Abstract
Isolated tremor in the elderly is commonly diagnosed as essential tremor (ET). The prevalence of tremor increases steeply with increasing age, whereas hereditary tremor is becoming less common. Moreover, late-manifesting tremor seems to be associated with dementia and earlier mortality. We hypothesize that different entities underlie tremor in the elderly. Two thousand four hundred forty-eight subjects from the Longitudinal Study of Aging Danish Twins older than 70 y answered screening questions for ET in 2001. Two thousan fifty-six (84%) participants drew Archimedes spirals to measure their tremor severity, and classical aging phenotypes were assessed. A subgroup of 276 individuals fulfilling either screening criteria for ET or being controls were personally assessed. Medications and mortality data are available. The spiral score increased with age. The spiral score correlated with tremor severity. For the whole cohort, mortality was significantly correlated with the spiral score, and higher spiral scores were associated with lower physical and cognitive functioning. Multivariate analysis identified higher spiral scores as an independent risk factor for mortality. In contrast, the ET patients did not show an increased but rather a lower mortality rate although it was not statistically significant. Consistent with a slower than normal aging, they were also physically and cognitively better functioning than controls. Because incident tremors beyond 70 y of age show worse aging parameters and mortality than controls and ET, we propose to label it ‘aging-related tremor’ (ART). This tremor starts later in life and is accompanied by subtle signs of aging both cognitively and physically. More detailed clinical features and pathogenesis warrant further assessment.
Keywords: Essential tremor, aging-related tremor, mortality, aging, predictors for mortality
Tremor in the elderly is a well-known feature but has never received adequate attention. Most studies include this condition under the umbrella of essential tremor (ET).1,2 Conversely, ET has long been considered to cover multiple diseases,3,4 and both clinical4 and pathological5 subclassifications have been proposed, but none has included tremor in the elderly as a separate entity. New data from independent sources are now challenging this view and suggest separating aging-related tremor (ART) from ET.
General consensus has been reached that ET is associated with a mild cerebellar functional deficit.6,7 Controversies, exist, however, on the question of whether a neurodegenerative process8 or functional abnormalities including possible receptor abnormalities with subsequent network changes underlie ET.3,9,10 One of the most convincing arguments in favor of a neurodegenerative origin would be a dementing process, but related findings remain controversial. Prevalence1,11 and incidence12 studies have demonstrated a cognitive decline in ET. However, such a cognitive decline was found for late-onset patients only.12,13 A shortening of life expectancy is another parameter often associated with neurodegenerative diseases: An early study of mortality in parents of ET patients suggested longevity of ET.14 Recent mortality studies found just the contrary, a significantly higher mortality in patients with ET.15 However, again late-onset tremors after the age of 65 y are showing the most convincing association with mortality and also are associated with increased frailty.16 Neuropathology is likewise controversial: The most far-reaching study17 is proposing 2 pathological patterns: a Lewy-body variant with Lewy bodies in the locus coeruleus and a cerebellar variant with a loss of Purkinje cells and increased numbers of torpedoes, enlargements of terminal buttons.18 This could be confirmed neither in a cohort of similar size19,20 nor in a smaller but clinically carefully described cohort,9 nor in the early reports since the last century.21
The most likely reason for these contradicting results is a too broad definition of ET covering multiple entities. In particular the large scatter of prevalence of ET in different studies spanning 3 orders of magnitude between 0.01% and 20.5%22 has raised this concern. Although ET increases with age, studies on hereditary ET found that hereditary tremor is fully penetrant after the age of 60 to 65 y.23,24 Therefore, hereditary tremor causing this increased prevalence beyond 60 y is unlikely, and the large increase in prevalence would therefore be attributable to sporadic cases.
Instead, we hypothesize that many of the tremors occurring late in life are not classical ET but a tremor related to aging (ART). If such a tremor variant is a sign of general aging, it should be an independent predictor of mortality and disability in epidemiological studies. We have tested these hypotheses in a large cohort of subjects older than 70 y embedded in the Danish Twin registry, 1 of the largest international twin registries.25 The first part of our study was planned to understand heritability of essential tremor with a twin study.26,27 The second part was designed to understand whether tremor measured with a spiral drawing can be used as a biomarker that is independently predicting survival and functioning. We found that a high spiral score in the whole cohort is an aging sign associated with increased mortality. However, subjects meeting the criteria of essential tremor did not exhibit such signs of motor and cognitive decline or increased mortality.
