Abstract
AIM: To determine if efficacy of chemotherapy on liver metastasis of gastrointestinal tract cancer can be predicted by apparent diffusion coefficient (ADC) values of diffusion-weighted imaging (DWI).
METHODS: In total, 86 patients with liver metastasis of gastrointestinal tract cancer (156 metastatic lesions) diagnosed in our hospital were included in this study. The maximum diameters of these tumors were compared with each other before treatment, 2 wk after treatment, and 12 wk after treatment. Selected patients were classified as the effective group and the ineffective group, depending on the maximum diameter of the tumor after 12 wk of treatment; and the ADC values at different treatment times between the two groups were compared. Spearman rank correlation was used to analyze the relationship between ADC value and tumor diameter. Receiver operating characteristic curve (ROC curve) was used to analyze the ADC values before treatment to predict the patient’s sensitivity and specificity degree of efficacy to the chemotherapy.
RESULTS: There was no difference in age between the two groups and in maximum tumor diameter before treatment and 2 wk after treatment. However, after 12 wk of treatment, maximum tumor diameter in the effective group was significantly lower than that in the ineffective group (P < 0.05). Before treatment, ADC values in the ineffective group were significantly higher than those in the effective group (P < 0.05). There was no difference in ADC values between the effective and ineffective groups after 2 and 12 wk of treatment. However, ADC values were significantly higher after 2 and 12 wk of treatment compared to before treatment in the effective group (P < 0.05). Spearman rank correlation analysis showed that ADC value before treatment and the reduced percentage of the maximum tumor diameter after 12 wk of treatment were negatively correlated, while the increase in the percentage of the ADC value 12 wk after treatment and the decrease in the percentage of the maximum tumor diameter were significantly positively correlated. The results of the ROC curve showed that ADC value with a chemotherapy ineffective threshold value of 1.14 × 10-3 mm2/s before treatment had a sensitivity and specificity of 94.3% and 76.7%, respectively.
CONCLUSION: DWI ADC values can be used to predict the response of patients with liver metastasis of gastrointestinal tract cancer to chemotherapy with high sensitivity and relatively high specificity.
Keywords: Chemotherapy, Liver metastatic tumor, Magnetic resonance imaging, Gastrointestinal tract cancer
Core tip:A total of 86 patients with liver metastasis of gastrointestinal tract cancer were assigned to one of two groups, effective group and ineffective group, according to the maximum diameter of the tumor after treatment. The apparent diffusion coefficient (ADC) values at different treatment times between the two groups were compared. The results revealed that ADC values before treatment can be used to predict chemotherapy response to liver metastasis of gastrointestinal tract cancer, with high sensitivity and relatively high specificity.
INTRODUCTION
Gastrointestinal cancer is the most common malignant tumor, and its incidence continues to rise[1-3]. Surgical resection is currently the primary treatment for gastrointestinal cancer. However, surgical resection alone has a low survival rate[1,4-6] due to the high incidence of invasion and metastasis. According to the statistics, liver metastasis occurs in approximately 45% of patients[7,8]. Patients with liver metastasis are not suitable for surgery. Thus, chemotherapy is the main treatment method used to improve patient survival, making patients more suitable for surgery[9,10]. Thus, the prediction and evaluation of the effectiveness of chemotherapy in patients with liver metastatic tumor is important for the survival status of patients and the development of treatment programs. Clinically, chemotherapy efficacy monitoring has been primarily made by measuring tumor size using computed tomography (CT), magnetic resonance imaging (MRI), and other imaging modalities. However, tumor size changes measured by radiological imaging methods are often detected later than functional changes; and it is difficult to predict the efficacy of chemotherapy at an early stage. In recent years, diffusion-weighted imaging (DWI) has been discovered as a functional magnetic resonance examination method, and the apparent diffusion coefficient (ADC) of DWI signal intensity can be accurately quantified to enable the evaluation of chemotherapeutic efficacy before tumor size changes[11,12]. Although using DWI to assess the efficacy of cancer treatments has already being applied clinically[13,14], there are few studies on its applications and its ability to predict results of therapy remains unclear. Therefore, this study investigated the prediction of chemotherapy efficacy in patients with liver metastasis of gastrointestinal tract cancer by DWI ADC, aiming to provide a new method for clinical evaluation.
