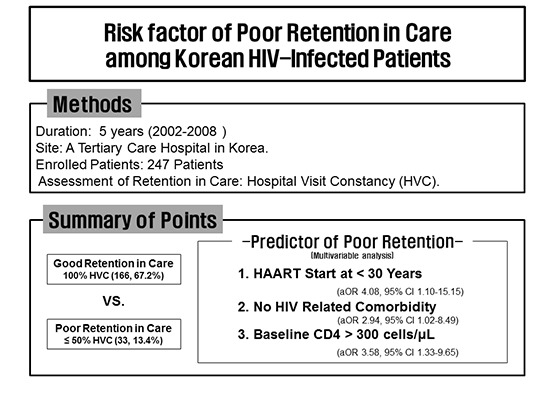
Abstract
Poor retention in care (RIC) is associated with higher antiretroviral therapy (ART) failure and worse survival. Identifying high risk patients for poor RIC is important for targeted intervention. A retrospective cohort study was conducted at a tertiary care hospital in Korea. HIV-infected patients initiating ART during 2002-2008 were included. 5 year-RIC was measured by hospital visit constancy (HVC) at 5 years after initiating ART. Among 247 enrolled patients, 179 (72.5%) remained in care, 20 (8.1%) were transferred to other hospitals, 9 (3.6%) died and 39 (15.8%) were lost to follow-up. We compared the demographic, psychosocial, and clinical characteristics between the groups with 100% HVC (n = 166, 67.2%) and ≤ 50% HVC (n = 33, 13.4%). In multivariable analysis, ART-starting age ≤ 30 years (odds ratio [OR] 4.08 vs. > 50; 95% confidence interval [CI] 1.10-15.15, P = 0.036), no non-HIV related comorbidity (OR 2.94 vs. comorbidity ≥ 1; 95% CI 1.02-8.49, P = 0.046), baseline CD4 cell count > 300 cells/μL (OR 3.58 vs. ≤ 200; 95% CI 1.33-9.65, P = 0.012) were significant predictable factors of poor RIC. HIV/AIDS care-givers should pay attention to young patients with higher baseline CD4 cell counts and no non-HIV related comorbidity.
Keywords: HIV, Antiretroviral Therapy, Retention, Visit Constancy
Graphical Abstract
INTRODUCTION
Retaining patients with human immunodeficiency virus (HIV) in medical care after initiation of effective antiretroviral therapy (ART) is essential for successful HIV treatment. Once patients are initiating ART, high levels of adherence to treatment are required to achieve sustained HIV suppression and to reduce risk of drug resistance. In addition to monitoring adherence to medication, regular clinical follow-up visits are crucial for scheduled laboratory tests, monitoring drug toxicity, timely immunization and prophylaxis for opportunistic infections (OIs), and to diagnose and treat new OIs that may occur and other comorbidities (1,2,3,4,5). Multiple studies indicate that poor retention in care is associated with higher rate of ART failure and worse survival (6,7,8,9,10,11,12,13).
The identification of risk factors for poor retention in care is of paramount importance to develop targeted interventions to improve optimal individual and public health outcomes and cost effectiveness. Some risk factors, including younger age, female sex, racial or ethnic minority status, low socioeconomic status, no usual source of health care, less advanced HIV disease, fewer non-HIV-related comorbidities, and greater unmet psychosocial needs, have been suggested as a predictors of poor retention in care (2). These risk factors are influenced by several factors, such as demographic, disease severity, psychosocial, and ancillary services use factors, and may be variable among different countries. The objective of this study was to determine the risk factors for suboptimal retention in care among HIV infected adults receiving ART in Korea.
