I. Introduction
The purpose of refractive surgery is to reduce dependence on corrective lenses. In laser keratorefractive procedures, this is achieved by altering the shape of the cornea to change the refractive state of the eye. Historically, sphere and cylinder were the only components of a refractive error that could be measured and systematically corrected. Procedures designed to treat these lower order aberrations often achieved emmetropia, but with bothersome visual symptoms such as glare, halos, starbursts and ghost images that could not be corrected with glasses or contact lenses. These symptoms have since been attributed to induction of higher-order aberrations (HOAs).1–4
Wavefront analysis is a method by which the aberrations of an optical system are measured. Several methods exist to assess ocular aberrations including Tscherning aberrometry, Shack-Hartmann wavefront sensing, ray-tracing, optical path difference aberrometry, and spatially resolved refractometry. Currently, it is most commonly performed using devices based on the Hartmann-Shack wavefront sensor. This technology was developed in the 1960’s and 70’s to improve the images of satellites captured by telescopes from Earth and later adapted to measure aberrations of the human eye.5 Such devices use a low-power laser beam focused on the retina and analyze the reflected rays of light. The light passes outward through the optical system of the eye, through an array of lenses and onto a detector. In a perfect optical system, these rays would emerge parallel and focus at a single plane. Given the complex nature of the eye’s optics, this is not the case. The degree that each image deviates from the expected focal point of an individual lens in the system represents the aberration or “wavefront error.”
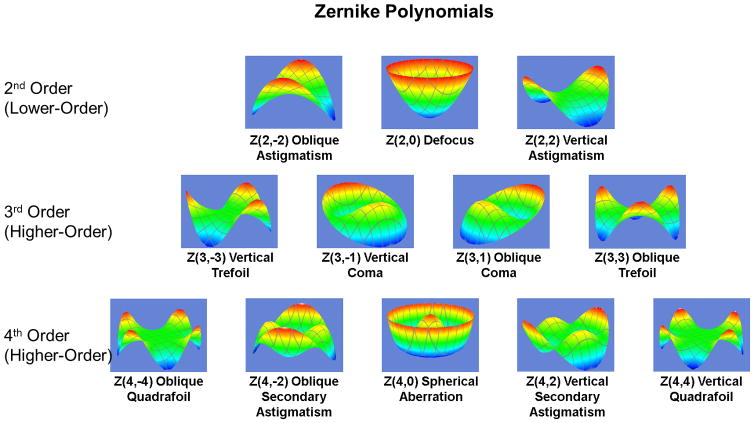
The wavefront error is computed and broken down into components that visually and mathematically describe distinct elements of the overall aberration. These components are most commonly expressed as Zernike polynomials, and encompass both lower- and higher-order aberrations. Lower (second) order aberrations include positive defocus (myopia), negative defocus (hyperopia), and regular astigmatism. Visually significant higher (third and fourth) order aberrations include coma, trefoil, and spherical aberration. These aberrations can have both positive and negative values. Coma, or comatic aberration occurs when light rays from one edge of the pupil comes to focus before those from the opposite edge. This third order aberration has the effect of “smearing” an image or making it appear to have a tail like a comet. Trefoil, another third order aberration, has less effect on image quality than an equal amount of coma. Spherical aberration is a fourth order aberration that occurs when rays from the peripheral pupil focus in front of those from the central cornea. Spherical aberration results in halos around point light sources and decreased contrast sensitivity. Other lower-order aberrations and those above fourth-order are considered to be relatively less visually significant.6 The most visually significant lower- and higher-order aberrations are shown in Figure 1. The Zernike polynomial system allows the deconstruction of any aberration structure, no matter how complex, into predefined, fundamental building blocks.
Figure 1.
Visually significant optical aberrations displayed as Zernike polynomials. Each is labeled with their common name and Z(x,y) notation where x is the order of the aberration and y is the angular frequency or number of times the wavefront pattern repeats itself. (Images courtesy of Dr. Ronald Krueger, M.D.)
The magnitude of total aberrations is measured as a Root Mean Square (RMS) error, with most normal patients having a value of less than 0.3 μm. A higher RMS value indicates a more highly aberrated cornea. Pupil size also influences the magnitude of HOAs. There is an increase in aberrations as the pupil becomes larger, with RMS error approximately doubling with each additional millimeter of mydriasis.10 Thus, pupil size has traditionally been an important consideration when planning a refractive procedure and evaluating post-operative HOAs.7 However, more recent studies have shown that pupil size does not necessarily have as significant an effect on visual quality as previously thought. Schallhorn et al. found that beyond the third postoperative month pupil size held no relationship to symptoms such as glare, haze or halo.8 In a separate study using wavefront-guided treatments, Schallhorn et al. found that there was no correlation between mesopic pupil size and reported visual symptoms following LASIK as early as the first postoperative month.9
Studies have shown that patients with subjective visual symptoms such as halo or monocular diplopia following refractive surgery have RMS HOAs 3.5 times greater than normal pre-operative eyes and 2.3 times greater than patients who underwent Laser in-situ keratomileusis (LASIK) and did not report persistent visual symptoms.10 Particular symptoms have also been linked with specific HOAs. By comparing visual symptoms with wavefront analysis, Chalita et al. found that monocular diplopia is correlated with total and horizontal coma, glare with spherical aberration and total aberrations, and starburst with spherical aberration.11 The application of wavefront analysis technology to the human eye has allowed refractive surgeons to identify and treat HOAs, in addition to sphere and cylinder. This has led to a significant improvement in the quality of vision following refractive surgery and the ability to correct induced post-surgical HOAs.
In the transition from incisional to laser refractive surgery, the quality of visual outcomes greatly increased. The ability to measure wavefront aberrations of the eye and incorporation of this data into treatment profiles further revolutionized laser refractive surgery through enhanced treatment algorithms. Topography-guided, wavefront-optimized, and wavefront-guided treatments are all methods currently employed to reduce the effect of HOAs post-operatively. As newer refractive techniques such as small incision lenticule extraction (SMILE) are developed, optical aberrations have become one of the primary visual quality endpoints studied.
Despite improvements in planning and technique, refractive surgery still carries a risk of unanticipated and sometimes undesirable optical consequences. At times, this may be due to unusual healing characteristics of a particular cornea and are unpredictable. The focus of this paper is to discuss the causes, prevention and management of unexpected lower- and higher-order aberrations following various forms of refractive surgery. Errors in lower order aberration within the surgeon’s control most commonly fall under two categories: (1) miscalculations in procedure planning, and (2) laser programming errors. These types of errors can leave a patient with a potentially devastating refractive result and the surgeon at risk for litigation. Induction of HOAs, on the other hand, is more often a function of laser beam profiles, centration during treatment and choices in ablation technique.
II. Lower-Order Aberrations
Miscalculations in Procedure Planning: Over- and Under-Correction of Lower-Order Aberrations
Over thirty years of collective knowledge has helped to refine treatment algorithms for LASIK and photorefractive keratectomy (PRK) to account for differences in patient age as well as type and extent of refractive error. Using this pooled data and power adjustments customized to a particular laser and surgeon, we are better able to predict how individual corneas will respond to treatments. This allows physicians to choose to fully or partially treat a patient’s lower-order aberrations, knowing that some will tend to stay stable while others will regress post-operatively. This concept is most relevant in conventional and wavefront-optimized treatments, where the patient’s refraction is the primary consideration used to program the laser.
FDA trials using conventional treatments have shown that PRK and LASIK are typically very accurate in correcting lower-order aberrations in myopia less than −6.00 diopters (D), with 77.8–100% of eyes achieving a final refraction within 1.00 D of emmetropia.12,13 There has been no significant difference found between LASIK and PRK in their ability to achieve emmetropia in this group of patients.14,15 Reports from the past 15 years have shown continued improvement in safety and accuracy with the use of wavefront-guided and wavefront-optimized systems being used to treat myopic astigmatism as high as −10.00 to −12.00 D. These trials have reported up to 100% of eyes achieving refractions within 1 D of emmetropia.16–18
Hyperopic treatments were found to be slightly less accurate, but still excellent with 84–91% of eyes up to +6.00 D achieving within 1.00 D of emmetropia.19,20 In early trials of LASIK for hyperopic astigmatism up to +6.00 D of hyperopia and +4.00 D of cylinder, 88–89% of treated eyes fell within 1.00 D of emmetropia.20 Studies treating above +4.00 D of hyperopia have shown significantly reduced predictability and stability.19 Wavefront-guided and wavefront-optimized treatments along with eye tracking software have improved accuracy of LASIK and PRK treatments with even higher levels of hyperopic ammetropia.21
Overcorrection in LASIK and PRK may occur from stromal dehydration and compaction during treatment. In this state, more corneal tissue is ablated per pulse. Dehydration may occur if the surgeon takes too long after removing the epithelium in PRK or lifting the flap in LASIK and initiating the excimer ablation. Atmospheric conditions such as low humidity or high temperature can similarly dehydrate the cornea. Older patients tend to have a higher incidence of overcorrection. This may be due to several factors including a less robust wound healing response and greater effect from the same amount of laser energy compared to younger eyes.22
Undercorrection typically results from an unexpected degree of regression postoperatively. In the first 3 to 6 months following PRK or LASIK, there is frequently some degree of regression in both myopic and hyperopic treatments. This is more pronounced in cases of higher myopia and any amount of hyperopia as well as in younger eyes.23–25 Even in the earliest clinical studies of PRK in normally sighted human eyes in the United States, McDonald et al. noted that attempted correction of 5 D or more regressed significantly more than corrections less than 5 D.26
Prevention
It is important to keep the temperature and humidity of the laser suite controlled and consistent within the laser manufacturer’s guidelines. This eliminates at least some variables that could cause the excimer laser to ablate corneal tissue at a higher or lower rate than expected. It may also help to prevent excessive dehydration of the cornea before and during treatment.
