Abstract
Purpose: The goals of the current study were to determine the in vitro antibacterial activity of tigecycline against multiple clinically relevant ocular pathogens and to evaluate the in vivo ocular tolerability and efficacy of 0.5% tigecycline in a methicillin-resistant Staphylococcus aureus (MRSA) keratitis model.
Methods: In vitro: Minimum inhibitory concentrations (MICs) were determined for 110 clinical conjunctivitis isolates, 26 keratitis isolates of Pseudomonas aeruginosa, and 10 endophthalmitis isolates each of MRSA, methicillin-susceptible S. aureus (MSSA), MR, and MS coagulase-negative Staphylococcus. Tolerability: Six uninfected rabbits were topically treated in both eyes with 0.5% tigecycline, vehicle, or saline every 15 min for 3 h. Efficacy: Thirty-two rabbits were intrastromally injected with 700 Colony Forming Units (CFU) of MRSA in both eyes and were separated into 4 groups (n = 8): tigecycline 0.5%; vancomycin 5%; saline; and no treatment (euthanized before treatment for baseline CFU). Four hours after MRSA challenge, topical treatment of 1 drop every 15 min for 5 h was initiated. One hour after treatment, the corneas were harvested for CFU. The data were analyzed nonparametrically.
Results: In vitro: Tigecycline demonstrated lower MICs than the other tested antibiotics against gram-positive organisms, especially MRSA, while MICs against gram-negative pathogens, including fluoroquinolone-resistant P. aeruginosa, appeared to be in the treatable range with aggressive topical therapy. Tolerability: 0.5% tigecycline was graded as minimally irritating. Efficacy: 0.5% tigecycline and vancomycin produced similar reductions in CFU and were less than saline (P < 0.05). Tigecycline and vancomycin demonstrated 99.9% reductions compared with baseline CFU.
Conclusions: Tigecycline is a potential candidate for a topical ocular antibiotic.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) ocular infections have been reported to be on the rise.1,2 The ophthalmic fluoroquinolones are frequently used as monotherapy for the empiric treatment of less than severe keratitis. However, fluoroquinolone monotherapy may not be optimal for MRSA keratitis because of the cross-resistance among the ophthalmic fluoroquinolones and of the methicillin resistance among ocular isolates of S. aureus, potentially making them ineffective for the treatment of MRSA keratitis.2 Currently, fortified vancomycin is a first-line antibiotic for the treatment of MRSA keratitis. As a fortified antibiotic, vancomycin must be compounded by a specialty pharmacy. The availability of a commercial antibiotic to treat MRSA ocular infections would be a beneficial addition to the ophthalmic antibiotic armamentarium.
Tigecycline is the first in a new class of glycylcycline antibiotics that is Food and Drug Administration (FDA) approved for the treatment of skin and soft tissue infections and intra-abdominal infections.3 Glycylcyclines are derivatives of tetracyclines and, as a class, are considered bacteriostatic. They act by binding to the 30S ribosomal subunit and blocking aminoacyl tRNA entry into the ribosome.3 As a result, amino acids are prevented from being incorporated into the peptide chains, thus halting protein synthesis.3 Specifically, tigecycline is a derivative of minocycline.4
Previously, tigecycline has been shown to have excellent in vitro activity against MRSA.5–7 Furthermore, there does not appear to be an increase in minimum inhibitory concentrations (MICs) over time,7 suggesting that there is little emerging resistance of MRSA to tigecycline. In vitro studies have also demonstrated that tigecycline is active against a number of other gram-positive and gram-negative pathogens. However, higher MICs against Pseudomonas aeruginosa preclude its use as treatment of systemic P. aeruginosa infections.3
A topical ophthalmic formulation of tigecycline [RPX-978; 0.5% tigecycline preserved with 0.005% benzalkonium chloride (BAK)] is in preclinical commercial development. The goals of the current study were to confirm the in vitro activity of tigecycline against multiple clinically relevant ocular pathogens; to evaluate the tolerability of topically instilled RPX-978 into uninfected rabbit eyes; and to determine the antibacterial efficacy of RPX-978 and the ability of antibacterial tigecycline contained in RPX-978 to penetrate the corneal epithelium in an NZW rabbit model of experimental MRSA keratitis.
Methods
In vitro antibacterial activity of tigecycline
Antibacterial agents
Tigecycline (Haorui Pharma-Chem, Inc., Irvine, CA) and besifloxacin (SPR01978b; Sequoia Research Products, Berkshire, UK) were supplied by Rempex Pharmaceuticals, San Diego, CA. Gatifloxacin (G0278), moxifloxacin (M5794), ciprofloxacin (C3262), and azithromycin (A9834) were purchased from LKT Laboratories, Inc., Saint Paul, MN. Tobramycin (T4014) was purchased from Sigma-Aldrich Co., Saint Louis, MO.
Bacterial isolates
All bacterial strains were isolated from patients with ocular disease at the Charles T. Campbell Ophthalmic Microbiology Laboratory at the University of Pittsburgh Medical Center (UPMC) Eye Center, University of Pittsburgh, Pittsburgh, PA. The isolates were retrieved from a frozen −80°C retrospective clinical collection that was deidentified and stored for antibiotic validations. One hundred ten clinical conjunctivitis isolates based on incidence at the Campbell Laboratory were used in this study. These included S. aureus (n = 36); coagulase-negative Staphylococcus (CNS) (14); Streptococcus pneumoniae (22); other gram-positive bacteria (8) (2 Streptococcus viridans group and 6 beta-hemolytic Streptococcus species); Haemophilus species (20); and other gram-negative bacteria (10) (2 Serratia marcescens, 2 Proteus mirabilis, 3 P. aeruginosa, 1 Enterobacter aerogenes, 1 Pseudomonas fluorescens, and 1 Klebsiella species).
