Abstract
The potential distribution of Amblyomma americanum ticks in Kansas was modeled using maximum entropy (MaxEnt) approaches based on museum and field-collected species occurrence data. Various bioclimatic variables were used in the model as potentially influential factors affecting the A. americanum niche. Following reduction of dimensionality among predictor variables using principal components analysis, which revealed that the first two principal axes explain over 87% of the variance, the model indicated that suitable conditions for this medically important tick species cover a larger area in Kansas than currently believed. Soil moisture, temperature, and precipitation were highly correlated with the first two principal components and were influential factors in the A. americanum ecological niche. Assuming that the niche estimated in this study covers the occupied distribution, which needs to be further confirmed by systematic surveys, human exposure to this known disease vector may be considerably under-appreciated in the state.
Key Words: : Lone star tick, Amblyomma americanum, MaxEnt, Climate, Soil moisture, Temperature, Precipitation
Introduction
Amblyomma americanum (Linn.) (Acari: Ixodidae), or the lone star tick, is a vector for multiple human and animal pathogens in the United States. The best-known diseases resulting from pathogens transmitted by this tick species include human monocytic ehrlichiosis and human ewingii ehrlichiosis (Centers for Disease Control and Prevention 2015a), tularemia (Centers for Disease Control and Prevention 2015b), southern tick-associated rash illness (STARI) (Centers for Disease Control and Prevention 2015c), and feline cytauxzoonosis (Reichard et al. 2010). Recent studies also identified yet another A. americanum–transmitted viral pathogen, Heartland virus from northwestern Missouri, causing a novel emerging disease in people (Savage et al. 2013, Centers for Disease Control and Prevention 2015d). The biology of A. americanum and the pathogens that it vectors have been reviewed by Childs and Paddock (2003) and Goddard and Varela-Stokes (2009). A. americanum–vectored diseases in humans are mostly endemic to regions where the ticks are known to occur, but the status of these diseases elsewhere in the United States remain poorly understood. The current geographic extent of A. americanum distribution estimated by the United States Centers for Disease Control and Prevention (CDC) covers all of the southeastern and eastern United States, including areas covering a large portion of eastern Kansas (www.cdc.gov/ticks/maps/lone_star_tick.html). However, a recent article by Springer and colleagues (Springer et al. 2014) indicated a wider but discontinuous distribution pattern for the state and noted some western counties in Kansas that have reported this species over the last century.
Some evidence suggests that the disease agents transmitted by A. americanum ticks in Kansas may be increasing (Raghavan et al. 2013, 2014). The spatio-temporal pattern of human monocytic ehrlichiosis, vectored by A. americanum, used to be concentrated in the southeastern counties in the state but is now reported commonly from most eastern and many central Kansas counties (Raghavan et al. 2014). Although other factors could influence this trend, such as human or animal movements, better awareness among physicians and patients of tick-borne illnesses, and improved and easy access to diagnostic techniques, the potential for A. americanum–vectored transmission from increasing abundance and geographic expansion is a concern. The current spatial distribution pattern estimated by the CDC for A. americanum in Kansas is based on acarological surveys conducted around 1945 (Bishopp and Trembley 1945), yet much may have changed in the ensuing years. Knowledge of the spatial extent of a vector species distribution is important for management and prevention of diseases that they transmit. One effective approach to understand species distribution is through correlative modeling (Phillips et al. 2006, Estrada-Peña and Venzal 2007).
Diverse methods have been developed for modeling Grinnellian niches of species and estimating their geographic distributions (Soberón 2007, Soberón and Nakamura 2009). Modeling approaches such as climate envelopes, logistic regression, multivariate regression splines, and boosted regression trees require absence data for modeling, but such data are difficult to obtain and may also be unreliable (Elith et al. 2006, Phillips et al. 2006). Other approaches such as the genetic algorithm for rule-set production (GARP) and maximum entropy (MaxEnt) do not require species absence data and have been used to model ecological niches and estimate potential distributions of a wide variety of species. Inclusion of species absence data, however, yields better information about prevalence than presence-only methods (Elith et al. 2009); such information is incorporated in GARP and MaxEnt methods as background or pseudo-absence data (Phillips and Dudik 2008, Stockwell 2009). The quality of predictions based on different modeling approaches and their interpretation has been discussed previously (Phillips et al. 2004, Peterson et al. 2007). Some studies have shown MaxEnt to produce consistently robust species distribution estimates among presence-only methods (Tsoar et al. 2007, Elith et al. 2009, Feria-Arroyo et al. 2014).
