Abstract
Background. The risk factors for Staphylococcus aureus (S. aureus) pneumonia are not fully identified. The aim of this work was to find out the clinical characteristics associated with S. aureus infection in patients with healthcare-associated pneumonia (HCAP) and hospital-acquired pneumonia (HAP), which may be applicable for more appropriate selection of empiric antibiotic therapy. Methods. From July 2007 to June 2010, patients who were admitted to the intensive care unit with severe HCAP/HAP and severe sepsis were enrolled in this study. Lower respiratory tract sample was semiquantitatively cultured. Initial broad-spectrum antibiotics were chosen by Taiwan or American guidelines for pneumonia management. Standard bundle therapies were provided to all patients according to the guidelines of the Surviving Sepsis Campaign. Results. The most frequently isolated pathogens were Pseudomonas aeruginosa, S. aureus, Acinetobacter baumannii, Klebsiella pneumoniae, and Escherichia coli. Patients with positive isolation of S. aureus in culture had significantly higher history of liver cirrhosis and diabetes mellitus, with odds ratios of 3.098 and 1.899, respectively. The S. aureus pneumonia was not correlated with history of chronic obstructive pulmonary disease, hypertension, and hemodialysis. Conclusion. Liver cirrhosis and diabetes mellitus may be risk factors for S. aureus infection in patients with severe HCAP or HAP.
1. Introduction
In present medical practice, severe pneumonia with CURB-65 score (confusion, uremia, respiratory rate, low blood pressure, and age 65 years or greater) of ≧ 3 points comprised more than 16% and 25% of 30-day mortality rate among community-acquired pneumonia (CAP) and healthcare-associated pneumonia (HCAP), respectively [1]. Initial broad-spectrum empiric antibiotic selection is important because inadequate or delayed antibiotic treatment results in high mortality. However, overuse of empirical broad-spectrum antibiotics can create multidrug-resistant (MDR) pathogens and contribute to antibiotic-induced complications.
Selection of antibiotics for initial empirical therapy is based on prediction of the most likely pathogens and knowledge of local susceptibility. Guidelines provide some risk factors for specific pathogens [2, 3]. This can help us decide initial empiric antibiotics. In general, physicians prescribe initial antibiotics which should be effective for Pseudomonas aeruginosa (P. aeruginosa) and MDR pathogens in patients combining HCAP and hospital-acquired pneumonia (HAP) with severe sepsis. If Staphylococcus aureus (S. aureus) is considered, vancomycin, linezolid, or teicoplanin is added due to high risk of methicillin-resistant S. aureus (MRSA). The Infectious Diseases Society of America and American Thoracic Society suggest risk factors of S. aureus pneumonia in patients with hemodialysis, lung abscess, structure lung disease, injection drug use, prior influenza, prior antibiotic therapy, and endobronchial obstruction [2].
However, the best indicator of S. aureus infection is that Gram staining shows a single/predominant Gram-positive coccus with clustered and polymorphonuclear cells. Then, S. aureus infection could be confirmed by following culture report. But culture report needs 3-4 days. Patients with late-onset HAP and HCAP are at greater risk for infection with MDR pathogens [3]. Thus, it becomes an important issue whether critically ill patients with severe HAP and HCAP have probability of S. aureus infection in present clinical practice.
We designed a prospective observational study to determine the prevalence and epidemiologic risk factor of S. aureus infection among patients admitted to intensive care unit (ICU) due to severe HCAP and HAP.
2. Materials and Methods
2.1. Subjects
From July 2007 to June 2010, patients who were admitted to the ICU at Chang Gung Memorial Hospital, Keelung, due to HCAP and HAP with severe sepsis or septic shock were enrolled in this study. The ICU is a medical and closed unit in our hospital. This study was approved by the Institutional Review Board at Chang Gung Memorial Hospital (96-0132B, 97-0121C, and 98-1682C). The following patient data were recorded within the first 3 days after admission: age; gender; medical history; lower respiratory tract sample for semiquantitative culture; Acute Physiology and Chronic Health Evaluation (APACHE) II score; and adverse events. History of intravenous drug use and prior antibiotic use within 30 days was recorded according to the statement of patient or patient's family. Endotracheal aspirates were used as lower respiratory tract samples for culture initially. If cultures were negative, bronchoalveolar lavages were performed to detect pathogens. Samples contaminated by upper airway secretions, as reflected by more than 10 squamous epithelial cells/low power field, were excluded.
