Introduction
With the rise in popularity of refractive surgery, there is an increasing number of patients with a history of laser vision correction that need cataract surgery. This population presents unique challenges to the cataract surgeon due to high expectations for excellent visual outcomes and spectacle independence and the difficulty of intraocular lens power (IOL) prediction. This chapter will discuss sources of error in IOL power determination in patients with a history of refractive surgery as well as new methods developed to improve accuracy.
Background
To determine optimal intraocular lens power, several pre-operative measurements are needed. These include at a minimum measuring axial length and corneal power, and when using newer generation formulas, anterior chamber depth and horizontal corneal diameter are also considered. In most cases these measurements can be obtained using optical coherence biometry and the values placed into regression formulas using optimized lens A constants to determine IOL power. In naïve corneas, traditional formulas such as the Holiday 1 and SRK/T do an excellent job in predicting post-surgical emmetropia. In most studies, a refractive error within 0.5 D of emmetropia was achieved in 70% of individuals.1 In post refractive eyes, however, these formulas are known to be inaccurate as both measurements of corneal curvature and regression formulas have sources which contribute to error in patients with a history of prior refractive surgery. In general, traditional IOL formulas usually result in a hyperopic surprise when applied to patients status post myopic correction and in a myopic surprise when applied to patients status post hyperopic correction.2–5
Sources of Error
A. Corneal Power
The main cause for refractive error in IOL prediction lies in the difficulty to accurately measure corneal power after refractive surgery. The general principle in keratometry is that the cornea acts as a convex mirror reflecting the light source off its surface producing a virtual image. The position and size of this image is measured and, as the light source size and distance to the cornea is known, the radius of the cornea can be calculated. Both manual and automated keratometers measure the intermediate areas around the central cornea and the central corneal power is then calculated. In manual keratometry, the reflected mires are approximately 3mm and in automated keratometry the reflections are at a 2.5mm optical zone. Thus, while corneal measurements are taken, the corneal power given is derived from calculations with assumptions of the corneal index and relationship of the anterior and posterior cornea. To measure true corneal power both the anterior and posterior surface must be considered.
The above methods of corneal power estimation are less accurate in an eye with refractive surgery for several reasons. First, corneal measurements are taken at a 2.5mm or a 3.0mm optical zone. However, after myopic (or hyperopic) ablation the power of the central cornea is flatter (or steeper) than the measured central power, respectively.2,3 In addition, the assumption of a sphero-cylindrical cornea leads to an overestimation of corneal power by 15–25% leading to a hyperopic outcome after myopic ablation and a myopic outcome after hyperopic ablation.3–5 And finally, by ablating the corneal surface the relationship between the anterior and posterior corneal curvature is changed after refractive surgery, thus the assumption of a refractive index of 1.3375 is no longer accurate.
B. Formula Error
One of the most common regression formulas used for IOL power estimation is the SRK formula, which is:
Where P is the dioptric power of the IOL, L is the axial length of the eye (mm), K is the average corneal power (K) and A represents a constant specified for the type of lens. Newer generation formulas (Holladay I, SRK/T, and Hoffer Q) also utilize the relationship between the steepness of the cornea and the anterior chamber depth to estimate the effective lens position (ELP). However, after a myopic ablation the formulas predict a falsely shallower anterior chamber depth and thus a more anterior effective lens position. This ultimately results in an underestimation of IOL power partially contributing to the hyperopic surprise. Conversely, after hyperopic ablation, these formulas predict a falsely deeper effective lens position is estimated resulting in an overestimated lens power with subsequent myopic surprise.
Newer Techniques/Formulas
Given these challenges, newer techniques and formulas have been applied to post refractive eyes in attempts to improve outcomes after cataract surgery.