Methods
Participants
The Longitudinal Study of Aging Danish Twins (LSADT) 2001 consists of an extensive face-to-face interview performed by trained lay interviewers from the Danish National Institute of Social Research. Surveys have been conducted every second year from 1995 through 2005. In 2001, all twins aged 70± years were invited. The LSADT 2001 wave comprised 2,448 twins, of which 1,398 were single twins and 1,050 from intact pairs.28
Special Tremor Assessments During the 2001 Survey
Non-proxy participants of the 2001 survey (n = 2,357; 96%) were asked to draw an Archimedes spiral, which is a reliable29 and valid30 tool for assessing tremor and has shown sensitivity to change31; 303 (12.9%) of these had missing spiral scores. The spiral drawings were evaluated by three experienced raters and classified according to a validated scale (range, 0–10).31,32
Moreover, all participants answered seven screening questions (see Supplemental Data). A positive screening result for essential tremor was defined by the following criteria: (1) a spiral score greater than 4 (mean of two raters) or (2) previously diagnosed ET, or (3) a positive answer to two or more of the questions listed in the Supplemental Data.33
Cohort With Expert Neurological Assessment
One or both members of 142 intact twin pairs and 25 from broken pairs (one nonparticipating twin), who accepted to participate in a neurologic examination by a specialized movement disorder neurologist (D.L.), met the screening criteria for essential tremor. Because of death, subsequent refusal, or remote living location, only 276 twins (126 intact pairs and 24 single twins) were examined. Fourteen were excluded from further analyses because of diagnosed Parkinsonism, and another nine individuals were classified as uncertain, leaving 134 with a positive screening assessment and 128 controls for analyses. They were diagnosed according to the Tremr Investigation Group (TRIG)-criteria and classified as definite, probable, and possible ET, or other tremor cases2 (Supplemental Data CONSORT-flowchart, Fig. A). Nine (6.7%) were not classifiable. Standard criteria were used for the diagnosis of Parkinson’s disease34 and tremors.2 Moreover, the 276 twins also performed a second test drawing an Archimedes spiral, which was rated similarly to the first test.
Spiral Score and Tremor Severity
The spiral score is related to tremor severity and can be regarded as a surrogate parameter for tremor severity, which has been shown previously.31 A tremor score above 3 is considered to be a clinically visible tremor. When using this criterion, 25.5% of the whole cohort (leaving out certain and suspected Parkinson patients) have tremor. In the group of definite ET patients, the rating of the spiral test at first visit classified 76.5% (P < 0.01) having symptomatic tremor. This relation is an application of the Weber-Fechner law and known for several other instrumental measures.35,36 In the present cohort, we used the data of the 276 subjects who had a complete neurologic examination. Tremor severity measured with the Fahn-tremor scale was exponentially related to the spiral score30,31,35 (Supplemental Data Fig. A).
Survival, Medication, and Morbidity
By means of a unique 10-digit personal number (CPR-number), the participants were linked to the Danish Civil Registration System, which includes complete information on migration as well as deaths, and all subjects were followed for 11 y.37 On 1 January 2013, 1,562 (63.8%) of the participants had died. Of these, 103 participants died before 1 March 2002, which was the date for the startup of the visits by an expert neurologist. To avoid bias, the Cox analyses of survival in ET patients were based on observation time and deaths from 1 March 2002 until January 2013.
As part of the interview, all participants were requested to line up their medication. The indication for use as well as the names of the medicaments were carefully noted by the interviewer and were subsequently assigned the proper Anatomical Chemical Classification code (ATC-code) (http://www.whocc.no/) by a professional pharmacist. Data are available on all prescribed medications for the whole cohort. Moreover, the participants were requested to answer whether a doctor had ever diagnosed them according to a list of 46 diseases or conditions, including Parkinsonism.
Aging Phenotypes and Statistics
The LSADT is using aging phenotypes that were extensively tested for reliability and validity, such as grip strength, a cognitive functioning score, and a composite score measuring activities of daily living.28,33 Details are provided in the Supplemental Data. For statistical analysis, see the Supplemental Data.
Results
The results are displayed separated for the whole group of LSADT 2001 participants with follow-up time from 2001 and the group with expert neurological assessment with follow-up time from 2002.
Whole Cohort of LSADT 2001 Participants
The current study excludes proxy-interviewed twins, self-reported Parkinson’s patients, Parkinson’s patients identified through their second visit, and users of antiparkinsonian drugs, leaving 2,327 (95,1%) participants for further analyses (Supplemental Data CONSORT-flowchart, Fig. B).
The first question was whether tremor measured with the spiral score is increasing with increasing age. Figure 1b (and Supplemental Data Table B) shows the whole data displayed for 5-y cohorts between 70 and 90 y and the 90- to 100-y subgroup. The tremor severity measured with the spiral score is steadily and significantly increasing (P < 0.001 for each change), with the highest scores in the oldest age-group. Also, the number of patients with symptomatic tremor defined as a tremor score greater than 3 shows an increase with each age group (Fig. 1a).
FIG. 1.