2.2. Disease Definitions
Pneumonia was defined as new abnormal infiltration on chest radiograph with respiratory symptoms or fever. Pneumonia was classified as HCAP and HAP according to guidelines [3]. HCAP includes any patient who was hospitalized in an acute care hospital for two or more days within 90 days of the infection; resided in a nursing home or long-term care facility; received recent intravenous antibiotic therapy, chemotherapy, or wound care within the past 30 days of the current infection; or attended a hospital or hemodialysis clinic. HAP is defined as pneumonia that occurs 48 hours or more after admission, which was not incubating at the time of admission. Severe sepsis and septic shock were defined according to the criteria established in the Consensus Conference [4]. Systemic inflammatory response syndrome (SIRS) was defined as fulfillment of two or more of the following criteria: (1) body temperature >38°C or <36°C; (2) respiratory rate >24 breaths/minute; (3) heart rate >90 beats/minute; and (4) white blood count >12,000/μL or <4000/μL or >10% bands. Sepsis was defined as SIRS according to a confirmed or suspected microbial etiology. Severe sepsis was defined as sepsis with one or more dysfunctional organs or hypotension. Septic shock was defined as sepsis with hypotension unresponsive to fluid resuscitation, which further required vasopressors to maintain blood pressure during the first three days following ICU admission. Acute renal failure was diagnosed as a rapidly rising serum creatine level ≥0.5 mg/dL over the baseline value [5]. Disease severity was assessed with the APACHE II score [6]. Survivors were defined as patients who were alive 30 days after ICU admission.
2.3. Treatment
Standard bundle therapies, including fluid resuscitation, broad-spectrum antibiotics, drainage, blood transfusion, sedation/paralysis, blood glucose control, hemodialysis, stress ulcer prophylaxis, and basic support, were provided to all patients according to the recommended guidelines [7]. Initial broad-spectrum antibiotics were chosen by the Taiwan Guidelines for Pneumonia Management (2007 version) or Guidelines of American Thoracic Society [3].
2.4. Statistical Analysis
Statistical analysis was performed with the Statistical Package for the Social Sciences (SPSS) V17.0 for Windows (SPSS, Inc., Illinois, USA). Differences for the continuous variables between the two groups were analyzed by Mann-Whitney test. Differences for categorical variables between the two groups were compared by Pearson chi-square test or Fisher's exact test. General linear model was used to determine the associations of S. aureus pneumonia among all factors. A p value of less than 0.05 was considered statistically significant. Odds ratio is the odds that a patient is exposed to the risk factor divided by the odds that a control is exposed to.