In addition, 26 keratitis isolates of P. aeruginosa and 10 endophthalmitis isolates each of MRSA, methicillin-susceptible S. aureus (MSSA), methicillin-resistant coagulase-negative Staphylococcus (MRCNS), and methicillin-susceptible coagulase-negative Staphylococcus (MSCNS) were also tested. In all, a total of 176 isolates were tested for MIC determinations.
MIC testing
MICs were determined for the ocular bacterial isolates using the recommended Clinical and Laboratory Standards Institute (CLSI) protocol for broth dilution.8 All antibacterials were tested with eleven 2-fold dilutions from 32 to 0.03125 μg/mL. All testing was performed in fresh Mueller-Hinton medium (Remel Products, Lenexa, KS) except for Streptococcus species (3% lysed horse red blood cells were added to the Mueller-Hinton medium) and Haemophilus species [fresh Haemophilus Test Medium (Remel)].
After 24 h of incubation, all plates were examined for positive growth in comparison with the control. The lowest antibacterial dilution that demonstrated no growth was deemed the MIC for that specific isolate. The data were placed in a Minitab file for MIC50, MIC90, and range calculations.
Ocular tolerability testing
Animals
Three to 4 pound female NZW rabbits were purchased from Covance Research Products, Denver, PA. These studies conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) approval (IACUC Protocol #1008951) was obtained.
Test agents
RPX-978 (tigecycline 0.5% solution preserved with 0.005% BAK), RPX-978 vehicle preserved with 0.005% BAK, and pharmaceutical grade saline were provided by Rempex Pharmaceuticals. The test articles were stored at 4°C until use. Thirty-seven microliter drops were instilled using a Rainin EDP1 electronic pipette (Mettler Toledo, Columbus, OH) set in the multidispense mode.
Tolerability testing
Six NZW rabbits were divided into 3 groups (RPX-978, RPX-978 vehicle, and saline) of 2 rabbits each. The rabbits were topically treated in both eyes with 1 drop every 15 min for 3 h (13 total doses). Thirty minutes after the final dose, the rabbits were evaluated in a masked manner for ocular toxicity by a board-certified ophthalmologist (F.S.M.) with specialty training in corneal and external disease. Ocular toxicity was also evaluated 2 days post-treatment for any delayed toxicity. The eyes were evaluated using the Draize scoring system.9 The maximum mean total score (MMTS) per group was calculated and categorized as previously described.10
In vivo antibacterial efficacy testing in the MRSA rabbit keratitis model
Animals
Three to 4 pound female NZW rabbits were purchased from Harlan, Indianapolis, IN. These studies also conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. IACUC approval was the same as the tolerability study.
Bacterial isolate
The S. aureus strain used in this study (isolate K950) was isolated from a patient with bacterial keratitis at the Charles T. Campbell Ophthalmic Microbiology Laboratory at the UPMC Eye Center, University of Pittsburgh, Pittsburgh, PA. The isolate was retrieved from a frozen −80°C retrospective clinical collection that was deidentified and stored for antibiotic validations. This S. aureus isolate was found to be resistant to gatifloxacin, moxifloxacin, and oxacillin by disk diffusion susceptibility testing. Therefore, the isolate was designated as fluoroquinolone resistant as well as methicillin resistant.
Test agents
RPX-978 and pharmaceutical grade saline were provided by Rempex Pharmaceuticals. Thirty-seven microliter drops were instilled using a Rainin EDP electronic pipette as described previously. Vancomycin (50 mg/mL) was purchased from the UPMC pharmacy as the fortified eye drop preparation used in patients. The vancomycin was administered using its supplied dropper bottle, delivering drops of undetermined size that would be used in the clinical situation. All test agents were stored at 4°C until use.
MRSA rabbit keratitis model
The MRSA rabbit keratitis model used in this study is a modification of the model used in previous studies.11–13 A total of 32 rabbits were used in duplicate trials comprising 16 rabbits each. The 32 rabbits were divided into 4 groups of 8 rabbits. Each group was then subdivided into 2 groups: (1) intact corneal epithelium and (2) abraded corneal epithelium. Using this methodology, we can determine whether tigecycline is effective in vivo and whether the corneal epithelium acts as a barrier for penetration of the drugs into the deeper portions of the cornea.
Briefly, a 6 mm area of the corneal epithelium was removed centrally with an Amoils epithelial scrubber in the left eye. The corneal epithelium in the right eye remained intact. Immediately afterward, both corneas of each rabbit were injected intrastromally with 645 (Trial 1) or 716 (Trial 2) CFU of the MRSA in 25 μL of tryptic soy broth following systemic anesthesia with a combination of 40 mg/kg of ketamine and 4 mg/kg of xylazine and topical anesthesia with 2 drops of 0.5% proparacaine.
Four hours after MRSA challenge, the rabbits were divided into 4 groups (n = 8): (1) RPX-978 (0.5% tigecycline), (2) vancomycin 50 mg/mL (used as a standard of therapy antibacterial efficacy control for MRSA keratitis and secondarily as a positive toxicity control), (3) saline, and (4) no treatment (euthanized before treatment for baseline CFU), and the topical treatment regimen of 1 drop every 15 min for 5 h (21 total doses) in both eyes was initiated. The no treatment group of rabbits was euthanized at 4 h post-MRSA challenge and the corneas harvested for baseline colony count determinations at the onset of therapy.
After treatment, the eyes were examined for signs of clinical disease by an ophthalmologist (F.S.M.) with corneal and external disease training. Eyes were evaluated for conjunctival redness, conjunctival chemosis, discharge, iritis, corneal edema, and corneal infiltrate using a 0–3 severity scoring system.