A. The historical method
The historical method of keratometry was first described by Holladay et al. to determine corneal power in patients with a history of radial keratotomy.6 This method relies on the patients’ pre-refractive surgery corneal power and spherical equivalent in combination with their post-refractive surgery spherical equivalent.6,7
It remains important to note this method provides only an estimation of corneal power which can then be used in regression formulas for IOL power prediction. Argento et al. compared the predictability of various methods of IOL power calculation in 7 cases (6 post-LASIK eyes and 1 post–radial keratotomy eye) using the Holladay 2, Hoffer Q, and SRK/T formulas and found that the clinical history method with the Hoffer Q formula provided the best results with a mean dioptric error of −0.98 +/− 0.87.9 While this may be more effective than standard methods of IOL prediction in patients with a history of refractive surgery, subsequent studies have shown large variations of IOL power prediction and large refractive errors still occur.6–9
B. Double-K Method
Aramberri described the double-k method to account for the IOL formulas use of corneal power to determine ELP.10 Similar to the clinical historical method, the double-k method utilizes pre-refractive surgery data to obtain the post-refractive surgery corneal power. However, the double-k method also relies on pre-refractive surgery corneal power data to determine the ELP. The Holladay 2 formula allows direct entry of 2 corneal power values for the double-k calculation. Subsequent studies have shown greater accuracy of IOL power prediction in third and fourth generation formulas when utilizing the double K method.11
C. ASCRS online
A commonly used tool from the website of the American Society of Cataract and Refractive surgery is the IOL power calculating tool. From this site, the type of refractive surgery is selected and then all known pre-operative and current data is entered. The IOL power is calculated by a variety of formulas, both those requiring historical data and those that do not. All predicted IOL powers are displayed when data required for them are entered, as well as an average of all available formulas. This remains a useful tool in estimation of IOL power calculation and can be utilized at http://iolcalc.org.
D. Tomography
With the advent of devices that can measure the tomography of the cornea with scheimflug images or fourier domain optical coherence tomography (OCT), the anterior and posterior surface powers can be more accurately calculated using Gaussian optics. Thus, a benefit of this methodology includes the possible elimination of regression formulas.12,13 Fourier domain optical coherence tomography (OCT) carries the advantage of higher resolution and speed of image acquisition to traditional scheimflug images, thereby decreasing potential motion artifact. However, no difference in predictive accuracy of scheimflug and OCT based IOL calculations have been demonstrated.14
E. Intraoperative Aberrometry
Intraoperative aberrometry is a relatively new technique for IOL power determination that’s shown promise in patients with a history of refractive surgery.15 This technique employs Talbot-Moire wavefront aberrometry to measure the refraction of the entire optical system in an aphakic eye during surgery. The independence of historical or even pre-operative data is an advantage to previously discussed methodologies. Recent studies found this to be statistically better at IOL power prediction to several techniques and can reach target refraction in similar percentages to virgin eyes undergoing cataract surgery.16–18 Even this technique has its limitations however, as aphakic measurements must be taken in the operating room, which can be altered depending on intraocular pressure, patient fixation, and external pressure from the speculum. In addition, determining the effective lens position still remains a source of error.
F. Pre-operative Planning
Given the imprecise nature of IOL prediction in eyes with a history of refractive surgery, several methods are typically employed. It is recommended to review the IOL prediction using several techniques and eliminate any outlier values. Even with intraoperative aberrometry it would be advisable to obtain multiple methods for IOL selection to minimize refractive surprise. Some surgeons will aim for slight myopia to avoid a hyperopic outcome in patients with a history of myopic procedures, however it’s important to note that in patients with previous high myopic ablations it may be easier to perform a hyperopic “touch up” ablation in a centrally thinned cornea.
Operative Considerations
A. Radial Keratotomy
Patients with previous radial keratotomy (RK) surgery have some intraoperative considerations to minimize post-operative complications. First, all incisions should be made in between the RK incisions to avoid wound dehiscence or leaks and to prevent irregular astigmatism post-operatively. However, some patients may have greater than 8 RK incisions and in this scenario, a scleral tunnel approach may be beneficial. Finally, RK incisions are weak points in the cornea and can open during surgery using standard phacoemulsification settings. Surgeons should be prepared to suture incisions and should consider lowering phacoemulsification parameters to minimize operative complications. Patients should be counseled at length regarding the risk of irregular astigmatism, and hyperopic drift depending on corneal stability.
B. LASIK/PRK
In patients with a history of LASIK the flap and hinge position should be determined and accounted for when planning surgical incisions. It remains important to not disrupt the flap during surgery as to keep the risk of epithelial ingrowth at a minimum. In the case of PRK, it’s important to note the degree of haze, if any pre-operatively. Given a potential for limited view during cataract surgery, trypan blue could be used to aid in visualization of the anterior capsule to make a capsulorrhexis.