Lasers should be checked and calibrated every surgical day and between procedures according to the protocol provided by the manufacturer. Failure to do so may lead to inconsistent ablation and unpredictable results.
In deciding what refraction to program into the laser for a conventional or wavefront-optimized treatment, the surgeon must be aware of the patient’s age. For older patients, less than the full refraction is typically used due to more robust responses to laser energy and a lower tendency to regress. The opposite is true for younger patients where a value closer to the full refraction is used to prevent an undertreatment.
Ensuring meticulous refractions with stability is integral in treating the correct amount of refractive error, particularly in contact lens wearers. When a patient’s manifest and cycloplegic refraction do not match, the surgeon must refer to their personal nomogram and historical outcomes to decide what to program into the laser. Furthermore, it is not uncommon for refractive astigmatic axis to differ from the topographic axis. If repeat measures give consistent results across the two modalities, it is assumed that there is an element of lenticular astigmatism and the refractive axis is preferentially used. Confidence in the axis is important, as a 15 degree offset in axis of treatment can result in a 35% reduction in astigmatic correction.
In high myopic or hyperopic corrections where regression is more likely, application of mitomycin C intraoperatively immediately following the ablation may help to modulate this response. Though LASIK and PRK are often performed bilaterally in the same session, occasionally eyes may be treated on different days due to scheduling or comfort reasons. If this is the case, the amount of regression in the first eye typically correlates with what will happen in the second eye and can be taken into consideration when choosing the amount of refraction to correct.
From a technical standpoint, the move to larger diameter ablation zones has reduced the tendency towards regression. This is postulated to be due to shifting the transitional zone effects further from the central optical zone and inducing less stromal and epithelial hyperplasia that led to higher rates of regression.27 Optical zone size will be discussed further in a later section.
Management
There is no set cutoff for residual refractive error that necessitates retreatment after laser vision correction. The primary indication is patient satisfaction, as different people vary in the amount of uncorrected sphere or cylinder that they can tolerate.
With the understanding that the corneal surface and refraction is dynamic for at least 3 months following photoablation, retreatment or enhancement should not be attempted before this time period has elapsed. Eyes treated with PRK tend to take longer to stabilize than LASIK and should be given up to 6 months to stabilize. If the patient expresses dissatisfaction in their vision during the post-operative period, careful measurements of lower- and higher-order aberrations, as well as topography should be performed at each visit to determine the underlying cause of the visual symptoms. The degree of change in refraction between follow-up visits allows the treating physician to determine when the cornea has stabilized and when it is appropriate to move ahead with further correction. A thorough slit lamp examination should be performed to ensure there is no ocular surface disease, epithelial ingrowth or haze formation influencing the measured refraction. If there appears to be continuous regression towards myopia, the topography should be reviewed carefully for any indication of ectasia.
In treating small amounts of overcorrection, corticosteroids may be abruptly discontinued to encourage a more robust healing response and regression. Conversely, undercorrection caused by robust regression may occasionally be reversed by aggressive treatment with topical steroids. Small refractive errors with ocular surface disease may respond to artificial tears28 or insertion of punctual plugs.29
If the post-operative refraction is determined to be stable and there is a large enough refractive error to warrant treatment, the physician needs to confirm that there is sufficient residual stromal bed thickness to carry out the required corrective treatment. Though there is no established guideline, most surgeons ensure that there will be a residual stromal bed (RSB) of at least 250–300 μm30 or a final percent tissue altered (PTA) value of 40% or less following the enhancement31 to reduce the risk of ectasia.
In cases of post-LASIK residual or induced second order aberrations, the stromal bed may be accessed in several ways. Most commonly, if the retreatment is within a year (or sometimes more) and the edge of the original flap is visible, it can be re-lifted. If the flap edge cannot be identified or the flap lift is difficult and at risk of tearing, other options include a larger flap recut, a side cut within the original flap, or a mini-flap that is smaller and thinner than the initial flap. Once the stromal bed is exposed via flap lift or creation as described, or epithelial removal is performed in cases of PRK retreatment, the corrective ablation may be applied. Using a wavefront-guided32 or wavefront-optimized ablation profile has the benefit of correcting both lower and higher-order aberrations. However, it must be kept in mind that these profiles remove more tissue than a conventional ablation. If the enhancement is for an eye that was initially overcorrected, 20–25% less correction should be programmed into the laser than if it was the first treatment. This is because these eyes are already known to have an exaggerated response to ablation.
In cases of errors in lower-order aberrations that are not amenable to repeat ablation due to insufficient RSB or high PTA, there are several enhancement modalities that preserve corneal tissue. In a post-LASIK eye, additional laser can be applied to the surface33 or undersurface34 of the LASIK flap. Corneal collagen crosslinking (CXL) is currently being developed as a refractive modality35, which would allow for correction of small amounts of post-refractive residual myopia without removing additional tissue while also strengthening a thinner stromal bed. Intraocular surgery with an implantable collamer lens (ICL, also known as a phakic intraocular lens) or clear lens exchange may be offered. Alternatively, if the patient does not want to undergo further procedures, contact lenses or glasses may be used depending on the extent of surface irregularity.
Laser Programming Errors
There is a scarcity of case reports or papers in the published literature discussing surgeon error in the refractive preoperative period. The largest published case series specifically addresses medical error in refractive surgery and identifies specific steps where this occurred in 18 patients.36 Only two other case reports describing errors in data entry in 4 eyes have been reported in the English literature.37,38 For this reason, it is difficult to know the incidence of these events, though extrapolating from internal data, Moshirfar et al. estimated that 280 to 400 cases of data entry errors in refractive surgery occur annually.36 Regardless of the actual incidence, the time period from when a surgeon first meets a patient for a screening examination to the application of the laser contains numerous opportunities for incorrect data entry or transfer.
The number of times that a patient’s refraction is entered and transferred from one source to another can be as high as 5 times or more. In our institution, for example, the manifest and cycloplegic refractions are first transferred from the phoropter to the electronic medical record by the technician. The record is then printed and given to another technician who enters the refraction data into a refractive database, which recommends optimized ablation profiles based on pooled data. The surgeon then selects the power to treat on a printout from the database and returns this to a technician who enters the information into the excimer laser on the day of surgery. Each of these steps represents a time point where an incorrect number, sign or decimal could potentially be entered or information could be recorded under the wrong patient’s name.
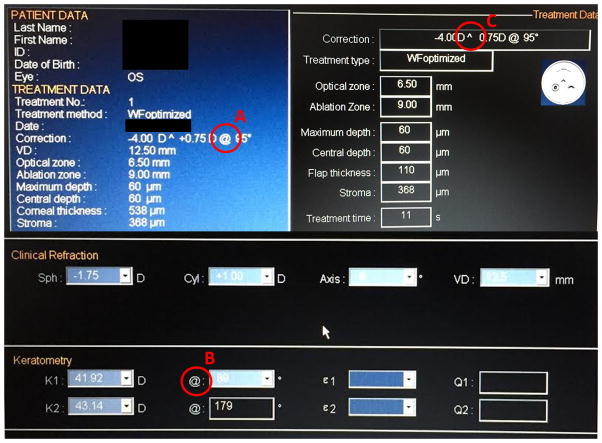
Another potential source of error is cylinder conversion. When various diagnostic equipment, electronic medical records or laser platforms require cylinder to be in either plus or minus notation, a conversion may be required. Simple errors in addition or subtraction, remembering to switch the sign or failing to rotate an axis can all lead to doubling of an astigmatic error post-operatively. The use of different symbols to denote axis by different laser platforms can also be confusing to a surgeon or technician not familiar with the interface and lead to error in data entry. For example, the software for one laser uses the “@” symbol to denote axis on some screens and meridian on others. Furthermore, on one screen the axis sign is replaced by a “^” symbol, making it unclear whether it is in plus or minus notation (Figure 2). By standard convention, “@” represents meridian while a “x” or the word “axis” denotes the axis location. Deviating from this convention creates potential confusion and incorrect treatment.
Figure 2.
Laser interface display of a single excimer laser system. Note that the “@” symbol is used to denote axis on one screen (A) and meridian on another (B). The “^” symbol replaces the sign for plus or minus cylinder on a third screen (C).
Prevention
Despite a reliance on technicians to make the clinic and operating room more efficient, it is ultimately the responsibility of the surgeon to verify that all information and treatments are accurate. Some laser platforms that perform custom treatments automatically transfer data from diagnostic equipment to the laser eliminating certain steps where a transcription error could occur. However, this is not standard across all platforms, particularly those performing conventional treatments. The final opportunity to recognize an error is the surgical “timeout” while the patient is in the laser suite awaiting ablation. At this time the patient and treatment are announced and confirmed by the physician and technicians. All clinical refractive data that was used to determine the ablation, including the medical record, should be available to compare to the treatment data. A consistent timeout protocol performed before every procedure will help to eliminate wrong patient and data entry errors.
Management
When a laser programming error occurs, and an incorrect treatment is carried out, the most important thing to do is communicate with the patient. The surgeon should inform the patient of what happened, and discuss the steps necessary to correct the error. The fear of litigation may tempt a surgeon to cover up the error and re-treat the patient without disclosing the mistake. However, evidence has shown that a perceived lack of communication and honesty following a surgical error is more likely to motivate a law suit than an error itself.39,40 In addition, if it is discovered that an error was intentionally covered up by a physician, then the financial and professional repercussions in a law suit become significantly worse.