The spatial distribution of most arthropods, including A. americanum ticks, are limited for the most part by climatic conditions and physical environment, such as landscape cover and landscape structure. Other influential factors that limit species' distributions include ecological forces such as predator availability and density, competition, and host abundance, which are difficult to incorporate in correlative models (Thuiller et al. 2006, Soberón and Nakamura 2009). In this study, we modeled the ecological niche of A. americanum in Kansas using a maximum entropy approach and evaluated important bioclimatic and physical environment determinants of that niche.
Materials and Methods
Species distribution data
Lone star ticks are widely present in the eastern, southeastern, and midwestern United States (Childs and Paddock 2003). Species distribution data were obtained from three sources. The Walter Reed Biosystematics Unit (WRBU), based in the Smithsonian Institution, made available historical collection data, which included presence records from across the species' range in North America. The Kansas State University Museum of Entomological and Prairie Arthropod Research (MEPAR) provided tick collection data from 1982 to 1995. Taxonomic label data in this collection were reviewed, and records with discernable textual location information were georeferenced using the MaNIS georeferencing calculator (http://manisnet.org/search.shtml). Only records that had an error radius of ≤1 km were used in analyses. Finally, we conducted tick surveys in central and eastern Kansas during May through August of 2012–2014. The presence of larval, nymphal, and both sexes of adult stages of A. americanum ticks in a given survey location was considered to indicate species presence in this study. In total, there were 461, 107, and 258 unique presence locations obtained from WRBU, MEPAR, and tick surveys, respectively. Counties in which A. americanum ticks were positively identified in the three data sources are shown in Figure 1. Duplicate presence locations within 1 km were removed to avoid redundancy; presence records were rarefied whenever clusters of points were noted by removing minimum necessary points until they were ≥2 km apart. These steps resulted in 682 unique presence records for modeling.
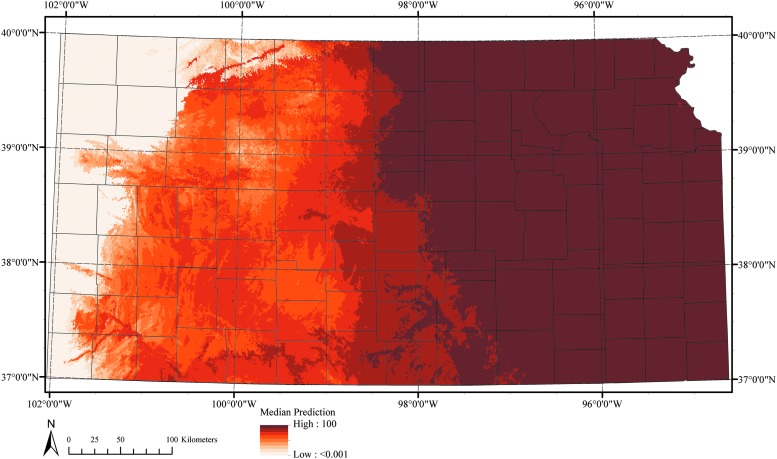
FIG. 1.
Abiotically suitable regions for A. americanum ticks in Kansas as modeled with MaxEnt.
Environmental variables
Environmental variables summarizing aspects of climate were prepared to summarize important potential drivers of the A. americanum ecological niche. That is, summaries of temperature, precipitation, and soil moisture index were obtained from the CliMond archive (Kriticos et al. 2012). CliMond contains gridded historical climate data at 10′ or 30′ resolution collectively, representing a statistical summary of temperature, precipitation, radiation, and soil moisture, primarily using historical data sourced from WorldClim (Hijmans et al. 2005) and Climate Research Unit datasets (www.cru.uea.ac.uk/cru/data/hrg/). We used CliMond as our source for climate data instead of WorldClim, which is used more frequently by others, because the latter does not include data on soil moisture estimates that are ecologically relevant in the A. americanum life cycle.
