Abstract
Objective:
The aim of this study was to determine the in vitro anti-inflammatory activities of solvent fractions from Chlorella vulgaris by inhibiting the production of pro-inflammatory mediators and cytokines.
Methods:
Methanolic extracts (80%) of C. vulgaris were prepared and partitioned with solvents of increasing polarity viz., n-hexane, chloroform, ethanol, and water. Various concentrations of the fractions were tested for cytotoxicity in RAW 264.7 cells using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, and the concentrations inducing cell growth inhibition by about 50% (IC50) were chosen for further studies. Lipopolysaccharide (LPS) stimulated RAW 264.7 cells were treated with varying concentrations of C. vulgaris fractions and examined for its effects on nitric oxide (NO) production by Griess assay. The release of prostaglandin E2 (PGE2), tumor necrosis factor-α (TNF-α), and interleukin 6 (IL-6) were quantified using enzyme-linked immunosorbent assay using Celecoxib and polymyxin B as positive controls.
Results:
MTT assay revealed all the solvent fractions that inhibited cell growth in a dose-dependent manner. Of all the extracts, 80% methanolic extract exhibited the strongest anti-inflammatory activity by inhibiting NO production (P < 0.01), PGE2 (P < 0.05), TNF-α, and IL-6 (P < 0.001) release in LPS induced RAW 264.7 cells. Both hexane and chloroform fractions recorded a significant (P < 0.05) and dose-dependent inhibition of LPS induced inflammatory mediators and cytokines in vitro. The anti-inflammatory effect of ethanol and aqueous extracts was not significant in the study.
Conclusion:
The significant inhibition of inflammatory mediators and cytokines by fractions from C. vulgaris suggests that this microalga would be a potential source of developing anti-inflammatory agents and a good alternate for conventional steroidal and nonsteroidal anti-inflammatory drugs.
SUMMARY
C. vulgaris extracts have potential anti-inflammatory activity
Solvent extraction using methanol, hexane, and chloroform has exhibited significant effect in LPS activated RAW 264.7 cells
C. vulgaris extracts reduce the production of NO, PGE2, TNF-α, and IL-6 in LPS activated RAW 264.7 cells.
Abbreviations Used: COX-2: Cyclooxygenase-2, DMSO: Dimethyl sulfoxide, FBS: Fetal bovine serum, IL-6: Interleukin 6, iNOS: Inducible nitric oxide synthase, L-NMMA: NG-methyl-L-arginine acetate salt, LPS: Lipopolysaccharide, MTT: 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, NO: Nitric oxide, PBS: Phosphate buffered saline, PGE2: Prostaglandin E2, TNF-α: Tumor necrosis factor-α
Keywords: Anti-inflammatory, Chlorella vulgaris, Microalgae, Pro-inflammatory cytokines, Pro-inflammatory mediators
INTRODUCTION
Inflammation is an important host defense mechanism and is characterized by a complex of interactions between mediators of inflammation and inflammatory cells.[1,2] Uncontrolled inflammation can lead to tissue injury and chronic diseases.[3] In general, treatment for inflammation is aimed at either inhibiting the activity of inflammatory cells or inhibiting the production of inflammatory mediators.[4] At present, most inflammatory diseases are treated with steroidal and nonsteroidal anti-inflammatory drugs which suppress the levels of pro-inflammatory cytokines, inducible nitric oxide synthase (iNOS), cyclooxygenase-2, and prostaglandin E2 (PGE2).[5] However, prolonged use of these conventional drugs may produce adverse side effects[6] in addition with long-term steroid use that suppresses the immune system.[7]
New anti-inflammatory agents from natural sources with fewer adverse effects are alternates that could be developed for long-term administration. Microalgae form the part of natural sources that could be a sustainable source of bioactive compounds, and anti-inflammatory activity of microalgae has been reported widely.[8,9,10,11,12,13,14] Pro-inflammatory cytokines activate immune cells to up-regulate inflammation and are, therefore, useful targets in the development of new anti-inflammatory drugs.[15,16] For this reason, we investigated the inhibitory activity of Chlorella vulgaris solvent fractions on pro-inflammatory cytokines and inflammatory mediators.
MATERIALS AND METHODS
Chemicals and reagents
Chemicals 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), lipopolysaccharide (LPS), NG-methyl-L-arginine acetate salt (L-NMMA), celecoxib, polymyxin B, and enzyme-linked immunosorbent assay (ELISA) kits were purchased from Sigma-Aldrich (India). All other solvents/chemicals used were of reagent or analytical grade.
