Abstract
The ascomycete family Nectriaceae (Hypocreales) includes numerous important plant and human pathogens, as well as several species used extensively in industrial and commercial applications as biodegraders and biocontrol agents. Members of the family are unified by phenotypic characters such as uniloculate ascomata that are yellow, orange-red to purple, and with phialidic asexual morphs. The generic concepts in Nectriaceae are poorly defined, since DNA sequence data have not been available for many of these genera. To address this issue we performed a multi-gene phylogenetic analysis using partial sequences for the 28S large subunit (LSU) nrDNA, the internal transcribed spacer region and intervening 5.8S nrRNA gene (ITS), the large subunit of the ATP citrate lyase (acl1), the RNA polymerase II largest subunit (rpb1), RNA polymerase II second largest subunit (rpb2), α-actin (act), β-tubulin (tub2), calmodulin (cmdA), histone H3 (his3), and translation elongation factor 1-alpha (tef1) gene regions for available type and authentic strains representing known genera in Nectriaceae, including several genera for which no sequence data were previously available. Supported by morphological observations, the data resolved 47 genera in the Nectriaceae. We re-evaluated the status of several genera, which resulted in the introduction of six new genera to accommodate species that were initially classified based solely on morphological characters. Several generic names are proposed for synonymy based on the abolishment of dual nomenclature. Additionally, a new family is introduced for two genera that were previously accommodated in the Nectriaceae.
Key words: Generic concepts, Nectriaceae, Phylogeny, Taxonomy
Taxonomic novelties: New family: Tilachlidiaceae L. Lombard & Crous
New genera: Aquanectria L. Lombard & Crous; Bisifusarium L. Lombard, Crous & W. Gams; Coccinonectria L. Lombard & Crous; Paracremonium L. Lombard & Crous; Rectifusarium L. Lombard, Crous & W. Gams; Xenoacremonium L. Lombard & Crous
New species: Mariannaea humicola L. Lombard & Crous, Neocosmospora rubicola L. Lombard & Crous, Paracremonium inflatum L. Lombard & Crous, P. contagium L. Lombard & Crous, Pseudonectria foliicola L. Lombard & Crous, Rectifusarium robinianum L. Lombard & Crous, Xenoacremonium falcatus L. Lombard & Crous, Xenogliocladiopsis cypellocarpa L. Lombard & Crous
New combinations: Aquanectria penicillioides (Ingold) L. Lombard & Crous; A. submerse (H.J. Huds.) L. Lombard & Crous; Bisifusarium biseptatum (Schroers, Summerbell & O'Donnell) L. Lombard & Crous; B. delphinoides (Schroers, Summerbell, O'Donnell & Lampr.) L. Lombard & Crous; B. dimerum (Penz.) L. Lombard & Crous; B. domesticum (Fr.) L. Lombard & Crous; B. lunatum (Ellis & Everh.) L. Lombard & Crous; B. nectrioides (Wollenw.) L. Lombard & Crous; B. penzigii (Schroers, Summerbell & O'Donnell) L. Lombard & Crous; Calonectria candelabra (Viégas) Rossman, L. Lombard & Crous; C. cylindrospora (Ellis & Everh.) Rossman, L. Lombard & Crous; Clonostachys apocyni (Peck) Rossman, L. Lombard & Crous; C. aurantia (Penz. & Sacc.) Rossman, L. Lombard & Crous; C. blumenaviae (Rehm) Rossman, L. Lombard & Crous; C. gibberosa (Schroers) Rossman, L. Lombard & Crous; C. manihotis (Rick) Rossman, L. Lombard & Crous; C. parva (Schroers) Rossman, L. Lombard & Crous; C. tonduzii (Speg.) Rossman, L. Lombard & Crous; C. tornata (Höhn.) Rossman, L. Lombard & Crous; Coccinonectria pachysandricola (B.O. Dodge) L. Lombard & Crous; C. rusci (Lechat, Gardiennet & J. Fourn.) L. Lombard & Crous; Hydropisphaera fusigera (Berk. & Broome) Rossman, L. Lombard & Crous; Ilyonectria destructans (Zinssm.) Rossman, L. Lombard & Crous; I. macroconidialis (Brayford & Samuels) Rossman, L. Lombard & Crous, Mariannaea catenulatae (Samuels) L. Lombard & Crous; Nectriopsis rexiana (Sacc.) Rossman, L. Lombard & Crous; Neocosmospora ambrosia (Gadd & Loos) L. Lombard & Crous; N. falciformis (Carrión) L. Lombard & Crous; N. illudens (Berk.) L. Lombard & Crous; N. ipomoeae (Halst.) L. Lombard & Crous; N. monilifera (Berk. & Broome) L. Lombard & Crous; N. phaseoli (Burkh.) L. Lombard & Crous; N. plagianthi (Dingley) L. Lombard & Crous; N. ramosa (Bat. & H. Maia) L. Lombard & Crous; N. solani (Mart.) L. Lombard & Crous; N. termitum (Höhn.) L. Lombard & Crous; N. tucumaniae (T. Aoki, O'Donnell, Yos. Homma & Lattanzi) L. Lombard & Crous; N. virguliformis (O'Donnell & T. Aoki) L. Lombard & Crous; Neonectria candida (Ehrenb.) Rossman, L. Lombard & Crous; Penicillifer diparietisporus (J.H. Miller, Giddens & A.A. Foster) Rossman, L. Lombard & Crous; Rectifusarium ventricosum (Appel & Wollenw.) L. Lombard & Crous; Sarcopodium flavolanatum (Berk. & Broome) L. Lombard & Crous; S. mammiforme (Chardón) L. Lombard & Crous; S. oblongisporum (Y. Nong & W.Y. Zhuang) L. Lombard & Crous; S. raripilum (Penz. & Sacc.) L. Lombard & Crous; Sphaerostilbella penicillioides (Corda) Rossman, L. Lombard & Crous; S. aurifila (W.R. Gerard) Rossman, L. Lombard & Crous; Volutella asiana (J. Luo, X.M. Zhang & W.Y. Zhuang) L. Lombard & Crous; Xenoacremonium recifei (Leão & Lôbo) L. Lombard & Crous
New name: Mariannaea pinicola L. Lombard & Crous
Typification: Epitypification (basionyms): Rectifusarium ventricosum Appel & Wollenw., Xenogliocladiopsis eucalyptorum Crous & W.B. Kendr.
Introduction
The order Hypocreales (Hypocreomycetidae, Sordariomycetes, Pezizomycotina, Ascomycota) includes approximately 2 700 fungal species from 240 genera, which are divided over eight families (Kirk et al., 2008, Crous et al., 2014), with some genera still classified as incertae sedis (Lumbsch & Huhndorf 2007). Members of this order are globally found in various environments and are of great importance to agriculture and medicine. They have been extensively exploited in industrial and commercial applications (Rossman 1996). These fungi are generally characterised by the production of lightly to brightly coloured, ostiolate, perithecial ascomata, containing unitunicate asci with hyaline ascospores; asexual morphs, the form most frequently encountered in nature, are moniliaceous and phialidic (Rogerson, 1970, Samuels and Seifert, 1987, Rossman, 1996, Rossman, 2000, Rossman et al., 1999). The taxonomic importance of these asexual morphs has only been recognised relatively recently (Rossman, 2000, Seifert and Samuels, 2000). The morphology of asexual forms is often crucial for the morphological identification of these fungi.
The family Nectriaceae is characterised by uniloculate ascomata that are white, yellow, orange-red or purple. These ascomata change colour in KOH, and are not immersed in a well-developed stroma. They are associated with phialidic asexual morphs producing amerosporous to phragmosporous conidia (Rossman et al., 1999, Rossman, 2000). This family includes around 55 genera that were originally based on asexual or sexual morphs. The genera include approximately 900 species (www.mycobank.org; www.indexfungorum.org). The majority of these species are soil-borne saprobes or weak to virulent, facultative or obligate plant pathogens, while some are facultatively fungicolous or insecticolous (Rossman et al., 1999, Rossman, 2000, Chaverri et al., 2011, Gräfenhan et al., 2011, Schroers et al., 2011). Several species have also been reported as important opportunistic pathogens of humans (Chang et al., 2006, Hoog et al., 2011, Guarro, 2013) while others produce mycotoxins of medical concern (Rossman 1996).
Prior to the advent of DNA sequencing studies, most sexual morph genera recognised in the Nectriaceae were placed in Nectria sensu lato (Rehner and Samuels, 1995, Rossman et al., 1999). The genus Nectria s. str., however, is restricted to the type species N. cinnabarina with tubercularia-like asexual morphs (Rossman, 2000, Hirooka et al., 2012). Recently, several studies have treated taxonomic concepts within Nectriaceae based on multi-gene phylogenetic inference (Lombard et al., 2010a, Lombard et al., 2010b, Lombard et al., 2012, Lombard et al., 2014a, Lombard et al., 2014b, Lombard and Crous, 2012, Chaverri et al., 2011, Gräfenhan et al., 2011, Schroers et al., 2011, Hirooka et al., 2012). In these studies, well-known and important plant and human pathogenic genera have been segregated into several new genera, with some older generic names resurrected (Chaverri et al., 2011, Gräfenhan et al., 2011, Schroers et al., 2011, Hirooka et al., 2011, Hirooka et al., 2012). This has resulted in debates (Geiser et al., 2013, O'Donnell et al., 2013, Aoki et al., 2014) about the prospects for continued use of certain well-known generic names, such as Fusarium, for species of agricultural and medical importance. Several genera traditionally classified in the Nectriaceae have been excluded from these studies. In the present study, the phylogenetic relationships of most of the genera known from culture and traditionally classified as Nectriaceae are evaluated based on DNA sequences of 10 loci. The goal is to provide a phylogenetic backbone for the family Nectriaceae. Nomenclatural changes due to the implementation of the new International Code of Nomenclature for algae, fungi and plants (ICN; McNiell et al. 2012), are also considered in this study. The taxonomy of some genera is re-evaluated.
Materials and methods
Isolates
Fungal strains were obtained from the culture collection of the CBS-KNAW Fungal Biodiversity Centre (CBS), Utrecht, The Netherlands and the working collection of Pedro W. Crous housed at the CBS (Table 1).
Table 1.
Details of strains included in the phylogenetic analyses. GenBank accessions numbers in italics were newly generated in this study.
T Ex-type and ex-epitype cultures.
AR: Collection of A.Y. Rossman; ATCC: American Type Culture Collection, U.S.A.; BBA: Biologische Bundesanstalt für Land- und Forstwirtschaft, Berlin-Dahlem, Germany; CBS: CBS-KNAW Fungal Biodiversity Centre, Utrecht, The Netherlands; CMW: Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa; CCT: Colecao de Culturas Tropical, Fundacao Tropical de Pesquisas e Technologia “André Tosello”, Campinas-SP, Brazil; CDC: Centers for Disease Control and Prevention, Atlanta, GA, USA; CLL: C. Lechat collection; CPC: P.W. Crous collection; CTR: C.T. Rogerson collection; DAOM: Agriculture and Agri-Food Canada National Mycological Herbarium, Canada; DSM: Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH, Braunschweig, Germany; FMR: Facultad de Medicina, Reus, Tarragona, Spain; GJS: Gary J. Samuels collection; HJS: Hans-Josef Schroers collection; HKUCC: University of Hong Kong Culture Collection, Department of Ecology and Biodiversity, Hong Kong, China; IFO: Institute for Fermentation, Osaka, Yodogawa-ku, Osaka, Japan; IHEM: Institute of Hygiene and Epidemiology-Mycology Laboratory, Brussels, Belguim; IMI: International Mycological Institute, CABI-Bioscience, Egham, Bakeham Lane, U.K.; IMUR: Institute of Mycology, University of Recife, Recife, Brazil; INIFAT: INIFAT Fungus Collection, Ministerio de Agricultura Habana; KAS: K.A. Seifert collection; MRC: National Research Institute for Nutritional Diseases, Tygerberg, South Africa; MUCL: Mycothèque de l’Université Catholique de Louvain, Belgium; NBRC: NITE Biological Resource Center, Japan; NRRL: Agricultural Research Service Culture Collection, USA; PD: Collection of the Dutch National Plant Protection Organization (NPPO-NL), Wageningen, The Netherlands; PPRI: Plant Protection Research Institute, Pretoria, South Africa; PREM: National collection of Fungi, Agriculture Department, Pretoria, South Africa; QM: Quatermaster Research and Development Center, US Army, Natick, MA, USA; TG: T. Gräfenhan collection; UAMH: University of Alberta Mold Herbarium and Culture collection, Edmonton, Canada; UFV: Universidade Federal de Viçosa, Brazil.
acl1: large subunit of the ATP citrate lyase; act: α-actin; cmdA: calmodulin; his3: histone H3; ITS: the internal transcribed spacer region and intervening 5.8S nrRNA; LSU: 28S large subunit; rpb1: RNA polymerase II largest subunit; rpb2: RNA polymerase II second largest subunit; tef1: translation elongation factor 1-alpha; tub2: β-tubulin.
DNA isolation, amplification and analyses
Total genomic DNA was extracted from 7-d-old single-conidial cultures growing on 2 % (w/v) malt extract agar (MEA) using the method of Damm et al. (2008). Partial gene sequences were determined for the 28S large subunit (LSU) nrDNA, the internal transcribed spacer region and intervening 5.8S nrRNA gene (ITS), the large subunit of the ATP citrate lyase (acl1), the RNA polymerase II largest subunit (rpb1), RNA polymerase II second largest subunit (rpb2), β-tubulin (tub2), histone H3 (his3), translation elongation factor 1-alpha (tef1), calmodulin (cmdA) and α-actin (act) using the primers and PCR protocols listed in Table 2. Integrity of the sequences was ensured by sequencing the amplicons in both directions using the same primer pairs as were used for amplification. A consensus sequence for each locus was assembled in MEGA v. 6 (Tamura et al. 2013) and additional sequences were obtained from GenBank (Table 1). Subsequent alignments for each locus were generated in MAFFT v. 7 (Katoh & Standley 2013) and manually corrected where necessary. Phylogenetic congruency of the 10 loci was tested using a 70 % reciprocal bootstrap criterion (Mason-Gamer & Kellogg 1996).
Table 2.
Information on loci used in the phylogenetic analyses.
| Locus1 | Primers | Nucleotide substitution models | Included sites (# excluded sites) | Phylogenetically informative sites (%) | Uninformative polymorphic sites | Invariable sites |
|---|---|---|---|---|---|---|
| acl1 | acl1-230up, acl1-1220low (Gräfenhan et al. 2011) | HKY+I+G | 1620 (1103) | 1281 (79 %) | 235 | 104 |
| act | ACT-512F (Carbone & Kohn 1999), ACT1Rd (Groenewald et al. 2013) | GTR+I+G | 985 (476) | 551 (56 %) | 114 | 320 |
| cmdA | CAL-228F (Carbone & Kohn 1999), CAL2Rd (Groenewald et al. 2013) | GTR+I+G | 1209 (846) | 919 (76 %) | 103 | 187 |
| his3 | CYLH3F, CYLH3R (Crous et al. 2004b) | GTR+I+G | 788 (431) | 530 (67 %) | 97 | 161 |
| ITS | ITS5, ITS4 (White et al. 1990) | GTR+I+G | 1008 (572) | 619 (61 %) | 184 | 205 |
| LSU | LR0R (Rehner & Samuels 1994), LR5 (Vilgalys & Hester 1990) | GTR+I+G | 874 (6) | 316 (36 %) | 101 | 457 |
| rpb1 | RPB1-Ac, RPB1-Cr (Matheny et al. 2002) | GTR+I+G | 1489 (879) | 1264 (85 %) | 202 | 23 |
| rpb2 | RPB2-5F2, RPB2-7cR (O'Donnell et al. 2007) | GTR+I+G | 1366 (557) | 919 (67 %) | 399 | 48 |
| tef1 | EF1-728F (Carbone & Kohn 1999), EF2 (O'Donnell et al. 1998) | GTR+I+G | 1049 (850) | 854 (81 %) | 101 | 94 |
| tub2 | T1 (O'Donnell & Cigelnik 1997), CYLTUB1R (Crous et al. 2004b) | GTR+I+G | 898 (561) | 650 (72 %) | 72 | 176 |
acl1: large subunit of the ATP citrate lyase; act: α-actin; cmdA: calmodulin; his3: histone H3; ITS: the internal transcribed spacer region and intervening 5.8S nrRNA; LSU: 28S large subunit; rpb1: RNA polymerase II largest subunit; rpb2: RNA polymerase II second largest subunit; tef1: translation elongation factor 1-alpha; tub2: β-tubulin.
Phylogenetic analyses were based on Bayesian inference (BI) and Maximum Likelihood (ML). For both analyses, the evolutionary model for each partition was determined using MrModeltest (Nylander 2004) and incorporated into the analyses. For the BI analysis, the software package BEAST v. 8.0 (Drummond et al. 2012) was used. The phylogenetic relationships were estimated by performing six independent repetitions of 100 M generations each, with sampling at every 1 000th generation. The Yule speciation algorithm with GTR substitution model and a lognormal uncorrelated relaxed clock were selected for the data. LogCombiner v. 8.0 (from the BEAST package) was used to combine the outputs of six independent runs. The resulting trees were summarised using Tree Annotator v. 1.8.0 (from the BEAST package) using the maximum clade credibility option. FigTree v. 1.4 was used to visualise the final tree.
The ML analysis was performed using RAxML v. 8.0.9 (randomised accelerated (sic) maximum likelihood for high performance computing; Stamatakis 2014) through the CIPRES website (http://www.phylo.org) to obtain a second measure of branch support. The robustness of the analysis was evaluated by bootstrap support (BS) with the number of bootstrap replicates automatically determined by the software. All novel sequences generated in this study were deposited in GenBank (Table 1) and the alignment(s) and tree(s) in TreeBASE.
Morphology
For morphological characterisation, single-conidial isolates were grown on synthetic nutrient-poor agar (SNA, Nirenberg 1981) with sterile toothpicks, filter paper or carnation leaves placed on the surface of the agar. Alternatively, isolates were also plated onto potato dextrose agar (2 % w/v, PDA), oatmeal agar (OA) and malt extract agar (2 % w/v, MEA) (recipes in Crous et al. 2009) to induce sporulation when this failed on SNA. Plates were incubated at room temperature (22–25 °C) under ambient light conditions. Some isolates were incubated at 12 h / 12 h fluorescent light and darkness at 25 °C. Gross morphological characters of the asexual morphs were examined after 7–10 d by mounting fungal structures in clear lactic acid and measurements were made at ×1 000 magnification using a Zeiss Axioscope 2 microscope with differential interference contrast (DIC) illumination. The 95 % confidence levels were determined for the conidial measurements with extremes given in parentheses while only extremes are provided for other structures. Colony morphology was assessed using 7-d-old cultures on MEA, OA and/or PDA and the colour charts of Rayner (1970). All descriptions, illustrations and nomenclatural data were deposited in MycoBank (Crous et al. 2004a).
Results
Phylogenetic relationships
The multi-gene alignment length was 11 286 bases including gaps, for the 10 gene regions. The phylogenetic analyses included 206 ingroup taxa, with Stachybotrys chartarum (CBS 129.13) as an outgroup taxon. The congruence analyses detected one conflict for the placement of Rodentomyces reticulatus (CBS 128675) and Sarocladium kiliense (CBS 400.52), which could not be resolved without excluding both from the analyses. However, as these conflicts only involved the placement of single species, this was ignored and all partitions were combined following the argument of Cunningham (1997) that combining incongruent partitions could increase phylogenetic accuracy. All ambiguously aligned regions were excluded from the analyses (Table 2). The number of polymorphic and parsimony informative sites, and evolutionary model selected for each gene region are indicated in Table 2.
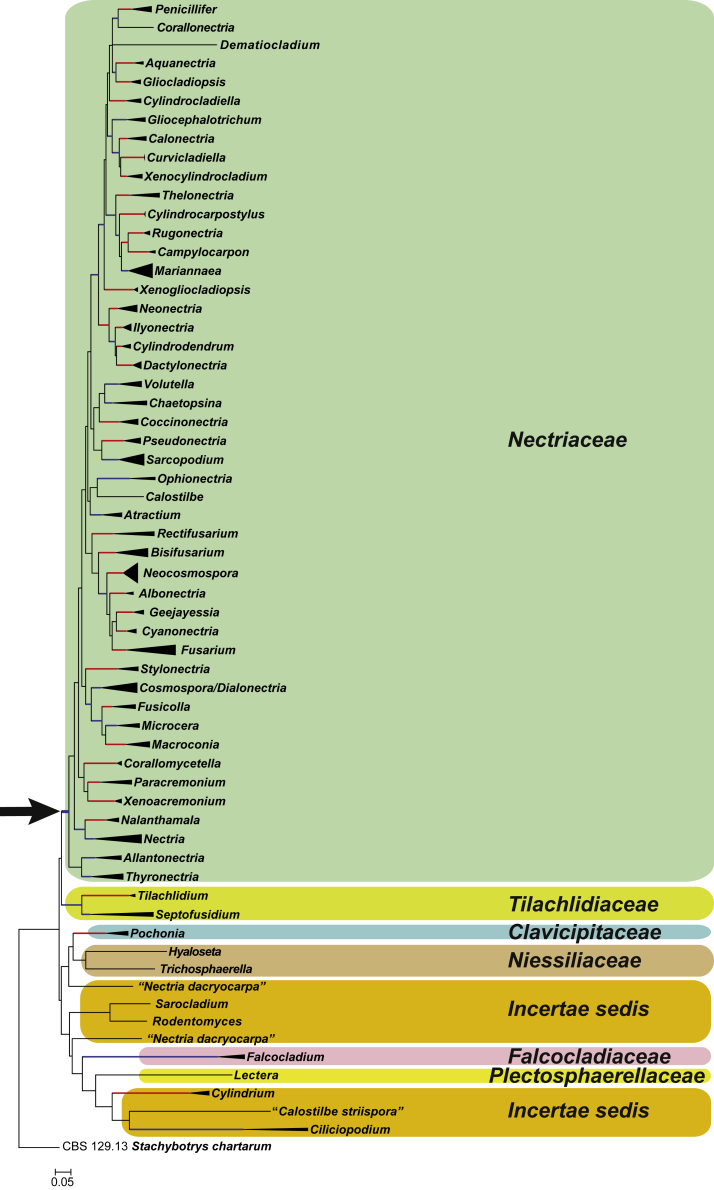
The Bayesian consensus tree confirmed the tree topology obtained from the ML analysis, and therefore only the ML consensus tree with bootstrap support values (BS) and posterior probability values (PP) are indicated for well-supported clades in Fig. 1, Fig. 2. Both Fig. 1, Fig. 2 represent the same underlying phylogenetic analyses, but are two different representations of the obtained phylogenetic tree with Fig. 1 providing a collapsed leaf overview of the genera and families, and Fig. 2 providing details at strain level. In Fig. 1, 44 well-supported clades (BS ≥ 75 %; PP ≥ 0.95) were resolved in the super-clade representing the Nectriaceae. Of these, 33 clades represent established genera with the remaining 11 clades representing possible new genera. Three separate single lineages were also resolved within the Nectriaceae super-clade, representing Corallonectria jatrophae (CBS 913.96), Calostilbe striispora (CBS 133491) and Dematiocladium celtidis (CBS 115994).
Fig. 1.
Maximum Likelihood (ML) consensus tree inferred from the combined 10 genes sequence data set providing a collapsed leaf overview of the genera and families. Thickened branches indicate branches present in both the ML and Bayesian consensus trees. Branches with BS = 100 % and PP = 1.0 are in red. Branches with BS ≥ 75 % and PP ≥ 0.95 are in blue. The tree is rooted to Stachybotrys chartarum (CBS 129.13). The arrow indicates the most basal node representing Nectriaceae.
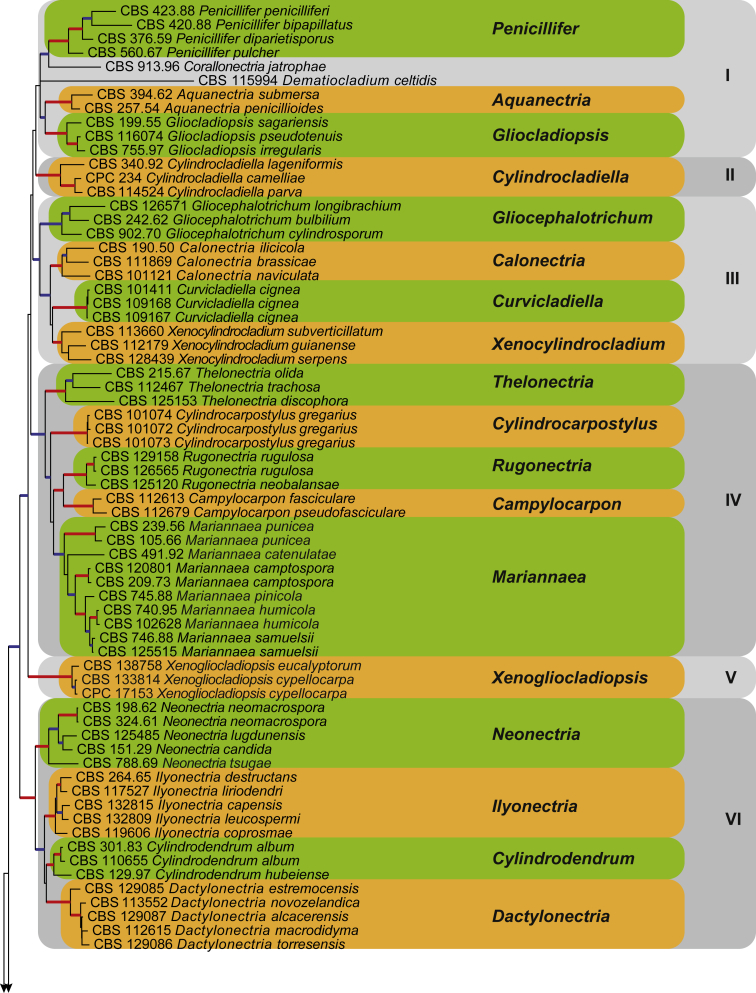
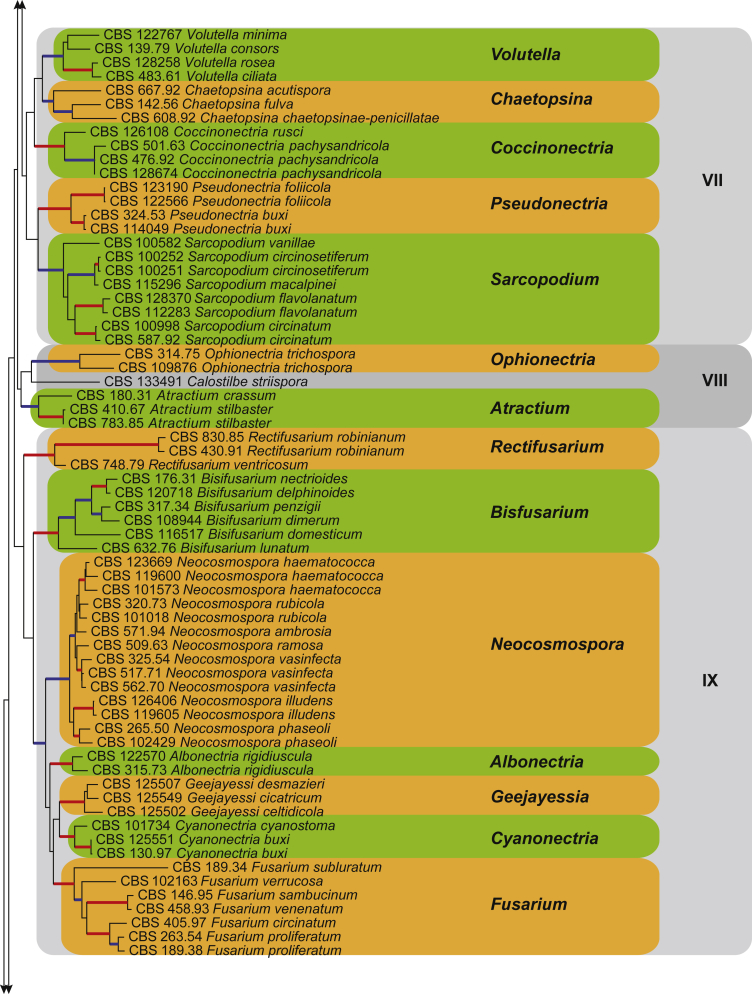
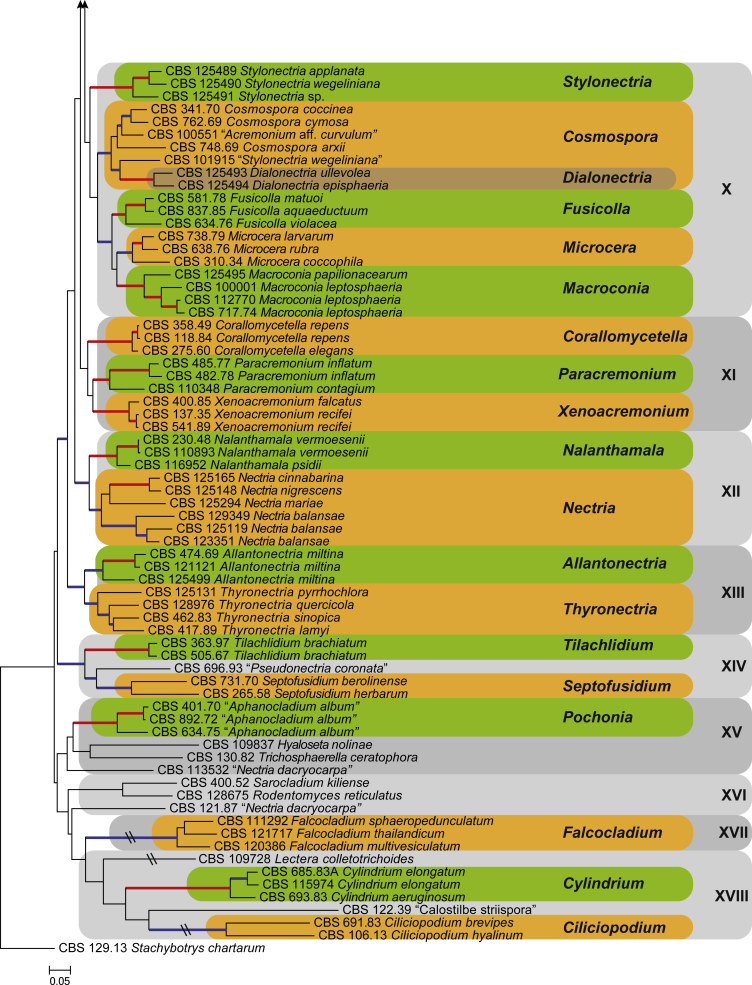
Fig. 2.
The ML consensus tree inferred from the combined 10 genes sequence data set. Thickened branches indicate branches present in both the ML and Bayesian consensus trees. Branches with BS = 100 % and PP = 1.0 are in red. Branches with BS ≥ 75 % and PP ≥ 0.95 are in blue. The tree is rooted to Stachybotrys chartarum (CBS 129.13). Clade numbers are provided to the right of the tree and these are used for reference in the Treatment of Genera section. Coloured blocks represent the accepted genera.
Several clades, representing genera traditionally classified in the Nectriaceae, resolved in well-supported sister clades (BS ≥ 75 %; PP ≥ 0.95) of the Nectriaceae super-clade. Isolates representing the species in the genera Tilachlidium (CBS 363. 97 & CBS 505. 67) and Septofusidium (CBS 265.58 & CBS 731.70), along with an isolate listed as “Pseudonectria coronata” (CBS 696.93), formed a well-supported clade (BS ≥ 75 %; PP ≥ 0.95) basal to the Nectriaceae super-clade. Representatives of the genera Aphanocladium (CBS 401.70, CBS 634.75 & CBS 892.72; BS = 100 %, PP = 1.0), Ciliciopodium (CBS 106.13 & CBS 691.83; BS ≥ 75 %, PP ≥ 0.95), Cylindrium (CBS 685.83A, CBS 693.83 & CBS 115974; BS = 100 %, PP = 1.0) and Falcocladium (CBS 111292, CBS 121717 & CBS 120386; BS ≥ 75 %, PP ≥ 0.95), each formed separate clades outside the Nectriaceae super-clade.
Treatment of genera (Fig. 2)
Based on phylogenetic inference supported by morphological observations, several novel taxa were identified in this study. Recognised clades, as well as novel families, genera and species are described and discussed below. Only generic circumscriptions are provided for known taxa where the descriptions are available in MycoBank, or in recently published scientific papers.
