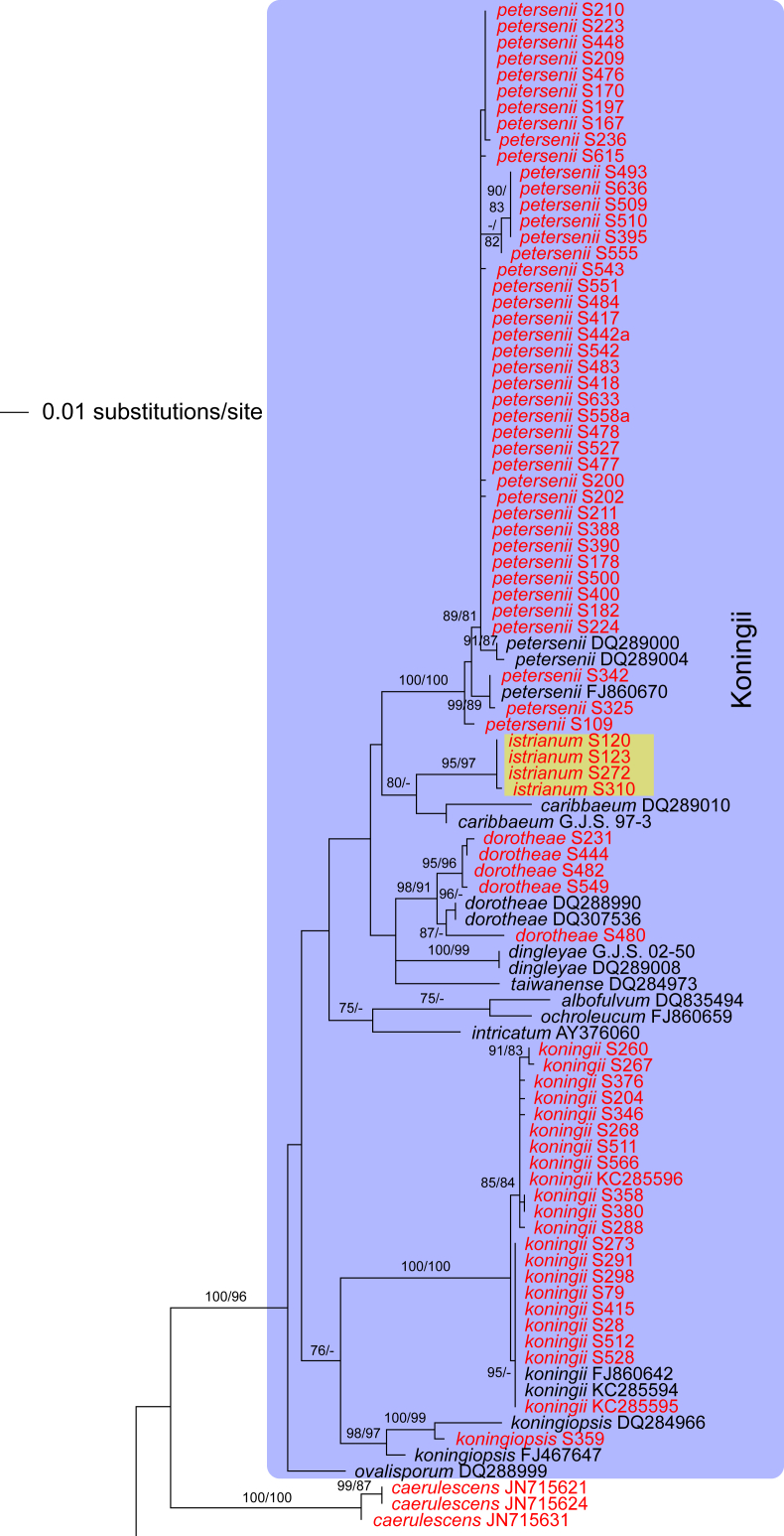
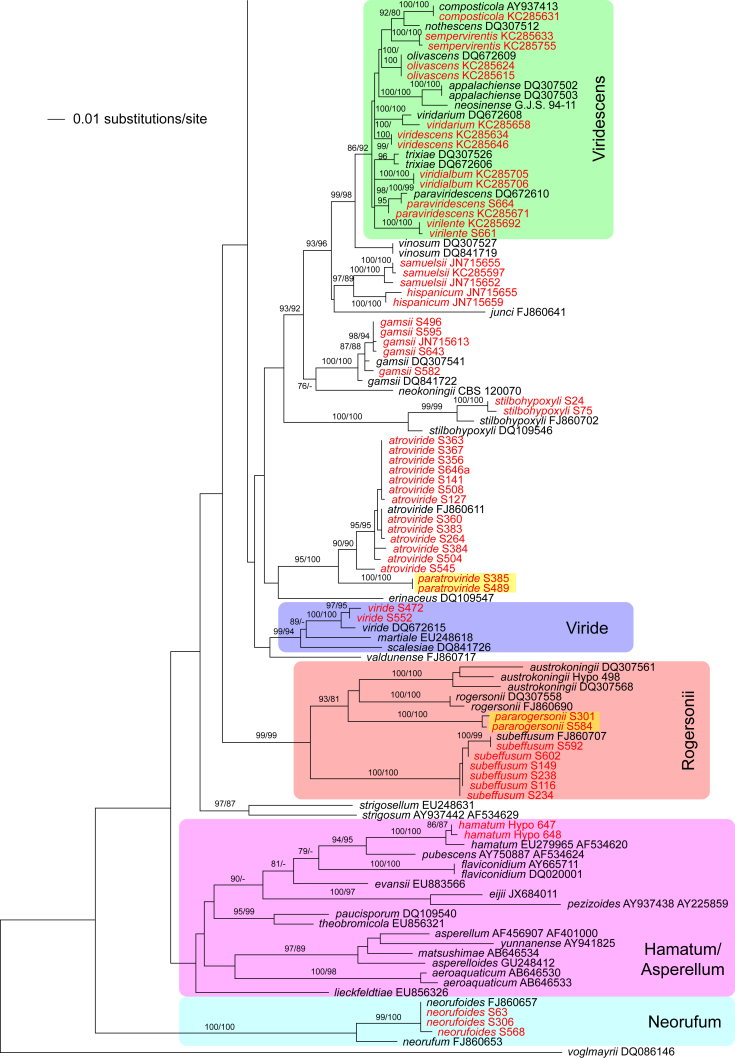
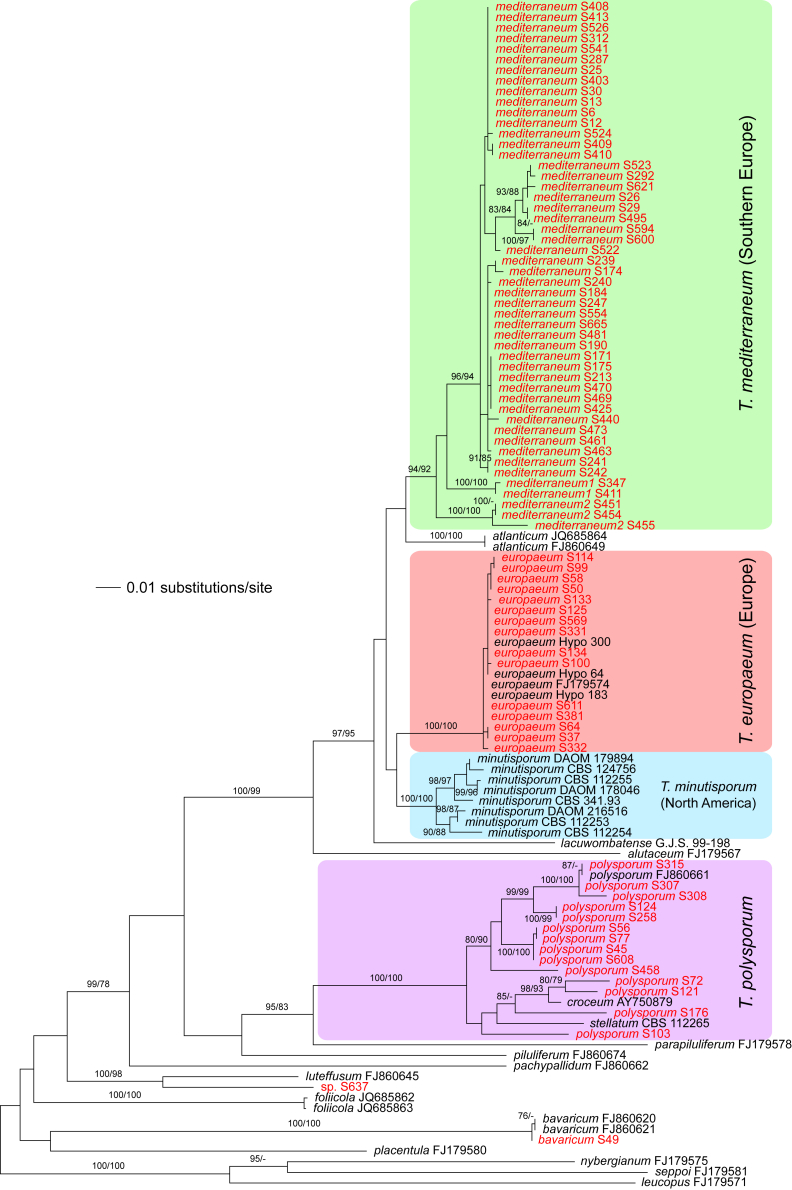
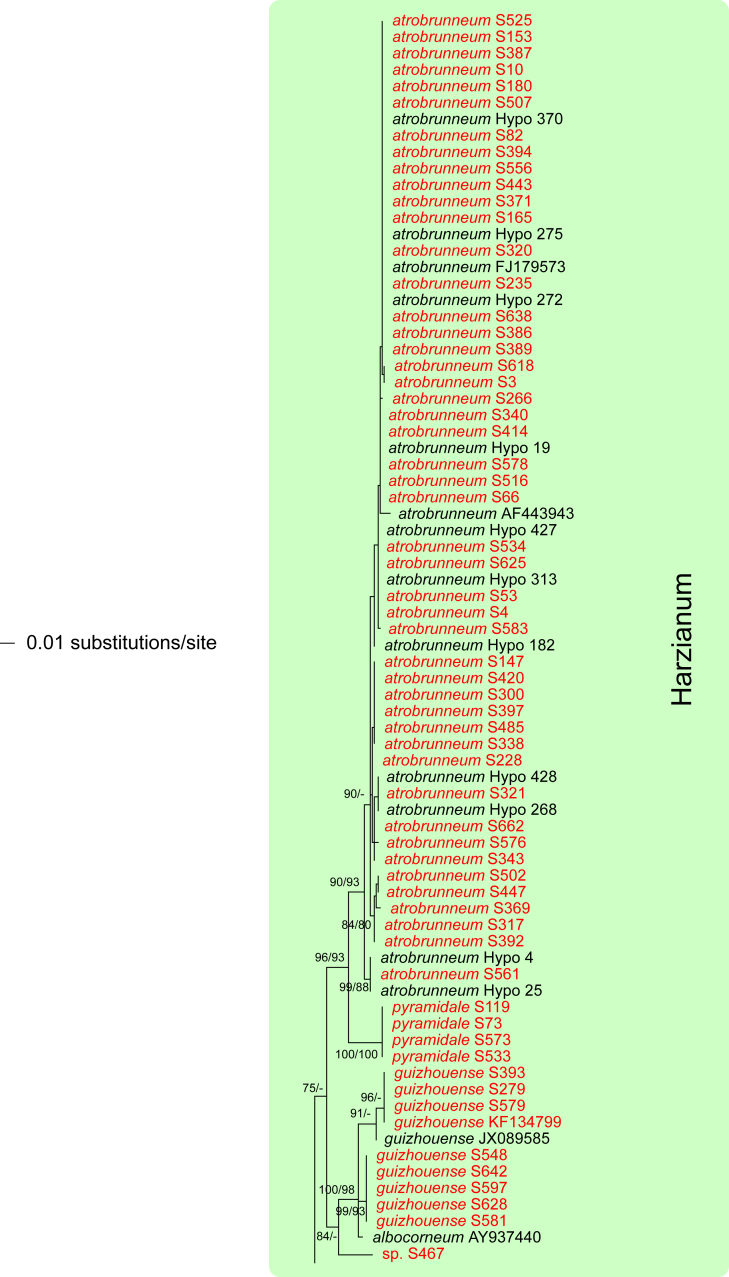
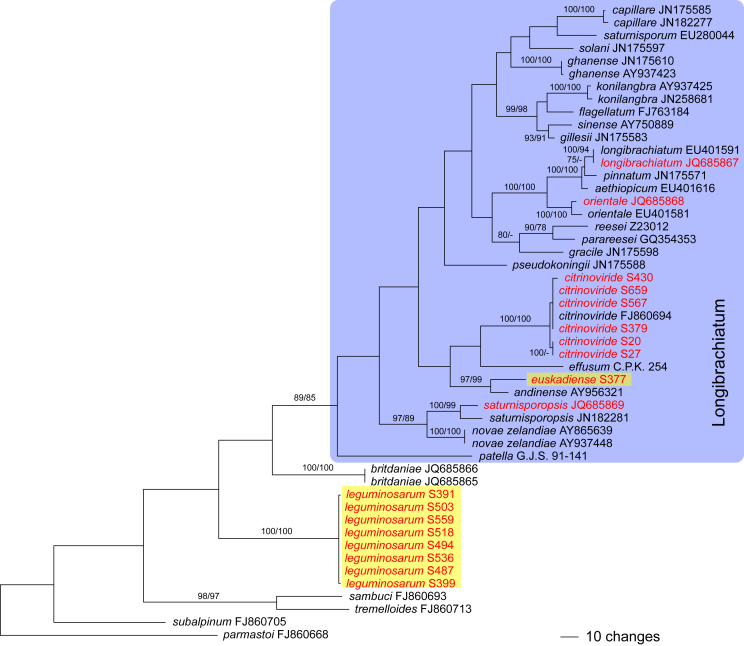
The three markers (acl1, rpb2, tef1) sequenced in the present study were analysed separately. For a universal phylogeny of Trichoderma, rpb2 sequences of representative strains obtained during the present study or in former works (Jaklitsch, 2009, Jaklitsch, 2011, Jaklitsch et al., 2012, Jaklitsch et al., 2013, Jaklitsch et al., 2014) were complemented with GenBank sequences. Likewise, a universal phylogeny of Trichoderma was produced with the acl1 sequences generated during the present study. Due to alignment issues, no universal phylogeny spanning the whole genus can be produced for tef1, the main marker currently used for phylogenetic species delimitation in Trichoderma. Therefore, four main subgroups of Trichoderma were analysed separately, according to the results of the rpb2 tree (Fig. 1). For these analyses, tef1 sequences obtained for numerous strains collected in Southern Europe and the Canary Islands during this study were aligned with sequences downloaded from GenBank to cover as much of the described biodiversity of the genus as possible.
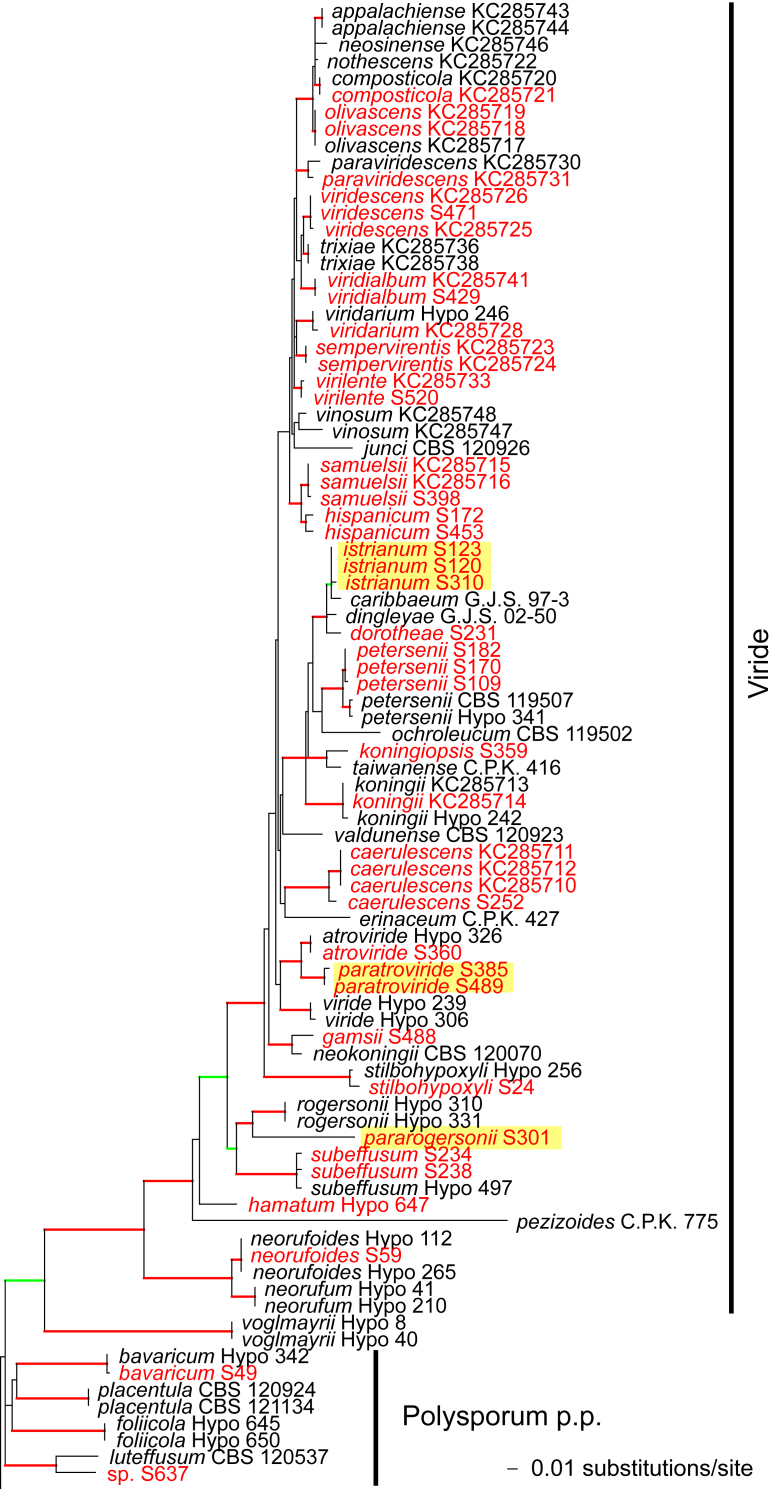
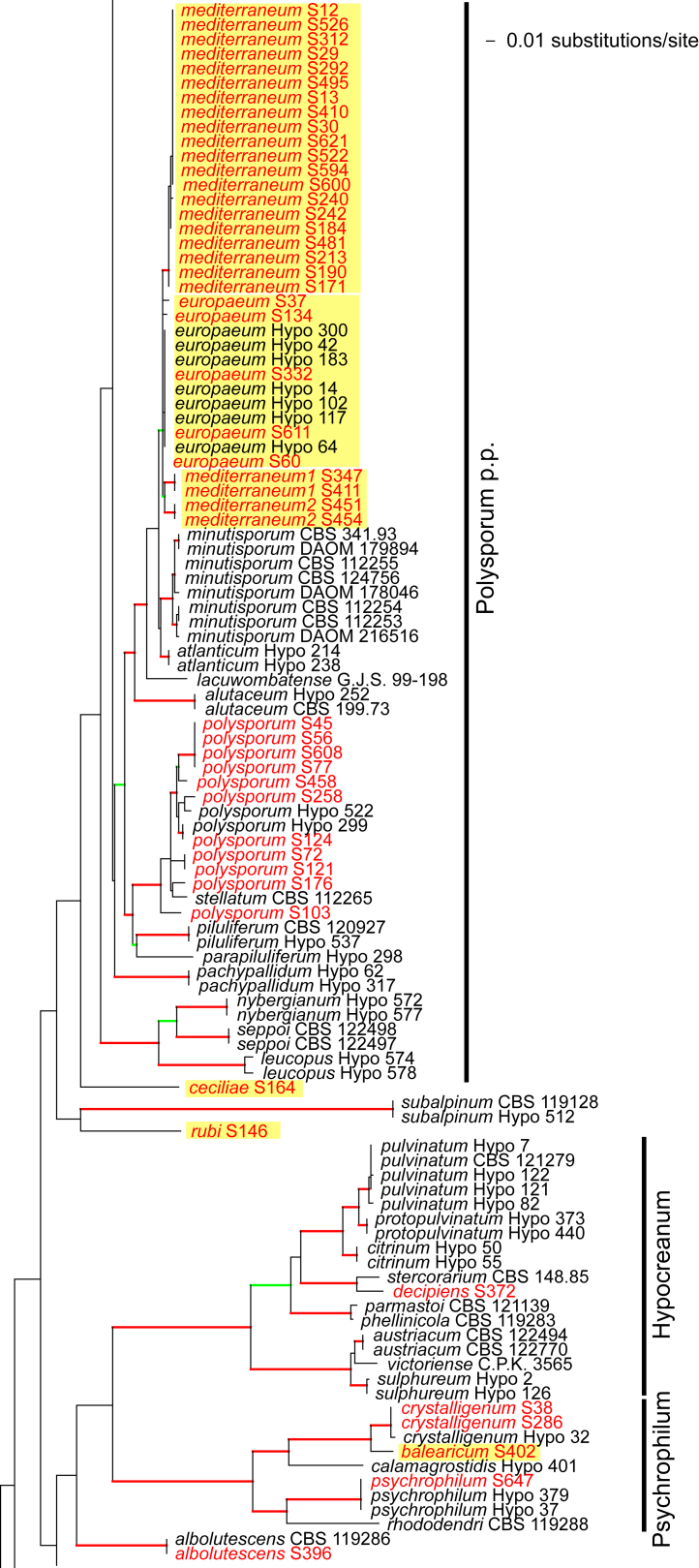
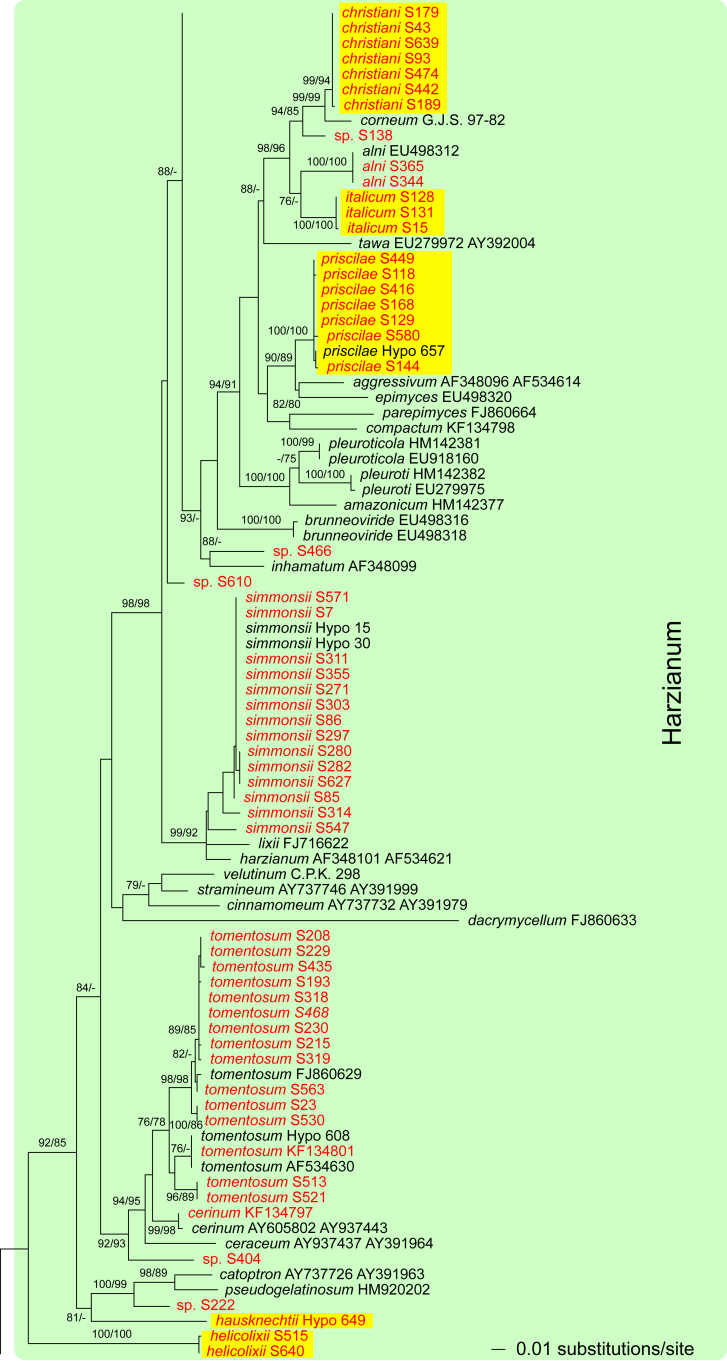
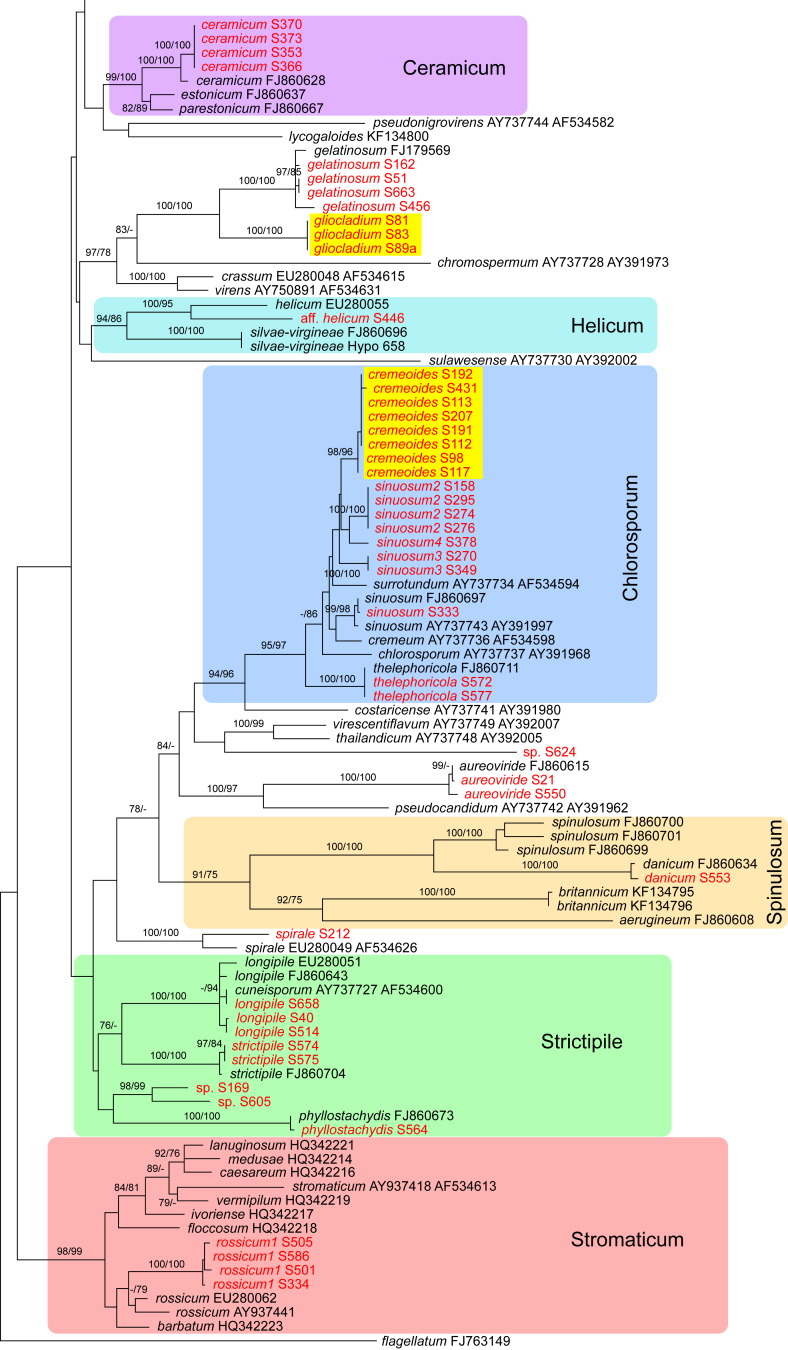
Fig. 1.
Phylogram of the best maximum likelihood tree (lnL = -34323.8842) revealed by RAxML from an analysis of the rpb2 sequence alignment. Red branches denote ML and/or MP bootstrap support values equal to or higher than 90 %, green branches those with ML and/or MP bootstrap support values between 75 and 90 %. Accessions collected during the present study are formatted in red, and new species described in the present work are highlighted in yellow.
All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft), with a gap open penalty of 1.0 and a gap extension penalty in the range of 0.05 to 0.1, with a tree building number = 100 and maxiterate = 100. The resulting alignments were checked and refined using BioEdit version v. 7.0.4.1 (Hall 1999).
For ML analyses, 500 rounds of random addition of sequences as well as 500 fast bootstrap replicates were computed with RAxML (Stamatakis 2006a) as implemented in raxmlGUI v. 1.3 (Silvestro & Michalak 2012) using the GTRGAMMAI and GTRCATI algorithms, respectively. The GTRCATI substitution model efficiently approximates the well-known general time-reversible model (GTR; Rodríguez et al. 1990) with gamma-distributed substitution rates, additionally assuming a proportion of invariant sites (GTR+I+G) (Stamatakis 2006b).
Maximum parsimony (MP) analyses were performed with PAUP v. 4.0 b10 (Swofford 2002), using 1000 replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect, COLLAPSE = MINBRLEN). All molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data. Bootstrap analyses with 500 replicates were performed in the same way, but using five rounds of random sequence addition and subsequent branch swapping during each bootstrap replicate; in addition, each replicate was limited to 1M rearrangements.
Results and discussion
Phylogeny
Overall phylogeny of Trichoderma based on rpb2 and acl1 sequence data
Of the 1 084 characters included in the rpb2 matrix, 450 were parsimony-informative. Fig. 1 shows the best ML tree (lnL = -34323.8842); the red branches denote ML and/or MP bootstrap support values equal to or higher than 90 %, green branches those with ML and/or MP bootstrap support values between 75 and 90 %.
The genus-wide phylogenetic tree based on rpb2 sequences is the hitherto most complete tree representing 228 named species of Trichoderma and several single isolates or groups, which are apparently additional, not yet taxonomically treated species. It supports the recognition of the following clades or groups, from the top of the phylogram shown in Fig. 1: the Viride Clade (= section Trichoderma), comprising several subclades, the paraphyletic Polysporum Group (re-labelled from the “pachybasium core group” of Jaklitsch, 2009, Jaklitsch, 2011), a clade containing the Hypocreanum (= section Hypocreanum) and the Psychrophilum Clades, a clade containing the Brevicompactum and the Deliquescens (= Lutea) subclades, the Semiorbis Clade, the so-called green-spored species clade with two major subclades, and the Stromaticum Clade and the Longibrachiatum Clade (= section Longibrachiatum). Sectional terms are historic and are here re-labelled as clades; also the clade names are modified to correspond to the Trichoderma epithets. The Longibrachiatum Clade in its strict sense (cf. Samuels et al. 2012a), includes only the subclade containing the species T. longibrachiatum to T. novae-zelandiae, while T. britdaniae forms a stable base to this clade. Basal species, sometimes loosely associated with the Longibrachiatum Clade, have variable positions, depending on the number of species included and the type of analysis.
Of the 1 379 characters included in the acl1 matrix, 777 were parsimony-informative. Fig. 2 shows the best ML tree (lnL = -34512.6549); the red branches denote ML and/or MP bootstrap support values equal to or higher than 90 %, green branches those with ML and/or MP bootstrap support values between 75 and 90 %.
Fig. 2.
Phylogram of the best maximum likelihood tree (lnL = -34512.6549) revealed by RAxML from an analysis of the acl1 sequence alignment. Colours as in Fig. 1.
The genus-wide phylogenetic tree based on acl1 sequences of 145 named species (Fig. 2) is in agreement with the rpb2 tree (Fig. 1) in that all clades are recognised and have largely the same positions, with the exception of the Semiorbis Clade, which is placed in a near-basal position in the acl1 tree. Positions of individual species within the clades and particularly of lone lineages differ substantially from the rpb2 tree. The latter (except T. britdaniae and T. voglmayrii) are so-called jumpers that vary their position depending on markers, included species numbers and the method of phylogenetic inference, while differences in relationships within clades may be caused by the limited number of species so far included in the acl1 tree. In effect, acl1 appears to be useful for the determination of phylogenetic relationships in Trichoderma; however, like rpb2, it does not always fully resolve at the species level. Sequences are generally easy to produce, although in rare cases, e.g. T. danicum, no amplicon was produced, which calls for development of additional primers for this gene.
Species numbers, diversity and distribution
Altogether 636 specimens of Trichoderma were identified to the species level using tef1 sequences and partly by morphology, yielding more than 90 putative species of Trichoderma, of which 74 are named. From these data, 17 species are described as new (see Taxonomy section below) and 10 isolates are singletons representing putative new species. In the 11 mixed specimens each containing two species, mostly sexual morphs were accompanied by asexual morphs of other species, which predominantly belong to the Viride Clade (T. atroviride, T. caerulescens, T. hispanicum, T. petersenii, and T. viridescens s.str.), frequently also T. polysporum, less commonly T. atrobrunneum, T. gliocladium or T. tomentosum.
Generally, in Mediterranean and submediterranean climates Trichoderma asexual morphs are much more common than sexual morphs, owing to short periods of moisture. Often immature stromata were found. The situation is different in Macaronesia, where sexual morphs of many species are common. We do not describe asexual morphic singletons as new species, due to insufficient information. However, four new species were based on single collections, because morphological data from both morphs clearly supported evidence of new species as suggested by DNA data.
All 636 identified specimens and isolates were allocated to the phylogenetic clades of Trichoderma. The distribution of the clades in the different studied regions is shown in Table 2. The clades with the highest percentages of isolates are the Viride Clade and the clade with green ascospores, of the latter in particular the Harzianum Clade. Our study clearly shows that the Viride Clade, represented by 43–62 % of all isolates, dominates in regions with Mediterranean climate. An excursion during an exceedingly cold period in Madeira revealed nearly 92 % of the specimens belonging to this clade (not explicitly shown). However, in northern Italy only 20 % of all Trichoderma isolates belonged to the Viride Clade, while in central and southern Italy they reached 36 %, in a region characterised by Central European climate in northern and by mostly submediterranean climate in central and southern Italy. For comparison, the distribution of Trichoderma isolates from sexual morphs in Central and Northern Europe (Jaklitsch, 2009, Jaklitsch, 2011; given as Central Europe below) is included. These figures corroborate the conclusion above, as only 15 % of the isolates belonged to the Viride Clade in Central Europe, although it should be noted that a study in these regions based on collected asexual morph colonies is missing, which might change this pattern. The most common species or rather species complexes in the Viride Clade are T. viridescens s.l., which varies among regions but may comprise more than 30 % of all isolates, T. petersenii s.l., which reaches 16 % in Macaronesia but is absent or rare in regions with Central European climate, and T. caerulescens amounting up to 18 % in Croatia or Sardinia, but is absent in northern regions. The second largest group is the clade comprising the species with green ascospores. As a whole, the percentage of this group in Southern Europe and Macaronesia is comparable with Central Europe, although the percentage tends to be higher in the North. Interestingly, 65 % of all isolates from northern Italy belong to this group. The Harzianum Clade generally ranged between 13 and 28 % and peaked at 35 % in northern Italy. Generally, the results presented here differ from all diversity studies of Trichoderma from soil (see Introduction for references), which listed T. harzianum s.l. as a predominant species complex, whereas the Viride Clade is clearly dominant on plant and fungal materials in all regions studied here. The Polysporum group turned out to be the third largest in the studied region, with a considerably varying percentage that was comparable or lower than in Central Europe, except for Sardinia, where the clade reached 25 % of all isolates. In Sardinia, the Longibrachiatum Clade peaked at nearly 11 %, in northern Spain it reached 8.7 %, while it was negligible in other regions. Remarkably, the Hypocreanum Clade is virtually absent from the Mediterranean; it was represented by only a single collection of T. decipiens in Southern Europe but outside the Mediterranean climate in southwestern France, whereas it amounted to 12 % in Central Europe.
Table 2.
Distribution of Trichoderma clades in Europe (in % of total isolates per region). Numbers do not always add up perfectly to 100 % due to rounding error.
| Clade/Group | Corfu | Crete | Mallorca | Sardinia | Mediterranean Islands | Macaronesia | Northern Spain | Southern Spain |
| Viride | 62.1 | 61.3 | 45 | 42.9 | 51.6 | 57.1 | 43.5 | 50.8 |
| T. caerulescens | 17.2 | 6.5 | 5 | 17.9 | 10.9 | 16.3 | 6.2 | |
| T. petersenii s.l. | 10.3 | 15 | 7.8 | 16.3 | 4.3 | 10.8 | ||
| T. viridescens s.l. | 31 | 29 | 10 | 7.1 | 18.8 | 13.6 | 30.4 | 13.8 |
| Green-spored spp. | 31 | 16.1 | 27.5 | 21.4 | 24.2 | 23.1 | 34.8 | 23.1 |
| Harzianum clade | 27.6 | 12.9 | 27.5 | 17.9 | 21.1 | 15 | 21.7 | 20 |
| Polysporum | 6.9 | 12.9 | 15 | 25 | 14.8 | 17.7 | 10.9 | 9.2 |
| Longibrachiatum | 10.7 | 2.3 | 1.4 | 8.7 | ||||
| Psychrophilum | 2.5 | 0.8 | 1.5 | |||||
| Brevicompactum | 1.5 | |||||||
| Hypocreanum | ||||||||
| Others | 9.7 | 10 | 5.5 | 0.7 | 2.2 | 13.8 | ||
| Clade/Group | Southern France |
Northern Italy |
Central and Southern Italy |
Croatia |
Southern Europe + Macaronesia |
Central Europe |
||
| Viride | 48.1 | 20 | 36.5 | 46.7 | 47.3 | 14.9 | ||
| T. caerulescens | 3.6 | 18.3 | 9.1 | |||||
| T. petersenii s.l. | 6.9 | |||||||
| T. viridescens s.l. | 22.2 | 5 | 21.9 | 6.7 | 17 | 3.9 | ||
| Green-spored spp. | 40.7 | 65 | 35.8 | 26.7 | 29.6 | 29.7 | ||
| Harzianum clade | 22.2 | 35 | 14.6 | 18.3 | 18.1 | 8.2 | ||
| Polysporum | 5 | 15.3 | 11.7 | 13.4 | 18.9 | |||
| Longibrachiatum | 5 | 1.7 | 1.9 | 1.2 | ||||
| Psychrophilum | 5.1 | 6.7 | 2 | 6.3 | ||||
| Brevicompactum | 3.7 | 5 | 2.9 | 6.7 | 1.7 | 3.9 | ||
| Hypocreanum | 12.2 | |||||||
| Others | 7.4 | 4.4 | 3.9 | 12.9 | ||||
As two interesting studies on soil-dwelling Trichoderma species in the investigated region were published, we want to compare our results briefly with those studies. One was a study by Migheli et al. (2009) in Sardinia, an island with a high proportion of endemic plants. The authors studied a remarkably high number of 482 isolates, in which they identified 15 species: T. asperellum, T. atroviride, T. gamsii, T. hamatum, T. harzianum (here identified as the two species T. atrobrunneum and T. harzianum s.str. based on the cited tef1 accessions), T. koningii, T. koningiopsis, T. semiorbis, T. spirale, T. tomentosum, T. velutinum, T. virens, T. viridescens, and Trichoderma sp. Vd2 (later described as T. samuelsii by Jaklitsch et al. 2012; see also Jaklitsch et al. 2013) and Trichoderma sp. (harzianum s.l.). In our study, a 1-wk excursion yielded 28 isolates representing 14 species: T. atrobrunneum, T. aureoviride, T. caerulescens, T. citrinoviride, T. italicum, T. koningii, T. mediterraneum, T. paraviridescens, T. samuelsii, T. saturnisporopsis, T. simmonsii, T. stilbohypoxyli, T. tomentosum, and T. viridescens. Therefore, only four species (T. atrobrunneum, T. koningii, T. samuelsii and T. tomentosum) were isolated from both soil and plant materials. One of our isolates turned out to be a new species and was described by Samuels et al. (2012a) as T. saturnisporopsis of the Longibrachiatum Clade. Based on these results, it is likely that more intense collecting on dead plant parts in Sardinia would result in a higher species number. On the Canary island Tenerife, Zachow et al. (2009) investigated fungal diversity in the rhizosphere of endemic plants in the different climatic and vegetation zones. They found eight species in 42 isolates, corresponding to low diversity of ubiquitous and widely distributed Trichoderma spp., and no correlation with plant communities or abiotic factors, in contrast to other fungi. They also found a marked dominance of T. harzianum, which is quite different from our study on plant material revealing 17 named and three putatively new species from 66 isolates, of which 62 % belong to the Viride Clade and only 16.7 % to T. harzianum s.l.