Preparation of algal extract
C. vulgaris was grown on Bold's medium for a period of 15 days under illumination and centrifuged at 10,000 ×g for 10 min and the pellet was washed with distilled water. The pellet was lyophilized and the dried powder was extracted by ultrasonication with 80% methanol (2 L) at room temperature for 30 min. The methanolic extracts were filtered by Whatman filter paper and the filtrate was concentrated using rotary vacuum evaporator. The evaporated extract was suspended in water (1 L) and partitioned with solvents of increasing polarity. At first, n-hexane (1 L) was added to the methanolic extract, agitated for 24 h at room temperature, and separated as hexane fraction. This was followed by extraction with chloroform and ethanol. Each fraction was dried under a rotary vacuum evaporator, lyophilized in a freeze drier and dissolved in dimethyl sulfoxide (DMSO), and stored at 4°C for further studies.
RAW 264.7 cell culture
RAW 264.7 cells, purchased from American Type Culture Collection (ATCC), were grown on Dulbecco's Modified Eagle Medium (DMEM), supplemented with 10% fetal bovine serum, penicillin (100 U/mL), and streptomycin (100 μg/mL). The cells were incubated and maintained in an atmosphere of 5% CO2 at 37°C. The cells were subcultured every 2 days and exponential phase cells were used throughout the experiments.
Cytotoxicity assessment using 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay
The cytotoxicity of the crude solvent extracts against the RAW 264.7 cells (ATCC, USA) was determined using the colorimetric MTT assay. Cells were seeded in a 24-well plate at a concentration of 1 × 105 cells/mL. After 24 h, the seeded cells were treated with microalgal extracts and incubated for an additional 24 h at 37°C. MTT stock solution (50 μl; 2 mg/mL in phosphate buffered saline) was added to each well to a total reaction volume of 250 μL. After incubating for 3 h at 37°C under humidified 5% CO2 atmosphere, the supernatants were aspirated and the formazan crystals in each well were dissolved in 200 μl DMSO. The resulting absorbance was measured with a microplate reader (Tecan Infinite, F 500) at 540 nm. The concentrations inducing cell growth inhibition by about 50% (IC50) were chosen for further studies.
Determination of nitric oxide production
RAW 264.7 cells (1 × 105 cells/mL) were placed in a 24-well plate and after 24 h, the cells were preincubated with different concentrations of C. vulgaris fractions at 37°C for 1 h. Further incubation was done at 37°C for another 24 h with LPS (1 μg/mL) and the nitrite that accumulated in the culture medium was measured[17] as indicative of the nitric oxide (NO) production. The culture medium was collected and centrifuged at 750 ×g to precipitate any remaining cell debris. 100 µl supernatant was mixed with equal volume of Griess reagent (1% sulphanilamide and 0.1% naphthylethylene diamine dihydrochloride in 2.5% phosphoric acid) and incubated at room temperature for 10 min. This was followed by measuring the absorbance at 540 nm in a microplate reader (Tecan Infinite, F 500) using L-NMMA (80 µM) as positive control. The nitrite concentration was estimated against a sodium nitrite standard calibration curve.
Assays of pro-inflammatory cytokines
The inhibitory effects of Chlorella extracts on the production of cytokines were measured by ELISA using culture supernatants collected from treated cells. RAW 264.7 cells (1.8 × 105 cells/mL) were plated in a 24-well plate containing 1 ml of DMEM medium for 18 h, followed by treatment with LPS (500 ng/ml) in the presence of solvent fractions of Chorella vulgaris. After another 24 h of incubation, the PGE2, tumor necrosis factor-α (TNF-α), and interleukin 6 (IL-6) in the cell culture medium were quantified using ELISA kits (Sigma-Aldrich, India) according to the manufacturer's instructions using Celecoxib (3 µM) and polymyxin B (100 U/mL) as positive controls.
Statistical analysis of data
All values were expressed as mean ± standard deviation. Statistical differences between the treatments and the control were evaluated by analysis of variance and Student's t-tests. Values of P < 0.05 were considered to be significant.
