Abstract
Background and Objectives:
Urinary tract infection (UTI) is one of the most frequent infectious diseases and can occur in all age groups. Escherichia coli is the main cause of this infection. Multiple resistances to antimicrobial agents are increasing quickly in E. coli isolates and may complicate therapeutic strategies for UTI. The aim of this study was to determine the antibiotic resistance pattern and the multidrug-resistance (MDR) phenotypes in uropathogenic E. coli (UPEC).
Materials and Methods:
A total of 135 UPEC isolates were collected from both outpatients (91 isolates) and inpatients (44 isolates) between September, 2012 and February, 2013. In order to determine the MDR among UPEC isolates, we have tested 15 antimicrobial agents and antibiotic susceptibility was done by Kirby-Bauer disk diffusion method.
Results:
The percentage of MDR isolates (resistant to at least three drug classes such as aminoglycosides, fluoroquinolones, penicillins, cephalosporins, or carbapenems) was 68% in the inpatients and 61% in the outpatients. Antibiotic resistance to ampicillin, ceftazidim, nalidixic acid, and trimethoprim/sulfamethoxazole were higher than 50%. Amikacin, nitrofurantoin, and gentamicin showed markedly greater activity (89.1%, 85.9%, and 82.4% sensitivity, respectively) than other antimicrobial agents. Resistance to meropenem did show either in outpatients or in inpatients.
Interpretation and Conclusions:
The high prevalence of drug resistance among UTI patients calls for continuous monitoring of the incidence of drug resistance for appropriate empiric selection of antibiotic therapy. Empirical treatment of UTIs should be relied on susceptibility patterns from local studies.
Keywords: Antimicrobial resistance, Escherichia coli, multidrug resistance, urinary tract infections
INTRODUCTION
Urinary tract infections (UTIs) are the most common infections and are mostly caused by Gram-negative bacteria.[1] Almost 150 million cases of UTIs per year were reported worldwide.[2] According to an estimation, almost 40-50% of women experience UTIs once in lifetime.[3] Recurrent UTI occurs in adult women and results in high healthcare costs.[4,5] Based on the surveys, UTI is an independent risk factor for renal cell carcinoma and bladder cancer.[6,7] According to the studies, uropathogenic Escherichia coli (UPEC) is the most common cause of UTIs.[8,9,10] UPEC isolates possess multiple virulence factors that promote colonization of the bacteria and infection in the urinary tract such as fimbrial, adhesins, afimbrialadhesin, toxins, siderophores, and capsular polysaccharide.[10,11,12] The clinical experiences have shown a high rate of antibiotic resistance among uropathogens.[13,14,15] Excessive use of antibiotics is the most important factor in the rise of multidrug resistance (MDR) in UPEC isolates.[16] Antibiotic resistance is a serious public health emergency leading to increased mortality and morbidity.[17] According to the European Centre for Disease Prevention and Control in 2007, antibiotic resistance caused about 25,000 deaths annually which was equal to about half the number of deaths in a road accident in Europe.[18] Due to the risk of kidney damage and complications, early diagnosis and treatment of disease is important.[19,20] Since UTI cause several complex symptoms, physicians begin empirical antibiotic treatment before getting the culture results because urine culture and antibiogram results take about 4 days to be prepared.[21,22,23] According to reports from the USA, Japan, China, India, Saudi Arabia, Brazil, and Nepal, the prevalence of MDR E. coli causing UTIs is increasing.[1,24,25,26,27,28,29] The knowledge of the main bacteria usually involved in the UTIs and their antimicrobial susceptibility are necessary for appropriate empirical therapy and prevention of the emergence of antibiotic resistance. Since these data are constantly changing and may vary from hospital to hospital, each institution should determine these information and update them regularly.[30,31] The present study aimed to define the current occurrence and phenotypes of MDR E. coli among UTI isolates from a university medical center, Alzahra Hospital, Isfahan, Iran.
