Abstract
HIV was first described in Kenya in 1984–1985. Currently, Kenya has an estimated HIV-1 prevalence of 6.2%. With the introduction of antiretroviral drugs, the survival of most HIV patients has been prolonged markedly. However, this is greatly threatened by increasing rates of antiretroviral dug resistance, which may eventually lead to suboptimal treatment outcomes. The objective of this study was to characterize currently occurring antiretroviral drug resistance mutations among drug-naive patients visiting two referral hospitals in Kenya. Using polymerase chain reaction, the HIV protease gene was amplified from blood samples of 63 study participants. The sequences were used to determine HIV-1 subtype and presence/prevalence of mutations associated with resistance to protease inhibitors. Finally, the protease gene was variably measured using Shannon entropy analysis. Analysis of frequency of HIV-1 subtypes revealed subtype A to be the predominant subtype, while the analysis of drug resistance mutations revealed the presence of four minor drug resistance mutations associated weakly with resistance to protease inhibitors. Among these mutations, L33I was the most prevalent mutation. Shannon entropy analysis revealed high genomic variability, especially in region spanning nucleotides 1–55, 113–170, and 205–240. This study warrants the need for dedicated efforts to improve compliance to antiretroviral therapy and reduce transmitted resistance rates, which will greatly ensure the therapeutic efficacy of antiretroviral drugs.
Introduction
Human immunodeficiency virus (HIV) is responsible for 34 million infections worldwide, and approximately 25 million deaths in the past three decades.1 Sub-Saharan Africa accounts for the largest global burden of HIV/AIDS with an estimated 1.8 million new infections and 1.8 million deaths in 2011, which is approximately 69% of the total global HIV/AIDS burden.2 Currently, Kenya has an estimated HIV-1 prevalence of 7.7% with a country population of about 40 million people.3
With the introduction of antiretroviral drugs, the survival of most HIV patients has been prolonged markedly. However, this is greatly threatened by increasing rates of antiretroviral dug resistance, which may eventually lead to suboptimal treatment outcomes.4 Development of resistance is aggravated by the fact that HIV replicates very rapidly and its reverse transcriptase lacks proofreading capabilities facilitating the occurrence of a large number of mutations.5 The prevalence of HIV-1 primary resistance varies from place to place and over time. In places that initiated antiretroviral therapy programs in the early 1990s,6,7 high rates of resistance have been reported as compared to most countries in developing world that scaled antiretroviral programs 10 years later.8
With continued use of antiretroviral agents, the emergence of resistance mutations is likely to occur. These viral mutants can be further transmitted to newly infected patients and can affect treatment outcomes.4,9 Previous studies from Kenya show an increasing prevalence of transmitted antiretroviral drug resistance in newly infected patients,10,11 advocating the need to monitor patterns of HIV-1 drug resistance in drug-treated and drug-naive patients to determine patterns of antiretroviral resistant mutations and to tailor the treatment accordingly.
The purpose of this study was to determine the prevalence of antiretroviral drug resistance mutations in a cohort of drug-naive HIV-1-positive adult patients visiting Aga Khan University Hospital and Thika Level 5 Hospital in Nairobi and Thika, Kenya, respectively. The study aimed to determine drug resistance mutations against protease inhibitors, which are among the most commonly used antiretroviral drugs in the country.
Materials and Methods
Study design and patients profile
This study was conducted on 121 drug-naive HIV-positive patients, aged 18 years or above, recruited prospectively at the Aga Khan University Hospital, Nairobi, Kenya and Thika Level 5 Hospital, Thika, Kenya, using a convenient sampling strategy. None of the patients reported having received antiretroviral therapy. A written informed consent was obtained from all study participants prior to carrying out any study procedures. Additionally, a questionnaire was used to obtain demographics and relevant clinical information from the study participants.
Sample collecting, RNA extracting, viral load, and CD4 counts
Viral genotyping was performed on patients with a viral load of more than 1,000 viral copies per ml. Approximately 8–10 ml of blood sample was collected from each patient, and plasma was separated from each blood sample and stored as 2-ml aliquots in microtubes. Viral RNA extraction was done from plasma using the Qiagen's QIAamp Viral RNA Mini Kit according to the manufacturer's instructions.