3. Results
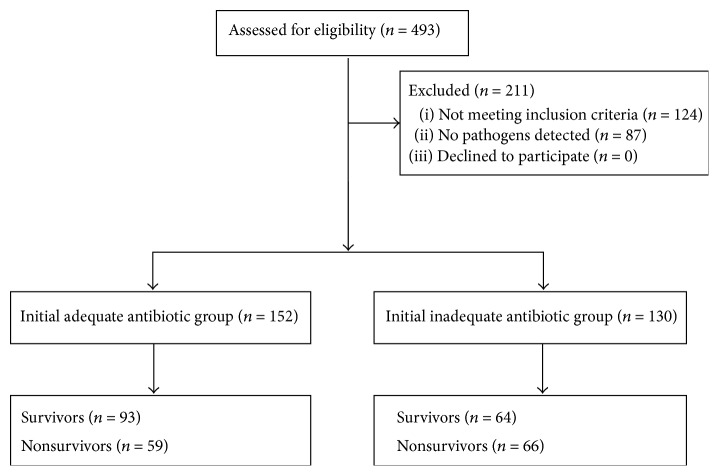
During the study period, 493 patients with severe sepsis or septic shock were screened (Figure 1). A total of 282 patients were enrolled for analysis, and 211 patients were excluded. The reasons for exclusion included nonpneumonia infection (n = 73), coisolated pathogens (n = 51), and no detectable pathogens (n = 87) in lower respiratory tract sample for culture. Patients with coisolated pathogens and no detectable pathogens were excluded to reduce the possible bias maximally due to concerns of pathogen colonization and unknown pathogens. No patients withdrew. Initially, 152 and 130 patients received adequate and inadequate antibiotic therapy, respectively. Fifty-nine and 66 patients died in adequate and inadequate antibiotic groups, respectively. Clinical characteristics of HCAP or HAP patients with severe sepsis are shown in Table 1. Patients with S. aureus pneumonia had higher percentage of liver cirrhosis and diabetes mellitus history. Percentages of history of chronic obstructive pulmonary disease (COPD), congestive heart failure (CHF), hypertension, and hemodialysis were similar between presence and absence of S. aureus pneumonia. There were no differences in age, APACHE II score, sex, adverse events, and 30-day mortality rates between patients with and without S. aureus infection.
Figure 1.
During the study period, 493 patients with severe sepsis and septic shock were screened, and 211 patients were excluded. The reasons for exclusion included nonpneumonia infection and no detectable pathogens in lower respiratory tract sample for culture. A total of 282 patients were enrolled for analysis. No patients withdrew. Initially, 152 and 130 patients received adequate and inadequate antibiotic therapy, respectively. Fifty-nine and 66 patients died in adequate and inadequate antibiotic groups, respectively.
Table 1.
Clinical characteristics between presence and absence of S. aureus infection in HCAP or HAP patients with severe sepsis.
| S. aureus (N = 63) | No S. aureus (N = 219) | p value | |
|---|---|---|---|
| Age, years∗ | 73.7 ± 14.4 | 73.9 ± 13.0 | 0.938 |
| APACHE II score∗ | 26.0 ± 6.7 | 27.0 ± 7.8 | 0.477 |
| Sex, number (%) | 0.916 | ||
| Male | 43 (68.3) | 151 (68.9) | |
| Female | 20 (31.7) | 68 (31.1) | |
| History, number (%) | |||
| Prior antibiotic use | 20 (31.7) | 64 (29.2) | 0.700 |
| Intravenous drug use | 0 (0.0) | 0 (0.0) | † |
| COPD | 9 (14.3) | 47 (21.5) | 0.208 |
| CHF | 10 (15.9) | 17 (7.8) | 0.054 |
| Hypertension | 26 (41.3) | 90 (41.1) | 0.980 |
| Liver cirrhosis | 11 (17.5) | 14 (6.4) | 0.006 |
| Hemodialysis | 4 (6.3) | 20 (9.1) | 0.485 |
| Diabetes mellitus | 27 (42.9) | 62 (28.3) | 0.029 |
| Adverse events, number (%) | |||
| RF with intubation and MV | 63 (100.0) | 219 (100.0) | † |
| GI bleeding | 8 (12.7) | 33 (15.1) | 0.638 |
| Shock | 31 (49.2) | 100 (45.7) | 0.619 |
| New arrhythmia | 4 (6.3) | 13 (5.9) | 1.000 |
| Acute renal failure | 27 (42.9) | 92 (42.0) | 0.904 |
| Jaundice | 7 (11.1) | 17 (7.8) | 0.401 |
| Thrombocytopenia | 23 (36.5) | 83 (37.9) | 0.841 |
| 30-day mortality, number (%) | 25 (39.7) | 100 (45.7) | 0.400 |
S. aureus: Staphylococcus aureus; HCAP: healthcare-associated pneumonia; HAP: hospital-acquired pneumonia; APACHE: Acute Physiology and Chronic Health Evaluation; COPD: chronic obstructive pulmonary disease; CHF: congestive heart failure; RF: respiratory failure; MV: mechanic ventilator; and GI: gastrointestinal
∗Data is shown as mean ± standard deviation.