MATERIALS AND METHODS
Bacterial isolates
A total of 135 strains of E. coli causing UTIs were isolated from both outpatients (91 isolates) and inpatients (44 isolates) from Alzahra Hospital (Isfahan, Iran). The isolates were collected between September, 2012 and February, 2013. Samples were isolated from the urine. Diagnosis of E. coli isolates has been done according to Bailey and Scott's diagnostic microbiological methods.[32] The samples were cultured on nutrient agar, MacConkey agar, blood agar, and eosin-methylene blue agar (purchased from Himedia Company). The plates were incubated at 35°C for 24 h and the pure isolates characterized and identified according to Gram-stains and biochemical tests such as catalase, oxidative, citrate utilization, indole production, methyl red-Voges Proskauer, triple iron sugar utilization, and urea test and as described in standard bacteriological methods.[32] Quality control was tested by E. coli ATCC25922.
Antibiotic susceptibility test
Bacterial susceptibility to antimicrobial agents was determined by using disk diffusion method as recommended by the Clinical and Laboratory Standards Institute guidelines.[33] The antibiotic disks used in this study were ciprofloxacin (CIP) (5 μg), norfloxacin (10 μg), ofloxacin (OFX) (5 μg), nalidixic acid (NAL) (30 μg), amikacin (30 μg), ampicillin (AMP) (10 μg), cefotaxime (CTX) (30 μg), gentamicin (GEN) (10 μg), nitrofurantoin (NOR) (300 μg), trimethoprim/sulfamethoxazole (SXT) (1.75/23.75 μg), cefoxitin (30 μg), meropenem (10 μg), cefepime (FEP) (30 μg), ceftazidime (30 μg), and cephalothin (30 μg).
E. coli ATCC25922 was used as a quality control strain. Then the data were entered into Whonet 5.6 (WHO, Geneva, Switzerland) software. An isolate was considered MDR if it was resistant to at least three of the antimicrobial classes such as aminoglycosides, fluoroquinolones, penicillins, cephalosporins, or carbapenems.
RESULTS
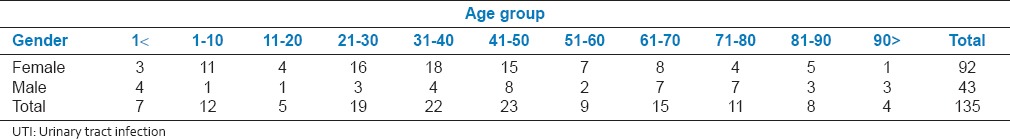
The age distribution was determined based on the decade, and different prevalence patterns were observed in each age range as described in Table 1. In total, 68% of the participants were female, and 14% of the samples belong to children.
Table 1.
Age and gender distribution of patients diagnosed with UTI
The resistance rates of UPEC isolates to antimicrobial agents in both outpatients and inpatients are shown in Figure 1. Antibiotic resistance to AMP, ceftazidim, NAL, and SXT were higher than 50%. The rates of resistance to AMP, SXT, ceftazidim, CTX, and FEP in outpatients were higher than in inpatients. Resistance to meropenem did show neither in outpatients nor in inpatients. Amikacin, NIT, and GEN showed markedly greater activity (89.1%, 85.9%, and 82.4% sensitivity, respectively) than other antimicrobial agents.
Figure 1.