One hour after the final dose (10 h after MRSA challenge), the rabbits from the RPX-978, vancomycin, and saline groups were euthanized and colony count determinations performed on each cornea; 9.5 mm buttons were removed from the area of the corneas, which contained the injection sites. The buttons were individually placed into tubes containing ice-cold phosphate-buffered saline (PBS). The corneal buttons were homogenized on ice using a motorized homogenizer (Pro Scientific, Oxford, CT) for 25 s. The homogenates were serially diluted in PBS and 0.1 mL of the dilutions was plated in duplicate onto trypticase soy agar containing 5% sheep's blood plates [BD (Becton, Dickinson and Company) Franklin Lakes, NJ]. The plates were incubated at 37°C overnight, at which time the colonies were counted.
Data analysis
The total ocular score (TOS) for each group was calculated by adding the scores of the 6 graded parameters. The data were analyzed nonparametrically using the Kruskal–Wallis ANOVA with Duncan's multiple comparisons method (True Epistat, Mesquite, TX).
The corneal colony counts +1 from each group were log transformed to the base 10. These data were also analyzed nonparametrically with the Kruskal–Wallis ANOVA with Duncan's multiple comparisons method. A bactericidal effect (99.9% or 3 Log10 decrease in colony counts) was assessed by comparing the final median colony count after treatment with the median colony count at the commencement of treatment.
Results
In vitro antibacterial activity of tigecycline
The results of the MIC testing are presented in Tables 1–3. Table 1 presents the data from the conjunctivitis isolates. Table 2 presents the data from the P. aeruginosa keratitis isolates, while Table 3 presents the data from the endophthalmitis isolates.
Table 1.
Antibiotic MIC Range, MIC50, and MIC90 (μg/mL) of Conjunctivitis Isolates Based on Incidence at the Campbell Laboratory
| Organism | Number | Drug | Range | MIC50 | MIC90 |
|---|---|---|---|---|---|
| Staphylococcus aureus | 36 | Tigecycline | 0.125 to 0.5 | 0.25 | 0.5 |
| Azithromycin | 0.5 to >32.0 | 8.0 | >32.0 | ||
| Besifloxacin | 0.03 to 2.0 | 0.06 | 1.0 | ||
| Gatifloxacin | <0.03 to 32.0 | 0.125 | 8.0 | ||
| Moxifloxacin | <0.03 to 8.0 | 0.06 | 4.0 | ||
| Ciprofloxacin | 0.125 to >32.0 | 0.5 | >32.0 | ||
| Tobramycin | 0.06 to >32.0 | 1.0 | >32.0 | ||
| Coagulase-negative Staphylococcus | 14 | Tigecycline | 0.05 to 1.0 | 0.25 | 0.5 |
| Azithromycin | 0.25 to >32.0 | >32.0 | >32.0 | ||
| Besifloxacin | <0.03 to 8.0 | 0.5 | 4.0 | ||
| Gatifloxacin | <0.03 to >32.0 | 4.0 | >32.0 | ||
| Moxifloxacin | <0.03 to >32.0 | 2.0 | 32.0 | ||
| Ciprofloxacin | 0.25 to >32.0 | >32.0 | >32.0 | ||
| Tobramycin | 0.25 to >32.0 | 16.0 | >32.0 | ||
| Streptococcus pneumoniae | 22 | Tigecycline | <0.03 to 0.25 | <0.03 | 0.125 |
| Azithromycin | 0.125 to >32.0 | 0.25 | >32.0 | ||
| Besifloxacin | <0.03 to 0.125 | 0.06 | 0.06 | ||
| Gatifloxacin | 0.06 to 0.5 | 0.125 | 0.125 | ||
| Moxifloxacin | 0.06 to 0.25 | 0.06 | 0.25 | ||
| Ciprofloxacin | 0.25 to 2.0 | 0.25 | 1.0 | ||
| Tobramycin | 8.0 to 32.0 | 16.0 | 16.0 | ||
| Other gram positives | 8 | Tigecycline | <0.03 to 1.0 | 0.125 | 0.5 |
| Azithromycin | 0.25 to >32.0 | 0.5 | 4.0 | ||
| Besifloxacin | 0.06 to 0.125 | 0.125 | 0.125 | ||
| Gatifloxacin | 0.25 to 0.5 | 0.25 | 0.5 | ||
| Moxifloxacin | 0.125 to 0.5 | 0.125 | 0.25 | ||
| Ciprofloxacin | 0.25 to 2.0 | 1.0 | 1.0 | ||
| Tobramycin | 16.0 to >32.0 | 16.0 | >32.0 | ||
| Haemophilus sp. | 20 | Tigecycline | 2.0 to 4.0 | 2.0 | 4.0 |
| Azithromycin | 0.25 to 8.0 | 2.0 | 4.0 | ||
| Besifloxacin | <0.03 to <0.03 | <0.03 | <0.03 | ||
| Gatifloxacin | <0.03 to 0.25 | <0.03 | <0.03 | ||
| Moxifloxacin | <0.03 to 0.5 | <0.03 | 0.125 | ||
| Ciprofloxacin | <0.03 to 2.0 | <0.03 | <0.03 | ||
| Tobramycin | 2.0 to 8.0 | 2.0 | 4.0 | ||
| Other gram negatives | 10 | Tigecycline | 0.5 to 8.0 | 1.0 | 4.0 |
| Azithromycin | 8.0 to >32.0 | 32.0 | >32.0 | ||
| Besifloxacin | 0.125 to 2.0 | 0.5 | 1.0 | ||
| Gatifloxacin | <0.03 to 2.0 | 0.125 | 0.5 | ||
| Moxifloxacin | 0.06 to 8.0 | 0.5 | 1.0 | ||
| Ciprofloxacin | <0.03 to 0.5 | <0.03 | 0.5 | ||
| Tobramycin | 0.5 to 4.0 | 0.5 | 4.0 |
MIC, minimum inhibitory concentration.
