Abstract
Data on vitamin D insufficiency as a cause of inflammation and metabolic dysfunction in HIV-infected individuals are conflicting. We examined the relationships between levels of 25-hydroxyvitamin D [25(OH)D] and biomarkers of inflammation and metabolism in stored blood samples from a prospective trial of vitamin D repletion. Blood samples from HIV-infected individuals on antiretroviral therapy (ART) with HIV-1 RNA <200 copies/ml enrolled in a prospective study were analyzed for 25(OH)D levels, a broad panel of cytokines, highly sensitive C-reactive protein, D-dimer, adiponectin, leptin, and insulin. Correlations between markers and 25(OH)D levels were determined. The Wilcoxon Rank Sum test was used to compare markers between individuals 25(OH)D insufficient and sufficient at baseline and before and after repletion among those who were insufficient and repleted to ≥30 ng/ml after 12 weeks. Of 106 subjects with stored plasma [66 with 25(OH)D <30 ng/ml and 40 ≥ 30 ng/ml], the median age was 50, the CD4 count was 515 cells/mm3, 94% were male, and the median baseline 25(OH)D was 27 ng/ml. Higher 25(OH)D levels were associated with lower tumor necrosis factor (TNF)-α (r = −0.20, p = 0.04) and higher adiponectin levels (r = 0.30, p = 0.002). Following successful repletion to 25(OH)D ≥30 ng/ml there were no significant changes in inflammatory or metabolic parameters. Our study found associations between low 25(OH)D levels and TNF-α and adiponectin. Repletion did not result in changes in markers of inflammation or metabolism. These data support continued study of the relationship between vitamin D, inflammation, and metabolism in treated HIV infection.
Introduction
Low serum 25-hydroxyvitamin D [25(OH)D] levels are common in HIV-infected individuals in all geographic regions, with studies showing rates in the range of 30–92% among patients on antiretroviral therapy (ART).1–3 In healthy, HIV-uninfected adults, vitamin D deficiency has been associated with increased production of proinflammatory cytokines4 and has been linked to morbidity including colon, breast, and prostate cancers,5,6 autoimmune disease,7,8 depression,9 chronic pain, and acute respiratory infections.10 Vitamin D levels may also have important implications for inflammation in patients with HIV infection. An emerging body of data in this population suggests associations between low vitamin D levels and elevated markers of inflammation including interleukin-6 (IL-6) and high-sensitivity C-reactive protein (hs-CRP),11–13 and in a random sample of 1,985 HIV-infected individuals in Europe, higher vitamin D levels were independently associated with a lower risk of mortality and AIDS events after adjusting for ART use, CD4+ T lymphocyte count, and viral load.14
Vitamin D deficiency has also been associated with metabolic dysregulation, with studies to date focusing largely on obesity in HIV-uninfected cohorts and the interaction between 25(OH)D levels and adiponectin, a peptide hormone secreted by adipose tissue. Low levels of adiponectin have been associated with high fat mass and obesity-related cardiometabolic complications. Higher adiponectin and 25(OH)D levels have been independently associated with a reduced risk for insulin resistance, atherosclerosis, other cardiometabolic risk factors, and cardiovascular disease.15–18 Data on the relationship between vitamin D levels and metabolic function are emerging from the HIV literature, but have been largely limited to cross-sectional studies and studies of heterogeneous populations in regard to the use of ART and degree of viral suppression.19–22
Because of uncertainty surrounding the association of vitamin D insufficiency with markers of inflammation and metabolism, we sought to evaluate differences in these parameters in HIV-infected adults on ART with viral suppression utilizing a cross-section of individuals with and without 25(OH)D insufficiency and via a prospective cohort study of changes in markers using those individuals insufficient at baseline (<30 ng/ml) and assessing markers after repletion to ≥30 ng/ml. We hypothesized that 25(OH)D-insufficient individuals would have higher levels of inflammation and markers suggesting metabolic dysregulation, and that vitamin D repletion to ≥30 ng/ml would result in improvements in these parameters.
Materials and Methods
Center for Clinical AIDS Research and Education (CARE) vitamin D cohort
The CARE Vitamin D cohort was a prospective study developed to assess the effectiveness of standard vitamin D repletion among HIV-infected patients on stable ART with HIV-1 RNA <200 copies/ml. The cohort enrolled participants ≥18 years of age from the University of California, Los Angeles (UCLA) CARE clinic in Los Angeles, California. Vitamin D-sufficient participants (≥30 ng/ml) had a single study visit that included biological specimen collection for storage and medical record review. Vitamin D-insufficient participants had a visit at baseline and underwent repletion with open-label, oral vitamin D3 50,000 IU twice weekly for 5 weeks, then 2,000 IU daily to complete 12 weeks, and completed follow-up visits at 12 and 24 weeks, with biological specimen collection and medical record review at each visit. All individuals enrolled in the study provided written informed consent and the study was approved by the UCLA Institutional Review Board. Detailed study schedules and evaluations and results of vitamin D repletion have been described in a previously published study.23
Study population and definitions for this analysis
The study population for the cross-sectional analysis included HIV-infected individuals enrolled in the CARE vitamin D cohort who were on ART with documented HIV-1 RNA <200 copies/ml within 6 months of enrollment, were not using vitamin D supplementation >400 IU daily at screening (the amount in a standard multivitamin), and had at least 3 ml of stored plasma available at the baseline visit. The study population for the prospective aim included individuals from the cross-sectional study who were 25(OH)D insufficient at baseline, achieved a vitamin D level ≥30 ng/ml after 12 weeks of vitamin D, and had at least 3 ml of stored plasma from both the baseline and 12 weeks study visits.