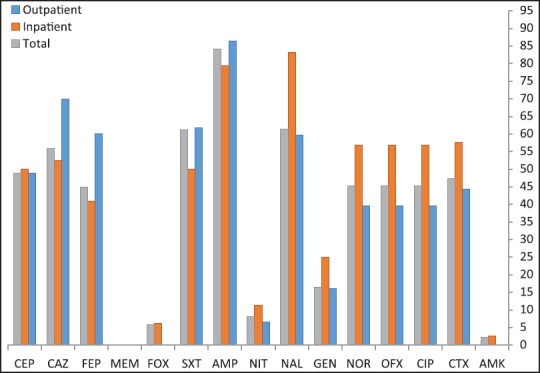
Percentages of antibiotic resistance in Escherichia coli isolated from outpatients and inpatients
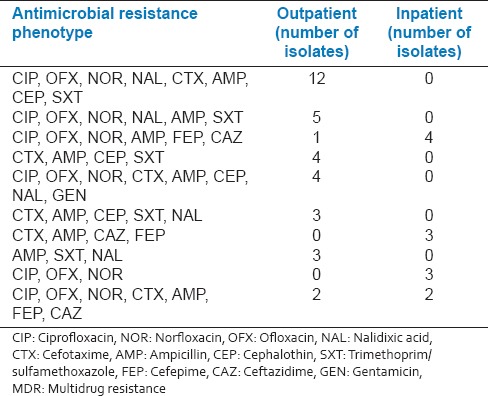
Antimicrobial susceptibility test showed that the rate of MDR isolates was 68% in inpatients and 61% in outpatients. The isolates were divided into seven groups according to their resistance patterns [Table 2]. The most common MDR phenotypes are shown in Table 3. The prevalent pattern among total UPEC isolates was CIP, OFX, NOR, NAL, CTX, AMP, FEP, SXT; and it was only among outpatients.
Table 2.
Resistance pattern of the Escherichia coli strains
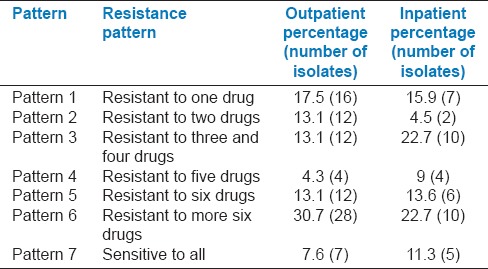
Table 3.
The most common antimicrobial resistance phenotypes showing MDR among Escherichia coli isolates
DISCUSSION
MDR in UPEC causing UTIs is an emerging and serious public health problem and results in treatment failure. This study provides current information about the antimicrobial resistance pattern in E. coli isolates from patients’ urine samples in the Alzahra Hospital, Isfahan, Iran. Based on the results of the present study, there is a high resistance rate to the commonly used antibiotics in the E. coli isolates. We found 63% of the isolates to be resistant to three or more antibiotics. The rates of antibiotic resistance in our study were different from some studies in other countries.[18,34,35,36,37] MDR rate was higher in inpatients isolates (68%). A significant high resistance to SXT (61.2%) was found in the present study while many guidelines recommend this drug for UTIs.[38,39] In addition, CIP and OFX antibiotics recommended for UTIs, showed high resistance in data from other surveys and European countries according to Annual report of the European Antimicrobial Resistance Surveillance Network in 2012.[40,41,42] Excessive use of AMP in the treatment of UTIs, especially in hospitalized patients, gives a possible explanation for the existence of high resistance rate (84.2%) to this antimicrobial agent. The rates of resistance to AMP, SXT and CTX in outpatients were higher than in inpatients while the rates of resistance to fluoroquinolones and NAL in outpatients were lower than in inpatients. According to experts, resistance level >20% is used as a cut-off in guidelines on UTIs, so these antibiotics should not be recommended for the treatment of UTIs.[43,44] However, low levels of resistance to amikacin, NIT, and GEN (2.2%, 8.1%, and 16.5%, respectively) and no resistance against meropenem was observed in the present study. Based on our results, the extremely high percentage of isolates showed an MDR phenotype. Some socioeconomic and behavioral factors can contribute to antibiotic resistance such as misuse of antimicrobial agents by hospital physicians or unskilled practitioners and easy access to antibiotics without a prescription.[45] These warning resistances to the commonly used antibiotics can affect the therapeutic strategies. The data of the current study cannot be translated to the international level. The successful empirical initial treatment is based on susceptibility and resistance patterns obtaining from local data. Since these susceptibility patterns are constantly changing and may vary in different geographical regions and institutions, regular monitoring of antimicrobial agents resistance seems necessary to formulate standard treatment guidelines for empirical therapy.
CONCLUSION
UTI is the most common infectious disease. As bacterial resistance to the common antimicrobial agents has increased considerably among E. coli causing UTIs, empirical antibiotic treatment should be reviewed periodically at a regional level.