Management of Refractive Error
A. Manage Expectations
The need to discuss potential refractive errors and expectations after cataract surgery cannot be overstated. Many patients will recall the speed of recovery and the freedom of corrective lenses after their refractive surgery and may expect the same results if not counseled appropriately. Patients may also request presbyopia correcting IOL’s without fully understanding the risk of glares associated with them, which could be especially problematic given their increased higher order aberrations from previous refractive surgery. In this scenario it may be beneficial to use IOL’s with no aberration or with negative spherical aberration.
B. Managing Post-operative Refractive Error
Despite the new advances in technology for IOL selection, patients may still have post-operative refractive errors. Small refractive errors can be managed with contact lenses or glasses if the patient is amendable to these therapies. Alternatively, for small amounts of sphero-cylindrical error, PRK or LASIK can be offered to reach the desired target refraction. For larger amounts of error, or in eyes where further corneal surgery would be contraindicated, piggyback IOL or even IOL exchange can be performed. Patients electing for a piggyback IOL or IOL exchange should be counseled regarding the risk of glaucoma, inflammation, and retinal tears.
Case presentations
The following cases highlight some of the challenges in IOL calculations when planning cataract surgery in post-refractive eyes.
Case 1
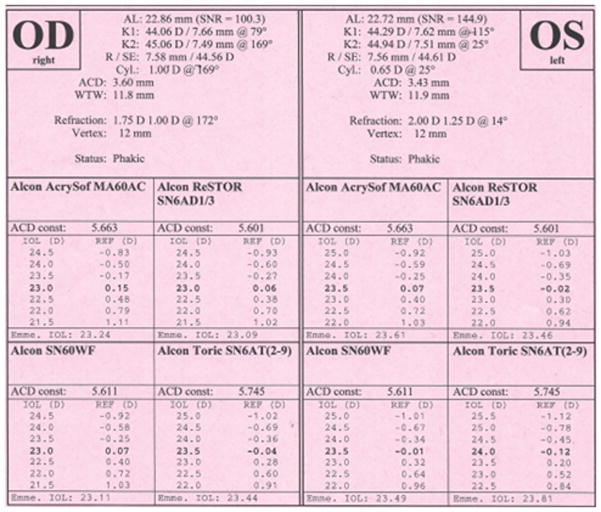
A 62 year old white male with a history of radial keratometry (RK) many years prior presented for cataract evaluation (Figure 1). Past ocular history was also significant for vitrectomy and scleral buckle surgery 6 months prior in the setting of a macula off retinal detachment with residual macular edema detected by ocular coherence tomography. Pre-operative BCVA was 20/400 with a 3+ NS and 3+ posterior subcapsular cataract. Pre-operative lens calculations with the ASCRS calculator were between 17 and 21 D (average 19D), with the Pentacam (Oculus, Arlington, WA) keratometry value in the SRK/T formula was 19.5D, and with straight SRK/T was 16.5D. Based on this information, the pre-operative plan was to place an 18.5D one piece acrylic lens into the capsular bag and to titrate this measurement with intraoperative wavefront aberrometry (ORA, Alcon, Fort Worth, Texas). Intra-operatively, bottle height, aspiration, and vacuum setting were all lowered to decrease the risk of inadvertent opening of the RK incisions. After cataract removal, the eye was prepared for ORA use by first filling the capsular bag and then the anterior chamber with viscoelastic until a pressure of 20–25 mm Hg was obtained. The eye was then kept well lubricated while 2 repeat measurements were taken with the ORA (Figure 2). The ORA suggested a 17 D lens for emmetropia and based on all measurement, an 18D single piece acrylic lens was placed in the capsular bag. To treat residual macular edema, an intravitreal triamcinolone injection was also given at the end of the case. Post-operative day 1, visual acuity was 20/400 due to a residual fibrotic capsule that could not be removed at the time of surgery. Post-operative month 3, after YAG capsulotomy and 2 additional triamcinolone injections, BCVA was 20/80 with minimal refractive error (0.50+0.50X075).
Figure 1.
