Abstract
Context:
The assessment of micronuclei (MN) in exfoliated oral epithelial cells is a promising tool for the study of epithelial carcinogens and can be used to detect chromosome breakage or mitotic interference, thought to be relevant to carcinogenesis.
Aims:
To detect MN in exfoliated oral mucosal cells in individuals using various tobacco forms and also to detect frequency of MN in premalignant lesions and conditions (potentially malignant diseases [PMD's]) and oral squamous cell carcinoma (OSCC). To correlate frequency of MN in oral exfoliated cells in clinically diagnosed cases of OSCC followed by a histopathological grading.
Materials and Methods:
A total of 90 subjects (30 smokeless tobacco users, 30 smokers and 30 nontobacco users) consisted of clinically diagnosed cases of PMD's and OSCC were selected for the study. Cytosmears from the groups were stained with rapid Papanicolaou stain. MN was identified according to the Tolbert et al. criteria.
Results:
MN cells were found to be significantly higher in smokeless tobacco users than in smokers. The frequency of MN was three to four times higher in patients with OSCC as compared to patients in PMD's (P < 0.0001). The frequency of MN correlated with the histopathological grade was statistically significant.
Conclusion:
MN index can be used as a biomarker/screening test among the high-risk groups particularly the smokeless tobacco users and PMD's. MN can be a candidate to serve as a biomarker for prediction of the grade of OSCC.
Keywords: Micronuclei, oral squamous cell carcinoma, potentially malignant diseases, smokeless tobacco, smoking tobacco
INTRODUCTION
Oral cancer is one of the most common causes of morbidity and mortality nowadays. In developing countries, both smoking, and smokeless tobacco have cancer causing behavior that continues to be increasing the global burden of oral cancer. The World Health Organization estimated that the proportion of deaths that result from tobacco-related diseases would rise in India from 1.4% of all in 1990 to 13.3% of all deaths in 2020. According to a report of Economic and Social Council, the models presented in 2002 showed that the number of persons consuming tobacco is also likely to rise.[1,2] The majority of the oral cancers preceded by the potentially malignant lesions and conditions (potentially malignant diseases [PMD's]).[3] These lesions clinically show premalignant mucosal changes that give a warning of risk and at hand an opportunity for detection and preventive measures. Early diagnosis of a potentially malignant lesions and sometimes cancerous lesions may improve the survival and the morbidity of patients, micronuclei (MN) are good prognostic indicators.[4]
The MN are round to oval extranuclear cytoplasmic bodies associated with chromosomal aberrations.[3] Cells often have errors in chromosomal segregation that lead to the formation of a lagging chromosome or chromosomal parts that become lost during the anaphase stage of cell separation and are excluded from the reforming nuclei. The laggards are observed in the cytoplasm as MN.[5] These MN are induced in oral exfoliated cells by a variety of substances, including genotoxic agents and carcinogenic compounds in tobacco, betel nut, and alcohol.[6] The induction of micronucleated cells by carcinogens and mutagens is a sign of the genotoxic effects of such substances.[3] Tobacco-specific nitrosamines have been reported to be potent clastogenic and mutagenic agents which are thought to be responsible for the induction of chromatid/chromosomal aberrations resulting in the production of MN. The genotoxic and carcinogenic chemicals released from betel nut and tobacco and also the calcium hydroxide content of lime present in the betel quid are thought to be responsible for the promotion of reactive oxygen species from areca nut extracts. These reactive oxygen species can in turn cause damage to the DNA.[6,7]
Oral cancer is characterized by complex karyotypes that involve many chromosomal deletions, translocations, and structural abnormalities.[5] Due to its association with chromosomal aberrations, MN has been used since 1937 as an indicator of genotoxic exposure, based on the radiation studies conducted by Brenneke and Mather.[7] The assay is reliable and technically easy to perform. The direct correlation between the MN formation and genomic damage make the MN assay an efficient alteration to the metaphase analysis.[8]
Based on these findings, suggested that MN can be used as a biomarker in the screening of PMD's. A considerable number of studies conducted in the past have confirmed a significant increase in MN frequency in oral exfoliated epithelial cells of PMD's, as compared with normal healthy mucosa. With this view in mind, the present study was carried out to detect MN in exfoliated oral mucosal cells in individuals using various tobacco forms and also to detect frequency of MN in PMD's and oral squamous cell carcinoma (OSCC). To correlate the frequency of MN in oral exfoliated cells in clinically diagnosed cases of OSCC followed by a histopathological grading.