Phylogenetic clades and groups of Trichoderma
The phylogenetic clades are revisited according to the position on the rpb2 tree (Fig. 1), starting at its top, and discussed on the basis of the tef1 trees (Fig. 3, Fig. 4, Fig. 5, Fig. 6). For more details see under the individual species in the Taxonomy section.
Fig. 3.
Phylogram of the best maximum likelihood tree (lnL = -12771.3005) revealed by RAxML from an analysis of the tef1 sequence alignment of the Viride Clade (formerly Trichoderma section Trichoderma). ML and MP bootstrap support values higher than 75 % are given at first and second position, respectively, above or below the branches. Accessions collected during the present study are formatted in red, and new species described in the present work are highlighted in yellow.
Fig. 4.
Phylogram of the best maximum likelihood tree (lnL = -9305.2705) revealed by RAxML from an analysis of the tef1 sequence alignment of the Polysporum Group (formerly pachybasium core group). Presentation as in Fig. 3.
Fig. 5.
Phylogram of the best maximum likelihood tree (lnL = -12771.3005) revealed by RAxML from an analysis of the tef1 sequence alignment of the green-spored clade. The tree was rooted with the Stromaticum Clade according to the rpb2/acl1 trees (Fig. 1, Fig. 2); note that the Helicum Clade is embedded within the Green-spored Clade. Presentation as in Fig. 3.
Fig. 6.
Phylogram of the single MP tree of 1594 steps revealed by PAUP from an analysis of the tef1 sequence alignment of the Longibrachiatum Clade. Presentation as in Fig. 3.
The Viride Clade (formerly section Trichoderma)
Of the 1 427 characters included in the tef1 matrix of the Viride Clade, 449 were parsimony-informative. Figure 3 shows the best ML tree (lnL = -12771.3005) with ML and MP bootstrap support values higher than 75 % given at first and second position, respectively, above or below the branches. This well (Fig. 1) to highly (Fig. 2) supported clade contains one of the most difficult groups of species, in terms of species recognition and distinction. Sexual morphs are characterised by inconspicuous ostiolar dots and generally hyaline ascospores, and all asexual morphs have green conidia that are often warted. Samuels et al. (2006) and Jaklitsch et al. (2006a) treated the two large subclades of this large clade, the Koningii and Viride Clades or species complexes, the composition of which has changed over time. Warted conidia occur in species in the Viride Clade, but Jaklitsch et al. (2012) added T. caerulescens, a species with verruculose conidia to the Koningii Clade. The structure of the overall clade is however more complex, as there are additional subclades, such as the Hamatum/Asperellum Clade, the Rogersonii Clade, the Neorufum Clade, and several smaller subclades. We here unite and address the entirety of these clades as Viride Clade.
In recent years several species have been added to the clade, e.g., T. aeroaquaticum and T. matsushimae by Yamaguchi et al. (2012), T. asperelloides by Samuels et al. (2010), T. caerulescens, T. hispanicum and T. samuelsii by Jaklitsch et al. (2012), T. eijii by Kim et al. (2013), and T. strigosellum by López-Quintero et al. (2013); most recently Jaklitsch et al. (2013) disentangled the T. viridescens species complex recognising 13 species, of which they (re-)described the 12 species T. appalachiense, T. composticola, T. neosinense, T. nothescens, T. olivascens, T. paraviridescens, T. sempervirentis, T. trixiae, T. viridarium, T. viridescens, T. viridialbum and T. virilente.
Here we add the three new species T. istrianum, T. pararogersonii and T. paratroviride, and for the first time we describe the sexual morph of T. hamatum from two specimens collected in La Réunion. Trichoderma hamatum is the type species of the formerly recognised genus Pachybasium, which Bissett (1992) reduced to a section of Trichoderma. This section turned out to be polyphyletic and T. hamatum was removed to the sect. Trichoderma.
Figure 3 shows the 61 currently recognised species in this clade, of which 25 species were identified in Southern Europe (including La Réunion). The most common species of the Viride Clade in this region include T. caerulescens, represented by 57 isolates, T. viridescens s.l. (altogether 106 isolates), in particular T. olivascens represented by 33 isolates, T. viridescens s.str. by 25 isolates and T. paraviridescens by 22 isolates. Also T. petersenii with 43 isolates, T. koningii with 21 isolates and T. atroviride by 12 isolates are common.
The Polysporum group
Of the 1 408 characters included in the tef1 matrix of the Polysporum Group, 396 were parsimony-informative. Figure 4 shows the best ML tree (lnL = -9305.2705) with ML and MP bootstrap support values higher than 75 % given at first and second position, respectively, above or below the branches.
The Polysporum Group corresponds to the pachybasium core group (Jaklitsch 2011); only one species, T. foliicola, was added by Jaklitsch & Voglmayr (2012). Because it does not form a monophyletic but a paraphyletic lineage in the rpb2 and acl1 trees due to the Viride Clade being nested within (Fig. 1, Fig. 2), it is here referred to as a group rather than a clade. However, due to lack of backbone support, monophyly of the Polysporum Group cannot be ruled out, and to enable comparison with previous studies (e.g. Jaklitsch, 2011, Jaklitsch and Voglmayr, 2012) it is here treated as a group of its own. In the current study numerous isolates of T. minutisporum s.l. were collected. Phylogenetic analyses using tef1, but also rpb2 and acl1 sequences (Fig. 1, Fig. 2, Fig. 4), clearly separated the strains into a European and an American clade. The American clade is T. minutisporum s.str., but may be subdivided in future at the species level. The European clade splits up into two main lineages. The first, corresponding to Hypocrea minutispora/T. minutisporum sensu Jaklitsch (2011), has a wide distribution in Europe; it has mostly been collected in Central and Northern Europe but occurs in Southern Europe as well; it is described as the new species T. europaeum below. The second lineage has only been found in Southern Europe and is described below as the new species T. mediterraneum. Within the latter the two subclades T. mediterraneum 1 and 2 were identified, which also differ in acl1 and rpb2 sequences from T. mediterraneum s.str. and may therefore be recognised as distinct species. Trichoderma mediterraneum 1 contains two isolates from Mallorca and the Basque Country in Spain, while T. mediterraneum 2 corresponds to three isolates, one conidial and two holomorphic, from specimens collected on the same day on Chamaecytisus proliferus in the northwestern corner of the Canary island La Palma. This distribution pattern suggests that the Mediterranean basin served as glacial refugia from which only T. europaeum managed to colonise Central and Northern Europe after glaciation. Also the high genetic diversity of T. mediterraneum, compared to T. europaeum (Fig. 4), is considered typical for glacial refugia (Feliner 2011) but may also be the result of wider ecological amplitude.
Trichoderma polysporum is not uncommon as its sexual morph but also as its (white) asexual morph in (sub-)Mediterranean habitats. This species had been infrequently collected as asexual morph on plant material, but was more commonly isolated from soil (Bissett 1992). Considerable variation of tef1 sequences was found among the isolates, which is in agreement with isolates from other regions (J. Bissett, unpubl. data; pers. comm.). These variants however form a compact clade, which contains the ex-type of T. croceum (already synonymised with T. polysporum by Lu et al. 2004) and the ex-type of T. stellatum (isolate received from the CBS and newly sequenced), a species described from New Zealand (Lu et al. 2004). The latter thus also seems to be a synonym of T. polysporum. Besides T. mediterraneum, T. polysporum and one specimen of T. bavaricum in Italy, a singleton was detected on the Greek island Corfu (T. sp. S637), whose tef1 sequence had 95 % similarity with T. luteffusum.
The Hypocreanum (formerly section Hypocreanum), the Psychrophilum, Brevicompactum and Deliquescens Clades
The section Hypocreanum was revised by Overton et al., 2006a, Overton et al., 2006b and augmented with two species by Jaklitsch (2011). Jaklitsch et al. (2008b) added T. decipiens (as Hypocrea decipiens), which had been mistaken for Protocrea farinosa (as Hypocrea farinosa) by Overton et al. (2006b). A recent addition is T. pseudolacteum (Kim et al. 2013), which is a substitute of Hypocrea lactea sensu Doi (1972), and Kim et al. (2012a) clarified the true identity of T. pseudostramineum. Hypocrea pseudostraminea (as T. pseudostramineum sensu Overton in Fig. 1) in the sense of Overton et al. (2006a) will need a new name and typification. Kim et al. (2012a) noted that Jaklitsch (2011: 244) had suggested that H. pseudostraminea sensu Overton et al. (2006a) was misidentified. However, the conclusion reached by Jaklitsch (2011) that the only European specimen listed under H. pseudostraminea by Overton et al. (2006a) was not this species, concerned only this specimen, while his whole concept of the species was primarily based on American material. For combinations of several species of this section in Trichoderma see Jaklitsch & Voglmayr (2014). In line with earlier analyses (see e.g., Jaklitsch, 2009, Jaklitsch, 2011), our phylogenetic analyses (Fig. 1, Fig. 2) clearly demonstrate that Aphysiostroma stercorarium, a fungus characterised by non-ostiolate ascomata in pulvinate stromata occurring on cow dung in central Spain near Madrid (Barrasa et al. 1986), belongs to the Hypocreanum Clade and is therefore combined in Trichoderma below. Based on this study, the Hypocreanum Clade is nearly non-existent in Southern Europe and absent from the Mediterranean, except for T. stercorarium. In the course of our study, only one specimen of T. decipiens was collected in southwestern France. In all of our analyses, the Hypocranum Clade was highly supported (Fig. 1, Fig. 2).
The Psychrophilum Clade, originally recognised as Megalocitrina Clade by Chaverri & Samuels (2004) and renamed and augmented by Jaklitsch, 2009, Jaklitsch, 2011, was highly supported in our analyses (Fig. 1, Fig. 2). Species of this clade are characterised by mostly light- or bright-coloured stromata and white-conidial gliocladium-like Trichoderma asexual morphs. Here we add the new species T. balearicum from Mallorca, which is morphologically similar to T. crystalligenum, but differs in gene sequences and a substantially more rapid growth. A remarkable finding is the collection of T. psychrophilum, which had been only known from Ericaceae (Rhododendron, Vaccinium) from the Alps, at a high elevation in Andalucía on Rubus ulmifolius.
The Brevicompactum Clade was defined by Degenkolb et al. (2008) and augmented by Jaklitsch (2011); it was highly supported in our analyses (Fig. 1, Fig. 2). In Southern Europe the two species T. auranteffusum (eight specimens) and T. margaretense (three specimens) were detected.
The small Deliquescens Clade (former Lutea Clade; sensu Jaklitsch 2011) also received high support in our analyses (Fig. 1, Fig. 2); only one sexual morph specimen of T. deliquescens (syn. Hypocrea lutea) was collected in the Gargano region of Apulia, Italy.
Species with green ascospores
Of the 1 434 characters included in the tef1 matrix, 512 were parsimony-informative. Fig. 5 shows the best ML tree (lnL = -12771.3005) with ML and MP bootstrap support values higher than 75 % given at the first and second position, respectively, above or below the branches. This large clade, here addressed as Green-spored Clade, includes both asexual and sexual morph species with green ascospores united by phylogenetic analyses, however, without significant statistical support. It is subdivided in two major groups, the Harzianum Clade and a paraphyletic group of species containing several subclades such as the Ceramicum, Chlorosporum and Spinulosum Clades and several smaller ones. Species of this clade were monographed by Chaverri & Samuels (2004) and augmented by Jaklitsch (2009). Recent additions include Trichoderma amazonicum (Chaverri et al. 2011), T. guizhouense (Li et al. 2013) and T. pseudogelatinosum (Kim et al. 2012a). Jaklitsch et al. (2014) synonymised Sarawakus with Trichoderma and recombined two species, the type species of Sarawakus, S. lycogaloides, and S. britannicus. The phylogenetic position of other former Sarawakus species remains undetermined. Druzhinina et al. (2010) analysed T. harzianum s.l. and anticipated taxonomic changes. In a separate analysis, Chaverri et al. (in press) recognised 15 species in the T. harzianum complex, of which T. atrobrunneum, T. guizhouense, T. harzianum, T. pyramidale and T. simmonsii occur in Europe. The tef1 GenBank accession labelled T. albocorneum (Table 1) is T. guizhouense; thus T. albocorneum should be restudied, based on fresh material from its original collection site in Japan. Trichoderma pyramidale is only known from Italy and Spain, whereas T. simmonsii, which has been found in warmer regions of most Southern European countries, but not in Macaronesia, also occurs in North America. With 48 isolates T. atrobrunneum (described as Hypocrea lixii/T. harzianum in Jaklitsch 2009) is by far the most common species of the whole clade in Southern Europe, just as in other parts of Europe.
Here we add the seven new species T. christiani, T. cremeoides, T. gliocladium, T. hausknechtii, T. helicolixii, T. italicum and T. priscilae to this clade. Fig. 5 includes 63 named species (32 in the Harzianum Clade), for which DNA data are currently available. Several species of Chaverri et al. (in press) that occur outside Europe are not included in the phylogenetic analysis, because that publication was not finished and data were not available at the time of computing our trees. The three new species T. rosulatum, T. rufobrunneum and T. stipitatum (Zhu & Zhuang 2015) are not included in our trees, because they have been described after submission of our work, but comparison of their tef1 and rpb2 sequences, kindly provided by the authors, with our data matrices revealed that they are distinct from all accessions included in our trees. Trichoderma rufobrunneum seems to be closely related to T. priscilae.
In this clade we found the highest number (nine) of so-called singletons, viz. single conidial isolates representing new lineages differing from other species, for which more sampling is necessary before they can be recognised as new species. Six of these singletons, isolates S138, S222, S404, S466, S467 and S610, belong to the Harzianum Clade, the isolate S624 is associated with the Spinulosum Clade, while the isolates S169 and S605 form a subclade in the Strictipile Clade (Fig. 5).
Studies in Southern Europe revealed new information regarding occurrence, distribution range and frequency of several species of this clade: in contrast to Central Europe, T. gelatinosum (19 isolates) is more common than T. strictipile (10 isolates). The latter seems to be absent from the Canary Islands and has not been detected in Mediterranean vegetation zones. The distribution range of T. aureoviride has to be revised from Atlantic to (sub-)Mediterranean regions, based on its detection in central Italy (Lazio), in Sardinia and even Serbia. Trichoderma ceramicum has not been recorded from Europe before, but was found four times in southwestern France in this study; T. danicum also occurs in Tenerife on pine wood, not only in Denmark on grasses; finally, T. phyllostachydis is not specific for bamboo (Phyllostachys), but was found on wood of Ostrya in northern Italy.
The Helicum Clade is apparently closely affiliated with the green-spored Clade, as it is either sister group to (Fig. 1, Fig. 2) or embedded within (Fig. 5) the green-spored Clade.
The Stromaticum and Semiorbis Clades
Samuels et al. (2012b) defined the Stromaticum Clade recognising nine species based on a fine-tuned scale, as judged from the low interspecific variation of tef1 sequences. Four isolates of our Southern European collections were identified as T. rossicum based on tef1 BLAST searches in GenBank. However, phylogenetic analysis (Fig. 5) suggests that these isolates may rather be interpreted as a new species.
The Semiorbis Clade is a small but interesting clade, as it contains both species with hyaline and green ascospores. Kim et al. (2012b) added the Japanese species T. mienum to the clade. In Southern Europe we detected an isolate that was identified as T. fertile via a BLAST search, but considering tef1 sequence differences again may represent a new species.
The Longibrachiatum Clade
Of the 1 320 characters included in the tef1 matrix, 364 were parsimony-informative. Fig. 6 shows the single MP tree of 1594 steps, with ML and MP bootstrap support values higher than 75 % given at the first and second position, respectively, above or below the branches.
This highly supported clade, which is the only one, apart from the Viride Clade, containing species with ornamented conidia, is one of the earliest that was assessed with molecular data (Samuels et al. 1998). Recently, Atanasova et al. (2010) added T. parareesei to the clade. Many additional isolates from various regions around the world led to a new treatment of this clade (Druzhinina et al., 2012, Samuels et al., 2012a), in which 21 taxa were recognised and named. Two isolates from Southern Europe were included in these studies; one was T. orientale (isolate S187), found as sexual morph on the island La Palma, the other is the asexual morphic T. saturnisporopsis (isolate S19) from Sardinia; see Samuels et al. (2012a) for descriptions and illustrations of these specimens and isolates. Yabuki et al. (2014) studied Japanese species of the Longibrachiatum Clade, recognising the two new species T. tsugarense and T. kunigamense, which are not included in our analyses due to unavailability of data. In our analyses using newly generated sequences, T. patella is remarkably also placed within the Longibrachiatum Clade (Fig. 1, Fig. 2, Fig. 6).
According to Druzhinina et al., 2008, Druzhinina et al., 2012, Samuels et al., 1998, Samuels et al., 2012a, W. Gams (pers. comm.) and the CBS collections database the following species have been reported from soil or mushroom compost in Europe: T. capillare (Austria, Hungary), T. citrinoviride (France), T. ghanense (Austria, France, Hungary, Italy, Netherlands), T. orientale (strain G.J.S. 91-157 = C.P.K. 1294 from the Hölloch caves in Switzerland) and T. saturnispoum (Italy). Jaklitsch (2011) found only T. citrinoviride (as Hypocrea schweinitzii) on plant material in Central Europe. In the current work we detected the four species T. citrinoviride, T. longibrachiatum, T. orientale and T. saturnisporopsis and describe the distinctive new species T. euskadiense based on a single holomorphic specimen from the Basque Country in Spain. In addition, a sexual morph specimen of T. reesei (H. jecorina) was collected on the French island Mayotte.
Lone Lineages/Miscellaneous species
Several species of Trichoderma form lone lineages. Some of them form stable phylogenetic sister group relationships independent of the marker used: T. britdaniae is connected to the Longibrachiatum Clade and T. voglmayrii to the Viride Clade. However, several species either form clades of variable positions or “jump” as solitary entities between several positions, depending on markers and type of analyses. The latter include T. albolutescens, T. alcalifuscescens, T. avellaneum, T. delicatulum, T. flavipes (syn. Hypocrea cinereoflava), T. parmastoi, T. peltatum, T. sambuci, T. subalpinum, T. taxi and T. tremelloides. Here we add the 3 new species T. ceciliae, T. leguminosarum and T. rubi. The reason for this variability of phylogenetic positions is not clear. Interestingly, none of these species forms green conidia, but either hyaline conidia or no asexual morph at all (T. peltatum). Trichoderma pseudolacteum was reported by Kim et al. (2013) as a lone lineage close to the Psychrophilum Clade, whereas it is associated with the Hypocreanum Clade in our analyses, though without significant support (Fig. 1).
Future prospects
In view of Jaklitsch, 2009, Jaklitsch, 2011 and the current work, Europe is the best-investigated continent with regard to species diversity of Trichoderma on plant and fungal material. Within the current work a short trip to Sri Lanka, planned to recollect T. lycogaloides and to gain a preliminary insight into the Trichoderma diversity, resulted in ten isolates mostly from sexual morphs, of which 70 % belonged to the Viride Clade and only one (T. stilbohypoxyli) revealed a tef1 sequence that is identical with those from Southern Europe. For all others 92–97 % similarities were retrieved by GenBank BLAST searches based on tef1, which suggests that they are potentially new species. Not only this result but studies in Colombia (Hoyos-Carvajal et al. 2009) and Ethiopia (Mulaw et al. 2010), which revealed new species, some of which still await taxonomic treatment (L. Atanasova & I. Druzhinina, pers. comm.), clearly show that detailed studies similar to our work on other continents, but also collecting of asexual morph colonies in other parts of Europe, will certainly reveal many more species than we currently know.
Important to note is also that one group of Trichoderma, the Polysporum Group, requires attention, because Lu et al. (2004) used only the tef1 intron 5 for the phylogenetic definition of species in this clade. We have implemented some changes resulting from the use of longer tef1 sequences that also include intron 4 and a part of the exon, by the recognition and description of T. europaeum and T. mediterraneum. There are however certainly more species to be recognised in this clade, if the analyses are based on larger sampling in future.
With the increasing importance of sequence data in species definition and recognition, morphological species definition and delimitation becomes less applicable or even impossible, especially in hyperdiverse lineages containing a high number of cryptic species. While tef1, the main and best-resolving marker used for species definition in Trichoderma, may overemphasize separation at the species level, it demonstrates the presence of a high biodiversity of genetically clearly separated cryptic species. In Trichoderma, hyperdiversity and cryptic speciation is considered to be connected with fungal parasitism (Kubicek et al., 2011, Chaverri and Samuels, 2013). This situation corresponds well with plant-parasitic lineages of other fungi in orders such as Capnodiales (e.g. Crous et al., 2013, Groenewald et al., 2013), Diaporthales (e.g. Gomes et al. 2013), Erysiphales (e.g. Takamatsu et al., 2007, Takamatsu et al., 2008), but also with the non-related downy mildews (Peronosporaceae, Chromista; e.g. García Blázquez et al., 2008, Göker et al., 2009a, Göker et al., 2009b, Thines et al., 2010, Voglmayr et al., 2014). In parasitic fungi, evolutionary diversification appears to be triggered by host specialisation resulting in high adaptation and rapid genetic isolation. As these cryptic species are genetically distinct, occupy different ecological niches and sometimes are important pathogens, it has become common practice to formally recognise and describe them at the species level.
Taxonomy
This section contains descriptions of new species, comments, specimen and culture information for all species of Trichoderma and subsequently for other species of Hypocreaceae found in Southern Europe. Species names are arranged in alphabetical order.
Trichoderma albolutescens Jaklitsch, Fungal Divers. 48: 202. 2011.
Material examined: Spain, Islas Baleares, Mallorca, east of Calviá, Ma-1016 roadside, asexual morph on Quercus ilex, 17 Nov. 2010, W.J. (culture S396).
Notes: An uncommon and atypical species of Trichoderma with white stromata and conidia; phylogenetically forming a lone lineage; found once in this study, as asexual morph associated with immature stromata in Mallorca.
Trichoderma alni Jaklitsch, Mycologia 100: 799. 2008.
Materials examined: France, Ariège, Rimont, Las Muros, on Alnus glutinosa, soc. Macrotyphula fistulosa, holomorph, stromata immature, 5 Nov. 2010, W.J. (culture S365). Spain, Gipuzkoa, Aralar, GI2133, Larraitz, deciduous forest between Abaltzisketa and Amezketa, on Alnus glutinosa, soc. Macrotyphula fistulosa, holomorph, stromata immature, 2 Nov. 2010, W.J. (culture S344).
Notes: That this species grows specifically in association with the basidiomycete Macrotyphula fistulosa on Alnus glutinosa as claimed by Jaklitsch et al. (2008a) was confirmed in the Basque Country. It has however not been found in Mediterranean habitats. The two closely related, newly described species T. christiani and T. italicum occur on other substrates.
Trichoderma atrobrunneum F. Branco-Rocha et al., Mycologia (in press).
Notes: A very common species in all parts of Europe; forty-eight isolates from various trees and shrubs in all studied countries were recorded in this study. See Chaverri et al. (in press) for specimen information and the taxonomic treatment of T. harzianum s.l. This species was described as Hypocrea lixii in Jaklitsch (2009).
Trichoderma atroviride P. Karst., Bidr. Känn. Finl. Nat. Folk 51: 363. 1892.
Materials examined: Twelve isolates, only one sexual morphic (all on dead twigs or branches): Croatia, Istrija, forest N of Barbariga, elev. ca. 20 m, on Quercus ilex, 24 Sep. 2010, W.J. (culture S264). France, Aquitaine, Pyrénées-Atlantiques, Osserain-Rivareyte, after crossing Gare de Mauleon from Guinarthe left at the river, on Phyllostachys sp., 4 Nov. 2010, W.J. (culture S356); S St. Palais, Château d'Uhart-Mixe, on Quercus robur, 4 Nov. 2010, W.J. (culture S363); ibid., on Sambucus nigra, 4 Nov. 2010, W.J. (WU 33363, culture S360). Ariège, Rimont, Las Muros, on Fraxinus excelsior, 5 Nov. 2010, W.J. (culture S367). Italy, Lazio, Corviano, on Hedera helix, 27 Nov. 2009, W.J., H.V. & W. Gams (culture S141); ibid., on Quercus cerris, soc. sexual morph of Trichoderma crystalligenum, 24 Oct. 2012, W.J. & H.V. (culture S646a); close to Magugnano, at the Strada Magugnano-Roccalvecce, left shortly before reaching the brook, on Quercus virgiliana, 25 Nov. 2009, W.J. & H.V. (culture S127). Spain, Andalucia, between El Bosque and Algar, on Quercus cf. canariensis, on bark, 24 Mar. 2011, W.J. (culture S545); Castellar de la Frontera, between the hotel Almoraima and the Castillo, on Alnus glutinosa, 19 Mar. 2011, W.J. & H.V. (culture S508); ibid., road to the Castillo, on Cytisus villosus, 19 Mar. 2011, W.J. & H.V. (culture S504); Basque country, Biskaia, Bengoetxea, at A2522/AP68, on Pinus cf. radiata, 7 Nov. 2010, W.J. (culture S383); Islas Baleares, Mallorca, Peguera, Carrer Savina, on Phillyrea angustifolia, 16 Nov. 2010, W.J. (culture S384).
Notes: This species is relatively common, but only rarely found in the sexual state. Good sexual morph material was found in France on a stump of Sambucus nigra.
Trichoderma auranteffusum Jaklitsch, Fungal Divers. 48: 162. 2011.
Materials examined: Croatia, Istrija, Bale, close to St. Golaš, on Acer monspessulanum, 26 Sep. 2010, W.J. (WU 33346, S284); forest north of Barbariga, elev. ca. 20 m, on Robinia pseudoacacia, 24 Sep. 2010, H.V. & W.J. (WU 33345, S269); Fažana, forest at Valbandon, on Carpinus orientalis, 17 Oct. 2010, W.J. (WU 33350, S302); ibid., on Fraxinus ornus, 26 Sep. 2010, W.J. & H.V. (culture S283). Italy, Basilicata, Parco Nazionale del Pollino, San Severino, Bosco Magnano, along the river Peschiera, on Alnus cordata, 17 Nov. 2009, H.V. & W.J. (WU 32177, culture S48); Lazio, Bomarzo, Santa Cecilia, on Cytisus scoparius, 29 Nov. 2009, W.J., H.V. & W. Gams (S161); Farnese, Selva del Lamone, hiking trail Roppozzo, on Quercus virgiliana, 28 Nov. 2009, W.J., H.V. & W. Gams (WU 33324, S159); Trentino, Vigolo Baselga, on Fagus sylvatica, 19 Oct. 2011, H.V. & W.J. (S565).
Note: This species reaches from Central Europe to Croatia and in Italy to the region of the Pollino national park, but was not found in Greece or Spain, including Macaronesia.
Trichoderma aureoviride Rifai, Mycol. Pap. 116: 34. 1969.
Materials examined: Italy, Lazio, Corviano, sexual morph on Cytisus scoparius, 27 Nov. 2009, W.J., H.V. & W. Gams (WU 33314, culture S142); Sardinia, at SS 392 from Lago di Coghinas, 20.5 km before Tempio Pausania, scant holomorph on Myrtus communis, 6 Nov. 2009, W.J. (culture S21). Spain, Canarias, La Palma, Cubo de La Galga (Punta Llana), 28RBS 2883, elev. 769 m, sexual morph on Myrica faya, 1 Dec. 2010, J. Fernández, P. Iglesias, M. Oyarzabal & R. Martinez, comm. P. Karasch (WU 33374, culture S426); Tenerife, Macizo de Anaga, Chinobre-La Ensillada, sexual morph on undetermined wood, 23 Jan. 2011, M.A. Ribes & L. Quijada 23011116 (TFCMic. 23081, culture S550).
Notes: Earlier concepts and conclusions about the distribution of this species had to be revised, as it also occurs on the Canary Islands, which is still in line with an Atlantic distribution, but it was quite unexpectedly also collected in Italy (Sardinia, Lazio) and is even reported from Serbia (Uzelac 2009, comm. D. Krstajić). Therefore, T. aureoviride is evidently confined to areas with mild winters rather than to humid climates.
Trichoderma balearicum Jaklitsch & Voglmayr, sp. nov. MycoBank MB809278. Fig. 7.
Fig. 7.
Trichoderma balearicum (WU 33314; S402 = CBS 133222). A–I. Sexual morph. A–C. Dry stromata (A, B. immature). D. Rehydrated stroma in 3 % KOH. E. Perithecium in section. F. Cortical and subcortical tissue in section. G. Subperithecial tissue in section. H. Asci with ascospores in cotton blue/lactic acid. I. Ascospores. J–S. Cultures and asexual morph. J. Asexual morph on the natural substrate. K–M. Cultures (K. CMD, 20 d, 25 °C; L. PDA, 7 d, 25 °C; M. SNA, 36 d, 15 °C). N–Q. Conidiophores (SNA, 36–43 d, 15 °C). R, S. Conidia (SNA, 37–43 d, 15 °C). Scale bars: A–D = 0.5 mm; E = 40 μm; F, G, N, O, Q = 15 μm; H, P = 10 μm; I = 3 μm; J = 0.7 mm; R, S = 7 μm.
Etymology: For its collection on the Balearic island Mallorca.
Stromata scattered or aggregated in small numbers, when fresh up to 4 mm diam and 1.5 mm high, pulvinate, first white, turning (reddish-)brown; ostiolar dots distinct. Stromata when dry (0.9–)1.4–2.4(–2.6) × (0.9–)1.1–1.9(–2.1) mm, (0.5–)0.7–1.1(–1.2) mm thick (n = 15), pulvinate with circular or oblong outline, narrowly attached, margin free, ostiolar dots (23–)35–68(–94) μm diam (n = 25), distinct, flat or convex, dark brown to nearly black; surface smooth to slightly tubercular, first white, then pale brown with a reddish tinge or brown with a thin whitish to greyish covering, appearing grey-brown, often covered by white spore powder; lower side white, papyraceous. Rehydrated stroma light yellow with distinct, light to medium brown ostiolar dots 78–130(–183) μm diam, not changing colour in 3 % KOH. Stroma anatomy: Stroma surface with short projecting cells or hairs (6–)7–15(–21) × 3–4(–5) μm (n = 20), 1–4 celled, cylindrical, subhyaline to pale brown, smooth, straight or curved. Cortical layer (12–)16–27(–35) μm thick (n = 30), comprising a light (yellow-)brown t. angularis of thin-walled cells (3.5–)5.0–11.0(–16.5) × (2.5–)3.5–6.0(–8.0) μm in section (n = 33), mixed with some hyphae arranged parallel to the surface, larger cells at the stroma sides (to 16.5 μm). Subcortical tissue a loose t. intricata of (sub)hyaline, thin-walled hyphae (2–)3–6(–9) μm wide (n = 30). Subperithecial tissue consisting of a t. angularis–epidermoidea of variable, hyaline, partly pale brownish, thin-walled cells (5–)7–21(–31) × (4–)6–9(–12) μm (n = 30), denser toward the base, mixed with (2.0–)2.5–4.8(–6.5) μm wide (n = 30) hyphae, often collapsing and entirely hyphal. Ostioles (47–)60–72(–74) μm long, projecting (11–)18–31(–37) μm, (27–)30–46(–52) μm wide at the apex inside (n = 21), periphysate, apical cells distinct, clavate to globose, to 7 μm wide. Perithecia (210–)225–260(–270) μm high, (140–)165–235(–295) μm wide (n = 21), crowded, to six per mm, (sub)globose-flask-shaped, peridium yellow, (12–)14–19(–22) μm thick at the base, (5–)9–16(–19) μm at the sides (n = 21). Asci (55–)60–68(–73) × (3.8–)4.0–5.0(–5.6) μm, stipe (3.5–)7–14(–20) μm long (n = 25), cylindrical, spores sometimes obliquely biseriate. Ascospores hyaline, finely spinulose, cells dimorphic, with 1–3 guttules, distal cells (2.7–)3.0–3.5(–3.7) × (2.5–)2.8–3.0(–3.3) μm, l/w (0.9–)1–1.2(–1.3) (n = 30), (sub)globose, ellipsoid or cuneate, proximal cells (2.7–)3.0–3.8(–4.2) × (2.3–)2.5–2.7(–3.0) μm, l/w (1.2–)1.2–1.5(–1.6) (n = 30), cuneate, oblong or subglobose. Asexual morph on the natural substrate: Stromata accompanied by white to pale yellowish, circular or oblong, 0.8–2.5 mm long, flat or pulvinate, farinose to crumbly colonies.
Cultures and asexual morph: Optimal growth at 25 °C, slow and limited at 30 °C; no growth at 35 °C. On CMD after 72 h colony radius 10–12 mm at 15 °C, 21–23 mm at 25 °C, 3–5 mm at 30 °C; mycelium covering the plate after 9–11 d at 25 °C. Colony hyaline, dense, circular, radial, homogeneous, not zonate, without conspicuous differences in hyphal width; centre soon becoming loose, with many surface hyphae appearing empty; margin becoming slightly wavy; colony turning first yellowish to brown 5CD4–7, centre lighter. Aerial hyphae inconspicuous, only appearing at the margin. Chlamydospores, autolytic excretions and coilings absent. Odour indistinct. Conidia only formed below 25 °C, observed at 15 °C after 30–40 d, in terminal heads on few small inconspicuous, whitish distant shrubs.
On PDA after 72 h colony radius 6–8 mm at 15 °C, 17–20 mm at 25 °C, 0.8–2 mm at 30 °C; mycelium not covering the plate within 42 d at 25 °C. Colony first hyaline, circular, dense, not zonate; margin becoming irregularly lobate. Surface covered by a white, downy to floccose mat formed by numerous, strongly branched aerial hyphae forming strands with thick connectives, eventually degenerating. Colony soon turning bright (3A7–8) to golden yellow, changing into yellow-brown from the centre and eventually dark brown 6F5–8. Yellow to brownish pigment diffusing into the agar. Autolytic excretions and coilings moderate. Odour indistinct. Conidiation starting after 2–3 d, colourless, effuse in the centre on surface and aerial hyphae around the plug, not spreading; conidiophores loose, simple, with few whorls of phialides.
On SNA after 72 h colony radius 8–10 mm at 15 °C, 19–22 mm at 25 °C, 3–4 mm at 30 °C; mycelium covering the plate after 10–11 d at 25 °C. Colony as described for CMD. Aerial hyphae only common at the margin. Autolytic excretions inconspicuous, coilings moderate. Chlamydospores and diffusing pigment absent. Odour indistinct. Conidia only formed below 25 °C, observed at 15 °C after 35–40 d, in thick white pustules scattered to aggregated mostly at the margin. Pustules up to 3(–6) mm diam, loose, finely granular due to minute peripheral terminal heads. Conidiophores peripheral, emerging from a loose, loose reticulum of perpendicular branches 3.5–7.5 μm wide (thickenings to 8.5 μm) mainly as stout radial sterile branches 2.5–4(–5.5) μm wide, with a terminal whorl or short terminal, often asymmetric tree-like structures of 1–5 levels plus terminal whorl of phialides. Side branches mostly in right angles, paired or unpaired, comprising 1–3 cells, not or once re-branching, with up to 4 branches in a whorl on the lowest cell of the side branch, resulting in dense structures. Phialides divergent or parallel, solitary or in whorls of 2–6, often on an intercalary cell, (4.7–)5.8–8.2(–10.5) × (2.2–)2.7–3.0(–3.2) μm, l/w (1.7–)2.0–2.9(–3.6), (1.2–)1.7–2.5(–2.8) μm wide at the base (n = 35), lageniform, inequilateral, straight or strongly curved with parallel necks, also sinuous. Conidia (2.5–)2.7–3.8(–5.2) × (1.5–)1.8–2.2(–2.5) μm, l/w (1.3–)1.4–1.9(–2.4) (n = 50), agglutinated in clusters, hyaline, ellipsoid or oblong, smooth, with 1–2 or more guttules; small fraction >4 μm long.
Habitat: On wood and bark of Quercus ilex and fungi growing on it.
Distribution: Southern Europe (Spain: Mallorca); only known from the holotype.
Typus: Spain, Islas Baleares, Mallorca, road Ma-10 above Fornalutx, opposite the property Monnaber, 39°47′45″ N, 2°46′00″ E, elev. 650 m, on a corticated twig of Quercus ilex on the ground, on a black crust, soc. Peniophora sp., Stereum sp., a hyphomycete and orange-brown rhizomorphs, holomorph, 17 Nov. 2010, W.J. (holotype WU 33371; ex-type culture S402 = CBS 133222).
Notes: Besides gene sequences the main feature that sets T. balearicum apart from the morphologically similar T. crystalligenum (Jaklitsch et al. 2006b) is its growth rate, which is more than double that of the latter species. Most other traits like a non-lobate colony, lack of conidiation and crystal formation at 25 °C on CMD, are within the variation of the numerous strains of T. crystalligenum, although the lack of colony zonation on CMD is also specific. The latter feature may be correlated with faster growth.
Trichoderma bavaricum Jaklitsch, Fungal Divers. 48: 87. 2011.
Material examined: Italy, Basilicata, Parco Nazionale del Pollino, San Severino, Bosco Magnano, along the river Peschiera, on Alnus cordata, 17 Nov. 2009, H.V. & W.J. (WU 32178, culture S49).
Note: An uncommon species of the Polysporum Group; in Italy known from a single collection in Basilicata.
Trichoderma caerulescens (Jaklitsch & Voglmayr) Jaklitsch & Voglmayr, Mycotaxon 126: 146. 2014 (2013).
Materials examined: (additional to Jaklitsch et al. (2012), all asexual morphs): Greece, Corfu, Kastellani near Troumpettas, 39°42′30″ N, 19°44′26″ E, elev. 250 m, on Ostrya carpinifolia, 23 Apr. 2012, H.V. & W.J. (culture S634); shortly before Skripero heading north, NE Poulades, opposite of the marble quarry, 39°40′58″ N, 19°48′4″ E, elev. 70 m, on Hippocrepis emerus, 21 Apr. 2012, H.V. & W.J. (culture S617); ibid., 39°41′0″ N, 19°47′59″ E, elev. 80 m, on Arbutus andrachne, 21 Apr. 2012, H.V. & W.J. (culture S622); between Troumpettas and Agia Anna, 39°42′22″ N, 19°44′41″ E, elev. 355 m, on Calicotome villosa, 21 Apr. 2012, W.J. & H.V. (culture S623); Agia Anna, 39°42′14″ N, 19°44′17″ E, elev. 420 m, on a corticiaceous fungus on Spartium junceum, 23 Apr. 2012, H.V. & W.J. (culture S629); Crete, Palaea Roumata, near Pananiana, 35°24′20″ N, 23°46′13″ E, elev. 370 m, on Platanus orientalis, 25 Nov. 2011, W.J. (culture S591); Vamvakades, 35°19′01″ N, 23°45′23″ E, elev. 745 m, on Quercus pubescens and yellow corticiaceous fungus, 27 Nov. 2011, W.J. (culture S609).
