Abstract
Much has changed since our survey of the landscape for myocardial regeneration powered by adult stem cells four years ago (Mohsin et al., Empowering adult stem cells for myocardial regeneration. Circ Res. 2011; 109(12):1415–1428) [1]. The intervening years since that first review has witnessed an explosive expansion of studies that advance both understanding and implementation of adult stem cells in promoting myocardial repair. Painstaking research from innumerable laboratories throughout the world is prying open doors that may lead to restoration of myocardial structure and function in the wake of pathologic injury. This global effort has produced deeper mechanistic comprehension coupled with an evolving appreciation for the complexity of myocardial regeneration in the adult context. Undaunted by both known and (as yet) unknown challenges, pursuit of myocardial regenerative medicine mediated by adult stem cell therapy has gathered momentum fueled by tantalizing clues and visionary goals. This concise review takes a somewhat different perspective than our initial treatise, taking stock of the business sector that has become an integral part of the field while concurrently updating “state of affairs” in cutting edge research. Looking retrospectively at advancement over the years as all reviews eventually must, the fundamental lesson to be learned is best explained by Jonatan Mårtensson: “Success will never be a big step in the future. Success is a small step taken just now.”
Keywords: Adult stem cells, therapy, cardiovascular disease, clinical trial
I. INTRODUCTION
Myocardial aging is a constellation of concurrent processes occurring at organ, cellular, and molecular levels. Despite this complexity, differences become readily identifiable by comparing phenotypic characteristics of mammalian cardiomyocytes in young versus old hearts. One hallmark unique to the early postnatal myocardium is increased cellular proliferation resulting in formation of new myocytes. Although research is ongoing to identify when proliferation subsides in the postnatal myocardium, current findings indicate the murine heart experiences a proliferative burst around two weeks [2], whereas the human heart experiences proliferation in smaller growth spurts throughout adolescence [3]. It is hypothesized that the regenerative capacity of the neonatal mammalian heart is dependent on the sympathetic nerve and poorly functioning nerve structure in adult myocardium impairs the heart’s capability to regenerate [4]. Later in life, de novo myocyte formation in mammals is severely limited by the sub-optimal milieu of struggling myocardium and accumulation of poorly functioning senescent myocytes. Senescence also exacts a toll upon the cardiac stem cell population that precipitates a loss of regenerative capacity. These multi-faceted issues developing from age and injury lead to reduced contractility and cardiac output, culminating in cardiovascular disease (CVD).
Simultaneously, CVD develops not only with age, but chronic disease (diabetes mellitus, obesity, hypertension), habitual stressors (smoking, poor diet, alcoholism) or genetic predisposition. Consequently, CVD is the leading cause of morbidity and mortality worldwide with approximately 400,000 new cases per year (a total diagnosis affecting over 28 million people) [5,6]. The total US healthcare cost for diagnosis and treatment of CVD exceeds $32 billion per year with a 50% mortality rate within 5 years of diagnosis [5,7]. Of individuals suffering with CVD, approximately 5 million people suffer heart failure (HF) [5]. Deaths from HF exceeds that of either breast or colon cancer [7]. The most common cause of HF in the Western World is ischemic heart disease fundamentally rooted in loss of functional cardiac tissue [8]. Therefore, regeneration of cardiac tissue to alleviate the underlying cause of CVD and HF is a major public health concern.
Despite medical interventional therapy (statins, beta-adrenergic blockers, angiotensin converting enzyme inhibitors, aspiring, clopidogrel, aldosterone antagonists, etc.), the prognosis of patients with ischemic heart failure remains poor [9]. Consequently, new approaches are needed to reduce mortality and morbidity of patients to account for considerable enthusiasm and interest in cell-based therapy. If cell therapy is beneficial in patients with ischemic cardiomyopathy, such benefits would likely be synergistic and complementary to those of standard therapy because mechanisms of action e.g., formation of new myocytes vs. secretion of paracrine factors limiting fibrosis, enhanced contractility, promote endogenous regeneration are completely different. These mechanisms do not involve neurohormonal pathways targeted by standard medical therapy (blockade of beta-adrenergic receptors, aldosterone antagonism, ACE inhibition, etc.). Differences in underlying mechanisms of action imply that cell therapy likely imparts benefits additive to those of standard medical therapy. Indeed, according to the latest and most authoritative meta-analysis [10], the trials of cell therapy in patients with ischemic cardiomyopathy produced beneficial effects in response to traditional medical therapy. However, salutary effects produced by stem cell therapies have been modest and variable. With a goal of full functional restoration in patients treated for heart failure, the pivotal question centers upon which direction(s) is (are) necessary to advance cellular therapy towards achieving greater returns on investment.
First-generation adult stem cell therapies focused upon single cell types, including bone marrow stem cells, mesenchymal stem cells, cardiac stem cells or similar. Paradoxically, clinical trial results were most promising in terms of improved cardiac function and viable cardiac tissue with the use of Cardiac Stem Cells (CSCs), although CPCs have not advanced as rapidly as other cell types in the clinical setting. Currently, clinical trials are gearing up for combinatory stem cell therapies, such as Mesenchymal Stem Cells (MSCs) and CSCs. Research in the use of MSCs and CSCs have also led to a new branch of potential therapy with secretome. Ongoing basic science and preclinical trials will continue to focus on enhanced stem cells, combinatory stem cells and secretome, which will lead to the next generation of clinical trials. Continued persistence, transparency in reporting, and avoidance of hyperbolic promises will be essential to drive cardiac stem cell research toward interventional therapies that provide long-term benefit.
This review is intended as an overview of progress of adult stem cell therapies toward treatment of heart failure. Use of induced pluripotent stem cells or embryonic stem cells as a means to treat heart failure will not be discussed due to the fundamental biologic differences that exist between these and adult stem cells, as well as markedly distinct challenges in clinical implementation. Assessments will focus instead upon current concerns regarding the use of adult stem cells to treat heart disease, including in private sector, which may lead to understanding mechanisms of stem cell action. Next, factors that influence clinical results for use of stem cells as a therapeutic treatment will be covered, culminating in an examination of future prospects for adult stem cells in treatment of heart failure.
II. STRATEGIC MANAGEMENT
THE MARKETPLACE
As is typical for technology and innovation, a degree of risk exists during testing and trial phases. For biotechnology, a great measure of protection and respect for life is established to minimize such risks during research development and clinical testing through internal and external regulatory bodies. Invasive stem cell therapies are regulated by the US Federal Drug Administration as a “drug” and therefore face very stringent pre-clinical studies to demonstrate safety and efficacy before approval for a Phase I Clinical Trial. The US has progressed at a steady pace towards development of stem cell therapies for treatment of heart failure. Progress of cellular therapies to treat heart disease continues to increase in clinical trials, dependent upon the cellular therapy applied [11]. With the overall population of heart disease continuing to grow and the average age of a person diagnosed with heart disease decreasing [5], responding successfully to this growing market becomes increasingly urgent.
The global market for adult stem cells is led by the US market, with an estimated $21.6B of $32.0B market share in 2013 and a projected $57.34B of $94.13B by 2018 [12]. The market is composed of broad categories including the technology (adult stem cell acquisition, production), products within the technology (cells, exosomes, mRNA etc), the application of the products (regenerative medicine, drug discovery and development, research etc), and the geographic regions (North America, South America, Asia-Pacific etc) involved with the development and implementation of the application. Within the field of stem cell therapies, adult stem cells constitute 80% of the total stem cell market. The appeal of adult stem cells is in the fact that they are multipotent and have plasticity, and different tissue types can arise from differentiation of these cells. This is in contrast to embryonic stem cells or induced pluripotent stem cells, which are pluripotent, can give rise to a greater number of tissue types that are not easily controlled during differentiation, and can potentially lead to abnormal growth and tumor formation [13–16,]. Advantages in the fundamental characteristics of adult compared to embryonic stem cells have led to rapid implementation of clinical trials with the former, whereas the latter remain in pre-clinical development.
With a large market for stem cell technology, the funding to support the research is drawn from a number of sources in the private sector (direct corporate research funds, contracted research from corporations at institutes, universities or start-up companies, and investors, through angel funding, venture capitalists, investment bankers) and the public sector (federal, state and nonprofit funding). MoneyTree™, a means to review venture capital investment activity in the United States, reports the Biotechnology sector holds approximately 13% of the investment marketplace with a Q3 2015 investment of $2.13B and Q4 2015 investment of $1.46B [17]. Historical data reported by MoneyTree™ displays an upward long-term growth trend in venture capital investment in the biotechnology sector but investment with respect to stem cell research or adult stem cell therapies in the treatment of heart failure is not reported. The private sector has consistently funded more medical and health research in the US, with a total R&D investment in 2012 for the combined pharmaceutical, biotechnology and medical technology industries at $69.17B, while the combination of federal government entities invested $41.02B in 2012 (NIH provides the greatest investment of $30.01B) [18].
Federal funding provided by the NIH for overall stem cell research can be viewed in the RePORT system [19]. Data can be obtained regarding total funding invested in heart disease or cardiovascular disease as well as regenerative medicine or stem cell research as a general search term, but specifics for NIH funding allocated to adult stem cells research for cardiovascular regeneration is not readily procured without data mining. Basic science at NIH is funded by classical research, training and fellowship grants and program project grants and the R43/R44 grant programs are specifically geared for start up funding of early stage small businesses. Small business programs are available at other federal agencies, including Department of Defense and Department of Energy but the NIH governs small business funding for biological or medical research involving the use of animals or human populations. State-level initiatives for funding adult stem cell research vary by state. California is heavily invested in stem cell research with approval of California Proposition 71 in 2004 to spend $3B in ten years, financed by state general obligation bonds and managed by the California Institute for Regenerative Medicine (CIRM). CIRM has invested over $1.95B in adult stem cell research and continues to operate for both education and research related initiatives [20]. In New York, the New York Stem Cell Foundation (NYSTEM) was founded in 2005 for basic and translational scientific research related to stem cell biology [21]. The Empire State Stem Cell Trust Fund funds NYSTEM with an initial 2007–08 appropriation of $100M with an additional $50M per year for 10 years [22]. Additional states actively funding stem cell research include, and not limited to, Connecticut, Ohio, Maryland, Massachusetts, Illinois, Minnesota, Wisconsin, Rhode Island and New Jersey, where many are members of the Interstate Alliance on Stem Cell Research [23]. Funding for early stage research business has been achieved through private or public funding but a blend of both mechanisms lends to the overall success of the business as the business transitions from fundamental R&D towards product commercialization.