High correlations among independent climatic variables are well known and their simultaneous presence in MaxEnt models has been shown to cause problems. To address this concern, a priori selection of variables (Medley 2010, Tonini et al. 2014) was carried out using the Band Collection Statistics tool in ArcGIS 10.1 to exclude pairs of highly correlated variables (r > 0.8). Dimensionality among independent variables was reduced by conducting principal component analysis using SPSS version 18 (IBM Corporation, Somers, NY), which yields uncorrelated axes of variance, or principal components. Principal components analysis was conducted by extracting climate and habitat data from 10,000 random points across the study area. The corresponding values were standardized such that each variable had a zero mean and standard deviation of 1. Data layers representing principal components were then used for modeling species distribution, and variable loading scores were used to interpret the importance of different variables to each factor, and ultimately A. americanum niche.
Niche modeling
We used the maximum entropy algorithm MaxEnt version 3.3.3k for modeling the ecological niche of A. americanum. A statistical explanation of the algorithm is provided in Elith et al. (2011). Briefly, the ecological niche and/or the spatial distribution of a species can be modeled in MaxEnt using correlative algorithms and known point-occurrences of a given species in relation to various environmental constraints. For this study, models were run mostly with default settings (Phillips et al. 2006), except as following. We set aside 25% of occurrence points for the binary omission rates tests, and the remaining were used to run 20 cross-validation replicates. The advantage of using cross-validation is that it uses all of the data for model building and validation, unlike the single training/test data split. We used the subsampling method in MaxEnt for randomly selecting test data points, and the model was iterated 5000 times to allow sufficient time for model convergence. Two complementary models were run, the first containing climatic variables only and the second with climatic and land cover variables.
We evaluated the model performance using area under the curve (AUC) scores, which is a measure of the area under a receiver operator characteristic curve (ROC) that plots the rate of true positives to false positives. It varies between 0.5 when the result is not better than random and 1.0 when the result is significantly better than random. Another evaluation method based on false negatives (omission error) was also used. MaxEnt calculates the omission error rate for training and test data, which indicates the percentage of test localities that falls into pixels not predicted as suitable for a given species (Phillips et al. 2006). Better models have low or nonsignificant omission rates. The AUC values and standard deviation of the replicated models, and the omission rates at threshold of M10, which have been suggested as an appropriate threshold (Pearson et al. 2006) were used.
Results
The principal components analysis of independent climatic variables defined an environmental space of reduced dimensionality that allowed modeling the ecological niche of A. americanum. The first two axes of the principal components analysis explained 87.9% of the total variance in the data (Table 1) and were significantly different from random (p < 0.01). The first principal component, consisting mostly of variables representing soil moisture index and also temperature, explained 61.4% of the variance; the second axis, consisting of variables primarily representing precipitation, explained 26.4% of the variance (Table 1).
Table 1.
Variables and Principal Component Analysis Loadings for the First Two Main Axes
| Factor loadingsa | |||
|---|---|---|---|
| Source | Variable | PC-1 | PC-2 |
| CliMond | (Bio01) Annual mean temperature (°C) | –0.75 | −0.18 |
| (Bio02) Mean diurnal temperature range | 0.68 | −0.24 | |
| (Bio07) Temperature annual range (Bio05-Bio06) (°C) | 0.19 | 0.01 | |
| (Bio11) Mean temperature of coldest quarter (°C) | −0.31 | −0.22 | |
| (Bio16) Precipitation of wettest quarter (mm) | 0.05 | 0.82 | |
| (Bio19) Precipitation of coldest quarter | −0.14 | 0.73 | |
| (Bio28) Annual mean moisture index | −0.51 | 0.13 | |
| (Bio30) Lowest weekly moisture index | 0.48 | −0.19 | |
| (Bio32) Mean moisture index of wettest quarter | 0.81 | 0.05 | |
| (Bio35) Mean moisture index of coldest quarter | 0.92 | −0.01 | |
| Eigenvalue | 6.24 | 3.88 | |
| % Explained variance | 61.42 | 26.48 | |
| Cumulative % variance | 87.90 | ||
Variables strongly correlated (r > 0.7) with the axes are shown in bold.