The present study aimed to detect MN in exfoliated oral mucosal cells in individuals using various tobacco forms such as smokeless tobacco users and smoking tobacco users. And also to detect the frequency of MN in PMD's and OSCC. To correlate the frequency of MN in oral exfoliated cells in clinically diagnosed cases of OSCC followed by a histopathological grading.
MATERIALS AND METHODS
Patient selection
A total of 90 patients, 30 patients with PMD's, 30 patients with OSCC, and 30 patients with normal mucosa were selected from the outpatients who attended the Department of Oral and Maxillofacial Pathology, MIDSR Dental College and Hospital, Latur, Maharashtra, from July 2013 to May 2015. Subjects with oral lesions suspected to be malignant were selected as the study group. Relevant history of each patient, including their oral habits was recorded thoroughly. Only those patients who were subsequently diagnosed histopathologically as squamous cell carcinoma (SCC) in addition to who had not received any prior therapy were included in the OSCC group. Thirty patients clinically and histopathologically proven cases of PMD's (Leukoplakia, oral submucous fibrosis, and lichen planus) were included in Group II. These two groups were divided according to habit as 30 patients as smokeless tobacco users and 30 patients as smoking tobacco users. Only those 30 were comprised in smokeless tobacco chewers who chewed five or more packets daily for at least 5 years and consumed 20-25 or more bidis/cigarettes in a day as smoking tobacco users. Age-and sex-matched healthy subjects having no obvious oral lesions or habits of consumption of tobacco, other tobacco-related substances, or other such substances were included in the control group.
Written informed consent from the patients was taken prior to the study. Four observers participated in the study for analysis as mentioned below:
Observer 1: Counting of MN in PMD's Group,
Observer 2: Counting of MN in OSCC Group,
Observer 3: Counting of MN for histopathological scoring and grading of OSCC,
Observer 4: Counting of MN in control group.
The slides of each group were exchanged among all the observers, providing each observer a participation in each type of observation. They were not provided with information regarding the study subjects to prevent observer bias. The average score of all the observations by the four observers was calculated for each group to resolve the inter-observer bias.
Collection of exfoliated cells
Subjects were asked to rinse their mouth gently with tap water. To obtain the smear of exfoliated cells from the oral cavity (buccal mucosa in control group), a slightly moistened wooden spatula was used. For PMD's, representative site selected for leukoplakia, oral submucous fibrosis and lichen planus was the lesional areas like buccal mucosa. In OSCC patients, oral mucosal cells were scraped from the margins of the lesion for obtaining the smear. The cells were immediately smeared on precleaned microscopic slides. Just prior to drying, the smears were fixed with commercially available spray fixative (available with the RAPIDPAPTM kit) for 15 min. The slides were coded and preserved in dust-free boxes until evaluation.
Biopsy procedure and histopathological grading
Incisional biopsies were taken from the representative sites with all aseptic precautions. The tissue specimens were labeled, fixed in 10% formalin for 24 h, and paraffin embedded. The wax blocks were cut to obtain two tissue sections of 4 μm thickness for each block and the sections stained by hematoxylin and eosin. Histopathological diagnosis was given as potentially malignant lesions and conditions (hyperkeratotic lesions, oral submucous fibrosis, and lichen planus) and OSCC. Histopathological grading of SCC was done according to the malignancy grading system proposed by Anneroth et al.[9]
Cytological preparation and evaluation
The smears were stained by Papanicolaou technique using a commercially available staining kit RAPIDPAP. From each slide, 500 cells were examined under the light microscope using low magnification (×400) for screening and high magnification (×1000) for counting of MN.
Scoring criteria
The criteria which was developed by Tolbert et al.[10] was used for counting the MN. Screening of each slide was made in a zigzag manner from one end, toward the other end of the slide.
Tolbert et al. criteria parameters for identifying MN are as follows:
Rounded smooth perimeter suggestive of a membrane.