STUDY LIMITATIONS
One limitation of this study was the lack of extended-spectrum beta-lactamase-testing to determine the minimum inhibitory concentration of antibiotics and further analysis for a more accurate measure of antibiotic resistance.
Financial support and sponsorship
This study was funded by the Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the members of Alzahra Hospital of Isfahan city for their technical assistance and help with the English language version of this paper.
REFERENCES
- 1.Niranjan V, Malini A. Antimicrobial resistance pattern in Escherichia coli causing urinary tract infection among inpatients. Indian J Med Res. 2014;139:945–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Mishra MP, Debata NK, Padhy RN. Surveillance of multidrug resistant uropathogenic bacteria in hospitalized patients in Indian. Asian Pac J Trop Biomed. 2013;3:315–24. doi: 10.1016/S2221-1691(13)60071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Momtaz H, Karimian A, Madani M, Safarpoor Dehkordi F, Ranjbar R, Sarshar M, et al. Uropathogenic Escherichia coli in Iran: Serogroup distributions, virulence factors and antimicrobial resistance properties. Ann Clin Microbiol Antimicrob. 2013;12:8. doi: 10.1186/1476-0711-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flower A, Bishop FL, Lewith G. How women manage recurrent urinary tract infections: An analysis of postings on a popular web forum. BMC Fam Pract. 2014;15:162. doi: 10.1186/1471-2296-15-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickling DR, Nitti VW. Management of recurrent urinary tract infections in healthy adult women. Rev Urol. 2013;15:41–8. [PMC free article] [PubMed] [Google Scholar]
- 6.Parker AS, Cerhan JR, Lynch CF, Leibovich BC, Cantor KP. History of urinary tract infection and risk of renal cell carcinoma. Am J Epidemiol. 2004;159:42–8. doi: 10.1093/aje/kwh014. [DOI] [PubMed] [Google Scholar]
- 7.Schaeffer EM. Re: Antimicrobial susceptibility of global inpatient urinary tract isolates of Escherichia coli: Results from the Study for Monitoring Antimicrobial Resistance Trends (SMART) program: 2009-2010. J Urol. 2012;187:1280. doi: 10.1016/j.juro.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 8.De Francesco MA, Ravizzola G, Peroni L, Negrini R, Manca N. Urinary tract infections in Brescia, Italy: Etiology of uropathogens and antimicrobial resistance of common uropathogens. Med Sci Monit. 2007;13:BR136–44. [PubMed] [Google Scholar]
- 9.Laupland KB, Ross T, Pitout JD, Church DL, Gregson DB. Community-onset urinary tract infections: A population-based assessment. Infection. 2007;35:150–3. doi: 10.1007/s15010-007-6180-2. [DOI] [PubMed] [Google Scholar]
- 10.Kucheria R, Dasgupta P, Sacks SH, Khan MS, Sheerin NS. Urinary tract infections: New insights into a common problem. Postgrad Med J. 2005;81:83–6. doi: 10.1136/pgmj.2004.023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tiba MR, Yano T, Leite Dda S. Genotypic characterization of virulence factors in Escherichia coli strains from patients with cystitis. Rev Inst Med Trop Sao Paulo. 2008;50:255–60. doi: 10.1590/s0036-46652008000500001. [DOI] [PubMed] [Google Scholar]
- 12.Asadi S, Kargar M, Solhjoo K, Najafi A, Ghorbani-Dalini S. The association of virulence determinants of uropathogenic Escherichia coli with antibiotic resistance. Jundishapur J Microbiol. 2014;7:e9936. doi: 10.5812/jjm.9936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soltani R, Ehsanpoor M, Khorvash F, Shokri D. Antimicrobial susceptibility pattern of extended-spectrum β-lactamase-producing bacteria causing nosocomial urinary tract infections in an Iranian referral teaching hospital. J Res Pharm Pract. 2014;3:6–11. doi: 10.4103/2279-042X.132703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sedighi M, Salehi-Abargouei A, Oryan G, Faghri J. Epidemiology of VIM-1-imipenem resistant Pseudomonas aeruginosa in Iran: A systematic review and meta-analysis. J Res Med Sci. 2014;19:899–903. [PMC free article] [PubMed] [Google Scholar]