Table 2.
Antibiotic MIC Range, MIC50, and MIC90 (μg/mL) of Pseudomonas aeruginosa Keratitis Isolates
| Organism | Number | Drug | Range | MIC50 | MIC90 |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa | 26 | Tigecycline | 2.0 to 32.0 | 4.0 | 8.0 |
| Azithromycin | >32 to >32.0 | >32.0 | >32.0 | ||
| Besifloxacin | 0.5 to 32.0 | 1.0 | 32.0 | ||
| Gatifloxacin | 0.25 to >32.0 | 1.0 | 32.0 | ||
| Moxifloxacin | 0.25 to >32.0 | 1.0 | >32.0 | ||
| Ciprofloxacin | 0.06 to >32.0 | 0.125 | 32.0 | ||
| Tobramycin | 0.125 to 32.0 | 0.25 | 2.0 |
Table 3.
Antibiotic MIC Range, MIC50, and MIC90 (μg/mL) of Endophthalmitis Isolates
| Organism | Number | Drug | Range | MIC50 | MIC90 |
|---|---|---|---|---|---|
| Methicillin-resistant Staphylococcus aureus | 10 | Tigecycline | 0.25 to 0.5 | 0.5 | 0.5 |
| Azithromycin | >32.0 to >32.0 | >32.0 | >32.0 | ||
| Besifloxacin | 0.5 to 2.0 | 1.0 | 2.0 | ||
| Gatifloxacin | 2.0 to >32.0 | 4.0 | 32.0 | ||
| Moxifloxacin | 2.0 to 16.0 | 4.0 | 16.0 | ||
| Ciprofloxacin | 8.0 to >32.0 | >32.0 | >32.0 | ||
| Tobramycin | 0.5 to >32.0 | >32.0 | >32.0 | ||
| Methicillin-susceptible Staphylococcus aureus | 10 | Tigecycline | 0.125 to 8.0 | 0.125 | 0.5 |
| Azithromycin | 1.0 to >32.0 | >32.0 | >32.0 | ||
| Besifloxacin | 0.06 to 8.0 | 0.5 | 1.0 | ||
| Gatifloxacin | 0.125 to 8.0 | 2.0 | 8.0 | ||
| Moxifloxacin | 0.06 to 16.0 | 2.0 | 8.0 | ||
| Ciprofloxacin | 0.5 to >32.0 | 8.0 | >32.0 | ||
| Tobramycin | 0.5 to >32.0 | >32.0 | >32.0 | ||
| Methicillin-resistant coagulase-negative Staphylococcus | 10 | Tigecycline | 0.125 to 0.25 | 0.25 | 0.25 |
| Azithromycin | 0.5 to >32.0 | 0.5 | >32.0 | ||
| Besifloxacin | 0.06 to 8.0 | 0.5 | 8.0 | ||
| Gatifloxacin | 0.125 to >32.0 | 2.0 | >32.0 | ||
| Moxifloxacin | 0.125 to >32.0 | 2.0 | 32.0 | ||
| Ciprofloxacin | 0.25 to >32.0 | >32.0 | >32.0 | ||
| Tobramycin | 0.06 to >32.0 | 0.06 | 0.125 | ||
| Methicillin-susceptible coagulase-negative Staphylococcus | 10 | Tigecycline | 0.125 to 0.5 | 0.25 | 0.5 |
| Azithromycin | 0.25 to >32.0 | 16.0 | >32.0 | ||
| Besifloxacin | <0.03 to 4.0 | 0.25 | 4.0 | ||
| Gatifloxacin | 0.125 to >32.0 | 0.25 | >32.0 | ||
| Moxifloxacin | 0.06 to 32.0 | 0.06 | 16 | ||
| Ciprofloxacin | 0.125 to >32.0 | 8.0 | >32.0 | ||
| Tobramycin | 0.06 to 16.0 | 0.25 | 16.0 |
Tigecycline demonstrated potent in vitro activity against the gram-positive strains among the conjunctivitis isolates (S. aureus, coagulase-negative Staphylococcus, S. pneumoniae, S. viridans group, and beta-hemolytic Streptococcus species) with MIC90s of 0.5 μg/mL or less (Table 1). These MIC90s were less than the comparator antibiotics for all strains except for those of the S. viridans group and beta-hemolytic Streptococcus species, for which moxifloxacin and besifloxacin had lower MIC90s, and for S. pneumoniae, for which besifloxacin had a lower MIC90.
Tigecycline demonstrated MIC90s of 4.0 μg/mL against the gram-negative conjunctivitis isolates of Haemophilus species and the other gram negatives tested (2 S. marcescens, 2 P. mirabilis, 3 P. aeruginosa, 1 E. aerogenes, 1 P. fluorescens, and 1 Klebsiella species) (Table 2). These values were comparable with azithromycin and tobramycin against Haemophilus species and tobramycin against the other gram negatives. Tigecycline had higher MIC90s than the fluoroquinolones tested against Haemophilus species and the other gram negatives.
Tigecycline produced an MIC90 of 8.0 μg/mL for the P. aeruginosa isolates tested. This MIC90 was lower than all of the comparator antimicrobial agents except tobramycin, a drug of choice to treat P. aeruginosa. Tigecycline demonstrated MIC90s of 0.5 μg/mL or less against the gram-positive endophthalmitis isolates (MRSA, MSSA, MRCNS, MSCNS) (Table 3). These MIC90s were lower than all of the comparator antibacterial agents.
