Abstract
Background:
Human influenza is a seasonal disease associated with significant morbidity and mortality. Anti-flu ayurvedic/herbal medicines have played a significant role in fighting the virus pandemic. Plumbagin and allicin are commonly used ingredients in many therapeutic remedies, either alone or in conjunction with other natural substances. Evidence suggests that these extracts are associated with a variety of pharmacological activities.
Objective:
To evaluate anti-influenza activity from Plumbago indica and Allium sativum extract against Influenza A (H1N1)pdm09.
Materials and Methods:
Different extraction procedures were used to isolate the active ingredient in the solvent system, and quantitative HPLTC confirms the presence of plumbagin and allicin. The cytotoxicity was carried out on Madin-Darby Canine kidney cells, and the 50% cytotoxic concentration (CC50) values were below 20 mg/mL for both plant extracts. To assess the anti-influenza activity, two assays were employed, simultaneous and posttreatment assay.
Results:
A. sativum methanolic and ethanolic extracts showed only 14% reduction in hemagglutination in contrast to P. indica which exhibited 100% reduction in both simultaneous and posttreatment assay at concentrations of 10 mg/mL, 5 mg/mL, and 1 mg/mL.
Conclusions:
Our results suggest that P. indica extracts are good candidates for anti-influenza therapy and should be used in medical treatment after further research.
SUMMARY
The search for natural antiviral compounds from plants is a promising approach in the development of new therapeutic agents. In the past century, several scientific efforts have been directed toward identifying phytochemicals capable of inhibiting virus. Knowledge of ethnopharmacology can lead to new bioactive plant compounds suitable for drug discovery and development. Macromolecular docking studies provides most detailed possible view of drug-receptor interaction where the structure of drug is designed based on its fit to three dimensional structures of receptor site rather than by analogy to other active structures or random leads. Our previous studies indicate that Allicin sand Plumbagin could be used as the potent multi drug targets against the Neuraminidase, Hemagglutinin and M2 protein channel of influenza A (H1N1) pdm09. This in-vittro study has shown that P. indica L. and A. sativum extracts can inhibit influenza A (H1N1)pdm09 virus by inhibiting viral nucleoprotein synthesis and polymerase activity.
Keywords: Allium sativum, Anti-influenza activity, Cytotoxicity, Hemagglutination, Plumbago indica
INTRODUCTION
Influenza is considered to be one of the life-threatening infectious diseases. Seasonal influenza affects up to 40% of the population annually in few countries, and people die from it worldwide every year. At present, the development of antiviral drugs represents a crucial strategy in the control and prevention of seasonal and pandemic influenza infections.[1,2,3,4]
The application of in silico techniques to antiviral research has already led to the design of several compounds approved by the US Food and Drug Administration and now used in clinical therapy. Indeed, one of the earliest successes of this technique is represented by the discovery of Zanamivir (a neuraminidase inhibitor).[5,6,7] Only after the determination of the three-dimensional structure of sialidase, did the breakthrough arise to design new selective inhibitors using structure-based approaches.[7,8]
In a previous study, we have used in silico subtractive genomics analysis to identify essential protein targets in influenza virus. Further, we proceed with comparative molecular docking analysis against neuraminidase, hemagglutinin, and M2 protein with well-known influenza drugs and virtual libraries of natural compounds. We selected Plumbago indica and Allium sativum plants for the present study based on dockability prediction, postdocking analysis, presence of phytochemicals in higher concentrations and availability of those natural plants. The complete analysis is discussed by Chavan et al., 2014.[9]
Recently, significant attention has been paid to utilize eco-friendly and bio-friendly plant-based products for the prevention and cure of different human diseases. Considering the adverse effects of synthetic drugs, many Ayurvedic experts are looking for natural remedies, which are safe and effective. It is recognized that most of the world's population have taken in traditional medicine; particularly, plant drug for the primary health care. The Indian flora offers a diversity of plants having medicinal properties. These plants can be exploited to find out an effective alternative to synthetic drugs.[10]
P. indica, belonging to the family Plumbaginaceae, is a family of flowering plants, with a cosmopolitan distribution. The family is occasionally referred to as the leadwort family or the plumbago family. Plumbaginaceae is distributed as a weed all over the tropical and subtropical countries of the world. The family Plumbaginaceae consists of 10 genera and 280 species. The genus Plumbago includes three species, namely P. indica. Linn, Procavia capensis. Linn, and Plumbago zeylanica. Linn, which are distributed in several parts of India.[11] P. indica root increases digestive power, promotes appetite, and has long been marked as a powerful antiseptic. A liniment made from bruised root mixed with some amount of bland oil is used in treating rheumatism, paralysis, leukoderma, enlarged glands and buboes and scorpion-sting.[12]
Allicin, one of the active ingredients of freshly crushed garlic (A. sativum) homogenates, has a variety of antimicrobial activities. Allicin in its pure form was found to exhibit antibacterial activity against a wide range of Gram-negative and Gram-positive bacteria, including multidrug-resistant enterotoxigenic strains of Escherichia coli; antifungal activity, particularly against Candida albicans; antiparasitic activity, including some major human intestinal protozoan parasites such as Entamoeba histolytica and Giardia lamblia; and antiviral activity. The main antimicrobial effect of allicin is due to its chemical reaction with thiol groups of various enzymes, e.g., alcohol dehydrogenase, thioredoxin reductase, and RNA polymerase.[13]
P. indica and A. sativum have wide antimicrobial effects[12,13] and have been extensively studied in various human disorders. Yet we could not find any research work that reports plumbagin and allicin as active anti-influenza agents. Therefore, in this report, we investigate anti-influenza activities of crude extracts obtained from P. indica and A. sativum and have shown a comparison between their activities in vitro.