Clade I
Aquanectria L. Lombard & Crous, gen. nov. MycoBank MB810949.
Etymology: Name refers to the aquatic niche of these fungi.
Ascomata perithecial, superficial, scattered or aggregated in groups, ovate to subglobose, collapsing laterally when old, brown-orange to orange-red, with papillate ostiolar region. Asci cylindrical to clavate, 8-spored. Ascospores ellipsoid to fusiform, hyaline, 1-septate, with a slight constriction at the septum. Conidiophores in aquatic environment erect, solitary, septate, hyaline, branched, with verticillate penicillus with 1–4 phialides. Phialides cylindrical, tip with periclinal thickening, collarette often tubular, not flared. Conidia filiform, curved to slightly sigmoid, aseptate to 1-septate, hyaline, smooth. Chlamydospores formed intercalary, pale to dark brown, containing a large oil guttule, aggregating to form sclerotia (adapted from Ingold 1942 and Ranzoni 1956).
Type species: Aquanectria penicillioides (Ingold) L. Lombard & Crous.
Notes: The aquatic genus Aquanectria is established here to accommodate two fungal species previously treated as members of the genera Flagellospora and Heliscus (Ingold, 1942, Ranzoni, 1956, Hudson, 1961). Recent studies (Baschien et al., 2013, Duarte et al., 2015) showed that species in the aquatic genus Flagellospora belongs to the Helotiales based on the type species, F. curvula. Furthermore, Lombard et al. (2014b) synonymised the genus Heliscus, based on the type species H. lugdunensis, under the genus Neonectria. In this study, CBS 257.54 (= F. penicillioides) clustered with the ex-type strain (CBS 394.62) of Heliscus submersus in a well-supported clade (BS = 100, PP = 1.0) sister to the clade representing the genus Gliocladiopsis. Therefore, new combinations are required to accommodate these fungi in the genus Aquanectria with A. penicillioides as type.
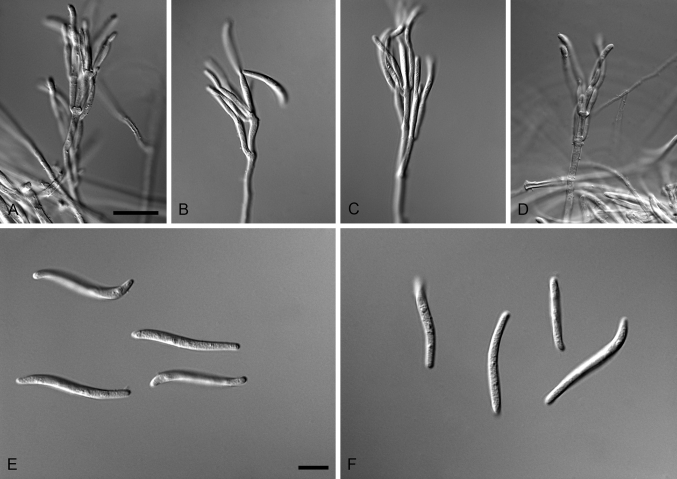
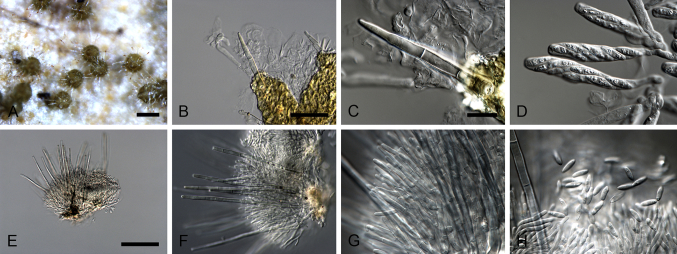
Aquanectria penicillioides (Ingold) L. Lombard & Crous, comb. nov. MycoBank MB810950. Fig. 3.
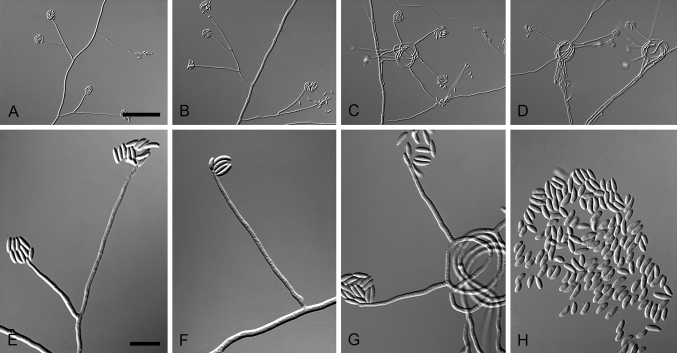
Fig. 3.
Aquanectria penicillioides (CBS 257.54). A–D. Conidiophores. E–F. Conidia. Scale bars: A = 20 μm (apply to B–D); E = 10 μm (apply to F).
Basionym: Flagellospora penicillioides Ingold, Trans. Brit. Mycol. Soc. 27: 44. 1942.
= Nectria penicillioides Ranzoni, Amer. J. Bot. 43: 17. 1956.
Material examined: USA, California, Napa County, Green Valley Falls, on decaying leaves of Acer sp. submerged in a stream, Dec. 1954, F.V. Ranzoni, culture CBS 257.54.
Descriptions and illustrations: Ingold, 1942, Ranzoni, 1956.
Aquanectria submersa (H.J. Huds.) L. Lombard & Crous, comb. nov. MycoBank MB810162.
Basionym: Heliscus submersus H.J. Huds., Trans. Brit. Mycol. Soc. 44: 91. 1961.
Material examined: Jamaica, St. Andrew, Hardwar Gap, on decaying leaves submerged in a stream, 1960, H.J. Hudson, (holotype IMI 76792 (not seen), culture ex-type CBS 394.62, sterile).
Description and illustration: Hudson (1961).
Notes: Based on the description provided by Hudson (1961), the fungus formerly known as Heliscus submersus belongs to the genus Aquanectria supported by phylogenetic inference in this study. Hudson (1961) placed this fungus in the aquatic fungal genus Heliscus, as the conidia formed two conical arms at the apex. Other members of the genus Heliscus, however, are known to produce three or more conical arms at the apex (Saccardo, 1880, Ingold, 1942, Webster, 1959). The two conical arms of A. submersa could either represent an atypical character for this species or the initiation of germination tubes at the apex of the conidia. The morphology of A. submersa could not be confirmed, as the ex-type strain could not be induced to sporulate by the addition of sterile water, carnation leaf pieces and/or toothpicks to the culture surface.
Corallonectria C. Herrera & P. Chaverri, Mycosystema 32: 539. 2013. MycoBank MB803108.
Ascomata perithecial, seated on short red stalks, in clusters of two or more, ovoid to obpyriform, not collapsing or collapsing when pinched laterally, orange-red to scarlet, with white to yellow furfuraceous coating below apex, apex acute, smooth, scarlet. Asci clavate, apex simple, 8-spored arranged biseriately. Ascospores smooth, fusiform-ellipsoid, sometimes reniform, 1-septate, often slightly constricted at septum, pale brown when discharged. Synnemata and rhizomorphs formed in culture. Synnemata cylindrical, slender to robust, straight to curved, rarely branching, appearing furfuraceous with loose, white hyphae, with a terminal cupulate capitulum, pale luteous. Rhizomorphs dichotomously branched, immersed in agar. Conidiophores unbranched or once simple monochasial or monoverticillate. Phialides cylindrical and hyaline. Conidial mass forming inside cupulate capitula, flame-shaped, luteous. Conidia fusarium-like, long-fusiform, slightly curved at the apical and basal ends, apical cell acute, basal cell pedicellate, hyaline, 3–4(–5)-septate (adapted from Herrera et al. 2013a).
Type species: Corallonectria jatrophae (A. Møller) C. Herrera & P. Chaverri, Mycosystema 32: 539. 2013. MycoBank MB803109.
≡ Corallomyces jatrophae A. Møller, Bot. Mitt. Trop. 9: 295. 1901, nom. illeg., Art. 53.
≡ Nectria jatrophae (A. Møller) Wollenw., Handb. Pflanzenkrank.: 560. 1931.
≡ Corallomycetella jatrophae (A. Møller) Rossman & Samuels, Stud. Mycol. 42: 114. 1999.
= Nectria madeirensis Henn., Hedwigia 43: 244. 1904.
= Macbridella amazonensis Bat., J.L. Bezerra & C.R. Almeida, Anais XIV Congr. Soc. Bot. Brasil: 118. 1964.
≡ Nectria amazonensis (Bat., J.L. Bezerra & C.R. Almeida) Samuels, Canad. J. Bot. 51: 1278. 1973.
Description and illustrations: Herrera et al. (2013b).
Notes: Corallonectria is a monotypic genus with C. jatrophae as type species. Our phylogeny placed the ex-type isolate (CBS 913.96) of C. jatrophae basal to the clade representing Penicillifer (= Viridispora).
Dematiocladium Allegr. et al., Mycol. Res. 109: 836. 2005. MycoBank MB28939.
Ascomatal state not known. Setae arising from pseudoparenchymatous cells in a basal stroma, adjacent to cells that give rise to conidiophore stipe, extending beyond the conidiophores; setae unbranched, straight to flexuous, brown, verruculose, thick-walled with basal cell initially smooth, becoming brown with age, tapering from a base which is either rounded and well-defined, or cylindrical and continuous with the cells in the pseudoparenchymatous stroma, to an acutely or subobtusely rounded apex, which is pale brown, thin-walled towards the apex; apical cell sometimes becoming fertile with age, forming an apical penicillate conidiophore. Conidiophores consist of a stipe, a penicillate arrangement of fertile branches, and rarely, an extension of the stipe, signifying continued growth and eventual branching of stipe and secondary penicillate conidiophores. Stipe septate, hyaline, smooth, brown at the base, arising from tightly arranged pale to medium brown pseudoparenchymous cells in a basal stroma, frequently terminating in a swollen, globose apical cell, giving rise to 1–6 primary branches. Conidiogenous apparatus branched (–4), hyaline, smooth, with terminal branches producing 1–6 phialides. Phialides elongate doliiform to reniform or subcylindrical, straight to slightly curved, aseptate; apex with minute periclinal thickening and inconspicuous collarette. Conidia cylindrical, rounded at both ends, straight, hyaline, 1(–2)-septate, lacking a visible abscission scar, held in parallel clusters by colourless slime. Chlamydospores globose, thick-walled, brown, in intercalary chains (adapted from Crous et al. 2005).
Type species: Dematiocladium celtidis Allegr. et al., Mycol. Res. 109: 836. 2005. MycoBank MB344508.
Description and illustrations: Crous et al. (2005).
Notes: Dematiocladium celtidis (ex-type CBS 115994) formed a single lineage basal to the clade representing the genus Penicillifer and the single lineage representing Corallonectria jatrophae. Recently, Crous et al. (2014) introduced a second species in this genus, D. celtidicola from China, which was not available for this study at the time.
Gliocladiopsis S.B. Saksena, Mycologia 46: 662. 1954. MycoBank MB8341.
= Glionectria Crous & C. L. Schoch, Stud. Mycol. 45: 58. 2000.
Ascomata perithecial, superficial, densely gregarious, seated on a thin basal stroma, obovoid to broadly obpyriform, collapsing laterally when drying, warted, red-brown with a dark red stromatic base, changing to dark red in KOH. Asci unitunicate, 8-spored, cylindrical, sessile, with a flattened apex, and a refractive apical apparatus. Ascospores uniseriate, overlapping, hyaline, ellipsoidal, smooth, medianly 1-septate. Conidiomata sporodochial, consisting of numerous aggregated penicillate conidiophores, or reduced to separate penicillate or subverticillate conidiophores. Conidiophores monomorphic, penicillate, consisting of a stipe and a penicillate arrangement of fertile branches, rarely dimorphic, penicillate and subverticillate. Stipe septate, hyaline, smooth. Conidiogenous apparatus with several series of aseptate or 1-septate branches, each terminal branch producing 2–6(–7) phialides. Phialides doliiform to cymbiform to cylindrical, hyaline, aseptate, apex with minute periclinal thickening and inconspicuous collarette. Conidia cylindrical, rounded at both ends, straight to curved, (0–)1-septate, lacking visible abscission scars, but frequently with a flattened base, held in fascicles by colourless slime (adapted from Saksena 1954 and Lombard & Crous 2012).
Type species: Gliocladiopsis sagariensis S.B. Saksena, Mycologia 46: 663. 1954. MycoBank MB297822.
Descriptions and illustrations: Saksena, 1954, Crous, 2002, Lombard and Crous, 2012.
Notes: Representative strains of the genus Gliocladiopsis formed a monophyletic clade (BS = 100 %, PP = 1.0) sister to the clade representing the aquatic genus Aquanectria. Interestingly, these two genera clustered together in a larger clade (BS ≥ 75 %, PP ≥ 0.95), even though they do not share the same ecological niche. Gliocladiopsis species are characteristically soil-borne (Lombard & Crous 2012). The genera do, however, share similar conidiophore morphology.
Penicillifer Emden, Acta Bot. Neerl. 17: 54. 1968. MycoBank MB9256.
= Viridispora Samuels & Rossman, Stud. Mycol. 42: 166. 1999.
Ascomata non-stromatic, superficial, solitary, globose to pyriform, red, orange-brown, tan, or brown, not reacting or changing to red in KOH, coarsely warted or glabrous. Asci clavate, apex simple. Ascospores green, 1-septate and smooth. Conidiophores erect, solitary, septate, hyaline, unbranched and monophialidic, or with a biverticillate penicillus. Phialides cylindrical, tip with periclinal thickening, collarette often tubular, not flared. Conidia cylindrical to slightly naviculate, 1-septate, hyaline, smooth, with blunt papilla at one or both ends (adapted from Samuels 1989 and Rossman et al. 1999).
Type species: Penicillifer pulcher Emden, Acta Bot. Neerl. 17: 54. 1968. MycoBank MB335703.
Descriptions and illustrations: Samuels, 1989, Polishook et al., 1991, Rossman et al., 1999.
Notes: The sexual genus Viridispora was established by Rossman et al. (1999) to accommodate species in the genera Nectria (Samuels, 1989, Watanabe, 1990) and Neocosmospora (Polishook et al. 1991) that had Penicillifer asexual morphs. Penicillifer was introduced by Emden (1968), typified by P. pulcher, for a fungus isolated from soil in the Netherlands. At present, the genus Viridispora accommodates four species, V. alata (= P. bipapillatus), V. diparietispora (= P. furcatus), V. fragariae (= P. fragariae) and V. penicilliferi (= P. macrosporus), each with its own Penicillifer asexual morphs (Samuels, 1989, Watanabe, 1990, Polishook et al., 1991, Rossman et al., 1999). So far, only P. japonicus (Matsushima 1985) has no associated sexual morph. Because the generic name Penicillifer (1968) is older than Viridispora (1999) for this monophyletic group of fungi (BS = 100 %, PP = 1.0), we propose that the sexual morph, Viridispora, be suppressed in favour of the asexual morph, Penicillifer. A new combination is, however, required for P. furcatus, as the epithet Pseudonectria diparietispora (1957) pre-dates that of Penicillifer furcatus (1991) and is provided below.
Penicillifer diparietisporus (J.H. Miller, Giddens & A.A. Foster) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810951.
Basionym: Pseudonectria diparietispora J.H. Miller, Giddens & A.A. Foster, Mycologia 49: 793. 1957 (1958, as ‘diparietospora’).
≡ Neocosmospora diparietispora (J.H. Miller, Giddens & A.A. Foster) Rossman, Samuels & Lowen, Mycologia 85: 699. 1993.
≡ Viridispora diparietispora (J.H. Miller, Giddens & A.A. Foster) Samuels & Rossman, Stud. Mycol. 42: 167. 1999.
= Neocosmospora arxii Udagawa, Horie & P. Cannon, Sydowia 41: 353. 1989.
= Neocosmospora endophytica Polishook, Bills & Rossman, Mycologia 83: 798. 1991.
= Penicillifer furcatus Polishook, Bills & Rossman, Mycologia 83: 798. 1991.
Clade II
Cylindrocladiella Boesew., Canad. J. Bot. 60: 2289. 1982. MycoBank MB7869.
= Nectricladiella Crous & C. L. Schoch, Stud. Mycol. 45: 54. 2000.
Ascomata perithecial, superficial, solitary, basal stroma absent, globose to obpyriform, collapsing laterally when dry, smooth, with several minute, brown setae arising from the perithecial wall surface, red, changing colour in KOH, ostiole consisting of clavate cells, lined with inconspicuous periphyses. Asci unitunicate, 8-spored, cylindrical, sessile, thin-walled, with a flattened apex, and a refractive apical apparatus. Ascospores uniseriate, overlapping, hyaline, ellipsoid to fusoid with obtuse ends, smooth, 1-septate. Conidiophores monomorphic, penicillate, or dimorphic (penicillate and subverticillate), mononematous, hyaline. Penicillate conidiophores consist of a stipe, a penicillate arrangement of fertile branches, a stipe extension, and a terminal vesicle. Subverticillate conidiophores consist of a stipe, and one or two series of phialides. Stipe septate, hyaline, smooth. Stipe extensions aseptate, straight, thick-walled, with one basal septum, terminating in a thin-walled vesicle of characteristic shape. Conidiogenous apparatus with primary branches aseptate to 1-septate, secondary branches aseptate, terminating in 2–4 phialides. Phialides cylindrical, straight or doliiform to reniform to cymbiform, hyaline, aseptate, apex with minute periclinal thickening and collarette. Conidia cylindrical, rounded at both ends, straight, (0–)1(–3)-septate, frequently slightly flattened at the base, held in asymmetrical clusters by colourless slime. Chlamydospores brown, thick-walled, more frequently arranged in chains than clusters (adapted from Boesewinkel 1982 and Lombard et al. 2012).
Type species: Cylindrocladiella parva (P.J. Anderson) Boesew., Canad. J. Bot. 60: 2289. 1982.
≡ Cylindrocladium parvum P.J. Anderson, Mass. Agric. Exp. Sta. Bull. 183: 37. 1919.
Descriptions and illustrations: Boesewinkel, 1982, Lombard et al., 2012.
Note: Representatives strains of the genus Cylindrocladiella formed a monophyletic clade (BS = 100 %, PP = 1.0), sister to the members of Clade I.
Clade III
Calonectria De Not., Comment. Soc. Crittog. Ital. 2: 477. 1867. MycoBank MB746.
= Cylindrocladium Morgan, Bot. Gaz. 17: 191. 1892.
= Candelospora Rea & Hawley, Proc. Roy. Irish Acad., B. 13: 11. 1912.
Ascomata perithecial, solitary or in groups, globose to subglobose to ovoid, yellow to orange to red or red-brown to brown, turning darker red to red-brown in KOH, rough-walled; perithecial apex consisting of flattened, thick-walled hyphal elements with rounded tips forming a palisade, discontinuous with warty wall, gradually becoming thinner towards the ostiolar canal, and merging with outer periphyses; perithecial base consisting of dark brown-red, angular cells, merging with a erumpent stroma, cells of the outer wall layer continuing into the pseudoparenchymatous cells of the erumpent stroma. Asci 8-spored, clavate, tapering to a long thin stalk. Ascospores aggregated in the upper third of the ascus, hyaline, smooth, fusoid with rounded ends, straight to sinuous, unconstricted, or constricted at the septa. Megaconidiophores if present, borne on the agar surface or immersed in the agar; stipe extensions mostly absent; conidiophores unbranched, terminating in 1–3 phialides, or sometimes with a single subterminal phialide; phialides straight to curved, cylindrical, seemingly producing a single conidium; periclinal thickening and an inconspicuous, divergent collarette rarely visible. Megaconidia hyaline, smooth, frequently remaining attached to the phialide, multi-septate, widest in the middle, bent or curved, with a truncated base and rounded apical cell. Macroconidiophores consist of a stipe, a penicillate arrangement of fertile branches, a stipe extension, and a terminal vesicle; stipe septate, hyaline or slightly pigmented at the base, smooth or finely verruculose; stipe extensions septate, straight to flexuous, mostly thin-walled, terminating in a thin-walled vesicle of characteristic shape. Conidiogenous apparatus with 0–1-septate primary branches; up to eight additional branches, mostly aseptate, each terminal branch producing 1–6 phialides; phialides cylindrical to allantoid, straight to curved, or doliiform to reniform, hyaline, aseptate, apex with minute periclinal thickening and inconspicuous divergent collarette. Macroconidia cylindrical, rounded at both ends, straight or curved, widest at the base, middle, or first basal septum, 1- to multi-septate, lacking visible abscission scars, held in parallel cylindrical clusters by colourless slime. Microconidiophores consist of a stipe and a penicillate or subverticillate arrangement of fertile branches. Primary branches 0–1-septate, subcylindrical; secondary branches 0–1-septate, terminating in 1–4 phialides; phialides cylindrical, straight to slightly curved, apex with minute periclinal thickening and marginal frill. Microconidia cylindrical, straight to curved, rounded at apex, flattened at base, 1(–3)-septate, held in asymmetrical clusters by colourless slime (adapted from Crous 2002).
Type species: Calonectria pyrochroa (Desm.) Sacc., Michelia 1: 308. 1878.
≡ Nectria pyrochroa Desm., Bull. Soc. Bot. France 4: 998. 1857.
= Calonectria daldiniana De Not., Comment. Soc. Crittog. Ital. 2: 477. 1867.
= Ophionectria puiggarii Speg., Bol. Acad. Nac. Ci. 11: 532. 1889.
= Nectria abnormis Henn., Hedwigia 36: 219. 1897.
= Cylindrocladium ilicicola (Hawley) Boedijn & Reitsma, Reinwardtia 1: 57. 1950.
≡ Candelospora ilicicola Hawley, Proc. Roy. Irish Acad., B. 31: 11. 1912.
Descriptions and illustrations: Rossman et al., 1999, Crous, 2002, Lombard et al., 2010b.
Notes: Representative strains of the genus Calonectria formed a monophyletic clade (BS = 100 %, PP = 1.0) closely related to the clades representing Curvicladiella and Xenocylindrocladium, respectively. Based on the ICN for algae, fungi and plants, new combinations are required for C. morganii and C. scoparia as there are older epithets available for both species.
Calonectria candelabra (Viégas) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810952.
Basionym: Cylindrocladium candelabrum Viégas, Bragantia 6: 370. 1946.
= Calonectria scoparia Ribeiro & Matsuoka, In: Ribeiro, M.Sc. Thesis, Heterotalismo em C. scoparium Morgan: 28. 1978 (nom. inval., Art. 29).
≡ Calonectria scoparia Peerally, Mycotaxon 40: 341. 1991.
Calonectria cylindrospora (Ellis & Everh.) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810953.
Basionym: Diplocladium cylindrosporum Ellis & Everh., Bull. Torrey Bot. Club 27: 58. 1900.
= Cylindrocladium scoparium Morgan, Bot. Gaz. 17: 191. 1892.
= Cylindrocladium pithecolobii Petch, Ann. Roy. Bot. Gard. (Peradeniya) 6: 244. 1917.
= Cylindrocladium ellipticum Alfieri, C.P. Seym. & Sobers, Phytopathology 60: 1213. 1970.
= Calonectria morganii Crous, Alfenas & M.J. Wingf. Mycol. Res. 97: 706. 1993.
Curvicladiella Decock & Crous, Stud. Mycol. 55: 225. 2006. MycoBank MB500866.
Ascomatal state unknown. Conidiomata sporodochial or synnematal, consisting of numerous penicillate conidiophores arising from a stroma of brown, thick-walled chlamydospores. Conidiophores consist of a thick-walled, smooth to finely verruculose, septate, pale brown to brown basal stipe, a conidiogenous apparatus and several sterile stipe extensions that have 1(–2) apical and one basal septum; stipe extensions avesiculate; apical cell thick-walled, verruculose, pale brown, prominently curved, tapering towards a bluntly rounded acute apex. Conidiogenous apparatus with several hyaline, smooth, subcylindrical, straight to slightly curved conidiophore branches; phialides hyaline, smooth, doliiform to reniform or subcylindrical, apex with minute periclinal thickening, and inconspicuous, flared collarette. Conidia cylindrical, septate, lacking a visible abscission scar, held in heads of colourless slime. Chlamydospores arranged intercalarily, often aggregating to form microsclerotia (adapted from Decock & Crous 1998 and Crous et al. 2006).
Type species: Curvicladiella cignea Decock & Crous, Stud. Mycol. 55: 225. 2006.
Descriptions and illustrations: Decock and Crous, 1998, Crous et al., 2006
Note: The monotypic genus Curvicladiella formed a well-supported clade (BS = 100 %, PP = 1.0) closely related to the genera Calonectria and Xenocylindrocladium.
Gliocephalotrichum J.J. Ellis & Hesselt., Bull. Torrey Bot. Club 89: 21. 1962. MycoBank MB8340.
Ascomata perithecial, superficial, globose to subglobose, scarlet, turning purple in KOH, with a white to pale luteous amorphous coating and hyphal stromatic base. Asci unitunicate, narrowly clavate, 8-spored, with flattened apex and a minute refractive ring. Ascospores hyaline, ellipsoidal, smooth, aseptate. Conidiophores consisting of a septate, hyaline, pale luteous to pale brown stipe and a penicillate arrangement of fertile branches subtended by septate stipe extensions. Stipe extensions hyaline, septate, terminating in narrowly to broadly clavate vesicles. Conidiogenous apparatus with a series of aseptate, hyaline to pale brown branches, each terminating in 2–8 phialides. Phialides clavate to cylindrical, hyaline, aseptate, constricted at the apex, with minute periclinal thickening. Conidia cylindrical to ellipsoidal, straight to slightly curved, aseptate, accumulating in a white to luteous mucoid mass above the phialides (adapted from Rossman et al. 1993 and Lombard et al. 2014a).
Type species: Gliocephalotrichum bulbilium J.J. Ellis & Hesselt., Bull. Torrey Bot. Club 89: 21. 1962.
Descriptions and illustrations: Rossman et al., 1993, Lombard et al., 2014a.
Notes: Species of Gliocephalotrichum are soil-borne fungi generally associated with post-harvest fruit spoilage of several important tropical fruit crops (Lombard et al. 2014a). Representatives of Gliocephalotrichum clustered in a monophyletic clade (BS ≥ 75 %, PP ≥ 0.95), basal to the clades representing Calonectria, Curvicladiella and Xenocylindrocladium.
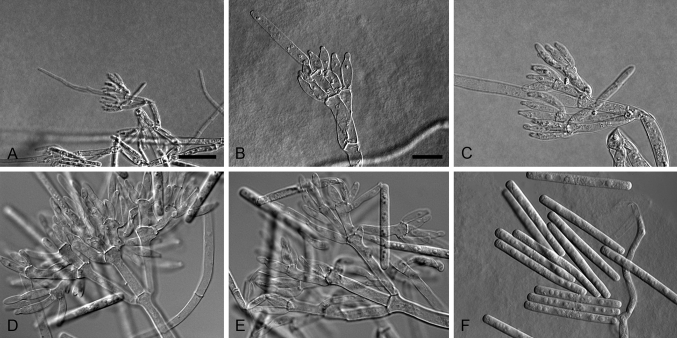
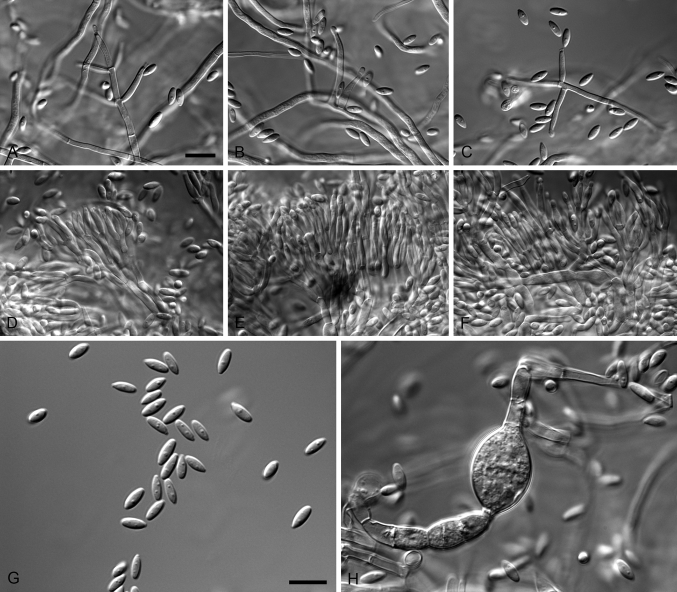
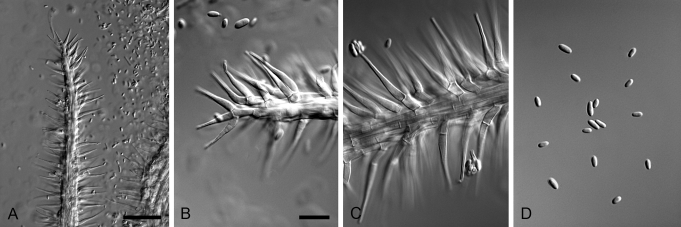
Xenocylindrocladium Decock et al., Mycol. Res. 101: 788. 1997. MycoBank MB27788. Fig. 4.
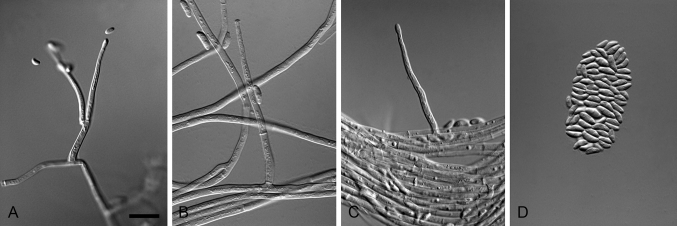
Fig. 4.
Xenocylindrocladium serpens (ex-type CBS 128439). A–C. Conidiophores. D–G. Conidiogenous apparatus with doliiform to reniform phialides. H–I. Avesiculate stipe extensions. J. Conidia. K. Chlamydospores. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–G); H = 10 μm (apply to I–K).
= Xenocalonectria Crous & C.L. Schoch, Stud. Mycol. 45: 50. 2000.
Ascomata perithecial, superficial, solitary or aggregated, globose to subglobose, warted, yellow to red and with a dark red stromatic base; ostiolar periphyses hyaline, tubular with rounded ends. Asci unitunicate, 8-spored, cylindrical, with long basal stalks, a flattened apex, and a refractive apical apparatus. Ascospores aggregate in the upper third of the ascus, hyaline, broadly to narrowly ellipsoidal, smooth, medianly 1-septate. Conidiophores consisting of a stipe, a penicillate arrangement of fertile branches, and an avesiculate stipe extension. Stipe septate, hyaline, smooth; stipe extensions septate, straight to flexuous or sinuous. Conidiogenous apparatus with aseptate or 1-septate primary branches; aseptate secondary, tertiary and quaternary branches, each terminal branch producing 2–6 phialides; phialides doliiform to reniform, hyaline, aseptate, apex with minute periclinal thickening and inconspicuous collarette. Conidia cylindrical, rounded at both ends, straight or curved, septate, lacking visible abscission scars, held in parallel cylindrical clusters by slime (adapted from Decock et al. 1997).
Type species: Xenocylindrocladium serpens Decock et al., Mycol. Res. 101: 788. 1997.
Notes: The genus Xenocylindrocladium includes three species described from the tropics, isolated from plant debris (Decock et al., 1997, Crous et al., 2001). At the same time, Decock et al. (1997) introduced the sexual morph of X. serpens as Nectria serpens, which was later transferred to the genus Xenocalonectria by Schoch et al. (2000). Given the name changes required if the genus name Xenocalonectria was used, we propose that the generic name Xenocalonectria be suppressed in favour of Xenocylindrocladium, which also has priority by date and therefore no new combinations are required. Representatives of the genus Xenocylindrocladium formed a monophyletic clade (BS = 100 %, PP = 1.0), closely related to the genera Curvicladiella and Calonectria.
Clade IV
Campylocarpon Halleen et al., Stud. Mycol. 50: 448. 2004. MycoBank MB28858.
Ascomatal state unknown. Asexual state cylindrocarpon-like. Conidiophores arise laterally from single or fasciculate aerial hyphae, carried singularly or aggregated, consisting of a stipe bearing several phialides or a penicillus of irregular branches with terminal branches bearing one or several phialides. Phialides cylindrical or narrowly flask-shaped. Macroconidia cylindrical, typically curved, (1–)3–4(–5)-septate, with minute tapering, obtuse ends, sometimes somewhat more strongly tapering at the base; base with or without an obscure hilum. Microconidia and chlamydospores not observed (adapted from Halleen et al. 2004).