Ocular tolerability testing
Both eyes of 2 rabbits were treated with 1 drop of RPX-978 (0.5% tigecycline solution preserved with 0.005% BAK), vehicle preserved with 0.005% BAK, and pharmaceutical grade saline every 15 min for 3 h (13 total doses). Ocular examination using the Draize scoring scale 30 min following the final dose revealed that 0.5% tigecycline produced an MMTS of 8.0 (of a 110-point scale), which was categorized as minimally irritating.10 Both the vehicle and saline controls produced an MMTS of 1.0, which was categorized as practically nonirritating. This demonstrates that the vehicle and preservative of the RPX-978 formulation had the same ocular toxicity as the saline negative toxicity control.
The most prevalent manifestation demonstrated with RPX-978 was ocular discharge with minimal conjunctival redness and chemosis in all eyes. No corneal manifestations were seen. Overall, the acute toxicity demonstrated with RPX-978 after this aggressive dosing was deemed acceptable.
The examination of the eyes 2 days after treatment revealed no delayed toxicity with RPX-978. These eyes demonstrated the same MMTS score of 0.5 as the vehicle and saline controls, which was categorized as nonirritating.
MRSA rabbit keratitis model
Clinical evaluation
The results of the clinical evaluations of eyes with abraded corneal epithelium demonstrated that eyes treated with RPX-978 (0.5% tigecycline) had a significantly lower TOS (median TOS = 5.5) compared with eyes treated with the saline control (median TOS = 6.5) (P ≤ 0.05). Both the RPX-978- and saline-treated eyes demonstrated a significantly lower TOS than 50 mg/mL vancomycin-treated eyes (median TOS = 8.0) (P ≤ 0.05).
The same was true for eyes with intact corneal epithelium. Eyes treated with RPX-978 had a significantly lower TOS (median TOS = 4.5) compared with eyes treated with the saline control (median TOS = 6.0) (P ≤ 0.05). RPX-978- and saline-treated eyes also demonstrated significantly lower TOS compared with vancomycin-treated eyes (median TOS = 8.25) (P ≤ 0.05).
Microbiological evaluation
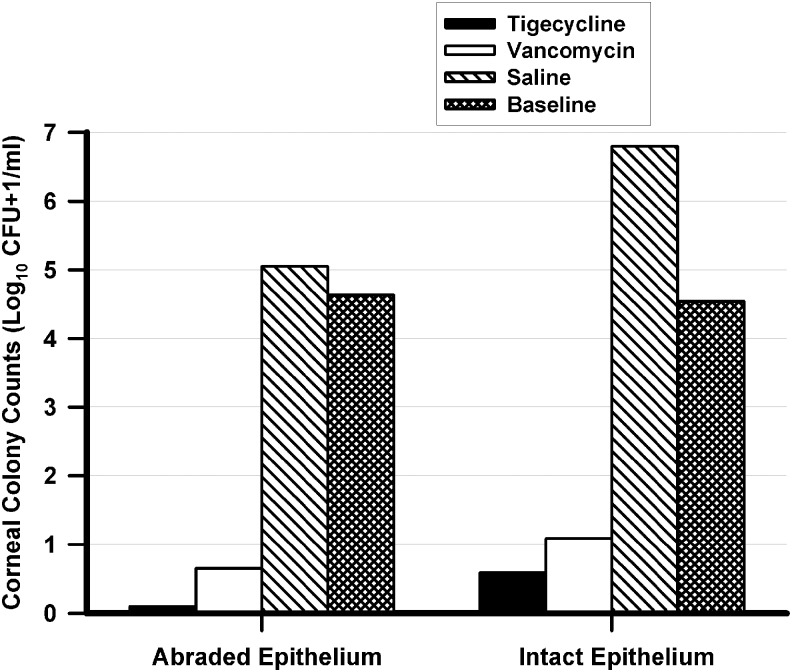
The results of the microbiological evaluations of eyes with abraded corneal epithelium are presented in Fig. 1. Eyes treated with RPX-978 (0.5% tigecycline) [median Log10 (CFU+1)/mL = 0.0; range of colony counts 0.0–1.7 Log10 (CFU+1)/mL] and 50 mg/mL vancomycin [median Log10 (CFU+1)/mL = 0.7; range of colony counts 0.0–2.0 Log10 (CFU+1)/mL] had significantly fewer MRSA colony counts in their corneas compared with eyes treated with the saline control [median Log10 (CFU+1)/mL = 5.1; range of colony counts 4.0–5.9 Log10 (CFU+1)/mL] (P ≤ 0.05). There was no significant difference in MRSA colony counts in abraded corneas treated with RPX-978 [median Log10 (CFU+1)/mL = 0.0] and vancomycin [median Log10 (CFU+1)/mL = 0.7] (P > 0.05). This analysis may indicate no significant differences, but this may be based on numbers of eyes per group that do not provide a high power of analysis. Both RPX-978 and vancomycin produced >3 Log10 decreases in median colony counts compared with the no treatment baseline control (median Log10 [CFU+1]/mL = 4.6; range of colony counts 4.3–4.9 Log10 [CFU+1]/mL).
FIG. 1.
Corneal MRSA colony counts after treatment. This figure presents the median number of MRSA corneal colony counts [Log10 (Colony Forming Units (CFU) + 1)/mL] for each treatment group (n = 8 per group) in eyes with abraded and intact corneal epithelium. Both 0.5% tigecycline and 50 mg/mL vancomycin demonstrated significantly fewer MRSA corneal colony counts compared with the saline controls (P ≤ 0.05 KW) in eyes both with abraded and intact corneal epithelium. Tigecycline and vancomycin produced >3 Log10 (>99.9%) bactericidal decreases in colony counts in eyes with both abraded and intact corneal epithelium compared with the baseline no treatment controls (baseline). MRSA, methicillin-resistant Staphylococcus aureus.
