Abstract
Objectives
This study assessed whether community mobilization and interventions to improve emergency obstetric and newborn care (EmONC) reduced perinatal mortality (PMR) and neonatal mortality rates (NMR) in Belgaum, India.
Methods
The cluster-randomised controlled trial was conducted in Belgaum District, Karnataka State, India. Twenty geographic clusters were randomized to control or the intervention. The intervention engaged and mobilized community and health authorities to leverage support; strengthened community-based stabilization, referral, and transportation; and aimed to improve quality of care at facilities.
Results
17,754 intervention births and 15,954 control births weighing ≥1000 g, respectively, were enrolled and analysed. Comparing the baseline period to the last 6 months period, the NMR was lower in the intervention vs. control clusters (OR=0.60, 95% CI 0.34–1.06, p=.076) as was the PMR (OR = 0.74, 95% CI 0.46–1.19, p=.20) although neither reached statistical significance. Rates of facility birth and caesarean section increased among both groups. There was limited influence on quality of care measures.
Conclusions
The intervention had large but not statistically significant effects on neonatal and perinatal mortality. Community mobilization and increased facility care may ultimately improve neonatal and perinatal survival, and are important in the context of the global transition towards institutional delivery.
Keywords: Emergency obstetric and newborn care, perinatal mortality, neonatal mortality, community mobilization, quality of care, India
Introduction
India contributes the largest number to the annual 3.1 million neonatal deaths and 2.6 million stillbirths worldwide, more than any other single country [1]. Even with a 54% reduction in the under-five mortality rate (U5MR), from 114 in 1990 to 61/1,000 live births in 2011, India is unlikely to reach its U5MR Millennium Development Goal 4 (MDG4) of 38/1,000 live births by 2015, largely because the country’s 2011 neonatal mortality rate (NMR) of 32 deaths/1,000 live births accounts for 52% of the U5MR [1,2]. Emergency obstetric and neonatal care (EmONC) has long been recognized as important to improve management of obstetric and neonatal conditions causing pregnancy-related morbidity and mortality [2–5]. Government policies and other efforts have been made to improve basic and comprehensive EmONC, including administration of intravenous antibiotics, oxytocics, and anticonvulsant drugs, manual removal of the placenta and retained products, assisted delivery, surgical delivery, blood transfusions, resuscitation and intensive care, have improved antenatal and delivery care in resource-constrained settings [6,7]. Quasi-experimental studies have suggested that community mobilization to improve problem recognition and transfer have increased the use of obstetric services in resource-limited settings [8–13]. However, in the context of a transition towards institutional delivery in India and worldwide, few studies have assessed the potential role of community mobilization for reducing perinatal mortality.
We sought to assess a community intervention to effectively respond to the labor and delivery complications associated with perinatal mortality. As part of a multi-country Eunice Kennedy Shriver National Institute of Child Health and Human Development Global Network for Women’s and Children’s Health Research (GN) project, we tested a composite intervention package designed to engage community members and health authorities, elicit their support and improve quality of care (QOC) to reduce the perinatal mortality rate (PMR) and neonatal mortality rate (NMR) [14,15]. Outcomes of the primary trial, conducted in 7 sites in 6 countries, are presented elsewhere [15]. As the largest site with a high facility delivery rate, we were interested in evaluating whether India might have unique findings. This manuscript presents the results of the composite intervention package, as implemented in the Belgaum District of Karnataka state in southern India.
Patients and Methods
The cluster-randomized trial was conducted in Belgaum, India in 20 geographically-defined clusters which were assigned to either the intervention group (n=10) or the comparison group (n=10) by computer generated randomization. The effects of the intervention including the primary mortality outcomes and measures of health care utilization were assessed by independent staff of the Maternal and Neonatal Health (MNH) Registry system that enrolled pregnant women and tracked their outcomes to 42-days post-partum[16]. Each of the 10 study and 10 control clusters had approximately 500 annual births.
Information on socio-demographic characteristics, implementation and use of antenatal care (ANC), transportation and EmONC services, and on primary and secondary outcomes from pre-pregnancy to 42-days post-partum were collected for consenting, eligible women and their offspring over a 6 month pre-intervention period (4/1/2009 through 9/30/2009) and for 24 months (10/1/2009 through 9/30/2011) during the intervention implementation. Pregnant women who were permanent residents of the study clusters and those living for at least four weeks during the antenatal period were eligible. This included 19,269 women in the intervention group and 17,188 women in the control group (Figure 2). Participants with miscarriages (intervention n=885, control n=757), medical termination of pregnancy (intervention n=596, control n=442), giving birth to infants weighing <1000 grams at birth (intervention n=164, control n=136), lost to follow-up (1 per group), and those with unknown birth weight (intervention n=4, control n=3) or missing delivery information (intervention n=8, control n=4) were excluded from the outcome analyses. PMR, defined as stillbirths and neonatal deaths < 7 days, and NMR, defined as neonatal deaths < 28 days, are the primary outcomes presented in this manuscript. Utilization of health services prior to and at delivery and quality of care (QOC) indicators were assessed to describe whether these factors influenced the NMR and PMR.