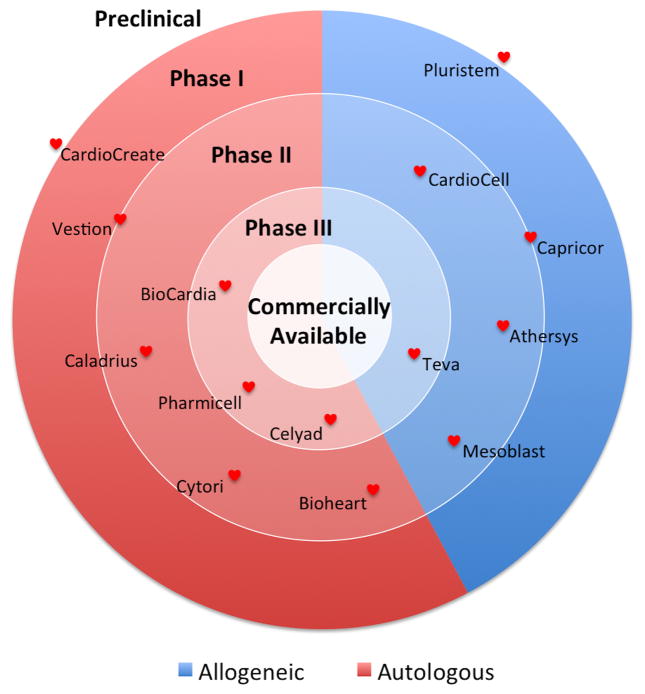
Development of adult stem cells as a therapeutic product to treat heart failure has led to a number of biotechnology start-up companies. Comparison of companies with adult stem cell therapies focused on increasing vascularization of heart tissue or building of new cardiac tissue to replace damaged cardiac tissue can be organized by various characteristics, such as the current clinical trial phase or the origin of the cell (see Figure 1 and Table 1). The Table includes a column titled “Clinical Trial” to reference recent trial(s) associated with the company based on information provided in ClinicalTrials.gov and the company website. Figure 1, in turn, reflects the company’s advancement to commercialization of its product based on the listed trial. For these and future companies to be successful in achieving FDA-approval with a cellular therapy to treat heart failure, a clear understanding of cellular mechanisms that influence incorporation into heart tissue is necessary. When we, as a research community, better understand stem cells, we will then be able to optimize clinical outcomes for patients using an approach of personalized medicine.
Figure 1. Cardiac Regenerative Medicine Product to Marketplace.
A number of companies focused on the treatment of heart failure and regenerative cardiac medicine have emerged over the past decade. At this time, no commercially available product is approved by the US FDA and commercially available in the United States. Each company is positioned based on its most current clinical status in ClinicalTrials.gov and the company website. This figure was last updated on February 1, 2016.
Table 1. Companies Focused on Adult Stem Cells to Treat Heart Disease.
Each company identified has a business model using adult stem cells focused on a therapy that either 1) increases vascularization of heart tissue or 2) builds new tissue to replace damaged heart tissue. Clinical Trials listed per company may not reflect all clinical trials sponsored by each company but provides a snapshot of the current stage of progress towards commercialization of a cellular therapy product. Each company was listed to best reflect the company’s predominant footprint with respect to a product in treating heart disease based on information obtained in ClinicalTrial.gov and each company’s website. This table was last updated on February 1, 2016.
| Company | Cell Type | Cell Origin | Clinical Phase | Clinical Trial |
|---|---|---|---|---|
| Autologous | ||||
| CardioCreate | Enhanced Cardiac Progenitor Cells | Heart | Late Preclinical | |
| Vestion | Mesenchymal Stem / Cardiac Stem Cells | Bone Marrow / Heart | Phase I/II | Pending Enrollment |
| Bioheart | Myoblasts | Skeletal Muscle | Phase II | NCT00526253 |
| Cytori | Adipose-Derived Stem Cells | Body Fat | Phase II | NCT02052427, NCT01216995 |
| Caladrius | Bone Marrow Stem Cells | Bone Marrow | Phase II | NCT01495364 |
| Pharmicell | Mesenchymal Stem Cells | Bone Marrow | Phase II, III | NCT01392105, NCT01652209 |
| BioCardia | Mesenchymal Stem Cells | Bone Marrow | Phase II, III | NCT02013674, NCT02438306 |
| Celyad | Mesenchymal Stem Cells | Bone Marrow | Phase III | NCT01768702, NCT02317458 |
| Allogeneic | ||||
| Pluristem | Placental expanded Cells | Placenta | Preclinical | |
| Capricor | Cardiospheres | Cardiac | Phase I/II | NCT02293603, NCT01458405 |
| Athersys | Bone Marrow Stem Cells | Bone Marrow | Phase II | NCT02277613 |
| CardioCell | Ischemic-Tolerant Mesenchymal Stem Cells | Bone Marrow | Phase II | NCT02467387, NCT01770613 |
| Mesoblast | Mesenchymal Progenitor Cells | Bone Marrow | Phase II | NCT01781390, NCT00877903 |
| Teva Pharmaceuticals | Mesenchymal Progenitor Cells | Bone Marrow | Phase II, III | NCT01781390, NCT02032004 |
THE PRODUCT
Stem cell therapy for cardiac repair holds great promise, but the ability of stem cells to repair damaged myocardium declines with age [24–26] and is characterized by impaired functional reserve of the endogenous stem cell pool due to exhaustion, senescence, depletion, or inability to cope with the environmental stressors [15]. Shortening of telomere length has been linked to both senescence [27] and cell death, further highlighting concerns related to aging and pathological stress. Therefore, stem cells derived from patients with advanced biological age and severe concurrent clinical features will require rejuvenation to reverse these deleterious effects of aging and disease. One means to bypass concerns with using “aged” autologous stem cells is to use “young” allogeneic stem cells. However, allogeneic stem cell use is complicated by multiple factors, including graft versus host rejection, infection, and arrhythmias, leading to poor survival and negligible persistence or engraftment [28,29]. Therefore, multiple additional considerations will need to be addressed if allogeneic stem cells are to be used as a viable therapeutic solution to improving heart function and reversing heart disease and failure.
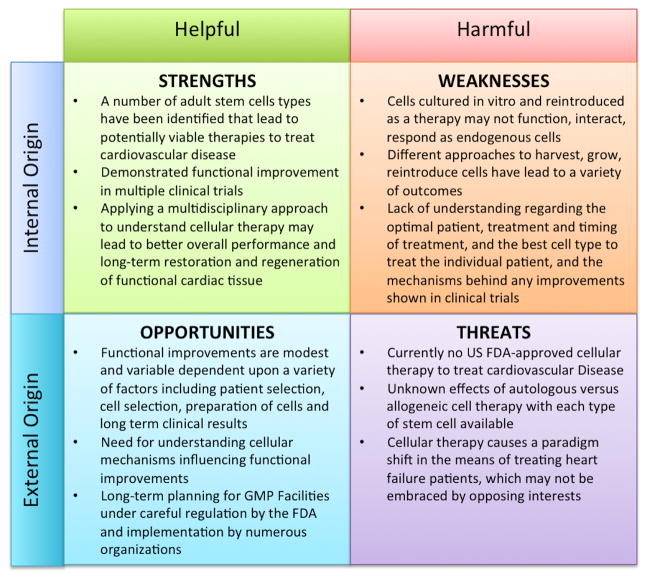
For adult stem cells to become a commercially available therapeutic product and treatment, the management and resolution regarding the uncertainty of their use as a therapeutic remains a key element. Adult stem cells have been in clinical testing since the early 2000’s and multiple strengths, such as the reduction of scar tissue and improvement cardiac output, demonstrate a potential for these cells to have a beneficial effect [30,31]. However, a number of concerns remain including: efficiency of the cells, understanding mechanisms responsible for therapeutic benefits, and understanding limitations for using stem cells as a therapeutic product and treatment. A SWOT (Strengths, Weaknesses, Opportunities and Threats) analysis shows benefits and concerns related to the use of adult stem cells, collectively, as a cellular therapy to treat heart disease (see Figure 2). In the SWOT analysis, internal origin refers to the cardiovascular community working with the adult stem cells at the basic science, preclinical and clinical trial level; external origin refers to the greater healthcare community, including the FDA, doctors, healthcare providers, insurance companies, pharmaceutical companies and the general public with a vested interest. Helpful attributes refer to the strengths and opportunities, whereas harmful attributes refer to the weakness and threats in using adult stem cells as a therapy to treat cardiovascular disease. Considering these four aspects of SWOT in evaluating the use of adult stem cells, a more strategic plan may be implemented to transition weaknesses and threats into strengths and opportunities for the treatment and elimination of heart failure.
Figure 2. SWOT Analysis of Adult Stems Cells as a Cardiovascular Therapy.
The analysis is an overall snapshot of the use of adult stems cells in the marketplace in the treatment and cure for cardiovascular disease. The analysis looks at both the internal workings of the field as well as the external origin, in terms of both helpful and harmful elements.
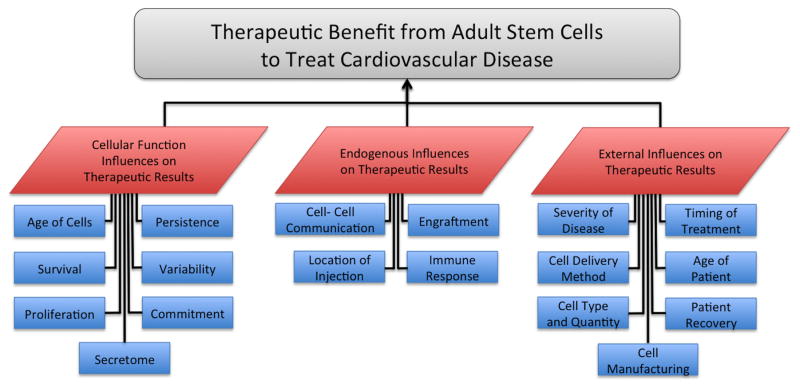
In assessing the therapeutic benefit of adult stem cells to treat cardiovascular disease, factors influencing outcome can be categorized into the stem cell attributes and function, the response of the endogenous tissue and cells post injection of the stem cells, and the external influences on the system, such as the patient and the procedure (see Figure 3). Factors influencing stem cell function include: age of the cells, persistence and survival of cells, proliferation and commitment of cells for long-term benefit, and variable homogeneity of the cells. Endogenous influences may include: location of the injection, engraftment of the injected cells, cell-cell communication of the injected cells to each other and to endogenous cells, and immune responses resulting from cell injection. External influences upon therapeutic efficacy include: severity of disease, patient age and recovery capacity, timing treatment with respect to disease onset and method of delivery, cell type and quantity used and manufacturing of the cell. Cell type plays one of the most critical roles in therapeutic success and cell biomanufacturing requires care with regard to oversight, planning, and implementation to be successful on a commercial level [32–35]. With a number of influencing factors all unique to the individual patient in dictating the success of the therapeutic treatment, use of stem cells as a therapeutic remains challenging, but possesses the potential to cure heart disease rather than merely maintain function a diseased cardiac function. As cell type is the primary factor influencing the potential therapeutic benefit, a clear understanding of various candidate cellular treatments is essential. A SWOT analysis for these individual therapies is summarized (Figure 4) and discussed in the next section.