MATERIALS AND METHODS
This study was conducted in the Department of Virology and Immunology, Haffkine Institute for Training, Research and Testing, Mumbai, India, and it was carried out for 9 months from August 2012 to March 2013. It was aimed to analyze the phytochemical constituents and the antiviral effects of the plant extracts on the basis of hemagglutination reduction assay. The study was approved by the Institutional Ethics Committee of Haffkine Institute.
Preparation of the plant extract
Fresh roots of P. indica Linn (red chitrak) were collected from Krishi vidyalaya, Pune and fresh A. sativum Linn (garlic) were collected from Dadar local market, Mumbai. The plant was authenticated by comparing with corresponding herbarium specimen at Herbarium at Sathaye College, Mumbai with the reference specimen (A. sativum L-M.15 and P. indica L-D/G-27). Leaves were washed with distilled water, shade-dried, and powdered. Thirty grams of the powdered sample of A. sativum bulbs were subjected to soxhlet (Borosil, Mumbai) extraction separately with 300 mL each with concentration of 70% ethanol and 98% methanol at room temperature for 24 h. Similarly, 30 gm of P. indica roots was subjected to cold maceration and 15 h of soxhlet extraction using methanol:chloroform (70:30 V/V). The solvent extract obtained was evaporated to dryness in a rotary evaporator in a vacuum. The extracts were filtered with Whatman No 1 filter paper and concentrated and reconstituted at 100 mg/mL in the minimum essential medium (MEM).
Reagents
All extraction reagents such as ethanol, methanol, were analytical reagent grade purchased from Hi-media Labs, Mumbai. Reagents for cell culture, such as MEM, trypsin-ethylenediaminetetraacetic acid (EDTA), and sodium bicarbonate, antibiotics, and oseltamivir phosphate were purchased from Sigma-Aldrich, India (Cata1og No-479304). Precoated silica gel 60G F254 TLC aluminum plates (10 cm × 10 cm, 0.2 mm thick) were obtained from E. Merck Ltd.,(Mumbai, India). Analytical grade toluene, ethyl acetate, methanol, chloroform, glacial acetic acid, diethylamine, and formic acid were obtained from SD Fine Chem. Ltd (Mumbai, India).
Cell line and viruses
Madin-Darby Canine Kidney (MDCK) cell lines were procured from Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGI, Lucknow, India) and were grown in MEM with L-glutamine (2 mM), penicillin (100 IU/mL), streptomycin (100 µg/mL), and gentamicin (10 µg/mL), and supplemented with 10% fetal bovine serum. The influenza A (H1N1)pdm09 virus was obtained from the Department of Microbiology, SGPGI, Lucknow, India. Reagents for cell culture, such as MEM, Trypsin-EDTA, and sodium bicarbonate, antibiotics, and oseltamivir phosphate were purchased from Life Technologies (C. A. USA).
Confluent MDCK cell monolayers in 96-well tissue culture plates were washed once with serum-free MEM before use. Serial 10-fold dilutions of virus in serum-free MEM containing 0.3% bovine serum albumin and 1 µg/mL L-(tosylamido 2-phenyl) ethyl chloromethyl ketone-treated trypsin (Sigma) were incubated in replicate wells (200 µL/well) for 2–3 days at 37°C temperature with 5% CO2. Wells positive for virus growth were identified by the presence of hemagglutinating activity in the supernatant, and hemagglutination units (HAU) were calculated. The virus stocks were stored at −80°C temperature for further use.
Qualitative phytochemical analysis
The basic phytochemical test such as the detection of alkaloids (Mayers test), carbohydrates (Molisch test), saponins (Froth test), phenols (ferric chloride test), tannins (gelatin test), flavonoids (lead acetate test), and amino acid (ninhydrin test) were carried out as per the standard test previously mentioned.[14]
Quantitative phytochemical analysis of the extract using high-performance thin layer chromatography
Quantitative analysis of allicin from A. sativum and Plumbagin from P. indica was performed using a Camag high performance thin layer chromatography (HPTLC) system equipped with sample applicator and a Camag TLC scanner at 254 nm wavelength and data filtering by win CATS V-1.2.3 at GNIRD laboratory, Guru Nanak Khalsa College, Mumbai. Table 1 illustrates the various phytochemicals screened with the respective solvent system and derivatizing agent.