Figure 2.
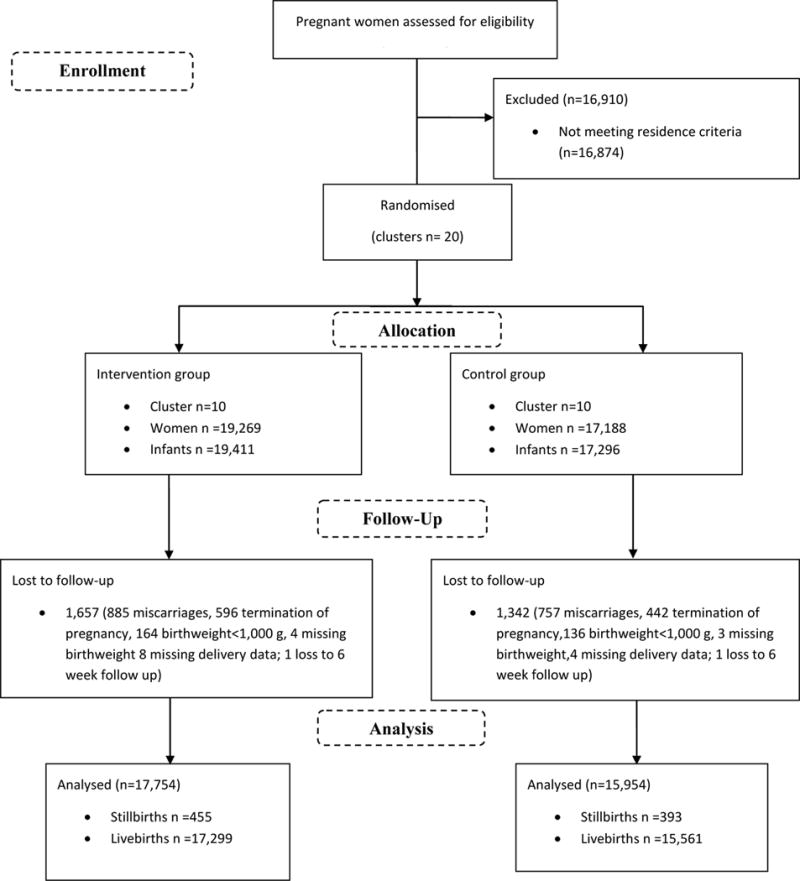
CONSORT Flow Diagram
Study intervention
A household-to-hospital continuum of care model was adopted in which preventable causes of maternal, fetal and early neonatal mortality and morbidity and existing resources for their management were identified and, where possible, available resources were leveraged to improve preparedness, knowledge of and timely access to transportation to care at higher level facilities for emergency care. The study interventions, described in detail elsewhere, sought to engage and mobilize community and health authorities to improve community recognition, stabilization, appropriate referral/transportation, and to improve the QOC at health facilities for pregnant women (Figure 1) [16,17]. The community-based components of the comprehensive intervention involved community mobilization efforts structured on the Community Action Cycle (CAC) paradigm that depend upon and empower communities to identify, prioritize and act upon MNH problems and existing practices. This process is fully described elsewhere [18]. The maternal conditions recognized by the communities as key contributors to maternal and neonatal morbidity and mortality were excess bleeding, prolonged labor, sepsis, pre-eclampsia/eclampsia and anemia. The newborn conditions identified as contributing most to PMR and NMR were breathing problems after birth, low birth weight, seizures and infection. The intervention strategies developed by the cluster teams to respond to these conditions included: strengthening antenatal care; early detection of high-risk conditions in mothers and newborns; preparing an effective birth plan; developing emergency funds through personal savings or local resources; arranging alternative emergency local transportation; seeking care at designated referral facilities; creating awareness of blood donations and of early identification and treatment of sexually-transmitted infections.
Figure 1.