†No statistic was computed because variable is a constant.
Table 2 shows the isolated pathogens with initial adequate or inadequate antibiotic treatment. The most frequently isolated pathogens, in decreasing order, were P. aeruginosa, S. aureus, Acinetobacter baumannii, Klebsiella pneumoniae (K. pneumoniae), Escherichia coli and so forth. All patients with Streptococcus pneumonia (S. pneumonia) infection were prescribed adequate antibiotic treatment initially. Only S. pneumonia infection was associated with 30-day mortality (Table 3). Patients with S. pneumonia infection had higher survivor rate. There was no difference in mortality between other pathogens and mortality.
Table 2.
Pathogens in severe pneumonia between adequate and inadequate antibiotic use.
| Pathogens | Adequate N (% of total) |
Inadequate N (% of total) |
Total N (% of pathogens) |
|---|---|---|---|
| Pseudomonas aeruginosa | 53 (74.6) | 18 (25.4) | 71 (25.1) |
| Staphylococcus aureus | 31 (49.2) | 32 (50.8) | 63 (22.3) |
| MSSA | 10 (15.9) | 3 (4.8) | 13 (4.6) |
| MRSA | 21 (33.3) | 29 (46.0) | 50 (17.7) |
| Acinetobacter baumannii | 18 (35.3) | 33 (64.7) | 51 (18.0) |
| Klebsiella pneumoniae | 32 (72.7) | 12 (27.3) | 44 (15.5) |
| Escherichia coli | 21 (67.7) | 10 (32.3) | 31 (11.0) |
| Streptococcus pneumonia | 11 (100.0) | 0 (0.0) | 11 (3.8) |
| Stenotrophomonas maltophilia | 3 (42.9) | 4 (57.1) | 7 (2.5) |
| Enterobacter | 3 (60.0) | 2 (40.0) | 5 (1.8) |
MSSA: Methicillin-Sensitive Staphylococcus aureus; MRSA: Methicillin-Resistant Staphylococcus aureus.
Table 3.
Difference in mortality among pathogens in severe pneumonia.
| Pathogens | Survivors | Nonsurvivors | p value |
|---|---|---|---|
| Pseudomonas aeruginosa | 0.338 | ||
| Yes | 34 | 28 | |
| No | 114 | 97 | |
| Staphylococcus aureus | 0.400 | ||
| Yes | 38 | 25 | |
| No | 119 | 100 | |
| Acinetobacter baumannii | 0.617 | ||
| Yes | 30 | 21 | |
| No | 127 | 104 | |
| Klebsiella pneumoniae | 0.868 | ||
| Yes | 25 | 19 | |
| No | 132 | 106 | |
| Escherichia coli | 0.776 | ||
| Yes | 18 | 13 | |
| No | 139 | 112 | |
| Streptococcus pneumonia | 0.026 | ||
| Yes | 10 | 1 | |
| No | 147 | 124 | |
| Stenotrophomonas maltophilia | 0.137 | ||
| Yes | 6 | 1 | |
| No | 151 | 124 | |
| Enterobacter | 0.658 | ||
| Yes | 2 | 3 | |
| No | 155 | 122 |
After regression analysis, patient's history of CHF, liver cirrhosis, and diabetes mellitus were independent factors associated with S. aureus pneumonia (Table 4). The odds ratios of CHF, liver cirrhosis, and diabetes mellitus were 2.242, 3.098, and 1.899, respectively. The S. aureus pneumonia was not correlated with history of prior antibiotic use, COPD, hypertension, and chronic renal failure. The S. aureus pneumonia was also not associated with age, gender, APACHE II score, adverse events, and mortality.
Table 4.