The ROC analysis of the resulting MaxEnt models based on all presence data and principal components as predictor variables indicated adequate performance without overfitting to training data (Table 2). When applying the M10 threshold to the cross-validation replicate with highest AUC values (0.84) for the models, they were found to perform significantly better than random (p < 0.01), with no records used for modeling falling outside the predicted suitable area. MaxEnt output includes a jackknife analysis of the contribution of each variable to the model (Table 2). Of the two principal components used as predictor variables in the model, the first component contributed most to the model, followed by the second component (Table 2).
Table 2.
Model Results with Bioclimatic Determinants of Amblyomma americanum Distribution in Kansas
| Jackknife results | |||||
|---|---|---|---|---|---|
| Model | Training AUC (mean ± SD) | Testing AUC (mean ± SD) | Avg. variable contribution (%) | Avg. AUC without the variable | Avg. AUC with only the variable |
| Climatic variables | 0.87 ± 0.01 | 0.82 ± 0.01 | |||
| PC-1 | 81.81 | 0.74 | 0.78 | ||
| PC-2 | 19.18 | 0.79 | 0.62 | ||
AUC, area under the curve; SD, standard deviation; PC, principal component axes.
On the basis of the average values of the 20 MaxEnt models generated, the median distribution for A. americanum using the three principal components as predictor variables is presented in Figure 2. The pixels with highest presence probabilities were concentrated in an east to west gradient, with the eastern region gradually more suitable than the west. Areas predicted to be suitable for this species in the present study covered a visibly larger area in the central and western areas of Kansas than currently estimated by the CDC.
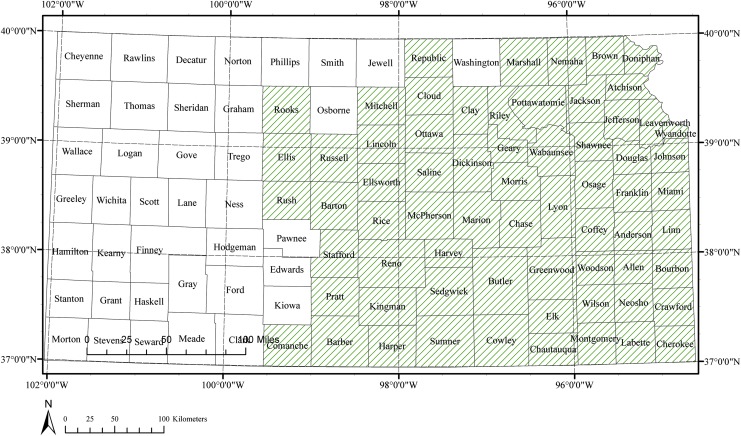
FIG. 2.
County-level presence map of A. americanum ticks in Kansas. Shaded counties include one or multiple locations where nymph, larvae, and adult stages of A. americanum were recorded by one or more presence data source in this study—the Walter Reed Biosystematics Unit, the Prairie Arthropod Research at Kansas State University, and field collections conducted between years 2012 and 2014.
Discussion
Most attempts to study the distribution and abundance of A. americanum and other tick species in Kansas have been made in the more populated eastern and central parts of the state, so few data exist from the western areas. Although MaxEnt and other niche modeling approaches are perhaps capable of predicting species' distributions in unsampled areas, extrapolations based on such spatially biased data should be interpreted with caution (Stockwell and Peterson 2002, Pearson et al. 2007). Presence-only models such as MaxEnt provide a robust and repeatable method for studying species distributions; however, limitations of such methods have been discussed elsewhere (Elith et al. 2011, Karplus 2011, Hijmans 2012). One particular problem with correlative modeling is the accidental introduction of false-positives (localities where species was collected but long-term population establishment is not feasible in such places) and false-negatives (conditions are suitable but the species has not reached due to natural barriers). This issue is also true for other statistical datasets and can be mitigated to a considerable extent through careful evaluation and ground-truthing, which we attempted in this study. The model output for A. americanum in this study should not be interpreted as the definitive limits of its range; cell-level occurrence probabilities should be used only as a guide for actual detailed field evaluations of this species' presence and abundance.