Viral loads were determined at the Aga Khan Laboratory, Nairobi (SANAS 15189 Accredited) using the Nuclisens EasyQ real-time assay (version 2.0 BioMerieux, France) according to the manufacturer's instructions, while CD4 counts of the participants, carried out within 90 days of the day of sampling, were obtained from the patent's record.
RNA reverse transcription
RNA reverse transcription and first polymerase chain reaction (PCR) were done using the QIAGEN One-Step RT-PCR Kit, which contains a blend of Sensiscript and Omniscript reverse transcriptases and HotStarTaq DNA polymerase in a one-tube setup. This reduces extra pipetting steps and also reduces the risk of contamination. A 1,030-base pair part of the pol gene containing the reverse transcriptase (1–330) and protease genes (1–99) was amplified using a nested PCR strategy. The primers used in the first round of PCR were Nyupol 7 (5′-GGGAATTTTCTTCAGAGCAG-3′) and Nyupol 8 (5′-TCTTCTGTCAATGGCCATTGT-3′) for the protease gene. For the second round of amplification, primers Nyupol 9 (5′-TCCTTAACTTCCCTCAAATCACT-3′) and Nyupol 10 (5′-CTGGCACGGTTTCAATAGGACT-3′) were used for the protease gene.
The following reagents and volumes were used in the first round of PCR reactions: first one-step PCR reaction, 5× PCR buffer 5 μl (also includes magnesium chloride), deoxynucleotides 1 μl containing 400 μM each deoxynucleotides, transcriptase gene; for each a volume of 0.3 μl at a final concentration of 0.6 μM was used for each reaction tube. In addition, there was an enzyme mix at 1 μl (Sensiscript and Omniscript Reverse Transcriptases, HotStarTaq DNA polymerase), a reaction tube having a final concentration of 5–10 units of the enzyme, Q Buffer to enhance PCR reactions in G-C-rich templates at a volume of 1 μl, water 11.4 μl, and an HIV-1 RNA template (minimum concentration of 1–2 pg for each reaction tube) to a total reaction volume of 25 μl.
The following reagents and volumes were used in the second round of PCR reactions: 2 μl of 10× PCR buffer (without magnesium chloride), 2 μl of 200 μM deoxynucleotides, 2.8 μl of 25 mM magnesium chloride (MgCl2), 0.4 μl of Nyupol 9 and Nyupol 10, primers, 0.2 μl of Taq polymerase, 10.2 μl water, and 2 μl of the Onestep PCR reaction products, in a total reaction volume was 20 μl.
The conditions used for reverse transcription included heating the reagents at 50°C for 30 min and deactivating the reverse transcriptase by heating to 95°C for 15 min. This heating step also activates the first PCR HotStarTaq DNA Polymerase to start the amplification.
The thermocycling conditions used for protease amplification in the first round were denaturation at 95°C for 10 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, with a final extension at 72°C for 15 min. The thermocycling conditions used for protease amplification in the second round were denaturation at 95°C for 10 min, followed by 35 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, with a final extension at 72°C for 15 min.
The amplified products were purified using the QiaQuick purification kit from Qiagen according to the manufacturer's instructions. The purified products were electrophoresed on 1.5% agarose gel, stained by ethidium bromide, and visualized under ultraviolet light.
PCR products of protease and reverse transcriptase genes were sequenced from Nyumbani Diagnostic Laboratory (NDL), Kenya, using primers Nyupol 9 and Nyupol 10 for protease.
The protease sequences were submitted to GenBank, where they were assigned the following accession numbers: KM376155–KM376216 and KM463084–KM463086.