Notes: With 57 isolates this is the most common species of the Viride Clade in Southern Europe. It was detected in all countries studied in this project except France and has so far not been found in Central Europe. See Jaklitsch et al. (2012).
Trichoderma ceciliae Jaklitsch & Voglmayr, sp. nov. MycoBank MB809279. Fig. 8.
Fig. 8.
Trichoderma ceciliae (WU33325, S164 = CBS 130010). A–L. Sexual morph. A–C. Dry stromata. D. Part of rehydrated stroma. E. Part of rehydrated stroma in 3 % KOH. F. Ostiole in section. G. Perithecium in section. H. Cortical and subcortical tissue in section. I. Subperithecial tissue in section. J. Stroma base in section. K, L. Asci (L. in cotton blue/lactic acid). M–S. Cultures and asexual morph (CBS 130010). M, N. Cultures at 25 °C (M. on PDA, 14 d; N. on SNA, 18 d). O, P. Conidia (O. CMD, 18 d; P. PDA, 19 d). Q–S. Conidiophores and phialides (PDA, 2 wk). M–S. All at 25 °C. Scale bars: A = 1 mm; B = 2 mm; C = 0.5 mm; D, E = 0.2 mm; F, H, J, Q = 15 μm; G = 30 μm; I, R, S = 10 μm; K, L, O, P = 5 μm.
Etymology: Named after the collection site Santa Cecilia, an excavated early Christian altar at Bomarzo, Italy.
Stromata effuse, when fresh ca. 2 cm long, 0.3–0.5 mm thick, following the contours of the wood, smooth, white, ostiolar dots yellowish. Stromata when dry 2–17(–24) × (0.8–)1.5–6.3(–9) mm, 0.1–0.4 mm (n = 10) thick, forming oblong or lobate, thinly effuse crusts with a finely farinose white surface (visible in the stereo-microscope) and slightly papillate, orange-brown to dark brown, circular or oblong ostiolar dots (31–)36–86(–118) μm (n = 30) wide; resulting in cream to pale brown 5CD4–5 colour. Margin fraying out, white, sometimes cottony; spore deposits white or yellow. After rehydration stroma surface mostly concealed by swollen, light brown, convex perithecia (65–)80–130 μm diam; further swelling of perithecia to ca. 200 μm diam after addition of 3 % KOH; colour turning slightly more orange. Stroma anatomy: True cortex lacking, tissue between projecting ostioles consisting of a (sub)hyaline pseudoparenchymatous layer (14–)20–33(–38) μm (n = 30) thick of thin-walled cells (2.2–)3.7–8.0(–14) × (2.0–)2.5–5.0(–6.5) μm (n = 35) in section, mixed with some hyphae, at places forming little subcortical t. intricata of thin-walled hyaline, (1.7–)3.0–5.5(–7.5) μm (n = 35) wide, often verruculose hyphae. Subperithecial tissue comprising a coarse t. angularis–epidermoidea of thin-walled cells (4.5–)6–21(–32) × (3.5–)5.5–11.5(–14.5) μm (n = 33) and some wide hyphae; hyaline, in places yellowish. Base not differentiated, but hyphae more common, 3–8(–12) μm (n = 30) wide. Perithecia (118–)155–205(–221) μm high, (81–)108–160(–180) μm wide (n = 20), globose or ellipsoid; peridium yellow, (10–)12–18(–23) μm wide at the base, (4–)7–15(–18) μm at the sides (n = 20). Ostioles (42–)52–68(–75) μm long, projecting by (4–)9–29(–43) μm, (18–)21–28(–34) μm wide at the apex inside, (35–)41–54(–60) μm outside (n = 20), bluntly conical, apical marginal cells cylindrical to distinctly clavate, 3–8 μm wide. Asci (52–)60–73(–78) × (3.8–)4.0–4.5(–4.7) μm, cylindrical, stipe (5–)11–21(–30) μm long (n = 30), apex truncate, slightly thickened and with minute refractive ring. Ascospores hyaline, spinulose, cells dimorphic, distal cells (2.5–)2.7–3.2(–3.5) × (2.3–)2.5–3.0(–3.3) μm, l/w 1.0–1.2(–1.4) (n = 30), globose or subglobose, proximal cells (2.2–)2.7–4.0(–4.5) × (1.8–)2.0–2.3(–2.7) μm, l/w (1.0–)1.2–1.8(–2.4) (n = 30), oblong or subglobose.
Cultures and asexual morph: optimal growth at 25 °C on all media, at 30 °C hyphae dying after short growth, no growth at 35 °C.
On CMD after 72 h colony radius 4–8 mm at 15 °C, 11–12 mm at 25 °C, 0–1.5 mm at 30 °C; mycelium covering the plate after 14 d at 25 °C. Colony hyaline, circular, thin, centre whitish, dense, larger outer part loose and thin, not or indistinctly zonate, surface becoming slightly whitish farinose due to conidial heads; hyphae narrow, without distinct differences in width. Autolytic excretions common within the colony, small; coilings absent; no pigment formed, odour indistinct; chlamydospores lacking. Conidiation starting after 2–3 d, effuse, spreading from the centre over the entire colony surface; conidia forming in wet heads of variable size, eventually growing to ca. 100 μm diam, on short erect conidiophores emerging from surface hyphae.
On PDA after 72 h colony radius 4–7 mm at 15 °C, 11–13 mm at 25 °C, 0–1 mm at 30 °C; mycelium covering the plate after 12–13 d at 25 °C. Colony circular, consisting of narrow hyphae, without distinct differences in width; centre dense, whitish, margin looser, hyaline, surface becoming whitish farinose by conidiation except for the plug and centre which become covered by a dense white cottony mat of long aerial hyphae. Autolytic excretions common; coilings absent. Colony reverse pale yellowish 3–4AB3–4, diffusing pigment lacking; odour indistinct. Conidiation starting after 3 d, effuse, spreading from the centre; conidiophores numerous, short, formed on surface and aerial hyphae; conidia forming in large wet heads. Conidiophores simple, verticillium-like, 45–100 μm long, branches (1.5–)2–4(–6) μm wide, 1–4-celled, unpaired, paired or in whorls of 3–4, in right angles or inclined upwards, sometimes substituted by phialides singly or in whorls. Phialides often solitary, e.g. directly on aerial hyphae or on a single spacer cell, or in whorls of (2–)3–5(–6), divergent, less commonly nearly parallel. Phialides (6–)8–14(–19) × (2.0–)2.3–3.0(–3.2) μm, l/w (2.3–)3.1–5.7(–7.7), (1.0–)1.5–2.5(–3.2) μm wide at the base (n = 46), lageniform, tending to be more subulate on PDA than on CMD, sometimes repetitive, forming chains. Conidia numerous, hyaline, ellipsoid, less commonly subglobose or oblong, (2.8–)3.0–5.0(–7.5) × (2.2–)2.5–3.0(–4.0) μm, l/w (1.1–)1.2–1.8(–2.6) (n = 67), smooth, finely multiguttulate, scar indistinct, sometimes truncate.
On SNA after 72 h colony radius 6–8 mm at 15 °C, 10–13 mm at 25 °C, 0–1.5 mm at 30 °C; mycelium covering the plate after 15–16 d at 25 °C. Colony and conidiation as on CMD, but mycelium entirely loose and conidiation less abundant than on CMD, wet conidial heads growing to ca. 120 μm diam and conidiation also occurring immersed in agar.
Habitat: On a corticiaceous fungus on wood of Quercus cerris.
Distribution: Known from a single collection in Italy.
Typus: Italy, Lazio, Bomarzo, walking path to Santa Cecilia, 42°28′31″ N, 12°15′49″ E, elev. 230 m, on a 10 cm thick branch of Quercus cerris, on well-decayed, crumbly wood and a yellowish corticiaceous fungus (?Trechispora sp.), soc. resupinate polypore, 29 Nov. 2009, W.J., W. Gams & H.V. (holotype WU 33325; ex-type culture CBS 130010 = S164).
Notes: Despite its effuse stromata that are similar to those of T. decipiens or T. phellinicola, phylogenetically T. ceciliae does not belong to the Hypocreanum Clade. The minute ascospore size agrees with T. decipiens but also T. delicatulum, which is also situated outside the Hypocreanum Clade. The submoniliform phialides of T. ceciliae are unusual for species with effuse stromata.
Trichoderma ceramicum P. Chaverri & Samuels, Stud. Mycol. 48: 47. 2004 (2003).
Materials examined: France, Aquitaine, Pyrénées-Atlantiques, SE Salies de Bearn, D430, deciduous forest at the junction to Quartier du Cout, on Corylus avellana, 3 Nov. 2010, W.J. (culture S353); Ariège, Rimont, Las Muros, on Corylus avellana, 5 Nov. 2010, W.J. & J. Fournier (cultures S370, S373); ibid., on Hymenochaete corrugata/Corylus avellana, holomorph, sexual morph immature/overmature, 5 Nov. 2010, W.J. & J. Fournier (WU 33364, culture CBS 132571 = S366).
Notes: An originally American species that was previously not known from Europe. Four specimens were collected in southwestern France, only one with (poor) sexual morphic material.
Trichoderma cerinum Bissett et al., Canad. J. Bot. 81: 581. 2003.
Material examined: France, Aquitaine, Pyrénées-Atlantiques, Laas, Parque du Chateau de Laas, asexual morph on Phyllostachys sp., 4 Nov. 2010, W.J. (culture CBS 136992 = S357).
Notes: This species is difficult to distinguish from T. tomentosum and therefore is often confused with the latter, particularly if relying on BLAST searches. Only one specimen identified as T. cerinum s.str. was found in southwestern France.
Trichoderma christiani Jaklitsch & Voglmayr, sp. nov. MycoBank MB809280. Fig. 9.
Fig. 9.
Trichoderma christiani. A–O. Sexual morph. A, B. Fresh stromata. C–E. Dry stromata. F. Rehydrated stroma in 3 % KOH. G. Ostiole in section. H. Cortex in face view. I. Cortex in section at stroma side. J. Perithecium in section. K. Cortical and subcortical tissue in section. L. Subperithecial tissue in section. M. Stroma base in section. N, O. Asci with ascospores. P–BB. Cultures and asexual morph (at 25 °C). P–R. Cultures after 7 d (P. CMD; Q. PDA; R. SNA). S–Y. Conidiophores and phialides (SNA, 4–6 d). Z–BB. Conidia (SNA, 4–6 d). A, C, O. WU 32194 (S93); B, D–G, I–N, P–R, T–Y, AA, BB. S442 = CBS 132572; H, S, Z. S138. Scale bars: A, B = 1 mm; C = 0.3 mm; D–F = 0.5 mm; G, J, S = 30 μm; H, I, K, L, V–X = 20 μm; M–O, T, Y = 10 μm; U = 50 μm; Z–BB = 5 μm.
Etymology: In honour of Christian Kubicek, for his important role in the development of molecular phylogeny of the genus Trichoderma.
Stromata solitary to aggregated in small groups, when fresh 1–5 mm diam, to 2 mm thick, pulvinate, smooth, brown to reddish brown; ostiolar dots minute, convex, dark green to black; margin free. Stromata when dry (0.8–)1–2.6(–3.7) × (0.7–)0.8–2.1(–3.0) mm, (0.3–)0.4–0.9(–1.1) mm thick (n = 20), discoid, undulate or flat pulvinate with roundish or oblong outline, often with a depressed area; margin dark reddish brown, free; sterile sides brown, hairy when young. Surface smooth, sometimes finely tubercular or rugose, dark reddish- to purplish brown, sometimes orange-brown. Ostiolar dots (25–)45–80(–102) μm diam (n = 40), distinct or more commonly diffuse, black, flat, convex or slightly projecting and nearly cylindrical. Spore deposits dark green. Rehydrated stromata more pulvinate, dark orange-brown with numerous densely disposed convex ostiolar dots 47–65 μm diam; colour not changing in 3 % KOH. Stroma anatomy: Cortical layer (16–)22–33(–39) μm thick (n = 30), comprising a t. angularis of distinct thin-walled cells (4–)7–12(–16) × (4–)5–8(–9.5) μm (n = 35) in section, (4.5–)7–12(–13.5) × (3.5–)5.5–9.5(–10.5) μm (n = 30) in face view, yellow to orange-brown; cells at stroma sides thick-walled (to ca. 1.5 μm) and larger, up to 27 × 18 μm in section. Subcortical tissue consisting of a subhyaline to brownish t. angularis of thin-walled cells (3.5–)5–8(–10) × (2.5–)3.5–5.0(–6.0) μm (n = 30), at places mixed with some (2.7–)3–5(–6.5) μm (n = 30) wide hyphae. Subperithecial tissue a coarse, hyaline to subhyaline/pale brownish t. epidermoidea of thin-walled cells (7–)10–24(–30) × (5–)8–13(–16) μm (n = 31). Stroma base comprising a dense, subhyaline to orange-brown t. intricata of thick-walled, (2.5–)3.5–5.2(–6.0) μm (n = 30) wide hyphae, merging into the host bark. Ostioles (75–)82–114(–138) μm long, projecting to 40(–60) μm, (18–)21–34(–42) μm wide at the apex inside (n = 26); wall colourless. Perithecia (170–)215–260(–270) μm high, (92–)115–180(–225) μm wide (n = 26), numerous, 8–10 per mm, crowded, ellipsoid, flask-shaped or subglobose; peridium (10–)12–18(–24) μm wide at the base, (6–)8–15(–21) μm at the sides (n = 26), yellow, slightly paler than the cortex. Asci (80–)87–100(–107) × (5–)6–7(–7.5) μm, stipe (6–)8–15(–22) μm long (n = 40). Ascospores green, turning brown in KOH, verruculose; cells dimorphic, distal cells subglobose or cuneate, (4.3–)4.8–5.7(–6.2) × (3.8–)4.2–4.8(–5.5) μm, l/w (1.0–)1.1–1.3(–1.5) (n = 60), proximal cells oblong or subglobose, (4.2–)5.0–6.2(–6.8) × (3.2–)3.7–4.5(–4.8) μm, l/w (1.1–)1.2–1.5(–1.7) (n = 60).
Cultures and asexual morph: Optimal growth at 25–30 °C on CMD and PDA, at 25 °C on SNA, no growth at 35 °C. On CMD after 72 h colony radius 22–23 mm at 15 °C, 48–56 mm at 25 °C, 53–56 mm at 30 °C; mycelium covering the plate after 4–5 d at 25 °C. Colony hyaline, dense, circular, loose, with conspicuous difference in hyphal width, homogeneous, not zonate. Aerial hyphae inconspicuous. Autolytic excretions lacking, coilings inconspicuous; diffusing pigment lacking, odour indistinct. Chlamydospores uncommon, terminal and intercalary. Conidiation first scant, effuse, short, soon in tufts or pustules 1–3 mm diam, confluent and aggregating up to 5 mm, in a broad zone in the middle of the colony spreading to more distant areas a broad zone, first white, after 4–5 d green, eventually dark blue-green 25E5–6, 25–27F5–8; conidia formed in wet heads to 30 μm diam.
On PDA after 72 h colony radius 17–21 mm at 15 °C, 43–47 mm at 25 °C, 43–45 mm at 30 °C; mycelium covering the plate after 5 d at 25 °C. Colony circular, dense. Aerial hyphae abundant, forming several white/hyaline alternating zones; zones first hairy, later farinose, turning yellowish green 28–30CD4–5, 26–27C3 from the near margin. Autolytic excretions and coilings inconspicuous; diffusing pigment lacking, odour indistinct. Conidiation effuse in shrubs and on aerial hyphae, yellow-green after 3–4 d.
On SNA after 72 h colony radius 19–21 mm at 15 °C, 50–54 mm at 25 °C, 40–42 mm at 30 °C; mycelium covering the plate after 4 d at 25 °C. Colony hyaline, loose, circular, with conspicuous variation in hyphal width, not zonate. Aerial hyphae common at the margin, long and high. Autolytic excretions from coilings common, coilings abundant; diffusing pigment lacking, odour indistinct. Chlamydospores rare, more common at 30 °C, predominantly intercalary, (5–)6–9(–10) × (3.5–)5–8(–10) μm, l/w 0.9–1.4(–1.7) (n = 30), (sub)globose, also oblong or ellipsoid. Conidiation effuse, on aerial hyphae and in pustules without a principal structural difference; shrubs, tufts, or pustules 0.4–1.4(–2.5) mm diam forming in irregular zones, aggregating to clusters of up to 6 mm in the centre and middle of the colony, turning green after 4 d, eventually dark green 26–27F5–8. Conidiophores emerging mostly in right angles from surface or aerial hyphae, often paired, short, typically with 1–3(–4) branching levels; side branches simple, rebranching 1–2 times or replaced by a phialide or whorl of phialides. Pustules with irregular outline, loose, when young often with a straight sterile elongation projecting to ca. 0.5 mm. Pustules comprising a smooth, thin-walled, 6–7 μm wide stipe, asymmetrically branched into primary branches forming a reticulum; peripheral conidiophores typically long, tree-like, i.e. consisting of a distinct main axis with mostly unpaired side branches in right-angles or inclined upwards; the latter with mostly paired terminal branches in right-angles, consisting of 1–3 long cells, once rebranching or bearing a whorl of 2–4 phialides, or phialides solitary, sometimes on an intercalary cell. Branches (2–)2.5–3.5(–5) μm wide. Phialides (5–)6–11(–16) × (2.5–)2.7–3.5(–3.8) μm, l/w (1.4–)1.8–3.9(–5.9), (1.1–)1.7–2.5(–3.0) μm wide at the base (n = 60), lageniform to nearly ampulliform, sometimes with narrow cylindrical neck, straight-curved, symmetric or inequilateral. Conidia produced in wet heads to 30 μm diam, (2.5–)3.0–3.7(–5.0) × (2.5–)2.7–3.0(–3.3) μm, l/w (1.0–)1.1–1.3(–1.6) (n = 68), subglobose or oval, green, smooth; scar indistinct.
Habitat: On wood and bark of Quercus and Ostrya.
Distribution: Canary Islands, Southern Europe.
Typus: Spain, Canarias, La Palma, Puntallana, El Corcho, 28°45′20″ N, 17°45′47.4″ W, elev. 435 m, sexual morph on a 8 cm thick branch of Castanea sativa, on bark and wood, soc. Annulohypoxylon multiforme, Cryphonectria radicalis, Trichoderma petersenii and a discomycete, holomorph, 3 Dec. 2010, W.J. (holotype WU 33379; ex-type culture S442 = CBS 132572).
Additional materials examined: Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra/Foresta Domaniale, 41°52′29″ N, 16°03′32″ E, elev. 230 m, on a branch 8–13 cm thick of Quercus cerris, on wood, soc. Lasiosphaeris hirsuta, a corticiaceous fungus and a brown hyphomycete, holomorph, 21 Nov. 2009, H.V. & W.J. (WU 32194, culture S93); Basilicata, SS653 east, 1.5 km before exit to Latrónico, 40°04′41″ N, 15°57′32″ E, elev. 670 m, on 1 cm thick twig of Quercus cerris, on a brown lichen, asexual morph, 17 Nov. 2009, W.J. & H.V. (culture S43). Spain, Canarias, La Palma, Cubo de la Galga, 28°46′00″ N, 17°46′15″ W, elev. 345 m, on deciduous wood, soc. Dacrymyces sp., corticiaceous fungus, 12 Dec. 2009, W.J. (culture S179); Cumbre Nueva, chestnut plantation at LP 301, close to crossing with LP 3, 28°38′23″ N, 17°49′46″ W, elev. 1080 m, on branch of Laurus novocanariensis, asexual morph, 13 Dec. 2009, W.J. (culture S189); Tenerife, Macizo de Anaga, Las Carboneras, walking path to El Batán from the road to Taborno, 28°32′22″ N, 16°16′34″ W, elev. 845 m, on 3 cm thick twig of Rhamnus glandulosa, at broken area, soc. black pyrenomycete in bark, asexual morph, 16 Dec. 2010, W.J. & H.V. (culture S474).
Notes: It is difficult to distinguish T. christiani from other species forming green ascospores in reddish brown stromata of the Harzianum Clade, esp. T. parepimyces or T. priscilae. Trichoderma alni may be distinguished by its simpler conidiophores, T. brunneoviride by more or less gliocladium-like conidiophores, T. italicum by bright yellow and more compact conidial pustules and different stroma colour. Cultures of T. christiani on PDA (Merck) yield yellow-green conidiation zones, whereas the yellow colour is absent in T. priscilae, and in T. parepimyces PDA cultures remain whitish or turn at most pale greenish.
Trichoderma citrinoviride Bissett, Canad. J. Bot. 62: 926. 1984.
= Hypocrea schweinitzii (Fr.) Sacc., Syll. Fung. 2: 522. 1883.
Materials examined: Croatia, Istrija, forest N of Barbariga, elev. ca. 20 m, on Quercus ilex, 18 Oct. 2010, immature stromata, W.J. (culture S313). Italy, Sardinia, at Aggius, on Quercus suber, 7 Nov. 2009, W.J. (culture S27); at the road SS392 from Lago di Coghinas, 20.5 km before Tempio Pausania, sexual morph on burnt wood of Quercus suber, 6 Nov. 2009, W.J. (WU 32173, culture S20); Trentino, Mattarello, near Villa Bertagnolli, asexual morph on Pinus nigra, 20 Oct. 2011, W.J. & H.V. (culture S567). Spain, Asturias, Saliencia, asexual morph soc. immature ?T. polysporum stromata, on Fagus sylvatica, 3 Jun. 2013, M. Pennanen (culture S659); Basque Country, Gipuzkoa, Oiartzun, BI3420 heading to Endara, nature park Aiako Harra, pasture with Betula and Ulex, asexual morph on Betula pendula, 6 Nov. 2010, W.J. (culture S379); Canarias, La Palma, “Märchenwald” below Refugio El Pilar approaching from the south, asexual morph on Pinus canariensis, 2 Dec. 2010, W.J. & R.M. Dähnke (culture S430).
Notes: This is the only species of the Longibrachiatum Clade that forms stromata in Central Europe (Jaklitsch 2011). In Southern Europe we found it twice as sexual morph, but it is more common as asexual morph.
Trichoderma composticola Samuels & Jaklitsch, Persoonia 31: 139. 2013.
Note: Apart from the holotype collected during this work in Crete, Greece, this species is also known from Mexico, The Netherlands, Russia and U.K. (see Jaklitsch et al. 2013).
Trichoderma cremeoides Jaklitsch & Voglmayr, sp. nov. MycoBank MB809281. Fig. 10.
Fig. 10.
Trichoderma cremeoides. A–Q. Sexual morph. A–C. Fresh stromata (A. immature). D–J. Dry stromata (D. immature; E. side view). K. Rehydrated stroma. L. Rehydrated stroma in 3 % KOH. M. Perithecium in section. N. Cortex and subcortical tissue in section. O. Subperithecial tissue in section. P. Stroma base in section. Q, R. Asci. S–AA. Cultures and asexual morph. S–V. Conidiophores and phialides (SNA, 9–10 d). W, AA. Conidia (SNA, 9–10 d). X–Z. Cultures (X. on CMD, 20 d; Y. on PDA, 28 d; Z. on SNA, 20 d). S–AA. All at 25 °C. A, B, E, G. S431. C. S192. D, I. S113. F. S98. H. S117. J–Q, S–AA. S112 = CBS 131486. R. S191 = CBS 136470. Scale bars: A = 1.5 mm; B = 1 mm; C, H, I = 0.5 mm; D, G, J–L = 0.3 mm; E, F = 0.2 mm; M = 40 μm; N–P = 25 μm; Q–U = 15 μm; V = 10 μm; W, AA = 5 μm.
Etymology: Denoting the similarity of the stromata to T. cremeum.
Stromata scattered, gregarious or aggregated in small numbers, when fresh 1(–3) mm diam, to 1 mm high, turbinate or pulvinate, outline variable, mostly irregularly angular, margin (edge of fertile elevated upper part) free, often conspicuously wavy or lobed, sterile sides smooth. Surface when young pale or bright yellow, with projecting perithecia, when mature dull yellow, pale to dull green and often smooth surface, with perithecia projecting or not; waxy, translucent. Stromata when dry (0.3–)0.4–1.3(–2) × (0.2–)0.4–1.1(–1.6) mm, (0.25–)0.3–0.5(–0.7) mm thick (n = 35), pulvinate, turbinate or irregularly discoid with variable outline, sometimes with white radiating base mycelium when young, often with a sterile, smooth or furrowed, white to yellow, cylindrical base or stipe; fertile upper part elevated above the substrate surface, typically with a coarsely wavy to crenate margin. Surface finely floccose, granulose or tubercular, with inconspicuous or distinctly prominent perithecia. Ostiolar or perithecial dots (38–)40–123(–200) μm diam (n = 45), densely disposed, distinct or diffuse, first brownish, turning green or grey due to translucent spore masses and often comprising a large part of the perithecium. Colour shades of yellow, yellow-brown or dull orange-brown, colour 1–4AB3, 2–3AB4, turning olive to green upon maturation and then macroscopically not determinable. Spore deposits dark green or dark grey-green. After rehydration stromata 20–40 % larger, pale yellow, with large olive-green ostiolar dots (67–)79–135(–146) μm diam, pulvinate; after addition of 3 % KOH turning dark yellow, dull yellow-brown to dull orange-brown from the margin. Stroma anatomy: Stroma surface smooth. Cortical layer (20–)23–34(–38) μm thick (n = 30), comprising a t. angularis of thin-walled, hyaline to faintly yellowish cells (3.5–)7–16(–19) × (2.5–)4.5–9.0(–11.5) μm in section (n = 30), slightly larger at stroma sides. Subcortical tissue a similar t. angularis, but cells thin-walled, hyaline, mixed with hyphae or replaced by a t. intricata of thin-walled hyphae (3–)4–7(–8.5) μm wide (n = 30). Subperithecial tissue comprising a t. angularis–epidermoidea of thin-walled, hyaline cells (9–)10–27(–40) × (6–)8–16(–25) μm (n = 30), slightly smaller at the base and mixed with hyaline hyphae 5–10 μm wide, incorporating diverse fungal material. Ostioles (60–)65–80(–90) μm long, projecting (6–)13–27(–36) μm, (10–)15–27(–31) μm wide at the apex inside (n = 27); apical cells cylindrical or narrowly clavate. Perithecia (169–)193–251(–267) μm high, (110–)118–185(–230) μm diam (n = 27), subglobose or flask-shaped, peridium (10–)14–21(–24) μm wide at the base, (6.5–)9–17(–20) at the sides (n = 27), hyaline in lactic acid and KOH. Asci (85–)98–118(–130) × (6.0–)6.5–7.5(–8.2) μm, stipe (1.5–)7.3–20(–31) μm long (n = 50); apex thickened to ca. 1.5 μm. Ascospores dull green to brown-green in water, clear brown in KOH, distinctly warted, cells dimorphic, distal cells (5.0–)5.5–6.4(–7.7) × (4.7–)5.2–6.0(–6.7) μm, l/w (0.9–)1.0–1.1(–1.3) (n = 60), (sub)globose, proximal cells (4.8–)5.5–7.3(–8.7) × (3.8–)4.3–5.2(–5.8) μm, l/w (1.0–)1.2–1.6(–1.9) (n = 60), oblong or slightly attenuated downward or subglobose.
Cultures and asexual morph: optimal growth at 25 °C on all media, no growth at 35 °C.
On CMD after 72 h colony radius 18–20 mm at 15 °C, 41–43 mm at 25 °C, 26–31 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony hyaline, loose or dense, with primary and secondary hyphae conspicuously differing in width, surface hyphae soon becoming empty. Aerial hyphae nearly lacking; no autolytic excretions, no coilings apparent; no pigment diffusing and distinct odour lacking. Chlamydospores uncommon or common, terminal and intercalary. Conidiation starting after 1 wk, first effuse, soon followed by formation of pustules 1–2 mm diam particularly at the margin, after 8–10 d green 27–28D4–8, 28C4–6, 27–29F4–8. Pustules thick, sometimes large, up to 5 mm diam, after prolonged storage (165 d) at 15 °C up to 11 mm diam, with irregular outline, transparent; with straight, fertile, collapsing elongations.
On PDA after 72 h colony radius 16–17 mm at 15 °C, 29–30 mm at 25 °C, 6–9 mm at 30 °C; mycelium covering the plate after 1wk at 25 °C. Colony circular, dense. Autolytic excretions inconspicuous, coilings absent; diffusing pigment and distinct odour not formed. Aerial hyphae abundant, forming a thick white cottony mat ascending up to the lid in concentric zones. Conidiation apparent after 4–5 d in small pustules and ascending on aerial hyphae, turning bluish green after 6–7 d; pustules spreading in several concentric rings, eventually grey-green 28–30E3–8, 29–30F4–8. At 30 °C little growth, colony irregularly lobed, autolysis abundant.
On SNA after 72 h colony radius 18–20 mm at 15 °C, 38–39 mm at 25 °C, 18–22 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony hyaline, dense, with primary and secondary hyphae of quite different width, the latter short and finely curly. Aerial hyphae common and long and high in a marginal zone. Autolytic excretions and coilings absent; diffusing pigment and distinct odour not formed. Chlamydospores common, terminal and intercalary, (6–)7–11(–13.5) × (4.5–)5.5–10(–13) μm, l/w (0.8–)0.9–1.6(–2.6) (n = 30), globose, sometimes oblong. At 30 °C colony irregularly lobed, autolysis and coilings abundant. Conidiation at 25 °C apparent after 6–7 d in numerous pustules forming in a broad marginal zone, turning green 29–30E4–8 after 7 d. Pustules (0.4–)0.7–1.2(–1.6) mm diam, often semiglobose to nearly globose, dense, compact, easily detachable, comprising a right-angled reticulum arising on a single thick-walled stipe. Conidiophores typically straight, verticillium-like or regularly tree-like, mostly narrow, only rarely forming long distinct main axes, radially emerging from the reticulum, sometimes projecting as short straight fertile elongations with 1–3 long terminal phialides. Branches (2–)2.5–4.5(–5) μm wide, sometimes widening to 8 μm; side conidiophores arranged in right angles or slightly inclined upward, loose, short, often consisting of a single cell terminated by a single phialide, or, mostly at higher positions on the conidiophore, rebranching on 2–3 levels. Phialides solitary or divergent in whorls of 2–5, (6.2–)7.3–13.0(–18.5) × (2.8–)3.0–3.5(–3.8) μm, l/w (2.0–)2.2–4.1(–6.1), (1.8–)2.0–2.7(–3.2) μm wide at the base (n = 31), lageniform, with or without distal thickening, often distinctly longer when terminal in the whorl, straight, less commonly curved. Conidia (4.0–)4.3–5.7(–6.8) × (3.0–)3.2–3.5(–3.7) μm, l/w (1.2–)1.3–1.8(–1.9) (n = 30), green, oblong or ellipsoid, sometimes with pinched sides, smooth, with few small guttules; scar indistinct or slightly truncate.
Habitat: On wood and bark, always associated with other fungi, particularly Hyphodontia or Hymenochaete.
Distribution: Southern Europe and Macaronesia.
Typus: Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, 41°50′31″ N, 16°02′56″ E, elev. 460 m, on partly corticated, 6–15 cm thick, superficially black, well-decayed log of Carpinus betulus, on/soc. Hyphodontia sp. and another corticiaceous fungus, soc. dark green Trichoderma, brown hyphomycete, Mollisia sp., Stemonitis cf. fusca, 22 Nov. 2009, W.J. & H.V. (holotype WU 33300, ex-type culture CBS 131486 = S112).
Additional materials examined: Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, Riserva biogenetica Falascone, 41°48′22″ N, 15°58′54″ E, elev. 760 m, on a burnt 20 cm diam thick log of Fagus sylvatica, soc. little light green asexual morph and Oligoporus subcaesius, 21 Nov. 2009, W.J. & H.V. (WU 32195, culture S98); same region, 41°50′36″ N, 16°02′50″ E, elev. 425 m, on 4–5 cm thick Fagus sylvatica, on wood, bark and on/soc. Hyphodontia sp. and a dark brown hyphomycete, soc. white corticiaceous fungus and effuse, green Trichoderma, 22 Nov. 2009, H.V. & W.J. (WU 33301, culture S113); same region, ca. 100 m after the military station, heading to Peschici, 41°49′35″ N, 15°59′44″ E, elev. 745 m, on 15 cm thick branch of Carpinus betulus, on wood, soc. Hypocrea subeffusa, corticiaceous fungi, effete pyrenomycete, 22 Nov. 2009, W.J. & H.V. (WU 33304, culture S117). Portugal, Madeira, Portela, PR5, close to its start, 32°44′48″ N, 16°49′16″ W, elev. 560 m, on 2 cm thick, well-decayed stalk of Rubus sp., only asexual morph, 21 Feb. 2010, W.J. & O. Sükösd (culture S207). Spain, Canarias, La Palma, Cumbre Nueva, old chestnut plantation at LP 301, close to crossing with LP 3, 28°38′23″ N, 17°49′50″ W, elev. 1040 m, on a 8 cm thick branch of Castanea sativa, on well-decayed, crumbly wood, 2 Dec. 2010, W.J. (WU 33376, culture S431); Montaña Tagoja, 28°43′18″ N, 17°47′08″ W, elev. 1040 m, on 5–7 cm thick, decorticated Erica arborea, on wood, on/soc. Hymenochaete sp. and Biscogniauxia sp., soc. Hypocrea minutispora, brown hyphomycete, white corticiaceous fungus, and Terana caerulea, 14 Dec. 2009, W.J. (WU 33335, cultures S191 and S192).
Notes: Stromata of T. cremeoides are typical of the Chlorosporum clade and resemble those of e.g., T. cremeum, T. sinuosum and T. thelephoricola, particularly when young, in age also T. strictipile, but typically appearing more waxy. The perithecial wall remains hyaline in KOH contrary to T. thelephoricola, although the usual association with corticiaceous fungi like Hyphodontia or Hymenochaete spp. would rather point to T. thelephoricola. The fungus was also found directly on Biscogniauxia stromata. Conidiophores of T. cremeoides are straight, unlike those of T. sinuosum; curvatures in images are mounting artifacts.
Trichoderma crystalligenum Jaklitsch, Mycologia 98: 502. 2006.
Materials examined: Croatia, Cres, at Prašče Brdo between Orline and Dragozetići, on Carpinus orientalis, 15 Oct. 2010, W.J., N. Matočec & I. Kušan (WU 33349, culture S293); Istrija, forest N of Barbariga, elev. ca. 20 m, on Carpinus orientalis, 24 Sep. 2010, H.V. & W.J. (WU 33344, culture S265); 1.4 km before Barbariga from Peroj, on Quercus pubescens, 18 Oct. 2010, W.J. (culture S309); Vrsar, beach forest at Petalon Resort, elev. 10 m, on Quercus ilex, 26 Sep. 2010, W.J. (WU 33347, culture S286). Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra/Foresta Domaniale, on Quercus cerris, 21 Nov. 2009, W.J. & H.V. (WU 32191, culture S90); Campania, left roadside of Via Provinciale del Corticato shortly after the highest point heading to Sacco, Parco Nazionale del Cilento, on Alnus cordata, 16 Nov. 2009, H.V. & W.J. (WU 32175, culture S38); Lazio, Corviano, on Cytisus scoparius, 27 Nov. 2009, W.J., H.V. & W. Gams (culture S143); same area and collectors, on Quercus cerris, 24 Oct. 2012 (WU 33412, culture S646); Farnese, Selva del Lamone, hiking trail Roppozzo, on Quercus cerris, 28 Nov. 2009, W.J., W. Gams & H.V. (WU 33323, culture S156); close to Magugnano, at the Strada Magugnano-Roccalvecce, left shortly before reaching the brook, on Castanea sativa, 25 Nov. 2009, H.V. & W.J. (culture S132); ibid., on Quercus virgiliana (WU 33309, culture S130).
Note: Based on 11 collections, all as sexual morphs, this species is common in Croatia and in central to southern Italy, but seems to be absent from other Southern European regions.
Trichoderma danicum (Jaklitsch) Jaklitsch & Voglmayr, Mycotaxon 126: 148. 2014 (2013).
Material examined: Spain, Canarias, Tenerife, Archifira (Fasnia), UTM: 28R355738 3128654, elev. 1295 m, pine forest, on wood of Pinus canariensis, 18 Feb. 2011, L. Quijada & J. Díaz, TFCMic.23127 (WU 33403, culture CBS 132575 = S553).
Notes: This species was described from a single specimen collected on the grass Calamagrostis epigejos in Denmark. In this study the species was collected in Tenerife on pine wood, which is an unexpected finding regarding both host and geography.
Trichoderma decipiens (Jaklitsch et al.) Jaklitsch & Voglmayr, Mycotaxon 126: 148. 2014. (2013).
Material examined: France, Ariège, Rimont, Las Muros, on Hymenochaete corrugata/Corylus avellana, 5 Nov. 2010, W.J. & J. Fournier (WU 33366, culture CBS 132861 = S372).
Note: In Europe only known from southwestern France (Jaklitsch et al., 2008b, Jaklitsch, 2011).
Trichoderma deliquescens (Sopp) Jaklitsch, Fungal Divers. 48: 176. 2011.
= Hypocrea lutea (Tode : Fr.) Petch, J. Bot. (Lond.) 75: 231. 1937.
Material examined: Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra/Foresta Domaniale, on Quercus cerris, holomorph, 21 Nov. 2009, H.V. & W.J. (WU 32192, culture S91).
Note: This is a generally uncommon species with a distinctive gliocladium-like asexual morph; the species was only found once as holomorph in Southern Europe.
Trichoderma dorotheae Samuels & Dodd, Stud. Mycol. 56: 112. 2006.
Materials examined: Spain, Canarias, La Palma, Pista El Corcho, 28°45′22″ N, 17°45′47″ W, elev. 450 m, albino stromata on Myrica faya, 3 Dec. 2010, W.J. (WU 33380, culture CBS 136995 = S444); Tenerife, Macizo de Anaga, El Pijaral, Chinobre, on Erica platycodon, 15 Apr. 2010, W.J. (WU 33338, culture S231); ibid., on Laurus novocanariensis, 16 Dec. 2010, H.V. & W.J. (WU 33395, culture S482); ibid., on undetermined wood, 16 Jun. 2008, L. Quijada 16060851 (culture S549); Montaña Chamuscada, on Erica platycodon, 16 Dec. 2010, W.J. & H.V. (WU 33393, culture S480).
Notes: Previously only known from Australia and New Zealand (Samuels et al. 2006). In this study collected on the Canary Islands, predominantly as sexual morphs, which differ from the closely related T. petersenii by an orange-brown colour. Trichoderma petersenii is common in the same region. On La Palma T. dorotheae was found in an albino form, i.e., having white stromata with yellowish ostiolar dots.
Trichoderma europaeum Jaklitsch & Voglmayr, sp. nov. MycoBank MB809282.
= Hypocrea minutispora sensu Jaklitsch (2011).
= Trichoderma minutisporum sensu Jaklitsch (2011).
Etymology: For its abundant occurrence in Europe.
See Jaklitsch (2011) for a more detailed description, illustrations (figs 41, 42) and additional examined material.