Type species: Campylocarpon fasciculare Schroers et al., Stud. Mycol. 50: 448. 2004.
Description and illustrations: Halleen et al. (2004).
Notes: The monophyletic clade (BS = 100 %, PP = 1.0) representing the asexual genus Campylocarpon is closely related but separate from the clade representing the genus Rugonectria. Both these genera share several morphological characters, such as having cylindrocarpon-like asexual states. Neither is known to produce chlamydospores in culture.
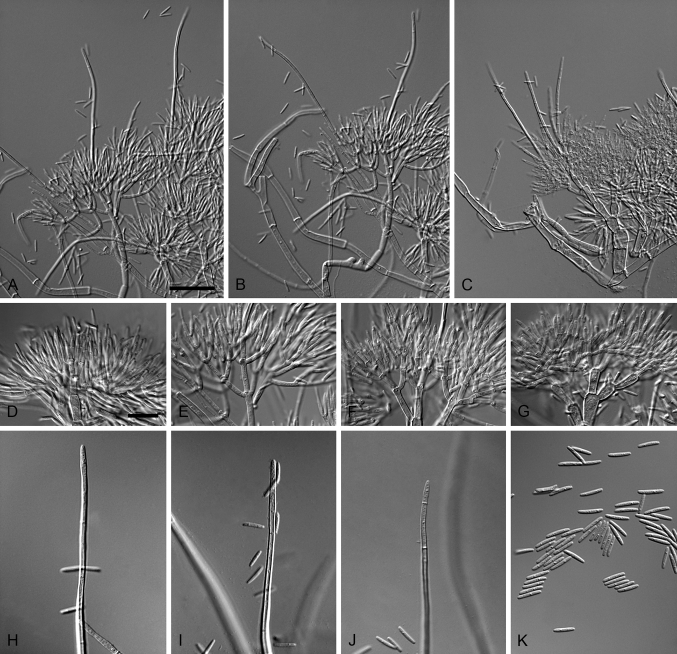
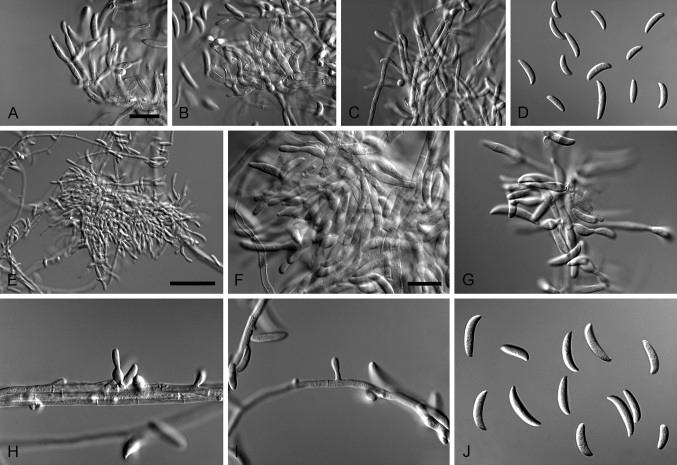
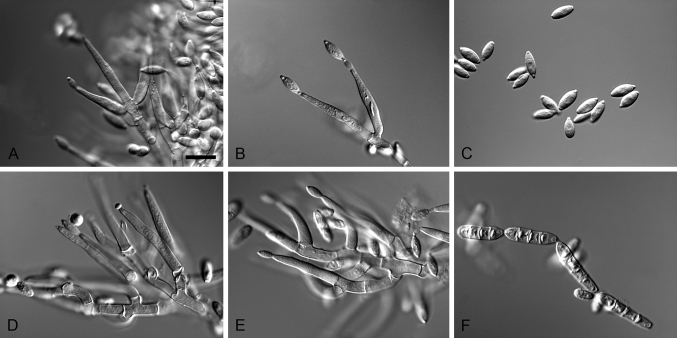
Cylindrocarpostylus R. Kirschner & Oberw., Mycol. Res. 103: 1155. 1999. MycoBank MB28330. Fig. 5.
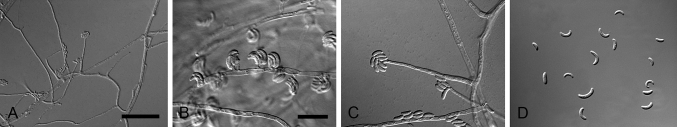
Fig. 5.
Cylindrocarpostylus gregarius (ex-type CBS 101072). A–C. Conidiophores. D–E. Conidiogenous apparatus with cylindrical to allantoid phialides. F. Conidia. Scale bars: A = 50 μm; B = 10 μm (apply to C–F).
Ascomatal state unknown. Conidiophores arise from hyphae, consisting of a stipe and penicillate arrangement of fertile branches. Stipe septate, smooth, becoming verruculose with age, initially hyaline, turning yellow to brown. Conidiogenous apparatus with aseptate primary, secondary, tertiary and quaternary branches, each terminal branch producing 2–4 phialides; phialides cylindrical to allantoid, hyaline, aseptate, apex with minute periclinal thickening and inconspicuous collarette. Conidia hyaline, smooth, cylindrical, rounded at both ends, straight or slightly curved, 0–3-septate, lacking visible abscission scars (adapted from Kirschner & Oberwinkler 1999).
Type species: Cylindrocarpostylus gregarius (Bres.) R. Kirschner & Oberw., Mycol. Res. 103: 1155. 1999.
≡ Diplocladium gregarium Bres., Ann. Mycol. 1: 127. 1903.
≡ Cylindrocladium gregarium (Bres.) de Hoog, Persoonia 10: 75. 1978.
Description and illustrations: Kirschner & Oberwinkler (1999).
Note: Representatives of the monotypic genus Cylindrocarpostylus formed a monophyletic clade (BS = 100 %, PP = 1.0), separate from all other members of Clade IV.
Mariannaea G. Arnaud ex Samson, Stud. Mycol. 6: 74. 1974. MycoBank MB8846.
Ascomata perithecial with inconspicuous or absent stroma, solitary, globose with a flat apex, not collapsing or collapsing laterally by pinching when dry, pale yellow, orange or brown, not reacting in KOH. Perithecial wall smooth or finely roughened. Asci cylindrical to narrowly clavate, sometimes with an inconspicuous apical ring, 8-spored. Ascospores 1-septate, hyaline, smooth to spinulose. Conidiophores verticillate to penicillate, hyaline, with phialides arising directly from the stipe or forming whorls of metulae on lower parts of the stipe. Stipe hyaline, becoming yellow-brown at the base. Phialides monophialidic, flask-shaped, hyaline, usually with obvious periclinal thickening and inconspicuous collarettes. Conidia aseptate, hyaline, in chains that collapse to form slimy heads. Chlamydospores globose to ellipsoidal, hyaline, formed in intercalary chains (adapted from Samson 1974).
Type species: Mariannaea elegans (Corda) Samson, Stud. Mycol. 6: 75. 1974.
≡ Penicillium elegans Corda, Icones Fung. 2: 17. 1838.
≡ Hormodendron elegans (Corda) Bonorden, Handb. Allg. Mykol.: 76. 1851.
≡ Spicaria elegans (Corda) Harz., Bull. Soc. Imp/Nat. Moscou 44: 238. 1871.
≡ Paecilomyces elegans (Corda) Mason & Hughes apud Hughes, Mycol. Pap. 45: 27. 1951.
Descriptions and illustration: Samson, 1974, Gräfenhan et al., 2011.
Note: Unfortunately no culture or sequences of M. elegans were available to be included in this phylogenetic study.
Mariannaea catenulatae (Samuels) L. Lombard & Crous, comb. nov. MycoBank MB810163.
Basionym: Chaetopsina catenulata Samuels, Mycotaxon 22: 28. 1985.
≡ Nectria chaetopsinae-catenulatae Samuels, Mycotaxon 22: 28. 1985.
≡ Cosmospora chaetopsinae-catenulatae (Samuels) Rossman & Samuels, Stud. Mycol. 42: 119. 1999.
≡ Chaetopsinectria chaetopsinae-catenulatae (Samuels) J. Luo & W.Y. Zhuang, Mycologia 102: 979. 2010.
Description and illustration: Samuels (1985).
Notes: Based on phylogenetic inference in this study, the ex-type culture CBS 491.92, previously known as Chaetopsinectria chaetopsinae-catenulatae (Samuels, 1985, Luo and Zhuang, 2010), clustered in the monophyletic clade (BS ≥ 75 %, PP ≥ 0.95) representing the genus Mariannaea. Therefore, a new combination is provided in the genus Mariannaea. This is the first study to include this ex-type strain in a molecular phylogeny.
Mariannaea pinicola L. Lombard & Crous, nom. nov. MycoBank MB810164.
≡ Nectria mariannaea Samuels & Seifert, Mycotaxon 110: 101. 2009.
≡ Nectria mariannaea Samuels & Seifert, Sydowia 43: 257. 1991. (nom. Inval., Art 23.4).
Etymology: Name derived from the plant host Pinus sp., from which it was collected.
Descriptions and illustrations: Samuels & Seifert (1991).
Notes: Gräfenhan et al. (2011) refrained from transferring Nectria mariannaea to the genus Mariannaea based on insufficient taxonomic information available at that time. As the use of the same epithet would create a tautonym (Art. 23.4), we choose to provide this species with a new epithet.
Mariannaea humicola L. Lombard & Crous, sp. nov. MycoBank MB810165. Fig. 6.
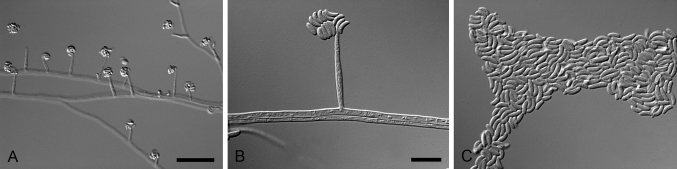
Fig. 6.
Mariannaea humicola (ex-type CBS 740.95). A–C. Conidiophores with verticillate phialides. D. Conidia. Scale bars: A = 50 μm; B = 10 μm (apply to C–D).
Etymology: Name refers to the soil substrate from which this fungus was isolated.
Ascomatal state not observed. Conidiophores arising from the agar surface from aerial hyphae or fascicles, mostly 80–100 μm long, axis 3–7 μm wide, branching verticillately at 2–3 levels, with a terminal whorl of 1–5 phialides, and 1–2 lower nodes of 1–3 phialides, rarely with single phialides. Phialides subulate, sometimes with base slightly swollen, 10–20 μm, 2–4 μm at the broadest part, with periclinal thickening and inconspicuous collarette. Conidia fusiform to ellipsoidal to obovoid, hyaline, smooth, (3–)4–6 × 2–3 μm (av. 5 × 3 μm), with a distinct hilum at both or at one end. Chlamydospores not seen.
Culture characteristics: Colonies slow growing on MEA, 45–50 mm diam in 14 d at 24 °C. Surface dirty white in the centre becoming tan to sienna towards the margins with dirty white, irregularly distributed tuffs of fascicles; aerial mycelium abundant. Reverse chestnut becoming umber at the margins.
Materials examined: Brazil, Sao Paulo, from rhizosphere soil under Araucaria angustifolia, Apr. 1995, S. Baldini (holotype CBS H-21953, culture ex-type CBS 740.95 = CCT 4534). Spain, Canary Islands, La Gomera, on decaying wood of unknown tree, Oct. 1999, R.F. Castañeda, culture CBS 102628 = INIFAT C99/130-2.
Notes: Mariannaea humicola is introduced here for two isolates (CBS 740.95 & CBS 102628), which were listed as “Nectria mariannaea” (= M. pinicola) in the CBS collection. Both isolates clustered together in a clade (BS = 100 %, PP = 1.0) separate from the ex-type culture (CBS 754.88) of M. pinicola. The conidia of M. humicola [(3–)4–6 × 2–3 μm (av. 5 × 3 μm)] are smaller than those of M. pinicola [5–9(–17) × (2–)2.5–4.5 μm; Samuels & Seifert 1991] and no chlamydospores were observed for M. humicola, which are readily formed by M. pinicola (Samuels & Seifert 1991).
Rugonectria P. Chaverri & Samuels, Stud. Mycol. 68: 73. 2011. MycoBank MB518563.
Ascomata perithecial, formed on or partially immersed within a stroma, globose to subglobose, warted, orange to red, turning dark red in KOH. Asci cylindrical to clavate, 8-spored. Ascospores 1-septate, ellipsoidal to oblong, hyaline or sometimes yellow. Asexual state cylindrocarpon-like. Microconidiophores monophialidic or sparsely branched, terminating in cylindrical phialides. Microconidia 0–1-septate, ovoid to cylindrical, with rounded ends, hyaline, lacking a prominent basal hilum. Macroconidiophores irregularly branched or in fascicles, terminating in cylindrical phialides. Macroconidia (3–)5–7(–9)-septate, fusiform, curved, tapering towards the ends with an inconspicuous basal hilum. Chlamydospores absent (adapted from Chaverri et al. 2011).
Type species: Rugonectria rugulosa (Pat. & Gaillard) Samuels et al., Stud. Mycol. 68: 73. 2011.
≡ Nectria rugulosa Pat. & Gaillard, Bull. Soc. Mycol. France 4: 115. 1888.
≡ Neonectria rugulosa (Pat. & Gaillard) Mantiri & Samuels, Canad. J. Bot. 79: 339. 2001.
= Cylindrocarpon rugulosum Brayford & Samuels, Sydowia 46: 146. 1994.
Description and illustration: Chaverri et al. (2011).
Note: Representatives of the genus Rugonectria formed a monophyletic clade (BS = 100 %, PP = 1.0), closely related but separate from the clade representing Campylocarpon.
Thelonectria P. Chaverri & C. Salgado, Stud. Mycol. 68: 76. 2011. MycoBank MB518567.
Ascomata perithecial formed superficial or seated on an immersed inconspicuous stroma, globose, subglobose, or pyriform to elongated, smooth or warted, with a prominently darkened papilla or darkly pigmented apex. Asci cylindrical and 8-spored. Ascospores 1-septate, hyaline, ellipsoidal to oblong, becoming pigmented with age. Asexual morph cylindrocarpon-like; microconidiophores and microconidia rare. Macroconidiophores irregularly branched or in fascicules, terminating in cylindrical phialides; macroconidia (3–)5–7(–9)-septate, curved, often broadest at upper third, with rounded apical cell and flattened or rounded basal cells with inconspicuous hilum. Chlamydospores rare, abundant in one species (adapted from Chaverri et al. 2011).
Type species: Thelonectria discophora (Mont.) P. Chaverri & C. Salgado, Stud. Mycol. 68: 76. 2011.
≡ Sphaeria discophora Mont., Ann. Sci. Nat., Bot. II 3: 353. 1835.
≡ Neonectria discophora (Mont.) var. discophora Mantiri & Samuels, Canad. J. Bot. 79: 339. 2001.
= Nectria tasmanica Berk. in Hooker, Flora Tasmaniae 2: 279. 1860.
= Nectria mammoidea W. Phillips & Plowr., Grevillea 3: 126. 1875.
≡ Creonectria mammoidea (W. Phillips & Plowr.) Seaver, Mycologia 1: 188. 1909.
= Nectria nelumbicola Henn., Verh. Bot. Ver. Prov. Brandenb. 40: 151. 1898.
= Nectria umbilicata Henn., Hedwigia 41: 3. 1902.
= Nectria mammoidea var. rugulosa Weese, Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1, 125: 552. 1916.
= Cylindrocarpon ianthothele var. majus Wollenw., Z. Parasitenk. 1: 161. 1928.
= Nectria mammoidea var. minor Reinking, Zentbl. Bakt. Parasitenk., Abt. II, 94: 135. 1936.
= Cylindrocarpon ianthothele var. minus Reinking, Zentbl. Bakt. Parasitenk., Abt. II, 94: 135. 1936.
= Creonectria discostiolata Chardón, Bol. Soc. Venez. Ci. Nat. 5: 341. 1939.
= Cylindrocarpon ianthothele var. rugulosum C. Booth, Mycol. Pap. 104: 25. 1966.
= Cylindrocarpon pineum C. Booth, Mycol. Pap. 104: 26. 1966.
Description and illustration: Chaverri et al. (2011).
Note: Representatives of the genus Thelonectria formed a monophyletic clade (BS = 100 %, PP = 1.0), distinct from the other member genera in Clade IV even though this genus shares some morphological characters with the genera Campylocarpon and Rugonectria.
Clade V
Xenogliocladiopsis Crous & W.B. Kendr., Canad. J. Bot. 72: 63. 1994. MycoBank MB27282.
Ascomatal state unknown. Conidiophores separate or aggregated in sporodochia, consisting of a stipe, a penicillate arrangement of fertile branches, and an avesiculate stipe extension; stipe septate, hyaline, smooth; stipe extensions septate, straight to flexuous. Conidiogenous apparatus with aseptate primary, secondary, tertiary and additional branches, each terminal branch producing 2–6 phialides. Phialides cylindrical to cymbiform, hyaline, aseptate; collarette absent. Conidia hyaline, aseptate, cylindrical to fusiform with acutely rounded ends (adapted from Crous & Kendrick 1994).
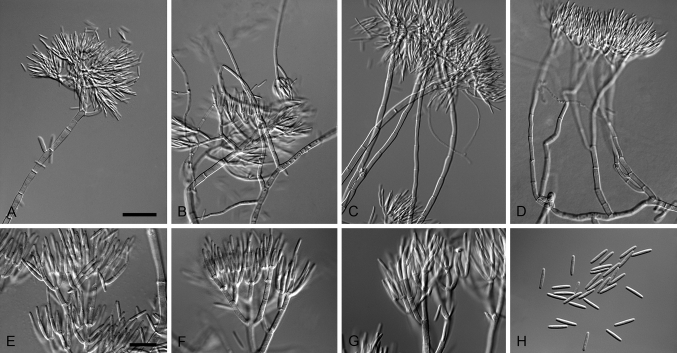
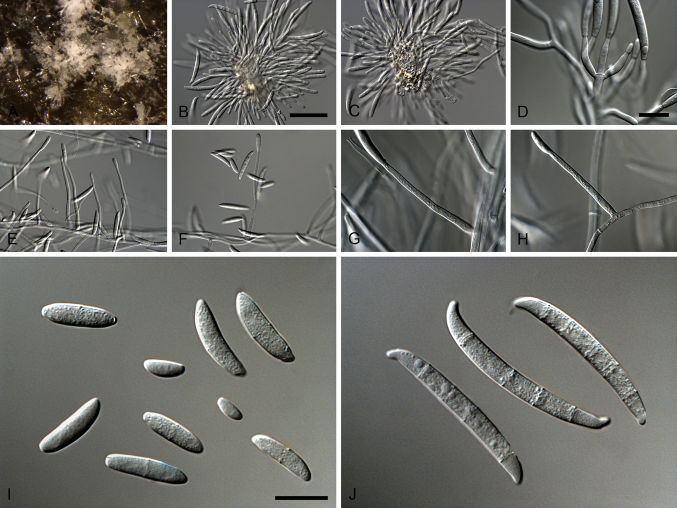
Type species: Xenogliocladiopsis eucalyptorum Crous & W.B. Kendr., Canad. J. Bot. 72: 63. 1994. Fig. 7.
Fig. 7.
Xenogliocladiopsis eucalyptorum (ex-epitype CBS 138758). A–D. Conidiophores. E–G. Conidiogenous apparatus with cylindrical to cymbiform phialides. F. Conidia. Scale bars: A = 50 μm (apply to B–D); E = 10 μm (apply to F–H).
Materials examined: South Africa, Limpopo Province, Gold River Game Resort, Eucalyptus leaf litter, May 1991, P.W. Crous, holotype PREM 51299; Northern Cape Province, Kleinzee, on leaves of Eucalyptus sp., 27 Feb. 2009, leg. Z.A. Pretorius, isol. P.W. Crous (epitype designated here CBS H-21952, MBT198395, culture ex-epitype CBS 138758 = CPC 16271).
Notes: When Crous & Kendrick (1994) introduced the asexual genus Xenogliocladiopsis based on X. eucalyptorum, they incorrectly linked it to the Dothidiomycete sexual morph Arnaudiella eucalyptorum. Phylogenetic inference in the current study clearly shows that the genus Xenogliocladiopsis belongs to the Nectriaceae, forming a well-supported clade (BS = 100 %, PP = 1.0) basal to Clades I–IV.
Xenogliocladiopsis cypellocarpa L. Lombard & Crous, sp. nov. MycoBank MB810166. Fig. 8.
Fig. 8.
Xenogliocladiopsis cypellocarpa (ex-type CBS 133814). A–C. Conidiophores. D–G. Conidiogenous apparatus with cylindrical to cymbiform phialides. H–J. Avesiculate stipe extensions. K. Conidia. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–K).
Etymology: Name derived from the plant host Eucalyptus cypellocarpa, from which it was isolated.
Ascomatal state not observed. Conidiophores hyaline, separate or aggregated in sporodochia, consisting of a stipe bearing a penicillate arrangement of fertile branches, and an avesiculate stipe extension; stipe septate, hyaline, smooth, 19–105 × 4–11 μm; stipe extension septate, straight to flexuous, 70–190 μm long, 2–4 μm wide at the apical septum. Conidiogenous apparatus 70–115 μm wide, and 65–105 μm long; primary branches aseptate, 15–30 × 3–7 μm; secondary branches aseptate, 10–20 × 2–6 μm; tertiary branches aseptate, 7–22 × 2–5 μm; quaternary branches and additional branches (–8) aseptate, 6–15 × 1–4 μm, each terminal branch producing 2–6 phialides; phialides cylindrical to cymbiform, hyaline, aseptate, 8–11 × 1–3 μm, collarette absent. Conidia cylindrical to fusiform, rounded at both ends, straight, 8–10 × 1–2 μm (av. 9 × 1 μm).
Culture characteristics: Colonies moderately fast growing on MEA, 60–80 mm diam after 10 d at 24 °C. Surface white to pale luteous with pale luteous to yellow tuffs of sporodochia forming at the margins; aerial mycelium abundant in the centre becoming immersed towards the margins, with conidiophores forming on the aerial mycelium and on the surface at the margins. Reverse similar in colour.
Material examined: Australia, Northern territories, Darwin, Kurralong Height, on leaves of Eucalyptus cypellocarpa, 25 Apr. 2011, P.W. Crous (holotype CBS H-21951, culture ex-type CBS 133814 = CPC 19417); Queensland, Slaughter Falls, on leaves of Eucalyptus sp., 16 Jul. 2009, P.W. Crous, culture CPC 17153.
Notes: Xenogliocladiopsis cypellocarpa is introduced here as a new species in the genus Xenogliocladiopsis. This species forms shorter stipe extensions (up to 190 μm) than X. eucalyptorum (up to 220 μm), and the conidia of X. cypellocarpa are also slightly smaller than those of X. eucalyptorum (7.5–11 × 1–1.5 μm; Crous & Kendrick 1994).
Clade VI
Cylindrodendrum Bonord., Handb. allg. Mykol.: 98. 1851. MycoBank MB7873.
Ascomatal state unknown. Conidiophores initially as lateral phialides on somatic hyphae, sometimes verticillate, hyaline. Phialides monophialidic, elongate doliiform to reniform to obpyriform, with the terminal part frequently having a swollen tip, apex with minute periclinal thickening and inconspicuous collarette. Conidia cylindrical, rounded at both ends, straight, 0–1-septate, with visible abscission scars (adapted from Lombard et al. 2014b).
Type species: Cylindrodendrum album Bonord., Handb. Allg. Mykol.: 48. 1851.
Description and illustrations: Lombard et al. (2014b).
Notes: Chaverri et al. (2011) suggested that the asexual morph-typified genus Cylindrodendrum could be considered as a synonym of “Cylindrocarpon”. Morphologically however, members of Cylindrodendrum more closely resemble the asexual morphs of fungal species in the genera Atractium, Cosmospora, Dialonectria, Fusicolla, Macroconia and Stylonectria, with the exception of conidium morphology (Gräfenhan et al. 2011). Based on phylogenetic inference, Cylindrodendrum isolates included in this study formed a monophyletic clade (BS = 100 %, PP = 1.0), sister to the monophyletic clade representing Dactylonectria.
Dactylonectria L. Lombard & Crous, Phytopathol. Medit. 53: 348. 2014. MycoBank MB810142.
Ascomata perithecial, superficial, solitary or aggregated in groups, ovoid to obpyriform, dark red, becoming purple-red in KOH, smooth to finely warted, with papillate apex; without recognisable stroma. Asci clavate to narrowly clavate, 8-spored; apex rounded, with a minutely visible ring. Ascospores ellipsoidal to oblong-ellipsoidal, somewhat tapering towards the ends, medianly septate, smooth to finely warted. Conidiophores simple or aggregated to form sporodochia; simple conidiophores arising laterally or terminally from aerial mycelium, solitary to loosely aggregated, unbranched or sparsely branched, septate, bearing up to three phialides. Phialides monophialidic, more or less cylindrical, tapering slightly in the upper part towards the apex. Macroconidia cylindrical, hyaline, straight to slightly curved, 1–4-septate, apex or apical cell typically slightly bent to one side and minutely beaked, base with visible, centrally located or laterally displaced hilum. Microconidia ellipsoid to ovoid, hyaline, straight, aseptate to 1-septate, with a minutely or clearly laterally displaced hilum. Chlamydospores rarely formed, globose to subglobose, smooth but often appear rough due to deposits, thick-walled, mostly occurring in chains.
Type species: Dactylonectria macrodidyma (Halleen, et al.) L. Lombard & Crous, Phytopathol. Medit. 53: 352. 2014.
≡ Neonectria macrodidyma Halleen et al., Stud. Mycol. 50: 445. 2004.
≡ Ilyonectria macrodidyma (Halleen et al.) P. Chaverri & C. Salgado, Stud. Mycol. 68: 71. 2011.
= Cylindrocarpon macrodidymum Halleen et al., Stud. Mycol. 50: 446. 2004.
Notes: Species in the genus Dactylonectria were initially regarded as members of the genus Ilyonectria. However, phylogenetic studies (Cabral et al., 2012a, Lombard et al., 2014b), showed that the genus Ilyonectria, as originally conceived, was paraphyletic. This led to the introduction of the genus Dactylonectria to accommodate Ilyonectria species isolated from grapevines (Cabral et al., 2012a, Lombard et al., 2014b). The clade representing the genus Dactylonectria (BS = 100 %, PP = 1.0) is monophyletic, and is sister to the clade representing Cylindrodendrum. Both clades are distinct from Ilyonectria.
Ilyonectria P. Chaverri & C. Salgado, Stud. Mycol. 68: 69. 2011. MycoBank MB518558.
Ascomata perithecial, superficial, solitarily or in groups, loosely attached to substrate, red, turning purple-red in KOH, globose to subglobose, or ovoid to obpyriform with a broadly conical papilla or flattened apex, scaly to slightly warted. Asci narrowly clavate or cylindrical, 8-spored; apex subtruncate, with a minutely visible ring. Ascospores ellipsoidal, 1-septate, smooth hyaline. Asexual morph cylindrocarpon-like. Conidiophores simple or complex or sporodochial. Simple conidiophores arising laterally or terminally from aerial mycelium, solitary or loosely aggregated, unbranched or sparsely branched, bearing up to three phialides. Complex conidiophores solitary or aggregated in small sporodochia, repeatedly and irregularly branched. Phialides cylindrical, tapering towards the apex. Microconidia 0–1-septate, oval to ovoid to fusiform to ellipsoid, with a minutely or clearly laterally displaced hilum, formed in heads on solitary conidiophores or as masses on sporodochia. Macroconidia straight, cylindrical, 1–3(–4)-septate, with both ends obtusely rounded, base sometimes with a visible, centrally located to laterally displaced hilum, forming flat domes of slimy masses. Chlamydospores globose to subglobose, thick-walled, intercalary or solitary, initially hyaline, becoming brown with age (adapted from Chaverri et al. 2011).
Type species: Ilyonectria destructans (Zinssm.) Rossman, L. Lombard & Crous.
Description and illustration: Chaverri et al. (2011).
Notes: Representatives of the genus Ilyonectria clustered together in a well-supported clade (BS = 100 %, PP = 1.0), distinct from the clades representing Cylindrodendrum and Dactylonectria. Chaverri et al. (2011) applied the epithet ‘radicicola’ (1963) to the type of this genus, whereas the older epithet ‘destructans’ (1918) is available. Therefore, a new combination is provided below for the type species of Ilyonectria. Furthermore, a new combination is provided for Neonectria macroconidialis, which Cabral et al. (2012a) showed to belong to this genus.
Ilyonectria destructans (Zinssm.) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810954.
Basionym: Ramularia destructans Zinssm., Phytopathology 8: 570. 1918.
≡ Cylindrocarpon destructans (Zinssm.) Scholten, Netherl. J. Plant Path. 70 suppl. (2): 9. 1964.
= Cylindrocarpon radicicola Wollenw., Fus. Autogr. Del. 2: 651. 1924.
= Nectria radicicola Gerlach & L. Nilsson, Phytopathol. Z. 48: 225. 1963.
≡ Neonectria radicicola (Gerlach & L. Nilsson) Mantiri & Samuels, Canad. J. Bot. 79: 339. 2001.
≡ Ilyonectria radicicola (Gerlach & L. Nilsson) P. Chaverri & C. Salgado, Stud. Mycol. 68: 71. 2011.
Ilyonectria macroconidialis (Brayford & Samuels) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810955.
Basionym: Cylindrocarpon macroconidialis Brayford & Samuels, Mycol. Res. 94: 440. 1990.
≡ Nectria radicicola var. macroconidialis Samuels & Brayford, Mycol. Res. 94: 440. 1990.
≡ Neonectria macroconidialis (Samuels & Brayford) Seifert, Phytopathology 93: 1541. 2003.
Neonectria Wollenw., Ann. Mycol. 15: 52. 1917. MycoBank MB3469.
= Chitinonectria Morelet, Bull. Soc. Sci. Nat. Archéol. Toulon & Var 178: 6. 1969.
= Heliscus Sacc., Michelia 2: 35. 1880.
Ascomata perithecial, solitary or in groups, seated on an erumpent stroma, red, turning dark red in KOH, smooth to scruffy, subglobose to broadly obpyriform, with a blunt or acute apex. Asci narrowly clavate to cylindrical, 8-spored. Ascospores ellipsoidal, smooth or finely verruculose, 1-septate, hyaline becoming pale brown with age. Paraphyses septate when present, slightly constricted at each septum. Conidiophores simple or complex forming sporodochia. Simple conidiophores solitary or loosely aggregated, unbranched or sparsely branched. Complex conidiophores irregularly branched, solitary or aggregated to form sporodochia. Phialides cylindrical, tapering towards the apex. Microconidia formed by simple conidiophores, hyaline, smooth, ellipsoidal to oblong, 0–1-septate. Macroconidia mostly formed by complex conidiophores, hyaline, smooth, straight or slightly curved towards the ends, 3–7(–9)-septate, lacking a scar or basal hilum. Chlamydospores globose to subglobose, hyaline (adapted from Chaverri et al. 2011).
Type species: Neonectria candida (Ehrenb.) Rossman, L. Lombard & Crous.
Description and illustration: Chaverri et al. (2011).
Notes: The genus Neonectria is monophyletic, forming a well-supported clade (BS = 100 %, PP = 1.0), distinct from the genera included in Clade VI. A new combination is required for N. ramulariae (1917) as there is an older epithet Fusarium candidum (1818), available for this species.
Neonectria candida (Ehrenb.) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810956.
Basionym: Fusarium candidum Ehrenb., Syl. Mycol. Berol: 24. 1818.
≡ Ramularia candida (Ehrenb.) Wollenw., Phytopatology 1: 220. 1913.
≡ Cylindrocarpon ehrenbergii Wollenw., Fus. Autogr. Del.: 461. 1916.
= Fusarium obtusiusculum Sacc., Michelia 2: 297. 1881.
≡ Fusarium oxysporum var. obtusiusculum (Sacc.) Cif., Ann. Bot., Roma 16: 221. 1924.
≡ Cylindrocarpon obtusiusculum (Sacc.) U. Braun, Cryptog. Bot. 4: 113. 1993.
= Fusarium eichleri Bres., Ann. Mycol. 1: 130. 1903.
= Neonectria ramulariae Wollenw., Ann. Mycol. 15: 52. 1917.
≡ Nectria ramulariae (Wollenw.) E. Müll., Beitr. Kryptogamenfl. Schweiz 11: 634. 1962.
= Cylindrocarpon magnusianum Wollenw., Z. Parasitenk. 1: 172. 1928.