Once the error has been disclosed, treatment is based on the residual refractive error, topographic changes, and the patient’s motivation to undergo additional procedures. In some cases, the patient may elect to simply wear glasses or contact lenses. If the incorrect treatment was small in magnitude and the patient has sufficient residual stromal thickness, they may benefit from an additional ablation. If the residual cornea is insufficient for further ablation, a lens-based refractive procedure such as an ICL or clear lens exchange may be performed. In severe cases where the extent of induced corneal irregularity is not correctable by any of the above methods, a corneal transplant alone or in combination with additional refractive procedures may be necessary to achieve an acceptable level of vision.
III. Higher Order Aberrations
Decentered or Rotated Ablations
Aberrations following refractive surgery may be caused by intraoperative issues, such as eye movements or poor fixation by the patient during treatment. This may result in the ablation pattern being misaligned or decentered over the visual axis (Figure 3). Eye movements, such as cyclotorsion of the eye in the supine position compared to sitting upright, can theoretically cause poorly-aligned ablation profiles and astigmatic axis shifts resulting in a poor visual outcome.41 Chernyak noted that 56% of eyes undergoing PRK or LASIK experienced more than 2 degrees of cyclorotation from the upright to supine position, with 21% experiencing more than 5 degrees of cyclorotation.41 Cyclotorsion has been postulated, though not clinically proven, to induce clinically-significant HOAs following laser refractive surgery as well.42,43 In contrast, centration error has been shown to induce much more significant total aberrations (both lower-order and higher-order) than cyclotorsional misalignment.43 Despite a reduction in gross decentrations with the advent of eye-tracking software, subclinical (<1.0mm) decentrations may still occur. Aberrations induced by these decentered ablations are a major risk factor for poor patient outcome.
Figure 3.
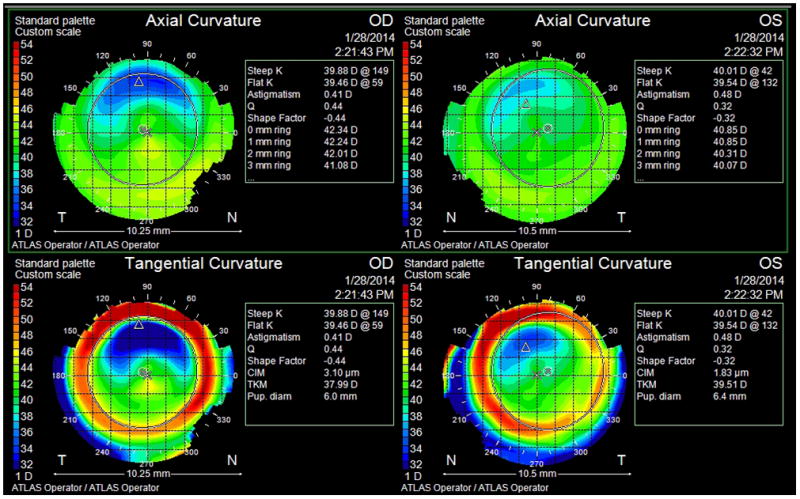
Axial and tangential topography maps of patient with a bilateral decentered ablation following conventional LASIK for high myopia. Note significantly flatter superior corneal surfaces indicating that the laser was centered superior to the visual axis in both eyes.
The exact incidence of decentered ablations in refractive surgery is not known, but may be inferred from studies analyzing their effects. One such study was a retrospective chart review of 3719 eyes undergoing LASIK for myopia, which found a total of 46 eyes (1.2%) with topographically-determined decentration.44 Decentration of the ablation profile can be caused by a number of intraoperative factors, including treatment displacement (a shift in the specified treatment location), eye movements such as drift, or poor patient fixation.45 Stromal drying has also been suggested to contribute to decentration risks, as this may induce poor patient visualization of the fixation beam. Misplacement of the ablation profile with respect to the pupil can also pose a risk, particularly if the pupil is miotic or mydriatic at the time of surgery.46
Decentered ablations can result in significant decrease in both visual acuity and visual quality with glare, halos, star bursts, irregular astigmatism, and poor contrast.47 Mrochen et al found that subclinical (<1.0mm) decentered ablation profiles produced a significant increase in HOAs compared to pre-operative aberration profiles, specifically coma and spherical-like aberrations.48 A study by Padmanabhan et al later confirmed a higher incidence of aberrations like tilt, vertical coma, oblique astigmatism, and spherical aberration in patients with decentered ablations compared to patients without decentered ablations. To support suspicion of clinical relevance, they also correlated ablation profile decentration with a lower uncorrected visual acuity.44
The direction and magnitude of decentration has been suspected to dictate type and magnitude of the aberration induced. Padmanabhan and colleagues suggested the largest increase in vertical coma was secondary to decentrations predominantly in the vertical direction.44 A linear correlation between the magnitude decentration and the magnitude of induced aberrations of tilt, coma, and secondary astigmatism has also been demonstrated.44 The effect of the transition zone in the aberrations produced by decentered ablations has also been evaluated and are suggested to contribute to the development of coma and spherical aberration.49
With the increasing popularity of small incision lenticule extraction (SMILE) in refractive surgery a number of reports on induced aberrations following this procedure have emerged.50,51 Despite the lack of active eye tracking software and theoretically higher risk of decentration in SMILE, it appears to induce few HOAs.51,52 One study specifically reporting on the effect of decentered SMILE failed to show significant correlation between induced aberration and extent of decentration, although all decentrations were notably small (<0.5mm).50 There are no SMILE studies to date demonstrating a significant impact of decentration on visual outcome.
Prevention
It is generally accepted that once the decentered ablation profile is noted to be present, it will usually remain and will not change with corneal remodeling, making prevention critical in improving patient outcomes.46 Prevention of decentered ablations should be focused on controlling intraoperative factors that may lead to poor centration, such as using brighter fixation beams with lower illumination lights, and decreasing operative time to prevent stromal drying.45–46 Verbal encouragement to the patient is often helpful for patient relaxation and cooperation with fixation as well. Eyes with large angle kappa are thought to be better managed by centering between the entrance pupil and the visual axis, rather than directly on the pupil.45,46 Of course, observing the eye while ablating and stopping ablation at the first sign of drift and poor centration is critical in preventing decentered profiles and irregular astigmatism.
Management
After making the diagnosis of a decentered ablation, it is generally recommended to observe for a period of 3 to 6 months, as there may be remodeling that can occur in the treatment zone that reduces subjective symptoms.45 Since aberrations may increase with larger pupil diameter, miotic drugs can be used to reduce optical aberration and symptoms of poor visual quality. Surgical management of decentered ablations has been previously reviewed by Jarade and Azar and have included arcuate keratotomy in the opposite direction of the decentration, laser retreatment with viscous impediment to the previously ablated cornea, and retreatment with intent on correcting any additional refractive error.45
A number of newer methods have been proposed to reduce symptoms and aberrations in eyes with decentered ablations. One technique described by Lafond et al. is to retreat with a combination hyperopic profile (in the direction of the initially-decentered myopic treatment) and myopic profile (in the opposite direction). They reported reduced symptoms and improved centration of the ablation zone in 15 of 16 eyes.53 Topography-guided retreatments, or topographic neutralizing techniques, have proven effective in highly-aberrated eyes with irregular corneas.54 Wavefront-guided ablation can also be used to correct for the abnormal aberration profiles created by a decentered ablation, and has been shown to reduce symptoms of monocular diplopia and halos for moderate decentrations.47 Wavefront-guided ablations have been shown to reduce total HOAs in the setting of persistently symptomatic eyes after LASIK as well.55 Recently, an algorithm has been proposed for the recentration of the optical zone, with promising simulations of optical zone recentration, expansion, and tissue preservation.56 However, this technique has yet to be clinically evaluated.
Central Islands
A central island is an area within a treatment zone that is steeper than the surrounding cornea following myopic PRK or LASIK (Figure 4). This occurs when a central area of the cornea is insufficiently ablated relative to the surrounding tissue. Several investigators have proposed a unifying definition of a central island, based both on post-operative topography and differential maps. Hersh suggested a standardized classification of corneal topographic findings after laser refractive surgery based on the differential topography map. He defined a central island as a central area of relatively less flattening greater than 1.0 mm in diameter and greater than 1.00 D in power not extending to the periphery.57 Comments following this article warned that a standard definition may be difficult to establish given the variability between topographic devices and even between measurements by the same device. Definitions in the literature vary from 1 to 3 mm in diameter and 1 to 3 D in height, with the incidence of central islands depending on the definition and follow-up time period used.45,46, 58–62
Figure 4.