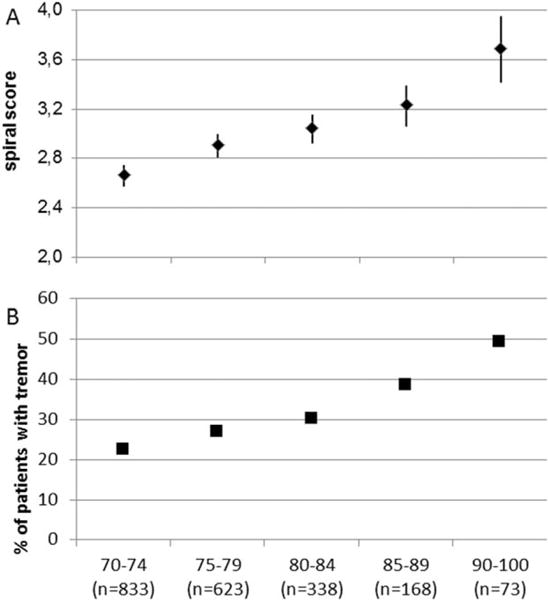
Spiral score severity and age. (A) Mean spiral scores for all subjects (men and women) with confidence limits according to age groups. The differences between all age groups are highly significant: P < 0.001). (B) Percentage of subjects with a spiral score of 4 or more, which can be considered symptomatic tremors. The percentage increases with each age group. This shows that the spiral score increases with age, and this is attributable to increasing numbers of patients with high spiral scores.
Mortality measured over the 11-y period was highly significantly dependent on the initial spiral score obtained in 2001 (Supplemental Data Table C) also when age and sex is controlled for. Kaplan-Meier curves show that this difference is found over the whole age range (Fig. 2) and depended on the severity of tremor. This result stands even if only intact twins were taken into account.
FIG. 2.
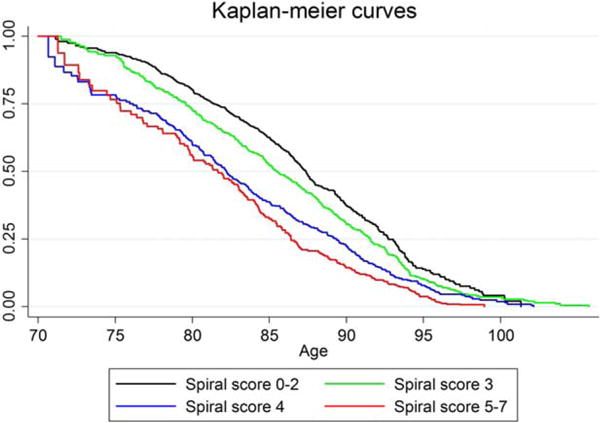
Kaplan-Meier curves for mortality in the whole cohort. Subjects with higher spiral scores have increased mortality. The reference population has a spiral score of 0 to 2 (n = 783 subjects). Subjects with a spiral score of 3 (n = 690), a score of 4 (n = 365), or 5–7 do have significantly worse hazard rations for mortality. All groups are significantly different at P < 0.01 from the reference population. This indicates that the survival of subjects is dependent on the tremor spiral score. The higher the tremor score is, the shorter is the life expectancy. Because this group is dominated by patients with aging-related tremor (ART), this shows that they have a shortened life expectancy. Instead of calculating the Kaplan-Meier curves, this also can be analyzed with hazard ratios for mortality. This shows the same result and is shown in Supplemental Data Table C.
Besides mortality, aging also can be estimated in such population with aging parameters. The LSADT study used grip strength, cognitive, or activity of daily living (see Methods). We have therefore compared these measures with the spiral score. The spiral score demonstrated a positive association with these known aging phenotypes (Table 1), that is, the presence of higher spiral scores was associated with lower grip strength measured with a dynamometer, lower cognitive functioning measured with the cognitive composite, and lower activities of daily living measured with the ADL-strength score.
TABLE 1.
Mean difference of age- and sex-adjusted physical and mental scores in participants with spiral scores > 2 compared with the reference group (spiral scores 0–2)a
| Spiral score | Nb | Grip strength (95%CI) | Cognitive composite (95%CI) | ADL Strength score (95%CI) |
|---|---|---|---|---|
| 0–2 | 783 | Reference | ||
| 3 | 690 | −0.75** (−1.36; −0.15) | −1.12** (−1.46; −0.77) | −0.09** (−0.15; −0.03) |
| 4 | 365 | −0.86** (−1.63; −0.10) | −1.58** (−2.01; −1.16) | −0.13** (−0.20; −0.06) |
| 5–7 | 191 | −2.95** (−4.09; −1.80) | −2.58** (−3.11; −2.04) | −0.35** (−0.47; −0.24) |
All differences are statistically significant (ie, P < 0.05). Confidence limits in brackets.