Our model indicates that the area suitable for A. americanum in Kansas is likely larger than the area currently suggested by the CDC. Particularly, the central and western portions of the state are likely suitable for A. americanum (Fig. 2), which is to some extent consistent with Springer et al. (2014), wherein a few western counties in Kansas were highlighted for A. americanum presence. That study was based on historic tick collection records covering the entire United States for over a century, and although it leaves many central Kansas counties without information, the authors noted that true absence needs confirmation in systematic surveys. The contrast with CDC's distribution map could be a result of many factors, including methods used for distribution modeling and adequacy of presence data used for predictions. The current CDC prediction is based on acarological surveys conducted over a broad region around 1945 (Bishopp and Trembley 1945), and the distribution of A. americanum has likely changed since that time owing to environmental, climatic, and anthropogenic influences.
The model evaluation criteria used in the study indicated satisfactory performance. Although similar studies evaluating the ecological niche or the spatial distribution for other species have achieved higher AUC values up to and above 0.9, our models consistently performed around 0.8. The significant variables in our models, based on variable associations with the first two axes (r > 0.7), were consistent with the biology of A. americanum ticks. The first principal component was primarily associated with variables representing soil moisture index and temperature. Soil moisture and temperature are important aspects in tick ecology, and the availability of soil moisture has been suggested as an important factor in characterizing tick habitats (Randolph 2000, Berger et al. 2013). Higher soil moisture content, lower midday temperature, and increased cloud cover are linked to increased questing activity of a similar hard tick, Ixodes ricinus (Medlock et al. 2013). Conditions that negatively affect soil moisture, such as droughts, can result in reduced host-seeking behavior and may increase mortality among quiescent A. americanum.
The second component of principal component analysis was associated with precipitation, which could be linked to soil moisture availability, but also may be a proxy for other factors, such as availability of vegetation and host density. Large variations exist in annual rainfall across Kansas, with eastern Kansas receiving up to three times more rainfall than the western portion (Goodin et al. 2004). As a result, climate and vegetation are transitional between the wetter east and semiarid western Kansas, which may explain the relatively higher probability for A. americanum habitat suitability in the east versus central and western portions of the state. The normalized difference vegetation index (NDVI) has been suggested, in general, as a better predictor for some tick spatial distributions compared to precipitation variables because it better captures water availability (Randolph 2000, Estrada-Peña et al. 2013). However, precipitation variables may perform adequately for regional studies such as the present one (Estrada-Peña et al. 2013).
The potential for a broader distribution of A. americanum ticks in Kansas and the wider region in general is worrisome because of the number of human and animal diseases these ticks are known to vector. A recent study (Raghavan et al. 2014a) has shown a steady spatio-temporal progression of human monocytic ehrlichiosis (HME) in Kansas during years 2005–2012. The spatial distribution noted in that study for a steady HME spatiotemporal progression has high visible concordance with the predicted distribution for A. americanum in the present study. Other diseases vectored by these ticks could also be increasing in the region. The number of cases of feline tularemia (Raghavan et al. 2013) and cytauxzoonosis (Raghavan et al. 2014b) in the region diagnosed at the Kansas State Veterinary Diagnostic Laboratory (KSVDL) have increased steadily over the years, at least partly owing to the wider geographic distribution of A. americanum. An important contributing factor for the current distribution of this species in the state and increases in A. americanum–vectored diseases could be the almost exponential population increase of their primary host, the white-tailed deer, in Kansas in the past two decades (Paddock and Yabsley 2007, Kansas Department of Wildlife, Parks and Tourism [KDWP] 2015). Studies evaluating nonstationary ecological processes (e.g., changes in climate patterns, landscape fragmentation) that may influence vector–host and as well as vector–human contact rates cannot be found for A. americanum ticks and are worthy of consideration.