- 15.Baral P, Neupane S, Marasini BP, Ghimire KR, Lekhak B, Shrestha B. High prevalence of multidrug resistance in bacterial uropathogens from Kathmandu, Nepal. BMC Res Notes. 2012;5:38. doi: 10.1186/1756-0500-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miles TD, McLaughlin W, Brown PD. Antimicrobial resistance of Escherichia coli isolates from broiler chickens and humans. BMC Vet Res. 2006;2:7. doi: 10.1186/1746-6148-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyar OJ, Hoa NQ, Trung NV, Phuc HD, Larsson M, Chuc NT, et al. High prevalence of antibiotic resistance in commensal Escherichia coli among children in rural Vietnam. BMC Infect Dis. 2012;12:92. doi: 10.1186/1471-2334-12-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linhares I, Raposo T, Rodrigues A, Almeida A. Frequency and antimicrobial resistance patterns of bacteria implicated in community urinary tract infections: A ten-year surveillance study (2000-2009) BMC Infect Dis. 2013;13:19. doi: 10.1186/1471-2334-13-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori R, Lakhanpaul M, Verrier-Jones K. Diagnosis and management of urinary tract infection in children: Summary of NICE guidance. BMJ. 2007;335:395–7. doi: 10.1136/bmj.39286.700891.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management, Roberts KB. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 21.Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med. 2012;366:1028–37. doi: 10.1056/NEJMcp1104429. [DOI] [PubMed] [Google Scholar]
- 22.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: A 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–20. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 23.Lo DS, Shieh HH, Ragazzi SL, Koch VH, Martinez MB, Gilio AE. Community-acquired urinary tract infection: Age and gender-dependent etiology. J Bras Nefrol. 2013;35:93–8. doi: 10.5935/0101-2800.20130016. [DOI] [PubMed] [Google Scholar]
- 24.Sahm DF, Thornsberry C, Mayfield DC, Jones ME, Karlowsky JA. Multidrug-resistant urinary tract isolates of Escherichia coli: Prevalence and patient demographics in the United States in 2000. Antimicrob Agents Chemother. 2001;45:1402–6. doi: 10.1128/AAC.45.5.1402-1406.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafay AM, Nsanze HN. Multi-drug resistance of Escherichia coli from the urinary tract. Saudi Med J. 2003;24:261–4. [PubMed] [Google Scholar]
- 26.Mathai E, Grape M, Kronvall G. Integrons and multidrug resistance among Escherichia coli causing community-acquired urinary tract infection in southern India. APMIS. 2004;112:159–64. doi: 10.1111/j.1600-0463.2004.apm1120301.x. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa K, Hayakawa S, Miyakawa S, Kusaka M, Shiroki R, Hoshinaga K. Survey of the susceptibility of urinary isolates to antibacterial agents in 2003. J Infect Chemother. 2005;11:44–7. doi: 10.1007/s10156-004-0356-9. [DOI] [PubMed] [Google Scholar]
- 28.Ho PL, Wong RC, Yip KS, Loke SL, Leung MS, Mak GC, et al. Antimicrobial resistance in Escherichia coli outpatient urinary isolates from women: Emerging multidrug resistance phenotypes. Diagn Microbiol Infect Dis. 2007;59:439–45. doi: 10.1016/j.diagmicrobio.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Kumari N, Ghimire G, Magar JK, Mohapatra TM, Rai A. Antibiogram pattern of isolates from UTI cases in Eastern part of Nepal. Nepal Med Coll J. 2005;7:116–8. [PubMed] [Google Scholar]
- 30.Wagenlehner F, Naber K. Antibiotics and resistance of uropathogens. EAU Update Ser. 2004;2:125–35. [Google Scholar]