Less than a third the diameter of associated nucleus, but large enough to discern shape and color.
Staining intensity similar to nucleus.
Same focal plane as nucleus.
Texture similar to nucleus.
The absence of overlap with or bridge to the nucleus.
Only those structures fulfilling the above-mentioned criteria were recorded as MN.
Inclusion and exclusion criteria
Micronucleated cells were counted out of 500 intact epithelial cells, and they were expressed as percentages. Nuclear blebbing (MN-like structure connected with the main nucleus with a bridge) were not considered. Clumps of cells with obscured nuclear or cytoplasmic boundaries and overlapping of cells were avoided and separated or cells lying singly were preferred for counting of MN. Dead or degenerated cells, apoptotic cells, and cytoplasmic fragments were excluded from evaluation.
Data entry and statistical analysis
Ethical clearance was taken by the institute before commencing the study.
All calculations were performed using Microsoft 2007 version for windows for Excel. The data obtained was statistically analyzed with the help of ANOVA-test and Tukey's post-hoc test.
RESULTS
The MN cells observed are shown in Figure 1.
Figure 1.

Photomicrograph showing exfoliated cells with four (a), Five (b) And six (c) Micronuclei with Papanicolaou stain (×400)
The present study comprised of 30 cases of histopathologically diagnosed OSCC and 30 as potentially malignant group and 30 as healthy control subjects having no obvious oral lesions or habits of consumption of tobacco, other tobacco-related substances, or other such substances. Potentially malignant group comprises of 10 cases of leukoplakia, 10 cases of oral submucous fibrosis and 10 cases of lichen planus. While OSCC cases were 15 (50%) of Grade I carcinoma, 10 (33.33%) cases were of Grade II carcinoma, and 5 (16.66%) cases were of Grade III carcinoma.
The results of our study are summarized in Tables 1–3.
Table 1.
Comparison of mean numbers of micronuclei among control, tobacco chewers and smokers

Table 3.
Grade wise comparison of mean numbers of micronuclei among the grades of squamous cell carcinoma patients
The mean distribution of MN in smoking tobacco users and smokeless tobacco users and control group showed significant difference [Table 1]. The mean number of MN cells in smokeless tobacco users, smoking tobacco users and controls are 171.63 ± 24.14, 114.53 ± 10.96 and 19.07 ± 8.45 respectively. In comparison, the mean difference between the number of MN in smokeless tobacco users and smoking tobacco users was statistically significant (P < 0.0001) [Table 1].
When the mean number of MN cell distribution was compared in potentially malignant group, OSCC group and control group, following observations were recorded as 116.57 ± 12.29, 177.93 ± 13.73 and 19.07 ± 8.45, respectively [Table 2]. In comparison, the mean difference between the number of MN in potentially malignant group, OSCC group was statistically significant (P < 0.0001) [Table 2].
Table 2.
Comparison of mean numbers of micronuclei among control subjects with potentially malignant diseases and oral squamous cell carcinoma

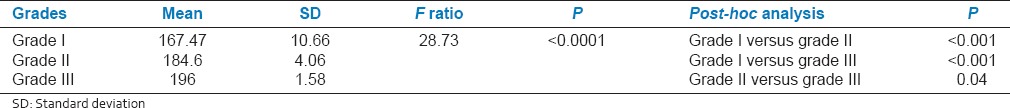
In OSCC group, the frequencies of MN in different histological grades of OSCC were tabulated in Table 3. The MN frequencies were found to increase from Grade I (167.47 ± 10.66) to Grade II (184.6 ± 4.06) and Grade III (196 ± 1.58), the difference being statistically highly significant (P < 0.0001) [Table 3].
Each slide was observed by four observers. The statistical analysis showed no significant difference between mean no. of nuclei observed by all the observers.