RESULTS
Cytotoxicity assay
Tetrazolium dye colorimetric test (MTT assay) was performed as a preliminary test to determine whether the solvent fractions from C. vulgaris caused cytotoxicity in RAW 264.7 cells. The absorbance of the cells exposed to different concentrations of solvent fractions for 24 h. All the fractions inhibited cell growth in a dose-dependent manner and the concentrations inducing cell growth inhibition by about 50% (IC50) were 125 µg/mL (methanol) and 250 µg/mL (hexane and chloroform) [Figure 1]. Treatment of RAW 264.7 cells with ethanol and aqueous fractions had no significant effect on cell viability after 24 h incubation. Based on cytotoxicity effects, IC50 was chosen as the highest concentration on the production of NO, TNF-α, and IL-6 in LPS stimulated RAW 264.7 cells.
Figure 1.
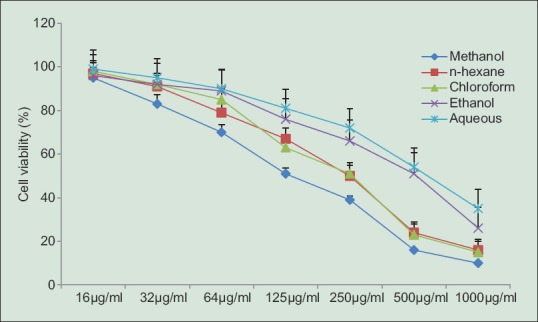
Cell viability of RAW 264.7 cells by fractions from C. vulgaris. Cells were incubated with various concentration of fractions (16, 32, 64, 125, 250, 500, 1000 μg/ml). Values were expressed as mean ± standard deviation for three independent experiments performed in triplicate
Inhibitory effects on inflammatory mediators
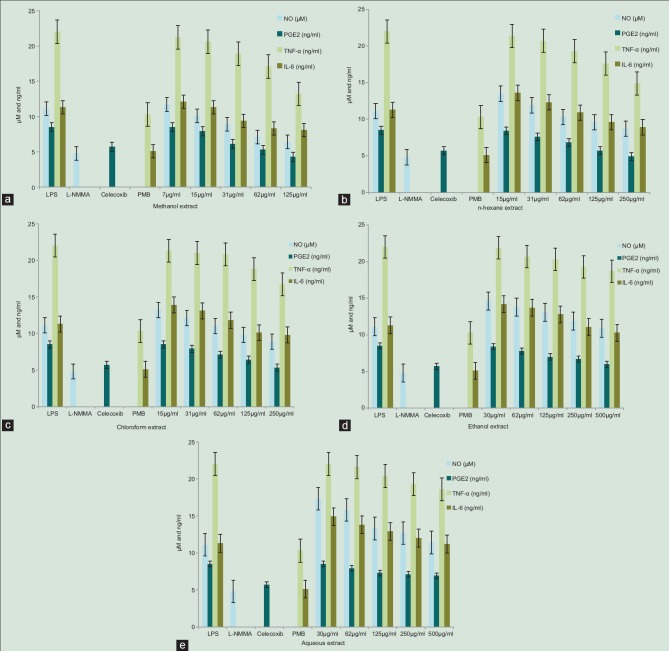
RAW 264.7 macrophages activated by LPS would generate massive inflammatory mediators (NO and PGE2) and cytokines (TNF-α and IL-6), which would cause kinds of inflammatory diseases.[18,19] Hence, LPS-activated RAW 264.7 cell model was chosen to reveal the anti-inflammatory mechanism of C. vulgaris extracts in this study. Treatment of RAW 264.7 cells with LPS alone resulted in significant increases in inflammatory mediators and cytokines. However, methanolic, hexane and chloroform extracts reduced the NO production significantly in a dose-dependent manner and methanol extract of C. vulgaris strongly inhibited the NO production (P < 0.01) at a concentration of 125 µg/mL. It was observed that ethanol and aqueous fractions had less activity in reducing NO production even at 500 µg/mL. The ability of C. vulgaris extracts to modulate the production of PGE2 was determined by ELISA and was found to significantly inhibit PGE2 production as compared to the LPS-treated group in a dose-dependent manner. RAW 264.7 cells were stimulated with LPS and then incubated with the solvent fractions of C. vulgaris and a significant inhibition of PGE2 production was observed in cells treated with methanol (P < 0.05), hexane and chloroform (P < 0.05) extracts.
