Abstract
Our objective was to evaluate the association of plasma inflammatory biomarkers with MetS in an older population of treated HIV-infected (HIV+) as compared to age-matched HIV-negative (HIV−) adults. This was done in a retrospective observational study. Plasma concentrations of complement component 3 (C3), cystatin C, fibroblast growth factor 1, interleukin 6, oxidized LDL, soluble RAGE, soluble CD163, soluble CD14, and osteopontin were measured in 79 HIV+ participants on combination antiretroviral treatment (cART) with a suppressed HIV viral load and 47 HIV− participants with a median age of 59 (range 50 to 79). Outcomes were individual MetS components (hypertension, type II diabetes, dyslipidemia, and obesity) and MetS. Covariates were screened for inclusion in multivariable models. Odds ratios are reported per 50 mg/dl increase in C3. In the HIV+ group, higher C3 levels were associated with MetS (OR 3.19, p = 0.004), obesity (OR 2.02, p = 0.01), type II diabetes (OR 1.93, p = 0.02), and at a trend level with dyslipidemia (OR 1.87, p = 0.07) and hypertension (OR 1.66, p = 0.09). C3 levels were significantly higher in HIV+ participants with MetS compared to those without MetS (p = 0.002). C3 was higher among HIV+ patients with three or four MetS components as compared to those with one or two (p = 0.04) and those with none (p = 0.002). No associations were found between C3 and the outcomes for HIV− participants. C3 is strongly associated with both MetS and MetS components in an older HIV+ sample on cART compared to HIV− controls. C3 warrants further investigation as a marker of cardiometabolic risk among persons aging with HIV.
Introduction
Despite the reduction in HIV-related mortality with the use of combined antiretroviral therapy (cART), metabolic abnormalities in the treated HIV-positive (HIV+) population are increasing in prevalence.1 Components of the metabolic syndrome (MetS), including dyslipidemia, hypertension, insulin resistance, and central obesity, have all been observed in this aging population, though the underlying causes of HIV-associated cardiometabolic disease have not been firmly established.2–4 Hypotheses include cART-related toxicities and persisting effects of HIV disease such as immune dysregulation and chronic low-level inflammation.3,5–7
In this study we aimed to better understand the link between chronic inflammation and MetS in older, treated HIV+ individuals compared to age-matched HIV negative (HIV−) controls. We measured nine inflammatory biomarkers chosen to reflect a broad range of inflammatory mechanisms in peripheral blood of 79 HIV+ and 47 HIV− participants and analyzed their association with MetS and individual MetS component conditions (hypertension, type II diabetes, dyslipidemia, and obesity). We hypothesized that MetS and its components are associated with distinct inflammatory profiles in HIV+ versus HIV− populations and that such biomarker profiles may be useful tools to assess cardiometabolic disease risk in the treated HIV+ population.
Materials and Methods
Participants
The present study included 79 HIV+ and 47 HIV− community-dwelling adults from the California HIV/AIDS Research Program Successfully Aging Seniors with HIV (SASH) study administered by the UCSD HIV Neurobehavioral Research Program (HNRP). Inclusion criteria for SASH were an age of at least 50 years, receiving stable cART (for HIV+), and plasma HIV RNA less than 48 copies/ml (for HIV+). Study participants were recruited from ongoing studies being conducted at the HNRP focused on HIV and cognition, wherein participants with a known history of non-HIV-related neurologic disorders or any conditions known to be associated with impaired cognitive functioning were excluded (e.g., severe TBI). The study was approved by the UCSD Institutional Review Board and all participants provided written informed consent.
Biomarker quantification
All inflammatory biomarker assays were measured by immunoassay in duplicate in EDTA plasma derived from peripheral blood samples collected by routine phlebotomy. Commercial immunoassay suppliers were R&D Systems [soluble CD163 (sCD163), soluble CD14 (sCD14), soluble receptors for advanced glycation end-products (RAGE), and cystatin C; Minneapolis, MN], Immuno-Biological Laboratories-America (osteopontin; Minneapolis, MN) Meso Scale Discovery [interleukin (IL)-6, fibroblast growth factor (FGF)-1; Rockville, MD], Assaypro (complement C3; St. Charles, MO), and ALPCO (oxidized LDL; Salem, NH). Measurements were repeated if the coefficient of variation was greater than 20% or if the measurement was greater than four standard deviations from the mean. A total of at least 10% of samples in each assay was repeated to further assess batch consistency.
Outcomes
Primary outcomes were MetS, dyslipidemia, type II diabetes, hypertension, and obesity. Obesity was defined as a body mass index (BMI) greater than or equal to 30 kg/m2. Dyslipidemia, type II diabetes, and hypertension were defined by the presence of both self-reported diagnosis and current, specific drug treatment for the condition. In a small number of patients, dyslipidemia, type II diabetes, and hypertension were defined by self-reported diagnosis only (hypertension: six patients; type II diabetes: five patients; dyslipidemia: seven patients). The primary definition of MetS was the International Diabetes Federation (IDF) 2006 definition, requiring increased central adiposity (or BMI greater than or equal to 30 kg/m2) plus at least two of the following factors: treatment for raised triglycerides or reduced HDL cholesterol, treatment of previously diagnosed hypertension, and previously diagnosed type II diabetes.8 Due to the prevalence of differing MetS definitions, we also tested our results using two versions of the Adult Treatment Panel (ATP) III criteria.9 The first, termed “standard ATP III,” used the ATP criteria for waist circumference (>102 cm for men, >88 cm for women, measured in a subset of 53 participants) and replaced the ATP quantitative criteria of raised triglycerides/low HDL cholesterol, blood pressure, and fasting glucose with our outcomes of dyslipidemia, hypertension, and type II diabetes, respectively. At least three of these four factors (waist circumference, dyslipidemia, hypertension, or type II diabetes) constituted a diagnosis of MetS. The second definition, termed “modified ATP III,” was identical to Standard ATP III but replaced minimum waist circumference with our outcome of obesity.