DISCUSSION
OSCC accounts for 90-95% of all oral malignancies.[11] Biomonitering, the patient with the pathological changes that may lead to the development of cancer, is becoming increasingly popular, and may be the most rapidly growing area. Epithelial cells are highly proliferative and are the origin of more than 90% of all human cancers. Therefore, the application of MN test in epithelial cells is considered to be a sensitive tool for biomonitoring the genetic damage in human population.[12] Oral exfoliative cytology has been used broadly for screening cellular alteration in OSCC cases.[13] MN in oral exfoliated cells is a marker of chromosomal damage caused through genotoxic agents from tobacco and tobacco-related substances, alcohol, etc.[6] The MN assay has been used to evaluate the genotoxic damage in OSCC and oral premalignancies.[14,15] The MN assay has been reported to correlate well with the histological grading of OSCC and leukoplakia.[16] The incidence of MN has been analyzed by various studies in normal patients, oral premalignancies, and OSCC. The most widely used procedure for staining MN analysis involves a feulgen and acridine orange reaction, fluorescence in situ hybridization with a centromeric probe, Giemsa staining method and H and E stain. However, we used Papaniculaou stain, which is the most commonly used cytological stain, very simple to use, less time consuming, economical and has resulted good clarity and transparency of epithelial cells which enables to identify MN easily.[4,5]
The present study evaluated the mean number of MN in smokeless tobacco users, smoking tobacco users and healthy control group. The results showed that the mean number of MN in smokeless tobacco (171.63 ± 24.14) were higher as compared to smoking tobacco (114.53 ± 10.96) and control group (19.07 ± 8.45). This finding was similar to those reported by Palaskar and Jindal,[5] Ozkul et al.,[17] Patel et al.,[18] and Bansal et al.,[19] when all the groups were further compared with each other for the mean difference, the result was highly statistically significant (P < 0.0001), which was in accordance with the previous studies by Bansal et al.[19] and Patel et al.[18] whereas Ozkul et al.[17] found no difference between the mean percentage of MN cells for the groups considered (P > 0.05). The mean number of MN was higher in smokeless tobacco users indicating more genotoxic effects of smokeless tobacco compared to smoking tobacco. This can be explained on the basis of tobacco-chewing habit in which the mucosa is in constant contact with the tobacco for longer period causing DNA damage to the mucosal cells by the local absorption of genotoxic agent, that is, nitrosamine present in the tobacco. Hence, increase in MN count might be due to the damage caused by genotoxic agents especially N-nitrosamine released from the tobacco which causes nuclear damage in the form of separation of small fragments leading to MN.[20]
In our present study, MN frequency was seen in increasing from the control group (19.07 ± 8.45) to potentially malignant (116.57 ± 12.29) and OSCC group (177.93 ± 13.73). And also, in comparison, the mean difference between the numbers of MN in potentially malignant group, OSCC group was statistically significant (P < 0.0001). These observations indicate cytogenic damage of the epithelial cells. We got similar results with Parvathi et al.,[12] Halder et al.[3] According to the literature, there are studies[3,11,12,14,21,22,23,24] being conducted in the past, in which statistically significant difference was found, between mean percentage of MN in PMD's and healthy subjects as controls. The same results were also observed in our study. According to Samanta and Dey, the various possible explanations for MN formation in preneoplastic conditions include chromosome loss/breakage, chromosomal aberrations, mitotic apparatus dysfunctions, aneuploidy, and genetic instability. Hence, as a biomarker of genomic damage, MN has been proved to be an important upcoming marker of tumorogenesis.[25] There was stepwise increase found in the percentage of micronucleated cells and MN from control to precancer patients, and from precancer to cancer patients in a study by Saran et al.[21] It is evident that our findings agree with those of Casartelli et al.,[14] a gradual increase in MN frequency from normal to precancerous to cancerous lesions. They concluded that the gradual increase in MN counts from normal mucosal to precancerous lesions to carcinoma suggested a link of this biomarker with neoplastic progression.[14]
A significant correlation of MN frequency with histopathological grading was observed in this study. The MN frequencies were found to increase from Grade I (167.47 ± 10.66) to Grade II (184.6 ± 4.06) and Grade III (196 ± 1.58), the difference being statistically highly significant (P < 0.0001). This observation was similar to those reported by Kumar et al.[6] and Palve and Tupkari,[4] where the frequency of MN increased significantly from Grade I to Grade II to Grade III, respectively, in SCC group. Thus, concluding that this could be due to chromosomal breakage associated with chromosomal translocation which may in turn lead to transposition and activation of oncogenes.[6] Hence, it can be put forward that the significant correlation of frequency of MN in oral exfoliated cells with the histopathological grading of OSCC was observed in this study.