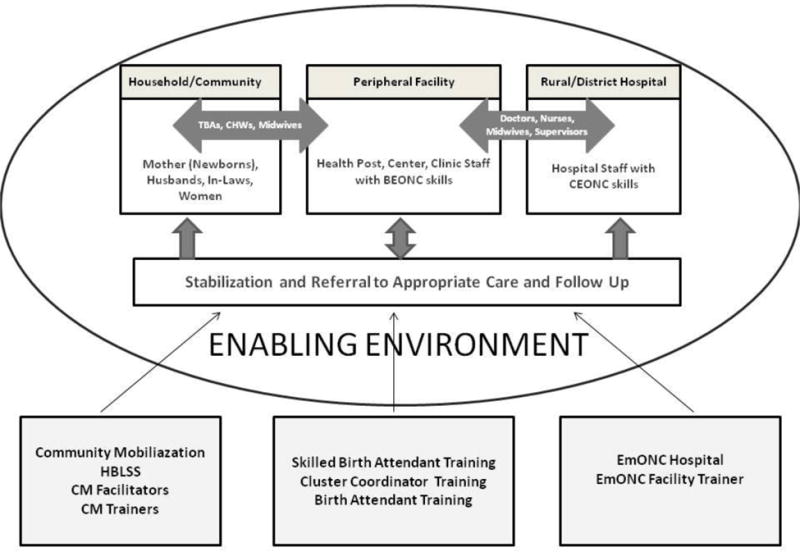
Study Intervention Diagram
Intervention activities to implement these strategies in intervention communities were: early registration of pregnancy, educating women about antenatal care (ANC) services and ensuring regular antenatal care; involving family decision makers and health care providers in preparing an effective birth plan; establishing emergency funds and use of local resources (especially transportation); teaching Home Based Life Saving Skills (HBLSS) to educate community members about danger signs during pregnancy and postpartum period and providing care at home; arranging blood donation camps; identifying and timely access of appropriate maternal and newborn emergency referral facilities; death audits; and using this information to develop intervention strategies.
An assessment of referral hospitals serving the intervention clusters was conducted at baseline and every 6 months during the study [14, 17]. This assessment examined availability of key health care providers, equipment, essential medications, and the performance of critical procedures. Based on the assessment, facilities were encouraged to expand their provision of EmONC, to improve their acceptance of referrals, and to implement quality assurance procedures. Training for hospitals included obstetric drills to strengthen emergency preparedness, essential newborn care, newborn resuscitation as well as skilled birth attendant training focused on active management of the third stage of labor, management of pre-eclampsia/eclampsia, sepsis, and prolonged Labor. All community birth attendants and community health workers were trained to screen, stabilize and refer women and neonates experiencing serious health conditions. After stabilization, communities were also encouraged to identify and arrange referral to higher facilities that could provide appropriate emergency care. Community-based workers received kits with essential items for the mother’s management at the facility and were taught to effectively communicate with transportation facilitators and with hospital staff to improve timely care. The birth attendants working at Primary Health Centers, Community Health Centers, Taluka Hospitals, General Hospitals and Private Hospitals were trained every six months over the two and a half year project period. A quality assurance process with a multi-disciplinary team including physicians, the Ministry of Health, and community members was established in each intervention cluster to review all cases of maternal, fetal and neonatal death and to discuss strategies to prevent similar deaths in the future.
Based on a cluster RCT in Bangladesh, where the effects of an essential newborn care training program were observed only in the last six months of a thirty-month intervention period, we evaluated the outcomes by time period [18]. We compared outcomes of the pre-intervention period (4/1/2009 – 9/30/2009) to the full intervention period (10/1/2009 – 9/30/2011) and to the final 6 months of the intervention period (4/1/2011 – 9/30/2011). For both PMR and NMR, regression models were developed to assess the relative change between 6 months before the intervention and the last 6 months of the intervention if the implementation of the intervention met four criteria: ≥80% deaths were audited, ≥80% of cluster and core group meetings were held at least monthly, and 80% of core group meetings included at least one quarterly “ACT Stage” implementation meeting, the meeting in which activities were planned to implement the intervention; these four criteria defined excellent implementation.
In Belgaum, Karnataka, all measures of implementing the community mobilization intervention were achieved in the first 12 months of the intervention. Therefore, regression analyses evaluated the relative change in the primary outcomes from the baseline compared to the final 6 months of the trial period, e.g., excluding the first 18 months when the intervention was being implemented and established. Two models were developed for each of these regression analyses; the first is adjusted only for cluster and the second is adjusted for cluster, infant gender, and infant birth weight <1500 grams. Birth weight <1500 grams was used as a surrogate measure for very preterm birth, those with lowest likelihood of survival. Because the gender composition differed between study groups and the association of gender with birth weight and survival is well established, adjusted analyses also controlled for gender [19]. Tests of difference in cluster-level rates or averages of background characteristics were obtained from linear models. Estimated marginal means from generalized estimating equation extensions of binomial Poisson regression models adjusted for cluster are presented for QOC, neonatal characteristics, PMR and NMR. All analyses were conducted using SAS v.9.3 (Cary, NC).
The study was approved by the Institutional Review Boards of the study site, Jawaharlal Nehru Medical College, Belgaum, Karnataka, India, and participating investigators, the University of Missouri at Kansas City and Christiana Care Health Services, Columbia University, Aga Khan University, and the Research Triangle Institute. The multi-country trial is registered with ClinicalTrials.gov NCT01073488.