Covariates
Candidate covariates for multivariable models predicting the outcomes included demographic factors, noncardiometabolic comorbidities, and cART variables for HIV+ participants. All multivariable logistic regression models predicting comorbidities included age, sex, ethnicity (white vs. nonwhite), smoking (ever and current), and cART drug classes for HIV+ participants [present exposure to protease inhibitors (PIs) or nonnucleoside reverse transcriptase inhibitors (NNRTIs)]. Other covariates were included if they showed an association with the comorbidity or biomarker at the α = 0.10 level. Screened covariates included AIDS diagnosis, estimated HIV infection duration, current cART regimen duration, duration of exposure to all cART drugs, self-reported nadir CD4+ T cell count, current CD4+ T cell count, lifetime history of drug or alcohol abuse, HCV serostatus, and chronic pulmonary disease.
Statistical analysis
For each biomarker, outliers greater than seven standard deviations above the mean were removed (one removed for cystatin C, one for IL-6). Correlations were performed with the nonparametric Spearman method. C3, IL-6, and cystatin C were log10 transformed to reduce skewness for comparison across MetS and comorbidity burden groups (one-tailed t-test and one-way ANOVA with Tukey's post hoc test, respectively). Univariable and multivariable logistic regression on untransformed biomarker data were used to test the association of biomarkers with the outcomes (dyslipidemia, type II diabetes, hypertension, obesity, and MetS). Receiver operating characteristic (ROC) curve analysis was performed on untransformed biomarker data. Covariate screens were conducted with univariable logistic regression, Spearman correlation, or Fisher's exact test, as appropriate. Unit odds ratios are calculated for an increase of 50 mg/dl (C3), 1 pg/ml (IL-6), or 0.1 ng/ml (cystatin C). Comparison of demographic and biomarker data across HIV+ and HIV− groups was performed with Fisher's exact test or two-tailed t-test, as appropriate. All statistical tests were performed with JMP 11.0.0 (SAS, 2013).
Results
Participants
HIV+ participants in this study were predominately men (68/79, 86%) and white (64/79, 81%) with a median age of 59 years (IQR = 9) (Table 1). The median estimated duration of HIV infection was 19.8 years, the median duration of exposure to all cART drugs was 133 months (11.1 years), and the mean nadir and current CD4+ T cell counts were 188 cells/mm3 and 639 cells/mm3, respectively (Table 2). HIV+ and HIV− participants had a similar prevalence of lifetime substance abuse, and there was no difference in current or lifetime alcohol, cannabis, cocaine, methamphetamine, or opioid use between the groups (p > 0.05 for all). Of the primary outcomes in the HIV+ group, obesity was observed in 16 (20.2%) participants, type II diabetes in 20 (25.3%), hypertension in 40 (50.6%), dyslipidemia in 48 (60.8%), and MetS in 10 (12.8%) (Table 3). Compared to HIV+ participants, the HIV− population included fewer males (57% vs. 86%, p < 0.001) and fewer cases of dyslipidemia (34% vs. 60.8%, p = 0.006) but a similar prevalence of MetS (p = 0.79). HIV+ participants had higher triglycerides (201 vs. 160 mg/dl, p = 0.05), lower HDL cholesterol (46 vs. 57 mg/dl, p = 0.04), and lower total cholesterol (180 vs. 197 mg/dl, p = 0.04), but similar systolic BP, diastolic BP, BMI, waist circumference, random serum glucose, and LDL cholesterol compared to HIV− participants (p > 0.05 for all, Table 3). Compared to HIV+ participants, HIV− participants showed lower plasma cystatin C (p < 0.001), lower plasma sCD163 (p = 0.01), and lower sCD14 (p < 0.001) but similar C3 (p = 0.43) (Table 1)
Table 1.