Figure 3. Factors Influencing Therapeutic Outcomes when using Stem Cells.
Factors influencing the therapeutic benefit from stem cells to treat cardiovascular disease can be broken down into three main categories of: 1) the individual cell’s function, 2) the surrounding tissue function in response to the therapeutic cell treatment, and 3) the external influences on the cellular and tissue response towards repair and regeneration.
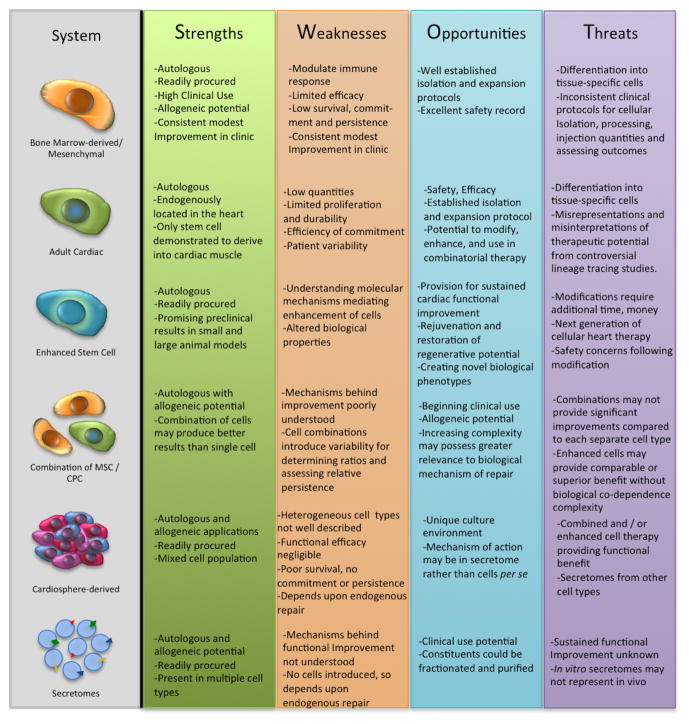
Figure 4. SWOT Analysis of Individual Adult Stems Cells as a Cardiovascular Therapy.
The analysis focus is the individual adult stem cell therapeutic treatment options. The analysis focuses on the internal workings of the individual treatment options based on current clinical trial results and ongoing pre-clinical research.
III. CURRENT STATUS OF ADULT STEM CELL THERAPIES
BONE MARROW-DERIVED / MESENCHYMAL
Bone marrow-derived stem cells (BMSCs) are composed of a diverse cell population including mononuclear cells [36], MSCs [37], hematopoietic stem cells [38], side population cells [39] and very small embryonic-like stem cells [40–43]. With this diverse population of stem cells originating in bone marrow, a wide variety of approaches have been implemented for isolation and expansion [44–46] as well as variation in number of cells injected, means of delivery, delivery post injury, type of injury, and means of follow-up screening [47–50]. Meta-analysis of BMSCs therapy from multiple clinical trials [47–53] concludes the impact is beneficial, albeit modest with a 2–5% ejection fraction improvement in the first 3 months from BMSC in patients with acute MI as well as chronic IHD despite variation in both therapeutic approach and means of analysis for results of the therapy.
Bone marrow mononuclear stem cells appeared as the first stem-cell-based clinical trial cell therapy for MI between 2002 and 2005 [54] with numerous studies initiated thereafter. In one study, improvement in global and regional myocardial function in the short term (6 months) was reported [55], while in the long term (18 months), a mixed result was reported with no significant differences in ejection fraction or improvement in diastolic function [56]. In another study using mononuclear bone marrow stem cells, no significant functional changes between 3 and 6 months post-injection in patients was found [57]. These early clinical trial results cast doubts as to whether there were any beneficial effects of bone marrow therapy for MI due to fleeting positive impact upon heart function long-term and wide-ranging efficacy [58], prompting a need for additional preclinical studies focused upon these and other stem cell treatment options [59]. One of the recent studies involves swine cortical bone stem cells (CBSCs), demonstrating increased proliferation, migration, cardiac lineage commitment, functional gap junctions and differential response to ATP and histamine stimuli as compared to MSCs or cardiac-derived stem cells [60].
As an enriched subpopulation of bone marrow-derived cells, MSCs provide functional improvement in cardiac function, both in basic research and in clinical trials. MSCs were first analyzed in a clinical trial for acute MI using an intravenous cell injection method in a dose dependent study, which provided pivotal safety and efficacy data [61]. In the clinical trial Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) (clinicaltrials.gov: NCT00587990), 2 or 20 MSCs or a placebo were used to determine therapeutic response to heart failure following a heart attack. After 9 of 45 patients enrolled, the trial was suspended due to slow accrual. Six patients received MSCs (2 low dose and 4 high dose; no placebo patients) and, at the 18 month follow-up, findings suggested increased ejection fraction, decreased scar mass and overall contractile improvement after MSCs treatment compared to baseline of these patients [61]. Additional clinical trials using MSCs include: Phase I/II, Randomized Pilot Study of the Comparative Safety and Efficacy of Transendocardial Injection of Autologous Mesenchymal Stem Cells Versus Allogeneic Mesenchymal Stem Cells in Patients with Nonischemic Dilated Cardiomyopathy (POSEIDON-DCM) (clinicaltrials.gov: NCT01392625) and The Transendocardial Stem Cell Injection Delivery Effects of Neomyogenesis Study (TRIDENT) (clinicaltrials.gov: NCT02013674). Interestingly, from the POSEIDON-DCM study and TRIDENT study, research was performed to compare EPC function after either allogeneic or autologous MSC administration in patients with HF, with results indicating allogeneic MSC administration enhances proliferation of fuctional EPCs and improved vascular reactivity [62]. Additional studies found MSC injections stimulated growth of endogenous cardiac progenitor cells (CPCs) and CSCs through cell-cell communication [63,64] and paracrine signaling [65,66], but did not demonstrate persistence to engraft in the endogenous tissue after a month [67]. A recent study has also analyzed functional affects of autologous MSCs compared to autologous cardiac-derived stem cell in a feline model after isoproterenol-induced cardiomyopathy [68]. The beneficial effects of MSCs has more recently been explained through the exocytosis of secretome, composed of cytokines, growth factors, paracrine factors microRNAs and exosomes, to protect intact tissue, prevent additional damage and support the endogenous repair of damaged tissue [69–72].
CARDIAC PROGENITOR CELLS
Myocardial repair, regeneration and functional processes in the heart are supported by CPCs localized within specific cardiac niches [73]. Intrinsic and extrinsic factors regulate CPC turnover within the niche [74], thus affecting the potential for CPC reparability in response to myocardial injury [75]. Accumulation of age-related changes [76], such as DNA damage, telomere attrition [77,78], epigenetic dysregulation, and environmental stress, impair CPC function [79]. Heart-related pathologies primarily occur in the aged population [80], along with a compromised repair capability by the endogenous CPC pool. Senescent CPCs have limited capacity to expand and generate de novo cardiomyocytes, resulting in diminished cell turnover and acceleration of myocardial aging [81]. Indeed, CPCs isolated from multiple patients exhibit variable growth kinetics, telomere length, and expression of cell cycle regulators [82,83]. In addition to effects of chronological age, disease pathogenesis as well as combined genetic and environmental factors also impact CPC function. Analysis of patient characteristics and their respective CPCs revealed fast-, medium-, and slow-growing CPCs with growth rates inversely related to expression of senescent markers [83]. Human CPCs (hCPCs) represent an attractive target cell population to employ in regenerative cell therapy, yet all samples from patients with heart failure fell short of exhibiting phenotypic characteristics comparable with fetal hCPCs, which are used as the gold standard for a healthy cardiogenic cell [83]. Nevertheless, small animal in vivo studies demonstrated regeneration of cardiac tissue post MI using human CPCs and improved cardiac performance [82,84], prompting the initiation of clinical trials.
SCIPIO in 2009 was the first CSC clinical trial to use autologous CSCs in a randomized controlled study (clinicaltrials.gov: NCT00474461). After the first year of the SCIPIO trial, 16 treatment group and 7 control group patients were pooled from a sub-population of patients who underwent elective coronary artery bypass graft (CABG) surgery post infarction and demonstrated a LVEF less than 40% at 4 months after the intervention [85]. The right atrial appendage, resected during the operation, was used for isolation and expansion of the patient’s own CSCs. In total, 20 patients were treated with CSCs and 13 patients served as the placebo group. In the treated group, 1 million autologous CPCs were infused via a balloon catheter into the graft vessel. Results indicated the control group did not experience improved average LVEF between baseline, 12 and 24 months post treatment (30% (n=12), 32% (n=12) and 32% (n=5), respectively), whereas the CSC treated patients experienced an average LVEF from 30% at baseline (n=12) to 38% (n=17) at year one and 42% by year two (n=12) post injection [85, 86]. The overall improved change in ejection fraction in the treatment group between injection and the two-year follow-up was 12%, compared to the placebo group of 3.7% [86]. Additionally, the infarct size decreased by 30% in the first year for treated patients [85], and the NYHA cardiac functional classification in patients was both downgraded from baseline to one-year post-injection and sustained two-years post-injection [86,87]. This trial was one of the most successful trials to date regarding functional improvement, over the long-term, along with structural improvement of the myocardium [88] and patient quality of life based on the MLHF Questionnaire score. This study warrants additional in-depth clinical assessment through additional studies. It also prompted an introduction to a combinatorial therapeutic approach through use of both MSCs with CPCs.