MATERIALS AND METHODS
Clinical data
From June 2012 to April 2015, a total of 86 patients treated for liver metastasis of gastrointestinal tract cancer in Taishan Medical College Affiliated Liaocheng Second People’s Hospital and Liaocheng Third People’s Hospital were included in this study. There were 50 male patients and 36 female patients. Age ranged from 44-75 years old, with an average of 58.2 ± 6.1 years. Among these 86 patients, 28 patients with gastric cancer had 52 metastases and 58 patients with colorectal cancer had 104 metastases. In total, 156 metastatic lesions were included in this study.
Examination method
GE 1.5T HDX superconducting MRI (Chalfont St. Giles, United Kingdom) and GE SIGNA HDe 1.5T MR scanner were used for testing. The patient was placed in supine position so that the coil cans wraparound the upper abdomen. The patient was instructed to breathe uniformly and located at the xiphoid. Then, DWI scanning was carried out (Figure 1A and B). DWI scan results were analyzed to generate the ADC values, and the images were reviewed in a blinded fashion by two radiologists.
Figure 1.
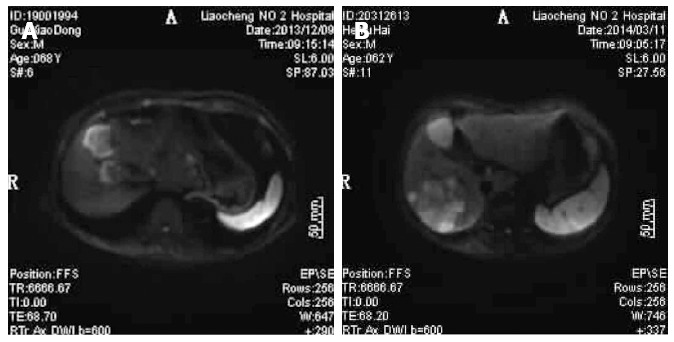
Diffusion-weighted imaging results for patients with liver metastasis of gastrointestinal tract cancer. A: Diffusion-weighted imaging (DWI) figure shows right anterior lobe of liver with surrounding high signal, clear boundary, and significant contrast; B: DWI figure shows the right posterior lobes of livers with huge and irregular high signal intensity; surrounding small satellite focus were visible with a blending tendency, clear boundary, and significant contrast.
Treatment regimen
All patients underwent chemotherapy based on the following specific regimen (LV5FU2 plan): CF 200 mg/m2 per day iv bolus, 1-2 d; 5-FU 400 mg/m2/iv bolus, first 1-2 d; 5-FU 600 mg/m2 per day iv bolus, first 1-2 d, repeated every 2 wk.
Efficacy assessment of chemotherapy on tumors
Tumor size (maximum diameter) was measured after 12 wk of each treatment and during the last week of chemotherapy treatment. Valid chemotherapy was considered as either disappearance of lesions or reduction of the sum of maximum diameter to > 30%. Otherwise, chemotherapy was considered invalid.
Observation indexes
Maximum diameters of metastatic tumors were measured before treatment, after 2 wk of treatment, and after 12 wk of treatment. Patients were classified into effective group and ineffective group based on tumor size after 12 wk of treatment. ADC values measured before treatment, after 2 wk of treatment, and after 12 wk of treatment were compared between the effective group and the ineffective group. Spearman’s rank correlation analysis was used to determine the correlation between ADC value and tumor diameter. Receiver operating characteristic curve (ROC curve) analysis of ADC values before treatment was used to predict patients with or without sensitivity and specificity for chemotherapy.
Statistical analysis
SPSS16.0 software (Chicago, IL, United States) was used for statistical analyses. Data are presented as mean ± SD. Differences between groups were analyzed using a t-test. P < 0.05 was considered statistically significant. Spearman’s rank correlation analysis was used to analyze the correlation between ADC value changes and diameter changes of metastasis tumor. ROC curve analysis of ADC values before treatment was used to predict the sensitivity and specificity of metastatic tumors to chemotherapy.