Sequence alignment and HIV-1 subtyping
The sequences obtained were aligned using MEGA6 software.12 The alignments were cleaned to remove nonnucleotide characters. HIV-1 subtyping was performed using the NCBI genotyping tool 9 (www.ncbi.nlm.nih.gov/pmc/articles/PMC441557/) and REGA subtyping software version 2.0.13,14
Determination of drug resistance mutations and analysis of genomic variability
The presence of drug resistance mutations and the impact of these mutations on antiretroviral therapy were assessed using the Stanford HIV Resistance Database (http://hivdb.stanford.edu/). The significant mutations were assessed using the International Antiviral Society-U.S mutation list using the version updated in March 2013.15
To evaluate the genomic variability, Shannon entropy analysis was performed using an online tool available at the Los Alamos National Laboratory (LANL) HIV Sequence Database: www.hiv.lanl.gov/content/ sequence/ENTROPY/entropy_one.html.
Results
Subject profile
Out of 121 patients, the protease gene could be amplified for only 63 samples. The protease sequences were amplified from other samples as well, but possibly owing to sequencing error or analysis, or circulating quasispecies of the virus in the study cohort, the sequences had stop codons, deletions, etc., that rendered them unsuitable for further analysis. Therefore, data from these 63 patients were used for further analyses. Among these 63 study subjects, 24 were males and 39 were females, aged between 22 and 70 years (mean 35). All participants were Kenyan citizens. Twenty participants reported that their partners had a concordant HIV status, while 21 reported a discordant HIV status from their partners; the rest of the participants were not aware of the HIV status of their partners (Table 1). At the time of their first visit, most of the study participants were in the chronic phase of HIV infection.
Table 1.
Profile of Study Participants
| Parameters | Number of participants | Percentage |
|---|---|---|
| Age | ||
| Mean age in years (age range) | 35 (22–70) | |
| Gender | ||
| Male | 24 | 38 |
| Female | 39 | 62 |
| Marital status | ||
| Married | 39 | 62 |
| Divorced | 2 | 3 |
| Separated | 8 | 13 |
| Single | 14 | 22 |
| Nationality | ||
| Kenyan | 63 | 100 |
| Partner's HIV status | ||
| Concordant HV status | 20 | 32 |
| Discordant HIV status | 21 | 33 |
| Don't know | 22 | 35 |
| Partner on antiretroviral therapy | ||
| Yes | 16 | 25 |
| No | 5 | 8 |
| Don't know | 42 | 67 |
| Sexual preferences | ||
| Heterosexual | 60 | 95 |
| Homosexual | 1 | 2 |
| Bisexual | 0 | 0 |
| Abstaining | 2 | 3 |
| Undeclared | 0 | 0 |
| WHO staging | ||
| Stage 1 | 58 | 92 |
| Stage 2 | 3 | 5 |
| Stage 3 | 2 | 3 |
| Stage 4 | 0 | 0 |
The details are presented for 63 treatment-naive patients from whom the protease gene was successfully amplified.
Disease stage, viral loads, and CD4 counts
Approximately 58 (94%) participants were classified as WHO stage I, three (6.5%) were classified as WHO stage II, two (3.1%) were classified as WHO stage III, and none was classified as WHO stage IV (Table 1).