Table 1.
List of targeted phytochemicals, solvent systems, and derivatizing agents used in HPTLC analysis

Cytotoxicity assessment
The evaluation of cytotoxic activity of plant extracts cell cytotoxicity (CC50) was carried out using MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. MDCK cells were cultured onto 96-well plate at the density of 1.0 × 105 cells/mL. Different concentrations prepared in MEM (10–0.01 mg/mL) of aqueous and methanolic crude extract were added to each culture wells at a final volume of 100 µL, in triplicate., After incubation at 37°C temperature with 5% CO2 for 16–18 h, 10% of 5 mg/mL MTT (100 µL) was added to each well. After 4 h of further incubation at 37°C temperature, the formazan was solubilized by adding DMSO to each well and the absorbance was read at 550 nm by an ELISA reader.[15]
Antiviral assays
Before starting the antiviral assay, the hemagglutination of virus titer is calculated in terms of HAU. The protocol is mentioned in Hemagglutination assay as provided later. And after carrying out the simultaneous and posttreatment assay, hemagglutination is carried out once again to analyze for the complete or partial reduction in virus titer.
Simultaneous treatment assay
In simultaneous treatment assay, 50 μL of virus (64 HAU) was first exposed to 50 µL of different dilutions of plant extract prepared in MEM without phenol red (aqueous and methanolic extracts) and was incubated at 37°C temperature for 1 h. Following incubation, 100 µL of the above mixture was added to the 96-wells plate containing confluent monolayer of MDCK cell line (1 × 105 cells/well). After 1 h of incubation at 37°C temperature, the supernatants were removed, and the cells were washed with MEM. After washing, the media was discarded and then 100 µL of virus growth medium was added, and the plate was kept at 37°C temperature in 5% CO2 incubator.[16]
Posttreatment assay
In posttreatment assay, confluent monolayer of MDCK cell line was washed twice with 50 µL of virus growth medium and then the medium was removed and 100 µL of virus (64 HAU) was added to the 96-wells plate. The virus was allowed to adsorb for 1 h at 37°C temperature in the 5% CO2 incubator. After incubation, the virus was removed from each well by washing with MEM. The media was then removed, and 100 µL of different dilutions of plant extracts prepared in virus growth medium was added to the monolayer and the plate was kept at 37°C temperature in 5% CO2 incubator.[16]
Hemagglutination assay
For carrying out hemagglutination assay, “V bottom” 96-well microtiter plate was used and 50 µL phosphate buffer saline (pH = 7.2) was added as a diluent in each well using a multichannel auto pipette. A 50 µL of sample (cell-free supernatant of simultaneous and posttreatment assay) was added in the first well of each row. Two-fold dilutions of the sample were made by transferring 50 µL suspension from the first well of each column to the next well by using a multichannel auto pipette. This procedure was repeated till the last column of the 96-well microtiter plate. After serially diluting the sample, 50 µL of 0.75% guinea pig red blood cells (RBCs) was added to each well and the plate was incubated at 4°C temperature for 1 h. After incubation, cell control was checked for complete settling of RBCs and results of hemagglutination assay, that is, the virus titer were recorded as HAU.[17]
Statistical analysis
Sampling proceeded on three independent replication (n = 3) for each test. Data were subjected to Graph Pad Prism v5.04 and v6.0,[18] and the HAUs were calculated by two-tailed t-test with P < 0.05 as significance.
RESULTS
Phytochemical analysis
The extraction procedure found A. sativum extracts to be pale yellow in color with transparency whereas P. indica extracts were found to be dark yellow in color. Preliminary photochemical screening tests mainly concluded the presence of carbohydrates, flavonoids, proteins, saponins, fats and oils, alkaloids, steroids, phenols, and tannins. The constituents are reported in Table 2. The presence of proteins, carbohydrates, and tannins was consistent in the all the extracts tested; as these are the plant building materials. Flavonoids, fats, oils, and steroids were present in A. sativum extracts, and alkaloids and phenols were found in P. indica extracts.
Table 2.
Qualitative phytochemical analysis (Methanol:Chloroform (70:30 V/V) used for both in cold maceration and 15 h soxhlet P. indica extraction)
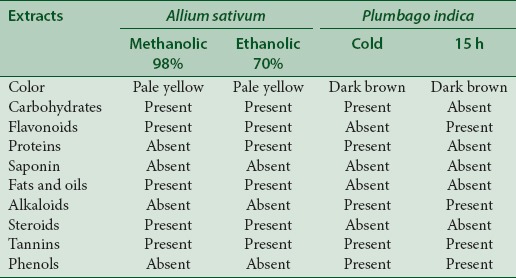
Figure 1 shows the chromatographic separation of phytochemicals from extracts A: Lane1: 70% ethanol and lane 2: 98% methanol extracts of A. sativum (the boxes are showing bands of allicin) and B: Lane 1: Cold extraction and lane 2: Fifteen hours extraction of P. indica (the boxes are showing bands of plumbagin).