MATERIALS AND METHODS
Study design
A retrospective cohort study was conducted to assess the risk factors associated with suboptimal retention in care among HIV-infected patients receiving ART. The characteristics of this cohort and details of the methodology have been previously described (11). Briefly, Pusan National University Hospital is a 1,220 bed, university-affiliated teaching hospital and provides HIV care for HIV infected patients in the southeastern area of Korea, in close collaboration with the local Public Health Centers (PHCs) in this area. The study included HIV infected patients aged 18 years and older who started ART at the study hospital between 2002 and 2008. Patients who had been started on ART in other hospitals before they referred to the study hospital were excluded. Patients who died or were transferred to other hospitals within 1 year after ART initiation were also excluded.
First, based on the follow-up status of patients to the study hospital as of 5 years after ART initiation, each patient was initially classified as remained in care, dead at the study hospital, transfer-out to other hospitals, or lost. And then, we traced the patients initially categorized as lost to ascertain their survival status in collaboration with local PHCs. After tracing, each patient was reclassified as alive or dead.
The observation periods were measured from the date of ART initiation to the earliest of the following dates: 5 years if the patients was still alive during 5 years after start of ART regardless of whether or not they remained in care, the date of death if the patients died within 5 years after ART initiation, the date of the last follow-up visit if the patients were transferred out to other health facility within 5 years after starting ART.
Retention in care was measured by hospital visit constancy (HVC) during the observation period after initiating ART (2,7,14,15). The observation period after start of ART was broken down into 3-month periods, and the number of 3-month periods in which patients had at least 1 hospital visit for HIV care was examined. HVC was calculated by using the equation HVC = (numbers of 3-month periods with ≥1 completed hospital visit) / (total numbers of 3-month periods during the observational periods of interest) × 100. Medical subspecialty appointment except HIV care visit was excluded, but urgent care visit for HIV care was included. AIDS-defining illness and clinical categories were defined by the 1993 Centers for Disease Control and Prevention (CDC) classification criteria (16). Non-HIV related comorbidity was assessed with Charlson Comorbidity Index (CCI) (17). We excluded AIDS as a co-morbidity (18). To determine the predictable factors of poor retention in care, we compared the demographic, psychosocial, and clinical characteristics between the patients with 100% HVC and the patients with ≤ 50% HVC, by using multiple logistic regression analysis.
Statistical analysis
Categorical variables were compared using Pearson’s chi-square test or Fisher’s exact test, whereas non-categorical variables were tested with the Mann-Whitney U-test or Kruskal wallis test. Logistic regression analysis was used to determine risk factors for poor retention in care. All variables with P < 0.25 in univariate analysis were assessed in multivariate models using stepwise backward election. All tests were considered statistically significant at P < 0.05. The statistical analyses were conducted using IBM SPSS Statistics version 22 (IBM, Armonk, NY, USA).
Ethics statement
This study protocol was approved by the institutional review board of Pusan National University Hospital (IRB No. E-2014115). Informed consent was waived by the board.
RESULTS
Between 2002 and 2008, a total of 328 patients were first prescribed ART in the study hospital. Of these, 32 patients (9.8%) who had taken ART before visiting the study hospital were excluded from the analysis. We excluded 14 patients (4.3%) who were transferred out to other hospitals within 1 year after ART initiation and 33 patients (10.1%) who died within 1 year after ART initiation. Two patients (0.6%) were unable to be traced after loss to follow-up (LTFU) and were also excluded from the analysis. Thus, 247 patients (75.3%) were included in the analysis.
As of 5 years after ART initiation, 179 patients (72.5%) remained in care in the study hospital, 20 patients (8.1%) were transferred out to other hospitals, 9 patients (3.6%) died in the study hospital, and 39 patients (15.8%) were lost. Of the 39 patients initially categorized as lost, after tracing, 8 patients (20.5%) were known to have died and 31 patients (79.5%) were alive.
The median age of patients was 42 years [interquartile range (IQR) 36-50] and 85.8% were male. Median CD4 lymphocyte count was 130 cells/μL (IQR 44-249) and 123 (48.8%) were in CDC clinical category B or C. The baseline characteristics of the study population and a comparison by HVC are presented in Table 1.
