Abstract
AIM: To explore the impact of body mass index (BMI) on surgical outcomes in patients undergoing laparoscopic liver resection (LLR).
METHODS: From January 2010 to February 2015, sixty-eight patients who underwent primary partial liver resection in our institute were retrospectively reviewed. Surgical outcomes of LLR were compared with those of open liver resection (OLR). In addition, we analyzed associations with BMI and surgical outcomes.
RESULTS: Among 68 patients, thirty-nine patients underwent LLR and 29 were performed OLR. Significant difference in operation time, blood loss, and postoperative hospital stay was observed. There were no significant differences in mortality and morbidity in two groups. Twenty-two patients (32.4%) were classified as obese (BMI ≥ 25). A statistically significant correlation was observed between BMI and operation time, between BMI and blood loss in OLR, but not in LLR. The operation time and blood loss of OLR were significantly higher than that of LLR in obese patients. Open liver resection and BMI were independent predictors for prolonged operation time and increased blood loss in multivariate analysis.
CONCLUSION: The present study demonstrated that BMI had influenced to surgical outcomes of OLR. LLR was less influenced by BMI and had great benefit in obese patients.
Keywords: Laparoscopic liver resection, Obesity, Body mass index, Prolonged operation time, Increased blood loss
Core tip: This study presented the correlation between body mass index (BMI) and surgical outcomes of 68 cases performed laparoscopic liver resection (LLR) and open liver resection (OLR). A statistically significant correlation was observed between BMI and operation time, between BMI and blood loss in OLR, but not in LLR. Open liver resection and BMI were independent predictors for prolonged operation time and increased blood loss in multivariate analysis. LLR in obese patients was safe and had great benefit without prolonged operation time and increased blood loss.
INTRODUCTION
Obese patients have been increasing worldwide[1,2]. They have multiple co-morbidities such as diabetes mellitus, dyslipidemia and cardiovascular disease[3,4]. Obesity induce various liver diseases, including fatty liver, ranging from steatosis to non-alcoholic steatohepatitis (NASH). NASH induce liver cirrhosis and hepatocellular carcinoma (HCC) with it progress[5,6]. Moreover, obesity is known as independent risk factor for several malignancies, including liver cancer[6,7]. Regarding the surgical outcomes of liver resection for obesity patients, some studies showed obesity to be a significant predictor of an adverse postoperative course after liver resection[8,9]. However, other studies have demonstrated that obesity did not increase the risk of morbidity[10].
Laparoscopic surgery has several advantages, such as less destruction of abdominal wall, early postoperative recovery and less postoperative morbidity as compared to open surgery[11,12]. More than thirty years ago, obesity had been generally regarded as a contraindication for laparoscopic surgery because of associated technical difficulties[13]. Approximately ten years ago, several reports had suggested obesity as a risk factor in conversion[14,15]. Recently, it comes to be reported that laparoscopic surgery brings good results even in obese patients, including cholecystectomy, gastrectomy and colectomy[16-18]. The impact of obesity on the outcomes of laparoscopic liver resection (LLR), however, still remains a controversial matter as on the outcomes of open liver resection (OLR).
The aim of this study was to evaluate the surgical outcomes of LLR in obese/non-obese patients and OLR in obese/non-obese patients to clarify the benefit of LLR in obese patients.
MATERIALS AND METHODS
Patients characteristics and classification according to BMI
We retrospectively reviewed medical data from charts and surgical records of 255 patients who underwent liver resection at the Department of Gastroenterological and Pediatric Surgery, Oita University Faculty of Medicine, Oita, Japan, from January 2010 through February 2015. During the period, partial liver resection was performed in 111 patients. The patients with repeat liver resection (n = 24) and had multiple site of liver resections (n = 19) were excluded. Finally, we reviewed the records of 68 patients. The patients were divided into following two groups: an OLR group, performed until September 2012, and a LLR group, performed after October 2012.