Clade VII
Chaetopsina Rambelli, Atti Accad. Sci. Ist. Bologna, Cl. Sci. Fis., Rendiconti: 5. 1956. MycoBank MB7584.
= Chaetopsinectria J. Luo & W.Y. Zhuang, Mycologia 102: 979. 2010.
Ascomata perithecial, solitary, non-stromatic, superficial, obpyriform, with an acute apex, red, becoming dark red in KOH, smooth. Asci unitunicate, clavate, 8-spored, with a simple apex or an apical ring. Ascospores ellipsoid to fusiform, 1-septate, hyaline, smooth to striate. Conidiophores erect, setiform, tapering towards acutely rounded apex, mostly flexuous, yellow-brown, turning red-brown in KOH, fertile in mid region, unbranched, verruculose, thick-walled, base bulbous. Fertile region consisting of irregularly branched dense aggregated conidiogenous cells. Conidiogenous cells ampulliform to lageniform, hyaline, smooth, mono- to polyphialidic. Conidia hyaline, smooth, guttulate, subcylindrical, aseptate, apex and base bluntly rounded, base rarely with flattened hilum (adapted from Rambelli 1956 and Luo & Zhuang 2010).
Type species: Chaetopsina fulva Rambelli, Atti Accad. Sci. Ist. Bologna, Cl. Sci. Fis. Rendiconti: 5. 1956.
= Nectria chaetopsinae Samuels, Mycotaxon 22: 18. 1985.
≡ Cosmospora chaetopsinae (Samuels) Rossman & Samuels, Stud. Mycol. 42: 119. 1999.
≡ Chaetopsinectria chaetopsinae (Samuels) J. Luo & W.Y. Zhuang, Mycologia 102: 979. 2010.
Descriptions and illustrations: Rambelli, 1956, Samuels, 1985, Luo and Zhuang, 2010.
Notes: Chaetopsinectria, a sexual genus based on Cosmospora chaetopsinae (Samuels 1985), was established by Luo & Zhuang (2010) for a group of fungi having Chaetopsina asexual morphs. We propose that the sexual genus Chaetopsinectria (2010) be suppressed in favour of asexual genus Chaetopsina (1956), which has priority by date and would require no new combinations. The clade representing Chaetopsina (BS ≥ 75 %, PP ≥ 0.95), which includes the type species, C. fulva (ex-type CBS 142.56), is closely related to but separate from the clade representing the genus Volutella. In addition, these two genera do not share any morphological characters.
Coccinonectria L. Lombard & Crous, gen. nov. MycoBank MB810176.
Etymology: Name refers to the scarlet ascomata produced by these fungi.
Ascomata perithecial, superficial, solitary or aggregated in groups, developing on old sporodochia of volutella-like asexual morphs, subovoid to subglobose, orange to orange-red to carmine red, becoming pink to purple in KOH, initially rough surface due to short, thick-walled setae, with a short papillate ostiole; perithecial wall consists of two regions; inner region composed of thin-walled, flattened, hyaline cells; outer region composed of thick-walled ellipsoid to elongated cells. Setae scattered on surface of the perithecia except at the ostiolar region, hyaline, thick-walled, straight to curved, aseptate, narrowing toward the apex. Asci unitunicate, clavate, 8-spored, apex simple, truncate with hyaline, thin-walled moniliform paraphyses between the asci. Ascospores narrowly ellipsoid to fusiform, aseptate or medianly septate, slightly constricted at the septum, hyaline, becoming dark yellow with age, finely verrucose. Conidiophores sporodochial, ochraceous to amber or light russet, with hyaline to lightly coloured aseptate setae. Conidia aseptate, hyaline, guttulate, ellipsoidal to fusiform.
Type species: Coccinonectria pachysandricola (B.O. Dodge) L. Lombard & Crous.
Notes: The sexual genus Coccinonectria is established here to accommodate fungal species previously incorrectly treated as members of the genus Pseudonectria (Rossman et al., 1999, Gräfenhan et al., 2011). Coccinonectria is distinguished from Pseudonectria by its orange to scarlet ascomata with short, thick-walled setae extending from the ascomatal surface (Dodge, 1944, Rossman et al., 1999). The latter genus is characterised by yellow to greyish yellow-green ascomata with longer setae on the ascomatal surface (Rossman et al. 1999). Phylogenetic inference also shows that the genus Coccinonectria is closely related to the genera Chaetopsina and Volutella, but clearly distinct from the genus Pseudonectria.
Coccinonectria pachysandricola (B.O. Dodge) L. Lombard & Crous, comb. nov. MycoBank MB810177.
Basionym: Pseudonectria pachysandricola B.O. Dodge, Mycologia 36: 536. 1944.
≡ Volutella pachysandricola B.O. Dodge, Mycologia 36: 536. 1944.
Description and illustrations: Dodge (1944).
Coccinonectria rusci (Lechat, Gardiennet & J. Fourn.) L. Lombard & Crous, comb. nov. MycoBank MB810179.
Basionym: Pseudonectria rusci Lechat et al., Persoonia 32: 297. 2014.
Description and illustrations: Crous et al. (2014).
Note: Coccinonectria rusci (ex-type CBS 126108) clustered in a monophyletic clade representing the genus Coccinonectria, and therefore a new combination is proposed for this species.
Pseudonectria Seaver, Mycologia 1: 48. 1909. MycoBank MB4460. emend. L. Lombard & Crous.
≡ Nectriella Sacc., Michelia 1: 51. 1877.
≡ Nectriella subgen. Notarisiella Sacc., Syll. Fung. 2: 452. 1883.
≡ Notarisiella (Sacc.) Clements & Shear, The genera of Fungi: 280. 1931.
Ascomata perithecial, superficial, solitary, with an inconspicuous basal stroma, globose to pyriform, with a pointed apex, pale yellow to greyish yellow-green, not changing in KOH; ascomatal wall smooth, with or without sparse to numerous hyaline to orange setae; ascomatal surface of cells with irregularly thickened walls and joined by pores. Asci cylindrical to narrowly clavate, 8-spored. Ascospores aseptate, fusiform to ellipsoidal. Conidiophores simple or sporodochial. Simple conidiophores as lateral phialides on somatic hyphae or monochasial or verticillate, hyaline. Sporodochial conidiophores consist of a stipe and a penicillate arrangement of fertile branches. Conidiogenous apparatus consists of aseptate primary, secondary and rarely tertiary branches with each terminal branch producing 2–4 phialides. Phialides hyaline, cylindrical to allantoid, tapering towards the apex, with obvious periclinal thickening and inconspicuous collarettes. Conidia aseptate, hyaline, fusiform to ellipsoidal. Chlamydospores hyaline, globose to subglobose, formed intercalarily in chains (adapted from Rossman et al., 1993, Rossman et al., 1999).
Type species: Pseudonectria buxi (DC.) Seifert et al., Stud. Mycol. 68: 107. 2011. Fig. 9.
Fig. 9.
Pseudonectria buxi (CBS 324.53). A. Ascomata on the leaf of Buxus sempervirens. B–C. Setae on ascomatal surface. D. Asci with ascospores. E–F. Sporodochial conidiophores. G. Conidiogenous apparatus with cylindrical to allantoid phialides. H. Conidia. Scale bars: A = 500 μm; B = 50 μm (apply to F); C = 10 μm (apply to D, G–H); E = 100 μm.
≡ Tubercularia buxi DC., Flore française, Edn. 3 (Paris) 6: 110. 1815.
≡ Chaetostroma buxi (DC.) Corda, Icon. Fung. 2: 30. 1838.
≡ Volutella buxi (DC.) Berk., Ann. Mag. Nat. Hist. 5: 465. 1850.
≡ Chaetodochium buxi (DC.) Höhn., Mitt. Bot. Lab. TH Wien 9: 45. 1932.
= Psilonia rosea Berk., The English Flora, Fungi 5-2: 353. 1836.
= Pseudonectria rousseliana (Mont.) Clements & Shear, The genera of Fungi: 280. 1931.
≡ Nectria rousseliana Mont. in Castagne, Cat. Pl. Marseille Suppl.: 44. 1851.
≡ Stigmatea rousseliana (Mont.) Fuckel, Jahrb. Nassauischen Vereins Naturk. 23/24: 97. 1870.
≡ Notarisiella rousseliana (Mont.) Clements & Shear, The genera of Fungi: 280. 1931.
= Nectria rousseliana Mont. var. viridis Berk. & Br., Ann. Mag. Nat. Hist. ser. 3, 3: 21. 1859, fide Lowen 1991.
Descriptions and illustrations: Rossman et al., 1993, Rossman et al., 1999.
Note: Representatives of the genus Pseudonectria formed a monophyletic clade (BS = 100 %, PP = 1.0), sister to the clade representing the genus Sarcopodium.
Pseudonectria foliicola L. Lombard & Crous, sp. nov. MycoBank MB810180. Fig. 10.
Fig. 10.
Pseudonectria foliicola (ex-type CBS 123190). A–C. Simple conidiophores. D–F. Sporodochial conidiophores. G. Conidia. H. Chlamydospores. Scale bars: A = 10 μm (apply to B–F); G = 10 μm (apply to H).
Etymology: Name refers to the natural habitat of this species, which is a foliar pathogen.
Ascomatal state not observed. Conidiophores simple or sporodochial. Simple conidiophores monochasial or verticillate or as lateral phialides on somatic hyphae; phialides aseptate hyaline, cylindrical to allantoid, 12–35 × 2–3 μm. Sporodochial conidiophores without setae, consisting of a stipe and a penicillate arrangement of fertile branches; stipe hyaline, smooth, 0–1-septate, 10–25 × 2–3 μm. Conidiogenous apparatus 75–95 μm wide, 85–100 μm long; primary branches aseptate, 25–40 × 2–4 μm, secondary branches aseptate, 15–20 × 2–4 μm, tertiary branches rare, aseptate, 12–15 × 2–3 μm, each terminal branch producing 2–4 phialides; phialides hyaline, cylindrical to allantoid, 9–14 × 2–4 μm, tapering towards the apex, with obvious periclinal thickening and inconspicuous collarettes. Conidia hyaline, aseptate, fusiform to ellipsoidal, (5–)6.5–7.5(–8) × 2–3 μm (av. 7 × 3 μm), forming flat domes of pink to salmon slimy masses on the sporodochia. Chlamydospores hyaline, globose to subglobose, 35–60 μm diam, formed intercalarily in chains or solitary.
Culture characteristics: Colonies fast growing on MEA, reaching 90 mm in 10 d at 24 °C. Surface white with abundant aerial mycelium, with scattered pink to salmon slimy masses of conidia on sporodochia at the margins. Reverse white.
Material examined: New Zealand, South Auckland, Ardmore, on leaves of Buxus sempervirens, 1 May 2008, S. Trower (holotype CBS H-21950, culture ex-type CBS 123190 = CPC 15385). USA, Maryland, Beltsville, Prince George's Co., on leaves of B. sempervirens, 10 May 1992, A.Y. Rossman, culture CBS 122566 = AR 2709.
Notes: Pseudonectria foliicola can be distinguished from P. buxi by the formation of simple conidiophores in the asexual state, something not reported for P. buxi (Bezerra, 1963, Rossman et al., 1993). Also, no setae were observed surrounding the sporodochia of P. foliicola, while setae formation is characteristic of P. buxi (Bezerra, 1963, Rossman et al., 1993). The conidia of P. foliicola are also smaller than those of P. buxi, which are 8–12 × 2.5–3 μm (Bezerra 1963).
Sarcopodium Ehrenb. ex Schlecht., Synop. Pl. Crypt. 2: 101. 1824. MycoBank MB9788.
≡ Sarcopodium Ehrenb., Syl. Mycol. Berol. 23. 1818.
= Tricholeconium Corda, Icon. Fung. 1: 17. 1837.
= Cyphina Sacc., Syll. Fung. 3: 623. 1884.
= Periolopsis Maire, Ann. Mycol. 11: 357. 1913.
= Actinostilbe Petch, Ann. Roy. Bot. Gard. (Peradeniya) 9: 327. 1925.
= Kutilakesa Subram., J. Indian Bot. Soc. 35: 478. 1956.
= Kutilakesopsis Agnihoth. & Barua, J. Indian Bot. Soc. 36: 308. 1957.
= Lanatonectria Samuels & Rossman, Stud. Mycol. 42: 137. 1999.
Ascomata perithecial, solitary or in groups, superficial on a minute stroma, on an erumpent, previously conidial stroma, or at the base of a synnema, subglobose to broadly obpyriform, red, turning dark red in KOH, non-papillate or with a minute papilla, with hyaline to yellow hyphal hairs; hairs smooth, spinulose, hooked or straight, septate, thin-walled, arising from the surface of the ascomatal wall and forming around the ascomatal base, sometimes forming a tomentum on the ascomatal surface. Asci clavate to fusiform, 8-spored, apex simple or with a ring. Ascospores ellipsoid to fusiform, 1-septate, hyaline to pale yellow-brown, striate. Conidiomata sporodochial, cupulate to synnematal, superficial. Setae simple, septate, rarely branched, smooth or verruculose, straight or circinate, brown. Conidiophores macronematous, irregularly, verticillately, or penicillately branched, hyaline, smooth. Phialides hyaline, smooth, cylindrical or doliiform to reniform. Conidia aggregated in slimy masses, straight, cylindrical to ellipsoid, hyaline, 0–1-septate (adapted from Sutton 1981 and Rossman et al. 1999).
Type species: Sarcopodium circinatum Ehrenb. ex Schlecht., Synop. Pl. Crypt. 2: 101. 1824. Fig. 11.
Fig. 11.
Sarcopodium circinatum (CBS 100998). A–B. Sporodochial conidiomata. C. Circinate setae. D–E. Conidiophores. F. Conidia. Scale bars: A, B = 100 μm; C = 10 μm (apply to D–F).
≡ Sarcopodium circinatum Ehrenb., Syl. Mycol. Berol. 12 & 23. 1818.
≡ Thelephora circinata (Ehrenb.) Fr., Elenchus Fung. 1: 226. 1828.
≡ Corticium circinatum (Ehrenb.) Fr., Epi. Syst. Mycol.: 556. 1838.
≡ Hymenochaete circinata (Ehrenb.) Lév., Ann. Sci. Nat., Bot. 5: 133. 1846.
Descriptions and illustrations: Sutton, 1981, Rossman et al., 1999.
Notes: Representatives of the genus Sarcopodium formed a monophyletic clade (BS ≥ 75 %, PP ≥ 0.95), closely related to the genus Pseudonectria. Rossman et al. (1999) established the sexual genus Lanatonectria, based on L. flocculenta, for nectriaceous fungi with Actinostilbe asexual morphs. Later, Rossman et al. (2013) proposed that the genus name Lanatonectria be suppressed in favour of Actinostilbe based on priority, as per the ICN (McNiell et al. 2012). However, Sutton (1981) had already synonymised Actinostilbe under the asexual morph genus Sarcopodium. Furthermore, Rossman et al. (2013) synonymised L. flocculenta (= A. macalpinei) under A. flocculenta. Actinostilbe flocculenta should be regarded as a synonym of S. macalpinei as proposed by Sutton (1981). Phylogenetic inference in this study clearly supports the findings of Sutton (1981). Therefore, we regard Actinostilbe as a synonym of Sarcopodium and introduce several new combinations below.
Sarcopodium flavolanatum (Berk. & Broome) L. Lombard & Crous, comb. nov. MycoBank MB810181.
Basionym: Nectria flavolanata Berk. & Broome, J. Linn. Soc., Bot. 14: 114. 1873.
≡ Actinostilbe flavolanata (Berk. & Broome) Rossman, Samuels & Seifert, IMA Fungus 4: 46. 2013.
= Nectria radians Penz. & Sacc., Malpighia 11: 510. 1897.
= Nectria tjibodensis Penz. & Sacc., Malpighia 11: 512. 1897.
= Chilonectria javanica Penz. & Sacc., Malpighia 11: 508. 1897.
= Calonectria sulphurella Starbäck, Bih. Kungl. Svenska Vetenskapakad. Handl. 25: 30. 1899.
= Sphaerostilbe ochracea Pat., in Duss, Énum. Champ. Guadeloupe: 79. 1903.
Sarcopodium mammiforme (Chardón) L. Lombard & Crous, comb. nov. MycoBank MB810182.
Basionym: Sphaerostilbe mammiformis Chardón, Sci. Surv. Porto Rico & Virgin Islands 8: 46. 1926.
≡ Nectria mammiformis (Chardón) Samuels, Caldasia 13: 393. 1982.
≡ Lanatonectria mammiformis (Chardón) Samuels & Rossman, Stud. Mycol. 42: 139. 1999.
= Actinostilbe mammiformis (Cif.) Seifert & Samuels, Stud. Mycol. 42: 139. 1999.
≡ Stromatographium mammiforme Cif., Sydowia 8: 264. 1954.
Sarcopodium oblongisporum (Y. Nong & W.Y. Zhuang) L. Lombard & Crous, comb. nov. MycoBank MB810183.
Basionym: Lanatonectria oblongispora Y. Nong & W.Y. Zhuang, Fungal Diversity 19: 98. 2005.
≡ Actinostilbe oblongispora (Y. Nong & W.Y. Zhuang) Rossman et al., IMA Fungus 4: 46. 2013.
Sarcopodium raripilum (Penz. & Sacc.) L. Lombard & Crous, comb. nov. MycoBank MB810184.
Basionym: Nectria raripila Penz. & Sacc., Malpighia 15: 228. 1901.
= Lanatonectria raripila (Penz. & Sacc.) Samuels & Rossman, Stud. Mycol. 42: 140. 1999.
Volutella Tode 1790: Fr. 1832. Fungi Mecklenb. Sel. 1: 28. 1790: Syst. Mycol. 3: 458, 466. 1832. MycoBank MB7573.
= Volutellonectria J. Luo & W.Y. Zhuang, Phytotaxa 44: 3. 2012.
Ascomata perithecial, solitary, on a thin basal stroma, superficial, obpyriform to pyriform, with an acute apex, orange to red, turning dark red in KOH, smooth or hairy. Asci unitunicate, subcylindrical to clavate, 8-spored, with an apical ring. Ascospores 1-septate, hyaline, fusiform to biconic, smooth or finely roughened. Conidiophores aggregated into sporodochia or synnemata, with an inconspicuous stroma; unbranched, hyaline setae around the margin of conidiomata. Synnemata when produced, determinate, pale, composed of a stipe of parallel hyphae and a divergent capitulum of conidiophores giving rise to a slimy conidial mass. Conidiophore branching once or twice monochasial, 2-level verticillate, monoverticillate or irregularly biverticillate. Conidiogenous cells monophialidic, hyaline, subulate, usually with conspicuous periclinal thickening. Conidia aseptate, hyaline, ellipsoidal, ovate or oblong, forming slimy white, yellow, orange or pink masses (adapted from Gräfenhan et al. 2011 and Luo & Zhuang 2012).
Type species: Volutella ciliata (Alb. & Schw.: Fr.) Fr., Syst. Mycol. 3: 466. 1832. Fig. 12.
Fig. 12.
Volutella ciliate (CBS 483.61). A–B. Sporodochial conidiomata. C. Conidiophores. D–E. Setae. F. Conidia. Scale bars: A = 100 μm; B = 50 μm; C = 10 μm (apply to D–F).
Descriptions and illustrations: Gräfenhan et al., 2011, Luo and Zhuang, 2012.
Notes: Representatives of the genus Volutella formed a monophyletic clade (BS ≥ 75 %, PP ≥ 0.95), distinct from the clades representing Coccinonectria and Pseudonectria. Volutella shares several morphological characters of the asexual morph with these genera.
Volutella asiana (J. Luo, X.M. Zhang & W.Y. Zhuang) L. Lombard & Crous, comb. nov. MycoBank MB810185.
Basionym: Volutellonectria asiana J. Luo, X.M. Zhang & W.Y. Zhuang, Phytotaxa 44: 5. 2012.
Notes: Luo & Zhuang (2012) established the sexual genus Volutellonectria (Vo.), with Vo. consors as type, and indicated that Volutella (V.) minima represents the asexual morph. However, Gräfenhan et al. (2011) synonymised V. minima under Vo. consors. Additionally, Luo & Zhuang (2012) introduced two more species in the genus Volutellonectria, namely Vo. asiana as a new species, and Vo. ciliata (= V. ciliata) as a new combination. Given the obscurity of Volutellonectria and the number of name changes that would be required if the use of this name were perpetuated, we propose that the sexual genus Volutellonectria be suppressed in favour of the asexual genus Volutella, which also has priority by date. Therefore only the single new combination proposed in this study is required.
Clade VIII
Atractium Link: Fr., Mag. Ges. naturf. Freunde, Berlin 3: 10. 1809: Fries, Syst. Mycol. 1: xli. 1821. MycoBank MB7291.
Ascomatal state unknown. Conidiophores aggregated into sporodochia or synnemata, non-stromatic. Synnemata determinate, pale brown, composed of a stipe of parallel hyphae and a divergent capitulum of conidiophores giving rise to a slimy conidial mass. Conidiophore branching once or twice monochasial, 2-level verticillate, monoverticillate or irregularly biverticillate. Conidiogenous cells monophialidic, hyaline, subulate with conspicuous periclinal thickening. Conidia (0–)1–5-septate, clavate, obovoid or gently curved, rarely ellipsoidal, with a rounded apical cell, and somewhat conical basal cell, lacking a differentiated foot, forming yellow to orange masses (adapted from Gräfenhan et al. 2011).
Type species: Atractium stilbaster Link, Mag. Ges. naturf. Freunde, Berline 3: 10. 1809.
≡ Fusarium stilbaster (Link) Link, Caroli Linné Sp. Pl. Ex. Pl. Rite Cogn. Gen. Relat. 6: 106. 1825.
= Atractium fuscum Sacc., Syll. Fung. 2: 514. 1883.
≡ Stilbella fusca (Sacc.) Seifert, Stud. Mycol. 27: 77. 1985.
= Atractium flavoviride Sacc., Syll. Fung. 2: 514. 1883.
= Stilbum madidum Peck, Annual Rep. New York St. Mus. Nat. Hist. 46: 35. 1893.
= Didymostilbe eichleriana Bres. & Sacc., C. r. Congr. Bot. Palermo: 59. 1902.
= Didymostilbe capillacea Bres. & Sacc., Annls Mycol. 1: 28. 1903.
= Didymostilbe obovoidea Matsush. Icon. Microfung. Matsush. Lect.: 60. 1975.
Description and illustrations: Gräfenhan et al. (2011).
Note: Representatives of the genus Atractium formed a monophyletic clade (BS ≥ 75 %, PP ≥ 0.95), closely related to the genera Calostilbe and Ophionectria.
Calostilbe Sacc. & Syd., Syll. Fung. 16: 591. 1902. MycoBank MB758.
= Nectria subgen. Phaeonectria Sacc., Syll. Fung. 11: 359. 1895.
≡ Phaeonectria (Sacc.) Sacc. & Trotter, Syll. Fung. 22: 485. 1913.
= Calostilbella Höhn., Ber. Deutsch. Bot. Ges. 37: 160. 1919.
Stromata well-developed, originating from a central point, pseudoparenchymatous below the ascomata, giving rise to synnemata, ascomata forming at the base and on rhizoids that arise from the stromata, growing under bark and breaking through at points. Ascomata perithecial, superficial, densely aggregated, ovoid, not collapsing or collapsing laterally when dry, orange, turning sienna in KOH, apical region with acute papilla. Ascomata surface prosenchymatous, walls thickened. Asci clavate, apex simple, base pointed to pedicellate. Ascospores fusiform to ellipsoidal, 1-septate, slightly constricted or not, yellow-brown, coarsely striate, appearing as longitudinal furrows. Asexual morph synnematal, arising throughout the stromata. Hyphae of the synnemata parallel, branched, with the ends of the hyphae at the surface with small “cork screws”, giving the surface a granular-crystalline aspect. Phialides formed in a well-defined, hemispherical cluster, with a swollen, often slightly flared apex at the tip and cylindrical base. Sterile elements interspersed with phialides, straight, smooth, thin-walled, septate. Conidia ellipsoidal, 1-septate, yellow-brown, thick-walled in the centre becoming hyaline and thin-walled at the ends, held in a solitary, brown drop of liquid at the apex (adapted from Rossman et al. 1999).
Type species: Calostilbe striispora (Ellis & Everh.) Seaver, Mycologia 20: 248. 1928.
≡ Nectria striispora Ellis & Everh., Bull. Iowa Univ. Lab. Nat. Hist. 2: 398. 1893.
≡ Macbridella striispora (Ellis & Everh.) Seaver, Mycologia 1: 196. 1909.
≡ Letendraea striispora (Ellis & Everh.) Weese, Sitzungsber. Kaiserl. Akad. Wiss., Math.- Naturwiss. Cl., Abt. 1, 125: 514. 1916.
= Sphaerostilbe longiasca Möller, Bot. Mitt. Tropen 9: 122. 1901.
≡ Calostilbe longiasca (Möller) Sacc. & P. Syd., Syll. Fung. 16: 591. 1902.
≡ Letendraea longiasca (Möller) Weese, Sitzungsber. Kaiserl. Akad. Wiss., Math.- Naturwiss. Cl., Abt. 1, 128: 742. 1919.
≡ Nectria longiasca (Möller) E. Müll., Beitr. Kryoptogamenfl. Schweiz 11: 636. 1962.
= Sphaerostilbe musarum Ashby, Bull. Dept. Agric. Jamaica 2: 118. 1914.
= Calostilbella calostilbe Höhn., Ber. Deutsch. Bot. Ges. 37: 160. 1919.
= Xenostilbum sydowii Petr., Sydowia 13: 106. 1919.
= Calostilbe ledermannii Syd., Engl. Bot. Jahrb. 57: 322. 1922.
Description and illustrations: Rossman et al. (1999).
Notes: We recommend that the generic name Calostilbe be protected over the generic name Calostilbella based on priority. Therefore, Calostilbella calostilbe should be regarded as a synonym of Calostilbe striispora.
Ophionectria Sacc., Michelia 1: 323. 1878. MycoBank MB3608.
= Antipodium Piroz., Canad. J. Bot. 52: 1143. 1974.
Ascomata perithecial, solitary or aggregated in groups, sometimes seated on a white to bright yellow subiculum of thick-walled, minutely warted septate hyphae, each cell swollen at one end, superficial, ovoid to elongate-ovoid to cylindrical, often truncate at the apex, red-orange to scarlet, turning dark red to bay in KOH, covered with conspicuous, concolorous warts of loosely compacted, irregularly globose, pigmented cells; ascomata often naked towards the apex. Asci clavate, 8-spored, with simple apex. Ascospores long-fusiform, often somewhat bent, vermiform, multiseptate, the proximal end slightly inflated and bluntly rounded, the distal end tapering and narrowly rounded, thick-walled, hyaline, with faint longitudinal striations or smooth. Conidiophores arise laterally from hyphae, septate, unbranched, erect, straight, thin-walled, hyaline tapering toward the apex, terminating in a cylindrical phialide. Conidia (2–)3–5(–6)-septate, with the two middle cells larger than the end cells, fusiform and not constricted at the septa, or broadly ellipsoidal to ovoid and somewhat constricted at the median septum, initially hyaline turning olive-yellow with age (adapted from Pirozynski 1974 and Rossman et al. 1999).
Type species: Ophionectria trichospora (Berk. & Broome) Sacc., Michelia 1: 323. 1878.
≡ Nectria trichospora Berk & Broome, J. Linn. Soc., Bot. 14: 115. 1875.
≡ Dialonectria trichospora (Berk. & Broome) Cooke, Grevillea 12: 111. 1884.
≡ Tubeufia trichospora (Berk. & Broome) Petch, Ann. Roy. Gard. Peradeniya 5: 285. 1912.
= Calonectria ornata A.L. Smith, J. Linn. Soc., Bot. 35: 8. 1901.
= Calonectria cinnabarina P. Henn., Hedwigia 36: 220. 1897.
≡ Ophionectria cinnabarina (P. Henn.) P. Henn., Hedwigia 41: 7. 1902.
= Calonectria theobromae Pat., in Duss, Énum. Champ. Guadeloupe: 81. 1903.
= Ophionectria portoricensis Chardón, Mycologia 13: 285. 1912.
= Antipodium spectabile Piroz., Canad. J. Bot. 52: 1143. 1974.
Descriptions and illustrations: Pirozynski, 1974, Rossman, 1977, Rossman et al., 1999.
Notes: Ophionectria trichospora, the type of the genus (Rossman 1977), is directly linked to the type of the asexual genus Antipodium (Pirozynski 1974), known as A. spectabile. Rossman (1977) re-evaluated the generic status of Ophionectria and retained only the type species. Later, Rossman (1983) added O. magniverrucosa to the genus. A second species isolated from Arechae catechu, A. arechae, was added to the genus Antipodium by Matsushima (1980). However, based on the description and illustrations provided, this species should be considered a member of the genus Trichothecium (Summerbell et al. 2011). Since the generic name Ophionectria (1878) has priority over the generic name Antipodium (1974), we recommend that the generic name Ophionectria be protected against Antipodium.
Clade IX
Albonectria Rossman & Samuels, Stud. Mycol. 42: 105. 1999. MycoBank MB27953.
Ascomata perithecial, solitary to gregarious on a sparse to well-developed stroma, superficial, globose to subglobose to ellipsoidal or ovoid to obovoid, white to pale yellow to pale ochraceous, not changing colour in KOH, warty, with or without a small pointed papilla. Asci narrowly clavate or broadly clavate to ellipsoidal, 4–8-spored. Ascospores ellipsoidal to long-ellipsoidal or fusiform to long-fusiform, multiseptate, hyaline to yellow-brown, smooth to striate. Conidiophores monophialidic, polyphialidic or sporodochial. Microconidia variable in shape, 0–1-septate, hyaline, smooth, with or without a flattened basal papilla, or with or without a poorly developed foot cell. Macroconidia cylindrical to broadly fusiform or long fusiform to clavate, multiseptate, curved, with curved, pointed tip and foot-cell, or distinctly beaked at both ends (adapted from Gerlach & Nirenberg 1982 and Rossman et al. 1999).
Type species: Albonectria rigidiuscula (Berk. & Broome) Rossman & Samuels, Stud. Mycol. 42: 105. 1999.
≡ Nectria rigidiuscula Berk. & Broome, J. Linn. Soc., Bot. 14: 116. 1873.
≡ Calonectria rigidiuscula (Berk. & Broome) Sacc., Michelia 1: 313. 1878.
= Calonectria lichenigena Speg., Bol. Acad. Nac. Ci. 11: 530. 1889.
= Calonectria eburnean Rehm., Hedwigia 37: 196. 1898.
= Calonectria sulcata Starbäck, Bih. Kongl. Svenska Vetenskapsakad. Handl. 25: 29.1899.
= Calonectria meliae Zimm., Centralbl. Bakteriol. Parasitenk. 7: 106. 1901.
= Calonectria cremea Zimm., Centralbl. Bakteriol. Parasitenk. 7: 140. 1901.
= Calonectria hibiscicola Henn., Hedwigia 48: 105. 1908.
= Fusarium decemcellulare Brick, Jahresber. Vereinigung. Angew. Bot. 6: 277. 1908.
= Scoleconectria tetraspora Seaver, North Amer. Flora 3: 27. 1910.
≡ Calonectria tetraspora (Seaver) Sacc. & Trotter, Syll. Fung. 22: 487. 1913.
Descriptions and illustrations: Gerlach and Nirenberg, 1982, Rossman et al., 1999.
Notes: The sexual genus Albonectria was introduced by Rossman et al. (1999) to accommodate species with white to pale yellow ascomata associated with Fusarium asexual morphs. Representatives of this genus formed a monophyletic clade (BS = 100 %, PP = 1.0) closely related but separate from the clades representing Cyanonectria, Geejayessia and Fusarium.
Bisifusarium L. Lombard, Crous & W. Gams, gen. nov. MycoBank MB810226. Fig. 13.
Fig. 13.
Bisifusarium. A–D. B. dimerum (ex-type CBS 108944). A–C. Sporodochia. D. Conidia. E–J. B. delphinoides (ex-type CBS 120718). E–G. Sporodochia. H–I. Lateral phialidic pegs. J. Conidia. Scale bars: A = 10 μm (apply to B–D); E = 50 μm; F = 10 μm (apply to G–J).
Etymology: Name refers to the 2-celled macroconidia characteristically formed by these fungi.