Stromata solitary, gregarious or aggregated, sometimes formed as compound stromata disintegrating into several parts; when fresh 1–7(–11) mm diam, 0.5–2.5(–3) mm thick, pulvinate or semiglobose, sometimes turbinate or discoid, sometimes with white base mycelium. Surface smooth, sometimes with white or silvery covering layer. Stromata when dry (0.8–)1.8–4.5(–7.5) × (0.5–)1.5–3.5 (–5.4) mm, (0.2–)0.5–1.4(–2.5) mm thick (n = 140), pulvinate or discoid. Margin or edges adnate or free, often lobed or undulate, smooth, sterile, sometimes white. Outline circular, oblong, ellipsoid or irregular. Stroma surface smooth or rugose or finely tubercular. Ostiolar dots (20–)30–70(–173) μm (n = 250) diam, numerous, minute but well-defined, plane or convex, reddish to brown, nearly black when old. Stromata first white, typically turning rosy from the centre, to greyish orange, pale red, greyish red to reddish brown; reddish pigment sometimes absent or often disappearing and yellow to brown colours emerging, stromata becoming yellow-brown, brown-orange, brown, less commonly dark brown, mostly in the range 6–8A2–5, 6AD6–7, 6–9BD4–7, 9A4, 9CE5–8, 7–8CE4–8. Spore deposits white. Mature stromata after rehydration brown with yellow surface and reddish brown dots 47–80(–95) μm diam; no colour change after addition of 3% KOH noticeable, only the brown to reddish perithecial colour becoming more prominent. Stroma anatomy: cortical layer (10–)15–25(–30) μm (n = 30) thick, yellow, of a thin amorphous layer and below a dense t. angularis of thick-walled cells (3–)4–9 (–12) × (2–)3–6(–7) μm (n = 30) in face view and in vertical section. Subcortical tissue a hyaline t. intricata of (2.0–)2.5–4.5(–6.0) μm (n = 30) wide hyphae. Subperithecial tissue a dense hyaline t. epidermoidea of mostly elongate, thick-walled cells (5–)7–34(–63) × (4–)7–13(–16) μm (n = 35). Ostioles (50–)56–80(–105) μm long, plane or projecting to 15 μm, (20–)26–40(–47) μm wide at the apex (n = 30), conical or cylindrical, periphysate. Perithecia (190–)210–270 (–320) × (115–)130–200(–240) μm (n = 30), numerous, crowded, 6–9 per mm stroma length, ellipsoidal, flask-shaped or globose; peridium (11–)14–17(–19) μm (n = 30) thick at the base, (5–)10–15(–17) μm (n = 30) thick at the sides, yellow, orange in 3 % KOH. Asci (77–)90–110 (–120) × (5.0–)5.5–6.5(–7.0) μm, stipe (3–)9–20(–27) μm long (n = 100). Ascospores hyaline, verruculose; cells dimorphic; distal cell (3.7–)4.0–4.8(–6.0) × (3.2–)3.5–4.0(–5.0) μm, l/w 1.0–1.3(–1.8) (n = 170), subglobose, ellipsoid or wedge-shaped; proximal cell (4.2–) 4.8–6.0(–7.2) × (2.7–)3.0–3.5(–4.0) μm, l/w (1.2–)1.4–1.9 (–2.4) (n = 170), wedge-shaped or oblong.
Cultures and asexual morph: optimal growth at 25 °C on all media; no growth at 35 °C.
On CMD colony radius 22–24 mm at 15 °C, 46–51 mm at 25 °C, 24– 36 mm at 30 °C after 72 h; mycelium covering the entire plate after 4–5 d at 25 °C. Colony hyaline, thin, circular; mycelium loose, not zonate; broad marginal zone becoming downy by long aerial hyphae. Autolytic activity and coilings lacking or inconspicuous. No diffusing pigment, no distinct odour noted. Chlamydospores terminal and intercalary. Conidiation noted after 2–3 d, green after 4–5 d; effuse, short, on surface hyphae and aerial hyphae, forming broad, diffuse concentric zones of shrubs. Conidia produced in minute wet heads.
On PDA colony radius 18–20 mm at 15 °C, 39–42 mm at 25 °C, 11– 22 mm at 30 °C after 72 h; mycelium covering the plate after 5–6 d at 25 °C. Colony dense, zonate, becoming hairy to floccose by abundant aerial hyphae forming a white to yellowish mat and radial strands. Autolytic excretions and coilings inconspicuous. No diffusing pigment produced, reverse yellowish, 2–4A3. Odour inconspicuous or unpleasant, rancid. Conidiation noted after 2 d, effuse, poor, e.g. on solitary phialides on aerial hyphae, colourless to white, not becoming green.
On SNA colony radius 18–21 mm at 15 °C, 36–42 mm at 25 °C, 8– 22 mm at 30 °C after 72 h; mycelium covering the plate after 5–6 d at 25 °C. Colony similar to CMD. Chlamydospores uncommon; terminal and intercalary, (5–)6–9(–11) × (4–)5–8(–10) μm, l/w (0.9–) 1.0–1.5(–2.1) (n = 27), globose or pyriform, when intercalary sometimes to 20 μm long andor fusoid or rectangular. Conidiation starting after 2 d, pale green after 5–6 d; effuse, on simple, short, erect conidiophores and in numerous small shrubs to 0.3 mm diam with up to 5 main axes, usually in broad, diffuse concentric zones. Simple conidiophores mostly unpaired, in shrubs tending to be paired in terminal side branches; generally short, 1–3 celled. Phialides formed solitarily or in whorls of 2–3(–5) on 3–4.5 μm wide cells. Conidia formed in minute wet green heads. Shrubs growing to circular or oblong tufts to 1.5 mm diam mostly along the distal margin after ca. 10 d, aggregating to 4 mm. Tufts or pustules circular, loose, of a stipe to 11 μm wide, with unpaired primary branches 6–9 μm wide, and several straight, radial main axes 200–400 μm long, typically with short paired side branches emerging at right angles; main axes and side branches fertile to the tips, attenuated upwards to 2–4(–5) μm. Side branches often pyramidal or slender with short side branches 20–80 μm long, sometimes 1- or 2-fold re-branching, forming dense structures. Cells supporting the phialides (1.5–)2.0–3.5 μm wide and often apically thickened. Phialides divergent in whorls of 2–5(–6), lageniform and long in effuse conidiation, ampulliform and short in tufts or pustules, (4–)6–10(–17) × (2.7–)3.2–4.0(–4.8) μm, l/w (1.2–)1.5–2.8(–4.3), (1.3–)1.7–2.5(–3.3) μm wide at the base (n = 63), often inequilateral and curved, with abruptly narrowed, thin, cylindrical neck. Conidia ellipsoid, less commonly subglobose, (2.8–)3.3–4.0(–5.0) × (2.5–)2.7–3.2(–3.8) μm, l/w (1.1–)1.2–1.4(–1.7) (n = 63), green, smooth, with minute guttules; scar indistinct.
Habitat: On hard, little degraded or medium-decayed wood and bark of trees, mostly Fagus sylvatica, and fungi growing on it.
Distribution: The most common Trichoderma species with hyaline ascospores in temperate Europe.
Typus: Austria, Kärnten, Klagenfurt Land, St. Margareten im Rosental, Schwarzgupf, above Umwiese, MTB 9452/4, 46°31′40″ N, 14°25′26″ E, elev. 870 m, on partly decorticated, 2–8 cm thick branches of Fagus sylvatica, soc. Melanomma sanguinarium, and Peniophora cinerea, 21 Oct. 2003, W.J. Hypo 117 (holotype WU 29250; ex-type culture CBS 121276 = C.P.K. 1607).
Additional materials from southern Europe examined (all on branches or twigs): Greece, Crete, between Omalos and the Samaria gorge, close to the road leading to the Kallergi lodge, 35°19′05″ N, 23°54′56″ E, elev. 1160 m, on Quercus coccifera, 28 Nov. 2011, W.J. (culture S611). Italy, Abruzzo, L'Aquila, Quattro Vie, 24 Nov. 2009, on Fagus sylvatica, 24 Nov. 2009, W. Gams (WU 33307, culture S125); Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, Riserva biogenetica Falascone, on Fagus sylvatica, 21 Nov. 2009, W.J. & H.V. (WU 32196, culture S99); ibid., on Datronia mollis/Fagus sylvatica, 21 Nov. 2009, W.J. & H.V. (culture S100); ibid., near military station, on Fagus sylvatica, 22 Nov. 2009, W.J. & H.V. (WU 33302, culture S114); Basilicata, Parco Nazionale del Pollino, San Severino, Bosco Magnano, along the river Peschiera, on Fagus sylvatica, 17 Nov. 2009, H.V. & W.J. (WU 32179, culture S50); Calabria, Mormanno, Parco Nazionale del Pollino, Valle di Fiume Argentino, Cielafforcato, on Fagus sylvatica, 18 Nov. 2009, W.J. & H.V. (culture S60); ibid. (WU 32184, culture S64); Campania, Parco Nazionale del Cilento, left roadside of Via Provinciale del Corticato shortly after the highest point heading to Sacco, on a polypore on Ostrya carpinifolia, 16 Nov. 2009, H.V. & W.J. (culture S37); Lazio, Soriano, Faggeta del Cimino, on branch of Castanea sativa, 26 Nov. 2009, W.J., H.V. & W. Gams (culture S133); ibid., on Fagus sylvatica (WU 33311, culture S134); Trentino, Mattarello, near Folgaria, on Fagus sylvatica, 20 Oct. 2011, H.V. & W.J. (culture S569). Spain, Basque Country, Gorbeia Natural Park, Álava, Murua, on Quercus petraea, 7 Nov. 2010, W.J. (culture S381); Bizkaia, forest at the road A624 3 km before Altube heading southeast, on Fagus sylvatica, 1 Nov. 2010, W.J. (culture S331); ibid., on Fagus sylvatica (culture S332).
Notes: Lu et al. (2004) considered that T. minutisporum in their sense was probably a species complex. Based on our phylogenetic analyses, T. europaeum cannot be subsumed under the American T. minutisporum s.str., which has a virtually identical phenotype, but seems to differ by the formation of some yellow pigment on CMD. Characteristic of T. europaeum is a more or less effuse conidiation in continuous concentric rings, i.e., a much reduced tendency to produce conidial pustules on CMD as compared to closely related species like T. mediterraneum. Trichoderma europaeum is common in Central and Northern Europe, while in the Mediterranean region it is nearly entirely replaced by T. mediterraneum.
Trichoderma euskadiense Jaklitsch & Voglmayr, sp. nov. MycoBank MB809283. Fig. 11.
Fig. 11.
Trichoderma euskadiense (WU33367; S377 = CBS 130013). A–Q. Sexual morph. A–C. Fresh stromata (A. with asexual morph). D–F. Dry stromata. G. Rehydrated stroma in 3 % KOH. H. Ostiole in section (in lactic acid). I. Perithecium in section. J. Cortical and subcortical tissue in section. K. Subperithecial tissue in section. L. Stroma base in section. M, N. Asci (N. in cotton blue/lactic acid). O–Y. Cultures and asexual morph. O. Conidiation pustule (SNA, 25 °C, 23 d). P–T. Conidiophores (SNA, 30 °C, 14 d; S. young, with sterile elongation). U, Y. Conidia (SNA, 30 °C, 14 d). V–X. Cultures (V. on CMD, 35 °C, 14 d; W. on PDA, 30 °C, 21 d; X. on SNA, 25 °C, 28 d). Scale bars: A, B = 1 mm; C = 1.5 mm; D, E, G = 0.5 mm; F, O = 150 μm; H, J–L, P–R, T = 15 μm; I, S = 25 μm; M, N, U, Y = 5 μm.
Etymology: Named after Euskadi, the Basque Country in the Basque language Euskera.
Stromata scattered or aggregated in small groups of 2–3, when fresh ca. 1–5 mm diam, to 1.5 mm thick, pulvinate, dots distinct, dark brown, surface smooth, (rosy-)reddish-brown to greyish brown, rarely dark grey to nearly black; spore deposits white. Stromata when dry (0.9–)1.5–3.6(–5.2) × (0.7–)1.3–2.8(–3.6) mm, (0.4–)0.5–1.0(–1.2) mm thick (n = 17), pulvinate, less commonly discoid with angular or circular outline, free, often acute margin, dark brown, less commonly dark grey to nearly black surface, smooth apart from mostly distinct black convex ostiolar dots (16–)26–54(–80) μm (n = 30) diam and some tubercles; spore deposits white. Stromata after rehydration ca. 25 % larger, lighter, more orange-red, smooth; no conspicuous change after addition of 3 % KOH, only slightly darker and more intensely orange- or reddish brown with hyaline ostiolar openings. Stroma anatomy: Cortical layer (14–)19–28(–34) μm thick (n = 30), comprising a dense orange-brown t. angularis of small but distinct, thin-walled cells (3.5–)5.0–8.5(–12.0) × (2.5–)3.5–6.0(–7.0) μm (n = 40) in section; in places covered by a thin irregular amorphous layer of compressed orange-brown cells; cortical cells larger, lighter and with thicker walls at stroma sides and base. Subcortical tissue partly like the cortex, but cells (sub)hyaline, or replaced by a short-celled thin-walled t. intricata of hyaline, thin-walled hyphae (2.3–)2.7–4.3(–5.6) μm wide (n = 33). Subperithecial tissue consisting of a t. angularis-epidermoidea of thin-walled cells (4.0–)5.0–17(–31) × (3.0–)5.0–8.5(–11) μm (n = 62) and some wide hyphae with up to 1.5 μm thick walls; hyphae frequently toward the base forming a dense compressed t. intricata firmly attached to the bark. Perithecia (145–)170–212(–230) μm high, (110–)125–160(–175) μm wide (n = 26), globose or flask-shaped, densely crowded to 9–12 per mm; peridium yellow, (13–)14–18(–21) μm wide at the base, (5–)8–13(–14) μm wide at the sides (n = 26). Ostioles (54–)60–80(–97) μm long, plane or projecting up to 25 μm, (19–)21–32(–37) μm wide at the apex inside (n = 26), with periphyses 0.7–1.7 μm wide; marginal cells at the apex turning green in lactic acid. Asci (50–)56–67(–71) × (4.2–)4.7–5.5(–5.8) μm with a stipe up to 19 μm long (n = 30), cylindrical, apex truncate, thickened to 1–1.5 μm, with a flat ring. Ascospores hyaline, verruculose, cells dimorphic, distal cells (2.8–)3.2–4.0(–4.7) × (2.7–)3.0–3.3(–3.7) μm, l/w (0.9–)1–1.3(–1.6) (n = 45), subglobose or ellipsoid, proximal cells (3.5–)3.7–4.7(–6.0) × (2.0–)2.5–2.8(–3.2) μm, l/w (1.2–)1.3–1.9(–2.6) (n = 45), ellipsoid, subglobose or wedge-shaped, to oblong and tending to be longer in the ascus base.
Asexual morph on natural substrates: white to pale green colonies to 6 mm long, also occurring on stromata.
Cultures and asexual morph: growth fastest on CMD, slowest on PDA, optimal at 30 °C on PDA, at 30–35 °C on CMD.
On CMD after 72 h colony radius 18–19 mm at 15 °C, 51–56 mm at 25 °C, 64–70 mm at 30 °C, 63–67 mm at 35 °C; mycelium covering the plate after 4(–5) d at 25–35 °C. Colony hyaline, centre dense, margin looser, hyphae with conspicuous variation in width, local aggregation causing a mottled appearance or whitish spots. Aerial hyphae only frequent along the plate margin. At 30–35 °C conspicuous curvatures or large coilings developing in wide primary surface hyphae. Conidiation scant, effuse, eventually also in some small, ill-defined, green shrubs composed of aggregated conidiophores along the plate margin and few in other areas. Autolytic excretions, coilings, diffusing pigment, and distinct odour lacking. Chlamydospores appearing after 4–5 d, frequent after 2 wk, terminal and intercalary, (5.5–)6.7–9.3(–10.5) × (5.5–)6.3–9.0(–10.7) μm, l/w (0.9–)1.0–1.1(–1.3) (n = 32), globose or pyriform.
On PDA after 72 h colony radius 9–11 mm at 15 °C, 27–33 mm at 25 °C, 51–54 mm at 30 °C, 39–41 mm at 35 °C; mycelium covering the plate after 7–8 d at 25 °C, after 4 d at 30 °C and after 5–6 d at 35 °C. Colony circular with wavy margin, dense, surface hyphae aggregating to strands, surface becoming whitish, downy, finely floccose to mottled, eventually turning faintly green 27C3–4, 28CG4–6, centrally 27DE4–6. Aerial hyphae forming a loose stellate mat of strands and eventually white spots. Conidiation starting after 2–4 d, effuse and in shrubs. Autolytic excretions frequent, coilings uncommon, diffusing pigment lacking, odour indistinct.
On SNA after 72 h colony radius 16–17 mm at 15 °C, 40–43 mm at 25 °C, 49–52 mm at 30 °C, 51–53 mm at 35 °C; mycelium covering the plate after 5 d at 25–35 °C. Colony as on CMD, but hyphal aggregations less conspicuous and coilings frequent, particularly at higher temperatures. Conidiation noticeable after 2–6 d, first effuse, later in small circular pustules mostly 0.5–1 mm diam, hairy when young, turning dark green, forming flat aggregates to ca. 5 mm in length. Conidia first formed within pustules. Elongations present on the periphery of pustules, straight, up to 150 μm long in young shrubs, inconspicuous in older pustules, appearing warted under low magnification, smooth in microscopic mounts. Conidiophores mostly tree-like, with branches decreasing in length from the base to the tip, with up to 5 levels of mostly 1-celled branches or phialides, solitary or in whorls of solitary or in whorls of 2–3(–4) around a common axis, perpendicular to the axis or slightly inclined upwards; branches (2.0–)2.5–4.0(–5.0) μm wide. Phialides (4.0–)4.8–8.2(–11.0) × (2.3–)2.5–3.0(–3.3) μm, l/w (1.4–)1.7–3(–4.5), (1.2–)1.4–2.0(–2.5) μm wide at the base (n = 42), lageniform, mostly inequilateral, straight or curved. Conidia (3.0–)3.3–3.8(–4.2) × (1.8–)2.0–2.5(–2.7) μm, l/w (1.3–)1.4–1.8(–1.9) (n = 50), pale green, oblong with parallel sides, smooth, eguttulate, scar indistinct; in older pustules conidia enclosed in a drop surrounded by a pellicle, e.g. 0.8 mm diam.
Habitat: On twigs of Ulex europaeus lying on the ground.
Distribution: Europe (Spain), only known from the holotype collection.
Typus: Spain, Gipuzkoa, Oiartzun, nature park Aiako Harra, at the road BI 3420 heading to Endara, pasture with Betula and Ulex, 43°16′25″ N, 1°48′17″ W, elev. 370 m, on 2–4 cm thick corticated twigs of Ulex europaeus lying in grass and moss, on bark, 6 Nov. 2010, W.J. (holotype WU 33367; ex-type culture CBS 130013 = S377).
Notes: Trichoderma euskadiense differs from all other species of the Longibrachiatum Clade by dimorphic ascospore cells and the light reddish brown stromata, which are similar to those of T. minutisporum s.l., when young and fresh. Stromata however turn brown to nearly black during development. The green discoloration of the ostioles by lactic acid is typical of the Longibrachiatum Clade.
Trichoderma cf. fertile Bissett, Canad. J. Bot. 69(11): 2382. 1992 (1991).
Material examined: Greece, Crete, Plemeniana, 35°19′31″ N, 23°43′25″ E, elev. 345 m, asexual morph on Platanus orientalis, 27 Nov. 2011, W.J. (culture CBS 137003 = S606).
Notes: Usually isolated from soil. One asexual morph specimen was found on plant material in Crete, for which a tef1 intron 4 BLAST search yielded a 96 % match with T. fertile strain DAOM 167070. Phylogenetic analysis using tef1, however, suggests a new species.
Trichoderma gamsii Samuels & Druzhin., Stud. Mycol. 56: 168. 2006.
Materials examined: Greece, Crete, Armeni, Neo Chorio, 35°24′53″ N, 24°08′15″ E, elev. 110 m, asexual morph on Olea europaea subsp. sylvestris, 26 Nov. 2011, W.J. (culture S595). Italy, Bomarzo, Santa Cecilia, on fruit of Quercus petraea, asexual morph soc. immature sexual morph, 21 Oct. 2012, H.V. & W.J. (culture S643); Veneto, Fontanafredda, H.V. & W.J., asexual morph on Vitis vinifera, 23 Oct. 2011, W.J. & H.V. (culture S582). Spain, Andalucia, Alcalá de los Gazules, via de servicio south of the exit at km 54 off the A7 (A381), asexual morph on Teline linifolia, 17 Mar. 2011, W.J. & H.V. (culture S488); Puerto del Castaño, asexual morph on Ulex parviflorus, 17 Mar. 2011, W.J. & H.V. (culture S496).
Notes: Originally isolated from soil in various regions and as endophytes from a fern and from Ricinus, T. gamsii was detected as conidial colonies on five different plants in three different Mediterranean countries in this study. No sexual morph is known.
Trichoderma gelatinosum P. Chaverri & Samuels, Stud. Mycol. 48: 68. 2004 (2003).
Materials examined: Croatia, Istrija, forest N of Barbariga, elev. ca. 20 m, on Quercus pubescens, 14 May 2010, W.J. & H.V. (culture S257). Greece, Corfu, Acharavi, forest opposite to the Hydropolis Park, 39°47′45″ N, 19°49′31″ E, on Quercus macrolepis, 24 Apr. 2012, W.J. & H.V. (culture S641). Italy, Apulia, Foggia, Gargano, SW of Mandrione, Foresta Umbra/Foresta Domaniale, on Radulomyces molaris/Quercus cerris, 21 Nov. 2009, H.V. & W.J. (WU 32189, culture S87); ibidem, on Carpinus betulus, 22 Nov. 2009, W.J. & H.V. (WU 32199, culture S104); ibid., Riserva biogenetica Falascone, on Fagus sylvatica, 21 Nov. 2009, W.J. & H.V. (culture S101). Basilicata, Parco Nazionale del Pollino, San Severino, Bosco Magnano, along the river Peschiera, on Fagus sylvatica, 17 Nov. 2009, H.V. & W.J. (WU 32180, culture S51). Calabria, Mormanno, Parco Nazionale del Pollino, Valle di Fiume Argentino, Conte Orlando, on Fagus sylvatica, 18 Nov. 2009, W.J. & H.V. (WU 32185, culture S65). Campania, Parco Nazionale del Cilento, left roadside of Via Provinciale del Corticato shortly after the highest point heading to Sacco, on Acer opalus, 16 Nov. 2009, W.J. & H.V. (WU 32174, culture S35). Lazio, Bomarzo, Santa Cecilia, on Quercus cerris, 29 Nov. 2009, W.J., H.V. & W. Gams (culture S162). Farnese, Selva del Lamone, hiking trail Roppozzo, on Radulomyces molaris/Quercus cerris, 28 Nov. 2009, W.J., H.V. & W. Gams (WU 33320, culture S152) and on Radulomyces molaris/Quercus virgiliana (culture S160). Lago di Vico, west side, on Carpinus betulus, 26 Nov. 2009, W.J., H.V. & W. Gams (culture S137 and WU 33313, culture S139). Spain, Andalucia, at Rio Hozgarganta shortly after entering Provincia de Málaga on the road C3331 from SE/Jimena, on Eucalyptus globulus, 21 Mar. 2011, W.J. & H.V. (culture S529). Asturias, Soto de los Infantes, near Viescas, asexual morph on Corylus avellana, 4 Jun. 2013, H.V. & W.J. (culture S663). Basque Country, Bizkaia, Gorbeia, forest at the road A624 3 km before Altube heading southeast, on Fagus sylvatica, 1 Nov. 2010, W.J. (WU 33358, culture S330). Canarias, La Palma, Cumbre Nueva, Castanea plantation at LP 301, close to crossing with LP 3, on Chamaecytisus proliferus, 2 Dec. 2010, W.J. (WU 33377, culture S437). Garafía, close to Estación de Guaguas Llano Negro, on Chamaecytisus proliferus, 4 Dec. 2010, W.J. (WU 33386, culture S456); Montaña Tagoja, on ?Laurus novocanariensis, 14 Dec. 2009, W.J. (WU 33336, culture S194).
Note: In contrast to Central Europe, this species is more common than T. strictipile in the south, based on 19 specimens collected in many different areas.
Trichoderma gliocladium Jaklitsch & Voglmayr, sp. nov. MycoBank MB809284. Fig. 12.
Fig. 12.
Trichoderma gliocladium. A–D. Cultures (A. on PDA, 15 °C, 28 d; B. on SNA, 15 °C, 28 d; C. on MEA, 25 °C, 28 d; D. on MEA, 25 °C, 33 d). E. Conidiation pustule (MEA, 15 °C, 42 d). F–I. Conidiophores and phialides from MEA at 15 °C (F, G, I. 13–14 d; H. 18 d). J. Chlamydospores (SNA, 25 °C, 9 d). K, L. Conidia (K. SNA, 25 °C, 9 d; L. MEA, 15 °C, 23 d). A–E, G, J–L. S81 = CBS 130009; F, I. S89a; H. S83. Scale bars: E = 0.1 mm. F, J = 25 μm. G, H = 15 μm. I, K, L = 10 μm.
Etymology: The epithet denotes the gliocladium-like conidiophores.
Colonies in nature conspicuous, forming extensive, continuous, often compact, dark green crusts or mats to more than 10 cm long. Growth in culture, as determined in two experiments on CMD, PDA, SNA and MEA, fastest on MEA, also conidiation best developed on this medium. Optimal growth, colony propagation and conidiation at 15 °C on CMD and MEA, between 15 and 25 °C on PDA and SNA; at 25 °C hyphae forming pegs; not growing at 30 °C and above.
On CMD after 72 h colony radius 8–9 mm at 15 °C; at 25 °C 5–7 mm after 72 h and 13–15 mm after 7 d. At 15 °C colony circular, dense, radial, margin becoming diffuse, not covering the plate within 1 mo; conidiation absent, pigment lacking, odour indistinct, no chlamydospores formed. At 25 °C colony dense, growth slow, hyphae forming pegs, conidiation scant.
On PDA after 72 h colony radius 5–6 mm at 15 °C, ca. 5 mm at 25 °C; mycelium nearly covering the plate after 1 mo at 15 °C. At 15 °C colony circular, dense, finely zonate, turning bright to dull orange 5–7B7–8; surface in places with whitish downy or granular patches, margin lobate and radially ribbed. Aerial hyphae inconspicuous, short. Conidiation scant, after 3 d, effuse, forming gliocladium-like conidiophores; chlamydospores abundant. Odour indistinct. At 25 °C hyphae homogeneous in width, forming pegs, colony dense, turning orange-brown, no conidiation noticeable within 18 d.
On SNA after 72 h colony radius 5–6 mm at 15 °C, ca. 5 mm at 25 °C; mycelium covering the plate after 4 wk at 15 °C. At 15 °C colony hyaline, dense, with wavy or sublobate margin. Diffusing pigment lacking, odour indistinct. Conidiation starting after 3–4 d at plug margins, spreading from the centre across the entire plate, appearing as green granules or small pustules <1 mm diam due to numerous aggregating gliocladium-like conidiophores and large conidial heads up to ca. 300 μm diam. At 25 °C growth slow, hyphae forming numerous pegs and chlamydospores, conidiation scant, turning green around the plug and in some small pustules. Chlamydospores (after 9 d at 25 °C) conspicuously abundant on virtually all hyphae, intercalary and terminal, (10–)11–14(–16) × (10–)11–13.5(–15.5) μm, l/w = 0.9–1.1(–1.2) (n = 30), (sub)globose, rarely 2-celled.
On MEA after 72 h colony radius ca. 10 mm at 15 °C, ca. 8 mm at 25 °C; mycelium covering the plate after ca. 17 d at 15 °C, ca. 6–7 wk at 25 °C. At 15 °C colony hyaline, turning slightly yellowish after 1 mo; odour indistinct; chlamydospores numerous. Conidiation starting after 3 d, forming small dark green granules mostly along the margin, with large wet heads as on SNA. At 25 °C colony hyaline, dense, radial, comprising hyphae similar in width, continuous, margin becoming lobate. Aerial hyphae inconspicuous, short. Colony turning pale yellowish brown or rosy; odour indistinct. Chlamydospores formed after 4 d, becoming conspicuously abundant after ca. 11 d. Conidiation starting after 3–4 d at the near margin and the plug on minute gliocladium-like conidiophores, effuse or aggregating to numerous shrubs or small pustules arranged in several farinose to granular, well- or ill-defined concentric zones, or in radial patches, turning dark green to nearly black. After prolonged storage (2–3 mo) at 15 °C agar sometimes turning orange and conidial pustules losing the green colour, turning rosy. Shrubs 0.3–0.6 mm diam often aggregated to pustules 0.6–2 mm diam, branched at or near the base into several to numerous radially emerging gliocladium-like conidiophores, ter- to quaterverticillate, i.e. above a typically asymmetrical branching with 3–4 levels of 3–4 branches in verticils in steep angles; fourth level mostly comprising whorls of 2–5 phialides (also solitary), but sometimes a further branch replacing a phialide, terminated by another phialide whorl. Conidiophores 6–10(–11.5) μm wide at the thick-walled base, with outer layer swelling in KOH, gradually attenuated to 3–5 μm at the apices. Phialides (9.0–)11.5–17.0(–23.3) × (3.0–)3.5–4.3(–5.0) μm l/w = (1.6–)2.3–3.4(–4.2), (2.2–)3.0–4.5(–6.8) μm wide at the base (n = 110), narrowly lageniform, inequilateral, aequilateral only central in whorls, green when old, straight, sometimes sinuous. Conidia formed in large green drops, (4.5–)5.3–8.0(–10.3) × (3.3–)3.7–4.5(–5.7) μm, l/w = (1.2–)1.4–1.8(–2.4) (n = 160), green, smooth, ellipsoid, less commonly oblong, with inconspicuous or truncate scar, with minute guttules or sometimes 1 large guttule, sometimes laterally constricted.
Habitat: On twigs and branches of Quercus spp.
Distribution: Southern Europe (southern France; Italy: Gargano).
Typus: Italy, Apulia, Foggia, Gargano, Monte Barone, 41°46′19″ N, 16°08′06″ E, elev. 360 m, on a corticiaceous fungus on a branch of Quercus ilex lying on the ground, 20 Nov. 2009, W.J. & H.V. (holotype dried culture WU 32187; ex-type culture CBS 130009 = S81).
Additional materials examined: France, Dept. Alpes-de-Haute-Provence, Gorge du Verdon SE Rougon, ca. 150 m SW Pont du Tusset at hiking trail to Encastel, elev. 620 m, on branch of Quercus pubescens, 29 Jul. 2011, H.V. (S560). Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, Foresta Domaniale, 41°52′41″ N, 16°03′30″ E, elev. 200 m, on Radulomyces molaris on a branch of Quercus cerris lying in leaf litter, 21 Nov. 2009, W.J. & H.V. (culture S83); ibid., 41°52′37″ N, 16°03′34″ E, elev. 205 m, on the same substrate, closely soc. Hypocrea tremelloides, 21 Nov. 2009, H.V. & W.J. (culture S89a).
Notes: Trichoderma gliocladium has the largest conidia of all known green-conidial species of Trichoderma that have gliocladium-like conidiophores. Phylogenetically, its closest relative is T. gelatinosum. No sexual morph has been detected for this species.
Trichoderma guizhouense Q.R. Li, McKenzie & Yong Wang bis, Mycol. Progr. 12: 170. 2012.
Materials examined: Trichoderma guizhouense s.str.: Croatia, Istrija, Fažana, forest at Valbandon, on Acer monspessulanum, and Carpinus orientalis 26 Sep. 2010, W.J. & H.V. (cultures S278 and S279). Italy, Veneto, Galzignano, Turri, on Acer campestre, 23 Oct. 2011, H.V. & W.J. (culture S579). Spain, Mallorca, Puigpunyent, on Ulmus minor, 16 Nov. 2010, W.J. (culture S393). Trichoderma guizhouense tef1-variant 2: Greece, Corfu, Gouvia, hotel Fiori, 39°40′01″ N, 19°49′37″ E, elev. 38 m, on Quercus frainetto, 23 Apr. 2012, W.J. & H.V. (culture S628); Crete, between Agioi Pantes and Vrysses, 35°23′37″ N, 24°09′48″ E, elev. 140 m, on Platanus orientalis, 26 Nov. 2011, W.J. (culture S597). Italy, Lazio, Farnese, Selva del Lamone, on Corticiaceae/?Fagus, 24 Sep. 2012, T. Gräfenhan & W. Gams (culture S642); Veneto, Galzignano, Turri, on a basidioma of a Phellinus sp. on Robinia, 23 Oct. 2011, H.V. & W.J. (culture S581). Spain, Andalucia, El Puerto de Santa Maria, Playa de la Muralla, on Retama monosperma, 25 Mar. 2011, H.V. & W.J. (culture S548).
Note: Occurs in Southern Europe in two distinct tef1 variants, all collected as asexual morphs.
Trichoderma hamatum (Bonord.) Bainier, Bull. Soc. mycol. Fr. 22: 131. 1906, emend. Fig. 13.
Fig. 13.
Trichoderma hamatum. A–M. Sexual morph. A. Fresh stromata. B–E. Dry stromata (E. lower side). F. Rehydrated stroma. G. Rehydrated stroma in 3 % KOH. H. Perithecium in section. I. Cortical and subcortical tissue in section. J. Subperithecial tissue in section. K. Stroma base in section. L. Ascus. M. Ascospores in cotton blue/lactic acid. N–V. Asexual morph after 5–7 d at 25 °C. N, S. Conidiophores. O. Conidiation pustule. P. Elongations on pustule. Q, R. Sterile elongations. T. Phialides. U, V. Conidia. N–P, S, U. From SNA. Q, R, T, V. From CMD. A, B, N–P, S, U. WU 31629 (Hypo 647). C–M, Q, R, T, V. WU 31630 (Hypo 648 = CBS 132565). Scale bars: A = 2.5 mm; B–G = 0.5 mm; H = 40 μm; I, J, N, Q, R = 20 μm; K–M, S, T = 10 μm; O = 0.3 mm; P = 100 μm; U, V = 5 μm.
Stromata scattered or aggregated in small groups, when fresh up to 1.5 mm thick, yellow 6CD5, when dry (1.1–)1.3–2.5(–3) × (0.8–)1.1–2.0(–2.4) mm, (0.5–)0.6–1.0(–1.2) mm thick (n = 20), pulvinate or discoid with convex, smooth or tubercular surface, broadly attached or lower side gradually attenuated to a short central stipe; outline variable, circular or angular; margin free, often wavy or distinctly ribbed; lower side sometimes radially rugose, darker brown. Ostiolar dots distinct, circular, (39–)52–78(–102) μm diam (n = 40), brown.
Stroma surface yellow-brown or dull orange. Entostroma white to light brownish. Spore deposits white. Stromata after rehydration ca. 30 % larger, surface smooth, yellow, ostiolar dots ochre, 60–100 μm diam, after addition of 3 % KOH deeply ochre to dull orange-brown, colour difference between surface and dots fading. Stroma anatomy: Cortical layer (19–)22–33(–39) μm thick (n = 30), comprising a narrow t. angularis of 3–5 layers of large, distinct, yellow, thin-walled (max. 1 μm) cells (4–)6–18(–29) × (3–)5–12(–18) μm (n = 40) in section; surface glabrous, uppermost layer of compressed cells. Subcortical tissue a hyaline t. intricata of thin-walled hyphae (2.0–)3.5–6.5(–7.5) μm wide (n = 30), partly short-celled and appearing as t. angularis. Subperithecial tissue a dense hyaline t. angularis–epidermoidea of thin-walled, toward the base thicker-walled and more oblong cells (4.5–)8–22(–30) × (4.5–)6–12(–14) μm (n = 32) and broad hyphae 4–10 μm wide. Stroma base similar to the cortex, but cells often compressed and mixed with a compressed layer of light hyphae (1.5–)2.0–3.5(–4.5) μm wide (n = 30), inside the stipe of partly compressed, thick-walled hyaline to yellowish hyphae 3.5–12 μm wide. Perithecia (208–)220–260(–285) μm high, (108–)145–220(–237) μm wide (n = 20), globose or ellipsoid; peridium (12–)15–20(–22) μm thick at the base, (6.5–)8–17(–21) μm at the sides (n = 20), yellow, lighter to hyaline at the ostioles, in 3 % KOH slightly more orange. Ostioles (74–)79–114(–137) μm long, projecting to 17(–26) μm, (23–)26–39(–45) μm wide inside (n = 20), apical cells cylindrical to narrowly clavate, 2–4 μm wide. Asci (92–)100–115(–128) × (5.5–)5.8–6.5(–6.7) μm, stipe (4–)7–15(–18) μm long (n = 35), fasciculate on long and distinctly sinuous ascogenous hyphae. Ascospores hyaline, distinctly warted, warts to ca. 0.7 μm long, cells dimorphic, distal cells subglobose, ellipsoid or cuneate, (4.3–)4.7–5.8(–7.0) × (3.8–)4.3–5.0(–5.7) μm , l/w (0.9–)1.0–1.2(–1.5) (n = 72), proximal cells oblong or slightly attenuated downward, (5.0–)5.5–7.0(–8.5) × (3.3–)3.7–4.3(–4.8) μm, l/w (1.3–)1.4–1.8(–2.2) (n = 72), often elongated in the lowest position in the ascus.
Cultures and asexual morph: On CMD and SNA cultures lacking a diffusing pigment and distinct odour; conidiation first effuse, scant or concentrated in diffuse concentric zones, after 4–5 d in numerous, nearly globose, compact pustules 0.4–2 mm diam, sometimes aggregating up to 4 cm, turning pale green 27CD4–6, lacking yellow tones. Pustules arising on a stipe to 11 μm wide, comprising a reticulum of 5–6 μm wide branches with thickenings to 6–7 μm. Conidiophores radiating from the reticulum, pachybasium-like, either entirely fertile or, more commonly, at the base of ca. 50–200 μm long, 2–4 μm wide, persistent, smooth, thin-walled, straight, sinuous or helically twisted, distally slightly pointed elongations, sometimes symmetrically or asymmetrically branched above their fertile bases. Phialides arising from short lateral branches at elongation bases. Lateral branches (2.5–)3–4.5(–5.5) μm wide, typically comprising 1–3 cells with phialides arising at the tip in whorls of 2–6, less commonly solitarily along branches. Branches paired or not, disposed perpendicular to the axis, only rarely rebranching with 1-celled branches in right angles. Phialides (4.0–)5.0–7.7(–10.8) × (2.8–)3.0–4.0(–4.8) μm, l/w (1.1–)1.3–2.3(–3.6), (1.7–)2.0–2.5(–3.0) μm wide at the base (n = 62), lageniform or ampulliform, often inequilateral, often with long neck and (sub-)globose body, often longer and narrower in terminal position, particularly on young conidiophores. Conidia (3.5–)4.0–4.7(–5.3) × (2.5–)2.7–3.0(–3.3) μm, l/w (1.3–)1.4–1.6(–1.9) (n = 63), oblong or ellipsoid, often with parallel sides, green, smooth, scar indistinct or truncate, nearly eguttulate, brown in KOH. Chlamydospores common, terminal and intercalary, (6.5–)8–12(–15) × (5–)7–11(–13) μm, l/w (0.8–)0.9–1.3(–1.5) (n = 30), globose, oblong or oval, sometimes 2–celled in ca. 11–12 μm wide hyphae.
Material examined: Canada, Quebec, isolated from spruce forest soil, Jun. 1977, P. Widden [Neotype, proposed by Bissett (1992), DAOM 167057].
Habitat: Soil, wood and herbaceous tissue; also commonly isolated as endophyte of Theobroma cacao sapwood (Samuels & Ismaiel 2009).
Known distribution: Probably cosmopolitan.