To summarize the results, MN in exfoliated cells is an innovative genotoxicity technique, which holds promise for the study of epithelial carcinogens. The induction in vivo and in vitro, of micronucleated cells by carcinogens and mutagens is a sign of the genotoxic effect. Various groups have found analysis of MN in buccal cells to be a sensitive method for monitoring genetic damage in human populations. Hence, the usefulness of MN assay as a screening and early detection technique for cancer susceptibility has been suggested.
CONCLUSION
The present study showed that the significant increase in MN frequency in smokeless tobacco users as compared to smoking tobacco users. The mean MN frequency in oral exfoliated cells was significantly elevated in malignant and potentially malignant group as compared to the control group with different grades of OSCC. Thus, from the present study it is evident that the MN frequency is elevated in increasing grades of the tumor, suggesting a strong cytogenetic damage of oral epithelium. To conclude, although our numbers are small, the oral mucosal MN frequency may be a marker of epithelial carcinogenic progression. Further studies are required for determining its usefulness in this role.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
I would like to thank Dr. Wadde, Assistant Professor, Department of Preventive Medicine and Social Sciences, MIMSR Medical College, Ambajogai Road, Latur, Maharashtra, India, for their help in carrying out this study.
REFERENCES
- 1.2nd ed. Atlanta: American Cancer Society; 2011. International Agency for Research on Cancer. Global cancer facts and figures; pp. 1–57. [Google Scholar]
- 2.Jandoo T, Mehrotra R. Tobacco control in India: Present scenario and challenges ahead. Asian Pac J Cancer Prev. 2008;9:805–10. [PubMed] [Google Scholar]
- 3.Halder A, Chakraborty T, Mandal K. Comparative study of exfoliated oral mucosal cell micronuclei frequency in normal, precancerous and malignant epithelium. Int J Hum Genet. 2004;4:257–60. [Google Scholar]
- 4.Palve DH, Tupkari JV. Clinicopathological correlation of micronuclei in oral squamous cell carcinoma by exfoliative cytology. J Maxillofac Pathol. 2008;12:2–7. [Google Scholar]
- 5.Palaskar S, Jindal C. Evaluation of micronuclei using Papanicolaou and may Grunwald-Giemsa stain in individuals with different tobacco habits: A comparative study. J Clin Diagn Res. 2011;4:3607–13. [Google Scholar]
- 6.Kumar V, Rao NN, Nair NS. Micronuclei in oral squamous cell carcinoma. A marker of genotoxic damage. Indian J Dent Res. 2000;11:101–6. [PubMed] [Google Scholar]
- 7.Heddle JA, Hite M, Kirkhart B, Mavournin K, MacGregor JT, Newell GW, et al. The induction of micronuclei as a measure of genotoxicity. A report of the U.S. Environmental Protection Agency Gene-Tox Program. Mutat Res. 1983;123:61–118. doi: 10.1016/0165-1110(83)90047-7. [DOI] [PubMed] [Google Scholar]
- 8.Fenech M, Denham J, Francis W, Morley A. Micronuclei in cytokinesis-blocked lymphocytes of cancer patients following fractionated partial-body radiotherapy. Int J Radiat Biol. 1990;57:373–83. doi: 10.1080/09553009014552471. [DOI] [PubMed] [Google Scholar]
- 9.Anneroth G, Batsakis J, Luna M. Review of the literature and a recommended system of malignancy grading in oral squamous cell carcinomas. Scand J Dent Res. 1987;95:229–49. doi: 10.1111/j.1600-0722.1987.tb01836.x. [DOI] [PubMed] [Google Scholar]
- 10.Jois HS, Kale AD, Kumar KP. Micronucleus as potential biomarker of oral carcinogenesis. Indian J Dent Adv. 2010;2:1–5. [Google Scholar]