Axial topography of an eye with a central island. The central 2 mm of the treatment zone has a power of curvature 2 to 3 diopters steeper than the surrounding corneal surface. (Image courtesy of author WJD)
The first description of central islands was published by Lin and initially termed a “central bump.”63 In his series of 502 eyes undergoing PRK, a central island was present in 26% of eyes at the 1-month postoperative visit.58 The incidence of central islands has been reported to be as high as 80% with PRK.62,64 The reported incidence of central islands following LASIK is lower, between 0 and 20%, also with various definitions of size and height.45,60 However, there may be a much higher incidence of transient central islands following LASIK due to flap or bed hydration that resolve within hours of the procedure.46
The precise cause of central island formation is unknown and may be multifactorial. Several mechanisms have been suggested. Non-uniform fluid distribution with excess fluid in the center of the ablation zone may decrease effective ablation by reflecting or absorbing laser energy.59 One theory explaining this central accumulation of fluid is that the acoustic shockwave from a broad-beam laser shifts water within the stroma.65 Nonhomogeneous broad-beam laser profiles with cooler central areas relative to the periphery could also lead to less effective ablation centrally.46,66 The most commonly cited cause is the plume of ejected corneal material that is produced by each laser pulse. This airborne material tends to concentrate over the central ablation zone and then settles in this area, re-depositing on the cornea and partially blocking subsequent laser pulses.67 Central islands have also been found to be more common with larger ablation zone diameters and higher preoperative refractive errors.45,60
McCormick et al. found that when patients with uncorrectable visual complaints following LASIK were categorized by topographic pattern, those with central islands had the greatest higher-order RMS wavefront error compared to decentered ablations, irregularly irregular astigmatism and “baby bowties”. The dominant HOAs in this group were negative vertical coma and positive spherical aberration.10 As a result of inducing HOAs, central islands cause delayed visual rehabilitation, reduced BCVA, monocular diplopia, ghost images and decreased contrast sensitivity.45,46,60,68
Prevention
Central islands are a complication almost exclusively associated with broad-beam lasers. Frequent maintenance of the laser with replacement of the optics and ensuring proper calibration and homogeneity of the beam profile can help to maintain a consistent ablation profile. Keeping the laser suite at a stable temperature and humidity with minimization of air flow in the room may also lessen island formation.46 The technique of blowing nitrogen gas across the ocular surface during ablation significantly reduced the rate of central island formation. In one series, this technique decreased the incidence of central islands at 3 months from 64% to 20%.62 The gas accomplishes this by drying the cornea and creating a more uniform surface while also removing ablation byproducts. Conflicting data regarding induction of corneal haze has made use of nitrogen gas less popular.69 An aspiration suction device can also reduce the accumulation of the laser plume over the ablation zone. Additional strategies employed for use with broad-beam lasers included wiping the surface of the corneal bed with a sponge before and during ablation to remove excess moisture,46,61 using a lower frequency ablation to allow the laser plume to dissipate,46 and using specialized software programs that provide additional laser treatment to the center of the ablation zone.45,46,61
With modern laser technology, including scanning-slit and flying-spot lasers, the incidence of central islands has been drastically reduced and they are rarely encountered. These laser patterns mitigate the problem of a plume blocking ablation by constantly moving across the corneal surface. They are programmed to avoid continuously ablating in the same position where the plume may be accumulating.70 Additionally, scanning-slit and flying-spot lasers do not produce a centripetal shock wave and thus do not push fluid towards the center of the ablation zone. The recall of the Alcon LADARVision 6000 excimer laser in 2007 is a cautionary example of how faster scanning spot lasers that do not implement a staggered spot strategy can generate central islands similar to those seen in older broad beam systems.
Management
Before proceeding to treatment of a central island, the patient must be monitored with topography for a minimum of 3 months and ideally longer. With time, central islands may resolve on their own. This is particularly true in cases following PRK, where the majority of cases resolve within 1 year. In a large series of patients with a central island noted 1 month following PRK, 39% had resolved at month 3, 88% at month 6, and 98% at month 12.58 Regression is likely indicative of epithelial remodeling resulting in a smoother corneal surface. Central islands tend to regress to a lesser degree following LASIK.61 This may be due to lack of epithelial remodeling to compensate for the central elevation45, or a greater tendency for intrastromal fluid to accumulate under the flap during treatment.61 At each follow-up visit, topography and visual symptoms should be evaluated. During this period of observation, if the patient is intolerant of their symptoms, a soft or rigid gas-permeable contact lens can be fit for temporary relief. However, contact lens wear must be taken into account and discontinued 2 to 4 weeks before each visit when deciding whether progression or regression has occurred, as the contact lens may cause alterations in topography.71 If the central elevation is improving or the patient’s symptoms are less bothersome or resolved, no treatment is indicated.
If after 3 months or more the central island is persistent on topography and the patient continues to have visual symptoms, surgical intervention may be considered. Various modalities have been described to treat central islands, with corrective ablations re-applied to the corneal surface in post-PRK patients and to the flap or stromal bed surface in post-LASIK cases. The combination of phototherapeutic keratectomy and PRK showed a modest improvement in central island power and significant improvement in mean UCVA in a small series.69 If the power of the central island matches the patient’s refraction, Munnerlyn’s equation may be used to plan PTK45,71: ablation depth = (S2 × D)/3, where S = diameter of the ablation zone and D = diopteric correction.72 The number of PTK pulses necessary can be calculated based on the knowledge that each pulse ablates approximately 0.25 μm of tissue. Masking agents such as methylcellulose may be used to prevent ablation of the flatter surrounding tissue. If the power of the central island does not match that of the refraction, the corneal surface is likely more irregular and will require additional ablation 3 to 6 months after PTK.45,71
Topography-guided ablations, developed for the treatment of irregular corneal surfaces, are in theory the ideal treatment for central islands. However, results thus far have been mixed. After encouraging initial clinical results73, one group treated 6 eyes with post-PRK/LASIK central islands using a topography-guided system. While patients with irregular corneal surfaces due to other reasons had significant improvements in topography and symptoms, they found the results to be sufficiently poor in the central island group to advise against using their technique in this patient population.74 Alío et. al had similarly disappointing results treating central islands with their topography-guided system.75 Alessio et al. successfully treated one patient with a symptomatic post-PRK central island using a system that transferred topographic data from a topography mapping system to a flying-spot excimer laser.76 More recently, Hafezi et al. reported a series of 3 successful treatments using a customized topography-guided ablation algorithm.77
Wavefront-guided treatments have not been found to be effective for correction of central islands. This is likely due to inaccuracies in measuring such a significant curvature change across a small area.78 Integration of live aberrometry or topography into an excimer laser system may be the most effective means of treating central islands, though such systems are not yet commercially available. While not encountered frequently, refractive surgeons must be aware of how to identify and treat central islands so that they are able to properly council patients regarding the variability of treatment results and the possible need for multiple revisions.
Small Ablation Zones (Ablation zone size and peripheral blend zones spherical aberrations)
When ablation profiles were initially developed, there was a belief that the optical zone diameter should be small to minimize depth of tissue ablation and decrease the risk of stromal scarring.79 The Munnerlyn formula dictates that the required depth of ablation increases by the square of the treatment zone.72 While successful in treating lower-order aberrations, it quickly became evident that small optical zones were a major cause of HOAs and poor visual outcomes.80,81 Complaints of glare, halo and disturbances in night vision resulted from the induced HOAs.27,82,83
This effect is in part a function of the pupil size in relation to the treatment zone.7,81 In a dilated pupil following a small-zone myopic ablation, peripheral rays incident upon the relatively steeper cornea outside the treatment zone may pass through the pupil and come into focus in front of the retina, resulting in spherical aberration.1 Due to this relationship, larger pupil sizes and scotopic conditions tend to produce more HOAs (particularly spherical aberration) following myopic refractive surgery, especially with small treatment zones.81 Higher myopic corrections are also associated with increased HOAs, attributed to a larger transition in refractive power between the treated and untreated cornea and loss of asphericity.4,45,81,84,85 Early ablation profiles compounded this effect by reducing the natural negative asphericity of the cornea within the treatment zone. With broad-beam pulses, the ablation rate drops off peripherally due to a more oblique angle of incidence. The net result is that the cornea becomes oblate rather than prolate, increasing spherical aberration.83
Prevention
Over time, the understanding that smaller ablation zones induced HOAs and bothersome visual symptoms led to increasing the standard ablation zone diameter from 4.0 mm to 6.0 mm and larger for PRK and LASIK. In studies comparing ablation zone sizes, incidence and magnitude of nighttime glare and halo were found to be significantly reduced with a 5 mm versus 4 mm ablation diameter27,86 and even more so when the ablation zone was expanded to 6 mm.27,87–90 In one large, prospective, randomized, double-blind study, 6 months post-PRK 17.5% of patients treated with a 5 mm zone complained of severe night vision disturbances compared with none in the 6 mm zone group.88 Another study comparing night vision symptoms of patients undergoing PRK with 4.0-, 5.0- and 6.0-mm optical zones also found that no patients in the 6.0 mm group experienced significant night vision disturbance versus 12% and 14% of patients in the 5.0 mm and 4.0 mm groups, respectively.27 In a study of 1488 eyes that underwent LASIK for myopia, optical zones smaller than 6.0 mm resulted in a 2.5-times increase in night vision complaints.91
Though increasing the ablation zone diameter initially resulted in higher incidence of central islands, adjustments in laser beam profiles eliminated this problem as discussed in the section above.27 When Oliver et al. looked specifically at HOAs induced by PRK, they found that the spherical aberration coefficient increased significantly more following a 5 mm ablation compared to a 6 mm ablation.2 Seo et al. also found that a larger, 6.5mm optical zone with an 8mm blend zone reduced HOAs compared to a conventional 6.0 mm optical zone following laser subepithelial keratomileusis (LASEK).81 Delivering more ablation to the peripheral optical zone also helps to counteract the positive asphericity induced by the reduced laser energy delivered to this area.83 This idea has been integrated into modern wavefront-guided and wavefront-optimized algorithms that aim to reduce overall aberrations.
While even larger ablation zones would theoretically reduce HOAs further, the maximum size of the ablation zone is limited by ablation depth required for larger diameters. If a high correction is necessary, PRK should be the procedure of choice rather than reducing the size of the treatment zone to preserve tissue or risking a thin residual stromal bed with LASIK.