For the study, the primary exposure of interest was vitamin D status defined as insufficient [25(OH)D <30 ng/ml] or sufficient [25(OH)D ≥30 ng/ml]. The primary outcomes of interest were biomarkers of inflammation [IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-1β, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and hs-CRP], coagulation (D-dimer), and metabolism (glucose, insulin, adiponectin, and leptin).
Determination of 25(OH)D levels and inflammation, coagulation, and metabolism biomarkers
Serum 25(OH)D was measured via DiaSorin Liaison direct competitive chemiluminescence immunoassay at the UCLA Clinical Laboratories. This assay has a lower limit of detection of 4 ng/ml and within- and between-assay coefficients of variation of <7.7% and <12.6%, respectively (DiaSorin, Stillwater, MN). Cytokine analyses (IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, IL-1β, TNF-α, and IFN-γ) were performed at UCLA using a Luminex platform high sensitivity multiplex assay (R&D systems Inc., Minneapolis, MN) on samples stored at −70°C. The lower limit of detection for each cytokine was determined by extrapolation from the lowest standard concentration using Bio-Plex Software (Bio-Rad, Hercules, CA). Zero was imputed when the lowest standard was indistinguishable from background. Samples for hs-CRP and D-dimer were transported from UCLA to Quest Diagnostics (Chantilly, VA). Testing for hs-CRP was performed with nephelometry24 and D-dimer with STA-R analyzer/immunoturbidometric method.25 Metabolic markers were also performed by Quest, as follows: insulin (immunoassay),26 adiponectin (ELISA),27 and leptin (radioimmunoassay).28 The Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was determined using a standard calculator.29
Statistical analysis
Sociodemographic characteristics, CD4+ T lymphocyte counts, ART regimens, and non-AIDS comorbidities were abstracted from medical records, summarized for the overall cohort, and compared by vitamin D status at baseline (insufficient versus sufficient). The Wilcoxon Rank Sum test was used to compare continuous variables and the Chi-square or Fisher's exact test was used to compare categorical variables. To determine whether the subgroup of participants with plasma samples was representative of the overall CARE vitamin D cohort we compared sociodemographic and clinical characteristics of those with and without stored plasma. We compared biomarkers of inflammation and metabolism using Wilcoxon Rank Sum tests in two ways: first between those who were 25(OH)D insufficient and sufficient at baseline (cross-sectional aim) and second before and after repletion among those who were insufficient at baseline and repleted to ≥30 ng/ml after 12 weeks (prospective aim). Finally, in the cross-sectional aim, Spearman's correlation was used to evaluate the association between markers of inflammation and metabolism and 25(OH)D levels among all participants. In the prospective aim, we evaluated correlations between changes in vitamin D levels and changes in marker levels from baseline to 12 weeks.
All statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC). A two-sided p-value <0.05 was considered statistically significant. Due to the exploratory nature of the analysis, corrections for multiple statistical tests were not performed. Based on prior studies of vitamin D and inflammation in HIV-infected cohorts,30,31 our sample size provided at least 80% power to detect baseline differences between 25(OH)D-insufficient and 25(OH)D-sufficient individuals of 0.54 and 0.58 in effect size for IL-6 and TNF-α, respectively.
Results
Size and characteristics of the study population
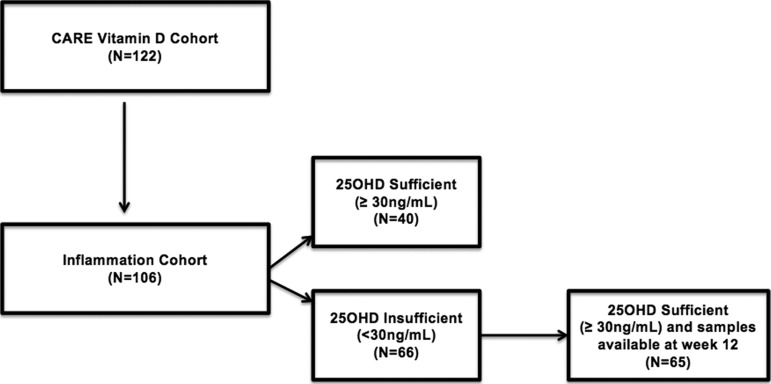
Of 122 individuals screened and enrolled in the CARE Vitamin D cohort, 106 had stored samples and were included in the analysis. Comparisons of those with and without stored plasma samples are shown in Table 1. Those excluded due to lack of stored plasma had significantly lower median 25(OH)D levels (15 ng/ml versus 27 ng/ml, p < 0.01) and were more likely to be of nonwhite race (69% versus 36%, p = 0.01). Of the 106 individuals included in the inflammation cohort, 40 were 25(OH)D sufficient and 66 insufficient at baseline and all had samples available for inclusion in the analysis. Of those insufficient at baseline, all achieved 25(OH)D levels ≥30 ng/ml at 12 weeks, and all but one had stored samples available for inclusion in the analysis. Figure 1 illustrates the number of participants with samples available from the inflammation cohort for both the cross-sectional and prospective aims.