RESULTS
Comparison of clinical data between the effective and ineffective group
Among the 156 metastatic lesions found in 86 patients with gastrointestinal cancer, 27 (17.3%) lesions were located in the left lobe of the liver, and 129 (82.7%) lesions were located in the right lobe of the liver. After chemotherapy, 73 lesions (46.8%) were classified into the effective group, and 83 lesions (53.2%) were classified into the ineffective. There was no significant difference in average age between the effective group and the ineffective group (P > 0.05), as shown in Table 1. There was no significant difference in the maximum diameter of tumors before chemotherapy between the effective group and ineffective group (P > 0.05), as shown in Table 1. Two weeks after chemotherapy, tumor diameter in the effective group was smaller than that in the ineffective group; but the difference was not statistically significant (P > 0.05), as shown in Table 1. Moreover, after 12 wk of chemotherapy, tumor size in the effective group was significantly smaller than that in the ineffective group (P < 0.05), as shown in Table 2.
Table 1.
Comparison of clinical data between the effective and ineffective groups
| Groups | Average age (yr) |
Maximum tumor diameter (cm) |
||
| Before treatment | After 2 wk of treatment | After 12 wk of treatment | ||
| Effective group (n = 73) | 57.7 ± 5.9 | 3.45 ± 0.81 | 3.29 ± 0.75 | 1.87 ± 0.38 |
| Ineffective group (n = 83) | 59.6 ± 6.2 | 3.62 ± 0.85 | 3.47 ± 0.88 | 3.45 ± 0.62 |
| t value | 1.953 | 1.274 | 1.365 | 18.874 |
| P value | 0.053 | 0.205 | 0.174 | 0 |
Table 2.
Changes in apparent diffusion coefficient values before and after chemotherapy treatment between the effective and ineffective groups
| Groups | Before treatment | After 2 wk of treatment | After 12 wk of treatment |
After 2 wk of treatment |
After 12 wk of treatment |
||
| t value | P value | t value | P value | ||||
| Effective group | 1.01 ± 0.06 | 1.26 ± 0.11 | 1.34 ± 0.18 | 17.047 | 0.000 | 14.86 | 0.000 |
| Ineffective group | 1.24 ± 0.08 | 1.26 ± 0.05 | 1.22 ± 0.17 | 1.931 | 0.055 | 0.97 | 0.334 |
| t value | 2.747 | 1.491 | 1.783 | / | / | / | / |
| P value | 0.007 | 0.138 | 0.077 | / | / | / | / |
Changes in ADC values between the effective group and ineffective group
ADC values before treatment in the ineffective group were significantly higher than those in the effective group (P < 0.05). There was no significant difference in ADC values after 2 wk and 12 wk of treatment between the effective group and the ineffective group (P > 0.05). Moreover, ADC values were significantly increased in the effective group after 2 wk and 12 wk of treatment compared with those before treatment (t = 17.047, 14.860; P = 0.000, 0.000). ADC value after 2 wk and 12 wk of treatment increased by 24.8% and 32.7%, respectively, in the effective group; while ADC value after 2 wk and 12 wk of treatment increased by 3.2% and 4.0%, respectively, in the ineffective group.
Relationship between ADC value and tumor diameter change
Spearman rank correlation analysis revealed that before treatment, there was no significant correlation between ADC values and the mean value of the maximum diameter of tumor (P > 0.05). After 2 wk and 12 wk of treatment, there was also no significant correlation between ADC values and the mean maximum diameter of tumors (P > 0.05). ADC values before treatment and the reduced percentage of the maximum diameter of tumor after 12 wk of treatment were significantly negatively correlated (P < 0.05). The increase percentage of ADC values after 12 wk of treatment and the reduced percentage of the maximum tumor diameter was significantly positively correlated (P < 0.05). Moreover, the reduced percentage of tumor size and the maximum diameter of metastatic tumors before treatment did not reveal any significant correlation (P > 0.05), as shown in Table 3.
Table 3.