In an attempt to increase the size of treatment zones while preserving corneal tissue, several groups tried variations of a compound or multizone ablation profile. Both Oliver et al.2 and Corbett et al.90 (Corbett Br J oph 1996) attempted a multizone treatment with −5.00 D correction over 4.6 mm and −1.00 D over 6 mm. In terms of improving visual symptoms such as nighttime glare and halos, they had limited success and were comparable to smaller zone ablations. Vinciguerra et al. had similarly poor results with a compound ablation profile created by increasing preoperative sphere 25% and applying hyperopic ablation of 25% of preoperative sphere.92 A high incidence of central islands was seen post-operatively in these treatments as well.90,92
During the pre-operative evaluation, pupil size measurements should be taken in photopic and scotopic conditions to ensure the planned treatment and blend zone extends beyond the mydriatic pupil. In one study evaluating pupil size and visual complaints, all patients who reported significant night vision disturbances had a scotopic pupil size larger than the ablation zone.27 Even with larger treatment zones now being the standard of care, patients with pupils that dilate to 6 mm or more in mesopic conditions should be warned of the possibility of an increased risk for night vision disturbances following ablative surgery. However, more recent data supports the idea that the relationship between large pupil size and an increase in subjective postoperative visual symptoms may be limited in duration and less prominent with modern laser technologies.8,9
Management
Patients who have previously undergone myopic PRK or LASIK with a small optical zone and have disabling visual symptoms may benefit from additional procedures to enlarge the optical zone as long as there is sufficient residual stromal thickness. When residual myopia is present, an additional myopic treatment with a larger optical zone can be applied. Eggink et al found that by employing this strategy, even if there was still a small amount of residual myopia, subjective reports of halos and night-driving problems were significantly reduced.93 If the eye is already near-plano following the first surgery, the larger-diameter treatment must be applied in a way so as not to change the overall refraction. This may be done by subtracting the desired correction in the small zone from that which would be required to achieve the same correction in a larger 6 or 7 mm zone. In this way, the peripheral zone receives an effective refractive treatment, while the original central zone is only deepened.94 Another method is to apply an equivalent small myopic and hyperopic treatment in the same session over a wider zone.53 Topographically-guided spot scanning laser ablations have also been shown to improve corneal regularity in eyes with small optical zones.74
Patients with small optical zones following PRK for high myopia often do not have enough residual stromal bed to safely perform an additional customized myopic ablation. In these cases, Hafezi et al.80 proposed a 2-step bioptics approach. First, a clear lens exchange is performed to shift the patient to hyperopia, then a topography-based hyperopic correction with up to a 7mm optical zone and minimal ablation in the center of the cornea is applied. If the patient is young and pre-presbyopic, the clear lens exchange step can be replaced by an ICL.
Reduction or Minimization of Higher Order Aberrations: Comparison of Techniques
Wavefront-optimized ablation, Wavefront-guided ablation, Topography-guided ablation, and SMILE
In the evolution of laser refraction techniques, one of the most significant advances came in the transition from static broad-beam laser ablations to scanning-slit and flying-spot laser systems. With smaller beam diameters (0.5–2mm), higher frequency, and less tissue removal per pulse, newer lasers allow for highly customizable treatment algorithms and integration with wavefront and topographic data. Wavefront-optimized treatments apply additional treatment to the peripheral ablation zone in order to maintain a prolate corneal surface and reduce induction of higher order aberrations. Wavefront-guided systems take a patient’s individual wavefront data and create a customized ablation profile to address pre-existing aberrations and prevent the formation of new ones. Topography-guided ablations, which are currently gaining popularity in the U.S., link topography data to the ablation profile, which specifically targets surface elevations. Finally, SMILE is the newest of the laser refractive surgery modalities. In SMILE, a femtosecond laser makes two passes through the cornea to create a lenticule that is removed from a small side incision. The remaining cap of stroma and epithelium collapses into the newly created pocket, inducing a predictable myopic shift. With strong support for each of these refractive procedures worldwide, the question remains, which induces the least HOAs and provides the best quality of vision?
Many studies and meta-analyses have been conducted comparing wavefront-guided to wavefront-optimized ablations.95–98 In a contralateral eye-to-eye study where one eye received a wavefront-optimized treatment while the other received a wavefront-guided treatment for hyperopia, HOAs decreased overall in both groups, but there was no difference in coma, trefoil or RMS error between the groups at 1 year.96 Another eye-to-eye study of predominantly myopic eyes showed that although there was a trend towards increasing HOAs in the wavefront-optimized group and decreasing HOAs in the wavefront-guided group post-operatively, there was no significant difference in HOAs between the groups.97 He and Manche similarly found no difference between the treatment modalities in post-operative HOAs amongst higher myopes.98 Feng et al. conducted a meta-analysis of seven articles comprising 930 eyes and also found no difference in HOAs following wavefront-optimized and wavefront-guided treatments.95 However, they did note that postoperative HOAs were significantly lower in the wavefront-guided treatment group amongst patients with preoperative RMS HOAs greater than 0.3 μm, indicating that this may be a better treatment modality for patients with higher preexisting HOAs.95
To our knowledge, only one contralateral eye-to-eye study has been performed thus far comparing topography-guided to wavefront-optimized surface ablation in myopic PRK. Although they did not directly analyze HOAs, Falavarjani et al. found that contrast sensitivity, which is correlated with HOAs, improved equally in both groups 6 months post-operatively.99 Topography-guided ablations may have the largest benefit in cases where wavefront data cannot be obtained due to corneal opacities or highly irregular corneal surfaces such as post-penetrating keratoplasty or radial keratotomy.100
SMILE is a promising refractive procedure that is not yet widely available in the U.S. In a comparison with wavefront-optimized LASIK, Wu and Wang found that SMILE had significantly lower 3rd to 6th-order HOAs, spherical aberration, and horizontal coma of the anterior surface and total cornea compared to the LASIK group. The only HOA that was higher in the SMILE group was vertical coma.101 The authors suggest that this may be a result of the flapless technique. In a retrospective analysis of moderately myopic eyes undergoing SMILE or wavefront-optimized femtosecond (FS) LASIK, Gyldenkerne et al. found that FS-LASIK induced significantly more HOAs, in particular coma and spherical aberration.52 In a similar study by Lin et al, patients in both the SMILE and wavefront-optimized FS-LASIK groups both had HOAs induced by their respective procedures. However, at 1 month and 3 months post-op, the SMILE group had significantly lower total HOAs, spherical aberration, and coma. This may be due to the maintenance of corneal structural integrity and construction of a larger optical zone in SMILE.51
LASIK Flap Construction: Microkeratome vs. Femtosecond Laser
In 2002, when a microkeratome was the only available method of flap formation for LASIK, Pallikaris et al. demonstrated that creating a nasally hinged flap alone significantly increased total HOAs, though it did not affect lower-order aberrations.102 The author felt that hinge position may play a role, as there was an increase in coma along the horizontal axis towards the hinge. Porter et al. also found a small but significant increase in HOAs, primarily trefoil, induced by the microkeratome.103
Since the introduction of the femtosecond laser as a method of creating LASIK flaps in 2000, the literature has been mixed concerning which method induces fewer HOAs. Chen et al. conducted a meta-analysis on the topic and found no difference in HOAs induced by femtosecond and microkeratome flaps.104 Interestingly, another meta-analysis was published one year prior that concluded that femtosecond flaps may in fact induce fewer HOAs, including spherical aberration. This may be due to the meniscus shape of a microkeratome flap versus the planar, uniform shape of a femtosecond flap.105 Xia et al. also found, in a head-to-head trial, increased HOAs in the microkeratome group as well as worse contrast sensitivity at 6 months.106 One author felt that flap thickness may be responsible for induction of HOAs since hinge direction did not influence coma contrary to prior reports and the more uniform femtosecond flaps induced fewer HOAs than microkeratomes.107 However, Cheng et al. demonstrated that there was no difference in HOAs induced by flaps of different thickness.108 Despite relatively increased HOAs in the microkeratome groups, none of the studies reported worse uncorrected or corrected vision or disabling visual symptoms amongst these patients.
Acknowledgments
Funding Sources: Supported in part by NIH R01 EY023381 and an Unrestricted Grant from Research to Prevent Blindness to the Department of Ophthalmology of the Cleveland Clinic Lerner College of Medicine of Case Western Reserve University
Footnotes
Conflicts of Interest: WJD is a consultant for Ziemer, a member of the medical advisory board of Avedro, and has received research support from Avedro and Zeiss. WJD is listed as an inventor on intellectual property through Cleveland Clinic Innovations related to computational modeling of the eye and biomechanical measurement.