Table 1. Baseline characteristics of 247 patients included in analyses at the start of ART.
| Characteristics | No (%) of patients by visit constancy | P value | |||
|---|---|---|---|---|---|
| Total (n = 247) |
100% (n = 166) |
51-99% (n = 48) |
≤ 50% (n = 33) |
||
| Sex | 0.597 | ||||
| Male | 212 (85.8) | 142 (85.5) | 43 (89.6) | 27 (81.8) | |
| Female | 35 (14.2) | 24 (14.5) | 5 (10.4) | 6 (18.2) | |
| Age at ART start, median (IQR), yr | 42 (36-50) | 44 (38-51) | 40 (33-47) | 38 (31-49) | 0.006 |
| > 50 | 63 (25.5) | 47 (28.3) | 9 (18.8) | 7 (21.2) | 0.014 |
| 41-50 | 88 (35.6) | 66 (39.8) | 13 (27.1) | 9 (27.3) | |
| 31-40 | 69 (27.9) | 43 (25.9) | 16 (33.3) | 10 (30.3) | |
| ≤ 30 | 27 (10.9) | 10 (6.0) | 10 (20.8) | 7 (21.2) | |
| Route of transmission | 0.095 | ||||
| Heterosexual | 134 (54.3) | 92 (55.4) | 20 (41.7) | 22 (66.7) | |
| Homo/bisexual | 108 (43.7) | 70 (42.2) | 28 (58.3) | 10 (30.3) | |
| IDU/transfusion | 5 (2.0) | 4 (2.4) | 0 (0) | 1 (3.0) | |
| Marriage | 0.185 | ||||
| Unmarried | 102 (41.3) | 61 (36.7) | 24 (50.0) | 17 (51.5) | |
| Married | 92 (37.2) | 70 (42.2) | 14 (29.2) | 8 (24.2) | |
| Divorced/separated by death | 53 (21.5) | 35 (21.1) | 10 (20.8) | 8 (24.2) | |
| Health security system | 0.345 | ||||
| Health insurance | 168 (68.0) | 114 (68.7) | 29 (60.4) | 25 (75.8) | |
| Medical aid | 79 (32.0) | 52 (31.3) | 19 (39.6) | 8 (24.2) | |
| Residential area | 0.485 | ||||
| Busan | 180 (72.9) | 122 (73.5) | 31 (64.6) | 27 (81.8) | |
| Surrounding satellite city | 44 (17.8) | 30 (18.1) | 10 (20.8) | 4 (12.1) | |
| Other city or region | 23 (9.3) | 14 (8.4) | 7 (14.6) | 2 (6.1) | |
| Non-HIV related comorbidity (Charlson comorbidity index)* | 0.039 | ||||
| 0 | 165 (66.8) | 104 (62.7) | 33 (68.8) | 28 (84.8) | |
| ≥ 1 | 82 (33.2) | 62 (37.3) | 15 (31.3) | 5 (15.2) | |
| Psychiatry disorder | 0.875 | ||||
| No | 228 (92.3) | 153 (92.2) | 45 (93.8) | 30 (90.9) | |
| Yes | 19 (7.7) | 13 (7.8) | 3 (6.3) | 3 (9.1) | |
| History of substance abuse | 0.801 | ||||
| No | 242 (98.0) | 163 (67.4) | 47 (19.4) | 32 (13.2) | |
| Yes | 5 (2.0) | 3 (60.0) | 1 (20.0) | 1 (20.0) | |
| CD4 cell counts on ART initiation, median (IQR), cells/µL | 130 (44.0-249.0) | 118 (37.8-227.8) | 109.5 (39.3-249.8) | 229 (77.0-326.0) | 0.012 |
| ≤ 200 | 158 (64.0) | 114 (68.7) | 29 (60.4) | 15 (45.5) | 0.075 |
| 201-300 | 54 (21.9) | 32 (19.3) | 13 (27.1) | 9 (27.3) | |
| > 300 | 35 (14.2) | 20 (12.0) | 6 (12.5) | 9 (27.3) | |
| Clinical category at ART start | 0.080 | ||||
| A | 124 (50.2) | 82 (49.4) | 22 (45.8) | 20 (60.6) | |
| B | 46 (18.6) | 30 (18.1) | 7 (14.6) | 9 (27.3) | |
| C | 77 (31.2) | 54 (32.5) | 19 (39.6) | 4 (12.1) | |
| Duration from HIV diagnosis to ART initiation, yr | 0.36 (0.15-2.65) | 0.28 (0.12-1.49) | 1.31 (0.24-5.11) | 0.42 (0.2-3.21) | 0.002 |