Figure 1.

Thin layer chromatography of the extracts
Quantitative high-performance thin layer chromatography analysis
Quantitative photochemical analysis was carried out by HPTLC method wherein the silica plates were subjected to win CATS V-1.2.3 at 254 nm; it revealed that the 20.56% allicin was present in the 70% ethanol [Figure 2] extract and 26.8% in the 98% methanol [Figure 3] extract as Retention Factor (RF) of allicin in A. sativum extract found between 0.26 and 0.34. Similarly, approximately 14.03% plumbagin were present in the cold extract [Figure 4] and 14% in 15 h extracts [Figure 5] as RF value for plumbagin ranges from 0.85 to 0.95.
Figure 2.
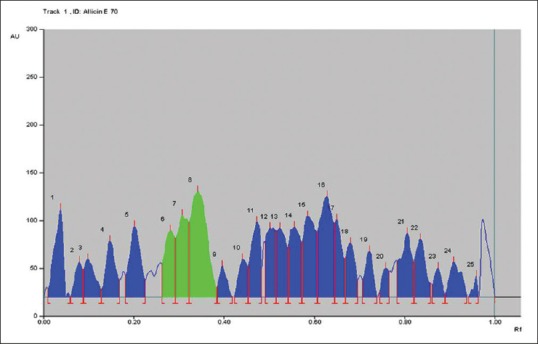
High-performance thin layer chromatography graph of Allium sativum 70% ethanol extract
Figure 3.
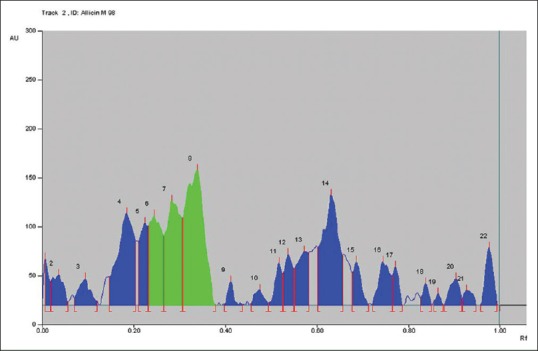
High-performance thin layer chromatography graph of Allium sativum 98% methanolic extract
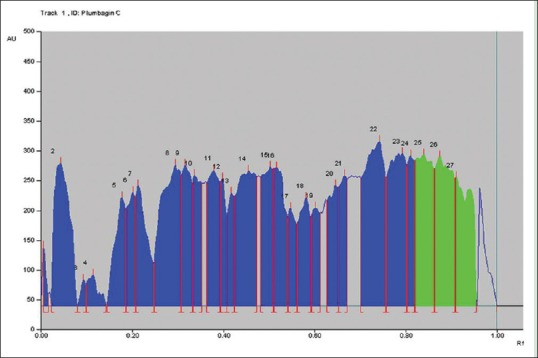
Figure 4.
High-performance thin layer chromatography graph of Plumbago indica cold extract
Figure 5.
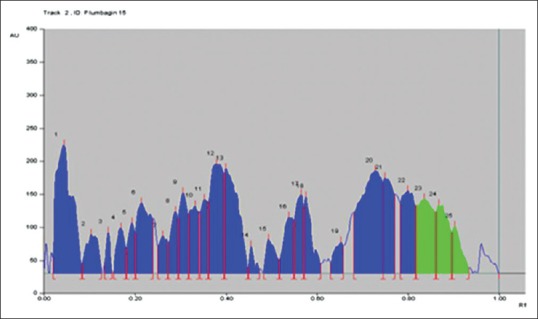
High-performance thin layer chromatography graph of Plumbago indica 15 h extract
Figure 2 represents the chromatogram peaks by win CATS V-1.2.3 at 254 nm allicin in the A. sativum 70% ethanol extract. The green peaks of allicin (Peak No. 6, 7, 8) are highlighted in green and are in the RF range of 0.24–0.34.
Figure 3 represents the chromatogram peaks by winCATS V-1.2.3 at 254 nm allicin in the A. sativum 98% methanolic extract. The green peaks of allicin (Peak No. 6, 7, 8) are highlighted in green and are in the RF range of 0.24–0.34.
Figure 4 represents the chromatogram peaks by winCATS V-1.2.3 at 254 nm plumbagin in the P. indica cold extract. The green peaks of plumbagin (Peak No. 25, 26, 27) are highlighted in green and are in the RF range of 0.83–0.94.
Figure 5 represents the chromatogram peaks by winCATS V-1.2.3 at 254 nm plumbagin in the P. indica 15 h extract the green peaks of plumbagin (Peak No. 23, 24, 25) are highlighted in green and are in the RF range of 0.83–0.94.