The results of the microbiological evaluations of eyes with intact corneal epithelium are also presented in Fig. 1. Similar to the eyes with abraded epithelium, eyes with intact corneal epithelium that were treated with RPX-978 (median Log10 [CFU+1]/mL = 0.6; range of colony counts 0.0–2.4 Log10 [CFU+1]/mL) and vancomycin (median Log10 [CFU+1]/mL = 1.1; range of colony counts 0.0–2.2 Log10 [CFU+1]/mL) had significantly fewer MRSA colony counts in their corneas compared with eyes treated with the saline control (median Log10 [CFU+1]/mL = 6.8; range of colony counts 4.8–7.1 Log10 [CFU+1]/mL) (P ≤ 0.05). Again, there was no significant difference in MRSA colony counts in intact corneas treated with RPX-978 and vancomycin (P > 0.05). As previously stated, this analysis may indicate no significant differences, but this may be based on numbers of eyes per group that do not provide a high power of analysis. As with eyes with abraded corneal epithelium, RPX-978 and vancomycin produced >3 Log10 decreases in median colony counts compared with the no treatment baseline control [median Log10 (CFU+1)/mL = 4.6; range of colony counts 4.0–4.7 Log10 (CFU+1)/mL] in eyes with intact corneal epithelium.
There were no significant differences in MRSA colony counts between corneas with intact or abraded corneal epithelium for both RPX-978 and vancomycin (P > 0.05). This analysis may indicate no significant differences, but this may be based on numbers of eyes per group that do not provide a high power of analysis.
Discussion
In the current study, we took a 3-pronged approach for the initial evaluation of tigecycline as a potential ocular antibacterial. We evaluated the in vitro antibacterial activity of tigecycline and a number of comparator ophthalmic antibiotics against a variety of bacterial pathogens isolated from conjunctivitis, keratitis, and endophthalmitis patients. We then evaluated the ocular tolerability of topical RPX-978, a formulation of 0.5% tigecycline that is in commercial development. Last, we evaluated the antibacterial efficacy of topical RPX-978 and the ability of tigecycline to penetrate the corneal epithelium in an MRSA rabbit keratitis model.
We started the in vitro evaluation with bacteria that cause conjunctivitis. We used a different approach for choosing the isolates to be tested. Instead of choosing a set number of isolates of the most common conjunctivitis pathogens (S. aureus, S. pneumoniae, Haemophilus species), we chose the isolates based on the incidence of bacterial species seen at the Charles T. Campbell Ophthalmic Microbiology Laboratory at the UPMC. This way, we could look at a real-world scenario of how tigecycline may fair in the treatment of common bacterial conjunctivitis. For the gram-positive conjunctivitis isolates, tigecycline produced MIC90s of 0.5 μg/mL or less. The tigecycline MIC90s were less than the comparator antibiotics for all strains except for those of the S. viridans group and beta-hemolytic Streptococcus species, for which moxifloxacin and besifloxacin had lower MIC90s, and for S. pneumoniae, for which besifloxacin had a lower MIC90. Tigecycline produced an MIC90 of 0.5 μg/mL for all the S. aureus conjunctivitis isolates tested, which included both MRSA and MSSA strains.
Tigecycline demonstrated MIC90s of 4.0 μg/mL against Haemophilus sp. and the other gram-negative conjunctivitis isolates, although these MIC90s were higher than those of the fluoroquinolones. Overall, these data suggest that tigecycline could possibly be an effective antibiotic for the treatment of bacterial conjunctivitis.
It has been well established in the systemic literature that tigecycline produces higher MICs against P. aeruginosa for which it is considered to be ineffective systemically.3,4,14–17 However, with aggressive topical therapy, it is presumed that higher concentrations of tigecycline would be produced locally in ocular tissue than would be produced in the serum from systemic treatment.
An MIC90 of 8.0 μg/mL was determined for tigecycline for the P. aeruginosa isolates tested. While this MIC would not be achieved in the serum after systemic therapy, it is conceivable that this concentration could be reached in the eye following topical therapy. This MIC90 was lower than all of the comparator antibiotics except tobramycin, a drug of choice to treat P. aeruginosa. Among the keratitis isolates were a number of fluoroquinolone-resistant P. aeruginosa isolates for which tigecycline demonstrated lower MIC90s than the fluoroquinolones tested. With aggressive topical therapy, tigecycline could be a possible treatment for fluoroquinolone-resistant P. aeruginosa infections. We previously demonstrated that aggressive topical therapy with an antibiotic can overcome high in vitro MICs to successfully treat resistant S. aureus and P. aeruginosa infections in rabbit keratitis models.11,12
Tigecycline also demonstrated MIC90s of 0.5 μg/mL or lower against the gram-positive endophthalmitis isolates (MRSA, MSSA, MRCNS, MSCNS). These results support the activity against S. aureus, in particular MRSA, and suggest that tigecycline could be used as a topical antibiotic for surgical prophylaxis.
These data provide evidence of possible broad-spectrum coverage of gram-positive and gram-negative pathogens that can infect the eye. Tigecycline demonstrated coverage of conjunctivitis isolates with incidence as seen in our clinical laboratory as well as pathogens that commonly cause endophthalmitis. Based on these results, tigecycline possesses the pathogen coverage of a viable topical antibiotic for ocular use.