| < 1 | 160 (64.8) | 119 (71.7) | 23 (47.9) | 18 (54.5) | 0.014 |
| 1-5 | 48 (19.4) | 25 (15.1) | 13 (27.1) | 10 (30.3) | |
| > 5 | 39 (15.8) | 22 (13.3) | 12 (25.0) | 5 (15.2) | |
| First ART | 0.463 | ||||
| Unboosted PIs | 59 (23.9) | 37 (22.3) | 10 (20.8) | 12 (36.4) | |
| Boosted PIs | 111 (44.9) | 75 (45.2) | 22 (45.8) | 14 (42.4) | |
| NNRTI | 77 (31.2) | 54 (32.5) | 16 (33.3) | 7 (21.2) | |
| ART regimen during the 5 yr after ART initiation | 0.080 | ||||
| PIs | 124 (50.2) | 82 (49.4) | 22 (45.8) | 20 (60.6) | |
| NNRTIs | 46 (18.6) | 30 (18.1) | 7 (14.6) | 9 (27.3) | |
| Mixed | 77 (31.2) | 54 (32.5) | 19 (39.6) | 4 (12.1) | |
Data are number (%) of patients, unless otherwise indicated. *Excluding AIDS as a co-morbidity. HIV, human immunodeficiency virus; IQR, interquartile range; ART, anti-retroviral therapy; IDU, injection drug user; PI, protease inhibitor; NNRTI, non-nucleotide reverse transcriptase inhibitor.
Among the included 247 patients, 166 patients (67.2%) was regular clinic attendance (HVC 100%), whereas 81 patients (32.8%) had various durations of LTFU at some points in their observation periods. Of these 81, 48 patients (59.3%) had 51-99% HVC and 33 patients (40.7%) had HVC ≤ 50%. Overall, 32 of 81(39.5%) were lost to follow-up within 6 months after ART initiation. Among the 81 patients who were lost to follow-up, 63 (77.8%) returned to care, however, 46 of 63 (73%) were lost to follow-up again. Of the 46 patients who were lost to follow-up again after return to care, 20 (43.5%) did not return to care. Among the 81 patients who were lost to follow-up, 30 (37%) had a cyclical pattern of being in and out of care at irregular intervals.
When we compared 166 patients (67.2%) with HVC 100% with 33 patients (13.4%) with HVC ≤ 50%, age at start of ART ≤ 30 years (odds ratio [OR], 4.70 vs. > 50; 95% confidence interval [CI], 1.35-16.41, P = 0.015), no non-HIV related comorbidity (OR, 3.25 vs. CCI ≥ 1; 95% CI, 0.19-8.87, P = 0.021), CD4 cell count > 300 cells/μL at ART initiation (OR, 3.42 vs. ≤ 200; 95% CI, 1.32-8.877, P = 0.011), CDC clinical category B (OR, 3.29 vs. C; 95% CI, 1.07-10.16, P = 0.038) or A (OR, 4.05 vs. C; 95% CI, 1.15-14.27, P = 0.030), duration from HIV diagnosis to ART initiation 1-5 years (OR, 2.64 vs. < 1; 95% CI, 1.09-6.41, P = 0.031), use of single class of ART during observational period nonnucleoside reverse transcriptase inhibitors (NNRTIs) (OR, 3.29 versus switch to another class of ART; 95% CI, 1.07-10.16, P = 0.038) or protease inhibitor (PIs) (OR, 4.05 vs. switch to another class of ART; 95% CI, 1.15-14.27, P = 0.030) were associated with a higher risk of poor retention in care (HVC ≤ 50%) in univariate analysis (Table 2).