Demographics and Biomarkers
| HIV+ | HIV− | p value | |
|---|---|---|---|
| All subjects (N) | 79 | 47 | |
| Demographics and comorbidities | |||
| Age, median (IQR, N) | 59 (9, 79) | 59 (10, 47) | 0.34 |
| Sex, male | 68/79 (86%) | 27/47 (57%) | <0.001 |
| Ethnicity | |||
| white | 64/79 (81%) | 30/47 (64%) | 0.04 |
| black | 10/79 (12.7%) | 11/47 (23%) | 0.14 |
| hispanic | 2/79 (2.5%) | 4/47 (9%) | 0.19 |
| other | 3/79 (3.8%) | 2/47 (4%) | 0.99 |
| Hepatitis C virus | 19/79 (24.1%) | 7/47 (15%) | 0.26 |
| Chronic pulmonary disease | 19/79 (24.1%) | 5/45 (9%) | 0.09 |
| Smoking, current | 28/79 (35.4%) | 13/47 (28%) | 0.43 |
| Smoking, ever | 32/79 (40.5%) | 19/47 (40%) | 0.99 |
| Lifetime substance abuse or dependence | 54/78 (69.2%) | 25/46 (54%) | 0.12 |
| Biomarkers | |||
| Complement C3, mean (SD, N) (mg/dl) | 118 (48, 79) | 115 (56, 47) | 0.43 |
| Cystatin C, mean (SD, N) (ng/ml) | 0.75 (0.18, 78) | 0.64 (0.19, 47) | <0.001 |
| FGF-1, mean (SD, N) (pg/ml) | 7.42 (5.42, 79) | 8.46 (7.20, 47) | 0.48 |
| IL-6, mean (SD, N) (pg/ml) | 1.31 (1.32, 78) | 1.05 (0.85, 47) | 0.07 |
| Oxidized LDL, mean (SD, N) (ng/ml) | 71.5 (115, 58) | 73.7 (151, 18) | 0.51 |
| RAGE, mean (SD, N) (pg/ml) | 1372 (629, 78) | 1230 (451, 47) | 0.25 |
| sCD163, mean (SD, N) (ng/ml) | 1473 (1045, 79) | 1146 (737, 45) | 0.01 |
| sCD14, mean (SD, N) (pg/ml) | 2264 (751, 79) | 1745 (627, 45) | <0.001 |
| Osteopontin, mean (SD, N) (ng/ml) | 408 (223, 79) | 324 (168, 45) | 0.05 |
FGF-1, fibroblast growth factor 1; RAGE, receptor for advanced glycation endproducts.
Table 2.
HIV+ Group Characteristics
| HIV disease factors | |
| AIDS | 53/79 (67%) |
| Estimated duration of HIV infection, median (IQR, N) (years) | 19.8 (13.2, 78) |
| Duration of current cART regimen, median (IQR, N) (months) | 33.1 (50.0, 78) |
| Duration of exposure to all cART drugs, median (IQR, N) (months) | 133 (123, 78) |
| Nadir CD4+ T cell count, mean (SD, N) (cells/mm3) | 188 (191, 78) |
| Current CD4+ T cell count, mean (SD, N) (cells/mm3) | 639 (339, 76) |
| cART regimen | |
| Protease inhibitor | 41/79 (51.9%) |
| NNRTI | 39/79 (49.3%) |
NNRTI, nonnucleoside reverse transcriptase inhibitor.
Table 3.
Cardiometabolic Outcomes and Continuous Measures
| HIV+ | HIV− | p value | |
|---|---|---|---|
| Primary outcomes | |||
| Metabolic syndrome | 10/78 (12.8%) | 7/45 (15.5%) | 0.79 |
| Obesity | 16/78 (20.5%) | 13/45 (29%) | 0.38 |
| Type II diabetes | 20/79 (25.3%) | 8/47 (17%) | 0.38 |
| Hypertension | 40/79 (50.6%) | 20/47 (43%) | 0.46 |
| Dyslipidemia | 48/79 (60.8%) | 16/47 (34%) | 0.006 |
| Cardiometabolic measures | |||
| Systolic BP, mean (SD) (mmHg) | 133 (21) | 136 (20) | 0.6 |
| Diastolic BP, mean (SD) (mmHg) | 76 (10) | 77 (13) | 0.5 |
| BMI, mean (SD) (kg/m2) | 27 (5.6) | 28 (5.0) | 0.7 |
| Waist circumference, mean (SD) (cm) | 101 (15) | 99 (19) | 0.7 |
| Random serum glucose, mean (SD) (mg/dl) | 109 (45) | 112 (54) | 0.7 |
| Total cholesterol, mean (SD) (mg/dl) | 180 (37) | 197 (46) | 0.04 |
| HDL cholesterol, mean (SD) (mg/dl) | 46 (17) | 57 (24) | 0.007 |
| LDL cholesterol, mean (SD) (mg/dl) | 96 (31) | 108 (38) | 0.08 |
| Triglycerides, mean (SD) (mg/dl) | 201 (121) | 160 (84) | 0.05 |
HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Correlations
Correlations were performed between C3 and BMI, systolic BP, diastolic BP, waist circumference, total cholesterol, LDL, HDL, triglycerides, and random plasma glucose. In the HIV+ group, C3 was correlated with total cholesterol (r = 0.36, 95% CI 0.15–0.54, p = 0.001), LDL cholesterol (r = 0.30, 95% CI 0.07–0.50, p = 0.01), and at a trend level with glucose (r = 0.21, 95% CI −0.01–0.42, p = 0.06) and triglycerides (r = 0.19, 95% CI −0.03–0.41, p = 0.08) but not systolic BP, diastolic BP, or waist circumference (p > 0.05). In the HIV− group, C3 was correlated with BMI (r = 0.32, 95% CI 0.03–0.56, p = 0.03) and triglycerides (r = 0.37, 95% CI 0.08–0.60, p = 0.01) but not with systolic BP, diastolic BP, waist circumference, total cholesterol, LDL, HDL, or random plasma glucose (p > 0.05 for all).
Finally, correlations were performed among inflammatory biomarkers in the HIV+ group. Statistically significant correlations were found between sCD14 and either IL-6 (r = 0.11, p = 0.04) or OPN (r = 0.30, p = 0.03), and between cystatin C and either RAGE (r = 0.19, p = 0.02), sCD14 (r = 0.15, p = 0.045), or OPN (r = 0.18, p = 0.03).