Results
Of the 53,331 pregnant women in the 20 study clusters during the study period, 19,269 in the intervention clusters (19, 411 live births including multiples) and 17,188 in the control clusters (17,296 live births including multiples) were eligible for the study (Figure 2). Of the pregnancies, 1,657 in the intervention clusters and 1,342 in the control clusters were excluded, primarily due to miscarriage, pregnancy termination or birth weight <1000 g.
Maternal education, age, parity, and amount of antenatal care were similar in the intervention and control clusters (Table 1). Ninety-eight percent of all deliveries were classified as live births in both study groups. Only 1.3% of intervention and 1.2% of control cluster newborns weighed 1000 – 1500 g at birth (Table 2). There were more female than male offspring (48.3% vs. 47.0%, respectively) in the intervention than control clusters (p=.03).
Table 1.
Socio-Demographic Characteristics of Women in the Intervention and Control Clusters in Belgaum, 2009 – 2011
| Intervention | Control | ||||
|---|---|---|---|---|---|
| % | n | % | n | p-Value | |
| Clusters | 10 | 10 | |||
| Deliveries | 17,628 | 15,857 | 0.22 | ||
| Maternal education | 17,350 | 15,620 | 0.50 | ||
| No formal schooling | 24.7 | 4,283 | 21.5 | 3,362 | |
| Primary | 36.5 | 6,500 | 33.7 | 5,266 | |
| Secondary | 32.0 | 5,547 | 36.8 | 5,749 | |
| ≥University | 5.8 | 1,020 | 8.0 | 1,243 | |
| Maternal age | 17,587 | 15,809 | 0.88 | ||
| < 20 | 12.3 | 2,164 | 10.5 | 1,654 | |
| 20 – 35 | 87.5 | 15,387 | 89.3 | 14,121 | |
| > 35 | 0.2 | 36 | 0.2 | 34 | |
| Parity | 17,469 | 15,621 | 0.58 | ||
| 0 | 37.6 | 6,566 | 39.2 | 6,116 | |
| 1 – 4 | 61.6 | 10,755 | 60.1 | 9,384 | |
| > 4 | 0.8 | 148 | 0.8 | 121 | |
| Antenatal care | 17,627 | 15,856 | 0.33 | ||
| No antenatal care | 0.0 | 5 | 0.5 | 75 | |
| ≥1visit | 100.0 | 17,622 | 99.5 | 15,781 | |
Table 2.
Characteristics of Neonates in the Intervention and Control Clusters in Belgaum, 2009 – 2011
| Intervention | Control | ||||
|---|---|---|---|---|---|
| % | n | % | n | p-value | |
| Clusters | 10 | 10 | |||
| Deliveries | 17,754 | 15,953 | |||
| Birth weight | |||||
| 1000–1499g | 1.3 | 231 | 1.2 | 191 | 0.74 |
| ≥1500 g | 98.7 | 17,523 | 98.8 | 15,762 | |
| Gender | |||||
| Male | 51.7 | 9,167 | 53.0 | 8,458 | 0.03 |
| Female | 48.3 | 8,587 | 47.0 | 7,495 | |
| Multiple Births | |||||
| Yes | 1.4 | 249 | 1.2 | 191 | 0.19 |
| No | 98.6 | 17,505 | 98.8 | 15,762 | |
Based on information provided by the study participants, the levels of QOC were similar between the intervention and control clusters (Table 3). However several key indicators improved in both groups over time. From the baseline period to the final 6 months, identification of transportation prior to birth increased similarly in both groups: from 75% in intervention and 78% in control clusters to 87% and 98%, (p=0.04), respectively. Similarly, the Caesarean section rates increased from about 9% to about 13% in both groups. The rates of women who were delivered by a physician or nurse midwife increased from 87% in the intervention clusters and 85% in controls to about 94% and 93%, respectively, with a corresponding decrease in the use of traditional birth attendants for delivery, from 7% in the intervention clusters and 10% in the controls to about 3% in both groups.
Table 3.