Ascomatal state unknown. Conidiophores macronematous, lateral phialidic pegs, simple or sporodochial. Later phialidic pegs arising from superficial or submerged hyphae. Simple conidiophores monophialidic, rarely polyphialidic, cylindrical and slightly tapering towards the apex, or flask-shaped, solitary or aggregated when forming terminally or laterally on hyphae. Sporodochia pionnotal or hemispherical. Pionnotal sporodochia poorly developed, consisting of densely arranged phialides or short supporting cells with whorls of phialides; whorls arising laterally from hyphae or from irregularly branched conidiophores. Hemispherical sporodochia consisting of a core of angular, uniformly thin-walled, hyaline cells bearing cylindrical phialide-subtending cells or monophialides. Microconidia 0(–1)-septate, ellipsoidal and straight or allantoid, broadly lunate to reniform or curved and tapering at both ends, mostly formed by monophialidic conidiophores and lateral phialidic pegs as inconspicuous heads. Macroconidia (0–)1–2(–3)-septate, curved to lunate, with a distal end slightly more bent than the proximal end or with both ends equally bent, both ends tapering, the proximal end typically slightly pedicellate, mostly formed as masses on poorly or well-developed sporodochia. Chlamydospores, if present, globose to subglobose to ellipsoidal, solitary or in chains, sometimes aggregated into sclerotia (adapted from Schroers et al. 2009).
Type species: Bisifusarium dimerum (Penz.) L. Lombard & Crous.
Notes: The genus Bisifusarium is established here to accommodate fusarium-like species previously classified in the genus Fusarium. Species of Bisifusarium can be distinguished from species in Fusarium by their short, (0–)1–2(–3)-septate macroconidia and the formation of lateral phialidic pegs arising from the hyphae (Gerlach and Nirenberg, 1982, Schroers et al., 2009), rarely seen in the genus Fusarium. Past phylogenetic studies (Schroers et al., 2009, O'Donnell et al., 2013) showed that species of Bisifusarium (as the Fusarium dimerum species group; Schroers et al. 2009) formed a well-supported monophyletic clade, closely related but separate to “the Fusarium terminal clade” (Geiser et al. 2013). Phylogenetic inference in this study further supports this observation, with representatives of Bisifusarium forming a well-supported clade (BS = 100 %, PP = 1.0) closely related but separate from the clade representing the genus Fusarium.
Bisifusarium biseptatum (Schroers, Summerbell & O'Donnell) L. Lombard & Crous, comb. nov. MycoBank MB810227.
Basionym: Fusarium biseptatum Schroers, Summerbell & O'Donnell, Mycologia 101: 59. 2009. [non Fusarium biseptatum Sawada, Special Publ. Coll. Agric. Natl. Taiwan Univ. 8: 228. 1959, nom. inval.]
Description and illustrations: Schroers et al. (2009).
Bisifusarium delphinoides (Schroers, Summerbell, O'Donnell & Lampr.) L. Lombard & Crous, comb. nov. MycoBank MB810228.
Basionym: Fusarium delphinoides Schroers, Summerbell, O'Donnell & Lampr., Mycologia 101: 57. 2009.
? = Fusarium dimerum var. majusculum Wollenw., Fus. Autogr. Del. 1: 90. 1916.
Description and illustrations: Schroers et al. (2009).
Bisifusarium dimerum (Penz.) L. Lombard & Crous, comb. nov. MycoBank MB810229.
Basionym: Fusarium dimerum Penz., Michelia 2: 484. 1882.
≡ Fusarium pusillum Wollenw., Fus. Autogr. Del. 2: 550. 1924.
≡ Fusarium aquaeductuum var. dimerum (Penz.) Raillo, Fungi of the genus Fusarium: 279. 1950.
≡ Microdochium dimerum (Penz.) Arx, Trans. Brit. Mycol. Soc. 83: 374. 1984.
? = Fusarium dimerum var. pusillum Wollenw., Fus. Autogr. Del. 3: 851. 1930.
Descriptions and illustrations: Gerlach and Nirenberg, 1982, Schroers et al., 2009.
Bisifusarium domesticum (Fr.) L. Lombard & Crous, comb. nov. MycoBank MB810230.
Basionym: Trichothecium domesticum Fr., Syst. Mycol. 3: 427. 1832.
≡ Fusarium domesticum (Fr.) Bachm., LWT – Food Sci. Tech. 38: 405. 2005.
Description and illustrations: Schroers et al. (2009).
Bisifusarium lunatum (Ellis & Everh.) L. Lombard & Crous, comb. nov. MycoBank MB810231.
Basionym: Gloeosporium lunatum Ellis & Everh., Proc. Acad. Nat. Sci. Philadelphia. 43: 82. 1891.
≡ Fusarium lunatum (Ellis & Everh.) Arx, Verh. Kon. Akad. Wetensch., Afd. Natuurk. 51: 101. 1957.
≡ Microdochium lunatum (Ellis & Everh.) Arx, Trans. Brit. Mycol. Soc. 83: 374. 1984.
= Fusarium dimerum var. violaceum Wollenw., Fus. Autogr. Del. 3: 854. 1930.
Description and illustrations: Schroers et al. (2009).
Bisifusarium nectrioides (Wollenw.) L. Lombard & Crous, comb. et stat. nov. MycoBank MB810232.
Basionym: Fusarium dimerum var. nectrioides Wollenw., Fus. Autogr. Del. 3: 855. 1930.
= Fusarium nectrioides (Wollenw.) Schroers, Summerbell & O'Donnell, Mycologia 101: 59. 2009.
Description and illustrations: Schroers et al. (2009).
Bisifusarium penzigii (Schroers, Summerbell & O'Donnell) L. Lombard & Crous, comb. nov. MycoBank MB810233.
Basionym: Fusarium penzigii Schroers, Summerbell & O'Donnell, Mycologia 101: 61. 2009.
Description and illustrations: Schroers et al. (2009).
Cyanonectria Samuels & Chaverri, Mycol. Progress 8: 56. 2009. MycoBank MB537057.
Ascomata perithecial, gregarious or caespitose, with a reduced or well-developed stroma, smooth, thin-walled, ampulliform to obpyriform or pyriform, dark bluish or red to red-brown, becoming darker in KOH, with darker bluish purple to black apex. Asci cylindrical to narrowly clavate, with rounded or flattened apex, with or without refractive ring, 8-spored. Ascospores ellipsoidal, 1-septate, not or slightly constricted at the septum, pale yellow-brown, smooth or finely warted. Conidiophores monophialidic, polyphialidic or sporodochial. Macroconidia (1–)5–7(–8)-septate, long-fusiform, with gently curving ends, pedicellate foot cell, with a hooked apical cell. Chlamydospores formed from cells of macroconidia, subglobose, not formed by hyphae.
Type species: Cyanonectria cyanostoma (Sacc. & Flageolet) Samuels & Chaverri, Mycol. Progress 8: 56. 2009.
≡ Nectria cyanostoma Sacc. & Flageolet, Atti Congr. Bot. Palermo: 53. 1902.
≡ Fusarium cyanostomum (Sacc. & Flageolet) O'Donnell & Geiser, Phytopathology 103: 404. 2013.
Description and illustrations: Samuels et al. (2009), Schroers et al. (2011).
Notes: Samuels et al. (2009) introduced the sexual genus Cyanonectria, based on C. cyanostoma, to accommodate the sexual morphs of an unnamed Fusarium sp., characterised by bicoloured perithecia. Later, Schroers et al. (2011) synonymised F. buxicola under C. buxi, recognising that the genus Cyanonectria formed a strongly supported clade distinct from other sexual genera associated with Fusarium asexual morphs. Phylogenetic inference in this study supports the findings of Samuels et al. (2009) and Schroers et al. (2011) with representatives of Cyanonectria forming a well-supported monophyletic clade (BS = 100 %, PP = 1.0).
Fusarium Link, Mag. Ges. Naturf. Freunde Berlin 3: 10. 1809. MycoBank MB8284.
= Selenosporium Corda, Icon. Fungorum H. Cogn. 1: 7. 1837.
= Pionnotes Fr., Summa Veg. Scand. 2: 481. 1849.
= Gibberella Sacc., Michelia 1: 43. 1877.
= Sporotrichella P. Karst., Meddeland. Soc. Fuana Fl. Fenn. 14: 96. 1887.
= Ustilaginoidella Essed, Ann. Bot. 25: 351. 1911.
= Rachisia Linder, Deutsche Essigind.: 467. 1913.
= Stagonostroma Died., Kryptog. Fl. Mark Brandenburg 9: 561. 1914.
= Discofusarium Petch, Trans. Brit. Mycol. Soc. 7: 143. 1921.
= Pseudomicrocera Petch, Trans. Brit. Mycol. Soc. 7: 164. 1921.
= Fusidomus Grove, J. Bot. 67: 201. 1929.
= Botryocrea Petr., Sydowia 3: 140. 1949.
= Bidenticula Deighton, Trans. Brit. Mycol. Soc. 59: 425. 1972.
= Pycnofusarium Punith., Trans. Brit. Mycol. Soc. 61: 63. 1973.
Ascomata perithecial, solitary or aggregated into groups, non-stromatic or on a thin stroma, superficial, globose to subglobose to pyriform, white, yellow, orange, red, bluish purple, bluish black or black, changing colour or not changing colour in KOH, slightly rugose to tuberculate to warted. Asci narrowly clavate to clavate to cylindrical, 8-spored, with or without an apical ring. Ascospores (0–)1–3-septate, mostly ellipsoidal, hyaline or pale yellow-brown. Conidiophores mono- or polyphialidic or sporodochial. Microconidia absent or present, 0–1(–2) septate. Macroconidia 1–multiseptate, straight or curved, with or without a hooked apical cell. Chlamydospores absent or present, globose, subglobose to ellipsoidal, formed terminally or intercalarily in chains or singular, sometime aggregating to form sclerotia (adapted from Gerlach & Nirenberg 1982).
Type species: Fusarium sambucinum Fuckel, Hedwigia 2: 135. 1863.
= Fusarium roseum Link, Mag. Ges. Naturf. Freunde Berlin 3: 10. 1809.
≡ Fusidium roseum (Link) Link, Mag. Ges. Naturf. Freunde Berlin 8: 31. 1816.
= Sphaeria pulicaris Kunze, Mykol. Hefte 2: 37. 1823.
≡ Gibbera pulicaris (Kunze) Fr., Summa Veg, Scand. 2: 402.
≡ Botryosphaeria pulicaris (Kunze) Ces. & De Not. 1963.
≡ Nectria pulicaris (Kunze) Tul. & C. Tul. Selec. Fung. Carpol. 3: 63. 1865.
≡ Cucurbitaria pulicaris (Kunze) Quél. Mém. Soc. Émul. Montbéliard. 5: 511. 1875.
≡ Gibberella pulicaris (Kunze) Sacc., Michelia 1: 43. 1877.
See Wollenweber and Reinking, 1935, Booth, 1971 and Index Fungorum (www.indexfungorum.org) for more synonymies.
Notes: The genus Fusarium as treated here accommodates Fusarium spp. belonging to the Gibberella clade (O'Donnell et al. 2013). This genus includes many important plant pathogenic and medically important species, and includes various Fusarium species groups, which could result in the segregation of this genus into more genera. However, a monographic study, which includes a more robust phylogeny, is required to identify and introduce these genera. In this study, representatives of this genus formed a well-supported monophyletic clade (BS = 100 %, PP = 1.0) distinct from the clades representing Albonectria, Cyanonectria and Geejayessia. A new combination is required for F. sambucinum (1863), the type species of the genus, as the epithet of Sphaeria pulicaris (1823) is older. However, we refrain from doing so here as F. sambucinum is extensively used in literature and better known among plant pathologists and other applied biologists.
Description and illustrations: Gerlach & Nirenberg (1982).
Geejayessia Schroers et al., Stud. Mycol. 68: 124. 2011. MycoBank MB519479.
Ascomata perithecial, aggregated into groups of five or more, broadly ampulliform with a short necks, or broadly ellipsoidal, pale orange, brownish to reddish orange, bright red or black, changing colour in KOH if not black. Asci cylindrical or clavate, with a broadly rounded or flattened apex, with or without a minute refractive ring, 8-spored. Ascospores 1-septate, broadly ellipsoidal to ellipsoidal, slightly constricted at the septum, verruculose, hyaline to pale brown. Conidiophores monophialidic, polyphialidic or sporodochial. Microconidia usually absent, when present, then oblong ellipsoidal, gently curved. rounded at both ends or with an asymmetrical hilum. Macroconidia formed in white to pale yellow slimy masses, gently curved, with pronounced pedicellate foot cell, and more or less inequilaterally fusoid, hooked apical cell (adapted from Schroers et al. 2011).
Type species: Geejayessi cicatricum (Berk.) Schroers, Stud. Mycol. 68: 124. 2011.
≡ Sphaeria sanguinea var. cicatricum Berk., Mag. Zool. Bot. 1: 48. 1837.
≡ Nectria cicatricum (Berk.) Tul. & C. Tul., Selec. Fung. Carpol. 3: 77. 1865.
≡ Fusarium cicatricum (Berk.) O'Donnell & Geiser, Phytopathology 103: 404. 2013.
Description and illustrations: Schroers et al. (2011).
Notes: The sexual genus Geejayessia was introduced to accommodate fusarium-like species characterised by their broadly ampulliform to broadly ellipsoidal, multicoloured ascomata (Schroers et al. 2011), and represents a well-supported monophyletic clade (BS = 100 %, PP = 1.0) distinct from the Fusarium clade.
Neocosmospora E.F. Sm., U.S.D.A. Div. Veg. Pathol. Bull. 17: 45. 1899. MycoBank MB3447.
= Lachnidium Giard., Compt. Rend. Hebd. des Séances Acad. Sci.: 1520. 1891. (nom. conf.)
= Hyaloflorea Bat. & H. Maia, Anais Soc. Biol. Pernambuco 13: 154. 1955.
? = Haematonectria Samuels & Nirenberg, Stud. Mycol. 42: 134. 1999.
Ascomata perithecial solitary, or aggregated in groups, non-stromatic or with a basal stroma, superficial, globose to pyriform, yellow to orange-brown to red, darkening in KOH, smooth to roughly warted. Asci narrowly clavate to cylindrical, simple apex or with a refractive ring, 8-spored. Ascospores 0–1-septate, globose to ellipsoidal, hyaline to yellow to yellow-brown, finely striate. Conidiophores generally simple, arising laterally from hyphae, rarely polyphialidic or forming poorly developed sporodochia. Microconidia 0–1-septate, oval, ellipsoidal to subcylindrical, hyaline, sometimes aggregated in slimy masses. Macroconidia subcylindrical, slightly curved with the tips cell slightly hooked, basal cell somewhat pedicellate, multiseptate. Chlamydospores when present hyaline to pale yellow, globose to obovoid, terminal or intercalary (adapted from Rossman et al. 1999 and Nalim et al. 2011).
Type species: Neocosmospora vasinfecta E.F. Sm., U.S.D.A. Div. Pathol. Bull. 17: 45. 1899.
≡ Fusarium neocosmosporiellum O'Donnell & Geiser, Phytopathology 103: 405. 2013.
= Neocosmospora vasinfecta var. tracheiphila E.F. Sm., U.S.D.A. Div. Veg. Pathol. Bull. 17: 45. 1899.
= Neocosmospora vasinfecta var. nivea E.F. Sm., U.S.D.A. Div. Veg. Pathol. Bull. 17: 45. 1899.
= Pseudonectria ornata Bat. & Maia, Anais Soc. Biol. Pernambuco 13: 74. 1955.
= Neocosmospora vasinfecta var. major Rama Rao, Mycopathol. Mycol. Appl. 21: 218. 1963.
= Neocosmospora vasinfecta var. conidiifera Kamyschko, Novoti Sist. Nizsh. Rast. 2: 115. 1965.
= Neocosmospora ornamentata M.A.F. Barbosa, Garcia de Orta, sér. Est. Argon.: 17. 1965.
= Neocosmospora vasinfecta var. africana (Arx) P.F. Cannon & D. Hawksw., Trans. Brit. Mycol. Soc. 82: 676. 1984.
Descriptions and illustrations: Rossman et al., 1999, Nalim et al., 2011.
Notes: Three generic names, Haematonectria (1999), Lachnidium (1891) and Neocosmospora (1899) could be applied to this group of fungi (Rossman et al., 1999, Summerbell and Schroers, 2002). However, the generic name Lachnidium is based on a nomen confusum (see Madelin 1966 and Kendrick 1974), and can therefore not be used. The genus Neocosmospora includes fusarium-like spp. also associated with the sexual genus Haematonectria. Rossman et al. (1999) could distinguish these genera based on ascomatal morphology and the reduced asexual morph of Neocosmospora. O'Donnell (1996) argued that the asexual morphs of Neocosmospora are microconidial Fusarium spp. that lost the ability to produce macroconidia and septate ascospores. Recent phylogenetic studies (Gräfenhan et al., 2011, Nalim et al., 2011, O'Donnell et al., 2013), which included representatives of both genera, showed that these genera are congeneric. As the generic name Neocosmospora (1899) is older than the generic name Haematonectria (1999), the name Neocosmospora takes priority for these fungi. Further support is provided by Nalim et al. (2011) whom stabilised the name Nectria haematococca through epitypification and provided a new combination for this species under the genus name Neocosmopora (as Neo. haematococca). Phylogenetic inference in this study supported these findings with the clade representing the sexual genus Neocosmospora being well-supported (BS ≥ 75 %, PP ≥ 0.95). However, as with the genus Fusarium, a monographic study is required to identify all the species belonging to this genus, and therefore only a few new combinations are introduced at this time. The ex-type strain of Hyaloflorea ramosa (CBS 509. 63), the type species of the genus Hyaloflorea (Batista & Maia 1955) clustered within the Neocosmospora clade, and therefore this genus is regarded as a synonym of Neocosmospora and a new combination is provided. Two isolates listed in the CBS collection as “F. ventricosum” (CBS 320.73 and CBS 101018) also clustered within this clade, separate from other known species, and are therefore described here as new.
Neocosmospora ambrosia (Gadd & Loos) L. Lombard & Crous, comb. nov. MycoBank MB810957.
Basionym: Monacrospium ambrosium Gadd & Loos, Trans. Brit. Mycol. Soc. 30: 13. 1947.
≡ Fusarium ambrosium (Gadd & Loos) Agnihothr. & Nirenberg, Stud. Mycol. 32: 98. 1990.
≡ Dactylella ambrosia (Gadd & Loos) K.Q. Zhang, X.Z. Liu & L. Cao, Mycosystema 7: 112. 1995.
= Fusarium bugnicourtii Brayford, Trans. Brit. Mycol. Soc. 89: 350. 1987.
Neocosmospora falciformis (Carrión) L. Lombard & Crous, comb. nov. MycoBank MB810958.
Basionym: Cephalosporium falciforme Carrión, Mycologia 43: 523. 1951.
≡ Acremonium falciforme (Carrión) W. Gams, Cephalosporium-artige Schimmelpilze: 139. 1971.
≡ Fusarium falciforme (Carrión) Summerb. & Schroers, J. Clin. Microbiol. 40: 2872. 2002.
Neocosmospora illudens (Berk.) L. Lombard & Crous, comb. nov. MycoBank MB810959.
Basionym: Nectria illudens Berk., in Hooker, Botany of the Antarctic Voyage II. Flora of New Zealand 7: 203. 1855.
≡ Cucurbitaria illudens (Berk.) Kuntze, Rev. Gen. Plant. 3: 461. 1898.
≡ Haematonectria illudens (Berk.) Samuels & Nirenberg, Stud. Mycol. 42: 136. 1999.
= Fusarium illudens C. Booth, The genus Fusarium: 53. 1971.
Neocosmospora ipomoeae (Halst.) L. Lombard & Crous, comb. nov. MycoBank MB810960.
Basionym: Nectria ipomoeae Halst., Rep. New Jersey Agric. Exp. Sta. 12: 281. 1891.
≡ Cucurbitaria ipomoeae (Halst.) Kuntze, Rev. Gen. Plant. 3: 461. 1898.
≡ Creonectria ipomoeae (Halst.) Seaver, N. Amer. Flora 3: 22. 1910.
≡ Hypomyces ipomoeae (Halst.) Wollew., Phytopathology 3: 34. 1913.
≡ Haematonectria ipomoeae (Halst.) Samuels & Nirenberg, Stud. Mycol. 42: 136. 1999.
= Fusarium javanicum Koord., Verh. Kon. Ned. Akad. Wetensch., Afd. Natuurk. 13: 247. 1907.
= Hypomyces solani f. cucurbitae W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 741. 1941.
Neocosmospora monilifera (Berk. & Broome) L. Lombard & Crous, comb. nov. MycoBank MB810961.
Basionym: Nectria monilifera Berk. & Broome, J. Linn. Soc., Bot. 14: 114. 1875.
≡ Nectriella monilifera (Berk. & Broome) Sacc., Michelia 1: 279. 1878.
≡ Dialonectria monilifera (Berk. & Broome) Cooke, Grevillea 12: 110. 1884.
≡ Neoskofitzia monilifera (Berk. & Broome) Höhn., Ann. Mycol. 8: 467. 1910.
≡ Haematonectria monilifera (Berk. & Broome) Samuels & Rossman, Stud. Mycol. 42: 137. 1999.
Neocosmospora phaseoli (Burkh.) L. Lombard & Crous, comb. nov. MycoBank MB810962.
Basionym: Fusarium martii f. phaseoli Burkh., Mem. Cornell Univ. Agric. Exp. Sta. 26: 1007, 1919.
≡ Fusarium solani f. phaseoli (Burkh.) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 28: 740. 1941.
≡ Fusarium phaseoli (Burkh.) T. Aoki & O'Donnell, Mycologia 95: 671. 2003.
Neocosmospora plagianthi (Dingley) L. Lombard & Crous, comb. nov. MycoBank MB810963.
Basionym: Nectria plagianthi Dingley, Trans. Roy. Soc. New Zealand 79: 196. 1951.
≡ Fusarium plagianthi (Dingley) O'Donnell & Geiser, Phytopathology 103: 404. 2013.
Neocosmospora ramosa (Bat. & H. Maia) L. Lombard & Crous, comb. nov. MycoBank MB810242.
Basionym: Hyaloflorea ramosa Bat. & H. Maia, Anais Soc. Biol. Pernambuco 13: 155. 1955.
Neocosmospora rubicola L. Lombard & Crous, sp. nov. MycoBank MB810243. Fig. 14.
Fig. 14.
Neocosmospora rubicola (ex-type CBS 101018). A–C. Sporodochial conidiophores. D. Conidiogenous apparatus with cylindrical to allantoid phialides. E–H. Simple conidiophores. I. Microconidia. J. Macroconidia. Scale bars: B = 50 μm (apply to C, E–F); D = 10 μm (apply to G–H); I = 10 μm (apply to J).
Etymology: Name derived from the plant host Rubus idaeus, from which it was collected.
Ascomatal state not observed. Conidiophores mononematous, simple, unbranched or aggregated into sporododochia. Mononematous conidiophores 13–129 μm long, 3–7 μm at the base, hyaline, aseptate or septate, terminating in a single phialide or a penicillate or verticillate arrangement of 2–4 phialides; single phialides 17–60 × 3–5 μm, cylindrical, tapering towards the apex, with periclinal thickening and slightly flared collarette; penicillate or verticillate phialides, 13–43 × 3–4 μm, cylindrical to allantoid, tapering towards the apex, with periclinal thickening and slightly flared collarette. Sporodochial conidiophores irregularly branched, sometimes slightly stipitate; sporodochial phialides cylindrical to allantoid, tapering towards the apex, 11–25 × 3–4 μm, with periclinal thickening, with or without slightly flared collarette. Microconidia mostly produced by mononematous conidiophores, 0–1(–2)-septate; 0-septate microconidia ellipsoidal to fusiform or obovoid, (8–)9–13(–19) × (2–)3–4(–5) μm (av. 11 × 4 μm); 1-septate microconidia, ellipsoidal to fusiform, straight to slightly curved, apex acutely rounded, base sometime flattened (13–)15–20(–22) × (3–)4–6 μm (av. 18 × 5 μm); 2-septate microconidia rarely formed, ellipsoidal to fusiform, straight to slightly curved, 20–22(–24) × 4–6 μm (av. 22 × 5 μm). Macroconidia 3–5-septate, cylindrical, straight or curving at both ends, beaked at both ends: 3-septate macroconidia (27–)32–44(–47) × 4–6 μm (av. 38 × 5 μm); 4-septate macroconidia (35–)38–48(–53) × 4–6 μm (av. 43 × 5 μm); 5-septate macroconidia (44–)45–49(–51) × 5–6 μm (av. 47 × 5 μm). Chlamydospores not observed.
Culture characteristics: Colony on PDA reaching 35–40 mm after 7 d at 24 °C, forming abundant white to pale luteous aerial mycelium, arranged in concentric rings, richly sporulating on the aerial mycelium; reverse concolorous. On SNA with sterile carnation leaf pieces, aerial mycelium absent, mononematous conidiophores arising on the surface of the agar; white sporodochia formed abundantly on the surface of the carnation leaf pieces.
Materials examined: Italy, on Rubus idaeus, Jun. 1998, A. Zazzerini (holotype CBS H-21949, culture ex-type CBS 101018); Sudan, isolated form soil, Feb. 1973, M.M. Musa, culture CBS 320.73 = ATCC 24395 = IMI 131652 = NRRL 22107 = NRRL 22122.
Notes: Neocosmospora rubicola is described here as a new species in the genus Neocosmospora. Sequence comparisons on the FUSARIUM-ID (http://isolate.fusariumdb.org; O'Donnell et al. 2010) and Fusarium MLST (http://www.cbs.knaw.nl/fusarium; O'Donnell et al. 2012) databases were inconclusive, identifying both isolates (CBS 101018 & CBS 320.73) as part of the F. solani complex only.
Neocosmospora solani (Mart.) L. Lombard & Crous, comb. nov. MycoBank MB810964.
Basionym: Fusisporium solani Mart., Die Kartoffel-Epidemie der letzten Jahre oder die Stockfäule und Räude der Kartoffeln: 20. 1842.
≡ Fusarium solani (Mart.) Sacc. Michelia 2: 296. 1881.
= Fusarium martii Appel & Wollew. Arb. Kaiserl. Biol. Anst. Ld.-u. Forstw. 8: 83. 1910.
= Nectria cancri Rutgers, Ann. Jard. Bot. Buitenzorg 2: 59. 1913.
= Fusarium striatum Sherb., Mem. Cornell Univ. Agric. Exp. Sta. 6: 255. 1915.
(See Index Fungorum (www.indexfungorum.org) and MycoBank (www.mycobank.org) for more synonyms).
Note: Nalim et al. (2011) concluded that Neocosmospora solani (= F. solani) is not congeneric with Neo. haematococca (= Haematonectria haematococca) and therefore a new combination is provided here.
Neocosmospora termitum (Höhn.) L. Lombard & Crous, comb. nov. MycoBank MB810965.
Basionym: Neoskofitzia termitum Höhn., Sitzungsber. Akad. Wiss. Wien, Math.-Naturwiss. Kl. 117: 998. 1908.
≡ Haematonectria termitum (Höhn.) Samuels & Rossman, Stud. Mycol. 42: 137. 1999.
Neocosmospora tucumaniae (T. Aoki, O'Donnell, Yos. Homma & Lattanzi) L. Lombard & Crous, comb. nov. MycoBank MB810966.
Basionym: Fusarium tucumaniae T. Aoki, O'Donnell, Yos. Homma & Lattanzi, Mycologia 95: 664. 2003.
Neocosmospora virguliformis (O'Donnell & T. Aoki) L. Lombard & Crous, comb. nov. MycoBank MB810967.
Basionym: Fusarium virguliforme O'Donnell & T. Aoki, Mycologia 95: 667. 2003.
Rectifusarium L. Lombard, Crous & W. Gams, gen. nov. MycoBank MB810252.
Etymology: Name refers to the erect, acremonium-like conidiophores characteristic of these fungi.
Ascomata perithecial, aggregated in groups, dark red, smooth-walled, globose to subglobose, with a papillate ostiolar region, ringed by a short collar of hyphal tips, smooth. Asci clavate, 8-spored, with a rounded apex containing a refractive apical ring. Ascospores ellipsoidal, 1-septate, constricted at the septum, verrucose, light brown. Conidiophores simple, mononematous, straight to flexuous, hyaline, septate, unbranched or rarely branched, terminating in a single phialide. Phialides cylindrical, tapering towards the apex, with periclinal thickening and flared collarettes. Sporodochia not formed. Microconidia rare, ellipsoidal to fusiform, 0–1-septate, hyaline. Macroconidia 3-septate, hyaline, ellipsoidal to fusiform, with both ends slightly curved, sometimes with a basal foot cell, apex acutely rounded. Chlamydospores hyaline, forming laterally or terminally, globose to subglobose (adapted from Gerlach & Nirenberg 1982).
Type species: Rectifusarium ventricosum (Appel & Wollenw.) L. Lombard & Crous.
Notes: The genus Rectifusarium is established here to include the fusarium-like species previously treated as F. ventricosum. Wollenweber (1913) established the section Ventricosum to accommodate F. ventricosum, recognising this Fusarium sp. as unique in the genus in having no sporodochia. Phylogenetic inference in this study showed that representatives of this group of fungi formed a distinct well-supported clade (BS = 100 %, PP = 1.0), basal to the other clades included in Clade IX.
Rectifusarium robinianum L. Lombard & Crous, sp. nov. MycoBank MB810258. Fig 15.
Fig. 15.
Rectifusarium robinianum (ex-type CBS 430.91). A–F. Conidiophores. G. Macroconidia. H. Microconidia. I. Chlamydospores. Scale bars: A = 50 μm (apply to B–C); D = 10 μm (apply to E–F); G = 10 μm (apply to H–I).
Etymology: Name derived from the plant host Robinia pseudoacacia, from which it was isolated.
Ascomatal state not observed. Conidiophores arising laterally from hyphae, simple, unbranched or sparsely branched, mononematous, straight to flexuous, septate, 110–197 × 4–7 μm, terminating in a single phialide; phialides cylindrical, tapering towards the apex, 40–85 × 2–6 μm, with periclinal thickening and slightly flared collarette. Microconidia rare, (0–)1-septate, straight and fusiform, or slightly curved and ellipsoidal, 12–16(–17) × 3–4 μm (av. 14 × 3 μm). Macroconidia (1–)3-septate, straight or slightly curved, fusiform to ellipsoidal, (22–)25–31(–33) × 5–7 μm (av. 28 × 6 μm), with rounded apex and flattened basal cell. Chlamydospores hyaline, verruculose, globose to subglobose, 6–10 μm diam, forming laterally or terminally.
Culture characteristics: Colony on PDA reaching 90 mm after 7 d at 24 °C, forming abundant white to pale luteous aerial mycelium, richly sporulating on the aerial mycelium; reverse concolorous.
Materials examined: Germany, Köln, on twig of Robinia pseudoacacia, May 1991, U. Kuchenbäcker (holotype CBS H-21948, culture ex-type CBS 430.91 = NRRL 25729); Berlin, from Solanum tuberosum, Dec. 1985, H. Nirenberg, culture CBS 830.85 = BBA 64246 = NRRL 13953.
Note: Rectifusarium robinianum can be distinguished from R. ventricosum by its smaller macroconidia and rarely branching acremonium-like conidiophores.
Rectifusarium ventricosum (Appel & Wollenw.) L. Lombard & Crous, comb. nov. MycoBank MB810253. Fig. 16.
Fig. 16.
Rectifusarium ventricosum (ex-epitype CBS 748.79). A–D. Conidiophores. E. Macroconidia. F. Chlamydospores. Scale bars: A = 50 μm (apply to B); C = 10 μm (apply to D–F).
Basionym: Fusarium ventricosum Appel & Wollenw., Phytopathology 3: 32. 1913.
≡ Fusarium solani var. ventricosum (Appel & Wollenw.) Joffe, Plant and Soil 38: 440. 1973.
= Fusarium cuneiforme Sherb., Mem. Cornell Univ. Agric. Exp. Sta. 6: 129. 1915.
Materials examined: Germany, Berlin, on tuber of Solanum tuberosum, Oct. 1909, H.W. Wollenweber [holotype B 700021849 (as Fusarium argillaceum)]; (epitype designated here: Germany, Kiel, from soil in wheat field, Dec. 1979, W. Gerlach, epitype CBS H-21947, MB198380, culture ex-epitype CBS 748.79 = BBA 62452 = NRRL 20846 = NRRL 22113).