Univariable analyses
For each biomarker, univariable logistic regression was used to determine the association with MetS, hypertension, dyslipidemia, type II diabetes, and obesity. In the HIV+ group, a higher C3 was associated with type II diabetes (OR 1.72, 95% CI 1.03–2.97, p = 0.04), dyslipidemia (OR 2.02, 95% CI 1.17–3.86, p = 0.02), obesity (OR 2.00, 95% CI 1.16–3.61, p = 0.02), and MetS (OR 2.79, 95% CI 1.47–5.92, p = 0.003). In addition, a higher IL-6 was associated with MetS (OR 1.61, 95% CI 1.07–2.72, p = 0.04) and showed a trend-level association with hypertension (OR 1.79, 95% CI 1.08–3.69, p = 0.06) and obesity (OR 1.49, 95% CI 1.02–2.44, p = 0.06). Other biomarkers were not associated with any of the five outcomes. In the HIV− group, C3 was not associated with diabetes, hypertension, dyslipidemia, obesity, or MetS (IDF 2006) (p > 0.05 for all) and higher IL-6 was associated with obesity (OR 2.61, 1.18–8.28, p = 0.04).
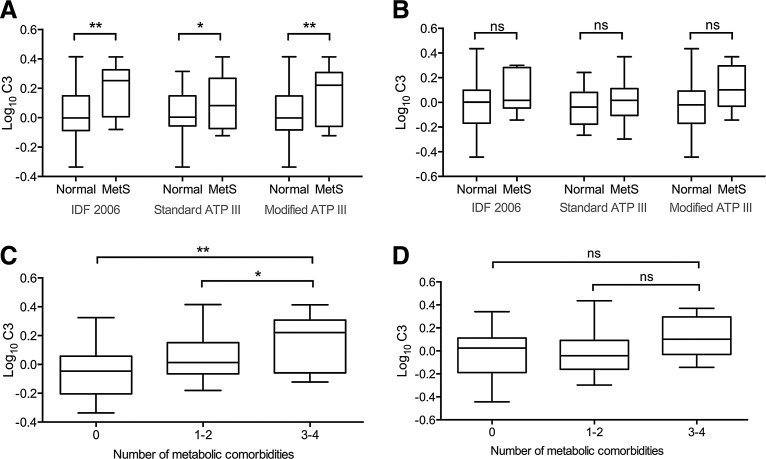
IL-6 and C3 were each higher in HIV+ participants with MetS compared to those without (log10 IL-6: 0.26 vs. −0.034, Cohen's d = 1.13, p = 0.005; log10 C3: 0.19 vs. 0.019, Cohen's d = 1.08, p = 0.008) (Fig. 1). HIV+ participants were divided into three groups based on the number of comorbid metabolic conditions (dyslipidemia, type II diabetes, hypertension, or obesity): no metabolic comorbidities (14 participants), one or two comorbidities (48), and three or four comorbidities (16). C3 values differed between groups (overall p = 0.004) and increased with the number of comorbidities (log10 C3: 0: −0.045; 1–2: 0.036; 3–4: 0.15) (Fig. 1). Pairwise comparison identified differences between those with three or four comorbidities and either the reference group (no comorbidities, p = 0.002) or those with one or two comorbidities (p = 0.04). IL-6 values tended to differ between groups (p = 0.06) and pairwise comparison reached a trend level (three or four comorbidities vs. no comorbidities: p = 0.07; three or four comorbidities vs. one or two comorbidities: p = 0.09). In the HIV− group, C3 levels showed no difference between MetS versus no MetS by any definition (p > 0.05) and did not differ between comorbidity burden groups (p > 0.05; Fig. 1).
FIG. 1.
Comparison of C3 among metabolic syndrome (MetS) and comorbidity groups. (A) Comparison of log10 C3 values among HIV+ participants using three clinical definitions of MetS. Log10 C3 values are higher in MetS cases with each definition (IDF 2006: p = 0.008; Standard ATP III: p = 0.048; Modified ATP III: p = 0.009; one-tailed t-test). (B) Comparison of log10 C3 values for MetS versus no MetS in HIV− participants. No differences were identified (p > 0.05 for all comparisons). (C) Comparison of log10 C3 values across HIV+ participants with 0, 1–2, or 3–4 MetS component comorbidities (hypertension, dyslipidemia, obesity, type II diabetes). Individuals with 3–4 comorbidities showed higher log10 C3 than those with either 1–2 (p = 0.039) or 0 (p = 0.002) comorbidities (one-way ANOVA followed by Tukey's post hoc test). (D) Comparison of log10 C3 values across HIV− participants with 0, 1–2, or 3–4 MetS component comorbidities. No differences were found (p > 0.05 for all comparisons). *p < 0.05; **p < 0.01.
HIV+: multivariable analyses
The associations of IL-6 and C3 with the outcomes among HIV+ participants were further investigated in multivariable models adjusting for age, sex, ethnicity, cART drug classes, smoking, and additional covariates that passed the univariable screen (see Materials and Methods). Among screened covariates, current CD4+ T cell count (p = 0.046) was associated with IL-6 and no associations were found for C3. For outcomes, both nadir (p = 0.03) and current (p = 0.005) CD4+ T cell counts were associated with hypertension. Diagnosis of AIDS (p = 0.06), HCV serostatus (p = 0.07), and total duration of exposure to cART drugs (p = 0.06) were associated with dyslipidemia. None of the screened covariates was associated with type II diabetes, obesity, or MetS.