Finally, ongoing warming of global temperatures will likely influence the ecology and distribution of such medically important ticks. Changes in climatic patterns, including regional increases in temperatures and shifts in precipitation, are altering the structure and function of ecosystems globally (Parmesan and Yohe 2003). These changes can favor emergence of new parasites and new disease agents transmitted by ticks (Epstein 2001, Harvell et al. 2002), and also alter host–parasite relationships (Kutz et al. 2005). Climate conditions considered typical for the Central Plains region (including Kansas) have already been noted to have changed in noticeable ways (Schoof 2013, Hayhoe et al. 2015), and many such conditions are known to affect tick phenology and spatial distribution either directly or indirectly. For instance, diurnal temperature range, a climate-change index, has been decreasing steadily since the 1950s, particularly in the midwestern United States but also other regions of North America (Karl et al. 1991, 1993). Increased atmospheric humidity during spring and summer months over the Northern Plains was noted for roughly the same time period (Schwartz 1995). Other ixodid ticks (Ixodes scapularis and I. ricinus) occurring in northern latitudes have already shown shifts in their distribution and abundance that have been linked to warming climate (Daniel et al. 2003, Leighton et al. 2012, Descamps 2013). Any such effect on A. americanum distribution in the midwestern United States is yet to be documented and requires further studies.
Acknowledgements
We are grateful to Dr. Townsend Peterson, University of Kansas, and two anonymous reviewers who provided generous comments to improve earlier versions of the manuscript. Bryanna Pockrandt and Mal Hoover, College of Veterinary Medicine, Kansas State University, provided excellent technical assistance. This article is Contribution No. 15-419-J from the Kansas Agricultural Experiment Station (KAES) and was supported in part by the PHS grant number AI070908 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA; and KAES Hatch Project No. 353, Insect Systematics. Funds for this research were also provided by Kansas State Veterinary Diagnostic Laboratory (KSVDL).
Author Disclosure Statement
No competing financial interests exist.
References
- Berger KA, Wang Y, Mather TN. MODIS-derived land surface moisture conditions for monitoring blacklegged tick habitat in southern New England. Int J Remote Sens 2013; 34:73–85 [Google Scholar]
- Bishopp FC, Trembley HL. Distribution and hosts of certain North American ticks. J Parasitol 1945; 31:1–54 [Google Scholar]
- Centers for Disease Control and Prevention. Ehrlichiosis Website. 2015a. Available at www.cdc.gov/ehrlichiosis/
- Centers for Disease Control and Prevention. Tularemia Website. 2015b. Available at www.cdc.gov/tularemia/transmission/
- Centers for Disease Control and Prevention. Southern Tick–Associated Rash Illness Website. 2015c. Available at www.cdc.gov/stari/
- Centers for Disease Control and Prevention. Division of Vector-Borne Diseases (DVBD) Website. 2015d. Available at www.cdc.gov/ncezid/dvbd/heartland/
- Childs JE, Paddock CD. The ascendancy of Amblyomma americanum as a vector of pathogens affecting humans in the United States. Annu Rev Entomol 2003; 48:307–337 [DOI] [PubMed] [Google Scholar]
- Daniel M, Danielova V, Kriz B, Jirsa A, Nozicka J. Shift of the tick Ixodes ricinus and tick-borne encephalitis to higher altitudes in Central Europe. Eur J Clim Microbiol 2003; 22:327–328 [DOI] [PubMed] [Google Scholar]
- Descamps S. Winter temperature affects the prevalence of ticks in an Arctic seabird. PLoS One 2013; e65374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elith J, Leathwick JR. Species distribution models: Ecological explanation and prediction across space and time. Annu Rev Ecol Syst 2009; 40:677–697 [Google Scholar]
- Elith J, Graham CH, Anderson RP, Dudík M, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography 2006; 29:129–151 [Google Scholar]
- Elith J, Phillips SJ, Hastie T, Dudik M, et al. A statistical explanation of MaxEnt for ecologists. Diversity Distrib 2011; 17:43–57 [Google Scholar]