Table 1.
Baseline Characteristics of the Parent CARE Vitamin D Cohort (N = 122) and Comparisons of Those Included (N = 106) Versus Excluded (N = 16) in the inflammation Cohort
| CARE vitamin D cohort N = 122 | Excluded from inflammation cohort due to lack of plasma N = 16 | Included in inflammation cohort N = 106 | p-value | |
|---|---|---|---|---|
| Median age (years) (IQR) | 49 (42, 55) | 46 (37, 52) | 50 (44, 56) | 0.10 |
| Median CD4 count (cells/mm3) (IQR) | 520 (391, 662) | 597 (450, 689) | 515 (390, 638) | 0.30 |
| Gender | ||||
| Male % (N) | 95 (116) | 100 (16) | 94 (100) | |
| Female % (N) | 5 (6) | 0 (0) | 6 (6) | 0.99 |
| Nonwhite race % (N) | 40 (49) | 69 (11) | 36 (38) | 0.01 |
| Median body mass index (kg/m2), IQR | 26 (25, 29) | 27 (25, 29) | 26 (25, 30) | 0.60 |
| Regimen | ||||
| NNRTI % (N) | 57 (70) | 69 (11) | 56 (59) | 0.32 |
| PI % (N) | 34 (41) | 25 (4) | 35 (37) | 0.43 |
| Integrase % (N) | 16 (20) | 13 (2) | 17 (18) | 0.99 |
| Median 25(OH)D level (ng/ml) (IQR) | 26 (17, 33) | 15 (9, 19) | 27 (20, 34) | <0.01 |
| Current smoker % (N) | 12 (15) | 25 (4) | 10 (11) | 0.11 |
| Diabetes mellitus % (N) | 3 (4) | 6 (1) | 3 (3) | 0.43 |
| Hypertension % (N) | 42 (51) | 44 (7) | 42 (44) | 0.87 |
| Hepatitis C coinfectiona % (N) | 4 (5) | 0 (0) | 4.7 (5) | 0.99 |
Defined by positive hepatitis C antibody.
NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; 25(OH)D, 25-hydroxyvitamin D.
FIG. 1.
Flow chart illustrating the number of participants with samples available from the inflammation cohort for both the cross-sectional aim (comparing individuals sufficient versus deficient at baseline) and prospective aim (comparing deficient individuals before and after repletion)
The median age of the inflammation cohort was 50 years (IQR 44,56), the median CD4+ T lymphocyte count was 515 cells/mm3 (IQR 390, 638), 6% of the cohort were female, and 36% were nonwhite race. The most common ART regimens included nonnucleoside reverse transcriptase inhibitors (56%), protease inhibitors (35%), and integrase inhibitors (17%). Eighty-one percent of individuals were on a tenofovir-containing nucleoside analogue regimen with the remainder on abacavir. Ten percent of the overall cohort were current smokers, 42% were hypertensive, and 4.7% had positive hepatitis C antibody (Table 1). At baseline, the median 25(OH)D level among the participants with insufficiency was 21.0 ng/ml (IQR 16.0, 27.0) and median level among the participants without insufficiency was 36.0 ng/ml (IQR 33.0, 41.5). There were no significant differences between the 25(OH)D-sufficient and 25(OH)D-insufficient groups in regard to age, gender, race, median CD4 T lymphocyte count, ART regimen, and comorbidities (smoking, hypertension, hepatitis C). Approximately 3% of the cohort had diabetes (N = 3) and all were in the 25(OH)D-insufficient group.