In the multivariable models C3 remained significantly associated with MetS (OR 3.19, 95% CI 1.54–7.98, p = 0.004), type II diabetes (OR 1.93, 95% CI 1.1–3.57, p = 0.02), obesity (OR 2.02, 95% CI 1.14–3.75, p = 0.01), and at a trend level with dyslipidemia (OR 1.87, 95% CI 0.98–4.03, p = 0.07) and hypertension (OR 1.66, 95% CI 0.94–129, p = 0.09) (Table 4). In the model for dyslipidemia, the outcome was associated with white ethnicity (OR 10.7, 95% CI 2.39–68, p = 0.004), PI use (OR 8.01, 95% CI 1.24–81, p = 0.04), and NNRTI use (OR 8.01, 95% CI 1.28–83, p = 0.04). In separate models, IL-6 was associated at a trend level with MetS (OR 1.42, 95% CI 0.93–2.24, p = 0.09) and obesity (OR 1.49, 95% CI 0.98–2.55, p = 0.09) but not with hypertension (p = 0.14). ROC curve analysis identified a threshold C3 concentration of 163.5 mg/dl for diagnosis of MetS (AUC = 0.71, 95% CI 0.54–0.88, p = 0.02) with a sensitivity of 57.1% and specificity of 90.6%.
Table 4.
Results of Multivariable Logistic Regression Models for HIV+
| Outcome | Covariatea | ORb | 95% CI | p-value |
|---|---|---|---|---|
| Metabolic syndrome (IDF 2006) | C3 | 3.19 | 1.54–7.98 | 0.004 |
| Type II diabetes | C3 | 1.93 | 1.10–3.57 | 0.02 |
| Age | 1.12 | 1.01–1.26 | 0.02 | |
| Obesity | C3 | 2.02 | 1.14–3.75 | 0.01 |
| Dyslipidemia | C3 | 1.87 | 0.98–4.03 | 0.07 |
| Ethnicity—white | 10.7 | 2.39–68 | 0.004 | |
| PI use | 8.01 | 1.24–81 | 0.04 | |
| NNRTI use | 8.01 | 1.28–83 | 0.04 | |
| Hypertensionc | C3 | 1.66 | 0.94–3.12 | 0.09 |
All models included age, sex, ethnicity, cART drug classes, and smoking as covariates in addition to those that passed the covariate screen. Reported here are terms that reached significance (p < 0.05).
Odds ratios reported per 50 mg/dl increase in C3 level and per year increase for age (see Materials and Methods).
No terms reached significance in the model for hypertension.
Alternate MetS definitions
We tested the robustness of the association of C3 and MetS in HIV+ participants using two alternate definitions of MetS (see Materials and Methods). Using our Standard ATP III criteria in a subset of participants with waist circumference data (n = 53, see Materials and Methods), C3 was associated with MetS in univariable analysis (OR 2.02, 95% CI 1.06–4.27, p = 0.04) and at a trend level in multivariable analysis (OR 1.92, 95% CI 0.91–4.29, p = 0.08). Using our Modified ATP III criteria, replacing minimum waist circumference with obesity (n = 78, see Materials and Methods), C3 was associated with MetS in univariable analysis (OR 1.98 95% CI 1.04–4.11, p = 0.04) and at a trend level in multivariable analyses (OR 2.06, 95% CI 0.99–4.66, p = 0.06). Higher C3 concentrations were also associated with MetS (versus no MetS) by either Standard ATP III criteria (p = 0.048) or Modified ATP III criteria (p = 0.009) (Fig. 1).
HIV−: univariable and multivariable analyses
In the covariate screen for the HIV− group, hypertension showed a marginal association with HCV infection (p = 0.09). Univariable logistic regression in this group showed that higher cystatin C was associated with hypertension (OR 1.55, 95% CI 1.08–2.46, p = 0.03), obesity (OR 1.66, 95% CI 1.14–2.69, p = 0.02), and MetS (IDF 2006; OR 1.74, 95% CI 1.16–2.93, p = 0.01). Higher RAGE was associated with lower odds of hypertension (OR 0.85, 95% CI 0.72–0.98, p = 0.04), and higher sCD163 was associated with MetS (IDF 2006; OR 1.13, 95% CI 1.02–1.27, p = 0.02).