- Epstein PR. Climate change and emerging infectious diseases. Microbes Infection 2001; 3:747–754 [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Venzal JM. Climate niches of tick species in the Mediterranean region: Modeling of occurrence data, distributional constraints, and impact of climate change. J Med Entomol 2007; 44:1130–1138 [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Estrada-Sánchez A, Estrada-Sánchez D, de la Fuente J. Assessing the effects of variables and background selection on the capture of the tick climate niche. Int J Health Geogr 2013; 12:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feria-Arroyo TP, Castro-Arellano I, Gordillo-Perez G, Cavazos AL, et al. Implications of climate change on the distribution of the tick vector Ixodes scapularis and risk for Lyme disease in the Texas–Mexico transboundary region. Parasites Vectors 2014; 7:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J, Varela-Stokes AS. Role of the lone star tick, Amblyomma americanum (L.), in human and animal diseases. Vet Parasitol 2009; 160:1–12 [DOI] [PubMed] [Google Scholar]
- Goodin DG, Mitchell JE, Knapp MC, Bivens RE. Climate and weather atlas of Kansas. An Introduction. 2004. Available at https://www.k-state.edu/ksclimate/documents/kgsed.pdf/
- Harvell CD, Mitchell CE, Ward JR, Altizer S, et al. Climate warming and disease risks for terrestrial and marine biota. Science 2002; 296:2158–2162 [DOI] [PubMed] [Google Scholar]
- Hayhoe K, VanDorn J, Naik V, Wuebbles D. Climate change in the Midwest. Projections of future temperature and precipitation. Available at www.ucsusa.org/sites/default/files/legacy/assets/documents/global_warming/midwest-climate-impacts.pdf
- Hijmans RJ. Cross-validation of species distribution models: Removing spatial sorting bias and calibration with a null model. Ecology 2012; 93:679–688 [DOI] [PubMed] [Google Scholar]
- Hijmans RJ, Cameron SE, Parra JL, Jones PG, et al. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 2005; 25:1965–1978 [Google Scholar]
- Karl TR, Kukla G, Razuvayev VN, Changery MJ, et al. Global warming—evidence for asymmetric diurnal temperature change. Geophys Res Lett 1991; 18:2253–2256 [Google Scholar]
- Karl TR, Jones PD, Knight RW, Kukla G, et al. A new perspective on recent global warming—asymmetric trends of daily maximum and minimum temperature. Bull Am Meteorolog Soc 1993; 74:1007–1023 [Google Scholar]
- Karplus K. Better than chance: The importance of null models, University of California, Santa Cruz, in Proceedings of the First International Workshop on Pattern Recognition in Proteomics, Structural Biology and Bioinformatics, 2011 [Google Scholar]
- Kansas Department of Wildlife, Parks and Tourism (KDWP). 2015. kdwpt.state.ks.us/KDWPT-Info/Locations/Hunting-Fishing-Atlas/Spring-Hunting-Atlas/
- Kriticos DJ, Webber BL, Leriche A, Ota N, et al. CliMond: Gobal high resolution historical and future scenario climate surfaces for bioclimatic modelling. Methods Ecol Evol 2012; 3:53–64 [Google Scholar]
- Kutz SJ, Hoberg EP, Polley L, Jenkins EJ. Global warming is changing the dynamics of Arctic host-parasite systems. Proc R Soc Lond B 2005; 272:2571–2576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighton PA, Koffi JK, Pelcat Y, Lindsay LR, et al. Predicting the speed of tick invasion: An empirical model of range expansion for the Lyme disease vector Ixodes scapularis in Canada. J Appl Ecol 2012; 49:457–464 [Google Scholar]
- Medley KA. Niche shifts during the global invasion of the Asian tiger mosquito, Aedes albopictus Skuse (Culicidae), revealed by reciprocal distribution models. Global Ecol Biogeogr 2010; 19:122–133 [Google Scholar]
- Medlock JM, Hansford KM, Bormane A, Derdakova M, et al. Driving forces for changes in geographical distribution of Ixodes ricinus ticks in Europe. Parasites Vectors 2013; 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock CD, Yabsley MJ. Ecological havoc, the rise of white-tailed deer, and the emergence of Amblyomma americanum-associated zoonoses in the United States. In: Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission. Berlin Heidelberg: Springer, 2007:289–324 [DOI] [PubMed] [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003; 421:37–42 [DOI] [PubMed] [Google Scholar]