Quality of Care Metrics: Baseline 6 Months and Last 6 Months of the Study in the Intervention and Control Groups
| Baseline 6 Months 4/1/2009 through 9/30/2009 |
Last 6 Months Study 4/1/2011 through 9/30/2011 |
|||||
|---|---|---|---|---|---|---|
|
| ||||||
| Intervention | Control | P-value | Intervention | Control | P-value | |
| Women (N) | 3,311 | 3,272 | 3,645 | 3,173 | ||
|
| ||||||
| Attended ≥1 ANC class | 86.9 | 94.3 | 0.21 | 86.5 | 99.8 | 0.06 |
| Had emergency fund/plan for hospitalization | 78.1 | 79.3 | 0.91 | 92.8 | 85.9 | 0.5 |
| Birth attendant identified prior to birth | 91.5 | 85.4 | 0.34 | 93.1 | 92.1 | 0.91 |
| Identified birth attendant assisted birth | 89.3 | 86.1 | 0.59 | 73.5 | 62.1 | 0.37 |
| Transport identified prior to birth | 74.9 | 77.9 | 0.8 | 87.1 | 98 | 0.04 |
| Delivery by cesarean section | 8.6 | 8.2 | 0.79 | 13.1 | 13.4 | 0.9 |
| Physician or nurse/midwife assisted deliveries | 86.8 | 85.2 | 0.66 | 93.5 | 93.4 | 0.96 |
| TBA deliveries | 6.9 | 10.4 | 0.18 | 3.3 | 3.2 | 0.92 |
| Facility deliveries | 84.2 | 84.1 | 0.98 | 92.8 | 93 | 0.91 |
| Clean razor used to cut cord | 99.1 | 98.4 | 0.32 | 99.7 | 99.6 | 0.61 |
| Birth attendant used new gloves | 95.8 | 96.6 | 0.66 | 96.3 | 97.8 | 0.38 |
| Resuscitated with bag and mask | 11.7 | 5 | 0.28 | 5.3 | 4 | 0.56 |
| Placed on mother’s chest after delivery | 66.7 | 63.5 | 0.8 | 68.6 | 58.1 | 0.53 |
| Bathed within 6 hours after delivery | 6.9 | 11.2 | 0.39 | 4.1 | 8.7 | 0.45 |
The intervention health facility assessments conducted every 6 months suggested that a lower proportion of hospitals and health centers had blood products for transfusion and availability of Caesarean sections and manual removal of the placenta at all times at a final assessment (July–September, 2011), compared with their status in August–September 2009 (Table 4). Facility QOC data also indicated a temporal decline in the availability of a physician to attend deliveries in the facilities that served the intervention populations. Fewer hospital facilities had a physician, always available to conduct deliveries at the final compared with baseline assessment, while the ‘always’ rates of nurse or midwives ‘always’ remained similar. While all intervention facilities had stethoscopes, blood pressure cuffs and resuscitation bag/masks throughout the study period, many of the health centers did not have such basic supplies as anti-hypertensives or antiseptics. Availability of misoprostol for prevention of postpartum haemorrhage increased from baseline to final assessment.
Table 4.
Hospitals and Health Centres: Availability of Staff and Services During the Baseline 6 Months and Last 6 Months of the Study in the Intervention Clusters Only
| Hospital % | Health Center % | |||
|---|---|---|---|---|
| Baseline1 | Final2 | Baseline1 | Final2 | |
| Facilities, N | 47 | 47 | 16 | 16 |
| Physician: Always | 94 | 78 | 73 | 62 |
| Sometimes | 6 | 22 | 0 | 23 |
| Never | 0 | 0 | 27 | 15 |
| Nurse: Always | 91 | 92 | 60 | 73 |
| Sometimes | 9 | 0 | 7 | 0 |
| Never | 0 | 8 | 33 | 27 |
| Antibiotics | 89 | 100 | 80 | 80 |
| Magnesium sulfate | 70 | 91 | 6 | 33 |
| Misoprostol | 85 | 96 | 19 | 50 |
| Oxytocics | 96 | 100 | 81 | 69 |
| Anti-hypertensives | 83 | 98 | 25 | 56 |
| Anesthetics | 85 | 77 | 25 | 19 |
| Antiseptic | 96 | 100 | 75 | 100 |
| Blood products | 24 | 17 | 6 | 13 |
| Dilation and curettage | 83 | 73 | 13 | 14 |
| Forceps/Suction | 57 | 70 | 13 | 7 |
| Cesarean Section | 89 | 84 | 13 | 7 |
| Manual removal of placenta | 55 | 71 | 0 | 7 |
Baseline reviews occurred August – September 2009.
Final reviews occurred July – September 2011.
In the intervention clusters, the PMR declined from 52.7/1,000 births (95% CI 42.9, 64.4) at baseline to 37.8/1,000 (95% CI 28.7, 49.6) during the last six months; the decline was smaller (from 47.9/1,000 births [95% CI 41.1, 55.7] to 44.2/1,000 [95% CI 32.1, 60.5]) in the control clusters (Figure 3). Neonatal mortality declined from 26.7/1,000 (95% CI 21.4, 33.1) live births to 18.4/1,000 (95% CI 14.4, 23.9) in the intervention group whereas the control group experienced a small increase from 21.2/1,000 (95% CI 16.8, 28.9) live births to 24.1/1,000 (95% CI 17.3, 33.4) live births over the same period. The relative decreases in PMR and NMR from baseline to the last 6 months of the intervention period were larger in the intervention clusters than in the control clusters (Figure 3); however, this decrease did not reach statistical significance for PMR (OR=0.74, 95% CI 0.67 – 1.19, p=.20, adjusted for cluster) nor for NMR (OR=0.60, 95% CI 0.34 – 1.06, p=0.08, adjusted for cluster).