Cross-sectional and prospective cohort comparisons of inflammation, coagulation, and metabolic markers
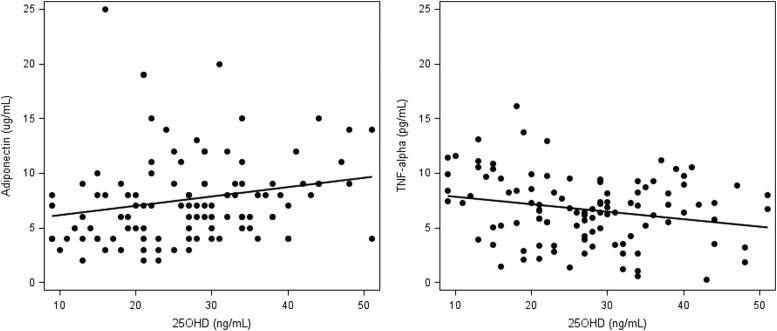
Median biomarker levels are presented in Table 2 for the comparison of those sufficient versus insufficient individuals at baseline. Low levels were seen for hs-CRP (median 1.1 mg/liter, reference range of <1.0 mg/liter low cardiovascular risk, 1.0–3.0 mg/liter average cardiovascular risk, and >3–10 mg/liter high cardiovascular risk32) and D-dimer (median 0.2 μ/ml, reference range <0.5 μ/ml). Median levels were undetectable for several cytokines including IL-2, IL-4, IL-12, and IL-1β. No significant differences in median biomarker levels were detected at baseline for 25(OH)D-insufficient versus 25(OH)D-sufficient individuals. There was a trend toward significance for adiponectin (8.0 μg/ml in sufficients and 6.5 μg/ml among insufficients, p = 0.06) that strengthened when the analysis was limited only to individuals with 25(OH)D <20 ng/ml (N = 26, p = 0.005), with no significant differences in other markers of metabolism including glucose, insulin, leptin, and HOMA-IR. In the analysis of correlations between baseline 25(OH)D levels and biomarkers, higher 25(OH)D levels were associated with lower TNF-α (r = −0.20, p = 0.04) and higher adiponectin levels (r = 0.30, p = 0.002) (Fig. 2). None of these results significantly changed when participants with diabetes were excluded from the analysis.
Table 2.
Median Baseline Levels (Interquartile Range) for Markers of Inflammation, Coagulation, and Metabolism in Participants with Sufficient (≥30 ng/ml) Versus Insufficient (<30 ng/ml) 25-Hydroxyvitamin D Levels
| 25-Hyroxy vitamin D sufficient (≥30 ng/ml) N = 40 | 25-Hyroxy vitamin D insufficient (<30 ng/ml) N = 66 | p-valuea | |
|---|---|---|---|
| IFN-γ pg/ml | 0.2 (0, 0.4) | 0.1 (0, 0.4) | 0.72 |
| IL-2 pg/ml | 0 (0, 0.4) | 0 (0, 0.4) | 0.38 |
| IL-4 pg/ml | 0 (0, 5.1) | 0 (0, 0.1) | 0.08 |
| IL-6 pg/ml | 1.6 (1.1, 2.4) | 1.7 (1.2, 2.4) | 0.68 |
| IL-8 pg/ml | 13.0 (8.9, 25.0) | 14.0 (9.6, 24.0) | 0.66 |
| IL-10 pg/ml | 0.7 (0.5, 1.1) | 0.7 (0.4, 1.1) | 0.65 |
| IL-12 pg/ml | 0 (0, 0) | 0 (0, 0) | 0.62 |
| IL-1β pg/ml | 0 (0, 0.1) | 0 (0, 0) | 0.62 |
| TNF-α pg/ml | 6.8 (3.5, 8.2) | 6.7 (5.0, 9.5) | 0.34 |
| hs-CRP mg/liter | 1.4 (0.6, 2.7) | 1.0 (0.7, 1.7) | 0.33 |
| D-dimer μ/ml | 0.2 (0.2, 0.3) | 0.2 (0.2, 0.3) | 0.69 |
| Adiponectin μg/ml | 8.0 (6.0, 9.0) | 6.5 (4.0, 9.0) | 0.06 |
| Leptin ng/ml | 4.8 (3.2, 9.9) | 5.2 (3.2, 9.9) | 0.42 |
| Glucose mg/dl | 91 (88, 102) | 95 (89, 102) | 0.20 |
| Insulin μIU/ml | 6.0 (2.5, 11.0) | 6.0 (4.0, 11.0) | 0.24 |
| HOMA-IR | 1.3 (0.5, 2.2) | 1.5 (0.9, 2.5) | 0.24 |
p-values determined by the Wilcoxon Rank Sum test.
IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; hs-CRP, high-sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance.
FIG. 2.
Left: Scatter plot showing the correlation between adiponectin and 25-hydroxy vitamin D [25(OH)D] levels (r = 0.30, p = 0.002). Right: Scatter plot showing the correlation between tumor necrosis factor (TNF)-α and 25(OH)D levels (r = −0.20, p = 0.04).
Among those with vitamin D insufficiency who achieved levels ≥30 ng/ml after 12 weeks (N = 65), the median change in 25(OH)D was 23.5 ng/ml (IQR 16.0, 31.0). There were no significant postrepletion changes in cytokines, including TNF-α, and no change in hs-CRP, D-dimer, or metabolic parameters (adiponectin, leptin, HOMA-IR, glucose, insulin). There were no significant correlations between change in 25(OH)D levels and change in any of the inflammatory or metabolic markers.
Discussion
Vitamin D insufficiency and inflammation in chronic HIV infection
We found an inverse correlation between TNF-α and 25(OH)D levels at baseline but no change in TNF-α levels with repletion. A relationship between vitamin D levels and TNF-α has been demonstrated in vitro, with binding of the vitamin D receptor resulting in downregulation of several cytokines, including TNF-α.33 The association with 25(OH)D and TNF-α has not been reported from other studies of HIV-infected cohorts; however, in a randomized study of HIV-uninfected adults with congestive heart failure, TNF-α was stable over 9 months in the vitamin D repletion group but increased by 12% in the placebo group (p = 0.017) suggesting a protective benefit in the inflammatory milieu of heart failure.34 While at least one cross-sectional study has shown no association between low 25(OH)D levels and systemic inflammation,22 several other cross-sectional studies have shown associations with IL-6 and hs-CRP11–13 as well as with soluble TNF receptor 1 (sTNFR1),35 soluble TNF receptor 2 (sTNFR2), and resistin.13 These studies have included adults both on and off ART and most did not require virologic suppression for study inclusion, making direct comparisons with our cohort difficult.