The next step in our evaluation of tigecycline was to test the ocular tolerability of the topical formulation of 0.5% tigecycline, RPX-978. Using a more aggressive treatment regimen than would be used for conjunctivitis, prophylaxis, and even keratitis, we demonstrated that RPX-978 was minimally irritating according to the Kay interpretation of Draize testing.9,10 This minimal irritation was manifested by minimal conjunctival redness and chemosis and moderate ocular discharge. No corneal adverse effects were demonstrated. The rabbits showed no adverse behavior after instillation. From these results, we can conclude that RPX-978 is tolerable to rabbit eyes. These results were confirmed in the clinical evaluation of the efficacy study.
We next tested the efficacy of RPX-978 in a previously described MRSA rabbit model.10–12 However, we modified the model to evaluate the penetration of tigecycline through the corneal epithelium. Removal of the corneal epithelium in 1 eye can provide information on whether tigecycline penetrates the corneal epithelium to the site of infection in the corneal stroma. For example, equivalent bacterial counts in eyes with abraded and intact corneal epithelium would suggest penetration of tigecycline through the corneal epithelium, while significantly more colony counts in the eyes with intact corneal epithelium compared with eyes with abraded corneal epithelium would suggest a lack of penetration of tigecycline through the corneal epithelium.
The clinical evaluation of the eyes after the cessation of treatment showed that RPX-978 reduced the TOS compared with saline, which in turn had a significantly lower TOS than vancomycin. These results demonstrate that tigecycline is effective in preventing the formation of signs of bacterial keratitis by limiting the bacterial infection. More importantly, these results show that 0.5% tigecycline is less toxic than fortified 50 mg/mL vancomycin, which produced toxicity that caused a significant increase in TOS compared with the saline control.
The microbiological results from the studies in the MRSA rabbit model demonstrated that RPX-978 had efficacy not distinguishable from fortified vancomycin in eyes with intact and abraded corneas. Both produced >99.9% decreases in colony counts compared with the baseline no treatment control in intact and abraded corneas. Therefore, tigecycline and vancomycin were both considered bactericidal in this model regardless of whether the corneal epithelium was intact or abraded.
This is a surprising finding for tigecycline since the glycylcyclines, as a class, are considered bacteriostatic.3 There are some reports that tigecycline is bactericidal against S. pneumoniae, but none were found for S. aureus.4 A true bacteriostatic agent should produce no increases in corneal colony counts above the no treatment baseline. This result was reproducible as tigecycline demonstrated bactericidal decreases in both trials. Perhaps the frequent dosing produced a sufficiently high concentration of drug in the cornea that increased killing of the bacteria. Additional studies must be performed to further investigate the mechanisms involved in producing the bactericidal effect of tigecycline against MRSA in this rabbit model.
Comparing the MRSA colony counts in eyes treated with tigecycline or vancomycin revealed no significant difference in MRSA colony counts between eyes with intact corneal epithelium and abraded epithelium for either drug. These analyses may have indicated no significant differences, but these may be based on numbers of eyes per group that do not provide a high power of analysis. Nevertheless, these results suggest good penetration of tigecycline and vancomycin through the corneal epithelium to the corneal stroma where the infection resides in the keratitis model used. These data provide evidence that tigecycline may have the ability to treat infections deep in the stroma, without having to remove the corneal epithelium.
The current study differs from a previous study, in which 1% and 5% tigecycline were evaluated in a different MRSA keratitis model.18 Goktas et al. used a model of MRSA keratitis, in which treatment was initiated at 16 h postinoculation18 rather than at 4 h postinoculation in the current study. Although there were significant decreases in MRSA corneal colony counts, the decreases compared with the negative control were 1.15 Log10 or less for 1% and 5% tigecycline, respectively.18 The previously published model is not the optimal model to evaluate the ocular efficacy of topical antibacterial agents, as is the current model, in which 0.5% tigecycline demonstrated bactericidal decrease compared with a baseline control and >6 Log10 decrease in colony counts compared with the negative control. Furthermore, the previous study did not evaluate the decrease in colony counts compared with the baseline number of bacteria present in the corneas at the onset of therapy, nor the ability of tigecycline to penetrate through the corneal epithelium. Finally, the previous study evaluated higher concentrations of tigecycline (1% and 5%) prepared from the systemic version of the medication rather than a lower concentration (0.5%) in a formulation that is in commercial development as an ocular antibiotic. Therefore, the current study provides valuable new information on the antibacterial efficacy of an ocular formulation of tigecycline in commercial development.
In conclusion, the results of this study demonstrated that tigecycline possesses broad-spectrum in vitro activity against a variety of ocular pathogens. Tigecycline demonstrated lower MICs against gram-positive organisms, especially MRSA, while MICs against gram-negative pathogens, including fluoroquinolone-resistant P. aeruginosa, appeared to be in the treatable range with aggressive topical therapy. The in vitro activity, especially against gram-positive bacteria, was comparable or better than the comparator antibiotics, including the fourth-generation fluoroquinolones tested, based on MICs.
In vivo, RPX-978 (0.5% tigecycline) proved to be minimally irritating and as efficacious, but less toxic, than 50 mg/mL fortified vancomycin in an MRSA rabbit keratitis model. It was also shown that the tigecycline effectively penetrates the corneal epithelium. This has important implications for treatment and prophylaxis. Further studies evaluating the efficacy of RPX-978 in rabbit keratitis models of various other gram-positive and gram-negative pathogens are indicated.
Acknowledgments
Support for this study was provided by Rempex Pharmaceuticals, San Diego, CA, through a research agreement with the University of Pittsburgh, Pittsburgh, PA; National Institutes of Health CORE Grant for Vision Research EY08098; National Institutes of Health Grant AI085570; The Eye and Ear Foundation of Pittsburgh; an unrestricted grant to the University of Pittsburgh Department of Ophthalmology from Research to Prevent Blindness; and a Career Development Award from Research to Prevent Blindness (RMQS). Rempex Pharmaceuticals did not design, participate, collect data, or analyze or interpret the data for this study. Rempex Pharmaceuticals did not aid in the preparation of this manuscript, but did have the opportunity to review the manuscript before submission. The other sponsors had no input on any aspect of this study.