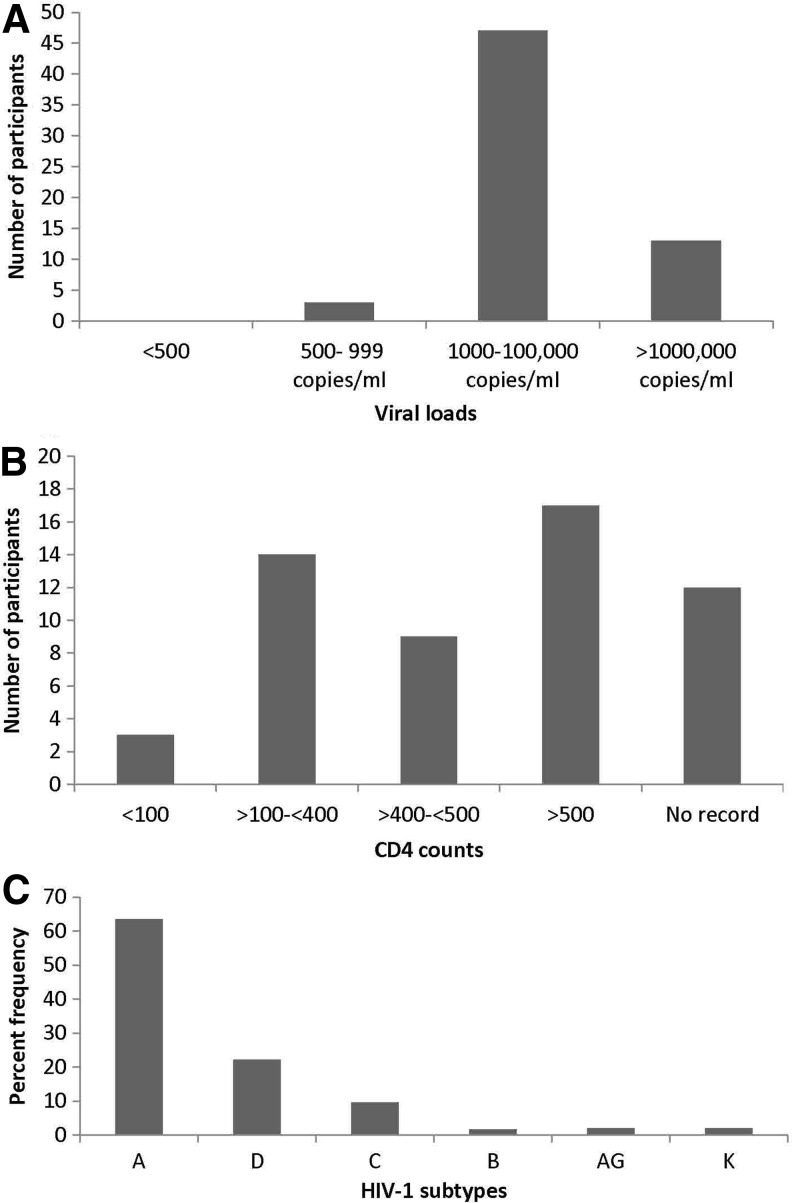
Out of 63 participants, 13 had viral loads of more than 100,000 copies/ml, while the majority of the participants (n = 47) had viral loads in the range of 1,000–100,000 copies/ml (Fig. 1A). The records for CD4 counts showed that the patients had CD4 counts ranging from 3 × 103/ml to 1,130 × 103/ml; only three participants had CD4 counts less than 100 × 103/ml (Fig. 1b).
FIG. 1.
Details of the patient's viral load, CD4 count, and infecting HIV-1 subtype. The figure shows the distribution of participants on the basis of (A) viral loads and (B) CD4 count. (C) Prevalence of HIV-1 subtype in the study cohort.
HIV-1 subtypes
Analysis of the frequency of HIV-1 subtypes revealed subtype A to be the predominant subtype (prevalence 63%), followed by subtypes D and C with prevalences of 22% and 10%, respectively (Fig. 1c).
Protease drug resistance mutations and genomic variability
The analysis of drug resistance mutations revealed the presence of four minor drug resistance mutations (classified according to the Stanford drug resistance database) associated weakly with resistance to protease inhibitors (Table 2). The most prevalent mutation was L33I, which was observed in 22% of the samples. Additionally, one patient each exhibited the K43T, T74S, and A71T mutations. In addition to minor mutations, several other minor protease drug resistance mutations were also observed. Of these, L89M, M36I, H69K, I64V, L63P, and V771 were highly prevalent (Table 2).
Table 2.