We compared clinical parameters and surgical outcomes between the two groups. The correlation between body mass index (BMI) and surgical outcomes in each group was also investigated. Each patient’s height and weight were measured preoperatively, and BMI (kg/m2) was calculated as the weight (in kg) divided by the height squared (in m). According to the World health Organization, body weight was divided into three type; underweight (BMI < 18.5), normal weight (BMI 18.5-24.9), over weight (BMI 25.0-29.9), and obese (BMI > 30)[19]. In Japan, BMI ≥ 25 is considered as obese based on the definition by the Japan Society for the Study of Obesity and World Health Organization expert consultation[20]. In the present study, we defined patients with BMI ≥ 25 as obesity. We also used the difficulty scoring system for LLR. The difficulty was scored by the extent of liver resection, tumor location, tumor size, liver function, and tumor proximity to major vessels, as described previously[21].
Surgical procedure
The surgical technique of LLR has been described previously[22]. Ultrasonography was routinely performed to confirm the tumor. Liver parenchyma transection was performed by the combination of a cavitation ultrasonic surgical aspirator, ultrasonic scalpel, and monopolar soft coagulation system. OLR was performed with same positioning and with same devises during liver parenchyma transaction under intermittent Pringle maneuver.
Statistical analysis
All variables are expressed as mean ± SD for continuous data and as number with percentages for categorical data. Statistical analysis was performed using Student’s t for continuous variables and χ² test for categorical variables. The correlation between continuous variables was investigated by Pearson’s rank correlation. Multivariate logistic regression analyses were performed to identify predictors associated with prolonged operation time and increased blood loss. In these analyses, the cutoff point for operation time and blood loss was determined using receiver operating characteristic (ROC) curve analysis. Statistical significance was set at P < 0.05. All statistical analyses were performed using SPSS for Windows software (IBM-SPSS, Inc., Chicago, IL, United States).
RESULTS
Patient characteristics
Among those 68 patients, thirty-nine underwent LLR and 29 were performed OLR. Patients’ characteristics and surgical outcomes are shown in Table 1. Patients’ characteristics were similar between the two groups. There were no open conversions in LLR group. LLR had lower operation time and blood loss than OLR, significantly. +LLR had also lower postoperative hospital stay than OLR. There were no significant differences in morbidity rate between the two groups.
Table 1.
Comparison of patients’ characteristics and surgical outcomes n (%)
| LLR (n = 39) | OLR (n = 29) | P value | |
| Age (yr) | 69.4 ± 10.2 | 68.3 ± 8.1 | NS |
| Sex (Male/Female) | 26/13 | 22/7 | NS |
| HBV | 6 (15.4) | 4 (13.8) | NS |
| HCV | 19 (48.7) | 13 (44.8) | NS |
| Diagnosis | |||
| Hepatocellular carcinoma | 33 (84.6) | 22 (75.9) | |
| Intrahepatic cholangiocarcinoma | 2 (5.1) | 1 (3.4) | |
| Metastatic liver tumor | 2 (5.1) | 6 (20.7) | |
| Hemangioma | 2 (5.1) | 0 | |
| Body mass index (kg/m2) | 24.1 ± 4.1 | 23.9 ± 3.9 | NS |
| Tumor size (mm) | 27.3 ± 14.5 | 27.6 ± 11.6 | NS |
| Surgical outcome | |||
| Operation time (min) | 226.1 ± 117.2 | 292.8 ± 86.7 | 0.009 |
| Blood loss (mL) | 109.3 ± 162.0 | 406.9 ± 425.6 | 0.001 |
| Postoperative hospital stay (d) | 12.8 ± 10.4 | 23.6 ± 30.1 | 0.042 |
| Overall morbidity | 4 (10.2) | 7 (24.1) | NS |
| Grade I | 0 | 1 | |
| Grade II | 3 | 4 | |
| Grade III (a/b) | 1/0 | 1/1 | |
| Grade IV | 0 | 0 | |
| Grade V | 0 | 0 | |
| Bile leakage | 1 (2.7) | 2 (6.9) | NS |
LLR: Laparoscopic liver resection; OLR: Open liver resection.
Correlation between BMI and surgical outcomes
Correlation between BMI and surgical outcomes using Pearson’s rank correlation test are shown in Figure 1. Significant correlations were found in the OLR group between BMI and operation time (R = 0.432, P = 0.019; Figure 1A) and between BMI and blood loss (R = 0.573, P = 0.001; Figure 1B). Otherwise, in the LLR group correlation between BMI and operation time was not significant (R = 0.209, P = 0.201; Figure 1C) and between BMI and blood loss also was not significant (R = 0.156, P = 0.344; Figure 1D).