In our cohort, levels of inflammatory biomarkers were lower compared to other HIV studies of inflammation in which participants with elevated viral loads were not excluded36–38 but similar to studies in virologically suppressed individuals.39–41 Whether further reductions in already low levels of inflammation in HIV-infected individuals with suppressed viral load translate into meaningful clinical benefit remains unclear; however, studies have shown associations between low 25(OH)D levels and important clinical endpoints. In a random sample of 1,985 individuals in the EuroSIDA cohort, lower vitamin D levels were independently associated with a higher risk of mortality and AIDS events after adjusting for ART use, CD4+ T lymphocyte count, and HIV-1 viral load.14 A nested case-control study of 250 individuals in this cohort showed that among individuals with 25(OH)D levels <10 ng/ml, IL-6 concentrations increased by 4.7% annually (95% CI 0.2, 9.3, p = 0.04) among cases (those with AIDS, non-AIDS events, or death) compared to controls who did not experience these endpoints.42 Notably, the odds of death decreased by 46.0% (95% CI 2.0, 70.0, p = 0.04) for a 2-fold increase in the latest 25(OH)D level. This study was composed of individuals with more advanced HIV infection (baseline CD4+ T lymphocyte count 289 cells/mm3) with high rates of prior AIDS-defining illnesses, and was not limited to those with suppressed viral load. The prognostic value of 25(OH)D levels may be less powerful among individuals with higher CD4+ T lymphocyte counts who are suppressed on ART.
While longitudinal cohort data on vitamin D repletion and inflammation are less abundant, several cohorts have recently been described. The Chicago Women's Interagency HIV Study (WIHS) identified 40 HIV-infected women who were vitamin D insufficient and evaluated baseline and postrepletion levels of hs-CRP, IL-6, and TNF-α.43 They found no changes after vitamin D repletion. Their cohort was composed of a mixed population of women in regard to ART, with approximately half suppressed to <48 copies/ml, limiting conclusions that can be drawn about residual inflammation in the setting of viral suppression. A recently completed randomized study of vitamin D on bone health for individuals initiating ART (AIDS Clinical Trials Group 5280) will provide data on vitamin D repletion and changes in inflammation over 48 weeks (IL-6, sTNFR1, sTNFR2, and soluble CD14).44
Vitamin D levels, adiponectin, and metabolic disease
We found lower adiponectin levels in participants with vitamin D insufficiency and a positive correlation between 25(OH)D and adiponectin levels. Cross-sectional data in HIV-infected populations have shown vitamin D deficiency to be associated with a decrease in pancreatic β cell function, reduced insulin sensitivity, and type 2 diabetes mellitus.19–21 A proposed mechanism for vitamin D's role in improving insulin sensitivity is through reducing inflammation, direct effects on peripheral and hepatic glucose uptake,45–47 and regulation of secretion of insulin by pancreatic β cells.48–50
The precise role by which vitamin D and adiponectin interact along these pathways is unknown; however, the vitamin D receptor has been identified in adipocytes and it has been hypothesized that 1,25(OH)D regulates gene expression of adiponectin.51 We found largely normal glucose, insulin, and HOMA-IR levels, precluding an analysis of the relationships of 25(OH)D and adiponectin with insulin resistance and diabetes. Given prior descriptions of adiponectin as an antiinflammatory adipokine,52–56 we performed exploratory analyses on the relationship of adiponectin to selected inflammatory biomarkers in our study (data not shown). In keeping with a hypothesized antiinflammatory role, we observed an inverse relationship between adiponectin and IL-6 levels among the baseline cohort (r = −0.27, p = 0.005). We did not find an association between adiponectin and TNF-α.
Few studies in HIV-infected populations have explored the benefits of vitamin D repletion on metabolic parameters. A study of vitamin D repletion in 20 HIV-infected individuals from the Netherlands found that despite an increase in 25(OH)D levels and an early signal for a decrease in insulin sensitivity at 14 weeks, there were no significant changes in insulin sensitivity after 48 weeks and no sustained benefits on adipokines, bone mineral density, and triglycerides.57
Studies of vitamin D repletion in HIV-uninfected individuals have shown metabolic benefits including improved insulin sensitivity in obese adolescents,58 improved measures of pancreatic function in type 2 diabetics based on the homeostasis model of assessment of β cell activity,59 and decreases in glycosylated hemoglobin and other cardiovascular risk factors (blood pressure, total cholesterol, and low-density lipoprotein), also in type 2 diabetics.60 Larger studies in HIV-infected patients are needed to determine if these benefits can be achieved in the more complicated inflammatory and metabolic milieu of treated HIV infection and to characterize how vitamin D may mediate metabolic risk through adiponectin, with adjustment for body fat composition, gender, race, and HIV disease and treatment factors.