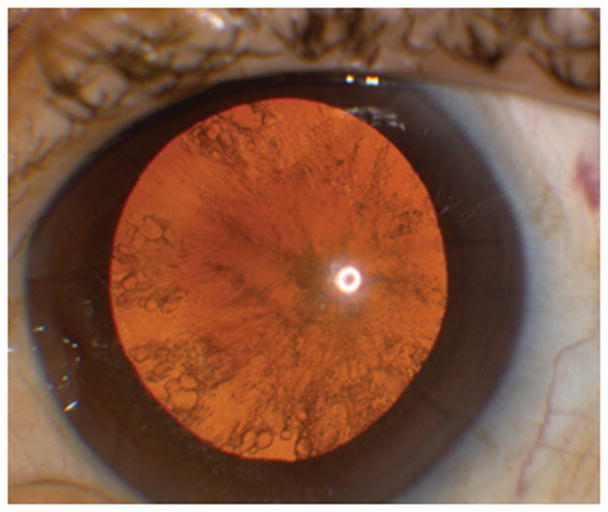
Posterior subcapsular cataract with radial keratotomy (RK) incisions in the cornea
Figure 2.
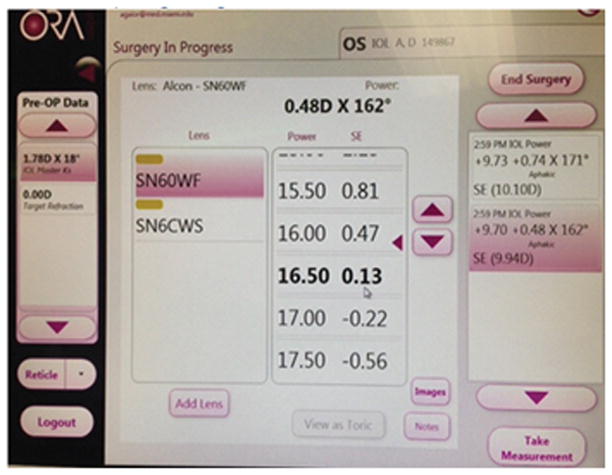
Intraoperative aberrometry screen suggesting intraocular lens power of 17.0D with a refractive error of −0.22.
Case 2
A patient with a history of RK presented to clinic for a cataract evaluation. Past ocular history was significant for a previous gasoline related trauma to the right eye. She presented with 20/50 vision in the affected eye with a refraction of −2.25+1.00×025. On examination, she was noted to have mild dryness of the ocular surface with 8 RK incisions as well as 2 astigmatic keratotomy (AK) incisions. She was also noted to have a 3+ nuclear sclerotic cataract and the retinal examination was within normal limits. The average values obtain from the automated keratometer was 41.25D and those obtained from the pentacam (Oculus, Arlington, WA) were 41.5D. The SRK/T formula from the biometry obtained suggested a 23.5 power acrylic lens for an approximate refractive error of −0.5D. The decision was made to proceed to surgery with the plan to use the ORA intraoperative aberrometer to titrate the results. The main incision was made between two RK incisions to avoid splaying of the wound. However, intraoperatively the RK incision opened during phacoemulsification (Figure 3). Phacoemulsification was still performed successfully, however given the anterior chamber instability intraoperative aberrometry could not be accurately performed. Decision was made to proceed with implantation of the lens power as predicted by pre-operative methodology, however upon insertion of the lens a crack was noted just off center (Figure 4). Given its clearance of the visual axis and the risk associated with explanation of the IOL in an eye with a splayed RK incision and reduced visualization (due to corneal edema), it was decided to leave the IOL in place (Figure 5). In the immediate post-operative period the patient had corneal edema which ultimately resolved. The final refraction was −0.25+0.50×180 with an uncorrected visual acuity of 20/25.
Figure 3.
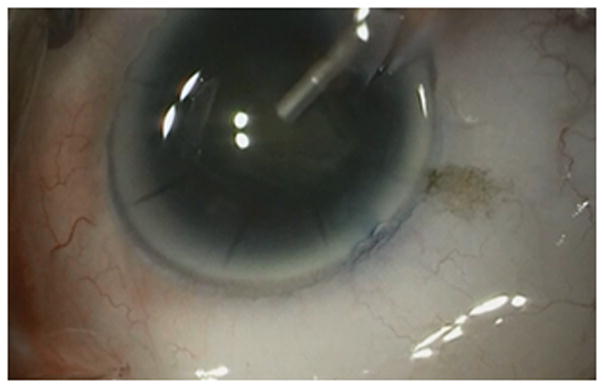
Radial keratotomy (RK) incision splay during phacoemulsification.