Figure 1.
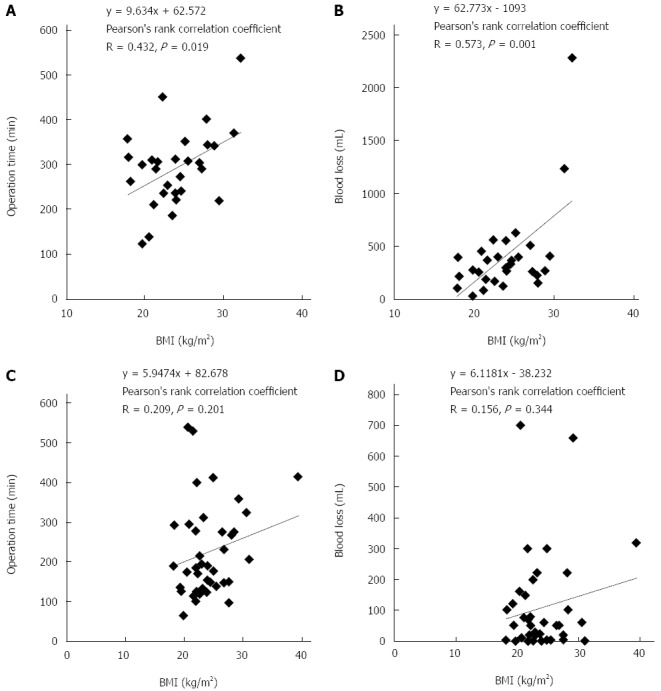
Scatter plot. A: Scatter plot showed the relationship between BMI and operation time in open liver resection; B: Scatter plot showed the relationship between BMI and blood loss in open liver resection; C: Scatter plot showed the relationship between BMI and operation time in laparoscopic liver resection; D: Scatter plot showed the relationship between BMI and blood loss in laparoscopic liver resection. BMI: Body mass index.
Association with obesity and surgical outcomes in each group
In LLR group, twelve of 39 patients were high BMI (BMI ≥ 25). No significant differences in age, sex, viral status, tumor size, or preoperative laboratory data between obese patients and non-obese patients were found in LLR group. There was no significant difference in the operation time between non-obese patients and obese patients in the LLR group. Similarly, the blood loss of non-obese patients was not significantly different from that of obese patients. There were no significant differences in the incidence of postoperative complication among BMI in patients performed LLR. Distribution of difficulty score was not different between patients that were high BMI and patients that were not (data not shown). In the non-obese patients, LLR required a similar operation time, whereas operation time in LLR group was significantly shorter than that in OLR group in the obese patients (Figure 2A). Blood loss was significantly lower than that of OLR in the non-obese and obese patients (Figure 2B).
Figure 2.
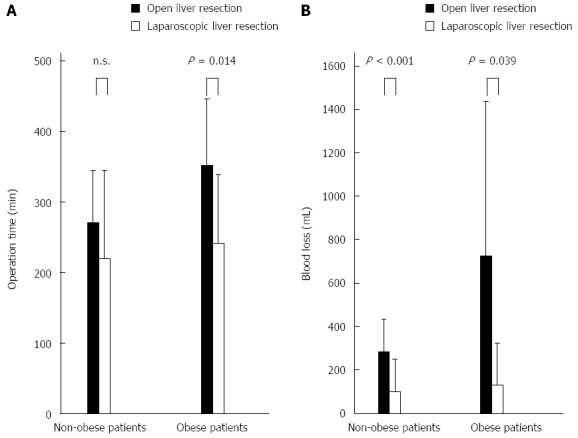
Surgical approach in obese and non-obese patients. A: Comparison of operation time according to the surgical approach in obese and non-obese patients; B: Comparison of blood loss according to the surgical approach in obese and non-obese patients.
In OLR group, ten of 29 patients were high BMI (BMI ≥ 25). There were no differences in patients’ characteristics between non-obese patients and obese patients who underwent OLR unless all obese patients were male.
Comparison between LLR and OLR in obese patients
In comparison between LLR and OLR in obese patients, there were similar in age, virus status, and preoperative laboratory data between LLR and OLR in obese patients, while gamma-GTP was significantly higher in the OLR group (Table 2). The operation time and blood loss of obese patients were significantly lower in LLR group. There was no significant difference in the incidence of complication between LLR and OLR.