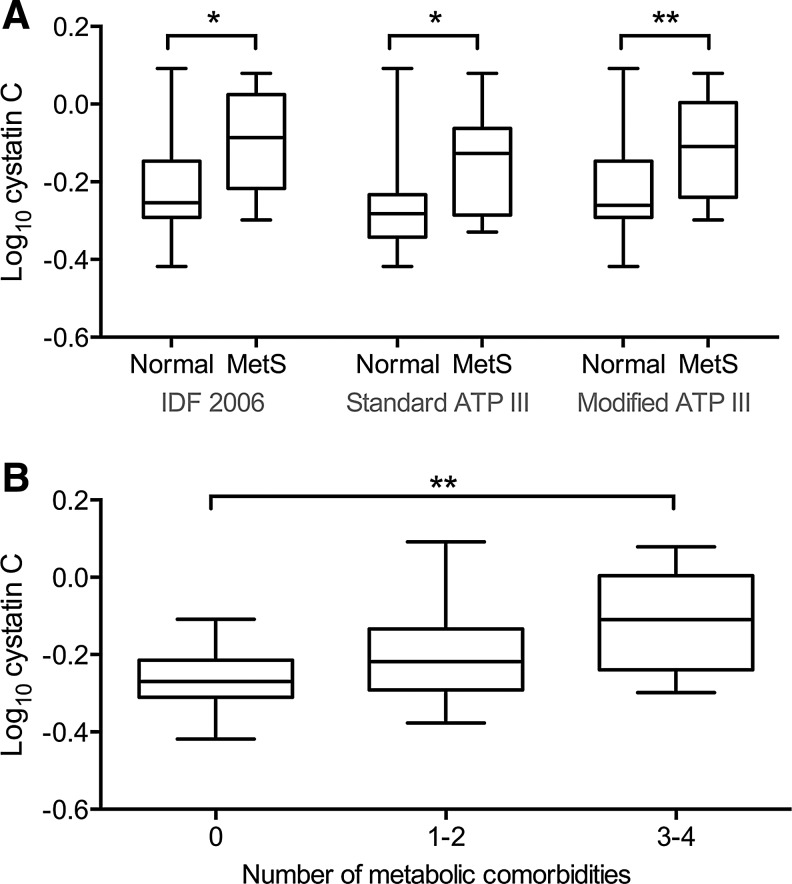
In multivariable logistic regression, cystatin C remained associated with obesity (OR 1.81, 95% CI 1.19–3.21, p = 0.02), MetS (IDF 2006; OR 1.89, 95% CI 1.18–3.49, p = 0.02), and at a trend level with hypertension (OR 1.62, 95% CI 1.02–2.85, p = 0.06). In the model for hypertension, HCV infection was associated with the outcome (OR = 13.8, 95% CI 1.48–223, p = 0.02). Cystatin C was higher in HIV− participants with MetS versus no MetS by all three definitions (IDF 2006: −0.01 vs. −0.23, Cohen's d = 0.11, p = 0.02; Standard ATP III: −0.12 vs. −0.23, Cohen's d = 0.16, p = 0.02; Modified ATP III: −0.15 vs. −0.26, Cohen's d = 0.11, p = 0.01) (Fig. 2). Cystatin C increased with the number of metabolic comorbidities (log10 cystatin C, 0 comorbidities: −0.26; 1–2: −0.02; 3–4: −0.12). Differences in cystatin C among comorbidity burden groups were significant overall (p = 0.007) and pairwise comparison was significant for three to four comorbidities vs. 0 (p = 0.005).
FIG. 2.
Comparison of cystatin C among MetS and comorbidity groups. (A) Comparison of log10 cystatin C values among HIV− participants using three clinical definitions of MetS. Log10 cystatin C values are higher in MetS cases with each definition (IDF 2006: p = 0.02; Standard ATP III: p = 0.02; Modified ATP III: p = 0.01; one-tailed t-test). (B) Comparison of log10 cystatin C values across HIV− participants with 0, 1–2, or 3–4 MetS component comorbidities. Individuals with 3–4 comorbidities showed higher log10 cystatin C than those with 0 (p = 0.002) but not 1–2 comorbidities (p > 0.05; one-way ANOVA followed by Tukey's post hoc test).
Discussion
In this study we found an association between higher plasma complement C3 levels and MetS, obesity, type II diabetes, and dyslipidemia in an older cohort of cART-treated HIV+ individuals. This association was present in univariable analysis and after adjustment for potential confounders including age, sex, ethnicity, and cART drug class, among others. The association was specific to the HIV+ group versus HIV− controls. We found limited evidence of similar associations in the HIV+ group for IL-6, which was associated with the composite MetS variable but not with individual MetS components. We found that the association between C3 and MetS in HIV+ individuals was robust to several definitions of MetS. In ROC curve analysis, a threshold C3 value of 163.5 mg/dl resulted in 90% specificity for the diagnosis of MetS among HIV+ participants. Finally, we performed similar analyses for the HIV− control group and found associations between cystatin C and multiple cardiometabolic outcomes.
C3 is the central component of the complement system, a primary mechanism of innate immunity. C3 is primarily produced by hepatocytes but is also produced by adipocytes as an adipokine; in the setting of obesity, higher C3 levels are associated with a low-level systemic inflammatory state and are hypothesized to contribute to the development of insulin resistance, though the exact causal mechanisms remain unclear.10–15 Higher C3 is also associated with nonalcoholic fatty liver disease, the primary liver manifestation of MetS, and may contribute to the impaired liver function and dyslipidemia observed in cardiometabolic disease.16,17 The complement system has been hypothesized to play a role in the development of MetS and may be involved in deranged postprandial lipid metabolism in this setting.18–20
C3 may play a particularly strong role in HIV-associated cardiometabolic disease. In this study, C3 was higher in HIV+ patients with MetS but was not correlated with other inflammatory biomarkers, potentially indicating a unique role for C3 in this population. HIV both directly and indirectly activates complement pathways during active infection; however, the sequelae of virus-induced inflammation in the context of long-term treated HIV disease remain unclear.7 Chronic treated HIV disease is associated with immune dysregulation and ongoing low-level systemic inflammation, which may further increase the levels of proinflammatory cytokines, increase C3 production, and heighten subsequent risk for MetS. The etiology of this chronic inflammation is poorly understood but may include residual viral replication, coinfections, or leakage of bacteria from the gut.26 The lipodystrophy syndrome associated with cART (fat redistribution from the periphery to primarily the abdomen and dorsocervical regions) has been proposed to cause an increase in proinflammatory adipokine production, including C3, that may underlie HIV-associated MetS, and increased visceral adiposity is highly predictive of cardiovascular complications across populations.4,21–23
In this study, we found little evidence for a relationship between C3 and MetS or its components in our HIV− control group; instead, cystatin C was most strongly associated with the outcomes in this group. As our participants were older (median age 59 years), this may reflect the increasing cardiometabolic importance of cystatin C with advancing age in the HIV− population. Others have reported a link between higher cystatin C and the nexus of advanced age, cardiometabolic disease, and declining renal function in the general population.24,25
We found that dyslipidemia was more prevalent in the HIV+ group while the prevalence of MetS and the other components (hypertension, obesity, hyperglycemia) did not differ among groups. This difference appears to be driven by higher average triglycerides and lower HDL cholesterol among HIV+ participants. In correlating C3 to these measures, we found associations with total cholesterol and LDL that were present in HIV+ but not HIV− individuals. In the HIV+ participants, C3 was not correlated with continuous measures underlying other components of MetS (blood pressure, BMI, waist circumference, plasma glucose). Finally, in the multivariable model for dyslipidemia in HIV+ individuals, PI use, NNRTI use, and white ethnicity were strongly associated with the outcome. Taken together, these data point to an interaction between HIV infection and/or cART, complement, and lipid regulation that could be partly responsible for driving MetS in this population.