CARDIOSPHERE-DERIVED
Cells found in the heart capable of forming spherical clusters in vitro (cardiospheres-derived cells, CDCs) have also been studied for regenerative capacity [89]. In the CADUCEUS trial (clinicaltrials.gov: NCT00893360), patients with ischemic heart disease, percutaneous coronary revascularization, LVEF dysfunction (mean baseline <39%) and a myocardial infarction that is two – four weeks old were included in the patient population. CDCs were obtained from each patient through endomyocardial biopsy and were then cultured between one and two months after the biopsy until up to 25 million cells were obtained and reintroduced to each patient [90]. Scar size between placebo and treatment group from baseline to 6 months and 12 months is reported as unchanged for the placebo group (n=8), and a decrease from baseline to 6 months as 7.7% and baseline to 12 months as 12.3% for the treatment group (n=17) [90]. Additionally, regional systolic wall thickening was reported and regional contractility increased at the six months follow-up between treated versus non-treated patients. However, unlike the SCIPIO study, functional parameters of cardiac hemodynamics including left ventricular ejection fraction, end-diastolic volume, and end-systolic volume did not improve compared to untreated patients. Furthermore, NYHA classification was unchanged between the treated patient population and the untreated population, although MLHF score decreased in treated patients relative to untreated patients with increased walking distance during the 6-min walk test and peak oxygen consumption [91]. Importantly, these data indicate there are distinguishable differences between CPCs and CDCs, despite both originating in the heart. The comparatively homogeneous CPC population may be better suited for persistence that results in long-term functional cardiac improvement of the heart, whereas heterogeneous CDCs rapidly disappear from the myocardium and fail to confer long-term functional benefit. CADUCEUS results were likely also consequential to study design, including a limited patient population, the optimization of delivery method and cell dose, and the specific stem cell choice used in the study [92].
CDCs have found their way into multiple clinical trials including 1) ALCADIA where autologous CDC, from CABG patients diagnosed with ischemic heart failure, were implanted back into the patients with a biodegradable gelatin-hydrogel releasing basic fibroblast growth factor (clinicaltrials.gov: NCT00981006); 2) TICAP where patients with a single ventricular chamber were treated with autologous CDCs (clinicaltrials.gov: NCT01273857) and 3) ALLSTAR, a phase I/II randomized double-blinded study using allogeneic CDCs (clinicaltrials.gov: NCT01458405). Results from ALCADIA for five of six treated patients (one patient was excluded after acute occlusion of the graft three weeks after surgery) demonstrated mixed results (no placebo group reported), with one patient’s heart failure symptoms worsening while the other four patients experiencing an average 9% (echocardiography) and 12% (MRI) LVEF improvement between baseline and six months post treatment and a infarct size decrease by 3.3% of the total LV volume [93]. TICAP results, from fourteen infants (1.8±1.5 years) were found to have improved RVEF by an average 5.2% at three months post-treatment and at 18 months, treated patients demonstrated higher RVEF compared to non-treated patients (average 40.4% versus 31.5%) [94]. Results from the ALLSTAR trial are awaited.
COMBINATION OF STEM CELLS
Combining MSCs together with CPCs has evolved from earlier single cell trials with the rationale that a combination of cells possessing complementary effects will be better suited than any single cell type to provide enhanced functional and structural improvement within the failing heart. MSCs secrete cytokines to activate c-kit+ resident CPCs within the heart [95]. A preclinical study using a mixture of 200 million MSCs together with 1 million CSCs in swine with MI demonstrated cardiac recovery to levels near baseline [96]. LVEF, as well as LV chamber dynamics, were improved in all treatment groups and a 21.1% reduction in scar size was measured with the combinatory therapy, as compared to a 10.4% reduction in CSC or 9.9% reduction using MSCs alone. A second preclinical study involving the combination of autologous MSCs and CPCs was performed in a porcine model of Gottingen swine 3 months after ischemia/reperfusion and received transendocardial injections of MSCs alone or a combination of MSC and cardiac-derived CSC and compared to placebo [97]. Treatments with either MSCs or the combination of MSC and CSC resulted in a significant reduction in scar size, increased viable tissue, improved wall motion, improved ejection fraction and improved cardiac output 3 months after treatment. The synergistic effect of the dual cell therapy has recently been expanded into two scheduled clinical trials. One trial, CONCERT-HF (clinicaltrials.gov: NCT02501811), is a randomized placebo-controlled phase II trial, where ischemic heart failure patients will receive either 150 million MSCs, 5 million CSC, a combination of 150 million MSC with 5 million CSC or a placebo. The other is the clinical phase I/II trial TAC-HFT II (clinicaltrials.gov: NCT02503280), where idiopathic DCM patients will receive autologous transplantation of either 200 million MSCs, a mixture of 199 million MSC with 1 million CSC or a placebo. Both of these studies are in early implementation stages with study start dates of October 2015 and March 2020, respectively.
A recent variation of combinatorial cell therapy employing MSC and CPC uses saphenous vein-derived pericytes (SCPs) with CSCs [98]. In a mouse model, researchers showed combinatorial SVP / CSC treatment reduces infarct size and promotes vascular proliferation and arteriogenesis but does not demonstrate a greater contractility than that shown with the individual cellular treatments described above. Another combinatorial cell therapy approach involves the fusion of two stem cells [99] to capture favorable attributes of each cell towards the genetic design and functional characteristics resulting in improved repair and regeneration in pathophysiologic heart conditions. Fused cells have been identified to occur naturally in vivo, in particular with stem cells [100,101] and are therefore another potential approach to treating cardiomyopathies. Another next-generation combinatorial stem cells approach is to use CPCs, MSCs, and endothelial progenitor cells (EPCs) together into a cluster. This approach includes the use of EPCs, which are known to secrete autocrine, paracrine and immunomodulatory factors that mediate cellular survival, persistence and communication [102], as well as increase the vascularization within the cardiac tissue [103]. The use of these three distinct stem cell populations and their respective cellular traits is purported to enhance endogenous repair within the heart for long-term improvement [104]. This “CardioCluster” has already been shown to be efficacious in vitro and small animal models studies are currently underway.
ENHANCED STEM CELLS
Findings of poor survival, engraftment, and persistence of adult stem cells in adoptive transfer therapy have prompted investigation into various “enhancements” to create a more optimized stem cell for cardiac regeneration. Stem cells can be enhanced through preconditioning, pharmacological intervention and/or genetic modification. Preconditioning is the use of in vitro treatment of the stem cells with growth factors, hypoxic shock or anti-aging compounds to improve cellular potency. Pharmacological intervention also enhances stem cell survival, engraftment, endurance and commitment, similar to preconditioning techniques. Genetic modification enhances endurance, anti-apoptosis, survival, engraftment and commitment of the cell. Use of these different approaches has been ongoing for both MSC and CPCs.
Preconditioning stem cells through multiple signaling pathways is advantageous because it simplifies treatment and because no genetic modification is needed. MSCs factors used for preconditioning include: vascular endothelial growth factor (VEGF) [105], treatment with hypoxia [106] to induce hypoxia-inducible factor (HIF) 1α and stem cell derived factor -1 (SDF-1) [107,108], fibroblast growth factor -2 (FGF-2) [109], hepatocyte growth factor (HGF) [110], insulin growth factor (IGF) [111] and transforming growth factor alpha (TGF-α) [112]. In CPCs, preconditioning with VEGF and SDF-1 [113], connexin-43 [114], hydrogen peroxide [115], hypoxia [116], and beta-adrenergic signaling [117] have all shown beneficial effects. Preconditioning serves as a means to enhance the cells towards favorable characteristics, but preconditioning results in a transiently altered state that does not benefit the cells long-term.
Pharmacological treatment of stem cells in vitro has shown improvement in phenotypic characteristics and potential therapeutic treatment during / after injection into tissue, enhancing activation, engraftment, commitment etc. Examples of in vitro pharmacological treatment include 5-azacytidine to demethylase DNA towards stem cell differentiation, as shown in MSCs [118,119] and CPCs [120] as well as dexamethasone to induce stem cell differentiation, particularly for CPCs [121]. Combinatory pharmacological and cellular treatment to improve stem cell properties include: trimetazidine and MSCs to decrease fibrosis and improve myocardial recovery [122], simvastatin to increase systolic wall thickening and increase MSC engraftment [123], and catecholamines to stimulate proliferation of endogenous CPCs through the beta 2 adrenergic receptor signaling pathway [124].
Genetic modification to enhance stem cell function has also been reported for a number of cells including MSCs and CPCs. In MSCs, Bcl-2 has been engineered to activate survival pathways capable of suppressing hypoxia-induced apoptosis [125], VEGF and Ang1 for the promotion and formation of new blood vessels [126], survivin to increase cellular survival after introduction into the damaged tissue [127] and the stem cell homing factor SDF-1 [128]. In CPCs, genetic modified targets include nuclear AKT [129], PIM-1 [130–132], ILK [133], nucleostemin [134], notch [135] and beta-adrenergic tolerance through 6-betaARKct [136]. PIM-1, a pro-survival and proliferation gene kinase, has been used to enhance CPCs with demonstrated long-term engraftment, increased cardiac function and reduced fibrotic scar as compared to regular CPCs or placebo in both small [130] and large animal models [132]. On a molecular level, a bi-functional genetically engineered fusion protein can be created to augment engraftment and binding of the functional stem cell(s) to damaged tissue, such as modification of αCD133-GPVI to bind CD133+ progenitor cells to damaged vessels producing higher capillary density and tissue vascularization [137]. Through a variety of approaches, the use of genetic modification provides a means to amplify and enhance traits associated with therapeutic success. This advanced approach to cellular therapies may be applicable to the clinical therapies currently tried in non-modified stem cells alone.
SECRETOME BYPRODUCTS
Collectively, the secretome is the entirety of bi-product secreted by a cell, parasite or similar entity and includes both molecules that influence phenotypic behavior of cells and molecules signaling for inflammation, stress response, apoptosis, and similar responses [138]. The secretome is composed of proteins, growth factors, cytokines, chemokines, microRNAs and similar soluble factors, which together may mediate underlying MSCs therapeutic benefit, despite lack of cellular engraftment. An early account of secretome released promoting beneficial effects was reported as a byproduct of MSCs genetically modified to overexpress AKT, which reduced infarct size and cardiomyocyte apoptosis in an adult rat model, as compared to controls of non-modified MSC [139]. Another early account of the beneficial byproduct effects of MSCs was reported from release of cytokines from bone marrow stem cells promoting new vessel formation, inhibiting cardiomyocyte apoptosis, and maintaining myocardial contractility [140].