- 11.Ramaesh T, Ratnatunga N, Mendis BR, Rajapaksa S. Exfoliative cytology in screening for malignant and premalignant lesions in the buccal mucosa. Ceylon Med J. 1998;43:206–9. [PubMed] [Google Scholar]
- 12.Parvathi D, Thimmarasa VB, Vishal M, Pallak A. Micronucleus assay for evaluation of genotoxicity in potentially malignant and malignant disorders. JIAOMR. 2011;23:97–100. [Google Scholar]
- 13.Tolbert PE, Shy CM, Allen JW. Micronuclei and other nuclear anomalies in buccal smears: A field test in snuff users. Am J Epidemiol. 1991;134:840–50. doi: 10.1093/oxfordjournals.aje.a116159. [DOI] [PubMed] [Google Scholar]
- 14.Casartelli G, Bonatti S, De Ferrari M, Scala M, Mereu P, Margarino G, et al. Micronucleus frequencies in exfoliated buccal cells in normal mucosa, precancerous lesions and squamous cell carcinoma. Anal Quant Cytol Histol. 2000;22:486–92. [PubMed] [Google Scholar]
- 15.Sun Z, Li N, Zhang Z. The correlation analysis between frequency of micronucleated cells of exfoliated oral mucosa cells and oral mucosa cells in different grading of oral leukoplakia lesions. Zhonghua Kou Qiang Yi Xue Za Zhi. 2000;35:439–41. [PubMed] [Google Scholar]
- 16.Rajendran R. Benign and Malignant Tumors of the Oral Cavity. In: Shafer WG, Hine MK, Levy BM, editors. A Textbook of Oral Pathology. 4th Edition. Philadelphia, PA: WB Saunders Co; 1993. pp. 115–9. [Google Scholar]
- 17.Ozkul Y, Donmez H, Erenmemisoglu A, Demirtas H, Imamoglu N. Induction of micronuclei by smokeless tobacco on buccal mucosa cells of habitual users. Mutagenesis. 1997;12:285–7. doi: 10.1093/mutage/12.4.285. [DOI] [PubMed] [Google Scholar]
- 18.Patel BP, Trivedi PJ, Brahmbhatt MM, Shukla SN, Shah PM, Bakshi SR. Micronuclei and chromosomal aberrations in healthy tobacco chewers and controls: A study from Gujrat, India. Arch Oncol. 2009;17:7–10. [Google Scholar]
- 19.Bansal H, Sandhu VS, Bhandari R, Sharma D. Evaluation of micronuclei in tobacco users: A study in Punjabi population. Contemp Clin Dent. 2012;3:184–7. doi: 10.4103/0976-237X.96825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Proia NK, Paszkiewicz GM, Nasca MA, Franke GE, Pauly JL. Smoking and smokeless tobacco-associated human buccal cell mutations and their association with oral cancer — A review. Cancer Epidemiol Biomarkers Prev. 2006;15:1061–77. doi: 10.1158/1055-9965.EPI-05-0983. [DOI] [PubMed] [Google Scholar]
- 21.Saran R, Tiwari RK, Reddy PP, Ahuja YR. Risk assessment of oral cancer in patients with pre-cancerous states of the oral cavity using micronucleus test and challenge assay. Oral Oncol. 2008;44:354–60. doi: 10.1016/j.oraloncology.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Delfino V, Casartelli G, Garzoglio B, Scala M, Mereu P, Bonatti S, et al. Micronuclei and p53 accumulation in preneoplastic and malignant lesions of the head and neck. Mutagenesis. 2002;17:73–7. doi: 10.1093/mutage/17.1.73. [DOI] [PubMed] [Google Scholar]
- 23.Buajeeb W, Kraivaphan P, Amornchat C, Triratana T. Frequency of micronucleated exfoliated cells in oral lichen planus. Mutat Res. 2007;627:191–6. doi: 10.1016/j.mrgentox.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Desai SS, Ghaisas SD, Jakhi SD, Bhide SV. Cytogenetic damage in exfoliated oral mucosal cells and circulating lymphocytes of patients suffering from precancerous oral lesions. Cancer Lett. 1996;109:9–14. doi: 10.1016/s0304-3835(96)04390-x. [DOI] [PubMed] [Google Scholar]
- 25.Samanta S, Dey P. Micronucleus and its applications. Diagn Cytopathol. 2012;40:84–90. doi: 10.1002/dc.21592. [DOI] [PubMed] [Google Scholar]