Notes: Wollenweber (1917) synonymised F. ventricosum and F. cuneiforme under F. argillaceum. This decision was based on Fuckel's Fungi Rhenani no. 226, which Booth (1971) rejected as a misdetermination of F. argillaceum as it did not agree with the description of Fries (1832) for F. argillaceum. Comparisons of the type material (B 700021849; as F. argillaceum) and Wollenweber's Fusaria autographice delineate no. 431 agree with the description and illustrations provided by Booth (1971) for F. ventricosum based on the isolate CBS 748.79, and therefore we agree with Booth's argument that F. ventricosum is not synonymous with F. argillaceum.
Clade X
Cosmospora Rabenh., Hedwigia 2: 59. 1862. MycoBank MB1273.
= Crysogluten Briosi & Farneti, Atti Is. Bot. Univ. Lab. Critt. Pavia 8: 117. 1904.
? = Dialonectria (Sacc.) Cooke, Grevillea 12: 109. 1884.
≡ Nectria subgen. Dialonectria Sacc., Syll. Fung. 2: 490. 1883.
Ascomata perithecial, scattered or gregarious, with inconspicuous or absent stroma, obpyriform with an acute or papillate apex, orange red or bright red, turning dark red in KOH, smooth walled. Asci narrowly clavate to cylindrical, with an apical ring, 8-spored. Ascospores initially hyaline, becoming yellow brown to reddish brown, 1-septate, becoming tuberculate when mature. Conidiophores acremonium-like, consisting of lateral phialides on somatic hyphae, or with one or two levels of monochasial branching, or verticillate, hyaline. Phialides monophialidic, cylindrical to subulate to subclavate, hyaline. Microconidia ellipsoidal, oblong or clavate or slightly allantoid, aseptate, hyaline, forming slimy heads. Macroconidia absent or rare, subcylindrical, curved, slightly narrowing towards each end, apical cell often slightly hooked with a more or less pointed tip, basal cell not or scarcely pedicellate, 3–5-septate, hyaline (adapted from Rossman et al. 1999 and Gräfenhan et al. 2011).
Type species: Cosmospora coccinea Rabenh., Hedwigia 2: 59. 1862 [non Nectria coccinea (Pers.) Fr. 1849].
= Nectria cosmariospora Ces. & De Not., Comment. Soc. Crittog. Ital. 1: 195. 1863.
≡ Dialonectria cosmariospora (Ces. & De Not.) Moraves, Česká Mykol. 8: 92. 1954.
= Verticillium olivaceum W. Gams, Cephalosporium-artige Schimmelpilze: 129. 1971.
Descriptions and illustrations: Rossman et al., 1999, Gräfenhan et al., 2011.
Notes: Representatives of the genus Cosmospora formed a well-supported clade (BS ≥ 75 %, PP ≥ 0.95), which also included representatives of the genus Dialonectria (CBS 125493 & CBS 125494; Gräfenhan et al. 2011). Samuels et al. (1991) revised the genus Dialonectria (as Nectria subgen. Dialonectria) and assigned it to Cosmospora sensu Rossman. Gräfenhan et al. (2011) later resurrected the genus Dialonectria and restricted its generic concept around the type species, D. episphaeria, recognising that this species represents a species complex of at least five phylogenetic lineages. Although the phylogenetic inference in this study supports the findings of Samuels et al. (1991) that Dialonectria should be seen as a synonym of Cosmospora, we select not to introduce new combinations at present. A monographic study for both genera is required to stabilise the taxonomy of these genera. Furthermore, isolates listed in the CBS collection as “Acremonium cf. curvulum” (CBS 100551) and “Stylonectria wegeliniana” (CBS 101915) clustered within the Cosmospora clade. Both isolates appear to be sterile, and therefore their taxonomic status cannot be determined at present.
Fusicolla Bonord., Handb. Allg. Mykol.: 150. 1851. MycoBank MB8294.
Ascomata perithecial, stroma erumpent, fully or partially immersed in a slimy, pale orange sheet of hyphae over the substrate, scattered to gregarious, or in small groups, globose to pyriform with a short acute or disk-like papilla, yellow, pale buff to orange, not changing in KOH, smooth walled. Asci cylindrical to narrowly clavate, with an apical ring, 8-spored. Ascospores hyaline to pale brown, 1-septate, smooth or slightly verrucose when mature. Conidiophores initially as lateral phialides on somatic hyphae, sometimes monochasial, verticillate or penicillate, hyaline. Phialides monophialidic, cylindrical to subulate, hyaline. Microconidia absent or sparse, ellipsoidal to allantoid, aseptate, hyaline. Macroconidia falcate, straight to curved, narrowing towards the ends, apical cell often hooked with a pointed tip, basal cell slightly pedicellate 1–3-septate or 3–5-septate or up to 10-septate, hyaline. Chlamydospores absent to abundant, globose, single, in pairs or chains, sometimes forming in macroconidia (adapted from Gerlach & Nirenberg 1982 and Gräfenhan et al. 2011).
Type species: Fusicolla betae (Desm.) Bonord. Handb. Allg. Mykol.: 150. 1851.
≡ Fusisporium betae Desm., Ann. Sci. Nat., Bot. 19: 436. 1830.
≡ Fusarium betae (Desm.) Sacc., Michelia 2: 132. 1880.
≡ Pionnotes betae (Desm.) Sacc., Syll. Fung. 4: 726. 1886.
≡ Pionnotes rhizophila var. betae (Desm.) de Wild. & Durieu, Prodrome de la flore belge 2: 367. 1898.
Descriptions and illustrations: Gerlach and Nirenberg, 1982, Gräfenhan et al., 2011.
Notes: Representatives of the genus Fusicolla formed a monophyletic clade (BS = 100 %, PP = 1.0) closely related to but separate from the clades representing the genera Macroconia and Microcera. Unfortunately, no cultures or sequences of F. betae were available to be included in the present study.
Macroconia (Wollenw.) Gräfenhan et al., Stud. Mycol. 68: 101. 2011. MycoBank MB519441.
≡ Nectria sect. Macroconia Wollenw., Angew. Bot. 8: 179. 1926.
Ascomata perithecial, stroma inconspicuous or absent, solitary, subglobose with or without a small apical papilla, orange to carmine red, turning dark red to violet in KOH, sometimes with hyphal hairs arising from the outer wall. Asci cylindrical to narrowly clavate, with a simple apex, 8-spored. Ascospores yellowish, 1-septate, smooth, sometimes becoming striate when mature. Conidiophores initially as lateral phialides on somatic hyphae, later monochasial to verticillate, hyaline. Phialides monophialidic, cylindrical to subulate, hyaline. Microconidia rare or absent, ellipsoidal to allantoid, hyaline. Macroconidia subcylindrical to curved, apical cell conical or hooked, basal cell mostly conspicuously pedicellate, 3–7(–14)-septate, hyaline. Chlamydospores absent to rare, globose, single, in pairs or chains in hyphae (adapted from Gräfenhan et al. 2011).
Type species: Macroconia leptosphaeriae (Niessl.) Gräfenhan, & Schroers, Stud. Mycol. 68: 102. 2011.
≡ Nectria leptosphaeriae Niessl., Fungi Saxonici Exsiccati. Die Pilze Sachsen's: no. 165. 1886.
≡ Cucurbitaria leptosphaeriae (Niessl.) Kuntze, Rev. Gen. Plant. 3: 461. 1898.
≡ Hypomyces leptosphaeriae (Niessl.) Wollenw., Fus. Autog. Del. 1: 57. 1916.
≡ Lasionectria leptosphaeriae (Niessl.) Petch, Trans. Brit. Mycol. Soc. 21: 268. 1938.
≡ Cosmospora leptosphaeriae (Niessl.) Rossman & Samuels, Stud. Mycol. 42: 122. 1999.
Description and illustrations: Gräfenhan et al. (2011).
Notes: The genus Macroconia was raised from section name to genus level by Gräfenhan et al. (2011) for fusarium-like species having large macroconidia and minute perithecia. Phylogenetic inference in this study supports this decision, with representatives of this genus forming a well-supported clade (BS = 100 %, PP = 1.0) closely related to but separate from the genera Fusicolla and Microcera.
Microcera Desm., Ann. Sci. Nat. Bot. 10: 359. 1848. MycoBank MB8920.
= Pseudomicrocera Petch, Trans. Brit. Mycol. Soc. 7: 164. 1921.
Ascomata perithecial with stroma and/or byssus covering host, solitary or in groups, globose, with a blunt papilla, orange to dark red, turning dark red or violet in KOH, finely roughened. Asci cylindrical to narrowly clavate, with an apical ring, 8-spored. Ascospores hyaline to pale yellow-brown, 1(–3)-septate, smooth, sometimes becoming tuberculate when mature. Conidiophores as lateral phialides on somatic hyphae, becoming monochasial, verticillate to penicillate, hyaline, forming discrete sporodochia or synnemata on the host. Phialides monophialidic, cylindrical to subulate to subclavate, hyaline. Macroconidia pale, orange, pink or bright red in mass, subcylindrical, moderately or conspicuously curved, apical cell often slightly or conspicuously hooked, basal cell scarcely to conspicuously pedicellate, (0–)3–5(–12)-septate, hyaline (adapted from Gräfenhan et al. 2011).
Type species: Microcera coccophila Desm., Ann. Sci. Nat. Bot. 10: 359. 1848.
≡ Tubercularia coccophila (Desm.) Bonord., Abh. Naturf. Ges. Halle 8: 96. 1864.
≡ Fusarium coccophila (Desm.) Wollenw. & Reinking, Die Fusarien, ihre Beschreibung, Schadwirkung und Bekämpfung: 34. 1935.
≡ Fusarium coccophilum (Desm.) Wollenw. & Reinking, Die Fusarien, ihre Beschreibung, Schadwirkung und Bekämpfung: 34. 1935.
≡ Nectria episphaeria f. coccophila (Desm.) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 32: 662. 1945.
≡ Fusarium episphaeria f. coccophilum (Desm.) W.C. Snyder & H.N. Hansen, Amer. J. Bot. 32: 662. 1945.
= Atractium flammeum Berk. & Ravenel, Ann. Mag. Nat. Hist. 13: 461. 1854.
= Microcera pluriseptata Cooke & Massee, Grevillea 17: 43. 1888.
Description and illustrations: Gräfenhan et al. (2011).
Notes: The genus Microcera includes fusarium-like species generally regarded as entomogenous fungi associated with scale insects, although they can also be found on other substrates (Gräfenhan et al. 2011). Gräfenhan et al. (2011) resurrected this genus based on DNA sequence data and its ecological association, after Wollenweber & Reinking (1935) placed all Microcera spp. in Fusarium. Our phylogenetic inference supports this decision, as representatives of the genus Microcera clustered in a well-supported clade (BS ≥ 75 %, PP ≥ 0.95) distantly related to Fusarium but closely related to the genera Fusicolla and Macroconia.
Stylonectria Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Math.-Naturwiss. Cl., Abt. 1, 124: 52. 1915. MycoBank MB5301.
Ascomata perithecial on a thin, white to yellow, hyphal or subiculum-like stroma, gregarious in groups of up to 20, subglobose, pyriform to subcylindrical, with a rounded or broad, circular, flat disc on a venter-like neck, pale yellow, orange-red, orange-brown, or pale to dark red, becoming dark red to purple in KOH, smooth. Asci cylindrical to clavate, apex simple or with a ring, 8-spored. Ascospores hyaline or yellow to pale brown, 1-septate, cylindrical to allantoid to ellipsoidal, smooth or tuberculate. Conidiophores initially formed as unbranched phialides on somatic hyphae, sometimes loosely branched, sometimes forming small sporodochia. Phialides monophialidic, cylindrical to subcylindrical, with a distinct collarette. Microconidia sparse, allantoid to lunulate, slightly or strongly curved, aseptate, in slimy heads. Macroconidia orange in mass, subcylindrical or moderately to strongly curved, falcate, 0–1-septate, apex narrower than base, apical cell blunt or hooked, basal cell not or scarcely pedicellate (adapted from Höhnel 1915 and Gräfenhan et al. 2011).
Type species: Stylonectria applanata Höhn., Sitzungsber. Kaiserl. Akad. Wiss., Wien, Math.-Naturwiss. Kl., Abt. 1, 124: 52. 1915.
Descriptions and illustration: Höhnel, 1915, Weese, 1916, Gräfenhan et al., 2011.
Notes: Species of Stylonectria are host-specific fungicolous fungi, which Rossman et al. (1999) considered as a synonym of Cosmospora. Phylogenetic inference in this study and Gräfenhan et al. (2011) showed that the genus Stylonectria formed a well-supported clade (BS = 100 %, PP = 1.0) basal to the other genera included in Clade X.
Clade XI
Corallomycetella Henn., Hedwigia 43: 245. 1904. MycoBank MB1237.
= Corallomyces Berk & M.A. Curtis, J. Acad. Nat. Sci. Philadelphia, Ser. 2, 2: 289. 1853 [non Fr. 1849].
= Rhizostilbella Wolk, Mykol. Zentralbl. 4: 237. 1914.
Ascomata perithecial, solitary or gregarious, associated with reddish rhizomorphs or synnemata, obpyriform, orange-red to red, changing to purple in KOH, slightly scruffy, smooth around the ostiole. Asci clavate to cylindrical, with an apical ring, 8-spored. Ascospores ellipsoidal, 1-septate, constricted at the septum, hyaline to yellow-brown, finely striate. Asexual morph synnematous. Synnemata solitary or gregarious, 2–5 caespitose, arising laterally or as terminal extension of the rhizomorphs or directly from the substrate, cylindric-capitate, subulate-capitate, cylindrical, slender to robust, straight to curved to sinuous, unbranched or branched, hirsute, pale luteous to luteous, turning red to purple in KOH. Marginal hyphae echinulate to verrucose, pale luteous, turning bright red in KOH, with clavate terminal cells, covering the entire surface of stipe. Conidiophores unbranched, or once simple monochasial or monoverticillate. Phialides cylindrical, terminal, collarettes not flared, periclinal thickening conspicuous. Conidia ellipsoidal, ovoidal with a truncate base, aseptate, smooth, forming white to yellow, subglobose conidial masses (adapted from Rossman et al. 1999 and Herrera et al. 2013b).
Type species: Corallomycetella repens (Berk. & Broome) Rossman & Samuels, Stud. Mycol. 42: 113. 1999.
≡ Sphaerostilbe repens Berk. & Broome, J. Linn. Soc., Bot. 14: 114. 1875.
= Corallomycetella heinsenii Henn., Hedwigia 43: 245. 1904.
≡ Corallomyces heinsenii Henn., Hedwigia 43: 245. 1904.
= Corallomycetella heinsenii Henn. Hedwigia 43: 245. 1904.
= Nectria mauritiicola Henn., Seifert & Samuels, Stud. Mycol. 27: 161. 1985.
= Stilbum incarnatum Wakker, Ziekten van het Suikerriet op Java, Leiden: 197. 1898.
= Nectria coccinea (Pers.: Fr.) Fr. var. platyspora Rehm, Ann. Mycol. 7: 137. 1900.
≡ Nectria platyspora (Rehm) Weese, Ann. Mycol. 8: 464. 1910.
= Rhizostilbella rubra Wolk, Mykol. Zentralbl. 4: 237. 1914.
= Stilbum incarnatum var. dioscoreae Sacc., Bull. Orto Bot. Regia Univ. Napoli 6: 63. 1918.
= Cephalosporium kashiense R.Y. Roy & G.N. Singh, Curr. Sci. 37: 535. 1968.
≡ Acremonium kashiense (R.Y. Roy & G.N. Singh) W. Gams, Cephalosporium-artige Schimmelpilze: 138. 1971.
= Rhizostilbella hibisci (Pat.) Seifert, Stud. Mycol. 27: 162. 1985.
≡ Stilbum hibisci Pat., J. Bot. (Morot): 320. 1891.
Description and illustrations: Herrera et al. (2013b).
Notes: Species of Corallomycetella are tropical fungi characterised by the formation of brightly coloured rhizomorphs of their rhizostilbella-like asexual morphs (Seifert, 1985, Rossman et al., 1999, Herrera et al., 2013b). These fungi are associated with rotting diseases of various woody tropical plant hosts (Rossman et al., 1999, Herrera et al., 2013b). Phylogenetic inference in this study showed that the species of Corallomycetella formed a distinct monophyletic clade (BS = 100 %, PP = 1.0).
Paracremonium L. Lombard & Crous, gen. nov. MycoBank MB810267.
Etymology: Name refers to the acremonium-like morphology of these fungi.
Ascomatal morph not observed. Mycelium consisting of hyaline, septate, branched hyphae, sometimes forming sterile coils with conidiophores radiating outwards, hyphal septa inconspicuously swollen. Conidiophores arising laterally from somatic hyphae, erect, cylindrical to subcylindrical, unbranched or rarely branched, aseptate or septate, smooth, hyaline. Conidiogenous cell terminal, monophialidic, hyaline, smooth, elongate-ampulliform or subcylindrical, tapering towards the apex, with periclinal thickening and inconspicuous collarette. Conidia aseptate, fusiform to ellipsoidal to cylindrical, straight to slightly or strongly curved, forming slimy heads on the conidiophore.
Type species: Paracremonium inflatum L. Lombard & Crous.
Notes: The genus Paracremonium is established here for different strains from a group of fungi previously treated as Acremonium recifei (Gams 1971; also see Xenoacremonium below). Species of Paracremonium are distinguished from other acremonium-like genera by the formation of sterile coils from which conidiophores radiate and having inconspicuously swollen septa in the hyphae. All species in Paracremonium are associated with human infections (see below). Phylogenetic inference in this study showed that representatives of this genus formed a monophyletic clade (BS = 100 %, PP = 1.0) closely related but separate from the clades representing Corallomycetella and Xenoacremonium.
Paracremonium inflatum L. Lombard & Crous, sp. nov. MycoBank MB810268. Fig. 17.
Fig. 17.
Paracremonium inflatum (ex-type CBS 485.77). A–B, E–F. Conidiophores arising laterally from somatic hyphae with swollen hyphal septa. C–D, G. Conidiophores arising laterally from somatic hyphae in sterile coils. H. Conidia. Scale bars: A = 50 μm (apply to B–D); E = 10 μm (apply to F–H).
Etymology: Name refers to the inconspicuous swollen septa of the hyphae formed by this fungus.
Ascomatal state unknown. Mycelium consisting of hyaline, septate, branched, 2–4 μm diam hyphae, inconspicuously swollen at the hyphal septa, sometimes forming sterile coils with conidiophores radiating outwards. Conidiophores arising laterally from somatic hyphae, erect, subcylindrical, unbranched or rarely branched, 0–1-septate, up to 115 μm tall, 2–3 μm diam, hyaline, smooth, terminating in one or two conidiogenous cells. Conidiogenous cells terminal, elongate-ampulliform, tapering towards apex, 20–85 × 2–4 μm, apex 1.5–2 μm diam, with prominent periclinal thickening and inconspicuous collarette, hyaline, smooth. Conidia formed in heads at apex of conidiogenous cells, aseptate, ellipsoidal to fusiform, smooth, slightly to strongly curved, 5–6 × 1–2 μm (av. 5 × 2 μm). Chlamydospores not seen.
Culture characteristics: Colony on PDA reaching 50–65 mm diam after 7 d at 24 °C; colony consists of semi-immersed aerial mycelium; surface with pink to salmon centre becoming white at the margins; reverse concolorous.
Materials examined: India, from a granulomatous lesion on the right hand of a male Homo sapiens, Oct. 1977, A.A. Padhye (holotype CBS H-21946, culture ex-type CBS 485.77 = CDC 77-043179). Colombia, Dep. de Meta, Municipio de Villavicencio, 25 km from road Villavicencio-Acacías, 550°m alt., from soil in maize-field, 18 Feb. 1978, O. Rangel, culture CBS 482.78.
Paracremonium contagium L. Lombard & Crous, sp. nov. MycoBank MB810269. Fig. 18.
Fig. 18.
Paracremonium contagium (ex-type CBS 110348). A–C. Conidiophores arising laterally from somatic hyphae. D. Conidia. Scale bar: A = 10 μm (apply to B–D).
Etymology: Name refers to the ability of this fungus to cause a subcutaneous infection of humans.
Ascomatal state unknown. Mycelium consisting of hyaline, septate, branched, 2–4 μm diam hyphae, sometimes inconspicuously swollen at the hyphal septa. Conidiophores arising laterally from somatic hyphae, erect, subcylindrical, unbranched or rarely branched, 0–1-septate, up to 75 μm tall, 1–2 μm diam, hyaline, smooth, terminating in one or two conidiogenous cells. Conidiogenous cells terminal, elongate-ampulliform, tapering towards apex, 25–50 × 2–3 μm, apex 1.5–2 μm diam, with prominent periclinal thickening and inconspicuous collarette, hyaline, smooth. Conidia formed in heads at apex of conidiogenous cells, aseptate, ellipsoidal to fusiform, smooth, slightly to strongly curved, 4–6(–7) × 2–3 μm (av. 5 × 2 μm). Chlamydospores not seen.
Culture characteristics: Colony on PDA reaching 45–50 mm diam after 7 d at 24 °C; colony consists of semi-immersed aerial mycelium; surface with pink to salmon centre becoming white at the margins; reverse apricot in centre becoming salmon to pale pink to white towards the margin.
Material examined: Canada, Ontario, Toronto, from a subcutaneous lesion in the left thigh of a male Homo sapiens, S. Mohan (holotype CBS H-21945, culture ex-type CBS 110348 = UAMH 10141).
Note: Paracremonium contagium can be distinguished from P. inflatum by its shorter conidiophores and the absence of sterile coils from which conidiophores radiate.
Xenoacremonium L. Lombard & Crous, gen. nov. MycoBank MB810270.
Etymology: Name refers to the acremonium-like morphology of these fungi.
Ascomatal state not observed. Mycelium consisting of hyaline, septate, branched hyphae. Conidiophores either as lateral phialidic pegs or arising laterally from somatic hyphae, erect, cylindrical to subcylindrical, unbranched or rarely branched, aseptate or septate, smooth, hyaline. Conidiogenous cells terminal, monophialidic, hyaline, smooth, elongate-ampulliform or subcylindrical, tapering towards the apex, with periclinal thickening and inconspicuous collarette. Conidia aseptate, fusiform to ellipsoidal to cylindrical, slightly or strongly curved, forming slimy heads on the conidiophore.
Type species: Xenoacremonium recifei (Leão & Lôbo) L. Lombard & Crous.
Notes: The genus Xenoacremonium is established here for a group of fungi previously treated as Acremonium recifei (Gams 1971), which includes the ex-type of A. recifei (CBS 137.35). Phylogenetic inference in this study showed that representatives of this genus formed a monophyletic clade (BS = 100 %, PP = 1.0) closely related but separate from the clades representing Corallomycetella and Paracremonium.
Xenoacremonium falcatus L. Lombard & Crous, sp. nov. MycoBank MB810271. Fig. 19.
Fig. 19.
Xenoacremonium falcatus (ex-type CBS 400.85). A, C. Conidiophores arising laterally from somatic hyphae. B. Lateral phialidic pegs. D. Conidia. Scale bars: A = 50 μm; B = 10 μm (apply to C–D).
Etymology: Name refers to the strongly curved conidia produced by this fungus.
Ascomatal morph unknown. Mycelium consisting of hyaline, septate, branched, 2–3 μm diam hyphae. Conidiophores either as lateral phialidic pegs, 2–4 × 1–2 μm, or arising laterally from somatic hyphae, erect, subcylindrical, unbranched or rarely branched, 0–1-septate, up to 80 μm tall, 2–4 μm diam, hyaline, smooth, terminating in one or two conidiogenous cells. Conidiogenous cells terminal, elongate-ampulliform, tapering towards apex, 25–80 × 2–3 μm, apex 1–2 μm diam, with prominent periclinal thickening and inconspicuous collarette, hyaline, smooth. Conidia formed in heads at apex of conidiogenous cells, aseptate, ellipsoidal to fusiform or reniform, smooth, slightly to strongly curved, 4–8(–10) × 1–2 μm (av. 6 × 2 μm). Chlamydospores not seen.
Culture characteristics: Colony on PDA reaching 55–60 mm diam after 7 d 24 °C; colony consists of semi-immersed aerial mycelium; surface with pale luteous to luteous centre becoming white towards the margins; reverse pale luteous with pale luteous or luteous pigment throughout the medium.
Material examined: New Zealand, North Island, Woodhill Forest, Compartment 75, on Pinus radiata, 14 May 1982, J. Reid (holotype CBS H-21944, culture ex-type CBS 400.85).
Notes: The conidia of Xenoacremonium falcatus [4–8(–10) × 1–2 μm (av. 6 × 2 μm)] are slightly larger than those of X. recifei [4–6(–7.5) × 1–2 μm; Gams 1971]. Furthermore, X. falcatus produces lateral phialidic pegs on its somatic hyphae, a feature not observed in this study or reported for X. recifei by Gams (1971).
Xenoacremonium recifei (Leão & Lôbo) L. Lombard & Crous, comb. nov. MycoBank MB810272. Fig. 20.
Fig. 20.
Xenoacremonium recifei (ex-type CBS 137.35). A–B. Conidiophores arising laterally from somatic hyphae. C. Conidia. Scale bars: A = 50 μm; B = 10 μm (apply to C).
Basionym: Cephalosporium recifei Leão & Lôbo, C.R. Soc. Biol. R. Janeiro: 205. 1934.
≡ Hyalopus recifei (Leão & Lôbo) Leão & M.A.J. Barbosa, Sub. Stud. Parasitol. Genero Hyalopus Corda 1838: 39. 1941.
≡ Acremonium recifei (Leão & Lôbo) W. Gams, Cephalosporium-artige Schimmelpilze: 133. 1971.
= Hyalopus furcatus Bat. & C. Ram., Atas Inst. Micol. Univ. Recife 4: 290. 1967.
= Hyalopus furcatum Bat. & C. Ram., Atas Inst. Micol. Univ. Recife 4: 290. 1967.
Material examined: Brazil, from mycetoma on Homo sapiens, 1934, A.E. de Area Leão (culture ex-type CBS 137.35).
Clade XII
Nalanthamala Subram., J. Indian Bot. Soc. 35: 478. 1956.
= Rubrinectria Rossman & Samuels, Stud. Mycol. 42. 1999.
Ascomata perithecial on an erumpent stroma, aggregated in groups, superficial, globose to broadly ovate or broadly pyriform, with a short, rounded, obtuse papilla, orange-red with orange, rarely green scales, turning dark red in KOH. Asci cylindrical, apex simple or with a small, refractive ring, 8-spored. Ascospores broadly ellipsoidal to fusiform, 1-septate, slightly constricted at the septum, pale brown to golden-brown, coarsely striate. Conidiophores sporodochial or penicillate, stalked, mononematous. Sporodochia hyaline, erumpent, hemispherical or flat; cells of well-developed sporodochia angular to globose, forming pseudoparenchymatous tissue, evenly thin-walled. Phialides formed singly or in whorls on cylindrical cells that arise from pseudoparenchymatous tissue of sporodochia or in whorls on penicillately branched conidiophores, elongate, widest at the base or in the lower third, narrowing towards the apex or cylindrical and narrowing below the apex. Conidia ovoid, frequently with somewhat truncated ends, hyaline, aseptate, smooth, held in dry chains (adapted from Rossman et al. 1999 and Schroers et al. 2005).
Type species: Nalanthamala madreeya Subram., J. Indian Bot. Soc. 35: 478. 1956.
Descriptions and illustrations: Rossman et al., 1999, Schroers et al., 2005.
Notes: Species of Nalanthamala are tropical fungi associated with wilt and blight diseases of various economically important tropical crops (Schroers et al., 2005, Rossman et al., 2013). Representatives of this genus formed a monophyletic clade (BS = 100 %, PP = 1.0) closely related to the clade representing the genus Nectria. Unfortunately cultures or sequences of N. madreeya were not available for the molecular phylogeny.
Nectria (Fr.) Fr., Summa Veg. Scand. 2: 387. 1849. MycoBank MB3431.
≡ Hypocrea sect. Nectria Fr., Syst. Orbis Veg.: 105. 1825.
= Ephodrosphaera Dumort., Commentat. Bot.: 90. 1822.
= Sphaerostilbe Tul. & C. Tul., Sel. Fung. Carpol. 1: 130. 1861.
= Megalonectria Speg., Anales Soc. Ci. Argent. 12: 217. 1881.
= Stilbonectria P. Karst., Hedwigia 28: 194. 1889.
= Creonectria Seaver, Mycologia 1: 183. 1909.
= Rhodothrix Vain., Ann. Acad. Sci. Fenn. 15: 31. 1921.
= Styloletendraea Weese, Mitt. Bot. Inst. Techn. Hochsch. Wien 1: 60. 1924.
= Ochraceospora Fiore, Boll. Soc. Naturalisti Napoli 41: 90. 1930.
Ascomata perithecial on or nearly or completely immersed in an erumpent stroma, aggregated in groups, red to bay to sienna, turning bright red to blood red to purple in KOH, subglobose to globose, surface smooth to warted. Asci cylindrical to narrowly clavate or clavate, with an inconspicuous ring, 8-spored. Ascospores ellipsoidal, oblong, fusiform, pyriform or allantoid, rounded at both ends, smooth or spinulose, hyaline, straight to slightly curved, up to 4-septate. Conidiophores pycnidial, sporodochial, lateral phialidic pegs or acropleurogenous. Microconidia hyaline, ellipsoid to fusoid, rarely curved, aseptate. Macroconidia hyaline, ellipsoidal, oblong, cylindrical to allantoid or subglobose to ellipsoidal, 0–1-septate, smooth, straight to slightly curved, rounded at both ends. Chlamydospores rare (adapted from Hirooka et al. 2012).
Type species: Nectria cinnabarina (Tode: Fr.) Fr., Summa Veg. Scand. 2: 388. 1849.
≡ Sphaeria cinnabarina Tode: Fr., Tode, Fungi Mecklenburg. Selecti. 2: 9. 1791: Fries, Syst. Mycol. 2: 412. 1823.
≡ Cucurbitaria cinnabarina (Tode: Fr) Grev., Scot. Crypt. Fl. 3: 135. 1825.
= Sphaeria tremelloides Weigel, Observ. Bot.: 46. 1772.
= Tubercularia vulgaris Tode: Fr., Tode, Fungi Mecklenburg. Selecti. 1: 18. 1790: Fries, Syst. Mycol. 3: 464. 1832.
= Sphaeria decolorans Pers.: Fr., Persoon, Neues Mag. Bot. 1: 83. 1794: Fries, Syst. Mycol. 2: 412. 1823.
= Sphaeria celastri Fr., Elenchus Fung. 2: 81. 1827.
= Nectria russellii Berk. & M.A. Curtis, Grevillea 4: 45. 1875.
= Nectria offuscata Berk. & M.A. Curtis, Grevillea 4: 45. 1875.
= Creonectria purpurea (L.) Seaver, Mycologia 1: 183. 1909.
≡ Tremella purpurea L., Species Pl.: 1158. 1753.
Description and illustration: Hirooka et al. (2012).
Notes: Hirooka et al. (2012) recently revised Nectria, recognising 29 species within the genus. Representatives of this genus included in this study formed a monophyletic clade (BS ≥ 75 %, PP ≥ 0.95) closely related to the genus Nalanthamala.
Clade XIII
Allantonectria Earle, In: Greene, Pl. Baker. 2: 11. 1901. MycoBank MB128.
Ascomata perithecial on a well-developed, erumpent stroma, superficial, scattered to aggregated, subglobose to globose, sometimes with a depressed apical region, bay to scarlet, turning blood-red in KOH, sometimes surface scruffy or scaly, slightly orange. Asci narrowly clavate with an inconspicuous ring at the apex, 8-spored. Ascospores allantoid to cylindrical with rounded corners, straight to slightly curved, aseptate, hyaline, smooth. Lateral phialidic pegs abundant, enteroblastic, monophialidic, flask-shaped. Conidiophores abundant, unbranched, sometimes trichoderma-like. Conidiogenous cells monophialidic, cylindrical, tapering towards the apex or slightly flask-shaped. Conidia oblong or ellipsoidal with strongly constricted centre, hyaline, straight or slightly curved, rounded at both ends (adapted from Hirooka et al. 2012).
Type species: Allantonectria miltina (Mont.) Weese, Ann. Mycol. 8: 464. 1910.
≡ Sphaeria miltina Mont., Explor. Sci. Algérie, Bot. I, 1: 477. 1848.
≡ Nectria miltina (Mont.) Mont., Syll. Gen. Sp. Pl. Cryptog.: 225. 1856.
≡ Nectriella miltina (Mont.) Sacc., Michelia 1: 278. 1878.
= Allantonectria yuccae Earle, In: Greene, Pl. Baker. 2: 11. 1901.