Table 2. Univariate and multivariate analyses of characteristics predictive of poor retention in care among 247 HIV infected patients included in analyses.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | aHR (95% CI) | P value | |
| Sex | ||||
| Male | 1 | - | - | - |
| Female | 1.32 (0.49-3.52) | 0.586 | - | - |
| Age at ART start (yr) | ||||
| > 50 | 1 | - | 1 | |
| 41-50 | 0.91 (0.32-2.63) | 0.916 | 0.82 (0.27-2.48) | 0.722 |
| 31-40 | 1.56 (0.55-4.47) | 0.406 | 1.15 (0.38-3.49) | 0.808 |
| ≤ 30 | 4.70 (1.35-16.41) | 0.015 | 4.08 (1.10-15.15) | 0.036 |
| Route of transmission | ||||
| Homo/bisexual | 1 | - | - | - |
| Heterosexual | 1.67 (0.75-3.76) | 0.212 | - | - |
| IDU/transfusion | 1.75 (0.18-17.27) | 0.632 | - | - |
| Marriage | ||||
| Married | 1 | - | - | - |
| Unmarried | 2.44 (0.98-6.04) | 0.054 | - | - |
| Divorced/separated by death | 2.00 (0.69-5.78) | 0.200 | - | - |
| Health security system | ||||
| Medical aid | 1 | - | - | - |
| Health insurance | 1.43 (0.60-3.37) | 0.420 | - | - |
| Residential area | ||||
| Busan | 1 | - | - | - |
| Surrounding satellite city | 0.60 (0.20-1.85) | 0.377 | - | - |
| Other city or region | 0.65 (0.14-3.01) | 0.577 | - | - |
| Non-HIV related comorbidity (Charlson comorbidity index) * | ||||
| ≥ 1 | 1 | - | 1 | - |
| 0 | 3.25 (0.19-8.87) | 0.021 | 2.94 (1.02-8.49) | 0.046 |
| Psychiatry disorder | ||||
| No | 1 | - | - | - |
| Yes | 0.83 (0.18-3.89) | 0.811 | - | - |
| History of substance abuse | ||||
| No | 1 | - | - | - |
| Yes | 1.18 (0.32-4.39) | 0.808 | - | - |
| CD4 cell counts on ART initiation, cells/µL | ||||
| ≤ 200 | 1 | - | 1 | - |
| 201-300 | 2.14 (0.86-5.34) | 0.104 | 2.13 (0.81-5.59) | 0.123 |
| > 300 | 3.42 (1.32-8.87) | 0.011 | 3.58 (1.33-9.65) | 0.012 |
| Clinical category at ART start | ||||
| C | 1 | - | ||
| B | 3.29 (1.07-10.16) | 0.038 | ||
| A | 4.05 (1.15-14.27) | 0.030 | ||
| Duration from HIV diagnosis to ART initiation, yr | ||||
| < 1 | 1 | - | - | |
| 1-5 | 2.64 (1.09-6.41) | 0.031 | - | - |
| > 5 | 1.50 (0.51-4.47) | 0.464 | ||
| First ART | ||||
| NNRTI | 1 | - | - | |
| Boosted PIs | 2.50 (0.90-6.95) | 0.079 | - | - |
| Unboosted PIs | 1.44 (0.55-3.81) | 0.462 | - | - |
| ART regimen during the 5 yr after ART initiation | ||||
| Mixed | 1 | - | - | |
| NNRTIs | 3.29 (1.07-10.16) | 0.038 | - | - |
| PIs | 4.05 (1.15-14.27) | 0.030 | - | - |
*Excluding AIDS as a co-morbidity. HR, hazard ratio; aHR, adjusted hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus; ART, anti-retroviral therapy; IDU, injection drug user; PI, protease inhibitor; NNRTI, non-nucleotide reverse transcriptase inhibitor.