Figure 3.
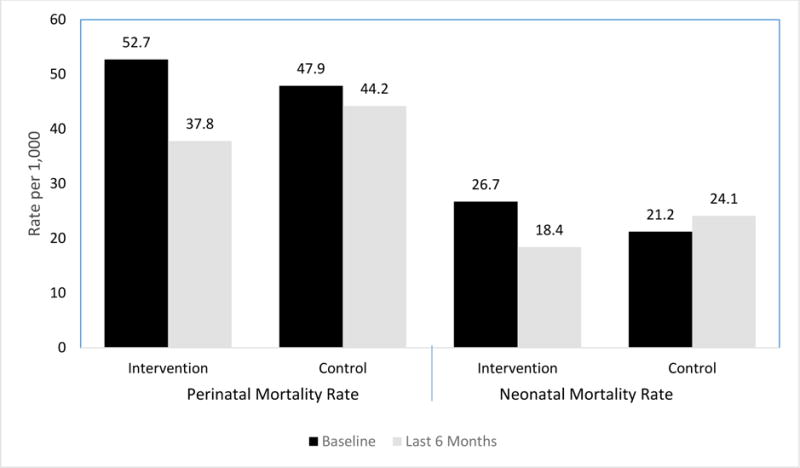
Comparison of Perinatal Mortality Rate (PMR) and Neonatal Mortality Rate (PMR) for Intervention and Control clusters at Baseline and Last 6 months of the Trial Period*
*Relative change (Intervention Δ vs Control Δ) in perinatal mortality rate (PMR) OR = 0.74 (0.46, 1.19), P=0.20
Relative change in neonatal mortality rate (NMR) OR = 0.60 (0.34, 1.06), P=0.08
Conclusions
This cluster-randomized trial of a composite package of interventions implemented to improve birth outcomes in the community and across the continuum of care suggested improvement in neonatal survival, when comparing the results at baseline to those during the final 6-month period of the study in rural Belgaum, India. Both intervention groups had increasing rates of Caesarean section, transportation for delivery, and facility delivery over the intervention period. During the same period, the Ministry of Health implemented programs, including implementation of cash-transfers for facility births as well as ambulance services in the region, which were beyond the scope of the study intervention [20, 21]. We posit that the increase in transportation and facility delivery led to higher rates of Caesarean delivery that may have improved neonatal survival and the stillbirth rate and hence perinatal survival. The intervention did not measurably improve the QOC compared to the control clusters (for example, only 60 to 70% of either group identified a birth attendant). Furthermore, the assessment of health facilities suggested that with a few exceptions, overall, there were similar or decreasing availability of services over the course of the trial; while training was a component of the intervention, provision of supplies and equipment for health facilities was not.
This study was designed as a part of a larger multi-site trial that had limited power to detect the effects of the community mobilization and increased use of institutional obstetric care for maternal and newborn complications within individual Global Network sites. The PMR and NMR risk reductions of 30% to 40%, respectively, in the intervention clusters found in Belgaum suggest that even with limited influence on QOC, in the context of increasing institutional delivery these interventions may be associated with improvements in neonatal and perinatal survival rates that have important public health implications. The study observed an impressive increase in rate of facility births; however, while rates of Cesarean section rates increased over the trial period, indicators of supplies and services available at hospitals and health centers did not change or appeared to decline. Thus, while most women are now reaching health facilities for delivery in this region, additional efforts to improve QOC at health facilities are needed to further improve perinatal and newborn outcomes. It is likely that even though the Cesarean section rates increased over time in both intervention and control clusters, through community mobilization efforts in the intervention clusters, women were able to identify and access emergency obstetric care services in a timely manner by avoiding seeking care at referral facilities that are expected to provide but lack such services. This may explain the differential impact on mortality in the intervention clusters compared to the control clusters.
Other studies of intervention packages that include community mobilization and quality improvement activities have shown increased use of evidence-based newborn care practices associated with improvements in neonatal survival, significantly reducing NMR by 30% in rural Nepal,8 by 34% in Bangladesh, by nearly 50% in Uttar Pradesh, India, 30% in Pakistan, and 33% in Gambia [9,10,12]. These studies primarily focused on community-based strategies to improve newborn care. For example, in Uttar Pradesh, community health workers were trained to provide essential newborn care for a population that primarily delivered at home [9]. These studies examined basic newborn care and 28-day neonatal outcomes, rather than inclusion of early pregnancy loss. Furthermore, in each of these examples, rates of ANC use were significantly lower than our study (for example, 25% ANC in Bangladesh compared to over 90% at baseline in this Belgaum study) and the majority of the deliveries occurred at home or community settings. Other community-based intervention studies have focused on obstetric care and its impact on maternal and perinatal mortality. One study training birth attendants to better identify and refer women with pregnancy complications found a 68% lower PMR (p=.005) in Bolivia whereas another study found a 27% lower PMR but not significant in Guatemala, settings where home or community deliveries were the norm [11,13]. Since these studies, there have been global efforts that have significantly increased rates of facility delivery in many regions, especially in countries such as India [15].