Author Disclosure Statement
The first author, E.G.R., is fully responsible for the content of this article. The authors E.G.R., F.S.M., R.M.Q.S., and R.P.K. have been paid independent consultation fees by Rempex Pharmaceuticals, San Diego, CA, but not in the last 3 years. These fees were deemed not to produce a conflict of interest for the authors by the University of Pittsburgh. One of the coauthors, F.S.M., is currently a consultant for competing companies, but no competing financial interest exists. E.G.R., R.M.Q.S., R.P.K., T.A.K., K.E.O., and K.A.Y. have no financial interest or conflict of interest with this study. This study was presented at the 2013 Ocular Microbiology and Immunology Group annual meeting in New Orleans, LA, the 2014 ARVO meeting in Orlando, FL, and the 11th ISOPT Clinical meeting in Reykjavik, Iceland.
References
- 1.Solomon R., Donnenfeld E.D., Holland E.J., et al. . Microbial keratitis trends following refractive surgery: results of the ASCRS infectious keratitis survey and comparisons with prior ASCRS surveys of infectious keratitis following keratorefractive procedures. J. Cataract Refract. Surg. 37:1343–1350, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Asbell P.A., Sahm D.F., Shaw M., et al. . Increasing prevalence of methicillin resistance in serious ocular infections caused by Staphylococcus aureus in the United States: 2000 to 2005. J. Cataract Refract. Surg. 34:814–818, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Noskin G.A. Tigecycline: a new glycylcycline for treatment of serious infections. Clin. Infect. Dis. 41(Suppl. 5):s303–s314, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Pankey G.A. Tigecycline. J. Antimicrob. Chemother. 56:470–480, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Goff D.A., and Dowzicky M.J. Prevalence and regional variation in meticillin-resistant Staphylococcus aureus (MRSA) in the USA and comparative in vitro activity of tigecycline, a glycylcycline antimicrobial. J. Med. Microbiol. 56:1189–1195, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Kaya O., Akcam F.Z., and Temel E.N. In vitro activities of linezolid and tigecycline against methicillin-resistant Staphylococcus aureus strains. Microb. Drug Resist. 14:151–153, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Putnam S.D., Sader H.S., Farrell D.J., and Jones R.N. Sustained antimicrobial activity of tigecycline against methicillin-resistant Staphylococcus aureus (MRSA) from United States medical centers from 2004 through 2008. J. Chemother. 22:13–16, 2010 [DOI] [PubMed] [Google Scholar]
- 8.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard 8th ed. CLSI document M07-A8. Wayne, PA: Clinical Laboratory Standards Institute; 2009 [Google Scholar]
- 9.Draize J.H., Woodward G., and Calvery H.O. Methods for the study of irritation and toxicity of articles applied topically to the skin and mucous membranes. J. Pharmacol. Exp. Ther. 82:377–390, 1944 [Google Scholar]
- 10.Kay J.H., and Calandra J.C. Interpretation of eye irritation tests. J. Soc. Cosmet. Chem. 13:281–289, 1962 [Google Scholar]
- 11.Kowalski R.P., Romanowski E.G., Mah F.S., Shanks R.M.Q., and Gordon Y.J. Topical levofloxacin 1.5% overcomes in vitro resistance in rabbit keratitis models. Acta Ophthalmol. 88:e120–e125, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romanowski E.G., Mah F.S., Yates K.A., Kowalski R.P., and Gordon Y.J. The successful treatment of gatifloxacin-resistant Staphylococcus aureus keratitis with Zymar® (Gatifloxacin 0.3%) in a NZW rabbit model. Am. J. Ophthalmol. 139:867–877, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Romanowski E.G., Mah F.S., Kowalski R.P., Yates K.A., and Gordon Y.J. Benzalkonium chloride enhances the antibacterial efficacy of gatifloxacin in an experimental rabbit keratitis model. J. Ocul. Pharmacol. Ther. 24:380–384, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Bradford P.A., Weaver-Sands T., and Peterson P.J. In vitro activity of tigecycline against isolates from patients enrolled in phase 3 clinical trials of treatment of complicated skin and skin-structure infections and complicated intra-abdominal infections. Clin. Infect. Dis. 41:S315–S332, 2005 [DOI] [PubMed] [Google Scholar]
- 15.Hawkey P., and Finch R. Tigecycline: in-vitro performance as a predictor of clinical efficacy. Clin. Micro. Infect. 13:354–362, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Bouchillon S.K., Hoban D.J., Johnson B.M., et al. . In vitro evaluation of tigecycline and comparative agents in 3049 clinical isolates: 2001 to 2002. Diagn. Microbiol. Infect. Dis. 51:291–295, 2005 [DOI] [PubMed] [Google Scholar]
- 17.Hoban D.J., Bouchillon S.K., Johnson B.M., et al. . In vitro activity of tigecycline against 6792 Gram-negative and gram-positive clinical isolates from the global Tigecycline Evaluation and Surveillance Trial (TEST Program, 2004). Diagn. Microbiol. Infect. Dis. 52:215–227, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Goktas S., Kurtoglu M.G., Sakarya Y., et al. . New therapy option for treatment of methicillin-resistant Staphylococcus aureus keratitis: tigecycline. J. Ocul. Pharmacol. Ther. 31:122–127, 2015 [DOI] [PubMed] [Google Scholar]