Table 2.
Comparison of patients’ characteristics and surgical outcomes in patients with obesity
| LLR (n = 12) | OLR (n = 10) | P value | |
| Patients’ characteristics | |||
| Age | 70.5 ± 9.4 | 67.1 ± 6.2 | NS |
| Sex (Male/Female) | 7/5 | 10/0 | 0.020 |
| HBV | 1 | 2 | NS |
| HCV | 7 | 2 | NS |
| Body mass index (kg/m2) | 29.0 ± 3.7 | 28.2 ± 2.3 | NS |
| Tumor size (mm) | 23.8 ± 8.7 | 26.0 ± 11.6 | NS |
| Liver weight (g) | 47.1 ± 35.5 | 60.8 ± 61.2 | NS |
| Surgical outcome | |||
| Operation time (min) | 241.3 ± 97.6 | 346.7 ± 83.8 | 0.014 |
| Blood loss (mL) | 128.3 ± 193.1 | 635.5 ± 655.1 | 0.039 |
| Postoperative hospital stay (d) | 11.6 ± 3.3 | 20.8 ± 15.4 | 0.094 |
| Complication, n (%) | 1 (8.3) | 1 (10) | NS |
| Biloma | 0 | 0 | NS |
LLR: Laparoscopic liver resection; OLR: Open liver resection.
Independent predictive factors for prolonged operation time and increased blood loss
Cutoff point of prolonged operation time was 200 min using ROC curving analysis. Univariate analysis showed BMI, OLR, tumor size, and ICG to be factors indicative of prolonged operation time. Multivariate analysis demonstrated that OLR [P < 0.001, Odds ratio (OR) = 16.244, 95%CI: 3.648-72.340], BMI (P = 0.024, OR = 1.259, 95%CI: 1.031-1.538), and tumor size (P = 0.047, OR = 1.056, 95%CI: 1.001-1.114) were independent predictive factors for prolonged operation time in all patients (Table 3).
Table 3.
Univariate and multivariate analysis on prolonged operation time
|
Operation time |
Univariate |
Multivariate |
|||
| < 200 | ≥ 200 | P value | P value | OR (95%CI) | |
| Number of patients | 25 | 43 | |||
| Age (yr) | 71.5 ± 8.4 | 67.4 ± 9.6 | 0.082 | ||
| Sex (Male/Female) | 16/9 | 32/11 | 0.363 | ||
| Body mass index (kg/m2) | 22.7 ± 2.6 | 24.8 ± 4.5 | 0.019 | 0.024 | 1.259 (1.031-1.538) |
| HBV | 2 | 8 | 0.304 | ||
| HCV | 12 | 20 | 0.906 | ||
| Open liver resection < 0.001 | 3 | 26 | < 0.001 | < 0.001 | 16.244 (3.648-72.340) |
| Tumor size (mm) | 23.3 ± 9.9 | 29.8 ± 14.4 | 0.049 | 0.047 | 1.056 (1.001-1.114) |
| Total bilirubin (mg/dL) | 0.77 ± 0.28 | 0.79 ± 0.31 | 0.829 | ||
| Albumin (g/dL) | 3.94 ± 0.59 | 3.91 ± 0.46 | 0.848 | ||
| Prothrombin time (%) | 101.4 ± 21.9 | 98.2 ± 17.2 | 0.508 | ||
| Indocyanine green (%) | 11.8 ± 11.1 | 18.9 ± 14.8 | 0.042 | ||
| Aspartate transaminase (U/L) | 36.8 ± 20.5 | 39.1 ± 20.9 | 0.669 | ||
| Alanine transaminase (U/L) | 30.2 ± 22.8 | 33.3 ± 22.3 | 0.596 | ||
| γ-glutamy transpeptidase (U/L) | 57.5 ± 48.3 | 64.8 ± 51.3 | 0.567 | ||
| Alkaline phosphatase (U/L) | 315.6 ± 109.9 | 306.0 ± 105.5 | 0.725 | ||
| Cholinesterase (U/L) | 176.6 ± 127.0 | 188.5 ± 115.3 | 0.649 | ||
| White blood cell (/mm3) | 4342 ± 1383 | 4638 ± 1748 | 0.470 | ||
| Hemoglobin (g/dL) | 12.8 ± 1.9 | 13.1 ± 2.1 | 0.598 | ||
| Hematocrit (%) | 36.9 ± 7.3 | 39.0 ± 5.6 | 0.185 | ||
| Platelet count (× 103/mm3) | 14.5 ± 7.7 | 13.2 ± 6.0 | 0.433 | ||
LLR: Laparoscopic liver resection; OLR: Open liver resection.