One of the strengths of this trial was the high-quality maternal-newborn registry that independently registered nearly all pregnant women by 16 weeks gestation. The registry was cross-checked and validated with other sources (e.g., registry of couples expected to conceive) to ensure completeness of enrolment. All pregnant women had their outcomes followed to 6 –weeks postpartum, with nearly 100% outcomes obtained. Furthermore, the community intervention team had a high fidelity to the study intervention, with all cluster community mobilization activities implemented early, with high levels of participation of mothers and birth attendants.
Because of the increasing role of facility delivery, we included facility birth attendant training in the community intervention. Our trial examines the impact of community mobilization and birth attendant training across all levels of care in an emerging context of increased rates of institutional delivery. In Karnataka, where facility deliveries have substantially increased in both groups, we observed a much larger reduction in the rates of neonatal mortality in the intervention compared to control, although these did not reach statistical significance. The increased rates of facility births, availability of transport and access to Cesarean section for both intervention and control clusters may have overwhelmed the differences in effect on mortality from the community mobilization efforts. For example, anecdotally, women, particularly those requiring emergency care, in the intervention clusters may have been more likely to reach the appropriate level of care (e.g., by-passing first level health service in case of emergency), but we were unable to document this. One limitation was that although a large number of women were enrolled, the trial was cluster randomized and thus not powered to detect site-specific effects. Despite this, the study’s results demonstrate large differential reductions in PMR and NMR in the intervention clusters compared to the controls that are important for programmatic planning.
Another limitation of the study is that it can be difficult to determine the elements of a comprehensive strategy such as our intervention that are most effective. The fact that there was a large increase in facility deliveries while there was little evidence of improvement in QOC supports the suggestion that the observed effects are likely due to community mobilization efforts leading to identification of appropriate referral facilities and accessing emergency obstetric care at these facilities in a timely manner. While we have anecdotal observations to support this speculation, the data collection did not capture these subtle nuances resulting from the community mobilization efforts. This line of reasoning is also consistent with other studies in India and beyond that find increasing institutional delivery and Caesarean sections rates to be associated with improving perinatal and/or neonatal survival. [20, 21]
Finally, this multi-site study was not independently powered to detect site specific difference in the outcomes. [18] The results of the trial in the Belgaum site are distinct from the multi-site trial and this difference may be due to differences in the implementation of the intervention that may have happened because of the differences in settings and availability of resources such as institutional capacity, attitudes towards seeking institutional care and ability to get to institutional care.
Acknowledgments
This study was funded through grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U01 HD040636; U10 HD076457; U10 HD078438).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- 1.Lozano R, Wang H, Foreman KJ, Rajaratnam JK, Naghavi M, Marcus JR, Dwyer-Lindgren L, Lofgren KT, Phillips D, Atkinson C, Lopez AD, Murray CJ. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet. 2011;378(9797):1139–65. doi: 10.1016/S0140-6736(11)61337-8. [DOI] [PubMed] [Google Scholar]
- 2.Bhutta ZA, Darmstadt GL, Hasan BS, Haws RA. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics. 2005 Feb;115(2 Suppl):519–617. doi: 10.1542/peds.2004-1441. [DOI] [PubMed] [Google Scholar]
- 3.Lassi ZS, Haider BA, Bhutta ZA. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database of Systematic Reviews. 2010;11:CD00754. doi: 10.1002/14651858.CD007754.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Campbell OM, Graham WJ, Lancet Maternal Survival Series steering group Strategies for reducing maternal mortality: getting on with what works. Lancet. 2006;368(9543):1284–1299. [Google Scholar]