Relationship between apparent diffusion coefficient values and tumor diameter changes
| Correlation | r value | P value |
| Apparent diffusion coefficient (ADC) values before treatment vs mean maximum tumor diameter | 0.124 | 0.108 |
| ADC values 2 wk after treatment vs mean maximum tumor diameter after 2 wk of treatment | 0.093 | 0.183 |
| ADC values after 12 wk of treatment vs mean maximum tumor diameter after 12 wk of treatment | 0.052 | 0.118 |
| ADC values before treatment vs reduced percentage of the mean maximum tumor diameter after 12 wk of treatment | -0.718 | 0.001 |
| Increased percentage of ADC values after 12 wk of treatment vs reduced percentage of maximum tumor diameter | 0.742 | 0.002 |
| Percent decrease in tumor size vs mean maximum diameter of metastatic tumors before treatment | -0.015 | 0.279 |
Analysis of the sensitivity and specificity of ADC value for the evaluation of the effectiveness of chemotherapy
By ROC curve analysis, the area under the curve was 0.934 (95%CI: 0.878-0.990). With an ADC ineffectiveness chemotherapy threshold value before treatment of 1.14 × 10-3 mm2/s, the sensitivity and specificity for predicting the ineffectiveness chemotherapy to metastatic tumors were 94.3% and 76.7%, respectively (Figure 2).
Figure 2.
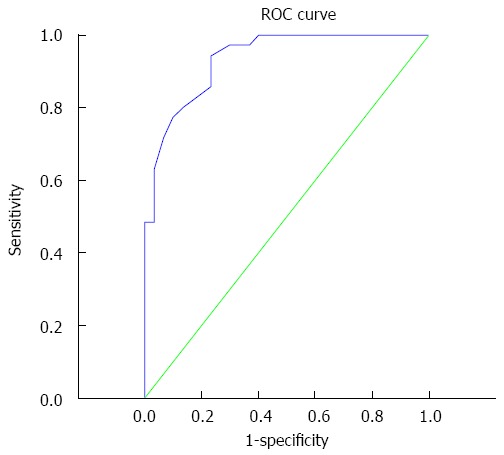
Receiver operating characteristic curve. Area under the curve is 0.934, the optimal diagnostic point is 1.14 × 10-3 mm2/s, and sensitivity and specificity are 94.3% and 76.7%, respectively.
DISCUSSION
Liver metastatic tumors are derived from gastrointestinal cancer and other digestive tract cancers and are also a common cause of death in gastrointestinal tumors[15]. Even though surgical resection is an effective treatment of gastrointestinal cancers, the proportion of patients who are suitable for surgery is very small, which is only about 15% of gastrointestinal cancers[16-18]. Therefore, chemotherapy is an important treatment for patients not suitable for surgical resection. Studies have shown that effective chemotherapy can significantly reduce the size of metastatic tumors so that patients will be suitable for surgery, thereby prolonging survival[19-22]. However, chemotherapy may be ineffective in some patients due to inter-individual variability. If we can predict the ineffectiveness of chemotherapy in patients at an early stage, the treatment plan could be changed in a timely manner. In recent years, DWI was found to be able to assess the efficacy of cancer treatment. ADC is an index used to measure the intensity of DWI. Animal studies have shown that ADC values for metastatic tumors in the effective group were significantly higher than the ineffective group[23,24]. However, there are few studies on the use of ADC values for predicting the effectiveness of chemotherapy on metastatic tumors in vivo. Therefore, in order to investigate whether ADC values could predict liver metastatic tumor response to chemotherapy, patients with liver metastases in gastrointestinal cancer at our hospital were selected for this study.
Correlation analysis of the efficacy of chemotherapy
It is very important to evaluate the efficacy of chemotherapy at an early stage. Early analysis of the effect of chemotherapy can provide guidance in selecting clinical therapeutic regimens, thereby improving the prognosis of patients. Although there was no significant difference in metastatic tumor size before treatment and after 2 wk of treatment between patients in the effective group and ineffective group, the maximum diameter of the metastatic tumors after 12 wk of treatment in the effective group was significantly smaller than the ineffective group (P < 0.05). As previously described, chemotherapy can cause liver metastatic tumor size to change. However, there is no correlation between the maximum metastatic tumor diameter before treatment and the reduced percentage of tumor diameter after treatment. The results have shown that the size of the lesion and chemotherapy response is not related.