In the analysis of increased blood loss, cutoff point was 215 mL using ROC curving analysis. There were significant difference in OLR, BMI, prothrombin time (PT), and indocyanine green (ICG) test for increased blood loss using univariate analysis. Multivariate analysis showed that OLR (P < 0.001, OR = 27.736, 95%CI: 5.926-129.811), BMI (P = 0.035, OR = 1.233, 95%CI: 1.015-1.498), and PT (P = 0.030, OR = 0.947, 95%CI: 0.902-0.995) were independent predictive factors for increased blood loss significantly (Table 4).
Table 4.
Univariate and multivariate analysis on increased blood loss
|
Blood loss |
Univariate |
Multivariate |
|||
| < 215 | ≥ 215 | P value | P value | OR (95%CI) | |
| Number of patients | 40 | 28 | |||
| Age (yr) | 68.5 ± 10.1 | 68.1 ± 4.5 | 0.558 | ||
| Sex (Male/Female) | 26/14 | 22/6 | 0.227 | ||
| Body mass index (kg/m2) | 23.1 ± 3.4 | 25.3 ± 4.5 | 0.027 | 0.035 | 1.233 (1.015-1.498) |
| HBV | 4 | 6 | 0.297 | ||
| HCV | 20 | 12 | 0.561 | ||
| Open liver resection | 8 | 21 | < 0.001 | < 0.001 | 27.736 (5.926-129.811) |
| Tumor size (mm) | 26.0 ± 11.4 | 29.4 ± 15.5 | 0.303 | ||
| Total bilirubin (mg/dL) | 0.76 ± 0.30 | 0.81 ± 0.30 | 0.490 | ||
| Albumin (g/dL) | 3.98 ± 0.55 | 3.84 ± 0.43 | 0.287 | ||
| Prothrombin time (%) | 103.2 ± 20.3 | 93.9 ± 15.6 | 0.046 | 0.030 | 0.947 (0.902-0.995) |
| Indocyanine green (%) | 13.4 ± 10.7 | 20.3 ± 16.9 | 0.044 | ||
| Aspartate transaminase (U/L) | 34.3 ± 19.8 | 43.9 ± 20.7 | 0.056 | ||
| Alanine transaminase (U/L) | 28.3 ± 19.6 | 37.6 ± 25.2 | 0.955 | ||
| γ-glutamy transpeptidase (U/L) | 52.6 ± 50.1 | 75.7 ± 47.4 | 0.060 | ||
| Alkaline phosphatase (U/L) | 310.1 ± 107.1 | 308.6 ± 107.3 | 0.955 | ||
| Cholinesterase (U/L) | 178.9 ± 130.0 | 191.6 ± 103.0 | 0.669 | ||
| White blood cell (/mm3) | 4339 ± 1540 | 4802 ± 1716 | 0.249 | ||
| Hemoglobin (g/dL) | 12.8 ± 1.9 | 13.4 ± 2.2 | 0.218 | ||
| Hematocrit (%) | 37.0 ± 6.5 | 40.0 ± 5.7 | 0.056 | ||
| Platelet count (× 103/mm3) | 14.7 ± 7.6 | 12.3 ± 4.6 | 0.114 | ||
LLR: Laparoscopic liver resection; OLR: Open liver resection.
DISCUSSION
Obese patients are predisposed to the development of various disease including diabetes mellitus, hypertension, coronary heart disease, airway obstruction and certain types of malignant tumors[6,7,23]. The real impact of obesity on the postoperative outcomes of surgical procedures was a controversial matter although obese patients are potentially at risk for poor outcomes of a wide variety of surgical procedures[24,25]. In liver resection, increased surgical risk was expected for obese patients underwent liver resection because of associated co-morbidities, underlying liver disease, and technical difficulties. However, recent reports had not demonstrated an increased risk of liver resection in obese patients[10,26,27]. Utsunomiya et al[10] reported that obesity alone might not have an adverse effect on the surgical outcomes of patients with primary HCC. Viganò et al[28] showed that severe morbidity rate and mortality were similar to between obese and non-obese patients, even in cirrhosis or after major liver resection.