The numbers vary because of missing information in outcome variables.
We defined symptomatic tremor by a spiral score greater than 3. The analyses were controlled for the established aging parameters sex, age, hand grip strength, cognition, daily activities, and use of medication (Table 2). The medication that the patients have taken at the time when they drew the spiral was known, and indeed we found some medications with a significant influence on tremor such as neuroleptics and bronchodilatators (Supplemental Data Table D). Medication alone has only a small influence (Table 2, columns 1 and 2). However, the classical aging parameters hand grip strength, activities of daily living, and cognitive functioning have a stronger influence (Table 2, columns 1 and 3). However, even after correcting for all of these parameters, a significant contribution of the spiral score to mortality occurs, finally indicating that the spiral score can be used as an independent aging parameter (Table 2, column 4). Table
TABLE 2.
Hazard ratios for mortality contrasting tremor (spiral score > 3) vs. nontremor (spiral score≤3) cases
| Column 1 | Column 2 | Column 3 | Column 4 | |
|---|---|---|---|---|
|
| ||||
| Adjusted for | Age, sex | Age, sex, and medication | Age, sex, grip strength, ADL strength score, and cognitive functioning | Age, sex, grip strength, ADL strength score, cognitive functioning, and all medications |
| N | 2,029 | 2,029 | 1,946 | 1,946 |
| HR (95% CI) | 1.45 (1.28; 1.66) | 1.44 (1.26; 1.64) | 1.25 (1.09; 1.44) | 1.25 (1.09; 1.44) |
| P | <0.01 | <0.01 | <0.01 | <0.01 |
Cohort With Expert Neurological Assessment
Within the cohort with personal assessment, 36 patients had definite ET, 69 had probable ET, and 20 had other tremors. The patients with ET did not show worse mortality and aging parameters than normal subjectes like the patients with ART did. The Cox regression for mortality demonstrated a 30% reduced mortality risk (HR = 0.70 [0.46–1.06], P = 0.09) (Table 3) in the ET group compared with the large group who were not assessed by an expert neurologist. The reduced risk failed statistical significance. The results were similar (but statistically nonsignificant) when we restricted the sample to intact twin pairs (i.e. both twins participated in the LSADT 2001 survey, results not shown).
TABLE 3.
Cox regression for mortality controlled for spiral score and age at spiral scorea
| Definite ET, Probable/Possible ET, Other Tremors vs. Remaining Participants | |||
|---|---|---|---|
|
| |||
| N (Control Group) | HR (95% CI) | P Value | |
| Definite ET (n = 34) | 1,831 | 0.70 (0.46; 1.06) | 0.09 |
| Possible/probable ET (n = 65) | 0.91 (0.66; 1.25) | 0.54 | |
| Other tremors (n = 17) | 1.23 (0.69; 2.21) | 0.49 | |
Although the ET patients have a lower mortality rate, the other tremors have a trend toward higher mortality than the aging-related tremor. These are not significant findings, most likely because of the small sample size.
Definite ET performed better than the control group on classical aging phenotypes (Table 4), and the differences reached statistical significance for the cognitive score and the ADL. This is also confirmed when controlling for intact pairs only (results not shown). In contrast, the group of other tremors performed significantly worse on grip strength compared with the control group. Aging parameters for the possible/probably ET were all insignificant.
TABLE 4.
Mean difference of age- and sex-adjusted physical and mental scores in definite ET, probable/possible ET, and other tremor groupsa
| Tremor group | Nb | Nb (ref. group) | Ref
|
||
|---|---|---|---|---|---|
| Grip Strength (95% CI) | Cognitive Composite (95% CI) | ADL Strength Score (95% CI) | |||
| Definite ET | 34 | 1,913 | 1.18 (−1.04; 3.40) | 1.11c (0.17; 2.05) | 0.24c (0.09; 0.39) |
| Possible/probable ET | 65 | 0.02 (−1.92; 1.96) | 0.16 (−0.69; 1.01) | 0.11 (−0.02; 0.24) | |
| Other tremors | 17 | −2.94c (−5.24; −0.64) | −0.23 (−2.15; 1.68) | −0.39 (−0.79; 0.00) | |
ET, essential tremor; AL, activitie of daily living.
The results demonstrate that ET and probable ET patients have better aging outcomes than the control population but the other tremors not.
P< 0.05.
The numbers vary because of missing information in outcome variables.
Control group: Not ET-cases among neurological assessed and individuals who did not have a neurological assessment.
We conclude from this that definite ET does not show evidence for an age-related decline of cognitive or motor functions. Thus, we do not have the same premature aging in the group of patients with ET as we found in the ART-dominated whole LSADT 2001 cohort.