- 5.Moss W, Darmstadt GL, Marsh DR, Black RE, Santosham M. Research priorities for the reduction of perinatal and neonatal morbidity and mortality in developing country communities. J Perinatol. 2002;22(6):484–95. doi: 10.1038/sj.jp.7210743. [DOI] [PubMed] [Google Scholar]
- 6.Ahluwalia IB. An evaluation of a community-based approach to safe motherhood in northwestern Tanzania. International Journal of Gynecology and Obstetrics. 2003;82:231–42. doi: 10.1016/s0020-7292(03)00081-x. [DOI] [PubMed] [Google Scholar]
- 7.Gazi R, Hossain SS, Zaman K, Koehlmoos TP. Community mobilization for safe motherhood. Cochrane Database of Systematic Reviews. 2011;(Issue 4) Art. No.: CD009091. [Google Scholar]
- 8.Manandhar DS, Osrin D, Shrestha BP, Mesko N, Morrison J, Tumbahangphe KM, et al. Effect of a participatory intervention with women’s groups on birth outcomes in Nepal: cluster randomized controlled trial. Lancet. 2004;364(9438):970–9. doi: 10.1016/S0140-6736(04)17021-9. [DOI] [PubMed] [Google Scholar]
- 9.Kumar V, Mohanty S, Kumar A, Misra RP, Santosham M, Awasthi S, et al. Effect of community-based behaviour change management on neonatal mortality in Shivgarh, Uttar Pradesh, India: a cluster-randomised controlled trial. Lancet. 2008 Sep 27;372(9644):1151–62. doi: 10.1016/S0140-6736(08)61483-X. [DOI] [PubMed] [Google Scholar]
- 10.Bhutta Z, Memon Z, Soofi S, Salat M, Cousens S, et al. Implementing community-based perinatal care: results from pilot study in rural Pakistan. Bull World Health Organ. 2008;86:452–459. doi: 10.2471/BLT.07.045849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Rourke K, Howard-Grabman L, Seoane G. Impact of community organization of women on perinatal outcomes in rural Bolivia. Rev Panam Salud Publica. 1998;3:9–14. doi: 10.1590/s1020-49891998000100002. [DOI] [PubMed] [Google Scholar]
- 12.Greenwood A, Bradley A, Byass P, et al. Evaluation of a primary health care programme in The Gambia. I. The impact of trained traditional birth attendants on the outcome of pregnancy. J Trop Med Hyg. 1990;93:58–66.13. [PubMed] [Google Scholar]
- 13.O’Rourke K. The effect of hospital staff training on management of obstetrical patients referred by traditional birth attendants. Int J Gynaecol Obstet. 1995;(48 suppl):S95–S102. doi: 10.1016/0020-7292(95)02324-6. [DOI] [PubMed] [Google Scholar]
- 14.Pasha O, Goldenberg RL, McClure EM, Saleem S, Goudar SS, Althabe F, et al. Communities, birth attendants and health facilities: a continuum of emergency maternal and newborn care (the Global Network’s EmONC trial) BMC Pregnancy Childbirth. 2010 Dec 14;10:82. doi: 10.1186/1471-2393-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasha O, McClure EM, Wright LL, Saleem S, Goudar SS, Chomba E, et al. A combined community- and facility-based approach to improve pregnancy outcomes in low-resource settings: a Global Network cluster randomized trial. BMC Med. 2013;11(1):215. doi: 10.1186/1741-7015-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goudar SS, Carlo WA, McClure EM, Pasha O, Patel A, Esamai F, et al. The Maternal and Newborn Health Registry Study of the Global Network for Women’s and Children’s Health Research. Int J Gynaecol Obstet. 2012;118(3):190–3. doi: 10.1016/j.ijgo.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manasyan A, Saleem S, Koso-Thomas M, Althabe F, Pasha O, Chomba E, et al. EmONC Trial Group Assessment of obstetric and neonatal health services in developing country health facilities. Am J Perinatol. 2013;30(9):787–94. doi: 10.1055/s-0032-1333409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baqui AH, El-Arifeen S, Darmstadt GL, Ahmed S, Williams EK, Seraji HR, et al. Projahnmo Study Group Effect of community-based newborn-care intervention package implemented through two service-delivery strategies in Sylhet district, Bangladesh: a cluster-randomised controlled trial. Lancet. 2008;371(9628):1936–44. doi: 10.1016/S0140-6736(08)60835-1. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO Child Growth Standards: Length/Height-for-age, Weight-for-age, Weight-for-length, Weight-for-height and Body Mass Index-for age. WHO; Geneva: 2006. [Google Scholar]
- 20.Lim SS, Dandona L, Hoisington JA, James SL, Hogan MC, Gakidou E. India’s Janani Suraksha Yojana, a conditional cash transfer programme to increase births in health facilities: an impact evaluation. Lancet. 2010;375(9730):2009–23. doi: 10.1016/S0140-6736(10)60744-1. [DOI] [PubMed] [Google Scholar]
- 21.Lagarde M, Haines A, Palmer N. The impact of conditional cash transfers on health outcomes and use of health services in low and middle income countries. Cochrane Database of Systematic Reviews. 2009;(Issue 4) doi: 10.1002/14651858.CD008137. Art. No.: CD008137. [DOI] [PMC free article] [PubMed] [Google Scholar]