General linear model to predict Staphylococcus aureus pneumonia.
| Variables | B | p value | 95% confidence interval | |
|---|---|---|---|---|
| Lower | Upper | |||
| Age, years | 0.000 | 0.839 | −0.004 | 0.004 |
| APACHE II score | −0.005 | 0.218 | −0.012 | 0.003 |
| Sex, male | −0.014 | 0.810 | −0.124 | 0.097 |
| History | ||||
| Prior antibiotic use | −0.010 | 0.859 | −0.119 | 0.099 |
| COPD | −0.053 | 0.434 | −0.185 | 0.080 |
| CHF | 0.175 | 0.043 | 0.006 | 0.345 |
| Hypertension | −0.026 | 0.639 | −0.135 | 0.083 |
| Liver cirrhosis | 0.334 | 0.003 | 0.117 | 0.552 |
| Hemodialysis | −0.080 | 0.398 | −0.267 | 0.107 |
| Diabetes mellitus | 0.113 | 0.049 | 0.001 | 0.225 |
| Adverse events | ||||
| GI bleeding | −0.076 | 0.317 | −0.224 | 0.073 |
| Shock | 0.060 | 0.304 | −0.054 | 0.173 |
| New arrhythmia | 0.006 | 0.957 | −0.205 | 0.216 |
| Acute renal failure | −0.012 | 0.831 | −0.121 | 0.097 |
| Jaundice | −0.097 | 0.392 | −0.319 | 0.125 |
| Thrombocytopenia | 0.000 | 0.989 | −0.111 | 0.110 |
| 30-day mortality, death | −0.023 | 0.692 | −0.136 | 0.090 |
4. Discussion
The present investigation is the first to find that history of CHF, liver cirrhosis, and diabetes mellitus increased the risk of S. aureus infection in patients with severe HAP or HCAP. Immunodeficiency in cirrhosis is multifactorial and might not be reversed by interventions. Polymorphonuclear leukocyte dysfunction and complement deficiency are well known as main causes leading to infection predisposition [8]. S. aureus has been reported to be the most frequently (27.4%) isolated pathogen from blood and ascites fluid in patients with liver cirrhosis [9]. From the database of a surveillance study of S. aureus infections, bacteremia and bone infection were more frequent in the chronic liver disease group when compared with the other-disease group [10]. After multivariate analysis, chronic liver disease was a factor significantly associated with 30-day mortality. However, there was no difference in pneumonia rate between the chronic liver disease and other-disease groups. This was dissimilar from the current study's findings. In this research, we found that HCAP or HAP patients with liver cirrhosis had higher percentage of S. aureus infection. More studies may be needed to clarify the association between S. aureus pneumonia and liver cirrhosis.
Compared with nondiabetes patients with severe pneumonia, diabetes patients had 1.899 of odds ratio for S. aureus infection in this study. Hyperglycemia promoted respiratory S. aureus infection, and metformin modified glucose flux across the airway epithelium to limit hyperglycemia-induced bacterial growth [11]. Shorr et al. developed a risk score to identify S. aureus in patients with HCAP [12]. Female with diabetes was one of eight variables in the final risk score. Furthermore, diabetes could be thought of as immunosuppressive disease, a risk factor of methicillin-resistant S. aureus infection (MRSA) [13]. However, in a study of healthcare-associated infection, Erben et al. found that diabetes mellitus was not a risk factor for S. aureus pneumonia [14]. Diabetes mellitus was a risk factor for S. aureus infection at surgical site. More studies may be necessary to determine the relationship between S. aureus pneumonia and diabetes mellitus.
In this work, S. aureus infection was the second most frequently found pathogen in patients with severe pneumonia. In Korea and Japan, the frequent pathogens in HCAP, in decreased order, were S. pneumonia, S. aureus, P. aeruginosa, and K. pneumoniae [15, 16]. Also, S. pneumonia, P. aeruginosa, and S. aureus were the frequently detected pathogens in HCAP in Spanish [17].