- 31.Dias Neto JA, Martins AC, Silva LD, Tiraboschi RB, Domingos AL, Cologna AJ, et al. Community acquired urinary tract infection: Etiology and bacterial susceptibility. Acta Cir Bras. 2003;18:33–6. [Google Scholar]
- 32.Tille P. China: Elsevier Health Sciences; 2013. Bailey & Scott's Diagnostic Microbiology. [Google Scholar]
- 33.Cockerill FR, Clinical, Institute LS. Wayne, USA: National Committee for Clinical Laboratory Standards; 2012. Performance Standards for Antimicrobial Disk Susceptibility Testing: Approved Standard. [Google Scholar]
- 34.Koningstein M, van der Bij AK, de Kraker ME, Monen JC, Muilwijk J, de Greeff SC, et al. Recommendations for the empirical treatment of complicated urinary tract infections using surveillance data on antimicrobial resistance in the Netherlands. PLoS One. 2014;9:e86634. doi: 10.1371/journal.pone.0086634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mowla R, Imam KM, Asaduzzaman M, Nasrin N, Raihan SZ, Chowdhury AK. Emergence of multidrug resistant extended-spectrum β-lactamase producing Eshcherichia coli associated with urinary tract infections in Bangladesh. J Basic Clin Pharm. 2011;3:225–8. doi: 10.4103/0976-0105.103829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jafri SA, Qasim M, Masoud MS, Rahman MU, Izhar M, Kazmi S. Antibiotic resistance of E. coli isolates from urine samples of Urinary Tract Infection (UTI) patients in Pakistan. Bioinformation. 2014;10:419–22. doi: 10.6026/97320630010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandal J, Acharya NS, Buddhapriya D, Parija SC. Antibiotic resistance pattern among common bacterial uropathogens with a special reference to ciprofloxacin resistant Escherichia coli. Indian J Med Res. 2012;136:842–9. [PMC free article] [PubMed] [Google Scholar]
- 38.Wuorela M, Kouri T, Laato M, Lipponen P, Sammalkorpi K, Uhari M, et al. Update on current care guidelines: Urinary tract infections. Duodecim. 2011;127:2334–5. [PubMed] [Google Scholar]
- 39.van Pinxteren B, van Vliet SM, Wiersma TJ, Goudswaard AN. Nederlands Huisartsen Genootschap. Summary of the practice guideline ‘Urinary-tract infections’ (second revision) from the Dutch College of General Practitioners. Ned Tijdschr Geneeskd. 2006;150:718–22. [PubMed] [Google Scholar]
- 40.Saha S, Nayak S, Bhattacharyya I, Saha S, Mandal AK, Chakraborty S, et al. Understanding the patterns of antibiotic susceptibility of bacteria causing urinary tract infection in West Bengal, India. Front Microbiol. 2014;5:463. doi: 10.3389/fmicb.2014.00463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmiemann G, Gágyor I, Hummers-Pradier E, Bleidorn J. Resistance profiles of urinary tract infections in general practice: An observational study. BMC Urol. 2012;12:33. doi: 10.1186/1471-2490-12-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stockholm: ECDC; 2013. European Centre for Disease Prevention and Control. Antimicrobial Resistance Surveillance in Europe 2012. Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS-Net) [Google Scholar]
- 43.Grabe M, Bishop M, Bjerklund-Johansen T, Botto H, Çek M, Lobel B, et al. Management of urinary and male genital tract infections. Update. 2008;5:47–60. doi: 10.1159/000049840. [DOI] [PubMed] [Google Scholar]
- 44.Wagenlehner FM, Schmiemann G, Hoyme U, Fünfstück R, Hummers-Pradier E, Kaase M, et al. National S3 guideline on uncomplicated urinary tract infection: Recommendations for treatment and management of uncomplicated community-acquired bacterial urinary tract infections in adult patients. Urologe A. 2011;50:153–69. doi: 10.1007/s00120-011-2512-z. [DOI] [PubMed] [Google Scholar]
- 45.Okeke IN, Lamikanra A, Edelman R. Socioeconomic and behavioral factors leading to acquired bacterial resistance to antibiotics in developing countries. Emerg Infect Dis. 1999;5:18–27. doi: 10.3201/eid0501.990103. [DOI] [PMC free article] [PubMed] [Google Scholar]