ADC value before chemotherapy in the ineffective group was significantly higher than the effective group, and ADC values before treatment and the reduced percentage of metastatic tumor diameter were negatively correlated. These results show that ADC values before chemotherapy and chemotherapy response are correlated. A high ADC value indicates that patients with liver metastatic tumors may be unresponsive to chemotherapy. Some studies have reported that ADC values can reflect the tissue density. The higher the ADC values, the lower the tissue density[15,25,26]. In addition, we showed that ADC value increased with chemotherapy effectiveness, which is consistent with that study in which tissue density was reduced after chemotherapy and tended to be normal. This may be due to the strong ability of tumor cells to proliferate before chemotherapy, an abundant cytoplasm, and a reduced extracellular space. Therefore, as density becomes greater, ADC value becomes lower. In addition, after effective chemotherapy on tissues, tumor cells are injured and raptured and dies. Therefore, tissues become less dense, diffusion motion of water molecules in the tissue increases, and ADC value increases[27,28]. We found that early treatment in the effective group can significantly increase ADC values, whereas the ineffective group did not exhibit this phenomenon. This may be due to the occurrence of tumor cells necrosis after chemotherapy in the effective group. First, early tumor cell necrosis swelling occurs, and then, cell walls burst and crack, leading to an increase in water molecular in the cell[29,30] and an increase in ADC value. Since treatment in the ineffective group does not injure or kill tumor cells; tumor tissues continue to increase, cell density increases or remains unchanged, diffusion motion of water molecules in the tumor tissues are reduced or remain unchanged, and ADC value decreases or remain unchanged.
ADC values before treatment predicts sensitivity and specificity of chemotherapy response to liver metastasis of gastrointestinal tract cancer
In addition, the ROC curve results showed that sensitivity and specificity for predicting the efficacy of chemotherapy on patients with liver metastatic tumors before treatment with 1.14 × 10-3 mm2/s as a threshold were 94.3% and 76.7%, respectively. The results have shown that sensitivity was high and specificity was relatively low. As described, this method has high clinical application value in predicting the efficacy of chemotherapy in patients with liver metastatic tumors due to high sensitivity. The relatively low specificity of this method, as described in other tumors, may also have a similar predictive effect. Studies have reported significant changes in ADC values in breast cancer, which are consistent with these results[31-33].
Limitations and outlook
The method of using ADC values before treatment to predict the efficacy of chemotherapy has the advantages of providing fast and accurate results as well as its noninvasiveness. In clinical practice, this method can be extensively applied to the patient with liver metastasis of gastrointestinal tract cancer to predict the efficacy of chemotherapy and to determine the corresponding changes in treatment for those patients who are unresponsive to chemotherapy at an early stage. A limitation of this study is that it evaluated only patients with liver metastasis of gastrointestinal tract cancer. It remains unclear whether this method will have a similar effect in other cancer patients. Thus, further research will need to focus on whether the evaluation of ADC values before treatment can also be applied to other cancers for chemotherapy reactions. In addition, this study did not take into account individual differences between each patient. Hence there is a need to further improve the experimental design of this study to confirm these results.
In conclusion, ADC values before treatment can be used to predict chemotherapy response to liver metastasis of gastrointestinal tract cancer, with high sensitivity and a relatively high specificity. Clinically, this approach has an important value in predicting the efficacy of chemotherapy on liver metastasis of gastrointestinal tract cancer.
COMMENTS
Background
Gastrointestinal cancer is the most common malignant tumor, and the incidence continues to rise. Surgical resection is currently the primary treatment for gastrointestinal cancer. However, surgical resection alone has a low survival rate due to its high incidence of invasion and metastasis. According to the statistics, liver metastasis occurs in approximately 45% of patients.
Research frontiers
In recent years, diffusion-weighted imaging (DWI) has been utilized to assess the efficacy of cancer treatment. Apparent diffusion coefficient values (ADCs) are used to measure the intensity of DWI. Animal studies have shown that ADCs for metastatic tumors in the effective group were significantly higher than the ineffective group.