Beneficial growth factors secreted by MSCs include: vascular endothelial growth factor (VEGF), transforming growth factor (TFG)-β, secreted frizzled-related protein (SFRP)-1 and SFRP-2 [141]. Growth factors secreted from MSCs, such as VEGF, endothelin, epiregulin, Smad-5, SFRP-1 and -4 and Galectin-3, have also been reported to improve myocardial function, reduce cardiomyocytes apoptosis, reduce fibrosis in the infarct zone and preserve myocardium after seven days when injected into swine with acute MI [142]. Likewise, MSC secretome with mobilizing factors HGF, LIF, SDF-1, SCF and VE-Cadherin was purported to increase mobilization and homing of endogenous MSCs in adult DCM hamsters [143]. Numerous microRNAs have been identified to modulate cardiac repair and regeneration [144,145] and these microparticles have also been identified as immature mRNA when secreted from MSCs [146]. Additional contents of MSC exosomes include CD81, CD9 and Alix, which reduced infarct after injection in an ischemia/reperfusion injury mouse model [147].
CPCs also secrete exosomes and similar by-products that promote beneficial effects in the cardiac microenvironment to maintain and promote myocardial repair. Exosome-like vesicles containing multi-vesicular bodies (MVBs) were recently shown to be present in CPCs by ultrastructure examination [148]. CPC exosomes contain miRNAs, such as miR-451, and can reduce cardiomyocyte apoptosis by over 50% when injected in an acute mouse myocardial ischemia / reperfusion model [149]. Other microRNAs recently identified in the CPC secretome under hypoxic conditions include: miR-292, mR-210, miR-103, miR-17, miR-199a, miR-20a and miR-15b [150]; microRNA exosomes generated after twelve hours under hypoxic conditions and injected demonstrate improved fractional shortening and reduced fibrosis in an adult rat ischemia / reperfusion model [150]. Likewise, microRNA 133a improves cardiac function through reduction of fibrosis and hypertrophy as well as increasing vascularization and cardiomyocyte proliferation in a rat myocardial infarction model [151]. Despite this relatively recent flurry of excitement regarding exosome-mediated effects, the mechanistic basis remains poorly understood and the long-term benefits in terms of restoring myocardial structure and function remain to be assessed. It is hypothesized that beneficial secretome is up regulated, promoting molecular reprogramming in surrounding cells and leading to the beneficial effects. This may also explain how functional benefits may be found despite the lack of therapeutic cells present. Future studies of the stem cell secretome may help to define necessary product(s) that may contribute to prevention of heart disease, maintenance of heart function and regeneration of healthy tissue to replace damaged heart myocardium.
IV. FUTURE DIRECTIONS
The future of stem cell therapies is bright as it embodies a revolutionary form of medicine to treat disease with the potential to regenerate tissue. For cardiac therapies, adult stem cells represent the majority of cells currently under investigation for clinical trials with a more realistic potential to regulate, enhance and utilize prior to and post reintroduction. Autologous stem cells have safety advantages as the cells obtained for the therapeutic treatment originate from the patient, demonstrating use for personalized medicine. Allogeneic therapies have demonstrated a mixture of success and failure within the patient, as these cells do not originate from the patient nor maintain long-term engraftment; successes with these cells may be due to the secretome released, as more recent research is demonstrating a similar effect in viable tissue from just exosome and/or growth factor introduction as compared to allogeneic therapies. A major unresolved issue remains dependency of allogeneic or secretome-based therapies upon recruitment and activation of endogenous responses to mediate regeneration. Patient populations most likely targeted for cell therapy will be predominantly elderly and / or suffering from co-morbidities (e.g. diabetes, hypertension, smoking, obesity, etc…) that compromise stem cell activity. If the efficacy of repair hinges upon recruitment of endogenous repair, then research will inevitably need to address and augment rehabilitation and rejuvenation of impaired endogenous stem cells. Importantly, very little attention is being paid to the relationship between endogenous stem cell competency in the clinical patient population to reparative potential, which is likely to be quite different from typical experimental animal models that are relatively young and overtly healthy. So too, emerging realities of current interventional approaches that offer positive structural remodeling in the absence of functional output improvement need to be acknowledged, as well as the unresolved durability of repair based upon a single treatment intended to mediate repair for months or even years after delivery. These future frontiers will require ever-more innovative and creative solutions to truly deliver upon the promise of restoring myocardial performance with stem cell therapy. Positive results of adult stem cell-based therapy will most likely continue to be revealed in both basic science and clinical trials through increasingly effective, consistently safe, and predictably small but importantly progressive steps.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
M.A. Sussman is supported by NIH grants: R01HL067245, R37HL091102, R01HL105759, R01HL113647, R01HL117163, P01HL085577, and R01HL122525, as well as an award from the Fondation Leducq.
Nonstandard Abbreviations Acronyms
- CVD
cardiovascular disease
- CSCs
Cardiac Stem Cells
- MSCs
Mesenchymal Stem Cells
- CIRM
California Institute for Regenerative Medicine (CIRM
- NYSTEM
New York Stem Cell Foundation (NYSTEM)
- SWOT
Strengths, Weaknesses, Opportunities and Threats
- BMSCs
Bone marrow-derived stem cells
- SCPs
saphenous vein-derived pericytes
Footnotes
DISCLOSURES
M.A. Sussman is a founder and co-owner of CardioCreate Inc. K.M. Broughton has a modest interest in CardioCreate Inc.
References
- 1.Mohsin S, Siddiqi S, Collins B, Sussman MA. Empowering adult stem cells for myocardial regeneration. Circ Res. 2011;109:1415–1428. doi: 10.1161/CIRCRESAHA.111.243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naqvi N, Li M, Calvert JW, Tejada T, Lambert JP, Wu J, Kesteven SH, Holman SR, Matsuda T, Lovelock JD, Howeard WW, Iismaa SE, Chan AY, Crawford BH, Wagner MB, Martin DIK, Lefer DJ, Graham RM, Husain A. A proliferative burst during preadolescence establishes the final cardiomyocyte number. Cell. 2014;157:795–807. doi: 10.1016/j.cell.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, dos Remedios CG, Graham D, Colan S, Kühn B. Cardiomyocyte proliferation contributes to heart growth in young humans. PNAS. 2013;110:1446–1451. doi: 10.1073/pnas.1214608110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White IA, Gordon J, Balkan W, Hare JM. Sympathetic Reinnervation Is Required for Mammalian Cardiac Regeneration. Circ Res. 2015;117:990–4. doi: 10.1161/CIRCRESAHA.115.307465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 2013;127:e1–e240. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kochanek KD, Murphy SL, Anderson RN, Scott C. National vital statistics reports. Deaths: Final data for 2011. National Center for Health Statistics. 2012;63:3. http://www.cdc.gov/nchs/data_access/Vitalstatsonline.htm. [PubMed] [Google Scholar]
- 7.Titler MG, Jensen GA, Dochterman JM, Xie XJ, Kanak M, Reed D, Shever LL. Cost of hospital care for older adults with heart failure: medical, pharmaceutical, and nursing costs. Health Services Research. 2008;43:635–655. doi: 10.1111/j.1475-6773.2007.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooley DA, Reul GJ, Wukasch DC. Ischemic contracture of the heart: “stone heart”. Am J Cardiol. 1972;29:575–577. doi: 10.1016/0002-9149(72)90454-7. [DOI] [PubMed] [Google Scholar]
- 9.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 10.Fisher SA, Doree C, Mathur A, Martin-Rendon E. Meta-analysis of cell therapy trials for patients with heart failure. Circ Res. 2015;116:1361–1377. doi: 10.1161/CIRCRESAHA.116.304386. [DOI] [PubMed] [Google Scholar]
- 11.Sanganalmath SK, Bolli R. Cell therapy for heart failure a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Regenerative Medicine Market. Frost and Sullivan. 2014 MA1E-52. [Google Scholar]
- 13.Gruen L, Grabel L. Concise review: scientific and ethical roadblocks to human embryonic stem cell therapy. Stem Cells. 2006;24:2162–2169. doi: 10.1634/stemcells.2006-0105. [DOI] [PubMed] [Google Scholar]
- 14.Hentze H, Soong PL, Wang ST, Phillips BW, Putti TC, Dunn NR. Teratoma formation by human embryonic stem cells: evaluation of essential parameters for future safety studies. Stem Cell Research. 2009;2:198–210. doi: 10.1016/j.scr.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Gutierrez-Aranda I, Ramos-Mejia V, Bueno C, Munoz-Lopez M, Real P, Mácia A, Sanchez L, Ligero G, Garcia-Parez JL, Menendez P. Human induced pluripotent stem cells develop teratoma more efficiently and faster than human embryonic stem cells regardless the site of injection. Stem Cells. 2010;28:1568–1570. doi: 10.1002/stem.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blum B, Benvenisty N. The tumorigenicity of human embryonic stem cells. Advances in cancer research. 2008;100:133–158. doi: 10.1016/S0065-230X(08)00005-5. [DOI] [PubMed] [Google Scholar]
- 17.Pwcmoneytree.com. [Accessed January 20, 2016];Current Quarter Data by Industry. 2016 Available at https://www.pwcmoneytree.com/CurrentQuarter/ByIndustry.
- 18.Katz AM. Truth and Consequences: Health R&D Spending in the US (FY11–12) 2014. [Google Scholar]
- 19.Report.nih.gov. [Accessed January 20, 2016];NIH Categorical Spending – NIH Research Portfolio Online Reporting Tools (RePORT) 2016 Available at: https://report.nih.gov/categorical_spending.aspx.
- 20.California’s Stem Cell Agency. [Accessed January 20, 2016];Adult Stem Cell CIRM Grants. 2016 Available at https://www.cirm.ca.gov/grants?field_public_web_stem_cell_use_tid%5B%5D=986.