Our study is subject to important limitations. Although we found a link between our inflammatory biomarkers and MetS using three MetS definitions, we did not use quantitative criteria (e.g., for low HDL) to categorize participants and relied instead on current drug treatment and self-reported diagnosis. Though the IDF criteria allow for replacing quantitative criteria in this way, the published ATP III definition does not (see Materials and Methods).8,9 A truly quantitative method of defining MetS and its component morbidities could reveal different associations with inflammatory biomarkers. Finally, our findings apply to a subset of patients whose HIV disease was well-controlled with an undetectable plasma viral load.
Findings from the present study point toward several lines of future investigation. C3 warrants additional prospective study as a marker of metabolic and cardiovascular disease risk in the aging, cART-treated HIV+ population. Moreover, data are needed to determine the precise role of C3 and potential interactions with latent virus, cART regimens or specific drugs, and lipid balance. Future studies will determine whether C3 plays a unique role in HIV-associated MetS compared to MetS in the general population and also compared to the other inflammatory molecules produced in HIV disease.
Acknowledgments
The San Diego HIV Neurobehavioral Research Center [HNRC] group is affiliated with the University of California, San Diego, the Naval Hospital, San Diego, and the Veterans Affairs San Diego Healthcare System, and includes Director: Robert K. Heaton, Ph.D., Co-Director: Igor Grant, M.D.; Associate Directors: J. Hampton Atkinson, M.D., Ronald J. Ellis, M.D., Ph.D., and Scott Letendre, M.D.; Center Manager: Thomas D. Marcotte, Ph.D.; Jennifer Marquie-Beck, M.P.H.; Melanie Sherman; Neuromedical Component: Ronald J. Ellis, M.D., Ph.D. (P.I.), Scott Letendre, M.D., J. Allen McCutchan, M.D., Brookie Best, Pharm.D., Rachel Schrier, Ph.D., Debra Rosario, M.P.H.; Neurobehavioral Component: Robert K. Heaton, Ph.D. (P.I.), J. Hampton Atkinson, M.D., Steven Paul Woods, Psy.D., Thomas D. Marcotte, Ph.D., Mariana Cherner, Ph.D., David J. Moore, Ph.D., Matthew Dawson; Neuroimaging Component: Christine Fennema-Notestine, Ph.D. (P.I.), Monte S. Buchsbaum, M.D., John Hesselink, M.D., Sarah L. Archibald, M.A., Gregory Brown, Ph.D., Richard Buxton, Ph.D., Anders Dale, Ph.D., Thomas Liu, Ph.D.; Neurobiology Component: Eliezer Masliah, M.D. (P.I.), Cristian Achim, M.D., Ph.D.; Neurovirology Component: David M. Smith, M.D. (P.I.), Douglas Richman, M.D.; International Component: J. Allen McCutchan, M.D., (P.I.), Mariana Cherner, Ph.D.; Developmental Component: Cristian Achim, M.D., Ph.D. (P.I.), Stuart Lipton, M.D., Ph.D.; Participant Accrual and Retention Unit: J. Hampton Atkinson, M.D. (P.I.), Jennifer Marquie-Beck, M.P.H.; Data Management and Information Systems Unit: Anthony C. Gamst, Ph.D. (P.I.), Clint Cushman; Statistics Unit: Ian Abramson, Ph.D. (P.I.), Florin Vaida, Ph.D. (Co-PI), Reena Deutsch, Ph.D., Anya Umlauf, M.S.
The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, the Department of Defense, or the United States Government.
D.J.M. designed the study. A.K.B. wrote the manuscript. S.L.L. and T.H.B. chose the biomarkers for assessment. P.L.F. conducted patient assessments and performed initial data screening. A.K.B. and S.L.L. performed the analysis. M.P. and T.H.B. performed biomarker assays. All authors contributed to the overall study conceptualization and provided critical revision of the manuscript.