= Nectriella bacillispora Traverso & Spessa, Bol. Soc. Brot. 25: 172. 1910.
Description and illustrations: Hirooka et al. (2012).
Notes: The genus Allantonectria is monotypic based on A. miltina, recently resurrected to generic level by Hirooka et al. (2012) after Rossman et al. (1999) placed the type species in Nectria. Isolates of A. miltina formed a monophyletic clade (BS ≥ 75 %, PP ≥ 0.95), distinct from the Nectria clade, but closely related to the clade representing the genus Thyronectria.
Thyronectria Sacc., Grevillea 4 (no. 29): 21. 1875. MycoBank MB5469.
= Pleonectria Sacc., Nuovo Giom. Bot. Ital. 8: 78. 1876.
= Chilonectria Sacc., Michelia 1: 279. 1878.
= Nectria subgenus Aponectria Sacc., Michelia 1: 296. 1878.
≡ Aponectria (Sacc.) Sacc., Syll. Fung. 2: 516. 1883.
= Mattirolia Berl. & Bres., Annuario Soc. Alpinisti Tridentini 14: 55. 1889.
= Scoleconectria Seaver, Mycologia 1: 197. 1909.
= Thyronectroidea Seaver, Mycologia 1: 206. 1909.
Ascomata perithecial, immersed in a stroma or superficial, densely aggregated, subglobose to globose to flask-shaped, apex obtuse, red to umber, turning slightly purple in KOH. Asci oblong or clavate, with undifferentiated apex or with an inconspicuous ring, 8-spored. Ascospores ellipsoidal, fusiform, long-cylindrical to filiform, hyaline, (0–)1-septate, multiseptate to muriform, smooth or striate, sometimes budding in the ascus to produce oblong to allantoid, aseptate, hyaline, ascoconidia. On natural substrate asexual morph sometimes pycnidial. Pycnidia co-occurring with ascomata, solitary or aggregated in groups, superficial, subglobose to irregularly discoid to cupulate or elongate and erect, rosy, orange, red, violaceous brown to nearly black. Conidiophores densely packed, simple, irregularly or verticillately branched. Conidia formed on lateral phialidic pegs or cylindrical to subulate phialides, conidial formation enteroblastic. Conidia hyaline, oblong, ellipsoid or allantoid, aseptate. In culture, asexual morph forming verticillate conidiophores or pycnidia. Conidiophores unbranched or branched, but sometimes densely branched to form sporodochia. Conidiogenous cells monophialidic, cylindrical, slightly curved towards the apex. Conidia oblong, ellipsoidal, cylindrical or allantoid, hyaline (0–)1–2-septate, smooth (adapted from Hirooka et al. 2012 and Jaklitsch & Voglmayr 2014).
Type species: Thyronectria rhodochlora (Mont.) Seeler, J. Arnold Arbor. 21: 455. 1940.
≡ Sphaeria rhodochlora Mont., Ann. Sci. Nat., Bot. 1: 307. 1834.
≡ Mattirolia rhodochlora (Mont.) Berl. (as “rhodoclora”), Atti Congr. Bot. Int., (Genova): 574. 1892.
≡ Pleosphaeria rhodochlora (Mont.) Sacc., Syll. Fung. (Abellini) 2: 306. 1883.
≡ Trichosphaeria rhodochlora (Mont.) Sacc., Syll. Fung. (Abellini) 1: 454. 1882.
= Pleosphaeria mutabilis Sacc., Syll. Fung. 2: 306. 1883.
≡ Mattirolia mutabilis (Sacc.) Checa, M.N. Blanco & G. Moreno, Mycotaxon 125: 153. 2013.
≡ Strickeria mutabilis (Sacc.) G. Winter, Rabenh. Krypt.-Fl., ed. 2, 1: 288. 1885.
= Thyronectria patavina Sacc., Atti Soc. Veneto-Trentino Sci. Nat. 4: 123. 1875.
≡ Nectria patavina (Sacc.) Rossman, Mem. New York Bot. Gard. 49: 260. 1989.
≡ Valsonectria patavina (Sacc.) Cooke, Grevillea 12: 105. 1884.
= Nectria pyrrhochlora Auersw., (as “pyrrochlora”) in Rabenhorst, Hedwigia 8: 88. 1869.
≡ Calonectria pyrrhochlora (Auersw.) Sacc., (as “pyrrochlora”) Michelia 1: 251. 1878.
≡ Thyronectria pyrrhochlora (Auersw.) Sacc., Michelia 2: 325. 1881.
≡ Valsonectria pyrrochlora (Auersw.) Cooke, Grevillea 12: 105. 1884.
≡ Pleonectria pyrrhochlora (Auersw.) G. Winter, Rabenh. Krypt.-Fl. Ed. 2, 1, II. Abt.: Ascomyc.: Gymnoasceen: 108. 1884.
≡ Mattirolia pyrrochlora (Auersw.) Starbäck, Bih. Kungl. Svenska Vetenskapsakad. Handl. 19: 43. 1894.
Descriptions and illustrations: Hirooka et al., 2012, Jaklitsch and Voglmayr, 2014.
Notes: Recently, Hirooka et al. (2012) revised this group of fungi, placing them in the genus Pleonectria, with P. lamyi as type, stating that this generic name was the oldest available name for these fungi. Jaklitsch & Voglmayr (2014), however, argued that the generic name Thyronectria represents the oldest name for these fungi based on phylogenetic inference. Previously, these fungi were incorrectly placed in the fungal family Thyridiaceae due to the presence of paraphyses, but have now been shown to belong to the Nectriaceae (Jaklitsch & Voglmayr 2014). Phylogenetic inference in the present study supports this conclusion with representatives of Thyronectria forming a monophyletic clade (BS ≥ 75 %, PP ≥ 0.95) closely related to but separate from the clade representing Allantonectria.
Clade XIV
Tilachlidiaceae L. Lombard & Crous, fam. nov. MycoBank MB810273.
Ascomatal state unknown. Conidiophores synnematous or acremonium-like. Synnemata terete, simple to branched, cylindrical, narrowing towards the apex, consisting of bundles of parallel longitudinal, closely compacted hyphae with 1–4 scattered phialides terminating the hyphae of the synnema. Phialides cymbiform to cylindrical, hyaline, aseptate, with obvious collarettes, narrowing towards the apex. Conidia hyaline, fusiform to ellipsoid to subcylindrical, aseptate becoming 1–3-septate in culture, smooth to finely ornamented, with or without mucoid sheath, formed in chains or agglutinating into large spherical or irregular white clumps. Parasitic or saprobic on living or dead foliicolous or entomogenous fungi.
Type genus: Tilachlidium Preuss.
Type species: Tilachlidium brachiatum (Batsch) Petch.
Notes: The fungal family Tilachlidiaceae is introduced here to include species of the synnematous genera Septofusidium and Tilachlidium. Gams (1971) placed the genus Septofusidium in the family Nectriaceae based on morphological characters, whereas the genus Tilachlidium was classified as incertae sedis in the order Hypocreales (Gams 1971). No records could be located where Septofusidium has been treated in a molecular or phylogenetic analysis and neither are there any DNA sequence records available for this genus on NCBI's GenBank sequence database. Only one record for T. brachiatum (CBS 506.67; HQ232177) could be found on GenBank. Therefore, this study represents the first molecular phylogenetic inference to include Septofusidium. Representatives of both genera clustered together in a well-supported clade (BS ≥ 75 %, PP ≥ 0.95) basal to the clades (Clades I–XIII) representing the family Nectriaceae, supporting the introduction of the new family Tilachlidiaceae.
Tilachlidium Preuss, Linnaea 24: 126. 1851. MycoBank MB10236. Fig. 21.
Fig. 21.
Tilachlidium brachiatum (CBS 505.67). A. Synnema of bundled, parallel, compacted hyphae with lateral and terminal phialides. B. Phialides terminating hyphae of synnema. C. Lateral phialides extending from synnema. D. Conidia. Scale bars: A = 50 μm; B = 10 μm (apply to C–D).
Ascomatal state unknown. Synnemata cylindrical, simple or branched, narrowing towards the apex, consisting of bundles of parallel, longitudinal, usually closely compacted hyphae. Phialides scattered, hyaline, subulate, gradually narrowing to an acute apex, usually terminating hyphae of the synnema, or as lateral cells of the hyphae, single or in groups. Conidia oblong to ellipsoidal, aseptate, hyaline, smooth, covered by a mucoid layer, aggregating into large spherical or irregular masses.
Type species: Tilachlidium brachiatum (Batsch) Petch, Trans. Brit. Mycol. Soc. 21: 66. 1937.
≡ Clavaria brachiata Batsch., Elenchus Fung. 1: 233. 1786.
≡ Isaria brachiata (Batsch) Schum., Enum. Fl. Saell. 2: 443. 1803.
= Isaria agaricina Pers., Disp. Meth. Fung.: 111. 1794.
= Isaria citrine Pers., Icon. Descr. Fung. Minus Cognit., Lipsiae: 9. 1798.
= Isaria intricata Fr., Syst. Mycol. 3: 278. 1829.
= Isaria filiformis Wallr., Fl. Cryptog. German. 2: 307. 1833.
= Tilachlidium pinnatum Preuss, Linnaea 24: 127. 1851.
= Corethropsis epimyces Massee, J. R. Microbiol. Soc. 5: 759. 1885.
= Tilachlidium subulatum A.L. Smith, Trans. Brit. Mycol. Soc. 3: 122. 1908.
= Hirsutella ramosa Mains, Mycologia 41: 308. 1949.
= Tilachlidium ramosum (Mains) Mains, Mycologia 43: 714. 1952.
= Tilachlidium setigerum Malençon, Bull. Soc. Hist. Natr. Afr. N. 44: 148. 1953.
Descriptions and illustrations: Mains, 1951, Gams, 1971.
Notes: Species of Tilachlidium are saprophytic fungi growing on dried fungi or entomogenous on lepidopterous insects (Petch, 1931, Mains, 1951). Representatives of the genus Tilachlidium formed a monophyletic clade (BS = 100 %, PP = 1.0) closely related to but separate from the clade representing the genus Septofusidium.
Septofusidium W. Gams, Cephalosporium-artige Schimmelpilze: 147. 1971. MycoBank MB9882. Fig. 22.
Fig. 22.
Septofusidium. A–C. S. herbarum. A–B. Conidiophores. C. Conidia. D–F. S. berolinese. D–E. Conidiophores. F. Conidia. Scale bar: A = 10 μm (apply to B–F).
Ascomatal state unknown. Conidiophores basitonously verticillate, arising laterally from submerged hyphae. Phialides sometimes integrated in septate branches, cylindrical to allantoid, smooth, becoming verrucose, hyaline to yellow. Conidia formed in long divergent chains, cylindrical to fusiform, 0–7-septate, hyaline to yellow, smooth or roughened to verrucose, sometimes with distinct hilum at both ends.
Type species: Septofusidium elegantulum (Pidopl.) W. Gams, Cephalosporium-artige Schimmelpilze: 147. 1971.
≡ Fusidium elegantulum Pidopl., Mykrobiol. Zh. Kiew 9: 53. 1948.
Descriptions and illustrations: Gams, 1971, Samson, 1974.
Notes: Species of Septofusidium are regarded as parasitic on foliicolous fungi (Gams, 1971, Samson, 1974). Representatives of this genus formed a monophyletic clade (BS ≥ 75 %, PP ≥ 0.95) within the larger clade representing the new family Tilachlidiaceae. Unfortunately sequences or cultures of S. elegantulum were not available for study. One isolate (CBS 696.93) listed as “Pseudonectria coronata” in the CBS collection also clustered within the Tilachlidiaceae clade. However, this isolate was sterile and analyses of the DNA sequences were inconclusive. Therefore this isolate cannot be identified at present and might be a contaminant of the original culture.
Clade XV
This weakly-supported clade includes representatives of the hypocrealean families Clavicipitaceae and Niessliaceae. The family Clavicipitaceae is represented here by isolates previously treated as Aphanocladium album (CBS 401.70, CBS 892.72 & CBS 634.75; Gams 1971) which formed a well-supported clade (BS = 100 %, PP = 1.0). Based on the illustration provided by Gams (1971) for CBS 401.70 and confirmed by comparisons of DNA sequences on NCBI’s GenBank sequence database, these isolates represent unknown species in the genus Pochonia. Unfortunately, all three isolates appear to be sterile and are therefore tentatively treated as undetermined species of Pochonia pending further investigation. The family Niessliaceae is represented by Hyaloseta nolinae (CBS 109837) and Trichosphaerella ceratophora (CBS 130.82). An isolate listed in the CBS collection as “Nectria dacryocarpa” (CBS 113532) also clustered within this clade, but is also sterile and no conclusive identification could be made based on DNA sequence comparisons, and is therefore not treated further here.
Clade XVI
This weakly-supported clade includes the ex-type of Rodentomyces reticulatus (CBS 128675; Doveri et al. 2010) and an authentic strain of Sarocladium kiliense (CBS 400.52; Herrera et al. 2013b). The monotypic genus Rodentomyces was initially placed in the Nectriaceae based on ITS and LSU sequence data (Doveri et al. 2010). However, this was not supported in the phylogenetic inference in this study. Analyses of the individual gene regions used here clustered both R. reticulatus and S. kiliense as a weakly-supported clade in the Nectriaceae (Clades I–XIII) using the tub2, ITS, LSU and tef1 gene regions (results not shown) basal to the Nectria clade (Clade XII). The remaining six genes regions used here, however, placed both these isolates at the basal position represented in Fig. 1, Fig. 2. At present, the genus Sarocladium is classified as incertae sedis in the order Hypocreales (Summerbell et al., 2011, Giraldo et al., 2014), and therefore based on the weak relationship between R. reticulatus and S. kiliense in this study, both are considered incertae sedis pending further investigation. An isolated listed in the CBS collection as “Nectria dacryocarpa” (CBS 121.87) also clustered within this clade, but is also sterile and no conclusive identification could be made based on DNA sequence comparisons, and is therefore not treated further here.
Clade XVII
Falcocladium S.F. Silveira et al., Mycotaxon 50: 447. 1994. MycoBank MB25800.
Ascomatal state unknown. Conidiophores sporodochial, synnematal, or penicillate when formed on aerial mycelium, hyaline, solitary or aggregated in groups, arising laterally from somatic hyphae, or from a stroma of thick-walled, red-brown chlamydospores. Stipe extensions hyaline to pale brown, straight to flexuous, aseptate, thick-walled, originating from any position on a conidiophore branch, or in the position of a phialide, frequently with more than one occurring in the same conidiogenous apparatus, terminating in an ellipsoidal, sphaeropedunculate or turbinate vesicle. Conidiogenous apparatus hyaline, aseptate to multi-septate, consisting of up to three series of branches. Phialides hyaline, arising from ends of each terminal branch in groups of 2–6, ampulliform or lageniform to subulate, with inconspicuous collarettes. Conidia hyaline, 0(–1)-septate, falcate with acute, short apical and basal appendages (adapted from Crous et al. 1994).
Type species: Falcocladium multivesiculatum S.F. Silveira et al., Mycotaxon 50: 448. 1994.
Descriptions and illustrations: Crous et al., 1994, Crous et al., 1997, Crous et al., 2007, Somrithipol et al., 2007.
Notes: The family Falcocladiaceae was recently introduced for the genus Falcocladium (Jones et al. 2014), which includes four species, namely F. multivesiculatum (Crous et al. 1994), F. sphaeropedunculatum (Crous et al. 1997), F. thailandicum (Crous et al. 2007) and F. turbinatum (Somrithipol et al. 2007). Crous et al. (2007) judged the genus to be polyphyletic (but allied with the Hypocreales) after the ITS sequence of F. thailandicum was included in a phylogenetic analysis of this species with F. multivesiculatum, F. sphaeropedunculatum and other related sequences downloaded from GenBank. Phylogenetic inference in the present study showed that the ex-type of F. thailandicum (CBS 121717) clustered within the monophyletic clade (BS ≥ 75 %, PP ≥ 0.95) representing the genus Falcocladium, but distinct from the Nectriaceae clade (Clade I–XIII), therefore supporting the introduction of the family Falcocladiaceae.
Clade XVIII
This unsupported clade includes Lectera colletotrichoides (CBS 109728) of the Plectosphaerellaceae (Hypocreomycetidae, incertae sedis, Sordariomycetes), representatives of the genera Cylindrium and Ciliciopodium, and a single isolate (CBS 122.39) listed as “Calostilbe striispora” in the CBS collection. Both Cylindrium and Ciliciopodium are classified in the family Nectriaceae by Index Fungorum and MycoBank and limited literature is available for both genera. Phylogenetic inference in this study excluded both genera from Nectriaceae and they are therefore considered as incertae sedis.
Untreated or excluded genera
Bacillispora Sv. Nilsson, Bot. Not. 115: 77. 1962. MycoBank MB7304.
Type species: Bacillispora aquatica Sv. Nilsson, Botaniska Notiser 115: 77. 1962.
Descriptions and illustrations: Nilsson (1962), Iqbal & Bhatty (1980).
Notes: Bacillospora is an aquatic asexual genus established by Nilsson (1962) with B. aquatica as type. Based on the descriptions provided by Nilsson (1962) and Iqbal & Bhatty (1980) (for B. inflata), members of this genus closely resemble the asexual morphs of the genera Neonectria and Thelonectria. However, no cultures were available at this time to determine the phylogenetic position of Bacillospora in the Nectriaceae.
Peziotrichum (Sacc.) Lindau, In: Engler & Prantl, Natürl. Pflanzenfam. 1(1): 467. 1900. MycoBank MB9285.
≡ Botryotrichum subgenus Peziotrichum Sacc., Hedwigia 32: 58. 1893.
Type species: Peziotrichum lachnella (Sacc.) Lindau, In: Engler & Prantl, Natürl. Pflanzenfam. 1(1): 467. 1900.
≡ Botryotrichum lachnella Sacc., Hedwigia 32: 58. 1893.
Description and illustration: Subramanian (1971).
Notes: Peziotrichum is an entomogenous asexual genus, based on P. lachnella, which was initially linked to Ophionectria coccorum (Petch, 1927, Subramanian, 1971). Rossman (1977) synonymised O. coccorum under Podonectria coccorum, which belongs to the Tubeufiaceae (Pleosporales, Pleosporomycetidae, Dothideomycetes; Rossman 1987), a genus also linked to the asexual genus Tetracrium (Tubeufiaceae, Pleosporales, Pleosporomycetidae, Dothideomycetes; Kodsueb et al. 2006). Since there are no living cultures available representing Peziotrichum that would allow for molecular studies, the link of this genus to Podonectria and Tetracrium cannot be confirmed. Peziotrichum could be considered as a member of the Tubeufiaceae, based on the descriptions and illustrations provided by Petch (1927) and Subramanian (1971).
Pleogibberella Sacc., In: Berl. & Voglino, Syll. Fung. Addit. 1–4: 217. 1886. MycoBank MB4211.
Type species: Pleogibberella calami (Cooke) Berl. & Voglino, Syll. Fung. Addit. 1–4: 217. 1886 (as “calamia”).
≡ Gibberella calami Cooke, Grevillea 13: 8. 1884.
Description and illustration: Rossman et al. (1999).
Notes: Rossman et al. (1999) studied the type specimen of Pleogibberella calami, the only species in this genus, and concluded that this genus is most similar to members of the genus Nectria based on the ascomatal wall structure, well-developed stroma and large, muriform ascospores. The type specimen also did not include asexual structures. No living cultures are available to allow this genus to be included in molecular studies.
Pleurocolla Petr., Ann. Mycol. 22: 15. 1924. MycoBank MB9458.
Type species: Pleurocolla tiliae Petr. Ann. Mycol. 22: 15. 1924.
Description and illustration: Diehl (1933).
Notes: No living cultures were available for molecular studies.
Pseudocosmospora C. Herrera & P. Chaverri, Mycologia 105: 1291. 2013. MycoBank MB802432.
Type species: Pseudocosmospora eutypellae C. Herrera & P. Chaverri, Mycologia 105: 1293. 2013.
Description and illustration: Herrera et al. (2013a).
Notes: Representatives of Pseudocosmospora have not been included in this study as no cultures were available to us. Herrera et al. (2013a), however, clearly indicated this sexual genus to form a monophyletic lineage sister to Dialonectria and Cosmospora.
Stalagmites Thiess. & Syd., Ann. Mycol. 12: 189. 1914. MycoBank MB5182.
Type species: Stalagmites tumefaciens (Syd. & P. Syd.) Thiess. & Syd., Ann. Mycol. 12: 189. 1914.
≡ Dothidea tumefaciens Syd. & P. Syd., Ann. Mycol. 5: 360. 1907.
Description and illustration: Rossman et al. (1999).
Notes: This monotypic genus, based on Stalagmites tumefaciens, is associated with galls on branches of a Serjania sp. Rossman et al. (1999) concluded that this genus belongs in the Nectriaceae based on morphological similarities to the sexual morphs of Fusarium (as Gibberella) and Pleogibberella. No living cultures were available for molecular studies.
Discussion
To our knowledge, this study represents the largest sampling of nectriaceous fungi subjected to multi-locus sequence analyses to date. It provides a broad phylogenetic backbone and framework for future studies of the Nectriaceae. Members of this family are commonly found in various environments, where they play important socio-economic roles in human endeavours in agriculture, industry and medicine. The phylogenetic foundation set in this study will form the basis for further investigation of several genera, and will allow identification of novel taxa in existing and new fungal groups in this family. Although taxonomic issues have been clarified in some genera in this study, it also highlights some taxonomic problems in the Nectriaceae.
Members of the Nectriaceae are pleomorphic fungi, displaying both asexual and sexual morphs during their life cycles. This originally resulted in the separate naming of each fungal morph, providing a considerable challenge to fungal systematics (Cannon & Kirk 2000). The implementation of The International Code of Nomenclature for algae, fungi and plants (ICN; McNiell et al. 2012), stipulating that only one scientific name should be used for a fungal species, resulted in the abolishment of dual nomenclature (ICN Art. 59; McNiell et al., 2006, Hawksworth et al., 2011) for pleomorphic fungi. Although selecting the correct generic name for a group of fungi should be based on priority of the oldest generic name, several fungal groups are considered exceptions to this principle based on the need for reasonable nomenclatural stability in fungi of economic or health significance (Rossman et al. 2013). Therefore, Hawksworth, 2011, Hawksworth, 2012 proposed several criteria to be applied for determining the status of a generic name. These criteria include (1) the number of name changes required, (2) the clarity of the generic concept, (3) the frequency of use of each generic name and (4) the vote of interested members of the scientific community. Applying these criteria, Rossman et al. (2013) proposed the conservation or protection of several generic names in the Nectriaceae. Also following this approach, we propose the conservation or protection of the generic names Penicillifer (= Viridispora), Sarcopodium (=Actinostilbe = Lanatonectria) and Xenocylindrocladium (=Xenocalonectria) based on priority of the generic name and the number of name changes required if the alternative generic name is applied. However, the implementation of ICN has already sparked intensive debate, especially where well-established generic names in literature, such as Fusarium s. lat. (Geiser et al., 2013, O'Donnell et al., 2013, Aoki et al., 2014), have now been segregated into more narrowly defined genera, with newly introduced and older generic names being applied for these newly segregated fungal groups (Gräfenhan et al. 2011, Schroers et al. 2011).
The generic name Fusarium is well-embedded in mycological literature, representing the fourth most commonly published fungal name (see Geiser et al. 2013). The segregation of the genus Fusarium by Gräfenhan et al. (2011) and Schroers et al. (2011) was therefore met by strong opposition from the general Fusarium working community (Geiser et al., 2013, O'Donnell et al., 2013, Aoki et al., 2014), although the genus Fusarium s. lat. clearly has internal phylogenetic structure supporting these divisions. A similar debate within the general plant pathological community surrounded the segregation of Cylindrocarpon and Neonectria into several genera by Chaverri et al. (2011). These changes have ultimately been widely accepted (Cabral et al., 2012a, Cabral et al., 2012b, Cabral et al., 2012c, Lombard et al. 2013). We therefore choose to retain the generic names Albonectria, Cyanonectria, Geejayessia and Neocosmospora as proposed by Gräfenhan et al., 2011, Nalim et al., 2011 and Schroers et al. (2011) for fungal groups previously treated in the genus Fusarium. This approach allows for consistency in the taxonomic treatment of genera in the Nectriaceae, as several clades, which include important plant pathogens (e.g. Clade III & IV) are shown here to display a similar genetic structure and ecology (e.g. Campylocarpon, Dactylonectria, Ilyonectria and Neonectria on Vitis vinifera; Cabral et al., 2012a, Cabral et al., 2012b, Cabral et al., 2012c, Lombard et al. 2013, Lombard et al., 2014a, Lombard et al., 2014b).
In this study, we were able to resolve 47 genera in the Nectriaceae, of which three genera, namely Calostilbe, Corallonectria and Dematiocladium, are represented by single lineages due to the paucity of cultures. For several of these genera there has been little or no DNA sequence data available prior to this study. These genera include Aquanectria, Curvicladiella, Cylindrocarpostylus, Cylindrodendrum, Ophionectria, Paracremonium, Penicillifer, Sarcopodium, Septofusidium, Tilachlidium, Xenoacremonium, Xenocylindrocladium, and Xenogliocladiopsis. All these genera were shown to form monophyletic clades. New studies will be needed on these groups, especially since two of them, Paracremonium and Xenoacremonium, represent important human pathogens (Gams 1971). The remaining genera are for the most part regarded as either foliicolous or entomogenous fungi or endophytes and saprobes of mostly woody plant hosts (Ranzoni, 1956, Gams, 1971, Crous and Kendrick, 1994, Kirschner and Oberwinkler, 1999, Rossman et al., 1999) which might play an important role in industrial applications in future.
Six new genera, which were previously treated as members of the genera Acremonium, Flagellospora, Fusarium and Pseudonectria, are introduced here in the family Nectriaceae. Species in the new genus Coccinonectria were initially regarded as members of the genus Pseudonectria mostly based on plant host association (Rossman et al., 1999, Gräfenhan et al., 2011, Crous et al., 2014). Morphologically, Coccinonectria species can be distinguished from Pseudonectria by their scarlet ascomata, although their asexual morphs share several morphological features. Phylogenetic inference in this study also supported segregation of Coccinonectria from Pseudonectria, and therefore two new combinations are made in Coccinonectria.
Bisifusarium, Neocosmospora and Rectifusarium were previously treated as members of the genus Fusarium. Phylogenetic inference in this study showed that these genera are monophyletic and distinct from each other and Fusarium. Bisifusarium includes fusarium-like species previously treated as the “Fusarium dimerum species complex” (Schroers et al., 2005, Geiser et al., 2013, O'Donnell et al., 2013). They are distinguished by the formation of lateral phialidic pegs, which are not commonly found in Fusarium, and by producing 1–2-septate macroconidia. These fungi are mostly isolated from clinical samples (Schroers et al. 2009). Species of Rectifusarium are soil-borne fungi and have been isolated from various agricultural crops, but are not regarded as important pathogens or post-harvest pathogens of these crops (Wollenweber, 1913, Gerlach and Nirenberg, 1982). This genus is distinguished from Fusarium by its simple, erect, almost cylindrocarpon-like conidiophores, and the absence of sporodochia. Members of the new genera Paracremonium and Xenoacremonium were previously treated as Acremonium recifei (Gams, 1971, Summerbell et al., 2011), which have been shown to be paraphyletic (Summerbell et al. 2011). Both genera include important human subcutaneous and opportunistic pathogens (Gams, 1971, Hoog et al., 2011). Phylogenetic inference guided the recognition of subtle morphological distinctions between the genera. Species of Paracremonium can be distinguished by the formation of sterile coils in culture and their pink to salmon coloured colonies on PDA. Xenoacremonium species do not form sterile coils in culture, but readily release a pale luteous to luteous pigment into the growth medium, a phenomenon that is not observed in Paracremonium.
A new family, Tilachlidiaceae, is introduced here in the order Hypocreales for two genera, Septofusidium and Tilachlidium, previous classified in the family Nectriaceae. These genera share several morphological characters and are known to be saprobic or parasitic on other fungi (Petch, 1931, Mains, 1951, Gams, 1971, Samson, 1974). Some species of Tilachlidium have been shown to produce important antibiotics (Gottshall et al., 1951, Roberts, 1952) as well as novel compounds that are cytotoxic to leukemia cells (Feng et al. 2004), discoveries highlighting the potential for exploitation of these fungi in medical applications.
Comparisons of the phenotypic and ecological characters of genera in the Nectriaceae included in this study showed marginal correlations with some of the clades identified in the phylogenetic tree. Genera in Clade I are characterised by their penicillate arrangement of fertile branches but do not all share the same ecological niche. Clade III includes genera that also have a penicillate arrangement of fertile branches but have a sterile stipe extension extending beyond the conidiogenous apparatus and are generally regarded as soil-borne fungi. Clade IV and VI include genera, with the exception of Cylindrocarpostylus and Mariannaea, having soil-borne cylindrocarpon-like asexual morphs. They are associated with basal rot and canker diseases of their plant hosts. Genera in Clade VII are characterised by their sporodochial asexual morphs with characteristic straight to circinate setae surrounding the sporodochia. They are associated mostly with leaf and stem blight diseases of plant hosts in the Buxaceae. Clade X includes genera with fusarium-like asexual morphs. They are generally pathogens of other fungi or of insects.
The ten gene regions used in this study were chosen based on their extensive use in molecular mycology. They have proved suitable to explore phylogenetic relationships within and between genera in the Nectriaceae (Chaverri et al., 2011, Gräfenhan et al., 2011, Hirooka et al., 2012, Lombard et al., 2010a, Lombard et al., 2010b, Lombard et al., 2012, Lombard et al., 2014a, Lombard et al., 2014b, Lombard and Crous, 2012, Herrera et al., 2013a, Herrera et al., 2013b, O'Donnell et al., 2013). Although phylogenetic analyses of the individual gene regions (results not shown) were able to resolve all the genera in the Nectriaceae with varying statistical support, none of these gene regions can be considered as the “silver bullet” for the Nectriaceae. An illustration of the unreliability of individual genes is found in the placement of Rodentomyces reticulatus and Sarocladium kiliense within the Nectriaceae clade by tub2, ITS, LSU and tef1, but not by the other six genes studied. The best statistical support for each genus was obtained using rpb1 and rpb2, and therefore these loci should be further studied in attempts to determine phylogenetic relationships in the Nectriaceae. However, the ability of these two loci to serve as barcodes for species in these genera still needs to be determined for each genus on an individual basis.
The present study, as mentioned previously, should serve as backbone for future taxonomic studies of genera in the Nectriaceae. More loci need to be identified and screened with an eye to finding a more robust single locus – a process that might be expedited by using whole genome sequences. Presently there is an under-representation of Nectriaceae in the available whole genome sequences (nine genomes; http://genome.jgi.doe.gov). More genomic studies are urgently needed in the Nectriaceae. Our study also highlights the importance of maintaining living cultures in public culture collections, as many of the genera included in this study were subjected to molecular analysis for the first time based on cultures collected at various times in history, while, on the other hand, several recently described taxa were unavailable for inclusion.
Acknowledgements
This study was financially supported by the NWO Joint Scientific Thematic Research Programme – Joint Research Projects 2012 ALW file number 833.13.2005 titled “Building the fungal quarantine & quality barcode of life database to ensure plant health”. The authors thank the technical staff, A. van Iperen and Y. Vlug for their invaluable assistance with cultures and Prof. W. Gams for valuable discussions.
Footnotes
Peer review under responsibility of CBS-KNAW Fungal Biodiversity Centre.
Contributor Information
L. Lombard, Email: l.lombard@cbs.knaw.nl.
P.W. Crous, Email: p.crous@cbs.knaw.nl.
Appendix
Recently Rossman et al. (2013) proposed generic names for acceptance or rejection in the families Bionectriaceae, Hypocreaceae and Nectriaceae. In this treatment, Clonostachys was recommended above Bionectria in the Bionectriaceae. Within the Hypocreaceae, Hypomyces was recommended over Cladobotryum, Sphaerostilbella over Gliocladium, and Trichoderma over Hypocrea. In keeping with these proposals and in line with the International Code of Nomenclature for algae, fungi and plants (ICN; McNiell et al. 2012), new combinations are required in the genera Clonostachys, Hydropisphaera, Nectriopsis (Bionectriaceae), and Sphaerostilbella (Hypocreaceae), which are provided here.
Bionectriaceae
Clonostachys apocyni (Peck) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810968.
Basionym: Nectria apocyni Peck, Bull. Buffalo Soc. Nat. Sci. 1: 71. 1873.