Laparoscopic surgeries for obese patients were considered as difficult because of limited visualization of surgical fields with cumbersome fat tissue. In laparoscopic colectomy, obesity has been reported to raise the risk of conversion to laparotomy[14,15]. However, some reports showed that obesity did not have an adverse impact on the technical difficulty and postoperative outcomes of laparoscopic colectomy[18,29]. There were several reports of obesity in laparoscopic gastrectomy and laparoscopic cholecystectomy and results were similar to laparoscopic colectomy[16,17].
Over the last decade, the number of LLR has rapidly increased. An indication of LLR has been extended to major liver resection[30,31]. With advances in instruction and technique, LLR had been performed safely. Nguyen et al[32] summarized that LLR was safe with acceptable morbidity and mortality for minor and major liver resection. Furthermore, the survival rate after LLR for hepatic malignancies was not inferior to that of open liver resection[30-32]. There were few reports have indeed analyzed the impact of obesity on the postoperative outcomes of patients undergoing LLR although LLR is considered as a safe and effective procedure for the management of surgical liver disease[33,34]. Toriguchi et al[33] reported that LLR in obese patients resulted in decreased intraoperative blood loss and shorter postoperative hospital stay compared with OLR. And Nomi et al[34] demonstrated that BMI did not negatively affect the postoperative short-term outcomes. Similar to OLR, it is not clear whether LLR is safe and feasible for obese patients.
In the present study, our results suggested that LLR in obese patients could be performed as safely as non-obese patients with the same risk of postoperative complication and with lower operation time and blood loss than that of non-obese patients. Additionally, BMI significantly correlated with operation time and blood loss in OLR, however, no such correlation was demonstrated in LLR. Operation time and blood loss of LLR in obese patients were not increased compared with those of OLR in obese patients. In addition, multivariate analysis showed that both BMI and OLR were independent predictive factors for prolonged operation time and increased blood loss. These result suggested that LLR was less influenced by BMI, and it was thought that LLR was suitable procedure for not only non-obese patients but also obese patients. However, there was a limitation in the present study. A few patients in this study were classified as obese (BMI ≥ 30 kg/m2) according to the WHO classification. Therefore, whether or not the present study was applicable to the group including many obese patients was still not clear and needs to be determined in future studies.
In conclusion, it was found that BMI was correlated with operation time and blood loss in OLR, but not in LLR. Therefore, LLR was less influenced by BMI and had a greater benefit in obese patients.
COMMENTS
Background
Obesity has been associated with worse surgical outcomes than those of non-obese patients. The impact of body weight on surgical outcomes of laparoscopic liver resection (LLR) remains poorly evaluated, although LLR has been widely adopted.
Research frontiers
Obesity is known as independent risk factor for several malignancies including liver cancer. Therefore, it is important to clarify the correlation between obesity and surgical outcomes of LLR.
Innovations and breakthroughs
This study indicated that LLR rather than open liver resection should be chosen in the obese patients. LLR was less influenced by body mass index (BMI).
Applications
In Japan, obese is considered as BMI ≥ 25 based on the definition by the Japan Society for the Study of Obesity and World Health Organization expert consultation.
Terminology
According to the WHO, obesity is defined as BMI ≥ 30. However, in this study, we defined obesity as BMI ≥ 25.
Peer-review
The manuscript entitled “The Benefit of Laparoscopic Liver Resection in High Body Mass Index Patients” by Uchida et al was well-presented and written. The authors revealed the correlation between BMI and surgical outcomes of 68 cases performed LLR and open liver resection.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Oita University Institutional Review Board.
Informed consent statement: All study participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement: The authors have no conflict of interest.