Sexual morphs examined: France, Île de la Réunion, St. Philippe, Sentier Botanique de Mare Longue, 200–300 m, tropical rain forest, on hardwood, possibly Sideroxylon sp., soc. white corticiaceous fungus, 6 Mar. 2011, A. & I. Hausknecht (WU 31629, culture Hypo 647); same area, on cutting area of hardwood, 6 Mar. 2011, A. & I. Hausknecht (WU 31630, culture Hypo 648 = CBS 132565).
Notes: The specimens cited above represent the first find of the sexual morph of T. hamatum, a species that is frequently isolated from soil and as an endophyte. As noted earlier (Bissett, 1992, Chaverri et al., 2003, Samuels and Ismaiel, 2009, Jaklitsch, 2011), asexual morph morphology of T. hamatum is typical for pachybasium-type conidiophores characterised by stout branches with ampulliform phialides and frequent occurrence of well-differentiated, sterile or fertile elongations of conidiophores. The sexual morph is morphologically similar to other species of Trichoderma that have the pachybasium morphology, i.e. distinct ostiolar dots, glabrous stroma surface, and the distinctly subglobose cells of the cortex. Although both morphs are typical of the Polysporum Group, and are atypical in the Viride Clade, DNA data however place it in that clade, albeit in a clade of its own, the Hamatum Clade, in which only three other species are known to form stromata, viz. T. eijii, T. flaviconidium and T. pezizoides.
Trichoderma hausknechtii Jaklitsch & Voglmayr, sp. nov. MycoBank MB809285. Fig. 14.
Fig. 14.
Trichoderma hausknechtii (WU 32168, Hypo 649 = CBS 133493). A–J. Sexual morph. A. Fresh stromata. B, C. Dry stromata. D. Rehydrated stroma in 3 % KOH. E. Perithecium in section. F. Cortical and subcortical tissue in section. G. Subperithecial tissue in section. H. Ascospores showing warts. I, J. Asci with ascospores. K–W. Cultures and asexual morph. K–O. Conidiophores. P. Phialides and conidia. Q, R. Conidia. S. Conidiation pustule. T–V. Cultures after 28 d at 25 °C (T. CMD; U. PDA; V. SNA). W. Chlamydospores (after 20 d at 25 °C). K–S. After 10–14 d at 23–25 °C. Scale bars: A = 1 mm; B–D = 0.5 mm; E = 50 μm; F, G, K, L, N = 20 μm; I, J, M, W = 15 μm; H, O, P = 10 μm; Q, R = 5 μm; S = 0.2 mm; Image A by I. Hausknecht.
Etymology: In honour of the collector Anton Hausknecht, for his long and valuable service as president of the Austrian Mycological Society.
Stromata scattered or gregarious, when fresh pulvinate, pale yellow, with conspicuous green ostiolar dots when mature, when dry (0.8–)1.1–1.8(–2.0) × (0.6–)0.9–1.5(–1.7) mm, (0.3–)0.4–0.6(–0.8) mm thick (n = 17), pulvinate to discoid, with even or concave upper side, centre often strongly depressed; outline irregular-angular or rounded, partly irregularly lobed; margin often for a large part free and stroma only centrally attached); surface smooth, sometimes rugose, yellow-brown, pale orange-brown or greenish due to spore powder. Ostiolar dots (47–)55–92(–118) μm wide (n = 30), conspicuous, numerous, brown to dark green or black when mature. Rehydrated stromata turbinate, more yellow, slightly more orange in 3 % KOH, i.e. without considerable change; sides turning more brownish. Stroma anatomy: Cortical layer (11–)16–27(–37) μm thick (n = 30), consisting of a textura angularis of yellow, thin-walled cells (7.5–)10–19(–23) × (4.0–)5.5–11.5(–17.0) μm (n = 30) in section, often only of 1–2 layers of coarse cells; uppermost layer partly collapsing. Subcortical tissue a t. angularis–epidermoidea of subhyaline, thin-walled cells (5.5–)7.5–18(–24.5) × (3.7–)5.5–12(–15) μm (n = 30). Subperithecial tissue a t. epidermoidea of subhyaline, thin-walled cells (5.5–)10–25(–31) × (4.0–)6.5–13.5(–18.5) μm (n = 30). Stroma base as cortex; where attached, with hyaline, (3.0–)4.0–5.5(–6.5) μm wide (n = 30) hyphae penetrating the substrate. Ostioles (67–)78–113(–134) μm long, projecting up to 25 μm, at the apex (13–)21–37(–42) μm wide inside, (43–)50–72(–80) μm wide including walls (n = 21), periphysate. Perithecia ca. 5 per mm stroma length, flask-shaped to globose, (185–)220–290(–320) μm high, (160–)200–255(–295) μm wide (n = 21); peridium (9–)12–19(–23) μm wide at the base, (9–)10–14(–16) μm at the sides (n = 21), yellow to yellow-brown, slightly darker, (reddish) brown in 3 % KOH. Asci cylindrical, (93–)100–114(–118) × (6.2–)6.5–7.3(–7.7) μm, stipe (7–)8–17(–25) μm long (n = 20), containing 6–8 ascospores; apex thickened to 1–1.5 μm. Ascospores dark olivaceous-green, coarsely tuberculate, with warts ca. 0.5–1 μm wide, cells dimorphic with little difference in shape and size, distal cells subglobose, (4.3–)5.3–6.5(–7.2) × (4.5–)5.0–6.0(–6.3) μm, l/w = (0.9–)1.0–1.2(–1.4) (n = 40), proximal cells subglobose to oblong, (5.0–)5.5–7.0(–8.7) × (4.2–)4.7–5.5(–6.0) μm, l/w = (0.9–)1.0–1.5(–1.7) (n = 40), including warts.
Cultures and asexual morph: growth slow, optimal at 25 °C on all media, negligible at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 1–2 mm at 15 °C, 6–7 mm at 25 °C, <1 mm at 30 °C; mycelium (nearly) covering the plate after 6 wk at 25 °C. Colony hyaline, dense, irregular, sublobate, centre denser, otherwise not zonate; surface mycelium scant. Aerial hyphae virtually absent. Autolytic excretions and coilings absent; colony becoming faintly yellowish; odour indistinct. Chlamydospores abundant, terminal and intercalary, globose to subglobose, (9–)11–18(–25) × (8–)11–16(–19) μm, l/w = 0.9–1.2(–1.5) μm (n = 30). Conidiation starting after 3 d in numerous clustered or densely aggregated gliocladium-like conidiophores, growing to small shrubs, granules or pustules <1 mm diam, with crystalline aspect, whitish, turning yellowish or green after ca. 1 wk, eventually dark green to nearly black 27F4–8, spreading from the centre over the entire colony surface, following colony growth. Shrubs/pustules 0.5–1 mm diam, more or less circular, overall of an erect, radial, fan-shaped architecture, starting within the agar by localized condensation and enhanced branching of submerged mycelium, arising on one or several stipes, i.e. often several erect conidiophores united to a compound structure. Stipe 9–17 μm wide, thick-walled, warted, asymmetrically branched into warted, up to 11 μm wide primary branches at narrow angles, the latter giving rise to a loose reticulum of abruptly narrowed, asymmetric branches. Conidiophores steeply ascending from the reticulum or as continuation of primary branches, comprising a straight, 5–7(–9) μm wide main axis and (2–)2.5–3.5(–4) μm wide side branches arranged in whorls up to 4 levels around the axis at steep angles, divergent at lower levels to more or less parallel at higher ones; side branches not or mostly once rebranching, terminating in a whorl of (2–)3(–5) curved, more or less parallel phialides or a single phialide, sometimes on an intercalary cell. Phialides narrowly lageniform or subulate, (6.5–)8.0–13.3(–17.7) × (2.3–)2.5–3.3(–3.8) μm, l/w = (2.2–)2.8–4.6(–6.0) μm, (1.8–)2.0–2.7(–3.5) μm wide at the base (n = 40). Conidia forming in wet heads to ca. 100 μm diam, ellipsoidal or oblong, (3.7–)4.5–6.5(–8.3) × (2.8–)3.0–3.7(–4.2) μm, l/w = (1.2–)1.4–1.9(–2.7) μm (n = 50), subhyaline to pale green, with 1–2 larger or many minute guttules and indistinct scar; more rarely long cylindrical and up to 10.7 μm long.
On PDA after 72 h colony radius 1 mm at 15 °C, 4 mm at 25 °C, <1 mm at 30 °C; mycelium not covering the plate after 6 d at 25 °C. Colony circular, dense, finely zonate, with hyper-branching within the agar, surface becoming farinose by conidiophores, first whitish, turning light green 27BC2–4. Aerial hyphae virtually absent. Autolytic excretions and coilings absent; colony turning pale yellowish 4B4; odour indistinct. Conidiation starting after 3d in densely disposed gliocladium like conidiophores, short-effuse, turning green after ca. 1 wk, conidia forming in wet heads; sometimes also irregular, thick greenish pustules to 5 mm diam forming in the centre, stroma-like.
On SNA after 72 h colony radius 0 mm at 15 °C, 1.5 mm at 25 °C, 0.1 mm at 30 °C; mycelium covering the plate after 5–6 wk at 25 °C. Colony similar to CMD but with more regular growth rate and shape, not zonate; pigment absent, odour indistinct. Chlamydospores abundant in the centre, terminal and intercalary. Conidiation starting after 4d in clustered gliocladium like conidiophores, turning green after 10 d; small pustules loosely spreading from the centre, dark green to nearly black 27F4–8, resembling fine black grains.
Habitat: On wood and bark and fungi growing on it.
Distribution: France, La Réunion, only known from the holotype collection.
Typus: France, Île de la Réunion, St. Paul, Bois de Sans-Souci, elev. ca. 1400 m, on well-decayed wood and bark of Acacia heterophylla, soc. various fungi, 19 Mar. 2011, A. Hausknecht Re 66/11 (holotype WU 32168, ex-type culture CBS 133493 = Hypo 649).
Notes: Trichoderma hausknechtii belongs phylogenetically to the Harzianum Clade. The nearly gliocladium-like conidiophores resemble those of T. thelephoricola or less so those of T. brunneoviride, the latter which also belongs to the Harzianum Clade, while the pale yellowish to pale orange stromata of T. hausknechtii are unlike those of this clade, but rather recall sexual morphs of e.g. T. strictipile or the Chlorosporum Clade.
Trichoderma helicolixii Jaklitsch & Voglmayr, sp. nov. MycoBank MB809295. Fig. 15.
Fig. 15.
Trichoderma helicolixii (WU 33410, S640 = CBS 133499). A–E. Sexual morph. A–C. Dry stromata. D, E. Asci with ascospores. F–Q. Cultures and asexual morph at 25 °C. F–H, K, L, N. Conidiophores and phialides (G, N. with sterile elongations). I. Conidiation pustule. J. Sterile conidiophore elongations. M. Conidia. F–N. After 6–10 d at 25 °C. O–Q. Cultures at 25 °C (O. CMD, 14 d; P. PDA, 14 d; Q. SNA, 10 d). Scale bars: A, C, I = 0.5 mm; B = 0.2 mm; D–F, H, K, L = 10 μm; G, J = 20 μm; M = 5 μm; N = 15 μm.
Etymology: Descriptive epithet reflecting typical green stromata of T. lixii and helical elongations of conidiation pustules.
Stromata flat pulvinate, placentiform or lentiform, when fresh ca. 4–5 mm diam, smooth, green, when dry (0.5–)0.8–2.5(–3.0) × (0.5–)0.8–1.8(–2.0) mm, (0.2–)0.4–0.8(–1.2) mm thick (n = 16), with circular, oblong or angular outline, sometimes with white to yellowish radiating mycelium around the base; surface smooth to finely tuberculate, dark green to black or dark grey, sometimes with reddish tints, or dark brown; outer side (on short base) pale yellowish brown; ostiolar dots absent, ostioles sometimes visible under strong magnification, minute, ca. 15–47 μm diam; spore deposits dark green. Asci cylindrical, (88–)93–108(–115) × (5.2–)5.5–6.5(–6.7) μm, stipe up to 23 μm long (n = 25). Ascospores olivaceous-green, spinulose, with one to several guttules per cell, dimorphic, distal cells ellipsoidal or subglobose, (4.2–)4.5–5.5(–6.0) × (4.0–)4.2–4.7(–5.0) μm, l/w 1.0–1.2(–1.3) (n = 32), proximal cells oblong to cuneate, (4.5–)5.0–6.5(–7.0) × (3.3–)3.5–4.0(–4.2) μm, l/w (1.2–)1.3–1.8(–2.0) (n = 32).
Cultures and asexual morph: optimal growth at 25 °C on all media, slow growth at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 23–27 mm at 15 °C, 51–53 mm at 25 °C, 6–8 mm at 30 °C; mycelium covering the plate after 5–6 d at 25 °C. Colony hyaline, dense, circular, loose, with distinct differences in hyphal width, hyphae with many curvatures, secondary hyphae short, narrow, curly. Aerial hyphae inconspicuous, more abundant, long and high after 5 d in distant areas: autolytic excretions and coilings inconspicuous. Diffusing pigment absent, odour indistinct. Chlamydospores terminal and intercalary. Conidiation starting after 3 d, first effuse, comprising short, simple conidiophores; phialides solitary or in whorls of 2–3, often terminally on surface or aerial hyphae; conidia accumulating in wet heads. Compact, pulvinate-semiglobose, velutinous, sometimes laterally fused pustules (0.6–)0.9–2.4 mm diam formed mostly in distant areas after 1 wk, white, soon turning pale to medium green 27–28E4–6. Pustules comprising a well-developed, dense reticulum with many right angles, terminating in the periphery mostly in sterile helical elongations to 250(–350) μm long, 2–3.5 μm wide terminally, 4–5 μm towards base, simple or with 1–2 branches near the base, the latter also terminating in sterile, curved to helical elongations. Conidiophores short and wide, firmly integrated in the pustule, their main axis either entirely fertile or terminating in elongations; mostly consisting of a terminal whorl and 1 branching level, but sometimes up to 6 branching levels with 3–4 side branches in whorls or unpaired around the common axis; side branches 3–6 μm long, branching points to 7 μm wide, typically 1-celled, not or once rebranching, rarely at lower levels up to nearly 200 μm long. Phialides solitary or in whorls of 2–4, arising directly on the conidiophore axis or on a plump, 4–6 μm wide intercalary cell, ampulliform, plump, rarely with long neck, (3.0–)4.3–7.0(–9.7) × (2.5–)3.0–3.7(–4.0) μm, l/w (1.1–)1.3–2.0(–2.5), base (1.5–)2.0–3.0(–3.5) μm wide (n = 33). Conidia oblong or ellipsoidal, (3.2–)3.5–4.2(–5.3) × (2.0–)2.3–2.5(–2.7) μm, l/w (1.4–)1.5–1.8(–2.1) (n = 33), green, smooth, small, often agglutinated and densely packed in large groups.
On PDA after 72 h colony radius 18–19 mm at 15 °C, 35–38 mm at 25 °C, 4–5 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony circular with diffuse margin, dense, with distinct variation in hyphal width and many curvatures in all hyphae. Aerial hyphae abundant, forming an even, downy to floccose whitish mat, not zonate. Autolytic excretions and coilings common, reverse faintly yellowish, no diffusing pigment formed; odour indistinct. Conidiation starting after 3d, effuse, spreading from the centre, after 7–8 d turning pale to medium green 28CD4–6.
On SNA after 72 h colony radius 19–23 mm at 15 °C, 40–43 mm at 25 °C, 4–5 mm at 30 °C; mycelium covering the plate after 5 d at 25 °C. Colony as on CMD, but soon becoming conspicuously transparent due to hyphal degeneration; autolytic excretions uncommon, coilings common. Chlamydospores abundant but loosely disposed, terminal and intercalary, (sub-)globose, sometimes ellipsoidal or pyriform, (6.5–)7.5–10.5(–12) × (6–)7–9(–11) μm, l/w (0.9–)1–1.3(–1.8) (n = 30). Conidiation starting after 3 d, first effuse, pustules formed after 5 d and turning green after 1 wk; pustules as on CMD, but more irregularly disposed, aggregating to ca. 5 mm diam and medium green 27DE3–4, 28DE4–6; with sinuous to helical sterile verrucose elongations sometimes branched at several levels or anastomosing at higher levels.
Habitat: On wood and bark of hardwood trees and shrubs.
Distribution: Europe, Mediterranean (Greece, Spain), uncommon.
Typus: Greece, Corfu, Acharavi, forest opposite to the Hydropolis Park, 39°47′45″ N, 19°49′31″ E, elev. 20 m, on 5–6 cm thick branch of Spartium junceum, on bark, soc. Hysteriaceae, holomorph, asexual morph pale bluish green with white hairs (elongations), 24 Apr. 2012, H.V. & W.J. (holotype WU 33410, ex-type culture CBS 133499 = S640).
Additional material examined: Spain, Andalucia, Castellar de la Frontera, road A2100 north of the village, 36°17′40″ N, 5°24′09″ W, elev. 80 m, on 2 cm thick, corticated branch of Erica arborea, on bark, asexual morph, 20 Mar. 2011, W.J. & H.V. (culture S515).
Notes: This species is only known from one holomorphic and one asexual morph specimen. Stromata of this species are indistinguishable from those of T. lixii s.l. and were therefore collected as such. Also ascospore sizes are similar. Most stromata of the holotype were immature or overmature and therefore not sectioned. Conidiation in pustules on CMD or SNA with sterile helical elongations forming green conidia recall T. cerinum, T. helicum, T. rossicum, T. tomentosum and T. velutinum among others. It was extremely difficult to separate conidiophores in order to make good microscope preparations because of their firm integration in pustules. Phylogenetically this species is isolated at or near the base of the Harzianum Clade, despite the lixii-like stromata.
Trichoderma aff. helicum Bissett et al., Canad. J. Bot. 81: 575. 2003.
Material sequenced: Spain, Canarias, La Palma, Cubo de la Galga, asexual morph on Persea indica, 3 Dec. 2010, W.J. (culture CBS 136996 = S446).
Note: One isolate from La Palma, which differs from true T. helicum in gene sequences.
Trichoderma hispanicum (Jaklitsch & Voglmayr) Jaklitsch & Voglmayr, Mycotaxon 126: 149. 2014.
Materials examined: Greece, Crete, Armeni, Neo Chorio, 35°24′53″ N, 24°08′15″ E, elev. 110 m, asexual morph on Calicotome villosa, 26 Nov. 2011, W.J. (culture S596); south of Platanos, 35°26′49″ N, 23°35′03″ E, elev. 305 m, asexual morph on Platanus orientalis, 27 Nov. 2011, W.J. (culture S603).
Note: Described as Hypocrea hispanica by Jaklitsch et al. (2012).
Trichoderma istrianum Jaklitsch & Voglmayr, sp. nov. MycoBank MB809286. Fig. 16.
Fig. 16.
Trichoderma istrianum (WU 33354, S310 = CBS 130539). A–J. Sexual morph. A–D. Dry stromata. E. Rehydrated stroma in 3 % KOH. F, G. Asci with ascospores (G. in cotton blue/lactic acid). H. Perithecium in section. I. Cortical and subcortical tissue in section. J. Subperithecial tissue in section. K–X. Cultures and asexual morph (at 25 °C). K, M, W. Phialides. L, P, Q, R. Conidiophores. N, X. Conidia. O. Conidiation pustule (SNA, 10 d). S–V. Cultures (S. CMD, 14 d; T. CMD, 35 d; U. PDA, 7 d; V. SNA, 21 d). K–N, P–R, W, X. SNA, 7–8 d. Scale bars: A = 0.3 mm; B, D, E = 0.5 mm; C = 0.2 mm; F, G = 7 μm; H, P = 30 μm; I, L = 20 μm; J, K, M, Q, R, W = 10 μm; N = 5 μm; O = 0.7 mm; X = 3 μm.
Etymology: Named after its first collection area, the Croatian peninsula Istrija.
Stromata scattered or forming aggregates up to 6 mm in length, when fresh light to bright yellow-orange to orange-brown, with downy to nearly smooth surface and minute hyaline ostiolar dots; when dry (0.4–)0.6–2.1(–3.4) × (0.3–)0.5–1.6(–2.5) mm, (0.15–)0.2–0.5(–0.7) mm thick (n = 20), first effuse, becoming disintegrated into discoid to flat pulvinate part stromata with variable, irregular outline; margin first attached, becoming free; surface homogeneous, scarcely tubercular, velvety with brown hairs; ostioles invisible, rarely dark diffuse dots 50–120 μm diam present. Surface yellow 4A4–5 to orange or orange-brown, rarely reddish brown, mostly 8CE6–8, also 6–7CD6–8. Spore deposits white. Rehydrated stromata more pulvinate, ochre, with minute, triangular to stellate, hyaline ostiolar dots 40–65 μm diam, in 3 % KOH slowly turning darker, orange to more reddish brown. Stroma anatomy: Cortex well-defined, (13–)17–24(–28) μm thick (n = 30), comprising a dense, orange-brown t. angularis of distinct, thin- to thick-walled cells (3.0–)4.5–8.5(–11) × (2.0–)3.3–6.5(–7.8) μm in section (n = 30). Hairs on the stroma surface common, (6–)9–22(–33) × (2.8–)3.0–4.5(–5.4) μm (n = 30), cylindrical, 1(–5)-celled, straight or curved, thin-walled, light brown, smooth or verruculose. Subcortical tissue a hyaline t. intricata comprising thin-walled, (2.5–)3.5–6.0(–8.5) μm wide (n = 30) hyphae. Subperithecial tissue a hyaline t. angularis-epidermoidea of cells (4.5–)8–21(–28) × (4–)6–13(–18) μm (n = 33) with walls 0.7–2 μm thick; partly hyphal just below the perithecia. Perithecia (120–)145–205(–225) μm high, (95–)105–180(–220) μm wide (n = 20), ellipsoid or flask-shaped to globose; peridium hyaline, (15–)16–21(–24) μm thick at the base, (9–)12–20(–24) μm at the sides (n = 20). Ostioles (51–)57–82(–101) μm long, umbilicate, rarely projecting up to 20 μm, (10–)16–28(–33) μm wide inside, (16–)26–47(–55) μm wide including walls (n = 20); apical cells cylindrical to narrowly clavate, to 2.5 μm wide. Asci (66–)76–91(–98) × (4.5–)5.0–5.5(–6.0) μm, stipe (1.5–)3.5–13(–18) μm long (n = 35). Ascospores hyaline, verruculose, cells dimorphic; distal cells (3.3–)3.7–4.5(–5.2) × (3.2–)3.5–4.0(–4.2) μm, l/w (0.9–)1.0–1.2(–1.4) (n = 40), subglobose, ellipsoid or cuneate, proximal cells (3.8–)4.5–6.0(–6.7) × (2.5–)2.8–3.3(–3.7) μm, l/w (1.3–)1.5–2.0(–2.3) (n = 40), oblong to almost cuneate.
Cultures and asexual morph: optimal growth at 25 °C on all media, good growth at 30 °C, no growth at 35 °C.
On CMD after 72 h colony radius 23–24 mm at 15 °C, 48–51 mm at 25 °C, 42–43 mm at 30 °C; mycelium covering the plate after 4–5 d at 25 °C. Colony hyaline, dense, circular with wavy margin, radial, not zonate; hyphae of diverging width. Aerial hyphae inconspicuous; autolytic excretions and coilings lacking. Odour indistinct. Agar turning dilute greenish yellow 2–3A2–3 to bright yellow 3A3–8 after ca. 10 d. Chlamydospores rare, terminal and intercalary. Conidiation effuse and in small, dark green, 27E4–5, pustules 1–2(–3) mm diam, concentrated in a marginal zone; conidia often adhering in chains. Chlamydospores scant, mostly central, mostly terminal, globose or pyriform or ellipsoid, (5.5–)7–9(–10) × (4.5–)6–8(–9) μm, l/w 1.0–1.3(–1.5) (n = 25).
On PDA after 72 h colony radius 17–19 mm at 15 °C, 48–51 mm at 25 °C, 35–38 mm at 30 °C; mycelium covering the plate after 4–5 d at 25 °C. Colony circular with wavy margin, dense, surface hyphae comparatively wide, aggregating and forming radial white streaks. Aerial hyphae abundant, loosely arranged, forming a flat, floccose mat. Surface below the mat turning faintly and diffusely greenish, 29CD4–6 to 26–27DE3–5, from the centre due to effuse, farinose conidiation. Autolytic excretions and coilings inconspicuous. Diffusing pigment lacking, odour indistinct.
On SNA after 72 h colony radius 17–19 mm at 15 °C, 32–41 mm at 25 °C, 27–30 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony hyaline, dense, circular with wavy margin, radial, not zonate; hyphal width varying distinctly. Aerial hyphae common, long and high at the margin; autolytic excretions lacking, coilings abundant. Pigment lacking, odour indistinct. Chlamydospores uncommon, only formed at 30 °C. Conidiation first effuse, concentrated in a marginal downy zone and later in velutinous, relatively compact, dark green, 26–27EF4–8, pustules 1–3 mm diam concentrated in a broad distant zone or irregularly disposed. Conidiophores on aerial hyphae and at the periphery of pustules similar, simple, narrowly or broadly tree-like, consisting of a distinct main axis up to 4.5 μm wide and loosely arranged, unpaired, paired or verticillate, delicate, narrow, 1.5–3 μm wide side branches typically increasing in length downwards, often perpendicular to the main axis, not or once re-branching. Phialides solitary or in whorls of 2–4(–5), rarely repetitive, i.e. 1–2 cells widening below. Phialides (5.8–)7.5–12.5(–17.2) × (2.0–)2.3–3.0(–3.3) μm, l/w (1.8–)2.7–5.2(–8.8), (1.3–)1.7–2.2(–2.5) μm wide at the base (n = 54), narrowly lageniform, straight or slightly curved, often inequilateral. Conidia (3.3–)3.5–4.0(–4.5) × (2.5–)2.7–3.0(–3.5) μm, l/w (1.1–)1.2–1.4(–1.5) (n = 64), ellipsoid or subglobose, green, smooth, scar indistinct, sometimes distinct.
Habitat: On wood and bark of Carpinus orientalis and Quercus pubescens.
Distribution: Southern Europe (Croatia, Italy).
Typus: Croatia, Istrija, between Peroj and Barbariga, 44°59′08″ N, 13°46′18″ E, elev. 30 m, on wood chips of Carpinus orientalis partly buried in leaf litter, on wood, a black crust and a white corticiaceous fungus, soc. Propolis versicolor, holomorph, asexual morph pale greenish, 18 Oct. 2010, W.J. (holotype WU 33354; ex-type culture CBS 130539 = S310).
Additional materials examined: Croatia, Istrija, between Peroj and Barbariga, 44°59′06″ N, 13°46′15″ E, elev. 30 m, on Quercus pubescens, effuse asexual morph and immature yellowish stromata, 24 Sep. 2010, H.V. & W.J. (culture S272). Italy, Abruzzo, Sulmona, Vallelarga, close to N village sign, 42°00′12″ N, 13°55′48″ E, elev. 540 m, submediterranean forest, asexual morph on a branch of Quercus pubescens, soc. immersed pyrenomycete, 24 Nov. 2009, W.J. & H.V. (culture S120); same area, 42°00′09″ N, 13°55′42″ E, elev. 585 m, same host, asexual morph, light blue-green, effuse to 6 mm, on wood, on/soc. Hymenochaete rubiginosa, 24 Nov. 2009, W.J. & H.V. (culture S123).
Notes: Trichoderma istrianum is so far only known from two areas in Croatia and Italy. Phylogenetically and morphologically it is most closely related to T. petersenii. CMD cultures of both species produce a bright yellow diffusing pigment.
Trichoderma italicum Jaklitsch & Voglmayr, sp. nov. MycoBank MB809287. Fig. 17.
Fig. 17.
Trichoderma italicum. A–F. Sexual morph; A–D. Dry stromata (A, B. immature). E, F. Asci with ascospores. G–R. Cultures and asexual morph (at 25 °C.). G–I. Cultures after 7 d (G. CMD; H. PDA; I. SNA). J. Conidiation pustules (SNA, 7 d). K–P. Conidiophores and phialides (SNA, 5–6 d). Q, R. Conidia (SNA, 7 d). M, N. S15, all others WU 33310, S131 = CBS 132567. Scale bars: A, B = 0.2 mm; D, J = 0.5 mm; E, F, K, L, O, P = 10 μm; M, N = 20 μm; Q, R = 5 μm.
Etymology: Named for its occurrence in Italy.
Stromata scattered or aggregated in small groups of 2–3, when fresh up to 3 mm diam, aggregates to 7 mm, pulvinate to undulate, ostioles invisible, dull reddish brown, young also greyish-rosy due to whitish scurf. Stromata when dry (0.5–)0.8–2.1(–3) × (0.5–)0.7–1.8(–3) mm, (0.2–)0.3–0.8(–1.2) mm thick (n = 23), mostly discoid, also pulvinate, rarely turbinate; sides often vertical, brown, granular; surface distinctly tubercular or rugose; ostiolar dots only rarely visible under strong magnification, 30–65 μm diam. Stromata first whitish, turning light to dark or greyish brown, sometimes with olivaceous tone, eventually dark reddish brown to black, brown inside when old. Spore deposits dark greyish green. Cortex comprising a t. angularis of thin-walled brown cells (3–)4–9(–12) × (2.2–)3–7(–10) μm in face view (n = 32), with inhomogeneously distributed pigment. Asci (75–)82–100(–109) × (4.7–)5.3–6.3(–7.5) μm, stipe up to 25 μm long (n = 30). Ascospores (olive-)green, brown in KOH, distinctly warted, cells dimorphic, distal cells cuneate, ellipsoid or oblong, (3.7–)4.2–5.5(–7.0) × (3.5–)3.7–4.5(–4.8) μm, l/w (0.9–)1.0–1.4(–1.7) (n = 40), proximal cells oblong or cuneate, (4.3–)5.0–6.5(–8.0) × (3.0–)3.3–4.0(–4.7) μm, l/w (1.1–)1.3–1.9(–2.4) (n = 40).
Asexual morph on natural substrates: Colonies thinly effuse to 2 cm, sometimes pulvinate, velvety or farinose, bright or dull green.
Cultures and asexual morph: optimal growth at 25 °C on all media, no growth at 35 °C.
On CMD after 72 h colony radius 13–15 mm at 15 °C, 37–41 mm at 25 °C, 19–34 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony hyaline, circular, dense, radial, with conspicuous difference in hyphal width, not zonate. Aerial hyphae scant. Autolytic excretions, coilings, diffusing pigment and chlamydospores absent, odour indistinct. Conidiation starting after 2–3 d, first effuse, scant, on short erect conidiophores, soon in thick ca. 1–3 mm diam pustules appearing in loosely and irregularly disposed in a large central zone and later in distant areas of the colony, first white, after 5 d turning yellow and eventually dark green, 28–29E5–6, 27–28F5–8. Conidia formed in minute wet heads up to 15 μm diam. At 30 °C colony circular with ill-defined margin, conidiation abundant in yellow to green pustules mostly in the colony centre.
On PDA after 72 h colony radius 12–13 mm at 15 °C, 32–38 mm at 25 °C, 23–33 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony circular, dense, radial, of more or less uniform, finely wavy hyphae, indistinctly zonate; surface of a broad central zone becoming condensed, farinose, turning pale yellowish-greenish, 30AB4, 30C4–5, 1B3–4, 1C4–5, 2AB3–4, residual part becoming covered by a varying, whitish to yellowish, mat of aerial hyphae with radial-stellate pattern. Autolytic excretions and coilings inconspicuous, diffusing pigment lacking, reverse yellowish-greenish 3B4–7, odour indistinct. Conidiation starting after 2 d, effuse, on aerial hyphae and in numerous shrubs spreading from the centre, farinose to floccose, turning yellow-green after 4–5 d.
On SNA after 72 h colony radius 13–15 mm at 15 °C, 30–38 mm at 25 °C, 16–28 mm at 30 °C; mycelium covering the plate after 6–7 d at 25 °C. Colony circular with, wavy to nearly lobed margin, hyaline, radial, with conspicuous difference in hyphal width; hyphae finely wavy. Aerial hyphae inconspicuous or common and long at the colony margin making a downy appearance. Autolytic excretions absent, coilings common to abundant, diffusing pigment lacking, odour indistinct. Chlamydospores uncommon, mostly globose and intercalary in narrow hyphae, (5–)6–9(–12) × (3.5–)4–6(–8) μm, l/w 1.0–1.7(–2.4) (n = 20). Conidiation starting after 2 d, first inconspicuous, effuse on short erect conidiophores to ca. 150 μm and on aerial hyphae, soon followed by similarly structured but denser conidiation in numerous pustules 1–2 mm diam with granular surface in a broad central zone, after 4–5 d turning first yellow, later green, 29–30CE5–6, eventually dark green 27–28F5–8, densely aggregating to several mm, later also in distant areas of the plate. Pustules arising on a thick-walled, ca. 5 μm wide stipe, first loose but soon comprising a densely intricate reticulum; main axes recognizable, but mostly integrated into the reticulum. Peripheral conidiophores and side branches from main axes arising asymmetrically, perpendicular to the axis, short, typically regularly tree-like, 1–2 times rebranching, terminal branches 1–3 celled, often paired or in verticils, straight or curved, less commonly sinuous; all branches 2–3.5 μm wide, thickened joints to 5 μm. Phialides formed in whorls of 2–4(–5), less commonly singly on an intercalary cell, lageniform, (4.7–)7–12(–18.5) × (2.0–)2.5–3.0(–3.5) μm, l/w (1.7–)2.3–4.6(–7.5), (1.5–)1.7–2.3(–2.8) μm wide at the base (n = 109). Conidia formed in minute wet heads, subglobose or oval, (2.7–)3.0–3.5(–4.3) × (2.3–)2.5–2.8(–3.2) μm, l/w (1.0–)1.1–1.3(–1.4) (n = 151), subhyaline to green, smooth, with minute guttules; scar indistinct.
Habitat: On wood and bark of Quercus and Ostrya.
Distribution: Only known from central Italy.
Typus: Italy, Lazio, close to Magugnano, at the Strada Magugnano-Roccalvecce, left shortly before the brook, 42°32′21″ N, 12°10′00″ E, elev. 195 m, holomorph on 3–4 cm thick branches of Quercus virgiliana, on wood and bark, soc. Hypocrea crystalligena, 25 Nov. 2009, W.J. & H. V. (holotype WU 33310; ex-type culture CBS 132567 = S131).
Additional materials examined: Italy, Lazio, close to Magugnano, at the Strada Magugnano-Roccalvecce, left shortly before the brook, 42°32′22″ N, 12°10′02″ E, elev. 195 m, asexual morph on 1.5 cm thick twig of Ostrya carpinifolia, on bark, 25 Nov. 2009, W.J. (culture S128); Sardinia, NE Fonni, at the road SP2ter to Pratobello, 40°07′55″ N, 9°16′27″ E, elev. 950 m, asexual morph on 2.5 cm thick twig of Quercus virgiliana, 4 Nov. 2009, W.J. (culture S15).
Notes: Stromata of the only sexual morph material WU 33310 are mostly immature or old and depauperate, with many asci aberrantly developed or immature, often with less than eight spores. Therefore they were not sectioned but saved for the holotype. They resemble a miniature form of T. alni or T. brunneoviride. Characteristic of T. italicum is the bright yellow colour of conidiation pustules (pronounced at 30 °C), before they eventually turn green. Colony patterns on PDA resemble T. alni, but in contrast to T. alni the conidiophores are more regularly tree-like. Judging from the three known isolates, submediterranean habitats seem to be typical of the species. The isolate S128 deviates by somewhat slower colony development and pustules formed mostly in distant areas of the plate. In comparison to T. christiani, where pustules are never yellow, pustules of T. italicum are also more rounded, more regularly shaped and compact, and it grows more slowly than T. christiani.
Trichoderma koningii Oudem., Arch. néerl. Sci., Sér. 2 7: 291. 1902.
Materials examined: All from Southern Europe (all asexual morphs). Croatia, Cres, at Prašče Brdo (between Orline and Dragozetići), on fruit of Quercus pubescens, 15 Oct. 2010, W.J., N. Matočec & I. Kušan (culture S291); Istrija, forest N of Barbariga, elev. ca. 20 m, on Quercus pubescens, 14 May 2010, W.J. & H.V. (culture S260); ibid., on Phellinus ferruginosus/Quercus ilex, 24 Sep. 2010, H.V. & W.J. (culture S267); ibid., on Erica arborea, 24 Sep. 2010, W.J. & H.V. (culture S268); between Peroj and Barbariga, on Carpinus orientalis, 24 Sep. 2010, H.V. & W.J. (culture S273); Vrsar, beach forest at Petalon Resort, elev. 10 m, on Quercus ilex, 26 Sep. 2010, W.J. & H.V. (culture S288); Lošinj, Jamna Uvala, on Quercus ilex, 16 Oct. 2010, W.J., N. Matočec & I. Kušan (culture S298). France, Aquitaine, Pyrénées-Atlantiques, Laas, Parque du Chateau de Laas, on Phyllostachys sp., 4 Nov. 2010, W.J. (culture S358). Italy, Apulia, Foggia, Gargano, Mattinata, on Hippocrepis emerus, 20 Nov. 2009, W.J. (culture S79); Sardinia, near Aggius, on Quercus suber, 7 Nov. 2009, W.J. (culture S28); at SS392 from Lago di Coghinas, 20.5 km before Tempio Pausania, on ?Myrtus communis, 6 Nov. 2009, W.J. (culture S22); Trentino, Mattarello, near Villa Bertagnolli, on Robinia pseudoacacia, 20 Oct. 2011, H.V. & W.J. (culture S566). Portugal, Madeira, Funchal, Botanical Garden, on deciduous wood, 19 Feb. 2010, W.J. (culture S204). Spain, Andalucia, Castellar de la Frontera, path from the Castillo to Fuente Viejo, on Calicotome villosa, 19 Mar. 2011, W.J. & H.V. (culture S511); north from Castellar de la Frontera, road A2100, on Cytisus sp., 20 Mar. 2011, H.V. & W.J. (culture S512); at Rio Hozgarganta shortly after entering Provincia de Málaga on the road C3331 from SE/Jimena, on Eucalyptus globulus, 21 Mar. 2011, H.V. & W.J. (culture S528); Basque Country, Gipuzkoa, BI3440, Jaizkibel, parking place close to road leading to golf course Justiz, on Quercus pyrenaica, 3 Nov. 2010, W.J. (culture S346); Basque Country, Gipuzkoa, Oiartzun, BI3420 heading to Endara, nature park Aiako Harra, pasture with Betula and Ulex, on Ulex europaeus, 6 Nov. 2010, W.J. (culture S376); ibid., on Scleroderma citrinum, 6 Nov. 2010, W.J. (culture S380); Canarias, Tenerife, above Farrobillo, on Castanea sativa, 14 Apr. 2010, W.J. (culture S227); Islas Baleares, Mallorca, Ma-10, near Esporles, heading to Banyalbufar, on corticiaceous fungus on Quercus ilex, 20 Nov. 2010, W.J. (culture S415).
Note: This is a common European member of the Viride Clade.
Trichoderma koningiopsis Samuels et al., Stud. Mycol. 56: 117. 2006.
Material examined: France, Aquitaine, Pyrénées-Atlantiques, Laas, Parque du Chateau de Laas, asexual morph on Phyllostachys sp., 4 Nov. 2010, W.J. (culture CBS 132570 = S359).
Notes: In Europe this species was previously only known from soil in Germany and Sardinia (Migheli et al., 2009, Samuels et al., 2006). We detected it once on plant material in France.
Trichoderma leguminosarum Jaklitsch & Voglmayr, sp. nov. MycoBank MB809288. Fig. 18.
Fig. 18.