Inhibitory effects on proinflammatory cytokines
Since C. vulgaris was found to potently inhibit the pro-inflammatory mediators, we further investigated its effect on LPS induced TNF-α and IL-6 production. The concentrations of cytokines TNF α and IL-6 in cell supernatants was measured by ELISA and treatment of RAW 264.7 cells with LPS alone resulted in higher cytokine production. Methanol extracts significantly decreased the LPS induced TNF-α at 125 µg/mL, whereas the concentration was 250 µg/mL for hexane and chloroform fractions (P < 0.05). However, ethanol and aqueous fractions decreased TNF-α at higher concentrations (500 µg/mL). Similar results were observed in reducing IL-6 production by C. vulgaris extracts.
DISCUSSION
Inflammation is the normal physiological and immune response to tissue injury. Macrophages play important roles in inflammation by overproducing inflammatory mediators, including NO and PGE2.[20] NO is synthesized by the iNOS and has been reported as a mediator of inflammation.[21] It is as an important molecule to regulate the biological activities in vascular, neural, and immune systems.[22] However, its uncontrolled release can cause target tissue destruction during an infection.[23] Inhibition of inflammatory mediators is a useful strategy for the treatment of acute or chronic inflammatory disorders. LPS and proinflammatory cytokines activate immune cells to up-regulate inflammatory states and are therefore useful targets in the development of anti-inflammatory agents. To investigate the effect of C. vulgaris on NO production, Griess assay was used to measure the accumulation of nitrite in culture media. RAW 264.7 cells were stimulated with LPS in the presence or absence of C. vulgaris extracts and the nitrite levels were increased significantly in LPS induced cells. Further, fractions of 80% methanolic extracts were evaluated on NO production by RAW 264.7 cells. Prostaglandins (PG) are involved in various pathophysiological processes including inflammation and PGE2 involved in inflammatory responses is generated by the sequential metabolism of arachidonic acid by cycloxygenase.[24] PGE2 is a pleiotropic mediator that causes pain, swelling, and stiffness.[25] In this study, the release of pro-inflammatory mediators were prevented as the amount of NO and PGE2 production was reduced as depicted in Figure 2a-e revealing the potential anti-inflammatory activities of C. vulgaris.
Figure 2.
Effects of C. vulgaris extracts and fractions on the production of lipopolysaccharide-induced nitric oxide, prostaglandin E2, tumor necrosis factor-α, interleukin 6. RAW 264.7 cells were incubated with various concentrations of (a) methanolic extracts, (b) hexane extracts, (c) chloroform extracts, (d) ethanol extracts, (e) aqueous extracts in the presence of 500 ng/ml lipopolysaccharide. Three independent assays were performedin triplicate and the data shown are the mean ± standard deviation
Pro-inflammatory cytokines (TNF-α, IL-1 β, and IL-6) are important initiators of the inflammatory response and mediators.[26,27] The cytokines IL-1, IL-6, and TNF-α are produced mainly by activated monocytes or macrophages which stimulate cell proliferation in various types of cells.[28] TNF was shown to affect various biological processes including the regulation and the production of other cytokines.[29] TNF-α activates macrophages and promotes inflammation and expression of cell adhesion molecules to inflammatory tissue thereby plays a key role in the induction and perpetuation of inflammation.[30,31] IL-6 is regarded as an endogenous mediator of LPS-induced fever.[32] In this study, we found that C. vulgaris extracts significantly inhibit proinflammatory cytokines production in a dose-dependent manner in LPS stimulated RAW 264.7 cells. Anti-inflammatory activity of bioactive compounds from marine Chlorella was reported earlier[33,34,35,36,37,38] however, inhibition of inflammatory mediators and cytokines by fresh water C. vulgaris is reported first time in this study.
CONCLUSION
In this study, 80% methanolic extract of C. vulgaris was used to prepare various fractions to investigate the in vitro anti-inflammatory activity using RAW 264.7 cells. The results showed that treatment with C. vulgaris extracts inhibits the inflammatory response by reducing the production of NO, PGE2, TNF-α and IL-6 in LPS activated RAW 264.7 cells in vitro. It suggests that C. vulgaris possesses significant anti-inflammatory activity and could be a potential source of anti-inflammatory agents of natural origin.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS

Dr. G Sibi, is an Associate Professor at the Department of Biotechnology, Indian Academy Degree College, Bangalore. His research interest is in the field of Phycology, which involves the characterization of microalgal bioactive compounds for health care and biofuel production for sustainable environment. He is the recipient of projects from National agencies and Editorial Board Member of various International Journals.