Details of the Drug Resistance Mutations Present in Study Participants
| Mutations | Number of patients (%) | Mutation classification | Association with resistance to protease inhibitors |
|---|---|---|---|
| L33I | 14 (22) | *+Minor/Nonpolymorphic | *ATV/RTV |
| T74S | 1 (2) | *+Minor/Polymorphic | +NFV |
| A71T | 1 (2) | *+Minor/Polymorphic | *ATV/RTV/IDV/LPV/NFV/SQV |
| K43T | 1 (2) | *+Minor/Nonpolymorphic | *TPV |
| R41K | 55 (87) | +Other/Polymorphic | None |
| M36I | 50 (79) | +Other or *Minor/Polymorphic | *ATV/RTV/IDV/NFV/TPV |
| H69K | 45 (71) | +Other or *Minor/Polymorphic | *TPV/RTV |
| L89M | 43 (68) | +Other or *Minor/Polymorphic | *TPV/RTV |
| E35D | 28 (44) | +Other/Polymorphic | None |
| L63A | 13 (21) | +Other/Polymorphic | None |
| L63P | 13 (21) | +Other or *Minor/Polymorphic | *LPV/RTV |
| I64V | 11 (17) | +Other or *Minor/Polymorphic | *ATV/RTV |
| V77I | 10 (16) | +Other or *Minor/Polymorphic | *IDV/RTV/NFV/SQV |
| I62V | 7 (11) | +Other or *Minor/Polymorphic | *ATV/RTV/SQV |
| I93L | 7 (11) | +Other or *Minor/Polymorphic | *ATV/RTV |
| V82I | 4 (6) | +Other or *Minor/Highly polymorphic | *ATV/RTV |
| L89I | 3 (5) | +Other or *Minor/Polymorphic | *TPV/RTV |
| L33V | 2 (3) | +Other or *Minor/Polymorphic | *ATV/RTV |
| H69R | 2 (3) | +Other or *Minor/Polymorphic | *TPV/RTV |
| I64L | 2 (3) | +Other or *Minor/Polymorphic | *ATV/RTV |
| E34Q | 1 (2) | +Other or *Minor/Polymorphic | *ATV/RTV |
The table presents the prevalence of different drug resistance mutations observed in the study participants. In the mutation classification column, + indicates mutations that are classified as minor or other drug resistance mutations by the Stanford drug resistance database, while * indicates mutations that are classified as minor drug resistance mutations by the International AIDS Society.15 In the association with resistance to protease inhibitors column, + shows the association of a mutation with resistance to a particular drug, as indicated in the Stanford drug resistance database, while * shows the association of a mutation with a particular drug, as indicated by the International AIDS Society.15 The abbreviations of the antiretroviral drugs are as follows: atazanavir (ATV), ritonavir (RTV), nelfinavir (NFV), indinavir (IDV), lopinavir (LPV), saquinavir (SQV), and tipranavir (TPV).
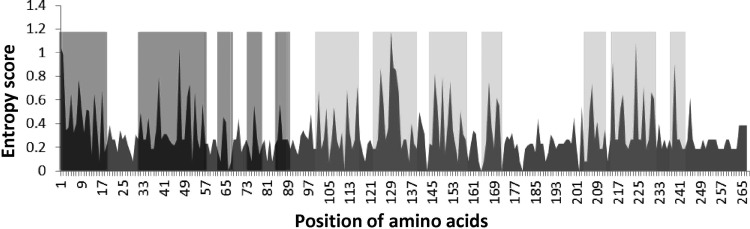
To analyze variability in treatment-naive protease gene sequences Shannon entropy analysis was performed. The analysis revealed that certain regions of protease exhibited high genomic variability, especially in regions spanning nucleotides 1–18, 28–57, 63–67, 74–80, 82–89, 98–115, 120–140, 145–160, and 205–235 (Fig. 2, gray highlighted areas).
FIG. 2.
Analysis of genetic variability in protease sequences. To analyze the variability in the protease gene, we performed Shannon entropy analysis on patient-derived protease sequences. The Shannon entropy analysis revealed that the region of protease spanning nucleotides 1–18, 28–57, 63–67, 74–80, 82–89, 98–115, 120–140, 145–160, and 205–235 exhibited high genomic variability (highlighted gray). The high entropy areas highlighted in dark gray show the region of protease (spanning nucleotides 1–90; HBX2 amino acids 20–50) where certain major PI resistance mutations tend to occur more frequently.
Discussion
In this study, in a cohort of treatment-naive HIV-1-positive patients from Nairobi and Thika, we determined the prevalence of HIV-1 subtypes and the presence of transmitted mutations in protease genes associated with resistance to protease inhibitors (PI).