Trichoderma leguminosarum. A–Q. Sexual morph. A–D. Fresh stromata (A. immature). E–H. Dry stromata. I. Rehydrated stroma. J. Rehydrated stroma in 3 % KOH. K. Perithecium in section. L. Stroma surface and subcortical tissue in section. M. Subperithecial tissue in section. N. Stroma base in section. O, P. Asci (P. in cotton blue/lactic acid). Q. Ejected ascospores. R–Z. Cultures and asexual morph. R. Conidiation pustules (CMD, 27 d). S, T. Cultures (S. on CMD, 14 d; T. on PDA, 31 d). U–W. Conidiophores and phialides (CMD, 13–19 d). X–Z. Conidia (CMD, 3–4 wk). R–Z. All at 25 °C. A, B. S487. C, F, G. WU 33397, S494 = CBS 130014. D. S532. E, I–P, V, Y. S557 = CBS 132576. H. S518. Q–T, X, Z. S503. U, W. S391. Scale bars: A, C–E, G, I, J, R = 0.7 mm; B, F, H = 0.5 mm; K = 30 μm; L, O, P, V–X = 10 μm; M, N, U = 20 μm; Q, Y, Z = 5 μm.
Etymology: Reflecting its host preference of woody legumes.
Stromata with little difference between fresh and dry except for shrinking in thickness; developing from white mycelial tufts, solitary, scattered or aggregated in numbers of 2–3, (0.5–)1.6–4.0(–5.6) × (0.4–)1.4–3.2(–4.0) mm, (0.2–)0.5–1.2(–1.8) mm thick (n = 46), pulvinate, subglobose, lenticular, placentiform or discoid, less commonly undulate; outline variable, circular, angular or oblong; margin often widely free, stromata often narrowly attached; outer side sterile, pale ochre. Surface usually convex, often irregularly tuberculate, comprising a thick chalky or calcareous, white, sometimes pale to bright yellow covering layer, initially homogeneous, becoming cracked into polygonal plates, eventually turning cream, pale-, yellowish- or dull brown. Ostioles (23–)26–62(–102) μm (n = 60) diam, numerous, densely disposed, minute but distinct, less commonly appearing as diffuse dots, slightly projecting, convex, rounded, ochre or pale brown, surrounded by stellate cracks; spore deposits white or yellow. Stromata after rehydration ca. 30 % larger than dry, distinctly thicker, turning pale red in 3 % KOH. Stroma anatomy: Superficial cortex lacking, surface appearing as an amorphous hyaline mucous layer in lactic acid; tissue between the ostioles comprising a mixture of cells (2.8–)4.3–8.0(–10) × (2.5–)3.5–5.3(–6.2) μm (n = 30) in section and/or a t. intricata of thin-walled hyphae (2.0–)3.0–5.5(–6.5) μm (n = 30) wide. Marginal cortex comprising a yellow t. angularis of cells (6–)8–21(–34) × (4–)6–12(–15) μm (n = 30) in section, walls up to 1.5 μm thick; overlain by some hyaline amorphous matter and collapsed hyphae. Subperithecial tissue a hyaline t. angularis-epidermoidea of thick-walled (up to 3 μm) cells (6–)7–25(–49) × (5–)6–12(–18) μm (n = 33); cells tending to be smaller and more regular in lower regions; with some brown inclusions of brown hyphae and spores of another fungus in the base. Stroma base consisting of yellow cells similar to the marginal cortex and some hyphae; attached to the substrate via thin-walled, hyaline, (2.3–)2.8–5.0(–6.5) μm (n = 30) wide hyphae. Ostioles (35–)43–57(–59) μm long, distinctly projecting up to 30 μm, (12–)18–32(–40) μm wide inside (n = 22), periphyses 2–3 μm wide; marginal cells apically up to 6 μm wide, cylindrical or slightly clavate. Perithecia crowded, numerous, 8–9 per mm stroma length, (170–)187–225(–250) μm high, (80–)115–183(–190) μm wide (n = 22), globose, ellipsoid or flask-shaped; peridium (10–)13–18(–20) μm wide at the base, (4–)7–14(–16) μm at the sides (n = 22), yellow in lactic acid. Asci (53–)58–72(–87) × (3.8–)4.0–4.5(–5.0) μm; stipe lacking or short, up to 13 μm long (n = 61); apex truncate, slightly thickened. Ascospores hyaline, often yellow after ejection, spinulose or verruculose, cells dimorphic, distal cells (2.5–)3.0–4.0(–5.3) × (2.5–)2.8–3.3(–3.7) μm, l/w (0.9–)1–1.3(–1.6), globose, subglobose to nearly wedge-shaped, proximal cells (2.5–)3.3–4.7(–6.5) × (2.2–)2.4–2.8(–3.2) μm, l/w (0.9–)1.3–1.7(–2.3) (n = 130), oblong or subglobose.
Cultures and asexual morph: Growth on CMD slow, after 72 h colony radius 4–6 mm at 25 °C, after 19 d reaching 35–37 mm; mycelium typically not covering the entire plate within a mo, often limited. Colony hyaline, dense, with irregular outline, commonly lobed; aerial hyphae inconspicuous, no chlamydospores detectable, no diffusing pigment formed, areas sometimes turning yellow between pustules; odour indistinct. Sterile, light-coloured stromata produced in the colony centre of some strains. Conidiation on CMD (after 12–27 d) first effuse in minute shrubs, later in numerous minute granules and amorphous pustules 0.4–2(–2.4) mm diam with irregular, granulose or plumose surface due to projecting conidiophores, turning pale to dark green 28–29CD5–8, 28E7–8 from the centre, additional new pustules consecutively produced in distal areas. Pustule stipe and primary branches 6–9(–11) μm wide, thickened cells to 13 μm wide, walls to ca. 2 μm thick, irregularly swelling to ca. 3 μm; primary branches rather abruptly narrowed, forming a simple or complex reticulum, main branches with long side branches (conidiophores), forming broad structures. Conidiophores densely disposed, mostly asymmetrically arranged, below terminal whorl also paired or in verticils of 2–3, not or only once again branched, mostly 1–3-celled, straight, stout, often perpendicular to the main axis, short, ca. 30–120 μm long, 3–5 μm wide, terminally 2–3(–3.5) μm. All branches including phialides coarsely warted with age. Phialides divergent in whorls of 2–5, also solitary or paired along conidiophores, (6.5–)9–15(–21.5) × (2.4–)2.7–3.3(–4.0) μm, l/w (2.0–)2.9–5.2(–7.3), at the base (1.3–)1.8–2.7(–3.3) μm wide (n = 110), narrowly lageniform or subulate, often asymmetric, straight, less commonly curved or sinuous, typically with a long neck, warted when old. Conidia formed in minute wet heads, (2.8–)3.5–4.5(–6.5) × (2.0–)2.3–2.8(–3.0) μm, l/w (1.1–)1.3–1.8(–2.6) (n = 105), green, variable, mostly ellipsoid, less commonly subglobose or oblong, smooth, containing one large or several minute guttules, scar indistinct, distinctly truncate or projecting.
Colonies on PDA turning brown, covered by whitish floccules, growth slow, radius after 1 mo 19–22 mm; on SNA slightly faster, colony radius e.g. 34–37 mm after 1 mo, numerous pale green shrubs forming across the entire colony, loosely and more or less equidistantly disposed.
Habitat: On twigs of Fabaceae, particularly Calicotome spp.
Distribution: Southern Europe (France, Greece, Spain).
Typus: Spain, Andalucia, Puerto del Castaño, 36°19′17″ N, 5°35′56″ W, elev. 250 m, on 3–4 cm thick twigs of Calicotome spinosa in mud, on crumbly wood, moss and dark corticiaceous fungus, 17 Mar. 2011, H.V. & W.J. (holotype WU 33397, ex-type culture CBS 130014 = S494).
Additional materials examined: France, Dept. Alpes-de-Haute-Provence, Rougon, along the hiking trail north of the village to the plateau of Suech, elev. ca. 1080 m, on twigs of Cytisus scoparius, soc. Trichoderma viridescens, 25 Jul. 2011, H.V. (WU 33405, culture S559). Greece, Crete, pine forest above Rethymno, 35°21′25″ N, 24°29′21″ E, elev. 145 m, on branches and twigs of Calicotome villosa, also on ochre corticiaceous fungus, 30 Jun. 2011, W.J. (WU 33404, culture S557 = CBS 132576). Spain, Andalucia, Alcalá de los Gazules, via de servicio shortly after the exit at km 54 off the A7 (A381), 36°22′31″ N, 5°39′00″ W, elev. 50 m, on 0.5–2 cm thick twigs of Teline linifolia lying on muddy soil, mostly on wood, also on an ochre corticiaceous fungus, 17 Mar. 2011, H.V. & W.J. (WU 33396, culture S487); Castellar de la Frontera, northeast from the village at the road A2100, 36°17′55″ N, 5°21′53″ W, elev. 185 m, on 2–7 cm thick twigs of Calicotome villosa, 20 Mar. 2011, H.V. & W.J. (WU 33400, culture S518); El Colmenar, roadside of MA9300 at km 8, 36°31′39″ N, 5°22′39″ W, elev. 330 m, on twigs of Calicotome villosa, also on a light corticiaceous fungus, soc. dark green T. hispanicum asexual morph, 22 Mar. 2011, W.J. & H.V. (WU 33402, culture S536); Rural road near Puerto del Castaño leading to Cerro del Palmito, 36°19′45″ N, 5°35′40″ W, elev. 240 m, on 2–7 cm thick twigs of Calicotome villosa in mud, on wood and bark and on a light-coloured corticiaceous fungus, soc. a Hysterium sp., immersed pyrenomycetes and a ?Phlebia sp., 18 Mar. 2011, H.V. & W.J. (WU 33398, culture S503); San Pablo de Buceite, 36°27′58″ N, 5°24′08″ W, elev. 70 m, on 2–4 cm thick corticated twigs of Calicotome villosa, soc. white corticiaceous fungus, 22 Mar. 2011, W.J. & H.V. (old, discarded); Islas Baleares, Mallorca, east of Calviá, Ma-1016 roadside, 39°34′56″ N, 2°32′41″ E, elev. 190 m, on 3 cm thick corticated twig of Calicotome spinosa, soc. Eutypa sp., ?Olla sp., Steccherinum ochraceum, partly overgrown by a Cosmospora sp., 17 Nov. 2010, W.J. (WU 33369, culture S399); Es Capdella, along the Torrent de Galatzó, 39°35′14″ N, 2°28′54″ E, elev. 120 m, on 1.5–6 cm thick twigs of Calicotome spinosa, soc. corticiaceous fungi, Eutypa sp. in wood and bark, Nectriopsis oropensoides, partly attacked by a white hyphomycete, 16 Nov. 2010, W.J. (WU 33368, culture S391); same area, 39°35′13″ N, 2°29′14.5″ E, elev. 140 m, on 1–2 cm thick corticated twig of Calicotome spinosa, immature, on an ochre corticiaceous fungus, soc. Eutypa sp. and an asexual Trichoderma caerulescens colony, 21 Nov. 2010, W.J. (part of WU 33368, culture S424).
Notes: A specimen collected by C. Gelpi on Cistus spp. in Extremadura, Spain and communicated by E. Rubio, may also be this species. Trichoderma leguminosarum is easily identifiable by sight due to the thick, cracked covering layer of the stromata that is unknown in any other species. The fungus is not uncommon in the Mediterranean, occurring on medium- to well-decayed wood and bark of leguminous shrubs, typically in moist or muddy places. Frequent association, however, suggests that the true host is a corticiaceous fungus, which may be specific for legumes. No asexual morph has been found in nature. When a green Trichoderma was associated with stromata it turned out to belong to other species. In one instance (S536) ochre to medium brown, aggregated pseudoparenchymatous stromata were formed after 18 mo at 15 °C.
Trichoderma longibrachiatum Rifai, Mycol. Pap. 116: 42. 1969.
Material examined: Spain, Bizkaia, 2.5 km north of Amorebieta, at roadside, on Biscogniauxia nummularia/Corylus avellana, 31 Oct. 2010, W.J. (culture S328).
Notes: A common soil fungus, which seems to be rare on plant material. No sexual morph is known for this species.
Trichoderma longipile Bissett, Canad. J. Bot. 69: 2395. 1992 (1991).
Materials examined: Italy, Campania, left roadside of Via Provinciale del Corticato shortly after the highest point heading to Sacco, Parco Nazionale del Cilento, on ?Acer obtusatum, sexual morph overmature, 16 Nov. 2009, W.J. & H.V. (culture CBS 135570 = S40). Spain, Andalucia, Castellar de la Frontera, road A2100 north from the village, on Cytisus sp., 20 Mar. 2011, W.J. & H.V. (culture S514); Asturias, Fuensanta, on decorticated wood of ?Castanea sativa, holomorph, stromata immature, 21 Sep. 2012, E. Rubio ERD-5670 (culture S658).
Notes: Analyses of both rpb2 (Fig. 1) and tef1 (Fig. 5) sequences clearly suggest synonymy of T. cuneisporum with T. longipile; see also Chaverri & Samuels (2004). The sexual morph of T. longipile is now known from Italy, Spain (Asturias), Sweden and USA (Louisiana, Virginia; as T. cuneisporum).
Trichoderma margaretense Jaklitsch, Fungal Divers. 48: 167. 2011.
Materials examined: France, Ariège, Rimont, Las Muros, sexual morph on Fraxinus excelsior, 5 Nov. 2010, J. Fournier & W.J. (WU 33365, culture CBS 136993 = S368). Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, in a ditch, sexual morph on Acer obtusatum, 22 Nov. 2009, H.V. & W.J. (WU 32200, culture S106). Spain, Andalucia, between El Bosque and Algar, asexual morph and immature stromata on Rhamnus sp., 24 Mar. 2011, H.V. & W.J. (culture S544).
Note: Originally Jaklitsch (2011) reported this species from a single area in Austria, but in the present work we found it in three additional countries.
Trichoderma mediterraneum Jaklitsch & Voglmayr, sp. nov. MycoBank MB809289. Fig. 19.
Fig. 19.
Trichoderma mediterraneum. A–I. Sexual morph. A–C. Fresh stromata (A. largely immature). D–G. Dry stromata. H, I. Asci (I. in cotton blue/lactic acid). J–T. Cultures and asexual morph (at 25 °C). J–L. Cultures at 25 °C (J. CMD, 14 d; K. PDA, 14 d; L. SNA, 11 d). M, N, P, Q. Conidiophores and phialides (SNA, 5–6 d). O. Conidiation pustule (SNA, 7 d). R–T. Conidia (SNA, 6–8 d). A, D, F–I, M, R, T. WU 33334, S190 = CBS 136469. B. S454. C. S12. E. S174. J–L, N–Q, S. S240. Scale bars: A, C = 2.5 mm; B = 2.0 mm; D–G, O = 1.0 mm; H, I = 15 μm; M, N = 20 μm; P, Q = 10 μm; R–T = 5 μm.
Etymology: For its occurrence in Mediterranean Europe including the Canary Islands.
Stromata solitary, gregarious or densely aggregated in groups of up to 7; when fresh often more pulvinate with smooth surface and paler in colour than when dry. Stromata when dry (0.7–)1.5–4.5(–7.0) × (0.5–)1.3–3.6(–6.2) mm, (0.3–)0.4–1.5(–2.5) mm high (n = 60), pulvinate, discoid or turbinate, often with flat top, sometimes on a thick white base or basally surrounded by white mycelium; outline circular, oblong or irregular; margin rounded or sharp, edges mostly free. Stroma surface smooth or rugose, sometimes slightly velutinous when young. Ostiolar dots (24–)32–66(–118) μm diam (n = 85), distinct, plane or convex, pale reddish when young, darkening with age and virtually black when old. Stroma colour mostly a mixture of rosy and brown or rosy and yellow, resulting in reddish brown, sometimes yellow- or orange-brown shades, mostly 8DE4–8, 7CD4–6, sometimes with a whitish covering layer; margin often white or yellowish. Spore deposits white or yellowish. Asci (86–)98–116 (–126) × (5.7–)6.0–6.5(–7.2) μm, stipe (4–)9–19(–27) μm long (n = 50). Ascospores hyaline, finely spinulose; cells dimorphic, distal cell (4.0–)4.5–5.5(–6.8) × (3.2–)3.7–4.7(–5.3) μm, l/w (1.0–)1.1–1.4(–1.7) (n = 80), subglobose, ellipsoid or wedge-shaped; proximal cell (4.0–)5.0–6.0(–7.2) × (2.5–)3.0–4.0 (–5.0) μm, l/w (1.1–)1.4–1.8 (–2.2) (n = 80), oblong or subglobose.
Cultures and asexual morph: Optimal growth at 25 °C on all media, good but considerably variable growth at 30 °C, not growing at 35 °C.
On CMD after 72 h colony radius 25–28 mm at 15 °C, 42–47 mm at 25 °C, 16–35 mm at 30 °C; mycelium covering the plate after 5 d at 25 °C. Colony hyaline, circular, mycelium dense, of wide primary surface hyphae and narrow secondary hyphae. Aerial hyphae only common and sometimes abundant at the colony margin, causing a downy zone. Autolytic excretions and coilings virtually absent; diffusing pigment lacking; odour indistinct. Chlamydospores uncommon, terminal and intercalary. Conidiation starting after 2–3 d, turning green after 5 d from lateral zone ends, eventually dark green 26–28F4–8; first effuse to shrubby in distant areas of the colony, spreading in 2–4 farinose to floccose concentric zones; shrubs with long, straight, radial, regularly tree-like conidiophores, some growing to pustules; conidia formed in densely disposed minute wet heads; after a week or later conidiation also in pustules 1–2 mm diam, sometimes to 3–5 mm diam.
On PDA after 72 h colony radius 20–21 mm at 15 °C, 35–43 mm at 25 °C, 17–27 mm at 30 °C; mycelium covering the plate after 6 d at 25 °C. Colony circular, whitish, farinose to downy; mycelium dense, with conspicuously wide primary surface hyphae. Aerial hyphae abundant, causing a floccose mat. Autolytic excretions and coilings scant; diffusing pigment lacking, reverse dull yellow 4AB3–5; odour indistinct to unpleasant. Conidiation starting after 2 d, turning pale (greenish-)yellow 3B4, 3A3 from the centre after 1 wk or later, effuse and in numerous floccose pustules spreading from the centre and eventually covering more or less the entire plate, with poor conidial yield. At 30 °C colony formed in pale irregular zones.
On SNA after 72 h colony radius 18–21 mm at 15 °C, 33–39 mm at 25 °C, 10–25 mm at 30 °C; mycelium covering the plate after 6–7 d at 25 °C. Colony similar to CMD, but margin often ill-defined, aerial hyphae reduced, only common at the margin, becoming fertile. Chlamydospores uncommon, sometimes abundant around distant conidiation pustules, mainly intercalary, globose, ellipsoid or oblong, (6.5–)7–10(–10.5) × (4.0–)5.5–9(–10), l/w = (0.9–)1.0–1.5(–2.2) (n = 33). Conidiation starting after 3 d, turning green after 4–5 d, eventually 27E3–7, 28E5–8 to 27–28F5–8, first more or less effuse and in loose shrubs with long radial tree-like conidiophores compacting to flat pustules 1–3 mm diam with finely granular to plumose surface; disposed in up to 5 often ill-separated, concentric zones spreading with the growth. Shrubs and pustules consisting of a wide stipe with asymmetric primary branches spanning a loose reticulum with radial, regular tree-like conidiophores of long main axes and side branches emerging from the main axes mostly at right angles, often paired or in verticils, also unpaired, straight or slightly curved, often 1-celled with a single whorl of phialides at upper levels, increasing in length from the top downward; branches 2–5 μm wide. Phialides in whorls of (2–)3–4(–6), less commonly solitary, lageniform or ampulliform with long neck, (5.3–)6–10.5(–18) × (2.0–)2.7–3.3(–3.8) μm, l/w (1.6–)1.8–4(–7.6), basal width (1.0–)1.5–2.2(–2.7) μm (n = 71), symmetric or inequilateral, straight, rarely sigmoid. Conidia ellipsoid to oval, less commonly oblong, (3.0–)3.2–4.3(–6.3) × (2.2–)2.5–2.8(–3.0) μm, l/w (1.1–)1.2–1.6(–2.1) (n = 76), green, smooth, with minute guttules; scar indistinct or slightly protruding.
Habitat: Wood and bark of trees and shrubs and fungi growing on them.
Distribution: Mediterranean Europe and Canary Islands.
Typus: Spain, Canarias, La Palma, Montaña Tagoja, on wood and bark of Myrica faya and Erica arborea, soc. corticiaceous fungus and effete pyrenomycete in bark, 14 Dec. 2009, W.J. (holotype WU 33334, ex-type culture CBS 136469 = S190).
Additional materials examined: All from branches or twigs. Croatia, Cres, at Prašče Brdo (between Orline and Dragozetići), holomorph on wood of Quercus pubescens, also on a white corticiaceous fungus, 15 Oct. 2010, W.J., N. Matočec & I. Kušan (WU 33348, culture CBS 136476 = S292); Istrija, 1.4 km before Barbariga from Peroj, holomorph on wood of Carpinus orientalis, soc. Propolis versicolor, a brown corticiaceous fungus, effete pyrenomycetes, 18 Oct. 2010, W.J. (WU 33355, culture S312); Vrsar, beach forest at Petalon Resort, elev. 10 m, on Quercus ilex, 26 Sep. 2010, W.J. & H.V. (culture S287). Greece, Corfu, shortly before Skripero heading north, NE Poulades, opposite of the marble quarry, 39°41′0″ N, 19°47′59″ E, elev. 80 m, on Spartium junceum, soc. corticiaceous fungus, 21 Apr. 2012, W.J. & H.V. (culture S621); Crete, Askyfou, road to the Halara peak, 35°17′48″ N, 24°12′29″ E, elev. 780 m, on Quercus coccifera, 26 Nov. 2011, W.J. (culture S600); Palaea Roumata, near Pananiana, 35°24′20″ N, 23°46′13″ E, elev. 370 m, on Olea europaea subsp. sylvestris, 25 Nov. 2011, W.J. (Culture S594). Italy, Sardinia, Oliena, Badde Orgolese, along the road SP22, on wood and bark of Viburnum tinus, soc. Rosellinia sp., Hysterium sp., and other fungi, 4 Nov. 2009, W.J. (WU 32170, culture S12); same place, date and host, soc. Hypomyces rosellus (culture CBS 135567 = S13); Monte Macchione, on Quercus ilex, 2 Nov. 2009, W.J. (culture S6); Tempio Pausania, Fonti di Rinaggiu, on a deciduous tree, 6 Nov. 2009, W.J. (culture S25); ibid., on branch of Pinus sp., 6 Nov. 2009, W.J. (culture S26); near Littigheddu shortly after leaving the SS127 from Tempio Pausania, on blackened wood of Cistus monspeliensis, soc. diverse fungi, partly on a ?Hyphoderma sp., 7 Nov. 2009, W.J. (cultures S29, S30). Spain, Andalucia, El Colmenar, forest road to Ubrique, on Quercus cf. faginea, holomorph, soc./on a pale corticiaceous fungus, 23 Mar. 2011, W.J. & H.V. (culture S541); Jimena de la Frontera, NW of the village at the road C3331, on Arbutus unedo, 21 Mar. 2011, W.J. (culture S524); same area and date, on Calicotome villosa, W.J. & H.V. (culture S523); same area and date, on Erica arborea, holomorph, on a corticiaceous fungus, bark and wood, W.J. & H.V. (WU 33401, culture S526); same area and date, on Olea europaea, soc. corticiaceous fungus, W.J. & H.V. (culture S522); Puerto del Castaño, on Calicotome villosa, 17 Mar. 2011, W.J. & H.V. (culture S495); Asturias, walking path above Villar de Vildas, on Quercus petraea and a corticiaceous fungus, 6 Jun. 2013, H.V. & W.J. (culture S665); Canarias, La Gomera, TF713, shortly after junction to Hermigua, on wood and bark of Myrica faya and Erica arborea, soc. effete pyrenomycete and moss, 9 Dec. 2010, W.J. (WU 33388, culture S463); La Palma, Cumbre Nueva, branching off LP-301 at Area Recreativa del Pilar, on dark wood of Myrica faya and a corticiaceous fungus, 11 Dec. 2009, W.J. (WU 33329, culture S174); same place, date and host, asexual morphic material (culture S175); old chestnut plantation at LP 301, close to crossing with LP 3, holomorph on bark of Erica arborea, 2 Dec. 2010, W.J. (WU 33378, culture S440); Cumbre Vieja, Pista Cabrito, on a brown corticiaceous fungus on Myrica faya, 13 Dec. 2009, W.J. (culture CBS 136468 = S184); Los Sauces, Los Tilos, on a black fungal crust on Ocotea foetens, 10 Dec. 2009, W.J. (culture S171); Montaña Tagoja, on blackened bark and wood of Erica arborea, soc. various fungi, 7 Dec. 2010, W.J. (WU 33387, culture S461); close to Refugio El Pilar, few stromata on wood and bark of Myrica faya, 28 Nov. 2010, J. Fernández Vicente, comm. P. Karasch (culture S425); Tenerife, Archifira (Fasnia), UTM: 28R355738 3128654, elev. 1295 m, on Pinus canariensis, 18 Feb. 2011, L. Quijada & J. Díaz (TFCMic.23128, culture S554); Bosque de La Esperanza, asexual morph on Adenocarpus foliolosus, 16 Apr. 2010, W.J. (culture S239); same area, asexual morph on Eucalyptus globulus, 18 Apr. 2010, W.J. (culture S247); same area, on Eucalyptus globulus, 14 Dec. 2010, H.V. & W.J. (culture S469); same area, date and host, scant holomorph on wood, W.J. & H.V. (culture S470); Macizo de Anaga, Las Carboneras, walking path to El Batán from the road to Taborno, on dark wood and bark of Myrica faya, 16 Dec. 2010, H.V. & W.J. (WU 33389, culture S473); Montaña Chamuscada, holomorph on dark wood and bark of Laurus novocanariensis, soc. Biscogniauxia sp., black discomycete, corticiaceous fungus and moss, 16 Dec. 2010, W.J. & H.V. (WU 33394, culture S481); Pico del Ingles, on wood of Myrica faya, scant sexual morph, soc. black discomycete, 11 Apr. 2010, W.J. (culture S213); Orotava, slightly above Pista de Benijos/camping place La Caldera, above Aguamansa/La Orotava, on darkened wood of Eucalyptus globulus, holomorph, on/soc. corticiaceous fungi, soc Orbilia sp. and several other fungi, overmature material from several branches, 16 Apr. 2010, W.J. (cultures S240, S241 and S242). Islas Baleares, Mallorca, Escorca, several sites near the village, all on wood and bark of Quercus ilex, also on a thick brown corticiaceous fungus, a Stereum sp. and a Diatrype sp., 18 Nov. 2010, W.J. (WU 33373, cultures S408, S409, S410); Fornalutx, at the road Ma-10 above the village, opposite the property Monnaber, on a thick brown corticiaceous fungus on Quercus ilex, 17 Nov. 2010, W.J. (WU 33372, culture S403); near Lluc, shortly after the crossing to Pollenca, asexual morph on Pinus halepensis, 18 Nov. 2010, W.J. (culture S413). Trichoderma mediterraneum 1: Spain, Basque Country, Gipuzkoa, BI3440, Jaizkibel, parking place close to road leading to golf course Justiz, 43°22′16.6″ N, 1°49′58″ W, elev. 175 m, on Ulex europaeus, 3 Nov. 2010, W.J. (culture CBS 136477 = S347); Islas Baleares, Mallorca, Escorca, 39°49′08″ N, 2°52′31″ E, elev. 705 m, asexual morph on ?Hymenochaete sp. on well-decayed wood of Quercus ilex , 18 Nov. 2010, W.J. (culture S411). Trichoderma mediterraneum 2: Spain, Canarias, La Palma, Garafía, at LP1 close to the junction to El Tablado, 28°47′48″ N, 17°53′39.6″ W, elev. 1110 m, on Chamaecytisus proliferus, 4 Dec. 2010, W.J. (culture S451); close to Estación de Guaguas Llano Negro, 28°48′17.5″ N, 17°55′34″ W, elev. 910 m, on Chamaecytisus proliferus, 4 Dec. 2010, W.J. (WU 33384, culture CBS 136998 = S454); ibid. (WU 33385, culture S455).
Notes: Stromata were not sectioned due to the morphological conservation of the sexual morphs of several closely related species in this group, viz. T. atlanticum, T. europaeum, and T. minutisporum. Stromata of T. mediterraneum often have virtually black ostiolar dots when mature and can therefore be taken for a green-spored species at first sight. The formation of rather distinct conidial pustules in CMD cultures may be characteristic for T. mediterraneum, but it varies among isolates, especially in the extent and time of the appearance of pustules. More useful for the distinction from T. europaeum is the formation of numerous flocks or pustules on PDA. In contrast to T. mediterraneum, which does not form a pigment on CMD, T. minutisporum s.str. from North America and occasionally also T. europaeum tend to develop a pale yellow pigment on CMD. Trichoderma mediterraneum has often been collected as asexual morph in this study, and sexual morphs when present were usually scant, but the specimens S174, S190, S312, S454 and S461 contain good sexual morphic material.
Phylogenetically, T. mediterraneum shows some substructure. Particularly the isolates S347 and S411, both only collected as asexual morphs and here called T. mediterraneum 1, and the three isolates S451, S454 and S455, collected as asexual or holomorphs in Garafía, La Palma, on Chamaecytisus proliferus and here called T. mediterraneum 2, may be recognised as separate species, as they yielded also distinct acl1 and rpb2 sequences.
Trichoderma mediterraneum is much more common (50 vs. 16 specimens) than T. europaeum in the south and occurs in typical Mediterranean habitats, but also on the Canary Islands on many different trees and shrubs, but never on Fagus. Only one specimen of T. mediterraneum (S665) was found on the deciduous oak Quercus petraea in Asturias, northern Spain. Trichoderma europaeum occurs in the Central European vegetation zone characterised by the occurrence of Fagus sylvatica. This zone extends to Calabria (Italy) in Southern Europe. Trichoderma europaeum is nearly exclusively confined to hosts of the Fagaceae, mainly Fagus and a few collections from Castanea, Quercus, sometimes Ostrya (Betulaceae). Only once it was found in a Mediterranean habitat in Crete on Quercus coccifera.
Trichoderma neorufoides Jaklitsch, Fungal Divers. 48: 25. 2011.
Materials examined: Croatia, Istrija, Fažana, forest at Valbandon, on Carpinus orientalis, 17 Oct. 2010, W.J. (WU 33352, culture S306). Italy, Calabria, Cosenza, Parco Nazionale del Pollino, above Morano Calabro, on Fagus sylvatica, 18 Nov. 2009, H.V. & W.J. (WU 32182, culture S59); Mormanno, Parco Nazionale del Pollino, Valle di Fiume Argentino, Conte Orlando, on Fagus sylvatica, 18 Nov. 2009, H.V. & W.J. (WU 32183, culture S63); Trentino, Mattarello, near Folgaria, on Fagus sylvatica, 20 Oct. 2011, W.J. & H.V. (WU 33408, culture S568).
Note: While T. neorufum was not detected in Southern Europe, T. neorufoides was collected four times, always as sexual morph or holomorph, chiefly on Fagus sylvatica.
Trichoderma olivascens Jaklitsch et al., Persoonia 31: 121. 2013.
Notes: A common species; 33 specimens are known from Croatia, France, Greece, Italy, Portugal and Spain. Trichoderma olivascens is also common in Central Europe (see Jaklitsch et al. 2013).
Trichoderma orientale (Samuels & Petrini) Jaklitsch & Samuels, Mycotaxon 126: 151. 2014 (2013).
≡ Hypocrea orientalis Samuels & Petrini in Samuels et al., Stud. Mycol. 41: 30. 1998.
Materials examined: Spain, Canarias, La Palma, Cumbre Nueva, Castanea plantation at LP 301, close to crossing with LP 3, sexual morph on Castanea sativa, 13 Dec. 2009, W.J. (WU 31609, culture CBS 131488 = S187).
Notes: Stromata of this common soil-inhabiting species was only known from a single sexual morph collection in China, until we found it also as sexual morph in La Palma in two subsequent years on the same log. Sequences differ slightly from the original isolate. See Samuels et al. (2012a) for description and illustration.
Trichoderma pararogersonii Jaklitsch & Voglmayr, sp. nov. MycoBank MB809290. Fig. 20.
Fig. 20.
Trichoderma pararogersonii. A–C. Cultures after 10 d at 25 °C (A. on CMD; B. on PDA; C. on SNA). D–J. Conidiophores and phialides. K, L. Conidia. D–L. From CMD at 25 °C after 5 d. A–E, H–J, L. S301 = CBS 133496; F, G, K. S584. Scale bars: D = 20 μm; E–G = 15 μm; H–J = 10 μm; K, L = 5 μm.
Etymology: Reflecting the similarity and close relationship to T. rogersonii.
Optimal growth at 25 °C on all media, slow and restricted growth at 30 °C, not growing at 35 °C. Colony radius on CMD after 72 h 15–19 mm at 15 °C, 29–32 mm at 25 °C, 7–9 mm at 30 °C; mycelium covering the plate after 6–7 d at 25 °C. Colony circular with well-defined margin, hyaline, mycelium dense; aerial hyphae scant; autolytic excretions rare, coilings absent; no diffusing pigment formed; odour indistinct. Chlamydospores uncommon. Conidiation starting after 22 h, first effuse, spreading from the centre, later in small, scattered, variably aggregated to confluent shrubs 0.3–0.9 mm diam arranged in several poorly defined concentric zones, first white, after 3 d starting to turn pale green 27C2–3 to 28–29CD3–4. Shrubs (after 5 d at 25 °C) consisting of a loose reticulum entirely or sometimes with a dense central part, with branches mostly at right angles. Conidiophores 2–5 μm wide and widening at the base to 7 μm, mostly radially emerging from the reticulum, well-defined, comprising a well-discernible main axis and rather few distantly placed side branches of varying length, inserted at right angles or slightly inclined upward, asymmetric, paired or in whorls of 3, often increasing in length downwards, but sometimes short at the base or long at higher levels, most branches only 1–2-celled, nor further branching, less commonly once rebranching. Phialides solitary, paired or in cruciform whorls of three, narrowly lageniform, (5.5–)7.7–12.8(–18.5) × (2.3–)2.5–3.0(–3.7) μm, l/w = (1.8–)2.7–4.7(–6.2), (1.3–)2.0–2.5(–3.2) μm wide at the base (n = 65), inequilateral, straight or slightly curved. Conidia formed in minute wet heads, ellipsoid to oblong, (3.7–)4.0–5.0(–5.5) × (2.8–)3.0–3.5(–3.7) μm, l/w = (1.1–)1.2–1.5(–1.8) (n = 63), green, smooth, thick-walled, eguttulate or with few guttules, scar indistinct or truncate.
Colony radius on PDA after 72 h 8–10 mm at 15 °C, 18–20 mm at 25 °C, 5–6 mm at 30 °C; mycelium covering the plate after 12–14 d at 25 °C. Colony well-defined, circular, dense, homogeneous, not zonate. Aerial hyphae abundant, forming a dense flat radial mat comprising thick strands and numerous short conidiophores, becoming radially textured and finely zonate, white, slowly turning dull greenish yellow 3B3–4 to 3C4–5 from the centre. Autolytic excretions and coilings inconspicuous; no diffusing pigment formed; odour indistinct to unpleasant. Conidiation starting after 22 h, effuse, turning greenish in the centre after 10 d.
Colony radius on SNA after 72 h 11–14 mm at 15 °C, 26–27 mm at 25 °C, 4–5 mm at 30 °C; mycelium covering the plate after 8 d at 25 °C. Colony as on CMD, but with increased autolytic activity, less mycelium and reduced conidiation starting after 22 h but developing more slowly, turning greenish after 6–7 d. Chlamydospores uncommon, terminal and intercalary, (sub)globose, (6.5–)8–11.5(–14) × (6–)7–10.5(–14) μm (n = 30).
Habitat: On wood and bark of broadleaf trees.
Distribution: (Sub)Mediterranean Europe, known from Croatia and Greece.
Typus: Croatia, Istrija, Vodnjan, near Divšići, on a branch of Quercus pubescens, 17 Oct. 2010, W.J. (holotype CBS 133496, culture permanently preserved in a metabolically inactive state; other culture number: S301).
Additional material examined: Greece, Crete, Fournés, citrus plantation along the river Keritis, on Ficus carica, 24 Nov. 2011, W.J. (culture S584).
Notes: Trichoderma pararogersonii differs from T. rogersonii in colony morphology (cf. Jaklitsch 2011) and enhanced conidiation on CMD, but is otherwise morphologically indistinguishable. These species differ from each other in the sequences of all phylogenetic markers used in this study, and even in ITS.
Trichoderma paratroviride Jaklitsch & Voglmayr, sp. nov. MycoBank MB809291. Fig. 21.
Fig. 21.
Trichoderma paratroviride. A–D. Cultures (A. on CMD, 25 °C, 7 d; B. on SNA, 25 °C, 10 d; C. on PDA, 25 °C, 10 d; D. on PDA, 30 °C, 7 d). E–K. Conidiophores and phialides. L–O. Conidia. E–O. From CMD at 25 °C after 4 d. A–E, G, I–N. S385 = CBS 136489; F, H, O. S489. Scale bars: E, F = 25 μm; G, H = 15 μm; I–K = 10 μm; L–O = 5 μm.
Etymology: Reflecting the similarity and close relationship with T. atroviride.
Optimal growth at 25 °C on all media, considerably more slowly on SNA than on CMD and PDA, slow and restricted growth at 30 °C, none at 35 °C.
Colony radius on CMD after 72 h 25–29 mm at 15 °C, 49–62 mm at 25 °C, 36–40 mm at 30 °C; mycelium covering the plate after 4–5 d at 25 °C. Colony well-defined, hyaline, dense with loose centre, not zonate, cells of surface hyphae around conidiation structures becoming conspicuously enlarged. Aerial hyphae, autolytic excretions and coilings absent; no diffusing pigment formed; odour strongly coconut-like. Chlamydospores uncommon. Conidiation starting after 2 d in numerous small shrubs aggregating to loosely floccose tufts or pustules to ca. 2(–5) mm length and eventually radially arranged within 2–3 ill-defined concentric zones in the outer half of the colony, white, turning green after 3–4 d, eventually dark green 27–28F4–8. Tufts/pustules (after 4 d at 25 °C) arising on a thick-walled, warted, up to 11 μm wide stipe with wide asymmetric primary branches spanning a loose reticulum at right angles. Conidiophores emerging radially from the reticulum, well-defined, mostly long and slender, consisting of a main axis and often distantly spaced side branches mostly at right angles or slightly inclined upward; branches 2–5 μm wide, branching points widened up to ca. 7 μm, straight or curved, side branches mostly 1–2-celled, often only longer in basal positions, not re-branching, solitary, paired or in whorls of three. Phialides solitary or commonly in whorls of 2–4, often 3 in cruciform configuration, often on a widened supporting cell, variable in shape, either narrowly lageniform to subulate, particularly when terminal on the main axis, or stout to nearly ampulliform and distinctly swollen, (5.2–)6.2–11(–14) × (2.0–)2.5–3.2(–3.5) μm, l/w = (1.6–)2–4(–7), (1.0–)1.5–2.3(–3.0) μm wide at the base (n = 61), symmetric or inequilateral, often with a long neck, straight to distinctly curved. Conidia (sub)globose, (3.0–)3.3–3.7(–4.0) × (3.0–)3.2–3.5(–3.7) μm, l/w = (0.9–)1.0–1.1(–1.2) (n = 60), green, smooth, eguttulate or with 1–2 large guttules, thick-walled; scar indistinct.