- Pearson RG, Thuiller W, Araújo MB, Martinez‐Meyer E, et al. Model‐based uncertainty in species range prediction. J Biogeogr 2006; 33:1704–1711 [Google Scholar]
- Pearson RG, Raxworthy CJ, Nakamura M, Peterson AT. Predicting species distributions from small numbers of occurrence records: A test case using cryptic geckos in Madagascar. J Biogeogr 2007; 34:102–117 [Google Scholar]
- Peterson AT, Papeş M, Eaton M. Transferability and model evaluation in ecological niche modeling: A comparison of GARP and Maxent. Ecography 2007; 30:550–560 [Google Scholar]
- Phillips SJ, Dudík M. Modeling of species distributions with MaxEnt: New extensions and a comprehensive evaluation. Ecography 2008; 31:161–175 [Google Scholar]
- Phillips SJ, Dudík M, Schapire RE. A maximum entropy approach to species distribution modeling. In: Proceedings of the Twenty-First International Conference on Machine Learning, Association for Computing Machinery, 2004:83 [Google Scholar]
- Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecol Model 2006; 190:231–259 [Google Scholar]
- Raghavan RK, Harrington JA, Jr, Anderson GA, Shawn Hutchinson JM, et al. Environmental, climatic, and residential neighborhood determinants of feline tularemia. Vector Borne Zoonotic Dis 2013; 13:449–456 [DOI] [PubMed] [Google Scholar]
- Raghavan RK, Neises D, Goodin DG, Andresen DA, et al. Bayesian spatio-temporal analysis and geospatial risk factors of human monocytic ehrlichiosis. PLoS One 2014a; e100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan RK, Almes K, Goodin DG, Harrington JA Jr, et al. Spatially heterogeneous land cover/land use and climatic risk factors of tick-borne feline cytauxzoonosis. Vector Borne Zoonotic Dis 2014b; 14:486–495 [DOI] [PubMed] [Google Scholar]
- Randolph SE. Ticks and tick-borne disease systems in space and from space. Adv Parasitol 2000; 47:217–243 [DOI] [PubMed] [Google Scholar]
- Reichard MV, Edwards AC, Meinkoth JH, Snider TA, et al. Confirmation of Amblyomma americanum (Acari: Ixodidae) as a vector for Cytauxzoon felis (Piroplasmorida: Theileriidae) to domestic cats. J Med Entomol 2010; 47:890–896 [DOI] [PubMed] [Google Scholar]
- Savage HM, Godsey MS, Jr, Lambert A, Panella NA, et al. First detection of Heartland virus (Bunyaviridae: Phlebovirus) from field collected arthropods. Am J Trop Med Hyg 2013; 89:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof JT. Historical and projected changes in human heat stress in the Midwestern United States. In: Pryor SC, ed. Climate Change in the Midwest: Impacts, Risks, Vulnerability and Adaptation. Bloomington, IN: Indiana University Press, 2013:146–157 [Google Scholar]
- Schwartz MD. Detecting structural climate change: an air mass-based approach in the north central United States, 1958–1992. Annals of the Association of American Geographers, 1995; 85:553–568 [Google Scholar]
- Soberón J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett 2007; 10:1115–1123 [DOI] [PubMed] [Google Scholar]
- Soberón J, Nakamura M. Niches and distributional areas: Concepts, methods, and assumptions. Proc Natl Acad Sci USA 2009; 106:19644–19650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer YP, Eisen L, Beati L, James AM, et al. Spatial distribution of counties in continental United States with records of Amblyomma americanum (Ixodida: Ixodidae). J Med Entomol 2014; 51:342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockwell D. The GARP modelling system: Problems and solutions to automated spatial prediction. Int J Geogr Information Sci 2009; 13:143–158 [Google Scholar]
- Stockwell D, Peterson AT. Effects of sample size on accuracy of species distribution models. Ecol Model 2002; 148:1–13 [Google Scholar]
- Thuiller W, Richardson DM, Rouget M, Procheş Ş, et al. Interactions between environment, species traits, and human uses describe patterns of plant invasions. Ecology 2006; 87:1755–1769 [DOI] [PubMed] [Google Scholar]
- Tonini F, Divino F, Lasinio GJ, Hochmair HH, et al. Predicting the geographical distribution of two invasive termite species from occurrence data. Environ Entomol 2014; 43:1135–1144 [DOI] [PubMed] [Google Scholar]
- Tsoar A, Allouche O, Steinitz O, Rotem D, et al. A comparative evaluation of presence-only methods for modelling species distribution. Diversity Distributions 2007; 13:397–405 [Google Scholar]