- 21.Stemcell.ny.gov. [Accessed January 20, 2016];NYSTEM. 2016 Available at: http://www.stemcell.ny.gov/
- 22.New York State Finance Law. 2007. Empire State Stem Cell Trust Fund; p. Article 6 §99. [Google Scholar]
- 23.Nas-sites.org. [Accessed January 20, 2016];IASCR Participants / Interstate Alliance on Stem Cell Research. 2016 Available at: http://nas-sites.org/iascr/about-iascr/iascr-participants/
- 24.Cesselli D, Beltrami AP, D’Aurizio F, Marcon P, Bergamin N, Toffoletto B, Pandolfi M, Puppato E, Marino L, Signore S, Livi U, Verardo R, Piazza S, Marchionni L, Fiorini C, Schneider C, Hosoda T, Rota M, Kajstura J, Anversa P, Beltrami CA, Leri A. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol. 2011;179:349–66. doi: 10.1016/j.ajpath.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capogrossi MC. Cardiac stem cells fail with aging: a new mechanism for the age-dependent decline in cardiac function. Circ Res. 2004;94:411–413. doi: 10.1161/01.RES.0000122070.37999.1B. [DOI] [PubMed] [Google Scholar]
- 26.Dimmeler S, Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102:1319–1330. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fyhrquist F, Saijonmaa O, Strandberg T. The roles of senescence and telomere shortening in cardiovascular disease. Nat Rev Cardiol. 2013;10:274–283. doi: 10.1038/nrcardio.2013.30. [DOI] [PubMed] [Google Scholar]
- 28.Mohty B, Mohty M. Long-term complications and side effects after allogeneic hematopoietic stem cell transplantation: an update. Blood Cancer J. 2011;1:e16. doi: 10.1038/bcj.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rovó A, Tichelli A. Cardiovascular complications in long-term survivors after allogeneic hematopoietic stem cell transplantation. Seminars in hematology. 2012;49:25–34. doi: 10.1053/j.seminhematol.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 31.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. PNAS. 2005;102:3766–3771. doi: 10.1073/pnas.0405957102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Halme DG, Kessler DA. FDA regulation of stem-cell–based therapies. N Engl J Med. 2006;355:1730–1735. doi: 10.1056/NEJMhpr063086. [DOI] [PubMed] [Google Scholar]
- 33.Burger SR. Commercial Manufacturing of Cell Therapy Products–Considering the Options. World Stem Cell Report: Genetics Policy Institute. 2009:149–153. [Google Scholar]
- 34.Knoepfler PS. From bench to FDA to bedside: US regulatory trends for new stem cell therapies. Advanced drug delivery reviews. 2015;82:192–196. doi: 10.1016/j.addr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee MH, Au P, Hyde J, Johnson CG, Heidaran M, Karandish S, Boxer L, Mendicino M, Yoon D, Tull L, Arcidiacono J, McCright B, Kaplan DS, Fink D, Durfor CN, McFarland R, Witten C. Translation of Regenerative Medicine Products Into the Clinic in the United States: FDA Perspective. Translational Regenerative Medicine. 2015 [Google Scholar]
- 36.Ellis WM, Georgiou GM, Roberton DM, Johnson GR. The use of discontinuous Percoll gradients to separate populations of cells from human bone marrow and peripheral blood. J Immunol Methods. 1984;66:9–16. doi: 10.1016/0022-1759(84)90242-4. [DOI] [PubMed] [Google Scholar]
- 37.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 38.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 39.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183:1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, et al. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 41.Zuba-Surma EK, Kucia M, Abdel-Latif A, Dawn B, Hall B, Singh R, et al. Morphological characterization of very small embryonic-like stem cells (VSELs) by Image Stream system analysis. J Cell Mol Med. 2008;12:292–303. doi: 10.1111/j.1582-4934.2007.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zuba-Surma EK, Kucia M, Dawn B, Guo Y, Ratajczak MZ, Bolli R. Bone marrow-derived pluripotent very small embryonic-like stem cells (VSELs) are mobilized after acute myocardial infarction. J Mol Cell Cardiol. 2008;44:865–873. doi: 10.1016/j.yjmcc.2008.02.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuba-Surma EK, Wojakowski W, Ratajczak MZ, Dawn B. Very small embryonic-like stem cells: biology and therapeutic potential for heart repair. Antioxid Redox Signal. 2011;15:1821–1834. doi: 10.1089/ars.2010.3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seeger FH, Tonn T, Krzossok N, Zeiher AM, Dimmeler S. Cell isolation procedures matter: a comparison of different isolation protocols of bone marrow mononuclear cells used for cell therapy in patients with acute myocardial infarction. Eur Heart J. 2007;28:766–772. doi: 10.1093/eurheartj/ehl509. [DOI] [PubMed] [Google Scholar]
- 45.Dawn B, Bolli R. Bone marrow for cardiac repair: the importance of characterizing the phenotype and function of injected cells. Eur Heart J. 2007;28:651–652. doi: 10.1093/eurheartj/ehm009. [DOI] [PubMed] [Google Scholar]
- 46.Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, et al. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–997. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 48.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, et al. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 49.Hristov M, Heussen N, Schober A, Weber C. Intracoronary infusion of autologous bone marrow cells and left ventricular function after acute myocardial infarction: a meta-analysis. J Cell Mol Med. 2006;10:727–733. doi: 10.1111/j.1582-4934.2006.tb00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenzweig A. Cardiac cell therapy– mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 51.Martin-Rendon E, Brunskill SJ, Hyde CJ, Stanworth SJ, Mathur A, Watt SM. Autologous bone marrow stem cells to treat acute myocardial infarction: a systematic review. Eur Heart J. 2008;29:1807–1818. doi: 10.1093/eurheartj/ehn220. [DOI] [PubMed] [Google Scholar]
- 52.Zhang SN, Sun AJ, Ge JB, Yao K, Huang ZY, Wang KQ, et al. Intracoronary autologous bone marrow stem cells transfer for patients with acute myocardial infarction: a meta-analysis of randomised controlled trials. Int J Cardiol. 2009;136:178–185. doi: 10.1016/j.ijcard.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 53.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Gulherme VS, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopouos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD for the Cardiovascular Cell Therapy Research Network (CCTRN) Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: the FOCUS-CCTRN trial. Jama. 2012;307(16):1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, et al. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 55.Meyer GP, Wollert KC, Lotz J, Steffens J, Lippolt P, Fichtner S, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months’ follow- up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006;113:1287–1294. doi: 10.1161/CIRCULATIONAHA.105.575118. [DOI] [PubMed] [Google Scholar]
- 56.Ruan W, Pan CZ, Huang GQ, Li YL, Ge JB, Shu XH. Assessment of left ventricular segmental function after autologous bone marrow stem cells transplantation in patients with acute myocardial infarction by tissue tracking and strain imaging. Chin Med J (Engl) 2005;118:1175–1181. [PubMed] [Google Scholar]
- 57.Wollert KC, Drexler H. Cell therapy for the treatment of coronary heart disease: a critical appraisal. Nature Reviews Cardiology. 2010;7:204–215. doi: 10.1038/nrcardio.2010.1. [DOI] [PubMed] [Google Scholar]
- 58.Chavakis E, Koyanagi M, Dimmeler S. Enhancing the outcome of cell therapy for cardiac repair: progress from bench to bedside and back. Circulation. 2010;121:325–335. doi: 10.1161/CIRCULATIONAHA.109.901405. [DOI] [PubMed] [Google Scholar]
- 59.Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, Gerstenblith G, DeMaria AN, Denktas AE, Gammon RS, Hermiller JB, Reisman MA, Schaer GL, Sherman W. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. JACC. 2009;54:2277–2286. doi: 10.1016/j.jacc.2009.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohsin S, Troupes CD, Starosta T, Sharp TE, Agra EJ, Smith S, Duran JM, Zalavadia N, Zhou Y, Kubo H, Berretta RM. Unique Features of Cortical Bone Stem Cells Associated With Repair of the Injured Heart. Circ Res. 2015;117:1024–33. doi: 10.1161/CIRCRESAHA.115.307362. [DOI] [PubMed] [Google Scholar]
- 61.Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Kaas TZ, Brynes J, Lowery M, Heldman AW, Hare JM. Autologous Mesenchymal Stem Cells Produce Concordant Improvements in Regional Function, Tissue Perfusion, and Fibrotic Burden When Administered to Patients Undergoing Coronary Artery Bypass Grafting The Prospective Randomized Study of Mesenchymal Stem Cell Therapy in Patients Undergoing Cardiac Surgery (PROMETHEUS) Trial. Circ Res. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, Dermarkarian CR, DiFede DL, Balkan W, Khan A, Hare JM. Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine. 2015;2:467–75. doi: 10.1016/j.ebiom.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mazhari R, Hare JM. Mechanisms of action of mesenchymal stem cells in cardiac repair: potential influences on the cardiac stem cell niche. Nature Clinical Practice Cardiovascular Medicine. 2007;4:S21–S26. doi: 10.1038/ncpcardio0770. [DOI] [PubMed] [Google Scholar]
- 64.Loffredo FS, Steinhauser ML, Gannon J, Lee RT. Bone marrow-derived cell therapy stimulates endogenous cardiomyocyte progenitors and promotes cardiac repair. Cell Stem Cell. 2011;8:389–398. doi: 10.1016/j.stem.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee RH, Pulin AA, Seo MJ, Kota DJ, Yiostalo J, Larson BL, Stemprun-Prieto L, Delafontaine P, Prockop DJ. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nature Medicine. 2005;11:367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 67.Han J, Kim B, Shin JY, Ryu S, Noh M, Woo J, Park JS, Lee Y, Lee N, Hyeon T, Choi D, Kim BS. Iron Oxide Nanoparticle-Mediated Development of Cellular Gap Junction Crosstalk to Improve Mesenchymal Stem Cells’ Therapeutic Efficacy for Myocardial Infarction. ACS nano. 2015;9:2805–2819. doi: 10.1021/nn506732n. [DOI] [PubMed] [Google Scholar]