In this study, patients with chronic obstructive pulmonary disease (COPD) did not have higher risk of S. aureus infection or colonization. That is similar to Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults [2]. However, in a retrospective chart review study, the risk for MRSA was increased by tobacco use (OR = 2.31, CI 1.23–4.31) and COPD (OR = 3.76, CI 1.74–8.08) in CAP patients [18], and the risk of COPD disappeared in HAP patients. Thus, whether COPD increases risk for S. aureus in HCAP is still unclear. About the history of CHF, we did not find published reports or studies showing the presence of correlation between CHF and S. aureus. Hemodialysis was not associated with S. aureus pneumonia in this work. This may be due to the fact that more than 90% identified S. aureus events were bloodstream infection [19]. Although hemodialysis did not significantly increase the risk of S. aureus pneumonia, patients with end-stage renal disease and hemodialysis patients had increased risk of S. aureus bacteremia [20].
As we know, inadequate empiric antimicrobial treatment may be associated with excess hospital mortality in pneumonia [3]. However, this point might not be constant after bundle therapy of severe sepsis. In our previous study, there was no difference in mortality between initial adequate and inadequate antibiotic therapy in patients with severe sepsis [21]. In this work, even though most of patients were prescribed initial adequate antibiotic therapy for P. aeruginosa and K. pneumoniae, their mortality rates did not decrease significantly. Only patients with S. pneumonia infection had significantly higher survival rate. The most likely reason might be that all patients with S. pneumonia infection were prescribed adequate empiric antibiotic therapy.
There are two limitations in this study. First, the accuracy of diagnosing pathogens is based on lower respiratory tract quantitative cultures of endotracheal aspirates, protected specimen brush, or bronchoalveolar lavage samples. The lower respiratory tract culture in our hospital is semiquantitative. Further, a positive culture cannot always distinguish a pathogen from a colonizing organism [3]. The culture results in this work may include colonizing pathogens. Second, there is a possible bias in statistical analysis. The percentage of history of CHF was low in this study. That made the statistical power low in regression analysis.
In conclusion, the traditional risk factors for S. aureus infection may change or vary. This study concludes that liver cirrhosis and diabetes mellitus may be risk factors for S. aureus infection in patients with HCAP or HAP. Using these features may allow us to select adequate empiric antibiotics.
Acknowledgment
The authors would like to thank all the members of the medical intensive care units for providing clinical assistance.
Conflict of Interests
The authors confirm that no author has any financial or personal relationships with other organizations that could inappropriately influence this work.
References
- 1.Jeong B.-H., Koh W.-J., Yoo H., et al. Performances of prognostic scoring systems in patients with healthcare-associated pneumonia. Clinical Infectious Diseases. 2013;56(5):625–632. doi: 10.1093/cid/cis970. [DOI] [PubMed] [Google Scholar]
- 2.Mandell L. A., Wunderink R. G., Anzueto A., et al. Infectious Diseases Society of America/American Thoracic Society Consensus Guidelines on the management of community-acquired pneumonia in adults. Clinical Infectious Diseases. 2007;44(supplement 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Thoracic Society; Infectious Diseases Society of America. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. American Journal of Respiratory and Critical Care Medicine. 2005;171(4):388–416. doi: 10.1164/rccm.200405-644st. [DOI] [PubMed] [Google Scholar]
- 4.Levy M. M., Fink M. P., Marshall J. C., et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Critical Care Medicine. 2003;31(4):1250–1256. doi: 10.1097/01.ccm.0000050454.01978.3b. [DOI] [PubMed] [Google Scholar]