Cell cytotoxicity
Cell cytotoxicity was performed by MTT assay on MDCK cell line. Percent cytotoxicity was calculated using following formula:[15]
Percent cytotoxicity = 100–percent cell survival
Percent cell survival = ([At–Ab]/[Ac–Ab]) × 100
Where,
Absorbance value of test compound - At
Absorbance value of blank - Ab
Absorbance value of control - Ac
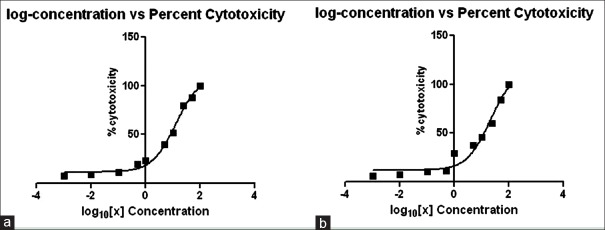
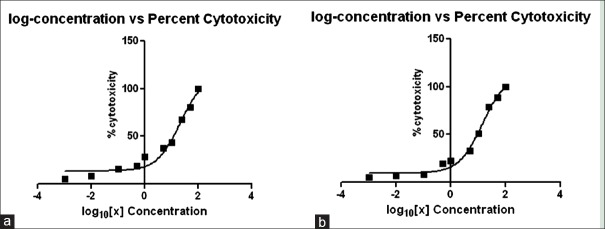
A cytotoxicity assay was performed to check the toxicity levels of the extracts prepared. In the 50% CC50 for A. sativum, 70% ethanolic extract was found to be 12.5 mg/mL and for 98% methanolic it was 20.90 mg/mL [Figure 6]. Similarly, the CC50 values for P. indica cold extract were 20.66 mg/mL and for 15 h extract, they were 14.17 mg/mL [Figure 7]. As this concentration indicated 50% cell cytotoxicity, concentration lower than the CC50 values was selected for the anti-viral assay.
Figure 6.
Log concentration versus percent cytotoxicity for Allium sativum. (a) 70% ethanolic extract and (b) 98% methanolic extract
Figure 7.
Log concentration versus percent cytotoxicity for Plumbago indica. (a) cold extract and (b) 15 hour extract
Figure 6 represents the CC50 graph of A: 70% ethanolic extract, value was found to be 12.5 mg/mL and B: 98% methanolic extract value was found to be 20.90 mg/mL. The concentrations of the extract were calculated in terms of Log10 when analyzed statistically on the graph pad prism software.
Figure 7 represents the CC50 graph of A: Cold extract, the value was found to be 20.66 mg/mL and B: Fifteen hours extract, the value was found to be 14.17 mg/mL. The concentrations of the extract were calculated in terms of Log10 when analyzed statistically on the GraphPad Prism Software.
Antiviral assay
The antiviral assay was based on percent reduction in hemagglutination activity and was calculated, and the HAU was calculated using the following formula:[17]
Percent (%) log2 HAU reduction= (1–A/B) × 100
Where,
A - log2HAU titer of virus control
B - log2HAU titer of sample.
Figure 8 shows the hemagglutination percent reduction versus concentration of A. sativum ethanolic 70% extract on the simultaneous and posttreatment assay.
Figure 8.
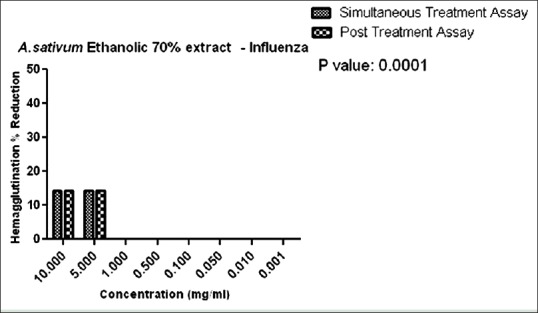
Hemagglutination percent reduction versus concentration of Allium sativum ethanolic 70% extract
Figure 9 indicates the hemagglutination percent reduction versus concentration of A. sativum methanolic 98% extract on the simultaneous and posttreatment assay.
Figure 9.

Hemagglutination percent reduction versus concentration of Allium sativum methanolic 98% extract
Figure 10 indicates the hemagglutination percent reduction versus concentration P. indica cold extracts on the simultaneous and posttreatment assay.
Figure 10.
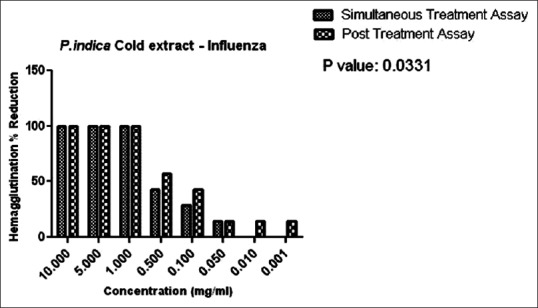
Hemagglutination Percent Reduction versus concentration of Plumbago indica cold extract
Figure 11 indicates the hemagglutination percent reduction versus concentration P. indica 15 h extracts on the simultaneous and posttreatment assay.