Figure 4.
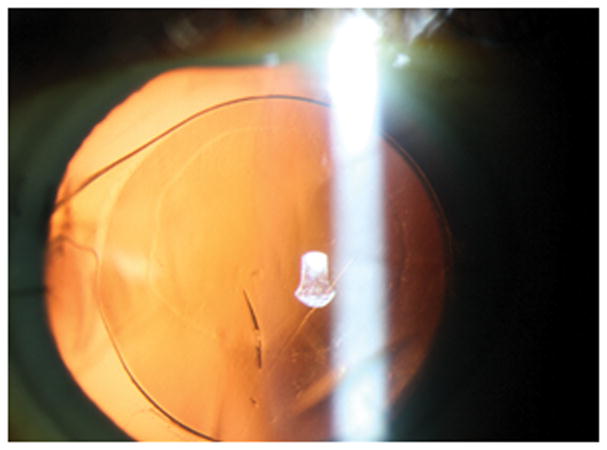
Red reflex highlighting mark on intraocular lens at post-operative day 1 visit.
Figure 5.
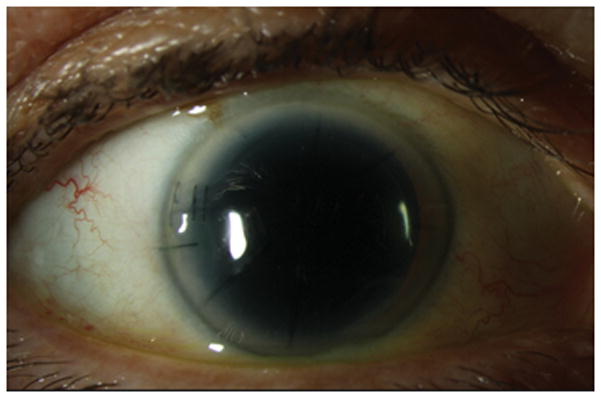
External Slit lamp photograph with sutured main incision and RK wound.
Case 3
A 68 year old female presented to clinic for cataract surgery evaluation of the left eye. The patient had a history of hyperopic LASIK performed several years prior. On initial examination her visual acuity was best corrected 20/40 in her left eye with a refraction of +2.50 +0.50×010. On examination she was noted to have a well healed LASIK flap and a 2+ nuclear sclerotic cataract with cortical changes. On the IOL master (Figure 6), the patients’ keratometry values were 44.29 and 44.94. The SRK/T formula predicted an acrylic lens powers of 23.5 for a predicted refractive error of −0.01. Given the known error of keratometry measurements post LASIK the decision was made to place a 24.5 power lens to err on the side of myopia. The patient underwent successful cataract extraction with posterior chamber intraocular lens implantation. At the 1 month postoperative exam, the patient was noted to have significant myopic surprise with a refraction of −2.00 +0.50×125. The patient preferred distance vision and thus it was decided to proceed with advanced surface ablation to treat the residual myopia. At the 1 month post-operative period the patient’s unaided visual acuity was 20/25 with a refraction of −0.50 +0.25×105 and the patient was happy with her refractive outcome.
Figure 6.
IOL Master using SRK/T formula for lens power prediction.
Acknowledgments
Financial Support:
Supported by Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development’s Career Development Award CDA-2-024-10S (Dr. Galor), NIH Center Core Grant P30EY014801 and Research to Prevent Blindness Unrestricted Grant NIH Center Core Grant P30EY014801, Research to Prevent Blindness Unrestricted Grant, Department of Defense (DOD-Grant#W81XWH-09-1-0675). The Ronald and Alicia Lepke Grant, The Lee and Claire Hager Grant, The Jimmy and Gaye Bryan Grant.