Study limitations and weaknesses
The 25(OH)D-insufficient participants in the CARE vitamin D cohort were followed for 24 weeks; however, sample availability was not sufficient at this time point for inclusion in the analysis. Benefits of repletion may take longer than 12 weeks and our study lacks long-term follow-up (24 weeks and beyond) to answer this question. Our cohort did not have a high proportion of individuals who had very high (>50 ng/ml) or very low (<15 ng/ml) vitamin D levels and therefore we could not perform subanalyses at the extreme ends of the vitamin D range. The greatest benefits of vitamin D may be for those who start with very low levels and achieve a threshold not represented in our study population. Our cohort was largely older white men and results may not be generalizable to other groups. Our study was an exploratory analysis to gain preliminary data on markers of inflammation and metabolism and 25(OH)D levels, and therefore we did not correct for multiple statistical testing.
Conclusions
Consistent with an emerging body of evidence, our study found associations between 25(OH)D levels and markers of inflammation (TNF-α) and metabolism (adiponectin). Repletion did not result in improvements of these or other parameters of inflammation or metabolic function. Future studies are needed to explore whether targeting higher 25(OH)D levels results in significant changes in biomarkers and to correlate changes in inflammatory and metabolic markers with clinical outcomes of interest.
Acknowledgments
The authors would like to thank the study participants, as well as Maricela Gonzalez, Zane Ashman, and the staff and providers at the UCLA CARE Center for their assistance with this project. These data were presented in part as a poster at the 13th International Workshop on Adverse Drug Reactions and Co-Morbidities in Rome, Italy (July 15, 2011) and as a poster at the 7th IAS Conference on HIV Pathogenesis and Treatment, Rome, Italy (July 17–20, 2011). Abstract number PUB002.
This work was supported by the National Institutes of Health (awards K24 AI56933 to J.S. Currier, K23AI110532 to J.E. Lake, P30 AG028748, T32 MH080634, UL1 TR000124, M01 RR00865, and 5P30 AI028697), the UCLA AIDS Institute to J.E. Lake, and the California HIV/AIDS Research Program to R.M. Hoffman.
Author Disclosure Statement
Jordan E. Lake has served as a consultant to Gilead Sciences and GlaxoSmithKline.
References
- 1.Rodriguez M, Daniels B, G'unawardene S, and Robbins GK: High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res Hum Retroviruses 2009;25(1):9–14 [DOI] [PubMed] [Google Scholar]
- 2.Van Den Bout-Van Den Beukel CJ, Fievez L, Michels M, et al. : Vitamin D deficiency among HIV type 1-infected individuals in the Netherlands: Effects of antiretroviral therapy. AIDS Res Hum Retroviruses 2008;24(11):1375–1382 [DOI] [PubMed] [Google Scholar]
- 3.Dao CN, Patel P, Overton ET, et al. : Low vitamin D among HIV-infected adults: Prevalence of and risk factors for low vitamin D levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin Infect Dis 2011;52(3):396–405 [DOI] [PubMed] [Google Scholar]
- 4.Peterson CA. and Heffernan ME: Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gorham ED, Garland CF, Garland FC, et al. : Vitamin D and prevention of colorectal cancer. J Steroid Biochem Mol Biol 2005;97(1–2):179–194 [DOI] [PubMed] [Google Scholar]
- 6.Giovannucci E, Liu Y, Rimm EB, et al. : Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006;98(7):451–459 [DOI] [PubMed] [Google Scholar]
- 7.Ponsonby AL, McMichael A, and van der Mei I: Ultraviolet radiation and autoimmune disease: Insights from epidemiological research. Toxicology 2002;181–182:71–78 [DOI] [PubMed] [Google Scholar]
- 8.VanAmerongen BM, Dijkstra CD, Lips P, and Polman CH: Multiple sclerosis and vitamin D: An update. Eur J Clin Nutr 2004;58(8):1095–1109 [DOI] [PubMed] [Google Scholar]
- 9.Gloth FM, 3rd, Alam W, and Hollis B: Vitamin D vs broad spectrum phototherapy in the treatment of seasonal affective disorder. J Nutr Health Aging 1999;3(1):5–7 [PubMed] [Google Scholar]
- 10.Walker VP. and Modlin RL: The vitamin D connection to pediatric infections and immune function. Pediatr Res 2009;65(5 Pt 2):106R–113R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poudel-Tandukar K, Poudel KC, Jimba M, et al. : Serum 25-hydroxyvitamin D levels and C-reactive protein in persons with human immunodeficiency virus infection. AIDS Res Hum Retroviruses 2013;29(3):528–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansemant T, Mahy S, Piroth C, et al. : Severe hypovitaminosis D correlates with increased inflammatory markers in HIV infected patients. BMC Infect Dis 2013;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legeai C, Vigouroux C, Souberbielle JC, et al. : Associations between 25-hydroxyvitamin D and immunologic, metabolic, inflammatory markers in treatment-naive HIV-infected persons: the ANRS CO9 “COPANA” cohort study. PLoS One 2013;8(9):e74868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viard JP, Souberbielle JC, Kirk O, et al. : Vitamin D and clinical disease progression in HIV infection: Results from the EuroSIDA study. AIDS 2011;25(10):1305–1315 [DOI] [PubMed] [Google Scholar]