Colony radius on PDA after 72 h 19–21 mm at 15 °C, 54–56 mm at 25 °C, 42–43 mm at 30 °C; mycelium covering the plate after 4–5 d at 25 °C. Colony well-defined, circular, dense. Aerial hyphae numerous, forming a thick loose mat in the distal part of the colony; colony surface white, particularly in a large condensed, mottled central zone, the latter turning greyish green, residual part white and pale yellow 4A3–4 in ill-defined, coarse concentric zones, the distal margin also turning greyish green 28–29CD4–6. Autolytic excretions common, coilings uncommon; no diffusing pigment formed, reverse slightly yellowish; odour unpleasant, pungent. Conidiation effuse in small condensed shrubs, particulalry in the colony centre, starting after 2 d, turning green in the centre after 3 d. Green concentric conidiation zones more well-defined at 30 °C.
Colony radius on SNA after 72 h 17–20 mm at 15 °C, 30–33 mm at 25 °C, 24–31 mm at 30 °C; mycelium covering the plate after 10–14 d at 25 °C. Colony similar to CMD, but growth strongly reduced; conidiation starting after 2–4 d, first effuse, then in scattered large pustules to 9 mm diam, turning dark green 26–27F4–8 after 4–5 d; odour indistinct. Chlamydospores uncommon, terminal and intercalary, (sub)globose or angular, (5–)6–9(–11) × (4–)5–8(–10) μm (n = 20).
Habitat: On wood and bark of broadleaf trees and shrubs.
Distribution: Mediterranean Europe (Spain).
Typus: Spain, Islas Baleares, Mallorca, Es Capdella, between Camí del Graner del Delme and Torrent de Galatzó, on branch of Phillyrea angustifolia, 16 Nov. 2010, W.J. (holotype CBS 136489, culture permanently preserved in a metabolically inactive state; other culture number: S385).
Additional material examined: Spain, Andalucia, Alcalá de los Gazules, via de servicio south of the exit at km 54 off the A7 (A381), on branch of Teline linifolia, 17 Mar. 2011, W.J. & H.V. (culture S489).
Notes: Conidiophores and micromorphological details are indistinguishable from those of T. atroviride, but the colony characteristics of the two species differ, as well as the sequences of all phylogenetic markers used. The distinctly unpleasant, pungent or rancid odour of PDA cultures is reminiscent of the unrelated species T. bavaricum, and a coconut-like odour is absent. The extremely short tef1 sequences (HM920193, HM920194 and HM920195) for three cultures taken from shiitake mushrooms in Korea (respectively CNUN112, CNUN121 and CNUN192; Kim et al. 2012c) suggest that they are this species.
Trichoderma paraviridescens Jaklitsch et al., Persoonia 31: 128. 2013.
Material examined: Italy, Lazio, Bomarzo, near the Etruscan excavation “Pyramide”, on Quercus virgiliana, holomorph, sexual morph immature, 22 Oct. 2012, W.J., H.V. & W. Gams (culture S645).
Notes: This species is common in Europe and occurs also on other continents. Jaklitsch et al. (2013) cited 22 Southern European collections, including France, Greece, Italy and Spain.
Trichoderma petersenii Samuels et al., Stud. Mycol. 56: 122. 2006.
Materials examined: Greece, Corfu, Gouvia, hotel Fiori, 39°40′3.6″ N, 19°49′37″ E, elev. 40 m, on Cercis siliquastrum, 24 Apr. 2012, H.V. & W.J. (culture S636); Kanakades, 39°39′25″ N, 19°45′35″ E, elev. 85 m, on Quercus ilex, 20 Apr. 2012, H.V. & W.J. (culture S615); Prinilas, 39°42′06″ N, 19°41′28″ E, elev. 300 m, on Quercus frainetto, 23 Apr. 2012, H.V. & W.J. (culture S633); Crete, near Prines, on ?Ceratonia siliqua, 27 Jun. 2011, W.J. (culture S555). Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, in a ditch, on Quercus ilex, 22 Nov. 2009, W.J. & H.V. (culture CBS 135576 = S109). Portugal, Madeira, Ribeiro Frio, Levada to Portela, on Ocotea foetens, 16 Feb. 2010, W.J. & O. Sükösd (culture S197); Seixal, Chao da Ribeira, on Ocotea foetens, 17 Feb. 2010, W.J. & O. Sükösd (WU 33337, culture CBS 136471 = S200, and from other twigs at the same place cultures S199, S201 and S202). Spain, Andalucia, road A2226 to Benalup, at km 10, on Rhamnus sp., 18 Mar. 2011, W.J. & H.V. (culture S500); Castellar de la Frontera, between the hotel Almoraima and the Castillo, on Fraxinus angustifolia, 19 Mar. 2011, W.J. (culture S509); same area, on ?Olea europaea, 19 Mar. 2011, H.V. & W.J. (culture S510); between El Bosque and Algar, on Rhamnus sp., 24 Mar. 2011, H.V. & W.J. (culture S543); N El Bosque, S Cortijo de la Borrega, on Quercus sp., 24 Mar. 2011, H.V. & W.J. (culture S542); El Jautor, near Cortijo de la Naranjuela, on Fraxinus angustifolia, 17 Mar. 2011, W.J. & H.V. (culture S493); at Rio Hozgarganta shortly after entering Provincia de Málaga on the road C3331 from SE/Jimena, on hardwood, 21 Mar. 2011, H.V. & W.J. (culture S527); Basque Country, Bizkaia, at the road BI2238 to Ibarrangelu, shortly after Basetxeta, on Alnus glutinosa, 31 Oct. 2010, W.J. (culture S325); Gipuzkoa, Aralar, GI2133, Larraitz, deciduous forest between Abaltzisketa and Amezketa, on Hypoxylon fuscum/Corylus avellana, 2 Nov. 2010, W.J. (culture S342); Canarias, La Palma, Cubo de la Galga, on Laurus novocanariensis, 12 Dec. 2009, W.J. (culture S178); same place and date, on ?Laurus or Ocotea; on/soc. Creosphaeria sassafras, 12 Dec. 2009, W.J. (WU 33332, culture S182); same area, on Ocotea foetens, soc. Xylaria discolor, 3 Dec. 2010, W.J. (WU 33382, culture S448); Los Sauces, Los Tilos, on Ocotea foetens, 10 Dec. 2009, W.J. (WU 33326, culture CBS 136466 = S167); ibid. (WU 33328, culture S170); Tenerife, Bosque de las Mercedes, Llano de los Viejos, on ?Laurus novocanariensis, 11 Apr. 2010, W.J. (culture S209); ibid., on Ilex canariensis, 11 Apr. 2010, W.J. (culture S210); ibid., on ?Ilex canariensis, 11 Apr. 2010, W.J. (culture S211); Macizo de Anaga, walking path right before descending to Batán de Arriba, on Diatrype flavovirens/Persea indica, 13 Apr. 2010, W.J. (culture S223); ibid., on Persea indica (culture S224); El Pijaral, on Diplazium caudatum, 21 Jun. 2011, L. Quijada TFC Mic 23206 (culture S558); El Pijaral, Chinobre, on Hypoxylon canariense/Laurus novocanariensis, 16 Dec. 2010, H.V. & W.J. (culture S483); Chinobre-La Ensillada, on undetermined wood, 23 Jan. 2011, M.A. Ribes & L. Quijada 23011123 (culture S551); Las Carboneras, walking path to El Batán from the road to Taborno, on ?Myrica faya, 15 Apr. 2010, W.J. (culture S236); ibid., on Xylaria discolor/Laurus, 16 Dec. 2010, W.J. & H.V. (culture S484); ibid., on Biscogniauxia cf. capnodes/Laurus, 16 Dec. 2010, H.V. & W.J. (WU 33390, culture S476); ibid., on Erica platycodon, 16 Dec. 2010, H.V. & W.J. (WU 33391, culture S477); ibid., on Prunus lusitanica, 16 Dec. 2010, W.J. & H.V. (WU 33392, culture S478); Islas Baleares, Mallorca, Algaida, between Cas Brusca and Cas Brau, asexual morph on Ceratonia siliqua, 21 Nov. 2010, W.J. (culture S418); east of Calviá, Ma-1016 roadside, asexual morph on Quercus ilex, 17 Nov. 2010, W.J. (culture S400); Es Capdella, between Camí del Graner del Delme and Torrent de Galatzó, asexual morph on Quercus ilex, 16 Nov. 2010, W.J. (cultures S388 and S390); Ma-10, 1.6 km before Banyalbufar, from Esporles, asexual morph on Pistacia lentiscus, 20 Nov. 2010, W.J. (culture S417); Puigpunyent, asexual morph on Platanus × hispanica, 16 Nov. 2010, W.J. (culture S395).
Notes: Very common in Southern Europe. The frequent detection of T. petersenii is quite unexpected, judging from the few records in Central Europe. Based on BLAST searches in GenBank initially four groups of isolates differing in tef1 sequences were identified as T. petersenii. One of these, which was only found on the Canary islands Tenerife and La Palma, was finally identified as T. dorotheae, a species that had formerly only been known from Australia and New Zealand (Samuels et al. 2006), another is described as the new species T. istrianum and the remaining two groups form a well-supported T. petersenii clade with a substructure. Trichoderma petersenii is very common on the Canary Islands, predominantly as sexual morph forming dark brown stromata, often on stromata of Xylariaceae.
Trichoderma phyllostachydis P. Chaverri & Samuels, Stud. Mycol. 48: 80. 2004 (2003).
Material examined: Italy, South Tyrol, NE corner of Kalterer See at the road to Laimburg, on Ostrya carpinifolia, 18 Oct. 2011, H.V. & W.J. (WU 33407, culture CBS 132577 = S564).
Notes: This rare species was only known from the bamboo Phyllostachys bambusoides in southwestern France (Chaverri & Samuels 2004). Our collection on Ostrya in northern Italy is therefore remarkable.
Trichoderma polysporum (Link) Rifai, Mycol. Pap. 116: 18. 1969.
Materials examined: Croatia, Istrija, Bale, close to St. Golaš, on Quercus pubescens, 18 Oct. 2010, W.J. (WU 33356, culture S315); 1.4 km before Barbariga from Peroj, on Carpinus orientalis, 18 Oct. 2010, W.J. (WU 33353, culture S307); ibid., asexual morph on a different branch (culture S308); forest N of Barbariga, elev. ca. 20 m, on Quercus pubescens, holomorph, sexual morph scant, 14 May 2010, W.J. (culture S258). Greece, Crete, Vamvakades, 35°19′1″ N, 23°45′23″ E, elev. 745 m, on Quercus pubescens, 27 Nov. 2011, W.J. (culture CBS 137004 = S608). Italy, Abruzzo, Sulmona, Vallelarga, close to N village sign, submediterranean forest, on Ostrya carpinifolia, 24 Nov. 2009, H.V. & W.J. (WU 33305, culture CBS 136463 = S121); ibid., on Steccherinum ochraceum/Quercus pubescens, 24 Nov. 2009, W.J. & H.V. (WU 33306, culture CBS 136464 = S124); Apulia, Andria, Parco Nazionale dell'Alta Murgia, Castel del Monte, between SP234 and Masseria Savignano, on Crataegus laciniata, 19 Nov. 2009, W.J. (culture CBS 135573 = S72); Foggia, Gargano, SW from Mandrione, Foresta Umbra, Riserva biogenetica Falascone, on Fagus sylvatica, 21 Nov. 2009, W.J. (WU 32198, culture CBS 135575 = S103); Mattinata, asexual morph on Quercus ilex, 20 Nov. 2009, W.J. & H.V. (WU 32186, culture S77); same area, asexual morph on Hippocrepis emerus, soc. T. koningii, 20 Nov. 2009, W.J. (culture S79a); Basilicata, SS653 east, 1.5 km before exit to Latrónico, on ?Tomentella sp. and bark of Quercus cerris, 17 Nov. 2009, W.J. & H.V. (culture CBS 135571 = S45); Calabria, Cosenza, Parco Nazionale del Pollino, above Morano Calabro, on Ostrya carpinifolia, 18 Nov. 2009, H.V. & W.J. (culture S56). Spain, Canarias, La Palma, Cumbre Nueva, branching off LP-301 at Area Recreativa del Pilar, sexual morph on Myrica faya and a white corticiaceous fungus, 11 Dec. 2009, W.J. (WU 33330, culture CBS 136467 = S176); Mazo, road LP2062, beginning of the walking path Camino La Banda, on Laurus novocanariensis, 5 Dec. 2010, W.J. (culture CBS 136999 = S458).
Note: This species is not uncommon in (sub-)Mediterranean habitats on twigs, either forming stromata or white asexual morph colonies; varying in tef1 sequences.
Trichoderma priscilae Jaklitsch & Voglmayr, sp. nov. MycoBank MB809292. Fig. 22.
Fig. 22.
Trichoderma priscilae. A–M. Sexual morph. A–C. Fresh stromata (A. immature). D–F. Dry stromata. G. Rehydrated stroma in 3 % KOH. H. Perithecium in section. I. Cortical and subcortical tissue in section. J. Subperithecial tissue in section. K. Stroma base in section. L, M. Asci with ascospores. N–U. Cultures and asexual morph at 25 °C. N–P. Cultures (N. CMD, 20 d; O. PDA, 10 d; P. SNA, 20 d). Q–T. Conidiophores and phialides (SNA, 5–6 d). U. Conidia (6 d). D, F, L. S129. E. S580. A–C, G–K, M–U. WU 33327, S168 = CBS 131487. Scale bars: A, C = 1.3 mm; B = 1 mm; D, G = 0.4 mm; E = 0.2 mm; F = 0.6 mm; H = 40 μm; I, J, R = 20 μm; K–M, Q, S, T = 10 μm; U = 5 μm.
Etymology: Named after Priscila Chaverri for her contributions to Trichoderma taxonomy.
Stromata scattered or aggregated in small numbers, when fresh 1–7 mm diam, to 1.5 mm thick, discoid or undulate, often with depressed centre or horseshoe-shaped, also pulvinate with circular outline, margin rounded, free; surface smooth; ostiolar dots inconspicuous, fine, olive, green to black. Colour when young pale reddish, then reddish brown or orange-brown, dull olive, eventually nearly black. Stromata when dry (0.8–)1.3–3.1(–3.6) × (0.7–)1.1–2.6(–3.2) mm, (0.3–)0.4–1.0(–1.5) mm thick (n = 40), discoid; outline circular or angular, often narrowly attached, margin free, often acute, sides cream, beige or reddish brown, smooth, scurfy or granular. Surface smooth or slightly tubercular, when young sometimes with whitish or silvery scurf. Ostiolar dots often invisible, less commonly distinct, minute, first reddish, later black, flat, convex or umbilicate, (30–)35–60(–80) μm wide (n = 40). Surface first whitish-yellowish, turning brown from the centre, later dark greyish brown (immature), eventually dark orange- or reddish brown to dark olive or nearly black. Spore deposits dark green. Stromata after rehydration 30–40 % larger, bright orange-brown, dots black, 40–65 μm diam; not changed after addition of 3% KOH. Stroma anatomy: Cortical layer (13–)24–37(–42) μm thick (n = 30), orange-brown, comprising a t. angularis of distinct, thin-walled cells (5–)6–15(–18) × (3.5–)5–10(–12) μm (n = 30) in section, below a thin, compressed, amorphous layer. Subcortical tissue comprising similar cells as the cortex, but hyaline, mixed with hyaline, thin-walled, (2–)3–6(–7.5) μm wide (n = 30) hyphae or replaced by them in places. Subperithecial tissue a t. epidermoidea of hyaline, thin-walled cells 5–25(–44) × (4–)6–10(–12) μm (n = 30). Stroma base brown, incorporating fungal spores and hyphae, tissue penetrating deeply into the bark in attachment areas, comprising hyaline, partly pale brownish cells and some hyphal elements (2–)3–5(–6.5) μm wide (n = 30); non-attached areas orange-brown t. epidermoidea mixing subperithecial with cortical tissue. Perithecia numerous, crowded, (140–)180–255(–280) μm high, (90–)115–175(–180) μm wide (n = 20), subglobose, ellipsoid or flask-shaped, peridium (14–)16–20(–22) μm thick at the base, (6–)9–16(–19) μm at the sides (n = 20), pale yellow in lactic acid, more intense in 3% KOH. Ostioles (63–)68–112(–146) μm long, projecting up to 20 μm, (20–)25–36(–42) μm wide at the apex inside (n = 20), periphysate. Asci (80–)88–105(–115) × (5.3–)5.7–6.7(–7.5) μm, stipe (4–)7–14(–17) μm long (n = 30), apex to 1.5 μm thick. Ascospores dull brown-green, warted, warts ca. 0.5 μm long; cells dimorphic, distal cells (4.0–)4.5–5.2(–6.0) × (3.5–)4.0–4.7(–5.5) μm, l/w 1.0–1.2(–1.3) (n = 95), (sub)globose, proximal cells (3.8–)4.7–6.0(–7.5) × (3.0–)3.5–4.2(–4.7) μm, l/w (1.1–)1.2–1.6(–2) (n = 95), oblong or subglobose.
Asexual morph on natural substrates: Colonies often large, up to 10 mm long, effuse or pustular, light to dark bluish green 24BC3 to 24EF4–5.
Cultures and asexual morph: Optimal growth at 25–30 °C on all media, no growth at 35 °C.
On CMD after 72 h colony radius 17–20 mm at 15 °C, 57–62 mm at 25 °C, 52–60 mm at 30 °C; mycelium covering the plate after 4 d at 25 °C. Colony hyaline, dense, circular; primary surface hyphae conspicuously wide at the margin; aerial hyphae, autolytic excretions and coilings inconspicuous, chlamydospores, pigment and distinct odour lacking. Conidiation first short-effuse, soon followed by the formation of shrubs growing to fluffy tufts and eventually compact, confluent pustules 1–4 mm diam with non-persistent straight elongations in a broad distal zone or in part irregularly distributed, after 5–6 d green, eventually dark green 28E5–7, 27F3–8. At 30 °C surface hyphae rapidly degenerating; conidiation in green pustules to 4 mm diam; some chlamydospores formed.
On PDA after 72 h colony radius 12–14 mm at 15 °C, 40–43 mm at 25 °C, 36–38 mm at 30 °C; mycelium covering the plate after 5 d at 25 °C. Colony circular, conspicuously dense; margin well defined and stellate due to parallel, aggregated surface hyphae. Aerial hyphae abundant, forming strands with irregular connectives in a flat, dense, zonate, downy to farinose, whitish mat. Autolytic excretions inconspicuous, coilings common, reverse faintly yellowish, no diffusing pigment formed; odour indistinct. Conidiation effuse, spreading from the centre, forming several ill-defined zones, turning green after 6–7 d, eventually 28C4–7 to 26–27F4–8. At 30 °C colony white, turning only faintly greenish.
On SNA after 72 h colony radius 15–18 mm at 15 °C, 52–54 mm at 25 °C, 48–53 mm at 30 °C; mycelium covering the plate after 4 d at 25 °C. Colony hyaline, circular with irregular margin, hyphae with conspicuous variation in width. Aerial hyphae common, forming a loose floccose mat, absent in the colony centre. Autolytic excretions inconspicuous, coilings common, pigment and distinct odour lacking. Chlamydospores uncommon, terminal and intercalary, globose, angular, ellipsoid or pyriform, small, (4–)5–9(–10.5) × (3.5–)5–9(–11) μm, l/w (0.8–)0.9–1.3(–1.7) (n = 30). Conidiation first scant, effuse and on aerial hyphae, soon abundant in numerous shrubs in a poorly defined zone or irregularly disposed, growing to fluffy tufts with long straight elongations, which later become fertile and integrated, turning green after 4–5 d, becoming compacted to pustules 1–2 mm diam aggregating to groups up to 9 mm, eventually dark green 27F3–8. Pustules hairy, comprising a densely intricate reticulum and long narrow axes with variable, often asymmetric branching mostly perpendicular to the axis or slightly inclined upwards; side branches similarly rebranching; terminal side branches mostly tree-like, i.e. branches gradually longer downwards, mostly 25–60 μm long, branched at 1–3 levels, mostly paired or in verticils. Conidiophores (side branches) difficult to separate, pachybasium-like, but often sinuous and branches only 2.0–3.5(–4.0) μm wide, thickenings up to 5.5 μm. Phialides solitary or produced in whorls of 2–6, ampulliform or lageniform, particularly in terminal position on the axis, (3.8–)5.5–8.2(–9.8) × (2.5–)3.0–3.5(–4.0) μm, l/w (1.1–)1.6–2.7(–3.1), (1.3–)1.5–2.3(–3.3) μm wide at the base (n = 32), neck variable, often long. Conidia (2.3–)2.8–3.2(–3.5) × (2.3–)2.5–2.8(–3.0) μm, l/w 1.1–1.2(–1.3) (n = 32), subglobose, green, smooth, scar indistinct, with no or few minute guttules. At 30 °C coilings abundant, conidiation in amorphous confluent green pustules.
Habitat: On wood and bark of broadleaf trees and shrubs.
Distribution: Southern Europe and Canary Islands.
Typus: Spain, Canarias, La Palma, Los Sauces, Los Tilos, 28°47′13″ N, 17°48′18″ W, elev. 550 m, on corticated, 5–10 cm thick branches of Ocotea foetens, on bark and wood, on bark, wood and moss, soc. lichens, Crepidotus sp., Stereum sp., 10 Dec. 2009, W.J. (holotype WU 33327; ex-type culture CBS 131487 = S168).
Additional materials examined: Italy, Abruzzo, Sulmona, Le Marane, riverine forest, 42°04′26″ N, 13°56′25″ E, elev. 380 m, on decorticated, 1–3 cm thick branch of Populus nigra, on well-decayed wood, asexual morph, 23 Nov. 2009, W.J. (culture S118); Lazio, Corviano, 42°28′30″ N, 12°11′50″ E, elev. 280 m, on 1 cm thick branch of Cytisus scoparius, on well-decayed wood, holomorph, 27 Nov. 2009, W.J. & H.V. (WU 33315, culture S144); close to Magugnano, at the Strada Magugnano-Roccalvecce, left shortly before reaching the brook, 42°32′21″ N, 12°10′00″ E, elev. 195 m, on 2–5 cm thick Cytisus scoparius in leaf litter, on medium to well-decayed wood and bark, holomorph, soc. ?Diaporthe sp. + Cosmospora sp., Crepidotus sp., Bisporella citrina, Lasiosphaeria ovina, ?Calycellina sp., 25 Nov. 2009, H.V. & W.J. (WU 33308, culture S129); Veneto, Galzignano, Turri, 45°18′58″ N, 11°46′08″ E, elev. 95 m, on decorticated twig of Sambucus nigra, on wood, soc. Hyphodontia sambuci, 23 Oct. 2011, H.V. & W.J. (WU 33409, culture S580). Spain, Baleares, Mallorca, Ma-10, 1.6 km before Banyalbufar approaching from Esporles, 39°40′58″ N, 02°32′29″ E, elev. 325 m, on corticated, 1.5 cm thick twig of Quercus ilex, asexual morph, 20 Nov. 2010, W.J. (culture S416); Canarias, La Palma, Cubo de la Galga, 28°45′43″ N, 17°46′34″ W, elev. 475 m, on 5 cm thick branch of Ocotea foetens, scattered on wood and bark, holomorph, soc. corticiaceous fungus and Xylaria discolor, 3 Dec. 2010, W.J. (WU 33383, culture S449).
Notes: Trichoderma priscilae is similar to other members of the Harzianum Clade forming reddish- or orange-brown stromata with green ascospores. See notes under T. christiani for comparisons.
Trichoderma psychrophilum Jaklitsch, Fungal Divers. 48: 195. 2011.
Material examined: Spain, Andalucia, Valdepenas de Jaén, UTM 30SVG26, elev. 960 m, on a twig of Rubus ulmifolius, 5 Dec. 2012, S. Tello 05121202 (WU 33413, culture CBS 137008 = S647).
Notes: This species is typically found at elevations of 1 000–2 000 m. Prior to now, the species was only known from members of the Ericaceae (Rhododendron, Vaccinium) in the Alps (Müller et al., 1972, Jaklitsch, 2011). Thus the present report of the species from the Mediterranean region in Spain on Rubus is an unexpected extension of range and hosts. Two other species that are known only from high elevations are T. andinense and T. konilangbra (Samuels et al. 1998).
Trichoderma pyramidale Jaklitsch & Chaverri, Mycologia (in press).
Notes: This species segregated from T. harzianum s.l. is based on four specimens and isolates from Calicotome villosa, Olea europaea, Quercus pubescens and Robinia pseudoacacia in Italy and Spain. See Chaverri et al. (in press) for details.
Trichoderma reesei E.G. Simmons, in Bigelow & Simmons, Abstracts, 2nd Int. Mycol. Congress (Tampa) 2: 618. 1977.
= Hypocrea jecorina Berk. & Broome, J. Linn. Soc. Bot. 14: 112. 1873.
Material examined: France, Mayotte, Chembenyoumba, on stromata on Rosellinia cf. corticium, 15 Aug. 2013, M. Pelissier (WU 33418, culture S666, isol. C. Lechat).
Notes: An economically important species common in tropical regions. One sexual morph specimen was collected in the French island Mayotte, east of Africa.
Trichoderma rossicum Bissett et al., Canad. J. Bot. 81: 578. 2003.
Materials sequenced: Greece, Crete, Lakki, asexual morph on Olea europaea subsp. sylvestris, 24 Nov. 2011, W.J. (culture CBS 137001 = S586). Spain, Andalucia, road A2226 to Benalup, at km 10, on Pistacia lentiscus, 18 Mar. 2011, W.J. & H.V. (culture S501); Castellar de la Frontera, road to the Castillo, on Quercus cf. faginea, 19 Mar. 2011, W.J. & H.V. (culture S505); Basque Country, Bizkaia, Urkiola, close to the village, on Corylus avellana, 1 Nov. 2010, W.J. (culture CBS 136991 = S334).
Notes: The four isolates collected during this study in Greece and Spain differ from true T. rossicum of the Stromaticum Clade in gene sequences and may be recognised as a separate species in future.
Trichoderma rubi Jaklitsch & Voglmayr, sp. nov. MycoBank MB809293. Fig. 23.
Fig. 23.
Trichoderma rubi (WU 33316, S146 = CBS 127380). A–L. Sexual morph. A. Fresh stromata. B–D. Dry stromata (B. immature, with white asexual morph). E. Rehydrated stroma. F. Rehydrated stroma in 3 % KOH. G. Perithecium in section. H. Cortical and subcortical tissue in section. I. Subperithecial tissue in section. J. Stroma base in section. K, L. Asci (L. in cotton blue/lactic acid). M–W. Cultures and asexual morph. M. Asexual morph on the natural substrate (colony margin). N. Conidiation pustule (SNA, 19 d). O–Q. Cultures (O. on CMD, 18 d; P. on PDA, 50 d; Q. on SNA, 24 d). R–U. Conidiophores (SNA, 17–19 d). V, W. Conidia (SNA, 20 d). N–W. All at 25 °C. Scale bars: A = 1 mm; B–D, M, N = 0.3 mm; E, F = 0.5 mm. G = 30 μm; H, J–L, T = 10 μm; I, R, S, U = 15 μm; V = 3 μm; W = 5 μm.
Etymology: Reflecting its occurrence on Rubus.
Stromata scattered or aggregated in groups of 2–3, when fresh 1–3 mm diam, ca. 0.5–1 mm thick, pulvinate, surface smooth, orange resulting from bright yellow surface and well-defined, plane, reddish ostiolar dots. Stromata when dry (0.7–)1.0–2.0(–2.3) × (0.6–)0.8–1.3(–1.6) mm, (0.2–)0.3–0.5(–0.7) mm thick (n = 20), developing in close association with the asexual morph, sometimes below an orange pellicle when young, flat pulvinate to discoid with circular, oblong or angular outline, narrow attachment and free margin, smooth surface apart from some wrinkles. Ostiolar dots (24–)25–52(–70) μm diam (n = 30), flat or slightly convex, well-defined, red, reddish brown to nearly black when old, on a bright yellow to orange surface; white inside; peridium yellow-orange; spore deposits white to yellowish. Rehydrated stroma 25–30 % larger than dry, more pulvinate, smooth, yellow-orange with brown dots, bright orange with hyaline ostiolar openings in 3 % KOH. Stroma anatomy: Cortical layer (13–)14–20(–24) μm (n = 30) thick, comprising a yellow t. angularis-globulosa of few layers of small, thick-walled cells (3.2–)4.0–8.5(–13.5) × (2.5–)3.0–5.5(–8.0) μm (n = 32) in section, larger, to 17 × 15 μm, at the stroma sides and base. Subcortical tissue hyaline to subhyaline, consisting of cells similar to the cortex, but thin-walled, (4.0–)5.5–10(–13.2) × (3.5–)4.5–6.7(–8.5) μm (n = 30), and/or of (2.5–)3.0–6.5(–8) μm (n = 30) wide hyphae with slightly constricted septa. Subperithecial tissue hyaline, comprising an ill-defined t. epidermoidea of thin-walled cells (5–)8–22(–41) × (4–)6–12(–17) μm (n = 30). Stroma base of yellow, similar cells as the cortex, but slightly larger and thinner-walled, in addition containing subhyaline to yellow, thin-walled, (2.0–)3.5–5.7(–7.5) μm (n = 30) wide hyphae. Perithecia (130–)170–235(–250) μm (n = 30) high, (100–)125–200(–270) μm (n = 30) wide, globose to subglobose; peridium (12–)14–21(–26) μm (n = 30) thick at the base, (8.5–)10–17(–23) μm (n = 30) at the sides, yellow. Ostioles (43–)51–65(–70) μm (n = 30) long, not projecting, (17–)23–36(–43) μm (n = 30) wide at the apex inside, periphysate, with some clavate apical cells to 5 μm wide. Asci (73–)78–90(–93) × (3.8–)4.3–5.0(–5.5) μm (n = 25), stipe (4–)7–13(–16) μm long (n = 25), cylindrical, apex thickened. Ascospores hyaline, spinulose, cells dimorphic, distal cells (3.5–)3.7–4.3(–5.3) × (3.0–)3.2–3.7(–4.2) μm, l/w (1.0–)1.1–1.2(–1.4) (n = 32), subglobose, proximal cells (4.0–)4.2–5.3(–6.5) × (2.5–)2.7–3.0(–3.2) μm, l/w (1.3–)1.4–1.9(–2.4) (n = 32), oblong, less commonly subglobose, sometimes considerably elongated in ascus base (up to 6.5 μm long).
Asexual morph on the natural substrate: white crumbly, 1–5 mm long patches, effuse or pulvinate, often directly connected with young stromata, ends fertile, hydrophobic.
Cultures and asexual morph: optimal growth at 25 °C on all media, at 30 °C hyphae dying after short growth, no growth at 35 °C. Growth on PDA distinctly slower than on CMD and SNA. On CMD after 72 h colony radius 2–8 mm at 15 °C, 10–15 mm at 25 °C, 0.1–1.5 mm at 30 °C; mycelium covering the plate after ca. 2 wk at 25 °C. At 25 °C colony circular with well-defined margin and indistinct alternating zones conspicuously differing in width, hyaline, dense, hyphae uniform, narrow, providing a radial structure; turning yellow 3A2–3 at the distal margin, with minute crystals in the agar and long needles projecting above the agar surface, crystals eventually disintegrating. Aerial hyphae virtually absent. Autolytic excretions inconspicuous, coilings absent; odour indistinct. No chlamydospores formed. Conidiation noticeable after 5–7 d, effuse, short, simple, conidia produced in minute wet heads to ca. 25 μm diam on often distinctly parallel phialides.
On PDA after 72 h colony radius 1–4 mm at 15 °C, 3–8 mm at 25 °C, 0–1 mm at 30 °C; mycelium covering the plate after ca. 2 mo at 25 °C. Colony at 25 °C dense, first circular with well-defined margin, becoming irregularly lobate; hyphae uniform, narrow; centre becoming farinose. Aerial hyphae short, inconspicuous. Autolytic excretions numerous, minute, immersed in the agar; coilings lacking. Colony turning bright yellow 3A5–8, 4A5–6, 4B6–8 from the centre, eventually brownish; pigment diffusing and masses of yellow crystals forming in the agar. Odour indistinct to slightly unpleasant. Conidiation noticeable after 3–5 d, effuse, conidia formed in minute wet heads on mostly solitary lageniform phialides on short erect simple conidiophores spreading from the centre.
On SNA after 72 h colony radius 3–5.5 mm at 15 °C, 8–12 mm at 25 °C, 0.5–1.5 mm at 30 °C; mycelium covering the plate after 3 wk at 25 °C. At 25 °C colony as on CMD, but margin often becoming ill-defined/wavy, zones more uniform in breadth, surface hyphae soon degenerating, appearing empty, no pigment formed, conidiation pustular, noticeable after 3–5 d, first effuse, on widely scattered, loosely disposed, short, erect, simple, verticillium-like, rarely gliocladium-like conidiophores with asymmetric branching, appearing warted under 10× objective. Conidia formed in minute wet heads. Effuse conidiation followed by the formation of pustules mainly in a broad distal zone, scattered or aggregated in small groups, 0.5–1.5(–2) mm diam, confluent to several mm, white, eventually turning yellow when old. Pustules consisting of a loosely branched reticulum with long stout radial main axes ending in projecting, straight to sinuous, sterile elongations in their periphery; elongations with fertile tips, more or less incorporated in the pustule. Conidiation starting within the pustules, on solitary phialides or phialide whorls attached by single cells to the main axes and on side branches emerging perpendicular to long axes (conidiophores). Conidiophore axis 2–5 μm wide, at branching points to 7 μm, forming regular trees or broad structures. Phialides solitary or, mostly in divergent whorls of 2–4(–6), often with a central phialide on an additional cell. Phialides (5.2–)6.5–10.5(–12) × (2.2–)2.5–3.0(–3.3) μm, l/w (1.7–)2.2–3.8(–5.2), (1.5–)2.0–2.5(–3.0) μm wide at the base (n = 50), lageniform, often inequilateral, gradually narrowing into a long and narrow neck, often sigmoid, broadly attached, broadest at the base or middle; ends often curved, appearing parallel. Conidia (2.2–)2.5–3.3(–4.3) × 2.0–2.7(–3.7) μm, l/w (0.9–)1.1–1.4(–1.7) (n = 65), subglobose to ellipsoid, hyaline, smooth, with one or few guttules, scar indistinct.
On MEA also yellow crystals formed, odour unpleasant (rancid or mushroomy); culture from conidia similar to the ascospore-derived culture. Conidiation first effuse, verticillium-like, followed by the formation of white pustules.
Habitat: On stems of Rubus ulmifolius.
Distribution: Southern Europe (Italy); only known from the holotype.
Typus: Italy, Lazio, Corviano, 42°28′53″ N, 12°12′12″ E, elev. 250 m, on the basal part of a 1–1.5 cm thick stem of Rubus ulmifolius covered by leaves, soc. white pulvinate asexual morph, 27 Nov. 2009, W.J., W. Gams & H.V. (holotype WU 33316; ex-type culture CBS 127380 = S146).
Notes: The accompanying white asexual Trichoderma was confirmed as the asexual morph by culture and tef1 sequences. Dry stromata may be flat and orange and may be mistaken for the sexual morph of T. auranteffusum, which has the same ascospore size. Conidiophores of T. rubi are similar to those of T. crystalligenum or T. psychrophilum, but phialides tend to be more divergent. A collection with similar stromata on the same host from Spain was identified as T. psychrophilum, a species that only grows below 25 °C.
Trichoderma samuelsii Jaklitsch & Voglmayr, Mycologia 104: 937. 2012.
Note: This species was described based on four isolates from Italy and Spain by Jaklitsch et al. (2012), with additional isolates listed by Jaklitsch et al. (2013).
Additional materials examined: All conidial. France, Dept. Alpes-de-Haute-Provence, Gorge du Verdon SW Rougon, at the end of the 2nd tunnel at the hiking trail through the gorge, elev. ca. 620 m, on Acer opalus, 29 Jul. 2011, H.V. (culture S562). Greece, Corfu, SE Liapades, shortly before Kanakades heading south, 39°39′35″ N, 19°46′0″ E, elev. 60 m, on Olea europaea, 20 Apr. 2012, H.V. & W.J. (culture S614); Crete, Palaea Roumata, near Pananiana, 35°24′20″ N, 23°46′13″ E, elev. 370 m, on Olea europaea subsp. sylvestris, 25 Nov. 2011, W.J. (culture S593). Spain, Andalucia, El Colmenar, at Rio Guadiaro, on Phyllostachys sp., 23 Mar. 2011, H.V. & W.J. (culture S537).
Trichoderma saturnisporopsis Samuels & Jaklitsch, Fungal Divers. 55: 103. 2012.
Material examined: Italy, Sardinia, at the road SP17, between junctions to Burgos and Foresta di Burgos, on Quercus virgiliana, 5 Nov. 2009, W.J. (WU 32172, culture CBS 128829 = S19).
Note: Only known as asexual morph. Collected only once during the present study.
Trichoderma sempervirentis Jaklitsch & Voglmayr, Persoonia 31: 143. 2013.
Note: Only known from two specimens from Acer sempervirens from Crete, Greece (see Jaklitsch et al. 2013).
Trichoderma simmonsii P. Chaverri et al., Mycologia (in press).
Notes: This species belongs to T. harzianum s.l. Fifteen specimens were collected from various trees and shrubs, predominantly Quercus spp., in Croatia, France, Greece, Italy and Spain. See Chaverri et al. (in press). This species occurs also in Austria, Central Europe (WU 29083, culture C.P.K. 1596, and WU 29087, culture C.P.K. 2391, listed under Hypocrea lixii in Jaklitsch 2009).
Trichoderma sinuosum P. Chaverri & Samuels, Stud. Mycol. 48: 81. 2004 (2003).
Materials examined (all collected as asexual morphs except variant 4): T. sinuosum s.str. (= T. sinuosum 1): Spain, Bizkaia, Gorbeia, forest at the road A624 3 km before Altube heading southeast, asexual morph on Fagus sylvatica, 1 Nov. 2010, W.J. (culture S333). T. sinuosum 2: Croatia, Cres, Crna, on Ostrya carpinifolia, 15 Oct. 2010, W.J. (culture S295); Istrija, between Vareški and Krnica, on Phanerochaete sanguinea/Quercus pubescens, 25 Sep. 2010, H.V. & W.J. (culture S274); same place, on Quercus pubescens, 25 Sep. 2010, W.J. & H.V. (culture S276). Italy, Lazio, Farnese, Selva del Lamone, hiking trail Roppozzo, on Acer monspessulanum, 28 Nov. 2009, W.J., H.V. & W. Gams (culture S158). T. sinuosum 3: Croatia, Istrija, forest N of Barbariga, elev. ca. 20 m, on Quercus pubescens, 24 Sep. 2010, H.V. & W.J. (culture S270). Spain, Basque Country, Gipuzkoa, BI3440, Jaizkibel, parking place close to road leading to golf course Justiz, on a branch of Castanea sativa, 3 Nov. 2010, W.J. (culture S349). T. sinuosum 4: Spain, Basque Country, Gipuzkoa, Oiartzun, BI3420 heading to Endara, nature park Aiako Harra, pasture with Betula and Ulex, on Ulex europaeus, holomorph, sexual morph scant, 6 Nov. 2010, W.J. (culture CBS 136478 = S378).
Notes: Based on tef1 BLAST searches in GenBank the isolates listed here were identified as T. sinuosum. However, there is considerable variation among sequences, and phylogenetic analyses showed that the variants T. sinuosum 2, 3 and 4 each form highly supported clades separate from T. sinuosum s.str. (Fig. 5), and thus are likely to represent distinct species.
Trichoderma spirale Bissett, Canad. J. Bot. 69: 2408. 1992 (1991).
Material examined: Spain, Canarias, Tenerife, Macizo de Anaga, Pico del Ingles, asexual morph on Laurus novocanariensis, 11 Apr. 2010, W.J. (culture CBS 136472 = S212).
Note: Only found once in Southern Europe.
Trichoderma stercorarium (Barrasa et al.) Jaklitsch & Voglmayr, comb. nov. MycoBank MB809294.