Footnotes
Conflict of interest: None
References
- 1.Aristodemou P, Knox Cartwright N, Sparrow J, et al. Formula Choice: Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8018 eyes after cataract surgery with biometry with partial coherence interferometry. J Cataract Refract Surg. 2011;37:63–71. doi: 10.1016/j.jcrs.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 2.Seitz B, Langenbucher A. Intraocular lens power calculation in eyes after corneal refractive surgery. J Refract Surg. 2000;16(3):349–61. doi: 10.3928/1081-597X-20000501-09. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Jackson DW, Koch DD. Methods of estimating corneal refractive power after hyperopic laser in situ keratomileusis. J Cataract Refract Surg. 2002;28(6):954–61. doi: 10.1016/s0886-3350(02)01318-4. [DOI] [PubMed] [Google Scholar]
- 4.Awwad ST, Manasseh C, Bowman RW, et al. Intraocular lens power calculation after myopic laser in situ keratomileusis: estimating the corneal refractive power. J Cataract Refract Surg. 2008;34(7):1070–6. doi: 10.1016/j.jcrs.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 5.Latkany RA, Chokoshi AR, Speaker MG, et al. Intraocular lens calculation after refarctive surgery. J Cataract Refract Surg. 2005;31:562–770. doi: 10.1016/j.jcrs.2004.06.053. [DOI] [PubMed] [Google Scholar]
- 6.Holladay JT. Consultations in refractive surgery. J Refract Corneal Surg. 1989;5:203. [Google Scholar]
- 7.Hoffer KJ. Intraocular lens power calculation for eyes after refractive keratotomy. J Refract Surg. 1995;11:490–3. doi: 10.3928/1081-597X-19951101-17. [DOI] [PubMed] [Google Scholar]
- 8.Gimbel HV, Sun R. Accuracy and predictability of intraocular lens power calculation after laser in situ keratomileusis. J Cataract Refract Surg. 2001;27:571–6. doi: 10.1016/s0886-3350(00)00795-1. [DOI] [PubMed] [Google Scholar]
- 9.Argento C, Cosentino MJ, Badoza D. Intraocular lens power calculation after refractive surgery. J Cataract Refract Surg. 2003;29:1346–51. doi: 10.1016/s0886-3350(03)00351-1. [DOI] [PubMed] [Google Scholar]
- 10.Aramberri J. Intraocular lens power calculation of corneal refractive surgery: double-K method. J Cataract Refract Surg. 2003;29:2063–8. doi: 10.1016/s0886-3350(03)00957-x. [DOI] [PubMed] [Google Scholar]
- 11.Koch DD, Wand L. Calculating IOL power in eyes that have had refractive surgery. J Cataract Refract Surg. 2003;29:2039–2042. doi: 10.1016/j.jcrs.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Booth MA, Koch DD. Comparison of intraocular lens power calculation methods in eyes that have undergone LASIK. Ophthalmology. 2004;111(10):1825–31. doi: 10.1016/j.ophtha.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 13.Borasio E, Stevens J, Smith GT. Estimation of true corneal power after keratorefractive surgery in eyes requiring cataract surgery: BESSt formula. J Cataract Refract Surg. 2006;32(12):2004–14. doi: 10.1016/j.jcrs.2006.08.037. [DOI] [PubMed] [Google Scholar]
- 14.Tang M, Wang L, Koch DD, Li Y, Huang D. Intraocular lens power calculation after previous myopic laser vision correction based on corneal power measured by Fourier-domain optical coherence tomography. J Cataract Refract Surg. 2012;38(4):589–94. doi: 10.1016/j.jcrs.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ianchulev T, Salz J, Hoffer K, et al. Intraoperative optical refractive biometry for intraocular lens power estimation without axial length and keratometry measurements. J Cataract Refract Surg. 2005;31:1530–6. doi: 10.1016/j.jcrs.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 16.Canto AP, Chhadva P, Cabot F, et al. Comparison of IOL power calculation methods and intraoperative wavefront aberrometry in eyes after refractive surgery. J Refract Surg. 2013;29:484–9. doi: 10.3928/1081597X-20130617-07. [DOI] [PubMed] [Google Scholar]
- 17.Fram N, Masket S, Wang L. Comparison of intraoperative aberrometry, OCT-based IOL formula, Haigis-L, and Maskey formulae for IOL power calculation after laser vision correction. Ophthalmology. 2015;122:1096–1101. doi: 10.1016/j.ophtha.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 18.Ianchulev T, Hoffer K, Yoo S, et al. Intraoperative refractive biometry for predicting intraocular lens power calculation after prior myopic refractive surgery. Ophthalmology. 2014;121:56–60. doi: 10.1016/j.ophtha.2013.08.041. [DOI] [PubMed] [Google Scholar]