Data sharing statement: Technical appendix, statistical code, and dataset available from the corresponding author at ucchy@oita-u.ac.jp. Participants gave informed consent was not obtained but the presented data are anonymized and risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 14, 2015
First decision: November 13, 2015
Article in press: December 8, 2015
P- Reviewer: Liu EQ, Sazci A S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
References
- 1.McCurry J. Japan battles with obesity. Lancet. 2007;369:451–452. doi: 10.1016/S0140-6736(07)60214-1. [DOI] [PubMed] [Google Scholar]
- 2.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 3.Berkalp B, Cesur V, Corapcioglu D, Erol C, Baskal N. Obesity and left ventricular diastolic dysfunction. Int J Cardiol. 1995;52:23–26. doi: 10.1016/0167-5273(95)02431-u. [DOI] [PubMed] [Google Scholar]
- 4.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119:655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 5.Ascha MS, Hanouneh IA, Lopez R, Tamimi TA, Feldstein AF, Zein NN. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 6.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology. 2004;127:S97–103. doi: 10.1053/j.gastro.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 7.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 8.Dindo D, Muller MK, Weber M, Clavien PA. Obesity in general elective surgery. Lancet. 2003;361:2032–2035. doi: 10.1016/S0140-6736(03)13640-9. [DOI] [PubMed] [Google Scholar]
- 9.Mathur AK, Ghaferi AA, Osborne NH, Pawlik TM, Campbell DA, Englesbe MJ, Welling TH. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg. 2010;14:1285–1291. doi: 10.1007/s11605-010-1232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Utsunomiya T, Okamoto M, Kameyama T, Matsuyama A, Yamamoto M, Fujiwara M, Mori M, Aimitsu S, Ishida T. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol. 2008;14:1553–1558. doi: 10.3748/wjg.14.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen KT, Marsh JW, Tsung A, Steel JJ, Gamblin TC, Geller DA. Comparative benefits of laparoscopic vs open hepatic resection: a critical appraisal. Arch Surg. 2011;146:348–356. doi: 10.1001/archsurg.2010.248. [DOI] [PubMed] [Google Scholar]
- 12.Martin RC, Scoggins CR, McMasters KM. Laparoscopic hepatic lobectomy: advantages of a minimally invasive approach. J Am Coll Surg. 2010;210:627–634, 634-636. doi: 10.1016/j.jamcollsurg.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 13.Loffer FD, Pent D. Laparoscopy in the obese patient. Am J Obstet Gynecol. 1976;125:104–107. doi: 10.1016/0002-9378(76)90902-9. [DOI] [PubMed] [Google Scholar]
- 14.Senagore AJ, Delaney CP, Madboulay K, Brady KM, Fazio VW. Laparoscopic colectomy in obese and nonobese patients. J Gastrointest Surg. 2003;7:558–561. doi: 10.1016/s1091-255x(02)00124-5. [DOI] [PubMed] [Google Scholar]
- 15.Pikarsky AJ, Saida Y, Yamaguchi T, Martinez S, Chen W, Weiss EG, Nogueras JJ, Wexner SD. Is obesity a high-risk factor for laparoscopic colorectal surgery? Surg Endosc. 2002;16:855–858. doi: 10.1007/s004640080069. [DOI] [PubMed] [Google Scholar]
- 16.Farkas DT, Moradi D, Moaddel D, Nagpal K, Cosgrove JM. The impact of body mass index on outcomes after laparoscopic cholecystectomy. Surg Endosc. 2012;26:964–969. doi: 10.1007/s00464-011-1978-5. [DOI] [PubMed] [Google Scholar]
- 17.Yamada H, Kojima K, Inokuchi M, Kawano T, Sugihara K. Effect of obesity on technical feasibility and postoperative outcomes of laparoscopy-assisted distal gastrectomy--comparison with open distal gastrectomy. J Gastrointest Surg. 2008;12:997–1004. doi: 10.1007/s11605-007-0374-x. [DOI] [PubMed] [Google Scholar]