Figure 11.
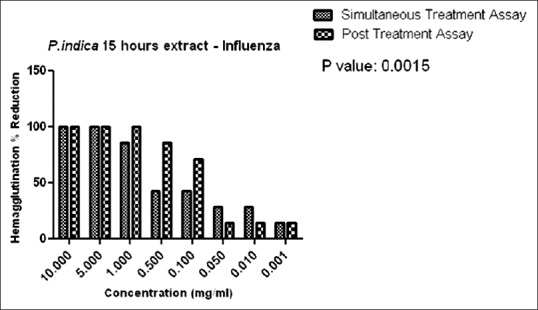
Hemagglutination percent reduction versus concentration of Plumbago indica 15 h extract
In the present study, anti-influenza activity was carried out by simultaneous and posttreatment assays. Simultaneous anti-influenza treatment was used to identify whether A. sativum and P. indica extracts block the viral adsorption to cells. As observed in Figures 8 and 9, in the simultaneous assay, 14% reduction in HA were observed at a concentration of 10 mg/mL in both the ethanolic and methanolic extracts. In P. indica extracts, as in Figures 10 and 11, 100% reduction was observed at a concentration of 10 mg and 5 mg/mL in cold and 15 h extraction. As the concentration was further lowered, the HA reduction was lowered to 14% till 0.001 mg/mL in 15 h extract. These data suggest that A. sativum and P. indica extracts may directly interfere with the viral envelope protein and not with the SA receptor at the cell surface.
We employed the posttreatment assay to evaluate the anti-influenza activity after virus infection. In this assay, 70% ethanolic extract and 98% methanolic extract of A. sativum exhibited only 14% HA reduction at concentrations of 10 mg/mL and 5 mg/mL only as seen in Figures 8 and 9. Alternatively, cold extract and 15 h extract of P. indica showed 100% reduction at concentrations of 10 mg/mL to 1 mg/mL. Reduction in hemagglutination was also observed at lower concentrations from 0.5 to 0.001 mg/mL as compared to A. sativum extracts. We found that only cold and 15 h extract of P. indica inhibited influenza virus infection suggesting the possible ways of viral inhibitions by blockage of viral attachment by inhibition of viral HA protein.
DISCUSSION
The emergence of drug-resistant variants of the influenza virus has led to a need to identify novel and effective antiviral agents. As an alternative to synthetic drugs, the consolidation of empirical knowledge with ethnopharmacological evidence of medicinal plants offers a novel platform for the development of antiviral drugs. The aim of this study was assessment of anti-influenza activity of P. indica (lal chitrak) and A. sativum (garlic) which was selected on the basis of the in silico analysis which represented them as capable antiviral agents.[9]
Allicin is one of the most active principal ingredients of freshly crushed garlic homogenates first discovered in 1944 by the scientific experts who noted its potent antimicrobial activity.[19] Similarly, other scientific reports suggest that preprocessed dried garlic samples were not active while freshly crushed samples showed better antibacterial activity on bacteria and that ethanolic and methanolic extracts actually enhanced garlic's antibacterial activity.[20] On the similar basis, we carried out 70% ethanolic extraction and 98% methanolic extraction for A. sativum with the aim that it may contain major concentrations of allicin which is reported to have antibacterial activity against Gram-positive and Gram-negative bacteria.[21]
Plumbagin (2-methyl-5-hydroxy-1,4-naphthoquinone) is a yellow crystalline bioactive phytoconstituent present in the roots isolated from P. indica by soxhlet apparatus, followed by silica gel column chromatography, cold maceration, and then by preparative thin layer chromatography techniques; and subsequently various other chromatographic methods.[22] Similarly, plumbagin has also been a principal bioactive compound of Plumbago Rosea (indica). Limited research has been carried out on P. indica L. wherein the presence of basic phytochemicals like reducing sugars, alkaloids, steroids, flavonoids, and gums has been reported. Since P. indica belongs to Plumbaginaceae family where Zeylancia and Rosea are also its species which reports the presence of plumbagin as an important bioactive component, we carried out the cold maceration and 15 h soxhlet extraction with the aim of plumbagin being extracted as the major bioactive component.
The previous reports suggest that that plumbagin content in P. rosea is the highest corresponding to 0.17%, while in P. capensis and P. zeylanica, it is 0.04 and 0.01%, respectively.[23] Three locally available plumbago species (P. auriculata, P. rosea, and P zeylanica) have been quantitatively screened for the bioactive marker plumbagin by high-performance thin layer chromatography. In general, the root parts were found to be the richest sources of plumbagin. Significantly, P. rosea was found to accumulate maximum plumbagin in the roots, while P. auriculata and P. zeylanica are high yielding species for the leaf and stem parts, respectively.[24] In contrast to the above reports, we have reported approximately 14% in both cold extract and 15 h extract of P. indica which is the first time this has been reported.