Basionym: Aphysiostroma stercorarium Barrasa et al., Canad. J. Bot. 63: 2441. 1986 (1985).
Notes: This species was placed in a separate genus Aphysiostroma because of its non-ostiolate ascomata and its growth on dung. Otherwise, in characters of its asexual morph and sexual morph it is consistent with members of the Hypocreanum Clade. Barrasa et al. (1986) mentioned its resemblance to species such as T. pulvinatum and T. americanum. Molecular phylogenies consistently place this species within the Hypocreanum Clade with high support (see also Jaklitsch, 2009, Jaklitsch, 2011); thus we transfer the species to Trichoderma. Trichoderma stercorarium is apparently rare as it is only known from the holotype collected in central Spain.
Trichoderma stilbohypoxyli Samuels & Schroers, Stud. Mycol. 56: 128. 2006.
Materials sequenced: Italy, Apulia, Foggia, Gargano, Mattinata, asexual morph on Rubus ulmifolius, 20 Nov. 2009, H.V. & W.J. (culture S75); Sardinia, at the road SS392 from Lago di Coghinas, 20.5 km before Tempio Pausania, asexual morph on ?Myrtus communis, 6 Nov. 2009, W.J. (culture S24).
Notes: As already noted by Jaklitsch (2011), European isolates differ consistently from American isolates in gene sequences and thus may represent a distinct species. We found the European genotype in Italy (Gargano and Sardinia) and Sri Lanka (W. Jaklitsch, unpubl. data).
Trichoderma strictipile Bissett, Canad. J. Bot. 69: 2410. 1992 (1991).
Materials examined (all containing stromata except where noted): Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra/Foresta Domaniale, on Quercus cerris, 21 Nov. 2009, W.J. & H.V. (WU 32190, culture S88); ibid., at Riserva biogenetica Falascone, on Fagus sylvatica, 21 Nov. 2009, H.V. & W.J. (culture S97); Basilicata, Parco Nazionale del Pollino, San Severino, Bosco Magnano, along the river Peschiera, on Alnus cordata, 17 Nov. 2009, H.V. & W.J. (WU 32181, culture S52); Calabria, Mormanno, Parco Nazionale del Pollino, Valle di Fiume Argentino, Cielafforcato, on Fagus sylvatica, 18 Nov. 2009, H.V. & W.J. (culture S61); Lazio, Bomarzo, Santa Cecilia, on Quercus cerris, 29 Nov. 2009, W.J., H.V. & W. Gams (culture S163); ibid., 21 Oct. 2012 (WU 33411, culture S644); Corviano, on Alnus glutinosa, 27 Nov. 2009, H.V., W.J. & W. Gams (culture S148); Farnese, Selva del Lamone, hiking trail Roppozzo, on Quercus cerris, 28 Nov. 2009, W.J., H.V. & W. Gams (WU 33322, culture S155); Veneto, near Teolo, asexual morph on Corylus avellana, 22 Oct. 2011, H.V. &W.J. (culture S574); ibid., on Castanea sativa (culture S575).
Notes: This species is common in temperate climate zones; in Southern Europe we found it, however, only in Italian areas of submediterranean to oro-mediterranean vegetation, but not in Mediterranean zones.
Trichoderma subeffusum Jaklitsch, Fungal Divers. 48: 55. 2011.
Materials examined: Croatia, Istrija, Vrsar, beach forest at Petalon Resort, elev. 20 m, asexual morph on Ulmus minor, 13 May 2010, H.V. &W.J. (culture S251). Greece, Crete, Palaea Roumata, near Pananiana, 35°24′20″ N, 23°46′13″ E, elev. 370 m, asexual morph on Olea europaea subsp. sylvestris, 25 Nov. 2011, W.J. (culture S592); south of Platanos, roadside, 35°27′09″ N, 23°35′19″ E, elev. 275 m, asexual morph on Platanus orientalis, 27 Nov. 2011, W.J. (culture S602). Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, ca. 100 m below military station, heading to Peschici, sexual morph on Carpinus betulus, soc. Skeletocutis nivea, T. cremeoides, corticiaceous fungi, 22 Nov. 2009, W.J. & H.V. (WU 33303, culture S116); Lazio, Corviano, holomorph on Alnus glutinosa, 27 Nov. 2009, W.J., H.V. & W. Gams (WU 33318, culture S149). Spain, Canarias, Tenerife, Bosque de La Esperanza, sexual morph on Adenocarpus foliolosus, 16 Apr. 2010, W.J. (WU 33341, culture CBS 136989 = S238); Macizo de Anaga, walking path to El Batán from the road to Taborno, sexual morph on Laurus novocanariensis, 15 Apr. 2010, W.J. (WU 33339, culture S234).
Notes: This hitherto uncommon species, previously known from Austria and the Ukraine, occurs also in Mediterranean and submediterranean habitats including Tenerife, where it occurs on seven unrelated genera of woody plants.
Trichoderma thelephoricola P. Chaverri & Samuels, Stud. Mycol. 48: 96. 2004 (2003).
Material examined: Italy, Veneto, Teolo, on Castanea sativa, 22 Oct. 2011, H.V. & W.J. (culture S577); ibid., on Steccherinum ochraceum/Ulmus minor (culture S572).
Notes: This species was described from Maryland, USA. In Europe it seems to be confined to Central European climate and vegetation zones, as in this study we detected it only in northern Italy, although its host Steccherinum ochraceum is common in the Mediterranean. On some superficially resembling corticiaceous fungi, e.g., Hyphodontia spp., we detected the green-spored species T. cremeoides instead.
Trichoderma tomentosum Bissett, Canad. J. Bot. 69: 2412. 1992 (1991).
Materials sequenced: Italy, Campania, Teggiano, chestnut plantation at San Marco, on Castanea sativa, 16 Nov. 2009, W.J. & H.V. (culture CBS 135569 = S33); Sardinia, at SS 392 from Lago di Coghinas, 20.5 km before Tempio Pausania, on root of ?Myrtus communis, 6 Nov. 2009, W.J. (culture CBS 135568 = S23); South Tyrol, NE corner of Kalterer See at road to Laimburg, on Ostrya carpinifolia, 18 Oct. 2011, H.V. & W.J. (culture S563). Spain, Andalucia, Castellar de la Frontera, road A2100 north from the village, on Quercus sp., 20 Mar. 2011, H.V. & W.J. (culture CBS 137000 = S513); Jimena de la Frontera, NW of the village at the road C3331, on Calicotome villosa, 21 Mar. 2011, W.J. & H.V. (culture S521); near La Sauceda, at the road C3331 from Jimena, on Calicotome villosa, 21 Mar. 2011, Voglmayr & W.J. (culture S530); Basque Country, Bizkaia, Uarka Auzoa, on Fagus sylvatica, 31 Oct. 2010, W.J. (culture S318); at the road BI2238 to Ibarrangelu, shortly after Basetxeta, on Pinus cf. radiata, 31 Oct. 2010, W.J. (culture S319); Canarias, La Palma, Cumbre Nueva, Castanea plantation at LP 301, close to crossing with LP 3, on Castanea sativa, 2 Dec. 2010, W.J. (culture S435); Montaña Tagoja, on Hymenochaete sp./Ocotea foetens, 14 Dec. 2009, W.J. (culture S193); Tenerife, Bosque de las Mercedes, Llano de los Viejos, on Apollonias barbujana, 11 Apr. 2010, W.J. (culture S208); above Farrobillo, on ?Laurus novocanariensis, 14 Apr. 2010, W.J. (culture S229); ibid., on Castanea sativa, (culture CBS 136474 = S230); Macizo de Anaga, pine forest above Batán de Arriba, on Phaeolus schweinitzii, 13 Dec. 2010, H.V. & W.J. (culture S468); San Juan de la Rambla, Barranco Rambla de Ruíz, on Phellinus sp., 12 Apr. 2010, W.J. (culture S215).
Notes: A species that is similar to T. cerinum and much more common than that species. It occurs in all climatic zones of Europe. No sexual morph is known. See under T. cerinum for additional comments.
Trichoderma tremelloides Jaklitsch, Fungal Divers. 48: 232. 2011.
Materials examined: Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra/Foresta Domaniale, on Radulomyces molaris/Quercus cerris, 21 Nov. 2009, H.V. & W.J. (WU 30192, culture S89); Lazio, Farnese, Selva del Lamone, hiking trail Roppozzo, on Quercus cerris, 28 Nov. 2009, W. Gams, W.J. & H.V. (WU 30193, culture S154). Spain, Andalucia, Castellar de la Frontera, road to the Castillo, on Quercus sp., 19 Mar. 2011, H.V. & W.J. (WU 33399, culture S506).
Note: Originally described from Denmark and later found in Central Europe, this uncommon species also occurs in (sub-)Mediterranean regions, typically on deciduous oak species.
Trichoderma viridarium Jaklitsch et al., Persoonia 31: 126. 2013.
Note: Described from eight specimens from France, Greece, Italy and Spain, Southern Europe, but also occurring in Central and Northern Europe (Jaklitsch et al. 2013).
Trichoderma viride Pers., Neues Mag. Bot. 1: 92. 1794.
Materials examined: Spain, Canarias, Tenerife, Macizo de Anaga, Montaña Chamuscada, near Roque de los Pasos, on Erica platycodon, 23 Jan. 2011, M.A. Ribes & L. Quijada 23011154 (TFCMic. 23079, culture S552); Pico del Ingles, on Erica platycodon, 16 Dec. 2010, W.J. (culture S472).
Notes: In contrast to early taxonomy, when every Trichoderma species with green conidia was identified as T. viride, this species is uncommon and occurs in central and northern regions (cf. Jaklitsch et al. 2006a). One exception, however, is Tenerife, where it was detected twice in the laurisilva forest of the Anaga massif. Zachow et al. (2009) also reported T. viride from a laurisilva habitat on this island.
Trichoderma viridescens (A.S. Horne & H.S. Will.) Jaklitsch & Samuels, Stud. Mycol. 56: 156. 2006.
Additional materials of T. viridescens s.str.: Spain, Asturias, Pola de Somiedo, at the road to Aiginu, on Quercus pyrenaica, 3 Jun. 2013, H.V. & W.J. (culture S660); same area, on Genista florida, 3 Jun. 2013, H.V. & W.J. (culture S661); Soto de los Infantes, near Viescas, on Quercus petraea, 5 Jun. 2013, H.V. & W.J. (culture S664).
Notes: Trichoderma viridescens, as it was recognised by Jaklitsch et al., 2006a, Jaklitsch et al., 2006b, was a species complex within which Jaklitsch et al. (2013) recognised 13 species, of which 12 were named. Trichoderma viridescens s.str. is common in Europe.
Trichoderma viridialbum Jaklitsch et al., Persoonia 31: 135. 2013.
Notes: Jaklitsch et al. (2013) reported this species from the Canary Islands La Palma and Tenerife. It is also known from Mexico, Norway and Sardinia.
Trichoderma virilente Jaklitsch & Voglmayr, Persoonia 31: 131. 2013.
Note: Jaklitsch et al. (2013) reported 12 specimens of this species from Croatia, Greece, Italy and Spain.
Trichoderma sp. S138
Material examined: Italy, Lazio, Lago di Vico, west side, 42°19′48″ N, 12°09′08″ E, elev. 560 m, on a 7 cm thick, decorticated, well-decayed branch of Acer campestre, holomorph, 26 Nov. 2009, W.J., H.V. & W. Gams (WU 33312, culture S138).
Notes: This singleton resembles T. christiani, but according to the phylogenetic analyses (Fig. 1, Fig. 2, Fig. 5) it represents a different taxon in a clade that contains T. christiani and T. corneum. It also has smaller ascospores than T. christiani, with distal cells (3.5–)3.8–4.7(–5.0) × (3.2–)3.5–4.3(–4.5) μm, l/w (0.9–)1.0–1.2(–1.3) (n = 30) and proximal cells (3.5–)4.0–5.0(–5.7) × (2.8–)3.0–4.0(–4.3) μm, l/w (1.0–)1.1–1.5(–1.9) (n = 30) and slightly slower growth rate. Otherwise no morphological difference was detected (cf. Fig. 8). However, the material is depauperate and insufficient for sectioning.
Trichoderma sp. S169
Material examined: Spain, Canarias, La Palma, Los Sauces, Los Tilos, asexual morph on ?Erica arborea, 10 Dec. 2009, W.J. (culture CBS 133494 = S169).
Note: This singleton forms a clade (Fig. 1, Fig. 2, Fig. 5) with Trichoderma sp. S605 that is affiliated with the Strictipile Clade.
Trichoderma sp. S222
Material examined: Spain, Canarias, Tenerife, Macizo de Anaga, walking path right before descending to Batán de Arriba, asexual morph on Erica arborea, 13 Apr. 2010, W.J. (culture CBS 136473 = S222).
Note: This singleton forms a clade (Fig. 1, Fig. 2, Fig. 5) with T. catoptron and T. pseudogelatinosum.
Trichoderma sp. S404
Material examined: Spain, Mallorca, Ma-10 above Fornalutx, opposite the property Monnaber, asexual morph on Quercus ilex, 17 Nov. 2010, W.J. (culture CBS 136994 = S404).
Note: This singleton is related (Fig. 1, Fig. 2, Fig. 5) to T. ceraceum, T. cerinum and T. tomentosum.
Trichoderma sp. S466
Material examined: Spain, Canarias, Tenerife, Teno Alto, asexual morph on Chamaecytisus proliferus, 10 Dec. 2010, W.J. (culture CBS 135581 = S466).
Note: This singleton is apparently related (Fig. 2, Fig. 5) to T. inhamatum within T. harzianum s.l.
Trichoderma sp. S467
Material examined: Spain, Canarias, Tenerife, Pista de Benijos/camping place La Caldera, above Aguamansa, asexual morph on Erica arborea, 12 Dec. 2010, W.J. (culture CBS 135582 = S467).
Note: This singleton is apparently related (Fig. 2, Fig. 5) to T. guizhouense within T. harzianum s.l.
Trichoderma sp. S605
Material examined: Greece, Crete, between Myloi and Strovles, 35°22′37″ N, 23°39′45″ E, elev. 370 m, asexual morph on Castanea sativa, 27 Nov. 2011, W.J. (culture CBS 137002 = S605).
Note: This singleton forms a clade (Fig. 2, Fig. 5) with Trichoderma sp. S169 that is related to the Strictipile Clade.
Trichoderma sp. S610
Material examined: Greece, Crete, Fournés, citrus plantation along the river Keritis, 35°26′33.5″ N, 23°55′45″ E, elev. 75 m, asexual morph on Juglans regia, 28 Nov. 2011, W.J. (culture CBS 135585 = S610).
Note: Representing an isolated lineage within the Harzianum Clade (Fig. 5).
Trichoderma sp. S624
Material examined: Greece, Corfu, between Troumpettas and Agia Anna, 39°42′22″ N, 19°44′41″ E, elev. 355 m, asexual morph on Spartium junceum, 21 Apr. 2012, W.J. & H.V. (culture CBS 137006 = S624).
Note: This singleton is phylogenetically isolated within the Green-spored Clade (Fig. 1, Fig. 2, Fig. 5).
Trichoderma sp. S637
Material examined: Greece, Corfu, Faiakes, 39°41′41″ N, 19°48′17″ E, elev. 100 m, asexual morph on Quercus frainetto, 24 Apr. 2012, W.J. (culture CBS 137007 = S637).
Note: This singleton is phylogenetically closely related to T. luteffusum (Fig. 2, Fig. 4).
Other species of Hypocreaceae outside Trichoderma:
Apart from a few observations of Hypomyces aurantius and H. rosellus we found the following hypocreaceous fungi in the studied region.
Arachnocrea stipata (Fuckel) Z. Moravec, Bull. trimest. Soc. mycol. Fr. 72: 161. 1956.
Materials examined: Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra, Riserva biogenetica Falascone, on Fagus sylvatica, 21 Nov. 2009, W.J. S102 (WU 32197). Spain, Canarias, Tenerife, Bosque de La Esperanza, on Eucalyptus globulus, 18 Apr. 2010, W.J. (WU 33343, culture S249).
Notes: This fungus is uncommon, but widespread in Europe. Preliminary studies have shown a considerable genetic variation (Jaklitsch & Voglmayr, unpubl. results), suggesting that this is a species complex.
Hypocreopsis rhododendri Thaxt., Proc. Amer. Acad. Arts & Sci. 57: 429. 1922.
Material examined: France, Aquitaine, Pyrénées-Atlantiques, SE Salies de Bearn, D430, deciduous forest at the junction to Quartier du Cout, on Corylus avellana, 3 Nov. 2010, W.J. (WU 33362, culture S354); no Hymenochaete detected on the plant host.
Note: A hypocreaceous fungus typically occurring on Corylus avellana colonised by Hymenochaete tabacina in Atlantic Western Europe (Ainsworth 2003).
Protocrea farinosa (Berk. & Broome) Petch, J. Bot., Lond. 75: 219. 1937.
Materials examined (all on Skeletocutis nivea or Skeletocutis sp. growing on the plants listed here): Croatia, Istrija, Fažana, forest at Valbandon, on Carpinus orientalis, 17 Oct. 2010, W.J. (WU 33351, S305). France, Aquitaine, Pyrénées-Atlantiques, SE Salies de Bearn, D430, deciduous forest at the junction to Quartier du Cout, on Quercus robur, 3 Nov. 2010, W.J. (WU 33361, S352). Italy, Apulia, Foggia, Gargano, SW from Mandrione, Foresta Umbra/Foresta Domaniale, on Ostrya carpinifolia, 21 Nov. 2009, W.J. (WU 32193, culture S92); Campania, left roadside of Via Provinciale del Corticato shortly after the highest point heading to Sacco, Parco Nazionale del Cilento, on Fraxinus ornus, 16 Nov. 2009, W.J. (WU 32176, culture S39); Lazio, Farnese, Selva del Lamone, hiking trail Roppozzo, on Quercus cerris, 28 Nov. 2009, W.J. (culture S157); Sardinia, at the road SP17, between junctions to Burgos and Foresta di Burgos, on Quercus virgiliana, 5 Nov. 2009, W.J. (WU 32171, culture S18). Spain, Basque Country, Bizkaia, Atxurra, on Corylus avellana, 30 Oct. 2010, W.J. (WU 33357, culture S316); Gipuzkoa, Aralar, east from San Martin/Ataun, at the road BI4151, Atseden Toki, on Fagus sylvatica, 2 Nov. 2010, W.J. (WU 33359, culture S337); Jaizkibel, road BI3440, parking place close to road leading to golf course Justiz, on Ulex europaeus, 3 Nov. 2010, W.J. (WU 33360, culture S348). Canarias, La Palma, Cumbre Nueva, Castanea plantation at LP 301, close to crossing with LP 3, on Castanea sativa, 13 Dec. 2009, W.J. (WU 33333, culture S186); Malgarida (above San Isidro), on Persea indica, 2 Dec. 2010, W.J. (WU 33375, culture S427); Pista El Corcho, on Myrica faya, 3 Dec. 2010, W.J. (WU 33381, culture S445); Tenerife, Bosque de La Esperanza, on Eucalyptus globulus, 18 Apr. 2010, W.J. (culture S246); Orotava, near Pista de Benijos/camping place La Caldera, above Aguamansa/La Orotava, on ?Adenocarpus foliolosus, 16 Apr. 2010, W.J. (WU 33342, culture S243); Islas Baleares, Mallorca, Ma-1016 roadside, 2.8 km after crossing with Ma-1043 heading east, on Arbutus unedo, 17 Nov. 2010, W.J. (WU 33370, culture S401).
Note: With 15 specimens collected in various regions, P. farinosa seems to be the most common member of the Hypocreaceae outside Trichoderma; it follows the range of its host, Skeletocutis nivea.
Acknowledgements
We are grateful to C.P. Kubicek, I. Druzhinina, M. Komon-Zelazowska and L. Atanasova from the TU Vienna for collaboration and providing strains, to G.J. Samuels and W. Gams for their helpful comments and suggestions to improve the text, to J. Bissett, E. Ismaiel, T. Merkx, G.J. Samuels and G. Verkleij for strains and data, to W. Gams, J. Fournier and E. Beltran for generous and pleasant support during collection trips, to L. Bernardo, M. Contu, R.M. Dähnke, I. Kušan, N. Matočec and O. Sükösd for excursion support, to R. Marcucci for support in the PAD herbarium, and to A. Akulov, J. Fernández, A. & I. Hausknecht, P. Karasch, M. Pelissier, L. Quijada, M.A. Ribes, E. Rubio and S. Tello for fresh specimens. Financial support by the Austrian Science Fund (FWF; project P22081-B17) is gratefully acknowledged.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
References
- AEMET . Agencia Estatal de Meteorología; Madrid: 2012. Atlas climático de los archipiélagos de Canarias, Madeira y Azores.http://www.aemet.es/documentos/es/conocermas/publicaciones/2Atlas_climatologico/Atlas_Clima_Macaronesia___Baja.pdf Online publication available at. [Google Scholar]
- Ainsworth M. Report on hazel gloves Hypocreopsis rhododendri, a UK BAP ascomycete fungus (with reference to willow gloves H. lichenoides) English Nature Research Reports. 2003;541:1–23. [Google Scholar]
- Atanasova L., Druzhinina I.S., Jaklitsch W.M. Two hundred Trichoderma species recognized based on molecular phylogeny. In: Mukherjee P.K., Singh U.S., Horwitz B.A., Schmoll M., Mukherjee M., editors. Trichoderma: biology and applications. CABI; Nosworthy Way, Wallingford, Oxon, UK: 2013. [Google Scholar]
- Atanasova L., Jaklitsch W.M., Komon-Zelazowska M. Clonal species Trichoderma parareesei sp. nov. likely resembles the ancestor of the cellulase producer Hypocrea jecorina/T. reesei. Applied and Environmental Microbiology. 2010;76:7259–7267. doi: 10.1128/AEM.01184-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrasa J.M., Martínez A.T., Moreno G. [1985]. Aphysiostroma, a new nonostiolate hypocrealean fungus. Canadian Journal of Botany. 1986;63:2439–2443. [Google Scholar]
- Bissett J. [1991]. A revision of the genus Trichoderma. III. Section Pachybasium. Canadian Journal of Botany. 1992;69:2373–2417. [Google Scholar]
- Błaszczyk L., Popiel D., Chełkowski J. Species diversity of Trichoderma in Poland. Journal of Applied Genetics. 2011;52:233–243. doi: 10.1007/s13353-011-0039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Chaverri P., Castlebury L.A., Overton B.E. Hypocrea/Trichoderma: species with conidiophore elongations and green conidia. Mycologia. 2003;95:1100–1140. [PubMed] [Google Scholar]
- Chaverri P., Gazis R.O., Samuels G.J. Trichoderma amazonicum, a new endophytic species on Hevea brasiliensis and H. guianensis from the Amazon basin. Mycologia. 2011;103:139–151. doi: 10.3852/10-078. [DOI] [PubMed] [Google Scholar]
- Chaverri P., Samuels G.J. [2003]. Hypocrea/Trichoderma (Ascomycota, Hypocreales, Hypocreaceae): species with green ascospores. Studies in Mycology. 2004;48:1–116. [Google Scholar]
- Chaverri P., Samuels G.J. Evolution of habitat preference and nutrition mode in a cosmopolitan fungal genus with evidence of interkingdom host jumps and major shifts in ecology. Evolution. 2013;67:2823–2837. doi: 10.1111/evo.12169. [DOI] [PubMed] [Google Scholar]
- Chaverri P., Branco-Rocha F., Jaklitsch W.M. Systematics of the Trichoderma harzianum species complex and the identification of commercial biocontrol strains. Mycologia. 2015 doi: 10.3852/14-147. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Braun U., Hunter G.C. Phylogenetic lineages in Pseudocercospora. Studies in Mycology. 2013;75:37–114. doi: 10.3114/sim0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenkolb T., Dieckmann R., Nielsen K.F. The Trichoderma brevicompactum clade: a separate lineage with new species, new peptaibiotics, and mycotoxins. Mycological Progress. 2008;7:177–219. [Google Scholar]
- Doi Y. Revision of the Hypocreales with cultural observations IV. The genus Hypocrea and its allies in Japan. (2) Enumeration of the species. Bulletin of the National Science Museum Tokyo, series B. 1972;15:649–751. [Google Scholar]
- Druzhinina I.S., Komoń-Zelazowska M., Kredics L. Alternative reproductive strategies of Hypocrea orientalis and genetically close but clonal Trichoderma longibrachiatum, both capable of causing invasive mycoses of humans. Microbiology. 2008;154:3447–3459. doi: 10.1099/mic.0.2008/021196-0. [DOI] [PubMed] [Google Scholar]
- Druzhinina I.S., Komoń-Zelazowska M., Ismaiel A. Molecular phylogeny and species delimitation in the section Longibrachiatum of Trichoderma. Fungal Genetics and Biology. 2012;49:358–368. doi: 10.1016/j.fgb.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzhinina I.S., Kubicek C.P., Komon-Zelazowska M. The Trichoderma harzianum demon: complex speciation history resulting in coexistence of hypothetical biological species, recent agamospecies and numerous relict lineages. BMC Evolutionary Biology. 2010;10:94. doi: 10.1186/1471-2148-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliner G.N. Southern European glacial refugia: a tale of tales. Taxon. 2011;60:365–372. [Google Scholar]
- García Blázquez G., Göker M., Voglmayr H. Phylogeny of Peronospora, parasitic on Fabaceae, based on ITS sequences. Mycological Research. 2008;112:502–512. doi: 10.1016/j.mycres.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Göker M., Voglmayr H., García Blázquez G. Species delimitation in downy mildews: the case of Hyaloperonospora in the light of nuclear ribosomal ITS and LSU sequences. Mycological Research. 2009;113:308–325. doi: 10.1016/j.mycres.2008.11.006. [DOI] [PubMed] [Google Scholar]
- Göker M., Voglmayr H., García Blázquez G. Molecular taxonomy of phytopathogenic fungi: a case study in Peronospora. PLoS One. 2009;4:e6319. doi: 10.1371/journal.pone.0006319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes R.R., Glienke C., Videira S.I.R. Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia. 2013;31:1–41. doi: 10.3767/003158513X666844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräfenhan T., Schroers H.J., Nirenberg H.I. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology. 2011;68:79–113. doi: 10.3114/sim.2011.68.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewald J.Z., Nakashima C., Nishikawa J. Species concepts in Cercospora: spotting the weeds among the roses. Studies in Mycology. 2013;75:115–170. doi: 10.3114/sim0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hoyos-Carvajal L., Bissett J. Biodiversity of Trichoderma in Neotropics, the dynamical processes of biodiversity – case studies of evolution and spatial distribution. In: Grillo Oscar., editor. InTech; China: 2011. ISBN: 978-953-307-772-7. [Google Scholar]
- Hoyos-Carvajal L., Orduz S., Bissett J. Genetic and metabolic biodiversity of Trichoderma from Colombia and adjacent neotropic regions. Fungal Genetics and Biology. 2009;46:615–631. doi: 10.1016/j.fgb.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W.M. European species of Hypocrea – Part I. Studies in Mycology. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M. European species of Hypocrea – Part II. Fungal Diversity. 2011;48:1–250. doi: 10.1007/s13225-011-0088-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Komon M., Kubicek C.P. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W.M., Komon M., Kubicek C.P. Hypocrea crystalligena sp. nov., a common European species with a white-spored Trichoderma anamorph. Mycologia. 2006;98:499–513. doi: 10.3852/mycologia.98.3.499. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W.M., Kubicek C.P., Druzhinina I.S. Three European species of Hypocrea with reddish brown stromata and green ascospores. Mycologia. 2008;100:796–815. doi: 10.3852/08-039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Lechat C., Voglmayr H. The rise and fall of Sarawakus (Hypocreaceae, Ascomycota) Mycologia. 2014;106:133–144. doi: 10.3852/13-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Põldmaa K., Samuels G.J. Reconsideration of Protocrea (Hypocreales, Hypocreaceae) Mycologia. 2008;100:962–984. doi: 10.3852/08-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Samuels G.J., Dodd S.L. Hypocrea rufa/Trichoderma viride: a reassessment, and description of five closely related species with and without warted conidia. Studies in Mycology. 2006;56:135–177. doi: 10.3114/sim.2006.56.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Samuels G.J., Ismaiel A. Disentangling the Trichoderma viridescens complex. Persoonia. 2013;31:112–146. doi: 10.3767/003158513X672234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Stadler M., Voglmayr H. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia. 2012;104:925–941. doi: 10.3852/11-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Hypocrea britdaniae and H. foliicola: two remarkable new European species. Mycologia. 2012;105:1213–1221. doi: 10.3852/11-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. [2013]. New combinations in Trichoderma (Hypocreaceae, Hypocreales) Mycotaxon. 2014;126:143–156. doi: 10.5248/126.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C.S., Park M.S., Kim S.C. Identification of Trichoderma, a competitor of Shiitake Mushroom (Lentinula edodes), and competition between Lentinula edodes and Trichoderma species in Korea. Plant Pathology Journal. 2012;28:137–148. [Google Scholar]
- Kim C.S., Shirouzu T., Nakagiri A. Trichoderma mienum sp. nov., isolated from mushroom farms in Japan. Antonie van Leeuwenhoek. 2012;102:629–641. doi: 10.1007/s10482-012-9758-3. [DOI] [PubMed] [Google Scholar]
- Kim C.S., Shirouzu T., Nakagiri A. Trichoderma eijii and T. pseudolacteum, two new species from Japan. Mycological Progress. 2013;12:739–753. [Google Scholar]
- Kim C.S., Yu S.H., Nakagiri A. Re-evaluation of Hypocrea pseudogelatinosa and H. pseudostraminea isolated from shiitake mushroom (Lentinula edodes) cultivation in Korea and Japan. Plant Pathology Journal. 2012;28:341–356. [Google Scholar]
- Köppen W. Das geographische System der Klimate. In: Köppen W., Geiger R., editors. Handbuch der Klimatologie. Gebrüder Borntraeger; Berlin: 1936. pp. 1–44. [Google Scholar]
- Kornerup A., Wanscher J.H. 3rd edn. Sankt Jørgen Tryk; Copenhagen: 1978. Methuen handbook of colour. [Google Scholar]
- Kubicek C.P., Herrera-Estrella A., Seidl-Seiboth V. Comparative genome sequence analysis underscores mycoparasitism as the ancestral life style of Trichoderma. Genome Biology. 2011;12:R40. doi: 10.1186/gb-2011-12-4-r40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q.-R., Tan P., Jiang Y.-L. A novel Trichoderma species isolated from soil in Guizhou, T. guizhouense. Mycological Progress. 2013;12:167–172. [Google Scholar]
- Liu Y.L., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- López-Quintero C.A., Atanasova L., Franco-Molano A.E. DNA barcoding survey of Trichoderma diversity in soil and litter of the Colombian lowland Amazonian rainforest reveals Trichoderma strigosellum sp. nov. and other species. Antonie van Leeuwenhoek. 2013;104:657–674. doi: 10.1007/s10482-013-9975-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Druzhinina I.S., Fallah P. Hypocrea/Trichoderma species with pachybasium-like conidiophores: sexual morphs for T. minutisporum and T. polysporum and their newly discovered relatives. Mycologia. 2004;96:310–342. [PubMed] [Google Scholar]
- Migheli Q., Balmas V., Komon-Zelazowska M. Soils of a Mediterranean hot spot of biodiversity and endemism (Sardinia, Tyrrhenian Islands) are inhabited by pan-European, invasive species of Hypocrea/Trichoderma. Environmental Microbiology. 2009;11:35–46. doi: 10.1111/j.1462-2920.2008.01736.x. [DOI] [PubMed] [Google Scholar]
- Mukherjee P.K., Singh U.S., Horwitz B.A. CABI; Nosworthy, Way, Wallingford, Oxon, UK: 2013. Trichoderma: biology and applications. [Google Scholar]
- Mulaw T.B., Kubicek C.P., Druzhinina I.S. The Rhizosphere of Coffea arabica in its native highland forests of Ethiopia provides a niche for a distinguished diversity of Trichoderma. Diversity. 2010;2:527–549. [Google Scholar]
- Müller E., Aebi B., Webster J. Culture studies on Hypocrea and Trichoderma V. Hypocrea psychrophila sp. Nov. Transactions of the British Mycological Society. 1972;58:1–4. [Google Scholar]
- Myers N., Mittermeier R.A., Mittermeier C.G. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- Naeimi S., Khodaparast S.A., Javan-Nikkhah M. Species patterns and phylogenetic relationships of Trichoderma strains in rice fields of Southern Caspian Sea, Iran. Cereal Research Communications. 2011;39:560–568. [Google Scholar]
- Nirenberg H.I. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium-Sektion Liseola. Mitteilungen aus der Biologischen Bundesanstalt für Land- und Forstwirtschaft Berlin-Dahlem. 1976;169: i–v:1–117. [Google Scholar]
- Overton B.E., Stewart E.L., Geiser D.M. Systematics of Hypocrea citrina and related taxa. Studies in Mycology. 2006;56:1–38. doi: 10.3114/sim.2006.56.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton B.E., Stewart E.L., Geiser D.M. Taxonomy and phylogenetic relationships of nine species of Hypocrea with anamorphs assignable to Trichoderma section Hypocreanum. Studies in Mycology. 2006;56:39–65. doi: 10.3114/sim.2006.56.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel M.C., Finlayson B.L., McMahon T.A. Updated world map of the Köppen-Geiger climate classification. Hydrology and Earth System Sciences. 2007;11:1633–1644. [Google Scholar]
- Pidwirny M. 2011. Moist mid-latitude climates with mild winters – C climate type.http://www.eoearth.org/view/article/162272 Retrieved from. [Google Scholar]
- Reisigl H. Vegetationslandschaften und Flora des Mittelmeerraumes. In: Hofrichter R., editor. Das Mittelmeer, Vol. 1. Allgemeiner Teil: Fauna, Flora, Ökologie. Spektrum-Akademischer Verlag; Heidelberg: 2001. pp. 196–257. [Google Scholar]
- Rodríguez F., Oliver J.F., Martín A. The general stochastic model of nucleotide substitution. Journal of Theoretical Biology. 1990;142:485–501. doi: 10.1016/s0022-5193(05)80104-3. [DOI] [PubMed] [Google Scholar]
- Rossman A.Y., Seifert K.A., Samuels G.J. Genera in Bionectriaceae, Hypocreaceae, and Nectriaceae (Hypocreales) proposed for acceptance or rejection. IMA Fungus. 2013;4:41–51. doi: 10.5598/imafungus.2013.04.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels G.J., Dodd S., Lu B.-S. The Trichoderma koningii aggregate species. Studies in Mycology. 2006;56:67–133. doi: 10.3114/sim.2006.56.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels G.J., Ismaiel A. Trichoderma evansii and T. lieckfeldtiae: two new T. hamatum-like species. Mycologia. 2009;101:142–156. doi: 10.3852/08-161. [DOI] [PubMed] [Google Scholar]
- Samuels G.J., Ismaiel A., Bon M.-C. Trichoderma asperellum sensu lato consists of two cryptic species. Mycologia. 2010;102:944–966. doi: 10.3852/09-243. [DOI] [PubMed] [Google Scholar]
- Samuels G.J., Ismaiel A., de Souza J. Trichoderma stromaticum and its overseas relatives. Mycological Progress. 2012;11:215–254. [Google Scholar]
- Samuels G.J., Ismaiel A., Mulaw T.B. The Longibrachiatum Clade of Trichoderma: a revision with new species. Fungal Diversity. 2012;55:77–108. doi: 10.1007/s13225-012-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels G.J., Petrini O., Kuhls K. The Hypocrea schweinitzii complex and Trichoderma sect. Longibrachiatum. Studies in Mycology. 1998;41:1–54. [Google Scholar]
- Schuster A., Schmoll M. Biology and biotechnology of Trichoderma. Applied Microbiology and Biotechnology. 2010;87:787–799. doi: 10.1007/s00253-010-2632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestro D., Michalak I. RaxmlGUI: a graphical front-end for RAxML. Organisms, Diversity & Evolution. 2012;12:335–337. [Google Scholar]
- Smith A., Beltrán C.A., Kusunoki M. Diversity of soil-dwelling Trichoderma in Colombia and their potential as biocontrol agents against the phytopathogenic fungus Sclerotinia sclerotiorum (Lib.) de Bary. Journal of General Plant Pathology. 2013;79:74–85. [Google Scholar]
- Stamatakis E. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Stamatakis E. Proceedings of the 20th International Parallel and Distributed Processing Symposium 2006, Rhodos, Greece. 2006. Phylogenetic models of rate heterogeneity: a high performance computing perspective. [Google Scholar]
- Sun R.-Y., Liu Z.-C., Fu K. Trichoderma biodiversity in China. Journal of Applied Genetics. 2012;53:343–354. doi: 10.1007/s13353-012-0093-1. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2002. PAUP*: phylogenetic analysis using parsimony (*and other methods). Version 4.0 Beta. [Google Scholar]
- Takamatsu S., Braun U., Limkaisang S. Phylogeny and taxonomy of the oak powdery mildew Erysiphe alphitoides sensu lato. Mycological Research. 2007;111:809–826. doi: 10.1016/j.mycres.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Takamatsu S., Inagaki M., Niinomi S. Comprehensive molecular phylogenetic analysis and evolution of the genus Phyllactinia (Ascomycota: Erysiphales) and its allied genera. Mycological Research. 2008;112:299–315. doi: 10.1016/j.mycres.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Taylor J.W., Jacobson D.J., Kroken S. Phylogenetic species recognition and species concepts in Fungi. Fungal Genetics and Biology. 2000;31:21–31. doi: 10.1006/fgbi.2000.1228. [DOI] [PubMed] [Google Scholar]
- Thines M., Runge F., Telle S. Phylogenetic investigations in the downy mildew genus Bremia reveal several distinct lineages and a species with a presumably exceptional wide host range. European Journal of Plant Pathology. 2010;128:81–89. [Google Scholar]
- Uzelac B. BGV Logik; Crvenih Hrastova, Cerak, Beograd: 2009. Gljive Srbije i zapadnog Balkana [Fungi of Serbia and the Balkans] 464 pp. ISBN 978-86-912677-0-4. [Google Scholar]
- Voglmayr H., Jaklitsch W.M. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae) Fungal Diversity. 2011;46:133–170. [Google Scholar]
- Voglmayr H., Montes-Borrego M., Landa B.B. Disentangling Peronospora on Papaver: species delimitation, taxonomy and host range inferred from molecular phylogeny and morphology. PLoS One. 2014;9:e96838. doi: 10.1371/journal.pone.0096838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuczkowski M., Druzhinina I., Gherbawy Y. Species pattern and genetic diversity of Trichoderma in a mid-European, primeval floodplain-forest. Microbiological Research. 2003;158:125–133. doi: 10.1078/0944-5013-00193. [DOI] [PubMed] [Google Scholar]
- Yabuki T., Miyazaki K., Okuda T. Japanese species of the Longibrachiatum Clade of Trichoderma. Mycoscience. 2014;55:196–212. [Google Scholar]
- Yamaguchi K., Tsurumi Y., Suzuki R. Trichoderma matsushimae and T. aeroaquaticum: two aero-aquatic species with Pseudaegerita-like propagules. Mycologia. 2012;104:1109–1120. doi: 10.3852/11-253. [DOI] [PubMed] [Google Scholar]
- Zachow C., Berg C., Müller H. Fungal diversity in the rhizosphere of endemic plant species of Tenerife (Canary Islands): relationship to vegetation zones and environmental factors. The ISME Journal. 2009;3:79–92. doi: 10.1038/ismej.2008.87. [DOI] [PubMed] [Google Scholar]
- Zhu Z.X., Zhuang W.Y. Trichoderma (Hypocrea) species with green ascospores from China. Persoonia. 2015;34:113–129. doi: 10.3767/003158515X686732. [DOI] [PMC free article] [PubMed] [Google Scholar]