- 5.Bellomo R., Ronco C., Kellum J. A., Mehta R. L., Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Critical Care. 2004;8(4):R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Knaus W. A., Draper E. A., Wagner D. P., Zimmerman J. E. APACHE II: a severity of disease classification system. Critical Care Medicine. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Dellinger R. P., Carlet J. M., Masur H., et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Critical Care Medicine. 2004;32(3):858–873. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 8.Christou L., Pappas G., Falagas M. E. Bacterial infection-related morbidity and mortality in cirrhosis. American Journal of Gastroenterology. 2007;102(7):1510–1517. doi: 10.1111/j.1572-0241.2007.01286.x. [DOI] [PubMed] [Google Scholar]
- 9.Campillo B., Richardet J.-P., Kheo T., Dupeyron C. Nosocomial spontaneous bacterial peritonitis and bacteremia in cirrhotic patients: impact of isolate type on prognosis and characteristics of infection. Clinical Infectious Diseases. 2002;35(1):1–10. doi: 10.1086/340617. [DOI] [PubMed] [Google Scholar]
- 10.Kang C.-I., Song J.-H., Ko K. S., Chung D. R., Peck K. R. Clinical significance of Staphylococcus aureus infection in patients with chronic liver diseases. Liver International. 2010;30(9):1333–1338. doi: 10.1111/j.1478-3231.2010.02270.x. [DOI] [PubMed] [Google Scholar]
- 11.Garnett J. P., Baker E. H., Naik S., et al. Metformin reduces airway glucose permeability and hyperglycaemia-induced Staphylococcus aureus load independently of effects on blood glucose. Thorax. 2013;68(9):835–845. doi: 10.1136/thoraxjnl-2012-203178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shorr A. F., Myers D. E., Huang D. B., Nathanson B. H., Emons M. F., Kollef M. H. A risk score for identifying methicillin-resistant Staphylococcus aureus in patients presenting to the hospital with pneumonia. BMC Infectious Diseases. 2013;13(1, article 268) doi: 10.1186/1471-2334-13-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung W. J., Kang Y. A., Park M. S., et al. Prediction of methicillin-resistant Staphylococcus aureus in patients with non-nosocomial pneumonia. BMC Infectious Diseases. 2013;13, article 370 doi: 10.1186/1471-2334-13-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erben N., Ozgunes I., Aksit F., Doyuk Kartal E., Colak E., Usluer G. Healthcare-associated infections and the distribution of causative pathogens in patients with diabetes mellitus. European Journal of Clinical Microbiology and Infectious Diseases. 2013;32(6):821–825. doi: 10.1007/s10096-013-1816-x. [DOI] [PubMed] [Google Scholar]
- 15.Park H. K., Song J.-U., Um S.-W., et al. Clinical characteristics of health care-associated pneumonia in a Korean teaching hospital. Respiratory Medicine. 2010;104(11):1729–1735. doi: 10.1016/j.rmed.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Ishida T., Tachibana H., Ito A., Yoshioka H., Arita M., Hashimoto T. Clinical characteristics of nursing and healthcare-associated pneumonia: a Japanese variant of healthcare-associated pneumonia. Internal Medicine. 2012;51(18):2537–2544. doi: 10.2169/internalmedicine.51.7987. [DOI] [PubMed] [Google Scholar]
- 17.Giannella M., Pinilla B., Capdevila J. A., et al. Pneumonia treated in the internal medicine department: focus on healthcare-associated pneumonia. Clinical Microbiology and Infection. 2012;18(8):786–794. doi: 10.1111/j.1469-0691.2011.03757.x. [DOI] [PubMed] [Google Scholar]
- 18.Wooten D. A., Winston L. G. Risk factors for methicillin-resistant Staphylococcus aureus in patients with community-onset and hospital-onset pneumonia. Respiratory Medicine. 2013;107(8):1266–1270. doi: 10.1016/j.rmed.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen D. B., Lessa F. C., Belflower R., et al. Invasive methicillin-resistant Staphylococcus aureus infections among patients on chronic dialysis in the United States, 2005–2011. Clinical Infectious Diseases. 2013;57(10):1393–1400. doi: 10.1093/cid/cit546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nielsen L. H., Jensen-Fangel S., Benfield T., et al. Risk and prognosis of Staphylococcus aureus bacteremia among individuals with and without end-stage renal disease: a Danish, population-based cohort study. BMC Infectious Diseases. 2015;15(1, article 6) doi: 10.1186/s12879-014-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu H.-P., Chung K., Lin C.-Y., Jiang B.-Y., Chuang D.-Y., Liu Y.-C. Associations of T helper 1, 2, 17 and regulatory T lymphocytes with mortality in severe sepsis. Inflammation Research. 2013;62(8):751–763. doi: 10.1007/s00011-013-0630-3. [DOI] [PMC free article] [PubMed] [Google Scholar]