Innovations and breakthroughs
ADC values before treatment can be used to predict chemotherapy response to liver metastasis of gastrointestinal tract cancer, with high sensitivity and a relatively high specificity.
Applications
Clinically, this work has important value for predicting the efficacy of chemotherapy on liver metastasis of gastrointestinal tract cancer.
Peer-review
Patients with liver metastases in gastrointestinal cancer at our hospital were selected for this study to investigate whether ADC values could predict liver metastatic tumor response to chemotherapy. The results demonstrated that ADC values before treatment can be used to predict chemotherapy response to liver metastasis of gastrointestinal tract cancer, which has high sensitivity and a relatively high specificity.
Footnotes
Institutional review board statement: This study was reviewed by and approved by the Department of Radiology, Second People’s Hospital of Liaocheng city, Shandong Province Institutional review board statement.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: We declare that there are no conflicts of interest to disclose.
Data sharing statement: No additional data are available.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 25, 2015
First decision: November 27, 2015
Article in press: December 30, 2015
P- Reviewer: Chorny M, Siddiqui I S- Editor: Qi Y L- Editor: Filipodia E- Editor: Ma S
References
- 1.Dwivedi AN, Jain S, Dixit R. Gall bladder carcinoma: Aggressive malignancy with protean loco-regional and distant spread. World J Clin Cases. 2015;3:231–244. doi: 10.12998/wjcc.v3.i3.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schüle S, Altendorf-Hofmann A, Dittmar Y, Rauchfuß F, Settmacher U. [Incidence of non-metastatic liver lesions in tumor patients: consequences for chemotherapy and local ablative procedures] Chirurg. 2014;85:806–811. doi: 10.1007/s00104-013-2660-3. [DOI] [PubMed] [Google Scholar]
- 3.Levic K, Bulut O, Hesselfeldt P. Transanal endoscopic microsurgery for giant polyps of the rectum. Tech Coloproctol. 2014;18:521–527. doi: 10.1007/s10151-013-1069-9. [DOI] [PubMed] [Google Scholar]
- 4.Lambertz A, Klink CD, Röth A, Schmitz D, Pich A, Feher K, Bremus-Köbberling E, Neumann UP, Junge K. Laser-induced drug release for local tumor control--a proof of concept. J Surg Res. 2014;192:312–316. doi: 10.1016/j.jss.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 5.Gourtsoyianni S, Goh V. MRI of anal cancer: assessing response to definitive chemoradiotherapy. Abdom Imaging. 2014;39:2–17. doi: 10.1007/s00261-013-0032-6. [DOI] [PubMed] [Google Scholar]
- 6.Tajima N, Utano K, Kijima S, Kawai A, Fujita A, Sakuma K, Sugimoto H, Fujii H. Intraductal papillary mucinous neoplasm penetrating to the stomach, duodenum, and jejunum demonstrated on MR cholangiopancreatography with an oral negative contrast agent. J Magn Reson Imaging. 2013;38:206–209. doi: 10.1002/jmri.23915. [DOI] [PubMed] [Google Scholar]
- 7.Busby RW, Bryant AP, Bartolini WP, Cordero EA, Hannig G, Kessler MM, Mahajan-Miklos S, Pierce CM, Solinga RM, Sun LJ, et al. Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol. 2010;649:328–335. doi: 10.1016/j.ejphar.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Ghevariya V, Malieckal A, Ghevariya N, Mazumder M, Anand S. Carcinoid tumors of the gastrointestinal tract. South Med J. 2009;102:1032–1040. doi: 10.1097/SMJ.0b013e3181b67356. [DOI] [PubMed] [Google Scholar]