Santa Rabina, is a Masters Degree Student at Department of Genetics, Indian Academy Degree College, Bangalore.
REFERENCES
- 1.Villarreal G, Zagorski J, Wahl SM. Encyclopedia of Life Sciences (ELS) London: Nature Publishing Group; 2001. Inflammation: Acut. [Google Scholar]
- 2.Sacca R, Cuff CA, Ruddle NH. Mediators of inflammation. Curr Opin Immunol. 1997;9:851–7. doi: 10.1016/s0952-7915(97)80189-6. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Benjamini E, Sunshine G, Leskowitz S. 3rd ed. New York: John Wiley and Sons, Inc; 1996. Immunology: A Short Course. [Google Scholar]
- 5.Pohanka M, Snopkova S, Havlickova K, Bostik P, Sinkorova Z, Fusek J, et al. Macrophage-assisted inflammation and pharmacological regulation of the cholinergic anti-inflammatory pathway. Curr Med Chem. 2011;18:539–51. doi: 10.2174/092986711794480140. [DOI] [PubMed] [Google Scholar]
- 6.Lin L, Hu K. Tissue plasminogen activator and inflammation: From phenotype to signaling mechanisms. Am J Clin Exp Immunol. 2014;3:30–6. [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng M, Pan ZY, Yang J, Gao YD. Corticosteroid therapy for severe community-acquired pneumonia: A meta-analysis. Respir Care. 2014;59:557–63. doi: 10.4187/respcare.02758. [DOI] [PubMed] [Google Scholar]
- 8.El Gamal AA. Biological importance of marine algae. Saudi Pharm J. 2010;18:1–25. doi: 10.1016/j.jsps.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhakuni DS, Rawat DS. New Delhi, India: Springer; 2005. Bioactive Marine Natural Products. [Google Scholar]
- 10.Awad NE. Biologically active steroid from the green alga Ulva lactuca. Phytother Res. 2000;14:641–3. doi: 10.1002/1099-1573(200012)14:8<641::aid-ptr668>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Tan LT, Williamson RT, Gerwick WH, Watts KS, McGough K, Jacobs R. Cis, cis- and trans, trans-ceratospongamide, new bioactive cyclic heptapeptides from the Indonesian red alga Ceratodictyon spongiosum and symbiotic sponge Sigmadocia symbiotica. J Org Chem. 2000;65:419–25. doi: 10.1021/jo991165x. [DOI] [PubMed] [Google Scholar]
- 12.Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. J Neurosci. 2002;22:6114–20. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng R, Chow TJ. Hypolipidemic, antioxidant, and antiinflammatory activities of microalgae Spirulina. Cardiovasc Ther. 2010;28:e33–45. doi: 10.1111/j.1755-5922.2010.00200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung HA, Jin SE, Ahn BR, Lee CM, Choi JS. Anti-inflammatory activity of edible brown alga Eisenia bicyclis and its constituents fucosterol and phlorotannins in LPS-stimulated RAW264.7 macrophages. Food Chem Toxicol. 2013;59:199–206. doi: 10.1016/j.fct.2013.05.061. [DOI] [PubMed] [Google Scholar]
- 15.Zeilhofer HU, Brune K. Analgesic strategies beyond the inhibition of cyclooxygenases. Trends Pharmacol Sci. 2006;27:467–74. doi: 10.1016/j.tips.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Jachak SM. PGE synthase inhibitors as an alternative to COX-2 inhibitors. Curr Opin Investig Drugs. 2007;8:411–5. [PubMed] [Google Scholar]
- 17.Lee MH, Lee JM, Jun SH, Lee SH, Kim NW, Lee JH, et al. The anti-inflammatory effects of Pyrolae herba extract through the inhibition of the expression of inducible nitric oxide synthase (iNOS) and NO production. J Ethnopharmacol. 2007;112:49–54. doi: 10.1016/j.jep.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Park HH, Kim MJ, Li Y, Park YN, Lee J, Lee YJ, et al. Britanin suppresses LPS-induced nitric oxide, PGE2 and cytokine production via NF-κB and MAPK inactivation in RAW 264.7 cells. Int Immunopharmacol. 2013;15:296–302. doi: 10.1016/j.intimp.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Barbour SE, Wong C, Rabah D, Kapur A, Carter AD. Mature macrophage cell lines exhibit variable responses to LPS. Mol Immunol. 1998;35:977–87. doi: 10.1016/s0161-5890(98)00070-4. [DOI] [PubMed] [Google Scholar]