This study was supported by the California HIV/AIDS Research Program IDEA Award (ID10-SD-057) and NIH K24 M097673. The study was more broadly supported by NIH R01MH099987 (PI: Dilip Jeste/David J.Moore) and the HIV Neurobehavioral Research Center (HNRC) Center Award P30MH062512 (PI: Robert Heaton).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Worm SW, Friis-Møller N, Bruyand M, et al. : High prevalence of the metabolic syndrome in HIV-infected patients: Impact of different definitions of the metabolic syndrome. AIDS 2010;24:427–435 [DOI] [PubMed] [Google Scholar]
- 2.Nix LM. and Tien PC: Metabolic syndrome, diabetes, and cardiovascular risk in HIV. Curr HIV/AIDS Rep 2014;11:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palios J, Kadoglou NPE, and Lampropoulos S: The pathophysiology of HIV-/HAART-related metabolic syndrome leading to cardiovascular disorders: The emerging role of adipokines. Exp Diabetes Res 2012;2012:103063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pao V, Lee GA, and Grunfeld C: HIV therapy, metabolic syndrome, and cardiovascular risk. Curr Atheroscler Rep 2008;10(1):61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anuurad E, Semrad A, and Berglund L: Human immunodeficiency virus and highly active antiretroviral therapy-associated metabolic disorders and risk factors for cardiovascular disease. Metab Syndr Relat Disord 2009;7:401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kotler DP: HIV and antiretroviral therapy: Lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr 2008;49(Suppl 2):S79–S85 [DOI] [PubMed] [Google Scholar]
- 7.Reeds DN: Metabolic syndrome risks of cardiovascular disease: Differences between HIV-positive and HIV-negative? J Cardiometabolic Syndr 2008;3:79–82 [DOI] [PubMed] [Google Scholar]
- 8.Alberti KGMM, Zimmet P, and Shaw J: Metabolic syndrome–a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet Med 2006;23(5):469–480 [DOI] [PubMed] [Google Scholar]
- 9.Grundy SM, Brewer HB, Cleeman JI, et al. : Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation 2004;109(3):433–438 [DOI] [PubMed] [Google Scholar]
- 10.Ricklin D. and Lambris JD: Complement in immune and inflammatory disorders: Pathophysiological mechanisms. J Immunol 2013;190(8):3831–3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wojciech B, Budkowska M, Sa D, et al. : Clinical analysis of selected complement-derived molecules in human adipose tissue. J Transl Med 2013;11:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nesargikar PN, Spiller B, and Chavez R: The complement system: History, pathways, cascade and inhibitors. Eur J Microbiol Immunol (Bp) 2012;2(2):103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernández-Mijares A, Jarabo-Bueno MM, López-Ruiz A, et al. : Levels of C3 in patients with severe, morbid and extreme obesity: Its relationship to insulin resistance and different cardiovascular risk factors. Int J Obes 2007;31(6):927–932 [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Llamas G, Szalowska E, de Vries MP, et al. : Characterization of the human visceral adipose tissue secretome. Mol Cell Proteom 2007;6(4):589–600 [DOI] [PubMed] [Google Scholar]
- 15.Hertle E, Stehouwer CD, and van Greevenbroek MMJ: The complement system in human cardiometabolic disease. Mol Immunol 2014;61(2):135–148 [DOI] [PubMed] [Google Scholar]
- 16.Bellentani S, Scaglioni F, Marino M, and Bedogni G: Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010;28:155–161 [DOI] [PubMed] [Google Scholar]
- 17.Hertle E, van Greevenbroek MMJ, and Stehouwer CDA: Complement C3: An emerging risk factor in cardiometabolic disease. Diabetologia 2012;55(4):881–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips CM, Goumidi L, Bertrais S, et al. : Complement component 3 polymorphisms interact with polyunsaturated fatty acids to modulate risk of metabolic syndrome. Am J Clin Nutr 2009;90:1665–1673 [DOI] [PubMed] [Google Scholar]
- 19.Ohsawa I, Inoshita H, Ishii M, et al. : Metabolic impact on serum levels of complement component 3 in Japanese patients. J Clin Lab Anal 2010;24:113–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajjan R, Carter AM, Somani R, et al. : Ethnic differences in cardiovascular risk factors in healthy Caucasian and South Asian individuals with the metabolic syndrome. J Thromb Haemost 2007;5:754–760 [DOI] [PubMed] [Google Scholar]
- 21.Bergersen BM, Schumacher A, Sandvik L, et al. : Important differences in components of the metabolic syndrome between HIV-patients with and without highly active antiretroviral therapy and healthy controls. Scand J Infect Dis 2006;38:682–689 [DOI] [PubMed] [Google Scholar]
- 22.Bonfanti P, Giannattasio C, Ricci E, et al. : HIV and metabolic syndrome: A comparison with the general population. J Acquir Immune Defic Syndr 2007;45:426–431 [DOI] [PubMed] [Google Scholar]
- 23.Estrada V, Martínez-Larrad MT, González-Sánchez JL, et al. : Lipodystrophy and metabolic syndrome in HIV-infected patients treated with antiretroviral therapy. Metabolism 2006;55:940–945 [DOI] [PubMed] [Google Scholar]
- 24.Liu P, Sui S, Xu D, et al. : Clinical analysis of the relationship between cystatin C in the elderly. Port J Cardiol 2014;33:411–416 [DOI] [PubMed] [Google Scholar]
- 25.Cepeda J, Tranche-Iparraguirre S, Marín-Iranzo R, et al. : Cystatin C and cardiovascular risk in the general population. Rev Esp Cardiol 2010;63:415–422 [PubMed] [Google Scholar]
- 26.Deeks S: Immune dysfunction, inflammation, and accelerated aging in patients on antiretroviral therapy. Top HIV Med 2015;13:118–123 [PubMed] [Google Scholar]