≡ Cucurbitaria apocyni (Peck) Kuntze, Rev. Gen. Plant. 3: 460. 1898.
≡ Bionectria apocyni (Peck) Schroers & Samuels, Z. Mykol. 63: 153, 1997.
= Nectria rugispora Pat., Bull. Trimestriel Soc. Mycol. France 8: 133. 1892.
≡ Cucurbitaria rugispora (Pat.) Kuntze, Rev. Gen. Plant. 3: 461. 1898.
= Nectria carneoflavida Penz. & Sacc., Malpighia 11: 511. 1897.
= Dendrodochium macrosporum Sacc. & Ellis, Michelia 2: 580. 1882.
≡ Clonostachys macrospora (Sacc. & Ellis) Schroers & W. Gams, Stud. Mycol. 46: 62. 2001.
= Dendrodochium roseomucosum Matsush., Matsush. Mycol. Mem. 8: 17. 1995.
Clonostachys aurantia (Penz. & Sacc.) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810969.
Basionym: Nectriella aurantia Penz. & Sacc., Malpighia 11: 509. 1897.
≡ Bionectria aurantia (Penz. & Sacc.) Rossman, Samuels & Lowen, Mycologia 85: 698. 1993.
Clonostachys blumenaviae (Rehm) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810970.
Basionym: Nectria blumenaviae Rehm, Hedwigia 37: 192. 1898.
Clonostachys gibberosa (Schroers) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810971.
Basionym: Bionectria gibberosa Schroers, Stud. Mycol. 46: 198. 2001.
Clonostachys manihotis (Rick) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810972.
Basionym: Nectria manihotis Rick, Ann. Mycol. 8: 458. 1910.
Clonostachys parva (Schroers) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810973.
Basionym: Bionectria parva Schroers, Stud. Mycol. 46: 143. 2001.
Clonostachys tonduzii (Speg.) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810974.
Basionym: Bionectria tonduzii Speg., Bol. Acad. Nac. Ci. 579: 563. 1919.
≡ Nectria tonduzii (Speg.) Samuels, Mem. New York Bot. Gard. 48: 22. 1988.
Clonostachys tornata (Höhn.) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810975.
Basionym: Pseudonectria tornata Höhn., Sitzungsber. Akad. Wiss. Wien, Mat.-Naturwiss. Kl. 118: 1470. 1909.
≡ Bionectria tornata (Höhn.) Schroers, Stud. Mycol. 46. 184. 2001.
= Nectria sesquiphialis Samuels, Mem. New York Bot. Gard. 49: 276. 1989.
= Sesquicillium asymmetricum Samuels, Mem. New York Bot. Gard. 49: 276. 1989.
≡ Clonostachys asymmetrica (Samuels) Schroers, Stud. Mycol. 46: 184. 2001.
Note: The sexual-asexual morph connections for these species in Clonostachys are based on the monograph of Bionectria by Schroers (2001).
Hydropisphaera fusigera (Berk. & Broome) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810976.
Basionym: Monotospora fusigera Berk. & Broome, J. Linn. Soc., Bot. 14: 99. 1873.
≡ Gliomastix fusigera (Berk. & Broome) C.H. Dickinson, Mycol. Pap. 115: 7. 1968.
≡ Acremonium fusigera (Berk. & Broome) W. Gams, Cephalosporium-artige Schimmelpilze: 94. 1971.
= Hydropsisphaera bambusicola Lechat, Mycotaxon 111: 96. 2010.
Notes: Lechat et al. (2010) linked the sexual morph Hydropisphaera bambusicola to the asexual morph Gliomastix fusigera. The epithet of G. fusigera (≡ Monotospora fusigera (1973) is older, therefore takes priority, and the new combination is provided.
Nectriopsis rexiana (Sacc.) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810977.
Basionym: Verticillium nanum subsp. rexianum Sacc., Michelia 2: 577. 1882.
≡ Verticillium rexianum (Sacc.) Sacc., Syll. Fung. 4: 153. 1886.
=Verticillium niveostratosum Lindau, Rabenh. Kryptogam.-Fl., Pilze – Fungi imperfecti 1: 316. 1905.
= Hypomyces exiguus Pat., Bull. Soc. Mycol. France 18: 180. 1902.
≡ Nectriopsis exigua (Pat.) W. Gams, Netherlands J. Pl. Pathol. 88: 73. 1982.
=Nectria myxomyceticola Samuels, Mem. New York. Bot. Gard. 48: 48. 1988.
Hypocreaceae
Sphaerostilbella aurifila (W.R. Gerard) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810979.
Basionym: Stilbum aurifilum W.R. Gerard, Bull. Torrey Bot. Club. 5: 39. 1874.
≡ Ciliciopodium aurifilum (W.R. Gerard) Cooke, Grevillea 19: 14. 1890.
≡ Dendrostilbella aurifilia (W.R. Gerard) Seifert & J.A. Mackinnon, Mycologia 75: 324. 1983.
= Sphaerostilbe lutea Henn., Bot. Jahrb. Syst. 30:40. 1901.
≡ Sphaerostilbella lutea (Henn.) Sacc., Syll. Fung. 17: 778. 1905.
= Stilbum zacalloxanthum R.T. Moore, Amer. Naturalist 93: 41. 1959.
= Stilbum mycetophilum S. Ahmad, Biologia (Lahore) 6: 136. 1961.
Sphaerostilbella penicillioides (Corda) Rossman, L. Lombard & Crous, comb. nov. MycoBank MB810978.
Basionym: Gliocladium penicillioides Corda, Icon. Fungorum hucusque Cogn. 4: 31. 1840.
= Hypomyces aureonitens Tul. & C. Tul., Selecta Fungorum Carpologia: Nectriei- Phacidiei- Pezizei 3: 64. 1865.
≡ Hypolyssus aureonitens (Tul. & C. Tul.) Kuntze, Rev. Gen. Plant. 3: 488. 1898.
≡ Nectriopsis aureonitens (Tul. & C. Tul.) Maire, Ann. Mycol. 9: 323. 1911.
≡ Hyphonectria aureonitens (Tul. & C. Tul.) Petch, J. Bot. 74: 220. 1937.
≡ Sphaerostilbella aureonitens (Tul. & C. Tul) Seifert, Samuels & W. Gams, Stud. Mycol. 27: 145. 1985.
References
- Aoki T., O'Donnell K., Geiser D.M. Systematics of key phytopathogenic Fusarium species: current status and future challenges. Journal of General Plant Pathology. 2014;80:189–201. [Google Scholar]
- Baschien C., Tsui C.K.-M., Gulis V. The molecular phylogeny of aquatic hyphomycetes with affinity to the Leotiomycetes. Fungal Biology. 2013;117:660–672. doi: 10.1016/j.funbio.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Batista A.C., Maia H. da Silva. Novos fungos do ar atmosférico. Anais da Sociedade de Biologia de Pernambuco. 1955;13:149–156. [Google Scholar]
- Bezerra J.L. Studies on Pseudonectria rousseliana. Acta Botanica Neerlandica. 1963;12:58–63. [Google Scholar]
- Boesewinkel H.J. Cylindrocladiella, a new genus to accommodate Cylindrocladium parvum and other small-spored species of Cylindrocladium. Canadian Journal of Botany. 1982;60:2288–2294. [Google Scholar]
- Booth C. International Mycological Institute; Kew: 1971. The genus Fusarium; pp. 1–234. [Google Scholar]
- Cabral A., Groenewald J.Z., Rego C. Cylindrocarpon root rot: multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycological Progress. 2012;11:655–688. [Google Scholar]
- Cabral A., Rego C., Crous P.W. Virulence and cross-infection potential of Ilyonectria species to grapevine. Phytopathologia Mediterranea. 2012;51:340–354. [Google Scholar]
- Cabral A., Rego C., Nascimento T. Multi-gene analysis and morphology reveal novel Ilyonectria species associated with black foot disease of grapevines. Fungal Biology. 2012;116:62–80. doi: 10.1016/j.funbio.2011.09.010. [DOI] [PubMed] [Google Scholar]
- Cannon P.F., Kirk P.M. The philosophy and practicalities of amalgamating anamorph and teleomorph concepts. Studies in Mycology. 2000;45:19–25. [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Chang D.C., Grant G.B., O'Donnell K. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. Journal of the American Medical Association. 2006;296:953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- Chaverri P., Salgado C., Hirooka Y. Delimitation of Neonectria and Cylindrocarpon (Nectriaceae, Hypocreales, Ascomycota) and related genera with Cylindrocarpon-like anamorphs. Studies in Mycology. 2011;68:57–78. doi: 10.3114/sim.2011.68.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous . APS Press; St. Paul, Minnesota, U.S.A.: 2002. Taxonomy and pathology of Cylindrocladium (Calonectria) and allied genera. [Google Scholar]
- Crous P.W., Allegrucci N., Arambarri A.M. Dematiocladium celtidis gen. sp. nov. (Nectriaceae, Hypocreales), a new genus from Celtis leaf litter in Argentina. Mycological Research. 2005;109:833–840. doi: 10.1017/s0953756205002911. [DOI] [PubMed] [Google Scholar]
- Crous P.W., Decock C., Schoch C.L. Xenocylindrocladium guianense and X. subverticillatum, two new species of hyphomycetes from plant debris in the tropics. Mycoscience. 2001;42:559–566. [Google Scholar]
- Crous P.W., Gams W., Stalpers J.A. MycoBank: an online initiative to launch mycology into the 21st century. Studies in Mycology. 2004;50:19–22. [Google Scholar]
- Crous P.W., Groenewald J.Z., Himaman W. Falcocladium thailandicum. Fungal Planet. 2007;18 [Google Scholar]
- Crous P.W., Groenewald J.Z., Risède J.-M. Calonectria species and their Cylindrocladium anamorphs: species with clavate vesicles. Studies in Mycology. 2006;55:213–226. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Groenewald J.Z., Risède J.-M. Calonectria species and their Cylindrocladium anamorphs: species with sphaeropedunculate vesicles. Studies in Mycology. 2004;50:415–430. doi: 10.3114/sim.55.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Kendrick W.B. Arnaudiella eucalyptorum sp. nov. (Dothideales, Ascomycetes), and its hyphomycetous anamorph Xenogliocladiopsis gen. nov., from Eucalyptus leaf litter in South Africa. Canadian Journal of Botany. 1994;72:59–64. [Google Scholar]
- Crous P.W., Kendrick W.B., Alfenas A.C. New species of hyphomycetes associated with Eucalyptus. South African Journal of Botany. 1997;63:286–290. [Google Scholar]
- Crous P.W., Shivas R.G., Quaedvlieg W. Fungal Planet description sheets: 214–280. Persoonia. 2014;32:184–306. doi: 10.3767/003158514X682395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Verkley G.J.M., Groenewald J.Z., editors. Fungal biodiversity. CBS-KNAW Fungal Biodiversity Centre; Utrecht: 2009. (CBS laboratory manual series no. 1). [Google Scholar]
- Crous P.W., Wingfield M.J., Alfenas A.C. Cylindrocladium naviculatum sp. nov., and two new vesiculate Hyphomycete genera, Falcocladium and Vesicladiella. Mycotaxon. 1994;50:441–458. [Google Scholar]
- Cunningham C.W. Can three incongruence tests predict when data should be combined? Molecular Biology and Evolution. 1997;14:733–740. doi: 10.1093/oxfordjournals.molbev.a025813. [DOI] [PubMed] [Google Scholar]
- Damm U., Mostert L., Crous P.W. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia. 2008;20:87–102. doi: 10.3767/003158508X324227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decock C., Crous P.W. Curvicladium gen. nov., a new hyphomycete genus from French Guiana. Mycologia. 1998;90:276–281. [Google Scholar]
- Decock C., Hennebert G.L., Crous P.W. Nectria serpens sp. nov. and its hyphomycetous anamorph Xenocylindrocladium gen. nov. Mycological Research. 1997;101:786–790. [Google Scholar]
- Diehl W.W. Thelebolus lignicola and the genus Pleurocolla (Fungi) Journal of the Washington Academy of Sciences. 1933;23:58–61. [Google Scholar]
- Dodge B.O. A new Pseudonectria on Pachysandra. Mycologia. 1944;36:532–537. [Google Scholar]
- Doveri F., Pecchia S., Sarrocco S. Rodentomyces, a new hypocrealean genus from Italy. Fungal Diversity. 2010;42:57–69. [Google Scholar]
- Drummond A.J., Suchard M.A., Xie D. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution. 2012;29:1969–1973. doi: 10.1093/molbev/mss075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte S., Batista D., Bärlocher F. Some new DNA barcodes of aquatic hyphomycete species. Mycoscience. 2015;56:102–108. [Google Scholar]
- van Emden JH. Penicillifer, a new genus of Hyphomycetes from soil. Acta Botanica Neerlandica. 1968;17:54–58. [Google Scholar]
- Feng Y., Blunt J.W., Cole A.L.J. Novel cytotoxic thiodiketopiperazine derivatives from a Tilachlidium sp. Journal of Natural Products. 2004;67:2090–2092. doi: 10.1021/np030326w. [DOI] [PubMed] [Google Scholar]
- Fries E.M. Systema mycologicum. 1832;3:261–524. [Google Scholar]
- Gams W. Gustav Fischer Verlag; Stuttgart, Germany: 1971. Cephalosporium-artige Schimmelpilze (Hyphomycetes) [Google Scholar]
- Geiser D.M., Aoki T., Bacon C.W. One fungus, one name: defining the genus Fusarium in a scientifically robust way that preserves longstanding use. Phytopathology. 2013;103:400–408. doi: 10.1094/PHYTO-07-12-0150-LE. [DOI] [PubMed] [Google Scholar]
- Gerlach W., Nirenberg H.I. The genus Fusarium – a pictorial atlas. Mitteilungen der Biologischen Bundesanstalt für Land- und Forstwirtschaft. 1982;209:1–406. [Google Scholar]
- Giraldo A., Gené J., Sutton D.A. Phylogeny of Sarocladium (Hypocreales) Persoonia. 2014;34:10–24. doi: 10.3767/003158515X685364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottshall R.Y., Roberts J.M., Portwood L.M. Synnematin, an antibiotic produced by Tilachlidium. Experimental Biology and Medicine. 1951;76:307–311. doi: 10.3181/00379727-76-18473. [DOI] [PubMed] [Google Scholar]
- Gräfenhan T., Schroers H.-J., Nirenberg H.I. An overview of the taxonomy, phylogeny, and typification of nectriaceous fungi in Cosmospora, Acremonium, Fusarium, Stilbella, and Volutella. Studies in Mycology. 2011;68:79–113. doi: 10.3114/sim.2011.68.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewald J.Z., Nakashima C., Nishikawa J. Species concepts in Cercospora: spotting the weed among the roses. Studies in Mycology. 2013;75:115–170. doi: 10.3114/sim0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarro J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. European Journal of Clinical Microbiology & Infectious Diseases. 2013;32:1491–1500. doi: 10.1007/s10096-013-1924-7. [DOI] [PubMed] [Google Scholar]
- Halleen F., Schroers H.-J., Groenewald J.Z. Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. associated with black foot disease of grapevines (Vitis spp.) Studies in Mycology. 2004;50:431–456. [Google Scholar]
- Hawksworth D.L. A new dawn for the naming of fungi: impacts of decisions made in Melbourne in July 2011 on the future publication and regulation of fungal names. IMA Fungus. 2011;2:155–162. doi: 10.5598/imafungus.2011.02.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth D.L., Crous P.W., Redhead S.A. The Amsterdam declaration on fungal nomenclature. IMA Fungus. 2011;2:105–112. doi: 10.5598/imafungus.2011.02.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawksworth D.L. Managing and coping with names of pleomorphic fungi in a period of transition. Mycosphere. 2012;2:143–155. doi: 10.5598/imafungus.2012.03.01.03. IMA Fungus3: 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C.S., Rossman A.Y., Samuels G.J. Pseudocosmospora, a new genus to accommodate Cosmospora vilior and related species. Mycologia. 2013;105:1287–1305. doi: 10.3852/12-395. [DOI] [PubMed] [Google Scholar]
- Herrera C.S., Rossman A.Y., Samuels G.J. Revision of the genus Corallomycetella with Corallonectria gen. nov. for C. jatrophae (Nectriaceae, Hypocreales) Mycosystema. 2013;32:518–544. [Google Scholar]
- Hirooka Y., Rossman A.Y., Chaverri P. A morphological and phylogenetic revision of the Nectria cinnabarina complex. Studies in Mycology. 2011;68:35–56. doi: 10.3114/sim.2011.68.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirooka Y., Rossman A.Y., Samuels G.J. A monograph of Allantonectria, Nectria, and Pleonectria (Nectriaceae, Hypocreales, Ascomycota) and their pycnidial, sporodochial, and synnematous anamorphs. Studies in Mycology. 2012;71:1–210. doi: 10.3114/sim0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höhnel F. von. Fragmente zur Mykologie (XVII. Mitteilung, Nr. 876 bis 93) Sitzungsberichte der mathematisch-naturwissenschaftlichen Klasse der Kaiserlichen Akademie der Wissenschaften, Wien. 1915;124:49–159. [Google Scholar]
- Hoog G.S. de, Guarro J., Gené J. 3rd edn. CBS-KNAW Fungal Biodiversity Centre; Utrecht, The Netherlands: 2011. Atlas of clinical fungi. (CD-ROM) [Google Scholar]
- Hudson H.J. Heliscus submersus sp. nov., an aquatic hyphomycete from Jamaica. Transactions of the British Mycological Society. 1961;44:91–94. [Google Scholar]
- Ingold C.T. Aquatic hyphomycetes of decaying Alder leaves. Transactions of the British Mycological Society. 1942;25:339–417. [Google Scholar]
- Iqbal S.H., Bhatty S.F. New freshwater hyphomycetes from Pakistan. Transactions of the Mycological Society of Japan. 1980;21:71–75. [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia. 2014;33:182–211. doi: 10.3767/003158514X685211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E.B.G., Suetrong S., Cheng W.H. An additional fungal lineage in the Hypocreomycetidae (Falcocladium species) and the taxonomic re-evaluation of Chaetosphaeria chaetosa and Swampomyces species, based on morphology, ecology and phylogeny. Cryptogamie Mycologie. 2014;35:119–138. [Google Scholar]
- Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick B. The generic iceberg. Taxon. 1974;23:747–753. [Google Scholar]
- Kirk P.M., Cannon P.F., Minster D.W. 10th edn. CABI; Europe, UK: 2008. Dictionary of fungi. [Google Scholar]
- Kirschner R., Oberwinkler F. Cylindrocarpostylus, a new genus based on a hyphomycete rediscovered from bark beetle galleries. Mycological Research. 1999;103:1152–1156. [Google Scholar]
- Kodsueb R., Jeewon R., Vijaykrishna D. Systematic revision of Tubeufiaceae based on morphological and molecular data. Fungal Diversity. 2006;21:105–130. [Google Scholar]
- Lechat C., Farr D.F., Hirooka Y. A new species of Hydropisphaera, H. bambusicola, is the sexual state of Gliomastix fusigera. Mycotaxon. 2010;111:95–102. [Google Scholar]
- Lombard L., Bezuidenhout C.M., Crous P.W. Ilyonectria black foot rot associated with Proteaceae. Australasian Plant Pathology. 2013;42:337–349. [Google Scholar]
- Lombard L., Crous P.W. Phylogeny and taxonomy of the genus Gliocladiopsis. Persoonia. 2012;28:25–33. doi: 10.3767/003158512X635056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D. Phylogeny and systematics of the genus Calonectria. Studies in Mycology. 2010;66:31–69. doi: 10.3114/sim.2010.66.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Crous P.W., Wingfield B.D. Species concepts in Calonectria (Cylindrocladium) Studies in Mycology. 2010;66:1–14. doi: 10.3114/sim.2010.66.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Shivas R.G., To-Anun C. Phylogeny and taxonomy of the genus Cylindrocladiella. Mycological Progress. 2012;11:835–868. [Google Scholar]
- Lombard L., Serrato-Diaz L.M., Cheewangkoon R. Phylogeny and taxonomy of the genus Gliocephalotrichum. Persoonia. 2014;32:127–140. doi: 10.3767/003158514X680261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombard L., Merwe N.A. van der, Groenewald J.Z. Lineages in Nectriaceae: re-evaluating the generic status of Ilyonectria and allied genera. Phytopathologia Mediterranea. 2014;53 [Google Scholar]
- Lumbsch T.H., Huhndorf S.M. Outline of Ascomycota. Myconet. 2007;13:1–58. [Google Scholar]
- Luo J., Zhuang W.-Y. Chaetopsinectria (Nectriaceae, Hypocreales), a new genus with Chaetopsina anamorphs. Mycologia. 2010;102:976–984. doi: 10.3852/09-263. [DOI] [PubMed] [Google Scholar]
- Luo J., Zhuang W.-Y. Volutellonectria (Ascomycota, Fungi), a new genus with Volutella anamorphs. Phytotaxa. 2012;44:1–10. [Google Scholar]
- Madelin M.F. Trichothecium acridiorum (Trabut) comb. nov. on red locusts. Transactions of the British Mycological Society. 1966;49:275–288. [Google Scholar]
- Mains E.B. Entomogenous species of Hirsutella, Tilachlidium and Synnematium. Mycologia. 1951;43:691–718. [Google Scholar]
- Matheny P.B., Liu Y.J., Ammirati J.F. Using RPB1 sequences to improve phylogenetic inference among mushrooms (Inocybe, Agaricales) American Journal of Botany. 2002;89:688–698. doi: 10.3732/ajb.89.4.688. [DOI] [PubMed] [Google Scholar]
- Mason-Gamer R., Kellogg E. Testing for phylogenetic conflict among molecular datasets in the tribe Triticeae (Graminae) Systematic Biology. 1996;45:524–545. [Google Scholar]
- Matsushima T. Saprophytic microfungi from Taiwan. Matsushima Mycological Memoirs. 1980;No. 1:1–82. Published by the author. [Google Scholar]
- Matsushima T. Matsushima Mycological Memoirs. 1985;No. 4:1–68. Published by the author. [Google Scholar]
- McNeill J., Barrie F.F., Buck W.R., editors. International Code of Nomenclature for algae, fungi and plants (Melbourne Code) A.R.G. Gantner Verlag KG; 2012. [Regnum Vegetabile no. 154] [Google Scholar]
- McNeill J., Barrie F.F., Burdet H.M., editors. International Code of Botanical Nomenclature (Vienna Code) A.R.G. Gantner Verlag KG; 2006. [Regnum Vegetabile no. 146.] [Google Scholar]
- Nalim F.A., Samuels G.J., Wijesundera R.L. New species from the Fusarium solani species complex derived from perithecia and soil in the Old World tropics. Mycologia. 2011;103:1302–1330. doi: 10.3852/10-307. [DOI] [PubMed] [Google Scholar]
- Nilsson S. Second note on Swedish freshwater Hyphomycetes. Botaniska Notiser. 1962;115:73–86. [Google Scholar]
- Nirenberg H.I. A simplified method for identifying Fusarium spp. occurring on wheat. Canadian Journal of Botany. 1981;59:1599–1609. [Google Scholar]
- Nylander J.A.A. Evolutionary Biology Centre, Uppsala University; 2004. MrModeltest v. 2. Programme distributed by the author. [Google Scholar]
- O'Donnell K. Progress towards a phylogenetic classification of Fusarium. Sydowia. 1996;48:57–70. [Google Scholar]
- O'Donnell K., Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Molecular Phylogenetics and Evolution. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- O’Donnell K., Humber R.A., Geiser D.M. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia. 2012;104:427–445. doi: 10.3852/11-179. [DOI] [PubMed] [Google Scholar]
- O'Donnell K., Kistler H.C., Cigelnik E. Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell K., Rooney A.P., Proctor R.H. Phylogenetic analyses of RPB1 and RPB2 supports a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genetics and Biology. 2013;52:20–31. doi: 10.1016/j.fgb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- O'Donnell K., Sarver B.A., Brandt M. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. Journal of Clinical Microbiology. 2007;45:2235–2248. doi: 10.1128/JCM.00533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell K., Sutton D.A., Rinaldi M.G. Internet-accessible DNA sequence database for identifying Fusaria from human and animal infections. Journal of Clinical Microbiology. 2010;48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petch B.A. Studies in entomogenous fungi. XII. Peziotrichum lachnella; Ophionectria coccorum; Volutella epicoccum. Transactions of the British Mycological Society. 1927;12:44–52. [Google Scholar]
- Petch T. Studies in entomogenous fungi. Transactions of the British Mycological Society. 1931;16:55–75. [Google Scholar]
- Pirozynski K.A. Antipodium, a new genus of Hyphomycetes. Canadian Journal of Botany. 1974;52:1143–1146. [Google Scholar]
- Polishook J.D., Bills G.F., Rossman A.Y. A new species of Neocosmospora with a Penicillifer anamorph. Mycologia. 1991;83:797–804. [Google Scholar]
- Rambelli A. Chaetopsina nuovo genere di ifali demaziacei. Atti della Accademia delle Scienze dell'Istituto di Bologna. 1956;11:1–6. [Google Scholar]
- Ranzoni F.V. The perfect stage of Flagellospora penicillioides. American Journal of Botany. 1956;43:13–17. [Google Scholar]
- Rayner R.W. Commonwealth Mycological Institute, Kew, Surrey. British Mycological Society; 1970. A mycological colour chart. [Google Scholar]
- Rehner S.A., Samuels G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycological Research. 1994;98:625–634. [Google Scholar]
- Rehner S.A., Samuels G.J. Molecular systematics of the Hypocreales: a teleomorph gene phylogeny and the status of their anamorphs. Canadian Journal of Botany. 1995;73:S816–S823. [Google Scholar]
- Roberts J.M. Antibiotic substances produced by species of Cephalosporium, with a description of a new species. Mycologia. 1952;44:292–306. [Google Scholar]
- Rogerson C.T. The hypocrealean fungi (Ascomycetes, Hypocreales) Mycologia. 1970;62:865–910. [PubMed] [Google Scholar]
- Rossman A.Y. The genus Ophionectria (Euascomycetes, Hypocreales) Mycologia. 1977;69:355–391. [Google Scholar]
- Rossman A.Y. The phragmosporous species of Nectria and related genera. Mycological Papers. 1983;150:1–164. [Google Scholar]
- Rossman A.Y. The Tubeufiaceae and similar Loculoascomycetes. Mycological Papers. 1987;157:1–66. [Google Scholar]
- Rossman A.Y. Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia. 1996;88:1–19. [Google Scholar]
- Rossman A.Y. Towards monophyletic genera in the holomorphic Hypocreales. Studies in Mycology. 2000;45:27–34. [Google Scholar]
- Rossman A.Y., Samuels G.J., Lowen R. Leuconectria clusiae gen. nov. and its anamorph Gliocephalotrichum bulbilium with notes on Pseudonectria. Mycologia. 1993;85:685–704. [Google Scholar]
- Rossman A.Y., Samuels G.J., Rogerson C.T. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes) Studies in Mycology. 1999;42:1–248. [Google Scholar]
- Rossman A.Y., Seifert K.A., Samuels G.J. Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales) proposed for acceptance and rejection. IMA Fungus. 2013;4:41–51. doi: 10.5598/imafungus.2013.04.01.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccardo P.A. Conspectus generum fungorum Italiae inferiorum nempe ad Sphaeropsideas, Melanconieas et Hyphomyceteas pertinentium systemate sporologicol dispositorum. Michelia. 1880;2:1–38. [Google Scholar]
- Saksena S.B. A new genus of Moniliaceae. Mycologia. 1954;46:660–666. [Google Scholar]
- Samson R.A. Paecilomyces and some allied Hyphomycetes. Studies in Mycology. 1974;6:1–119. [Google Scholar]
- Samuels G.J. Four new species of Nectria and their Chaetopsina anamorphs. Mycotaxon. 1985;22:13–32. [Google Scholar]
- Samuels G.J. Nectria and Penicillifer. Mycologia. 1989;81:347–355. [Google Scholar]
- Samuels G.J., Lu B., Chaverri P. Cyanonectria, a new genus for Nectria cyanostoma and it Fusarium anamorph. Mycological Progress. 2009;8:49–58. [Google Scholar]
- Samuels G.J., Rossman A.Y., Lowen R. A synopsis of Nectria subgen. Dialonectria. Mycological Papers. 1991;164:1–48. [Google Scholar]
- Samuels G.J., Seifert K.A. Taxonomic implications of variation among hypocrealean anamorphs. In: Sugiyama J., editor. Pleomorphic fungi: the diversity and its taxonomic implications. Kodansha, Tokyo, Japan and Elsevier, Amsterdam, The Netherlands; 1987. pp. 29–56. [Google Scholar]
- Samuels G.J., Seifert K.A. Two new species of Nectria with Stilbella and Mariannaea anamorphs. Sydowia. 1991;43:249–263. [Google Scholar]
- Schoch C.L., Crous P.W., Wingfield M.J. Phylogeny of Calonectria and selected hypocrealean genera with cylindrical macroconidia. Studies in Mycology. 2000;45:45–62. [Google Scholar]
- Schroers H.-J. A monograph of Bionectria (Ascomycota, Hypocreales, Bionectriaceae) and its Clonostachys anamorph. Studies in Mycology. 2001;46:1–211. [Google Scholar]
- Schroers H.-J., Geldenhuis M.M., Wingfield M.J. Classification of the guava wilt fungus Myxosporium psidii, the palm pathogen Gliocladium vermoesenii and the persimmon wilt fungus Acremonium diospyri in Nalanthamala. Mycologia. 2005;97:375–395. doi: 10.3852/mycologia.97.2.375. [DOI] [PubMed] [Google Scholar]
- Schroers H.-J., Gräfenhan T., Nirenberg H.I. A revision of Cyanonectria and Geejayessia gen. nov., and related species with Fusarium-like anamorphs. Studies in Mycology. 2011;68:115–138. doi: 10.3114/sim.2011.68.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroers H.-J., O'Donnell K., Lamprecht S.C. Taxonomy and phylogeny of the Fusarium dimerum species group. Mycologia. 2009;101:44–70. doi: 10.3852/08-002. [DOI] [PubMed] [Google Scholar]
- Seifert K.A. A monograph of Stilbella and some allied Hyphomycetes. Studies in Mycology. 1985;27:1–224. [Google Scholar]
- Seifert K.A., Samuels G.J. How should we look at anamorphs? Studies in Mycology. 2000;45:5–18. [Google Scholar]
- Somrithipol S., Sudhom N., Tippawan S. A new species of Falcocladium (Hyphomycetes) with turbinate vesicles from Thailand. Sydowia. 2007;59:148–153. [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014 doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian C.V. Indian Council of Agricultural Research; New Delhi, India: 1971. Hyphomycetes: an account of Indian species, except Cercosporae. [Google Scholar]
- Summerbell R.C., Gueidan C., Schroers H.-J. Acremonium phylogenetic overview and revision of Gliomastix, Sarocladium, and Trichothecium. Studies in Mycology. 2011;68:139–162. doi: 10.3114/sim.2011.68.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerbell R.C., Schroers H.-J. Analysis of phylogenetic relationships of Cylindrocarpon lichenicola and Acremonium falciforme species complex and a review of similarities in the spectrum of opportunistic infections caused by these fungi. Journal of Clinical Microbiology. 2002;40:2866–2875. doi: 10.1128/JCM.40.8.2866-2875.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton B.C. Sarcopodium and its synonyms. Transactions of the British Mycological Society. 1981;76:97–102. [Google Scholar]
- Tamura K., Stecher G., Peterson D. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T. Three new Nectria species from Japan. Transactions of the Mycological Society of Japan. 1990;31:227–236. [Google Scholar]
- Webster J. Nectria lugdunensis sp. nov., the perfect state of Heliscus lugdunensis. Transactions of the British Mycological Society. 1959;42:322–327. [Google Scholar]
- Weese J. Beiträge zur Kenntnis der Hypocreaceen. Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften, Mathematisch-Naturwissenschaftlichen Klasse, Abt. 1. 1916;125:465–575. [Google Scholar]
- White T.J., Burns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., editors. PCR protocols: a guide to methods and applications. Academic Press; U.S.A.: 1990. pp. 282–287. [Google Scholar]
- Wollenweber H.W. Studies on the Fusarium problem. Phytopathology. 1913;3:24–50. [Google Scholar]
- Wollenweber H.W. Fusaria autographice delineate. Annales Mycologici. 1917;15:1–56. [Google Scholar]
- Wollenweber H.W., Reinking O.A. P. Parey; Berlin, Germany: 1935. Die Fusarien: ihre Beschreibung, Schadwirkung und Bekämpfung. [Google Scholar]