Discussion
Tremor in the elderly is not well understood and has never received much attention. Almost no formal studies have been published, and almost all of the available epidemiological studies are classifying action tremors in the elderly as “essential tremor.” Indeed, these studies show that the prevalence of ET in the elderly is strongly increasing with age. A recent review22 identified 11 studies that provide prevalence rates in different age groups. Figure 3 summarizes the prevalence rates of these studies.38–48 This meta-analysis suggests that almost 10% of subjects older than 90 y have symptomatic tremor. Whether these late-onset tremors can be considered the same disease as early-onset ET is unknown. This cannot be hereditary tremor, because ET is almost fully penetrant after the age of 65 y. Bain et al.23 found this in their 20 families with hereditary tremor at the age of 65 y, as did Larssen and Sjögren24 in their group of 169 families. Therefore, the current classification of ET would classify the incident cases above approximately 65 y as “sporadic ET.” Instead we propose to consider this condition as a separate entity, aging-related tremor. The term senile tremor has been used in the past49 but is now possibly too unspecific.
FIG. 3.
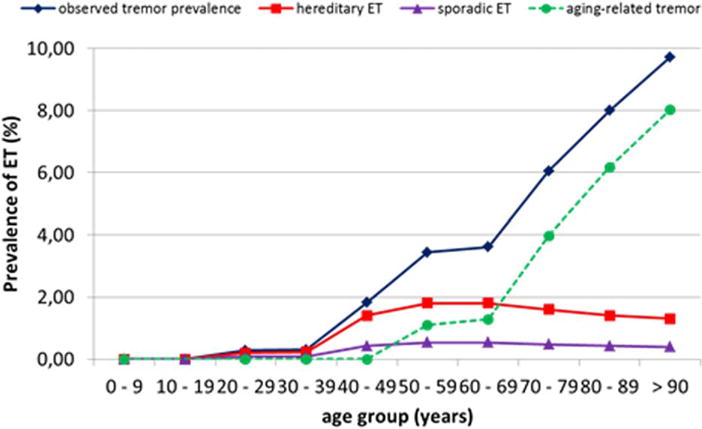
Hypothetical model of the prevalence of tremor in the community. Essential tremor (ET) is more prevalent at younger age, and hereditary ET reaches complete penetrance in the 7th decade, but the observed tremor prevalence continuously increases. The group responsible for this growth is hypothetically assumed to represent the aging-related tremor, which increases until late life. The observed tremor prevalence was calculated based on data from Louis and Ferreira22 (Supplemental Data Fig. C). The prevalence of hereditary and sporadic ET is estimated from epidemiologic studies. The aging-related tremor curve is calculated by subtracting hereditary and sporadic ET cases from the observed tremor prevalence.
The present study has contributed two main findings in favor of the existence of an ART. It has more precisely defined this ART epidemiologically. This tremor has an increasingly higher prevalence with older age and increases in severity with age. Other aging parameters show a coincident worsening with higher tremor scores. This tremor is associated with higher mortality. Tremor-producing medications increase the likelihood for symptomatic tremor, but neither medication nor the other aging parameters can explain the positive relation between tremor and mortality. Second, because only very strong survival predictors will be detectable when mortality risk is high (e.g., at ages 70±), we were surprised not to observe a similar negative effect for classical ET at ages 70+ based on a quite small sample of ET patients (n = 34), which limits strong conclusions: The average survival of classical ET patients was longer but without statistical significance, and the patients were significantly fitter measured with the classical aging parameters cognitive score and activities of daily living. This provides evidence against the notion that classical ET does have a worse prognosis than nontremor cases but certainly warrants confirmation by further studies.15 Longevity already was proposed by L. Minor almost 100 years ago50 and was also found in a previous, frequently criticized study by J. Jankovic et al.14 We interpret this to be evidence against lumping ET into the large group of subjects suffering from tremor at old age, and we provide a rationale to subdivide tremor in the elderly into the group with ET and an even larger group that we call “aging-related tremor.”
We interpret the worsening of the spiral score to represent a decline of fine motor control capabilities during aging. A slow worsening with age of the spiral score has even been found in a population-based cohort between age 20 and 60 y,51 although at a much lower scale. At older age, a significant acceleration of this aging parameter appears to occur. Aging-related tremor is an aging symptom in only a subgroup of aging persons and is not a conditio-sine-qua-non of aging. This subgroup may therefore share a common neuropathologic52 or even genetic background.