In multivariate analysis, age at start of ART ≤ 30 years (OR, 4.08 vs. > 50; 95% CI, 1.10-15.15, P = 0.036], no non-HIV related comorbidity (OR, 2.94 vs. CCI ≥ 1; 95% CI, 1.02-8.49, P = 0.046), CD4 cell count > 300 cells/μL at ART initiation (OR, 3.58; 95% CI, 1.33-9.65, P = 0.012) were significant predictable factors of poor retention in care (HVC ≤ 50%) during up to 5-year observational period after ART initiation (Table 2).
DISCUSSION
In this study, we evaluated the factors predicting poor retention in care after initiating ART in a retrospective cohort. Identifying which patients are at greatest risk for not being retained is important to develop targeted interventions to improve optimal individual clinical outcomes and potential public health benefit (1). At present, there is no gold standard for assessing retention in care and selection of the most appropriate measurement method is challenging (3,15). The length of the follow-up periods as well as differences in healthcare systems and characteristics of the study populations are likely to influence the estimates.
In our study, almost one-third of patients (32.8%) had LTFU at some points in up to 5-year observation periods. The pattern of healthcare usage and duration of LTFU of these patients were considerably variable. About three fourths of patients (77.8%) were returned to care, however, about three fourths of patients (73%) were lost to follow-up again. Overall, approximately half of patients (46.9%) did not return after varying duration of LTFU, and more than one third of patients (37%) had a cyclical pattern of being in and out of care at irregular intervals. This heterogeneity in pattern of LTFU makes it difficult to compare regular users with sporadic users or nonengagers as dichotomous variables (1,19).
In a recent study, we evaluated the mortality rate and risk factors for death in 327 HIV infected patients initiating ART during 1998-2005 in a tertiary hospital of Korea (11). Among patients who survived more than 12 months after starting ART, patients with ≤ 50% HVC were about 13 times more likely to die than those who attended hospital regularly during a 5-year observation period.
In the present study, we measured retention in care by HVC during the 5-year observation period after ART initiation (2,7,14,15) and compared regular users with HVC 100% with sporadic users or nonengagers with HVC ≤ 50% (1,19). Although this measure is less detailed to assess retention in care than appointment adherence, it is known to be preferable for research for longer observation periods, particularly relevant for patients starting ART, and is better accounts for LTFU (15). In this study, we included urgent care visit for HIV care to measure the retention because patients who returned after LTFU were frequently hospitalized via urgent care, and thereafter successfully retained in care if they survived (11).
The main strength of this study is the 5-year observation period and active tracing the patients who are LTFU. The risk of LTFU was highest during the first 6 months after initiating ART (39.5%). More than half of patients (59.3%) were lost to follow-up within 1 year after starting ART. Our study also revealed that younger patients (≤ 30 years), those who had higher CD4 cell counts (> 300 cells/μL), and those who had no non-HIV related comorbidity were less likely to be retained after starting ART. These findings might be associated with health care seeking behavior. Younger patients appear to be more difficult to retain in long-term follow-up, probably due to a youthful sense of invulnerability, feeling healthier, or lifestyle issues, such as work or school, that prevent them from maintaining regular scheduled visits (20,21). In addition, patients with higher CD4 cell counts and without medical comorbidity are more likely to be lost to follow-up after starting ART, perhaps because they did not feel sick enough to be motivated to visit the hospital regularly (2,22,23).
In this study, other sociodemographic and clinical characteristics reported by other studies were not associated with poor retention in care. Of note, we did not find that the association between economic factor and poor retention. This finding can be explained by the fact that all medical cost for HIV care including antiretroviral drugs was provided free of charge by the government through medical aid or national health insurance program in Korea. The effect of injecting drug on poor retention in care was also not significant in our analysis, possibly because of low prevalence of HIV infection among injecting drug users in Korea (24,25).