The majority of study participants in the cohort were females representing 61.9% of all study participants. This is consistent with recent national surveys that have shown that there are more HIV-1 infections in females than males.16 The majority of the participants (95%) were in heterosexual relationships. This is consistent with previous reports in which heterosexual transmission has been shown to be the most common mode of HIV-1 transmission in sub-Saharan Africa.17
In this study 20% of all couples reported having discordant HIV status. This figure is higher than reported in other studies.18,19 This might be due to (1) reporting bias, since actual testing was not done to confirm the reported serostatus or/and (2) the small sample size employed in this study as compared to other previous studies.
Analysis of HIV subtypes in the cohort revealed subtype A to be the most prevalent subtype, infecting 63% of all study participants. This is consistent with previous reports on HIV subtypes in Kenya, where subtype A has been shown to be the most common HIV subtype.20–23 The second most common viral subtype was D with a prevalence of 22%. This is also consistent with previous studies, where subtype D has been reported to be the second most prevalent subtype in Kenya (www.hiv.lanl.gov/).
The WHO guidelines for antiretroviral therapy in Kenya suggest a combination of three drugs from at least two different classes. This may be two nucleoside reverse transcriptase inhibitors (NRTIs) + nonnucleoside reverse transcriptase inhibitors (NNRTI) or two NRTIs + PI/r (ritonavir boosted PI).24 Additionally, in Kenya, the PI lopinavir/ritonavir is given to adults and children as part of the standardized national second-line antiretroviral drug regimen.24 A study conducted to explore the coverage of antiretroviral therapy in HIV-infected patients residing in 39 low-income and middle-income countries (LMIC), including Kenya, reported that the majority of adults (98%) from LMIC were receiving first-line regimens, while only 2% of patients were receiving second-line regimens. Among the 2% of individuals on second-line treatment, lopinavir boosted with low-dose ritonavir (LPV/r) was the predominant protease inhibitor, received by 95% of adults.25
Analysis of the drug resistance mutations showed the presence of several minor mutations weakly associated with resistance to one or more PIs. Among the minor drug-resistant mutations, L33I was most prevalent, present in 22% of patients. L33I is a relatively nonpolymorphic PI-selected mutation that is weakly associated with resistance to atazanavir and ritonavir.15 The other minor mutations found to be prevalent in the cohort were M36I, H69K, L89M, I64V, and L63P. These mutations were found in 17–79% of the patients, and are weakly associated with resistance to one or more PIs, including atazanavir, ritonavir, tipranavir, nelfinavir, and indinavir.15
Since these patients were treatment naive, these mutant viruses are most likely to be transmitted to them from their partners. This is supported by an important observation that among the HIV concordant couples, 80% of their partners were on antiretroviral therapy; this can provide a window of transmitting a virus containing resistance mutations, especially if the partners are not compliant with their antiretroviral therapy and are still having unprotected sex, which contributes to the burden of transmitted HIV-1 resistance.
Minor mutations generally emerge later than major mutations.15 Since most of the patients in our cohort did not present during the acute phase of HIV infection, it might be possible that these patients have also acquired major mutations from their partners, but in the absence of drug pressures the major mutations have reverted to wild-type mutations.26,27 The minor mutations, in this case, might represent the footprint mutations acquired from the transmitted virus.28
The minor mutations have no major effect on drug resistance by themselves, but if a major mutation occurs in the presence of minor mutations, resistance to PIs may be drastically increased.29–31 To obtain an estimate of the probability of the future cooccurrence of major–minor mutations, we performed Shannon entropy analysis, which measures the probability of acquiring mutations in a given set of genomic sequences.22 This analysis revealed that certain regions of protease gene sequences in our study cohort exhibited high entropy, indicating a higher probability of the later occurrence of mutations. The region 1–90 (HBX2 amino acids 20–50), which contained five high entropy patches (Fig. 2, red highlighted area), with the possibility of acquiring further mutations, is especially interesting in this case, since certain major PI resistance mutations tend to occur in this region, for example, mutations D30N, M46I/N, I47V, and I50V, which are responsible for conferring resistance against indinavir, nelfinavir, fosamprenavir, ritonavir, etc.15 It is possible that any major PI resistance mutation that might occur in the genetic background of the minor mutations discussed above will augment the effect of the major PI resistance mutation.