References
- 1.Applegate RA, Howland HC. Refractive Surgery, Optical Aberrations, and Visual Performance. J Refract Surg. 1997;13:295–299. doi: 10.3928/1081-597X-19970501-16. [DOI] [PubMed] [Google Scholar]
- 2.Oliver KM, Hemenger RP, Corbett MC, et al. Corneal optical aberrations induced by photorefractive keratectomy. J Refract Surg. 1997;13:246–254. doi: 10.3928/1081-597X-19970501-10. [DOI] [PubMed] [Google Scholar]
- 3.Alió JL, Piñero DP, Espinosa MJ, Corral MJ. Corneal aberrations and objective visual quality after hyperopic laser in situ keratomileusis using the Esiris excimer laser. J Cataract Refract Surg. 2008;34:398–406. doi: 10.1016/j.jcrs.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 4.Yoon G, Macrae S, Williams DR, Cox IG. Causes of spherical aberration induced by laser refractive surgery. J Cataract Refract Surg. 2005;31:127–135. doi: 10.1016/j.jcrs.2004.10.046. [DOI] [PubMed] [Google Scholar]
- 5.Platt BC, Shack R. History and Principles of Shack-Hartmann Wavefront Sensing. J Refract Surg. 2001;17:S573–577. doi: 10.3928/1081-597X-20010901-13. [DOI] [PubMed] [Google Scholar]
- 6.Huang D, Arif M. Spot size and quality of scanning laser correction of higher-order wavefront aberrations. J Cataract Refract Surg. 2002;28:407–16. doi: 10.1016/s0886-3350(01)01163-4. [DOI] [PubMed] [Google Scholar]
- 7.Applegate RA, Gansel KA. The importance of pupil size in optical quality measurements following radial keratotomy. Refract Corneal Surg. 1990;6:47–54. [PubMed] [Google Scholar]
- 8.Schallhorn SC, Kaupp SE, Tanzer DJ, et al. Pupil size and quality of vision after LASIK. Ophthalmology. 2003;110(8):1606–1614. doi: 10.1016/S0161-6420(03)00494-9. [DOI] [PubMed] [Google Scholar]
- 9.Schallhorn S, Brown M, Venter J, et al. The role of the mesopic pupil on patient-reported outcomes in young patients with myopia 1 month after wavefront-guided LASIK. J Refract Surg. 2014;30:159–165. doi: 10.3928/1081597X-20140217-02. [DOI] [PubMed] [Google Scholar]
- 10.McCormick GJ, Porter J, Cox IG, MacRae S. Higher-Order Aberrations in Eyes with Irregular Corneas after Laser Refractive Surgery. Ophthalmology. 2005;112:1699–1709. doi: 10.1016/j.ophtha.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 11.Chalita MR, Chavala S, Xu M, Krueger RR. Wavefront Analysis in Post-LASIK Eyes and Its Correlation with Visual Sympotms, Refraction, and Topography. Ophthalmology. 2004;111:447–453. doi: 10.1016/j.ophtha.2003.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Sugar A, Rapuano CJ, Culbertson WW. Laser in situ keratomileusis for myopia and astigmatism: safety and efficacy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:175–187. doi: 10.1016/s0161-6420(01)00966-6. [DOI] [PubMed] [Google Scholar]
- 13.Hersh PS, Stulting RD, Steinert RF, et al. Results of phase III excimer laser photorefractive keratectomy for myopia. The Summit PRK Study Group. Ophthalmology. 1997;104:1535–1553. doi: 10.1016/s0161-6420(97)30073-6. [DOI] [PubMed] [Google Scholar]
- 14.El Danasoury MA, el Maghraby A, Klyce SD, Mehrez K. Comparison of photorefractive keratectomy with excimer laser in situ keratomileusis in correcting low myopia (from −2.00 to −5.50 diopters). A randomized study. Ophthalmology. 1999;106:411–420. doi: 10.1016/S0161-6420(99)90084-2. [DOI] [PubMed] [Google Scholar]
- 15.Shortt AJ, Allan BD. Photorefractive keratectomy (PRK) versus laser-assisted in-situ keratomileusis (LASIK) for myopia. Cochrane Database Syst Rev. 2006;19:CD005135. doi: 10.1002/14651858.CD005135.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Fares U, Otri AM, Al-Aqaba MA, et al. Wavefront-optimized excimer laser in situ keratomileusis for myopia and myopic astigmatism: refractive outcomes and corneal densitometry. J Cataract Refract Surg. 2012;38:2131–2138. doi: 10.1016/j.jcrs.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 17.Kulkarni SV, AlMahmoud T, Priest D, et al. Long-term visual and refractive outcomes following surface ablation techniques in a large population for myopia correction. Invest Ophthalmol Vis Sci. 2013;54:609–619. doi: 10.1167/iovs.12-10387. [DOI] [PubMed] [Google Scholar]
- 18.Kanellopoulos AJ, Asimellis G. Long-term bladeless LASIK outcomes with the FS200 Femtosecond and EX500 Excimer Laser workstation: the Refractive Suite. Clin Ophthalmol. 2013;7:261–269. doi: 10.2147/OPTH.S40454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varley GA, Huang D, Rapuano CJ, et al. LASIK for hyperopia, hyperopic astigmatism, and mixed astigmatism: a report by the American Academy of Ophthalmology. Ophthalmology. 2004;111:1604–1617. doi: 10.1016/j.ophtha.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Salz JJ, Stevens CA LADARVision LASIK Hyperopia Study Group. LASIK correction of spherical hyperopia, hyperopic astigmatism, and mixed astigmatism with the LADARVision excimer laser system. Ophthalmology. 2002;109:1647–1656. doi: 10.1016/s0161-6420(02)01133-8. [DOI] [PubMed] [Google Scholar]
- 21.Durrie DS, Smith RT, Waring GO, 4th, et al. Comparing conventional and wavefront-optimized LASIK for the treatment of hyperopia. J Refract Surg. 2010;26:356–363. doi: 10.3928/1081597X-20090617-07. [DOI] [PubMed] [Google Scholar]
- 22.Luger MH, Ewering T, Arba Mosquera S. Influence of patient age on high myopic correction in corneal laser refractive surgery. J Cataract Refract Surg. 2013;39:204–210. doi: 10.1016/j.jcrs.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 23.Tabbara KF, El-Sheikh HF, Islam SM. Laser in situ keratomileusis for the correction of hyperopia from +0.50 to +11.50 diopters with the Keracor 117C laser. J Refract Surg. 2001;17:123–128. doi: 10.3928/1081-597X-20010301-05. [DOI] [PubMed] [Google Scholar]
- 24.Guell JL, Muller A. Laser in situ keratomileusis (LASIK) for myopia from −7 to −18 diopters. J Refract Surg. 1996;12:222–228. doi: 10.3928/1081-597X-19960201-03. [DOI] [PubMed] [Google Scholar]
- 25.Helmy SA, Salah A, Badawy TT, Sidky AN. Photorefractive keratectomy and laser in situ keratomileusis for myopia between 6.00 and 10.00 diopters. J Refract Surg. 1996;2:417–421. doi: 10.3928/1081-597X-19960301-17. [DOI] [PubMed] [Google Scholar]
- 26.McDonald MB, Liu JC, Byrd TJ, et al. Central Photorefractive Keratectomy for Myopia. Partially Sighted and Normally Sighted Eyes. Ophthalmology. 1991;98:1327–1337. doi: 10.1016/s0161-6420(91)32128-6. [DOI] [PubMed] [Google Scholar]
- 27.Rajan MS, O’Brart D, Jaycock P, Marshall J. Effects of Ablation Diameter on Long-term Refractive Stability and Corneal Transparency after Photorefractive Keratectomy. Ophthalmology. 2006;113:1798–1806. doi: 10.1016/j.ophtha.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 28.Nilforoushan MR, Latkany RA, Speaker MG. Effect of artificial tears on visual acuity. Am J Ophthalmol. 2005;140:830–835. doi: 10.1016/j.ajo.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Khalil MB, Latkany RA, Speaker MG, Yu G. Effect of punctual plugs in patients with low refractive errors considering refractive surgery. J Refract Surg. 2007;23:467–471. doi: 10.3928/1081-597X-20070501-08. [DOI] [PubMed] [Google Scholar]
- 30.Randleman JB, Woodward M, Lynn MJ, Stulting RD. Risk assessment for ectasia after corneal refractive surgery. Ophthalmology. 2008;115:37–50. doi: 10.1016/j.ophtha.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 31.Santhiago MR, Smadja D, Gomes BF, et al. Association between the percent tissue altered and post-laser in situ keratomileusis ectasia in eyes with normal preoperative topography. Am J Ophthalmol. 2014;158:87–95. doi: 10.1016/j.ajo.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Alio JL, Pinero D, Moftuoglu O. Corneal wavefront-guided retreatments for significant night vision symptoms after myopic laser refractive surgery. Am J Ophthalmol. 2008;145:65–74. doi: 10.1016/j.ajo.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Beerthuizen JJ, Siebelt E. Surface ablation after laser in situ keratomileusis: retreatment on the flap. J Catarat Refract Surg. 2007;33:1376–1380. doi: 10.1016/j.jcrs.2007.04.024. [DOI] [PubMed] [Google Scholar]
- 34.Maldonado MJ. Undersurface ablation of the flap for laser in situ keratomileusis retreatment. Ophthalmology. 2002;109:1453–1464. doi: 10.1016/s0161-6420(02)01096-5. [DOI] [PubMed] [Google Scholar]
- 35.Park S, Chuck RS. Corneal collagen cross-linking for correction of low myopia? Curr Opin Ophthalmol. 2013;24:273–274. doi: 10.1097/ICU.0b013e3283622cb1. [DOI] [PubMed] [Google Scholar]
- 36.Moshirfar M, Simpson RG, Dave SB, et al. Sources of Medical Error in Refractive Surgery. J Refract Surg. 2013;29:303–310. doi: 10.3928/1081597X-20130415-01. [DOI] [PubMed] [Google Scholar]