- 68.Taghavi S, Sharp TE, Duran JM, Makarewich CA, Berretta RM, Starosta T, Kubo H, Barbe M, Houser SR. Autologous c-Kit+ Mesenchymal Stem Cell Injections Provide Superior Therapeutic Benefit as Compared to c-Kit+ Cardiac-Derived Stem Cells in a Feline Model of Isoproterenol-Induced Cardiomyopathy. Clinical and translational science. 2015 Feb 1; doi: 10.1111/cts.12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Phys—Heart Circ Phys. 2006;291:H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 70.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 71.Huang L, Ma W, Ma Y, Feng D, Chen H, Cai B. Exosomes in mesenchymal stem cells, a new therapeutic strategy for cardiovascular diseases? Int J Biol Sci. 2015;11:238–245. doi: 10.7150/ijbs.10725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gallina C, Turinetto V, Giachino C. A New Paradigm in Cardiac Regeneration: The Mesenchymal Stem Cell Secretome. Stem Cells Int. 2015 doi: 10.1155/2015/765846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frati C, Savi M, Graiani G, Lagrasta C, Cavalli S, Prezioso L, Rossetti P, Mangiaracina C, Ferraro F, Madeddu D, Musso E, Stilli D, Rossini A, Falco A, Angelis AD, Rossi F, Urbanek K, Leri A, Kajstura J, Anversa P, Quaini E, Quaini F. Resident cardiac stem cells. Curr Pharm Des. 2011;17:3252–3257. doi: 10.2174/138161211797904181. [DOI] [PubMed] [Google Scholar]
- 74.De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H, Hosoda T, Rota M, Urbanek K, Kajstura J, Leri A, Rossi F, Anversa P. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation. 2010;121:276–292. doi: 10.1161/CIRCULATIONAHA.109.895771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cesselli D, Beltrami AP, D’Aurizio F, Marcon P, Bergamin N, Toffoletto B, Pandolfi M, Puppato E, Marino L, Signore S, Livi U, Verardo R, Piazza S, Marchionni L, Fiorini C, Schneider C, Hosoda T, Rota M, Kajstura J, Anversa P, Beltrami CA, Leri A. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol. 2011;179:349–66. doi: 10.1016/j.ajpath.2011.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 77.Leri A, Franco S, Zacheo A, Barlucchi L, Chimenti S, Limana F, Nadal-Ginard B, Kajstura J, Anversa P, Blasco MA. Ablation of telomerase and telomere loss leads to cardiac dilatation and heart failure associated with p53 upregulation. EMBO J. 2003;22:131–139. doi: 10.1093/emboj/cdg013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leri A, Barlucchi L, Limana F, Deptala A, Darzynkiewicz Z, Hintze TH, Kajstura J, Nadal-Ginard B, Anversa P. Telomerase expression and activity are coupled with myocyte proliferation and preservation of telomeric length in the failing heart. Proc Natl Acad Sci USA. 2001;98:8626–8631. doi: 10.1073/pnas.151013298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yao EH, Yu Y, Fukuda N. Oxidative stress on progenitor and stem cells in cardiovascular diseases. Current pharmaceutical biotechnology. 2006;7:101–108. doi: 10.2174/138920106776597685. [DOI] [PubMed] [Google Scholar]
- 80.Lakatta EG. Cardiovascular regulatory mechanisms in advanced age. Physiol Rev. 1993;73:413–467. doi: 10.1152/physrev.1993.73.2.413. [DOI] [PubMed] [Google Scholar]
- 81.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res. 2003;93:604–613. doi: 10.1161/01.RES.0000093985.76901.AF. [DOI] [PubMed] [Google Scholar]
- 82.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, Sussman MA. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol. 2012;60:1278–1287. doi: 10.1016/j.jacc.2012.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mohsin S, Khan M, Nguyen J, Alkatib M, Siddiqi S, Hariharan N, Wallach K, Monsanto M, Gude N, Dembitsky W, Sussman MA. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ Res. 2013;113:1169–1179. doi: 10.1161/CIRCRESAHA.113.302302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hosoda T, D’Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas E, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano Sm, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, Leri A. Clonality of mouse and human cardiomyogenesis in vivo. PNAS. 2009;106:17169–17174. doi: 10.1073/pnas.0903089106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaugheter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Bolli R, Chugh AR, D’Amario D, Loughran JH, Stoddard MF, Ikram S, Wagner SG, Beache GM, Leri A, Hosoda T, Goichberg P, Fiorini C. Solankhi NK, Fahsah I, Elmore JB, Rokosh DG, Slaugheter MS, Kajstura J, Anversa P. Effect of cardiac stem cells in patients with ischemic cardiomyopathy: interim results of the SCIPIO trial up to 2 years after therapy. Circulation. 2012;126:2784. [Google Scholar]
- 87.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation. 2012;126:S54–64. doi: 10.1161/CIRCULATIONAHA.112.092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayashi E, Hosoda T. Therapeutic application of cardiac stem cells and other cell types. BioMed Res Int. 2013:Article ID 736815. doi: 10.1155/2013/736815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham R, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 90.Makkar RR, Smith RR, Cheng KE, Malliaras K, Thomson L, Berman D, Czer L, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. The Lancet. 2012;379:895–904. doi: 10.1016/S0140-6736(12)60195-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xie Y, Ibrahim A, Cheng K, Wu Z, Liang W, Malliaras K, Sun B, Liu W, Shen D, Cho HC, Li T, Lu L, Lu G, Marbán E. Importance of Cell-Cell Contact in the Therapeutic Benefits of Cardiosphere-Derived Cells. Stem Cells. 2014;32:2397–2406. doi: 10.1002/stem.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Schulman IH, Hare JM. Key developments in stem cell therapy in cardiology. Regenerative medicine. 2012;7:17–24. doi: 10.2217/rme.12.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakata M, Ogata T, Nakamura T, Matoba S, Gojo S, Sawada T, Yaku H, Matsubara H. The ALCADIA (AutoLogous Human CArdiac-Derived Stem Cell To Treat Ischemic cArdiomyopathy) Trial. 2012. [Google Scholar]
- 94.Ishigami S, Ohtsuki S, Tarui S, Ousaka D, Eitoku T, Kondo M, Okuyama M, Kobayashi J, Baba K, Arai S, Kawabata T, Yoshizumi K, Tateishi A, Kuroko Y, Iwasaki T, Sato S, Kasahara S, Sano S, Oh H. Intracoronary Autologous Cardiac Progenitor Cell Transfer in Patients With Hypoplastic Left Heart Syndrome The TICAP Prospective Phase 1 Controlled Trial. Circulation research. 2015;116:653–664. doi: 10.1161/CIRCRESAHA.116.304671. [DOI] [PubMed] [Google Scholar]
- 95.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and restore cardiac function after myocardial infarction. Circulation. 2012;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales A, Hatzistergos K, Natsumeda M, Margitich I, Schulman IH, Gomes SA, Mushtaq M, DiFede DL, Fishman JE, Pattany P, Zambrano JP, Heldman AW, Hare JM. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. JACC. 2015;66:1990–9. doi: 10.1016/j.jacc.2015.08.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, Oikawa A, Rodriguez-Arabaolaza I, Dang Z, Mitchell K, Reni C, Alvino VV, Rowlinson J, Livi U, Cesselli D, Angelini G, Emanueli C, Beltrami AP, Madeddu P. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116:e81–e94. doi: 10.1161/CIRCRESAHA.115.306146. [DOI] [PubMed] [Google Scholar]
- 99.Quijada P, Salunga HT, Hariharan N, Cubillo JD, El-Sayed FG, Moshref M, Bala KM, Emathinger JM, De La Torre A, Ormachea L, Alvarez R, Jr, Gude NA, Sussman MA. Cardiac stem cell hybrids enhance myocardial repair. Circ Res. 2015;117:695–706. doi: 10.1161/CIRCRESAHA.115.306838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang S, Wang D, Estrov Z, Raj S, Willerson JT, Yeh ET. Both cell fusion and transdifferentiation account for the transformation of human peripheral blood CD34-positive cells into cardiomyocytes in vivo. Circulation. 2004;110:3803–3807. doi: 10.1161/01.CIR.0000150796.18473.8E. [DOI] [PubMed] [Google Scholar]
- 101.Zhu W, Bender I, Yellamilli A, van Berlo J. Activation of Cardiogenic Fate of C-kit+ Cardiac Progenitor Cells in Doxorubicin Induced Cardiotoxicity. FASEB J. 2015;29(1 Suppl):670–2. [Google Scholar]
- 102.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. JMCC. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 103.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 104.Monsanto M, Mohsin S, Fisher K, Sussman M. Enhanced Myocardial Repair with CardioClusters. Circ Res. 2013;113:E159–E159. [Google Scholar]
- 105.Deuse T, Peter C, Fedak PW, Doyle T, Reichenspurner H, Zimmermann WH, Schrepfer S. Hepatocyte growth factor or vascular endothelial growth factor gene transfer maximizes mesenchymal stem cell–based myocardial salvage after acute myocardial infarction. Circulation. 2009;120:S247–S254. doi: 10.1161/CIRCULATIONAHA.108.843680. [DOI] [PubMed] [Google Scholar]
- 106.Hu X, Yu SP, Fraser JL, Lu Z, Ogle ME, Wang JA, Wei L. Transplantation of hypoxia-preconditioned mesenchymal stem cells improves infarcted heart function via enhanced survival of implanted cells and angiogenesis. J Thorac Cardiovasc Surg. 2008;135:799–808. doi: 10.1016/j.jtcvs.2007.07.071. [DOI] [PubMed] [Google Scholar]
- 107.Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: A role for AKT and hypoxia-inducible factor (HIF) 1α. J Cell Physiol. 2008;216:708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- 108.Zhang M, Mal N, Kiedrowski M, Chacko M, Askari AT, Popovic ZB, Koc ON, Penn MS. SDF-1 expression by mesenchymal stem cells results in trophic support of cardiac myocytes after myocardial infarction. FASEB J. 2007;21:3197–3207. doi: 10.1096/fj.06-6558com. [DOI] [PubMed] [Google Scholar]
- 109.Hahn JY, Cho HJ, Kang HJ, Kim TS, Kim MH, Chung JH, Bae JW, Oh BH, Park YB, Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. JACC. 2008;51:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 110.Guo Y, He J, Wu J, Yang L, Dai S, Tan X, Liang L. Locally overexpressing hepatocyte growth factor prevents post-ischemic heart failure by inhibition of apoptosis via calcineurin-mediated pathway and angiogenesis. Archives of medical research. 2008;39:179–188. doi: 10.1016/j.arcmed.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 111.Khan M, Akhtar S, Mohsin S, Khan SN, Riazuddin S. Growth factor preconditioning increases the function of diabetes-impaired mesenchymal stem cells. Stem cells and development. 2010;20:67–75. doi: 10.1089/scd.2009.0397. [DOI] [PubMed] [Google Scholar]
- 112.Herrmann JL, Wang Y, Abarbanell AM, Weil BR, Tan J, Meldrum DR. Preconditioning mesenchymal stem cells with transforming growth factor-alpha improves mesenchymal stem cell-mediated cardioprotection. Shock. 2010;33:24–30. doi: 10.1097/SHK.0b013e3181b7d137. [DOI] [PubMed] [Google Scholar]