This study suggests that in Kenyan HIV patients there is an increased prevalence of transmitted mutations associated with resistance to PIs. Our results also show that there is a high probability that these patients will acquire major PI resistance mutations at a later point in time, leading to the full-fledged development of drug resistance. This calls for dedicated efforts to improve compliance to antiretroviral therapy in Kenya, which will go a long way to ensuring the therapeutic efficacy of ARVs. Some of the recommended efforts include effective supply chain systems that ensure a constant supply of drugs to patients, careful choice of ARVs, consistent follow-up and strengthening of adherence patterns, and robust monitoring of viral loading to identify early treatment failure.
Acknowledgments
The study was cofunded by the Aga Khan University Research Council Grant (URC Grant Project 102001KEN) and Aga Khan University Postgraduate Medical Education Seed Funding.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Abidi SH, Shahid A, Lakhani LS, et al. : Population-specific evolution of HIV Gag epitopes in genetically diverged patients. Infect Genet Evol 2013;16:78–86 [DOI] [PubMed] [Google Scholar]
- 2.UNAIDS (2010) Global Report—UNAIDS Report on the Global AIDS Epidemic
- 3.Njeru MK, Blystad A, Shayo EH, et al. : Practicing provider-initiated HIV testing in high prevalence settings: Consent concerns and missed preventive opportunities. BMC Health Serv Res 2011;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poggensee G, Kucherer C, Werning J, et al. : Impact of transmission of drug-resistant HIV on the course of infection and the treatment success. Data from the German HIV-1 Seroconverter Study. HIV Med 2007;8:511–519 [DOI] [PubMed] [Google Scholar]
- 5.Preston BD, Poiesz BJ, and Loeb LA: Fidelity of HIV-1 reverse transcriptase. Science 1988;242:1168–1171 [DOI] [PubMed] [Google Scholar]
- 6.Skoura L, Metallidis S, Pilalas D, et al. : High rates of transmitted drug resistance among newly-diagnosed antiretroviral naive HIV patients in Northern Greece, data from 2009–2011. Clin Microbiol Infect 2013;19:E169–172 [DOI] [PubMed] [Google Scholar]
- 7.Kwena ZA, Bukusi EA, Ng'ayo MO, et al. : Prevalence and risk factors for sexually transmitted infections in a high-risk occupational group: The case of fishermen along Lake Victoria in Kisumu, Kenya. Int J STD AIDS 2010;21:708–713 [DOI] [PubMed] [Google Scholar]
- 8.Aghokeng AF, Kouanfack C, Laurent C, et al. : Scale-up of antiretroviral treatment in sub-Saharan Africa is accompanied by increasing HIV-1 drug resistance mutations in drug-naive patients. AIDS 2011;25:2183–2188 [DOI] [PubMed] [Google Scholar]
- 9.Van Vaerenbergh K: Study of the impact of HIV genotypic drug resistance testing on therapy efficacy. Verh K Acad Geneeskd Belg 2001;63:447–473 [PubMed] [Google Scholar]
- 10.Lihana RW, Khamadi SA, Lubano K, et al. : HIV type 1 subtype diversity and drug resistance among HIV type 1-infected Kenyan patients initiating antiretroviral therapy. AIDS Res Hum Retroviruses 2009;25:1211–1217 [DOI] [PubMed] [Google Scholar]
- 11.Sigaloff KC, Mandaliya K, Hamers RL, et al. : Short communication: High prevalence of transmitted antiretroviral drug resistance among newly HIV type 1 diagnosed adults in Mombasa, Kenya. AIDS Res Hum Retroviruses 2011;28:1033–1037 [DOI] [PubMed] [Google Scholar]
- 12.Tamura K, Stecher G, Peterson D, et al. : MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol 2013;30:2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alcantara LC, Cassol S, Libin P, et al. : A standardized framework for accurate, high-throughput genotyping of recombinant and non-recombinant viral sequences. Nucleic Acids Res 2009;37:W634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira T, Deforche K, Cassol S, et al. : An automated genotyping system for analysis of HIV-1 and other microbial sequences. Bioinformatics 2005;21:3797–3800 [DOI] [PubMed] [Google Scholar]