- 37.Karthikappallil J. Induced astigmatism after laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:940–941. doi: 10.1016/j.jcrs.2004.02.055. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez-Prats J, Ahmed AG, Ayala MJ, Alio JL. Induced astigmatism after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29:414–415. doi: 10.1016/s0886-3350(03)00019-1. [DOI] [PubMed] [Google Scholar]
- 39.Hickson GB, Clayton EW, Githens PB, Sloan FA. Factors that prompted families to file medical malpractice claims following perinatal injuries. JAMA. 1992;267:1359–1363. [PubMed] [Google Scholar]
- 40.Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553–559. doi: 10.1001/jama.277.7.553. [DOI] [PubMed] [Google Scholar]
- 41.Chernyak DA. Cyclotorsional eye motion occurring between wavefront measurement and refractive surgery. J Cataract Refract Surg. 2004;30:633–638. doi: 10.1016/j.jcrs.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 42.Arba Mosquera S, Merayo-Lloves J, De Ortueta D. Clinical Effects of Pure Cyclotorsional Errors during Refractive Surgery. Invest Ophthalmol Vis Sci. 2008;49:4828–4836. doi: 10.1167/iovs.08-1766. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Koch DD. Residual higher-order aberrations caused by clinically measured cyclotorsional misalignment or decentration during wavefront-guided excimer laser corneal ablation. J Cataract Refract Surg. 2008;34:2057–2062. doi: 10.1016/j.jcrs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 44.Padmanabhan P, Mrochen M, Viswanathan D, Basuthkar S. Wavefront aberrations in eyes with decentered ablations. J Cataract Refract Surg. 2009;35:695–702. doi: 10.1016/j.jcrs.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 45.Jarade EF, Azar DT. Management of Irregular Astigmatism after Laser In Situ Keratomileusis. Int Ophthalmol Clin. 2003;43:141–156. doi: 10.1097/00004397-200343030-00013. [DOI] [PubMed] [Google Scholar]
- 46.Duffey RJ. Central islands and decentered ablations after LASIK. Int Ophthalmol Clin. 2000;40:93–101. doi: 10.1097/00004397-200007000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Mrochen M, Krueger RR, Bueeler M, Seiler T. Aberration-sensing and Wavefront-guided Laser in situ Keratomileusis: Management of Decentered Ablation. J Refract Surg. 2002;18:418–428. doi: 10.3928/1081-597X-20020701-01. [DOI] [PubMed] [Google Scholar]
- 48.Mrochen M, Kaemmerer M, Mierdel P, Seiler T. Increased higher-order optical aberrations after laser refractive surgery. J Cataract Refract Surg. 2001;27:362–369. doi: 10.1016/s0886-3350(00)00806-3. [DOI] [PubMed] [Google Scholar]
- 49.Fang L, Wang Y, He X. Theoretical analysis of wavefront aberration caused by treatment decentration and transition zone after custom myopic laser refractive surgery. J Cataract Refract Surg. 2013;39:1336–1347. doi: 10.1016/j.jcrs.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 50.Li M, Zhao J, Miao H, Shen Y, Sun L, Tian M, Wadium E, Zhou X. Mild Decentration Measured by a Scheimpflug Camera and Its Impact on Visual Quality Following SMILE in the Early Learning Curve. Invest Ophthalmol Vis Sci. 2014;55:3886–3892. doi: 10.1167/iovs.13-13714. [DOI] [PubMed] [Google Scholar]
- 51.Lin F, Xu Y, Yang Y. Comparison of the visual results after SMILE and femtosecond laser-assisted LASIK for myopia. J Refract Surg. 2014;20:248–254. doi: 10.3928/1081597X-20140320-03. [DOI] [PubMed] [Google Scholar]
- 52.Gyldenkerne A, Ivarsen A, Hjortdal JØ. Comparison of corneal shape changes and aberrations induced by FS-LASIK and SMILE for myopia. J Refract Surg. 2015;31:223–229. doi: 10.3928/1081597X-20150303-01. [DOI] [PubMed] [Google Scholar]
- 53.Lafond G, Bonnet S, Solomon L. Treatment of previously decentered excimer laser ablation with combined myopic and hyperopic ablations. J Refract Surg. 2004;20:139–148. doi: 10.3928/1081-597X-20040301-08. [DOI] [PubMed] [Google Scholar]
- 54.Lin D, Holland SP, Rocha KM, Krueger RR. Method for Optimizing Topography-guided Ablation of Highly Aberrated Eyes With the ALLEGRETTO WAVE Excimer Laser. J Refract Surg. 2008;24:S439–S445. doi: 10.3928/1081597X-20080401-22. [DOI] [PubMed] [Google Scholar]
- 55.Kanellopoulos AJ, Pe LH. Wavefront-guided enhancements using the wavelight excimer laser in symptomatic eyes previously treated with LASIK. J Refract Surg. 2006;22:345–349. doi: 10.3928/1081-597X-20060401-08. [DOI] [PubMed] [Google Scholar]
- 56.Arba Mosquera S, Verma S. Numerical nonwavefront-guided algorithm for expansion or recentration of the optical zone. J Biomed Opt. 2014;19:088001. doi: 10.1117/1.JBO.19.8.088001. [DOI] [PubMed] [Google Scholar]
- 57.Hersh PS. A standardized Classification of Corneal Topography after Laser Refractive Surgery. J Refract Surg. 1997;13:571–578. doi: 10.3928/1081-597X-19970901-14. [DOI] [PubMed] [Google Scholar]
- 58.Lin DT. Corneal Topographic Analysis after Excimer Photorefractive Keratectomy. Ophthalmology. 1994;101:1432–1444. doi: 10.1016/s0161-6420(94)31154-7. [DOI] [PubMed] [Google Scholar]
- 59.Oshika T, Myce SD, Smolek MK, McDonald MB. Corneal hydration and central islands after excimer laser photorefractive keratectomy. J Cataract Refract Surg. 1998;24:1575–1580. doi: 10.1016/s0886-3350(98)80345-3. [DOI] [PubMed] [Google Scholar]
- 60.Kang SW, Chung ES, Kim WJ. Clinical analysis of central islands after laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:536–542. doi: 10.1016/s0886-3350(99)00458-7. [DOI] [PubMed] [Google Scholar]
- 61.Wilson SE. LASIK: Management of Common Complications. Cornea. 1998;17:459–467. doi: 10.1097/00003226-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Krueger RR, Saedy NF, McDonnell PJ. Clinical analysis of steep central islands after excimer laser photorefractive keratectomy. Arch Ophthalmol. 1996;114:377–381. doi: 10.1001/archopht.1996.01100130373002. [DOI] [PubMed] [Google Scholar]
- 63.Lin DT. Corneal topographic analysis after excimer photorefractive keratectomy. J Cataract Refract Surg. 1993;19:149–154. doi: 10.1016/s0886-3350(13)80399-9. [DOI] [PubMed] [Google Scholar]
- 64.Tsai YY, Lin JM. Natural history of central islands after laser in situ keratomileusis. J Cataract Refract Surg. 2000;26:853–858. doi: 10.1016/s0886-3350(00)00375-8. [DOI] [PubMed] [Google Scholar]
- 65.Hersh PS, Scher KS, Irani R. Corneal topography of photorefractive keratectomy versus laser in situ keratomileusis. Summit PRK-LASIK Study Group. Ophthalmology. 1998;105:612–619. doi: 10.1016/s0161-6420(98)94013-1. [DOI] [PubMed] [Google Scholar]
- 66.Förster W, Clemens S, Brüning S, et al. Steep central islands after myopic photorefractive keratectomy. J Cataract Refract Surg. 1998;24:899–904. doi: 10.1016/s0886-3350(98)80040-0. [DOI] [PubMed] [Google Scholar]
- 67.Noack J, Tönnies R, Hohla K, et al. Influence of ablation plume dynamics on the formation of central islands in excimer laser photorefractive keratectomy. Ophthalmology. 1997;104:823–830. doi: 10.1016/s0161-6420(97)30227-9. [DOI] [PubMed] [Google Scholar]
- 68.Castillo A, Romero F, Martin-Valverde JA, et al. Management and treatment of central steep islands after excimer laser photorefractive keratectomy. J Refract Surg. 1996;12:715–720. doi: 10.3928/1081-597X-19960901-15. [DOI] [PubMed] [Google Scholar]
- 69.Rachid MD, Yoo SH, Azar DT. Phototherapeutic Keratectomy for Decentration and Central Islands after Photorefractive Keratectomy. Ophthalmology. 2001;108:545–552. doi: 10.1016/s0161-6420(00)00595-9. [DOI] [PubMed] [Google Scholar]
- 70.Shimmick JK. This VISX Perspective on Fixed vs. Variable Spot Scanning Ablation. J Refract Surg. 2001;17:S594–595. doi: 10.3928/1081-597X-20010901-18. [DOI] [PubMed] [Google Scholar]
- 71.Johnson JD, Azar DT. Surgically induced topographical abnormalities after LASIK: management of central islands, corneal ectasia, decentration, and irregular astigmatism. Curr Opin Ophthalmol. 2001;12:309–317. doi: 10.1097/00055735-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 72.Munnerlyn CR, Koons SJ, Marshall J. Photorefractive keratectomy: a technique for laser refractive surgery. J Cataract Refract Surg. 1988;14:46–52. doi: 10.1016/s0886-3350(88)80063-4. [DOI] [PubMed] [Google Scholar]
- 73.Wiesinger-Jendritza B, Knorz MC, Hugger P, Liermann A. Laser in situ keratomileusis assisted by corneal topography. J Cataract Refract Surg. 1998;24:166–174. doi: 10.1016/s0886-3350(98)80196-x. [DOI] [PubMed] [Google Scholar]
- 74.Knorz MC, Jendritza B. Topographically-guided laser in situ keratomileusis to treat corneal irregularities. Ophthalmology. 2000;107:1138–1143. doi: 10.1016/s0161-6420(00)00094-4. [DOI] [PubMed] [Google Scholar]