- 9.Xie H, Sun T, Chen M, Wang H, Zhou X, Zhang Y, Zeng H, Wang J, Fu W. Effectiveness of the apparent diffusion coefficient for predicting the response to chemoradiation therapy in locally advanced rectal cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e517. doi: 10.1097/MD.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawai S, Nishida T, Hayashi Y, Ezaki H, Yamada T, Shinzaki S, Miyazaki M, Nakai K, Yakushijin T, Watabe K, et al. Choroidal and cutaneous metastasis from gastric adenocarcinoma. World J Gastroenterol. 2013;19:1485–1488. doi: 10.3748/wjg.v19.i9.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuang F, Yan Z, Wang J, Rao Z. The value of diffusion-weighted MRI to evaluate the response to radiochemotherapy for cervical cancer. Magn Reson Imaging. 2014;32:342–349. doi: 10.1016/j.mri.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Ippolito E, Mantini G, Morganti AG, Mazzeo E, Padula GD, Digesù C, Cilla S, Frascino V, Luzi S, Massaccesi M, et al. Intensity-modulated radiotherapy with simultaneous integrated boost to dominant intraprostatic lesion: preliminary report on toxicity. Am J Clin Oncol. 2012;35:158–162. doi: 10.1097/COC.0b013e318209cd8f. [DOI] [PubMed] [Google Scholar]
- 13.Wang CS, Du LJ, Si MJ, Yin QH, Chen L, Shu M, Yuan F, Fei XC, Ding XY. Noninvasive assessment of response to neoadjuvant chemotherapy in osteosarcoma of long bones with diffusion-weighted imaging: an initial in vivo study. PLoS One. 2013;8:e72679. doi: 10.1371/journal.pone.0072679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohno T, Yokoyama Y, Aihara R, Mochiki E, Asao T, Kuwano H. Sudden bilateral sensorineural hearing loss as the presenting symptom of meningeal carcinomatosis of gastric cancer: report of a case. Surg Today. 2010;40:561–565. doi: 10.1007/s00595-009-4099-1. [DOI] [PubMed] [Google Scholar]
- 15.Malik I, Hussein F, Bush D, Alqaisi M, Bernal P, Byrd J, Garberoglio C. A phase I study of capecitabine, irinotecan, celecoxib, and radiation as neoadjuvant therapy of patients with locally advanced rectal cancer. Am J Clin Oncol. 2010;33:242–245. doi: 10.1097/COC.0b013e3181a650fb. [DOI] [PubMed] [Google Scholar]
- 16.Heo SH, Shin SS, Kim JW, Lim HS, Jeong YY, Kang WD, Kim SM, Kang HK. Pre-treatment diffusion-weighted MR imaging for predicting tumor recurrence in uterine cervical cancer treated with concurrent chemoradiation: value of histogram analysis of apparent diffusion coefficients. Korean J Radiol. 2013;14:616–625. doi: 10.3348/kjr.2013.14.4.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takusagawa S, Ushigome F, Nemoto H, Takahashi Y, Li Q, Kerbusch V, Miyashita A, Iwatsubo T, Usui T. Intestinal absorption mechanism of mirabegron, a potent and selective β3-adrenoceptor agonist: involvement of human efflux and/or influx transport systems. Mol Pharm. 2013;10:1783–1794. doi: 10.1021/mp300582s. [DOI] [PubMed] [Google Scholar]
- 18.King AD, Chow KK, Yu KH, Mo FK, Yeung DK, Yuan J, Bhatia KS, Vlantis AC, Ahuja AT. Head and neck squamous cell carcinoma: diagnostic performance of diffusion-weighted MR imaging for the prediction of treatment response. Radiology. 2013;266:531–538. doi: 10.1148/radiol.12120167. [DOI] [PubMed] [Google Scholar]
- 19.Iwasa S, Ikeda M, Okusaka T, Ueno H, Morizane C, Nakachi K, Mitsunaga S, Kondo S, Hagihara A, Shimizu S, et al. Transcatheter arterial infusion chemotherapy with a fine-powder formulation of cisplatin for advanced hepatocellular carcinoma refractory to transcatheter arterial chemoembolization. Jpn J Clin Oncol. 2011;41:770–775. doi: 10.1093/jjco/hyr037. [DOI] [PubMed] [Google Scholar]
- 20.Wu CF, Chuang WP, Li AH, Hsiao CH. Cardiac magnetic resonance imaging in sunitinib malate-related cardiomyopathy: no late gadolinium enhancement. J Chin Med Assoc. 2009;72:323–327. doi: 10.1016/S1726-4901(09)70379-X. [DOI] [PubMed] [Google Scholar]