- 113.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY. 2VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovasc Res. 2011;91:402–411. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lu G, Haider HK, Jiang S, Ashraf M. Sca-1+ stem cell survival and engraftment in the infarcted heart dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pendergrass KD, Boopathy AV, Seshadri G, Maiellaro-Rafferty K, Che PL, Brown ME, Davis ME. Acute preconditioning of cardiac progenitor cells with hydrogen peroxide enhances angiogenic pathways following ischemia-reperfusion injury. Stem cells and development. 2013;22:2414–2424. doi: 10.1089/scd.2012.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yan F, Yao Y, Chen L, Li Y, Sheng Z, Ma G. Hypoxic preconditioning improves survival of cardiac progenitor cells: role of stromal cell derived factor-1alpha-CXCR4 axis. PloS one. 2012;7:e37948. doi: 10.1371/journal.pone.0037948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Khan M, Mohsin S, Avitabile D, Nguyen J, Gude N, Sussman MA. {beta}-adrenergic Signaling Promotes Survival and Proliferation of Mouse Cardiac Progenitor Cells Prior to Lineage Commitment. Circulation. 2012;126:A13590. [Google Scholar]
- 118.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Invest. 1999;103:697–705. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ye NS, Chen J, Luo GA, Zhang RL, Zhao YF, Wang YM. Proteomic profiling of rat bone marrow mesenchymal stem cells induced by 5-azacytidine. Stem Cells Dev. 2006;15:665–676. doi: 10.1089/scd.2006.15.665. [DOI] [PubMed] [Google Scholar]
- 120.van Vliet P, Roccio M, Smits AM, van Oorschot AA, Metz CH, van Veen TA, Sluijter JP, Doevendans PA, Goumans MJ. Progenitor cells isolated from the human heart: a potential cell source for regenerative therapy. Neth Heart J. 2008;16:163–169. doi: 10.1007/BF03086138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Leri A, Kajstura J, Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Reviews. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 122.Wisel S, Khan M, Kuppusamy ML, Mohan IK, Chacko SM, Rivera BK, Sun BC, Hideg K, Kuppusamy P. Pharmacological preconditioning of mesenchymal stem cells with trimetazidine (1-[2, 3, 4-trimethoxybenzyl] piperazine) protects hypoxic cells against oxidative stress and enhances recovery of myocardial function in infarcted heart through Bcl-2 expression. J Pharm and Exp Ther. 2009;329:543–550. doi: 10.1124/jpet.109.150839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang YJ, Qian HY, Huang J, Li JJ, Gao RL, Dou KF, Geng YJ. Combined therapy with simvastatin and bone marrow–derived mesenchymal stem cells increases benefits in infarcted swine hearts. Arterioscl Throm, Vas. 2009;29:2076–2082. doi: 10.1161/ATVBAHA.109.189662. [DOI] [PubMed] [Google Scholar]
- 124.Ellison GM, Torella D, Karakikes I, Purushothaman S, Curcio A, Gasparri C, Indolifi C, Cable NT, Goldspink DF, Nadal-Ginard B. Acute β-adrenergic overload produces myocyte damage through calcium leakage from the ryanodine receptor 2 but spares cardiac stem cells. J Biol Chem. 2007;282:11397–11409. doi: 10.1074/jbc.M607391200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Li W, Ma N, Ong LL, Nesselmann C, Klopsch C, Ladilov Y, Furlani D, Piechaczek C, Moebius JM, Lutzow K, Lendlein A, Stamm C, Li RK, Steinhoff G. Bcl-2 engineered MSCs inhibited apoptosis and improved heart function. Stem cells. 2007;25:2118–2127. doi: 10.1634/stemcells.2006-0771. [DOI] [PubMed] [Google Scholar]
- 126.Shujia J, Haider HK, Idris NM, Lu G, Ashraf M. Stable therapeutic effects of mesenchymal stem cell-based multiple gene delivery for cardiac repair. Cardiovasc Res. 2008;77:525–533. doi: 10.1093/cvr/cvm077. [DOI] [PubMed] [Google Scholar]
- 127.Fan L, Lin C, Zhuo S, Chen L, Liu N, Luo Y, Fang J, Huang Z, Lin Y, Chen J. Transplantation with survivin-engineered mesenchymal stem cells results in better prognosis in a rat model of myocardial infarction. Eur J Heart Fail. 2009;11:1023–1030. doi: 10.1093/eurjhf/hfp135. [DOI] [PubMed] [Google Scholar]
- 128.Tang J, Wang J, Guo L, Kong X, Yang J, Zheng F, Zhang L, Huang Y. Mesenchymal stem cells modified with stromal cell-derived factor 1α improve cardiac remodeling via paracrine activation of hepatocyte growth factor in a rat model of myocardial infarction. Mol Cells. 2010;29:9–19. doi: 10.1007/s10059-010-0001-7. [DOI] [PubMed] [Google Scholar]
- 129.Fischer KM, Din S, Gude N, Konstandin MH, Wu W, Quijada P, Sussman MA. Cardiac progenitor cell commitment is inhibited by nuclear Akt expression. Circ Res. 2010;108:960–970. doi: 10.1161/CIRCRESAHA.110.237156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fischer KM, Cottage CT, Wu W, Din S, Gude NA, Avitabile D, Quijada P, Collins BL, Fransioli J, Sussman MA. Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation. 2009;120:2077–2087. doi: 10.1161/CIRCULATIONAHA.109.884403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mohsin S, Khan M, Nguyen J, Alkatib M, Siddiqi S, Hariharan N, Wallach K, Monsanto M, Gude N, Sussman MA. Rejuvenation of human cardiac progenitor cells with Pim-1 kinase. Circ Res. 2013;113:1169–1179. doi: 10.1161/CIRCRESAHA.113.302302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Karantalis V, Suncion VY, McCall F, Wang B, Bagno L, Golpanian S, Wolf A, Williams A, Rodriguez J, Valdes D, Rosado M, Mohsin S, Sussman M, Da Silva J, Morales A, Hare J. Pim1 Kinase Overexpression Enhances ckit+ Cardiac Stem Cells Cardioreparative Ability After Intramyocardial Delivery in a Swine Model. Circulation. 2014;130:A18046–A18046. [Google Scholar]
- 133.Ling L, Bai J, Gu R, Jiang C, Li R, Kang L, Ferro A, Xu B. Sca-1+ cardiac progenitor cell therapy with cells overexpressing integrin-linked kinase improves cardiac function after myocardial infarction. Transplantation. 2013;95(10):1187–1196. doi: 10.1097/TP.0b013e31828a9423. [DOI] [PubMed] [Google Scholar]
- 134.Hariharan N, Quijada P, Mohsin S, Joyo A, Samse K, Monsanto M, De La Torre A, Avitabile D, Omachea L, McGregor MJ, Tsai EJ, Sussman MA. Nucleostemin rejuvenates cardiac progenitor cells and antagonizes myocardial aging. JACC. 2015;65(2):133–147. doi: 10.1016/j.jacc.2014.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gude N, Joyo E, Toko H, Quijada P, Villanueva M, Hariharan N, Sacchi V, Truffa S, Joyo A, Voelkers M, Alvarez R, Sussman MA. Notch activation enhances lineage commitment and protective signaling in cardiac progenitor cells. Basic Res Cardiol. 2015;110(3):1–15. doi: 10.1007/s00395-015-0488-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Khan M, Mohsin S, Toko H, Alkatib M, Nguyen J, Truffa S, Gude N, Chuprun K, Tilley DG, Koch WJ, Sussman MA. Cardiac progenitor cells engineered with βARKct have enhanced β-adrenergic tolerance. Mol Ther. 2014;22(1):178–185. doi: 10.1038/mt.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baumer Y, Leder C, Ziegler M, Schönberger T, Ochmann C, Perk A, Degen H, Schmid-Horch B, Elvers M, Munch G, Ungerer M, Scholsshauer B, Gawaz M. The recombinant bifunctional protein αCD133–GPVI promotes repair of the infarcted myocardium in mice. J Thromb Haemost. 2012;10(6):1152–1164. doi: 10.1111/j.1538-7836.2012.04710.x. [DOI] [PubMed] [Google Scholar]
- 138.Stastna M, Van Eyk JE. Investigating the Secretome Lessons About the Cells That Comprise the Heart. Circulation: Cardiovasc Gen. 2012;5(1):o8–o18. doi: 10.1161/CIRCGENETICS.111.960187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med. 2005;11(4):367–368. doi: 10.1038/nm0405-367. [DOI] [PubMed] [Google Scholar]
- 140.Takahashi M, Li TS, Suzuki R, Kobayashi T, Ito H, Ikeda Y, Matsuzaki M, Hamano K. Cytokines produced by bone marrow cells can contribute to functional improvement of the infarcted heart by protecting cardiomyocytes from ischemic injury. Am J Physiol Heart Circ Physiol. 2006;291(2):H886–H893. doi: 10.1152/ajpheart.00142.2006. [DOI] [PubMed] [Google Scholar]
- 141.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103(11):1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Nguyen BK, Maltais S, Perrault LP, Tanguay JF, Tardif JC, Stevens LM, Borie M, Harel F, Mansour S, Noiseux N. Improved function and myocardial repair of infarcted heart by intracoronary injection of mesenchymal stem cell-derived growth factors. J Cardiovasc Transl Res. 2010;3(5):547–558. doi: 10.1007/s12265-010-9171-0. [DOI] [PubMed] [Google Scholar]
- 143.Shabbir A, Zisa D, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888–H1897. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wen Z, Zheng S, Zhou C, Yuan W, Wang J, Wang T. Bone marrow mesenchymal stem cells for post-myocardial infarction cardiac repair: microRNAs as novel regulators. J Cell Mol Med. 2012;16(4):657–671. doi: 10.1111/j.1582-4934.2011.01471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sluijter JP. MicroRNAs in cardiovascular regenerative medicine: directing tissue repair and cellular differentiation. ISRN Vascular Medicine. 2013 [Google Scholar]
- 146.Chen TS, Lai RC, Lee MM, Choo ABH, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38(1):215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lai RC, Arslan F, Lee MM, Sze NSK, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, deKleijn DP, Lim SK. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4(3):214–222. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 148.Sahoo S, Losordo DW. Exosomes and cardiac repair after myocardial infarction. Circ Res. 2014;114(2):333–344. doi: 10.1161/CIRCRESAHA.114.300639. [DOI] [PubMed] [Google Scholar]
- 149.Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y. Cardiac progenitor-derived exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun. 2013;431(3):566–571. doi: 10.1016/j.bbrc.2013.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res. 2015;116(2):255–263. doi: 10.1161/CIRCRESAHA.116.304360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Izarra A, Moscoso I, Levent E, Cañón S, Cerrada I, Díez-Juan A, Blanca V, Nunez-Gil IJ, Valiente I, Ruiz-Sauri A, Sepulveda P, Tiburcy M, Zimmermann WH, Bernad A. miR-133a enhances the protective capacity of cardiac progenitor cells after myocardial infarction. Stem Cell Reports. 2014;3(6):1029–1042. doi: 10.1016/j.stemcr.2014.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.