The current study has weaknesses: This is a prevalence study, and we do not have incidence data for the spiral scores. However, the substantial increase of the prevalence depending on age is unlikely because of cohort effects or reasons other than age. Two hundred seventy-six (11.7%) persons out of the cohort of 2,327 subjects received expert neurological assessment. The selection was based on the result of the spiral score and additional screening questions and included a control group with normal spiral score and negative screening questions. Having clinical data from the whole cohort would have allowed us to compare the clinical presentations of ET and ART and their relation with the spiral score measurements. Longevity of ET patients was demonstrated only when the spiral of personal assessments were combined with the ones during the first assessment, but because all of the parameters (survival, other aging parameters) were better in the ET groups, we feel encouraged to propose this statement. Also, this result for ET was found despite the larger group being likely to be “contaminated” with undetected ET cases who have a lower mortality. Therefore, the negative effect of ART on mortality is presumably even bigger than we demonstrate. Most of the examined twins were from intact pairs, and they might have a better health status compared with broken pairs. However, the results persist using various control groups including one consisting of intact pairs only. Essential tremor is a well-known, mostly autosomal dominant disease, and ART is apparently a different condition accompanying aging in a much higher percentage of the elderly.
The Distinction of Essential and Aging-Related Tremor: A New Hypothesis
Our data can probably solve the ongoing controversy between the hypothesis of ET as a disease of abnormal function without neurodegeneration3,4,53 and ET as a neurodegenerative disorder.1,12,13,15,54 The latter hypothesis is based on the epidemiological finding of a higher prevalence of dementia and earlier mortality in subjects older than 65 y.15,55 The existence of two different variants of action tremor in the elderly (ET and ART) can explain in a simple way why this is the case. Our hypothesis proposes that ET and ART are different conditions (Fig. 3). Essential tremor is considered a tremor with some minor neurological findings such as mild cerebellar abnormalities and consists of 60% to 80% hereditary and 20% to 40% sporadic cases.10,56 Incident cases with hereditary ET can only rarely be expected after the age of 65 y.23,27,57 Thus, the steep increase in the number of patients with postural tremors after the age of 65 y cannot be attributable to increasing prevalence of classical hereditary ET. It could be explained as a steep increase of sporadic ET, patients who have the same clinical picture as ET but have no family history. Because of such a strong increase in this group of tremors late in life that differs epidemiologically from hereditary ET, we believe ourselves justified to assume a separate form of tremor, which we propose to call descriptively age-related tremor.
The clinical description of patients with ART is not yet fully clear. Our present data suggest that ART is an action tremor, as revealed by the abnormal spirals. It is characterized by its late onset. A decline of aging parameters, including a change of cognition, activities of daily living, and reduction of strength and thereby a faster aging may be further hallmarks of this condition. These are all clinical findings that need to be studied prospectively in a large group of elderly subjects with tremor.
Supplementary Material
Acknowledgments
Funding agencies: The study was supported by the German Research Council (SFB 855), the National Institute on Aging grant P01 AG08761; and The Danish Agency for Science Technology and Innovation (The National Programme for Research Infrastructure).
Footnotes
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
Relevant conflicts of interest/financial disclosures: Nothing to report. Full financial disclosures and author roles may be found in the online version of this article.
References
- 1.Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 2.Deuschl G, Bain P, Brin M, Ad-Hoc-Scientific-Committee Consensus statement of the Movement Disorder Society on Tremor. Mov Disord. 1998;13(Suppl 3):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 3.Critchley M. Observations on essential (heredofamial) tremor. Brain. 1949;72(Pt. 2):113–139. doi: 10.1093/brain/72.2.113. [DOI] [PubMed] [Google Scholar]
- 4.Marsden CD, Obeso JA, Rothwell J. Benign essential tremor is not a single entity. In: Yahr MD, editor. Current Concepts of Parkinson Disease and Related Disorders. Amsterdam: Excerpta Medica; 1983. pp. 31–46. [Google Scholar]