- 21.Winfield JM, deSouza NM, Priest AN, Wakefield JC, Hodgkin C, Freeman S, Orton MR, Collins DJ. Modelling DW-MRI data from primary and metastatic ovarian tumours. Eur Radiol. 2015;25:2033–2040. doi: 10.1007/s00330-014-3573-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nissan N, Furman-Haran E, Shapiro-Feinberg M, Grobgeld D, Degani H. Diffusion-tensor MR imaging of the breast: hormonal regulation. Radiology. 2014;271:672–680. doi: 10.1148/radiol.14132084. [DOI] [PubMed] [Google Scholar]
- 23.Han NY, Park BJ, Sung DJ, Kim MJ, Cho SB, Lee CH, Jang YJ, Kim SY, Kim DS, Um SH, et al. Chemotherapy-induced focal hepatopathy in patients with gastrointestinal malignancy: gadoxetic acid--enhanced and diffusion-weighted MR imaging with clinical-pathologic correlation. Radiology. 2014;271:416–425. doi: 10.1148/radiol.13131810. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F, Le T, Wu X, Wang H, Zhang T, Meng Y, Wei B, Soriano SS, Willis P, Kolokythas O, et al. Intrabiliary RF heat-enhanced local chemotherapy of a cholangiocarcinoma cell line: monitoring with dual-modality imaging--preclinical study. Radiology. 2014;270:400–408. doi: 10.1148/radiol.13130866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nogueira L, Brandão S, Matos E, Nunes RG, Ferreira HA, Loureiro J, Ramos I. Diffusion-weighted breast imaging at 3 T: preliminary experience. Clin Radiol. 2014;69:378–384. doi: 10.1016/j.crad.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Farjam R, Tsien CI, Feng FY, Gomez-Hassan D, Hayman JA, Lawrence TS, Cao Y. Investigation of the diffusion abnormality index as a new imaging biomarker for early assessment of brain tumor response to radiation therapy. Neuro Oncol. 2014;16:131–139. doi: 10.1093/neuonc/not153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang T, Zhang F, Meng Y, Wang H, Le T, Wei B, Lee D, Willis P, Shen B, Yang X. Diffusion-weighted MRI monitoring of pancreatic cancer response to radiofrequency heat-enhanced intratumor chemotherapy. NMR Biomed. 2013;26:1762–1767. doi: 10.1002/nbm.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ng TS, Wert D, Sohi H, Procissi D, Colcher D, Raubitschek AA, Jacobs RE. Serial diffusion MRI to monitor and model treatment response of the targeted nanotherapy CRLX101. Clin Cancer Res. 2013;19:2518–2527. doi: 10.1158/1078-0432.CCR-12-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwee RM, Kwee TC. Role of imaging in predicting response to neoadjuvant chemotherapy in gastric cancer. World J Gastroenterol. 2014;20:1650–1656. doi: 10.3748/wjg.v20.i7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen ZG, Xu L, Zhang SW, Huang Y, Pan RH. Lesion discrimination with breath-hold hepatic diffusion-weighted imaging: a meta-analysis. World J Gastroenterol. 2015;21:1621–1627. doi: 10.3748/wjg.v21.i5.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ellingson BM, Cloughesy TF, Lai A, Nghiemphu PL, Liau LM, Pope WB. Quantitative probabilistic functional diffusion mapping in newly diagnosed glioblastoma treated with radiochemotherapy. Neuro Oncol. 2013;15:382–390. doi: 10.1093/neuonc/nos314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentzel HJ, Reinsch S, Kurzai M, Stenzel M. Magnetic resonance imaging in children and adolescents with chronic inflammatory bowel disease. World J Gastroenterol. 2014;20:1180–1191. doi: 10.3748/wjg.v20.i5.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim HS, Kim CK, Park BK, Huh SJ, Kim B. Evaluation of therapeutic response to concurrent chemoradiotherapy in patients with cervical cancer using diffusion-weighted MR imaging. J Magn Reson Imaging. 2013;37:187–193. doi: 10.1002/jmri.23804. [DOI] [PubMed] [Google Scholar]