The influenza virus replication cycle can be divided into five steps: (1) Binding of viral haemagglutinin to sialic acid (SA) receptor on host cell surface (adsorption step), (2) internalization of virus by receptor-mediated endocytosis and fusion of viral HA2 with endosomal membranes triggered by influx of protons through M2 channel (endocytosis and fusion step), (3) release of viral genes into the cytoplasm (uncoating step), (4) packaging of viral proteins with viral genes after viral RNA replication, transcription and translation, and budding of new viruses (packaging and budding step), and (5) release of new viruses by Sialidase cleaving SA receptors (release step).[25,26]
An antiviral assay previously reported in which the simultaneous exposure assays were used to identify whether the extracts blocked the viral adsorption to cells, by synergistically binding to the free virus particles or by blocking the SA receptors to prevent virus entry into the cells.[27] From the postexposure treatment, they concluded that the extracts may be inhibiting the replication of influenza virus or virus budding from the infected MDCK cells.
The previous reports suggest that fresh garlic extracts in which allicin is known to be the main active component have been shown to have in vitro and in vivo antiviral activity. Among the viruses which are sensitive to garlic extracts are the human cytomegalovirus, influenza b, herpes simplex virus type 1 (HSV-1), HSV-2, parainfluenza virus type 3, vaccinia virus, vesicular stomatitis virus, and human rhinovirus type 2.[28]
Plumbagin is a simple plant secondary metabolite exhibiting highly potent biological activities that have been attributed to its effects on multiple signaling and apoptotic pathways, and its ability to undergo redox cycling property generating ROS. Its potent anticancer activity has, in particular, attracted a lot of attention lately. Studies have proved the efficacy of plumbagin against a number of different cancer models representing cancers of different origin. These findings indicate a possible use of plumbagin as a therapeutic tool against a wide variety of human malignancies.[29]
We have initially reported allicin and plumbagin as potent anti-influenza moieties depending on the in silico analysis. Further in this report, we carried out the anti-viral assay to confirm our findings with the in silico approach which states true that allicin and plumbagin have the potential to be used further as anti-influenza agents. The HPTLC analysis carried out on A. sativum and P. indica confirmed the presence of allicin and plumbagin, respectively. We confirm the findings of insilico analysis with the in vitro extract analysis of allicin and plumbagin as potent anti-influenza agents.
CONCLUSIONS
This study has shown that P. indica L. and A. sativum extracts can inhibit influenza A (H1N1)pdm09 virus by inhibiting viral nucleoprotein synthesis and polymerase activity. These results lead to a further investigation about the characterization of active compound and their specific mechanism against influenza virus. Given the urgent need for new and abundantly available anti-influenza drugs, these plant extracts appear to be a promising option as a replacement or supplemental strategy to currently available anti-influenza therapies.
Financial support and sponsorship
The study was funded by Intra-mural fund of Haffkine Institute for Training, Research and Testing, Mumbai, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Tapan Dhole, Department of Genetics, SGPGI, Lucknow for providing the standard influenza virus.
REFERENCES
- 1.Eyer L, Hruska K. Antiviral agents targeting the influenza virus: A review and publication analysis. Vet Med. 2013;58:113–85. [Google Scholar]
- 2.Air GM. Influenza neuraminidase. Influenza Other Respir Viruses. 2012;6:245–56. doi: 10.1111/j.1750-2659.2011.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gamblin SJ, Skehel JJ. Influenza hemagglutinin and neuraminidase membrane glycoproteins. J Biol Chem. 2010;285:28403–9. doi: 10.1074/jbc.R110.129809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okomo-Adhiambo M, Sleeman K, Ballenger K, Nguyen HT, Mishin VP, Sheu TG, et al. Neuraminidase inhibitor susceptibility testing in human influenza viruses: A laboratory surveillance perspective. Viruses. 2010;2:2269–89. doi: 10.3390/v2102269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorgensen WL. Efficient drug lead discovery and optimization. Acc Chem Res. 2009;42:724–33. doi: 10.1021/ar800236t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singh S, Sharma DK. In silico modeling in conjunction with natural products: Paving the way for rational drug design. Biotechnol Mol Biol Rev. 2011;6:88–93. [Google Scholar]