- 15.Johnson VA, Calvez V, Gunthard HF, et al. : Update of the drug resistance mutations in HIV-1: March 2013. Top Antivir Med 2013;21:6–14 [PMC free article] [PubMed] [Google Scholar]
- 16.Glynn JR, Carael M, Auvert B, et al. : Why do young women have a much higher prevalence of HIV than young men? A study in Kisumu, Kenya and Ndola, Zambia. AIDS 2001;15(Suppl 4):S51–60 [DOI] [PubMed] [Google Scholar]
- 17.Lamptey PR: Reducing heterosexual transmission of HIV in poor countries. BMJ 2002;324:207–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biraro S, Ruzagira E, Kamali A, et al. : HIV-1 transmission within marriage in rural Uganda: A longitudinal study. PLoS One 2013;8:e55060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaiser R, Bunnell R, Hightower A, et al. : Factors associated with HIV infection in married or cohabitating couples in Kenya: Results from a nationally representative study. PLoS One 2011;6:e17842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khamadi SA, Lihana RW, Osman S, et al. : Genetic diversity of HIV type 1 along the coastal strip of Kenya. AIDS Res Hum Retroviruses 2009;25:919–923 [DOI] [PubMed] [Google Scholar]
- 21.Lihana RW, Khamadi SA, Lwembe RM, et al. : The changing trend of HIV type 1 subtypes in Nairobi. AIDS Res Hum Retroviruses 2009;25:337–342 [DOI] [PubMed] [Google Scholar]
- 22.Abidi SH, Kalish ML, Abbas F, et al. : HIV-1 subtype A Gag variability and epitope evolution. PLoS One 2014;9:e93415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abidi SH, Shahid A, Lakhani LS, et al. : HIV-1 progression links with viral genetic variability and subtype, and patient's HLA type: Analysis of a Nairobi-Kenyan cohort. Med Microbiol Immunol 2013;203:57–63 [DOI] [PubMed] [Google Scholar]
- 24.WHO (2005): Guidelines for antiretroviral therapy in Kenya
- 25.Renaud-Thery F, Avila-Figueroa C, Stover J, et al. : Utilization patterns and projected demand of antiretroviral drugs in low- and middle-income countries. AIDS Res Treat 2011;2011:749041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbour JD, Hecht FM, Wrin T, et al. : Persistence of primary drug resistance among recently HIV-1 infected adults. AIDS 2004;18:1683–1689 [DOI] [PubMed] [Google Scholar]
- 27.Zaccarelli M, Tozzi V, Perno CF, and Antinori A: The challenge of antiretroviral-drug-resistant HIV: Is there any possible clinical advantage? Curr HIV Res 2004;2:283–292 [DOI] [PubMed] [Google Scholar]
- 28.Lemey P, Derdelinckx I, Rambaut A, et al. : Molecular footprint of drug-selective pressure in a human immunodeficiency virus transmission chain. J Virol 2005;79:11981–11989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borman AM, Paulous S, and Clavel F: Resistance of human immunodeficiency virus type 1 to protease inhibitors: Selection of resistance mutations in the presence and absence of the drug. J Gen Virol 1996;77(Pt 3):419–426 [DOI] [PubMed] [Google Scholar]
- 30.Carrillo A, Stewart KD, Sham HL, et al. : In vitro selection and characterization of human immunodeficiency virus type 1 variants with increased resistance to ABT-378, a novel protease inhibitor. J Virol 1998;72:7532–7541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nijhuis M, Schuurman R, de Jong D, et al. : Increased fitness of drug resistant HIV-1 protease as a result of acquisition of compensatory mutations during suboptimal therapy. AIDS 1999;13:2349–2359 [DOI] [PubMed] [Google Scholar]