- 20.Esposito E, Cuzzocrea S. The role of nitric oxide synthases in lung inflammation. Curr Opin Investig Drugs. 2007;8:899–909. [PubMed] [Google Scholar]
- 21.de las Heras B, Abad MJ, Silván AM, Pascual R, Bermejo P, Rodríguez B, et al. Effects of six diterpenes on macrophage eicosanoid biosynthesis. Life Sci. 2001;70:269–78. doi: 10.1016/s0024-3205(01)01402-3. [DOI] [PubMed] [Google Scholar]
- 22.Moncada S, Palmer RM, Higgs EA. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–42. [PubMed] [Google Scholar]
- 23.Kim HW, Murakami A, Williams MV, Ohigashi H. Mutagenicity of reactive oxygen and nitrogen species as detected by co-culture of activated inflammatory leukocytes and AS52 cells. Carcinogenesis. 2003;24:235–41. doi: 10.1093/carcin/24.2.235. [DOI] [PubMed] [Google Scholar]
- 24.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 25.Dinarello CA. Cytokines as endogenous pyrogens. J Infect Dis. 1999;179(Suppl 2):S294–304. doi: 10.1086/513856. [DOI] [PubMed] [Google Scholar]
- 26.Männel DN, Echtenacher B. TNF in the inflammatory response. Chem Immunol. 2000;74:141–61. [PubMed] [Google Scholar]
- 27.Glauser MP. The inflammatory cytokines.New developments in the pathophysiology and treatment of septic shock. Drugs. 1996;52(Suppl 2):9–17. doi: 10.2165/00003495-199600522-00004. [DOI] [PubMed] [Google Scholar]
- 28.Stein B, Sutherland MS. IL-6 as a drug discovery target. Drug Discov Today. 1998;3:202–13. [Google Scholar]
- 29.Hume DA, Underhill DM, Sweet MJ, Ozinsky AO, Liew FY, Aderem A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2:11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beutler B, Cerami A. The biology of cachectin/TNF – A primary mediator of the host response. Annu Rev Immunol. 1989;7:625–55. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- 31.Youn GS, Kwon DJ, Ju SM, Choi SY, Park J. Curcumin ameliorates TNF-α-induced ICAM-1 expression and subsequent THP-1 adhesiveness via the induction of heme oxygenase-1 in the HaCaT cells. BMB Rep. 2013;46:410–5. doi: 10.5483/BMBRep.2013.46.8.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van Snick J. Interleukin-6: An overview. Annu Rev Immunol. 1990;8:253–78. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- 33.Renju GL, Kurup GM. Anti-inflammatory activity of lycopene isolated from Chlorella marina on carrageenan induced rat paw edema. J Res Biol. 2011;3:886–94. [Google Scholar]
- 34.Renju GL, Kurup GM, Saritha Kumari CH. Anti-inflammatory activity of lycopene isolated from Chlorella marina on type II collagen induced arthritis in Sprague Dawley rats. Immunopharmacol Immunotoxicol. 2013;35:282–91. doi: 10.3109/08923973.2012.742534. [DOI] [PubMed] [Google Scholar]
- 35.Guzmán S, Gato A, Lamela M, Freire-Garabal M, Calleja JM. Anti-inflammatory and immunomodulatory activities of polysaccharide from Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res. 2003;17:665–70. doi: 10.1002/ptr.1227. [DOI] [PubMed] [Google Scholar]
- 36.Guzmán S, Gato A, Calleja JM. Antiinflammatory, analgesic and free radical scavenging activities of the marine microalgae Chlorella stigmatophora and Phaeodactylum tricornutum. Phytother Res. 2001;15:224–30. doi: 10.1002/ptr.715. [DOI] [PubMed] [Google Scholar]
- 37.Samarakoon KW, Ko JY, Shah MR, Lee JH, Kang MC, Nam KO, et al. In vitro studies of anti-inflammatory and anticancer activities of organic solvent extracts from cultured marine microalgae. Algae. 2013;28:111–9. [Google Scholar]
- 38.Soontornchaiboon W, Joo SS, Kim SM. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol Pharm Bull. 2012;35:1137–44. doi: 10.1248/bpb.b12-00187. [DOI] [PubMed] [Google Scholar]