- 15.Ouchi N, Shibata R, and Walsh K: Cardioprotection by adiponectin. Trends Cardiovasc Med 2006;16(5):141–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ouchi N, Kihara S, Arita Y, et al. : Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation 2000;102(11):1296–1301 [DOI] [PubMed] [Google Scholar]
- 17.Holick MF: Vitamin D status: Measurement, interpretation, and clinical application. Ann Epidemiol 2009;19(2):73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oda E: Obesity-related risk factors of cardiovascular disease. Circ J 2009;73(12):2204–2205 [DOI] [PubMed] [Google Scholar]
- 19.Moreno-Perez O, Portilla J, Escoin C, et al. : Impact of vitamin D insufficiency on insulin homeostasis and beta cell function in nondiabetic male HIV-infected patients. HIV Med 2013;14(9):540–548 [DOI] [PubMed] [Google Scholar]
- 20.Szep Z, Guaraldi G, Shah SS, et al. : Vitamin D deficiency is associated with type 2 diabetes mellitus in HIV infection. AIDS 2011;25(4):525–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammond E, McKinnon E, Glendenning P, et al. : Association between 25-OH vitamin D and insulin is independent of lipoatrophy in HIV. Clin Endocrinol (Oxf) 2012;76(2):201–206 [DOI] [PubMed] [Google Scholar]
- 22.Missailidis C, Hoijer J, Johansson M, et al. : Vitamin D status in well controlled Caucasian HIV patients in relation to inflammatory and metabolic markers—a cross sectional cohort study in Sweden. Scand J Immunol 2015;82(1), 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lake JE, Hoffman RM, Tseng CH, et al. : Success of standard dose vitamin D supplementation in treated HIV infection. Open Forum Infect Dis 2015;2(2), ofv068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Test Information for high sensitivity C-reactive protein. Available from Quest Diagnostics at www.questdiagnostics.com/testcenter/TestDetail.action?ntc=10124 Accessed May4, 2015
- 25.Test Information for D-dimer. Available from Quest Diagnostics at www.questdiagnostics.com/testcenter/TestDetail.action?ntc=8659 Accessed May4, 2015
- 26.Test Information for insulin. Available from Quest Diagnostics at www.questdiagnostics.com/testcenter/TestDetail.action?ntc=561 Accessed May4, 2015
- 27.Test Information for adiponectin. Available from Quest Diagnostics at www.questdiagnostics.com/testcenter/TestDetail.action?tabName=OrderingInfo&ntc=15060 Accessed May4, 2015
- 28.Test Information for leptin. Available from Quest Diagnostics at www.questdiagnostics.com/testcenter/TestDetail.action?ntc=90367 Accessed May4, 2014
- 29.HOMA Calculator. Available from Quest Diagnostics at www.dtu.ox.ac.uk/homacalculator Accessed May4, 2015
- 30.Villar-Garcia J, Hernandez JJ, Guerri-Fernandez R, et al. : Effect of probiotics (Saccharomyces boulardii) on microbial translocation and inflammation in HIV-treated patients: A double-blind, randomized, placebo-controlled trial. J Acquir Immune Defic Syndr 2015;68(3):256–263 [DOI] [PubMed] [Google Scholar]
- 31.Metkus TS, Timpone J, Leaf D, et al. : Omega-3 fatty acid therapy reduces triglycerides and interleukin-6 in hypertriglyceridemic HIV patients. HIV Med 2013;14(9):530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearson TA, Mensah GA, Alexander RW, et al. : Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003;107(3):499–511 [DOI] [PubMed] [Google Scholar]
- 33.Nagpal S, Na S, and Rathnachalam R: Noncalcemic actions of vitamin D receptor ligands. Endocr Rev 2005;26(5):662–687 [DOI] [PubMed] [Google Scholar]
- 34.Schleithoff SS, Zittermann A, Tenderich G, et al. : Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr 2006;83(4):754–759 [DOI] [PubMed] [Google Scholar]
- 35.Ross AC, Judd S, Kumari M, et al. : Vitamin D is linked to carotid intima-media thickness and immune reconstitution in HIV-positive individuals. Antivir Ther 2011;16(4):555–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford ES, Greenwald JH, Richterman AG, et al. : Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS 2010;24(10):1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duprez DA, Kuller LH, Tracy R, et al. : Lipoprotein particle subclasses, cardiovascular disease and HIV infection. Atherosclerosis 2009;207(2):524–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuller LH, Tracy R, Belloso W, et al. : Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erlandson KM, Allshouse AA, Jankowski CM, et al. : Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 2013;208(2):249–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown TT, Tassiopoulos K, Bosch RJ, et al. : Association between systemic inflammation and incident diabetes in HIV-infected patients after initiation of antiretroviral therapy. Diabetes Care 2010;33(10):2244–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson EM, Singh A, Hullsiek KH, et al. : Monocyte-activation phenotypes are associated with biomarkers of inflammation and coagulation in chronic HIV infection. J Infect Dis 2014;210(9):1396–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shepherd L, Souberbielle JC, Bastard JP, et al. : Prognostic value of vitamin D level for all-cause mortality, and association with inflammatory markers, in HIV-infected persons. J Infect Dis 2014;210(2):234–243 [DOI] [PubMed] [Google Scholar]
- 43.Adeyemi O, Hotton A, Aziz M, et al. : Vitamin D supplementation increases vitamin D levels but does not improve inflammatory markers in HIV-infected women: A Chicago Women's Interagency HIV Study (WIHS) study. Abstract 321. Presented as a poster at IDWeek, San Francisco, California October2–6, 2013 Available at https://idsa.confex.com/idsa/2013/webprogram/Paper41092.html Accessed May15, 2015 [Google Scholar]
- 44.Overton ET. and Yin MT: High dose vitamin D and calcium for bone health in individuals initiating HAART (AIDS Clinical Trials Group 5280), clinical trials identifier NCT01403051. Available at https://clinicaltrials.gov/ct2/show/NCT01403051 Accessed May25, 2015
- 45.Huang Y, Ishizuka T, Miura A, et al. : Effect of 1 alpha,25-dihydroxy vitamin D3 and vitamin E on insulin-induced glucose uptake in rat adipocytes. Diabetes Res Clin Pract 2002;55(3):175–183 [DOI] [PubMed] [Google Scholar]
- 46.Reusch JE, Begum N, Sussman KE, and Draznin B: Regulation of GLUT-4 phosphorylation by intracellular calcium in adipocytes. Endocrinology 1991;129(6):3269–3273 [DOI] [PubMed] [Google Scholar]
- 47.Maestro B, Davila N, Carranza MC, and Calle C: Identification of a vitamin D response element in the human insulin receptor gene promoter. J Steroid Biochem Mol Biol 2003;84(2–3):223–230 [DOI] [PubMed] [Google Scholar]
- 48.Mitri J, Dawson-Hughes B, Hu FB, and Pittas AG: Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: The Calcium and Vitamin D for Diabetes Mellitus (CaDDM) randomized controlled trial. Am J Clin Nutr 2011;94(2):486–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitri J, Muraru MD, and Pittas AG: Vitamin D and type 2 diabetes: A systematic review. Eur J Clin Nutr 2011;65(9):1005–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeitz U, Weber K, Soegiarto DW, et al. : Impaired insulin secretory capacity in mice lacking a functional vitamin D receptor. FASEB J 2003;17(3):509–511 [DOI] [PubMed] [Google Scholar]
- 51.Sun X. and Zemel MB: Calcium and 1,25-dihydroxyvitamin D3 regulation of adipokine expression. Obesity (Silver Spring) 2007;15(2):340–348 [DOI] [PubMed] [Google Scholar]
- 52.Choi KM, Ryu OH, Lee KW, et al. : Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract 2007;75(2):235–240 [DOI] [PubMed] [Google Scholar]
- 53.Ouchi N. and Walsh K: Adiponectin as an anti-inflammatory factor. Clin Chim Acta 2007;380(1–2):24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Engeli S, Feldpausch M, Gorzelniak K, et al. : Association between adiponectin and mediators of inflammation in obese women. Diabetes 2003;52(4):942–947 [DOI] [PubMed] [Google Scholar]
- 55.Mantzoros CS, Li T, Manson JE, et al. : Circulating adiponectin levels are associated with better glycemic control, more favorable lipid profile, and reduced inflammation in women with type 2 diabetes. J Clin Endocrinol Metab 2005;90(8):4542–4548 [DOI] [PubMed] [Google Scholar]
- 56.Berg AH. and Scherer PE: Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005;96(9):939–949 [DOI] [PubMed] [Google Scholar]
- 57.van den Bout-van den Beukel CJ, van den Bos M, Oyen WJ, et al. : The effect of cholecalciferol supplementation on vitamin D levels and insulin sensitivity is dose related in vitamin D-deficient HIV-1-infected patients. HIV Med 2008;9(9):771–779 [DOI] [PubMed] [Google Scholar]
- 58.Belenchia AM, Tosh AK, Hillman LS, and Peterson CA: Correcting vitamin D insufficiency improves insulin sensitivity in obese adolescents: A randomized controlled trial. Am J Clin Nutr 2013;97(4):774–781 [DOI] [PubMed] [Google Scholar]
- 59.Al-Sofiani ME, Jammah A, Racz M, et al. : Effect of vitamin D supplementation on glucose control and inflammatory response in type II diabetes: A double blind, randomized clinical trial. Int J Endocrinol Metab 2015;13(1):e22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bonakdaran S, Nejad AF, Abdol-Reza V, et al. : Impact of oral 1,25-dihydroxy vitamin D (calcitriol) replacement therapy on coronary artery risk factors in type 2 diabetic patients. Endocr Metab Immune Disord Drug Targets 2013;13(4):295–300 [DOI] [PubMed] [Google Scholar]