- 75.Alío JL, Belda JI, Osman AA, Shalaby AM. Topography-guided laser in situ keratomileusis (TOPOLINK) to correct irregular astigmatism after previous refractive surgery. J Refract Surg. 2003;19:516–527. doi: 10.3928/1081-597X-20030901-06. [DOI] [PubMed] [Google Scholar]
- 76.Alessio G, Boscia F, La Tegola MG, Sborgia C. Topography-driven photorefractive keratectomy: results of corneal interactive programmed topographic ablation software. Ophthalmology. 2000;107:1578–1587. doi: 10.1016/s0161-6420(00)00224-4. [DOI] [PubMed] [Google Scholar]
- 77.Hafezi F, Jankov M, Mrochen M, et al. Customized ablation algorithm for the treatment of steep central islands after refractive laser surgery. J Cataract Refract Surg. 2006;32:717–721. doi: 10.1016/j.jcrs.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 78.Cheng AC, Lam DS. Central island treatment using Technolas 217 based on Orbscan II assessment. J Refract Surg. 2005;21:294–296. doi: 10.3928/1081-597X-20050501-14. [DOI] [PubMed] [Google Scholar]
- 79.Maloney RK. Corneal topography and optical zone location in photorefractive keratectomy. Refract Corneal Surg. 1990;6:363–371. [PubMed] [Google Scholar]
- 80.Hafezi F, Mrochen M, Seiler T. Two-step procedure to enlarge small optical zones after photorefractive keratectomy for high myopia. J Cataract Refract Surg. 2005;31:2254–2256. doi: 10.1016/j.jcrs.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 81.Seo KY, Lee JB, Kang JJ, et al. Comparison f higher-order aberrations after LASEK with a 6.0mm ablation zone and a 6.5 mm ablation zone with blend zone. J Cataract Refract Surg. 2004;30:653–657. doi: 10.1016/j.jcrs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 82.Seiler T, Reckmann W, Maloney RK. Effective speherical aberration of the cornea as a quantitative descriptor in corneal topography. J Cataract Refract Surg. 1993;19:155–165. doi: 10.1016/s0886-3350(13)80400-2. [DOI] [PubMed] [Google Scholar]
- 83.Hersh PS, Fry K, Blaker JW. Spherical aberration after laser in situ keratomileusis and photorefractive keratectomy. Clinical results and theoretical models of etiology. J Cataract Refract Surg. 2003;29:2096–2104. doi: 10.1016/j.jcrs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 84.Baek TM, Lee KH, Tomidokoro A, Oshika T. Corneal ireegular astigmatism after laser in situ keratomileusis for myopia. Br J Ophthalmol. 2001;85:534–536. doi: 10.1136/bjo.85.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halladay JT, Dudeja DR, Chang J. Functional vision and corneal changes after laser in situ keratomileusis determined by contrast sensitivity, glare testing, and corneal topography. J Cataract Refract Surg. 1999;25:663–669. doi: 10.1016/s0886-3350(99)00011-5. [DOI] [PubMed] [Google Scholar]
- 86.O’Bart DP, Gartry DS, Lohmann CP, et al. Excimer laser photorefractive keratectomy for myopia: comparison of 4.00- and 5.00-millimeter ablation zones. J Refract Corneal Surg. 1994;10:87–94. [PubMed] [Google Scholar]
- 87.Kalski RS, Sutton G, Bin Y, et al. Comparison of 5-mm and 6-mm ablation zones in photorefractive keratectomy for myopia. J Refract Surg. 1996;12:61–67. doi: 10.3928/1081-597X-19960101-13. [DOI] [PubMed] [Google Scholar]
- 88.O’Brart DP, Corbett MC, Lohmann CP, et al. The effects of ablation diameter on the outcome of excimer laser photorefractive keratectomy. A prospective, randomized, double-blind study. Arch Ophthalmol. 1995;113:438–443. doi: 10.1001/archopht.1995.01100040054026. [DOI] [PubMed] [Google Scholar]
- 89.O’Brart DP, Corbett MC, Verma S, et al. Effects of ablation diameter, depth, and edge contour on the outcome of photorefractive keratectomy. J Refract Surg. 1996;12:50–60. doi: 10.3928/1081-597X-19960101-12. [DOI] [PubMed] [Google Scholar]
- 90.Corbett MC, Verma S, O’Brart DP, et al. Effect of ablation profile on wound healing and visual performance 1 year after excimer laser photorefractive keratectomy. Br J Ophthalmol. 1996;80:224–234. doi: 10.1136/bjo.80.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pop M, Payette Y. Risk factors for night vision complaints after LASIK for myopia. Ophthalmology. 2004;111:3–10. doi: 10.1016/j.ophtha.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 92.Vinciguerra P, Munoz MI, Camesasca FI. Reduction of spherical aberration: experimental model of photoablation. J Refract Surg. 2002;18:S366–370. doi: 10.3928/1081-597X-20020502-18. [DOI] [PubMed] [Google Scholar]
- 93.Eggink FA, Beekhuis WH, Trokel SL, den Boon JM. Enlargement of the photorefractive keratectomy optical zone. J Cataract Refract Surg. 1996;22:1159–1164. doi: 10.1016/s0886-3350(96)80064-2. [DOI] [PubMed] [Google Scholar]
- 94.Lafond G. Treatment of halos after photorefractive keratectomy. J Refract Surg. 1997;13:83–88. doi: 10.3928/1081-597X-19970101-18. [DOI] [PubMed] [Google Scholar]
- 95.Feng Y, Yu J, Wang Q. Meta-analysis of wavefront-guided vs. wavefront-optimized LASIK for myopia. Optom Vis Sci. 2011;88:1463–1469. doi: 10.1097/OPX.0b013e3182333a50. [DOI] [PubMed] [Google Scholar]
- 96.Sales CS, Manche EE. One-year outcomes from a prospective, randomized, eye-to-eye comparison of wavefront-guided and wavefront-optimized LASIK in myopes. Ophthalmology. 2013;120:2396–2402. doi: 10.1016/j.ophtha.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 97.Moshirfar M, Betts BS, Churgin DS, et al. A prospective, randomized, fellow eye comparison of WaveLight® Allegretto Wave ® Eye-Q versus VISX CustumVue™ STAR S4 IR™ in laser in situ keratomileusis (LASIK): analysis of visual outcomes and higher order aberrations. Clin Ophthalmol. 2011;5:1339–1347. doi: 10.2147/OPTH.S24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.He L, Manche EE. Contralateral eye-to-eye comparison of wavefront-guided and wavefront-optimized photorefractive keratectomy: a randomized clinical trial. JAMA Ophthalmol. 2015;133:51–59. doi: 10.1001/jamaophthalmol.2014.3876. [DOI] [PubMed] [Google Scholar]
- 99.Falavarjani KG, Hashemi M, Modarres M, et al. Topography-Guided vs. Wavefront-Optimized Surface Ablation for Myopia Using the WaveLight Platform: A Contralateral Eye Study. J Refract Surg. 2011;27:13–17. doi: 10.3928/1081597X-20100310-02. [DOI] [PubMed] [Google Scholar]
- 100.Pasquali T, Krueger R. Topography-guided laser refractive surgery. Curr Opin Ophthalmol. 2012;23:264–268. doi: 10.1097/ICU.0b013e328354adf0. [DOI] [PubMed] [Google Scholar]
- 101.Wu W, Wang Y. The Correlation Analysis between Corneal Buiomechanical Properties and the Surgically Induced Corneal High-Order Aberrations after Small Incision Lenticule Extraction and Femtosecond Laser In Situ Keratomileusis. J Ophthalmol. 2015;2015:758196. doi: 10.1155/2015/758196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pallikaris IG, Kymionis GD, Panagopoulou SI. Induced optical aberrations following formation of a laser in situ keratomileusis flap. J Cataract Refract Surg. 2002;28:1737–1741. doi: 10.1016/s0886-3350(02)01507-9. [DOI] [PubMed] [Google Scholar]
- 103.Porter J, MacRae S, Yoon G, et al. Separate effects of the microkeratome incision and laser ablation on the eye’s wave aberration. Am J Ophthalmol. 2003;136:327–337. doi: 10.1016/s0002-9394(03)00222-8. [DOI] [PubMed] [Google Scholar]
- 104.Chen S, Feng Y, Stojanovic A, et al. Intralase femtosecond laser vs mechanical microkeratomes in LASIK for myopia: a systematic review and meta-analysis. J Refract Surg. 2012;28:15–24. doi: 10.3928/1081597X-20111228-02. [DOI] [PubMed] [Google Scholar]
- 105.Zhang ZH, JHY, Suo Y, et al. Femtosecond laser versus mechanical microkeratome laser in situ keratomileusis for myopia: Metaanalysis of randomized controlled trials. J Cataract Refract Surg. 2011;37:2151–2159. doi: 10.1016/j.jcrs.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 106.Xia LK, Yu J, Chai GR, et al. Comparison of the femtosecond Laser and mechanical microkeratome for flap cutting in LASIK. Int J Ophthalmol. 2015;8:784–790. doi: 10.3980/j.issn.2222-3959.2015.04.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yvon C, Archer TJ, Gobbe M, Reinstein DZ. Comparison of higher-order aberration induction between manual microkeratome and femtosecond laser flap creation. J Refract Surg. 2015;31:130–135. doi: 10.3928/1081597X-20150122-09. [DOI] [PubMed] [Google Scholar]
- 108.Cheng ZY, He JC, Zhou XT, Chu RY. Effect of Flap Thickness on Higher Order Wavefront Aberrations Induced by LASIK: A Bilateral Study. J Refract Surg. 2008;24:524–529. doi: 10.3928/1081597X-20080501-11. [DOI] [PubMed] [Google Scholar]