This study has some limitations. First, our study might have some unmeasured confounding due to its retrospective design. In particular, detailed socio-economic status such as occupation, which was one of important variable, was not analyzed. Second, our study was conducted at a single center in the southeastern area of Korea, and the numbers of patients were relatively small, therefore our findings may not be generalized to other region of the country. Third, we did not measure the adherence to treatment. Although most patients got a refill prescription during routine follow-up visit after starting ART, patients can be retained in care, but not necessarily adhere to treatment (3,5). However, it was unable to assess the adherence of included patients precisely because our study was performed relatively and for a long time. Forth, we measured retention in care by 3 month HVC. The missed visit within an interval of interest could not be measured, and retention could be overestimated (2,15).
In conclusion, we found that almost one-third of patients had LTFU at some points in up to 5-year observation periods. The pattern of healthcare usage and duration of LTFU of these patients were considerably variable. More than half of patients were lost to follow-up within 1 year after starting ART. The risk of LTFU was highest during the first 6 months after initiating ART. Age at start of ART ≤ 30 years, no non-HIV related comorbidity, CD4 cell count > 300 cells/μL at ART initiation were risk factors predicting poor retention in care among HIV-infected patients receiving antiretroviral therapy in Korea. These results suggest that effective strategies for retaining the patients in care after starting ART in the long-term perspective are needed, particularly with special attention to these risk groups, focusing on the early period after ART initiation.
ACKNOWLEDGMENT
The authors thank the Department of Biostatistics, Clinical Trial Center of Pusan National University Hospital for statistical advice on this work.
Footnotes
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Study design: Lee S, Lee SH. Data collection: Lee S, Lee SH. Statistical analysis: Lee S, Lee SH. Writing the first draft: Lee S, Lee SH. Review and revision of manuscript: Lee SJ, Kim KH, Lee JE, Cho H, Lee SG, Chen DH, Chung JS, Kwak IS. Approval of final manuscript: all authors.
References
- 1.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: where are we? Where do we go from here? Clin Infect Dis. 2010;50:752–761. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 2.Giordano TP. Retention in HIV care: what the clinician needs to know. Top Antivir Med. 2011;19:12–16. [PMC free article] [PubMed] [Google Scholar]
- 3.Stricker SM, Fox KA, Baggaley R, Negussie E, de Pee S, Grede N, Bloem MW. Retention in care and adherence to ART are critical elements of HIV care interventions. AIDS Behav. 2014;18(Suppl 5):S465–S475. doi: 10.1007/s10461-013-0598-6. [DOI] [PubMed] [Google Scholar]
- 4.Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, Orrell C, Altice FL, Bangsberg DR, Bartlett JG, et al. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817–833. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, Hirschhorn L, Fullem A, Ojikutu B, Oser R. Adult adherence to treatment and retention in care. Arlington, VA: USAID/AIDSTAR-ONE; 2010. [Google Scholar]
- 6.Mugavero MJ, Lin HY, Willig JH, Westfall AO, Ulett KB, Routman JS, Abroms S, Raper JL, Saag MS, Allison JJ. Missed visits and mortality among patients establishing initial outpatient HIV treatment. Clin Infect Dis. 2009;48:248–256. doi: 10.1086/595705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano TP, Gifford AL, White AC, Jr, Suarez-Almazor ME, Rabeneck L, Hartman C, Backus LI, Mole LA, Morgan RO. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 8.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–87. doi: 10.7326/0003-4819-131-2-199907200-00002. [DOI] [PubMed] [Google Scholar]