- 18.Leroy J, Ananian P, Rubino F, Claudon B, Mutter D, Marescaux J. The impact of obesity on technical feasibility and postoperative outcomes of laparoscopic left colectomy. Ann Surg. 2005;241:69–76. doi: 10.1097/01.sla.0000150168.59592.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 20.Kanazawa M, Yoshiike N, Osaka T, Numba Y, Zimmet P, Inoue S. Criteria and classification of obesity in Japan and Asia-Oceania. World Rev Nutr Diet. 2005;94:1–12. doi: 10.1159/000088200. [DOI] [PubMed] [Google Scholar]
- 21.Ban D, Tanabe M, Ito H, Otsuka Y, Nitta H, Abe Y, Hasegawa Y, Katagiri T, Takagi C, Itano O, et al. A novel difficulty scoring system for laparoscopic liver resection. J Hepatobiliary Pancreat Sci. 2014;21:745–753. doi: 10.1002/jhbp.166. [DOI] [PubMed] [Google Scholar]
- 22.Uchida H, Iwashita Y, Watanabe K, Takayama H, Kawasaki T, Yada K, Ohta M, Kitano S, Inomata M. Surgical Outcomes of Laparoscopic Liver Resection in Elderly Patients: A Comparative Study From a Single Center. Surg Laparosc Endosc Percutan Tech. 2015;25:e109–e112. doi: 10.1097/SLE.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 23.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 24.Mullen JT, Davenport DL, Hutter MM, Hosokawa PW, Henderson WG, Khuri SF, Moorman DW. Impact of body mass index on perioperative outcomes in patients undergoing major intra-abdominal cancer surgery. Ann Surg Oncol. 2008;15:2164–2172. doi: 10.1245/s10434-008-9990-2. [DOI] [PubMed] [Google Scholar]
- 25.Benoist S, Panis Y, Alves A, Valleur P. Impact of obesity on surgical outcomes after colorectal resection. Am J Surg. 2000;179:275–281. doi: 10.1016/s0002-9610(00)00337-8. [DOI] [PubMed] [Google Scholar]
- 26.Pessaux P, van den Broek MA, Wu T, Olde Damink SW, Piardi T, Dejong CH, Ntourakis D, van Dam RM. Identification and validation of risk factors for postoperative infectious complications following hepatectomy. J Gastrointest Surg. 2013;17:1907–1916. doi: 10.1007/s11605-013-2226-1. [DOI] [PubMed] [Google Scholar]
- 27.Saunders JK, Rosman AS, Neihaus D, Gouge TH, Melis M. Safety of hepatic resections in obese veterans. Arch Surg. 2012;147:331–337. doi: 10.1001/archsurg.2011.1404. [DOI] [PubMed] [Google Scholar]
- 28.Viganò L, Kluger MD, Laurent A, Tayar C, Merle JC, Lauzet JY, Andreoletti M, Cherqui D. Liver resection in obese patients: results of a case-control study. HPB (Oxford) 2011;13:103–111. doi: 10.1111/j.1477-2574.2010.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makino T, Trencheva K, Shukla PJ, Rubino F, Zhuo C, Pavoor RS, Milsom JW. The influence of obesity on short- and long-term outcomes after laparoscopic surgery for colon cancer: a case-matched study of 152 patients. Surgery. 2014;156:661–668. doi: 10.1016/j.surg.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 30.Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825–830. doi: 10.1097/sla.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 31.Iwahashi S, Shimada M, Utsunomiya T, Imura S, Morine Y, Ikemoto T, Arakawa Y, Mori H, Kanamoto M, Yamada S. Laparoscopic hepatic resection for metastatic liver tumor of colorectal cancer: comparative analysis of short- and long-term results. Surg Endosc. 2014;28:80–84. doi: 10.1007/s00464-013-3165-3. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen KT, Gamblin TC, Geller DA. World review of laparoscopic liver resection-2,804 patients. Ann Surg. 2009;250:831–841. doi: 10.1097/SLA.0b013e3181b0c4df. [DOI] [PubMed] [Google Scholar]
- 33.Toriguchi K, Hatano E, Sakurai T, Seo S, Taura K, Uemoto S. Laparoscopic liver resection in obese patients. World J Surg. 2015;39:1210–1215. doi: 10.1007/s00268-014-2927-y. [DOI] [PubMed] [Google Scholar]
- 34.Nomi T, Fuks D, Ferraz JM, Kawaguchi Y, Nakajima Y, Gayet B. Influence of body mass index on postoperative outcomes after laparoscopic liver resection. Surg Endosc. 2015;29:3647–3654. doi: 10.1007/s00464-015-4121-1. [DOI] [PubMed] [Google Scholar]