- 5.Louis ED. Essential tremors: a family of neurodegenerative disorders? Arch Neurol. 2009;66:1202–1208. doi: 10.1001/archneurol.2009.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benito-Leon J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2:666–678. doi: 10.1038/ncpneuro0347. quiz 662p following 691. [DOI] [PubMed] [Google Scholar]
- 7.Elble R, Deuschl G. Milestones in tremor research. Mov Disord. 2011;26:1096–1105. doi: 10.1002/mds.23579. [DOI] [PubMed] [Google Scholar]
- 8.Benito-Leon J, Louis ED. Clinical update: diagnosis and treatment of essential tremor. Lancet. 2007;369:1152–1154. doi: 10.1016/S0140-6736(07)60544-3. [DOI] [PubMed] [Google Scholar]
- 9.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18:626–628. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Deuschl G, Elble R. Essential tremor: neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord. 2009;24:2033–2041. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- 11.Thawani SP, Schupf N, Louis ED. Essential tremor is associated with dementia: prospective population-based study in New York. Neurology. 2009;73:621–625. doi: 10.1212/WNL.0b013e3181b389f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bermejo-Pareja F, Louis ED, Benito-Leon J. Risk of incident dementia in essential tremor: A population-based study. Mov Disord. 2007;22:1573–1580. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 13.Benito-Leon J, Louis ED, Mitchell AJ, Bermejo-Pareja F. Elderly-onset essential tremor and mild cognitive impairment: a population-based study (NEDICES) J Alzheimers Dis. 2011;23:727–735. doi: 10.3233/JAD-2011-101572. [DOI] [PubMed] [Google Scholar]
- 14.Jankovic J, Beach J, Schwartz K, Contant C. Tremor and longevity in relatives of patients with Parkinson’s disease, essential tremor, and control subjects. Neurology. 1995;45:645–648. doi: 10.1212/wnl.45.4.645. [DOI] [PubMed] [Google Scholar]
- 15.Louis ED, Benito-Leon J, Ottman R, Bermejo-Pareja F. A population-based study of mortality in essential tremor. Neurology. 2007;69:1982–1989. doi: 10.1212/01.wnl.0000279339.87987.d7. [DOI] [PubMed] [Google Scholar]
- 16.Louis ED, Benito-Leon J, Vega S, Bermejo-Pareja F. Frailty in elderly persons with essential tremor: a population-based study (NEDICES) Eur J Neurol. 2011;18:1251–1257. doi: 10.1111/j.1468-1331.2011.03374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130:3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 18.Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136:3051–3061. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 20.Benito-Leon J, Louis ED, Sanchez-Ferro A, Bermejo-Pareja F. Rate of cognitive decline during the premotor phase of essential tremor: a prospective study. Neurology. 2013;81:60–66. doi: 10.1212/WNL.0b013e318297ef2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: a clinicopathologic study of 20 cases. Neurology. 2004;62:932–936. doi: 10.1212/01.wnl.0000115145.18830.1a. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 23.Bain PG, Findley LJ, Thompson PD, et al. A study of hereditary essential tremor. Brain. 1994;117:805–824. doi: 10.1093/brain/117.4.805. [DOI] [PubMed] [Google Scholar]
- 24.Larssen T, Sjögren T. Essential tremor: a clinical and genetic population study. Acta Psychiatr Scand. 1960;36:1–176. [PubMed] [Google Scholar]
- 25.Christensen K, Holm NV, McGue M, Corder L, Vaupel JW. A Danish population-based twin study on general health in the elderly. J Aging Health. 1999;11:49–64. doi: 10.1177/089826439901100103. [DOI] [PubMed] [Google Scholar]
- 26.Thier S, Lorenz D, Nothnagel M, et al. Polymorphisms in the glial glutamate transporter SLC1A2 are associated with essential tremor. Neurology. 2012;79:243–248. doi: 10.1212/WNL.0b013e31825fdeed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorenz D, Frederiksen H, Moises H, Kopper F, Deuschl G, Christensen K. High concordance for essential tremor in monozygotic twins of old age. Neurology. 2004;62:208–211. doi: 10.1212/01.wnl.0000103236.26934.41. [DOI] [PubMed] [Google Scholar]
- 28.McGue M, Christensen K. Social activity and healthy aging: a study of aging Danish twins. Twin Res Hum Genet. 2007;10:255–265. doi: 10.1375/twin.10.2.255. [DOI] [PubMed] [Google Scholar]
- 29.Bain PG. Clinical measurement of tremor. Mov Disord. 1998;13(Suppl 3):77–80. doi: 10.1002/mds.870131313. [DOI] [PubMed] [Google Scholar]
- 30.Haubenberger D, Kalowitz D, Nahab F, et al. Validity and reliability of computerized tremor spirography as outcome measure for clinical trials in essential tremor. Neurology. 2010;74(Suppl 2):A349. [Google Scholar]
- 31.Haubenberger D, Kalowitz D, Nahab FB, et al. Validation of digital spiral analysis as outcome parameter for clinical trials in essential tremor. Mov Disord. 2011;26:2073–2080. doi: 10.1002/mds.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bain PG, Findley LJ, Atchison P, et al. Assessing tremor severity. J Neurol Neurosurg Psychiatry. 1993;56:868–873. doi: 10.1136/jnnp.56.8.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lorenz D, Papengut F, Frederiksen H, et al. Evaluation of a screening instrument for essential tremor. Mov Disord. 2008;23:1006–1012. doi: 10.1002/mds.22010. [DOI] [PubMed] [Google Scholar]