- 9.Robbins GK, Daniels B, Zheng H, Chueh H, Meigs JB, Freedberg KA. Predictors of antiretroviral treatment failure in an urban HIV clinic. J Acquir Immune Defic Syndr. 2007;44:30–37. doi: 10.1097/01.qai.0000248351.10383.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Association between adherence to antiretroviral therapy and human immunodeficiency virus drug resistance. Clin Infect Dis. 2003;37:1112–1118. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Kim KH, Lee SG, Cho H, Chen DH, Chung JS, Kwak IS, Cho GJ. Causes of death and risk factors for mortality among HIV-infected patients receiving antiretroviral therapy in Korea. J Korean Med Sci. 2013;28:990–997. doi: 10.3346/jkms.2013.28.7.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi JY, Park YS, Kim CO, Park YS, Yoon HJ, Shin SY, Kim YA, Song YG, Kim JM. Correlation between adherence to antiretroviral treatment and virologic failure in HIV-infected Koreans. Infect Chemother. 2005;37:9–15. [Google Scholar]
- 13.Kim YJ, Lee WK, Kim SW, Chang HH, Lee JM, Kim SJ. Impact of self-efficacy on medication adherence among people living with human immunodeficiency virus. Korean J Med. 2015;89:305–311. [Google Scholar]
- 14.Mugavero MJ. Improving engagement in HIV care: what can we do? Top HIV Med. 2008;16:156–161. [PubMed] [Google Scholar]
- 15.Mugavero MJ, Davila JA, Nevin CR, Giordano TP. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24:607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Skiest DJ, Rubinstien E, Carley N, Gioiella L, Lyons R. The importance of comorbidity in HIV-infected patients over 55: a retrospective case-control study. Am J Med. 1996;101:605–611. doi: 10.1016/S0002-9343(96)00329-4. [DOI] [PubMed] [Google Scholar]
- 19.Mallinson RK, Relf MV, Dekker D, Dolan K, Darcy A, Ford A. Maintaining normalcy: a grounded theory of engaging in HIV-oriented primary medical care. ANS Adv Nurs Sci. 2005;28:265–277. doi: 10.1097/00012272-200507000-00008. [DOI] [PubMed] [Google Scholar]
- 20.Israelski D, Gore-Felton C, Power R, Wood MJ, Koopman C. Sociodemographic characteristics associated with medical appointment adherence among HIV-seropositive patients seeking treatment in a county outpatient facility. Prev Med. 2001;33:470–475. doi: 10.1006/pmed.2001.0917. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan S, Wu K, Smurzynski M, Bosch RJ, Benson CA, Collier AC, Klebert MK, Feinberg J, Koletar SL. ALLRT/A5001 Team. Incidence rate of and factors associated with loss to follow-up in a longitudinal cohort of antiretroviral-treated HIV-infected persons: an AIDS Clinical Trials Group (ACTG) Longitudinal Linked Randomized Trials (ALLRT) analysis. HIV Clin Trials. 2011;12:190–200. doi: 10.1310/HCT1204-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giordano TP, Hartman C, Gifford AL, Backus LI, Morgan RO. Predictors of retention in HIV care among a national cohort of US veterans. HIV Clin Trials. 2009;10:299–305. doi: 10.1310/hct1005-299. [DOI] [PubMed] [Google Scholar]
- 23.Lebouché B, Yazdanpanah Y, Gérard Y, Sissoko D, Ajana F, Alcaraz I, Boitte P, Cadoré B, Mouton Y. Incidence rate and risk factors for loss to follow-up in a French clinical cohort of HIV-infected patients from January 1985 to January 1998. HIV Med. 2006;7:140–145. doi: 10.1111/j.1468-1293.2006.00357.x. [DOI] [PubMed] [Google Scholar]
- 24.Min JA, Yoon Y, Lee HJ, Choi J, Kwon M, Kim K, Lee CU, Kim DJ, Yun H. Prevalence and associated clinical characteristics of hepatitis B, C, and HIV infections among injecting drug users in Korea. J Med Virol. 2013;85:575–582. doi: 10.1002/jmv.23523. [DOI] [PubMed] [Google Scholar]
- 25.Suguimoto SP, Techasrivichien T, Musumari PM, El-saaidi C, Lukhele BW, Ono-Kihara M, Kihara M. Changing patterns of HIV epidemic in 30 years in East Asia. Curr HIV/AIDS Rep. 2014;11:134–145. doi: 10.1007/s11904-014-0201-4. [DOI] [PubMed] [Google Scholar]