- 7.Zonta N, Coluccia A, Brancale A. Advanced in silico approaches in antiviral research. Antivir Chem Chemother. 2010;20:147–51. doi: 10.3851/IMP1500. [DOI] [PubMed] [Google Scholar]
- 8.Deyde VM, Xu X, Bright RA, Shaw M, Smith CB, Zhang Y, et al. Surveillance of resistance to adamantanes among influenza A (H3N2) and A (H1N1) viruses isolated worldwide. J Infect Dis. 2007;196:249–57. doi: 10.1086/518936. [DOI] [PubMed] [Google Scholar]
- 9.Chavan R, Shinde P, Girkar K, Mandage R, Chowdhary A. Identification of potent natural inhibitors against H1N1/A/2009 virus using in silico subtractive genomics approach and docking technology. Int J Pharm Res. 2014;6:105–13. [Google Scholar]
- 10.Gaikwadi SS, Vadlamudi P, Waghmaee SP, Maral VJ, Ranteke VD, Dhok AP. Phytochemical analysis of aqueous extract of few medicinal plants. PKV Res J. 2003;27:91–2. [Google Scholar]
- 11.van der Vijver LM. Distribution of plumbagin in the Plumbaginaceae. Phytochemistry. 1974;11:3247–8. [Google Scholar]
- 12.Rathinasabapathi B, Fouad WM, Sigua CA. beta-Alanine betaine synthesis in the Plumbaginaceae. Purification and characterization of a trifunctional, S-adenosyl-L-methionine-dependent N-methyltransferase from Limonium latifolium leaves. Plant Physiol. 2001;126:1241–9. doi: 10.1104/pp.126.3.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ankri S, Mirelman D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999;1:125–9. doi: 10.1016/s1286-4579(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 14.Tiwari P, Kumar B, Kaur M, Kaur G, Kaur H. Phytochemical screening and extraction: A review. Internationale pharmaceutica sciencia. 2011;1:98–106. [Google Scholar]
- 15.Yu Z, Li W, Liu F. Inhibition of proliferation and induction of apoptosis by genistein in colon cancer HT-29 cells. Cancer Lett. 2004;215:159–66. doi: 10.1016/j.canlet.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 16.He W, Han H, Wang W, Gao B. Anti-influenza virus effect of aqueous extracts from dandelion. Virol J. 2011;8:538. doi: 10.1186/1743-422X-8-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hussain M, Mehmood MD, Ahmad A, Shabbir MZ, Yaqub T. Factors affecting hemagglutination activity of avian influenza virus subtype H5N1. J Vet Anim Sci. 2004;35:31–6. [Google Scholar]
- 18.Graph Pad Prism Version 5.04 and Version 6.0, Graph Pad Software, La Jolla California USA. Available from: http://www.graphpad.com .
- 19.Cavallito C, Bailey JH. Allicin, the antibacterial principal of Allium sativum. Isolation, physical properties and antibacterial action. J Am Chem Soc. 1944;66:1944–52. doi: 10.1021/ja01207a039. [DOI] [PubMed] [Google Scholar]
- 20.Idoh E, Hovana K, James US, Egbobor EM, Egba AG, Eta ES, et al. Antibacterial and phytochemical studies of Allium sativum. N Y Sci J. 2011;28:4. [Google Scholar]
- 21.Uchida Y, Takahashi T, Sato N. The characteristics of the antibacterial activity of garlic (author's transl) Jpn J Antibiot. 1975;28:638–42. [PubMed] [Google Scholar]
- 22.Ganesan K, Gani SB. Ethnomedical and pharmacological potentials of Plumbago zeylanica L – A review. Am J Phytomed Clin Ther. 2013;1:313–37. [Google Scholar]
- 23.Aryanathan S. Thanjavur, India: PhD Thesis, Sastra University; 2009. Chemical Examination of Three Plumbago species. [Google Scholar]
- 24.Mallavadhani UV, Sahu G, Muralidhar J. Screening of Plumbago species for the bio-active marker plumbagin. Pharm Biol. 2002;40:508–11. [Google Scholar]
- 25.Palese P. Influenza: Old and new threats. Nat Med. 2004;10(12 Suppl):S82–7. doi: 10.1038/nm1141. [DOI] [PubMed] [Google Scholar]
- 26.Sugaya N, Nerome K, Ishida M, Matsumoto M, Mitamura K, Nirasawa M. Efficacy of inactivated vaccine in preventing antigenically drifted influenza type A and well-matched type B. JAMA. 1994;272:1122–6. [PubMed] [Google Scholar]
- 27.Kwon HJ, Kim HH, Yoon SY, Ryu YB, Chang JS, Cho KO, et al. In vitro inhibitory activity of Alpinia katsumadai extracts against influenza virus infection and hemagglutination. Virol J. 2010;7:307. doi: 10.1186/1743-422X-7-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai Y, Cole LL, Davis LE, Lockwood SJ, Simmons V, Wild GC. Antiviral properties of garlic: In vitro effects on influenza B, herpes simplex and coxsackie viruses. Planta Med. 1985;51:460–1. doi: 10.1055/s-2007-969553. [DOI] [PubMed] [Google Scholar]
- 29.Padhye S, Dandawate P, Yusufi M, Ahmad A, Sarkar FH. Perspectives on medicinal properties of plumbagin and its analogs. Med Res Rev. 2012;32:1131–58. doi: 10.1002/med.20235. [DOI] [PubMed] [Google Scholar]