- 34.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elble RJ, Pullman SL, Matsumoto JY, Raethjen J, Deuschl G, Tintner R. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain. 2006;129:2660–2666. doi: 10.1093/brain/awl190. [DOI] [PubMed] [Google Scholar]
- 36.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB, The Danish Civil Registration System A cohort of eight million persons. Dan Med Bull. 2006;53:441–449. [PubMed] [Google Scholar]
- 38.Hornabrook RW, Nagurney JT. Essential tremor in Papua, New Guinea. Brain. 1976;99:659–672. doi: 10.1093/brain/99.4.659. [DOI] [PubMed] [Google Scholar]
- 39.Salemi G, Savettieri G, Rocca WA, et al. Prevalence of essential tremor: a door-to-door survey in Terrasini, Sicily. Sicilian Neuro-Epidemiologic Study Group. Neurology. 1994;44:61–64. doi: 10.1212/wnl.44.1.61. [DOI] [PubMed] [Google Scholar]
- 40.Sur H, Ilhan S, Erdogan H, Ozturk E, Tasdemir M, Boru UT. Prevalence of essential tremor: a door-to-door survey in Sile, Istanbul, Turkey. Parkinsonism Relat Disord. 2009;15:101–104. doi: 10.1016/j.parkreldis.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 41.Dogu O, Sevim S, Camdeviren H, et al. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province, Turkey. Neurology. 2003;61:1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 42.Rautakorpi I, Takala J, Marttila RJ, Sievers K, Rinne UK. Essential tremor in a Finnish population. Acta Neurol Scand. 1982;66:58–67. doi: 10.1111/j.1600-0404.1982.tb03129.x. [DOI] [PubMed] [Google Scholar]
- 43.Glik A, Masarwa M, Abuful A, et al. Essential tremor might be less frequent than Parkinson’s disease in North Israel Arab villages. Mov Disord. 2009;24:119–122. doi: 10.1002/mds.22324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mancini ML, Stracci F, Tambasco N, Sarchielli P, Rossi A, Calabresi P. Prevalence of essential tremor in the territory of Lake Trasimeno, Italy: results of a population-based study. Mov Disord. 2007;22:540–545. doi: 10.1002/mds.21349. [DOI] [PubMed] [Google Scholar]
- 45.Bergareche A, De La Puente E, Lopez De Munain A, et al. Prevalence of essential tremor: a door-to-door survey in bidasoa, spain. Neuroepidemiology. 2001;20:125–128. doi: 10.1159/000054771. [DOI] [PubMed] [Google Scholar]
- 46.Louis ED, Thawani SP, Andrews HF. Prevalence of essential tremor in a multiethnic, community-based study in northern Manhattan, New York, N.Y. Neuroepidemiology. 2009;32:208–214. doi: 10.1159/000195691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18:389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 48.Louis ED, Marder K, Cote L, et al. Differences in the prevalence of essential tremor among elderly African Americans, whites, and Hispanics in northern Manhattan, NY. Arch Neurol. 1995;52:1201–1205. doi: 10.1001/archneur.1995.00540360079019. [DOI] [PubMed] [Google Scholar]
- 49.Chiu YL, Rubin DT, Vermeire S, et al. Serum adalimumab concentration and clinical remission in patients with Crohn’s disease. Inflamm Bowel Dis. 2013;19:1112–1122. doi: 10.1097/MIB.0b013e3182813242. [DOI] [PubMed] [Google Scholar]
- 50.Minor L. Über das erbliche Zittern. Zentralblatt der gesammten Neurologie und Psychiatrie. 1925;89:586–633. [Google Scholar]
- 51.Louis ED, Hafeman D, Parvez F, et al. Prevalence of essential tremor in Araihazar, Bangladesh: a population-based study. Neuroepidemiology. 2011;36:71–76. doi: 10.1159/000323389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shill HA, Adler CH, Beach TG, et al. Brain biochemistry in autopsied patients with essential tremor. Mov Disord. 2012;27:113–117. doi: 10.1002/mds.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Busenbark KL, Nash J, Nash S, Hubble JP, Koller WC. Is essential tremor benign? Neurology. 1991;41:1982–1983. doi: 10.1212/wnl.41.12.1982. [DOI] [PubMed] [Google Scholar]
- 54.Louis ED, Benito-Leon J, Vega-Quiroga S, Bermejo-Pareja F. Faster rate of cognitive decline in essential tremor cases than controls: a prospective study. Eur J Neurol. 2011;17:1291–1297. doi: 10.1111/j.1468-1331.2010.03122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benito-Leon J, Louis ED, Bermejo-Pareja F. Elderly-onset essential tremor is associated with dementia. Neurology. 2006;66:1500–1505. doi: 10.1212/01.wnl.0000216134.88617.de. [DOI] [PubMed] [Google Scholar]
- 56.Elble RJ. Diagnostic criteria for essential tremor and differential diagnosis. Neurology. 2000;54:S2–S6. [PubMed] [Google Scholar]
- 57.Larsson T, Sjogren T. Essential tremor: a clinical and genetic population study. Acta Psychiatr Scand Suppl. 1960;36:1–176. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


